Executive Summary
In February 2010, the Medical Advisory Secretariat (MAS) began work on evidence-based reviews of the literature surrounding three pharmacogenomic tests. This project came about when Cancer Care Ontario (CCO) asked MAS to provide evidence-based analyses on the effectiveness and cost-effectiveness of three oncology pharmacogenomic tests currently in use in Ontario.
Evidence-based analyses have been prepared for each of these technologies. These have been completed in conjunction with internal and external stakeholders, including a Provincial Expert Panel on Pharmacogenetics (PEPP). Within the PEPP, subgroup committees were developed for each disease area. For each technology, an economic analysis was also completed by the Toronto Health Economics and Technology Assessment Collaborative (THETA) and is summarized within the reports.
The following reports can be publicly accessed at the MAS website at: http://www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Gene Expression Profiling for Guiding Adjuvant Chemotherapy Decisions in Women with Early Breast Cancer: An Evidence-Based Analysis
Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: an Evidence-Based Analysis
K-RAS testing in Treatment Decisions for Advanced Colorectal Cancer: an Evidence-Based Analysis
Objective
The Medical Advisory Secretariat undertook a systematic review of the evidence on the clinical effectiveness and cost-effectiveness of epidermal growth factor receptor (EGFR) mutation testing compared with no EGFR mutation testing to predict response to tyrosine kinase inhibitors (TKIs), gefitinib (Iressa®) or erlotinib (Tarceva®) in patients with advanced non-small cell lung cancer (NSCLC).
Clinical Need: Target Population and Condition
With an estimated 7,800 new cases and 7,000 deaths last year, lung cancer is the leading cause of cancer deaths in Ontario. Those with unresectable or advanced disease are commonly treated with concurrent chemoradiation or platinum-based combination chemotherapy. Although response rates to cytotoxic chemotherapy for advanced NSCLC are approximately 30 to 40%, all patients eventually develop resistance and have a median survival of only 8 to 10 months. Treatment for refractory or relapsed disease includes single-agent treatment with docetaxel, pemetrexed or EGFR-targeting TKIs (gefitinib, erlotinib). TKIs disrupt EGFR signaling by competing with adenosine triphosphate (ATP) for the binding sites at the tyrosine kinase (TK) domain, thus inhibiting the phosphorylation and activation of EGFRs and the downstream signaling network. Gefitinib and erlotinib have been shown to be either non-inferior or superior to chemotherapy in the first- or second-line setting (gefitinib), or superior to placebo in the second- or third-line setting (erlotinib).
Certain patient characteristics (adenocarcinoma, non-smoking history, Asian ethnicity, female gender) predict for better survival benefit and response to therapy with TKIs. In addition, the current body of evidence shows that somatic mutations in the EGFR gene are the most robust biomarkers for EGFR-targeting therapy selection. Drugs used in this therapy, however, can be costly, up to C$ 2000 to C$ 3000 per month, and they have only approximately a 10% chance of benefiting unselected patients. For these reasons, the predictive value of EGFR mutation testing for TKIs in patients with advanced NSCLC needs to be determined.
The Technology: EGFR mutation testing
The EGFR gene sequencing by polymerase chain reaction (PCR) assays is the most widely used method for EGFR mutation testing. PCR assays can be performed at pathology laboratories across Ontario. According to experts in the province, sequencing is not currently done in Ontario due to lack of adequate measurement sensitivity. A variety of new methods have been introduced to increase the measurement sensitivity of the mutation assay. Some technologies such as single-stranded conformational polymorphism, denaturing high-performance liquid chromatography, and high-resolution melting analysis have the advantage of facilitating rapid mutation screening of large numbers of samples with high measurement sensitivity but require direct sequencing to confirm the identity of the detected mutations. Other techniques have been developed for the simple, but highly sensitive detection of specific EGFR mutations, such as the amplification refractory mutations system (ARMS) and the peptide nucleic acid-locked PCR clamping. Others selectively digest wild-type DNA templates with restriction endonucleases to enrich mutant alleles by PCR. Experts in the province of Ontario have commented that currently PCR fragment analysis for deletion and point mutation conducts in Ontario, with measurement sensitivity of 1% to 5%.
Research Questions
In patients with locally-advanced or metastatic NSCLC, what is the clinical effectiveness of EGFR mutation testing for prediction of response to treatment with TKIs (gefitinib, erlotinib) in terms of progression-free survival (PFS), objective response rates (ORR), overall survival (OS), and quality of life (QoL)?
What is the impact of EGFR mutation testing on overall clinical decision-making for patients with advanced or metastatic NSCLC?
What is the cost-effectiveness of EGFR mutation testing in selecting patients with advanced NSCLC for treatment with gefitinib or erlotinib in the first-line setting?
What is the budget impact of EGFR mutation testing in selecting patients with advanced NSCLC for treatment with gefitinib or erlotinib in the second- or third-line setting?
Methods
A literature search was performed on March 9, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment for studies published from January 1, 2004 until February 28, 2010 using the following terms:
Non-Small-Cell Lung Carcinoma
Epidermal Growth Factor Receptor
An automatic literature update program also extracted all papers published from February 2010 until August 2010. Abstracts were reviewed by a single reviewer and for those studies meeting the eligibility criteria full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist, and then a group of epidemiologists, until consensus was established. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
The inclusion criteria were as follows:
Population: patients with locally advanced or metastatic NSCLC (stage IIIB or IV)
Procedure: EGFR mutation testing before treatment with gefitinib or erlotinib
Language: publication in English
Published health technology assessments, guidelines, and peer-reviewed literature (abstracts, full text, conference abstract)
Outcomes: progression-free survival (PFS), Objective response rate (ORR), overall survival (OS), quality of life (QoL).
The exclusion criteria were as follows:
Studies lacking outcomes specific to those of interest
Studies focused on erlotinib maintenance therapy
Studies focused on gefitinib or erlotinib use in combination with cytotoxic agents or any other drug
Grey literature, where relevant, was also reviewed.
Outcomes of Interest
PFS
ORR determined by means of the Response Evaluation Criteria in Solid Tumours (RECIST)
OS
QoL
Quality of Evidence
The quality of the Phase II trials and observational studies was based on the method of subject recruitment and sampling, possibility of selection bias, and generalizability to the source population. The overall quality of evidence was assessed as high, moderate, low or very low according to the GRADE Working Group criteria.
Summary of Findings
Since the last published health technology assessment by Blue Cross Blue Shield Association in 2007 there have been a number of phase III trials which provide evidence of predictive value of EGFR mutation testing in patients who were treated with gefitinib compared to chemotherapy in the first- or second-line setting. The Iressa Pan Asian Study (IPASS) trial showed the superiority of gefitinib in terms of PFS in patients with EGFR mutations versus patients with wild-type EGFR (Hazard ratio [HR], 0.48, 95%CI; 0.36-0.64 versus HR, 2.85; 95%CI, 2.05-3.98). Moreover, there was a statistically significant increased ORR in patients who received gefitinib and had EGFR mutations compared to patients with wild-type EGFR (71% versus 1%). The First-SIGNAL trial in patients with similar clinical characteristics as IPASS as well as the NEJ002 and WJTOG3405 trials that included only patients with EGFR mutations, provide confirmation that gefitinib is superior to chemotherapy in terms of improved PFS or higher ORR in patients with EGFR mutations. The INTEREST trial further indicated that patients with EGFR mutations had prolonged PFS and higher ORR when treated with gefitinib compared with docetaxel.
In contrast, there is still a paucity of strong evidence regarding the predictive value of EGFR mutation testing for response to erlotinib in the second- or third-line setting. The BR.21 trial randomized 731 patients with NSCLC who were refractory or intolerant to prior first- or second-line chemotherapy to receive erlotinib or placebo. While the HR of 0.61 (95%CI, 0.51-0.74) favored erlotinib in the overall population, this was not a significant in the subsequent retrospective subgroup analysis. A retrospective evaluation of 116 of the BR.21 tumor samples demonstrated that patients with EGFR mutations had significantly higher ORRs when treated with erlotinib compared with placebo (27% versus 7%; P=0.03). However, erlotinib did not confer a significant survival benefit compared with placebo in patients with EGFR mutations (HR, 0.55; 95%CI, 0.25-1.19) versus wild-type (HR, 0.74; 95%CI, 0.52-1.05). The interaction between EGFR mutation status and erlotinib use was not significant (P=0.47). The lack of significance could be attributable to a type II error since there was a low sample size that was available for subgroup analysis.
A series of phase II studies have examined the clinical effectiveness of erlotinib in patients known to have EGFR mutations. Evidence from these studies has consistently shown that erlotinib yields a very high ORR (typically 70% vs. 4%) and a prolonged PFS (9 months vs. 2 months) in patients with EGFR mutations compared with patients with wild-type EGFR. Although having a prolonged PFS and higher respond in EGFR mutated patients might be due to a better prognostic profile regardless of the treatment received. In the absence of a comparative treatment or placebo control group, it is difficult to determine if the observed differences in survival benefit in patients with EGFR mutation is attributed to prognostic or predictive value of EGFR mutation status.
Conclusions
Based on moderate quality of evidence, patients with locally advanced or metastatic NSCLC with adenocarcinoma histology being treated with gefitinib in the first-line setting are highly likely to benefit from gefitinib if they have EGFR mutations compared to those with wild-type EGFR. This advantage is reflected in improved PFS, ORR and QoL in patients with EGFR mutation who are being treated with gefitinib relative to patients treated with chemotherapy.
Based on low quality of evidence, in patients with locally advanced or metastatic NSCLC who are being treated with erlotinib, the identification of EGFR mutation status selects those who are most likely to benefit from erlotinib relative to patients treated with placebo in the second or third-line setting.
Background
In February 2010, the Medical Advisory Secretariat (MAS) began work on evidence-based reviews of the literature surrounding three pharmacogenomic tests. This project came about when Cancer Care Ontario (CCO) asked MAS to provide evidence-based analyses on the effectiveness and cost-effectiveness of three oncology pharmacogenomic tests currently in use in Ontario.
Evidence-based analyses have been prepared for each of these technologies. These have been completed in conjunction with internal and external stakeholders, including a Provincial Expert Panel on Pharmacogenetics (PEPP). Within the PEPP, subgroup committees were developed for each disease area. For each technology, an economic analysis was also completed by the Toronto Health Economics and Technology Assessment Collaborative (THETA) and is summarized within the reports.
The following reports can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Gene Expression Profiling for Guiding Adjuvant Chemotherapy Decisions in Women with Early Breast Cancer: An Evidence-Based Analysis
Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: an Evidence-Based Analysis
K-RAS testing in Treatment Decisions for Advanced Colorectal Cancer: an Evidence-Based Analysis
Objective
In response to the request by Cancer Care Ontario (CCO), this report from the Medical Advisory Secretariat (MAS) was intended to evaluate the clinical effectiveness of epidermal growth factor receptor (EGFR) mutation testing as a predictor of response to single-agent therapy with the tyrosine kinase inhibitors (TKIs), gefitinib (Iressa®) or erlotinib (Tarceva®) in patients with advanced non-small cell lung cancer (NSCLC). This systematic review will provide information that will evaluate the effects of EGFR mutation testing on patient outcomes and also provide information to be used for cost-effectiveness analyses.
The scope of this review will be limited to the evaluating the predictive value of EGFR mutation testing in the following conditions:
Single-agent therapy with gefitinib compared with chemotherapy in the first-line setting;
Single-agent therapy with gefitinib compared with chemotherapy in the second- or third-line settings;
Single-agent therapy with erlotinib compared with best supportive care (BSC) in the second- or third-line settings.
Clinical Need and Target Population
Non-Small Cell Lung Cancer
Lung cancer is the most common cause of cancer mortality in Canada. (1) In 2009, the age-adjusted incidence of lung cancer in Ontario was 56 cases per 105 in men and 42 cases per 105 in women, respectively. With an estimated 7,800 new cases of lung cancer and 7,000 lung cancer deaths last year, it is the leading cause of cancer deaths in Ontario. (1) The overall 5-year survival rate of patients with lung cancer is 15 to 20% in North America. (1) Lung cancer is generally classified into two major histological types, small cell lung cancer and non-small cell lung cancer (NSCLC). NSCLC accounts for approximately 80-85% of all cases of lung cancer. (2) NSCLC is further divided into squamous-cell carcinoma (SSC), adenocarcinoma (AC), large cell carcinoma, and other. (3)
Treatment in Patients with Advanced Non-small Cell Lung Cancer
Treatment of patients with lung cancer depends on the tumour stage (4) and an assessment of the patient’s overall medical condition. Patients with stage I, II, or IIIA NSCLC are generally treated with surgery, chemotherapy and/ or radiation therapy. However, approximately 50 to 70% of patients with NSCLC present with advanced stage disease or present with early-stage disease that has subsequently relapsed. (5;6)
For patients with stage IIIB or stage IV disease treatment with multiple-agent chemotherapy has been shown to modestly improve survival, reduce disease-related symptoms, and improve quality-of life. (7) Systemic chemotherapy is also used for patients who have relapsed following prior definitive treatment. Combination chemotherapy, usually platinum-based, is currently the first-line therapy of choice Doublet regimens typically consist of Cisplatin or Carboplatin with paclitaxel, Gemcitabine, Docetaxel, Vinorelbine or irinotectan.
EGFR-Targeting TKIs in Advanced NSCLC
EGFR is a transmembrane protein kinase which consists of an extra and intracellular domain. The intracellular domain serves as the site of protein kinase activity and has emerged as important role in the cancer cell proliferation, apoptosis and angiogenesis. (8) Activation of the EGFR pathway initiates a process that promotes tumor cell proliferation, angiogenesis, decreased apoptosis, and the development of metastasis. (9;10) NSCLC is associated with a high rate of EGFR expression, and EGFR expression has been associated with a poor prognosis. (11)
As one of the developed strategies for targeting EGFR receptor, one being small molecule TKIs that interfere with activation/ phosphorylation of EGFR. (12) There are currently two EGFR TKIs approved by Health Canada for the treatment of advanced NSCLC: gefitinib (Iressa®) and erlotinib (Tarceva®). These two drugs disrupt EGFR signaling by competing with adenosine triphosphate (ATP) for the binding sites at the tyrosine kinase domain thus inhibiting the phosphorylation and activation of EGFRs and the downstream signaling network. (13) Gefitinib and erlotinib have been shown to be either non-inferior or superior to chemotherapy in the first- or second-line setting (gefitinib), or superior to placebo in the second- or third-line setting (erlotinib).
Predictive Biomarkers of Response to EGFR-Targeted TKI Drugs in NSCLC
Clinical trials of gefitinib and erlotinib reported significant responses in approximately 10% of unselected patients. (14) As TKIs have demonstrated definite activity in a subset of patients with NSCLC, predictive markers have been developed to forecast clinical outcomes in patients treated with these drugs. Moreover, certain patient clinical characteristics (adenocarcinoma, female gender, Asian ethnicity, non-smoking history) were found to predict for better response to TKIs. (14-17) EGFR-related biomarkers for the efficacy of EGFR-targeting TKIs include those related to the receptor itself, such as protein expression, gene copy number, or mutation status. Most of the studies have not shown consistent correlation between EGFR protein expression and EGFR gene amplification status. However, the current body of evidence shows that somatic mutations in the tyrosine kinase domain of the EGFR gene are the most robust biomarkers for EGFR-targeting therapy selection. (18;19) Evidence shows that nearly 90% of EGFR mutations occur as either deletions in exon 19 or as substitution in exon 21 and both of them have shown to be associated with increased sensitivity to TKIs. (19;20) EGFR-targeting TKI found to be effective in nearly 70% of patients with EGFR mutations, whereas the same treatment is effective in only 10% of patients with wild-type EGFR. (21) In addition, K-ras mutation or genetic polymorphisms might also be important contributors to variability in response to EGFR TKI therapy, but evidence is contradictory. (22)
1. EGFR Protein Overexpression
The EGFR protein is overexpressed in 50% to 90% of NSCLC tumours. (23;24) Although it has been hypothesized that the quantity of EGFR protein might be predictive of response to EGFR inhibitor therapy, the relationship between EGFR protein expression and tumour sensitivity to TKIs still is unclear. (17;25;26) Retrospective analyses of patients with NSCLC who received TKI therapy found no obvious relationship between the amount of EGFR protein assessed by immunohistochemistry (IHC) and objective response rates (ORR). (17;26) However, the National Cancer Institute of Canada Clinical Trials Group (BR.21) phase III study of erlotinib for patients with relapsed or refractory NSCLC found an association between EGFR protein expression and prolonged OS. (25) In the Iressa Survival Evaluation in Lung Cancer (ISEL) trial a phase III double-blind, randomized, placebo-controlled trial that compared gefitinib treatment with placebo, patients with EGFR protein expression-positive tumours who received gefitinib, demonstrated prolonged survival compared with patients with EGFR protein expression-negative tumours (HR, 0.77; 95%CI, 0.56-1.08). (27) EGFR protein expression-positive patients also seemed to achieve better response rates than EGFR protein expression-negative patients (8.2% versus 1.5%). (28)
1.1 EGFR Protein Overexpression Testing
There are several ways to detect and measure EGFR protein. These include radioactive-labelled ligand binding, competitive immunoassay, western blotting and immunohistochemistry (IHC). (22) IHC is the most commonly used. IHC is a quick and simple technique that utilizes available equipment and reagents. There are several advantages of using IHC such as the direct evaluation of EGFR expression level on tumour cells, performing the test on formalin-fixed, paraffin-embedded tumours and the ease of use in clinical pathology practice. Most laboratories use the indirect immunolabeling for IHC. The other methods for IHC require special laboratory setups that are more suitable for basic research.
Several commercial antibodies for EGFR are available. The two most commonly used are the DakoCytomatin (Carpentaria, CA, USA) clone 18C9, which is used in the pharmDx kit, and Zymed Laboratories Inc. (South San Francisco, CA, USA) clone 31G7. For better consistency in results, IHC studies need to be controlled and validated carefully.
However, there are some disadvantages to using IHC. Although it is often used for routine clinical testing, IHC is subject to many variables that decrease its reproducibility and quantitative value. The presence and intensity of staining is highly dependent on several factors including: techniques used for tissue collection and handling; time from when the slide is prepared from the tumour block; antigen retrieval techniques; antibody detection protocol; and size and quality of the tissue sections used. Moreover, there is currently no standard methodology for ensuring consistent handling and scoring of EGFR protein levels (e.g. cut off levels for positive or negative staining or cytoplasmic vs. membrane staining) between testing centers. Quantification of EGFR protein expression levels by visual inspection is semiquantitative compared with the other quantitative methods. The next important technical issue concerning IHC is the type of primary antibody used. Sensitivity and specificity of the primary antibody are crucial factors in the immunohistochemical reaction. In an analysis of 296 patients treated in the ISEL trial compared EGFR protein expression measured by using the EGFR pharmDx assay kit (Dako Denmark A/S, Glostrup, Denmark) or the Zymed EGFR kit (with the 31G7 mouse antihuman MoAb; Invitrogen Corporation, Carlsbad, CA) showed the discordance rate of 24% between results. (24;27) The increasing number of commercially available EGFR-antibodies by laboratories makes standardisation of IHC difficult. These technical issues are reason why EGFR IHC is not yet considered an optimal method in prediction of response to EGFR-targeting TKIs.
1.2 Effectiveness of EGFR Overexpression in predicting response to TKIs
There have been conflicting results with regard to the value of EGFR overexpression testing in prediction of response to EGFR TKIs. In patients enrolled in BR.21 trial, an IHC positive test result was associated with a better response to erlotinib. (27;29) In this trial erlotinib provided a prolonged OS compared with placebo in patients who were IHC positive (HR, 0.68; 95% CI, 0.49-0.95; P= 0.02) but not in patients who were IHC negative (HR, 0.93; 95% CI 0.63-1.36; P=0.70). However, the BR.21 study did not find that an IHC positive test was associated with survival in multivariate analysis (P=0.25). (24) Further evaluation of different cut points for defining the IHC positive test threshold revealed that EGFR protein expression was a weak predictive factor for survival benefit for erlotinib in the BR.21 study. (30)
In ISEL, IHC-positive patients had a significantly longer OS with gefitinib compared to patients with IHC-negative test (HR, 0.77; 95% CI 0.56 to 1.08) vs. (HR, 1.57; 95%CI 0.86-2.87; P=0.05). (28) Moreover, in a study evaluating pooled data from patients enrolled in two clinical trials of gefitinib, using the combination of EGFR protein overexpression and EGFR gene copy number testing, patients with positive IHC and fluorescence in situ hybridization (FISH) test had improved RR and OS compared with IHC and FISH- negative patients. (31) In a multivariate analysis both EGFR overexpression and high EGFR copy number were independent predictive variables: RR, 41 vs. 2% (P<0.001); PFS, 9 vs. 2 months (P<0.001); and, OS, 21 vs. 6 months (P<0.001). The updated results from the BR.21 study also showed a significant association between IHC/FISH-positive test and an increased response to erlotinib compared with patients who were IHC/FISH-negative (23 vs. 3%; P=0.03). (32) The OS of patients who were IHC/FISH positive was (HR, 0.37; 95%CI, 0.18-0.79) (P=0.01) vs. (HR, 0.62, 95%CI 0.31-1.24) (P=0.17). In INTEREST trial, a multi-centre, randomised, open-label, phase III trial of gefitinib versus docetaxel in patients who were pre-treated with platinum, no difference was found in OS irrespective of a patient’s EGFR protein expression status. (33)
2. EGFR gene copy number or dosage
The EGFR gene is located on the short arm of chromosome 7 (7p21). Gene dosage is the number of copies of gene present in the cell or nucleus. An increase in gene dosage means the genes is amplified and results in oncogene overexpression. The production of multiple gene copies which amplifies the phenotype that the gene confers is amplified in the cell. (13) EGFR amplification or high polysomy have been detected in approximately 30% of patients with NSCLC using FISH, and it is usually associated with a poor clinical prognosis. (34) It has been shown that a high copy of EGFR probably is an effective predictor for better response to treatment with EGFR-targeting TKIs. (33;35-37) Patients with high number of copies of the EGFR gene also show a significant survival benefit from EGFR-targeting TKI treatment. (25;28;36;38)
2.1 EGFR Amplification Testing
There are different laboratory methods for detecting and determining EGFR gene copy number and dosage, including fluorescence in situ hybridization (FISH), chromogenic in situ hybridization (CISH) and real-time quantitative polymerase chain reaction (qPCR). (22;24)
2.1.1 FISH
FISH is the primary method for detecting the chromosomal location and copy number of specific gene in tissue sections. (24) This method uses fluorescence-tagged DNA probes corresponding to EGFR to detect all cellular copies of the EGFR gene on tumour serial sections by fluorescence microscopy. Gene copy number is easily standardized because it can be quantitatively assessed by manual observation. The main barriers to routinely performing FISH include the lack of expertise in molecular techniques and lack of experience with dark-field fluorescence microscopy, needed to assess copy number.
2.1.2 CISH
CISH is an alternative assay to FISH for determining gene copy number amplification. It can be evaluated using bright-field light microscopic techniques to assess gene copy number. As with IHC, CISH-stained slides can be archived and stored permanently. (22)
2.1.3 qPCR
Another method for detecting increased EGFR copy number is quantitative real-time polymerase chain reaction (PCR). This technique was used to analyze tumour specimens from the Iressa NSCLC trial. (39) Of the 453 tumour specimens, 33 (7%) had EGFR DNA amplification, which was much lower than expected. (25;28) This is likely due to the inability of qPCR to control for contamination from normal host cells that results in the dilution of tumour cell gene copy.
2.2 Effectiveness of EGFR Amplification in Predicting Response to TKIs
In the BR.21, the ISEL and the Italian Expanded Access Study of gefitinib, an association was observed between EGFR FISH-positive test result and either increased RR or an improved survival benefit. Furthermore, the results of 3 retrospective studies suggest that an increased EGFR gene copy number is associated with enhanced TKI response in patients with advanced NSCLC. (25;28) However, studies that used quantitave PCR (qPCR) to assay EGFR gene copy number reported conflicting results with the FISH findings. (28;39;40) In studies conducted in Asian patients, FISH positivity was not associated with survival benefit. (41;42)
3. EGFR gene somatic mutations
All activating mutations that have been reported are on exon 18 through 21 and among all mutations, four predominately result in TKI drug sensitivity by in vitro and in vivo studies. These include point mutations in exon 18, (G719A/C) and 21 (L858R and L861Q) and in-frame deletions in exon 19, which eliminate four amino acids (LREA) downstream of the lysine residue at position 745. Other mutations appear to be associated with variable or less sensitivity. Exon 19 deletion and exon 21 L858R substitution account for 85-90% of drug-sensitive EGFR mutation seen in NSCLC. The mutations are common in adenocarcinoma, Asian ethnicity, women and never smokers. The prevalence of EGFR mutations varies by ethnicity with ranges from 20-40% in Asian populations to 5-20% among Caucasians. (22)
3.1 EGFR gene mutation testing
The most commonly used method to detect mutations in the past was direct sequencing of exons 18 to 24 of EGFR. This method consists of two steps. The first step consists of identification of the kinase domain by polymerase chain reaction (PCR). This method is the most widely used method that can be performed on a variety of samples, including DNA or RNA derived from whole blood, frozen cell pellets, or tissues. The second step consists DNA direct sequencing on the PCR products, and is used to determine the order of nucleotides within DNA.
A variety of new methods have been introduced to increase the sensitivity of the mutation assay that have advantages and disadvantages in terms of complexity, performance, and sensitivity. Some technologies such as single-stranded conformational polymorphism, denaturing high-performance liquid chromatography, and high-resolution melting analysis have the advantage of rapid mutation screening of large numbers of samples with high sensitivity but they require direct sequencing to confirm the identity of the detected mutations. Other techniques have been developed for the simple but highly sensitive detection of specific EGFR mutations, such as the amplification refractory mutations system (ARMS) and the peptide nucleic acid-locked PCR clamping. Others selectively digest wild-type DNA templates with restriction endonucleases to enrich mutant alleles by PCR. (24)
3.2 EGFR somatic mutations and response to TKIs
There is high variability in the degree and duration of response to TKIs in patients with EGFR mutations, about 70% of the patients with EGFR mutations respond to EGFR-targeting TKIs whereas only 10% of those without the mutations do so. (21)
Ontario Context
Currently, there is no published guideline on using EGFR mutation testing to prescribe TKIs (gefitinib, erlotinib). Recently, Health Canada has released indications for using gefitinib and erlotinib acknowledging the role of EGFR mutation testing before treatment with either gefitinib or erlotinib. However, according to the report by the EGFR mutation testing expert panel, no formal recommendation was made by CCO for EGFR mutation testing before the use of gefitinib or erlotinib in patients with advanced or metastatic NSCLC, and no physician request for EGFR mutation testing before erlotinib therapy. According to the Health Canada indication, erlotinib must be used as single-agent therapy for locally advanced or metastatic NSCLC after failure of at least one prior chemotherapy regimen, and in patients whose EGFR mutation status is positive or unknown. According to the CCO Guidelines, single-agent therapy with erlotinib is recommended as third-line treatment for patients with NSCLC who have failed previous chemotherapy and who maintain a good performance status. Erlotinib is also suggested as an option for second-line therapy for patients who are not candidates for second-line chemotherapy. Experts in the province of Ontario have commented that currently there is no CCO guideline for the use of gefitinib in the first-line setting (personal communication).
In Ontario, University Hospital Network (UHN) in Toronto has been designated to conduct EGFR testing for patients who may be eligible to receive gefitinib in the first-line setting. The EGFR gene sequencing by polymerase chain reaction (PCR) assays was previously the most widely used method. Experts in Ontario have commented that sequencing is not currently done in Ontario because this method is not sensitive enough (personal communication). A variety of new methods have been introduced to increase the sensitivity of the mutation assay. Experts have commented that currently PCR fragment analysis for deletion and point mutation has a measurement sensitivity of 1% to 5%.
Evidence-Based Analysis
Key Questions
The final key questions are as follows:
In patients with locally-advanced or metastatic NSCLC, what is the clinical effectiveness of EGFR mutation testing for prediction of patient response to treatment with TKIs in terms of improved progression-free survival, objective response rates, overall survival, quality of life?
What is the impact of EGFR mutation testing on overall clinical decision-making for patients with advanced or metastatic NSCLC?
What is the cost-effectiveness or budget impact of EGFR mutation testing in treatment of patients with advanced NSCLC?
Research Methods
Literature Search
Search Strategy
A literature search was performed on March 9, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 until February 28, 2010. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria; full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology. (43)
Inclusion Criteria
Each citation identified from the reach strategies was evaluated according to the following selection criteria. Abstracts were reviewed by a single reviewer and for those studies meeting the eligibility criteria full-text articles were obtained.
English-language studies and health technology assessments and literatures published between January 2004 and July 2010
All phase III clinical trials reporting the results of using single-agent gefitinib or erlotinib compared with conventional chemotherapy, or best supportive care alone and which provided adequate information about EGFR mutation status and important clinical outcomes such as progression-free survival (PFS), objective response rate (ORR), overall survival (OS), and quality of life (QoL)?
All phase II trials reporting the results of using single-agent gefitinib or erlotinib and which they provided adequate information about EGFR mutation status and outcomes of our interest such as PFS, ORR, OS, QoL
Prospective and retrospective observational studies as long as they provided adequate information about EGFR mutation status and clinical outcomes
Sample size of 20 or more
Abstracts or meeting proceedings as long as they provided adequate information about EGFR mutation status and important clinical outcomes
Exclusion Criteria
Studies in which outcomes were not specific to those of interest
Studies reporting on gefitinib or erlotinib in combination with any other cytotoxic or investigational agents
Studies focused on gefitinib or erlotinib as maintenance therapy
Case reports
Outcomes of Interest
Progression-free survival
Objective response rates determined by means of the Response Evaluation Criteria in Solid Tumours (RECIST)
Overall Survival
QoL
Quality of Evidence
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria as presented below. (43)
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
Literature Search
The search strategy yielded 2605 citations; a detailed literature search strategy is shown in Figure 1.
Fig 1: Flow Diagram of the Systematic Literature Search.
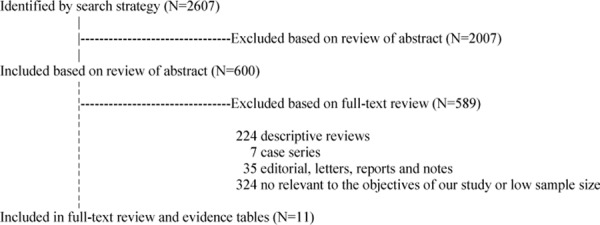
The 11 studies include 6 phase III randomized trials on gefitinib vs. chemotherapy used in the first- or second-line setting, 1 non-concurrent prospective study and 4 phase II trials (Table 1).
Table 1: Study Design and Level of Evidence Characteristics of Included Studies.
| Study Design | Level of Evidence† | Number of Eligible Studies | |
|---|---|---|---|
| Gefitinib | Erlotinib | ||
| Systematic review and meta-analyses | 1a | 0 | 0 |
| Large RCT, systematic review of RCTs | 1 | 0 | 0 |
| Large RCT | 1a | 5 | 0 |
| Large RCT unpublished but reported to an | 1(g) | 1 | 0 |
| Small RCT | 2 | 0 | 0 |
| Small RCT unpublished but reported to an | 2(g) | 0 | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 | 0 |
| Non-RCT with historical controls | 3b | 0 | 0 |
| Non-RCT presented at international conference | 3(g) | 0 | 0 |
| Surveillance (database or register) | 4a | 0 | 0 |
| Case series (multisite) | 4b | 0 | 0 |
| Case series (single site) | 4c | 0 | 0 |
| Retrospective review, modelling | 4d | 0 | 5 |
| Case series presented at international conference | 4(g) | 0 | 0 |
| Total | 6 | 5 | |
RCT refers to randomized controlled trial
For each included study, level of evidence was assigned according to a ranking system proposed by Goodman. (44)
Section 1. Published Systematic Evidence Reviews
The most recent systematic review by Blue Cross Blue Shield Association for the U.S.A in 2007 is based on twenty-three retrospective and 6 prospective studies. (45) According to this report, there is sufficient evidence to support the predictive value of EGFR mutation testing for ORR to gefitinib therapy based on sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of mutation presence compared to its absence. However, the authors did not make any conclusions concerning the clinical validity or utility of EGFR mutation testing to predict response to erlotinib in patients with advanced NSCLC.
An HTA review by CADTH in August 2010 was based on eleven observational studies and one evidence-based guideline. (46) The focus of the review was on the clinical effectiveness and diagnostic performance of polymerase chain reaction (PCR)-based methods to detect the presence of EGFR mutations in patients with advanced NSCLC. The authors concluded that PCR-based tests are likely to be useful for identifying patients with NSCLC who are likely to respond to treatment with a TKI.
Section 2. MAS Evidence Review
2.1 Clinical Effectiveness of EGFR Mutation testing in the First-Line Setting
2.1.1 Prediction of Patients’ Response to Gefitinib versus Chemotherapy
The literature search yielded four large randomized controlled phase III trials that compare single-agent therapy with gefitinib against standard chemotherapy in patients with advanced NSCLC (Table 2). Only one of them however provided adequate information regarding EGFR mutation status. The Iressa Pan-Asia Study (IPASS) study randomly assigned a selected group of 1,217 patients with previously untreated, advanced NSCLC to receive either gefitinib 250 mg orally per day until disease progression or unacceptable toxicity, or the doublet therapy of carboplatin and paclitaxel. (47) This study met its primary objective of demonstrating non-inferiority of gefitinib over chemotherapy in terms of PFS (HR, 0.74; 95%CI, 0.65-0.85;P <0.001). Median PFS was 5.7 versus 5.8 months in the gefitinib and chemotherapy group, respectively.
Table 2: Characteristics of the studies included in the review: EGFR mutation testing as a predictor of response to gefitinib compared with chemotherapy in the first-line setting.
|
Author, year |
Patient selection | Design | Tissue available/ pts treated |
Mutated/ assessable pts (%) |
Median PFS, ORR, OS [p-value] |
|||
|---|---|---|---|---|---|---|---|---|
| Subset analyses of the phase III trials for selected patients based on clinical characteristics | ||||||||
| EGFR Mutated | EGFR Wild-type | |||||||
| G | C+P | G | C+P | |||||
|
Mok, 2009 IPASS (n=1217) |
Asian 99.7% Female 79% Non- or former light smokers 99.8% Adenocarcinoma 96.3% |
Gefitinib vs. carboplatin/ paclitaxel Phase III RCT |
683/1217 (56%) |
261/437 (60%) |
PFS HR, 0.48 (0.36-0.64) ORR 71.2 vs. 47.3 OS HR, 0.78 (0.50-1.20) |
PFS HR, 2.85 (2.05-3.98) ORR 1.1 vs. 23.5 OS HR, 1.39 (0.92-2.09) |
||
| G | Cis+G | G | Cis+G | |||||
|
Lee, 2009 FIRST-SIGNAL (n=309) |
Asian Female Never-smokers Adenocarcinoma |
Gefitinib vs. cisplatin- gemcitabine Phase III RCT |
92/309 (30%) |
42/96 (44%) |
PFS 8.5 vs. 6.7 months HR, 0.61 (95%CI, 0.31-1.22) ORR 84.6 vs. 37.5 [0.002] OS 30.6 vs. 26.5 months HR, 0.82 (95%CI, 0.35-1.92) |
NR |
||
| Subset analyses of phase III trials pf patients selected according to EGFR mutation status | ||||||||
| G | C+P | G | C+P | |||||
|
Maemondo, 2010 NEJ002 (n=230) |
Asian 100% Female 64% Never-smokers 62% Adenocarcinoma 93% |
Gefitinib vs. Carboplatin/ paclitaxel Phase III RCT |
PFS 10.8 vs. 5.4 months HR, 0.30 (95%CI, 0.22-0.41) ORR 73.7% vs. 30.7% OS 30.5 vs. 23.6 months [NS] |
0 |
||||
| G | Cis+D | G | Cis+D | |||||
|
Mitsudomi, 2010 WJTOG3405 (n=177) |
Asian 100% Female 69% Never-smokers 69% Adenocarcinoma 98% |
Gefitinib vs. cisplatin- docetaxel Asian, female, adendocarcinoma Never-smoker |
PFS 9.2 vs. 6.3 months HR, 0.48 (95%CI, 0.33-0.71) ORR 62.1% vs. 32.2% [P<0.0001] |
0 |
||||
Abbreviations: PFS, Progression-free survival; ORR, Objective response rate; OS, overall survival; HR, Hazard ratio; G, gefitinib; C, Cisplatin; P, paclitaxel; Cis, Cisplatin; D, docetaxel; NR, Not reported.
Among 437 patients analyzed for EGFR mutation status an overall EGFR mutation rate of 60% has been detected. Those patients who had positive test and were treated with gefitinib had a longer PFS compared with patients who were treated with chemotherapy (9.5 vs. 6.3 months; HR, 0.48; 95%CI, 0.36-0.64). Conversely, in patients with a wild-type EGFR mutation test a longer PFS was observed in those who were treated with chemotherapy compared with gefitinib (HR, 2.85; 95%CI, 2.05-3.98; P<0.001). The ORR in the overall population was significantly higher with gefitinib than with carboplatin-paclitaxel (43% versus 32%). In the subgroup analysis based on EGFR mutation status, a higher ORR was observed in patients with EGFR mutation treated with gefitinib than with chemotherapy (71% versus 47%), and those patients with wild-type EGFR had higher ORR only if they were treated with chemotherapy compared with gefitinib (24% versus 1%). No differences in overall survival were seen in patients with EGFR mutations regardless of the treatment they received.
The Functional Assessment of Cancer Therapy-Lung (FACT-L), Trial Outcome Index (TOI) and lung-cancer subscale (LCS) scores of FACT-L; showed a significant improvement in QoL symptoms measures associated with gefitinib versus carboplatin/paclitaxel in patients with EGFR mutations compared with wild-type EGFR (total FACT-L, 70% vs. 15%; TOI, 70% vs. 38%; LCS, 76% vs. 20%
The first-line single agent Iressa versus gemcitabine and cisplatin (First-SIGNAL) trial was a study with a nearly identical design and patient population characteristics to the IPASS, which have only been presented in abstract form. (48) In a subgroup study for EGFR mutations in over 30% of patients with an overall EGFR mutation rate of 40%, ORR was 84.6% versus 37.5% with gefitinib versus chemotherapy in patients with EGFR mutations, and 30% with gefitinib versus 52% with chemotherapy in patients with wild-type EGFR. However, there was a non-significant difference in PFS favouring gefitinib in patients with EGFR mutations (8.5 versus 6.7 months; HR, 0.61; 95%CI, 0.31-1.22).
In the NEJ002 and WJTOG3405 trials, patients with advanced NSCLC were randomly assigned to receive gefitinib or chemotherapy with carboplatin-paclitaxel or cisplatin-docetaxel respectively. (49;50) Unlike IPASS and First-SIGNAL studies, these two studies included only patients with EGFR mutations. The NEJ002 trial, carried out in 230 patients by the North East Japan Gefitinib Study Group, compared gefitinib to chemotherapy as frontline therapy. In the interim analysis a median PFS of 10.8 months was shown in patients who received gefitinib, a statistically significant improvement over the median of 5.4 months in the chemotherapy group (HR, 0.30; 95%CI, 0.22-0.41). ORR was 74% with gefitinib versus 31% with chemotherapy. In the WJTOG3405, carried out by the West Japan Thoracic Oncology Group, 172 patients were randomized to receive gefitinib or chemotherapy with cisplatin-docetaxel. The study showed a median PFS of 9.2 months with gefitinib versus 6.3 months with chemotherapy (HR, 0.49; 95%CI, 0.33-0.71).
ORR was 62.1% and 32.2% with gefitinib versus chemotherapy, respectively. Both of these two studies showed a statistically significant improvement in PFS and ORR favouring gefitinib (Table 4).
Table 4: Characteristics of the studies included in the review: EGFR mutation testing as a predictor of response with gefitinib compared with chemotherapy in the second- or third-line setting.
| Author, year |
Patient selection |
Design | Tissue available/ pts treated |
Mutated/ assessa ble pts (%) |
Median PFS, ORR, OS [p-value] |
|||
|---|---|---|---|---|---|---|---|---|
| Mutated | Wild-type | |||||||
| G | D | G | D | |||||
|
Douilard, 2010 (n=1466) |
Caucasian 88% Female 35% Non- smokers 20% Adenocarcinoma 54% |
Gefitinib vs. docetaxel Phase III trial |
297/1466 (20%) |
44/297 (15%) |
PFS 7.0 vs. 4.1 months HR, 0.16 (95%CI, 0.05-0.49) ORR 42.1% vs. 21.1 [0.04] |
PFS 1.7 vs. 2.6 months HR, 1.24 (95%CI, 0.94-1.64) ORR 6.6% vs. 9.8% [0.37] |
||
|
Hirsch, 2006 (n=1692) |
Caucasian 93% Female 36% Non-smokers14% Adenocarcinoma 50% |
Gefitinib+ BSC vs. placebo+ BSC Phase III trial |
215/1692 (12%) |
26/215 (12%) |
ORR 37.5% vs. 2.6% [0.04] |
NR |
||
Abbreviations: PFS, Progression-free survival; ORR, Objective response rate; OS, overall survival; HR, Hazard ratio; G, gefitinib; D, Docetaxel; NR, not reported.
Taken together, the results of IPASS, First-SIGNAL, NEJ002 and WJTOG3405 trials confirm that the presence of EGFR mutation best identifies those patients who would derive the most benefit from gefitinib compared with chemotherapy in terms of PFS and ORR. The lack of difference in OS, both in the overall study population or EGFR mutation subgroups is most likely due to the cross-over effect caused by patients receiving gefitinib on progression in the chemotherapy arm.
2.2 Clinical Effectiveness of EGFR Mutation testing in the Second- or Third-Line Setting
2.2.1 Erlotinib versus Placebo for Treatment of Advanced NSCLC
One retrospective study based on a phase III trial and four phase II trials were identified which compared single-agent erlotinib in patients with and without EGFR mutations (Table 3). (32;51-54) A double-blind randomized phase III trial conducted by the National Cancer Institute of Canada Clinical Trials Group (BR.21), assigned 731 pre-treated patients in a 2:1 ratio to erlotinib at a dose of 150 mg daily until disease progression or unacceptable toxicity, or Best Supportive Care (BSC). (29) The results of this study revealed a statistically significant improvement in PFS (HR, 0.61; 95%CI, 0.51-0.74) and OS (HR, 0.73; 95%CI, 0.60-0.87) in patients who were treated by erlotinib monotherapy compared with BSC. In addition, erlotinib was associated with a superior ORR (9% versus <1%, p<0.0001). In a subsequence retrospective analysis of the data of BR.21 trial, EGFR mutation testing could be attempted for 204 (28%) of 731 study participants. (32) The analysis was performed for 169 patients and included the Scorpion Amplified Refractory Mutation System and fragment length EGFR mutation assays of exons 19 and 21 to identify EGFR gene mutations. The results showed a higher ORR with erlotinib compared with BSC (27% versus 7%; P= 0.03), although the observed survival advantage with erlotinib was not significantly different with EGFR mutation status due to possible type II error.
Table 3: Characteristics of the phase II trials included in the review: EGFR mutation testing as a predictor of response with erlotinib in the second- or third-line setting.
| Author, year |
Characteristics (%) | Design | Mutation of assessable treated patients (%) |
Median PFS, ORR, OS [p-value] |
|---|---|---|---|---|
| EGFR mutant vs. wild- type |
||||
|
Miller, 2008 (n=101) |
Female 65% Never-smoker 42% Adenocarcinoma 88% |
Multicenter, single-arm, two-stage, phase II |
18/81 (22%) |
PFS 13 vs. 2 months ORR 83% vs. 7% OS 23 months vs. 17 months |
|
Schneider, 2008 (n=393) TRUST |
Caucasian 99% Female 40% Adenocarcinoma 51% Never smoker 24% Second-line 80% |
Open-label single-arm |
6/91 (6.5) OR 6/195 (3.1%) |
PFS HR, .31 (95%CI .13-0.78) ORR 33% vs. 3% OS HR, 0.33 (95%CI, 0.12 to 0.91) |
|
Felip, 2008 (n=83) |
Female 28% Adenocarcinoma 43% SCC 18% PS 0-1 Never-smoker 13% |
Phase II |
5/39 (13) All mutation from Adenocarcinoma |
PFS 6.8 months vs. 1.4 months ORR 40% vs. 3% OS 6.8 months vs. 3.8 months |
|
Ahn, 2008 (n=120) |
All Asian Female 37% Adenocarcinoma 62% Never-smoker 39% |
Phase II |
24/92 (26%) |
ORR 58% vs. 16% |
Abbreviations: OS, overall survival; TTP, time to progression; DFS, disease free survival; PFS, progress free survival; NR: not reported; NS: not significant; ORR: objective response rate according to RECIST or ECOG criteria, comparing CR+PR; Wt: wild-type.
Furthermore, a total of 4 phase II trials were identified involving EGFR mutation testing for pre-treated patients with advanced NSCLC who received erlotinib (Table 5). The results of these retrospective studies indicated the superiority of PFS and ORR with erlotinib in EGFR mutated patients compared with EGFR wild-type.
Table 5: Base-case analysis results for the cost-effectiveness analysis.
| Strategy | Cost | Incr Cost | QALY | Incr QALY | ICER |
|---|---|---|---|---|---|
| No EGFR mutation testing | $14,368 | 0.4842 | |||
| EGFR mutation testing | $16,857 | $2,488 | 0.5383 | 0.0541 | $46,021 |
2.2.2 Gefitinib versus chemotherapy for Treatment of Advanced NSCLC
There is scarce evidence of the effectiveness EGFR mutation testing as predictor of response to gefitinib compared to chemotherapy in the second- or third-line setting. In the phase III randomized placebo-controlled Iressa NSCLC Trial Evaluating Response and Survival Versus Taxotere (INTEREST), 1466 previously treated patients with advanced NSCLC were randomly assigned to receive gefitinib (250 mg/d orally) or docetaxel (75 mg.m2 intravenously once very 3 weeks). (55) In a subgroup study for EGFR mutations in 297 patients (18%) with an overall EGFR mutation rate of about 15%, PFS was significantly longer with gefitinib than docetaxel in patients with EGFR mutations (HR, 0.16; 95%CI, 0.05-0.49) but not in patients with wild-type EGFR (HR, 1.24, 95%CI, 0.94-1.64). ORR was also higher with gefitinib than with docetaxel in patients with EGFR mutations (42% versus 21 %) (Table 4). In agreement with the findings of the INTEREST trial, patients with EGFR mutations had higher ORR with gefitinib, compared with patients without EGFR mutations. (26) Evaluation of survival outcomes was limited due to the low number of events in patients with EGFR mutations.
Conclusions
Based on moderate quality of evidence, patients with locally advanced or metastatic NSCLC with adenocarcinoma histology being treated with gefitinib in the first-line setting are highly likely to benefit from gefitinib if they have EGFR mutations compared to those with wild-type EGFR. This advantage is reflected in improved PFS, ORR and QoL in patients with EGFR mutation who are being treated with gefitinib relative to patients treated with chemotherapy.
Based on low quality of evidence, in patients with locally advanced or metastatic NSCLC who are being treated with erlotinib, the identification of EGFR mutation status may help to select those who are likely to benefit from erlotinib relative to patients treated with placebo in the second- or third-line setting.
Existing Guidelines for EGFR Mutation testing
Currently, there is no CCO guideline on using EGFR mutation as a predictor of response to TKIs (gefitinib, erlotinib). Recently, Health Canada has released indications for using gefitinib and erlotinib acknowledging the role of EGFR mutation testing before treatment gefitinib or erlotinib.
The National Comprehensive Cancer Network in U.S also acknowledges the effectiveness of using gefitinib or erlotinib in EGFR-positive patients; however, there is still no FDA guideline on using EGFR mutations to prescribe these drugs.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Study Question
About 20% of patients with non-small cell lung cancer (NSCLC) have an epidermal growth factor receptor (EGFR) gene mutation on exon 19 to 21.(56;57) Recently, NSCLC patients with an EGFR gene mutation were found to respond to EGFR tyrosine kinase inhibitors (TKIs), including gefitinib and erlotinib.(58;59) Therefore, EGFR gene mutation testing has been used to identify EGFR gene mutation status in patients with advanced NSCLC to guide the selection of first-line therapy and avoid the use of expensive EGFR TKIs among those without the mutation. The study question was whether applying EGFR gene mutation testing to guide the selection of first-line therapy among patients with advanced NSCLC living in Ontario was cost-effective when compared to no testing.
Economic Analysis Overview
A decision analytic model was developed to compare the lifetime benefits (life years and quality-adjusted life years (QALY)) and direct medical costs in 2010 Canadian dollars between the strategy of EGFR gene mutation testing and the strategy of no EGFR gene mutation testing in patients with advanced (stage 3b or 4) NSCLC. Under the strategy of EGFR gene mutation testing, tumor material from patients’ biopsies is assessed for mutations on exon 19 to 21 of EGFR gene. The patients having EGFR gene mutations would receive gefitinib as first-line therapy, followed by conventional chemotherapy. The patients having no EGFR mutations or undetermined mutation status were managed by conventional chemotherapy. For the no testing strategy, chemotherapy was offered as per the no EGFR mutation group.
The base case analysis was conducted using the baseline values of the variables in the model. Sensitivity analyses were conducted to explore uncertainty associated with the variables in the model. A budget impact analysis was also performed to illustrate the changes of future health care expenditure on patients with advanced NSCLC in Ontario after the adoption of EGFR gene mutation testing.
Economic Literature Review
No economic studies were found assessing the cost-effectiveness of EGFR gene mutation testing in guiding the selection of first-line therapy in patients with advanced NSCLC. Both MEDLINE and EMBASE medical databases were systematically searched up to July 2010.
A systematic literature review was also performed (using MEDLINE, EMBASE, The Cochrane Library) for data extraction regarding the following: epidemiology of advanced NSCLC in Ontario, sensitivity of EGFR gene mutation testing, prevalence of EGFR gene mutation in the population of patients with NSCLC, natural history of advanced NSCLC, patterns of care for NSCLC in Ontario, efficacy of the treatments, quality of life for patients, and health care expenditures on patients with advanced NSCLC in Ontario.
Target Population
Patients were defined as those diagnosed with advanced NSCLC (Stage IIIb or IV) and no thaving started any first-line therapy with TKIs or conventional chemotherapy.
Perspective
The analytic perspective was that of the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Comparators
The following two strategies were compared:
EGFR gene mutation testing: tumour tissues taken by biopsy were assessed to detect any mutation of EGFR gene from exon 19 to 21. Patients with EGFR gene mutations would receive gefitinib daily as first-line therapy, platinum based chemotherapy (cisplatin plus gemcitabine) as second-line therapy, and docetaxel or pemetrexed as third-line therapy before BSC. Patients without EGFR gene mutation would receive cisplatin plus gemcitabine as first-line therapy, docetaxel or pemetrexed as second-line therapy, and best supportive care. The other patients whose EGFR gene mutation status remained unknown after the testing (due to inadequate tissue or other technical limitations) would be treated with the combination of cisplatin and gemcitabine as first-line, docetaxel or pemetrexed as second-line, and erlotinib as third-line before BSC.
No EGFR gene mutation testing: all patients would not be assessed for EGFR gene mutations. Patients would receive cisplatin and gemcitabine as first-line, docetaxel or pemetrexed as second-line, and erlotinib as third-line before BSC.
Time Horizon
The time horizon used in the model was life-time in length (i.e. the model’s patient cohort was followed until death).
Discounting
5% per annum for both benefits (life years or QALY) and costs (Canadian dollars in 2010).
Model Structure
The Markov cohort model was constructed to reflect the natural history of advanced NSCLC and the patterns of care for patients with advanced NSCLC in Ontario starting from first-line therapy (Figure 2). The cycle length of the Markov cohort model was 3 weeks. The following health states in the model were stratified by type of treatment: daily dosing for gefitinib or erlotinib, four cycles of cisplatin and gemcitabine, four cycles of docetaxel or pemetrexed, and best supportive care. In addition, the health states related to the response to the treatments (no progression, progressive disease) and vital status (alive and dead) were specified in the model.
Figure 2: The structure of Markov model for patients tested positive for EGFR gene mutation.
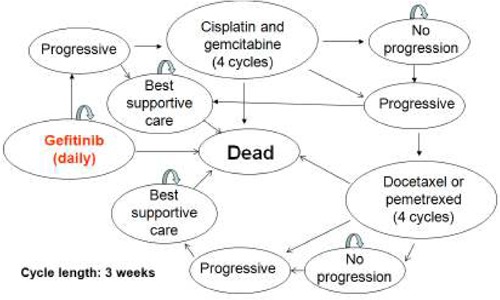
Outcomes
The incremental cost-effectiveness ratio (ICER) of the strategy of EGFR gene mutation testing (versus no testing) was calculated for the base-case analysis for both patient life years (cost per life year) and QALYs (cost per QALY).
Resource Use and Costs
All direct medical costs spent on patients with advanced NSCLC were collected using the perspective of the MOHLTC.
Direct medical costs were classified by the following items:
EGFR gene mutation testing: According to expert consultation, it costs $500 per test for the assessment of EGFR gene mutation status.
Treatment with gefitinib per cycle: the drug cost was $1514.31 at the dose of 250 mg daily. The cost for general care was assumed to be the same as the general care cost for patients taking erlotinib, which was estimated at $540.(60)
Treatment with the combination of cisplatin and gemcitabine per cycle: according to the average doses of cisplatin (80 mg/m2 at day 2) and gemcitabine (1250 mg/m2 at days 1 and 8) per cycle and an average body surface area of 1.75 m2, the total cost of the combination was $491.75. Treatment and general care costs were assumed to be the same as the costs for treating patients with docetaxel, which were estimated at $453 for treatment-related costs and $582 for general care.(61)
Treatment with docetaxel per cycle: according to an average dose of docetaxel (75 mg/m2) per cycle and average body surface area (as above), the total drug cost was $1499.33. The costs related to treatment and general care were estimated at $453 and $582, respectively.
Treatment with pemetrexed per cycle: according to an average doses of pemetrexed (500 mg/m2) per cycle and average body surface area (as above), the total drug cost was $4865. The costs related to treatment and general care were assumed to be the same as those for patients treated with docetaxel, which were estimated at $453 for treatment-related costs and $582 for general care.
Treatment with erlotinib per cycle: the cost of the drug was $1698.06 at a dose of 150 mg daily. The cost for general care for patients under the treatment with erlotinib was estimated at $540.
Best supportive care per cycle: The cost for BSC was estimated at $582.
Probabilities and Utilities
The parameters in the model included probability variables, utility variables and cost variables. The cost variables were described in the section Resources and Costs. The following parameter estimates were used for the probability and utility variables.
Probability parameter estimates:
Distribution of squamous cell carcinoma in patients with advanced NSCLC: According to the Canadian Cancer Registry 1992 to 2007, 63,199 cases out of 274,013 patients diagnosed with NSCLC were squamous cell carcinoma.(62;63) The proportion of squamous cell carcinoma in patients with NSCLC in Canada was estimated at 23.1%, with a 95% confidence interval (CI) from 22.9% to 23.2%.
Prevalence of EGFR gene mutation in patients with NSCLC: 16.6% (95% CI: 15.0% to 18.2%)(Rosell, Moran et al. 2009).
Failure rate of EGFR gene mutation testing: 32.3% (95% CI: 27.1% to 37.5%) of cases fail due to inadequate tissue and 1.8% (95% CI: 0% to 3.9%) of cases fail due to other reasons.(25)
-
Efficacy of treatments: Hazard ratios (HRs) for progressive disease and death per cycle were calculated by pooling the arms of identified RCTs. Meta-analyses were performed to estimate HRs for both progressive disease and death using the trial arms from identified RCTs, which had the same treatment in similar patient populations:
Gefitinib as first-line therapy in patients with EGFR gene mutation (2 RCTs with 202 patients)(49;50): HR for progressive disease per cycle: 0.0529 (95% CI: 0.0293 to 0.0938); HR for death per cycle: 0.0170 (95% CI: 0.0059 to 0.0478).
Cisplatin and gemcitabine as first-line therapy in patients with advanced NSCLC (25 RCTs with 4148 patients)(50;64-87): HR for progressive disease per cycle: 0.0982 (95% CI: 0.0886 to 0.1111); HR for death per cycle: 0.0513 (95% CI: 0.0447 to 0.0588).
Docetaxel as second-line therapy in patients with advanced NSCLC (15 RCTs with 1853 patients)(88-102): HR for progressive disease per cycle: 0.1888 (95% CI: 0.1625 to 0.2182); HR for death per cycle: 0.0748 (95% CI: 0.0636 to 0.0877).
Pemetrexed as second-line therapy in patients with non-squamous cell carcinoma NSCLC (2 RCTs with 578 patients(93;103): HR for progressive disease per cycle: 0.1900 (95% CI: 0.1601 to 0.2241); HR for death per cycle: 0.0706 (95% CI: 0.0524 to 0.0947).
Erlotinib as third-line therapy in patients with advanced NSCLC (2 RCTs with 513 patients)(29;104): HR for progressive disease per cycle: 0.2340 (95% CI: 0.2000 to 0.2730); HR for death per cycle: 0.0773 (95% CI: 0.0571 to 0.1038).
Best supportive care) (11 RCTs with 1333 patients)(65;105-114): HR for death per cycle: 0.1132 (95% CI: 0.0970 to 0.1318).
Utility estimates (health-related quality of life weights for QALY calculations):
-
The disease, the response after treatment, and the side effects of treatment could significantly affect the quality of life in patients with advanced NSCLC. One study applied multivariate linear regression analysis by taking the utility of patients with advanced NSCLC as the independent variable and calculating the intercept and coefficients for covariates, including the response after treatment and common side-effects caused by treatments. (115) The study arms of identified RCTs were reviewed to extract the distributions of clinical responses (stable disease, response and progressive disease) after treatment and the proportions of side-effects occurring during treatment. Meta-analyses were conducted on the extracted data to estimate the baseline value and 95% CI of the distributions of clinical responses after treatment and the proportions of side-effects. The results of the meta-analyses were applied using the above regression formula to derive the utilities for the following patients with advanced NSCLC:
Patients with EGFR gene mutation under the treatment with gefitinib as first-line therapy (2 RCTs with 202 patients): 0.5698.
Patients under the treatment with cisplatin and gemcitabine as first-line therapy (25 RCTs with 4148 patients): 0.5353.
Patients after the treatment with cisplatin and gemcitabine as first-line therapy (25 RCTs with 4148 patients): 0.6166.
Patients under the treatment with docetaxel as second-line therapy (15 RCTs with 1853 patients): 0.4537.
Patients after the treatment with docetaxel as second-line therapy (15 RCTs with 1853 patients): 0.5704.
Patients with non-squamous cell carcinoma under the treatment with pemetrexed as second-line therapy (2 RCTs with 578 patients): 0.5362.
Patients with non-squamous cell carcinoma after the treatment with pemetrexed as second-line therapy (2 RCTs with 578 patients): 0.5865.
Patients under the treatment with erlotinib as third-line therapy (2 RCTs with 513 patients): 0.4798.
Patients under best supportive care (11 RCTs with 1333 patients): 0.4734.
Sensitivity Analysis
One-way sensitivity analyses were conducted to identify the main variables affecting the ICER value associated with the strategy of EGFR gene mutation testing. Probabilistic sensitivity analyses (PSA) were also conducted to assess the relative percentage cost-effectiveness of the strategy of EGFR gene mutation testing under different willingness to pay (WTP) values (range of $0 to $100,000).
Results and Discussion
The base case analysis was conducted by applying the parameter point estimates (baseline values) in the model for the two analyses of interest, calculating the incremental cost per life year gained (cost-effectiveness analysis - CEA) (Table 5) and the incremental cost per QALY gained (cost-utility analysis - CUA) (Table 6).
Table 6: Base-case analysis results for the cost-utility analysis (cost per QALY).
| Strategy | Cost | Incr Cost | QALY | Incr QALY | ICER |
|---|---|---|---|---|---|
| No EGFR mutation testing | $14,368 | 0.2881 | |||
| EGFR mutation testing | $16,857 | $2,488 | 0.3188 | 0.0307 | $81,071 |
Under the strategy of no EGFR gene mutation testing, the average lifetime benefit associated with patients with advanced NSCLC was 0.4842 life years or 0.2881 QALY; the average lifetime direct medical cost spent on patients was $14,368.
Under the strategy of EGFR gene mutation testing, the average lifetime benefit collected for patients with advanced NSCLC was 0.5383 life years or 0.3188 QALY; the average lifetime direct medical cost consumed by patients was $16,857.
Compared to the strategy of no EGFR gene mutation testing, the ICER for the strategy of EGFR gene mutation testing was $46,021 per life year or $81,071 per QALY.
One-way sensitivity analysis was conducted to explore the impact of varying the range of each variable in the model by its 95% CI, or by increasing/decreasing the baseline value by 50%. Note that the latter was done only for variables without a reported CI. The one-way sensitivity analyses indicated that the ICER of EGFR gene mutation testing could increase over $10,000 per QALY based on the range in values of the following variables:
Cost of EGFR gene mutation testing: ICER increased $12, 387 when the variable ranged from $250 to $750.
Cost of medical care for patients taking gefitinib as first-line therapy: ICER increased $33,016 when the variable ranged from $270 to $810.
Probability of disease progression per cycle in patients taking gefitinib as first-line therapy: ICER increased $35,937 when the variable ranged from 0.0289 to 0.0895.
Probability of death per cycle in patients taking gefitinib as first-line therapy: ICER increased $67,588 when the variable ranged from 0.0059 to 0.0467.
Cost of gefitinib per day: ICER increased $92,586 when the variable ranged from $36.06 to $108.17.
Furthermore, one-way sensitivity analyses indicated that the ICER of EGFR gene mutation testing could decrease over $10,000 per QALY by varying the range of the following variables:
Cost of erlotinib per day: ICER decreased $16,866 when the variable ranged from $40.43 to $121.29.
Cost of medical care for patients taking cisplatin and gemcitabine: ICER decreased $18,052 when the variable ranged from $517.50 to $1552.50.
A PSA was also performed by varying the values of all variables based on certain distributions as a means to explore the impact of overall uncertainty on the ICER of EGFR gene mutation testing. A Monte Carlo simulation with 20,000 trials was run to generate the mean and 95% credible intervals for both benefits and lifetime direct medical costs associated with patients with advanced NSCLC under the two strategies.
The PSA projected that the average life years, QALY, and lifetime medical costs were 0.469 years (95% CI 0.387 to 0.562), 0.276 years (95% CI 0.223 to 0.337), and $13,543 (95% CI $6,081 to $23,533) for patients under the strategy of no EGFR gene mutation testing and 0.522 years (95% CI 0.421 to 0.670), 0.305 years (95% CI 0.242 to 0.392), and $16,067 (95% CI $8,741 to $25,866) for patients under the strategy of EGFR gene mutation testing respectively.
In addition, a cost-effectiveness acceptability curve was generated to explore the association between WTP per QALY and the percentage cost-effectiveness of EGFR gene mutation testing. The proportions of simulations in which EGFR gene mutation testing was cost-effective under the WTP of $50,000 and $100,000 were 5.2% and 56.1% respectively.
Supplementary Analysis
According to lung cancer clinical experts, erlotinib has been used as third or fourth-line therapy for patients with NSCLC irrespective of their EGFR gene mutation status in Ontario. Therefore, this supplementary analysis modified the structure of the Markov model by allowing all patients (EGFR gene mutation positive, negative, undetermined) to receive erlotinib after the failure of docetaxel or pemetrexed under the strategy of EGFR gene mutation testing. After the modifications, a base case analysis was conducted by applying the baseline value of the variables in the model. Compared to the strategy of no testing, the ICER for the strategy of EGFR gene mutation testing was $45,338 per life year or $81,807 per QALY.
Budget Impact Analysis
Budget impact analyses were conducted to explore the distribution of lifetime direct medical costs by using the average costs calculated by the Markov cohort model under the two strategies. Total yearly costs were projected by aggregating health care expenditures on newly diagnosed patients with advanced NSCLC from 2011 to 2015 in Ontario. Based on the estimated incidence of lung cancer in Canada in 2010 by the Canadian Cancer Society (57 per 100,000) (116), and the proportion of advanced NSCLC (50%) among patients with lung cancer (2;117), the estimated number of newly diagnosed advanced NSCLC cases in Ontario in 2010 was estimated at 3,535; the general population in Ontario reported by the 2006 Canada Census was used in this calculation (62).
By assuming that the yearly incidence of advanced NSCLC in Ontario from 2011 to 2015 would be the same as that in 2010, and EGFR gene mutation testing was performed once for any newly diagnosed patients with advanced NSCLC from 2011 to 2015, patients under the strategy of EGFR gene mutation testing in Ontario were projected to cost $4.6M, $7.0M, $7.9M, $8.1M, and $8.1M more a year from 2011 to 2015, respectively, when compared to the patients under the strategy of no testing (Table 7).
Table 7: The projected increase of health care expenditure on patients with NSCLC in Ontario by year from 2011 to 2015 under the strategy of EGFR gene mutation testing.
| Type of cost | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Testing | $1,343,300 | $1,343,300 | $1,343,300 | $1,343,300 | $1,343,300 |
| Gefitinib | $9,162,660 | $11,855,280 | $12,637,524 | $12,778,795 | $12,778,795 |
| Cisplatin plus gemcitabine | -$1,339,710 | -$1,146,106 | -$1,089,861 | -$1,079,703 | -$1,079,703 |
| Docetaxel | -$169,783 | -$160,540 | -$151,295 | -$149,378 | -$149,378 |
| Pemetrexed | -$1,320,419 | -$1,248,581 | -$1,176,673 | -$1,161,757 | -$1,161,757 |
| No progression | -$1,431,651 | -$1,410,989 | -$1,321,666 | -$1,302,277 | -$1,302,277 |
| Erlotinib | -$1,665,416 | -$2,344,001 | -$2,408,515 | -$2,412,453 | -$2,412,453 |
| Best supportive care | $1,543 | $68,840 | $39,141 | $63,700 | $65,009 |
| Total | $4,580,523 | $6,957,204 | $7,871,954 | $8,080,226 | $8,081,535 |
Economic Analysis Conclusion
Applying EGFR gene mutation testing to guide the use of gefitinib as first-line therapy for patients with advanced NSCLC is cost-effective if the willingness to pay is above $81,000 per QALY. The cost-effectiveness of EGFR gene mutation testing is sensitive to the efficacy and cost of gefitinib. The use of erlotinib after the failure of docetaxel or pemetrexed in patients with known EGFR gene mutation status does not affect the cost-effectiveness of EGFR gene mutation testing.
Appendix 1: Literature Search Strategies
Final Search-EGFR Mutation testing as Predictor of Response to Tyrosine Kinase inhibitors
EGFR – Final Search
Search date: March 9, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1996 to February Week 4 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Carcinoma, Non-Small-Cell Lung/ (17283)
(nsclc or non-small cell lung cancer).ti,ab. (15062)
1 or 2 (19597)
exp Receptor, Epidermal Growth Factor/ (14194)
(epidermal growth factor receptor* or EGFR or EGF receptor*).ti,ab. (18496)
"EC 2-7-10-1 (Receptor, Epidermal Growth Factor)".rn. (14194)
or/4-6 (21219)
3 and 7 (1977)
limit 8 to (english language and humans and yr="2004-Current") (1584)
Database: EMBASE <1980 to 2010 Week 09>
Search Strategy:
---------------------------------------------------------------
exp lung non small cell cancer/ (25172)
(nsclc or non-small cell lung cancer).ti,ab. (18614)
1 or 2 (27617)
exp epidermal growth factor receptor/ (21357)
(epidermal growth factor receptor* or EGFR or EGF receptor*).ti,ab. (23242)
4 or 5 (29269)
3 and 6 (2893)
limit 7 to (human and english language and yr="2004-Current") (2232)
Appendix 2: GRADE of Evidence
Table A1: GRADE table for EGFR mutation testing in the first-line setting.
| Studies | Quality Assessment | Summary | ||||
|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other Modifying Factors |
Overall Quality | |
| Mok, 2009 Lee, 2009 Maemondo, 2010, Mitsudomi, 2010 |
Observational, prospective |
None |
None |
None |
None |
MODERATE |
|
MODERATE |
MODERATE |
MODERATE |
MODERATE |
MODERATE |
||
Table A2: GRADE table for EGFR mutation testing in the second- or third-line setting.
| Studies | Quality Assessment | |||||
|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other Modifying Factors |
Overall Quality |
|
| Zhu, 2008 Miller, 2009 Schneider, 2009 Felip, 2008 Ahn, 2008 |
Observational, retrospective |
Some Limitations |
None |
None |
None |
LOW |
|
MODERATE |
(-1) LOW |
LOW |
LOW |
LOW |
||
Table A3: GRADE table for EGFR mutation testing in the second- or third-line setting.
| Studies | Quality Assessment | Summary of Findings |
||||
|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other Modifying Factors |
Overall Quality | |
|
Douilard, 2010 Hirsch, 2006 |
Observational, prospective |
Some limitations |
No inconsistency |
None |
None |
LOW |
| MODERATE | (-1) MODERATE |
LOW | LOW | LOW | ||
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Epidermal growth factor receptor (EGFR) genetic testing for prediction of response to EGFR-targeted (TKI) drugs in patients with advanced non-small-cell lung cancer: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 Dec [cited YYYY MM DD]; 10(24) 1-48. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/EGFR_2010_1209.pdf
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-4829-8 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Abbreviations
- OHTAC
Ontario Health Technology Advisory Committee
- MAS
Medical Advisory Secretariat
- NSCLC
Non-small cell lung cancer
- TKI
Tyrosine kinase inhibitor
- TK
Tyrosine kinase
- EGFR
Epidermal growth factor receptor
- PFS
Progression-free survival
- ORR
Objective response rate
- OS
Overall survival
- QoL
Quality of life
- CI
Confidence interval(s)
- OR
Odds ratio
- RCT
Randomized controlled trial
- HR
Hazard ratio
- ATP
Adenosine triphosphate
- BSC
Best supportive care
- NAA
Nucleic acid amplification
- IHC
Immunohistochemistry
- FISH
Fluorescence in situ hybridization
- CISH
Chromomeric in situ hybridization
- qPCR
Quantitative polymerase chain reaction
- ARMS
Amplification refractory mutations system
References
- 1.Canadian Cancer Society's Steering Committee. Canadian cancer statistics 2009. 2009. Apr, [[cited: 2010 Mar 3].]. [Internet]. Toronto: Canadian Cancer Society. 124 p. Available from: http://tinyurl.com/d885qh .
- 2.Franceschi S, Bidoli E. The epidemiology of lung cancer Ann Oncol. Suppl. 1999;5(10):S3–S6. doi: 10.1093/annonc/10.suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla E, Gazdar A. Pathogenesis of lung cancer signalling pathways: roadmap for therapies. Eur Respir J. 2009;33(6):1485–97. doi: 10.1183/09031936.00014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bülzebruck H, Bopp R, Drings P, Bauer E, Krysa S, Probst G, et al. New aspects in the staging of lung cancer. Prospective validation of the International Union Against Cancer TNM classification Cancer. 1992;70(5):1102–10. doi: 10.1002/1097-0142(19920901)70:5<1102::aid-cncr2820700514>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2010;128(1):452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 6.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the united states over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 7.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2009;346(2):92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S. Purification of the receptor for epidermal growth factor from A-431 cells: its function as a tyrosyl kinase. Methods Enzymol. 1983. pp. 379–87. [DOI] [PubMed]
- 9.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 10.Wells A. Molecules in focus: EGF receptor. Int J Biochem Cell Biol. 1999;31(6):637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 11.Alexander L. EGF receptor as a therapeutic target. Lung Cancer. 2003;41(1):S9–14. doi: 10.1016/s0169-5002(03)00134-x. Suppl. [DOI] [PubMed] [Google Scholar]
- 12.Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: A promising therapeutic target in solid tumors. Semin Oncol. 2003;1(1):3–11. doi: 10.1053/sonc.2003.50027. Suppl. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Chang A. Molecular predictors of EGFR-TKI sensitivity in advanced non-small cell lung cancer. Int J Med Sci. 2008;5(4):209–17. doi: 10.7150/ijms.5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2009;358(11):1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003;21(12):2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol. 2004;22(16):3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 18.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 19.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 20.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232–41. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 21.Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11(3):190–8. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- 22.John T, Liu G, Tsao M. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28(S1):S14–S23. doi: 10.1038/onc.2009.197. [DOI] [PubMed] [Google Scholar]
- 23.Giaccone G. HER1/EGFR-targeted agents: predicting the future for patients with unpredictable outcomes to therapy. Ann Oncol. 2005;16(4):538–48. doi: 10.1093/annonc/mdi129. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal growth factor receptor inhibitors in non-small-cell lung cancer working group: standardization for use in the clinical trial setting. J Clin Oncol. 2008;26(6):983–94. doi: 10.1200/JCO.2007.12.9858. [DOI] [PubMed] [Google Scholar]
- 25.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 26.Parra HS, Cavina R, Latteri F, Zucali PA, Campagnoli E, Morenghi E, et al. Analysis of epidermal growth factor receptor expression as a predictive factor for response to gefitinib ('Iressa', ZD1839) in non-small-cell lung cancer. Br J Cancer. 2004;91(2):208–12. doi: 10.1038/sj.bjc.6601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch FR, Dziadziuszko R, Thatcher N, Mann H, Watkins C, Parums DV, et al. Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced nonsmall-cell lung cancer. Cancer. 2008;112(5):1114–21. doi: 10.1002/cncr.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch FR, Varella-Garcia M, Bunn PA Jr, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24(31):5034–42. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 30.Clark GM, Zborowski DM, Culbertson JL, Whitehead M, Savoie M, Seymour L, et al. Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non-small cell lung cancer for treatment with erlotinib. J Thorac Oncol. 2006;1(8):837–46. [PubMed] [Google Scholar]
- 31.Hirsch FR, Varella-Garcia M, Cappuzzo F, McCoy J, Bemis L, Xavier AC, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2007;18(4):752–60. doi: 10.1093/annonc/mdm003. [DOI] [PubMed] [Google Scholar]
- 32.Zhu CQ, da Cunha SG, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26(26):4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 33.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremnes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21(20):3798–807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 35.Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(28):6829–37. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 36.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97(9):643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 37.Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23(22):5007–18. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23(28):6838–45. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]
- 39.Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23(31):8081–92. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 40.Dziadziuszko R, Witta SE, Cappuzzo F, Park S, Tanaka K, Danenberg PV, et al. Epidermal growth factor receptor messenger RNA expression, gene dosage, and gefitinib sensitivity in non-small cell lung cancer. Clin Cancer Res. 2006;12(10):3078–84. doi: 10.1158/1078-0432.CCR-06-0106. [DOI] [PubMed] [Google Scholar]
- 41.Sone T, Kasahara K, Kimura H, Nishio K, Mizuguchi M, Nakatsumi Y, et al. Comparative analysis of epidermal growth factor receptor mutations and gene amplification as predictors of gefitinib efficacy in Japanese patients with nonsmall cell lung cancer. Cancer. 2007;109(9):1836–44. doi: 10.1002/cncr.22593. [DOI] [PubMed] [Google Scholar]
- 42.Ichihara S, Toyooka S, Fujiwara Y, Hotta K, Shigematsu H, Tokumo M, et al. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer. 2007;120(6):1239–47. doi: 10.1002/ijc.22513. [DOI] [PubMed] [Google Scholar]
- 43.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: The Swedish Council on Technology Assessment in Health Care. 1993. 81 p. SBU-Report No 119E. [DOI] [PubMed]
- 45.Blue Cross and Blue Shield Association. Epidermal growth factor receptor mutations and tyrosine kinase inhibitor therapy in advanced non-small-cell lung cancer [Internet]. Chicago: Blue Cross and Blue Shield Association. 2007. [[cited: 2010 Jun 3]]. 21 p. Volume 22, No. 6. Available from: http://www.bcbs.com/blueresources/tec/vols/22/22_06.pdf .
- 46.Mujoomdar M, Moulton K, Spry C. Epidermal growth factor receptor mutation analysis in advanced non-small cell lung cancer: A review of the clinical effectiveness and guidelines [Internet] Ottawa: Canadian Agency for Drugs and Technologies in Health. 2010. 28 p. Available from: http://www.cadth.ca/media/pdf/M0017_EGFR_Testing_for_NSCLC_e.pdf .
- 47.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Park K, Kim S, Lee JS, Park K, Kim SW;, et al. A randomized phase III study of gefitinib (IRESSATM) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung. Presented at 13th World Conference on Lung Cancer. Jul, 2009. 2009. 31 to August 4th. San Francisco, CA. Pres. 4.
- 49.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 50.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 51.Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26(9):1472–8. doi: 10.1200/JCO.2007.13.0062. [DOI] [PubMed] [Google Scholar]
- 52.Schneider CP, Heigener D, Schott-von-Romer K, Gutz S, Laack E, Digel W, et al. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: an analysis of patients from German centers in the TRUST study. J Thorac Oncol. 2008;3(12):1446–53. doi: 10.1097/JTO.0b013e31818ddcaa. [DOI] [PubMed] [Google Scholar]
- 53.Felip E, Rojo F, Reck M, Heller A, Klughammer B, Sala G, et al. A phase II pharmacodynamic study of erlotinib in patients with advanced non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res. 2008;14(12):3867–74. doi: 10.1158/1078-0432.CCR-07-5186. [DOI] [PubMed] [Google Scholar]
- 54.Ahn MJ, Park BB, Ahn JS, Kim SW, Kim HT, Lee JS, et al. Are there any ethnic differences in molecular predictors of erlotinib efficacy in advanced non-small cell lung cancer? Clin Cancer Res. 2008;14(12):3860–6. doi: 10.1158/1078-0432.CCR-07-4608. [DOI] [PubMed] [Google Scholar]
- 55.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28(5):744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 56.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23(4):857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 57.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 58.Dahabreh IJ, Linardou H, Siannis F, Kosmidis P, Bafaloukos D, Murray S. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2010;16(1):291–303. doi: 10.1158/1078-0432.CCR-09-1660. [DOI] [PubMed] [Google Scholar]
- 59.De LA, Normanno N. Predictive biomarkers to tyrosine kinase inhibitors for the epidermal growth factor receptor in non-small-cell lung cancer. Curr Drug Targets. 2010;11(7):851–64. doi: 10.2174/138945010791320773. [DOI] [PubMed] [Google Scholar]
- 60.Bradbury PA, Tu D, Seymour L, Isogai PK, Zhu L, Ng R, et al. Economic analysis: randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer. J Natl Cancer Inst. 2010;102(5):298–306. doi: 10.1093/jnci/djp518. [DOI] [PubMed] [Google Scholar]
- 61.Leighl NB, Shepherd FA, Kwong R, Burkes RL, Feld R, Goodwin PJ. Economic analysis of the TAX 317 trial: docetaxel versus best supportive care as second-line therapy of advanced non-small-cell lung cancer. J Clin Oncol. 2002;20(5):1344–52. doi: 10.1200/JCO.2002.20.5.1344. [DOI] [PubMed] [Google Scholar]
- 62.Statistics Canada. Canada Census [Internet] 2006. 2006. [[cited: 2010 Dec 16]]. Statistics Canada. Available from: http://www.fin.gov.on.ca/en/economy/demographics/census/cenhi06-1.html .
- 63.Statistics Canada. Canadian Cancer Registry [Internet] Ottawa: Statistics Canada. 2007 [cited: 2010 Dec 16]. Available from: http://www.statcan.gc.ca/cgibin/imdb/p2SV.pl?Function=getSurvey&SurvId=3207&SurvVer=0&InstaId=15214&InstaVer=10&SDDS=3207&lang=en&db=imdb&adm=8&dis=2 .
- 64.Alberola V, Camps C, Provencio M, Isla D, Rosell R, Vadell C, et al. Cisplatin plus gemcitabine versus a cisplatin-based triplet versus nonplatinum sequential doublets in advanced non-small-cell lung cancer: a Spanish Lung Cancer Group phase III randomized trial. J Clin Oncol. 2003;21(17):3207–13. doi: 10.1200/JCO.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155–63. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Cardenal F, Lopez-Cabrerizo MP, Anton A, Alberola V, Massuti B, Carrato A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17(1):12–8. doi: 10.1200/JCO.1999.17.1.12. [DOI] [PubMed] [Google Scholar]
- 67.Ceribelli A, Gridelli C, De MF, Fabi A, Gamucci T, Cortesi E, et al. Prolonged gemcitabine infusion in advanced non-small cell lung carcinoma: a randomized phase II study of two different schedules in combination with cisplatin. Cancer. 2003;98(2):337–43. doi: 10.1002/cncr.11501. [DOI] [PubMed] [Google Scholar]
- 68.Chang JW, Tsao TC, Yang CT, Lin MC, Cheung YC, Liaw CC, et al. A randomized study of gemcitabine plus cisplatin and vinorelbine plus cisplatin in patients with advanced non-small-cell lung cancer. Chang Gung Med J. 2008;31(6):559–66. [PubMed] [Google Scholar]
- 69.Crino L, Scagliotti GV, Ricci S, De MF, Rinaldi M, Gridelli C, et al. Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced non-small-cell lung cancer: A randomized phase III study of the Italian Lung Cancer Project. J Clin Oncol. 1999;17(11):3522–30. doi: 10.1200/JCO.1999.17.11.3522. [DOI] [PubMed] [Google Scholar]
- 70.Grigorescu A, Ciuleanu T, Firoiu E, Muresan DR, Teodorescu G, Basson BR. A randomized phase II trial of sequential gemcitabine plus vinorelbine followed by gemcitabine plus ifosfamide versus gemcitabine plus cisplatin in the treatment of chemo-naive patients with stages III and IV non-small cell lung cancer (NSCLC) Lung Cancer. 2007;57(2):168–74. doi: 10.1016/j.lungcan.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Hsu C, Kuo SH, Hu FC, Cheng AL, Shih JY, Yu CJ, et al. Gemcitabine plus conventional-dose epirubicin versus gemcitabine plus cisplatin as first-line chemotherapy for stage IIIB/IV non-small cell lung carcinoma--a randomized phase II trial. Lung Cancer. 2008;62(3):334–43. doi: 10.1016/j.lungcan.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Kim JH, Kim SY, Jung KH, Park K, Suh CW, Lim HY, et al. Randomized phase II study of gemcitabine plus cisplatin versus etoposide plus cisplatin for the treatment of locally advanced or metastatic non-small cell lung cancer: Korean Cancer Study Group experience. Lung Cancer. 2006;52(1):75–81. doi: 10.1016/j.lungcan.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 73.Liao ML, Zhu YZ, Li LY, Wan HY, Yu SY, Hall B, et al. Gemcitabine and cisplatin treatment over a 3-week versus a 4-week dosing schedule: a randomized trial conducted in Chinese patients with nonsmall cell lung cancer. Chin Med J (Engl) 2008;121(10):892–7. [PubMed] [Google Scholar]
- 74.Martoni A, Marino A, Sperandi F, Giaquinta S, Di FF, Melotti B, et al. Multicentre randomised phase III study comparing the same dose and schedule of cisplatin plus the same schedule of vinorelbine or gemcitabine in advanced non-small cell lung cancer. Eur J Cancer. 2005;41(1):81–92. doi: 10.1016/j.ejca.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 75.Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18(2):317–23. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 76.Paz-Ares L, Douillard JY, Koralewski P, Manegold C, Smit EF, Reyes JM, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense, oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24(9):1428–34. doi: 10.1200/JCO.2005.04.3299. [DOI] [PubMed] [Google Scholar]
- 77.Pereira JR, Fein L, Del GA, Blajman CR, Richardet E, Schwartsmann G, et al. Gemcitabine administered as a short infusion versus a fixed dose rate in combination with cisplatin for the treatment of patients with advanced non-small cell lung cancer. Lung Cancer. 2007;58(1):80–7. doi: 10.1016/j.lungcan.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 78.Reck M, von PJ, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 79.Ricci S, Antonuzzo A, Galli L, Tibaldi C, Bertuccelli M, Lopes PA, et al. A randomized study comparing two different schedules of administration of cisplatin in combination with gemcitabine in advanced nonsmall cell lung carcinoma. Cancer. 2000;89(8):1714–9. doi: 10.1002/1097-0142(20001015)89:8<1714::aid-cncr10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 80.Rinaldi M, Crino L, Scagliotti GV, Mosconi AM, De MF, Gridelli C, et al. A three-week schedule of gemcitabine-cisplatin in advanced non-small-cell lung cancer with two different cisplatin dose levels: a phase II randomized trial. Ann Oncol. 2000;11(10):1295–300. doi: 10.1023/a:1008334610955. [DOI] [PubMed] [Google Scholar]
- 81.Rubio JC, Vazquez S, Vazquez F, Amenedo M, Firvida JL, Mel JR, et al. A phase II randomized trial of gemcitabine-docetaxel versus gemcitabine-cisplatin in patients with advanced non-small cell lung carcinoma. Cancer Chemother Pharmacol. 2009;64(2):379–84. doi: 10.1007/s00280-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 82.Sandler AB, Nemunaitis J, Denham C, von PJ, Cormier Y, Gatzemeier U, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18(1):122–30. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 83.Scagliotti GV, Parikh P, von PJ, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 84.Syrigos KN, Vansteenkiste J, Parikh P, von PJ, Manegold C, Martins RG, et al. Prognostic and predictive factors in a randomized phase III trial comparing cisplatin-pemetrexed versus cisplatin, gemcitabine in advanced non-small-cell lung cancer. Ann Oncol. 2010;21(3):556–61. doi: 10.1093/annonc/mdp392. [DOI] [PubMed] [Google Scholar]
- 85.Wachters FM, Van Putten JW, Kramer H, Erjavec Z, Eppinga P, Strijbos JH, et al. First-line gemcitabine with cisplatin or epirubicin in advanced non-small-cell lung cancer: a phase III trial. Br J Cancer. 2003;89(7):1192–9. doi: 10.1038/sj.bjc.6601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zatloukal P, Petruzelka L, Zemanova M, Kolek V, Skrickova J, Pesek M, et al. Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: a phase III randomized trial. Lung Cancer. 2003;41(3):321–31. doi: 10.1016/s0169-5002(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 87.Zwitter M, Kovac V, Smrdel U, Vrankar M, Zadnik V. Gemcitabine in brief versus prolonged low-dose infusion, both combined with cisplatin, for advanced non-small cell lung cancer: a randomized phase II clinical trial. J Thorac Oncol. 2009;4(9):1148–55. doi: 10.1097/JTO.0b013e3181ae280f. [DOI] [PubMed] [Google Scholar]
- 88.Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, et al. Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol. 2006;17(3):467–72. doi: 10.1093/annonc/mdj115. [DOI] [PubMed] [Google Scholar]
- 89.Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest. 2006;129(4):1031–8. doi: 10.1378/chest.129.4.1031. [DOI] [PubMed] [Google Scholar]
- 90.Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anticancer Drugs. 2006;17(4):401–9. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 91.Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, et al. Phase II randomised trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2005;16(1):90–6. doi: 10.1093/annonc/mdi018. [DOI] [PubMed] [Google Scholar]
- 92.Gridelli C, Gallo C, Di MM, Barletta E, Illiano A, Maione P, et al. A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer. 2004;91(12):1996–2004. doi: 10.1038/sj.bjc.6602241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De MF, von PJ, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 94.Jones S, Thompson D, Barton J, Patton J, Shipley D, Greco FA, et al. A randomized phase II trial of oral topotecan versus docetaxel in the second-line treatment of non-small-cell lung cancer. Clin Lung Cancer. 2008;9(3):154–9. doi: 10.3816/CLC.2008.n.023. [DOI] [PubMed] [Google Scholar]
- 95.Krzakowski M, Ramlau R, Jassem J, Szczesna A, Zatloukal P, von PJ, et al. Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. J Clin Oncol. 2010;28(13):2167–73. doi: 10.1200/JCO.2009.23.4146. [DOI] [PubMed] [Google Scholar]
- 96.Lai CL, Tsai CM, Chiu CH, Wang GS, Su WJ, Chen YM, et al. Phase II randomized trial of tri-weekly versus days 1 and 8 weekly docetaxel as a second-line treatment of advanced non-small cell lung cancer. Jpn J Clin Oncol. 2005;35(12):700–6. doi: 10.1093/jjco/hyi191. [DOI] [PubMed] [Google Scholar]
- 97.Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 98.Paz-Ares L, Ross H, O'Brien M, Riviere A, Gatzemeier U, von PJ, et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer. 2008;98(10):1608–13. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pectasides D, Pectasides M, Farmakis D, Kostopoulou V, Nikolaou M, Gaglia A, et al. Comparison of docetaxel and docetaxel-irinotecan combination as second-line chemotherapy in advanced non-small-cell lung cancer: a randomized phase II trial. Ann Oncol. 2005;16(2):294–9. doi: 10.1093/annonc/mdi053. [DOI] [PubMed] [Google Scholar]
- 100.Quoix E, Lebeau B, Depierre A, Ducolone A, Moro-Sibilot D, Milleron B, et al. Randomised, multicentre phase II study assessing two doses of docetaxel (75 or 100 mg/m2) as second-line monotherapy for non-small-cell lung cancer. Ann Oncol. 2004;15(1):38–44. doi: 10.1093/annonc/mdh005. [DOI] [PubMed] [Google Scholar]
- 101.Schuette W, Nagel S, Blankenburg T, Lautenschlaeger C, Hans K, Schmidt EW, et al. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol. 2005;23(33):8389–95. doi: 10.1200/JCO.2005.02.3739. [DOI] [PubMed] [Google Scholar]
- 102.Wachters FM, Groen HJ, Biesma B, Schramel FM, Postmus PE, Stigt JA, et al. A randomised phase II trial of docetaxel vs docetaxel and irinotecan in patients with stage IIIb-IV non-small-cell lung cancer who failed first-line treatment. Br J Cancer. 2005;92(1):15–20. doi: 10.1038/sj.bjc.6602268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cullen MH, Zatloukal P, Sorenson S, Novello S, Fischer JR, Joy AA, et al. A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2008;19(5):939–45. doi: 10.1093/annonc/mdm592. [DOI] [PubMed] [Google Scholar]
- 104.Lynch TJ, Fenton D, Hirsh V, Bodkin D, Middleman EL, Chiappori A, et al. A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(8):1002–9. doi: 10.1097/JTO.0b013e3181aba89f. [DOI] [PubMed] [Google Scholar]
- 105.Anderson H, Hopwood P, Stephens RJ, Thatcher N, Cottier B, Nicholson M, et al. Gemcitabine plus best supportive care (BSC) vs BSC in inoperable non-small cell lung cancer--a randomized trial with quality of life as the primary outcome. UK NSCLC Gemcitabine Group. 2000;83(4):447–53. doi: 10.1054/bjoc.2000.1307. Non-Small Cell Lung Cancer. Br J Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cellerino R, Tummarello D, Guidi F, Isidori P, Raspugli M, Biscottini B, et al. A randomized trial of alternating chemotherapy versus best supportive care in advanced non-small-cell lung cancer. J Clin Oncol. 1991;9(8):1453–61. doi: 10.1200/JCO.1991.9.8.1453. [DOI] [PubMed] [Google Scholar]
- 107.Goss G, Ferry D, Wierzbicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. 2009;27(13):2253–60. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennedy W, Reinharz D, Tessier G, Contandriopoulos AP, Trabut I, Champagne F, et al. Cost utility of chemotherapy and best supportive care in non-small cell lung cancer. Pharmacoeconomics. 1995;8(4):316–23. doi: 10.2165/00019053-199508040-00006. [DOI] [PubMed] [Google Scholar]
- 109.Neninger VE, de la TA, Osorio RM, Catala FM, Bravo I, Mendoza del PM, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–8. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 110.Rapp E, Pater JL, Willan A, Cormier Y, Murray N, Evans WK, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer--report of a Canadian multicenter randomized trial. J Clin Oncol. 1988;6(4):633–41. doi: 10.1200/JCO.1988.6.4.633. [DOI] [PubMed] [Google Scholar]
- 111.Roszkowski K, Pluzanska A, Krzakowski M, Smith AP, Saigi E, Aasebo U, et al. A multicenter, randomized, phase III study of docetaxel plus best supportive care versus best supportive care in chemotherapy-naive patients with metastatic or non-resectable localized non-small cell lung cancer (NSCLC) Lung Cancer. 2000;27(3):145–57. doi: 10.1016/s0169-5002(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 112.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 113.Thatcher N, Chang A, Parikh P, Rodrigues PJ, Ciuleanu T, von PJ, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 114.Thongprasert S, Sanguanmitra P, Juthapan W, Clinch J. Relationship between quality of life and clinical outcomes in advanced non-small cell lung cancer: best supportive care (BSC) versus BSC plus chemotherapy. Lung Cancer. 1999;24(1):17–24. doi: 10.1016/s0169-5002(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 115.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008. pp. 6–84. [DOI] [PMC free article] [PubMed]
- 116.Canadian Cancer Society. Lung cancer statistics [Internet]. Canadian Cancer Society. 2010. [[cited: 2010 Dec 16]]. Available from: www.cancer.ca/canada-wide/about%20cancer/cancer%20statistics/stats%20at%20a%20glance/lung%20cancer.aspx?sc_lang=en .
- 117.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(1):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. Suppl. [DOI] [PubMed] [Google Scholar]


