Executive Summary
Objective
The objective of this review was to determine the clinical effectiveness of oral appliances compared to ‘no treatment’, continuous positive airway pressure (CPAP), or surgery for the management of obstructive sleep apnea (OSA).
Clinical Need: Condition and Target Population
OSA is characterized by repeated occurrences of upper airway collapse and obstruction during sleep. The condition leads to excessive daytime sleepiness, diminished quality of life, and increased risks of accidents, cardiovascular disease and death. In the general population, the prevalence of OSA is estimated to be 4% in men and 2% in women. Risk factors for OSA include obesity, male gender, increasing age, alcohol use, sedative use, and a family history of OSA.
Description of Oral Appliances
Oral appliances for OSA fall into two broad categories: mandibular advancement splints (MAS), also known as mandibular repositioning devices, and tongue repositioning or retaining devices. The aim of MAS devices is to advance the mandible forward slightly to enlarge the upper airway and prevent it from collapsing. Similarly, tongue repositioning devices suction the tongue forward to prevent it from falling back and obstructing the airway during sleep.
The alternatives to oral appliances include continuous positive airway pressure (CPAP) devices, surgery, drug therapy, positional devices, and lifestyle modification. CPAP is the gold standard of treatment, but despite its effectiveness, compliance rates for CPAP have declined because required systems are noisy and because wearing the mask can be uncomfortable, causing claustrophobia in some users.
Evidence-Based Analysis Methods
Research Questions
Are oral appliances effective in improving sleep-disordered breathing in patients with OSA compared to alternative treatments?
Are there safety concerns with oral appliances?
What is the evidence regarding patient preference, quality of life, and compliance for oral appliances?
If effective, are oral appliances cost effective?
Literature Search
A literature search was conducted up to February 2009. Systematic reviews, meta-analyses and randomized controlled trials (RCTs) with more than 20 adults with OSA were eligible for inclusion. The primary outcomes of interest were the Apnea Hypopnea Index (AHI), measures of daytime sleepiness, patient preference, compliance, and adverse events.
Summary of Findings
Five systematic reviews and 16 RCTs that met the inclusion criteria were identified. The systematic reviews consistently concluded that CPAP was more effective than oral appliances at improving sleep disordered breathing, although there may be a niche area for the latter, especially among those with mild OSA as CPAP is difficult to tolerate by some users.
Based on the results of the RCTs analyzed for this review, MAS devices are less effective than CPAP when AHI is used as the outcome of interest. MAS devices were shown to decrease AHI levels, but whether this reduction is clinically meaningful is uncertain.
The Epworth Sleepiness Scale (ESS) was not able to achieve statistical significance in comparisons of MAS versus CPAP and MAS versus placebo. Nonetheless, after treatment with either MAS or CPAP, patients seem to be able to achieve normal ESS levels. The ESS has substantial limitations including its subjective nature and low construct validity (i.e. it is unclear if the scale is an accurate measure of sleepiness).
Adverse events among patients with MAS devices in the RCTs were common, but mostly mild and transient. Jaw discomfort was the most commonly reported adverse event.
Based on the results of the RCTs, compliance does not seem to be better or worse with MAS or CPAP. Similarly, there is no clear patient preference for MAS or CPAP among the studies reporting preference and satisfaction.
Keywords
Obstructive sleep apnea, oral appliances, mandibular advancement splints, tongue repositioning devices
Background
Clinical Need and Target Population
Obstructive sleep apnea (OSA) is the most common form of apnea (breathing disruption) and is characterized by repeated occurrences of upper airway collapse and obstruction during sleep. The condition can lead to excessive daytime sleepiness, diminished quality of life and an increased risk of accidents. (1) In addition, OSA is associated with higher risks of hypertension, ischemic heart disease, stroke and death. (2-5) The second most common form is central apnea, a condition in which the central nervous system does not properly maintain the breathing process. (1) Mixed apnea, as the name suggests, is a combination of obstructive and central apnea.
The prevalence of OSA is higher in men than in women (6;6), as demonstrated by Young et al (7) who randomly sampled of over 600 men and women between 30 and 60 years and found that OSA (AHI > 5 and symptoms of sleepiness) prevalence was 4% in men and 2% in women. The authors also reported that obesity was strongly associated with the presence of OSA, which has been widely reported elsewhere. (8) Other noted risk factors for OSA include increasing age, alcohol use, sedative use, and a family history of OSA. Anatomical conditions, such as craniofacial skeletal abnormalities or adenotonsillar hypertrophy, have also been found to be risk factors. (1)
Sleep apnea has been extensively examined in the Wisconsin Sleep Study, a prospective population-based study in which polysomnography was performed on more than 1,500 participants between the ages of 30 and 60 years. (3) At baseline, the study established that ~76% of participants did not have sleep apnea, ~14% had mild sleep apnea, ~5% had moderate sleep apnea and ~4% had severe sleep apnea. Sleep apnea was defined according to the Apnea Hypopnea Index (AHI), a count of the number of apneas, or hypopneas per hour. An AHI of 5 to <15 is considered mild sleep apnea, 15 to 30 is moderate sleep apnea, and an AHI greater than 30 is categorized as severe sleep apnea (an AHI less than 5 is the expected norm of a population). The authors of the Wisconsin Sleep Study have also estimated that 41% of adults can attribute their sleep-disordered breathing to BMI greater than 25. (3)
After a mean observation period of 13.8 years (20,963 person-years), the Wisconsin Sleep Study investigators reported that the risk of all-cause mortality is almost twice as high for people with mild or moderate sleep apnea compared to those without sleep apnea. For those with severe sleep apnea, the risk of all-cause mortality is more than five times greater than those with no sleep apnea. The risk of death is still higher in those with sleep apnea compared to those without when the risk is adjusted for age, body mass index (BMI), and sex.
The Busselton Health Study in Australia, a second prospective population-based study of sleep apnea, followed 400 participants for a mean of 13.4 years. (4) Its results were generally consistent with the Wisconsin Sleep Study for moderate and severe sleep apnea, finding that the risk of all-cause mortality was significantly higher among those in this group than those without sleep apnea. The Australian study did not, however, find an increased risk of all-cause mortality among those with mild sleep apnea.
Diagnosis and Therapeutic Evaluation of OSA
Several tools are used to diagnose OSA, measuring daytime sleepiness, respiratory variables, and sleep variables. When assessing OSA, however, it’s important to consider that severity can be subtly affected by many factors such as alcohol and sedative use prior going to sleep, overall tiredness, cold symptoms and sleeping position (the supine position increases likelihood of OSA).
There are a few tests to assess sleepiness, of which the gold standard is the Multiple Sleep Latency Test (MSLT). (9-11) It’s an objective measurement of a patient’s tendency to fall asleep. The theory of the test is that the more tired a person is, the faster he or she will fall asleep. It is a time consuming and expensive test that requires observing a patient falling asleep four to five times during the day in a sleep laboratory. (10;11)
An alternative to the MSLT is the Epworth Sleepiness Scale (ESS), an easily administered and widely available questionnaire (9) that is frequently reported in the literature. (12-22) The scale measures daytime sleepiness through a subjective score of an individual’s perceived likelihood of falling asleep under eight scenarios ranging from falling asleep while watching television to falling asleep while in a car stopped in traffic. (9) According to the scale, a score of 0 to 9 is within the normal range. A score of 10 to 24 would indicate a potential sleep condition requiring further investigation.
Despite the widespread use of the ESS, it has some limitations. (23) In particular, the scale is subjective and may lead to bias with patients potentially over-reporting an improvement in sleepiness once they have received treatment. This is especially important when considered from an Ontario perspective as untreated and unmanaged OSA can result in the suspension of a patient’s driver’s license. This threat may lead some patients exaggerating their response to treatment. (24) When reviewing the methodology regarding how the scale was created, it is unclear what evidence six of the eight scenarios are based on.(23) Further, none of the scenarios address patient consumption of caffeinated beverages, daytime naps, changes in work performance, memory, or energy levels. (23) In addition, the original study evaluating the ESS from 1991 included 55 patients with OSA, of which only two were women. (9) The study reported that the ESS scores were not significantly higher for those with moderate OSA compared to mild OSA, but the scores for patients with severe OSA were significantly higher than those with moderate OSA. (9) This finding questions whether the study was not sufficiently powered to detect a difference in ESS between the mild and moderate OSA sufferers, or whether the ESS tool itself is not sensitive enough to detect the difference. The validity of the ESS construct thus needs further investigation. (23;25)
Currently, the measurement of respiratory and sleep variables is conducted through polysomnography, a multi-parametric test (the product which is called a polysomnogram) that typically measures brain activity, airflow, chin movements, leg movements, eye movements, heart rate and rhythm, oxygen saturation and chest wall movement. AHI can be derived from the results of a polysomnography and is the most frequently reported measure of OSA assessment. (12-22)
One issue with the AHI is the lack of standardized criteria to define hypopnea. (26;27) The 2005 Practice Parameters by the American Academy of Sleep Medicine on polysomnography highlighted the lack of consensus regarding its definition. (28) The ambiguity lies in determining how restricted airflow must be in order to be classified as a hypopnea. In contrast, the definition of apnea is clearly defined as the cessation of airflow for more than 10 seconds. (28)
Other concerns of the AHI are that it does not correlate well with degree of sleepiness (though it does correlate well with the cardiovascular risk associated with long-term OSA) (29) and that intra-individual variability has been observed. (30) Aarab et al. assessed the variability of AHI in a 10-week study of 15 patients who underwent four at-home polysomnography tests. There was no significant difference in the mean AHI values across the four tests, however, there was a significant difference in individual AHI measurements. Since this review focuses on the mean AHI values from baseline to follow-up, the AHI variability is expected be minimal.
Description of Oral Appliances
There are many different brands of oral appliances for OSA available, all of which fall into two broad categories: mandibular advancement splints (MAS), also known as mandibular repositioning devices, and tongue repositioning (or retaining) devices. The aim of the MAS devices is to slightly advance the mandible forward to enlarge the upper airway and prevent it from collapse. Similarly, tongue repositioning devices suction the tongue forward to prevent it from falling back and obstructing the airway during sleep.
Many more studies have been published investigating the effectiveness of splints compared to the tongue repositioning devices. According to a clinical expert (Personal Communication, March 2009), the reasoning behind this is that tongue repositioning devices are not as effective as splints as the suction that holds the tongue forward must be gentle in order to be tolerated; but because it is so gentle, it is easy to pull the tongue out during sleep, rendering the device less effective. The advantage of tongue repositioning devices, however, is that dentition is not an issue, meaning that patients can use them regardless of how many teeth they have.
When the Medical Advisory Secretariat contacted four experts in the field, they each independently indicated that the ideal candidate for mandibular advancement splint would have no temporomandibular joint (TMJ) problems, a BMI less than 30, and enough teeth to hold the MAS in place. Their opinions were confirmed in a study by Liu et al., which aimed to identify the predictors of efficacy of splints. (31) They found that the patients with the greatest response to the splints (i.e. >75% decrease in AHI) were younger and had lower BMI.
Regulatory Status
As non-invasive devices, oral appliances for obstructive sleep apnea are classified as Type 1 devices by Health Canada and thus do not need to go through the rigorous review process like some of the other devices licensed by Health Canada. Type 1 devices have Establishment Licenses which must comply with safety, labeling, distribution, mandatory problem reporting, and recall requirements outlined by Health Canada.
Alternatives to Oral Appliances
The alternatives to oral appliances include drug therapy, positional devices, lifestyle modification (32-35), and continuous positive airway pressure (CPAP) devices, the gold standard treatment for OSA. (1) CPAP devices require users to wear a mask, while sleeping. The mask is connected to a machine via a hose, which provides continuous air pressure so that the airway does not collapse during sleep. Despite its effectiveness, however, compliance rates with CPAP have declined as the system is noisy and wearing the mask can be uncomfortable and cause claustrophobia in some users. A clinical expert (Personal Communication, April 2009) estimated that 20% to 25% of those prescribed CPAP were unable to tolerate it. This estimate was confirmed by a recent National Institute of Clinical Excellence (NICE) health technology assessment from the United Kingdom which indicated a pooled compliance rate of 79% in 4 studies with 2 to 7 years follow-up. (32)
Surgery may also be considered for some patients with OSA. Options include nasal surgery, uvulopalatopharyngoplasty (UPPP), maxillomandibular advancement, and tongue reduction. (36) In 2005, a Cochrane review published by Sundaram et al. (33) reported on various surgical techniques used to treat OSA. The authors identified a small sample of studies examining surgery for patients with OSA and concluded that, based on limited evidence, surgery did not seem to improve daytime sleepiness associated with OSA. In a similar manner, a Cochrane review of drug therapy option from 2006 concluded that none of the drug therapies identified have been proven to be widely effective in relieving the symptoms of OSA. (34) The review concluded that the effectiveness of drug therapy may be increased if therapies are tailored to a patient’s dominant mechanism of OSA.
Positional devices are designed to keep a person sleeping on his or her side. Sleeping in a supine position can cause the upper airway to collapse more easily, thus sleeping on one’s side may be beneficial in some cases. Positional devices include specially designed pillows and shirts with a lump sewn on the back (e.g. a sock with tennis balls) so that the person will avoid a supine position while sleeping. Although these devices are readily available, their effectiveness is unknown.
In 2001, a Cochrane review was released, which examined the evidence of the effects of lifestyle modification on OSA. (35) Specifically, the review looked at weight loss, sleep hygiene, and exercise. They limited their search to RCTs and were unable to identify any eligible studies for their review, highlighting a gap in the literature. The review was updated to April 2008 and still no eligible studies were found. (35)
Evidence-Based Analysis
Objective of Analysis
The objective of this review was to determine the clinical effectiveness of oral appliances compared to ‘no treatment’, CPAP, or surgery for the management of OSA.
Research Questions
Are oral appliances effective in improving sleep-disordered breathing in patients with OSA compared to the alternative treatments?
Are there safety concerns with oral appliances?
What is the evidence regarding patient preference, quality of life and compliance for oral appliances?
If effective, are oral appliances cost effective?
Methods
Search Strategy
A literature search was conducted up to February 2009 that included OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), The Cochrane Library, and the International Agency for Health Technology Assessment/Centre for Review and Dissemination. The full search strategy is listed in Appendix 1.
Inclusion Criteria
Systematic review, meta-analysis or randomized controlled trials (RCT)
Patients with OSA
Studies with >20 patients
Adults (> 18 years)
Intervention group treated with oral appliance (either a mandibular advancement splint or a tongue repositioning device)
Control group treated with CPAP, placebo, no treatment or surgery
Exclusion Criteria
Studies comparing one mandibular advancement splint to another splint
Case series, retrospective analyses, case reports
Grey literature, abstracts
Non-English studies
Outcomes of Interest
Apnea Hypopnea Index
Daytime sleepiness
Patient preference
Compliance
Adverse effects
Measuring Effectiveness
AHI is the most frequently reported measure assessing OSA reported in the literature (12-22), followed by the ESS. According to two clinical experts (Personal Communication April 2009), an adequate treatment for patients with OSA allows them to achieve ≥50% reduction in AHI from baseline, or an AHI of ≤10 – the latter target has been used in previous systematic reviews of OSA treatment. (37) For the purposes of this report, both targets were used.
Even though MSLT is considered the gold standard for measuring sleepiness (10), its expense and time demands make it an impractical test in most situations. Only one of the studies included in this review reported an outcome for sleep latency. (22)
Results of Evidence-Based Analysis
Literature Search Results
The literature search identified 274 citations for review. After analyzing these citations, 16 RCTs were identified for inclusion in this review (Figure 1). In addition to these, five systematic reviews of oral appliances for the treatment of OSA were found (Table 1).
Figure 1: Results of the literature search for the effective of oral appliances.
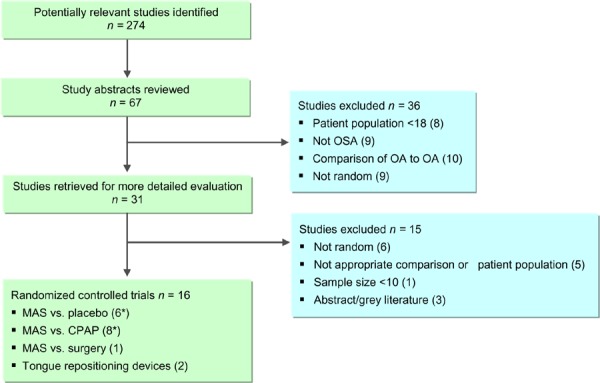
Table 1: Quality of Evidence of Included Studies*,†.
| Study Design | Level of Evidence† |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic review of RCTs | 1 | 5 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | 0 |
| Small RCT | 2 | 16 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | 0 |
| Case series (multisite) | 4b | 0 |
| Case series (single site) | 4c | 0 |
| Retrospective review, modelling | 4d | 0 |
| Case series presented at international conference | 4(g) | 0 |
| Total | 21 |
g refers to grey literature; RCT, randomized controlled trial.
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (38) An additional designation “g” was added for preliminary reports of studies that have been presented at international scientific meetings.
Summary of Existing Evidence
Systematic Reviews
McDaid et al. conducted a systematic review designed primarily to compare CPAP with other technologies including oral appliances. (1) The primary outcomes used were measures of sleepiness and ESS in particular. In their meta-analyses, they found no difference in ESS values between the CPAP and MAS treatment groups. They concluded that there was inconsistency in the results reported comparing CPAP to oral appliances.
Lim et al (2) published a Cochrane systematic review on oral appliances for OSA in 2006, which has since been updated to June 2007. They identified 17 RCTs that met their inclusion criteria and concluded that oral appliances improved subjective measures of sleepiness and sleep disordered breathing compared to no treatment or placebo. When oral appliances were compared with CPAP they concluded that CPAP was more effective at improving sleep disordered breathing, but it was unclear if CPAP improved quality of life compared to oral appliances.
In 2007, the Haute Autorité de Santé in France published a review of MAS for the treatment of OSA. It concluded that splints should be offered to patients with mild to moderate OSA and to those with moderate to severe OSA that are unable to tolerate CPAP. The review was not analyzed in detail as it was only published in French and the current review is limited publications to English.
The ECRI Institute published a systematic review of oral appliances in the treatment of OSA in 2002. (3) The review included five RCTs and five case series and concluded that oral appliances were effective in treating OSA, especially in those with mild OSA.
Ferguson et al. (4) published a systematic review of oral appliances on behalf of the American Academy of Sleep Medicine (AASM) in 2006. They concluded that oral appliances were not as effective as CPAP at reducing AHI, yet oral appliances were still preferable to CPAP (according to self-report). They concluded that there was a 52% success rate with oral appliances (success was defined as an AHI < 10).
Hoekema et al. (5) published a systematic review of oral appliances in 2004. Similar to the conclusions reported by Lim et al. (2), they found that CPAP was more effective than oral appliances at improving sleep disordered breathing, but because of the tolerability issues with CPAP, oral appliances may be a viable option for some patients, especially among those with mild OSA.
Overall, the systematic reviews published to date have concluded that CPAP is more effective than oral appliances at improving sleep disordered breathing, although there may be opportunity for oral appliances among patients with mild OSA who find CPAP difficult to tolerate.
Table 1: Overview of systematic reviews investigating oral appliances for sleep apnea.
| Systematic review |
Databases searched (years) |
Inclusion criteria | Studies eligible for inclusion |
|---|---|---|---|
| McDaid et al (NICE review), 2009 (1) | Medline Embase Cochrane Up to November 2006 |
|
29 studies (systematic review compares CPAP to other technologies; 6 RCTs comparing CPAP to MAS were identified) |
| Lim et al (Cochrane review), 2006 (2) updated to June 2007 | Medline Cochrane Up to June 2007 |
|
17 studies |
| Ferguson et al. 2006 (4) |
PubMed Up to July 2004 |
|
15 randomized trials |
| Hoekema et al. 2004 (5) |
Medline (1966-2002) Embase (1989-2002) Cochrane (1800-2002) |
|
16 (13 included in meta-analyses) |
| ECRI 2002 (3) |
Medline (up to Aug 2001) Embase (up to Aug 2001) Cochrane (up to Aug 2001) ECRI databases (up to Aug 2001) |
|
5 randomized trials |
AHI, apnea hypopnea index; ESS, Epworth Sleepiness Scale; MAS, mandibular advancement splints
Quality of the Evidence
Assessment of Quality of Evidence
The quality of the trials was examined according to the GRADE Working Group criteria. (6;7) Detailed tables of the GRADE assumptions for this review are listed in Appendix 2.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up, while consistency refers to the similarity of estimates of effect across studies. If there is important unexplained inconsistency in the results, confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the size of the differences in effect, and the significance of the differences guide the decision about whether important inconsistency exists. Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate |
| Very Low | Any estimate of effect is very uncertain |
Table 3 briefly outlines the quality of the studies included in this review. The studies are categorized according to comparison:
Table 3: Description of RCTs comparing MAS to placebo oral appliances for the treatment of OSA.
| Study | Inclusion criteria | N (# males) | Mean Age |
Mean BMI |
Duration of study (wash out period) |
|---|---|---|---|---|---|
| Petri et al. 2008 (8) Parallel arm study |
AHI >5 >20 years Sufficient teeth to hold splint All patients offered CPAP, but chose oral appliances |
93 (76) (12 dropouts) (29 in no intervention arm) |
50 (SD 10) |
31.3 (SD 1.3) |
4 weeks (NA) |
| Blanco et al. 2005 (9) Parallel arm study |
≥2 OSA symptoms AHI ≥10 Exclude BMI >40 |
24 (20) (9 dropouts) |
53.5 (SD 13.5) |
26.8 (SD 2.8) |
3 months (NA) |
| Barnes et al. 2004 (10) Crossover study |
Adequate dentition AHI 5−30 |
114 (91) | 47 (SD 9.6) |
31.1 (SD 5.3) |
3 months (2 weeks) |
| Gotsopoulos et al. 2004 (11-13)* Crossover study |
RDI >10/h At least 2 OSA symptoms 20 years Ability to protrude mandible at least 3 mm |
73 (59) | 48 (SD 11) |
29.0 (SD 4.7) |
4 weeks (1 week) |
| Johnston et al. 2002 (14) Crossover study |
AHI >10/h Absence of concurrent serious illness |
21 (16) (1 drop out) |
55.1 (SD 6.9) |
31.6 (SD 5.9) |
4−6 weeks (NR) |
| Mehta et al. 2001 (15) Crossover study |
AHI >10/h At least 2 OSA symptoms |
28 (22) (4 dropouts) |
48 (SD 9) |
29.4 (SD 3.1) |
1 week (1 week) |
Note: BMI, body mass index; CI, confidence interval; NA, not applicable; NR, not reported; RDI, respiratory distress index; SD, standard deviation
The same group of authors published results for a RCT between 2002-2005.
MAS versus placebo,
MAS versus CPAP,
MAS versus surgery, and
studies investigating tongue repositioning devices.
Blinding was inconsistent across the studies comparing MAS to placebo, and not possible in the MAS versus CPAP studies, nor in the MAS versus surgery study. The majority of the studies comparing MAS to placebo or to CPAP were crossover studies, thus participants had an opportunity to try MAS and the comparator. Intent-to-treat analyses were generally not used in this group of RCTs. The description of sample size determination and power calculations were also inconsistently reported in the literature.
Table 2: Quality of studies in review of oral appliances.
| Study | Study design | Randomization method | Blinding | Intent-to-treat | Adequate sample size |
|---|---|---|---|---|---|
| MAS versus Placebo Devices | |||||
| Petri et al. 2008 (8) | 3-armed, parallel group design | Computer generated | Yes§ | No | Yes |
| Blanco et al. 2005 (9) | Parallel group design | Unclear | No | No | Unclear |
| Barnes et al. 2004 (10)* | Randomized crossover study (3 arm) | Random draw | No | No | Yes |
| Gotsopoulos et al. 2004 † (11-13) | Randomized crossover study | Unclear | Yes | Yes | Unclear |
| Johnston et al. 2002 (14) | Randomized crossover study | Unclear | No | No | Yes |
| Mehta et al. 2001 (15) | Randomized crossover study | Unclear | No§ | No | Unclear |
| MAS versus CPAP | |||||
| Hoekema et al. 2008 † (16-19) | Parallel group design, Non-inferiority | Block randomization | No | Yes | Yes |
| Lam et al. 2007 (20) | Parallel group design | Unclear | No | Yes | Yes |
| Barnes et al. 2004 (10)* | Randomized crossover study (3 arm) | Random draw | No | No | Yes |
| Engleman et al. 2002 (21) | Randomized crossover study | Balanced blocks of 4 stratified by OSA severity | No | No | Yes |
| Randerath et al. 2002 (22) | Randomized crossover study | Unclear | No | N/A | Unclear |
| Tan et al. 2002 (23) | Randomized crossover study | Unclear | No | Yes | Unclear |
| Ferguson et al. 1997 (24) | Randomized crossover study | Unclear | No | No | Unclear |
| Ferguson et al. 1996 (25) | Randomized crossover study | Unclear | No | No | Unclear |
| MAS versus Surgery | |||||
| Walker-Engstrom et al. 2002 † (26-30) | Parallel group design | Closed envelope system | No | Inconsistent | Yes |
| Tongue Repositioning Devices | |||||
| Deane et al. 2009 (in press) (31) | Randomized crossover study | Unclear | No | No | Yes‡ |
| Dort et al. 2008 (32) | Randomized crossover study | Block randomization | Unclear | No | Unclear |
Barnes et al was a 3-arm trial that compared MAS, CPAP and placebo tablet.
These trials were reported in multiple publications.
The study was powered to detect difference between baseline and endpoints, not between group differences.
Contacted authors
Effectiveness of Oral Appliances
MAS versus Placebo
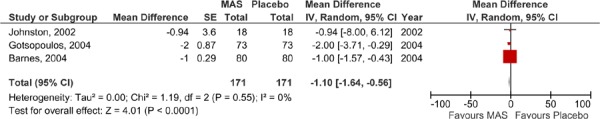
There were six RCTs identified that compared MAS to placebo for the treatment of OSA (summarized in Tables 3). Two of the RCTs were parallel group designs (8;9) and the other (10;11;14;15) were randomized crossover trials in which all patients tried both treatments for a defined period of time. Patients receiving placebo were given a sham oral appliance in five of the six studies, however, in one study by Barnes et al (10), the patients in the placebo group were instead given a placebo tablet.
The duration of the studies varied from 1 week to 3 months per treatment. Three of the four crossover studies described a 1-2 week washout period to minimize the risk of carry-over effect. The majority of study participants were men in all studies, which is unsurprising given the higher prevalence of OSA among men. The mean age of participants across the studies ranged from 47 to 55 years and the mean BMI from 26.8 to 31.6. The vast majority of participants thus were likely overweight or obese.
The baseline and follow-up AHI results for the included studies are reported in Table 4. The baseline and follow-up ESS results are reported in Table 5.
Table 4: AHI reported in RCTs comparing MAS to placebo devices for the treatment of OSA.
| Study | Treatment | Baseline AHI (h) | Follow-up AHI (h) |
Mean difference between baseline and follow-up |
Mean difference between MAS and Placebo |
Clinical significance |
|---|---|---|---|---|---|---|
| Petri et al. 2008 (8) Parallel arm study (N=93) |
MAS Placebo |
39.1 SD 23.8 32.6 SD 22.0 |
25.0 SD 27.5 31.7 SD 25.0 |
14.1 P <.001 0.9 P=.69 |
−6.7 P<.001 |
No No |
| Blanco et al. 2005 (9) Parallel arm study (N=15) |
MAS Placebo |
33.8 SD 14.7 24.0 SD 12.2 |
9.6 SD 12.1 11.7 SD 7.9 |
24.2 P <.01 12.3 P=.05 |
−2.1 NR |
Yes No |
| Barnes et al. 2004 (10) Crossover study (N=80) |
MAS Placebo tablet |
21.3 SD 13.9 21.3 SD 13.9 |
14.0 SD 11.7 20.3 SD 11.7 |
7.3 P <.001 1.0 P= NS (no value reported) |
−6.2 P<.001 |
No No |
| Gotsopoulos et al. 2004 (11-13)* Crossover study (N=73) |
MAS Placebo |
26.9 SD 15.4 26.9 SD 15.4 |
12.2 SD 12.3 25.4 SD 14.5 |
14.7 NR 1.5 P=NS (no value reported) |
−12.3 NR |
Yes No |
| Johnston et al. 2002 (14) Crossover study (N=20) |
MAS Placebo |
31.9 SD 21.2 31.9 SD 21.2 |
22.9 SD 22.8 37.7 SD 24.9 |
9.0 NR −5.8 NR |
−14.8 P=.011 |
No No |
| Mehta et al. 2001 (15) Crossover study (N=24) |
MAS Placebo |
27.0 SD17.0 27.0 SD 17.0 |
14.0 SD 9.8 30.0 SD 9.8 |
13.0 NR −3.0 P=NS (no value reported) |
−16.0 P<.0001 |
No No |
Note: AHI, apnea hypopnea index; MAS, mandibular advancement splint; NR, not reported; SD, standard deviation
The same group of authors published results for a RCT between 2002-2005.
Table 5: ESS reported in RCTs comparing MAS to placebo oral appliances for the treatment of OSA.
| Study | Treatment | Baseline ESS |
Follow-up ESS |
Mean difference between baseline and follow-up |
Mean difference between MAS and Placebo |
Clinical significance |
|---|---|---|---|---|---|---|
| Petri et al. 2008 (8) Parallel arm study (N=93) |
MAS Placebo |
11.7 SD 4.3 10.8 SD 4.6 |
8.4 SD 4.3 9.6 SD 4.2 |
3.3 P<.001 1.2 P=0.5 |
−1.2 P=.044 |
Yes No |
| Blanco et al. 2005 (9) Parallel arm study (N=15) |
MAS Placebo |
14.7 SD 5.1 16.3 SD 2.5 |
5.1 SD 1.9 13.6 SD 6.7 |
9.6 P <.05 2.7 NS (no value reported) |
−8.5 NR |
Yes No |
| Barnes et al. 2004 (10) Crossover study (N=80) |
MAS Placebo |
10.7 SD 3.6 10.7 SD 3.6 |
9.2 SD 3.6 10.2 SD 3.6 |
1.5 P<.001 0.5 NS (no value reported) |
−1.0 P<.001 P <.001 |
Yes No |
| Gotsopoulos et al. 2004 (11-13)* Crossover study (N=73) |
MAS Placebo |
10.9 SD 4.8 10.9 SD 4.8 |
7.1 SD 4.5 9.1 SD 5.1 |
3.8 NR 1.8 NR |
−2.0 P<.01 |
Yes Yes |
| Johnston et al. 2002 (14) Crossover study (N=20) |
MAS Placebo |
13.9 SD 6.4 13.9 SD 6.4 |
11.6 SD 6.7 12.6 SD6.7 |
2.3 NR 1.3 NR |
−1.0 P=.41 |
No No |
| Mehta et al. 2001 (15) Crossover study (N=24) |
MAS Placebo |
10.1 SD 5.4 10.1 SD 5.4 |
3.9 SD 2.9 NR |
6.2 P<.01 N/A |
N/A | Yes N/A |
Note: ESS, Epworth Sleepiness Scale; MAS, mandibular advancement splint; NR, not reported; SD, standard deviation
The same group of authors published results for a RCT between 2002-2005.
All of the studies defined a cut-off point for AHI or the respiratory distress index (RDI). The RDI is similar to the AHI, but includes other respiratory disturbances in addition to apneas and hypopneas. It is reported in the same way, as a value per hour averaged over several hours of sleep.
The RCT by Petri et al (8) enrolled 93 patients and randomized them to one of three arms: compared MAS, placebo or no intervention. The Medical Advisory Secretariat’s review focused specifically on the comparison of MAS to placebo in order to compare the results with similar RCTs. This is the most recent RCT identified comparing MAS to placebo. In this study, all participants were offered CPAP but chose oral appliances instead. It is unclear if the patients had tried CPAP or whether they chose the oral appliances without having first tried CPAP.
The parallel-arm RCT by Blanco et al. (9) involved 24 patients randomized to receive either MAS or placebo. Unfortunately, their analysis only included results for 15 patients as nine patients dropped out. Four dropped out immediately after randomization because three wanted CPAP instead, while the fourth joined a weight loss program. Within a month of starting the study, five additional patients withdrew due to side effects, including nausea and appliance displacement in patients in both the MAS and placebo group. Thus, in the end they reported results for eight patients receiving MAS and seven patients treated with placebo.
The crossover RCT by Barnes et al. (10) had three treatment arms: MAS, CPAP, and placebo. The results of the Barnes et al. study are reported in both the section comparing MAS to placebo and the results section comparing MAS to CPAP. The authors randomized 114 patients with AHI levels of 5-30 (i.e. mild to moderate OSA) to 3 months of treatment in each arm (with a 2 week washout between each period). This was the only study identified where patients were given a placebo tablet instead of a sham oral appliance. Eighty patients completed all three treatment arms. Since the study lasted almost a year, there were several drop-outs due to family and work commitments (n=15). One patient was unable to tolerate CPAP, while two were unable to tolerate MAS. Another five patients were lost in the MAS arm because they did not have sufficient teeth to hold the device in position.
The overall GRADE assessment for these studies was graded as low for the AHI outcome and very low for the ESS outcome. The GRADE scores were limited by inconsistent blinding in the studies, large losses to follow-up without intention-to-treat analyses, and large standard deviations and variability in the AHI and ESS outcomes (details in Appendix 2).
The AHI values before and after treatment are listed in Table 5 with the mean differences between and within groups also reported. AHI varied at baseline across the studies. The mean AHI at baseline for the MAS group in Petri et al. study (8), for example, was 39 compared to a baseline AHI of 21 among patients in Barnes et al RCT. (10) A large difference at baseline between the MAS and placebo group (33.8 versus 24.0) was also exhibited in the RCT by Blanco et al. The authors did not comment on this difference, which could have impacted the outcome given the small sample size of the study.
Four studies reported that MAS was significantly more effective than placebo for lowering AHI levels (the remaining two studies did not whether or not this difference was significant). In terms of mean difference from baseline to follow-up, three studies reported that there was a significant improvement in AHI levels in patients using the MAS devices at follow-up. The study by Blanco et al (9) reported a significant improvement between baseline and follow-up for both the MAS and placebo group, however, the sample population of this study was limited in size.
As mentioned previously, the clinically significant outcome was an AHI score after treatment of <10 or ≥50% reduction in AHI from baseline. Only the MAS treatment arms of the studies by Blanco et al. (9) and Gotsopotulos et al. (11-13) reported clinically significant results, although again, the small sample size and potentially unbalanced groups at baseline in Blanco et al. makes this result unreliable. Gotsopoulos et al. were unable to achieve a mean AHI <10, but they did report ≥50% reduction in AHI from baseline.
The results of those studies comparing MAS to placebo were pooled in a series of meta-analyses. Parallel arm and crossover studies are not easily pooled due to the possible carry-over effect in the crossover studies. For this reason, the Medical Advisory Secretariat analyzed data separately: a meta-analysis for the parallel-arm and the first arm of crossover studies where data was provided (2) and a second meta-analysis for the crossover studies alone. The AHI values of the studies comparing MAS to placebo are displayed in Figures 2 and 3; both show similar results in favour of MAS.
Figure 2: AHI for MAS versus placebo – parallel arm and first-arm of crossover studies.
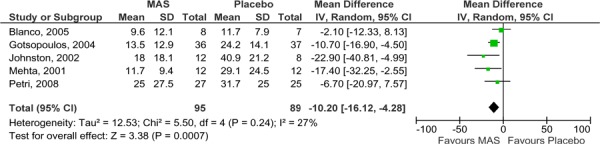
Figure 3: AHI for MAS versus placebo – crossover studies.
It is important to note that the studies by Petri et al. (8) and Blanco et al. (9) were parallel arm studies, while the others were crossover studies. In the parallel arm studies, using the difference in follow-up AHI scores between MAS and placebo may be subject to bias as the mean AHI scores of the study groups were unequal at baseline with the MAS groups having a higher mean baseline AHI. Yet despite this, the MAS group patients in both studies achieved a lower mean follow-up AHI those patients in the placebo groups.
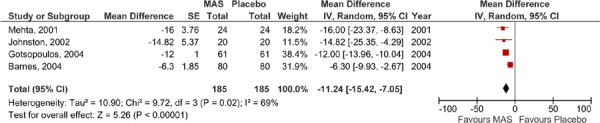
All six RCTs reported at least some description of ESS measures in their studies. As described earlier (page 10), an ESS score of less than 10 is considered to be within the normal range and thus a clinically meaningful result for study groups at follow-up. In five of the six studies, the mean ESS value at follow-up for the patients in the MAS group was within the normal range. In contrast, only one of the studies reporting ESS for the placebo group reported a mean ESS value within the normal range at follow-up among these patients.
The results for ESS were also pooled in meta-analyses: one for the parallel arm and the first arm of the crossover studies where data was available (2), and the other for the crossover studies alone (as displayed Figures 4 and 5). Neither analysis was able to demonstrate a difference in ESS values between the MAS and placebo groups.
Figure 4: ESS for MAS versus placebo—parallel arm and first arm of crossover studies.
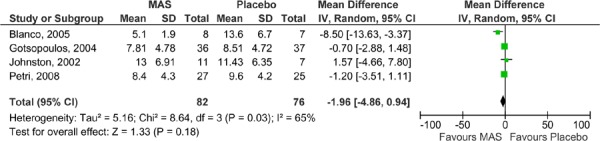
Figure 5: ESS for MAS versus placebo—crossover studies.
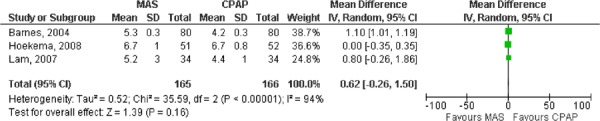
MAS versus CPAP
Eight RCTs comparing MAS with CPAP in OSA patients were identified (described in Table 7), two were parallel arm studies (16-20) and the remaining six were randomized crossover trials (10;21-25). The study groups were comprised primarily of men with a mean age ranging of 44 to 56 years and a mean BMI of 27.3 to 32.3 (thus most participants were overweight or obese). As with the studies comparing MAS with placebo, the majority of these studies focused on populations with mild to moderate OSA with a few exceptions. Hoekema et al. (16-19) and Engleman et al. (21) indicated that patients in their studies had to have an AHI level greater than 5, but did not limit the AHI level to an upper value. The mean baseline AHI in these studies was, therefore, slightly higher than in others (see Table 8).
Table 7: AHI reported in RCTs comparing MAS to CPAP for the treatment of OSA.
| Study | Treatment | Baseline AHI (h) |
Follow-up AHI (h) |
Mean difference between baseline and follow-up |
Mean difference between MAS and Placebo |
Clinical significance |
|---|---|---|---|---|---|---|
| Hoekema et al. 2008 (16-19)* |
MAS (n=51) | 39.4 SD 30.8 | 7.8 SD 14.4 | 31.6 NR |
5.4 P=.006 |
Yes |
| Parallel arm study (N=103) |
CPAP (n=52) |
40.3 SD 27.6 | 2.4 SD 4.2 | 37.9 NR |
Yes | |
| Lam et al. 2007 (20) |
MAS (n=34) | 20.9 SD 14.0 | 10.6 SD 14.0 | 10.6 P <.001 |
7.8 P<.05 |
Borderline |
| Parallel arm study (N=101) |
CPAP (n=34) |
23.8 SD 15.7 | 2.8 SD 9.1 | 21.0 P <.001 |
Yes | |
| Barnes et al. 2004 (10) |
MAS | 21.3 SD 11.6 | 14.0 SD 9.8 | 7.3 P <.001 |
9.2 P <.05 |
No |
| Crossover study (N=80) |
CPAP | 21.3 SD 11.6 | 4.8 SD 4.5 | 16.5 P <.001 |
Yes | |
| Engleman et al. 2002 (21) |
MAS | 30 SD 21 | 15 SD 16 | 15.0 NR |
7.0 P<.001 |
Yes |
| Crossover study (N=48) |
CPAP | 32 SD 29 | 8 SD 6 | 24.0 NR |
Yes | |
| Randerath et al. 2002 (22) |
MAS | 17.5 SD 7.7 | 13.8 SD 11.1 | 3.7 P <.05 |
10.6 P <.01 |
No |
| Crossover study (N=20) |
CPAP | 17.5 SD 7.7 | 3.2 SD 2.9 | 14.3 P <.01 |
Yes | |
| Tan et al. 2002 (23) |
MAS | 22.2 SD 9.6 | 8.0 SD 4.1 | 14.2 P <.001 |
4.9 P=NS |
Yes |
| Crossover study (N=24) |
CPAP | 22.2 SD 9.6 | 3.1 SD 2.8 | 19.1 P <.001 |
Yes | |
| Ferguson et al. 1997 (24) |
MAS | 25.3 SD 15.0 | 14.2 SD 14.7 | 11.1 P <.005 |
10.2 P <.01 |
No |
| Crossover study (N=24) |
CPAP | 23.5 SD 16.5 | 4.0 SD 2.2 | 19.5 P <.005 |
Yes | |
| Ferguson et al. 1996 (25) |
MAS | 19.7 SD 13.8 | 9.7 SD 7.3 | 10.0 P <.005 |
6.1 NR |
Yes |
| Crossover study (N=27) |
CPAP | 17.6 SD 13.2 | 3.6 SD 1.7 | 14.0 P <.005 |
Yes |
Note: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; MAS, mandibular advancement splint; NR, not reported; SD, standard deviation
The same group of authors published results for a RCT between 2007-2008.
Table 8: ESS reported in RCTs comparing MAS to CPAP for the treatment of OSA.
| Study | Treatment | Baseline ESS |
Follow-up ESS |
Mean difference between baseline and follow-up |
Mean difference between MAS and Placebo |
Clinical significance |
|---|---|---|---|---|---|---|
| Hoekema et al. 2008 (16-19)* |
MAS (n=51) | 12.9 SD 5.6 | 6.9 SD 5.5 | 6.0 NR |
1.0 P=.53 |
Yes |
| Parallel arm study (N=103) |
CPAP (n=52) |
14.2 SD 5.6 | 5.9 SD 4.8 | 8.3 NR |
Yes | |
| Lam et al. 2007 (20) |
MAS (n=34) | 12.0 SD 8.3 | 9.0 SD 8.3 | 3.0 P<.001 |
2.0 P<.05 |
Yes |
| Parallel arm study (N=101) |
CPAP (n=34) |
12.0 SD 8.3 | 7.0 SD 8.3 | 5.0 P<.001 |
Yes | |
| Barnes et al. 2004 (10) |
MAS | 10.7 SD 3.6 | 9.2 SD 3.6 | 1.5 P<.001 |
0 P=NS |
Yes |
| Crossover study (N=80) |
CPAP | 10.7 SD 3.6 | 9.2 SD 3.6 | 1.5 P<.001 |
Yes | |
| Engleman et al. 2002(21) |
MAS | 13.0 SD 4 | 12.0 SD 5 | 1.0 NR |
4.0 P<.001 |
No |
| Crossover study (N=48) |
CPAP | 15.0 SD 3 | 8.0 SD 5 | 7.0 NR |
Yes | |
| Randerath et al. 2002 (22) |
MAS | Not reported | ||||
| Crossover study (N=20) |
CPAP | |||||
| Tan et al. 2002 (23) |
MAS | 13.4 SD 4.6 | 9.0 SD 5.1 | 4.4 P<.001 |
0.9 P=NS |
Yes |
| Crossover study (N=24) |
CPAP | 13.4 SD 4.6 | 8.1 SD 4.1 | 5.3 P<.001 |
Yes | |
| Ferguson et al. 1997 (24) |
MAS | 10.3 SD 3.1 | 4.7 SD 2.6 | 5.6 P<.005 |
−0.4 P=NS |
Yes |
| Crossover study (N=24) |
CPAP | 11.0 SD 3.8 | 5.1 SD 3.3 | 5.9 P<.05 |
Yes | |
| Ferguson et al. 1996 (25) |
MAS | Not reported | ||||
| Crossover study (N=27) |
CPAP |
Note: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; MAS, mandibular advancement splint; NR, not reported; SD, standard deviation
The same group of authors published results for a RCT between 2007-2008.
In a crossover RCT, Tan et al (23) randomized 10 participants to MAS treatment first and 14 to receive CPAP first. They reported an intent-to-treat analysis that included three patients who dropped out of the study after the first arm of the study. In addition to AHI and ESS, they also reported quality of life and patient preference. Despite achieving better (although not statistically significant) outcomes for AHI and ESS for CPAP, 17 of 21 patients who completed the study (i.e. used both CPAP and MAS) preferred MAS over CPAP. Two separate RCTs were published by Ferguson et al. (24;25). The studies had similar designs, but they more recent reported more patient-centred outcomes such as compliance and measures of daytime sleepiness.
The overall GRADE assessment for the mandibular advancement versus CPAP studies was graded as low for the AHI outcome and very low for ESS. The most 2 for the GRADE predominant issues with these studies were that there were large losses to follow-up without intention-to-treat analyses and large reported standard deviations highlighting the variability in AHI and ESS outcomes (see Appendix tables).
Table 6: Description of RCTs comparing MAS to CPAP for the treatment of OSA.
| Study | Inclusion criteria | N (# males) |
Mean age |
Mean BMI |
Duration of study (wash out period) |
|---|---|---|---|---|---|
| Hoekema et al. 2008 (16-19)* |
AHI >5 | 103 | 48.8 (SD 9.5) |
32.3 (SD 6.1) |
8 weeks (NA) |
| Parallel arm study | |||||
| Lam et al. 2007 (20) |
AHI 5-40 ESS >9 for pts with AHI 5−20 |
101 (79) | 47.0 (SD 20.0) |
27.3 (SD 6.0) |
10 weeks (NA) |
| Parallel arm study | |||||
| Barnes et al. 2004 (10) |
Adequate dentition AHI 5−30 |
114 (91) | 47.0 (SD 9.6) |
31.1 (SD 5.3) |
3 months (2 weeks) |
| Crossover study | |||||
| Engleman et al. 2002 (21) |
AHI>5 and 2 or more OSA symptoms Adequate dentition |
51 (39) | 46 (SD 9) |
NR | 8 weeks (NR) |
| Crossover study | No patients working shift work | ||||
| Randerath et al. 2002 (22) |
AHI 5−30/h (mild to moderate) Clinical symptoms of OSA |
20 (16) | 56.5 (SD 10.2) |
31.2 (SD 6.4) |
6 weeks (NR) |
| Crossover study | |||||
| Tan et al. 2002 (23) |
AHI <50 (mild-moderate) >18 years Adequate dentition |
27 (20) (3 drop outs) |
50.9 (SD 10.1) |
31.9 (SD 6.8) |
2 months (2 weeks) |
| Crossover study | |||||
| Ferguson et al. 1997 (24) |
Symptomatic mild-moderate OSA (AHI 15-55/h) At least 10 teeth in maxillary |
24 (19) (4 dropouts) |
44 (SD 10.6) |
32.0 (SD 8.2) |
4 months (2 weeks) |
| Crossover study | and mandibular arches | ||||
| Ferguson et al. 1996 (25) |
Symptomatic mild-moderate OSA (AHI 15−50/h) |
27 (24) (2 dropouts) |
46.2 (SD 10.9) |
30.4 (SD 4.8) |
4 months (2 weeks) |
| Crossover study | |||||
Note: CPAP, continous positive airway pressure; OSA, obstructive sleep apnea SD, standard deviation; SEM, standard error of the mean; SF-36, short form health survey
The same group of authors published results for a RCT between 2007-2008.
At baseline, mean AHI values were moderate to severe in all studies. Clinical significance was achieved in the MAS arm in four of the eight studies comparing MAS to CPAP. In an additional study by Lam et al (20), clinical significance in the MAS treatment arm was borderline. In all eight RCTs CPAP reached clinical significance and in seven of these the mean AHI levels of patients in the CPAP arm was within normal range (i.e. AHI < 5). None of the studies reported that the mean AHI level reached normal range in patients in the MAS group.
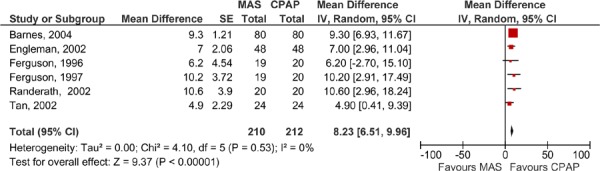
When the MAS and CPAP results for AHI were pooled across the studies, CPAP was significantly more effective than MAS (Figures 6 and 7). Again, two meta-analyses were run: one for the parallel arm studies and the first arm of the crossover studies where data were available (2), and another for the crossover studies alone.
Figure 6: AHI for MAS versus CPAP – parallel arm and first arm of crossover studies.
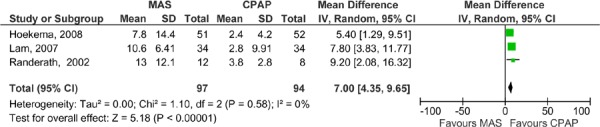
Figure 7: AHI for MAS versus CPAP – crossover studies.
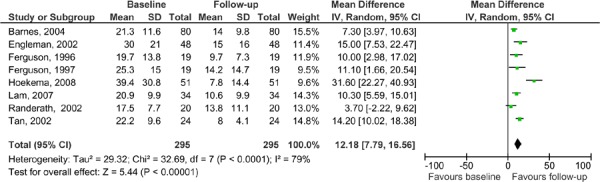
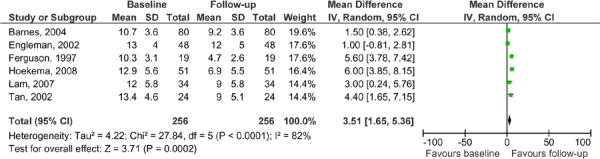
AHI measures were compared from baseline to follow-up for patients in the MAS arms. The results show that there is an improvement from baseline to follow-up, even though CPAP was shown to be significantly more effective than MAS. It is, though, important to note the limitations of this analysis. All of the studies were pooled, both the parallel arm and crossover studies. The patients in the crossover studies were ‘double-counted’ as they are represented in both arms of the study. The purpose of this is to demonstrate that even though CPAP is more effective than MAS in terms of AHI improvement, patients in the MAS group also improved with treatment compared to their baseline scores (see Figure 8).
Figure 8: AHI for MAS: baseline versus follow-up in MAS versus CPAP studies.
The ESS results for the MAS versus CPAP are presented in Table 8. Only six of eight RCTs comparing MAS to CPAP reported ESS and, with the exception of Engleman et al. (21), all reported clinically significant outcomes for both the MAS and CPAP (Engleman et al. only reported clinical significance for CPAP patients). When the ESS results were pooled, there were no significant differences between the MAS and CPAP groups (see Figures 9 and 10). When the baseline and follow-up ESS values were pooled for patients in the MAS arm there was an improvement in ESS score, however, there was also high statistical heterogeneity. It is important to note that in this analysis, all of the studies were pooled (both the parallel arm and crossover studies) with the patients in the crossover studies being ‘double-counted’ as they are represented in both study arms. The purpose of this analysis is to demonstrate that the ESS scores do not appear to change significantly between baseline and follow-up (see Figure 11).
Figure 9: ESS for MAS versus CPAP – parallel arm studies.
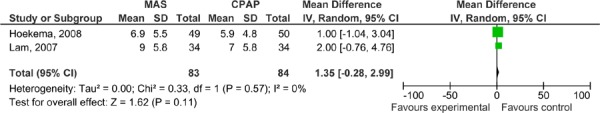
Figure 10: ESS for MAS versus CPAP – crossover studies.
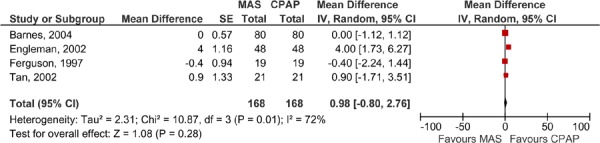
Figure 11: ESS for MAS: baseline versus follow-up in MAS versus CPAP studies.
MAS versus Surgery
The Medical Advisory Secretariat only identified one RCT that compared oral appliances to surgery. This trial has been published several times reporting various outcomes at different time points (26-30). Essentially, the trial compared MAS to uvulopalatopharyngoplasty (UPPP) in patients diagnosed with mild to moderate OSA, this was defined as an AHI of >5 and <25. The primary outcome of this trial was success rate at one year, defined as a ≥50% reduction in AHI. The authors reported that the results were presented using the intent-to-treat principle and presented according to the arm that the patient was initially randomized to, regardless of crossover. The results did not, however, include drop-outs and thus it wasn’t a true intent-to-treat analysis. Of the 95 randomized patients, 80 completed 1-year follow-up and 72 completed 4-year follow-up. The trial was powered to test the hypothesis that the oral appliance would have a success rate of 80% compared to 50% for UPPP. The results were found to be better than hypothesized for both arms with a 95% success rate for MAS and a 70% success rate for UPPP at 1 year. After 4 years, the success rates dropped to 81% for oral appliances and 53% for UPPP. (26) Ten of the 40 patients who underwent UPPP and completed 4-year follow-up where fitted for oral appliances during the follow-up period. There was a 60% success rate reported among these 10 patients.
The overall GRADE assessment for this study was ‘very low’ for the AHI outcome because of the large number of dropouts, the fact that only one RCT comparing MAS to surgery was identified, and because the study only included men (see Appendix 2 for detailed GRADE tables).
Table 9: Description of the RCT comparing MAS to surgery for the treatment of OSA.
| Study | N | Inclusion criteria | Age | Baseline AHI (mean) |
Baseline ESS (mean) |
Mean BMI |
Mean follow- up |
Success rate |
|---|---|---|---|---|---|---|---|---|
| Walker- Engstrom, 2002 (26-30)* |
95 (All male) |
Mild to moderate OSA, (<5 to >25 AHI) 20 to 65 years, Adequate dentition to hold MAS |
49.3 | 18.2 | Not reported |
26.9 | 4.1 years (3.8−5.4) |
1 year: 95% for MAS 70% for UPPP 4 years: 81% for MAS 53% for UPPP |
Note: AHI, apnea hypopnea index; BMI, body mass index; MAS, mandibular advancement splint; UPPP, uvulopalatopharyngoplasty
The same group of authors published results for a RCT between 1999-2003.
Tongue Repositioning Devices
The RCT by Deane et al. (in press) (31) comparing tongue repositioning devices to MAS was powered to detect a 50% reduction in both groups (see Table 11). The authors reported the results comparing the outcomes to baseline values, not by comparing TRD to MAS. Compared to baseline TRD and MAS both exhibited significant improvements in the AHI and ESS measures (Table 12), though it is unclear which is more effective at improving these outcomes. The Medical Advisory Secretariat calculated whether there was a significant difference between the mean AHI and ESS values based on data reported in the study. For AHI, no significant differences between TRD and MAS were found (P = .62), but for ESS, there was a significant difference in favour of MAS (P = .04). These calculations need to be interpreted with caution as they were calculated post hoc based on summary data, not the original raw data collected during the study.
Table 11: Outcomes of RCTs comparing tongue repositioning devices to MAS for the treatment of OSA.
| Study | Type of study | TRD | AHI (h) | Mean Difference |
TRD | ESS | Mean Difference |
|---|---|---|---|---|---|---|---|
| MAS | MAS | ||||||
| Deane, 2009 (in press) (31) | Crossover study | 13.2 (SD 10.8) |
11.7 (SD 8.9) |
1.5 P=0.62* |
5.9 (SD 4.6) |
3.5 (SD 2.4) |
2.4 P=0.04* |
Note: AHI, apnea hypopnea index; ESS, Epworth Sleepiness Scale; MAS, mandibular advancement splint; SD, standard deviation; TRD, tongue repositioning device
Medical Advisory Secretariat calculation based on summary data.
Table 12: Outcomes of RCTs comparing tongue repositioning devices to placebo devices for the treatment of OSA.
| Study | Type of study | Respiratory Disturbance Index (h) | ESS | ||||
|---|---|---|---|---|---|---|---|
| TRD | Placebo | Mean Difference |
TRD | Placebo | Mean Difference |
||
| Dort et al. 2008 (32) | Crossover study | 8.9 (SD 7.6) |
13.5 (SD 15.4) | −4.9 P=0.02 |
10.9 (SD 4.4) |
10.3 (SD 4.3) |
0.65 P=25 |
Note: ESS, Epworth Sleepiness Scale; SD, standard deviation; TRD, tongue repositioning device
The RCT by Dort et al (32) compared tongue repositioning devices to placebo devices in 38 patients with mild to moderate OSA. They reported a significant difference between the groups in terms of the respiratory disturbance index, but no significant changes between the groups in terms of ESS score (see Table 13).
Table 13: Adverse events reported in RCTs comparing MAS to placebo or CPAP for the treatment of OSA.
| Adverse events | ||
|---|---|---|
| Study | MAS | Placebo |
| Petri, 2008 (8) |
|
|
| Blanco, 2005 (9) |
|
|
| Gotsopoulos, 2004 (11-13) |
|
|
| Johnston, 2002 (14) |
|
|
| Mehta, 2001 (15) |
|
|
| Study | MAS | CPAP |
| Lam et al. 2007 (20) |
|
|
| Engleman et al. 2002 (21) |
|
|
| Randerath et al. 2002 (22) |
|
|
| Tan et al. 2002 (23) |
|
|
| Ferguson et al. 1997 (24) |
|
|
| Ferguson et al. 1996 (25) |
|
|
Adverse Events
Several studies reported adverse events associated with oral appliances (summarized in Table 14). The most common adverse events among patients using MAS were jaw or TMJ pain, excessive salivation and mouth dryness. Only Ferguson et al. (25) reported a severe adverse event in a patient using the splint, although details of the event were not described). All of the other studies that reported adverse events in patients using MAS reported that the events were generally mild and transient.
Table 14: Device compliance in RCTs of oral appliances.
| Study | Mean time (hours) device worn per night | Mean nights device worn per week | ||
|---|---|---|---|---|
| MAS vs. Placebo | MAS | Placebo | MAS | Placebo |
| Petri, 2008 (8) | Not reported | Not reported | Not reported | Not reported |
| Blanco, 2005 (9) | 7.7 SD 0.5 | 6.5 SD 1.4 | Not reported | Not reported |
| Barnes, 2004* (10) | 5.5 SD 0.3 | Not reported | 5.3 SD 0.3 | Not reported |
| Gotsopoulos, 2004 (11) | 6.7 SD 0.1 | 6.7 SD 0.1 | 97% of nights | 96% of nights |
| Johnston, 2002 (14) | 79% used MAS ≥4 hours/night |
Not reported | Not reported | Not reported |
| Mehta, 2001 (15) | Not reported | Not reported | Not reported | Not reported |
| MAS vs. CPAP | MAS | CPAP | MAS | CPAP |
| Hoekema, 2008 (17) | 6.9 SD 1.0 | 6.5 SD 1.6 | 6.7 SD 1.0 | 6.7 SD 0.8 |
| Lam, 2007 (20) | 6.4 SD 2.0 | 4.2 SD 1.0 | 5.2 SD 3.0 | 4.4 SD 1.0 |
| Barnes, 2004 (10) | 5.5 SD 0.3 | 3.6 SD 0.3 | 5.3 SD 0.3 | 4.2 SD 0.3 |
| Engleman et al. 2002 (21) | 21% reported >3 hours/night |
27% reported >3 hours/night |
Not reported | Not reported |
| Randerath et al. 2002 (22) | 33% reported >8 hours/night 53% 6−7 hours 7% 4−5 hours |
9% reported >8 hours/night 27% 6−7 hours 64% 4−5 hours |
Not reported | Not reported |
| Tan et al. 2002 (23) | Not reported | Not reported | Not reported | Not reported |
| Ferguson et al. 1997 (24) | ~96% used MAS >75% of the night† |
~78% used CPAP >75% of the night† |
~92% used MAS >75% of nights† |
~75% used CPAP >75% of nights† |
| Ferguson et al. 1996 (25) | Not reported | Not reported | Not reported | Not reported |
| MAS vs. surgery | MAS | Surgery | MAS | Surgery |
| Walker-Engstrom, 2002 (26) | Not reported | N/A | 6.1 SD not reported | N/A |
| TRD | TRD | Placebo | TRD | Placebo |
| Deane et al. 2009 (in press) (31) | 27% used the device every night for ≥6 hours |
MAS: 82% used the device every night for ≥6 hours |
Not reported | Not reported |
| Dort et al. 2008(32) | 6.7 SD 1.4 | 6.4 SD 1.7 | Not reported | Not reported |
The expected adverse events associated with CPAP were reported in the studies where MAS was compared with CPAP. Adverse events for CPAP include the noise, dryness in the nose and mouth, and nasal stuffiness.
Table 10: Description of RCTs investigating tongue repositioning devices for the treatment of OSA.
| Study | Inclusion criteria | N (# males) |
Age | Baseline AHI (mean) |
Baseline ESS (mean) |
Mean BMI |
Intervention | Control | Study Duration |
|---|---|---|---|---|---|---|---|---|---|
| Deane, 2009 (in press) (31) |
|
27 (20) |
49.4 (SD 11.0) |
27.0 (SD 17.2) |
8.6 (SD 5.1) |
29.3 (SD 5.6) |
TRD | MAS | 4 weeks with each device; 1 week washout in between |
| Dort et al. 2008 (32) |
|
38 (25) |
48 (SD 10.0) |
RDI 15.5 (SD 17.7) |
12.4 (SD 4.5) |
29.4 (SD 5.7) |
TRD | Placebo device | 1 week each device; 1 week washout n between |
Note: AHI, apnea hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; MAS, mandibular advancement splints; RDI, respiratory disturbance index; SD, standard deviation; TRD, tongue repositioning device
Compliance
In 2008 the UK’s National Institute for Health and Clinical Excellence (NICE) published a systematic review of the effectiveness of CPAP. One of the questions addressed was the issue of adherence or compliance with CPAP. They reported that across seven studies the mean rate of compliance was 71% up to 12 months (range 64%-83%) and the mean rate of compliance was 79% (range 68-90%) at 12 months or more. (1) Compliance was also examined in a systematic review by Ferguson et al. (4) who pooled compliance rates for oral appliances and reported a wide range of rates from 25% to 100%. When they pooled the results of 10 studies (sample sizes ranging from 8 to 121 patients), they found that the median use of the device was 77% at 12 months. In the studies comparing MAS to CPAP, three of the studies reported compliance in terms of the hours the device was worn per night and the nights per week the device was worn. Based on their results, there does not appear to be a significant difference in compliance between MAS and CPAP (Figures 12 and 13).
Figure 12: Comparison of the number of hours per night MAS and CPAP were worn.
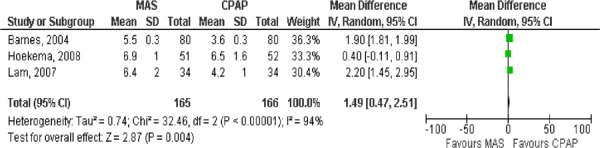
Figure 13: Comparison of the number of nights per week MAS and CPAP were used.
These results should be interpreted with caution as the follow-up periods are different across the studies – a difference that was not accounted for when calculating the weighted means listed above. It’s also important to note that under controlled study conditions, patients may be more likely to adhere to treatment (i.e. use of CPAP or MAS) than they would outside the setting of a clinical trial. (33)
Patient Preference
Eight of the RCTs included patient preference and/or satisfaction as an outcome (see Table 16). These were, however, differently measured in every study and thus it’s not possible to quantitatively summarize the results and there was no clear trend in preference for either MAS or CPAP. For instance, in the study by Engleman et al. (21), it was found that 52% of the sample population preferred CPAP over MAS, while in the studies by Ferguson et al. (24;25) a slight preference for MAS was reported (although patients were satisfied with both devices).
Table 16: Base case analysis results for dental device and CPAP costs (GBP), QALYs and ICERs.
| Conservative management, £ |
Dental device, £ | CPAP, £ | |
|---|---|---|---|
| Treatment costs | 21 | 1,726 | 2,465 |
| RTA costs | 2,201 | 1,138 | 904 |
| Cardiovascular costs (CHD + stroke) | 5,918 | 5,932 | 5,931 |
| Total costs | 8,140 | 8,797 | 9,301 |
| Total QALYs | 11.93 | 12.26 | 12.39 |
| ICER (GBP) | 2,000 | 3,899 | |
| ICER (CAD) | 4,420 | 8,617 |
Source: Table 36, McDaid et al., 2009. (1)
Table 15: Patient preference outcomes in RCTs of oral appliances.
| Study | Patient Preference/Satisfaction | |
|---|---|---|
| MAS versus Placebo | ||
| Gotsopoulos et al. 2004 (11) | MAS: | |
| ||
| ||
| ||
| Placebo: | ||
| ||
| ||
| ||
| Mehta et al. 2001 (15) |
|
|
| MAS versus CPAP | ||
| Hoekema et al. 2008 (17) | Satisfaction measured on 10-point scale. Mean scores: | |
| ||
| ||
| Barnes et al. 2004 (10) |
|
|
| ||
| Engleman et al. 2002 (21) |
|
|
| ||
| Tan et al. 2002 (23) |
|
|
| Ferguson et al. 1997 (24) |
|
|
| ||
| Ferguson et al. 1996 (25) | CPAP: | |
| ||
| ||
| MAS: | ||
| ||
| ||
Conclusion
In summary:
MAS were found to be less effective than CPAP when AHI is used as the outcome of interest. AHI, although not without limitations, is the most consistently reported objective outcome to measure OSA. MAS devices were also found to be significantly better at reducing AHI levels than placebo devices; however, whether this reduction is clinically meaningful is uncertain.
The ESS was unable to achieve statistical significance in comparisons between MAS and CPAP or between MAS and placebo. Nonetheless, after treatment with either MAS or CPAP, patients seem to be able to achieve normal ESS levels. Although, again, it’s important to note that there are substantial limitations to ESS, such as the subjective nature of the scale and its low construct validity (i.e. it’s unclear if the scale is an accurate measure of sleepiness).
The adverse events associated with MAS devices in the RCTs were common, but mostly mild and transient. Jaw discomfort was the most commonly reported adverse event.
Based on the results of the RCTs included in this analysis, compliance does not seem to be better or worse with MAS or CPAP. Similarly, no clear favourite was found among patients for MAS or CPAP in those studies reporting preference and satisfaction.
Existing Guidelines
The College of Physicians and Surgeons of Ontario (34) published clinical practice parameters for sleep medicine in Ontario. The practice parameters recommend that any patient that presents with signs and symptoms should be offered full overnight monitoring in a sleep laboratory. The conservative treatment options include weight loss, avoidance of alcohol and nocturnal sedation, avoidance of the supine position, smoking cessation and treatment of nasal obstruction. CPAP is listed as a medical treatment option and oral appliances are listed as a dental treatment option. Several surgical options are also listed including genioglossus advancement, somnoplasty, laser assisted uvuloplatoplasty, maxillofacial surgery and nasal surgery.
BlueCross BlueShield in Tennessee has published criteria for the use of oral appliances in the treatment of OSA. Its criteria include that the appliance must be ordered by a physician and custom-made. The patient must also have adequate dentition to anchor the appliance, an unobstructed nasal airway, and an AHI >5. They also stipulate that in order to be eligible to receive an oral appliance, patients must not have temporomandibular joint dysfunction, periodontal disease, severe OSA (which is not explicitly defined) or a systemic disease (examples given include cardiac, respiratory, hypertension, or neurological pathology). (35)
-
In 2005 Kushida et al. (36) updated the practice parameters for the treatment of OSA with oral appliances on behalf of the American Academy of Sleep Medicine (AASM). The authors made several recommendations including:
OSA should be clinical established through polysomnography.
Oral appliances should be fitted by a qualified dental professional trained in oral health care, including knowledge of temporomandibular joint pathology and dental occlusion.
Oral appliances are appropriate for patients who do not respond to behaviour modification (such as weight loss and sleep position change).
Oral appliances should be used when CPAP is not tolerated, although patients with severe OSA should try CPAP first.
Patients should be followed-up with polysomnography to assess the effectiveness of the oral appliance.
Patients should have follow-up visits with their dentist every 6 months for the first year, then annually thereafter to monitor patient adherence as well as to evaluate appliance deterioration and overall oral and temporomandibular joint health.
The Scottish Intercollegiate Guidelines Network published guidelines for the management of OSA in 2003. (37) They recommended that CPAP be offered first for the management of OSA, especially those with moderate or severe OSA, but if it was not tolerated then oral appliances may be considered. It also recommended that if the OSA was mild and the patient did not suffer from daytime sleepiness, then oral appliances may be considered.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for all in-hospital stay costs for the designated International Classification of Diseases-10 (ICD-10) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits for physician fees, laboratory fees from the Ontario Laboratory Schedule of Fees, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effectiveness analyses, a discount rate of 5% is used as per the Canadian Agency for Drugs and Technologies in Health.
Downstream costs: All costs reported are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature. In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Literature Review
A broad range of studies assessing the cost-effectiveness, economic evaluations, modelling studies and analysis of administrative data are considered in this systematic review. The items identified are based on the current review of clinical effectiveness of CPAP and oral appliances for OSA in this report. All chosen studies were identified as being CUAs (CEA using utilities) and compare CPAP to “no treatment” (no CPAP) or dental device. Results of the search yielded only one study comparing dental devices directly with CPAP. (1)
The HTA study by McDaid et al. uses HRQoL measurements based on effectiveness reviews of both CPAP and dental devices for the treatment of OSA. Specifically, an association is developed using regression models describing patient utility values, adjusting for ESS score, ESS baseline score and baseline utility as measured by EQ-5D or SF-6D. ESS measurements were chosen by McDaid et al. as they are most frequently reported in clinical effectiveness trials of both CPAP and dental devices. Several issues exist, however, with the interpretation of the ESS questionnaire items and responsiveness of the derived data. It is argued that the ESS eight-question measurement of sleepiness is not objective and it cannot be used to diagnose pathological sleepiness due to lack of construct validity and consistency of responses over time by the same individual. (38;39) In order to obtain more objective measurements of sleepiness, utilities to be used in a CUA could instead be derived from AHI scores.
Recent CUAs for Oral Appliances for OSA
Despite the limitation of using the ESS score, the HTA developed by McDaid et al. is presented in the current economic review and summarized below. The authors’ evaluation is designed for three interventions: 1) CPAP, 2) dental devices, and 3) conservative management, the latter being defined as usual care for OSA without assistive devices. This implies that only a general practitioner or family physician consultation(s) were available to patients. The perspective taken is that of a health care system (public payer), specifically that of the UK’s NHS and Personal Social Services (PSS).
Base case
The base case used in this analysis consists of males of average age 50, diagnosed with OSA (or equivalently Obstructive Sleep Apnea-Hypopnea Syndrome; OSAHS) with an AHI greater than 15. Subgroup analyses are also undertaken by gender, OSA severity as measured by ESS score (“mild” with mean ESS of 7, “moderate” with mean ESS of 13, “severe” with mean ESS of 16), and other baseline patient characteristics. Secondary analyses were performed to assess the sensitivity of model results to changes in parameters, including changes in cardiovascular events, road traffic events, lifespan of devices, female patients, and patients aged 35 or 65.
Health states
The model is as a Markov state transition cohort model. Four health states are defined:
1) OSA,
2) OSA post Coronary Heart Disease (CHD),
3) OSA post stroke, and
4) death.
The model characterizes lifetime costs and the effects of these health states in yearly cycles. Transitions between the health states take the form of CHD or stroke events, or Road Traffic Accidents (RTAs). CPAP and dental devices have a beneficial effect on sleepiness and blood pressure (i.e. lower blood pressure), which in turn affect the incidence of traffic accidents and cardiovascular-related events. Health states 3 and 4 are used to represent the increased mortality and morbidity associated with experiencing CHD or a stroke.
Costs
The costs of the three interventions (CPAP, dental device, and conservative management) for OSA include the initial cost of the intervention and the associated costs of continuing care. Overhead costs, staff time, and device costs with titration and maintenance are included in the list of resources used; outpatient visits, nurse and physician consultations are also included. CPAP machines are assumed to have a 7-year lifespan and dental devices a 2-year lifespan, with replacement of the devices being necessary thereafter for both interventions. Published references are used by McDaid et al. for the costs of stroke, CHD and RTAs events. The cost of non-compliance for CPAP machines is estimated as the proportion of machines not returned (10%), and for dental devices is estimated as the cost of devices no longer used. These non-compliance costs were taken from a survey of clinicians.
The UK’s health care system perspective for dental devices differs slightly from the public payer perspective of Ontario in the partial coverage of dental services by the NHS. The cost of a mandibular advancement device was estimated according to the national reimbursement rates of 12 units of dental service by the NHS (Band 3), for a total of 250.92 GBP (554.53 CAD). All costs converted to Canadian dollars use an average annual Bank of Canada exchange rate (GBP to CAD). (40)
Treatment effects
The effects of the three treatments/interventions for OSA are valued in terms of utilities (HRQoL weights) derived from two regression equations based on the responses to the EQ-5D and SF-6D (SF-36) questionnaires, respectively. The regression models predict the change in utility or disutility of OSA patients based on RCT data, adjusting for the covariates of ESS score (post intervention), baseline ESS (pre intervention), and baseline utility. The risk of CHD or stroke in the model is determined by the use of Framingham risk equations, where changes in systolic and diastolic blood pressure are affected by changes in ESS score based on treatment. The difference in risk of RTAs from treatment is taken from a meta-analysis of before-and-after studies reported by Ayas et al. (41), specifically for CPAP interventions. It is important to note that while utility estimates and risk differences are based on RCT or before-and-after studies, mean differences of effect for certain comparisons (i.e. CPAP versus dental device, or dental device versus conservative management) are obtained indirectly.
Compliance
The majority of the trial data reviewed by McDaid et al. lists compliance rates for CPAP use with a follow up time of 12 weeks or less. Estimation of compliance is based on an observational study over 6 years in a cohort of Scottish patients with a median age of 50 and an average baseline ESS score of 12. Continued use of CPAP in years 1, 2, 3, and 4 after treatment are 84%, 74%, 73%, and 68% respectively. Compliance rates for dental devices are assumed to follow the same distribution as that of CPAP.
Mortality rates
McDaid et al. uses mortality rate estimates from UK life tables produced by the Government Actuary Department. The age group-specific rates are derived by reducing all-cause hazard estimates by the proportion of deaths due to cardiovascular disease or ischemic heart disease. For OSA patients who experienced CHD or stroke, relative risks from a literature review are applied to the above non-cardiovascular/ischemic heart disease rates. RTA mortality is taken from the Department of Transportation statistics related to road traffic accidents in the UK in 2004.
Cost-effectiveness results
The results from of the HTA from McDaid et al. are shown in Table 16. Both CPAP and dental devices were found to be cost-effective, however, CPAP is associated with higher costs and greater effect (Quality Adjusted Life Years; QALYs). The CUA values reported in the table below are for a base case of men aged 50. The results concerning cost-effectiveness are found to be similar for a range of secondary analyses including the following model parameter changes:
a cohort of women aged 50,
a younger cohort with mean age 35,
an older cohort with mean age 65,
CPAP machine lifespan is shortened to 5 years, and
exclusion of CHD and stroke events.
In all scenarios, dental devices have the lower ICER value, although CPAP is the more effective intervention. It is important to mention the evaluation of dental devices versus CPAP performed by McDaid et al. is only for moderate OSA patients. Mild or severe OSA patients are not included in the base case analyses (for dental devices) as all trials comparing CPAP with dental devices had mean baseline ESS scores that would classify patients as having moderate OSA.
Discussion
The economic evaluation of McDaid et al. published in 2009 assessed CPAP and mandibular advancement oral appliances (dental devices) treatment options for OSA patients in the UK. Results of the study suggest that both oral appliances and CPAP are cost-effective interventions for moderate OSA patients. CPAP is more costly and more effective when compared to dental devices, with a strictly higher ICER value of approximately 4,000 GBP (9,000 CAD) per QALY compared to 2,000 GBP (4,800 CAD) per QALY.
It’s unclear whether dental devices are as cost-effective as CPAP in cases of severe OSA, however, CPAP shows the greatest benefit to patients according to both ESS and AHI outcome measures. The results of the McDaid et al. study are limited due to the use of only ESS to define OSA severity. No cost-effectiveness evaluation has used AHI to characterize the benefits of using dental devices or CPAP. Further research is required to determine the feasibility of using AHI with HRQoL weights and utilities.
In Ontario, the total cost of an oral appliance for the first year is approximately $2,000, which includes device and follow up consultation costs with an additional cost of $175 for the initial consultation (source: expert consultant). For CPAP machines, the Assistive Devices Program within Ontario’s Ministry of Health and Long Term Care covers 75% of the base CPAP device cost for OSA patients (approximately $900). Private insurance may cover the remaining CPAP device costs but will not cover any of the costs of oral appliances.
Ontario Health System Impact Analysis
Considerations
In Ontario, physicians have a legal obligation to notify the Ministry of Transportation of those patients who cannot safely operate a motor vehicle. Individuals diagnosed with OSA who are not compliant with treatment are at risk of having their licenses suspended until they can demonstrate improvement in their sleep disordered breathing. (42) The Canadian Medical Association publishes a guide for physicians to help them determine a patient’s medical fitness to drive. (43) According to the guide, patients with moderate to severe OSA who are not compliant with treatment are at increased risk of RTAs and should not drive any type of vehicle. Patients with mild OSA and no daytime somnolence are considered safe to drive motor vehicles.(43)
Diffusion on a national level is small because oral appliances are not covered under any provincial insurance plan. According to a clinical expert, private insurance companies typically do not cover the costs of oral appliances for OSA.
Ontario Context
The Assistive Devices Program within Ontario’s Ministry of Health and Long-Term Care received approximately 28,000 applications for CPAP in 2008. The number of applications has been steadily increasing over the past several years (Personal communication, April 2009). The Assistive Devices Program pays for a portion of the cost of CPAP and, in many cases, private insurance will cover the remainder of the cost, while, as mentioned previously, oral appliances are not covered by private insurance.
Appendices
Appendix 1: Search Strategy
Search date: February 3-4, 2009
Databases searched: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library (all via OVID); Ebsco CINAHL, CRD/INAHTA
Database: Ovid MEDLINE(R) <1950 to January Week 3 2009>
Search Strategy:
exp Sleep Apnea Syndromes/ (15446)
(sleep adj2 (apnea$ or hypopnea$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (16681)
1 or 2 (17067)
exp Tongue/ (14131)
exp Tongue Habits/ (754)
(tongue adj2 suction).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3)
exp Splints/ (6422)
exp Orthodontic Appliances/ (15545)
exp Mandibular Advancement/ (804)
exp “Prostheses and Implants”/ (270347)
((oral or intraoral or intra-oral or dental or orthodontic or mandibula or mandibular or tongue) adj2 (advancement or re?position* or device* or appliance* or splint* or retainer* or prosthes*)).mp. (26963)
(SomnoDent or Klearway or Nocturnal Airway Patency Appliance or Patency Appliance or SNOAR or Thornton Oral Appliance or TOA-TAP or Herbst Appliance or Z-Appliance or Silencer or EMA Titratable or Snore-Aid Plus or OASYS or Equilizer or Jasper Jumper or PM Positioner or Snore-no-more or Adjustable soft palate lifter or Elastometric or Snore Guard or Silent Night or TheraSnore or SnoreEx).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1765)
or/4-12 (310022)
limit 13 to (english language and humans and yr=“1990 - 2009”) (142778)
3 and 14 (910)
limit 15 to (controlled clinical trial or meta analysis or randomized controlled trial) (72)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (38983)
(health technology adj2 assess$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (719)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (76238)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (556834)
exp Double-Blind Method/ (98197)
exp Control Groups/ (1114)
exp Placebos/ (27289)
(RCT or placebo? or sham?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (167269)
or/16-24 (725897)
25 and 15 (124)
Database: EMBASE <1980 to 2009 Week 05>
Search Strategy:
exp Sleep Apnea Syndrome/ (15018)
(sleep adj2 (apnea$ or hypopnea$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (16318)
1 or 2 (16318)
exp Tongue/ (6709)
exp orthodontic device/ or exp periodontic device/ (535)
exp Dental Device/ or exp Mandible Reconstruction/ (1989)
exp Splint/ (1664)
((oral or intraoral or intra-oral or dental or orthodontic or mandibula or mandibular or tongue) adj2 (advancement or re?position* or device* or appliance* or splint* or retainer* or prosthes*)).mp. (1992)
(tongue adj2 (retain* or suction)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (30)
exp Maxillofacial Prosthesis/ or exp Prosthesis/ or exp Mandible Prosthesis/ (8647)
(SomnoDent or Klearway or Nocturnal Airway Patency Appliance or Patency Appliance or SNOAR or Thornton Oral Appliance or TOA-TAP or Herbst Appliance or Z-Appliance or Silencer or EMA Titratable or Snore-Aid Plus or OASYS or Equilizer or Jasper Jumper or PM Positioner or Snore-no-more or Adjustable soft palate lifter or Elastometric or Snore Guard or Silent Night or TheraSnore or SnoreEx).mp. (1848)
or/4-11 (22302)
3 and 12 (685)
limit 13 to (human and english language and yr=“1990 - 2009”) (510)
Randomized Controlled Trial/ (165071)
exp Randomization/ (26467)
exp RANDOM SAMPLE/ (1395)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (297798)
(health technology adj2 assess$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (670)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (64531)
Double Blind Procedure/ (71178)
exp Triple Blind Procedure/ (12)
exp Control Group/ (2779)
exp PLACEBO/ or placebo$.mp. or sham$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (212600)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (429709)
(control$ adj2 clinical trial$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (282757)
or/15-26 (795016)
27 and 14 (117)
CINAHL
# Query Limiters/Expanders Last Run Via Results
S18 (S12 and S17) Limiters - Published Date from: 199001-200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 41
S17 S13 or S14 or S15 or S16 Limiters - Published Date from: 199001-200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 109158
S16 (control* N2 clinical trial*) OR meta analy* or metaanaly* or pooled analysis or (systematic* N2 review*)
or published studies or medline or embase or data synthesis or data extraction or cochrane Limiters -
Published Date from: 199001-200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 28964
S15 (MH “Meta Analysis”) OR (MH “Systematic Review”) or (MH “Double-Blind Studies”) or (MH “Single-
Blind Studies”) or (MH “Triple-Blind Studies”) or (MH “Placebos”) or (MH “Control (Research)”) Limiters
- Published Date from: 199001-200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 27994
S14 random* or sham* or RCT or health technology N2 assess* Limiters - Published Date from: 199001-
200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 83626
S13 (MH “Random Assignment”) or (MH “Random Sample+”) Limiters - Published Date from: 199001-
200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 36449
S12 (S3 and S11) Limiters - Published Date from: 199001-200912; English Language
Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 270
S11 S4 or S5 or S6 or S7 or S8 or S9 or S10 Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 27036
S10 SomnoDent or Klearway or Nocturnal Airway Patency Appliance or Patency Appliance or SNOAR or
Thornton Oral Appliance or TOA-TAP or Herbst Appliance or Z-Appliance or Silencer or EMA Titratable or Snore-
Aid Plus or OASYS or Equilizer or Jasper Jumper or PM Positioner or Snore-no-more or Adjustable soft palate
lifter or Elastometric or Snore Guard or Silent Night or TheraSnore or SnoreEx Search modes - Boolean/Phrase
Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 37
S9 (oral or intraoral or intra-oral or dental or orthodontic or mandibula or mandibular or tongue) AND
(advancement or re?position* or device* or appliance* or splint* or retainer* or prosthes*) Search modes -
Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 4324
S8 (MH “Prostheses and Implants+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 22523
S7 (MH “Orthodontic Appliances+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 974
S6 (MH “Splints”) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 1060
S5 tongue N2 suction Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 0
S4 (MH “Tongue+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 831
S3 (S1 or S2) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 3253
S2 (sleep N2 apnea*) or (sleep N2 hypopnea*) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 3228
S1 (MH “Sleep Apnea Syndromes+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Database - CINAHL;Pre-CINAHL 2962
Appendix 2: GRADE Analysis
Appendix Table 1. MAS versus Placebo.
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | ||||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations |
MAS | Placebo | Absolute (95% CI) | Quality | Importance |
| Apnea-Hypopnea Index (follow-up 0−6 months; measured with: AHI at follow-up; range of scores: 0->30; Better indicated by less) | |||||||||||
| 5 | randomized trial | very serious1,2,3 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 95 | 89 | MD −10.2 (−16.12 to −4.28) | ⊕⊕ΟΟ LOW |
CRITICAL |
| Epworth Sleepiness Scale (follow-up 0-6 months; measured with: ESS at follow-up; range of scores: 0−24; Better indicated by less) | |||||||||||
| 4 | randomized trial | very serious1,2,3 | no serious inconsistency | serious4 | no serious imprecision | none | 82 | 76 | MD −1.96 (−4.86 to 0.94) | ⊕ΟΟΟ VERY LOW |
IMPORTANT4 |
No blinding in some studies
Many studies had large losses to follow-up with no intent-to-treat analyses
Although all studies had similar mean AHI values, the standard deviations were high for all studies indicating varying degrees of AHI severity in the sample.
The ESS is a subjective measure that may not be sensitive enough to detect subtle changes in daytime sleepiness.
Appendix Table 2. MAS versus CPAP.
| Quality assessment | Summary of findings | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | |||||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations |
MAS | CPAP | Relative (95% CI) |
Absolute | Quality | Importance |
| Apnea-Hypopnea Index--Parallel/First Arm (follow-up 0−6 months; measured with: AHI at follow-up; range of scores: 0->30; Better indicated by less) | ||||||||||||
| 3 | randomized trial | very serious1,2,3 | no serious inconsistency | no serious indirectness | no serious imprecision | none | 97 | 94 | − | MD 7 (4.35 to 9.65) |
⊕⊕ΟΟ LOW |
CRITICAL |
| Epworth Sleepiness Score--Parallel/First Arm (follow-up 0−6 months; measured with: ESS at follow-up; range of scores: 0-24; Better indicated by less) | ||||||||||||
| 2 | randomized trial | very serious1,2,3 | no serious inconsistency | serious4 | no serious imprecision | none | 83 | 84 | − | MD 1.35 (−0.28 to 2.99) |
⊕ΟΟΟ VERY LOW |
IMPORTANT4 |
Many studies had large losses to follow-up
Although all studies had similar mean AHI values, the standard deviations were high for all studies indicating varying degrees of AHI severity in the sample.
No blinding
The ESS is a subjective measure that may not be sensitive enough to detect subtle changes in daytime sleepiness.
Appendix Table 3. MAS versus Surgery (UPPP).
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | ||||||||||
| No of studies |
Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations |
MAS | UPPP | Absolute (95% CI) |
Quality | Importance |
| Apnea-Hypopnea Index (follow-up mean 4 years; measured with: AHI at follow-up; range of scores: 0->30; Better indicated by less) | |||||||||||
| 1 | randomized trial | serious1 | serious2 | no serious indirectness | serious3 | none | 49 | 46 | MD 7.0 (−8.4 to −5.6) |
⊕ΟΟΟ VERY LOW |
CRITICAL |
The study did not include the 23 dropouts in the final analysis
There was only RCT identified that compared MAS to surgery
There is only 1 RCT identified that compared MAS to surgery, and this RCT only included men. So there is no evidence specifically addressing women with OSA.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Oral appliances for obstructive sleep apnea: an evidence-based analysis. Ontario Health Technology Assessment Series 2009;9(5).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-9961-3 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: www.health.gov.on.ca/ohtas.
List of Abbreviations
- AASM
American Academy of Sleep Medicine
- ADP
Assistive Devices Program
- AHI
Apnea Hypopnea Index
- AUC
Area under curve
- BMI
Body Mass Index
- CAD
Canadian dollars
- CEA
Cost-effectiveness analysis
- CHD
Coronary Heart Disease
- CI
Confidence interval(s)
- CPAP
Continuous positive airway pressure
- CUA
Cost-utility analysis
- ESS
Epworth Sleepiness Scale
- GPS
Pound sterling
- HRQoL
Health-related quality of life
- HTA
Health technology assessment
- ICER
Incremental cost-effectiveness ratio
- MAS
Mandibular advancement splint
- NHS
National Health Service (United Kingdom)
- OHTAC
Ontario Health Technology Advisory Committee
- OR
Odds ratio
- OSA
Obstructive sleep apnea
- PSS
Personal Social Services
- QALY
Quality-adjusted life years
- RCT
Randomized controlled trial
- RDI
Respiratory distress index
- RR
Relative risk
- RTA
Road traffic accident
- SD
Standard deviation
- SROC
Summary receiver operating characteristic
- TMJ
Temoporomandibular joint
- TRD
Tongue repositioning device
- UPPP
Uvulopalatopharyngoplasty
References
- 1.McDaid C, Griffin S, Weatherly H, Duree K, van der BM, van HS, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13(4):1–119. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 2.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2008;(Issue 3) Art. No.: CD004435. DOI: 10.1002/14651858.CD004435.pub3. [Google Scholar]
- 3.ECRI. Mandibular advancement devices for obstructive sleep apnea -14 2002 Plymouth Meeting, PA, ECRI. [Google Scholar]
- 4.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep. 2006;29(2):244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 5.Hoekema A, Stegenga B, de Bont LG. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004;15(3):137–155. doi: 10.1177/154411130401500303. [DOI] [PubMed] [Google Scholar]
- 6.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grade Working Group. Grade. www.gradeworkinggroup.org/index.htm . [2006 [cited 2009 Apr. 5] Available from: URL: www.gradeworkinggroup.org/index.htm .
- 8.Petri N, Svanholt P, Solow B, Wildschiodtz G, Winkel P. Mandibular advancement appliance for obstructive sleep apnoea: Results of a randomised placebo controlled trial using parallel group design. J Sleep Res. 2008;17(2):221–229. doi: 10.1111/j.1365-2869.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanco J, Zamarron C, beleira Pazos MT, Lamela C, Suarez QD. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath. 2005;9(1):20–25. doi: 10.1007/s11325-005-0003-4. [DOI] [PubMed] [Google Scholar]
- 10.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 11.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: A randomized, controlled trial. Sleep. 2004;27(5):934–941. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 12.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: A randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(5):743–748. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 13.Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1(4):374–380. [PubMed] [Google Scholar]
- 14.Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ. Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. Eur J Orthod. 2002;24(3):251–262. doi: 10.1093/ejo/24.3.251. [DOI] [PubMed] [Google Scholar]
- 15.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(6):1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 16.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87(9):882–887. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 17.Hoekema A, Voors AA, Wijkstra PJ, Stegenga B, van der Hoeven JH, Tol CG, et al. Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. Int J Cardiol. 2008;128(2):232–239. doi: 10.1016/j.ijcard.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Hoekema A, Stegenga B, Bakker M, Brouwer WH, de Bont LGM, Wijkstra PJ, et al. Simulated driving in obstructive sleep apnoea-hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep Breath. 2007;11(3):129–138. doi: 10.1007/s11325-006-0093-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoekema A, Stel A-L, Stegenga B, van der Hoeven JH, Wijkstra PJ, van Driel MF, et al. Sexual function and obstructive sleep apnea-hypopnea: A randomized clinical trial evaluating the effects of oral-appliance and continuous positive airway pressure therapy. J Sex Med. 2007;4(4 II):1153–1162. doi: 10.1111/j.1743-6109.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 20.Lam B, Sam K, Mok WYW, Cheung MT, Fong DYT, Lam JCM, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–359. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 22.Randerath WJ, Heise M, Hinz R, Ruehle K-H. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002;122(2):569–575. doi: 10.1378/chest.122.2.569. [DOI] [PubMed] [Google Scholar]
- 23.Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24(3):239–249. doi: 10.1093/ejo/24.3.239. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short term controlled trial of ant adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52(4):362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109(5):1269–1275. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 26.Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121(3):739–746. doi: 10.1378/chest.121.3.739. [DOI] [PubMed] [Google Scholar]
- 27.Ringqvist M, Walker-Engstr+|m M, Tegelberg +, Ringqvist I. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: a prospective, randomized study. Am J Orthod Dentofacial Orthop. 2003;124(1):53–60. doi: 10.1016/s0889-5406(03)00240-3. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelmsson B, Tegelberg A, Walker-Engstrom M-L, Ringqvist M, Andersson L, Krekmanov L, et al. A prospective randomized study of a dental appliance compared with uvulopalatopharyngoplasty in the treatment of obstructive sleep apnoea. Acta Otolaryngol (Stockh) 1999;119(4):503–509. doi: 10.1080/00016489950181071. [DOI] [PubMed] [Google Scholar]
- 29.Walker-Engstrom M-L, Wilhelmsson B, Tegelberg A, Dimenas E, Ringqvist I. Quality of life assessment of treatment with dental appliance or UPPP in patients with mild to moderate obstructive sleep apnoea. A prospective randomized 1-year follow-up study. J Sleep Res. 2000;9(3):303–308. doi: 10.1046/j.1365-2869.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 30.Tegelberg A, Wilhelmsson B, Walker-Engstrom ML, Ringqvist M, Andersson L, Krekmanov L, et al. Effects and adverse events of a dental appliance for treatment of obstructive sleep apnoea. Swed Dent J. 1999;23(4):117–126. [PubMed] [Google Scholar]
- 31.Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler AM. Comparison of mandibular advancement splint and tongue stabilising device in obstructive sleep apnea. A randomized controlled trial. Sleep. 2009;32(5):648–653. doi: 10.1093/sleep/32.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dort L, Brant R. A randomized, controlled, crossover study of a noncustomized tongue retaining device for sleep disordered breathing. Sleep Breath. 2008;12(4):369–373. doi: 10.1007/s11325-008-0187-5. [DOI] [PubMed] [Google Scholar]
- 33.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(Issue 2) doi: 10.1002/14651858.CD001106.pub2. Art. No.: CD001106 DOI: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 34.College of Physicians and Surgeons of Ontario. Independent health facilities. Clinical practice parameters and facility standards: sleep medicine. -172. 2005. Toronto, ON, The College. 5-3-2009. [Google Scholar]
- 35.BlueCross BlueShield of Tennessee Medical Policy Manual. Oral appliances for management of mild to moderate obstructive sleep apnea (OSA) BlueCross BlueShield of Tennessee Medical Policy Manual. [2008 [cited 2009 May 29] Available from: URL: www.bcbst.com/mpmanual/Oral_Appliances_for_Management_of_Obstructive_Sleep_Apnea.htm .
- 36.Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29(2):240–243. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 37.Scottish Intercollegiate Guidelines Network. Management of obstructive sleep apnoea/hypopnoea syndrome in adults. A national clinical guideline [Internet]. Scottish Intercollegiate Guidelines Network Report No. 73, -36. 2003 Edinburgh, Scotland, The Network. 14-5-2009. Ref Type: Report. [Google Scholar]
- 38.Miletin MS, Hanly PJ. Measurement properties of the Epworth sleepiness scale. Sleep Med. 2003;4(3):195–199. doi: 10.1016/s1389-9457(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith SS, Oei TPS, Douglas JA, Brown I, Jorgensen G, Andrews J. Confirmatory factor analysis of the Epworth sleepiness scale (ESS) in patients with obstructive sleep apnoea. Sleep Med. 2008;9:739–744. doi: 10.1016/j.sleep.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Bank of Canada Rates and Statistics. Ten-year currency converter. Bank of Canada. [ 2009 [cited 2009 June 11] Available from: URL: www.bankofcanada.ca/en/rates/exchform.html .
- 41.Ayas NT, FitzGerald JM, Fleetham JA, White DP, Schulzer M, Ryan CF, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med. 2006;166(9):977–984. doi: 10.1001/archinte.166.9.977. [DOI] [PubMed] [Google Scholar]
- 42.The Lung Association. Sleep apnea. The Lung Association. [ 2009 [cited 2009 Apr. 30] Available from: URL:www.lung.ca/diseases-maladies/apnea-apnee/drivers-camionneures/index_e.php .
- 43.Canadian Medical Association. Determining medical fitness to operate motor vehicles, 7th edition [Internet] Ottawa, ON, The Association. 2006 -134. 30-4-2009. [Google Scholar]


