Executive Summary
Objective
The objective of the report is to examine the comparative effectiveness and cost-effectiveness of various intraocular lenses (IOLs) for the treatment of age-related cataracts.
Clinical Need: Target Population and Condition
A cataract is a hardening and clouding of the normally transparent crystalline lens that may result in a progressive loss of vision depending on its size, location and density. The condition is typically bilateral, seriously compromises visual acuity and contrast sensitivity and increases glare. Cataracts can also affect people at any age, however, they usually occur as a part of the natural aging process. The occurrence of cataracts increases with age from about 12% at age 50 years, to 60% at age 70. In general, approximately 50% of people 65 year of age or older have cataracts. Mild cataracts can be treated with a change in prescription glasses, while more serious symptoms are treated by surgical removal of the cataract and implantation of an IOL.
In Ontario, the estimated prevalence of cataracts increased from 697,000 in 1992 to 947,000 in 2004 (35.9% increase, 2.4% annual increase). The number of cataract surgeries per 1,000 individuals at risk of cataract increased from 64.6 in 1992 to 140.4 in 1997 (61.9% increase, 10.1% annual increase) and continued to steadily increase to 115.7 in 2004 (10.7% increase, 5.2% increase per year).
Description of Technology/Therapy
IOLs are classified either as monofocal, multifocal, or accommodative. Traditionally, monofocal (i.e.. fixed focusing power) IOLs are available as replacement lenses but their implantation can cause a loss of the eye’s accommodative capability (which allows variable focusing). Patients thus usually require eyeglasses after surgery for reading and near vision tasks. Multifocal IOLs aim to improve near and distant vision and obviate the need for glasses. Potential disadvantages include reduced contrast sensitivity, halos around lights and glare. Accommodating IOLs are designed to move with ciliary body contraction during accommodation and, therefore, offer a continuous range of vision (i.e. near, intermediate and distant vision) without the need for glasses. Purported advantages over multifocal IOLs include the avoidance of haloes and no reduction in contrast sensitivity.
Polymethyl methacrylate (PMMA) was the first material used in the fabrication of IOLs and has inherent ultraviolet blocking abilities. PMMA IOLs are inflexible, however, and require a larger incision for implantation compared with newer foldable silicone (hydrophobic) and acrylic (hydrophobic or hydrophilic) lenses. IOLs can be further sub-classified as being either aspheric or spheric, blue/violet filtered or non-filtered or 1- or 3-piece.
Methods of Evidence-Based Analysis
A literature search was conducted from January 2003 to January 2009 that included OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), The Cochrane Library, and the International Agency for Health Technology Assessment/Centre for Review and Dissemination.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Comparisons of Interest
The primary comparison of interest was accommodative vs. multifocal vs. monofocal lenses.
Secondary comparisons of interest included:
tinted vs. non-tinted lenses
aspheric vs. spheric lenses
multipiece vs. single piece lenses
biomaterial A (e.g. acrylic) vs. biomaterial B (e.g. silicone) lenses
sharp vs. round edged lenses
The quality of the studies was examined according to the GRADE Working Group criteria for grading quality of evidence for interventional procedures.
Summary of Findings
The conclusions of the systematic review of IOLs for age-related cataracts are summarized in Executive Summary Table 1.
Considerations for the Ontario Health System
Procedures for crystalline lens removal and IOL insertion are insured and listed in the Ontario Schedule of Benefits.
If a particular lens is determined to be medically necessary for a patient, the cost of the lens is covered by the hospital budget. If the patient chooses a lens that has enhanced features, then the hospital may choose to charge an additional amount above the cost of the usual lens offered.
An IOL manufacturer stated that monofocal lenses comprise approximately 95% of IOL sales in Ontario and premium lenses (e.g., multifocal/accomodative) consist of about 5% of IOL sales.
A medical consultant stated that all types of lenses are currently being used in Ontario (e.g., multifocal, monofocal, accommodative, tinted, nontinted, spheric, and aspheric). Nonfoldable lenses, rarely used in routine cases, are primarily used for complicated cataract implantation situations.
ES Table 1: Conclusions for the Systematic Review of IOLs for Age-Related Cataracts.
| Comparison | Conclusion | GRADE Quality |
|---|---|---|
| Multifocal vs. monofocal |
Objective Outcomes Significant improvement in BDCUNVA No significant difference in BCDVA Inconclusive evidence for contrast sensitivity Inconclusive evidence for glare Subjective Outcomes Inconclusive evidence for visual satisfaction Significant increase in glare/halos Significant increase in freedom from spectacles |
moderate moderate low very low low low/moderate low/moderate |
| Accommodative vs. multifocal/monofocal | Inconclusive due to Insufficient limited evidence for any effectiveness outcome | very low |
| Hydrophilic acrylic vs. other materials (hydrophobic acrylic, silicone) | Significant increase in PCO score | Low |
| Sharp edged compared to round edged | Significant reduction in PCO score | Low |
| One piece compared to three piece | No significant difference in PCO score | low |
| Hydrophobic acrylic compared to silicone | No significant difference in PCO score | moderate |
| Aspherical modified prolate anterior surface compared to spherical | No significant difference in VA Significant reduction in contrast sensitivity |
very low very low |
| Blue light filtering compared to non blue-light filtering | No significant difference in BCDVA No significant difference in contrast sensitivity No significant difference in HRQL |
low low high/moderate |
BCDVA refers to best corrected distance visual acuity; BDCUNVA, best distance corrected unaided near visual acuity; HRQL, health related quality of life; PCO, posterior capsule opacification; VA, visual acuity.
Background
Objective of Analysis
The objective of the report is to examine the comparative effectiveness and cost-effectiveness of various intraocular lenses (IOLs) for the treatment of age-related cataracts.
Clinical Need and Target Population
A cataract is a hardening and clouding of the normally transparent crystalline lens that may result in a progressive loss of vision depending on its size, location and density. The condition is typically bilateral, seriously compromises visual acuity and contrast sensitivity and increases glare. (1) Cataracts can also affect people at any age, however, they usually occur as a part of the natural aging process. The occurrence of cataracts increases with age from about 12% at age 50 years, to 60% at age 70. (1) In general, approximately 50% of people 65 year of age or older have cataracts. Mild cataracts can be treated with a change in prescription glasses, while more serious symptoms are treated by surgical removal of the cataract and implantation of an IOL. The most common cataract procedure is an extracapsular lens removal with implantation of a posterior chamber (behind the iris) IOL within the capsular bag.
In Ontario, the estimated prevalence of cataracts increased from 697,000 in 1992 to 947,000 in 2004 (35.9% increase, 2.4% annual increase). (2) The number of cataract surgeries per 1,000 individuals at risk of cataract increased from 64.6 in 1992 to 140.4 in 1997 (61.9% increase, 10.1% annual increase) and continued to steadily increase to 115.7 in 2004 (10.7% increase, 5.2% increase per year). (2) Another Ontario study showed that the number of cataract surgeries performed on patients over 65 more than doubled from 44,000 to 90,000 over a 10 year period (1994 to 2005), accounting for approximately 81% of all cataract surgeries in Ontario. For 2004 to 2005, rates including all cataract surgeries ranged from 4,300 to 6,600 cataract surgeries per 100,000 residents aged 65 or older. (3)
IOLs
IOL implants restore optical focusing power lost by removal of the clouded natural crystalline lens. The devices can be classified as monofocal, multifocal or accommodative. (4) Traditionally, monofocal (e.g., fixed focusing power) IOLs are available as replacement lenses but their implantation can cause a loss of the eye’s accommodative capability (which allows variable focusing). Patients thus usually require eyeglasses after surgery for reading and near vision tasks. (4) Multifocal IOLs aim to improve near and distant vision and obviate the need for glasses. Potential disadvantages include reduced contrast sensitivity, halos around lights and glare. Accommodating IOLs are designed to move with ciliary body contraction during accommodation and, therefore, offer a continuous range of vision (i.e. near, intermediate and distant vision) without the need for glasses. Purported advantages over multifocal IOLs include the avoidance of haloes and no reduction in contrast sensitivity. (5)
Accommodative lenses can be single optic or dual optic (6): Single optic lenses have one focal point, but they act as if they were multifocal. They were designed with a hinge similar to the mechanics of the eye’s natural lens. Using the eye’s muscles, the single focal point of an accommodative IOL can shift to bring objects at varying distances into focus. Dual optic devices have a fixed anterior optic and a second posterior lens that moves anteriorly towards the anterior lens.
Monovision is also an option for some patients requiring IOLs. Patients receive an IOL where one eye is fitted for distance vision and the other eye is fitted for near vision. Patients who have in the past had monovision contact lenses (one eye for distance and one eye for near) may prefer these.
Materials and Design
Polymethyl methacrylate (PMMA) was the first material was used in the fabrication of IOLs and has inherent ultraviolet blocking abilities. (7) PMMA IOLs are inflexible, however, and require a larger incision for implantation (5-7 mm requiring sutures) compared with newer foldable silicone (hydrophobic) and acrylic (hydrophobic or hydrophilic) lenses (2.8–3.5 mm and not requiring sutures). IOLs can be further subclassified as being either aspheric or spheric, blue/violet filtered, or non-filtered. Tables 1 and 2 summarize the subclassifications of IOLs.
Table 1: Classification of IOLs for Cataracts.
| Classification | Description | Material |
|---|---|---|
| Rigid | Large incision requiring sutures. | PMMA |
| Foldable | Smaller incision, no sutures required. Potentially less early postoperative inflammation and reduced surgically induced astigmatism. Implanted using either forceps or an injector. |
Silicone (hydrophobic) Hydrophobic acrylic Hydrophilic acrylic (hydrogel) Collagen/hydroxyl ethyl methacrylate copolymer |
Table 2: Subclassifications of IOLs for Cataracts.
| Subclassification | Description |
|---|---|
| Blue or violet filtering | May protect against macular toxicity and provide retinal protection. |
| Spherical or aspherical | Traditional spheric design induces spherical aberration that when added to positive corneal spherical aberration can reduce contrast sensitivity. Aspheric IOLs with negative spherical aberration may improve contrast sensitivity and quality of vision (including night driving). Aspheric lenses currently available each correct or reduce a different amount of spherical aberration. Optimal amount unclear. |
| 1 or 3 piece lens | 1 piece lens is manufactured from a single piece of material. 3 piece is made of the optic (either silicone or acrylic) and 2 attached haptics (the arms of the lens, often made of prolene) |
Complications
An ‘after cataract’, also called a posterior capsular opacification (PCO), is a cloudy membrane that sometimes forms on the membrane behind the IOL after cataract surgery. Although the membrane is untouched during the surgery, afterward lens epithelial cells may migrate along the posterior capsule leading to opacification. Symptoms of an after cataract include blurred vision and are similar to those of a normal cataract. Patients may also see streaks of light, halos, or excessive glare. Through the 1980s and 1990s, the 5 year incidence of PCO had been reported to be 28.4% (8), however, this rate has varied considerably with suggestions that the incidence has now decreased. (9)
Neodymium: yttrium-aluminum-garnet (ND:YAG) laser treatment for PCO involves cutting open the clouded posterior capsule allowing light to transmit normally. (7) This treatment can produce complications such as an increase in intraocular pressure, damage to the IOLs, ocular inflammation, cystoid macular edema, and retinal detachment.
Goals of IOL Insertion
In a population-based, cross-sectional study of people ≥65 years, the Salisbury Eye Evaluation (SEE) study indicated that visual acuity, contrast sensitivity, glare sensitivity, bilateral acuity and visual fields were risk factors for self-reported difficulty with everyday activities. (10) Though the study did not necessarily focus on patients with cataracts, it provides insight into outcomes that are important to an elderly population. In a separate study to determine the visual measures most predictive of falls in community-dwelling seniors, Lord et al. (11) found that multiple fallers had impaired depth perception, contrast sensitivity and low contrast visual acuity. Thus, although there is no single outcome measure that summarizes the effectiveness of an IOL, visual acuity and contrast sensitivity, are amongst the most commonly reported.
Visual Acuity
Visual acuity is a quantitative measure of the ability to resolve fine detail and the most common clinical measurement of visual function because it is easy to assess (i.e. using the Snellen chart) and because even small amounts of refractive error produce marked declines in acuity test performance. (10) The test also corresponds well with the normal daily activities that a person can handle and can evaluate their ability to do them. It should be noted, however, that those people with colour blindness, reduced contrast sensitivity or an inability to track fast moving objects, may still have ‘normal visual acuity’ as this does not necessarily correspond to ‘normal vision’.
There are different classifications of visual acuity with the following outcomes being commonly reported:
Best corrected near visual acuity: near visual acuity with the aid of spectacles.
Uncorrected near visual acuity: near visual acuity without the aid of any spectacles.
Best corrected distance visual acuity: distance visual acuity with the aid of spectacles.
Uncorrected distance visual acuity: distance visual acuity without the aid of any spectacles.
Best distance corrected near visual acuity: near vision that would be obtained without the use of any additional reading spectacles when any distance refractive error is corrected.
According to an expert whom the Medical Advisory Secretariat consulted, best distance corrected unaided near visual acuity (BDCUNVA) is an important outcome when measuring the effectiveness of multifocal lenses. This outcome is the near vision obtained, after correcting any distance refractive error, without the use of any additional reading spectacles. However, since monofocal IOLs do not correct for near vision, best corrected distance acuity (BCDA) is the best overall measure of visual outcome. Uncorrected acuity would be heavily dependent on preoperative biometric accuracy as well as individual patient preference. For example, some life long myopic patients prefer to continue to wear distance spectacles postoperatively and not use spectacles for reading. Also, in unilateral cataract cases, it is preferred to keep both eyes with similar postoperative refractions.
Contrast sensitivity
Contrast sensitivity determines the lowest contrast level that can be detected by a patient for a given size target. It varies between individuals and usually peaks at age 20, then declining with age. Normally, a range of targets is used to assess contrast sensitivity, but unlike acuity which measures size alone, contrast sensitivity measures size and contrast.
Typically, there is a decrease in contrast sensitivity for intermediate and high spatial frequencies that becomes more pronounced with age. (12) For example, with advancing age, increased contrast is needed to discriminate faces. (13) Owsley et al. found that age differences in contrast sensitivity were not eliminated when young subjects viewed objects under conditions of simulated ocular aging. (12) These results indicated that the age difference in contrast sensitivity represented an age-related change in the neural rather than optical characteristics of the visual process.
A person with poor contrast sensitivity (e.g. due to cataracts) may have vision difficulties such as:
Trouble seeing in rain, fog or at dusk/night.
Missing facial gestures
Tripping when using stairs or walking over curbs
Inability to discriminate objects in a cluttered environment
Ontario Schedule of Benefits
Procedures for crystalline lens removal and IOL insertion are insured and are listed in the Ontario Schedule of Benefits. (14) If a particular lens is determined to be medically necessary for a patient, the cost of the lens is covered by the hospital budget. If the patient chooses a lens with enhanced features, then hospitals may choose to charge an additional amount above the cost of the usual lens offered.
Regulatory Status
At least 38 IOLs are licensed by Health Canada for the treatment of cataracts. These include monofocal, multifocal, and accommodating lenses with the various subclassifications summarized in Table 2 (page 12).
Existing Guidelines
Currently existing guidelines for the use of IOLs in the treatment of age-related cataracts are limited to those issued by the American Academy of Ophthalmology, which state:
“The surgeon should have access to a variety of lens styles to select an appropriate IOL for an individual patient. Variations in the preoperative state of the eye, the surgical technique, patient expectation and surgeon experience and preference affect the decision.” (9)
“Whether the improvement in near unaided acuity outweighs the adverse effects of multifocal IOLs will vary among the patients, with motivation to achieve spectacle independence likely to be the definitive factor.” (9)
Evidence-Based Analysis (Methods)
Research Question(s)
What is the comparative effectiveness and cost-effectiveness of using the various IOLs for the treatment of age-related cataracts?
Literature Search
A literature search was conducted from January 2003 to January 2009 that included OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), The Cochrane Library, and the International Agency for Health Technology Assessment/Centre for Review and Dissemination. Details of the literature search strategy can be found in Appendix 1.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Comparisons of Interest
The primary comparison of interest is accommodative vs. multifocal vs. monofocal lenses.
Secondary comparisons of interest include:
tinted vs. nontinted
aspheric vs. spheric
multipiece vs. single piece
biomaterial A (e.g. acrylic) vs. biomaterial B (e.g. silicone)
sharp vs. round edged
Assessment of Quality of Evidence
The quality of the studies was examined according to the GRADE Working Group criteria for interventions. (15)
Results of Evidence-Based Analysis
The literature search identified 739 citations, of which 3 were systematic reviews and nine were studies that were published after the literature search cut-off dates in the systematic reviews. The quality of the literature is presented below in Table 3.
Table 3: Quality of Evidence of Included Studies.
| Study Design | Level of Evidence* |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic review of RCTs | 1 | 3 systematic reviews 8 RCTs |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | 0 |
| Small RCT | 2 | 1 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | 0 |
| Case series (multisite) | 4b | 0 |
| Case series (single site) | 4c | 0 |
| Retrospective review, modeling | 4d | 0 |
| Case series presented at international conference | 4(g) | 0 |
g refers to grey literature; RCT, randomized controlled trial.
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (16) An additional designation “g” was added for preliminary reports of studies that have been presented at international scientific meetings.
Summary of Existing Evidence
Descriptive Systematic Reviews from International Health Technology Assessment (HTA) Organizations
Three descriptive systematic reviews from Canada, Australia and the United Kingdom on accommodative lenses were identified in the literature search. (6; 17; 18) Table 4 summarizes the systematic reviews by date, country, organization, and overall conclusion.
Table 4: Conclusions of Descriptive Systematic Reviews from International HTA Organizations.
| Publication Date | Country | Organization | Overall Conclusion |
|---|---|---|---|
| August 2004 | Australia | Australia and New Zealand Horizon Scanning Network (17) |
|
| August 2006 | Canada | Canadian Agency for Drugs and Technologies in Health (6) |
|
| February 2007 | United Kingdom | National Institute for Health and Clinical Excellence (18) |
|
Meta-Analyses
IOL Materials and Design
Three meta-analyses (7; 19; 20) of the effect of IOLs on the development of PCO were identified (details of each are supplied in Appendix 2). The most recent systematic review from July 2007 on PCO was a Cochrane review by Findl et al. (19) As there were different types of PCO scores used in the primary studies within this review, Findl et al. converted the values to a ‘common score’ between 0 (no PCO) to 100 (maximum PCO score) in order to compare the values in forest plots. Further details of the scoring system were not included in their report.
Summary statistics were calculated, but there was statistical heterogeneity or ‘no studies available’ for analysis in many subgroups (e.g. hydrophilic acrylic lenses vs. silicone lenses).
The main findings of the Cochrane review (19) showed:
There was a significantly higher PCO score (mean difference 12.39; 95% CI 9.82 to 14.95), and Nd:YAG capsulotomy rate (OR 8.37; 95% CI 3.74 to 20.36) in hydrophilic acrylic IOLs compared to other materials (see Table 5); however, some studies compared sharp edge to round edge IOLs.
There was a significantly lower PCO score (mean difference -8.65; 95% CI -10.72 to -6.59; statistically significant heterogeneity) and Nd:YAG rate (OR 0.19; 95% CI 0.11 to 0.35) in sharp edged IOLs compared to round edged IOLs of any material (see Table 6).
There was no significant difference in PCO scores or Nd:YAG rates between 1 piece and 3 piece IOLs (see Table 7); however, data was limited to acrylic and PMMA IOLs
Table 5: Meta-Analytic Results for Comparisons of IOL Materials from Findl et al.
| PMMA vs. Silicone | PMMA vs. Acrylic | PMMA vs. Hydrophilic Acrylic | Acrylic vs. Silicone | Acrylic vs. Hydrophilic Acrylic | Silicone vs. Hydrophilic Acrylic | Hydrophilic Acrylic vs. All Other Materials | |
|---|---|---|---|---|---|---|---|
| BCDVA* | -0.11 (-0.16 to -0.06) 1 study |
No study | 0.06 (-0.02 to 0.14) 1 study |
2 studies‡ | 0.10 (0.05 to 0.16) 3 studies |
No study | -0.09 (-0.13 to -0.06) 4 studies |
| PCO score* | 4 studies‡ | 3 studies‡ | -17.0 (-27.69 to -6.31) 2 studies |
0.00 (-0.06 to 0.05) 5 studies |
2 studies‡ | No study | 12.39 (9.82 to 14.95) 5 studies |
| Nd:YAG rate† | 6 studies‡ | 7.19 (2.72 to 18.96) 2 studies |
0.43 (0.11 to 1.69) 1 study |
0.56 (0.25 to 1.28) 7 studies |
0.18 (0.02 to 1.38) 4 studies |
No study | 8.37 (3.74 to 20.36) 4 studies |
Mean difference (95% CI)
Odds ratio (95% CI)
Significant statistical heterogeneity
Table 6: Meta-Analytic Results for Comparisons of IOL Designs (Round and Sharp Edges) from Findl et al.
| Sharp vs. Round Edge PMMA | Sharp vs. Round Edge Acrylic | Sharp vs. Round Edge Silicone | Sharp vs. Round Edge Any Material | |
|---|---|---|---|---|
| BCDVA* | -0.05 (-0.18 to 0.08) 1 study |
0.06 (0.01 to 0.12) 2 studies |
2 studies‡ | 7 studies‡ |
| PCO score* | -28.3 (-40.95 to -15.65) 1 study |
3 studies‡ | 5 studies‡ | 12 studies‡ |
| Nd:YAG rate† | 0.24 (0.07 to 0.85) 1 study |
0.07 (0.02 to 0.32) 2 studies |
0.18 (0.04 to 0.72) 4 studies |
0.19 (0.11 to 0.35) 11 studies |
Mean difference (95% CI)
Odds ratio (95% CI)
Significant statistical heterogeneity
Table 7: Meta-Analytic Results for Comparisons of IOL Designs (One and Three Piece) from the Cochrane Systematic Review by Findl et al.
| 1 Piece vs. 3 Piece Acrylic |
1 Piece vs. 3 Piece PMMA |
1 Piece vs. 3 Piece Silicone |
|
|---|---|---|---|
| BCDVA* | 0.00 (-0.04 to 0.04) 2 studies |
No study | No study |
| PCO score* | Not reported 5 studies |
No study | No study |
| Nd:YAG rate† | 0.48 (0.02 to 10.24) 3 studies |
0.94 (0.59 to 1.51) 1 study |
No study |
Mean difference (95% CI)
Odds ratio (95% CI)
Significant statistical heterogeneity
Multifocal vs. Monofocal IOLs
The only systematic review examining the efficacy of multifocal versus monofocal IOLs was a Cochrane review by Leyland and Pringle in which ten trials were identified. (21) The primary outcomes were distance and near visual acuity and spectacle dependence. Overall, there was significant heterogeneity in how outcomes were reported.
Distance Visual Acuity
Best corrected distance visual acuity was similar between multifocal and monofocal IOLs (SMD 0.15; 95% CI –0.01 to 0.31).
Near Visual Acuity
Best distance corrected unaided near visual acuity is an important outcome in the assessment of multifocal efficacy, but it was reported in a manner that made comparison between studies difficult.
For studies, reading distances differed and it is unclear whether the reported print size had been corrected for reading distance to allow near acuity to be calculated.
Two studies explicitly calculated best distance corrected unaided near visual acuity after bilateral multifocal versus monofocal implantation. (22; 23) Both studies used logMAR reading charts, calculated acuity with a correction for reading distance and had a high Jadad score of methodological quality (5 out of 5). The first study (23) reported significantly improved unaided near visual acuity with multifocal IOLs while the other study (22) found no significant difference. The latter, however, by Leyland et al. (22) did not accrue the number of patients stipulated in the sample size calculation, thereby allowing the possibility of a type 2 error. Furthermore, the study included people ≥18 years of age and thus not all included patients had age-related cataracts.
Javitt et al. (23) found a significant difference in best distance corrected unaided near visual acuity between patients who had received multifocal IOLs compared to monofocal IOLs [mean visual acuity 0.14 (SD: 0.14) logMAR versus 0.35 (SD: 0.18) logMAR; p=0.0001].
Depth of Field (Defocus Test)
Four of the 10 studies measured depth of field. A meta-analysis was not conducted since the outcomes and methods were not similar across studies.
All studies described better acuity with lens defocus from the distance correction with the multifocal IOL.
Statistical (or clinical) significance was not discussed in the Cochrane review.
Contrast Sensitivity
Seven studies reported this outcome with data presented differently in each (e.g. visual acuity at different contrast levels; difference between high contrast and lower contrast acuity), precluding meta-analysis.
All seven studies reported lower contrast sensitivity with the multifocal IOL.
Statistical (or clinical) significance was not discussed in the Cochrane review.
Glare
Two studies reported numerical results for glare using a Brightness Acuity Tester.
One study found that acuity decreased as glare increased, but there was no significant difference between the lenses. (24) The other study found no significant decrease in acuity with glare for either type of lens. (22)
Subjective outcomes (satisfaction with vision, glare and spectacle dependence) were also reported in the Cochrane review.
Satisfaction with Vision
Seven of the 10 included studies involved patients with surgery in one eye only. Unilateral studies allow measurement on uni-ocular outcomes such as visual acuity but are of limited use when attempting to measure the effect of multifocal IOLs on quality of life, especially where the fellow eye has good vision.
Overall, the studies could not be combined for meta-analysis.
-
Validated instruments were used by four studies, of which two (assessing bilateral outcomes) used the same questionnaire.
- Javitt et al. (23) found a small but statistically significant increase in overall visual satisfaction with the multifocal (mean score 8.4) compared to the monofocal lens (mean score 7.9). Mean overall visual satisfaction ranged from 0 to 10; 0 being worst and 10 being best.
- Leyland et al. (22) found no difference in overall subjective satisfaction between groups (median score for both groups was 8); however, since the study was not powered to examine this outcome, the non-significant result may be a type 2 error.
Spectacle Dependence
In all 10 studies, most multifocal IOL patients still used spectacles for some tasks (e.g. small print).
-
Independence from spectacles was found in:
- 26% to 47% of multifocal IOL patients
- 1% to 11% of monofocal IOL patients
A summary statistic was calculated for eight studies. Independence of spectacles was achieved more frequently with multifocal than monofocal IOLs (OR: 0.17; 95% CI 0.12 to 0.24).
Glare
Four studies reported the proportion of patients with glare and halos.
Symptoms were significantly less frequent in the monofocal group (OR 3.55; 95% CI 2.11 to 5.96).
Complications
Complications are expected to be similar for multifocal and monofocal IOLs as they are similar in all but the design of the optics and require no modifications to surgical technique.
Pre- and post-operative complications were reported in five studies. The incidence of complications was reported to be low and similar in the multifocal and monofocal IOL groups.
Accommodating IOLs
Findl and Leydolt (25) reviewed studies that reported visual acuity after accommodating lens implantation. Of the three types of accommodative lenses included in the review, only the AT-45 Cyrstalens is licensed by Health Canada; therefore, only results pertaining to that IOL are discussed. No RCTs were identified by Findl and Leydolt. (25) The results of six nonrandomized studies (26–31) included in the review by Findl and Leydolt are shown in Appendix 2. Overall, Findl and Leydolt concluded that there were large discrepancies in VA data.
The overall limitation to the review by Findl and Leydolt was a lack of stringent inclusion criteria. For example, the objective of one of the study by Alio et al. (27) was to “investigate potential for near vision restoration using three IOL models (two multifocal and an accommodative) after presbyopic lens exchange.” Patients did not require cataracts for IOL implantation. Furthermore, some patients in each study group had laser in situ keratomileusis (LASIK) 6 months after IOL implantation. VA results were reported up to 1 year. Alio et al. did not report sample size calculations. A summary of the limitations of the individual studies included in the review by Findl and Leydolt is displayed in Table 8.
Table 8: Limitations of Studies Included in Findl and Leydolt, 2007.
| Study | Objective | Limitation |
|---|---|---|
| Alio et al. (27) |
|
|
| Cumming et al. (28) |
|
|
| Cumming et al. (29) |
|
|
| Koeppl et al. (26) |
|
|
| Marchini et al. (30) |
|
|
| Buratto et al. (31) |
|
|
Studies Published After Literature Search Cut-off Dates in Systematic Reviews
IOL Materials and Design
Four prospective randomized studies that had posterior capsule opacification as the primary endpoint were identified (32–35), of which two (and possibly three) were updates of studies that were included in the Cochrane review. (19) Detailed results of the four studies are supplied in Appendix 3.
Posterior opacification was the primary outcome for one study (32) comparing hydrophilic versus hydrophobic sharp edged IOLs; one study (33) comparing acrylic versus silicone IOLs of the same optic design and haptics; and two studies (34; 35) comparing 1- and 3-piece haptic hydrophobic acrylic IOLs (the results of each are shown in Table 9). Overall, results were consistent with the previous Cochrane review. There was significantly less PCO for hydrophobic compared to hydrophilic sharp edged IOLs, but no significant difference in PCO between 1- and 3- piece IOLs. One study compared acrylic versus silicone IOLs of the same optic and haptic designs and found no significant difference in PCO.
Table 9: Studies Comparing IOL Material and Design with PCO as the Primary Outcome (Published after Cochrane Review).
| Comparison | Results | Comments |
|---|---|---|
| Hydrophobic vs. hydrophilic sharp edged IOL (32) Update of study in Cochrane review |
|
|
| Acrylic vs. silicone IOL of same optic and haptic designs (33) |
|
|
| 1-piece vs. 3-piece hydrophobic acrylic IOL (34) Update of study in Cochrane review |
|
|
| 1 piece vs. 3 piece hydrophobic acrylic IOL (35) Update of study in Cochrane review |
|
|
Modified Prolate Anterior Surface IOLs
One prospective randomized trial was identified in which a modified prolate anterior surface IOL was compared to a spherical non-blue light filtering IOL and a spherical blue-light filtering IOL. (36) The primary endpoint was contrast sensitivity at 6 months post-implantation (detailed results of the study are found in Appendix 3). Compared with the spherical non-blue light filtering IOL, the modified prolate IOL showed significantly better results at 1 and 12 cycles per degree in photopic conditions; at 3, 12, and 18 cycles per degree, in photopic with glare; at 3, 12, and 18 cycles per degree in mesopic and at 12 and 18 cycles per degree in mesopic with glare. Compared with the spherical blue light filtering IOL, the modified prolate IOL provided significantly better contrast sensitivity at almost all spatial frequencies in any lighting condition.
The clinical significance of the contrast sensitivity results was not discussed by the authors. In a young eye, positive spherical aberration in the cornea is a partially compensated by the negative spherical aberration of the youthful lens. Positive spherical aberration of the cornea changes little with age, however, the lens changes from negative to positive spherical aberration, leading to a gradual loss of contrast sensitivity in elderly eyes.
The rationale behind the modified prolate anterior surface IOL is to compensate for corneal spherical aberration by creating a modified prolate front surface (flatter curve in the periphery of the IOL), which, in theory, provides better contrast sensitivity than a spherical IOL. It is claimed that the modified prolate produces an amount of negative spherical aberration similar to that of a young natural crystalline lens and “approximates the optical system of a youthful eye”. (36)
There was no significant difference in BCVA between the study groups. It was not reported if BCVA referred to near or distant VA. Limitations to the study included:
A lack of comparisons to aspherical IOLs that do not have a modified prolate anterior surface. Comparisons were only made with spherical IOLs.
No reported blinding of the patient or the examiner
No sample size calculation and no information about drop outs or if consecutive patients were randomized.
Three different lenses were examined with differing numbers of pieces and blue-light filtering.
Blue-Light Filtering IOLs
The importance of having an IOL that closely mimics the protection afforded by the natural crystalline lens led some researchers to suggest adding a yellow chromophore to IOLs (to block blue light). (37) Their rationale for this included:
Blue light has the highest amount of energy in the visible light spectrum.
The ability of the lens to filter blue light decreases with age.
Some experimental cell culture studies showed that exposure to blue light damaged retinal pigment epithelial cells. (37)
Epidemiologic studies are inconclusive regarding a correlation between exposure to phototoxic levels of blue light after cataract surgery (and the insertion of a non blue light filtering IOL) and the development of age-related macular degeneration. (37)
Filtering out short wavelength of visible light (up to 480 nm) eliminates intraocular scatter and enhances contrast sensitivity. (38)
It has been reported that the concentration of yellow chromophore in a blue filtering IOL results in a transmission curve that better resembles that of a 25 year old natural crystalline lens. (39) Others claim a transmission of light that mimics the natural lens of a 53-year old person without a cataract. (38)
Four prospective randomized trials (38–41) were identified in which blue-light filtering IOLs were compare to non-blue light filtering IOLs (detailed results of these studies are provided in Appendix 3). Overall, there was no significant difference between blue light filtering and non-blue light filtering IOLs in terms of contrast sensitivity and visual acuity (results shown in Table 10). Only one of the trials reported a sample size calculation, therefore there is the possibility of type 2 errors occurring in the other three studies. To date, there are no published clinical trials comparing the long-term effect of blue light filtering IOLs compared to non-blue light filtering IOLs on macular toxicity.
Table 10: Studies Comparing Blue Light Filtering to Non-Blue Light Filtering IOLs.
| Comparison | Results | Comments |
|---|---|---|
| Blue light filtering IOL vs. regular single piece IOL. (39) |
|
|
| Blue light filtering IOL vs. conventional non-blue light filtering IOL. (40) |
|
|
| Blue light filtering IOL vs. regular IOL. (38) |
|
|
| Blue light filtering IOL vs. regular single piece IOL. (41) |
|
|
Quality of the Evidence
Tables 11 to 19 show the quality of evidence for the studies published concerning the use of IOL for the treatment of age-related cataracts according to the GRADE quality-of-evidence criteria.
Table 11: GRADE Quality of Evidence for Interventions – Multifocal vs. Monofocal Lenses Objective Endpoints.
| Outcome | Quality Assessment | Summary of Findings (Objective Endpoints) | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| Best distance corrected unaided near visual acuity | RCT | High | Inconsistent (1 study) |
Direct | <6 months follow-up (3 months) | Multi: mean 0.14 [0.14] logMAR (Snellen equivalent 20/28) Mono: mean 0.35 [0.18] logMAR (Snellen equivalent 20/45) P=0.0001 |
Moderate* |
| Best corrected distance visual acuity | RCT | High | Consistent with Cochrane review | Direct | <6 months follow-up (3 months) | Multi: mean 8.40 [0.97] Regan lines (Snellen equivalent 20/18) Mono: mean 8.46 [0.94] Regan lines (Snellen equivalent 20/18) P=0.60 |
Moderate† |
| Contrast sensitivity | Cochrane systematic review | Low/ Moderate | Consistent (7 studies) | Direct | Precluded combined analysis. Data described as “contrast sensitivity” using different charts; visual acuity at different contrast levels; and difference between high contrast and lower contrast acuity. | All 7 studies reported lower contrast sensitivity with multifocal IOL. Statistical significance not reported in 6 studies. Clinical significance not addressed. |
Low‡ |
| Glare | Cochrane systematic review | Low/ Moderate | Inconsistent (2 studies) | Direct | Variability in how outcomes reported. |
Leyland: Effect on acuity (logMAR) Multi: -0.02 (0.06) logMAR Mono: -0.02 (0.06) logMAR Significance between lenses not reported. Steibert: Effect on acuity (Regan lines) Multi: -5.67 (SD 2.23) at high glare Mono: -6.42 (SD2.43) at high glare No significant difference between lenses. |
Very Low§ |
Downgraded due to inability to determine consistency and < 6 months follow-up. Unlikely to be important uncertainty.
Downgraded due to < 6 months follow-up. Unlikely to be important uncertainty.
Downgraded due to quality (3 out of the 10 studies included in the Cochrane review were double masked; 7 studies reported withdrawals; 5 reported method of randomization) and heterogeneity in how outcomes reported.
Downgraded due to quality, inconsistency and heterogeneity in how outcomes reported.
Table 19: GRADE Quality of Evidence for Interventions – Blue Light Filtering IOLs.
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| Contrast Sensitivity | RCTs | Moderate* | Consistent (2 studies) | Direct |
|
|
Low† |
| BCVA | RCTs | Moderate* | Consistent (3 studies) | Direct |
|
|
Low† |
| HRQL | RCT | High | Inconsistent (1 study) | Direct |
|
No significant difference in:
|
High/ Moderate‡ |
Downgraded due to study design (lack of details about randomization/blinding).
Downgraded due to uncertainty with methodological issues.
Downgraded due to uncertainty in consistency.
Table 12: GRADE Quality of Evidence for Interventions – Multifocal vs. Monofocal Lenses Subjective Endpoints.
| Outcome | Quality Assessment | Summary of Findings (Objective Endpoints) | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| Patient Satisfaction | Cochrane review | Low/ Moderate | Inconsistent (4 studies) | Direct |
|
|
Low* |
| Glare and Halos | Cochrane review | Low/ Moderate | Consistent (4 studies) | Direct |
|
|
Low/ Moderate |
| Spectacle dependence | Cochrane review | Low/ Moderate | Consistent (8 studies) | Direct |
|
|
Low/ Moderate |
Downgraded due to inconsistency and heterogeneity in how outcomes reported.
Table 13: GRADE Quality of Evidence for Interventions – Accommodating IOLs.
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| Best distance corrected unaided near visual acuity | Systematic review of observational studies | low* | Inconsistent† | Indirect‡ |
|
No summary statistic | Very low |
| Best corrected near visual acuity | Same systematic review of observational studies | Low* | Inconsistent† | Indirect‡ |
|
No summary statistic | Very low |
Downgraded due to study design (overall, lack of stringent inclusion criteria; for individual studies, no explanation how sample sizes arrived at, confounding)
Downgraded due to variability of reported outcomes and how reported.
Downgraded due to mixing of patient indications (e.g., presbyopia, non-age related cataracts).
Table 14: GRADE Quality of Evidence for Interventions – Hydrophilic Acrylic Compared to All Other Materials.
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| PCO | Cochrane systematic review | Moderate* | Consistent (5 studies) | Direct |
|
12.39 (9.82 to 14.95) Mean difference (95% CI) |
Low† |
| BCDVA | Cochrane systematic review | Moderate* | Consistent (4 studies) | Direct |
|
-0.09 (-0.13 to -0.06) Mean difference (95% CI) |
Low† |
| Nd:YAG rate | Cochrane systematic review | Moderate* | Consistent (4 studies) | Direct |
|
8.37 (3.74 to 20.36) Odds ratio (95% CI) |
Low† |
Downgraded due to study design (lack of details about randomization/blinding)
Downgraded due to mixing of round and sharp edge IOLs.
Table 15: GRADE Quality of Evidence for Interventions – Sharp Edged Compared to Round Edged IOLs Regardless of Lens Material.
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| PCO | Cochrane systematic review | Moderate* | Consistent (11 studies) | Direct |
|
No summary statistic | Low† |
| BCDVA | Cochrane systematic review | Moderate* | Inconsistent (7 studies) | Direct |
|
No summary statistic | Low† |
| Nd:YAG rate | Cochrane systematic review | Moderate* | Consistent (11 studies) | Direct |
|
0.19 (0.11 to 0.35) Odds ratio (95% CI) |
Moderate |
Downgraded due to study design (lack of details about randomization/blinding)
Downgraded due to significant heterogeneity.
Table 16: GRADE Quality of Evidence for Interventions – 1-Piece Compared to-3 Piece IOLs.
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| PCO | Cochrane systematic review | Moderate* | Consistency (5 studies) | Direct |
|
Results omitted from systematic review but 2 studies continued and reported 2 and 5 year outcomes after systematic review: Leydolt et al. (mean PCO score ±standard deviation at 5 year outcome) 1.7±1.7; vs. 1.3±1.4; p=0.30 Zemaitiene et al. (mean PCO score ±standard deviation at 2 year outcome) 0.15 ±0.19 vs. 0.14±0.22; p=0.18 |
Low† |
| BCDVA | Cochrane systematic review | Moderate* | Consistent (2 studies) | Direct |
|
0.00 (-0.04 to 0.04) Mean difference (95% CI) |
Low† |
| Nd:YAG rate | Cochrane systematic review | Moderate* | Consistent (3 studies) | Direct |
|
0.48 (0.02 to 10.24) acrylic 0.94 (0.59 to 1.51) PMMA Odds ratio (95% CI) |
Low† |
Downgraded due to study design (lack of details about randomization/blinding)
Downgraded due to limited number of materials (e.g., no silicone or acrylic only).
Table 17: GRADE Quality of Evidence for Interventions – Acrylic Compared to Silicone (Same Optic Design and Haptics).
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | |
| PCO | RCT | High | Consistent (1 study; consistent with Cochrane systematic review) | Direct |
|
No significant difference P=0.96 | Moderate* |
| BCDVA | Same RCT | High | Consistent (1 study; consistent with Cochrane systematic review) | Direct |
|
No significant difference No p value reported |
Low/Moderate*,† |
| Nd:YAG rate | Same RCT | High | Consistent (1 study; consistent with Cochrane systematic review) | Direct |
|
No significant difference P=0.19 | Low/Moderate*,† |
Downgraded due to lack of explicit data and no a priori same size calculation
Uncertainty since not primary outcome; possible type 2 error.
Table 18: GRADE Quality of Evidence for Interventions – Modified Prolate Anterior Surface IOLs.
| Outcome | Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect | Quality | ||
| Contrast Sensitivity | RCT | Moderate* | Uncertainty (1 study) | Direct |
|
|
Very Low† | |
| BCVA | Same RCT | Moderate* | Uncertainty (1 study) | Direct |
|
No significant difference Aspherical modified prolate Spherical Spherical blue light filtering |
1.00±0.13 0.97±0.12 0.99±0.13 |
Very Low† |
Downgraded due to study design (lack of details about randomization/blinding).
Downgraded due to uncertainty with methodological issues.
Conclusions
Table 20 shows conclusions for the systematic review of IOLs for age-related cataracts.
Table 20: Conclusions for the Systematic Review of IOLs for Age-Related Cataracts.
| Comparison | Conclusion | GRADE Quality |
|---|---|---|
| Multifocal vs. monofocal |
Objective Outcomes Significant improvement in BDCUNVA No significant difference in BCDVA Inconclusive evidence for contrast sensitivity Inconclusive evidence for glare Subjective Outcomes Inconclusive evidence for visual satisfaction Significant increase in glare/halos Significant incr0ease in freedom from spectacles |
moderate moderate low very low low low/moderate low/moderate |
| Accommodative vs. multifocal/ monofocal | Inconclusive due to Insufficient limited evidence for any effectiveness outcome | very low |
| Hydrophilic acrylic vs. other materials (hydrophobic acrylic, silicone) | Significant increase in PCO score | low |
| Sharp edged compared to round edged | Significant reduction in PCO score | low |
| One piece compared to three piece | No significant difference in PCO score | low |
| Hydrophobic acrylic compared to silicone | No significant difference in PCO score | moderate |
| Aspherical modified prolate anterior surface compared to spherical | No significant difference in VA Significant reduction in contrast sensitivity |
very low very low |
| Blue light filtering compared to non blue-light filtering | No significant difference in BCDVA No significant difference in contrast sensitivity No significant difference in HRQL |
low low high/moderate |
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for all in-hospital stay costs for the designated International Classification of Diseases-10 (ICD-10) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits for physician fees, laboratory fees from the Ontario Laboratory Schedule of Fees, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effectiveness analyses, a discount rate of 5% is used as per the Canadian Agency for Drugs and Technologies in Health.
Downstream costs: All costs reported are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature. In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Literature Review
A broad range of studies assessing cost-effectiveness, economic evaluations, modelling studies and analysis of administrative data were considered in this systematic review. (42–58) The items were identified based on the current review of clinical effectiveness of IOL implantation for the treatment of age-related cataracts. All chosen studies contained Cost-Utility Analyses (CUAs) and compared multifocal and/or monofocal IOLs for cataracts.
In general, IOL implantation for cataracts was found to be cost-effective. In a 2004 study by Baltussen et al. (42), extracapsular cataract extraction (ECCE) procedures using posterior chamber IOL implantation were shown to be cost-effective (specifically when compared to intracapsular cataract extraction (ICCE) in various countries around the world including Canada. In 2007, Lansingh et al. (43) also reported cost-effectiveness of IOLs for cataract surgery for both ECCE and small incision phacoemulsification procedures across various global jurisdictions. Although there was a large range of cost-effectiveness ratios in cost per quality adjusted life years (QALY) and cost per disability adjusted life years (DALY) found among the studies reviewed, each ratio showed the procedure(s) to be cost-effective in the respective country of analysis.
Cost utility analyses (CUAs) of IOL implants for cataracts undertaken by Busbee et al. (United States) and Kobelt et al. (Sweden) also showed cost-effectiveness in terms of an increase in utility and QALY linked to an improvement in post-operative visual acuity. (44; 45) It was unclear, however, whether visual outcomes were assessed using tests for best spectacle-corrected or uncorrected visual acuity. It is important to note that the utility of cataract surgery and IOL implantation was positively correlated with visual acuity of the better-seeing eye. (43–45)
Specific evaluation of multifocal versus monofocal IOLs for cataracts was performed in several studies that valued improvement in outcomes such as spectacle independence (i.e. no need of glasses for near or far vision). Maxwell et al. performed a cost-benefit analysis (CBA) of apodized, diffractive, presbyopia-correcting IOLs in 2008, which reported a greater net benefit (11,670 USD) of implanting multifocal over monofocal IOLs for cataract patients. (44) The data used in the decision-tree analysis reported an average age of 69 for cataract patients with a life expectancy of 14 years based on the general US population of age 70. In the study done by Orme et al. in 2002, the total direct medical care costs (hospital, physician, drug) of patients receiving multifocal and monofocal IOLs was similar, but multifocal IOLs were found to be more cost-effective for spectacle-free patients than monofocal IOLs. (45) In addition, cost per patient without overall limitation in vision-related function and cost per patient without limited night vision were found to be similar for both the multifocal and monofocal IOL patient groups.
Cost-effectiveness and Modelling of Multifocal versus Monofocal IOL
The current review of effectiveness of IOLs for age-related cataract patients was used to inform the selection of strategies and target population below. In particular, complications rates following IOL implantation (e.g. raised intraocular pressure, endophthalmitis, cystoid macular edema) were found to be similar between multifocal and monofocal IOLs, as no modifications to surgical technique were required for the different lenses. (19) The rate of PCO, however, was found to differ among lens materials used and was incorporated into the model for evaluation of the effect of lens material. (32; 46) Furthermore, as PCO complications were treated using Nd-YAG laser capsulotomy, retinal detachment was also included in the model following Nd-YAG laser treatment. Note that this complication was both the best documented adverse event of Nd-YAG treatment of PCO and the most costly with an incidence rate of approximately 1.2%. (47)
Target Population
The target population of interest in the current cost-effectiveness analysis consisted of cataract patients of age ≥65 years for whom IOL implantation (phacoemulsification) was performed after cataract extraction. As the population would have surgery indicated for age-related cataracts, patients with other ocular comorbidities or complicating conditions, such as diabetes or glaucoma, were not considered in the analysis.
Evaluation Strategies
Strategies that were evaluated in this CEA were based on ‘design’ and ‘material’ factors of currently manufactured IOLs. The first strategy (Strategy 1) evaluated the IOL design and compared multifocal and monofocal lenses, both made of hydrophobic acrylic material and foldable in form. The second strategy (Strategy 2) evaluated the choice of material of the implanted lens, comparing hydrophobic acrylic lenses with silicone lenses, specifically for multifocal (foldable) lenses.
Perspective
The analytic perspective taken of the current cost-effectiveness evaluation was that of the MOHLTC. It is important to note that while the cost-utility model incorporated the requirement of eyeglasses for near and far vision correction after IOL implantation, the associated costs were omitted in the economic analysis. Both direct and indirect health care costs (for hospital costs) were included in the analysis.
Model Overview: Base Case, Time Horizon and Discounting
A cost-utility analysis was performed focusing on whether eyeglasses were required for near and far vision correction after IOL implantation. A Markov model was then developed and used to evaluate both Strategies 1 and 2. The cycle length of the model was 1 year and a time horizon of 14 years was used, corresponding to the average life expectancy of the base case.
The base case used in this analysis consisted of an age-related cataract patient of age 65 years or older (average age of 70) without pre-existing eye disease or ocular comorbidities, who had cataract surgery with IOL implantation. These patients did not experience any complications from the cataract surgery itself, but may have had a PCO complication as a result of the IOL procedure. Costs and outcomes were discounted at a 5% annual rate as recommended by CADTH guidelines. (48)
Health states
The Markov model was designed with ten health states, as shown in Figure 1. Five health states were defined with no PCO complication after IOL implantation and five health states were associated with the existence of a PCO complication, with corresponding Nd-YAG laser capsulotomy. Each set of five health states incorporated the requirement of correction for near and far vision (if applicable) as follows:
Figure 1: Markov health states for the evaluation of IOL strategies for age-related cataracts.
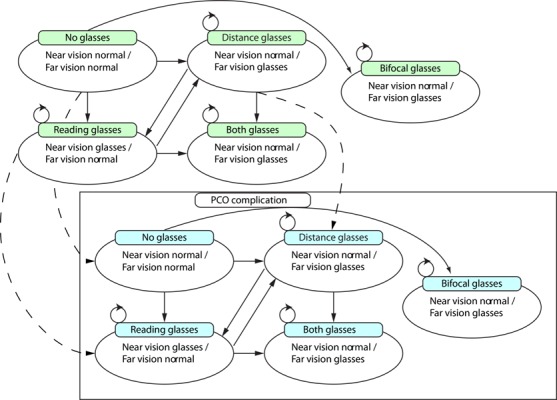
Note: The health state ‘death’ is present but not shown.
‘No glasses’ (near vision normal, far vision normal);
‘Distance glasses’ (near vision normal, far vision glasses);
‘Reading glasses’ (near vision glasses, far vision normal);
‘Both glasses’ (near vision and far vision glasses); and
‘Bifocal glasses’ (near vision / far vision glasses).
The difference between health states 4 and 5 was defined as patients having two separate pairs of glasses for reading and distance vision, and a pair of bifocal glasses, respectively. Health states 4 and 5 were used in the model to represent the cost difference of having two pairs of glasses versus one pair. However, this distinction had no effect on the cost-effectiveness of the evaluated strategies given the MOHLTC perspective defined above.
Although not shown in Figure 1, it should be noted that transition to the absorbing health state ‘death’ could be made from any of the 10 states. Transitions between health states (excluding death) occurred based on the need for near and/or far vision correction, represented by arrows in Figure 1. Arrows with dashed lines represented transitions from non-PCO health states to corresponding PCO health states that could only occur if a PCO complication was experienced by the patient. The occurrence of PCO necessarily implied the additional use of Nd-YAG laser capsulotomy for the treatment of PCO and an additional cost due (potentially) to retinal detachment. It is important to note the one-time cost associated with PCO and Nd-YAG laser treatment and retinal detachment was the only functional difference between the non-PCO and PCO-specific health states in the model.
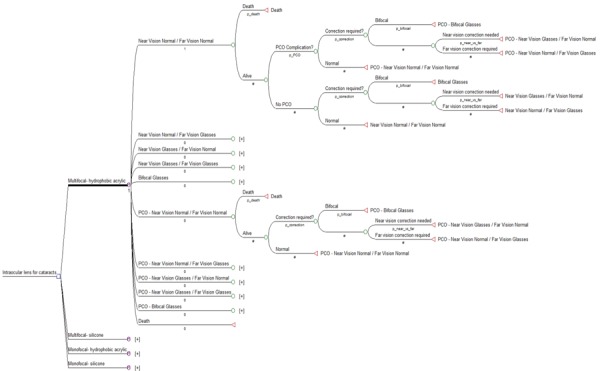
An overview of the Markov model used in the CUA evaluation of strategies 1 and 2 is shown in Figure 2. The decision trees located at each of the non-PCO-related health states were structured similarly to the branches shown under ‘No glasses’. Likewise, the decision trees located at each of the PCO-related health states were structured similarly to the expanded branches for health state ‘PCO- No glasses’. The decision node labelled intraocular lens for cataracts’ was designed to evaluate Strategies 1 and 2, with corresponding branches for each of the options in those strategies: ‘Multifocal- hydrophobic acrylic’ versus ‘Monofocal- hydrophobic acrylic’ for Strategy 1, and ‘Multifocal- silicone’ versus ‘Multifocal-hydrophobic acrylic’ for strategy 2. Note that evaluating the strategy ‘Multifocal- silicone’ versus ‘Monofocal- silicone’ would yield similar results to Strategy 1, and ‘Monofocal- silicone’ versus ‘Monofocal- hydrophobic acrylic’ would produce similar results to Strategy 2.
Figure 2: Markov model for CUA evaluating Strategies 1 and 2 for IOL implants for age-related cataracts.
Model Assumptions
Certain simplifying assumptions were made to the model, which are summarized below:
IOL implantation occurred in only one eye; bilateral IOL implantation was not included
Only PCO complication after cataract extraction and IOL implantation was incorporated into the model, as the importance of this complication has been studied in detail (49; 50)
Costs of cataract surgery (hospital, physician) and associated complications (excluding PCO) were not included, as the costs of surgery were identical and differed only in IOL device costs
Differences in PCO complication rates associated with round-edged or sharp-edged IOLs were not modelled; an overall PCO rate was used from observational data combining both types of lenses (47)
Conditional costs of retinal detachment were incorporated directly into the costs associated with PCO and were not modelled separately; the probability of retinal detachment given a PCO complication was used to distribute the additional cost among all patients with PCO
If PCO occurred as a complication of IOL implantation, it was assumed to occur in the first four years after cataract surgery; an average of four years was chosen due to the large variation in PCO incidence rates (46)
Costs
Total hospital costs (direct and indirect), physician costs and device costs were estimated for IOL devices and PCO complications, including retinal detachment and Nd-YAG laser capsulotomy for age-related cataracts. In the current CUA, costs for cataract surgery and cataract complications were not included, as the strategies evaluated had identical costs for surgery and IOL implantation. That is, the only cost difference between multifocal and monofocal IOLs (Strategy 1) was found to be in the cost of the device itself, given the MOHLTC perspective employed in the analysis. Similarly, the cost difference in Strategy 2 was in lens material (silicone versus hydrophobic acrylic) and costs associated with lens material-dependent PCO complication rates. The costs summarized in Table 1 are in 2009 Canadian dollars; the additional cost of silicone versus hydrophobic acrylic was estimated and 2009 Euros converted using the Bank of Canada rate. (51)
The cost of retinal detachment was also included in the model as an important potential complication of Nd-YAG laser capsulotomy and as a conditional cost of PCO complication. (46; 52) In order to simplify the Markov model, the conditional probability of retinal detachment given a PCO complication (44) was used to distribute the additional cost of vitrectomy among all patients with PCO. Hospital and physician costs of vitrectomy for retinal detachment were estimated and amounted to the addition of an average of $10 for patients developing a PCO complication in the model.
Table 21: Costs used in the CUA evaluation associated with IOL devices and PCO complications.
| Type of Cost | Description | Cost (CAD) | Reference |
|---|---|---|---|
| Hospital | PCO complication (retinal detachment-vitrectomy) PCO complication (Nd-YAG laser capsulotomy) |
$2,294 $58 |
CCI code 1.CM.89 (OCCI 2007-08) CCI code 1.CL.59.LA-AG (OCCI 2007-08) |
| Physician | PCO complication (retinal detachment-vitrectomy) PCO complication (Nd-YAG laser capsulotomy) |
$830 $104 |
ON Schedule of Benefits fee code E142 (50) BC Payment Schedule fee code 22115 (49) |
| IOL Device | Multifocal lens (hydrophobic acrylic material) Monofocal lens (hydrophobic acrylic material) Additional cost of silicone versus hydrophobic acrylic material (multifocal design) |
$950 $250 $8 |
Correspondence with IOL lens manufacturers Correspondence with IOL lens manufacturers Correspondence with IOL lens manufacturers |
Treatment effects
The measure of effect used in the Markov model was visual acuity in terms of needing glasses for near or far vision correction after cataract extraction and IOL implantation. A disutility of -0.03 was assigned to health states where glasses were necessary for vision correction, based on average time trade-off (TTO) and standard gamble (SG) values from studies reporting the (dis)utility of wearing glasses. (53; 54) Disutilities were also associated with IOL complications related to PCO. A disutility of -0.0004 was assigned to the conditional probability of retinal detachment based on values from the literature. (55; 56) This (dis)utility was distributed among all patients with PCO in a similar fashion to how additional costs associated with vitrectomy were distributed among patients for PCO costs.
The above decrements in utility were applied to base case values derived from the literature, which were based on average utility values of cataract patients in Canada. (57) The relative improvement in utility of having surgery for cataracts (and IOL implantation) was estimated as being 21%. (55; 56) This utility increase was applied to age-specific utilities of 0.77 for patients of age 60 to 69 years, 0.79 for patients of age 70 to 79 years, and 0.73 for patients of age 80 to 89 years, which provided base case utility values of 0.93, 0.96 and 0.88 for the model, respectively.
Transition rates and the probabilities of requiring reading, distance and bifocal eyeglasses were based on (retrospective) observational data. The proportion of cataract patients requiring vision correction (reading, distance, bifocal glasses) after IOL implantation was used from two studies: Javitt et al. 2000 and Maxwell et al. 2008. (23;44) Specifically, the proportion of ‘wearing glasses’ for near and far vision correction were based on observational data from Javitt et al., taken from the patients enrolled in that RCT, and from Maxwell et al. from a study of patients who underwent IOL implantation in a non-randomized trial. The proportion of patients requiring glasses was interpreted as the corresponding probability of requiring vision correction and, in the case of Maxwell et al., was re-weighted to derive a mutually exclusive probability of requiring near vision compared to far or distant vision correction. Note that the probability of ‘requiring vision correction’ remained constant over time, with the same probabilities used in each cycle of the Markov model (see Table 22).
Table 22: Probabilities of requiring near or far/distance vision correction after IOL implantation.
| Probability description | Multifocal IOL | Monofocal IOL | Reference |
|---|---|---|---|
| Requiring vision correction (either near or far vision) | 0.201 | 0.923 | Maxwell 2008 |
| Requiring bifocal glasses | 0.271 | 0.386 | Maxwell 2008 |
| Requiring near vision correction (versus far vision correction) | 0.882 | 0.722 | Maxwell 2008 |
| Requiring far vision correction | 0.250 | 0.400 | Javitt 2000 |
| Requiring near vision correction | 0.670 | 0.890 | Javitt 2000 |
The CUA and Markov model also made use of retrospective chart review data in order to estimate the rates of PCO complication. Smith et al. performed a chart review of patients with IOLs implanted in 1996 or 1997 in Europe (France, Italy, Germany, Spain) and analyzed the occurrence of PCO three years after surgery. Differences were found in the rate of PCO among different IOL materials and were incorporated into the model as follows: a rate of 8.9% for hydrophobic acrylic IOLs and 21.6% for silicone-based IOLs, for either multifocal or monofocal design. (47)
Mortality rates
Probabilities associated with transitions to the Markov state ‘death’ were estimated from tables summarizing deaths by age (and sex) and by province based on the Statistics Canada death database. (52) An average annual (fiscal year) rate was used for Ontario deaths combining sex-specific death rates in 2006-07.
Cost-effectiveness results
Both strategies evaluated in the current cost-utility analysis were found to be cost-effective. For hydrophobic acrylic, one of materials with the lowest rate of PCO complication, the multifocal IOL implantation for age-related cataracts (strategy 1) was found to have an ICER of about $8,000/QALY. As a result, using the commonly accepted threshold of $50,000/QALY implied cost-effectiveness of multifocal IOLs. For the second strategy, hydrophobic acrylic material was found to be the dominant strategy: it saved costs and improved quality of life (QALY) of patients receiving multifocal IOLs. Results are shown in Table 3 and Table 4.
One-way sensitivity analysis was performed on the Markov model used in the current CUA. The factors having the greatest impact on the ICER for strategy 1 were the device cost of the multifocal and the proportion of patients requiring glasses. If the lens device cost increased to $4,500, multifocal IOLs were no longer considered cost-effective, with resulting ICERs exceeding the threshold of $50,000/QALY. Likewise, if the proportion of patients requiring visual correction was increased from 20% to 63%, multifocal IOLs were not considered cost-effective.
For strategy 2 evaluating lens materials for multifocal IOLs, the factors having the greatest impact on the ICER were the difference in cost between silicone and hydrophobic acrylic and the rate of PCO complication. According to the model developed, the cost of silicone IOLs must be reduced by at least $60 for hydrophobic acrylic not to be found cost-effective. Also, the rate of PCO complication associated with hydrophobic acrylic must increase from about 9% to 24% for this material not to produce cost savings.
Table 23: Cost-effectiveness of Strategy 1 (multifocal vs. monofocal IOL made of hydrophobic acrylic).
| Design | Cost | Incremental Cost | Effect (QALY) | Incremental Effect | Cost per QALY |
|---|---|---|---|---|---|
| Multifocal | $755.46 | $700.00 | 8.902779 | 0.083536 | $8,380 |
| Monofocal | $55.46 | 8.819243 |
Table 24: Cost-effectiveness of Strategy 2 (hydrophobic acrylic vs. silicone for multifocal IOLs).
| Material | Cost | Incremental Cost | Effect (QALY) | Incremental Effect | |
|---|---|---|---|---|---|
| Hydrophobic acrylic | $755.46 | -$59.81 | 8.902779 | 0.000125 | Dominant |
| Silicone | $815.27 | 8.902654 |
Discussion
Both strategies evaluated in the current cost-utility analysis were found to be cost-effective. For age-related cataract patients with IOL implantation, Strategy 1 of using multifocal lenses made of hydrophobic acrylic (low PCO rate) was found to be cost-effective with an ICER of about $8,000/QALY. In Strategy 2 the use of hydrophobic acrylic material was found to be dominant: the material saved costs and improved the quality of life (QALY) of patients receiving multifocal IOLs (compared to silicone material). Further examination of the model used in the CUA suggested Strategy 1 was sensitive to increased cost of the multifocal lens and to the increased proportion of patients required near or far vision correction after IOL implantation. The associated cost and probability parameters, however, would need to be increased 3- to 5-fold in order to change the cost-effectiveness of Strategy 1. In a similar way, factors found to influence the cost-effectiveness of Strategy 2 were the cost difference between silicone and hydrophobic acrylic lens material and the rate of PCO complications. These parameters would need to be increased 3- to 7-fold to change the cost-effectiveness of Strategy 2.
In Ontario, the total cost of providing monofocal or multifocal IOL to patients with age-related cataracts would be approximately $27.4 million for monofocal and $113.2 million for multifocal lenses. These estimates were based on 90,183 cataract surgeries performed in Ontario in 2005 (3), with an average cost per patient of $304 for monofocal and $1255 for multifocal IOLs (Markov model estimates), both made of hydrophobic acrylic material.
The CUA and Markov model used to evaluate the two IOL strategies made use of observational data and patient chart reviews. The low quality evidence currently available in these areas suggests further research is needed to develop more accurate models evaluating multifocal IOLs and the type of material used in the manufacturing process.
Ontario Health System Impact Analysis
Considerations and Implications
Ontario
Procedures for crystalline lens removal and IOL insertion are insured and are listed in the Ontario Schedule of Benefits.
If a particular lens is determined to be medically necessary for a patient, the cost of the lens is covered by the hospital budget. If the patient chooses a lens that has enhanced features then the hospital may elect to charge an additional amount above the cost of the usual lens offered.
An IOL manufacturer stated that monofocal lenses comprise approximately 95% of IOL sales in Ontario and premium lenses (e.g. multifocal/accomodative) make up the remaining 5%.
A medical consultant stated that all types of lenses are currently being used in Ontario (e.g. multifocal, monofocal, accommodative, tinted, nontinted, spheric, and aspheric). Nonfoldable lenses, rarely used in routine cases, are primarily used in complicated cataract implantation situations.
Diffusion: International
United States
Aetna (September 2008)
Aetna considers standard fixed monofocal posterior chamber IOL medically necessary for aphakia.
Accommodating posterior chamber IOLs (e.g., Crystalens, Eyeonics Inc., Aliso Viejo, CA), apodized diffractive optic IOLs (e.g., AcrySof ReSTOR, Alcon, Inc., Fort Worth, TX), ultraviolet absorbing lenses (e.g., AcrySof Natural blue-light filtering IOL, Alcon, Inc., Fort Worth, TX, and C-flex IOL model 570C, Rayner Surgical Inc., Los Angeles, CA), multifocal posterior chamber IOLs, and other new technology lenses (e.g., the Sofport LI61AO aberration-neutral IOL, Bausch & Lomb, San Dimas, CA) are considered non-covered deluxe items.
Given that multifocal IOLs, accommodating IOLs, and apodized diffractive optic IOLs are intended to obviate the need for reading glasses post-surgery, these IOLs are considered convenience items.
For members who elect non-covered new technology IOLs, cataract removal and lens implantation would be considered medically necessary if the criteria for cataract surgery outlined above are met. The new technology lens itself would not be covered.
Cigna (December 2008)
CIGNA covers a standard monofocal or multifocal IOL implant as medically necessity for replacement of the crystalline lens as part of cataract surgery.
CIGNA does not cover an accommodating IOL implant (e.g. Crystalens Model AT-45) for the treatment of cataracts because they are considered experimental, investigational or unproven.
CIGNA does not cover IOL implants (clear lens extraction [CLE]) for the correction of presbyopia because the procedure is performed to correct refractive errors and is not medically necessary.
Centers for Medicare and Medicaid Services (May 2005)
One pair of conventional eyeglasses or contact lenses furnished subsequent to each cataract surgery with insertion of an IOL is covered.
-
A single presbyopia-correcting IOL essentially provides what is otherwise achieved by two separate items: an implantable conventional IOL (one that is not presbyopia-correcting) and eyeglasses or contact lenses. Although presbyobia-correcting IOLs may serve the same function as eyeglasses or contact lenses furnished following cataract surgery, IOLs are neither eyeglasses nor contact lenses.
Therefore, the presbyopia-correcting functionality of an IOL does not fall into the benefit category and is not covered. Any additional provider or physician services required to insert or monitor a patient receiving a presbyopia correcting IOL are also not covered. For example, eye examinations performed to determine the refractive state of the eyes following insertion of a presbyopia-correcting IOL are not covered.
United Kingdom
National Institute for Health and Clinical Excellence (February 2007)
Current evidence suggests that there are no major safety concerns associated with the implantation of accommodating lenses for cataracts. There is evidence of short-term efficacy in correcting visual acuity but there is inadequate evidence that the procedure achieves accommodation. Therefore, the procedure should not be used without special arrangements for consent and for audit or research.
Clinicians wishing to undertake implantation of accommodating lenses should take the following actions.
Ensure that patients understand the uncertainty about the procedure’s efficacy, and provide them with clear written information.
Audit and review clinical outcomes of all patients having implantation of accommodating lenses.
Publication of long-term efficacy outcomes of the procedure will be useful, particularly on the effects on accommodation. The Institute will review the procedure in due course.
It was noted that the evidence reviewed on this procedure relates to the treatment of cataract and not to the correction of presbyopia. It was also noted that accommodating lenses are at a relatively early stage of development and that the technology is evolving rapidly.
Glossary (optional)
- Aphakic eye
An eye that does not have a new lens after cataract extraction
- Contrast sensitivity
The ability to perceive differences between an object and its background
- Depth of field
The range of distances over which the eye cannot detect any change in focus
- Mesopic
Intermediate levels of light
- Night vision disturbances
Vision quality defects apparent in low light or darkness; the best-known examples are starbursts and haloes.
- Photopic
Full level of light.
- Pseudophakic eye
The substitution of the natural crystalline lens with a synthetic lens.
- Regeneratory posterior capsule opacification
Also referred to as ‘after cataract’, it’s the most common long-term complication of IOL implantation surgery for cataracts. It’s the result of migration of lens epithelial cells along the posterior capsule behind the IOL. These cells proliferate to form layers of lens material that leading to opacification and reduced visual function.
- Visual acuity
The ability of the visual system to discern fine detail, as measured by printed or projected visual stimuli
Appendix 1: Literature Search Strategies
Databases searched: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library (all via OVID); CRD/INAHTA
Database: Ovid MEDLINE(R) <1996 to January Week 3 2009>
Search Strategy:
exp Lens Implantation, Intraocular/ or exp Lenses, Intraocular/ (7114)
((implant* or intraocular) adj2 lens*).mp. (7878)
(iol or iols).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3550)
(Tek-Clear or Tetraflex or SmartLens or SmartIOL or FlexOptic or (Array adj3 lens*) or AcriTec or ReStor or ReZoom or Crystalens or Synchrony or Tecnis or SofPort or Acrysof).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3594)
exp Pseudophakia/ (769)
pseudophak*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1391)
or/1-6 (11750)
exp Cataract Extraction/ or exp Cataract/ (14261)
(Pseudoaphaki* or cataract*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17315)
(lens* adj (opacity or opacities or opacification)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (788)
or/8-10 (18998)
7 and 11 (6241)
limit 12 to (english language and humans and yr="2003 - 2009") (2643)
limit 13 to (controlled clinical trial or meta analysis or randomized controlled trial) (346)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (35585)
(health technology adj2 assess$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (650)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (67644)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (380904)
exp Double-Blind Method/ (54040)
exp Control Groups/ (823)
exp Placebos/ (9446)
(RCT or placebo? or sham?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (96228)
or/14-22 (490457)
23 and 13 (438)
limit 24 to "all child (0 to 18 years)" (25)
24 not 25 (413)
Database: EMBASE <1980 to 2009 Week 05>
Search Strategy:
exp lens implant/ (9976)
exp Lens Implantation/ (2309)
((implantable or intraocular) adj2 lens*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (8514)
(iol or iols).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (4809)
(Tek-Clear or Tetraflex or SmartLens or SmartIOL or FlexOptic or (Array adj3 lens*) or AcriTec or ReStor or ReZoom or Crystalens or Synchrony or Tecnis or SofPort or Acrysof).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (4509)
Pseudophakia/ (2508)
pseudophak*.mp. (3160)
or/1-7 (18259)
exp Cataract Extraction/ or exp Cataract/ (33442)
(Pseudoaphakia or cataract*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (36058)
(lens* adj (opacity or opacities or opacification)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1327)
or/9-11 (38013)
8 and 12 (10019)
limit 13 to (human and english language and yr="2003 - 2009") (2654)
Randomized Controlled Trial/ (165071)
exp Randomization/ (26467)
exp RANDOM SAMPLE/ (1395)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (297798)
(health technology adj2 assess$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (670)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (64531)
Double Blind Procedure/ (71178)
exp Triple Blind Procedure/ (12)
exp Control Group/ (2779)
exp PLACEBO/ or placebo$.mp. or sham$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (212600)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (429709)
(control$ adj2 clinical trial$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (282757)
or/15-26 (795016)
27 and 14 (510)
limit 28 to (embryo <first trimester> or infant <to one year> or child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) (32)
28 not 29 (478)
Appendix 2: Results of Published Meta-Analyses
A2: Results of Published Meta-Analyses.


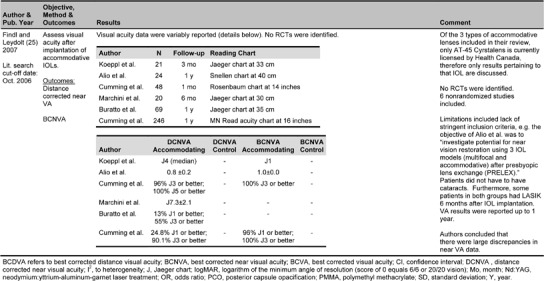
BCDVA refers to best corrected distance visual acuity; BCNVA, best corrected near visual acuity; BCVA, best corrected visual acuity; CI, confidence interval; DCNVA, distance corrected near visual acuity; I2, to heterogeneity; J, Jaeger chart; logMAR, logarithm of the minimum angle of resolution (score of 0 equals 6/6 or 20/20 vision); Mo, month; Nd:YAG, neodymium:yttrium-aluminum-garnet laser treatment; OR, odds ratio; PCO, posterior capsule opacification; PMMA, polymethyl methacrylate; SD, standard deviation; Y, year.
Appendix 3: Results of Studies Published After the Meta-Analyses
A3(1): Results of Studies on Posterior Opacification.
| Author & Pub. Year | Objective | Method | Outcomes | Results | Comment | ||
|---|---|---|---|---|---|---|---|
| Kugelberg et al. (32) 2008 | Evaluate PCO 2 years after cataract surgery following implantation of a hydrophilic or hydrophobic sharp edged IOL. Follow-up of study included in PCO Cochrane review (Kugelberg et al. (58)) |
N=120 patients Surgery performed in 1 eye per patient. Prospectively randomized. 2 year follow-up |
1° outcome: PCO 2° outcome: logMAR VA (2.5% and 100% contrast) glare disability |
Hydrophobic (n=58 patients) vs. Hydrophilic (n=57 patients) median (range) PCO area (%) PCO severity p<0.001 Nd:YAG rate total, n (%) BCVA (high contrast) Low contrast VA (2.5%) Glare |
4.5 (0 to 71) vs. 46 (0 to 100), p<0.001 0.045 (0 to 0.83) vs. 0.74 (0 to 2.2), 6 (10) vs. 24 (42), p<0.001) -0.02±0.09 vs. 0.05±0.14, p<0.01 0.50 ±0.15 vs. 0.61± 0.22, p<0.01 0.004± 0.11 vs. 0.12±0.18, p<0.001 |
Masking of patients/examiners unknown No sample size calculation 5 patients LTF (details provided) ITT not discussed POCOman software –calculates %PCO by area within whole capsulorhexis and a score for PCO severity. Low contrast VA (unknown if corrected or uncorrected). Glare measured with Brightness Acuity Test Not know how VA measured (assuming near VA). Partially supported by grant from manufacturer. |
|
| Hayashi and Hayashi (33) 2007 | Compare PCO and visual functions between eyes with an acrylic vs. silicone IOL of the same optic design and haptics up to 36 months after implantation. | N=100 patients Acrylic implanted in one eye and silicone implanted in fellow eye in each patient. Prospective Randomized. Patients and examiners masked. 3 year follow-up |
1° outcome: PCO 2° outcome: Nd:YAG logMAR BCVA Contrast VA Glare VA (contrast VA in presence of a glare source) |
9 patients lost to follow-up (89 remained). No significant difference found in: PCO density value (p=0.96) Nd:YAG capsulotomy (p=0.19) BCVA (no p value reported) Photopic (daylight vision) or mesopic (intermediate light vision) contrast sensitivity with or without glare. |
PCO density value measured using Scheimpflug videophotography. Post hoc power calculation showed 99% power to detect clinically meaningful PCO difference between acrylic and silicone. Actual data for PCO/VA not reported (comparisons presented in figures only). Not know how VA measured (what charts and if distance corrected). Patients and examiners masked. Nd:YAG performed when an eye lost 2 ore more decimal lines of VA or if patient complained of blurred vision. 9 patients LTF (details provided). No funding from manufacturer. |
||
| Leydolt et al. (34) 2007 | Compare PCO between 1-piece and 3-piece haptic designs of foldable hydrophobic acrylic IOL Follow-up of study included in PCO Cochrane review (Sacu et al. (53)) |
N=52 patients IOL bilaterally inserted. Prospective Randomized Double blind-stated in abstract only 5 year follow-up |
1° outcome: PCO score 2° outcome: Nd:YAG BCDVA |
24 patients not available for 5 year follow-up exam (25 remained). No significant difference in mean (standard deviation) PCO score at 5 years using image analysis software: 1 piece 1.7±1.7; 3 piece 1.3±1.4; p=0.30 No significant difference in PCO score at 5 years using slit lamp: 1 piece 1.6±1.9; 3 piece 1.1±1.5; p=0.24 No significant difference in BCDVA: 0.8±0.2 in both groups, p=0.40 No significant difference in Nd:YAG: 1 piece (4 patients); 3 piece (3 patients), p=0.40 |
No sample size calculation. VA measured with Snellen chart. Approximately 50% drop out PCO calculated subjectively using slit lamp and objectively using automated image analysis software developed by authors. Post hoc power calculation for the observed standard deviation of the 25 patients (50 eyes) showed that a clinically relevant difference of PCO score of 1 (i.e., 10%) could be calculated with a 90% power at an alpha level of 5%. Results by Sacu et al. showed a slight but significant difference with more PCO in 1 piece compared with 3 piece IOL eyes 1 year after surgery. This was not seen 2 and 5 years after surgery. Funding from manufacturer not specified. |
||
| Zemaitiene et al. (35) 2007 | Compare PCO between 1-piece and 3-piece haptic designs of hydrophobic acrylic IOL. Not explicitly stated by authors, but possible follow-up of study included in Cochrane review (Zemaitiene et al. (54) |
N=74 patients IOL bilaterally inserted. Prospective Randomized Not blinded 2 year follow-up |
1° outcome: PCO score 2° outcome: Nd:YAG BCVA |
16 patients did not participate in 2 year follow-up (58 remained). Mean (standard deviation) PCO values (measured with image analysis system) Significant difference at 6 months 3 piece 0.002±0.009; 1 piece 0.007±0.017; p=0.04 Significant difference at 1 year 3 piece 0.004±0.016; 1 piece 0.026±0.041; p=0.001 No significant difference in PCO score at 2 years 3 piece 0.136±0.223; 1 piece 0.154±0.190; p=0.18 No significant difference in BCVA at 2 years (no data provided). Not stated whether distance or near BCVA was examined. No significant difference in Nd:YAG at 2 years: No case of PCO with a decrease of ≥2 lines of VA that required Nd:YAG laser capsulotomy in either group. |
No sample size calculation. 16 LTF (details provided) PCO calculated using photographic image analysis system (EPCO2000) developed by one of the authors. No a priori or post hoc power calculation reported. Blinding of patient/examiner not reported. Results by Zemaitiene et al. in 2004 showed a slight but significant difference with more PCO in 1 piece compared with 3 piece IOL eyes 1 year after surgery. This was not seen at 2 years after surgery. Funding from manufacturer not specified. |
||
BCDVA refers to best corrected distance visual acuity; BCVA, best corrected visual acuity; IOL, intraocular lens; ITT, intent to treat analysis; logMAR, logarithm of the minimum angle of resolution (score of 0 equals 6/6 or 20/20 vision); Nd:YAG, neodymium:yttrium-aluminum-garnet laser treatment; PCO, posterior capsule opacification; VA, visual acuity.
A3(2): Results of Studies on Modified Prolate Anterior Surface IOLs.
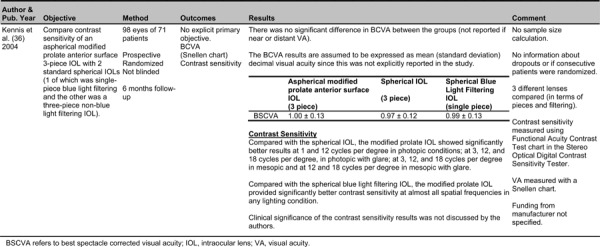
BSCVA refers to best spectacle corrected visual acuity; IOL, intraocular lens; VA, visual acuity.
A3(3): Results of Studies on Blue Light Filtering IOLs.
| Publication Date | Objective | Method | Outcomes | Results | Comment |
|---|---|---|---|---|---|
| Marshall et al. (39) 2005 | Evaluate safety and effectiveness of a blue light filtering IOL. | N=297 patients IOL bilaterally inserted. Prospective Randomized Patient masked 6 months to 1 year follow-up. |
No 1° outcome reported Contrast sensitivity (at 6 months) BCDVA (at 1 year) using Snellen chart. PCO (6 months) |
Blue filter IOL n=150 patients Regular single piece IOL (Acrysof SA30AL) n=147 patients PCO Blue filter: no cases of clinically significant PCO or PCO requiring Nd:YAG Regular IOL: 1 clinically significant PCO, none requiring Nd:YAG BCDVA No significant difference noted between the IOLs. Contrast Sensitivity No clinically significant differences under photopic or mesopic conditions (p=0.6220) Colour Perception No statistically significant differences noted between the IOLs (p=0.2669) |
Concentration of chromophore in blue filtering IOL results in a transmission curve that best resembles that of a 25 year old natural crystalline lens (ability of lens to filter blue light decreases with age). Contrast sensitivity measured with CSV1000E contrast sensitivity unit at 8 feet under photopic and mesopic conditions. No sample size calculation. Possible type 2 error. For contrast sensitivity, clinical significance was defined as a difference of ≥0.3 log units at ≥2 spatial frequencies. Inconclusive whether blue light is a risk factor for age related macular degeneration. No clinical trials comparing effect of blue filtering IOLs and non-blue filtering IOLs on macular toxicity performed to date. Funding from manufacturer not specified. |
| Barisic et al. (40) 2007 | Examine clinical effects of blue filter IOL. | N=60 patients IOL bilaterally implanted Prospective Randomized 6 months follow-up |
No 1° outcome reported. UCVA BCVA Nd:YAG rate Patient satisfaction Subjective colour perception |
UCVA No significant difference (UCVA better than 0.8 [20/25] was achieved in 86.7% of patients in the blue filter group and 85.0% of those in the control group), p=0.793 BCVA All patients achieved BCVA better than 0.8 (20/25), no p value reported. Nd:YAG Rate No significant difference between the groups (p=0.50). Patient satisfaction 96.7% of the blue filter group would implant the same IOL again. Subjective Colour Perception None of the patients reported any colour perception disturbances in photopic or mesopic conditions. |
No sample size calculation. Limited reporting of data. Possible type 2 error. Funding from manufacturer not specified. |
| Bhattacharjee et al. (38) 2006 | Compare effects of blue filter IOL with regular IOL. | N=13 patients (26 eyes) Blue filter IOL implanted in one eye and regular IOL implanted in fellow eye of each patient. Prospective Randomized Patients masked 18 months follow-up |
No 1° outcome reported. BCDVA (Snellen chart) Colour vision Contrast sensitivity (Pelli Robson chart) |
BCDVA No significant difference between groups (blue filter group: improved to 20/20 in 12 eyes and to 20/30 in 1 eye; regular IOL: improved to 20/20 in all cases, p=0.30). Colour Vision Postoperative improvement between groups was comparable without any significant difference in the total score (p=0.19), blue-yellow partial error scores (p=0.07), and the red-green partial error scores (p=0.66). Contrast Sensitivity No significant difference between groups (p=0.26). |
No sample size calculation. No reporting whether patients were consecutively randomized. Contrast sensitivity measured via Pelli Robson chart. Possible type 2 error. Funded by a Health and Educational Foundation in India. |
| Espindle et al. (41) 2005 | Compare patient reported vision related and health related functioning following implantation of a blue filter IOL and a non-blue light filtering (Acrysof single piece) IOL. | N=257 Bilateral implantation Prospective Randomized Double masked 6 month follow-up |
Health related quality of life questionnaires (National Eye Institute’s visual Functioning Questionnaire [NEI VFQ-39] and Short Form Health Survey [SF-12]). | N=257 Primary treatment comparisons performed for: VFQ composite score, colour vision driving scales SF-12 physical and mental component No significant difference in the 5 primary comparisons. |
Detailed sample size calculation reported. Last observation carried forward analysis. Patient and data collector masked. Funded by competing manufacturer. |
BCDVA refers to best corrected distance visual acuity; BSCVA, best spectacle corrected visual acuity; IOL, intraocular lens; Nd:YAG, neodymium:yttrium-aluminum-garnet laser treatment; PCO, posterior capsule opacification; UCDVA, uncorrected distance visual acuity; UCNVA, uncorrected near visual acuity; UCVA, uncorrected visual acuity; VA, visual acuity.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Intraocular lenses for the treatment of age-related cataracts: an evidence-based analysis. Ontario Health Technology Assessment Series 2009;9(15).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-0637-3 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit //www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables & Figures
| ES Table 1: Conclusions for the Systematic Review of IOLs for Age-Related Cataracts |
| Table 1: Classification of IOLs for Cataracts |
| Table 2: Subclassifications of IOLs for Cataracts |
| Table 3: Quality of Evidence of Included Studies |
| Table 4: Conclusions of Descriptive Systematic Reviews from International HTA Organizations |
| Table 5: Meta-Analytic Results for Comparisons of IOL Materials from Findl et al. |
| Table 6: Meta-Analytic Results for Comparisons of IOL Designs (Round and Sharp Edges) from Findl et al. |
| Table 7: Meta-Analytic Results for Comparisons of IOL Designs (One and Three Piece) from the Cochrane Systematic Review by Findl et al. |
| Table 8: Limitations of Studies Included in Findl and Leydolt, 2007 |
| Table 9: Studies Comparing IOL Material and Design with PCO as the Primary Outcome (Published after Cochrane Review) |
| Table 10: Studies Comparing Blue Light Filtering to Non-Blue Light Filtering IOLs. |
| Table 11: GRADE Quality of Evidence for Interventions – Multifocal vs. Monofocal Lenses Objective Endpoints |
| Table 12: GRADE Quality of Evidence for Interventions – Multifocal vs. Monofocal Lenses Subjective Endpoints |
| Table 13: GRADE Quality of Evidence for Interventions – Accommodating IOLs |
| Table 14: GRADE Quality of Evidence for Interventions – Hydrophilic Acrylic Compared to All Other Materials |
| Table 15: GRADE Quality of Evidence for Interventions – Sharp Edged Compared to Round Edged IOLs Regardless of Lens Material. |
| Table 16: GRADE Quality of Evidence for Interventions – 1-Piece Compared to-3 Piece IOLs |
| Table 17: GRADE Quality of Evidence for Interventions – Acrylic Compared to Silicone (Same Optic Design and Haptics) |
| Table 18: GRADE Quality of Evidence for Interventions – Modified Prolate Anterior Surface IOLs |
| Table 19: GRADE Quality of Evidence for Interventions – Blue Light Filtering IOLs |
| Table 20: Conclusions for the Systematic Review of IOLs for Age-Related Cataracts |
| Figure 1: Markov health states for the evaluation of IOL strategies for age-related cataracts |
| Figure 2: Markov model for CUA evaluating Strategies 1 and 2 for IOL implants for age-related cataracts |
| Table 21: Costs used in the CUA evaluation associated with IOL devices and PCO complications |
| Table 22: Probabilities of requiring near or far/distance vision correction after IOL implantation |
| Table 23: Cost-effectiveness of Strategy 1 (multifocal vs. monofocal IOL made of hydrophobic acrylic) |
| Table 24: Cost-effectiveness of Strategy 2 (hydrophobic acrylic vs. silicone for multifocal IOLs) |
| A2: Results of Published Meta-Analyses |
| A3(1): Results of Studies on Posterior Opacification |
| A3(2): Results of Studies on Modified Prolate Anterior Surface IOLs |
| A3(3): Results of Studies on Blue Light Filtering IOLs |
List of Abbreviations
- AUC
Area under the curve
- BCDVA
Best corrected distance visual acuity
- BCNVA
Best corrected near visual acuity
- BCVA
Best corrected visual acuity
- BDCUNVA
Best distance corrected unaided near visual acuity
- CI
Confidence interval(s)
- DCNVA
Distance corrected near visual acuity
- HRQL
Health related quality of life
- IOL
Intraocular lens
- MAS
Medical Advisory Secretariat
- Nd:YAG
Neodymium: yttrium-aluminum-garnet
- PCO
Posterior capsule opacification
- PMMA
Polymethyl methacrylate
- OR
Odds ratio
- OHTAC
Ontario Health Technology Advisory Committee
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SROC
Summary receiver operating characteristic
- UCVA
Uncorrected visual acuity
- VA
Visual acuity
References
- 1.Owsley C, Stalvey BT, Wells J, Sloane ME, McGwin G. Visual risk factors for crash involvement in older drivers with cataract. Arch Ophthalmol. 2001;119(6):881–7. doi: 10.1001/archopht.119.6.881. [DOI] [PubMed] [Google Scholar]
- 2.Rachmiel R, Trope GE, Chipman ML, Buys YM. Cataract surgery rates in Ontario Canada from 1992 to 2004: more surgeries with fewer ophthalmologists. Can J Ophthalmol. 2007;42:539–42. [PubMed] [Google Scholar]
- 3.Hatch W.V, Cernat G, Singer S, Bell C.M. A 10-year population-based cohort analysis of cataract surgery rates in Ontario. Can J Ophthalmol. 2007;42:552–446. [PubMed] [Google Scholar]
- 4.Cigna. Cigna medical coverage policy: intraocular lens implant. Philadelphia, PA: Cigna. 2008 Dec 15. [[cited: 2009 Mar 11]]. [Internet] 12 p. Available from: www.cigna.com/customer_care/healthcare_professional/coverage_positions/medical/mm_0125_coveragepositioncriteria_intraocular_lens_implant.pdf .
- 5.Bellan L, Ahmed IIK, MacInnis B, Mann C, Noel F, Sanmugasunderam S. Canadian Ophthalmological Society evidence-based clinical practice guidelines for cataract surgery in the adult eye. Can J Ophthalmol. 2008;43(SUPPL. 1):S7–S57. doi: 10.1139/i08-133. [DOI] [PubMed] [Google Scholar]
- 6.Scott A. Accommodative intraocular lenses for age-related cataracts. Issues Emerg Health Technol. 2006;85:1–6. [PubMed] [Google Scholar]
- 7.Li N, Chen X, Zhang J, Zhou Y, Yao X, Du L, et al. Effect of AcrySof versus silicone or polymethyl methacrylate intraocular lens on posterior capsule opacification. Ophthalmology. 2008;115(5):830–8. doi: 10.1016/j.ophtha.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Schaumberg D.A, Dana M.R, Christen W.G, Glynn R.J. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105:1213–21. doi: 10.1016/S0161-6420(98)97023-3. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology. Cataract in the adult eye: preferred practice pattern. San Francisco, CA: The Academy. 2006 Sep 16. [[cited: 2009 Apr 22]]. 70 pp. [Internet] Available from: http://one.aao.org/asset.axd?id=821cecfb-85c5-400d-a65f-7a9a727bc163 .
- 10.Rubin GS, Bandeen-Roche K, Huang GH, Munoz B, Schein OD, Fried L.P, et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Invest Ophthalmol Vis Sci. 2001;42(1):64–72. [PubMed] [Google Scholar]
- 11.Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001;49(5):508–15. doi: 10.1046/j.1532-5415.2001.49107.x. [DOI] [PubMed] [Google Scholar]
- 12.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23(7):689–99. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- 13.Ball KK. Real-world evaluation of visual function. Ophthalmol Clin North Am. 2003;16(2):289–98. doi: 10.1016/s0896-1549(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health and Long-Term Care. Schedule of benefits for physician services under the health insurance act: ocular and aural surgical procedures. Toronto, ON: Queen's Printer for Ontario. 2008 Jan 4. [[cited: 2009 Dec 3]]. [Internet] 16 p. Available from: www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/y_specia.pdf .
- 15.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman C. Literature searching and evidence interpretation for assessing health care practices. The Swedish council on Technology Assessment in Health Care. 1993 doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 17.Mundy L, Merlin T, Parrella A, CrystaLens TM. an accommodating intraocular lens replacement for patients with cataracts. [[cited: 2009 Apr 4]];Horizon scanning prioritising summary. 2004 Vol. 6:5 p. [Internet] Available from: www.horizonscanning.gov.au/internet/horizon/publishing.nsf/Content/829B55021D13FDE9CA2575AD0080F2EC/$File/v6_3.rtf . [Google Scholar]
- 18.National Institute for Health and Clinical Excellence. Implantation of accommodating intraocular lenses for cataract. [[cited: 2009 Aug 8]]. [Internet] 2007 Feb 28. Available from: http://guidance.nice.org.uk/IPG209/Guidance/pdf/English .
- 19.Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. Art. No.: CD003738. DOI: 10.1002/14651858.CD003738.pub2. 2007;(Issue 3) doi: 10.1002/14651858.CD003738.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JW, Wei RL, Cai JP, Xi GL, Zhu H, Li Y, et al. Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: a meta-analysis. Am J Ophthalmol. 2007;143(3):428–36. doi: 10.1016/j.ajo.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Leyland M, Pringle E. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2006;(Issue 3):Art. No.: CD003169. DOI: 10.1002/14651858.CD003169.pub2. doi: 10.1002/14651858.CD003169.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Leyland M, Langan L, Goolfee F, Lee N, Bloom PA. Prospective randomized double-masked trial of bilateral multifocal bifocal or monofocal intraocular lenses. Eye. 2002;16:481–90. doi: 10.1038/sj.eye.6700077. [DOI] [PubMed] [Google Scholar]
- 23.Javitt JC, Steinert RF. Cataract extraction with multifocal intraocular lens implantation: a multinational clinical trial evaluating clinical, functional and quality of life outcomes. Ophthalmology. 2000;107:2040–8. doi: 10.1016/s0161-6420(00)00368-7. [DOI] [PubMed] [Google Scholar]
- 24.Steinert RF, Post CT Jr, Brint SF, Fritch CD, Hall DL, Wilder LW, et al. A prospective randomized double-masked comparison of a zonal progressive multifocal intraocular lens and a monofocal intraocular lens. Ophthalmology. 1992;99:853–61. doi: 10.1016/s0161-6420(92)31864-0. [DOI] [PubMed] [Google Scholar]
- 25.Findl O, Leydolt C. Meta-analysis of accommodating intraocular lenses. J Cataract Refract Surg. 2007;33(3):522–7. doi: 10.1016/j.jcrs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Koeppl C, Findl O, Menapace R, Kriechbaum K, Wirtitsch M, Buehl W, et al. Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg. 2005;31(7):1290–7. doi: 10.1016/j.jcrs.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Alio JL, Tavolato M, De La HF, Claramonte P, Rodriguez-Prats J-L, Galal A. Near vision restoration with refractive lens exchange and pseudoaccommodating and multifocal refractive and diffractive intraocular lenses: Comparative clinical study. J Cataract Refract Surg. 2004;30(12):2494–503. doi: 10.1016/j.jcrs.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 28.Cumming JS, Slade SG, Chayet A. AT-45 Study Group. Clinical evaluation of the model AT-45 silicone accommodating intraocular lens. Results of feasibility and the initial phase of a Food and Drug Administration clinical trial. Ophthalmology. 2001;108(11):2005–10. doi: 10.1016/s0161-6420(01)00779-5. [DOI] [PubMed] [Google Scholar]
- 29.Cumming JS, Colvard DM, Dell SJ, Doane J, Fine IH, Hoffman RS, et al. Clinical evaluation of the Crystalens AT-45 accommodating intraocular lens. Results of the U.S. Food and Drug Administration clinical trial. J Cataract Refract Surg. 2006;32(5):812–25. doi: 10.1016/j.jcrs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Marchini G, Pedrotti E, Sartori P, Tosi R. Ultrasound biomicroscopic changes during accommodation in eyes with accommodating intraocular lenses: pilot study and hypothesis for the mechanism of accommodation. J Cataract Refract Surg. 2004;30(12):2476–82. doi: 10.1016/j.jcrs.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 31.Buratto L, Di Meglio G. Accommodative intraocular lenses: short term visual results of two different lens types. Eur J Ophthalmol. 2007;16(1):33–9. doi: 10.1177/112067210601600107. [DOI] [PubMed] [Google Scholar]
- 32.Kugelberg M, Wejde G, Jayaram H, Zetterstrom C. Two-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lens. Acta Opthalmologica. 2008;86(5):533–6. doi: 10.1111/j.1600-0420.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K, Hayashi H. Influence on posterior capsule opacification and visual function of intraocular lens optic material. Am J Ophthalmol. 2007;144(2):195–202. doi: 10.1016/j.ajo.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Leydolt C, Davidovic S, Sacu S, Menapace R, Neumayer T, Prinz A, et al. Long-term effect of 1-piece and 3-piece hydrophobic acrylic intraocular lens on posterior capsule opacification: a randomized trial. Ophthalmology. 2007;114(9):1663–9. doi: 10.1016/j.ophtha.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Zemaitiene R, Jasinskas V, Auffarth GU. Influence of three-piece and single-piece designs of two sharp-edge optic hydrophobic acrylic intraocular lenses on the prevention of posterior capsule opacification: a prospective, randomised, long-term clinical trial. Br J Ophthalmol. 2007;91(5):644–8. doi: 10.1136/bjo.2006.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennis H, Huygens M, Callebaut F. Comparing the contrast sensitivity of a modified prolate anterior surface IOL and of two spherical IOLs. Bull Soc Belge Ophtalmol. 2004;294:49–58. [PubMed] [Google Scholar]
- 37.Pierre A, Wittich W, Faubert J, Overbury O. Luminance contrast with clear and yellow-tinted intraocular lenses. J Cataract Refract Surg. 2007;33(7):1248–52. doi: 10.1016/j.jcrs.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharjee H, Bhattacharjee K, Medhi J. Visual performance: Comparison of foldable intraocular lenses. J Cataract Refract Surg. 2006;32(3):451–5. doi: 10.1016/j.jcrs.2005.12.136. [DOI] [PubMed] [Google Scholar]
- 39.Marshall J, Cionni RJ, Davison J, Ernest P, Lehmann R, Maxwell WA, et al. Clinical results of the blue-light filtering AcrySof Natural foldable acrylic intraocular lens. J Cataract Refract Surg. 2005;31(12):2319–23. doi: 10.1016/j.jcrs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 40.Barisic A, Dekaris I, Gabric N, Bosnar D, Lazic R, Martinovic ZK, et al. Blue light filtering intraocular lenses in phacoemulsification cataract surgery. Coll Antropol. 2007;31(Suppl 1):57–60. [PubMed] [Google Scholar]
- 41.Espindle D, Crawford B, Maxwell A, Rajagopalan K, Barnes R, Harris B, et al. Quality-of-life improvements in cataract patients with bilateral blue light-filtering intraocular lenses: clinical trial. J Cataract Refract Surg. 2005;31(10):1952–9. doi: 10.1016/j.jcrs.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 42.Baltussen R, Sylla M, Mariotti SP. Cost-effectiveness analysis of cataract surgery: a global and regional analysis. Bull World Health Organ. 2004;82(5):338–45. [PMC free article] [PubMed] [Google Scholar]
- 43.Lansingh VC, Carter MJ, Martens M. Global cost-effectiveness of cataract surgery. Ophthalmology. 2007;114(9):1670–8. doi: 10.1016/j.ophtha.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Maxwell WA, Waycaster CR, D'Souza AO, Meissner BL, Hileman K. A United States cost-benefit comparison of an apodized, diffractive, presbyopia-correcting, multifocal intraocular lens and a conventional monofocal lens. J Cataract Refract Surg. 2008;34(11):1855–61. doi: 10.1016/j.jcrs.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Orme ME, Paine AC, Teale CW, Kennedy LM. Cost-effectiveness of the AMOArray multifocal intraocular lens in cataract surgery. J Refract Surg. 2002;18(2002):162–8. doi: 10.3928/1081-597X-20020301-11. [DOI] [PubMed] [Google Scholar]
- 46.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127(4):555–62. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 47.Smith AF, Lafuma A, Berdeaux G, Berto P, Brueggenjuergen B, Magaz S, et al. Cost-effectiveness analysis of PMMA, silicone, or acrylic intra-ocular lenses in cataract surgery in four European countries. Ophthalmic Epidemiol. 2005;12(5):343–51. doi: 10.1080/09286580500180598. [DOI] [PubMed] [Google Scholar]
- 48.Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. Ottawa: CADTH. 2006. [[cited: 2009 Aug 8]]. [Internet] 46 p. Available from: hwww.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf .
- 49.British Columbia Ministry of Health Services. Medical Services Commission - payment schedule. [[updated 2009; cited 2009 Jun 18]]. [Internet] Available from: www.health.gov.bc.ca/msp/infoprac/physbilling/payschedule/
- 50.Ontario Ministry of Health and Long-Term Care. Ontario health insurance schedule of benefits and fees. [[updated 2008; cited 2009 Jun 18]]; [Internet] Available from: www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html . [Google Scholar]
- 51.Bank of Canada. [[updated 2009; cited 2009 Jun 25]];Ten-year currency converter. [Internet] Available from: www.bankofcanada.ca/en/rates/exchform.html . [Google Scholar]
- 52.Statistics Canada. [[updated 2009; cited 2009 Jun 25]];Vital statistics - death database. [Internet] Available from: www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDDS=3233&lang=en&db=imdb&adm=8&dis=2 . [Google Scholar]
- 53.Sacu S, Findl O, Menapace R, Buehl W, Wirtitsch M. Comparison of posterior capsule opacification between the 1-piece and 3-piece Acrysof intraocular lenses: two-year results of a randomized trial. Ophthalmology. 2004;111(10):1840–6. doi: 10.1016/j.ophtha.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Zemaitiene R, Jasinskas V, Barzdziukas V, Auffarth G.U. Prevention of posterior capsule opacification using different intraocular lenses (results of one-year clinical study) Medicina (Kaunas) 2004;40(8):721–30. [PubMed] [Google Scholar]
- 55.Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc. 1999;97:473–511. doi: 10.1016/s0002-9394(00)00513-4. [DOI] [PubMed] [Google Scholar]
- 56.Busbee BG, Brown MM, Brown GC, Sharma S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology. 2002;109(3):606–12. doi: 10.1016/s0161-6420(01)00971-x. [DOI] [PubMed] [Google Scholar]
- 57.Mittmann N. Utility scores for chronic conditions in a community-dwelling population. Pharmaco Economics. 1999;15(4):369–76. doi: 10.2165/00019053-199915040-00004. [DOI] [PubMed] [Google Scholar]
- 58.Kugelberg M, Wejde G, Jayaram H, Zetterstrom C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens: one-year follow-up. J Cataract Refract Surg. 2006;32(10):1627–31. doi: 10.1016/j.jcrs.2006.05.011. [DOI] [PubMed] [Google Scholar]


