Background
In June 2008, the Medical Advisory Secretariat began work on the Diabetes Strategy Evidence Project, an evidence-based review of the literature surrounding strategies for successful management and treatment of diabetes. This project came about when the Health System Strategy Division at the Ministry of Health and Long-Term Care subsequently asked the secretariat to provide an evidentiary platform for the Ministry’s newly released Diabetes Strategy.
After an initial review of the strategy and consultation with experts, the secretariat identified five key areas in which evidence was needed. Evidence-based analyses have been prepared for each of these five areas: insulin pumps, behavioural interventions, bariatric surgery, home telemonitoring, and community based care. For each area, an economic analysis was completed where appropriate and is described in a separate report.
To review these titles within the Diabetes Strategy Evidence series, please visit the Medical Advisory Secretariat Web site, http://www.health.gov.on.ca/english/providers/program/mas/mas_about.html,
Diabetes Strategy Evidence Platform: Summary of Evidence-Based Analyses
Continuous Subcutaneous Insulin Infusion Pumps for Type 1 and Type 2 Adult Diabetics: An Evidence-Based Analysis
Behavioural Interventions for Type 2 Diabetes: An Evidence-Based Analysis
Bariatric Surgery for People with Diabetes and Morbid Obesity: An Evidence-Based Summary
Community-Based Care for the Management of Type 2 Diabetes: An Evidence-Based Analysis
Home Telemonitoring for Type 2 Diabetes: An Evidence-Based Analysis
Application of the Ontario Diabetes Economic Model (ODEM) to Determine the Cost-effectiveness and Budget Impact of Selected Type 2 Diabetes Interventions in Ontario
Objective
The objective of this report series is to provide an evidentiary platform for the Ontario Diabetes Strategy developed by the Health System Strategy Division (HSSD).
Clinical Need: Target Population and Condition
Diabetes is a highly prevalent chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. The majority (90%) of diabetes patients have type 2 diabetes and in 2005, an estimated 8.8% of Ontario’s population had diabetes, representing more than 816,000 Ontarians. Clinically, diabetes is the leading causes of blindness, end-stage renal disease, and non-traumatic amputation in Canadian adults and is a significant cause of cardiovascular complications, hypertension, stroke, cataracts, and glaucoma. In 2000, the direct health care cost of diabetes was $1.76 billion, a total that’s projected to rise to $3.14 billion by 2016.
Based on the United Kingdom Prospective Diabetes Study (UKPDS), intensive blood glucose and blood pressure control lower the risk of microvascular and macrovascular complications in type 2 diabetics. Specifically, a 1% reduction in HbA1c has been associated with a 10% reduction in diabetes-related mortality and a 25% reduction in microvascular end-points. Likewise, intensive blood pressure control is associated with a 32% reduction in risk of mortality from diabetes-associated conditions, two-thirds of which are cardiovascular diseases. Furthermore, tight blood pressure control is associated with a 34% reduction in the risk of macrovascular disease (including myocardial infarction, sudden death, stroke, and peripheral vascular disease), a 44% reduction in the risk of stroke, and a 37% reduction in the risk of microvascular disease.
Project Scope
On July 22, 2008 the government of Ontario announced an investment of $741 million in new funding over four years for a comprehensive Diabetes Strategy. Key elements of the Strategy included an online registry, improving access to insulin pumps and supplies, expanding chronic kidney disease services, expanding access to bariatric surgery, educational campaigns in high risk populations, and increasing access to team-based care.
An analysis was undertaken by the Medical Advisory Secretariat (MAS) to identify components of the Diabetes Strategy as well as other relevant components of care which lacked clear evidence. The analysis identified five areas amenable to review: continuous subcutaneous insulin infusion (CSII) pumps for type 1 adults and type 2 diabetics, bariatric surgery for people with diabetes and morbid obesity, community-based care for the management of type 2 diabetes, behavioural interventions1 for type 2 diabetes, and home telemonitoring for type 2 diabetes. Some components of the Diabetes Strategy were not suited for an evidence-based analysis, for example, diabetes registries, and thus were not reviewed in this report.
The following is a summary of evidence-based analyses of available medical literature around the five key researchable areas of the Diabetes Strategy. Where possible, economic analyses were performed using an Ontario-specific economic model for type 2 diabetes. As indicated, while some analyses may focus on both type 1 and type 2 diabetes, the majority of analyses centre on type 2.
Assessment of Quality of Evidence
In all analyses, the quality of the evidence was assessed as high, moderate, low or very low according to the GRADE methodology and GRADE Working Group (see Appendix 1). As per GRADE the following definitions apply:
| High: | Further research is very unlikely to change confidence in the estimate of effect |
| Moderate: | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate |
| Low: | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate |
| Very low: | Any estimate of effect is very uncertain |
CSII Pumps for Type 1 and Type 2 Adult Diabetics
Objective
The objective of this analysis is to review the efficacy of continuous subcutaneous insulin infusion (CSII) pumps as compared to multiple daily injections (MDI) for the type 1 and type 2 adult diabetics.
Clinical Need and Target Population
Insulin therapy is an integral component of the treatment of many individuals with diabetes. Type 1, or juvenile-onset diabetes, is a life-long disorder that commonly manifests in children and adolescents, but onset can occur at any age. It represents about 10% of the total diabetes population and involves immune-mediated destruction of insulin producing cells in the pancreas. The loss of these cells results in a decrease in insulin production, which in turn necessitates exogenous insulin therapy.
Type 2, or ‘maturity-onset’ diabetes represents about 90% of the total diabetes population and is marked by a resistance to insulin or insufficient insulin secretion. The risk of developing type 2 diabetes increases with age, obesity, and lack of physical activity. The condition tends to develop gradually and may remain undiagnosed for many years. Approximately 30% of patients with type 2 diabetes eventually require insulin therapy.
Description of Technology
In conventional therapy programs for diabetes, insulin is injected once or twice a day in some combination of short- and long-acting insulin preparations. Some patients require intensive therapy regimes known as multiple daily injection (MDI) programs, in which insulin is injected three or more times a day. It’s a time consuming process and usually requires an injection of slow acting basal insulin in the morning or evening and frequent doses of short-acting insulin prior to eating. The most common form of slower acting insulin used is neutral protamine gagedorn (NPH), which reaches peak activity 3 to 5 hours after injection. There are some concerns surrounding the use of NPH at night-time as, if injected immediately before bed, nocturnal hypoglycemia may occur. To combat nocturnal hypoglycemia and other issues related to absorption, alternative insulins have been developed, such as the slow-acting insulin glargine. Glargine has no peak action time and instead acts consistently over a twenty-four hour period, helping reduce the frequency of hypoglycemic episodes.
Alternatively, intensive therapy regimes can be administered by continuous insulin infusion (CSII) pumps. These devices attempt to closely mimic the behaviour of the pancreas, continuously providing a basal level insulin to the body with additional boluses at meal times. Modern CSII pumps are comprised of a small battery-driven pump that is designed to administer insulin subcutaneously through the abdominal wall via butterfly needle. The insulin dose is adjusted in response to measured capillary glucose values in a fashion similar to MDI and is thus often seen as a preferred method to multiple injection therapy. There are, however, still risks associated with the use of CSII pumps. Despite the increased use of CSII pumps, there is uncertainty around their effectiveness as compared to MDI for improving glycemic control.
Analysis of Effectiveness Part A: Type 1 Diabetic Adults (≥19 years)
An evidence-based analysis on the efficacy of CSII pumps compared to MDI was carried out on both type 1 and type 2 adult diabetic populations.
Research Questions
Are CSII pumps more effective than MDI for improving glycemic control in adults (≥19 years) with type 1 diabetes?
Are CSII pumps more effective than MDI for improving additional outcomes related to diabetes such as quality of life (QoL)?
Inclusion Criteria
Randomized controlled trials, systematic reviews, meta-analysis and/or health technology assessments from MEDLINE, EMBASE, CINAHL
Adults (≥ 19 years)
Type 1 diabetes
Study evaluates CSII vs. MDI
Published between January 1, 2002 – March 24, 2009
Patient currently on intensive insulin therapy
Exclusion Criteria
Studies with <20 patients
Studies <5 weeks in duration
CSII applied only at night time and not 24 hours/day
Mixed group of diabetes patients (children, adults, type 1, type 2)
Pregnancy studies
Outcomes of Interest
The primary outcomes of interest were glycosylated hemoglobin (HbA1c) levels, mean daily blood glucose, glucose variability, and frequency of hypoglycaemic events. Other outcomes of interest were insulin requirements, adverse events, and quality of life.
Search Strategy
The literature search strategy employed keywords and subject headings to capture the concepts of:
1) insulin pumps, and
2) type 1 diabetes.
The search was run on July 6, 2008 in the following databases: Ovid MEDLINE (1996 to June Week 4 2008), OVID MEDLINE In-Process and Other Non-Indexed Citations, EMBASE (1980 to 2008 Week 26), OVID CINAHL (1982 to June Week 4 2008) the Cochrane Library, and the Centre for Reviews and Dissemination/International Agency for Health Technology Assessment. A search update was run on March 24, 2009 and studies published prior to 2002 were also examined for inclusion into the review. Parallel search strategies were developed for the remaining databases. Search results were limited to human and English-language published between January 2002 and March 24, 2009. Abstracts were reviewed, and studies meeting the inclusion criteria outlined above were obtained. Reference lists were also checked for relevant studies.
Summary of Findings
The database search identified 519 relevant citations published between 1996 and March 24, 2009. Of the 519 abstracts reviewed, four RCTs and one abstract met the inclusion criteria outlined above. While efficacy outcomes were reported in each of the trials, a meta-analysis was not possible due to missing data around standard deviations of change values as well as missing data for the first period of the crossover arm of the trial. Meta-analysis was not possible on other outcomes (quality of life, insulin requirements, frequency of hypoglycemia) due to differences in reporting.
HbA1c
In studies where no baseline data was reported, the final values were used. Two studies (Hanaire-Broutin et al. 2000, Hoogma et al. 2005) reported a slight reduction in HbA1c of 0.35% and 0.22% respectively for CSII pumps in comparison to MDI. A slightly larger reduction in HbA1c of 0.84% was reported by DeVries et al., however, this study was the only study to include patients with poor glycemic control marked by higher baseline HbA1c levels. One study (Bruttomesso et al. 2008) showed no difference between CSII pumps and MDI on Hba1c levels and was the only study using insulin glargine (consistent with results of parallel RCT in abstract by Bolli 2004). While there is statistically significant reduction in HbA1c in three of four trials, there is no evidence to suggest these results are clinically significant.
Mean Blood Glucose
Three of four studies reported a statistically significant reduction in the mean daily blood glucose for patients using CSII pump, though these results were not clinically significant. One study (DeVries et al. 2002) did not report study data on mean blood glucose but noted that the differences were not statistically significant. There is difficulty with interpreting study findings as blood glucose was measured differently across studies. Three of four studies used a glucose diary, while one study used a memory meter. In addition, frequency of self monitoring of blood glucose (SMBG) varied from four to nine times per day. Measurements used to determine differences in mean daily blood glucose between the CSII pump group and MDI group at clinic visits were collected at varying time points. Two studies use measurements from the last day prior to the final visit (Hoogma et al. 2005, DeVries et al. 2002), while one study used measurements taken during the last 30 days and another study used measurements taken during the 14 days prior to the final visit of each treatment period.
Glucose Variability
All four studies showed a statistically significant reduction in glucose variability for patients using CSII pumps compared to those using MDI, though one, Bruttomesso et al. 2008, only showed a significant reduction at the morning time point. Brutomesso et al. also used alternate measures of glucose variability and found that both the Lability index and mean amplitude of glycemic excursions (MAGE) were in concordance with the findings using the standard deviation (SD) values of mean blood glucose, but the average daily risk range (ADRR) showed no difference between the CSII pump and MDI groups.
Hypoglycemic Events
There is conflicting evidence concerning the efficacy of CSII pumps in decreasing both mild and severe hypoglycemic events. For mild hypoglycemic events, DeVries et al. observed a higher number of events per patient week in the CSII pump group than the MDI group, while Hoogma et al. observed a higher number of events per patient year in the MDI group. The remaining two studies found no differences between the two groups in the frequency of mild hypoglycemic events. For severe hypoglycemic events, Hoogma et al. found an increase in events per patient year among MDI patients, however, all of the other RCTs showed no difference between the patient groups in this aspect.
Insulin Requirements and Adverse Events
In all four studies, insulin requirements were significantly lower in patients receiving CSII pump treatment in comparison to MDI. This difference was statistically significant in all studies. Adverse events were reported in three studies. Devries et al. found no difference in ketoacidotic episodes between CSII pump and MDI users. Bruttomesso et al. reported no adverse events during the study. Hanaire-Broutin et al. found that 30 patients experienced 58 serious adverse events (SAEs) during MDI and 23 patients had 33 SAEs during treatment out of a total of 256 patients. Most events were related to severe hypoglycemia and diabetic ketoacidosis.
Quality of Life and Patient Preference
QoL was measured in three studies and patient preference was measured in one. All three studies found an improvement in QoL for CSII users compared to those using MDI, although various instruments were used among the studies and possible reporting bias was evident as non-positive outcomes were not consistently reported. Moreover, there was also conflicting results in two of the studies using the Diabetes Treatment Satisfaction Questionnaire (DTSQ). DeVries et al. reported no difference in treatment satisfaction between CSII pump users and MDI users while Brutomesso et al. reported that treatment satisfaction improved among CSII pump users.
Patient preference for CSII pumps was demonstrated in just one study (Hanaire-Broutin et al. 2000) and there are considerable limitations with interpreting this data as it was gathered through interview and 72% of patients that preferred CSII pumps were previously on CSII pump therapy prior to the study. As all studies were industry sponsored, findings on QoL and patient preference must be interpreted with caution.
Quality of Evidence
Overall, the body of evidence was downgraded from high to low due to study quality and issues with directness as identified using the GRADE quality assessment tool (see Table 1) While blinding of patient to intervention/control was not feasible in these studies, blinding of study personnel during outcome assessment and allocation concealment were generally lacking. Trials reported consistent results for the outcomes HbA1c, mean blood glucose and glucose variability, but the directness or generalizability of studies, particularly with respect to the generalizability of the diabetic population, was questionable as most trials used highly motivated populations with fairly good glycemic control. In addition, the populations in each of the studies varied with respect to prior treatment regimens, which may not be generalizable to the population eligible for pumps in Ontario. For the outcome of hypoglycaemic events the evidence was further downgraded to very low since there was conflicting evidence between studies with respect to the frequency of mild and severe hypoglycaemic events in patients using CSII pumps as compared to CSII (see Table 2). The GRADE quality of evidence for the use of CSII in adults with type 1 diabetes is therefore low to very low and any estimate of effect is, therefore, uncertain.
Table 1: GRADE Quality Assessment for CSII pumps vs. MDI on HbA1c, Mean Blood Glucose, and Glucose Variability For Adults with Type 1 Diabetes.
| Outcome | Study | Design | Study Quality |
Consistency | Directness | Other modifying factors |
Overall quality of evidence |
|---|---|---|---|---|---|---|---|
| HbA1c |
Hanaire-Broutin 2000 |
RCT |
Serious limitations* |
Consistency† |
Indirect‡ |
||
| Mean Blood Glucose | Brutomesso 2008 | RCT | Not applicable | ||||
| Glucose Variability | DeVries 2002 | RCT | LOW | ||||
| Hoogma 2005 | RCT HIGH |
MODERATE | MODERATE | LOW |
Inadequate or unknown allocation concealment (3/4 studies); Unblinded assessment (all studies) however lack of blinding due to the nature of the study; No ITT analysis (2/4 studies); possible bias SMBG (all studies)
HbA1c: 3/4 studies show consistency however magnitude of effect varies greatly; Single study uses insulin glargine instead of NPH; Mean Blood Glucose: 3/4 studies show consistency however magnitude of effect varies between studies; Glucose Variability: All studies show consistency but 1 study only showed a significant effect in the morning
Generalizability in question due to varying populations: highly motivated populations, educational component of interventions/ run-in phases, insulin pen use in 2/4 studies and varying levels of baseline glycemic control and experience with intensified insulin therapy, pumps and MDI.
Table 2: GRADE Quality Assessment for CSII pumps vs. MDI on Frequency of Hypoglycemic Events For Adults with Type 1 Diabetes.
| Outcome | Study | Design | Study Quality |
Consistency | Directness | Other modifying factors |
Overall quality of evidence |
|---|---|---|---|---|---|---|---|
| Frequency of Hypoglycemic Events |
Hanaire-Broutin 2000 |
RCT |
Serious limitations* |
Inconsistent† |
Indirect‡ |
||
| Brutomesso 2008 | RCT | Not applicable | VERY LOW | ||||
| DeVries 2002 | RCT | ||||||
| Hoogma 2005 | RCT HIGH |
MODERATE | LOW | VERY LOW |
Inadequate or unknown allocation concealment (3/4 studies); Unblinded assessment (all studies) however lack of blinding due to the nature of the study; No ITT analysis (2/4 studies); possible bias SMBG (all studies)
Conflicting evidence with respect to mild and severe hypoglycemic events reported in studies
Generalizability in question due to varying populations: highly motivated populations, educational component of interventions/ run-in phases, insulin pen use in 2/4 studies and varying levels of baseline glycemic control and experience with intensified insulin therapy, pumps and MDI.
Economic Analysis
One article was included in the analysis from the economic literature scan. Four other economic evaluations were identified but did not meet our inclusion criteria. Two of these articles did not compare CSII with MDI and the other two articles used summary estimates from a mixed population with Type 1 and 2 diabetes in their economic microsimulation to estimate costs and effects over time. Included were English articles that conducted comparisons between CSII and MDI with the outcome of Quality Adjusted Life Years (QALY) in an adult population with type 1 diabetes.
From one study, a subset of the population with type 1 diabetes was identified that may be suitable and benefit from using insulin pumps. There is, however, limited data in the literature addressing the cost-effectiveness of insulin pumps versus MDI in type 1 diabetes. Longer term models are required to estimate the long term costs and effects of pumps compared to MDI in this population.
Conclusions
CSII pumps for the treatment of adults with type 1 diabetes
Based on low-quality evidence, CSII pumps confer a statistically significant but not clinically significant reduction in HbA1c and mean daily blood glucose as compared to MDI in adults with type 1 diabetes (>19 years).
CSII pumps also confer a statistically significant reduction in glucose variability as compared to MDI in adults with type 1 diabetes (>19 years) however the clinical significance is unknown.
There is indirect evidence that the use of newer long-acting insulins (e.g. insulin glargine) in MDI regimens result in less of a difference between MDI and CSII compared to differences between MDI and CSII in which older insulins are used.
There is conflicting evidence regarding both mild and severe hypoglycemic events in this population when using CSII pumps as compared to MDI. These findings are based on very low-quality evidence.
There is an improved quality of life for patients using CSII pumps as compared to MDI however, limitations exist with this evidence.
-
Significant limitations of the literature exist specifically:
All studies sponsored by insulin pump manufacturers
All studies used crossover design
Prior treatment regimens varied
Types of insulins used in study varied (NPH vs. glargine)
Generalizability of studies in question as populations were highly motivated and half of studies used insulin pens as the mode of delivery for MDI
One short-term study concluded that pumps are cost-effective, although this was based on limited data and longer term models are required to estimate the long-term costs and effects of pumps compared to MDI in adults with type 1 diabetes.
Analysis of Effectiveness Part B: Type 2 Diabetic Adults
Research Questions
Are CSII pumps more effective than MDI for improving glycemic control in adults (≥19 years) with type 2 diabetes?
Are CSII pumps more effective than MDI for improving other outcomes related to diabetes such as quality of life?
Inclusion Criteria
Randomized controlled trials, systematic reviews, meta-analysis and/or health technology assessments from MEDLINE, Excerpta Medica Database (EMBASE), Cumulative Index to Nursing & Allied Health Literature (CINAHL)
Any person with type 2 diabetes requiring insulin treatment intensive
Published between January 1, 2000 – August 2008
Exclusion Criteria
Studies with <10 patients
Studies <5 weeks in duration
CSII applied only at night time and not 24 hours/day
Mixed group of diabetes patients (children, adults, type 1, type 2)
Pregnancy studies
Outcomes of Interest
The primary outcome of interest was a reduction in glycosylated hemoglobin (HbA1c) levels. Other outcomes of interest were mean blood glucose level, glucose variability, insulin requirements, frequency of hypoglycemic events, adverse events, and quality of life.
Search Strategy
A comprehensive literature search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published between January 1, 2000 and August 15, 2008. Studies meeting the inclusion criteria were selected from the search results. Data on the study characteristics, patient characteristics, primary and secondary treatment outcomes, and adverse events were abstracted. Reference lists of selected articles were also checked for relevant studies. The quality of the evidence was assessed as high, moderate, low, or very low according to the GRADE methodology.
Summary of Findings
The database search identified 286 relevant citations published between 1996 and August 2008. Of the 286 abstracts reviewed, four RCTs met the inclusion criteria outlined above. Upon examination, two studies were subsequently excluded from the meta-analysis due to small sample size and missing data (Berthe et al.), as well as outlier status and high drop out rate (Wainstein et al) which is consistent with previously reported meta-analyses on this topic (Jeitler et al 2008, and Fatourechi M et al. 2009).
HbA1c
The primary outcome in this analysis was reduction in HbA1c. Both studies demonstrated that both CSII pumps and MDI reduce HbA1c, but neither treatment modality was found to be superior to the other. The results of a random effects model meta-analysis showed a mean difference in HbA1c of -0.14 (-0.40, 0.13) between the two groups, which was found not to be statistically or clinically significant. There was no statistical heterogeneity observed between the two studies (I2=0%).
Figure 1: Forrest plot of two parallel, RCTs comparing CSII to MDI in type 2 diabetes.
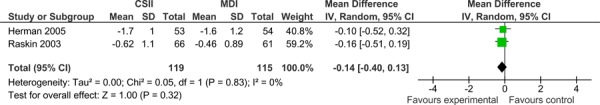
Secondary Outcomes
Mean Blood Glucose and Glucose Variability
Mean blood glucose was only used as an efficacy outcome in one study (Raskin et al. 2003). The authors found that the only time point in which there were consistently lower blood glucose values for the CSII group compared to the MDI group was 90 minutes after breakfast. Glucose variability was not examined in either study and the authors reported no difference in weight gain between the CSII pump group and MDI groups at the end of study. Conflicting results were reported regarding injection site reactions between the two studies. Herman et al. reported no difference in the number of subjects experiencing site problems between the two groups, while Raskin et al. reported that there were no injection site reactions in the MDI group but 15 such episodes among 8 participants in the CSII pump group.
Frequency of Hypoglycemic Events and Insulin Requirements
All studies reported that there were no differences in the number of mild hypoglycemic events in patients on CSII pumps versus MDI. Herman et al. also reported no differences in the number of severe hypoglycemic events in patients using CSII pumps compared to those on MDI. Raskin et al. reported that there were no severe hypoglycemic events in either group throughout the study duration. Insulin requirements were only examined in Herman et al., who found that daily insulin requirements were equal between the CSII pump and MDI treatment groups.
Quality of Life
QoL was measured by Herman et al. using the Diabetes Quality of Life Clinical Trial Questionnaire (DQOLCTQ). There were no differences reported between CSII users and MDI users for treatment satisfaction, diabetes impact, and worry-related scores. Patient satisfaction was measured in Raskin et al. using a patient satisfaction questionnaire, whose results indicated that patients in the CSII pump group had significantly greater improvement in overall treatment satisfaction at the end of the study compared to the MDI group. Although patient preference was also reported, it was only examined in the CSII pump group, thus results indicating a greater preference for CSII pumps in this groups (as compared to prior injectable insulin regimens) are biased and must be interpreted with caution.
Quality of Evidence
Overall, the body of evidence was downgraded from high to low according to study quality and issues with directness as identified using the GRADE quality assessment tool (see Table 3). While blinding of patient to intervention/control is not feasible in these studies, blinding of study personnel during outcome assessment and allocation concealment were generally lacking. ITT was not clearly explained in one study and heterogeneity between study populations was evident from participants’ treatment regimens prior to study initiation. Although trials reported consistent results for HbA1c outcomes, the directness or generalizability of studies, particularly with respect to the generalizability of the diabetic population, was questionable as trials required patients to adhere to an intense SMBG regimen. This suggests that patients were highly motivated. In addition, since prior treatment regimens varied between participants (no requirement for patients to be on MDI), study findings may not be generalizable to the population eligible for a pump in Ontario. The GRADE quality of evidence for the use of CSII in adults with type 2 diabetes is, therefore, low and any estimate of effect is uncertain.
Table 3: GRADE Quality Assessment for CSII pumps vs. MDI on HbA1c Adults with Type 2 Diabetes.
| Study | Design | Study Quality | Consistency | Directness | Other modifying factors | Overall quality of evidence |
|---|---|---|---|---|---|---|
|
Raskin 2003 |
RCT |
Serious limitations* MODERATE |
Consistent LOW |
Indirect† LOW |
||
| Herman 2005 | RCT HIGH |
Not applicable | LOW |
Inadequate or unknown allocation concealment (all studies); Unblinded assessment (all studies) however lack of blinding due to the nature of the study; ITT not well explained in 1 of 2 studies
Indirect due to lack of generalizability of findings since participants varied with respect to prior treatment regimens and intensive SMBG suggests highly motivated populations used in trials.
Economic Analysis
An economic analysis of CSII pumps was carried out using the Ontario Diabetes Economic Model (ODEM) and has been previously described in the report entitled “Application of the Ontario Diabetes Economic Model (ODEM) to Determine the Cost-effectiveness and Budget Impact of Selected Type 2 Diabetes Interventions in Ontario”, part of the diabetes strategy evidence series. Based on the analysis, CSII pumps are not cost-effective for adults with type 2 diabetes, either for age 65+ sub-group or for all patients in general. Details of the analysis can be found in the full report.
Conclusions
CSII pumps for the treatment of adults with type 2 diabetes
There is low quality evidence demonstrating that the efficacy of CSII pumps is not superior to MDI for adult type 2 diabetics.
There were no differences in the number of mild and severe hypoglycemic events in patients on CSII pumps versus MDI.
There are conflicting findings with respect to an improved quality of life for patients using CSII pumps as compared to MDI.
-
Significant limitations of the literature exist specifically:
All studies sponsored by insulin pump manufacturers
Prior treatment regimens varied
Types of insulins used in study varied (NPH vs. glargine)
Generalizability of studies in question as populations may not reflect eligible patient population in Ontario (participants not necessarily on MDI prior to study initiation, pen used in one study and frequency of SMBG required during study was high suggesting highly motivated participants)
Based on ODEM, insulin pumps are not cost-effective for adults with type 2 diabetes either for the age 65+ sub-group or for all patients in general.
Behavioural Interventions for Type 2 Diabetes
Objective
The objective of this analysis is to determine whether behavioural interventions2 are effective for improving glycemic control in adults with type 2 diabetes.
Background
Data from the UKPDS showed that tight glycemic control in type 2 diabetes significantly reduces the risk of developing serious complications of diabetes. Despite physicians’ and patients’ knowledge of the importance of glycemic control, Canadian data has shown that only 38% of patients with diabetes have HbA1c levels in the optimal range of 7% or less. This highlights the complexities involved in the management of diabetes, which is characterized by extensive patient involvement added to the support provided by physicians. In particular, an enormous demand is placed on patients to self-manage the physical, emotional, and psychological aspects of living with a chronic illness.
Despite differences in individual needs for coping with diabetes, there is general agreement for the necessity of supportive programs for patient self-management. While traditional programs were didactic models with the goal of improving patients’ knowledge of their disease, current models focus on behavioural approaches aimed at providing patients with the skills and strategies required to promote and change their behaviour.
Several meta-analyses and systematic reviews have demonstrated improved health outcomes with self-management support programs in type 2 diabetics. All these meta-analyses, however, have either looked at a specific component of self-management support programs (i.e. self-management education) or have been conducted among specific populations. Most reviews are also qualitative and do not clearly define the interventions of interest, making findings difficult to interpret. Moreover, heterogeneity in the interventions has led to conflicting evidence on the components of effective programs. Thus, there is much uncertainty regarding the optimal design and delivery of these programs.
Evidence-Based Analysis of Effectiveness
Research Questions
Are behavioural interventions effective in improving glycemic control in adults with type 2 diabetes?
Is the effectiveness of the intervention impacted by intervention characteristics (e.g. delivery of intervention, length of intervention, mode of instruction, interventionist etc…)?
Inclusion Criteria
English Language
Published between January 1996 to August 2008
Type 2 diabetic adult population (≥19 years)
RCTs
Systematic reviews, or meta-analyses
Describing a multi-faceted self-management support intervention as defined by the 2007 Self Management Mapping Guide
Reporting outcomes of HbA1c with extractable data
Studies with a minimum of 6 months of follow up
Exclusion Criteria:
Studies with a control group other than usual care2
Studies with a sample size <30
Studies without a clearly defined intervention
Outcomes of Interest
Primary outcome: HbA1c
Secondary outcomes: systolic blood pressure (SBP) control, lipid control, change in smoking status, weight change, quality of life, knowledge, self-efficacy, managing psychosocial aspects of diabetes, assessing dissatisfaction and readiness to change, and setting and achieving diabetes goals.
Search Strategy
A search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the CINAHL, The Cochrane Library, and the International Agency for Health Technology Assessment INAHTA for studies published between January 1996 and August 2008. Abstracts were reviewed by a single author and studies meeting the inclusion criteria outlined above were obtained. Data on population characteristics, glycemic control outcomes, and study design were extracted. Reference lists were also checked for relevant studies. The quality of the evidence was assessed as high, moderate, low, or very low according to the GRADE methodology.
Summary of Findings
The database search identified 638 citations published between 1996 and August 2008. Of the 638 abstracts reviewed, 12 studies met the inclusion criteria, one of which was a meta-analysis. All the remaining studies identified were RCTs and only one was categorized as small (total sample size N=47). Of the 11 RCTs, nine were used in a meta-analysis reported below.
Summary of Participant Demographics across studies
Across the 11 identified studies, a total of 2,549 participants were included with a mean reported age of approximately 58 years and a mean duration of diabetes of approximately 6 years. Most studies reported gender with a mean percentage of females of 67%. Of the eleven studies, two focused only on women and four included only Hispanic individuals. All studies evaluated type 2 diabetes patients exclusively.
Study Characteristics
The studies were conducted between 2002 and 2008 and six were carried out within the United States, with the remaining studies conducted in the United Kingdom (UK), Sweden, and Israel (sample sizes ranged from 47 to 824 participants). The quality of the studies ranged from moderate to low with four of the studies being of moderate quality and the remaining of low quality (based on the Consort Checklist).
Differences in quality were mainly due to methodological issues such as inadequate descriptions of randomization methods, sample size calculation, allocation concealment, blinding, and uncertainty of the use of intention-to-treat (ITT) analysis. Patients were recruited from several settings: six studies from primary or general medical practices, three studies from the community (e.g. via advertisements), and two from outpatient diabetes clinics. A usual care control group was reported in nine of the studies and two reported some type of minimal diabetes care in addition to usual care for the control group.
Intervention Characteristics
All of the interventions examined in the studies were mapped to the 2007 Self-management Mapping Guide. The interventions most often focused on problem solving, goal setting and encouraging participants to engage in activities that protect and promote health (e.g. modifying behaviour, change in diet, and increase physical activity). All of the studies examined comprehensive interventions targeting at least two self-care topics (e.g. diet, physical activity, blood glucose monitoring, foot care, etc.). Despite the homogeneity in the aims of the interventions, there was substantial clinical heterogeneity in other intervention characteristics such as duration, intensity, setting, mode of delivery (group vs. individual), interventionist, and outcomes of interest.
Interventionists and Setting
The following interventionists were reported (categories not mutually exclusive): nurse (36%), dietician (18%), physician (9%), pharmacist (9%), peer leader/community worker (18%), and other (36%). The ‘other’ category included interventionists such as consultants and facilitators with unspecified professional backgrounds. The setting of most interventions was community-based, followed by primary care practices.
Outcomes
Duration of follow up of the studies ranged from 6 months to 8 years with a median follow-up duration of 12 months, but whether the follow up was measured from participant entry into study or from the end of intervention was unclear in some of the studies. All studies reported measures of glycemic control using HbA1c levels and Body Mass Index (BMI) was measured in five studies and body weight in two studies. Cholesterol levels were examined in three studies and blood pressure reduction in two studies. Smoking status was only examined in one of the studies. Additional outcomes examined in the trials included patient satisfaction, quality of life, diabetes knowledge, diabetes medication reduction, and behaviour modification (i.e. daily consumption of fruits/vegetables, exercise etc). Meta-analysis of the studies identified a moderate but significant reduction in HbA1c levels -0.44%, 95%CI: -0.60, -0.29) for behavioural interventions in comparison to usual care for adults with type 2 diabetes. Subgroup analyses suggested that the largest effects were seen in interventions that were of at least one year in duration and in interventions in diabetics with higher baseline HbA1c (≥9.0). The quality of the evidence for the study outcomes is summarized in Table 5.
Table 5: Summary of GRADE Quality Assessment for Behavioural Interventions.
| Intervention | # of Studies | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | Effect (HbA1c) Mean Difference [95% CI] | Quality | ||
| Behavioural interventions (all studies) | 8 | RCT High |
Serious limitations† Moderate |
Consistent Moderate |
Direct Moderate |
None | -0.44 [-0.60, -0.29] |
Moderate |
| Behavioural interventions: where intervention duration = 1 year | 2 | RCT High |
Serious limitations‡ Moderate |
Consistent Moderate |
Some uncertainty about directness§ Low |
None | -0.68 [-1.22, -0.14] |
Low |
| Behavioural interventions: in patients with high baseline HbA1c (≥9.0) | 2 | RCT High |
Serious limitations¥ Moderate |
Consistent Moderate |
Some uncertainty about directness§ Low |
None | -0.79 [-1.23, -0.34] |
Low |
RCT refers to randomized controlled trial; CI, confidence interval; Int, intervention
Unclear allocation concealment, unclear if outcome assessors were blinded in 4 studies. Although 4 studies represented 50 % of body of evidence the sample size represented only 25% of the overall population therefore it was not downgraded any further.
Unclear allocation concealment, unclear if outcome assessors were blinded, unclear whether analysis was completed with Intention-to-treat (ITT)
One RCT contributes to the majority of the sample size and is based on a Hispanic population
Unclear allocation concealment in both RCTs, unclear whether ITT was used in one RCT, possible bias in one RCT due to enhanced follow-up of participants (outcome measured at 8 years)
Conclusions
Based on moderate quality evidence, behavioural interventions, as defined by the 2007 Self-management mapping guide (Government of Victoria, Australia), produce a moderate reduction in HbA1c levels in patients with type 2 diabetes compared with usual care.
Based on low quality evidence, the interventions with the largest effects are those:
- in diabetics with higher baseline HbA1c (≥9.0)
- in which the interventions were carried out for at least 1 year.
Table 4: Summary of Meta-Analysis of Studies Investigating the Effectiveness of Behavioural Interventions on HbA1c in Patients with Type 2 Diabetes.
| Group | Estimate of effect [95% Confidence Interval] |
Statistical heterogeneity (I2) |
|---|---|---|
| Overall | -0.44 [-0.60, -0.29] | 8% |
| Quality | ||
| High Quality | -0.50 [-0.75, -0.26] | 40% |
| Low Quality | -0.37 [-0.62, -0.13] | 0% |
| Intervention length | ||
| Intervention length < 6 weeks | -0.42 [-0.68, -0.15] | 28% |
| Intervention length 6 weeks to 1 year | -0.43 [-0.74, -0.12] | 47% |
| Intervention length = 1 year | -0.68 [-1.22, -0.14] | 0% |
| Setting of Care | ||
| Community-based setting | -0.48 [-0.70, -0.26] | 11% |
| Primary Care setting | -0.42 [-0.68, -0.15] | 28% |
| Interventionist | ||
| Interventionist < 2 disciplines | -0.44 [-0.66, -0.22] | 40% |
| Interventionist ≥ 2 disciplines | -0.51 [-0.84, -0.17] | 0% |
| Baseline HbA1c | ||
| Baseline HbA1c <9.0 | -0.40 [-0.55, -0.24] | 0% |
| Baseline HbA1c ≥9.0 | -0.79 [-1.23, -0.34] | 0% |
| Mode of Delivery | ||
| Group sessions | -0.47 [-0.66, -0.28] | 6% |
| Individual sessions* | -0.80 [-1.35, -0.25] | NA* |
| Combined | -0.30 [-0.57, -0.02] | 0% |
| Ethnicity | ||
| Hispanic Population | -0.42 [-0.71, -0.13] | 0% |
| Non-Hispanic Population | -0.46 [-0.66, -0.25] | 25% |
Based on one study
Bariatric Surgery for People with Diabetes and Morbid Obesity
Objective
The objective of this analysis is to examine the effectiveness and cost-effectiveness of bariatric surgery for the management of diabetes in morbidly obese people.
Clinical Need: Target Population and Condition
Obesity is defined as an excessive accumulation of body fat as measured by the body mass index (BMI). BMI is calculated as body weight in kilograms (kg) divided by height in metres squared (m2). People with a BMI over 30 kg/m2 are considered obese in most countries.
Obesity is associated with the development of several diseases, including hypertension, diabetes mellitus (type 2 diabetes), hyperlipidemia, coronary artery disease, obstructive sleep apnea, depression, and cancers of the breast, uterus, prostate, and colon. Clinically severe or morbid obesity is commonly defined by a BMI of at least 40 kg/m2, or a BMI of at least 35 kg/m2 with the presence of comorbid conditions such as diabetes, cardiovascular disease, or arthritis.
The prevalence of morbid obesity among people with type 2 diabetes was previously examined by Daousi in 2006: of 2,460 patients with type 2 diabetes, 52% (n = 1,279) were obese (BMI ≥ 30 kg/m2) and 23% (n = 561) had a BMI ≥ 35 kg/m2.
Description of Technology/Therapy
People with morbid obesity may be eligible for surgical intervention, of which there are numerous available options, each falling into one of two general categories (depending on how the stomach is remodelled):
malabsorptive - bypassing parts of the gastrointestinal tract to limit the absorption of food
restrictive - decreasing the size of the stomach in order for the patient to feel satiated with a smaller amount food.
Both of these surgical options can be performed either as open surgery or laparoscopically.
Surgery for obesity is usually considered a last resort for people who have attempted first-line medical management (e.g. diet, behaviour modification, increased physical activity, and drugs therapy) but who have not lost weight permanently. It is restricted to people with morbid obesity (BMI > 40 kg/m2), or with a BMI of at least 35 kg/m2 with comorbid conditions.
Evidence-Based Analysis of Effectiveness
A literature search was conducted from January 1996 to December 2004 that included OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, and the INAHTA and CRD databases. Details of the full literature search can be found in the 2005 evidence-based analysis of bariatric surgery located online at: http://www.health.gov.on.ca/english/providers/program/mas/tech/tech_mn.html
Inclusion Criteria
Data on effectiveness or cost-effectiveness of bariatric surgery for the improvement of diabetes
Systematic reviews, RCTs, observational controlled prospective studies that had ≥100 patients
Exclusion Criteria
Duplicate publications
Non-English-language articles
Non-systematic reviews, letters, and editorials
Animal and in-vitro studies
Case reports, case series
Studies that did not examine the outcomes of interest
Outcomes of Interest
The primary out come of interest was an improvement in, or resolution of, diabetes. The quality of the studies was examined according to the GRADE Working Group criteria for grading quality of evidence.
Summary of Findings
There is evidence that bariatric surgery is highly effective for improvement and resolution of diabetes in patients who are morbidly obese (BMI >35 kg/m2). According to the GRADE criteria, the quality of this evidence was found to be moderate (see Table 6).
Summary Table 6: GRADE Quality of Evidence for Bariatric Surgery for the Resolution or Improvement of Diabetes.
| Outcomes | Quality of Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|---|
| Design | Quality | Consistency | Directness | Other | No. of Patients | Effect | Quality | |
| Improvement in HbA1c in diabetic and glucose intolerant patients | Meta-analysis | Moderate* | Consistent | Direct | None | n=171 | -2.70% (-5.0% to -0.70%) weighted mean change (range) | Moderate |
| Resolution or improvement of diabetes | Meta-analysis | Moderate* | Consistent | Direct | None | 414/485 (n resolved or improved / n evaluated) | 86.0% (78.4% to 93.7%) mean% (95% CI) | Moderate |
| Resolution of diabetes (diabetes disappeared or no longer required therapy) | Meta-analysis | Moderate* | Consistent | Direct | None | 1417/1846 (n resolved / n evaluated) | 76.8% (70.7% to 82.9%) mean% (95% CI) | Moderate |
| Recovery of diabetes (fasting plasma glucose level of less than 126 mg per decilitre [7.0 mmol per litre]) | Observational prospective controlled study | Moderate* | Consistent | Some uncertainty† | Some uncertainty‡ | control n=84 intervention n=118 | 3.45 (1.64 to 7.28) OR (95% CI) at 10 yrs | Moderate |
Downgraded due to study design (not randomized controlled trial)
Unlikely to be an important uncertainty. Inclusion criteria for the SOS study not specific to conventional definition of “morbidly obese” patients (BMI ≥ 40 or ≥ 35 kg/m2 with comorbid conditions)
Unlikely to be an important uncertainty. Control group not standardized, however, this lends to the pragmatic nature of the study.
Comparison of various bariatric techniques:
No prospective, long-term direct comparison is available between malabsorptive and restrictive techniques.
Retrospective subgroup analyses from a large observational study showed greater improvement and resolution of diabetes using malabsorptive techniques rather than purely restrictive methods.
There is evidence from a meta-analysis of indirect, prospective studies, retrospective studies and case series that malabsorptive techniques are better than other banding techniques in terms of improvement and resolution of diabetes.
Conclusions
Based on moderate quality evidence, bariatric surgery is highly effective for the improvement and resolution of diabetes in patients who are morbidly obese (BMI >35 kg/m2). There is currently no prospective evidence comparing malabsorptive and restrictive techniques. Lower quality evidence suggests malabsorptive techniques are the superior alternative for diabetes improvement and resolution.
Community-Based Care for the Management of Type 2 Diabetes
Objective
The objective of this analysis is to determine the efficacy of specialized multidisciplinary community care for the management of type 2 diabetes compared to usual care.
Clinical Need: Target Population and Condition
Diabetes (i.e. diabetes mellitus) is a highly prevalent chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. The majority (90%) of diabetes patients have type 2 diabetes. Based on the United Kingdom Prospective Diabetes Study (UKPDS), intensive blood glucose and blood pressure control significantly reduce the risk of microvascular and macrovascular complications in type 2 diabetics. While many studies have documented that patients often do not meet the glycemic control targets specified by national and international guidelines, factors associated with glycemic control are less well studied, one of which is the provider(s) of care.
Multidisciplinary approaches to care may be particularly important for diabetes management. According guidelines from the Canadian Diabetes Association (CDA), the diabetes health care team should be multi- and interdisciplinary. Presently in Ontario, the core diabetes health care team consists of at least a family physician and/or diabetes specialist, and diabetes educators (registered nurse and registered dietician). Increasing the role played by allied health care professionals in diabetes care and their collaboration with physicians may represent a more cost-effective option for diabetes management. Several systematic reviews and meta-analyses have examined multidisciplinary care programs, but these have either been limited to a specific component of multidisciplinary care (e.g. intensified education programs), or were conducted as part of a broader disease management program, of which not all were multidisciplinary in nature. Most reviews also do not clearly define the intervention(s) of interest, making the evaluation of such multidisciplinary community programs challenging.
Evidence-Based Analysis Methods
Research Questions
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a registered nurse, registered dietician and physician (primary care and/or specialist) for the management of type 2 diabetes compared to usual care? [Henceforth referred to as Model 1]
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a pharmacist and a primary care physician for the management of type 2 diabetes compared to usual care? [Henceforth referred to as Model 2]
Inclusion Criteria
English language full-reports
Published between January 1, 2000 and September 28, 2008
Randomized controlled trials (RCTs), systematic reviews and meta-analyses
Type 2 diabetic adult population (≥18 years of age)
Total sample size ≥30
Describe specialized multidisciplinary community care defined as ambulatory-based care provided by at least two health care disciplines (of which at least one must be a specialist in diabetes) with integrated communication between the care providers.
Compared to usual care (defined as health care provision by non-specialist(s) in diabetes, such as primary care providers; may include referral to other health care professionals/services as necessary)
≥6 months follow-up
Exclusion Criteria
Studies where discrete results on diabetes cannot be abstracted
Predominantly home-based interventions
Inpatient-based interventions
Outcomes of Interest
The primary outcomes for this review were glycosylated hemoglobin (HbA1c) levels and systolic blood pressure (SBP).
Search Strategy
A literature search was performed on September 28, 2008 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published between January 1, 2000 and September 28, 2008. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist, then a group of epidemiologists until consensus was established. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
Given the high clinical heterogeneity of the articles that met the inclusion criteria, specific models of specialized multidisciplinary community care were examined based on models of care that are currently being supported in Ontario, models of care that were commonly reported in the literature, as well as suggestions from an Expert Advisory Panel Meeting held on January 21, 2009.
Summary of Findings
The initial search yielded 2,116 unique citations, from which 22 RCTs trials and nine systematic reviews published were identified as meeting the eligibility criteria. Of these, five studies focused on care provided by at least a nurse, dietician, and physician (primary care and/or specialist) model of care (Model 1; see Table ES 1), while three studies focused on care provided by at least a pharmacist and primary care physician (Model 2; see Table ES 2).
Based on moderate quality evidence, specialized multidisciplinary community care Model 2 has demonstrated a statistically and clinically significant reduction in HbA1c of 1.0% compared with usual care. The effects of this model on SBP, however, are uncertain compared with usual care, based on very-low quality evidence. Specialized multidisciplinary community care Model 2 has demonstrated a statistically and clinically significant reduction in both HbA1c of 1.05% (based on high quality evidence) and SBP of 7.13 mm Hg (based on moderate quality evidence) compared to usual care. For both models, the evidence does not suggest a preferred setting of care delivery (i.e., primary care vs. hospital outpatient clinic vs. community clinic).
Table 7: Summary of Results of Meta-Analyses of the Effects of Multidisciplinary Care Model 1.
| Outcome | Estimate of effect* (95% CI) |
Heterogeneity I2 (p-value) |
GRADE |
|---|---|---|---|
| Glycosylated Hemoglobin (HbA1c [%]) | -1.00 [-1.27, -0.73] | 4% (p=0.37) |
Moderate-quality |
| Subgroup: Moderate-to-High Quality | -0.91 [-1.19, -0.62] | 0% (p=0.74) | |
| Systolic Blood Pressure (mm Hg) | -2.04 [-13.80, 9.72] | 89% (p=0.002) | Very-low quality |
Mean change from baseline to follow-up between intervention and control groups
Table 8: Summary of Results of Meta-Analyses of the Effects of Multidisciplinary Care Model 2.
| Outcome | Estimate of effect* (95% CI) |
Heterogeneity I2 (p-value) |
GRADE |
|---|---|---|---|
| Glycosylated Hemoglobin (HbA1c [%]) | -1.05 [-1.57, -0.52] | 0% (p=0.75) | High-quality |
| Systolic Blood Pressure (mm Hg) | -7.13 [-11.78, -2.48] | 46% (p=0.17) | Moderate quality |
Mean change from baseline to follow-up between intervention and control groups
Conclusions
-
Model 1: Specialized multidisciplinary community care provided by at least a registered nurse, registered dietitian and physician (primary care and/or specialist) for the management of type 2 diabetes:
- has demonstrated a statistically and clinically significant reduction in HbA1c compared to usual care based on moderate quality evidence.
- has demonstrated an uncertain estimate of effect on SBP compared to usual care based on very-low quality evidence.
-
Model 2: Specialized multidisciplinary community care provided by at least a pharmacist and primary care for the management of type 2 diabetes:
- has demonstrated a statistically and clinically significant reduction in HbA1c compared to usual care based on high quality evidence.
- has demonstrated a statistically and clinically significant reduction in SBP compared to usual care based on moderate quality evidence.
For both models, the evidence does not suggest a preferred setting of care delivery (i.e. primary care vs. hospital outpatient clinic vs. community clinic).
Based on examination of an Ontario-specific multidisciplinary care program, specialized multidisciplinary community care for the management of type 2 diabetes is a cost-effective strategy.
Home Telemonitoring for Type 2 Diabetes
Objective
The objective of this analysis is to determine whether home telemonitoring and management of blood glucose is effective for improving glycemic control in adults with type 2 diabetes.
Background
An aging population coupled with a shortage of nurses and physicians in Ontario is increasing the demand for home care services for chronic diseases including diabetes. In recent years, there has also been a concurrent rise in the number of blood glucose home telemonitoring technologies available for diabetes management. The CDA currently recommends self-monitoring of blood glucose for patients with type 2 diabetes, particularly for individuals using insulin. With the emergence of home telemonitoring, there is potential for improving the impact of self-monitoring by linking patients with health care professionals who can monitor blood glucose values and provide guided recommendations remotely. MAS has, therefore, conducted a review of the available evidence on blood glucose home telemonitoring and management technologies for type 2 diabetes.
Evidence-Based Analysis of Effectiveness
Research Question
Is home telemonitoring of blood glucose for adults with type 2 diabetes more efficacious in improving glycemic control (i.e. can it reduce HbA1c levels) in comparison to usual care?
Inclusion Criteria
Intervention: Must involve the frequent transmission of remotely-collected blood glucose measurements by patients to health care professionals for routine monitoring through the use of home telemonitoring technology.
Intervention: Monitoring must be combined with a coordinated management and feedback system based on transmitted data.
Control: Usual diabetes care as provided by the usual care provider (usual care largely varies by jurisdiction and study).
Population: Adults ≥18 years of age with type 2 diabetes.
Follow-up: ≥6 months.
Sample size: ≥30 patients total.
Publication type: Randomized controlled trials (RCTs), systematic reviews, and/or meta-analyses.
Publication date range: January 1, 1998 to January 31, 2009.
Exclusion Criteria
Studies with a control group other than usual care.
Studies published in a language other than English.
Studies in which there is indication that the monitoring of patients’ diabetic measurements by a health care professional(s) was not occurring more frequently in intervention patients than in control patients receiving usual care.
Outcomes of Interest
The primary outcome of interest was a reduction in glycosylated hemoglobin (HbA1c) levels.
Search Strategy
A comprehensive literature search was performed in OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, The Cochrane Library, and INAHTA for studies published between January 1, 2007 and January 31, 2009. The search was designed as a continuation of a search undertaken for a systematic review by the Canadian Agency for Drugs and Technologies in Health originally completed from 1950 up until July of 2008, which reviewed home telemonitoring in comparison to usual care for the management of type 1 and type 2 diabetes.
Summary of Findings
A total of eight studies identified by the literature search were eligible for inclusion. One study was excluded post-hoc from analysis. Studies varied considerably on characteristics of design, population, and intervention/control. Of note, few trials limited populations to type 2 diabetics only, thus trials with mixed populations (type 1 and type 2) were included, though in such cases, the majority of patients (>60%) had type 2 diabetes. No studies restricted inclusion or analyses by diabetes treatment type (i.e. populations were mixed with respect to those on insulin therapy vs. not) and studies further varied on whether intervention was provided in addition to usual care or as a replacement. Lastly, trials often included blood glucose home telemonitoring adjunctive to other telemedicine components and thus the incremental value of adding home telemonitoring remains unclear. The overall grading of the quality of evidence was low, indicating that there is uncertainty in the findings (see Table 9).
Table 9: GRADE quality assessment for all included studies.
| Studies | Design | Quality | Consistency | Directness | Other Modifying Factors | Effect Size | Overall Quality |
|---|---|---|---|---|---|---|---|
| Ralston et al. 2009 | RCTs* |
|
|
-1.10 | |||
| Kim and Kim 2008 | -1.09 | ||||||
| Bond et al. 2007 | None | -0.57 | |||||
| Yoon and Kim 2007 | -0.9 |
LOW |
|||||
| Cho et al. 2007 | -0.12 | ||||||
| Harno et al. 2006 | -0.13 | ||||||
| Shea et al. 2006 | -0.40 | ||||||
| McMahon et al. 2005 |
HIGH |
MODERATE |
MODERATE |
LOW |
LOW |
-1.10 |
RCT refers to randomized controlled trial.
Meta-analysis of seven trials identified a moderate but significant reduction in HbA1c levels (~0.5% reduction) in favour blood glucose home telemonitoring compared to usual care for adults with type 2 diabetes). Subgroup analyses suggested differences in effect size depending on the type of intervention, however, these findings should be held under caution as the analyses were exploratory in nature and intervention components overlapped between subgroups (see Table 10).
Table 10: Meta-Analyses of Reduction in HbA1c Values for Analyzed Studies.
| Group | Estimate of Effect (95% Confidence Interval) |
Statistical Heterogeneity (I2) |
|
|---|---|---|---|
| Follow-up values | |||
| All studies | -0.48 [-0.70 to -0.26] | 45% | |
| Upload studies | -0.39 [-0.66 to -0.13] | 48% | |
| Web entry studies | -0.66 [-0.99 to -0.33] | 0% | |
| Change-from-baseline values (ρ=0.5) | |||
| All studies | 0.50 [-0.80 to -0.19] | 65% | |
| Upload studies | -0.26 [-0.55 to 0.02] | 45% | |
| Web entry studies | -0.78 [-1.14 to -0.43] | 0% | |
| Change-from-baseline values (ρ=0.65) | |||
| All studies | -0.52 [-0.82 to -0.21] | 73% | |
| Upload studies | -0.25 [-0.51 to 0.01] | 46% | |
| Web entry studies | 0.78 [-1.08 to -0.48] | 0% | |
| Change-from-baseline values (ρ=0.85) | |||
| All studies | -0.54 [-0.84 to -0.24] | 85% | |
| Upload studies | -0.21 [-0.41 to 0.00] | 47% | |
| Web entry studies | -0.81 [-1.11 to -0.51] | 49% | |
Conclusions
Based on low quality evidence, blood glucose home telemonitoring technologies confer a statistically significant reduction in HbA1c of ~0.50% in comparison to usual care when used adjunctively to a broader telemedicine initiative in adults with type 2 diabetes.
Regarding Subgroup analyses, exploratory analysis seems to suggest differences in effect sizes for the primary outcome when analyzing by subgroup; however, subgroup analyses should only be viewed as exploratory or hypothesis-generating.
Significant limitations and/or sources of clinical heterogeneity are present in the available literature, generating great uncertainty in conclusions.
More robust trials in type 2 diabetics only, utilizing more modern technologies, preferably performed in an Ontario or a similar setting (given the infrastructure demands and that the standard comparator is usual care), while separating out the effects of other telemedicine intervention components, are needed to clarify the effect of emerging remote blood glucose monitoring technologies.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Purpose
The Programs for Assessment of Technology and Health (PATH) was commissioned by MAS to predict the long-term costs and effects of strategies for successful management and treatment of type 2 diabetes, as well as their cost-effectiveness. This report summarizes the economic analyses of the following strategies: multi-disciplinary diabetes programs, CSII pumps, behavioural interventions, and bariatric surgery.
Objective
An assessment of type 2 diabetes interventions requires an evaluation of both short- and long-term costs and effectiveness. Early management of diabetes can help delay and even prevent complications that can have large impacts on patients’ quality of life and healthcare costs. Reductions in future complications may also offset ‘up-front’ medical resources invested in intensive disease management.
The objective of this economic analysis was to compare the lifetime costs, effects, and cost-effectiveness of the following treatments for type II diabetes using the Ontario Diabetes Economic Model (ODEM):
1) Primary care multidisciplinary diabetes program versus no program in adults with type 2 diabetes.
2) CSII pumps versus MDI of insulin in insulin-dependent adults with type 2 diabetes
3) Behavioural interventions versus no intervention in adults with type 2 diabetes
4) Bariatric surgery versus no surgery in morbidly obese adults with type 2 diabetes
Evidence-Based Analysis of Cost-Effectiveness
Research Questions
Are these interventions cost-effective in improving glycemic control in adults with type 2 diabetes?
What are the lifetime costs, effects, health events, and cost-effectiveness of these interventions in adults with type 2 diabetes?
Ontario Diabetes Economic Model
The recently developed UKPDS Outcomes Model is a computer simulation that uses a system of equations to predict the occurrence and timing of seven diabetes-related complications (i.e. fatal or non-fatal MI (myocardial infarction), other ischaemic heart disease, stroke, heart failure, amputation, renal failure and blindness) and death, to calculate life expectancy and quality-adjusted life expectancy for patients with Type 2 diabetes. To account for event-related dependencies, the model makes use of time-varying risk factors (e.g. blood pressure and HbA1c), which also facilitates its application to patient groups at different stages of the disease. The UKPDS Outcomes Model is based on data from over 5,000 patients with over 53,000 years of patient follow-up. To apply the model to other geographic areas (such as Ontario), however, it requires needs adaptation. Specifically, international differences may exist in: the incidence and prevalence of diabetes, baseline demographics, diabetes risk factors, overall mortality or mortality from diabetes-related complications, costs (e.g. treatment and management of complications), and the cost and effects of treatment programs. Accordingly, the UKPDS Outcomes Model was populated with Ontario-specific data for use in the province.
In brief, more than 734,000 patients with diabetes were identified in the Ontario Diabetes Database (ODD) and followed for up to 10 years. Various administrative databases were linked to this population in order to measure the prevalence and incidence of complications, healthcare resource utilization (i.e. inpatient and outpatient hospitalizations, outpatient visits, prescription drugs, emergency room visits, and home care), and death. Unit costs were collected and assigned to each of the different health care sectors. Complication-specific costs were divided into two time periods:
1) Immediate costs that accrue within the year in which a complication first occurs; and
2) Long-term costs that reflect ongoing costs in subsequent years associated with the management of the complication (including subsequent events of the same type).
Hospital inpatient and non-inpatient event and state costs were estimated for each of the seven complications. The perspective taken for estimating costs was that of the Ontario Ministry of Health and Long-term Care. All healthcare costs used in the model were based on direct costs as it was not possible to measure productivity costs or other patient costs from the data available. The ODEM was then used to conduct the cost-effectiveness analyses.
Summary of Findings
Table 11 summarizes the various diabetes treatment and management programs based on the ODEM analysis over a 40 year time horizon. Table 12 describes the population and health system impact based on the ODEM analysis over a 40 year time horizon and the various assumptions used to calculate the eligible population for each intervention.
Table 11: Summary of diabetes programs based on ODEM.
| Incremental Costs, QALYS, CE and Events per 1,000 |
Multi-disciplinary Diabetes Program |
Insulin Pumps |
Behavioural Interventions |
Bariatric Surgery |
|---|---|---|---|---|
| Δ HbA1c | -1.02% | -0.14% | -0.44% | -2.70% |
| Δ Costs | $7,551 | $39,840 | $285 | $9,188 |
| Δ QALYs | 0.390 | 0.021 | 0.008 | 0.585 |
| $/QALY gained | $19,869/QALY | $1.9M/QALY | $36,226/QALY | $15,697/QALY |
| Δ IHD | 20.5 | 1.0 | 0.6 | 16.1 |
| Δ MI | 54.9 | 2.8 | 0.7 | 80.8 |
| Δ Heart Failure | 11.5 | 2.3 | 0.8 | 181.8 |
| Δ Stroke | 18.9 | 1.8 | 0.5 | 52.3 |
| Δ Amputation | 17.7 | 1.0 | 0.5 | 17.5 |
| Δ Blindness | 8.3 | 1.4 | 0.7 | 24.4 |
| Δ Renal Failure | 1.1 | (0.04) | 0.1 | 0.1 |
Table 12: Summary of health system impact based on ODEM.
| Incremental Costs, QALYs, CE and Events per 1,000 |
Multi-disciplinary Diabetes Program* |
Insulin Pumps† |
Behavioural Interventions* |
Bariatric Surgery‡ |
|---|---|---|---|---|
| Δ HbA1c | -1.02% | -0.14% | -0.44% | -2.70% |
| Δ Costs | $5.623 | $8.010 | $0.212 | $1.573 |
| Δ QALYs | 290,424 | 4,222 | 5,957 | 100,196 |
| $/QALY gained | $19,869/QALY | $1.9M/QALY | $36,226/QALY | $15,697/QALY |
| Δ IHD | 15,265 | 201 | 446 | 2,757 |
| Δ MI | 40,882 | 562 | 521 | 13,839 |
| Δ Heart Failure | 8,563 | 462 | 595 | 31,137 |
| Δ Stroke | 14,074 | 361 | 372 | 8,957 |
| Δ Amputation | 13,180 | 201 | 372 | 2,997 |
| Δ Blindness | 6,180 | 281 | 521 | 4,179 |
| Δ Renal Failure | 819 | -8 | 74 | 17 |
All type 2 diabetes = 744,677
Insulin dependent type 2 diabetes = 201,062
Morbidly obese with type 2 diabetes = 171,275
Conclusions
Based on an analysis of an Ontario-specific model of diabetes care (ODEM) using data on clinical efficacy obtained from the above MAS systematic reviews, multi-disciplinary programs, behavioural interventions, and bariatric surgery would be considered cost-effective for the treatment and management of adults with type 2 diabetes.
Insulin pumps are not cost-effective for adults with type 2 diabetes, either for age 65+ sub-group or for all patients in general.
The determination of relative cost-effectiveness would require head-to-head field evaluation information. Such data is not available at this time.
The results of the ODEM model using data on clinical efficacy obtained from the above MAS systematic reviews can be used in order to prioritize funding. The following would be the direction of priority:
Community multi-disciplinary programs >> bariatric surgery >> behavioural interventions
Priority of funding is based on the cost per QALY ratio.
Barriers to Access Diabetes Care in Ontario
At the request of the MAS, the Institute for Clinical Evaluative Sciences (ICES) undertook a study of barriers to diabetes care as experienced by patients in Ontario. The study is described in a paper by Kwan et al. published in the Canadian Journal of Diabetes in 2008. The Institute for Social Research at York University conducted the survey for this study. A key objective in the study was to identify barriers that are amenable to policy change.
A random-digit dialing procedure was used to select households in Ontario with valid telephone numbers. A response rate of 71% yielded a sample of 1,150 (763 patients with self-reported diabetes sampled from representative Ontario population while 387 patients were sampled from low-income neighbourhoods).
The study identified low socioeconomic status (defined as household income less than $30,000) and absence of supplemental health insurance as key barriers to access of diabetes care in Ontario. Furthermore, household and even neighbourhood socioeconomic status were indentified as influencing both the access of diabetes care and the use of available care.
These data suggest the need for policy amendments, particularly to address apparent financial discrepancies in order to reduce disparities in diabetes-related health outcomes between socioeconomic groups.
Overall Conclusions
Clinical Efficacy
1. CSII Pumps
Type 1 Adult Diabetic Population
Based on low quality evidence, CSII pumps confer a statistically significant but not clinically significant reduction in HbA1c and mean daily blood glucose compared to MDI in adults with type 1 diabetes (>19 years).
CSII pumps also confer a statistically significant reduction in glucose variability as compared to MDI in adults with type 1 diabetes (>19 years) however the clinical significance is unknown.
There is evidence to suggest that the use of newer long-acting insulins (e.g. insulin glargine, detemir) increase the effectiveness of MDI treatment.
There is conflicting evidence regarding both mild and severe hypoglycemic events in this population when using CSII pumps as compared to MDI. Findings are based on very low quality evidence.
There is an improved quality of life for patients using CSII pumps as compared to MDI however, limitations exist with this evidence.
Type 2 Adult Diabetic Population
There is low quality evidence that demonstrates that the efficacy of CSII pumps is not superior to MDI for adults with type 2 diabetes. There are conflicting findings with respect to an improved quality of life for patients using CSII pumps as compared to MDI.
2. Behavioural Interventions
Based on moderate quality evidence, behavioural interventions as defined by the 2007 Self-management mapping guide (Government of Victoria, Australia) produce a moderate reduction in HbA1c levels in patients with type 2 diabetes compared with usual care.
Based on low quality evidence, the interventions with the largest effects are those used among diabetics with higher baseline HbA1c (≥9.0) and those carried out for at least 1 year.
3. Bariatric Surgery
Based on moderate quality evidence, bariatric surgery is highly effective for the improvement and resolution of diabetes in patients who are morbidly obese (BMI >35 kg/m2). There is currently no prospective evidence comparing malabsorptive and restrictive techniques. Lower quality evidence suggests malabsorptive techniques are superior in terms of improvement and resolution of diabetes.
4. Community Programs
There is moderate quality evidence that specialized multidisciplinary community care provided by at least a registered nurse, registered dietitian and physician (primary care and/or specialist) is an effective model of care for the improvement of glycemic control. The effects of this care model care on SBP control, however, are uncertain, based on very-low quality evidence. Furthermore, specialized multidisciplinary community care provided by at least a pharmacist and primary care physician is an effective model of care for the improvement of both glycemic control (based on high quality evidence) and SBP (based on moderate quality evidence).
5. Home Telemonitoring
There is insufficient evidence to evaluate the incremental clinical efficacy of home telemonitoring for type 2 diabetes above other home telemedicine initiatives.
6. Barriers to Access
Low socioeconomic status (defined as household income less than $30,000) and absence of supplemental health insurance are key barriers to access of diabetes care in Ontario.
Cost-effectiveness
1. Type 2 Diabetic Population – ODEM Analyses
Based on an analysis of an Ontario-specific model of diabetes care (ODEM), using data on clinical efficacy obtained from the above MAS systematic reviews, multi-disciplinary programs, behavioural interventions, and bariatric surgery would be considered cost-effective for the treatment and management of adults with type 2 diabetes.
CSII pumps are not cost-effective for adults with type 2 diabetes, either for age 65+ sub-group or for all patients in general.
The determination of relative cost-effectiveness would require head-to-head field evaluation information. Such data is not available at this time.
2. Type 1 Diabetic Population
One short term study concluded that CSII pumps are cost-effective compared to MDI in adults with type 1 diabetes, however this is based on limited data and longer term models are required to estimate the long-term costs and effects of CSII pumps compared to MDI in adults with type 1 diabetes.
-
The results of the ODEM model using data on clinical efficacy obtained from the above MAS systematic reviews can be used in order to prioritize funding. The following would be the direction of priority:
Community multi-disciplinary programs >> bariatric surgery >> behavioural interventions
Priority of funding is based on the cost per QALY ratio and impact on reducing downstream complications from diabetes
Appendix: GRADE Tool
Table A1: GRADE tool for grading the quality of evidence and strength of recommendations.
| Number of Studies | Study Design | Quality of Studies | Consistency | Directness | Other Modifying Factors |
|---|---|---|---|---|---|
| N | RCT = High Observational = Low Any other evidence = Very Low |
Serious limitation to study quality (-1) Very Serious Limitation to study quality (-2) |
Important inconsistency (-1) | Some uncertainty about directness (-1) Major uncertainty about directness (-2) |
Association Strong (+1) Association Very Strong (+2) Dose Response Gradient (+1) All plausible confounders would have reduced the effect (+1) Imprecise or sparse data (-1) High probability of reporting bias (-1) |
Source: Atkins D et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328(7454): 1490.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Diabetes strategy evidence platform: a summary of evidence-based analyses. Ontario Health Technology Assessment Series 2009;9(19).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/publicengageoverview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-9429-8 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit //www.health.gov.on.ca/english/providers/program/ohtac/publicengageoverview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Abbreviations
- BMI
Body Mass Index
- CDA
Canadian Diabetes Association
- CINAHL
Cumulative Index to Nursing & Allied Health Literature
- CSII
Continuous Insulin Infusion Pumps
- EMBASE
Excerpta Medica Database
- HbA1c
Glycosylated hemoglobin
- HSSD
Health System Strategy Division
- ICD
International Classification of Diseases
- IHD
Ischemic Heart Disease
- INAHTA
International Agency for Health Technology Assessment
- ITT
Intention-To-Treat Analysis
- MAS
Medical Advisory Secretariat
- MDI
Multiple Daily Injections
- MI
Myocardial Infarction
- NPH
Neutral Protamine Hagedorn
- ODD
Ontario Diabetes Database
- ODEM
Ontario Diabetes Economic Model
- OHTAC
Ontario Health Technology Advisory Committee
- PATH
Programs for Assessment of Technology and Health
- QALY
Quality-Adjusted Life Year
- RCT
Randomized Controlled Trial
- SBP
Systolic Blood Pressure
- UK
United Kingdom
- UKPDS
United Kingdom Prospective Diabetes Study
- USA
United States of America
Footnotes
Referred to as ‘self-management support interventions’ in the Strategy and in diabetes literature
Referred to in diabetes literature as ‘self-management support interventions’.


