Abstract
There are many of aspects of “mothering” that may provide information to the mammalian infant about environmental conditions during critical periods of development. One essential element of mothering involves the quantity and quality of milk that mothers provide for their infants, but little is known about the consequences of variation in milk production. Mother’s milk may affect infant behavior by contributing to brain development and to the development of behavioral dispositions. Here we present the first evidence for any mammal that natural variation in available milk energy (AME) from the mother is associated with later variation in infant behavior and temperament in rhesus macaques (Macaca mulatta, N=59). In the early post-natal period, heavier mothers with more reproductive experience produced greater AME, which is the product of milk energy density (kcal/g) and milk yield (g). Moreover, infants whose mothers produced greater AME in the early post-natal period showed higher activity levels and greater confidence in a stressful setting later in infancy. Our results suggest that the milk energy available soon after birth may be a nutritional cue that calibrates the infant’s behavior to environmental or maternal conditions. These data provide new insight into potential mechanisms for the development of behavior and temperament and illuminate new directions for investigating maternal effects, nutritional programming, and developmental plasticity.
Introduction
Early life experiences can exert substantial influence over offspring phenotype. From a functional perspective, it has been hypothesized that developmental plasticity affords offspring the ability to respond to maternal signals that are predictive of future environmental conditions [Metcalfe and Monaghan 2001, Lumaa and Clutton-Brock 2001, Barker et al. 2002, West-Eberhard 2003, Bateson 2004, Armitage et al. 2004, Wells 2007, Kuzawa 2007]. In this way, maternal cues during development have the potential to prime offspring for the world in which they will live, and fine-tune life-history trajectories and behavioral strategies [Kuzawa 2007, Bateson et al. 2004, Barker et al. 2002]. Extensive research has demonstrated that metabolism, growth rate, immunocompetence, reproduction, and risk for adult disease can be influenced by parental or environmental conditions during early development in insects, birds, and mammals [reviewed in Kuzawa 2007, Bateson et al. 2004, Barker et al. 2002, Lumaa and Clutton-Brock 2001]. It remains unclear if the resulting effects represent byproducts of early experience or an adaptation to that experience, but emerging theoretical and empirical research increasingly support an adaptationist interpretation [Kuzawa 2007].
Most research on developmental programming has focused on events that occur during fetal development because often, the earlier that an insult occurs, the greater the magnitude of subsequent phenotypic consequences [Desai and Hales 1997]. However, signals about environmental conditions during lactation may also provide valuable information for developing infants and programming may continue in species with maternal care. For example, Meaney and colleagues have demonstrated that infant rats whose mothers spend more time licking/grooming them and in an arched back nursing posture have better spatial learning abilities, better memory, and better behavioral adaptation to novel environments [Champagne et al. 2003, Weaver et al. 2004, Fish et al. 2004]. Under experimental conditions of unpredictable foraging demand, macaque mothers are less attentive and more rejecting toward their infants. Infants reared by these mothers grew normally, but were less playful and exploratory and as juveniles were less gregarious and more likely to be subordinate in newly formed groups [Andrews et al. 1993, Andrews and Rosenblum 1994, Rosenblum et al. 2001]. In humans, mothers under nutritional constraints have infants that are less active and alert and potentially at greater risk for cognitive deficits [Rahmanifar et al. 1993].
While these studies examined the consequences of variation in aspects of “mothering,” they did not directly examine the most crucial feature of maternal investment in mammals, milk production. Two fundamental aspects of lactation are the energy density of milk [the kilocalories derived from protein, carbohydrate, and fat concentrations) and the amount of milk that mothers produce. Together, the energy density and the amount of milk determine the available milk energy (AME) during lactation [Hinde 2009]. Mothers’ ability to produce milk is influenced by their condition [macaques, Hinde 2007a, Hinde et al. 2009, Hinde 2009; baboons, Roberts et al. 1985; common marmosets, Tardif et al. 2001] and there is considerable evidence across mammals that variation in the quality and quantity of milk from the mother influences infant growth and development [baboons, Roberts et al. 1985; common marmosets, Tardif et al. 2001; deer; Landete-Castillejos et al. 2001, Gomez et al. 2002; and seals, Mellish et al. 1999, Oftedal et al. 1993, Oftedal et al. 1996]. Infant mass and growth in the present sample are similarly associated with mother’s milk [Hinde 2007b, Hinde et al. 2009]. However, to date virtually nothing is known about the impact of AME on infant behavioral development.
Here we examine the impact of variation in AME on infant behavioral dispositions, a term that reflects an individual’s tendencies to behave in characteristic ways, and that fits into the overlapping literatures on temperament, personality, and behavioral syndromes in non-human animals [Réale et al. 2007, Smith and Blumstein 2008, Weinstein et al. 2008]. We hypothesize that AME provides a reliable signal to developing infants about the kind of environment in which they live, and helps to program infant behavioral development. From a life-history perspective, individuals with different expectations of future resources are expected to show different behavioral dispositions [Wolf et al. 2007]. We expected that particular aspects of infant behavior and temperament – activity, confidence/boldness, and playfulness – would be particularly sensitive to variation in AME as these behaviors are calorically expensive for the developing infant. Infants whose mothers produced greater available milk energy were therefore predicted to be more active and to be characterized as having a more playful, exploratory, and active temperament. Lastly if available milk energy functions as a form of maternal programming, then infant behavior and temperament are expected to be more closely related to AME shortly after birth than to AME later in infancy.
Methods
Subjects
We tested the above predictions in an Old World monkey, the rhesus macaque (Macaca mulatta). Rhesus macaques display substantial individual variation in behavior and temperament [Capitanio et al. 1986, Capitanio et al. 2006, Suomi and Ripp 1983, Golub et al. 2007], and adapt successfully to a broad range of habitats throughout Asia [Richard et al. 1989]. We studied 59 mother-infant dyads that lived in large social groups in outdoor 0.2 hectare enclosures at the California National Primate Research Center in 2006. Of the 59 mothers, 11 were primiparous and 23 were rearing sons. Social groups were provisioned twice daily with commercial monkey chow, twice weekly with fruits and vegetables, and water was available ad libitum. All subjects were of known age, parity, mass, and social rank from colony records.
Milk Collection
Milk was collected at one month of infant age to assess AME in the early post-natal period as infants became behaviorally active [Fairbanks 2000] and again at 3–4 months during peak lactation [Oftedal 1984, Riek 2008]. In the early post-natal period at one month of infant age (mean±SD=33±2 days), infants and mothers were captured in their outdoor enclosures between 7:30–9:00AM, and were relocated together to temporary housing. To prevent nursing for 3.5–4 hours (mean±SD=216±8 mins), mothers were placed in mesh jackets, which allowed infants to remain in contact with their mother during the period of milk accumulation and were only separated for 10–15 mins. for milk collection. Between 11:30 and 13:00, mothers were sedated with ketamine hydrochloride (5–10 mg/kg IM) and administered a non-physiological dose of exogenous oxytocin [2IU/kg(0.1ml/kg)IM] for myoepithelial cell contraction and milk letdown. Milk was collected by gentle hand stripping of the nipple and mammary glands were fully evacuated to prevent sampling bias [Oftedal 1984]. Mothers and infants were returned to their social group several hours following milk collection, after the mother had recovered from sedation.
During peak lactation, at 3.5 months of infant age (mean±SD=104±7 days), infants and mothers were again captured between 7:30 and 9:00AM and separated, and infants were relocated to a novel environment for 25 hours, during which infants’ behavioral responsiveness and temperament were assessed [Capitanio et al. 2006, Capitanio et al. 2005; see below]. Milk was again collected 3.5–4 hours (mean±SD=217±6 mins) after maternal-infant separation using identical techniques as at one month of infant age. Milk collection procedures for this study have been described in further detail elsewhere [Hinde et al. 2009].
Available Milk Energy
Proximate analyses of milk composition (concentrations of fat, protein, and sugar) were conducted at the Nutrition Laboratory, Smithsonian National Zoological Park, Washington DC using standard methods [Oftedal 1984, Oftedal and Iverson 1996]. The gross energy of milk, also referred to as energy density, was calculated as 9.11 kcal/g for fat, 3.95 kcal/g for sugars, and 5.86 kcal/g for protein which allowed for an estimate of the total energy density of milk (kcal/g) [Oftedal 1984]. A relative measure of milk yield was determined as the total mass in grams of the milk sample obtained by mammary evacuation after a standard time of milk accumulation (3.5–4 hours). This method of estimating relative differences in milk production among individuals has been used for other primates [Tardif et al. 2001, Ota et al. 1991] and has been associated with infant mass and growth in the present sample population [Hinde et al 2009, Hinde 2009]. An index of available milk energy (AME) was calculated as the product of milk gross energy (kcal/g) and milk yield (g) value for each mother at the one month and 3.5 month time points [Hinde 2009]. This index of AME represents relative differences in milk synthesis among mothers during the standardized period of milk accumulation rather than an absolute value for daily milk energy output.
Infant Biobehavioral Assessment: Behavior and Temperament
All 59 infant subjects were part of an ongoing biobehavioral assessment program at the CNPRC described in detail elsewhere [Golub et al. 2009, Capitanio et al. 2005, Capitanio et al. 2006]. Briefly, cohorts of up to eight animals at a time were relocated to an indoor testing area when infants were 3–4 months of age, and each animal in a cohort was housed in an individual holding cage (60 cm x 65 cm x 79 cm, Lab Products, Inc., Maywood, NJ), containing a cloth diaper, a stuffed terrycloth duck, and a novel, manipulable object. Over the next 25-hr. period, behavioral data were collected in a variety of standardized situations [described in Golub et al., 2009; Capitanio et al., 2006]; at the conclusion of this period, infants were returned to their mothers, and mother-infant pairs were returned to their outdoor enclosures. Two types of measures, derived from focal observations and from temperament ratings, were used in analyses for the present study.
Behavioral responsiveness
Five-minute focal behavioral observations were conducted while the animal was in its holding cage, using an ethogram comprising behavioral categories reflecting activity states (e.g., locomote, sleep, hang) and events such as vocalizations, facial expressions, and self directed behaviors [see full listing and definitions in Golub et al., 2009]. Focal observations were performed on two occasions, once approximately 15 min after the infants were placed in the cage (Day 1), and again the next morning, 22 hrs following separation from mother (Day 2). All behavioral data on infants were collected by trained observers that had demonstrated reliability of greater than 85% agreement. Owing to slight variations in the length of the observation periods, duration measures were converted to a proportion of the total observation time, and frequencies of states and events were converted to a rate per 60 s.
Data reduction for measures of behavioral responsiveness and temperament (see next section) was accomplished via exploratory and confirmatory factor analyses using MPlus statistical software [Muthen & Muthen, 2001] in order to derive reliable scales that could be used as outcome measures. Factor analytic procedures (which utilized data from more than 1,400 infants collected over a 5-year period), and all indices of factor model fit, are described in detail in Golub et al. [2009]. Briefly, an exploratory factor analysis, using weighted least squares with robust standard errors, identified a two-factor solution, which was given a promax rotation, and variables were retained if loadings were > 0.40. Based on this solution, separate confirmatory factor analyses were performed on data from subsequent years, and fit was excellent based on traditional fit statistics [see Golub et al., 2009]. Scales were constructed by summing z-scores for the items that loaded on a given factor. Cronbach’s alphas ranged from 0.6 to 0.8. The final scales were then z-scored and labeled “Activity” (comprising the proportion of time animals were locomoting; proportion of time animals were not in a hang position from the side or top of the cage; rate of environmental exploration; and whether animals ate food; drank water; and were in a crouched posture) and “Emotionality” (rate of vocalizing [coos and barks]; and whether the animal displayed threats, lipsmacks, and self-scratch). Identical scales were constructed for the Day 2 observations. As indicated by values for the Day 1 scales, the infants’ acute responses to the separation and relocation generally involved high Emotionality and low Activity, whereas 22 hours later (Day 2), many, but not all, infants were more active and less emotional. Thus, we consider that the variation in behavioral activity and emotionality on Day 2 as reflecting behavioral adjustment to the challenging situation and an indication of individual coping ability. As no analyses using the Day 1 composites were significant, they are not considered further.
Temperament ratings
Infant temperament was determined through subjective observer ratings [Weinstein et al., 2008], which reflected an overall “thumbnail” portrait of the animal’s functioning during the entire biobehavioral assessment. At the end of the 25-hr. period, an observer rated each infant using a list of 16 trait adjectives [Golub et al., 2009], and a seven point Likert scale for each trait. Assessment of inter-rater agreement and reliability for the data collection have been published [Weinstein & Capitanio, 2008]. Briefly, mean inter-rater reliability for the 16 items, assessed using an intra-class correlation, was 0.53. Inter-rater agreement, assessed using chi-square [Lawlis & Lu, 1972], was significantly greater than chance (P < 0.00001) for each item, and the mean T index, a kappa-based measure indicating the magnitude of agreement [Tinsley & Weiss 1975], was 0.64, when different observers’ ratings were allowed to vary from each other by one point.
As with the behavioral measures (above), scale development proceeded by conducting exploratory factor analysis on one sample (using maximum likelihood estimation and promax rotation) and confirmatory factor analyses on other samples (all fit indices, which indicated close fit, are described in Golub et al., 2009). Factor scores were calculated by summing the z-scores for all adjective items loading on a given factor, and then z-scoring each scale. Cronbach’s alpha values for the scales ranged from 0.6 to 0.9. The four scales, named for the adjective with the highest factor loading, were: Vigilant (vigilant, NOT depressed, NOT tense, NOT timid), Gentle (gentle, calm, flexible, curious), Confident (confident, bold, active, curious, playful), Nervous (nervous, fearful, timid, NOT calm, NOT confident). The traits preceded by the word “not” reflect a negative loading in the factor analysis.
Data Analysis
Multiple regression analyses were performed to assess the relationship between AME and maternal characteristics. Maternal parity, mass, and social rank were all included in the model, as was an interaction term of parity by maternal mass (both variables were centered prior to construction of the interaction term) as parity and maternal mass have an interaction effect on milk yield [Hinde et al 2009]which is a main component of AME [Hinde 2009]. Infant age was included as a covariate. Multiple regression was also used to examine the role of AME on measures of infant behavior and temperament controlling for maternal and infant characteristics that may influence behavior or have been associated with milk production [Hinde 2007b, Hinde et al. 2009, Hinde 2009]. The controlled covariates were maternal age, mass, parity, and social rank, and infant age, mass, and sex. Bivariate correlations of AME and the number of minutes of milk accumulation were non-significant at both one and 3.5 months of infant age (r=−0.03, p=0.819 and r=0.13, p=0.34, respectively, N=59). Milk synthesis is not linear and attenuates when the mammary is not regularly evacuated by infant nursing [Akers 2002]. The number of minutes of milk accumulation within the 3.5–4 hour window had no affect on AME and was not included as a covariate in the regression models. As found in other mammals, primiparous primate mothers differ from multiparous mothers in behavioral and physiological components of mothering [rhesus macaques; Bercovitch et al. 1998, Hinde et al. 2009, Hinde 2009, baboons; Cheney et al. 2006, gorillas; Robbins et al. 2006; humans; Fraser et al. 1995, reviewed in Maestripieri 2009, Bercovitch and Harvey 2004, Bentley 1999, Debyser 1995]. Therefore post-hoc modeling for multiparous subjects only (N=48) was performed to determine if data from the 11 primiparous mothers were having a disproportionate influence on our results. All statistical analyses were conducted using SPSS 16.0.
Results
The available milk energy produced by mothers (N=59) changed over the course of lactation, and substantial variation between individuals was evident at both time points. At one month of infant age, mean±SD AME was 9.44±4.6 kilocalories (kcal) during the 3.5–4 hours of milk accumulation (range=2.70–19.30 kcal). By 3.5 months of age, this value almost doubled (16.53±7.0, range=4.32–32.92 kcal), as mothers increased their milk production to meet the energetic needs of their growing infants. Mothers that produced milk with relatively high AME when their infants were one month of age also produced milk with relatively high AME when their infants were 3.5 months of age (Pearson r=0.696, p<0.001).
Individual variation in AME was linked to maternal characteristics. Maternal reproductive experience, quantified as the number of previous pregnancies, was significantly associated with AME at both one month (beta=0.383, partial r=0.364, p=0.006) and 3.5 months of infant age (beta=0.559, partial r=0.500, p<0.001). For each additional parity, mothers produced 0.4 and 0.9 additional kcal of milk at each time point, respectively. Although maternal mass did not have a main effect on AME, there was an interaction between parity and maternal mass during the first month of lactation (beta=−0.276, partial r=−0.357, p=0.007). Less experienced, lighter mothers produced lower AME in the early post-natal period compared to higher parity, heavier mothers. This interaction was not significant for mothers later in lactation (beta=−0.071, ns). There were no effects of maternal social rank at either time point.
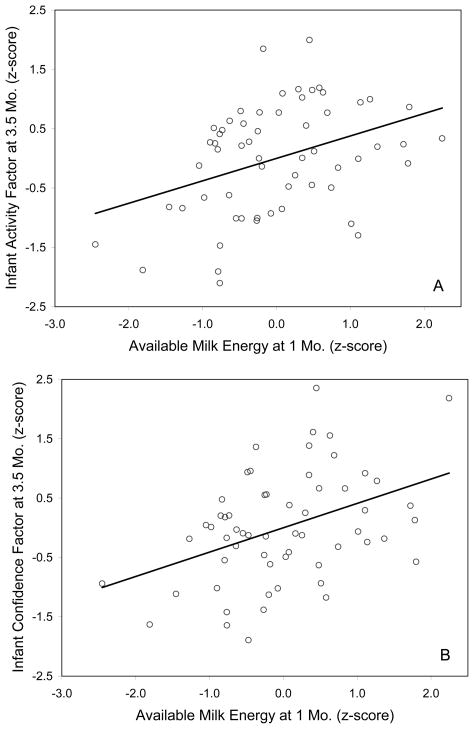
Infants whose mothers produced higher AME at one month of age were better able to cope with the novel, stressful situation 2–3 months later, as indicated by higher levels of behavioral Activity (beta=0.414, partial r=0.379, p=0.006). Infants that received higher AME at one month of age were also rated as having a more Confident temperament (beta=0.452, partial r=0.411, p=0.003). The behavioral composite of Activity and Confident temperament covaried (r=0.501, p<0.001). Additionally higher AME at one month was related, though not significantly, to lower ratings for Vigilant temperament (beta=−0.305, partial r=−0.268, p=0.06) and higher scores for Gentle temperament (beta=0.296, partial r=0.262, p=0.06). There was no relationship between AME at either time point on the behavioral composite of Emotionality (p=0.40) or Nervous temperament factor (p=0.79). All significant effects remained even after primiparous mothers were removed from the analyses (Activity: beta=0.430, partial r=0.433, p=0.005 and Confidence: beta=0.351, partial r=0.333, p=0.036, N=48)
Although available milk energy at one month and at 3.5 months of infant age were positively correlated, AME at one month of age had a more consistent impact on infants’ behavioral dispositions than did AME at 3.5 months of age. AME at 3.5 months of age was associated with the behavioral factor Activity (beta=0.333, partial r=0.284, p=0.04), but not with any of the temperament factors (Confident, beta=0.291, partial r=0.238, p=0.09; all other factors, p > 0.5). Because Activity at 3.5 months of infant age was predicted by AME at both one month and 3.5 months post-partum, we constructed a regression model to examine the relative contribution of the two AME measures: Activity remained significantly associated with AME at one month of age (beta=0.370, partial r=0.278, p=0.05) but not with AME at 3.5 months of age (beta=0.083, partial r=0.060, p=0.67).
Discussion
Our data show that maternal characteristics influence the milk produced by the mother, and that milk energy available in early infancy predicts infants’ behavior and temperament more than two months later. Importantly, the two different metrics that we used to assess infant behavioral dispositions, Activity (measured using objective behavioral observations) and Confidence (measured using subjective temperament ratings), were correlated, suggesting that each may be tapping into a single underlying trait.
Inexperienced mothers, especially those in poor condition, produced lower available milk energy than did more experienced, heavier mothers. Infants whose mothers produced greater available milk energy early in infancy coped more effectively (e.g., locomoted more, explored the environment more, ate, drank), and showed greater confidence (were more playful, exploratory, curious, active, and confident) in a stressful, novel situation months later. These responses suggest that infants may be adjusting their behavioral dispositions to expectations of energy resources available from the mother. For infants whose mothers produce ample available milk energy, Active and Confident behavioral dispositions (which presumably require more energy) are not risky inasmuch as infants receiving high AME during early development were likely to continue to receive high AME from the mother. Indeed AME at one month was significantly associated with AME at 3–4 months of infant age. In contrast, infants whose mothers produce less milk energy potentially face tradeoffs between growth, maintenance, and behavioral activity and may need to adjust behavioral patterns to minimize energy expenditure. As demonstrated in the present study, infants whose mothers produced low AME were characterized by lower activity levels, and less confident temperament during the stressful, novel conditions: they were less exploratory, playful, active, or curious.
We are able to rule out a number of infant and maternal characteristics that might mediate the effects of AME on behavioral dispositions. Early nutrition is also associated with infant size and growth rates in this population [Hinde et al 2009]. Similarly infant age and sex are associated with infant size [Hinde 2007b, Hinde et al 2009, Hinde 2009], which may influence behavior and temperament. However, infant age and mass were controlled in our hierarchical models and the AME effects on infant behavior and temperament persisted. Although infant sex is not associated with AME [Hinde 2009], it was included as a covariate as it is likely to influence behavior and temperament. The results presented here are likewise not attributable to maternal parity. Although AME was associated with parity, our hierarchical model controlled for this maternal characteristic as a continuous variable. Furthermore post-hoc analyses confirmed that this effect was not disproportionately due to primiparae who differ in their mothering behavior and lactational performance from reproductively experienced mothers [reviewed in Bercovitch and Harvey 2004, Maestripieri 2009]: the effects of AME on infant Activity and Confidence remained significant when only multiparous mothers were included in the analysis.
AME at one month was more strongly associated with infant behavioral outcomes during bio-behavioral assessment at 3.5 months of infant age than was the AME collected concurrently during BBA, suggesting that infant behavior and temperament are shaped to a greater extent by available milk energy in the first weeks after birth, than later in the postnatal period. The importance of the early postnatal period is consistent with the hypothesis that adaptive responses to environmental conditions are organized during critical periods [West-Eberhard 2003, Metcalfe and Monaghan 2001, Dewitt et al. 1998]. However we have not yet identified the mechanisms underlying the observed relationship between available milk energy and infant outcomes. It is possible that the behavioral effects of the nutritional value of milk are mediated through differential brain development, but to date no data are available on natural variation in maternal milk and brain development among individuals within a species. During the early post-natal period infants begin to explore their social and physical environment and object, social, and activity play emerge [Fairbanks 2000]. Activity play (non-social acrobatic play such as swinging, jumping, and twirling), in particular, is highest during the early months of lactation [Fairbanks 2000]. At this same time, cerebral glucose metabolism increases [Jacobs et al. 1995] as does synaptic density [Zecevic et al. 1989] and development of the hippocampus, crucial for memory and cognition [Lavenex et al. 2007]. Mothers may contribute to infant brain development and behavioral dispositions directly through milk constituents that build and fuel the brain (e.g. long-chain polyunsaturated fatty acids) or indirectly by providing the caloric energy for infant activities and experiences that in turn shape brain development.
We recognize that several other explanations may account for our results. The associations between AME and infant behavioral dispositions may reflect genetic correlations between mothers and infants. Alternatively, insofar as milk production responds to infant demand, confident, active infants may be better at extracting resources from the mother. However we note that available milk energy during the early post-natal period, but not during peak lactation, predicted infant behavioral dispositions later in infancy. If these effects were primarily due to underlying genetic correlations or infant ability to extract resources, we could predict that the effects would persist throughout lactation. It is also possible that the relationships between mother’s milk and infant behavioral dispositions represent a continuation of fetal nutritional programming into the post-natal period. Indeed functional development of the mammary occurs during gestation [Akers 2002]. Lastly we can not rule out the potential influences of individual variation in mothering behavior that are not accounted for by our control variables of maternal parity, weight, and social rank [Fairbanks 1996].
Increasingly, maternal influences on infant phenotype are not considered merely byproducts of variation in maternal condition or ability to invest in offspring, but rather as adaptations that have been shaped by natural selection [Räsänen and Kruuk 2007, Mosseau and Fox 1998]. The results presented here are first evidence suggesting that inter-individual variation in mother’s milk has the potential to shape infant behavioral dispositions. We show that naturally occurring individual variation in available milk energy is significantly associated with later infant temperament and behavior. This study represents a necessary first step in identifying potential lactational effects on infant behavioral phenotype. Future research should focus on the mechanisms by which available milk energy affects developmental trajectories.
Figure 1.
(A) Partial regression scatter plot of Activity and Available Milk Energy (AME). (B) Partial regression scatter plot of Confident temperament and AME.
Acknowledgments
We thank O. Oftedal, M. Power, K. Widaman, L. Del Rosso, L. Calonder, C. Stanko, J. Horrocks, S. Fisher, and the animal care and veterinary staffs of CNPRC for contributions to this project. Joan Silk, Lynn Fairbanks, and Fritz Trillmich and two anonymous reviewers provided valuable comments on earlier drafts of the manuscript. This work was supported by National Science Foundation (DDIG 0525025 to KH and Joan Silk), the American Society of Primatologists Small Research Grant (to KH), and National Institute of Health (RR019970 to JPC, and RR000169 to UC Davis). All procedures adhered to the American Society of Primatologists principles for the ethical treatment of nonhuman primates and were approved by the Institutional Animal Care and Use Committee at UC Davis and conducted in accordance with the laws of the United States of America.
Literature Cited
- Akers RM. Lactation and the mammary gland. Iowa: Blackwell Publishing; 2002. [Google Scholar]
- Andrews MW, Rosenblum LA. The development of affiliative and agonistic social patterns in differentially reared monkey. Child Develop. 1994;65:1398–1404. doi: 10.1111/j.1467-8624.1994.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Andrews MW, Sunderland G, Rosenblum LA. Impact of foraging demand on conflict within mother-infant dyads. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. SUNY Press; Albany: 1993. pp. 229–252. [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals. J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bentley GR. Aping our ancestors: Comparative aspects of reproductive ecology. Evol Anthro. 1999;7:175–185. [Google Scholar]
- Bercovich FB, Harvey NC. Reproductive life history. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. New York: Cambridge Univ Press; 2004. pp. 61–80. [Google Scholar]
- Bercovitch FB, Lebron MR, Martinez HS, Kessler MJ. Primigravidity, body weight, and costs of rearing first offspring in rhesus macaques. Am J Primatol. 1998;46:135–144. doi: 10.1002/(SICI)1098-2345(1998)46:2<135::AID-AJP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Biro PA, Stamps JA. Are animal personality traits linked to life-history productivity? Trends Ecol Evol. 2008;23:361–368. doi: 10.1016/j.tree.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Capitanio J. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. Am J Primatol. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. Springer; New York: 2006. pp. 191–214. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–30. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Rasmussen KLR, Snyder DS, Laudenslager M, Reite M. Long-term follow-up of previously separated pigtail macaques: Group and individual differences in response to novel situations. J Child Psychol Psychiat. 1986;27:531–538. doi: 10.1111/j.1469-7610.1986.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as mediating influence of the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner JC, Bergman TJ, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Reproduction, mortality, and female reproductive success in chacma baboons of the Okavango Delta, Botswana. In: Swedell L, Leigh SR, editors. Reproduction and fitness in baboons: behavioral, ecological, and life history perspectives. New York: Springer; 2006. pp. 147–176. [Google Scholar]
- Debyser IWJ. Platyrrhine juvenile mortality in captivity and in the wild. Int J Primatol. 1995;16:909–933. [Google Scholar]
- Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biol Rev. 1997;72:329–348. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- Dewitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol Evol. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Amer J Primatol. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in maternal style: causes and consequences for mothers and offspring. Adv Study Behav. 1996;25:579–611. [Google Scholar]
- Fairbanks LA. The developmental timing of primate play: a neural selection model. In: Parker ST, Langer S, McKinney ML, editors. Biology, Brains, and Behavior. James Currey, Inc; Oxford: 2000. pp. 131–158. [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann NY Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. New Engl J Med. 1995;332:1113–7. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–84. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobio. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JA, Landete-Castillejos T, Garcia AJ, Gallego L. Effect of calving advance on milk production and composition, and calf growth in Iberian deer (Cervus elaphus hispanicus) Sm Ruminant Res. 2002;44:213–218. [Google Scholar]
- Hinde K, Power ML, Oftedal OT. Rhesus macaque milk: magnitude, sources, and consequences of individual variation over lactation. Am J Phys Anth. 2009;138:148–57. doi: 10.1002/ajpa.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K. Milk composition varies in relation to the presence and abundance of Balantidium coli in the mother in captive rhesus macaques (Macaca mulatta) Am J Primatol. 2007a;69:625–634. doi: 10.1002/ajp.20373. [DOI] [PubMed] [Google Scholar]
- Hinde K. First-time macaque mothers bias milk composition in favor of sons. Curr Biol. 2007b;17:R958–R959. doi: 10.1016/j.cub.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Hinde K. Richer milk for sons but more milk for daughters: sex biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21:512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Chugan HT, Allad V, Chen S, Phelps ME, Pollack DB, Raleigh MJ. Developmental changes in brain metabolism in rhesus macaques and vervet monkeys revealed by positron emission tomography. Cereb Cortex. 1995;5:222–33. doi: 10.1093/cercor/5.3.222. [DOI] [PubMed] [Google Scholar]
- Kuzawa C. Developmental origins of life history: growth, productivity, and reproduction. Am J Hum Biol. 2007;19:654–661. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- Landete-Castillejos T, Garcia A, Gallego G. Calf growth in captive Iberian red deer (Cervus elaphus hispanicus): effects of birth date and hind milk production and composition. J Anim Sci. 2001;79:1085–1092. doi: 10.2527/2001.7951085x. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavanex PB, Amaral DG. Postnatal development of the primate hippocampal formation. Dev Neurosci. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- Lawlis GF, Liu E. Judgement of counseling process: Reliability, agreement, and error. Psychol Bull. 1972;78:17–20. doi: 10.1037/h0032935. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival, and reproduction in humans. Trends Ecol Evol. 2001;17:141–147. [Google Scholar]
- Maestripieri D. Maternal influences on offspring growth, reproduction, and behavior in primates. In: Maestripieri D, Mateo JM, editors. Maternal Effects in Mammals. Chicago: The University of Chicago Press; 2009. [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD. Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol Biochem Zool. 1999;72:67–690. doi: 10.1086/316708. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now and pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Mosseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 2. Los Angeles, CA: Muthen & Muthen; 1998–2001. [Google Scholar]
- Oftedal OT. Milk composition, milk yield and energy output at peak lactation: a comparative review. Zool Soc Lond. 1984;51:33–85. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ. Energy transfer by lactating hooded seals and nutrient deposition in their pups during the four days from birth to weaning. Physiol Zool. 1993;66:412–436. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ. Lactation performance and nutrient deposition in pups of the harp seal (Phoca groenlandica) on ice floes off southeast Labrador. Physiol Zool. 1996;69:635–657. [Google Scholar]
- Oftedal OT, Iverson SJ. Comparative analysis of non-human primate milks. In: Jenson RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 749–789. [Google Scholar]
- Ota K, Makino Y, Kimura M. Lactation in the Japanese monkey (Macaca fuscata): yield and composition of milk and nipple preference of young. Primates. 1991;32:35–48. [Google Scholar]
- Rahmanifar A, Kirksey A, Wachs TD, McCabe GP, Bishry Z, Galal OM, Harrison GG, Jerome NW. Diet during Lactation Associated with Infant Behavior and Caregiver-Infant Interaction in a Semirural Egyptian Village. J Nutr. 1993;123:164–175. doi: 10.1093/jn/123.2.164. [DOI] [PubMed] [Google Scholar]
- Räsänen K, Kruuk LEB. Maternal effects and evolution at ecological time-scales. Func Ecol. 2007;21:408–421. [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Richard AF, Goldstein SJ, Dewar RE. Weed macaques: the evolutionary implications of macaque feeding ecology. Int J Primatol. 1989;10:569–594. [Google Scholar]
- Riek A. Relationship between milk energy intake and growth rate in suckling mammalian young at peak lactation: an updated meta-analysis. J Zool. 2008;274:160–170. [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anth. 2006;131:511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Cole TJ, Coward WA. Lactational performance in relation to energy intake in the baboon. Am J Clin Nutr. 1985;41:1270–1276. doi: 10.1093/ajcn/41.6.1270. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Dev Psychobiol. 2001;39:40–45. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: a meta-analysis. Behav Ecol. 2008;19:448–455. [Google Scholar]
- Suomi SJ, Ripp C. A history of motherless mother monkey mothering at the University of Wisconsin Primate Laboratory. In: Reite M, Caine N, editors. Child Abuse: The Nonhuman Primate Data. Liss; New York: 1983. pp. 49–78. [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome, and phenotypic differences in behavior. Reprod Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power ML, Oftedal OT, Power RA, Layne DG. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): effects of maternal size and litter size. Behav Ecol Sociobiol. 2001;51:17–25. [Google Scholar]
- Tinsley HEA, Weiss DJ. Interrater reliability and agreement of subjective judgements. J Counseling Psychol. 1975;22:358–376. [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weinstein TAR, Capitanio JP. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Anima Behav. 2008;76:455–465. doi: 10.1016/j.anbehav.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein TAR, Capitanio JP, Gosling SD. Personality in animals. In: John OP, Robinns RW, Pervin LA, editors. Handbook of Personality: Theory and Research. 3. Guilford Press; New York: 2008. pp. 328–348. [Google Scholar]
- Wells JCK. The thrifty phenotype as an adaptive maternal effect. Biol Rev. 2007;82:143–172. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; Oxford: 2003. [Google Scholar]
- Wolf M, Sander van Doorn G, Leimar O, Weissing FJ. Life-history tradeoffs favor the evolution of animal personalities. Nature. 2007;447:581–584. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]