Executive Summary
In early August 2007, the Medical Advisory Secretariat began work on the Aging in the Community project, an evidence-based review of the literature surrounding healthy aging in the community. The Health System Strategy Division at the Ministry of Health and Long-Term Care subsequently asked the secretariat to provide an evidentiary platform for the ministry’s newly released Aging at Home Strategy.
After a broad literature review and consultation with experts, the secretariat identified 4 key areas that strongly predict an elderly person’s transition from independent community living to a long-term care home. Evidence-based analyses have been prepared for each of these 4 areas: falls and fall-related injuries, urinary incontinence, dementia, and social isolation. For the first area, falls and fall-related injuries, an economic model is described in a separate report.
Please visit the Medical Advisory Secretariat Web site, http://www.health.gov.on.ca/english/providers/program/mas/mas_about.html, to review these titles within the Aging in the Community series.
Aging in the Community: Summary of Evidence-Based Analyses
Prevention of Falls and Fall-Related Injuries in Community-Dwelling Seniors: An Evidence-Based Analysis
Behavioural Interventions for Urinary Incontinence in Community-Dwelling Seniors: An Evidence-Based Analysis
Caregiver- and Patient-Directed Interventions for Dementia: An Evidence-Based Analysis
Social Isolation in Community-Dwelling Seniors: An Evidence-Based Analysis
The Falls/Fractures Economic Model in Ontario Residents Aged 65 Years and Over (FEMOR)
This report features the evidence-based analysis on caregiver- and patient-directed interventions for dementia and is broken down into 4 sections:
Introduction
Caregiver-Directed Interventions for Dementia
Patient-Directed Interventions for Dementia
Economic Analysis of Caregiver- and Patient-Directed Interventions for Dementia
Caregiver-Directed Interventions for Dementia
Objective
To identify interventions that may be effective in supporting the well-being of unpaid caregivers of seniors with dementia living in the community.
Clinical Need: Target Population and Condition
Dementia is a progressive and largely irreversible syndrome that is characterized by a loss of cognitive function severe enough to impact social or occupational functioning. The components of cognitive function affected include memory and learning, attention, concentration and orientation, problem-solving, calculation, language, and geographic orientation. Dementia was identified as one of the key predictors in a senior’s transition from independent community living to admission to a long-term care (LTC) home, in that approximately 90% of individuals diagnosed with dementia will be institutionalized before death. In addition, cognitive decline linked to dementia is one of the most commonly cited reasons for institutionalization.
Prevalence estimates of dementia in the Ontario population have largely been extrapolated from the Canadian Study of Health and Aging conducted in 1991. Based on these estimates, it is projected that there will be approximately 165,000 dementia cases in Ontario in the year 2008, and by 2010 the number of cases will increase by nearly 17% over 2005 levels. By 2020 the number of cases is expected to increase by nearly 55%, due to a rise in the number of people in the age categories with the highest prevalence (85+). With the increase in the aging population, dementia will continue to have a significant economic impact on the Canadian health care system. In 1991, the total costs associated with dementia in Canada were $3.9 billion (Cdn) with $2.18 billion coming from LTC.
Caregivers play a crucial role in the management of individuals with dementia because of the high level of dependency and morbidity associated with the condition. It has been documented that a greater demand is faced by dementia caregivers compared with caregivers of persons with other chronic diseases. The increased burden of caregiving contributes to a host of chronic health problems seen among many informal caregivers of persons with dementia. Much of this burden results from managing the behavioural and psychological symptoms of dementia (BPSD), which have been established as a predictor of institutionalization for elderly patients with dementia.
It is recognized that for some patients with dementia, an LTC facility can provide the most appropriate care; however, many patients move into LTC unnecessarily. For individuals with dementia to remain in the community longer, caregivers require many types of formal and informal support services to alleviate the stress of caregiving. These include both respite care and psychosocial interventions. Psychosocial interventions encompass a broad range of interventions such as psychoeducational interventions, counseling, supportive therapy, and behavioural interventions.
Assuming that 50% of persons with dementia live in the community, a conservative estimate of the number of informal caregivers in Ontario is 82,500. Accounting for the fact that 29% of people with dementia live alone, this leaves a remaining estimate of 58,575 Ontarians providing care for a person with dementia with whom they reside.
Description of Interventions
The 2 main categories of caregiver-directed interventions examined in this review are respite care and psychosocial interventions. Respite care is defined as a break or relief for the caregiver. In most cases, respite is provided in the home, through day programs, or at institutions (usually 30 days or less). Depending on a caregiver’s needs, respite services will vary in delivery and duration. Respite care is carried out by a variety of individuals, including paid staff, volunteers, family, or friends.
Psychosocial interventions encompass a broad range of interventions and have been classified in various ways in the literature. This review will examine educational, behavioural, dementia-specific, supportive, and coping interventions. The analysis focuses on behavioural interventions, that is, those designed to help the caregiver manage BPSD. As described earlier, BPSD are one of the most challenging aspects of caring for a senior with dementia, causing an increase in caregiver burden. The analysis also examines multicomponent interventions, which include at least 2 of the above-mentioned interventions.
Methods of Evidence-Based Analysis
A comprehensive search strategy was used to identify systematic reviews and randomized controlled trials (RCTs) that examined the effectiveness of interventions for caregivers of dementia patients.
Questions
Section 2.1
Are respite care services effective in supporting the well-being of unpaid caregivers of seniors with dementia in the community?
Do respite care services impact on rates of institutionalization of these seniors?
Section 2.2
Which psychosocial interventions are effective in supporting the well-being of unpaid caregivers of seniors with dementia in the community?
Which interventions reduce the risk for institutionalization of seniors with dementia?
Outcomes of Interest
any quantitative measure of caregiver psychological health, including caregiver burden, depression, quality of life, well-being, strain, mastery (taking control of one’s situation), reactivity to behaviour problems, etc.;
rate of institutionalization; and
cost-effectiveness.
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology and GRADE Working Group. As per GRADE the following definitions apply:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very low | Any estimate of effect is very uncertain. |
Summary of Findings
Conclusions in Table 1 are drawn from Sections 2.1 and 2.2 of the report.
Executive Summary Table 1: Summary of Conclusions on Caregiver-Directed Interventions.
| Section | Intervention | Conclusion |
|---|---|---|
| 2.1 | Respite care for dementia caregivers | Assessing the efficacy of respite care services using standard evidence-based approaches is difficult.
|
| 2.2a | Behaviourai interventions (individual ≥ 6 sessions) |
|
| 2.2b | Multicomponent interventions |
|
RCT indicates randomized controlled trial.
Patient-Directed Interventions for Dementia
Objective
The section on patient-directed interventions for dementia is broken down into 4 subsections with the following questions:
3.1 Physical Exercise for Seniors with Dementia – Secondary Prevention
What is the effectiveness of physical exercise for the improvement or maintenance of basic activities of daily living (ADLs), such as eating, bathing, toileting, and functional ability, in seniors with mild to moderate dementia?
3.2 Nonpharmacologic and Nonexercise Interventions to Improve Cognitive Functioning in Seniors With Dementia – Secondary Prevention
What is the effectiveness of nonpharmacologic interventions to improve cognitive functioning in seniors with mild to moderate dementia?
3.3 Physical Exercise for Delaying the Onset of Dementia – Primary Prevention
Can exercise decrease the risk of subsequent cognitive decline/dementia?
3.4 Cognitive Interventions for Delaying the Onset of Dementia – Primary Prevention
Does cognitive training decrease the risk of cognitive impairment, deterioration in the performance of basic ADLs or instrumental activities of daily living (IADLs),1 or incidence of dementia in seniors with good cognitive and physical functioning?
Clinical Need: Target Population and Condition
Secondary Prevention2
Exercise
Physical deterioration is linked to dementia. This is thought to be due to reduced muscle mass leading to decreased activity levels and muscle atrophy, increasing the potential for unsafe mobility while performing basic ADLs such as eating, bathing, toileting, and functional ability.
Improved physical conditioning for seniors with dementia may extend their independent mobility and maintain performance of ADL.
Nonpharmacologic and Nonexercise Interventions
Cognitive impairments, including memory problems, are a defining feature of dementia. These impairments can lead to anxiety, depression, and withdrawal from activities. The impact of these cognitive problems on daily activities increases pressure on caregivers.
Cognitive interventions aim to improve these impairments in people with mild to moderate dementia.
Primary Prevention3
Exercise
Various vascular risk factors have been found to contribute to the development of dementia (e.g., hypertension, hypercholesterolemia, diabetes, overweight).
Physical exercise is important in promoting overall and vascular health. However, it is unclear whether physical exercise can decrease the risk of cognitive decline/dementia.
Nonpharmacologic and Nonexercise Interventions
Having more years of education (i.e., a higher cognitive reserve) is associated with a lower prevalence of dementia in crossectional population-based studies and a lower incidence of dementia in cohorts followed longitudinally. However, it is unclear whether cognitive training can increase cognitive reserve or decrease the risk of cognitive impairment, prevent or delay deterioration in the performance of ADLs or IADLs or reduce the incidence of dementia.
Description of Interventions
Physical exercise and nonpharmacologic/nonexercise interventions (e.g., cognitive training) for the primary and secondary prevention of dementia are assessed in this review.
Evidence-Based Analysis Methods
A comprehensive search strategy was used to identify systematic reviews and RCTs that examined the effectiveness, safety and cost effectiveness of exercise and cognitive interventions for the primary and secondary prevention of dementia.
Questions
Section 3.1: What is the effectiveness of physical exercise for the improvement or maintenance of ADLs in seniors with mild to moderate dementia?
Section 3.2: What is the effectiveness of nonpharmacologic/nonexercise interventions to improve cognitive functioning in seniors with mild to moderate dementia?
Section 3.3: Can exercise decrease the risk of subsequent cognitive decline/dementia?
Section 3.4: Does cognitive training decrease the risk of cognitive impairment, prevent or delay deterioration in the performance of ADLs or IADLs, or reduce the incidence of dementia in seniors with good cognitive and physical functioning?
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology. As per GRADE the following definitions apply:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very low | Any estimate of effect is very uncertain. |
Summary of Findings
Table 2 summarizes the conclusions from Sections 3.1 through 3.4.
Executive Summary Table 2: Summary of Conclusions on Patient-Directed Interventions*.
| Section | Intervention | 1° or 2° Prevention | Conclusion |
|---|---|---|---|
| 3.1 | Physical exercise for seniors with dementia | 2° Prevention |
Physical exercise is effective for improving physical functioning in patients with dementia. |
| 3.2 | Nonpharmacologic and nonexercise interventions to improve cognitive functioning in seniors with dementia | 2° Prevention |
|
| 3.3 | Physical exercise for delaying onset of dementia | 1° Prevention |
Long-term outcomes
|
Short-term Outcomes
|
|||
| 3.4 | Nonpharmacologic and nonexercise interventions for delaying onset of dementia | 1° Prevention |
For seniors with good cognitive and physical functioning:
|
1° indicates primary; 2°, secondary; CST, cognitive stimulation therapy; IADL, instrumental activities of daily living; RCT, randomized controlled trial.
Benefit/Risk Analysis
As per the GRADE Working Group, the overall recommendations consider 4 main factors:
the trade-offs, taking into account the estimated size of the effect for the main outcome, the confidence limits around those estimates, and the relative value placed on the outcome;
the quality of the evidence;
translation of the evidence into practice in a specific setting, taking into consideration important factors that could be expected to modify the size of the expected effects such as proximity to a hospital or availability of necessary expertise; and
uncertainty about the baseline risk for the population of interest.
The GRADE Working Group also recommends that incremental costs of health care alternatives should be considered explicitly alongside the expected health benefits and harms. Recommendations rely on judgments about the value of the incremental health benefits in relation to the incremental costs. The last column in Table 3 reflects the overall trade-off between benefits and harms (adverse events) and incorporates any risk/uncertainty (cost-effectiveness).
Executive Summary Table 3: Overall Summary Statement of the Benefit and Risk for Patient-Directed Interventions*.
| Intervention | Quality | Benefits | Risks/Burden | Overall Strength of Recommendation |
|
|---|---|---|---|---|---|
| Section 3.1: Physical Exercise for Seniors with Dementia – Secondary Prevention | Exercise – mix | Moderate | Improvement in functional, cognitive and behavioural outcomes | Short-term follow-up and heterogeneity in studies Unclear if leads to delayed institutionalization |
Moderate |
| Section 3.2. Nonpharmacologic & Nonexercise Interventions to Improve Cognitive Functioning in Seniors with Dementia – Secondary Prevention | Cognitive training | Very low | None | Intervention does not offer significant benefit (possible type 2 error) Unclear if leads to delayed institutionalization |
Very low |
| Cognitive stimulation therapy (CST) | Moderate/Low | Increased cognition and quality of life | Unclear how CST compares with past terminologies and methodologies. | Low | |
| Short-term results. | |||||
| Role and extent of maintenance CST. | |||||
| Unclear how CST may impact functional dependence. | |||||
| Unclear if leads to delayed institutionalization. | |||||
| Section 3.3. Physical Exercise for Delaying the Onset of Dementia – Primary Prevention | Exercise – walking only | High/Moderate | Short-term decreased incidence of dementia | Unknown if leads to delayed institutionalization. | High/Moderate |
| Exercise – mix | High/Moderate | Short-term reduced risk of subsequent cognitive decline | Unknown if leads to delayed diagnosis of dementia or institutionalization. | High/Moderate | |
| Exercise – mix | Moderate | Long-term decreased incidence of dementia | Unknown if leads to delayed institutionalization. | Moderate | |
| Section 3.4. Nonpharmacologic & Nonexercise Interventions for Delaying the Onset of Dementia – Primary Prevention | Cognitive interventions | Low | Cognitive improvements sustained after 5 years | Results addressing functional outcomes unclear. | Very low |
| Need more than 5-year follow-up. | |||||
| (however, none of these improvements had effects beyond the specific cognitive domains of the intervention) | No evidence to determine if cognitive training leads to: 1) delayed diagnosis of dementia 2) delayed institutionalization |
Economic Analysis
Budget Impact Analysis of Effective Interventions for Dementia
Caregiver-directed behavioural techniques and patient-directed exercise programs were found to be effective when assessing mild to moderate dementia outcomes in seniors living in the community. Therefore, an annual budget impact was calculated based on eligible seniors in the community with mild and moderate dementia and their respective caregivers who were willing to participate in interventional home sessions. Table 4 describes the annual budget impact for these interventions.
Executive Summary Table 4: Annual Budget Impact (2008 Canadian Dollars).
| Parameter | Unit Cost ($ Cdn) |
Unit | Annual Cost ($ Cdn) |
Population* | No. of Patients |
Annual Impact ($ Cdn) |
|---|---|---|---|---|---|---|
| Caregiver-Directed Behavioural Techniques† | ||||||
| Occupational Therapist | 120.22 | 1 hour session -12 total | 1,442.64 | Caregivers of seniors with mild to moderate dementia who are willing to participate | 56,629 | 81,695,125 |
| Nurse | 82.12 | 1 hour session - 12 total | 985.44 | Caregivers of seniors with mild to moderate dementia who are willing to participate | 56,629 | 55,804,389 |
| Patient-Directed Exercise Program‡ | ||||||
| Occupational Therapist | 120.22 | 1 hour session -32 total | 3,847.04 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 148,866,672 |
| Physiotherapist | 108.49 | 1 hour session -32 total | 3,471.68 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 134,341,585 |
| Personal Support Worker | 30.48 | 1 hour session -32 total | 975.36 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 37,742,939 |
| Recreation Therapist | 25.85 | 1 hour session -32 total | 827.20 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 32,009,678 |
| Caregiver- and Patient-Directed Behavioural Techniques§ | ||||||
| Occupational Therapist | 120.22 | 1 hour session -10 total | 1,202.20 | Caregivers and seniors with mild to moderate dementia willing to participate | 56,629 | 68,079,271 |
| Nurse | 82.12 | 1 hour session -10 total | 821.20 | Caregivers and seniors with mild to moderate dementia willing to participate | 56,629 | 46,503,658 |
Assumed 7% prevalence of dementia aged 65+ in Ontario.
Assumed 8 weekly sessions plus 4 monthly phone calls.
Assumed 12 weekly sessions plus biweekly sessions thereafter (total of 20).
Assumed 2 sessions per week for first 5 weeks. Assumed 90% of seniors in the community with dementia have mild to moderate disease. Assumed 4.5% of seniors 65+ are in long-term care, and the remainder are in the community. Assumed a rate of participation of 60% for both patients and caregivers and of 41% for patient-directed exercise. Assumed 100% compliance since intervention administered at the home. Cost for trained staff from Ministry of Health and Long-Term Care data source. Assumed cost of personal support worker to be equivalent to in-home support. Cost for recreation therapist from Alberta government Website.
Note: This budget impact analysis was calculated for the first year after introducing the interventions from the Ministry of Health and Long-Term Care perspective using prevalence data only. Prevalence estimates are for seniors in the community with mild to moderate dementia and their respective caregivers who are willing to participate in an interventional session administered at the home setting. Incidence and mortality rates were not factored in. Current expenditures in the province are unknown and therefore were not included in the analysis. Numbers may change based on population trends, rate of intervention uptake, trends in current programs in place in the province, and assumptions on costs. The number of patients was based on patients likely to access these interventions in Ontario based on assumptions stated below from the literature. An expert panel confirmed resource consumption.
In early August 2007, the Medical Advisory Secretariat began work on the Aging in the Community project, an evidence-based review of the literature surrounding healthy aging in the community. The Health System Strategy Division at the Ministry of Health and Long-Term Care subsequently asked the secretariat to provide an evidentiary platform for the ministry’s newly released Aging at Home Strategy.
After a broad literature review and consultation with experts, the secretariat identified 4 key areas that strongly predict an elderly person’s transition from independent community living to a long-term care home. Evidence-based analyses have been prepared for each of these 4 areas: falls and fall-related injuries, urinary incontinence, dementia, and social isolation. For the first area, falls and fall-related injuries, an economic model is described in a separate report.
Please visit the Medical Advisory Secretariat Web site, www.health.gov.on.ca/english/providers/program/mas/mas_about.html, to review these titles within the Aging in the Community series.
Aging in the Community: Summary of Evidence-Based Analyses
Prevention of Falls and Fall-Related Injuries in Community-Dwelling Seniors: An Evidence-Based Analysis
Behavioural Interventions for Urinary Incontinence in Community-Dwelling Seniors: An Evidence-Based Analysis
Caregiver- and Patient-Directed Interventions for Dementia: An Evidence-Based Analysis
Social Isolation in Community-Dwelling Seniors: An Evidence-Based Analysis
The Falls/Fractures Economic Model in Ontario Residents Aged 65 Years and Over (FEMOR)
This report features the evidence-based analysis on caregiver- and patient-directed interventions for dementia and is broken down into 4 sections:
Introduction
Caregiver-Directed Interventions for Dementia
Patient-Directed Interventions for Dementia
Economic Analysis of Caregiver- and Patient-Directed Interventions for Dementia
1. Introduction
Objective
To assess the effectiveness of patient- and caregiver-directed interventions in supporting seniors with dementia and their caregivers in the community.
Clinical Need: Target Population and Condition
Dementia Identified as a Predictor of Long-Term Care Home Admission
Dementia is a progressive and largely irreversible syndrome that is defined as the “loss of intellectual abilities (medically called cognitive function) of sufficient severity to interfere with social or occupational functioning”. (1) The components of cognitive function affected include memory and learning, attention, concentration and orientation, problem-solving, calculation, language, and geographic orientation. Dementia was identified as one of the key predictors in a senior’s transition from independent community living to admission to a long-term care (LTC) home since approximately 90% of individuals diagnosed with dementia will be institutionalized before death. (2) In addition, the cognitive decline linked to dementia is one of the most commonly cited reasons for institutionalization. (3) A study published in 2004 found a strong predictive effect, with a hazard ratio (HR) of 2.3 (95% confidence interval [CI]: 1.8-2.8) for severe dementia versus no dementia. (4)
Several patient and caregiver factors have been established as predictors of institutionalization for elderly patients with dementia. Factors identified from the Canadian Study of Health and Aging included type of dementia (Alzheimer’s disease), problematic behaviours, and severity of disabilities [activities of daily living (ADL) dependencies]. Caregiver factors included level of caregiver burden, old age, poor physical health, no first-degree kinship of the caregiver with the patients, use of services, and desire to institutionalize. (5) The study found that caregiver burden often resulted from the patient’s behavioural problems and that caregiver burden was associated with the caregiver’s depressive mood. (5)
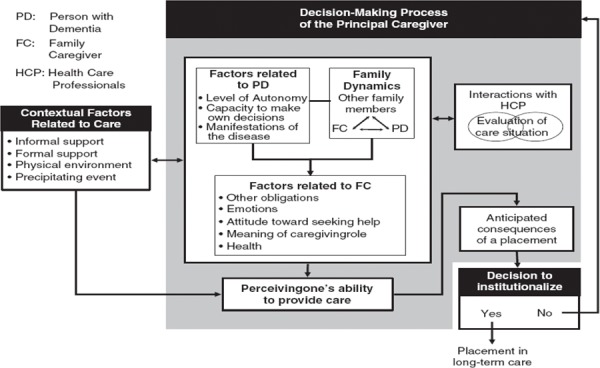
The decision to institutionalize, however, is impacted by many other factors. Contextual and psychosocial factors such as family dynamics, interactions with health care professionals, and the caregiver’s perception of their ability to provide care, play a large role in explaining a caregiver’s decision to institutionalize (Figure 1).
Figure 1: Emerging Theoretical Model Explaining Caregivers’ Decision to Institutionalize an Older Relative Living with Dementia (from Caron et al, 2006) (3).
Epidemiology of Dementia
Dementia has a significant global impact. It is estimated that there are 24.3 million people with dementia worldwide with 4.6 million new cases presenting each year. (6) In Canada, the most reliable prevalence estimates come from the Canadian Study of Health and Aging, a population-based survey conducted in 1991. (Table 1) displays the dementia prevalence by 5-year age groups in the Canadian population in 1994. Based on these estimates, it is projected that there will be approximately 165, 000 dementia cases in Ontario in the year 2008, and by 2010 the number of cases will increase by nearly 17% over 2005 levels. (7) By 2020 the number of dementia cases is expected to increase by nearly 55%, due to a rise in the number of people in the age categories with the highest prevalence (85+). The increase in dementia cases will cause a greater demand on health care resources including LTC, medical, social, and recreational services.
Table 1: Canada Dementia Prevalence by 5-Year Age Groups (1994).
| Age Group | Prevalence, % |
|---|---|
| 65–69 | 1.4 |
| 70–74 | 2.8 |
| 75–79 | 5.6 |
| 80–84 | 11.1 |
| 85+ | 24 |
Types of Dementia
Dementia can arise from a number of causes however the 2 most common are Alzheimer’s disease and vascular dementia, accounting for approximately 64% and 20%, respectively, of all dementia cases in Canada. (8;9) Other diseases and conditions identified to cause symptoms of dementia include Lewy body dementia, Huntington’s disease, Creutzfeld-Jakob Disease, Human Immunodeficiency Virus (HIV) dementia, alcohol-related dementia, Parkinson’s disease, stroke, and nutritional deficiencies.
The most common symptoms of dementia include confusion, agitation, forgetfulness, and sleep disturbance.
Less than 10% of cases are reversible. (10) As dementia progresses individuals are often disoriented with respect to time, place, and people they encounter. Dementia is often confused with delirium and other mental illnesses. Managing the cognitive and noncognitive symptoms of dementia is demanding and challenging. Individuals with moderate and advanced dementia typically require a full-time caregiver to help them with daily tasks such as eating, bathing, and dressing. Caregivers must also ensure that individuals with dementia are not harmful to themselves or others.
Dementia Risk Factors
Several risk factors have been identified that put one at a higher risk for developing dementia. These include age, genetics/family history, smoking, heavy alcohol use, abnormally high levels of plasma homocysteine, Down syndrome, diabetes, and mild cognitive impairment. Both atherosclerosis and hypercholesterolemia are significant risk factors for vascular dementia. (11)
Prevention of Dementia
Since the exact cause of dementia is not known, it is difficult to engage in prevention. Furthermore, few definitive studies exist and the majority of these focus on prevention of Alzheimer’s disease, making generalizability difficult to other dementias. However, factors which may possibly contribute to the prevention of Alzheimer’s include: lowering homocysteine, treatment of high blood pressure, lowering cholesterol, exercise, education, controlling inflammation, and the long-term use of non steroidal anti-inflammatory drugs (NSAIDS). Prevention of vascular dementia requires modification of lifestyle factors closely related to stroke including: maintaining a healthy weight, treatment of high blood pressure, smoking cessation, and lowering cholesterol. (11) Currently, researchers are investigating other preventative measures such as leisure activities (reading, playing board games, playing musical instruments, and dancing). (12) A few studies have also suggested that light to moderate alcohol use may reduce the risk of dementia in older people. (13;14)
Treatment and Management of Dementia
Pharmacological Treatment
While there are no drugs available to stop the progression of dementia, cholesterolase inhibitors are widely used to improve symptoms and slow its progression. Drug treatment may also enhance the quality of life (QOL) of dementia patients and ease the level of caregiver burden, thus potentially delaying admission to LTC. Other drugs such as antidepressants or antipsychotics may be prescribed to aid with the Behavioural and Psychological Symptoms of Dementia (BPSD) such as depression, anxiety, agitation, aggression, sleep disorders, and psychotic symptoms. (11)
Psychosocial Treatment
Due to the complex and challenging nature of dementia, treatment and management of patients goes well beyond pharmacological therapy. Both the cognitive features and noncognitive symptoms of the syndrome cause immense stress to both patients and caregivers. Psychosocial interventions designed to alleviate the burden and stress of caring are essential for caregivers in the management of dementia. These include respite care services, psychoeducational interventions, and counseling, as well as a host of other supportive services. Since BPSD is highly correlated with caregiver burden and in turn a major influence in a caregiver’s decision to institutionalize, interventions to help manage BPSD are essential to the caregiver. Typically, environmental and behavioural interventions are used to manage BPSD, and drugs are prescribed only if these are inadequate.
Use of Community Services
People with dementia who have severe functional disability receive far more services than those with mild to moderate disability. And, although the needs of patients and caregivers of dementia increase with increasing levels of patient disability, services remain underutilized in this population. Only 3.4% of dementia caregivers use respite services, a service identified by caregivers as a key formal support to alleviate the stress of caring. It has been documented that spousal caregivers use fewer support services than caregivers who are adult children. Despite the decreased utilization in services, dementia has a significant economic burden on the Canadian health care system. A main driver for these costs is the cost associated with caring for a dementia patient in LTC. In 1991, the total net costs of dementia in Canada were $ 3.9 billion (Cdn) with 2.18 billion coming from LTC (Table 2).
Table 2: Total Net Costs of Dementia in Canada From the Canadian Study on Health Aging (15).
| Source of Costs | Total Annual Net Costs, $ million |
|
|---|---|---|
| Long-term care | 2,180 | |
| Community | 1,250 | |
| Paid services | 615 | |
| Unpaid services | 636 | |
| Drugs | 60.6 | |
| Hospitals* | 0 | |
| Diagnosis | 13.5 | |
| Research | 9.8 | |
| People < 65 years | 389 | |
Costs did not differ significantly between dementia and control subjects
Role of the Caregiver for Dementia Patients
Caregivers play a crucial role in the management of dementia patients due to the high levels of dependency and morbidity that are associated with dementia. Although caregivers can be formal (paid), much of the burden of caregiving is often placed on informal (unpaid) caregivers, typically family caregivers. A family caregiver is defined a person who considers themselves to be a primary caregiver and who is providing care because of a prior relationship with the client. (16;17) They may be members of a biological family or friends, partners, and neighbours.
Data from the Canadian Study of Health and Aging provides us with caregiving patterns for seniors with dementia across Canada. According to the report, approximately 50% of seniors with dementia live in the community (at home); 97% of these people have a caregiver, 2.4% have no caregiver, 29% live alone but typically have a daughter living close by, and 8% have only 1 caregiver for support. (18)
Over 70% of informal caregivers are women, most often wives (24%) or adult daughters (29%). Half of the informal caregivers are over the age of 60 with 36% being over the age of 70. Ninety-two percent of people with dementia living in the community have 2 or more relatives or friends beyond their primary caregiver who provide assistance. Finally, spousal caregivers are less likely to have back-up support than others and yet are more likely to be caring for a person with severe dementia. (18)
It has been documented that there is a greater demand faced by dementia caregivers when they are compared with caregivers of persons with other chronic diseases. The increased burden of caregiving attributes to chronic health problems seen among informal dementia caregivers. According to the Canadian Study of Health and Aging, 16% of people caring for someone with mild dementia in the community report symptoms of depression. The rate is more than double for those caring for someone with moderate dementia (40%). The prevalence of depression in dementia caregivers is nearly twice that of caregivers of persons with other chronic diseases. (18)
Based on prevalence estimates from the Canadian Study of Health and Aging, it is projected that there will be approximately 165,000 dementia cases in Ontario in the year 2008. (7) Assuming that 50% of persons with dementia live in the community, (18) a conservative estimate of the number of informal caregivers is 82,500. Recognizing that 29% of people with dementia live alone(18), results in an estimate of 58,575 Ontarians providing care for a person with dementia with whom they co-reside.
Support for Seniors With Dementia and Their Caregivers in the Community
While it is recognized that some seniors with dementia will receive the best and appropriate care for their situation in a LTC home, there are many seniors with dementia who transition to LTC unnecessarily. These patients often have caregivers who are overburdened by the demands of caregiving and lack the support services required to manage the patient. Keeping seniors with dementia in the community requires a network of formal and informal support services for both the caregiver and patient.
The 2 main categories of interventions for dementia caregivers are respite care and psychosocial interventions. Respite care is identified by caregivers as one of the key formal supports to alleviate the stress of caring. (19) Respite care is defined as a break or relief for the caregiver. In most cases, respite is provided in the home, through day programs or at institutions (usually 30 days or less). Depending on a caregivers needs, respite services will vary in delivery and duration. A number of individuals carry out respite care including paid staff, volunteers, family, or friends.
Psychosocial interventions encompass a broad range of interventions and have been classified in various ways in the literature. They may include educational, behavioural, dementia-specific, supportive, and coping interventions. Multicomponent interventions may also be used which include at least 2 of the above-mentioned interventions. Patient interventions may be focused on promoting independence and maintaining cognitive function. In addition to pharmacological treatment to slow the progression of dementia, nonpharmacological interventions including occupational therapy (OT), physical therapy, exercise, and cognitive therapy may be explored.
It is hoped that by optimizing support services, we can improve the QOL and psychological health of seniors with dementia and their caregivers living in the community.
2. Caregiver-Directed Interventions for Dementia
2.1. Respite Care for Caregivers of Seniors With Dementia
Clinical Need: Target Population and Condition
Caregivers play a crucial role in the management of seniors with dementia due to the high level of dependency and morbidity that is associated with this condition. It has been documented that there is a greater demand faced by dementia caregivers as compared with caregivers of persons with other chronic diseases. Furthermore, the increased burden of caregiving attributes to a host of chronic health problems seen among many informal dementia caregivers. Much of this burden results from managing BPSD, which has been established as a predictor of institutionalization for elderly patients with dementia. (5) As dementia progresses, individuals typically require a full-time caregiver to help them with daily tasks such as eating, bathing, and dressing. Caregivers must also ensure that individuals are not harmful to themselves or others.
Respite care is a service identified by carers as one of the key formal supports to alleviate the stress of caring. (19) Respite care is defined as a break or relief for the caregiver. (20) In most cases, respite is provided in the home, through day programs or at institutions (usually 30 days or less). Depending on caregivers needs, respite services will vary in delivery and duration. A number of individuals may carry out respite care including paid staff, volunteers, family, or friends.
Evidence-Based Analysis of Effectiveness
Questions
Are respite care services effective in supporting the well-being of unpaid caregivers of seniors with dementia in the community?
Do respite care services impact on rates of institutionalization of these seniors?
Methods
Inclusion Criteria
English-language articles (January 2000–November 2007),
journal articles that report primary data on the effectiveness or cost-effectiveness of respite care services for dementia caregivers of seniors living in the community,
study design and methods must be clearly described,
systematic reviews, meta-analyses, or RCTs, and
primary outcome includes at least 1 measure of caregiver psychological health.
Exclusion Criteria
studies that are duplicate publications (superseded by another publication by the same investigator group, with the same objective and data),
non-English articles,
studies with less than 10 patients, and
formal (paid) carers.
Literature Search
A search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, PsycINFO, and the International Agency for Health Technology Assessment/Centre for Reviews and Dissemination (INAHTA/CRD) for studies published between January 2000 and November 2007 (Appendix 1). Abstracts were reviewed by a single author, and studies meeting the inclusion criteria were obtained. Reference lists were also checked for relevant studies.
Outcomes of Interest
caregiver: burden, depression, QOL, mood, and
care recipient: rate of institutionalization, functional outcomes, QOL.
Results of Literature Search
The search identified 530 articles published from January 1, 1998 to November 1, 2007. Of the 530 citations identified, 2 met the inclusion criteria. These were both systematic reviews evaluating the effectiveness of respite care for dementia caregivers and are outlined below:
Summary of Existing Evidence
NCCSDO - Arskey et al. 2004
The objective of this review was to evaluate the effectiveness and cost-effectiveness of respite care or short term breaks for caregivers of people with dementia. The review encompassed a broad spectrum of literature (published and grey literature) and included studies with both quantitative and qualitative designs. Out of the 45 studies examined, only 5 were RCTs (Table 3) and the majority of studies examined day care programs. Due to the heterogeneity in studies and quality of the trials, a narrative review was conducted to synthesize the evidence. In addition, the authors consulted with various stakeholders, including organizations offering respite services and dementia caregivers, to better understand the components of an effective respite care service.
Table 3: Numbers of Studies According to Research Design and Type of Respite Care and Short-Term Break for Carers for People with Dementia* (n=51)†.
| RCTs | Quasi- Experimental |
Before and After |
Survey/ Postrespite Intervention |
Qualitative interviews |
Mixed Methods |
Other | |
|---|---|---|---|---|---|---|---|
| Day care | 2 | 3 | 2 | 7 | 7 | ||
| Institutional respite | 5 | 1 | 6 | ||||
| In-home respite | 1 | 3 | 2 | 2 | |||
| Multi-dimensional carer-support packages | 3 | 1 | |||||
| Respite programmes | 1 | 2 | |||||
| Host-family respite | 1 | ||||||
| Video respite | 1 | 1 | |||||
| Total | 5 | 6 | 9 | 11 | 3 | 16 | 1 |
Adapted from Arskey H. et al. 2004 (21)
RCT indicates randomized controlled trials.
Note: Studies add up to 51 because 5 studies in the review evaluated 2 or more forms of respite.
Summary of Effectiveness and Cost-Effectiveness
Effectiveness
The primary outcomes of interest were the health and well-being of the caregiver and care recipient, dementia-related symptoms (care recipient), impact on use of other services, and cost-effectiveness. The findings were reported according to type of respite service including day care, in-home respite, host-family respite, institutional/overnight respite, respite programmes, multidimensional caregiver-support packages, and video respite. The authors concluded that the evidence on the effectiveness and cost-effectiveness of respite care services for dementia caregivers is limited. However, the review reported considerable qualitative evidence from carers (and some care recipients) of the perceived benefits of the use of respite services.
Delay of Entry Into Long-Term Care
The only studies to show a postponement in the entry into LTC of seniors with dementia in the study groups compared with those in the control groups were the 3 studies on multisupport caregiver packages. The length of the delays varied, and ranged between an average of 22 days (23) and 7 weeks. (24;25) Since respite care was offered as part of a package, it is difficult to discern the individual effects of services. Moreover, although multidimensional support packages seemed to delay entry into LTC, they did not necessarily impact the caregivers’ psychosocial health in terms of anxiety or QOL.
One of the major challenges with assessing the effectiveness of respite care using standard evidence-based practices is the lack of high-quality trials conducted in this field. Therefore, any conclusions must be interpreted with caution. However, the authors did find RCT evidence to suggest that the complex needs of dementia carers may be better addressed by multidimensional packages that allow carers access to a wide range of community-based services.
Cost-Effectiveness
There were 5 economic reports included in the NCCSDO review. Four of the reports examined day care services compared to standard care. All of these studies reported potential benefits of respite care offered through day care services; however, there was a discrepancy among the studies with respect to the costs associated with these benefits. Two of the 4 studies suggested that the benefits associated with day care services come at a higher cost than standard care and 2 of the 4 studies reported that the benefits come at a lower cost. With the exception of 1 of the 4 reports, there were no statistically significant differences found in the costs and benefits across groups in any of these studies; thus, findings must be interpreted with caution.
The fifth economic report included in the review examined the cost-effectiveness of multi-dimensional carer-support packages compared to standard community nursing care. The authors reported that the multi-dimensional carer-support packages were associated with higher benefits for the caregiver at a higher cost; however, differences were not statistically significant.
Limitations
There are several limitations described in detail by the authors of this review. Firstly, although 45 studies were included in the review, few were of high methodological quality. In addition, only a few studies assessed the medium- to long-term effects of respite care. The lack of significant findings is also attributable to the heterogeneity in studies with respect to outcome measures, patient and caregiver populations, duration of studies, amount and type of respite, timescales, weaknesses in study design, and inadequate or lack of control groups.
Cochrane Review – Lee et al. 2004
The objective of the review was to assess the effects of respite care for people with dementia and their caregivers, in particular the effects of respite care on rates of institutionalization. The review examined 3 RCTs but included only 2 in the analysis (Table 4).
Table 4: Summary of Key Characteristics of Studies Examined in the Cochrane Review of Respite Care.
| Grant et al. 2003 (n=55) (26) |
Lawton et al. 1989 (23)* (n=632) |
Wishart et al. 2000 (27) (n=24) |
|
|---|---|---|---|
| Type of respite | In-home | In-home, day-care, institutional† Funding was provided as needed |
Visiting/walking programme |
| Duration | 2 weeks | 1 year | 6 weeks |
| Intensity | 60 hours (no more than 6 hrs/day) |
As requested | 2.5 hrs/week |
| Delivered by | Trained professionals | Varied | Trained volunteer |
| Controls | No respite | No respite‡ | Wait-list |
Not included in the Cochrane analysis.
Not mutually exclusive.
Had higher use of respite services than intervention group.
Conclusions
The authors concluded that there are no significant effects of respite on caregiver outcomes; however, this is due to the lack of high-quality research in this area and thus, current evidence does not allow one to make any reliable conclusions about the efficacy of respite care for people with dementia and their caregivers.
Limitations
A lack of significant findings can be attributed to the many limitations of the studies included in the review. As seen in (Table 4), there is much heterogeneity among the 3 studies with respect to type of respite, duration, intensity and delivery of intervention. The 2 studies used in the analysis of the review (Grant et al. 2003 (26) and Wishart et al. 2000 (27)), both had small sample sizes (55 and 24 respectively). Both studies also had extremely short durations (2 and 6 weeks), so it is questionable whether the effects of respite care could be observed and evaluated in such a short time. Furthermore, with the exception of Grant et al. (26), the studies had inadequate control groups. In the Lawton et al. study (23), the control group had a higher use of respite services than the intervention group, making evaluation of the effectiveness of respite impossible. Wait-list controls were used in the Wishart et al. study (27), which are often questioned for their appropriateness in caregiver intervention studies. It is possible that any improvement in caregiver outcomes observed in the intervention arm of the study were not significant because caregivers in the control group knew that they would be receiving respite care services and thus had higher values of caregiver health at baseline.
Updates to Published Health Technology Assessments
There were no updates to these published health technology assessments (HTAs).
Ontario Health Systems Impact Analysis
Considerations and Implications
An expert panel on aging in the community met on February 29, 2008, and May 16, 2008 and discussed, in part, respite care for seniors with dementia in Ontario. In particular, the expert panel commented on the gaps in current understanding and delivery of respite care and methodological difficulties with evaluating respite care services for the senior population. Comments from the panel are found below.
Methodological and Quality Issues With Studies
Respite care is difficult to define.
Randomized controlled trials are very challenging to conduct in this population.
Caregivers of seniors with dementia have complex and diverse needs.
Patients differ greatly with respect to type of dementia, severity of disease, and limits in ADLs and IADLs.
Caregivers differ greatly with respect to characteristics, age, health status, relationship to care recipient, amount of formal or informal support available, and use/access of other supportive services.
Outcomes measured may not be sensitive/appropriate measures to detect effectiveness of respite.
Interventions are heterogeneous (type of respite, duration, intensity).
Study duration is typically short; therefore, it is difficult to assess medium- to long-term effects.
There are many forms of respite that are effective but have not been studied (i.e., respite provided through religious groups). One must be careful with how the results of the respite care literature are reported.
Current Delivery
-
Community Care Access Centres (CCACs) provide respite care in 3 ways:
informal in-home, 1-on-1 care for a couple of hours per day,
referral to community-support programs, and
referral to short-term nursing home stays.
Hours of respite are coordinated by CCACs and delivered by personal support workers (PSWs).
Informal agencies and religious groups provide some respite services (congregate driving, meals on wheels, and friendly visiting).
What seems to be useful is someone taking the senior with dementia for a walk for 1 to 2 hours per day since this gives the caregiver free time. This is often organized by a PSW from a CCAC.
In general, a short-term stay in a nursing home has less positive effects than other forms of respite since there is disruption of routine for the patient/caregiver.
System Pressures
Problem: not enough hours of respite provided by PSWs from CCACs.
Other issues are: high turnaround of staff, lack of flexibility, lack of knowledge to manage behavioural challenges, inconsistency in delivery of services.
Individuals with dementia need a familiar face and an individualized approach.
Large issue in evaluating effectiveness of interventions in the dementia population.
Often, informal arrangements are made (i.e., with neighbours/friends, etc.) to alleviate the burden of the caregiver.
Future Research/Direction
There exist caregiver-support programs that define the number of hours in-home and flexibility benchmarks for caregiver-support interventions.
In nursing homes, spouses of people with dementia support one another and help with the caregiving requirements, which is a form of respite for these caregivers.
Not enough research is done into what happens to caregivers once the care receiver dies.
Overall Summary Statement of the Efficacy of Respite Services
There is poor-quality and inconclusive evidence from RCTs surrounding the effectiveness and cost-effectiveness of respite care services. Due to the methodological difficulties with studying respite services, especially within an RCT design, alternate forms of research may need to be explored such as interviews with focus groups and organizations providing respite services to determine effectiveness and identify the caregiver population who would most benefit from these services. Consultation with experts reveals the value and importance of respite care services to caregivers in alleviating the burden associated with caring for seniors with dementia and the need to optimize current services.
2.2A. Psychosocial Interventions for Caregivers of Seniors With Dementia
Clinical Need: Target Population and Condition
Caregivers of seniors with dementia are often overburdened by the demands of caregiving and lack the support services they require. Keeping patients in the community requires the extension of formal and informal support services. In addition to respite care services, psychosocial interventions are essential to caregivers in the management of patients with dementia. Psychosocial interventions encompass a broad range of interventions including psychoeducational interventions, counseling, supportive therapy, and behavioural management interventions, as well as a host of other supportive services. Many studies have examined the effects of psychosocial interventions on caregivers’ psychological health, especially as it relates to caregiver burden and depression, which are key predictors of institutionalization of seniors with dementia. (5)
Evidence-Based Analysis of Effectiveness
Questions
Which psychosocial interventions are effective in supporting the well-being of unpaid caregivers of seniors with dementia in the community?
Which interventions reduce the risk for institutionalization of seniors with dementia?
Comparisons of Interest (and for which evidence of these comparisons exist)
Psychosocial intervention versus no intervention (control group receiving routine care or minimal support).
Methods
Inclusion Criteria
English-language articles (1996 – February 2008),
journal articles that report primary data on the effectiveness of dementia caregiver interventions,*
study design and methods must be clearly described, and
systematic reviews, meta-analyses, RCTs.
Exclusion Criteria
studies that are duplicate publications (superseded by another publication by the same investigator group, with the same objective and data),
nonsystematic reviews, letters, and editorials,
studies with less than 10 patients, and
formal (paid carers).
Literature Search
A search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, PsycINFO, and INAHTA/CRD for studies published between January 1996 and February 2008 (Appendix 2). Abstracts were reviewed by a single author, and studies meeting the inclusion criteria outlined above were obtained. Reference lists were also checked for relevant studies.
Outcomes of Interest
Caregiver: Burden, depression, reactivity to behaviour problems, QOL, mood, mastery, anxiety, physical health
Care recipient: Rate of institutionalization, functional outcomes, frequency of problem behaviours, QOL
Results of Literature Search
The Cochrane and INAHTA/CRD databases yielded 7 systematic reviews/meta-analyses on caregiver interventions for dementia caregivers.
Summary of Existing Evidence
Table 5: Summary of Systematic Reviews and Meta-Analyses on Dementia Caregiver Interventions*.
| Author, Year, Type of Study (# of trials) | Interventions Examined |
Outcome(s)† | Conclusion |
|---|---|---|---|
| Peacock et al. 2003 (28) Systematic review (n=11) |
|
|
|
| Brodaty et al. 2003 (29) Meta-analysis (n=30) |
|
|
|
| Thompson et al. 2007 (30) Systematic review (n=44) |
|
|
|
| Acton et al. 2001 (31) Meta-analysis (n=24) |
|
|
|
| Pusey et al. 2001 (32) Systematic review (n=30) |
|
|
|
| Pinquart et al. 2006 (33) Meta-analysis (n=127) |
|
|
|
| Selwood et al. 2007 (34) Systematic review (n=62) |
|
|
|
ADL indicates activities of daily living; CG, caregiver; CR, care receiver; QOL, quality of life.
Caregiver outcomes unless otherwise specified.
Despite the heterogeneity in outcomes and interventions examined in the reviews on caregiver interventions, there were common findings that emerged.
Ineffective interventions included:
education about dementia by itself,
supportive therapy, and
group behavioural therapy.
Effective interventions included:
reaching caregivers problem solving/coping strategies,
involving patients in addition to caregivers,
individual behavioural management therapy (≥6 sessions), and
multicomponent interventions.
The Medical Advisory Secretariat review intended to update behavioural management interventions and multicomponent interventions. The reasons are 3-fold:
Given the time frame of the project, an analysis of these 2 caregiver interventions was reasonable.
Evidence from the literature demonstrates that caregiver burden largely attributed to managing BPSD is an established predictor of institutionalization for elderly patients with dementia.
According to the systematic reviews and meta-analyses on caregiver interventions, multicomponent interventions are the only interventions to reduce the risk of institutionalization.
Upon confirmation of the scope with expert consultants, the Medical Advisory Secretariat performed an update to the most recent review examining behavioural management techniques. According to Selwood et al. 2007 (34), 6 sessions is the therapeutic minimum required for these interventions to be effective; therefore, this requirement was included in the search strategy.
Updates to Published Health Technology Assessments
Four RCTs were found, all of which focused on behavioural management therapy directed at the caregiver or both the caregiver and the care receiver.
Summary of Updated Studies
The first study retrieved involved behavioural management therapy directed at both the caregiver and patient, and was carried out by an occupational therapist. Details of the study are shown in (Table 6).
Table 6: Summary of Randomized Controlled Trial by Graff et al. 2006, 2007 (35;36).
| Study/ Year |
Population | Description of Intervention |
Outcome/ Follow-Up |
Results | Limitations |
|---|---|---|---|---|---|
| Graff et al.(35) 2006 Graff et al. (36) 2007 |
N=135 Mild to moderate dementia |
Intervention: 10 1-hour sessions of occupational therapy (OT) over 5 weeks (including cognitive and behavioural interventions) Total time: 18 hrs per patient and CG together Control: no OT |
CG burden Patients’ daily functioning assessment (determined by assessment of motor and process skills [AMPS] and interview of deterioration in daily activities in dementia [IDDD]) |
CGs: At 6 wks CGs who received OT felt significantly more competent than those who did not Mean competence score (assessed by sense of competence questionnaire [SCQ]) Difference at 6 weeks 11.0 (9.2–12.8) statistically and clinically significant Number needed to treat: 2.5 (2.3–2.7) |
Generalizability of results, due to recruitment of patients from a memory clinic and day clinic of a university hospital. Short study duration (12 weeks). Unclear if controls were on wait-list. Intervention was directed at patients and CGs – unclear. |
| Baseline, 6 weeks, and 3 months | Outcomes remained at 12 weeks | In 18% of cases (n=21) the assessors knew the treatment allocation. | |||
Outcomes(36) CG:
|
Patient: At 6 weeks, patients in the OT group significantly improved in daily functioning and outcomes remained significant at 3 months | ||||
| Results(36) At 6 weeks, CGs in the OT group had significantly improved outcomes for overall quality of life, health status, depression, and mastery than those in the control group (P < .0001). Outcomes remained significant at 3 months. |
CG indicates caregiver; QOL, quality of life.
Table 7 shows the results of the study at 6- and 12-week time points. At 6 weeks, caregivers in the OT group felt significantly more competent than those who did not (treatment difference 11.0; 95%CI, 9.2–12.8). Outcomes remained significant at 12 weeks. In addition, at 6 weeks, patients in the OT group significantly improved in daily functioning, and outcomes remained significant at 12 weeks.
Table 7: Outcomes in Patients with Dementia and Caregivers in Intention-to-Treat Population at 6-and 12-week Time Points*.
| 6 Weeks | 12 Weeks | |||||
|---|---|---|---|---|---|---|
| AMPS Process | IDDD Performance | Competence (SCQ) | AMPS Process | IDDD Performance | Competence (SCQ) | |
| Covariate adjusted treatment difference (95% CI) | 1.5 (1.3–1.7) | −11.7 (−13.6 to −9.7) |
11.0 (9.2–12.8) |
1.6 (1.3–1.8) |
−13.6 (−15.8 to −11.3) |
9.6 (4.7–14.5) |
| Difference in clinically relevant improvement | 75% | 66% | 40% | 66% | 72% | 24% |
| Number needed to treat (95% CI) | 1.3 (1.2–1.4) | 1.5 (1.4–1.6) | 2.5 (2.3–2.7) | 1.5 (1.4–1.6) | 1.4 (1.3–1.5) | 4.2 (4.0–4.4) |
| P value | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 |
| Effect size | 2.5 | 2.3 | 1.2 | 2.7 | 2.4 | 0.8 |
Adapted from Graff et al. 2006 (35)
AMPS indicates assessment of motor and process skills; CI, confidence interval; IDDD, interview of deterioration in daily activities in dementia; SCQ, sense of competence questionnaire.
As seen in Table 8, at 6 weeks, caregivers in the OT group had significantly improved outcomes for overall QOL, health status, depression and mastery than those in the control group (P< .0001). Outcomes remained significant at 12 weeks (Table 9).
Table 8: Additional Caregiver Outcomes at 6 Weeks*.
| Caregiver Outcomes | Covariate-Adjusted Treatment Difference (95% CI) |
P value | Effect Size |
|---|---|---|---|
| Dqol overall | 0.7 (0.5–0.9) | < .0001 | 1.2 |
| Dqol aesthetics | 4.1 (3.1–5.0) | < .0001 | 1.6 |
| Dqol positive affect | 1.3 (0.1–2.5) | .0270 | 0.4 |
| Dqol negative affect | −1.9 (−3.9 to 0.2) | .0690 | NS |
| Dqol feelings of belonging | 1.0 (0.5–1.5) | < .0001 | 1.0 |
| Dqol self-esteem | 3.7 (3.0–4.3) | < .0001 | 2.1 |
| GHQ-12 | −4.6 (−6.0 to −3.2) | < .0001 | 1.3 |
| CES-D | −7.6 (−9.7 to −5.4) | < .0001 | 1.3 |
| Mastery scale | 3.5 (2.7–4.4) | < .0001 | 1.6 |
Adapted from Graff et al. 200 (36)
CI indicates confidence interval; CES-D, Center for Epidemiologic Studies Depression Scale; Dqol, Dementia Quality of Life Instrument; GHQ, General Health Questionnaire; NS, not significant.
Table 9: Additional Caregiver Outcomes at 12 Weeks*.
| Caregiver Outcomes | Covariate-Adjusted Treatment Difference (95% CI) |
P Value | Effect Size |
|---|---|---|---|
| Dqol overall | 0.9 (0.6–1.1) | < .0001 | 1.5 |
| Dqol aesthetics | 4.0 (3.4–4.6) | < .0001 | 1.3 |
| Dqol positive affect | 0.9 (−0.4 to 2.3) | .163 | NS |
| Dqol negative affect | −2.0 (−2.1 to −1.9) | .069 | NS |
| Dqol feelings of belonging | 0.8 (0.1–1.5) | .022 | 0.5 |
| Dqol self-esteem | 3.8 (2.9–4.8) | < .0001 | 1.6 |
| GHQ-12 | −4.9 (−6.6 to −3.3) | <.0001 | 1.1 |
| CES-D | −8.4 (−11 to −5.8) | < .0001 | 1.3 |
| Mastery scale | 4.1 (3.2–4.9) | < .0001 | 2.0 |
Adapted from Graff et al. 2007 (36)
CI indicates confidence interval; CES-D, Center for Epidemiologic Studies Depression Scale; Dqol, Dementia Quality of Life Instrument; GHQ, General Health Questionnaire; NS, not significant.
Limitations
Overall the study had very good methodological design. Limitations of the study have been outlined in Table 6.
The next study identified was conducted by Teri et al. (37) and examined a standardized dementia management intervention in 95 caregivers designed to provide strategies for modifying consequences of problem behaviours (Table 10).
Table 10: Summary of Randomized Controlled Trial by Teri et al., 2005 (37)*.
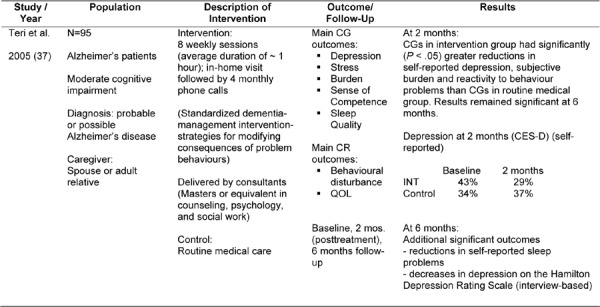
CES-D indicates Center for Epidemiologic Studies Depression Scale; CG, caregiver; CR, care receiver; INT, intervention; QOL, quality of life.
The authors found positive effects of the intervention on caregiver outcomes. At 2 months, caregivers in the intervention group had significantly greater reductions in self-reported depression, subjective burden, and reactivity to behaviour problems than caregivers in the control group. Results remained significant at 6 months. Additional significant outcomes at 6 months were: decreases in depression (Hamilton Depression scale (P= .041)), and a reduction in self-reported sleep problems (P= .033). When examining problem behaviours of the care recipient, overall 62% of the caregivers in the intervention group had improvement in caregiver-reactivity scores, 57% had reductions in frequency of problem occurrence, and 52% were reported to have reductions in problem severity.
Limitations
Consultants carrying out the intervention were heavily supervised, which may not reflect typical practice. In addition the study had a relatively small sample size. Follow-up was of only 6 months duration, making assessment of longer term effects difficult.
Mahoney et al.(38) report on a study which provided caregivers with 12-month access to an interactive voice response (IVR) mediated system designed to assist the caregiver in managing the BPSD of the patient (Table 11).
Table 11: Summary of Mahoney et al., 2003 (38)*.
| Study / Year | Population | Description of Intervention |
Outcome/ Follow-Up | Results |
|---|---|---|---|---|
| Mahoney et al. (38) 2003 |
N=100 Alzheimer’s Disease (AD) CG: Provided 4 or more hr/day of assistance or supervision for a minimum of 6 months to a family member with AD |
Intervention: Information technology: 12-month access to an interactive voice response (IVR) mediated system, which was designed to assist CG managing persons with disruptive behaviours related to AD Participants chose the type of component, freq, duration and timing |
Bothersome nature of CR disruptive behaviours
Mediating effect of CG mastery was also examined Baseline, 6, 12, and 18 months |
At 18 months: No significant main effect of the intervention in reducing bother scores, depression scores, or state anxiety. |
| Control: usual care (reference booklet containing similar content to module 1 of the intervention (strategies) |
CG indicates caregiver; CR, care recipient.
The authors found no significant main effect of the intervention in reducing bother scores, depression scores, or state anxiety at 18 months. Stratified analysis showed a significant intervention effect for caregivers with low- to mid-mastery at baseline (P< .05) for all 3 outcomes relative to controls. A significant effect was also found when caregivers were stratified by relationship status of the caregiver to care recipient. There was a significant reduction in bother scores for caregivers who were wives (P= .023).
It is important to note that there exist many models of information technology for caregiver interventions. This study only employed 1 model, which many not have been ideal for this population. The main limitation of this study is that it was inadequately powered. In addition, the intensity of the intervention differed greatly among users, and there was a possible floor effect as caregivers had low bother scores and depression scores at baseline.
Burgio et al. (39) investigated the use of a skills training program in 140 caregivers of patients with Alzheimer’s disease and related disorders (Table 12).
Table 12: Summary of Burgio et al. 2003 (39)*.
| Study / Year | Population | Description of Intervention |
Outcome/ Follow-Up |
Results | Comment |
|---|---|---|---|---|---|
| Burgio et al. (39) 2003 |
N=140 Analysis sample N=118 White (n=70) African American (n=48) AD and related disorders |
Intervention: Skills training condition – 3-hour group workshop followed by 16 in-home (1 hr) treatment sessions over 12 months Culturally appropriate (targets improvement of CG behaviour management skills, problem solving skills, and cognitive restructuring) |
CR problem behaviours, CG appraisal, CG social support and activity CG well-being Desire to institutionalize Baseline, 6 months (at 6 months, CG has received 8 home visits and 2 therapeutic phone calls) |
There were no significant main effects for treatment condition on the covariate adjusted 6-month outcome scores for any variable (P > .10). | No blinding of study personnel to group assignment Study duration: 6 months Difficult to separate effects of group versus individual sessions |
| Control: minimal support condition (general telephone support and written information) |
AD indicates Alzheimer’s disease; CG, caregiver, CR, care receiver.
The authors found that at 6 months, there were no significant main effects of the intervention on any of the outcomes (P> .10). Other findings were that spouses reported a significantly reduced number of problem behaviours in the care recipients as compared with nonspouses. In addition, white caregivers showed the most improvement in the minimal support group whereas African American caregivers showed greatest improvement in the intervention group. Caregivers in both groups reported significantly fewer problem behaviours, less behaviour bother, and an increase in satisfaction with leisure activities. The findings of this study suggest that cultural and relationship factors may be important considerations when designing caregiver interventions.
Limitations of the study can be seen in Table 12.
Summary of Findings
As stated by the GRADE Working Group, the following definitions were used in grading the quality of the evidence. The overall quality of the evidence is shown in Tables 13 and 14.
Table 13: Quality of Individual Behavioural Intervention Trials According to GRADE*.
| Outcome | Studies | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|---|
| CG burden and CG depression |
Graff et al., 2006; 2007 (35;36) |
RCT | No limitations High |
Consistent High |
Some uncertainty on directness† Moderate |
Moderate/High |
| Teri et al. 2005 (37) |
RCT |
CG indicates caregiver; RCT, randomized controlled trial
In 1 RCT, patients were recruited from a memory clinic; in 1 RCT consultants were heavily supervised.
Table 14: Quality of Individual Behavioural Intervention Trials According to GRADE*.
| Outcome | Studies | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|---|
| Other outcomes of CG psychosocial health |
Mahoney et al. 2003 (38) |
RCT | Some limitations† |
Not consistent | Direct | Low |
| Burgio et al. 2003 (39) | RCT | Moderate | Low | Low |
CG indicates caregiver; RCT, randomized controlled trial
One RCT was inadequately powered; 1 RCT had no blinding of outcome assessors; participants had low bother scores and low depression scores at baseline.
High: further research is very unlikely to change our confidence in the estimate of effect,
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate,
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate, and
Very low: any estimate of effect is very uncertain.
Conclusion
Previous systematic reviews and meta-analyses suggest that 6 or more sessions of individual behavioural management therapy centered on the care recipient’s behaviour can alleviate caregiver symptoms both immediately and for up to 32 months.
A recent RCT supports these findings concluding that individual behavioural interventions (≥ 6 sessions) directed at the caregiver (or combined with the patient) are effective in improving psychological health in dementia caregivers.
2.2B. Multicomponent Interventions for Caregivers of Seniors With Dementia
Clinical Need: Target Population and Condition
As mentioned previously, existing evidence from systematic reviews and meta-anlayses show that multicomponent interventions can significantly reduce caregiver burden (31) and the risk for institutionalization. (33) Moreover, dementia caregivers have complex needs, which may require a variety of interventions to provide adequate support.
A 2006 systematic review of multicomponent interventions by Pinquart et al.(33) was identified and a literature search was conducted in order to identify any RCTs subsequently published.
Evidence-Based Analysis of Effectiveness
Research Questions
Does new evidence since the last systematic review support existing findings that multicomponent interventions reduce caregiver burden?
Does new evidence support existing findings that multicomponent interventions delay entry into LTC settings?
Methods
Inclusion Criteria
English-language articles published after the search date (2005) of the systematic review by Pinquart et al.(33),
randomized controlled trials that report primary data on the effectiveness of multicomponent interventions (2 or more psychosocial interventions) for dementia caregivers of seniors with dementia living in the community,
study design and methods must be clearly described,
control group = routine care, and
primary outcome = any measure of caregiver psychological health (i.e., burden, depression, stress, QOL).
Exclusion Criteria
studies that are duplicate publications (superseded by another publication by the sameinvestigator group, with the same objective and data),
studies with less than 10 patients, and
formal (paid carers).
Literature Search
A search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, PsycINFO, and INAHTA/CRD for studies published between January 2005 and February 2008 (Appendix 2). Abstracts were reviewed by a single author, and studies meeting the inclusion criteria outlined above were obtained. Reference lists were also checked for relevant studies.
Results of Literature Search (Update To Existing Evidence)
The search identified 1 RCT on multicomponent interventions. Belle et al. (40) evaluated the effects of a structured multicomponent intervention on caregivers of 3 diverse racial groups. Five target areas of the intervention were: depression, burden, self-care and healthy behaviours, social support, and problem behaviours. The study was carried out in 642 caregivers of individuals with Alzheimer’s disease or related disorders (Table 15).
Table 15: Summary of Belle et al. 2006 (40)*.
| Study / Year | Population | Description of Intervention | Outcome/ Follow-Up |
Results | Comment |
|---|---|---|---|---|---|
| Belle et al. 2006 |
N=642 Hispanic (n=212) White (n=219) Black (n=211) Alzheimer’s disease or related disorders 5 US cities |
Intervention: Strategies: provision of information, role playing, problem solving, telephone support, etc. 5 target areas: depression, burden, self-care and healthy behaviours, social support, problem behaviours Delivered by interventionist with at least a BA 12 sessions (9 in-home [1.5 hrs. each] and 3 telephone sessions [30 min. each]), and 5 structured telephone support group sessions over 6 months Control: mailed basic info, 2 brief telephone calls at 3 and 5 months |
Primary outcomes:
|
Hispanic CGs:
Secondary outcomes: Prevalence of clinical depression was significantly greater among CGs in the control group than those in INT group (22.7% vs. 12.6%; P = .001); difference remained significant after adjustment for race & ethnicity. Number of CRs institutionalized did not differ statistically significantly between groups (7.2% control vs. 4.3% intervention). - no significant differences between groups in any racial or ethnic group. |
Loss to follow-up: 60% completed all 12 sessions; 5% did not complete any session. Long-term efficacy unknown. Assessing effects of the intervention on institutional placement typically requires 1-yr follow-up or longer. Larger effects seen in Hispanic CGs – probably due to the availability of intervention in Spanish versus otherwise limited access to community resources that are culturally appropriate. |
BA indicates Bachelor of Arts; CG, caregiver; CI, confidence interval; CR, care recipient; INT, intervention.
Results of the study were reported by ethnic group in each of the 5 domains examined: burden, depression, self-care, social support, and problem behaviour. In Hispanic participants, the net improvement across all 5 domains was greater in the intervention group than in the control. Clinically significant differences in net improvement in the Hispanic participants favoured the intervention for depression and problem behaviours, as 39% of participants in the intervention group lowered their depression scores compared with 0% in the control group. In the intervention group, 32% of participants experienced a clinically significant decrease in problem behaviours versus 5% of participants who reported a net increase in problem behaviours in the control group. In white or Caucasian participants, differences in net improvement favoured the intervention for social support. For black or African American participants there were no significant differences between the groups for any of the 5 domains.
The larger effects seen in Hispanic caregivers may be due to the fact that this intervention was delivered in Spanish (with translated materials) to a population of caregivers that may otherwise have limited access to community resources that are culturally appropriate.
Secondary outcomes examined in this study (Table 16) were the prevalence of clinical depression and institutional placement of care recipients. At 6 months, the prevalence of clinical depression was significantly greater among caregivers in the control group than those in the intervention group (22.7% vs. 12.6%; P = .001).
Table 16: Clinical Depression of Caregivers and Institutional Placement of Care Recipients at 6-Month Follow-Up*.
| Combined (Hispanic or Latino, White or Caucasian, Black or African American) |
||
|---|---|---|
| Control | Intervention | |
| Caregivers at follow-up, n | 289 | 293 |
| Caregivers with clinical depression* at follow-up, n% |
65 (22.7) | 37 (12.6) |
| Care recipient randomization, n | 319 | 323 |
| Care recipients institutionalized, n(%) | 23 (7.2) | 14 (4.3) |
Adapted from Belle et al. 2006 (40)
Clinical depression was defined as a CES-D score ≥15. CES-D indicates Center for Epidemiologic Studies Depression scale.
Note: 3 participants were missing CES-D scores.
There was no significant effect of the intervention on the number of care recipients institutionalized (7.2% control vs. 4.3% intervention; P = .118), and also no significant difference between the groups in any racial or ethnic group. However it must be noted that assessing the effects of an intervention on institutional placement typically requires 1-year follow-up or longer and thus this study was not adequately designed to assess this outcome.
Limitations
Since this study was of 6 months duration, the long-term efficacy is unknown. However, most studies conducted in seniors with dementia and caregiver populations assess short- to medium-term effects. Also, only 60% of participants completed all 12 sessions of the intervention and 5% of participants did not complete any sessions.
Summary of Findings
Table 17: Quality of Multicomponent Intervention Trials According to GRADE*.
| Outcome | Study | Design | Quality | Consistency | Directness | Quality |
|---|---|---|---|---|---|---|
| Caregiver burden |
Belle et al. 2006 |
RCT | No limitations |
Not consistent† | Direct | Moderate/High |
| High | Moderate | High |
RCT indicates randomized controlled trial.
Although the results of this study were not consistent with previous studies reporting a reduction in caregiver burden associated with multicomponent interventions, the current study shows that other measures of caregiver psychosocial health showed improvement such as depression, problem behaviours, and social support.
Ontario Health Systems Impact Analysis
Considerations and Implications
An expert panel on aging in the community met on May 16, 2008, and discussed in part, behavioural management interventions for seniors with dementia in Ontario. In particular, the expert panel commented on the challenges with conducting studies on caregiver interventions and the lack of programs/tools available to caregivers to help them manage BPSD. Comments from the panel are found below.
Behavioural Management Interventions
Current Delivery
Two groups generally provide behavioural management interventions: community occupational therapists and psychogeriatric nurses.
Psychogeriatric nurses counsel caregivers, and occupational therapists make environmental modifications to the home and provide case management.
Physicians are reluctant to prescribe medications to seniors with dementia for problem behaviours; however, when caregivers have major difficulties with managing the care recipient (i.e., wandering, sleep disruptions), physicians will prescribe medication.
Systems Pressures
Programs/tools are needed which will give caregivers the skills to manage and provide relief.
It is difficult to co-ordinate funding of technology and of research.
There are fundamental problems with studying caregiver interventions for dementia.
Future Research/Direction
Examine the research being done at the OT department at the University of Toronto around family caregivers and outcome measures; identify which interventions are most effective.
Field evaluations are required as different models and evaluations are needed.
Technological interventions such as websites and online networking for care providers can be effective.
It is important to focus on characteristics of people requiring services since response to interventions greatly differs according to type and severity.
Overall Conclusions for Caregiver-Directed Interventions
Respite Care
Assessing the efficacy of respite care services using standard evidence-based approaches is difficult.
There is limited evidence from RCTs that respite care is effective in improving caregiver outcomes for those caring for seniors with dementia.
There is considerable qualitative evidence of the perceived benefits of respite care.
Respite care is known as one of the key formal support services for alleviating caregiver burden in those caring for dementia patients.
Respite care services need to be tailored to individual caregivers needs since there are vast differences between caregivers and patients of dementia (severity, type of dementia, amount of informal/formal support available, housing situation, etc.)
Psychosocial Interventions – Behavioural Management Interventions
There is moderate- to high-quality evidence that individual behavioural interventions (≥ 6 sessions), directed at the caregiver (or combined with the patient) are effective in improving psychological health in dementia caregivers.
Multicomponent Interventions
There is moderate- to high-quality evidence that multicomponent interventions improve caregiver psychosocial health and may impact rates of institutionalization of dementia patients.
3. Patient-Directed Interventions for Dementia
Objective
This section on patient-directed interventions for dementia is broken down into 4 subsections with the following questions:
3.1 Physical Exercise for Seniors with Dementia – Secondary Prevention
What is the effectiveness of physical exercise for the improvement or maintenance of basic activities of daily living (ADLs), such as eating, bathing, toileting, and functional ability, in seniors with mild to moderate dementia?
3.2 Nonpharmacologic and Nonexercise Interventions to Improve Cognitive Functioning in Seniors With Dementia – Secondary Prevention
What is the effectiveness of nonpharmacologic interventions to improve cognitive functioning in seniors with mild to moderate dementia?
3.3 Physical Exercise for Delaying the Onset of Dementia – Primary Prevention
Can exercise decrease the risk of subsequent cognitive decline/dementia?
3.4 Cognitive Interventions for Delaying the Onset of Dementia – Primary Prevention
Does cognitive training decrease the risk of cognitive impairment, deterioration in the performance of basic ADLs or instrumental activities of daily living (IADLs),4 or incidence of dementia in seniors with good cognitive and physical functioning?
3.1. Physical Exercise for Seniors With Dementia – Secondary Prevention
Clinical Need: Target Population and Condition
Dementia is a general loss of cognitive abilities, including impairment of memory as well as 1 or more of the following: speech disorders; loss of ability to carry out familiar, purposeful movements; loss of the power to recognize the meaning of sensory stimuli; or disturbed planning, organizing, and abstract thinking abilities. Causes include a large number of conditions that result in widespread cerebral damage or dysfunction. The most common cause is Alzheimer’s disease (50%–60%) followed by cerebrovascular disease (20%).
Dementia adversely affects cognitive, emotional, and behavioural functioning. (41) There are also a number of studies that link dementia with physical deterioration. (42-46) Compared with age-matched controls, patients with Alzheimer’s disease show more signs of undernutrition (42), a higher risk of falls and fractures, (43-46) and more rapid decline on measures of mobility. (47;48) Once injured, patients with Alzheimer’s disease are at greater risk of subsequent injury than age- and sex-matched controls. (43)
Reduced muscle mass has been associated with loss of independence. (49) Decreased activity levels can lead to muscle atrophy, increasing the potential for unsafe mobility while performing the basic ADLs such as eating, bathing, toileting, and functional ability. (50)
Improved physical conditioning for seniors with dementia may extend their independent mobility and maintain performance of ADL. (51)
Evidence-Based Analysis of Effectiveness
Question
What is the effectiveness of physical exercise for the improvement or maintenance of ADLs in seniors with mild to moderate dementia?
Comparisons of Interest (and for which evidence of these comparisons exist)
physical exercise versus no physical exercise, and
physical exercise versus usual care.
Methods
Literature Review
A standard Medical Advisory Secretariat literature review was undertaken (Appendix 3).
Inclusion Criteria
elderly patients (≥65 years) with mild to moderate dementia,
inpatients or outpatients,
patients receive any type of physical exercise as the intervention,
systematic reviews, RCTs, and
primary outcome = any measure of physical functioning.
Exclusion Criteria
patients less than 65 years of age,
studies with less than 10 patients,
studies that examine the effectiveness of multitherapies (e.g., physical exercise + behavioural therapy),
studies that do not report physical exercise as the intervention.
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology and GRADE Working Group (52) As per GRADE the following definitions apply:
High: further research is very unlikely to change our confidence in the estimate of effect,
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate,
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate, and
Very low: any estimate of effect is very uncertain.
Results of Literature Search
A literature search from January 2003 to April 2008 (including OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library, International Agency for Health Technology Assessment/Centre for Review and Dissemination) identified 2 systematic reviews.
Heyn et al. (53) conducted a systematic review/meta-analysis to determine whether physical exercises are beneficial for people with dementia and related cognitive impairments. Law et al. from the Occupational Therapy Evidence-Based Practice Research Group at McMaster University (54) systematically reviewed the effectiveness of activity programs in improving occupational performance (i.e., participation in self-care, productivity, and leisure) and/or performance components (physical, affective, and cognitive).
Of 6 studies identified that were published after the most recent systematic review, 6 were excluded (patients did not have dementia; observational studies; multimodal therapy).
Summary of Existing Evidence
Summary of Systematic Reviews
Table 18 summarizes the 2 systematic reviews that were identified in the literature search.
Table 18: Summary of Systematic Reviews for Physical Activity in Seniors with Dementia*.
| Study/Year/Country | Type of Study |
Objective | Outcomes | Results | Comment |
|---|---|---|---|---|---|
| Heyn et al. (53) 2004 United States |
Meta-analysis (fixed effects) | To determine whether physical exercises are beneficial for people with dementia and related cognitive impairments |
|
30 RCTs met inclusion criteria Mix of community dwelling and LTC residents. N=2,020 Results (Summary Effect Size and 95% CI) Fitness 0.69 (0.58–0.80) Cognitive 0.57 (0.38–0.75) Functional 0.59 (0.43–0.76) Behaviour 0.54 (0.36–0.72) Overall 0.62 (0.55–0.70) |
Intervention delivered via occupational therapists Short-term studies Methodological issues (heterogeneity) Unclear whether patients maintained the intensity throughout or if additional devices were used to enhance motivation |
| Occupational Therapy Evidence-Based Practice Research Group, McMaster University (54) 1999 Canada Grey literature |
Systematic review | To determine effectiveness of activity programs in improving occupational performance (i.e., participation in self-care, productivity, and leisure) and or performance components (physical, affective, and cognitive) | “Occupational performance(participation in daily activities)” This was based on: Performance component areas (physical/psychologi cal/cognitive) Environmental factors (family/caregiver perspectives) |
4 RCTs met inclusion criteria; each had 4 different activity programs (planned walking, mental stimulation, physical activation, and purposeful activities). N=164; Mix of inpatients and outpatients Statistically significant results favoured the treatment group in all 4 studies. “They support the use of activity groups for older persons with dementia for improving their wellbeing, communication, mental status, and emotional state. Future research is needed in this area due to the small amount of evidence available.” |
Intervention delivered via occupational therapists Activity outcomes “include some sort of activity which may be physical, social cognitive or psychological behavioural in nature” Heterogeneity |
CI indicates confidence interval; LTC, long-term care; N, number; RCT, randomized controlled trial.
Economic Analysis
Literature Review
No economic analyses were identified that examined the cost-effectiveness of exercise programs for seniors with dementia.
Summary of Findings for Physical Activity in Seniors With Dementia
The overall quality of the evidence was determined by using GRADE (52) as shown in Table 19.
Table 19: Quality of Trials According to GRADE.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
| Physical functioning | Meta-analysis | Moderate (heterogeneity -variation in frequency intensity, duration of interventions) |
Consistent (mostly short-term follow-up) |
Mix of community dwelling and long-term care residents | Moderate |
Ontario Health Systems Impact Analysis
Considerations and Implications
An expert panel on aging in the community met on February 29, 2008, and, in part, discussed physical exercise for seniors in Ontario. In particular, it was discussed how physical exercise is made available to seniors and who provides the service. Comments from the panel are found below.
Long-Term Care Facilities
In-house occupational/physiotherapists and recreational therapists provide physical exercise interventions.
In the Community
Community Care Access Centres can provide referrals for occupational therapists, physiotherapists, and personal support workers to go to homes.
Community recreation centres – recreationalists can teach caregiver and client exercise programs.
Community agencies and religious groups offer exercise programs – volunteer-led informal exercise groups (e.g., “mall walkers”).
Exercise programs often provided in/around supportive housing units.
Exercise activities often organized outside of the formal health system.
Municipality websites often list services available within the area.
Benefit/Risk Analysis
As per the GRADE Working Group (52), the strength of a recommendation to use exercise as an intervention to improve functional outcomes is shown in Table 20.
Table 20: Overall Summary Statement of the Benefit and Risk.
| Outcome | Quality | Benefits | Risks/Burden | Overall Strength of Recommendation |
|---|---|---|---|---|
| Physical functioning | Moderate | Improvement in functional, cognitive, and behavioural outcomes | Short-term follow-up and heterogeneity in studies. Unclear if leads to delayed institutionalization. |
Moderate |
Conclusion
Physical exercise is effective for improving physical functioning in patients with dementia and the strength of a recommendation in this regard is moderate when weighing risks and benefits.
3.2. Nonpharmacologic & Nonexercise Interventions to Improve Cognitive Functioning in Seniors with Dementia – Secondary Prevention
Clinical Need: Target Population and Condition
Cognitive impairments, including memory problems, are a defining feature in patients with dementia. These impairments can have a major impact on the patient leading to anxiety, depression, and withdrawal from activities. (55) In addition, caregivers can be affected due to the practical impact of cognitive problems on daily activities.(55) Cognitive interventions aim to improve these impairments in people with mild to moderate dementia.
General reality orientation was first described in 1966 as a technique to improve the QOL of confused elderly people, although its origins lie in attempts to rehabilitate severely disturbed war veterans. (56) General reality orientation approaches were shown to produce improvements in cognition in a systematic review by Spector et al. (56); however, the overall quality of the studies was poor (6 studies; N=125; study publication range 1979 to 1994)). Most studies did not provide enough information to draw conclusions about contamination and blinding. Dropouts were not described well in some studies. A therapeutic protocol was not mentioned in any of the studies. Many studies used 1970s concepts of the neuropsychology of dementia. (56)
Progress in understanding the operation of memory and related cognitive functions, and of mechanisms of learning, has allowed the development of more specific approaches designed to help maintain or enhance cognitive functioning for people with dementia. (55) These include cognitive training and individualized cognitive rehabilitation. These are defined as follows:
Cognitive Training: Guided practice on a set of standard tasks designed to improve particular cognitive functions (e.g., memory, attention, problem solving). The underlying assumption is that practice has potential to improve or at least maintain functioning in the given domain and that any effects of practice will generalize beyond the immediate training context. (55)
Cognitive Rehabilitation: More individualized approach to help people with cognitive impairments in which those affected, and their families, work together with health care professionals to identify personally relevant goals and devise strategies for addressing these. Emphasis is not on enhancing performance on cognitive tasks, but on improving functioning in the everyday context. (55)
Cognitive training and rehabilitation have been used interchangeably in the literature. Some examples include:
memory therapy/retraining/support/stimulation; or
cognitive training/retraining/remediation/support/stimulation.
Evidence-Based Analysis of Effectiveness
Question
What is the effectiveness of nonpharmacologic interventions to improve cognitive functioning in seniors with mild to moderate dementia?
Comparisons of Interest (and for which evidence of these comparisons exist)
cognitive training versus usual care,
cognitive rehabilitation versus usual care, and
cognitive training versus cognitive rehabilitation.
Methods
Inclusion Criteria
elderly patients (≥65 years) with mild to moderate dementia,
inpatients or outpatients,
patients receiving cognitive or memory training/therapy/retraining/stimulation/support/remediation as intervention targeting cognitive functioning,
systematic reviews, RCTs, and
outcome being any measures of memory or other aspects of cognitive functioning for seniors with mild to moderate dementia.
Exclusion Criteria
studies with fewer than 10 patients.
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology. (52)
Results of Literature Search
A literature search from January 2006 to December 2007 (Appendix 4; including OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library, International Agency for Health Technology Assessment/Centre for Review and Dissemination) identified 1 Cochrane review that evaluated the effectiveness and impact of cognitive training and cognitive rehabilitation interventions aimed at improving memory and other aspects of cognitive functioning for people in the early stages of Alzheimer’s disease or vascular dementia (inpatients or outpatients). (55)
Of 7 studies identified that were published after the Cochrane review, 6 were excluded (N < 10 patients; patients did not have dementia; subgroup analysis of previous study). One RCT by Spector et al. (57) was included in this report.
The quality of the included article is presented below (Table 21).
Table 21: Quality of Evidence of Included Studies*.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic reviews of RCTs | 1 | 1 |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT, unpublished but reported to an international scientific meeting | 2(g) | |
| Nonrandomized study with contemporaneous controls | 3a | |
| Nonrandomized study with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multi-site) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (58) An additional sesignation “g” was added for preliminary reports of studies that have been presented at international scientific meetings.
Summary of Existing Evidence
Summary of Systematic Reviews
Table 22 summarizes the systematic review by Clare et al. (55) which concluded that there were no significant benefits associated with cognitive training. No RCTs of cognitive rehabilitation met the inclusion criteria.
Table 22: Summary of Systematic Reviews for Nonpharmacologic Interventions to Improve Cognitive Functioning in Seniors With Mild to moderate Dementia*.
| Study/Year/Country | Type of Study | Objective | Outcomes | Results | Comment |
|---|---|---|---|---|---|
| Clare et al. (55) 2003 United Kingdom |
Cochrane systematic review | To evaluate the effectiveness and impact of cognitive training and cognitive rehabilitation interventions aimed at improving memory and other aspects of cognitive functioning for people in the early stages of Alzheimer’s disease or vascular dementia | Any outcomes for the person with dementia and/or the family caregiver | 9 RCTs reporting cognitive training No RCTs of cognitive rehabilitation The diversity of outcome measures used in the studies did not allow meta-analysis. There were no significant positive effects of cognitive training. |
“Further well-designed studies of cognitive training and cognitive rehabilitation are required to provide more definitive evidence.” Consistency regarding type of therapies (Clare et al. terminology vs. original paper terminologies) Small sample sizes – possible type 2 errors No age restrictions Frequency / intensity / duration of interventions Baseline differences between studies |
RCT indicates randomized controlled trial.
Updated Studies
Table 23 shows the results of the RCT by Spector et al. (57) The authors concluded that cognitive stimulation therapy (CST) significantly improved cognitive function in people with dementia.
Table 23: Results of the Randomized Controlled Trial by Spector et al. (57)*.
| Study/Year/Country | Type of Study | Objective | Outcomes | Results | Comments |
|---|---|---|---|---|---|
| Spector et al. (57) 2003 United Kingdom |
RCT Single blind Multicentre ITT N=201 |
To determine if cognitive stimulation therapy (CST) for older people with dementia is effective in improving cognition and quality of life. CST based on “reality orientation” and cognitive stimulation. Also based largely on a trial (Breuil et al. 1994) that was identified as having the most significant results. |
Primary outcome:
|
CST: n=115 Control: n=86 Patients from day care centres or residential homes CST= 2 sessions a week for 7 weeks Primary outcome: CST had significantly higher scores on cognitive function testing Secondary outcomes: CST had significantly higher scores on ADASCog and quality of life than the control group No significant differences for communication, behaviour, depression or anxiety (Possible Type 2 errors) |
Powered to detect a difference in means of 2 points for cognitive functioning testing Study not powered to detect differences in secondary outcomes. Role of maintenance CST unclear Largest sample size to date |
ADAS-Cog indicates Alzheimer’s disease assessment scale – cognitive subscale; ITT, intention-to-treat; n, number; RCT, randomized controlled trial.
Economic Analysis
One study was identified that examined the cost-effectiveness of an evidence-based CST programme for people with dementia as part of a RCT. (59)
Ninety-one people with dementia, living in care homes or the community, received a group CST intervention twice weekly for 8 weeks. Seventy people with dementia received treatment as usual. A cost-effectiveness analysis was conducted with cognition as the primary outcome and QOL as the secondary outcome.
Cognitive stimulation therapy had benefits for cognition and QOL in dementia and costs were not different between the groups. According to Knapp et al. (59), under reasonable assumptions, there is a high probability that CST is more cost-effective than treatment as usual for both the primary and secondary outcomes.
Summary of Findings for Nonpharmacologic and Nonexercise Interventions to Improve Cognitive Functioning in Seniors With Dementia
The overall quality of the evidence as per GRADE (52) is shown in Table 24.
Table 24: Quality of Trials According to GRADE*.
| Outcome | Technique/Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Cognitive function | Cognitive training Systematic review of RCTs |
Low | Not consistent (Diversity of outcome measures) |
Mix of community dwelling and long-term care residents | Very low |
| Cognitive function | Cognitive stimulation therapy RCT |
High | Not applicable(1 trial) | Mix of community dwelling and long-term care residents | Moderate/Low |
RCT indicates randomized controlled trial.
Ontario Health Systems Impact Analysis
Considerations and Implications
An expert in cognitive interventions for people with dementia stated:
He was not aware of any provider in Ontario who was offering CST to people with mild to moderate dementia.
A variety of nonpharmacologic interventions to improve cognitive function in seniors with mild to moderate dementia are probably being used in the province.
Nonpharmacologic interventions to improve cognitive function in seniors with mild to moderate dementia are in the “artisan” stage (moving to becoming more evidence-based).
Benefit/Risk Analysis1
As per the GRADE Working Group (52), the strength of a recommendation to use cognitive training, rehabilitation or CST as an intervention to improve cognitive functioning is shown in Table 25.
Table 25: Overall Summary Statement of Benefit and Risk.
| Outcome/Technique | Quality | Benefits | Risks/Burden | Overall Strength of Recommendation |
|---|---|---|---|---|
| Cognitive functioning Cognitive training |
Very low | None | Intervention does not offer significant benefit (possible type 2 error) Unclear if leads to delayed institutionalization |
Very low |
| Cognitive functioning Cognitive stimulation therapy (CST) |
Moderate /Low | Increased cognition and quality of life | Unclear how CST compares with past terminologies and methodologies Short-term results Role and extent of maintenance Unclear how CST may impact functional dependence Unclear if leads to delayed institutionalization |
Low |
Conclusion
Previous systematic review indicated that “cognitive training” is not effective in patients with dementia.
-
Recent RCT suggests CST (up to 7 weeks) is effective for improving cognitive function and QOL in patients with dementia.
However:
unclear how CST compares with past terminologies and methodologies,
short-term results,
role and extent of maintenance CST unclear, and
unclear how CST may impact functional dependence.
3.3. Physical Exercise for Delaying the Onset of Dementia – Primary Prevention
Clinical Need: Target Population and Condition
Various vascular risk factors have been found to contribute to the development of dementia (e.g., hypertension, hypercholesterolemia, diabetes, overweight). (60;61)
Physical exercise is important in promoting overall and vascular health. (62) However, it is unclear if physical exercise can decrease the risk of cognitive decline/dementia. A possible biological basis for how physical exercise might preserve brain function includes improved cerebral blood flow and oxygen delivery. (63)
Evidence-Based Analysis of Effectiveness
Question
Can exercise decrease the risk of subsequent cognitive decline/dementia?
Comparisons of Interest (and for which evidence of these comparisons exist)
physical activity versus no physical activity, and
physical activity versus usual care.
Methods
Inclusion Criteria
elderly patients (≥65 years) without dementia,
patients participate in physical activity,
systematic reviews, RCTs, and
outcome = cognitive decline/dementia.
Exclusion Criteria
patients less than 65 years of age,
less than 10 patients, and
studies that do not report physical activity as the intervention.
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology. (52)
Results of Literature Search
A literature search from January 2003 to April 2008 (Appendix 5; including OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library, International Agency for Health Technology Assessment/Centre for Review and Dissemination) failed to identify any RCTs. Since no RCTs were identified, prospective observational studies were considered for inclusion. Five prospective observational studies were identified. (64-68) Four of these studies included seniors 65 years of age and older who were followed up for a short-term duration (mean ~5 year follow-up). (64-67) One study included seniors who had a mean age of 51 years at study onset. (68) The mean follow-up period for these participants was 21 years. (68)
Although the observational study by Rovio et al. (68) did not fit the a priori inclusion criteria because it included patients less than 65 years of age, it was included in this systematic review since it is the only study identified to date that investigated whether there may be a long-term association between midlife leisure activity and subsequent risk of dementia.
The quality of the included articles is presented below (Table 26).
Table 26: Quality of Evidence of Included Studies*.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT, unpublished but reported to an international scientific meeting | 2(g) | |
| Nonrandomized study with contemporaneous controls | 3a | 5 |
| Nonrandomized study with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multi-site) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (58) An additional sesignation “g” was added for preliminary reports of studies that have been presented at international scientific meetings.
Summary of Existing Evidence
Summary of Short-Term Observational Studies
Two studies examined cognitive decline (64;67) as an endpoint, and 2 studies assessed incidence of dementia as the endpoint. (65;66)
Effects of Exercise on Cognitive Decline
Lytle et al. (64) showed that “high exercise” defined by the authors as “aerobic exercise ≥30 min duration ≥3 times per week” or defined by the Surgeon General as “aerobic exercise >30 min duration >5 times per week,” was associated with a significantly reduced risk of cognitive decline over a 2-year follow-up (Table 11). According to the authors’ definition of high exercise, low exercise was not associated with a significantly reduced risk of cognitive decline. According to the Surgeon General’s definition, low exercise was marginally significant in terms of a reduced risk of cognitive decline (Table 11).
Weuve et al. (67) showed that over a 2-year follow-up, higher levels of activity were associated with less cognitive decline in women who participated in a substudy of the Nurses Health Study (Table 27).
Table 27: Summary of Observational Studies That Examine the Effect of Exercise on Cognitive Decline*.
| Study, Year Country | Type of Study | Patients | Outcomes | Results |
|---|---|---|---|---|
| Lytle et al., 2004 (64) United States |
Longitudinal analysis. Objective: Examine incidence, risk, and protective factors and outcomes of cognitive impairment and dementia among community-dwelling seniors (65+) Seniors assessed at study entry and at follow-up waves every 2 years using a cognitive battery Self-reported exercise data collected only at Waves 3 & 4 This study focused on people who survived to participate in Wave 3 and Wave 4 |
Initially N=1681 Mean age 72.9 years Wave 3 n=1146 Mean age 76.8 years Wave 4 n=929 Mean age 76.2 years |
Whether exercise level at Wave 3 associated with “cognitive decline” between Waves 3 and 4 Decline = decrease of ≥3 MMSE points Exercise stratification High exercise (authors): aerobic exercise of ≥30 min duration ≥3 times per week High exercise (Surgeon General): aerobic exercise of >30 min duration >5 times per week Low exercise Aerobic exercise <30 min duration <3 times per week No exercise |
After controlling for age, sex and education, Wave 3 MMSE score and self-rated health, logistic regression showed: ≥30 Min ≥3 Times Per Week: High exercise associated with reduced risk of subsequent cognitive decline at Wave 4. OR=0.39; 95% CI (0.19–0.78) Low exercise not significant. OR=0.69; 95% CI (0.43–1.10) >30 Min >5 Times Per Week: High exercise OR=0.45 95% CI (0.22–0.95) Low exercise OR=0.63 95% CI (0.39–0.99) Did not assess development of dementia |
| Weuve et al., 2004 (67) United States Nurses Health Study |
Prospective: Women reported participation in leisure physical activities on biennial mailed questionnaires starting in 1986. Each activity assigned a metabolic equivalent value. Overall activity assessed by average energy expenditure per week. Analyses based on average of energy expenditures from 1986 questionnaire through the questionnaire immediately preceding the baseline cognitive assessment. |
16,466 women aged 70 to 81 years | Validated telephone assessments of cognition administered twice ~ 2 years apart for participants ≥70 years 1995 to 2001 Cognition Test # 1997 to 2003 Cognition Test #2 |
Higher levels of activity associated with less cognitive decline Did not assess development of dementia |
CI indicates confidence interval; MMSE, Mini Mental State Exam; OR, odds ratio.
Effects of Exercise on Incidence of Dementia
Larson et al. (65) examined whether regular exercise in seniors was associated with a reduced risk for dementia. Table 12 shows that after a mean follow-up of 6.2 years, seniors who exercised at least 3 times per week (≥15 min at time during the past year) had a high probability of being dementia-free compared with those who exercised less than 3 times per week (HR 0.62, 95% CI 0.44–0.86, P = .03).
Abbott et al. (66) examined the association between self-reported walking in elderly men living in Honolulu and future risk of dementia (Table 28).
Table 28: Summary of Observational Studies That Examine the Effect of Exercise on the Incidence of Dementia.
Overall, exercise was associated with a reduced incidence of dementia. (66) After adjusting for age, men who walked the least (<0.25 mile per day) experienced a 1.8-fold excess of total dementia compared with those who walked more than 2 miles per day (17.8 vs. 10.3/1000 person-years; relative hazard [RH] 1.77; 95% CI 1.04–3.01). Compared with men who walked the most (>2 miles per day), an excess of dementia was also observed in those who walked 0.25 to 1 mile per day (17.6 vs. 10.3/1000 person-years; RH 1.71; 95% CI 1.02–2.86).
After adjustment, a 1.9-fold excess risk for total dementia occurred in men who walked less than 0.25 miles per day compared with men who walked more than 2 miles per day (RH 1.93; 95% CI, 1.11–3.34). Compared with the most active men, those who walked 0.25 to 1 mile per day experienced a 1.7-fold excess in dementia risk (RH 1.75; 95% CI, 1.03–2.99).
Summary of Long-Term Observational Studies
Rovio et al. (68) examined the association between leisure time physical activity at midlife and subsequent development of dementia. Overall, exercise at midlife was associated with a reduced risk of developing dementia (Table 29).
Table 29: Results from Roivio et al. (68).
| Study/Year/Country | Type of Study | Patients | Outcomes | Results |
|---|---|---|---|---|
| Roivo et al., 2005 (68) Finland |
Prospective cohort Investigate association between leisure time physical activity at midlife and subsequent development of dementia “Active” = participated in activity at least twice a week “Sedentary” = less than twice a week |
Having been examined once at midlife, 1499 people (72.5%) aged 65–79 years participated in the reexamination in 1998 (mean follow-up 21 years). | Development of dementia Leisure time assess on questionnaire |
Mean age at midlife exam was 50.6 years (range 39–64) Mean age at re-examination was 71.6 years (range 65–79) 115 people had dementia and 76 had Alzheimer’s disease Ascertained dementia cases from re-examination as well as hospital records for nonparticipants Comments: No follow-up measurements to assess changes occurring in physical activity |
In the final model, participants in the active group had 53% lower odds of dementia compared with the sedentary group.
One limitation to the study was that there were no follow-up measurements to assess any changes that may have occurred in physical activity.
Economic Analysis
No economic analyses were identified that examined the cost-effectiveness of exercise programs specifically for the primary prevention of dementia.
Munro et al. (69) assessed the cost-effectiveness of a community-based exercise program, as a population public health intervention for seniors via a pragmatic, cluster-randomized, community intervention trial. Participants were all those aged 65 and over in the least active four-fifths of the population responding to a baseline survey in the United Kingdom. Eligible candidates were invited to free locally held exercise classes made available for 2 years.
Twenty-six percent of the intervention group attended 1 or more exercise sessions. (69) There were no significant differences in mortality rates, survival times, or hospital admissions. After adjusting for baseline characteristics, seniors in the intervention group had a lower decline in health status, although this was statistically significant for only 1 out of 9 of the Short Form 36 Health Survey Questionnaire (SF-36) health dimension scores, and 2 out of 3 composite scores. The incremental average quality-adjusted life year gain of 0.011 per person in the intervention group resulted in an incremental cost per quality-adjusted life year ratio of €17,174 (95% CI €8,300–€87,120). (69)
Summary of Findings
As per the GRADE Working Group (52), the overall quality of the evidence is shown in (Table 30).
Table 30: Quality of Trials According to GRADE.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Short term Incidence of dementia |
Prospective cohort 2 studies |
High/Moderate* | Consistent | Direct (target population elderly) |
High/Moderate |
| Short term Cognitive decline |
Prospective cohort 2 studies |
High/Moderate† | Consistent | Direct (target population elderly) |
High/Moderate |
| Long term Incidence of dementia |
Prospective cohort 1 study |
Moderate‡ | Consistent (1 study but results consistent with short-term results) |
Not direct (middle aged) |
Moderate |
Ontario Health Systems Impact Analysis
Considerations and Implications
There is uncertainty regarding what type, frequency, intensity, or duration of physical activity is most beneficial in preventing cognitive deterioration.
There are implications for preventative health care for both seniors and pre-seniors:
There is evidence that regular exercise by seniors is associated with a reduced risk of cognitive decline and dementia.
There is evidence that regular midlife exercise is associated with a reduced risk of the development of dementia.
An expert panel on aging in the community met on February 29, 2008, and in part, discussed physical exercise for seniors in Ontario. In particular, it was discussed how physical exercise is made available to seniors and who provides the service. Comments from the panel are found below.
Long-Term Care Facilities
In-house occupational/physiotherapists and recreational therapists provide physical exercise interventions.
In the Community
Community Care Access Centres can provide referrals for occupational therapists, physiotherapists and personal support workers to go to homes.
Community recreation centres – recreationalists can teach caregiver and client exercise programs.
Community Agencies (e.g., SPRINT) and religious groups off exercise programs – volunteer led informal exercise groups (e.g., “mall walkers”).
Exercise programs often provided in/around supportive housing units.
Exercise activities often organized outside of the formal health system.
Municipality websites often list services available within the area.
Benefit/Risk Analysis
As per the GRADE Working Group (52), the strength of a recommendation to use physical activity as an intervention to reduce the risk of cognitive decline or dementia is shown in Table 31.
Table 31: Overall Summary Statement of Benefit and Risk.
| Outcome | Quality | Benefits | Risks/Burden | Overall Strength of Recommendation |
|---|---|---|---|---|
| Short term Incidence of dementia |
High/Moderate | Decreased incidence of dementia | Unknown if leads to delayed institutionalization | High/Moderate |
| Short term Cognitive decline |
High/Moderate | Reduced risk of subsequent cognitive decline | Unknown if leads to delayed diagnosis of dementia or institutionalization | High/Moderate |
| Long term Incidence of dementia |
Moderate | Decreased incidence of dementia | Unknown if leads to delayed institutionalization | Moderate |
Conclusion
Long-Term Outcomes
Regular leisure time physical activity in midlife is associated with a reduced risk of dementia in later life (mean follow-up 21 years).
Short-Term Outcomes
Regular physical activity in seniors is associated with a reduced risk of cognitive decline (mean follow-up 2 years).
Regular physical activity in seniors is associated with a reduced risk of dementia (mean follow-up 6–7 years).
3.4. Nonpharmacologic & Nonexercise Interventions for Delaying the Onset of Dementia – Primary Prevention
Clinical Need: Target Population and Condition
Cognitive impairments, including memory problems, are a defining feature in patients with dementia. (55) Declines in specific cognitive domains (e.g., memory, executive functions) are predictive of deficits in the performance of IADLs in older adults. (70;71)
Having more years of education (i.e., a higher cognitive reserve) is associated with a lower prevalence of dementia in crossectional population based studies and to a lower incidence of dementia in cohorts followed longitudinally. (72;73) However, it is unclear whether cognitive training can increase cognitive reserve or decrease the risk of cognitive impairment, deterioration in the performance of ADLs or IADLs, or incidence of dementia. (74)
Evidence Based Analysis of Effectiveness
Question
Does cognitive training decrease the risk of cognitive impairment, deterioration in the performance of ADLs or IADLs or incidence of dementia in seniors with good cognitive and physical functioning?
Comparisons of Interest (and for which evidence of these comparisons exist)
Cognitive training versus usual care/activity.
Methods
Inclusion Criteria
elderly patients (≥65 years) without dementia,
patients receive cognitive intervention targeting cognitive functioning,
systematic reviews, RCTs, and
outcome being any measures of cognitive functioning/ADL/IADL/incidence of dementia.
Exclusion Criteria
patients <65 years of age,
N < 10 patients, and
studies that do not report cognitive exercises as the intervention.
Assessment of Quality of Evidence
The quality of the evidence was assessed as High, Moderate, Low, or Very low according to the GRADE methodology and GRADE Working Group at www.Gradeworkinggroup.org. (52)
Results of Literature Search
A literature search from January 2006 to December 2007 (Appendix 4) including OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library, INAHTA/Centre for Review and Dissemination identified no systematic review that evaluated the effectiveness of cognitive training interventions to decrease the risk of cognitive impairment, deterioration in the performance of ADLs or IADLs or incidence of dementia in seniors with good cognitive and physical functioning
Two publications of a singe RCT were identified. (75;76) Ball et al. (75) examined whether 3 cognitive training interventions improved mental abilities and daily functioning in older independent living adults. Willis et al.(76) conducted a 5-year extension follow-up of the original trial by Ball et al. (75)
The quality of the included article is presented below (Table 32).
Table 32: Quality of Evidence.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic reviews of RCTs | 1 | 1 + 1 (original + extension) |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT, unpublished but reported to an international scientific meeting | 2(g) | |
| Nonrandomized study with contemporaneous controls | 3a | |
| Nonrandomized study with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multi-site) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (58) An additional sesignation “g” was added for preliminary reports of studies that have been presented at international scientific meetings.
Summary of Existing Evidence
Randomized Controlled Trials
The RCT reported by Bell et al. (75) tested if a 10-session training intervention for specific cognitive functions (memory, reasoning, and speed of processing) produced immediate improvements in these domains compared with a nonintervention control group (Table 33).
Table 33: Summary of the 2 Randomized Controlled Trials.
| Study/Year/Country | Type of Study |
Patients | Objective | Outcomes | Results |
|---|---|---|---|---|---|
| Ball et al. (75) 2002 United States |
RCT Single blind 4 arms 2 year follow-up Intervention conducted in small group settings in ten 60–75 min sessions over 5–6 weeks. |
N=2,802 Patients had good cognitive/functional status at enrollment Mean age (SD) = 73.6 (5.9) Range 65–94 years Age groups: 65–74 years 60.1% 75–84 years 35.0% >85 years 4.9% |
To evaluate whether 3 cognitive training interventions (memory, speed of processing, reasoning) improve mental abilities and daily functioning in older independent living adults Training lasted 10 sessions for each group |
Basic measures of cognition and on measures of cognitively demanding daily activities (e.g., food preparation, driving, medication use, financial management) | Tests of cognitive abilities given immediately after training showed significant improvement on the particular cognitive skill on which the individual had been trained, but no transfer to the other 2 cognitive domains. No significant training effects on everyday function were detected at 2 years. |
| Willis et al. (76) 2006 United States |
RCT Single blind 4 arms 5-year follow-up |
67% completed 5 year follow-up | To determine effects of cognitive training on daily function and durability of training on cognitive abilities | Self-reported and performance based measures of daily function and cognitive abilities | Training on cognitive abilities showed significant improvement on particular cognitive skill on which person was trained. No transfer to other domains. Training on functional abilities (IADLs; everyday problem solving; everyday speed of processing). No significant differences in functional outcomes for memory or speed processing training. Reasoning significantly improved IADL, but not the other 2 abilities) |
IADL, instrumental activities of daily living; RCT, randomized controlled trial; SD, standard deviation.
Cognitive improvements were sustained after 5 years of follow-up, but none of these improvements had effects beyond the specific cognitive domains of the intervention (Table 33). (76)
Results addressing the investigators’ primary hypothesis (cognitive training would delay declines in functional status measured by self-reported IADL scores and performance assessments) were unclear.(76)
Only participants who underwent reasoning training (verbal episodic) self-reported significantly higher IADL functioning compared with the control group.
The remaining 2 intervention groups had higher, but nonsignificant, self-reported IADL scores than the control group.
None of the groups demonstrated significant improvements in the performance-based measures(everyday problem solving and everyday speed of processing).
Comments/Limitations Regarding the Randomized Controlled Trials
-
Primary outcome (functional activities) versus proximal outcome (cognitive abilities): (75;76)
Prior studies showed cognitive interventions improve cognitive abilities in normal seniors but have not included functional outcome measures and have been limited by small homogenous samples and lack of randomization.
Authors expected to see transfer of training effects to affect functioning (e.g., IADL). (75;76)
Vast majority of patients remained functionally independent over the course of the 24-month observation period.
Study was powered to show an effect size of 0.20 at 95% power with a sample of 2,832, which should have been sufficient power to detect a significant effect of the cognitive training on functional outcomes. (75)
-
Why no transfer to functional outcomes?
A proportion of patients were already functioning at ceiling levels (43% had no room for improvement) on the daily functional composite. (75)
Strong practice or retest effects in the control group. Approximately 25% of control patients showed reliable gain on cognitive and functional composites. (75)
The control group did not experience functional decline over the 2-year follow-up. (75)
Individuals with functional or cognitive decline were screened out of the study. Study focused on patients whose future decline rates were likely to mimic or be less than rates for the general elderly population.
Prior longitudinal research on cognitively demanding measures of everyday functioning indicates that age related decline occurs later for these tasks than for more basic abilities that were the focus of training. (76) Age-related decline on everyday problem-solving tasks shown not to occur until mid-seventies. Declines on basic abilities such as reasoning and memory typically occur in mid-sixties.
Since the patients were functionally independent at baseline, the authors hypothesized that observations of training effects on IADL functioning would be delayed until the control group began to experience significant functional decline (not stated in the original 2002 study). This was observed at the 5-year follow-up.
Full extent of daily function would take longer than 5 years to observe in a population that was highly functioning at enrollment.
No information about physical activity of patients.
Economic Analysis
No economic analyses were identified that examined the cost-effectiveness of cognitive training for the primary prevention of dementia.
Summary of Findings
As per the GRADE Working Group (52), the overall quality of the evidence is shown in Table 34.
Table 34: Quality of Trials According to GRADE.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Cognitive functioning and performance of ADL | RCT | Moderate | Not consistent (1 RCT) | No People with functional or cognitive decline were screened out along with people with medical conditions associated with “imminent functional decline or death.” |
Low |
ADL indicates activities of daily living; RCT, randomized controlled trial.
Ontario Health Systems Impact Analysis
Considerations and Implications
The full extent of daily function would take longer than 5 years to observe in a population that was highly functioning at enrollment as was the case with the study by Ball et al. and Willis et al. (75;76)
According to Ball et al. (75), the 3 training interventions (memory, reasoning, and speed of processing) were selected because they showed the most promise in smaller laboratory studies and had been related to IADL. It is unclear if these particular cognitive training exercises encapsulate cognitive measures of importance for clinical settings.
The cognitive training results are very specific to the skills that are trained. It is unknown whether there is any effect on when or whether an individual develops dementia. (74)
Benefit/Risk Analysis
As per the GRADE Working Group (52), the strength of a recommendation to use cognitive training as an intervention to reduce the risk of cognitive decline is shown in (Table 35).
Table 35: Overall Summary Statement of Benefit and Risk*.
| Outcome | Quality | Benefits | Risks/Burden | Overall Strength of Recommendation |
|---|---|---|---|---|
| Cognitive functioning and performance of ADL | Low | Cognitive improvements sustained after 5 years (however, none of these improvements had effects beyond the specific cognitive domains of the intervention) | Results addressing functional outcomes unclear Need more than 5-year follow-up No evidence to determine if cognitive training leads to:
|
Very low |
ADL indicates activities of daily living.
Conclusion
For seniors with good cognitive and physical functioning, there is:
evidence that cognitive training for specific functions (memory, reasoning, and speed of processing) produces improvements in these specific domains, and
limited inconclusive evidence that cognitive training can offset deterioration in the performance of self-reported IADL scores and performance assessments.
Overall Summary of Results for Patient-Directed Interventions for Dementia
Summary
Table 36 summarizes the conclusions from Sections 3.1 through 3.4.
Table 36: Overall Conclusions on Patient-Directed Initiatives.
| Intervention | Target Population |
1° or 2° Prevention | Conclusion | Overall Quality (GRADE) |
|---|---|---|---|---|
| Physical exercise | Seniors with mild to moderate dementia | 2° | Physical exercise is effective for improving physical functioning in patients with dementia. | Moderate |
| Physical exercise | Seniors with good cognitive functioning (no dementia) | 1° |
Long-term outcomes
|
Moderate |
Short-term outcomes
|
High/Moderate High/Moderate |
|||
| Nonpharmacol ogic and nonexercise interventions | Seniors with mild to moderate dementia | 2° | Previous systematic review indicated that “cognitive training” is not effective in patients with dementia. Recent RCT suggests CST (up to 7 weeks) is effective for improving cognitive function and quality of life in patients with dementia. |
Low |
| Nonpharmacol ogic and nonexercise interventions | Seniors with good cognitive functioning (no dementia) | 1° | For seniors with good cognitive and physical functioning:
|
Low |
CST indicates cognitive stimulation therapy; IADL, instrumental activities of daily living, RCT, randomized controlled trial.
Benefit/Risk Analysis
The last column in Table 37 is the overall trade-off between benefits and harms, and incorporates any risk/uncertainty.
Table 37: Overall Summary Statement of the Benefit and Risk for Patient-Directed Initiatives*.
| Intervention | Target Population | 1° or 2° Prevention | Overall Quality (GRADE) | Benefits | Risks/Burden | Overall Strength of Recommendation (GRADE) |
|---|---|---|---|---|---|---|
| Physical exercise | Seniors with mild to moderate dementia | 2° | Moderate | Improvement in functional, cognitive, and behavioural outcomes |
|
Moderate |
| Physical exercise | Seniors with good cognitive functioning (no dementia) | 1° | High/Moderate | Reduced risk of subsequent cognitive decline |
|
High/Moderate |
| Short-term Cognitive decline | ||||||
| Short-term Incidence of dementia | High/Moderate | Decreased incidence of dementia |
|
High/Moderate | ||
| Long-term Incidence of dementia | Moderate | Decreased incidence of dementia |
|
Moderate | ||
| Nonpharmacologic and nonexercise interventions | Seniors with mild to moderate dementia | 2° | Very low | None |
|
Very low |
| Cognitive training | ||||||
| Cognitive stimulation therapy (CST) | Moderate/Low | Increased cognition and quality of life |
|
Low | ||
| Nonpharmacologic and nonexercise interventions | Seniors with good cognitive functioning (no dementia) | 1° | Low | Cognitive improvements sustained after 5 years, but none of these improvements had effects beyond the specific cognitive domains of the intervention) |
|
Very low |
1° indicates primary;2°, secondary; CST, cognitive stimulation therapy; IADL, instrumental activities of daily living; RCT, randomized controlled trial.
4. Economic Analysis
Literature Review
No economic analyses were identified that examined the cost-effectiveness of exercise programs for seniors with dementia.
Ontario-Based Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for all in-hospital stay costs for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits for physician fees, laboratory fees from the Ontario Laboratory Schedule of Fees, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, a discount rate of 5% is used as per the Canadian Agency for Drugs and Technologies in Health.
Downstream costs: All costs reported are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature. In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Budget Impact Analysis of Effective Interventions for Dementia
Caregiver-directed behavioural techniques and patient-directed exercise programs were found to be effective when assessing mild to moderate dementia outcomes in seniors living in the community. Therefore, an annual budget impact was calculated based on eligible seniors in the community with mild and moderate dementia and their respective caregivers who were willing to participate in interventional home sessions. Table 38 describes the annual budget impact for these interventions.
Table 38: Annual Budget Impact (2008 Cdn Dollars).
| Parameter | Unit Cost ($ Cdn) | Unit | Annual Cost ($ Cdn) |
Population* | No. of Patients | Annual Impact ($ Cdn) |
|---|---|---|---|---|---|---|
| Caregiver-Directed Behavioural Techniques† | ||||||
| Occupational Therapist | 120.22 | 1 hour session - 12 total | 1,442.64 | Caregivers of seniors with mild to moderate dementia who are willing to participate | 56,629 | 81,695,125 |
| Nurse | 82.12 | 1 hour session - 12 total | 985.44 | Caregivers of seniors with mild to moderate dementia who are willing to participate | 56,629 | 55,804,389 |
| Patient-Directed Exercise Program‡ | ||||||
| Occupational Therapist | 120.22 | 1 hour session - 32 total | 3,847.04 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 148,866,672 |
| Physiotherapist | 108.49 | 1 hour session - 32 total | 3,471.68 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 134,341,585 |
| Personal Support Worker | 30.48 | 1 hour session - 32 total | 975.36 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 37,742,939 |
| Recreation Therapist | 25.85 | 1 hour session - 32 total | 827.20 | Seniors with mild to moderate dementia who are willing to participate | 38,696 | 32,009,678 |
| Caregiver- and Patient-Directed Behavioural Techniques§ | ||||||
| Occupational Therapist | 120.22 | 1 hour session - 10 total | 1,202.20 | Caregivers and seniors with mild to moderate dementia willing to participate | 56,629 | 68,079,271 |
| Nurse | 82.12 | 1 hour session - 10 total | 821.20 | Caregivers and seniors with mild to moderate dementia willing to participate | 56,629 | 46,503,658 |
Assumed 7% prevalence of dementia aged 65+ in Ontario. (Numbers in Ontario from Statistics Canada and prevalence of dementia from Alzheimer’s Disease International April 1999.) (42)
Assumed 8 weekly sessions plus 4 monthly phone calls. (77)
Assumed 12 weekly sessions plus biweekly sessions thereafter (total of 20).(51)
Assumed 2 sessions per week for first 5 weeks. (35) Assumed 90% of seniors in the community with dementia have mild to moderate disease. (78) Assumed 4.5% of seniors 65+ are in long-term care, and the remainder are in the community. (79) Assumed a rate of participation of 60% for both patients and caregivers (36) and of 41% for patient-directed exercise. (51) Assumed 100% compliance since intervention administered at the home. Cost for trained staff from Ministry of Health and Long-Term Care data source. (Personal communication, June 2008) Assumed cost of personal support worker to be equivalent to in-home support. Cost for recreation therapist from Alberta government Website. (80)
Note: This budget impact analysis was calculated for the first year after introducing the interventions from the Ministry of Health and Long-Term Care perspective using prevalence data only. Prevalence estimates are for seniors in the community with mild to moderate dementia and their respective caregivers who are willing to participate in an interventional session administered at the home setting. Incidence and mortality rates were not factored in. Current expenditures in the province are unknown and therefore were not included in the analysis. Numbers may change based on population trends, rate of intervention uptake, trends in current programs in place in the province, and assumptions on costs. The number of patients was based on patients likely to access these interventions in Ontario based on assumptions stated below from the literature. An expert panel confirmed resource consumption.
Assumptions
There were several assumptions made to calculate the annual budget impact:
Assumed 7% prevalence of dementia in 65+ seniors in Ontario. (81)
Assumed 90% of seniors in the community with dementia have mild to moderate disease. (78)
Assumed 4.5% of seniors 65+ are in LTC facilities – the remainder are in the community. (36)
Assumed a participation rate of 60% for both caregivers and patients. (36)
Assumed a participation rate of 41% for patient directed exercise.(51)
Assumed 100% compliance.
Assumed an occupational therapist hourly cost of $120.22, a physiotherapist hourly cost of $108.49, a nurse hourly cost of $82.12, and a personal support worker hourly cost of $30.48 from the Ministry of Health and Long-Term Care data source for homecare costs (Personal communication, June 2008) and an hourly cost for a recreation therapist of $25.85 from the government of Alberta. (80) Assumed 8 weekly sessions plus 4 monthly phone calls thereafter for caregiver directed behavioural techniques.(77)
Assumed 12 weekly sessions plus biweekly sessions thereafter (20 in total) for patient-directed exercise program. (51)
Assumed 2 sessions per week for the first 5 weeks for combination therapy. (35)
As a result of these assumptions and due to the limited data available in the literature, uncertainty becomes an issue; if/when new evidence is presented, these results may change and may better predict health outcomes over time allowing for a more accurate analysis.
Appendices
Appendix 1: Literature Search – Respite Care
Search date: January 3, 2008
Databases searched: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, INAHTA/CRD, CINAHL, Cochrane Library
Database: Ovid MEDLINE(R) <1996 to November Week 2 2007>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ or exp Memory Disorders/ or exp Cognition Disorders/ (68097)
(alzheimer$ or dementia$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (57020)
1 or 2 (81807)
exp Caregivers/ (9236)
exp Home Nursing/ (2604)
exp Day Care/ (1134)
exp Community Health Services/ or exp Social Support/ (181196)
(daycare$ or day care$ or respite or caregiver$ or care giver$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (19925)
or/4-8 (195118)
3 and 9 (5708)
limit 10 to (humans and english language and yr=”2005 - 2008”) (1530)
limit 11 to (controlled clinical trial or meta analysis or randomized controlled trial) (130)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (29765)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (56269)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (332772)
exp Double-Blind Method/ (48766)
exp Control Groups/ (503)
exp Placebos/ (8499)
RCT.mp. (2098)
or/12-19 (397969)
11 and 20 (241)
Database: EMBASE <1980 to 2008 Week 01>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (98803)
exp Memory Disorder/ (21803)
(alzheimer$ or dementia$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (91427)
or/1-3 (125576)
exp caregiver/ (12536)
exp Home Care/ (14957)
exp Day Care/ (2942)
exp Community Care/ (21986)
exp Social Support/ (14769)
exp Caregiver Support/ (181)
exp caregiver burden/ (442)
(daycare$ or day care$ or respite or caregiver$ or care giver$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (21809)
or/5-12 (68161)
4 and 13 (5360)
limit 14 to (human and english language and yr=”2005 - 2008”) (1308)
Randomized Controlled Trial/ (152795)
exp Randomization/ (24783)
exp RANDOM SAMPLE/ (903)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (276427)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (55130)
Double Blind Procedure/ (67702)
exp Triple Blind Procedure/ (8)
exp Control Group/ (1257)
exp PLACEBO/ (108318)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (395692)
or/16-25 (601733)
15 and 26 (305)
Database: CINAHL - Cumulative Index to Nursing & Allied Health Literature <1982 to December Week 1 2007>
Search Strategy:
--------------------------------------------------------------------------------
exp DEMENTIA/ (14991)
exp Memory Disorders/ (1529)
exp Cognition Disorders/ (4710)
1 or 2 or 3 (19857)
exp Caregivers/ (7601)
exp Caregiver Support/ (1551)
exp Caregiver Burden/ (3201)
exp Day Care/ or exp Respite Care/ (1722)
exp Home Nursing/ (1588)
exp Community Health Services/ (131553)
exp Support, Psychosocial/ (18842)
(daycare$ or day care$ or respite or caregiver$ or care giver$).mp. [mp=title, subject heading word, abstract, instrumentation] (18799)
or/5-12 (159864)
4 and 13 (4272)
limit 14 to (english and yr=“2005 - 2007”) (1253)
random$.mp. or exp RANDOM ASSIGNMENT/ or exp RANDOM SAMPLE/ (62969)
17 RCT.mp. (785)
exp Meta Analysis/ (5947)
exp “Systematic Review”/ (3456)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or medline or embase or data synthesis or data extraction or cochrane).mp. (20908)
exp double-blind studies/ or exp single-blind studies/ or exp triple-blind studies/ (11977)
exp PLACEBOS/ (3902)
exp Medical Practice, Evidence-Based/ (3919)
health technology assessment.mp. (345)
or/16-24 (85257)
15 and 25 (143)
Appendix 2: Literature Search – Caregiver Support
Search date: March 3, 2008
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library, INAHTA/CRD, PsycINFO
Database: Ovid MEDLINE(R) <1996 to February Week 3 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (48778)
exp Memory Disorders/ (8295)
(dementia or demented or alzheimer$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (58996)
or/1-3 (71134)
exp Caregivers/ (9550)
exp Spouses/ (3084)
exp Family/ (73561)
(carer$ or caregiv$ or care-giv$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (20224)
or/5-8 (88930)
4 and 9 (4208)
exp Self-Help Groups/ (3315)
exp Adaptation, Psychological/ (35597)
exp behavior therapy/ or cognitive therapy/ (15601)
exp Psychotherapy/ (37496)
exp Counseling/ (10892)
exp Problem Solving/ (7134)
exp Social Support/ (21185)
exp Intervention Studies/ (2987)
exp Home Nursing/ (2671)
exp Teaching/ (22494)
((caregiv$ or carer$ or spouse or spousal or psyhological or psychosocial or education$ or psychoeducational or program$) adj4 (support$ or intervenion$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (8243)
exp Stress, Psychological/th [Therapy] (1019)
exp Anxiety/th [Therapy] (871)
or/11-23 (133233)
10 and 24 (1297)
limit 25 to (english language and humans and yr=”2003 - 2008”) (549)
limit 26 to (controlled clinical trial or meta analysis or randomized controlled trial) (89)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (31137)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (58887)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (343549)
exp Double-Blind Method/ (50073)
exp Control Groups/ (528)
exp Placebos/ (8685)
RCT.mp. (2210)
or/27-34 (411588)
26 and 35 (126)
Database: EMBASE <1980 to 2008 Week 09>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (100182)
exp Memory Disorder/ (22176)
(dementia or demented or alzheimer$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (93182)
or/1-3 (127770)
exp Caregiver/ or exp Caregiver Burden/ (13106)
exp SPOUSE/ (3204)
exp FAMILY/ (96271)
(carer$ or caregiv$ or care-giv$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (20420)
or/5-8 (111876)
4 and 9 (5928)
exp Self Help/ (2994)
exp Coping Behavior/ (18583)
exp Stress Management/ (253)
exp Behavior Modification/ or exp Behavior Therapy/ (24577)
exp PSYCHOTHERAPY/ or exp Distress Syndrome/th [Therapy] (73612)
exp counseling/ or exp Problem Solving/ (51165)
exp Social Support/ or exp Adaptation/ or exp Adaptive Behavior/ (54601)
exp Education Program/ or exp Intervention Study/ (25232)
exp Support Group/ (3626)
exp Caregiver Support/ or exp Home Care/ (15348)
exp Teaching/ (11165)
exp home mental health care/ or exp psychosocial care/ (5265)
((caregiv$ or care-giv$ or carer$ or spouse or spousal or psyhological or psychosocial or education$ or psychoeducational or program$) adj4 (support$ or intervenion$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (9243)
exp Stress Management/ (253)
exp ANXIETY/th [Therapy] (2)
exp Behavior Disorder/th [Therapy] (9429)
or/11-26 (240510)
10 and 27 (1855)
Randomized Controlled Trial/ (154967)
exp Randomization/ (25139)
exp RANDOM SAMPLE/ (990)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (280024)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (56485)
Double Blind Procedure/ (68397)
exp Triple Blind Procedure/ (8)
exp Control Group/ (1462)
exp PLACEBO/ (110517)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (401372)
or/29-38 (610648)
28 and 39 (343)
limit 40 to (human and english language and yr=”2003 - 2008”) (219)
Database: CINAHL - Cumulative Index to Nursing & Allied Health Literature <1982 to February Week 4 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (15489)
exp Memory Disorders/ (1631)
(dementia or demented or alzheimer$).mp. [mp=title, subject heading word, abstract, instrumentation] (16853)
or/1-3 (18563)
exp Caregivers/ (7792)
exp Caregiver Burden/ (3223)
exp Spouses/ (2754)
exp FAMILY/ (54157)
(carer$ or caregiv$ or care-giv$).mp. [mp=title, subject heading word, abstract, instrumentation](19962)
or/5-9 (68755)
4 and 10 (3862)
exp Support Groups/ (4075)
exp ADAPTATION, PSYCHOLOGICAL/ (7260)
exp Psychotherapy/ (44148)
exp Counseling/ (8177)
exp Learning/ (18675)
exp Support, Psychosocial/ (19251)
exp Caregiver Support/ (1562)
exp Home Nursing/ (1602)
((caregiv$ or carer$ or spouse or spousal or psyhological or psychosocial or education$ or psychoeducational or program$) adj4 (support$ or intervenion$)).mp. [mp=title, subject heading word, abstract, instrumentation] (27902)
Stress, Psychological/th [Therapy] (269)
exp Stress Management/ (2995)
exp Role Stress/th [Therapy] (1)
exp ANXIETY/th [Therapy] (532)
exp Coping/ (11410)
exp Behavior Modification/ (10092)
exp Problem Solving/ (3026)
or/12-27 (90851)
11 and 28 (1485)
limit 29 to (english and yr=”2003 - 2008”) (589)
random$.mp. or exp RANDOM ASSIGNMENT/ or exp RANDOM SAMPLE/ (65853)
RCT.mp. (826)
exp Meta Analysis/ (6098)
exp “Systematic Review”/ (3495)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or medline or embase or data synthesis or data extraction or cochrane).mp. (21778)
exp double-blind studies/ or exp single-blind studies/ or exp triple-blind studies/ (12919)
exp PLACEBOS/ (4067)
or/31-37 (86049)
30 and 38 (71)
Appendix 3: Literature Search – Exercise Therapy
Search date: May 13, 2008
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library, CINAHL; INAHTA/CRD
Database: Ovid MEDLINE(R) <1996 to April Week 5 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (49790)
exp Cognition Disorders/ (24181)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (60527)
or/1-3 (81338)
exp Exercise/ or exercise$.mp. or physical activit$.mp. or walk$.mp. or run$.mp. or yoga.mp. or tai chi.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (154985)
exp Physical Fitness/ or exp Motor Activity/ (45134)
5 or 6 (178167)
4 and 7 (1946)
limit 8 to (english language and humans and yr=”2003 - 2008”) (801)
limit 9 to (controlled clinical trial or meta analysis or randomized controlled trial) (75)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (32095)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (60836)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (352050)
exp Double-Blind Method/ (51030)
exp Control Groups/ (566)
exp Placebos/ (8862)
RCT.mp. (2313)
or/10-17 (422207)
9 and 18 (141)
Database: CINAHL - Cumulative Index to Nursing & Allied Health Literature <1982 to May Week 2 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (16608)
exp Cognition Disorders/ (5630)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, subject heading word, abstract, instrumentation] (18112)
or/1-3 (22838)
exp Exercise/ (26885)
exp Therapeutic Exercise/ (15906)
exp Physical Activity/ (7225)
exp Physical Fitness/ (4333)
(exercise$ or physical activit$ or walk$ or run$ or yoga or tai chi).mp. [mp=title, subject heading word, abstract, instrumentation] (68340)
or/5-9 (72103)
4 and 10 (802)
random$.mp. or exp RANDOM ASSIGNMENT/ or exp RANDOM SAMPLE/ (71326)
RCT.mp. (902)
exp Meta Analysis/ (6487)
exp “Systematic Review”/ (3681)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or medline or embase or data synthesis or data extraction or cochrane).mp. (23706)
exp double-blind studies/ or exp single-blind studies/ or exp triple-blind studies/ (14310)
exp PLACEBOS/ (4394)
exp “Control (Research)”/ (2347)
or/12-18 (93423)
11 and 20 (127)
limit 21 to (english and yr=”2003 - 2008”) (83)
Database: EMBASE <1980 to 2008 Week 19>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (101554)
exp Cognitive Defect/ (35519)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (94770)
or/1-3 (135596)
exp exercise/ or exp physical activity/ (159534)
exp kinesiotherapy/ (16933)
exp Fitness/ (9604)
exp Exercise/ or exercise$.mp. or physical activit$.mp. or walk$.mp. or run$.mp. or yoga.mp. or tai chi.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (265529)
or/5-8 (304796)
4 and 9 (3428)
limit 10 to (human and english language and yr=”2003 - 2008”) (1657)
Randomized Controlled Trial/ (157352)
exp Randomization/ (25458)
exp RANDOM SAMPLE/ (1083)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (283949)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (57934)
Double Blind Procedure/ (69149)
exp Triple Blind Procedure/ (10)
exp Control Group/ (1707)
exp PLACEBO/ (112938)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (407342)
or/12-21 (620138)
11 and 22 (406)
Appendix 4: Literature Search – Cognitive Training
Search date: December 29, 2007
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library
Database: Ovid MEDLINE(R) <1996 to November Week 2 2007>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ or exp Memory Disorders/ or exp Cognition Disorders/ (68097)
(alzheimer$ or dementia$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (57020)
1 or 2 (81807)
exp Cognitive Therapy/ (6287)
((cognitive or cognition or memory or reality) adj2 (therap$ or rehabilit$ or train$ or retrain$ or re-train$ or support$ or aid$ or stimulation or remediat$ or management or group$ or strateg$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (10495)
exp Reality Therapy/ (42)
(Reality Orientation or Reminiscence Therap$ or Validation Therap$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (92)
or/4-7 (10549)
3 and 8 (1575)
limit 9 to (humans and english language and yr=”2006 - 2008”) (361)
limit 10 to (controlled clinical trial or meta analysis or randomized controlled trial) (72)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (29765)
(meta analy$ or metaanaly$ or evidence-based medicine or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (80013)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (332772)
exp Double-Blind Method/ (48766)
exp Control Groups/ (503)
exp Placebos/ (8499)
RCT.mp. (2098)
or/11-18 (398704)
10 and 19 (117)
Database: EMBASE <1980 to 2007 Week 52>
Search Strategy:
--------------------------------------------------------------------------------
exp DEMENTIA/ (98673)
exp Memory Disorder/ (21760)
(alzheimer$ or dementia$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (91290)
or/1-3 (125391)
exp cognitive rehabilitation/ or exp cognitive therapy/ (13757)
((cognitive or cognition or memory or reality) adj2 (therap$ or rehabilit$ or train$ or retrain$ or re-train$ or support$ or aid$ or stimulation or remediat$ or management or group$ or strateg$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (23115)
(Reality Orientation or Reminiscence Therap$ or Validation Therap$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (161)
or/5-7 (23200)
4 and 8 (2261)
limit 9 to (human and english language and yr=”2006 - 2008”) (417)
exp Evidence Based Medicine/ (271508)
exp Biomedical Technology Assessment/ (5095)
Randomized Controlled Trial/ (152628)
exp Randomization/ (24752)
exp RANDOM SAMPLE/ (900)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (55006)
Double Blind Procedure/ (67654)
exp Triple Blind Procedure/ (8)
exp Control Group/ (1228)
exp PLACEBO/ (108111)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (395206)
or/11-21 (600930)
10 and 22 (143)
Database: CINAHL - Cumulative Index to Nursing & Allied Health Literature <1982 to December Week 1 2007>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (14991)
exp Memory Disorders/ (1529)
exp Cognition Disorders/ (4710)
(alzheimer$ or dementia$).mp. [mp=title, subject heading word, abstract, instrumentation](16201)
or/1-4 (21238)
exp Cognitive Therapy/ (3071)
exp Rehabilitation, Cognitive/ (595)
((cognitive or cognition or memory or reality) adj2 (therap$ or rehabilit$ or train$ or retrain$ or re-train$ or support$ or aid$ or stimulation or remediat$ or management or group$ or strateg$)).mp. [mp=title, subject heading word, abstract, instrumentation] (7659)
exp Reality Therapy/ (146)
(Reality Orientation or Reminiscence Therap$ or Validation Therap$).mp. [mp=title, subject heading word, abstract, instrumentation] (955)
or/6-10 (8448)
5 and 11 (1706)
limit 12 to (english and yr=”2006 - 2007”) (387)
exp Medical Practice, Evidence-Based/ (3919)
random$.mp. or exp RANDOM ASSIGNMENT/ or exp RANDOM SAMPLE/ (62969)
RCT.mp. (785)
exp Meta Analysis/ (5947)
exp “Systematic Review”/ (3456)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or medline or embase or data synthesis or data extraction or cochrane).mp. (20908)
exp double-blind studies/ or exp single-blind studies/ or exp triple-blind studies/ (11977)
exp PLACEBOS/ (3902)
or/14-21 (84982)
13 and 22 (108)
Appendix 5: Literature Search – Exercise for Prevention of Dementia
Search date: April 17, 2008
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Cochrane Library, INAHTA/CRD
Database: Ovid MEDLINE(R) <1996 to April Week 2 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (49503)
exp Cognition Disorders/ (23964)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (60160)
or/1-3 (80799)
exp Exercise/ or exercise$.mp. or physical activit$.mp. or walk$.mp. or run$.mp. or yoga.mp. or tai chi.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (153868)
exp Physical Fitness/ or exp Motor Activity/ (44688)
5 or 6 (176830)
4 and 7 (1931)
(prevent$ or delay$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (474740)
exp Primary Prevention/ (35366)
exp “Age of Onset”/ (15469)
or/9-11 (512070)
8 and 12 (364)
limit 13 to (english language and humans and yr=”2003 - 2008”) (149)
limit 14 to (controlled clinical trial or meta analysis or randomized controlled trial) (16)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (31803)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (60173)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (349434)
exp Double-Blind Method/ (50775)
exp Control Groups/ (559)
exp Placebos/ (8816)
RCT.mp. (2278)
or/15-22 (418963)
14 and 23 (33)
Database: EMBASE <1980 to 2008 Week 15>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (100981)
exp Cognitive Defect/ (35158)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (94234)
or/1-3 (134687)
exp exercise/ or exp physical activity/ (158574)
exp kinesiotherapy/ (16787)
exp Fitness/ (9568)
exp Exercise/ or exercise$.mp. or physical activit$.mp. or walk$.mp. or run$.mp. or yoga.mp. or tai chi.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (264114)
or/5-8 (303183)
4 and 9 (3390)
exp PREVENTION/ (453479)
exp Onset Age/ (25408)
(prevent$ or delay$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (765936)
or/11-13 (1039251)
10 and 14 (722)
limit 15 to (human and english language and yr=”1998 - 2008”) (496)
Randomized Controlled Trial/ (156348)
exp Randomization/ (25316)
exp RANDOM SAMPLE/ (1047)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (282291)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (57322)
Double Blind Procedure/ (68835)
exp Triple Blind Procedure/ (8)
exp Control Group/ (1607)
exp PLACEBO/ (111912)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (404873)
or/17-26 (616167)
16 and 27 (159)
Database: CINAHL - Cumulative Index to Nursing & Allied Health Literature <1982 to April Week 2 2008>
Search Strategy:
--------------------------------------------------------------------------------
exp Dementia/ (16322)
exp Cognition Disorders/ (5514)
(dement$ or alzheimer$ or predementia$ or pre-dementia$).mp. [mp=title, subject heading word, abstract, instrumentation] (17805)
or/1-3 (22441)
exp Exercise/ (26476)
exp Therapeutic Exercise/ (15752)
exp Physical Activity/ (7131)
exp Physical Fitness/ (4244)
(exercise$ or physical activit$ or walk$ or run$ or yoga or tai chi).mp. [mp=title, subject heading word, abstract, instrumentation] (67343)
or/5-9 (71058)
4 and 10 (784)
exp “Age of Onset”/ (1928)
(prevent$ or delay$).mp. [mp=title, subject heading word, abstract, instrumentation] (89830)
12 or 13 (91551)
11 and 14 (91)
random$.mp. or exp RANDOM ASSIGNMENT/ or exp RANDOM SAMPLE/ (70195)
RCT.mp. (880)
exp Meta Analysis/ (6389)
exp “Systematic Review”/ (3631)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or medline or embase or data synthesis or data extraction or cochrane).mp. (23203)
exp double-blind studies/ or exp single-blind studies/ or exp triple-blind studies/ (14096)
exp PLACEBOS/ (4281)
exp “Control (Research)”/ (2308)
or/16-22 (91868)
15 and 24 (21)
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ontario Health Technology Assessment Series 2008;8(4).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-7289-0 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables
| Executive Summary Table 1: Summary of Conclusions on Caregiver-Directed Interventions |
| Executive Summary Table 2: Summary of Conclusions on Patient-Directed Interventions* |
| Executive Summary Table 3: Overall Summary Statement of the Benefit and Risk for Patient-Directed Interventions* |
| Executive Summary Table 4: Annual Budget Impact (2008 Canadian Dollars) |
| Table 1: Canada Dementia Prevalence by 5-Year Age Groups (1994) |
| Table 2: Total Net Costs of Dementia in Canada From the Canadian Study on Health Aging (15) |
| Table 3: Numbers of Studies According to Research Design and Type of Respite Care and Short-Term Break for Carers for People with Dementia* (n=51)† |
| Table 4: Summary of Key Characteristics of Studies Examined in the Cochrane Review of Respite Care |
| Table 5: Summary of Systematic Reviews and Meta-Analyses on Dementia Caregiver Interventions* |
| Table 6: Summary of Randomized Controlled Trial by Graff et al. 2006, 2007 (35;36) |
| Table 7: Outcomes in Patients with Dementia and Caregivers in Intention-to-Treat Population at 6- and 12-week Time Points* |
| Table 8: Additional Caregiver Outcomes at 6 Weeks* |
| Table 9: Additional Caregiver Outcomes at 12 Weeks* |
| Table 10: Summary of Randomized Controlled Trial by Teri et al., 2005 (37)* |
| Table 11: Summary of Mahoney et al., 2003 (38)* |
| Table 12: Summary of Burgio et al. 2003 (39)* |
| Table 13: Quality of Individual Behavioural Intervention Trials According to GRADE* |
| Table 14: Quality of Individual Behavioural Intervention Trials According to GRADE* |
| Table 15: Summary of Belle et al. 2006 (40)* |
| Table 16: Clinical Depression of Caregivers and Institutional Placement of Care Recipients at 6-Month Follow-Up* |
| Table 17: Quality of Multicomponent Intervention Trials According to GRADE* |
| Table 18: Summary of Systematic Reviews for Physical Activity in Seniors with Dementia* |
| Table 19: Quality of Trials According to GRADE |
| Table 20: Overall Summary Statement of the Benefit and Risk |
| Table 21: Quality of Evidence of Included Studies* |
| Table 22: Summary of Systematic Reviews for Nonpharmacologic Interventions to Improve Cognitive Functioning in Seniors With Mild to moderate Dementia* |
| Table 23: Results of the Randomized Controlled Trial by Spector et al. (57)* |
| Table 24: Quality of Trials According to GRADE* |
| Table 25: Overall Summary Statement of Benefit and Risk |
| Table 26: Quality of Evidence of Included Studies* |
| Table 27: Summary of Observational Studies That Examine the Effect of Exercise on Cognitive Decline* |
| Table 28: Summary of Observational Studies That Examine the Effect of Exercise on the Incidence of Dementia |
| Table 29: Results from Roivio et al. (68) |
| Table 30: Quality of Trials According to GRADE |
| Table 31: Overall Summary Statement of Benefit and Risk |
| Table 32: Quality of Evidence |
| Table 33: Summary of the 2 Randomized Controlled Trials |
| Table 34: Quality of Trials According to GRADE |
| Table 35: Overall Summary Statement of Benefit and Risk* |
| Table 36: Overall Conclusions on Patient-Directed Initiatives |
| Table 37: Overall Summary Statement of the Benefit and Risk for Patient-Directed Initiatives*. |
| Table 38: Annual Budget Impact (2008 Cdn Dollars) |
List of Figures
Abbreviations
- ADL
Activities of daily living
- AMPS
Assessment of motor and process skills
- BPSD
Behavioural and psychological symptoms of dementia
- CCAC
Community Care Access Centres
- CI
Confidence interval
- CST
Cognitive stimulation therapy
- HTA
Health technology assessment
- IADL
Instrumental activities of daily living
- INAHTA/CRD
International Agency for Health Technology Assessment / Centre for Reviews and Dissemination
- IVR
Interactive voice response
- LTC
Long-term care
- NCCDSO
National Co-ordinating Centre for National Health Service Service Delivery and Organisation Research and Development
- NHS
National Health Service
- NSAID
Nonsteroidal anti-inflammatory drug
- OT
Occupational therapy
- PSW
Personal support worker
- QOL
Quality of life
- RCT
Randomized controlled trial
- RH
Relative hazard
- SF-36
Short Form 36 Health Survey Questionnaire
Footnotes
Activities of Daily Living (ADL) are basic but important general tasks required for day to day living such as bathing, dressing, grooming, eating, and toileting. Instrumental Activities of Daily Living (IADL) are activities that need to be done but on a less time sensitive schedule. These are activities related to independent living and include preparing meals, managing money, shopping, doing housework, and using a telephone.
Secondary prevention covers all activities to take care of early symptoms of a disease and to preclude the development of possible irreparable medical conditions.
Primary prevention covers all activities designed to preclude the development of a disease.
Activities of Daily Living (ADL) are basic but important general tasks required for day to day living such as bathing, dressing, grooming, eating, and toileting. Instrumental Activities of Daily Living (IADL) are activities that need to be done but on a less time sensitive schedule. These are activities related to independent living and include preparing meals, managing money, shopping, doing housework, and using a telephone.
including respite interventions
References
- 1.Mental health and substance abuse: facts and figures a[Internet] [[updated 2008; cited 2008 Jun 7]];World Health Organization. Available from: http://www.searo.who.int/en/Section1174/Section1199/Section1567/Section1823_8057.htm .
- 2.Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–25. doi: 10.1111/j.1532-5415.2000.tb04998.x. [DOI] [PubMed] [Google Scholar]
- 3.Caron CD, Ducharme F, Griffith J. Deciding on Institutionalization for a relative with dementia: the most difficult decision for caregivers. Can J Aging. 2006;25(2):193–205. doi: 10.1353/cja.2006.0033. [DOI] [PubMed] [Google Scholar]
- 4.Banaszak-Holl J, Fendrick MA, Foster NL. Cognitive function and chronic medical conditions as predictors of nursing home admission in a nationally representative sample of older Americans. Alzheimer Dis Assoc Disord. 2004;18(2):83–9. doi: 10.1097/01.wad.0000126619.80941.91. [DOI] [PubMed] [Google Scholar]
- 5.Hebert R, Dubois M-F, Wolfson C, Chambers L, Cohen C. Factors associated with long-term insitutionalization of older people with dementia: data from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2001;56(11):M693–M699. doi: 10.1093/gerona/56.11.m693. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins RW, Hopkins JF. Dementia projections for the counties, regional municipalities and districts of Ontario. PCCC Mental Health Services, Kingston, Ontario: Geriatric Psychiatry Programme Clinical/Research Bulletin No. 15. 2005 [Google Scholar]
- 8.People with alzheimer’s disease and related dementias. [[updated 2007 Nov 23; cited 2008 Jun 1]];Alzheimer’s Disease International. Available from: http://www.alzheimer.ca/english/disease/stats-people.htm .
- 9.Vasular dementia Alzheimer’s Disease International. [[updated 2007 Nov 23; cited 2008 Jun 1]]; Available from: http://www.alz.co.uk/alzheimers/vascular.html .
- 10.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;(163):2219–29. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 11.Dementia: hope through research. National Institute of Neurological Disorders and. [[updated 2008 Jun 20; cited 2008 Jun 1]];Stroke. Available from: http://www.ninds.nih.gov/disorders/dementias/detail_dementia.htm#1032919213 .
- 12.Verghese J. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 13.Mukamal K.J. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289(11):1405–13. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 14.Ruitenberg A. Alcohol consumption and risk of dementia: the Rotterdam study. Lancet. 2002;359(9303):281–6. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 15.Ostbye T, Crosse E. Net economic costs of dementia in Canada. Can Med Assoc J. 1994;151(10):1457–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Give me a break! Helping family caregivers of seniors overcome barriers to respite. Ottawa: Canadian Association for Community Care. 2002 [Google Scholar]
- 17.Hollander MJ, Walker ER. Report of continuing care organizations and terminology [Internet] [[updated 1998; cited 2008 Jun 1]]; Available from: http://dsp-psd.pwgsc.gc.ca/Collection/H88-3-23-1999E.pdf .
- 18.The Canadian Study of Health and Aging Working Group. Patterns of caring for people with dementia in Canada. Can J Aging. 1994;13(4):470–87. [Google Scholar]
- 19.Rudin D.J. Caregiver attitudes regarding utilisation and usefulness of respite services for peoploe with Alzheimer’s disease. J Gerontol Soc Work. 1994;23(1/2):85–107. [Google Scholar]
- 20.Dunbrack J. Respite for family caregivers: an environmental scan of publicly-funded programs in Canada [Internet] [[updated 2003; cited 2008 Jun 1]]; Available from: http://www.hc-sc.gc.ca/hcssss/alt_formats/hpb-dgps/pdf/pubs/2003-respite-releve/2003-respite-releve-eng.pdf .
- 21.Arskey H, Jackson K, Croucher K, Weatherly H, Golder S, Hare P, et al. Review of respite services and short-term breaks for carers for people with dementia [Internet] [[updated 2004 Jan 7; cited 2008 May 5]];National Coordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO) Available from: http://www.sdo.nihr.ac.uk/files/project/48-final-report.pdf .
- 22.Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database of Systematic Reviews. 2004;(2) doi: 10.1002/14651858.CD004396.pub2. Art. No.: CD004396. DOI:10.1002/14651858.CD004396.pub2:CD004396. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM, Saperstein AR. Respite for caregivers of Alzheimer patients: research and practice. New York: Springer Publishing Company. 1991 [Google Scholar]
- 24.Mohide EA, Pringle DM, Streiner DL, Gilbert JR, Muir G, Tew M. A randomized trial of family caregiver support in the home management of dementia. J Am Geriatr Soc. 1990;38(4):446–54. doi: 10.1111/j.1532-5415.1990.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 25.Chu P, Edwards J, Levin R, Thomson J. The use of clinical case management for early stage Alzheimer’s patients and their families. Am J Alzheimers Dis Other Demen. 2008;15(5):284–90. [Google Scholar]
- 26.Grant I, McKibbin CL, Taylor MJ, Mills P, Dimsdale J, Ziegler M, et al. In-home respite intervention reduces plasma epinephrine in stressed Alzheimer caregivers. Am J Geriatr Psychiatry. 2003;11(1):62–72. [PubMed] [Google Scholar]
- 27.Wishart L, Macerollo J, Loney P, King A, Beaumont L, Browne G, et al. “Special steps”: an effective visiting/walking program for persons with cognitive impairment. Can J Nurs Res. 2000;31(4):57–71. [PubMed] [Google Scholar]
- 28.Peacock SC, Forbes DA. Interventions for caregivers of persons with dementia: a systematic review. Can J Nurs Res. 2003;35(4):88–107. [PubMed] [Google Scholar]
- 29.Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51(5):657–64. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 30.Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr. 2007;7(18) doi: 10.1186/1471-2318-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acton GJ, Kang J. Interventions to reduce the burden of caregiving for an adult with dementia: a meta-analysis. Res Nurs Health. 2001;24(5):349–60. doi: 10.1002/nur.1036. [DOI] [PubMed] [Google Scholar]
- 32.Pusey H, Richards D. A systematic review of the effectiveness of psychosocial interventions for carers of people with dementia. Aging Ment Health. 2001;5(2):107–19. doi: 10.1080/13607860120038302. [DOI] [PubMed] [Google Scholar]
- 33.Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577–95. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]
- 34.Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord. 2007;101(1-3):75–89. doi: 10.1016/j.jad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ. 2006;333(7580) doi: 10.1136/bmj.39001.688843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Olderikkert MG. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(9):1002–9. doi: 10.1093/gerona/62.9.1002. [DOI] [PubMed] [Google Scholar]
- 37.Teri L, Huda P, Gibbons L, Young H, van LJ. STAR: a dementia-specific training program for staff in assisted living residences. Gerontologist. 2005;45(5):686–93. doi: 10.1093/geront/45.5.686. [DOI] [PubMed] [Google Scholar]
- 38.Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist. 2003;43(4):556–67. doi: 10.1093/geront/43.4.556. [DOI] [PubMed] [Google Scholar]
- 39.Burgio L, Stevens A, Guy D, Roth DL, Haley WE. Impact of two psychosocial interventions on white and African American family caregivers of individuals with dementia. Gerontologist. 2003;43(4):568–79. doi: 10.1093/geront/43.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(10):727–38. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small GN, Rabins PC, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimers Association and the American Geriatrics Society. JAMA. 1997;278(16):1363–71. [PubMed] [Google Scholar]
- 42.Wolf-Klein BP, Silverstone FA. Weight loss in Alzheimer’s disease: an international review of the literature. Int Psychogeriatr. 1994;6(2):135–42. doi: 10.1017/s1041610294001705. [DOI] [PubMed] [Google Scholar]
- 43.Buchner DM, Larson EB. Falls and fractures in patients with Alzheimer-type dementia. JAMA. 1987;257(11):1492–5. [PubMed] [Google Scholar]
- 44.Morris JC, Rubin EH, Morris EJ, Mandel SA. Senile dementia of the Alzheimer’s type: an important risk factor for serious falls. J Gerontol. 1987;42(4):412–7. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- 45.Oleske DM, Wilson RS, Bernard BA, Evans DA, Terman EW. Epidemiology of injury in people with Alzheimer’s disease. J Am Geriatr Soc. 1995;43(7):741–6. doi: 10.1111/j.1532-5415.1995.tb07042.x. [DOI] [PubMed] [Google Scholar]
- 46.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43(11):1214–21. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 47.Krenz C, Larson EB, Buchner DM, Canfield CG. Characterizing patient dysfunction in Alzheimer’s - type dementia. Med Care. 1988;26(5):453–61. doi: 10.1097/00005650-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101–M107. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 49.Dvorak RV, Poehlman ET. Appendicular skeletal muscle mass, physical activity and cogntive status in patients with Alzheimer’s disease. Neurology. 1998;51(5):1386–90. doi: 10.1212/wnl.51.5.1386. [DOI] [PubMed] [Google Scholar]
- 50.Miller PA, Butin D. The role of occupational therapy in dementia - COPE (Caregiver Options for Practical Experiences) Int J Geriatr Psychiatry. 2000;15(1):86–9. doi: 10.1002/(sici)1099-1166(200001)15:1<86::aid-gps124>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–22. doi: 10.1001/jama.290.15.2015. [see comment] [DOI] [PubMed] [Google Scholar]
- 52.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Law M, Stewart D, Letts L, Pollock N, Westmorland M, Bosch J, et al. Effectiveness of activity programmes for older persons etih dementia. [[updated 1999; cited 2008 May 4]];A critical review of the literature by the Occupational Therapy Evidence Based Practice Research Group, McMaster University, Hamilton, Ontario [Internet] Available from: http://www.srsmcmaster.ca/Portals/20/pdf/ebp/activity.pdf .
- 55.Clare L, Woods RT. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD003260. Art. No.: CD003260. DOI: 10.1002/14651858.CD003260. [DOI] [PubMed] [Google Scholar]
- 56.Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist. 2000;40(2):206–12. doi: 10.1093/geront/40.2.206. [DOI] [PubMed] [Google Scholar]
- 57.Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;(183):248–54. doi: 10.1192/bjp.183.3.248. [DOI] [PubMed] [Google Scholar]
- 58.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: The Swedish Council on Technology Assessment in Health Care. 1993 doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 59.Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry. 2006;(188):574–80. doi: 10.1192/bjp.bp.105.010561. [DOI] [PubMed] [Google Scholar]
- 60.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal population based study. BMJ. 2001;322(7300):1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1(1):61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 62.Paffenbarger RS Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 63.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38(2):123–8. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 64.Lytle ME, Bilt JV, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18(2):57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 65.Larson EB, Wang L, Bowen JD, McCormick WCc, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 66.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447–53. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 67.Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical activity including walking and cognitive function in older women. JAMA. 2004;292(12):1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 68.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 69.Munro JF, Nicholl JP, Brazier JE, Davey R, Cochrane T. Cost effectiveness of a community based exercise programme in over 65 year olds: cluster randomized trial. J Epidemiol Community Health. 2004;58(12):1004–10. doi: 10.1136/jech.2003.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community dwelling older individuals. Appl Neuropsychol. 2002;9(3):187–91. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 71.Shumaker SA, Legault C, Coker LH. Behavior based interventions to enhance cognitive functioning and independence in older adults. JAMA. 2006;296(23):2852–4. doi: 10.1001/jama.296.23.2852. [DOI] [PubMed] [Google Scholar]
- 72.Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, et al. Prevalence of Alzheimer’s disease and vascular dementia: association with education. BMJ. 1995;310(6985):970–3. doi: 10.1136/bmj.310.6985.970. The Rotterdam Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ott A, van Rossum CT, van Harskamp F, van de Mheen H, Hofman A, Breteler MM. Education and the incidence of dementia in a large population based study: The Rotterdam Study. Neurology. 1999;52(3):663–6. doi: 10.1212/wnl.52.3.663. [DOI] [PubMed] [Google Scholar]
- 74.Gatz M. Educating the brain to avoid dementia: Can mental exercise prevent Alzheimer disease? PLoS Med. 2005;2(1) doi: 10.1371/journal.pmed.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–14. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: a randomized controlled trial. Gerontologist. 2005;45(6):802–11. doi: 10.1093/geront/45.6.802. [DOI] [PubMed] [Google Scholar]
- 78.Canadian study of health and aging: study methods and prevalence of dementia. CMAJ. 1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- 79.Information Services Group. Long-term care home system report as of March 31, 2007. Toronto, ON: Ministry of Health and Long-Term Care; Long-Term Care Planning and Renewal Branch. 2007 [Google Scholar]
- 80.Alberta Learning Information Service. Alberta occupational profiles [Internet] [[updated 2008; cited 2008 Oct 6]];Alberta Government. Available from: http://www.alis.gov.ab.ca/occinfo/Content/RequestAction.asp?format=html&aspAction=GetTitleSearch&Page=TitleSearch .
- 81.The prevelance of dementia. Factsheet 3 [Internet] [[updated 1999 Apr; cited 2008 Apr 4]];London, UK: Alzheimer’s Disease International. Available from: http://www.alz.co.uk/adi/pdf/3preval.pdf .


