Executive Summary
In June 2008, the Medical Advisory Secretariat began work on the Diabetes Strategy Evidence Project, an evidence-based review of the literature surrounding strategies for successful management and treatment of diabetes. This project came about when the Health System Strategy Division at the Ministry of Health and Long-Term Care subsequently asked the secretariat to provide an evidentiary platform for the Ministry’s newly released Diabetes Strategy.
After an initial review of the strategy and consultation with experts, the secretariat identified five key areas in which evidence was needed. Evidence-based analyses have been prepared for each of these five areas: insulin pumps, behavioural interventions, bariatric surgery, home telemonitoring, and community based care. For each area, an economic analysis was completed where appropriate and is described in a separate report.
To review these titles within the Diabetes Strategy Evidence series, please visit the Medical Advisory Secretariat Web site, http://www.health.gov.on.ca/english/providers/program/mas/mas_about.html,
Diabetes Strategy Evidence Platform: Summary of Evidence-Based Analyses
Continuous Subcutaneous Insulin Infusion Pumps for Type 1 and Type 2 Adult Diabetics: An Evidence-Based Analysis
Behavioural Interventions for Type 2 Diabetes: An Evidence-Based Analysis
Bariatric Surgery for People with Diabetes and Morbid Obesity: An Evidence-Based Summary
Community-Based Care for the Management of Type 2 Diabetes: An Evidence-Based Analysis
Home Telemonitoring for Type 2 Diabetes: An Evidence-Based Analysis
Application of the Ontario Diabetes Economic Model (ODEM) to Determine the Cost-effectiveness and Budget Impact of Selected Type 2 Diabetes Interventions in Ontario
Objective
The objective of this report is to determine the efficacy of specialized multidisciplinary community care for the management of type 2 diabetes compared to usual care.
Clinical Need: Target Population and Condition
Diabetes (i.e. diabetes mellitus) is a highly prevalent chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. The majority (90%) of diabetes patients have type 2 diabetes. (1) Based on the United Kingdom Prospective Diabetes Study (UKPDS), intensive blood glucose and blood pressure control significantly reduce the risk of microvascular and macrovascular complications in type 2 diabetics. While many studies have documented that patients often do not meet the glycemic control targets specified by national and international guidelines, factors associated with glycemic control are less well studied, one of which is the provider(s) of care.
Multidisciplinary approaches to care may be particularly important for diabetes management. According guidelines from the Canadian Diabetes Association (CDA), the diabetes health care team should be multi-and interdisciplinary. Presently in Ontario, the core diabetes health care team consists of at least a family physician and/or diabetes specialist, and diabetes educators (registered nurse and registered dietician).
Increasing the role played by allied health care professionals in diabetes care and their collaboration with physicians may represent a more cost-effective option for diabetes management. Several systematic reviews and meta-analyses have examined multidisciplinary care programs, but these have either been limited to a specific component of multidisciplinary care (e.g. intensified education programs), or were conducted as part of a broader disease management program, of which not all were multidisciplinary in nature. Most reviews also do not clearly define the intervention(s) of interest, making the evaluation of such multidisciplinary community programs challenging.
Evidence-Based Analysis Methods
Research Questions
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a registered nurse, registered dietician and physician (primary care and/or specialist) for the management of type 2 diabetes compared to usual care? [Henceforth referred to as Model 1]
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a pharmacist and a primary care physician for the management of type 2 diabetes compared to usual care? [Henceforth referred to as Model 2]
Inclusion Criteria
English language full-reports
Published between January 1, 2000 and September 28, 2008
Randomized controlled trials (RCTs), systematic reviews and meta-analyses
Type 2 diabetic adult population (≥18 years of age)
Total sample size ≥30
Describe specialized multidisciplinary community care defined as ambulatory-based care provided by at least two health care disciplines (of which at least one must be a specialist in diabetes) with integrated communication between the care providers.
Compared to usual care (defined as health care provision by non-specialist(s) in diabetes, such as primary care providers; may include referral to other health care professionals/services as necessary)
≥6 months follow-up
Exclusion Criteria
Studies where discrete results on diabetes cannot be abstracted
Predominantly home-based interventions
Inpatient-based interventions
Outcomes of Interest
The primary outcomes for this review were glycosylated hemoglobin (rHbA1c) levels and systolic blood pressure (SBP).
Search Strategy
A literature search was performed on September 28, 2008 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published between January 1, 2000 and September 28, 2008. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist, then a group of epidemiologists until consensus was established. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
Given the high clinical heterogeneity of the articles that met the inclusion criteria, specific models of specialized multidisciplinary community care were examined based on models of care that are currently being supported in Ontario, models of care that were commonly reported in the literature, as well as suggestions from an Expert Advisory Panel Meeting held on January 21, 2009.
Summary of Findings
The initial search yielded 2,116 unique citations, from which 22 RCTs trials and nine systematic reviews published were identified as meeting the eligibility criteria. Of these, five studies focused on care provided by at least a nurse, dietician, and physician (primary care and/or specialist) model of care (Model 1; see Table ES 1), while three studies focused on care provided by at least a pharmacist and primary care physician (Model 2; see Table ES 2).
Based on moderate quality evidence, specialized multidisciplinary community care Model 2 has demonstrated a statistically and clinically significant reduction in HbA1c of 1.0% compared with usual care. The effects of this model on SBP, however, are uncertain compared with usual care, based on very-low quality evidence. Specialized multidisciplinary community care Model 2 has demonstrated a statistically and clinically significant reduction in both HbA1c of 1.05% (based on high quality evidence) and SBP of 7.13 mm Hg (based on moderate quality evidence) compared to usual care. For both models, the evidence does not suggest a preferred setting of care delivery (i.e., primary care vs. hospital outpatient clinic vs. community clinic).
Table ES1: Summary of Results of Meta-Analyses of the Effects of Multidisciplinary Care Model 1.
| Outcome | Estimate of effect* (95% CI) |
Heterogeneity I2 (p-value) |
GRADE |
|---|---|---|---|
| Glycosylated Hemoglobin (HbA1c [%]) | -1.00 [-1.27, -0.73] | 4% (p=0.37) |
Moderate-quality |
| Subgroup: Moderate-to-High Quality | -0.91 [-1.19, -0.62] | 0% (p=0.74) | |
| Systolic Blood Pressure (mm Hg) | -2.04 [-13.80, 9.72] | 89% (p=0.002) | Very-low quality |
Mean change from baseline to follow-up between intervention and control groups
Table ES2: Summary of Results of Meta-Analyses of the Effects of Multidisciplinary Care Model 2.
| Outcome | Estimate of effect* (95% CI) |
Heterogeneity I2 (p-value) |
GRADE |
|---|---|---|---|
| Glycosylated Hemoglobin (HbA1c [%]) | -1.05 [-1.57, -0.52] | 0% (p=0.75) | High-quality |
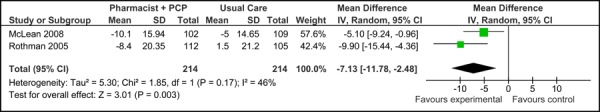
| Systolic Blood Pressure (mm Hg) | -7.13 [-11.78, -2.48] | 46% (p=0.17) | Moderate quality |
Mean change from baseline to follow-up between intervention and control groups
Background
In June 2008, the Medical Advisory Secretariat began work on the Diabetes Strategy Evidence Project, an evidence-based review of the literature surrounding strategies for successful management and treatment of diabetes. This project came about when the Health System Strategy Division at the Ministry of Health and Long-Term Care subsequently asked the secretariat to provide an evidentiary platform for the Ministry’s newly released Diabetes Strategy.
After an initial review of the strategy and consultation with experts, the secretariat identified five key areas in which evidence was needed. Evidence-based analyses have been prepared for each of these five areas: insulin pumps, behavioural interventions, bariatric surgery, home telemonitoring, and community based care. For each area, an economic analysis was completed where appropriate and is described in a separate report.
To review these titles within the Diabetes Strategy Evidence series, please visit the Medical Advisory Secretariat Web site, http://www.health.gov.on.ca/english/providers/program/mas/mas_about.html,
Diabetes Strategy Evidence Platform: Summary of Evidence-Based Analyses
Continuous Subcutaneous Insulin Infusion Pumps for Type 1 and Type 2 Adult Diabetics: An Evidence-Based Analysis
Behavioural Interventions for Type 2 Diabetes: An Evidence-Based Analysis
Bariatric Surgery for People with Diabetes and Morbid Obesity: An Evidence-Based Summary
Community-Based Care for the Management of Type 2 Diabetes: An Evidence-Based Analysis
Home Telemonitoring for Type 2 Diabetes: An Evidence-Based Analysis
Application of the Ontario Diabetes Economic Model (ODEM) to Determine the Cost-effectiveness and Budget Impact of Selected Type 2 Diabetes Interventions in Ontario
Objective
The objective of this report is to determine the efficacy of specialized multidisciplinary community care for the management of type 2 diabetes compared to usual care.
Clinical Need and Target Population
Diabetes is a highly prevalent chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. The majority (90%) of diabetes patients have type 2 diabetes and in 2005, an estimated 8.8% of Ontario’s population had diabetes, representing more than 816,000 Ontarians. (1) Clinically, diabetes is the leading causes of blindness, end-stage renal disease, and non-traumatic amputation in Canadian adults and is a significant cause of cardiovascular complications, hypertension, stroke, cataracts, and glaucoma. (2) In 2000, the direct health care cost of diabetes was $1.76 billion, a total that’s projected to rise to $3.14 billion by 2016.
Based on the United Kingdom Prospective Diabetes Study (UKPDS), intensive blood glucose and blood pressure control lower the risk of microvascular and macrovascular complications in type 2 diabetics. Specifically, a 1% reduction in HbA1c has been associated with a 10% reduction in diabetes-related mortality and a 25% reduction in microvascular end-points. (3) Likewise, intensive blood pressure control is associated with a 32% reduction in risk of mortality from diabetes-associated conditions, two-thirds of which are cardiovascular diseases. (4) Furthermore, tight blood pressure control is associated with a 34% reduction in the risk of macrovascular disease (including myocardial infarction, sudden death, stroke, and peripheral vascular disease), a 44% reduction in the risk of stroke, and a 37% reduction in the risk of microvascular disease. (4)
Diabetes Management and Organization of Diabetes Care
Due to poor compliance with evidence-based recommendations for diabetes management regimens, diabetes and its complications have significantly added to the cost of primary health care and prolonged waiting times for treatment in emergency and surgery departments. (1) While many studies have documented that patients often do not meet the glycemic control targets specified by national and international guidelines, the factors associated with glycemic control are less well studied, one of which is the provider(s) of care. (5)
Multidisciplinary teams refer to “individuals from different disciplines who contribute specialized knowledge in non-hierarchical relationships and who act according to situational demands rather than traditional organizational roles.” (6) Such approaches to care may be particularly important for the management of diabetes and its associated risk factors. Ideal collaborative relationships among health care professionals enable cooperative problem-solving and decision-making that result in synergistic benefits to patient care. (6)
Currently, chronic disease management approaches supported by government involve an interdisciplinary approach to diabetes care and associated risk factor management. According to CDA guidelines, a diabetes health care team should be multi- and interdisciplinary and sustain effective communication with the health care system at large. (1) The core team should consist of at least a family physician and/or diabetes specialist, as well as diabetes educators (registered nurse and registered dietician) (1). It has been noted, however, that increasing the role of allied health care professionals in diabetes care and their collaboration with physicians may represent a more cost-effective option for diabetes management. (7) Multidisciplinary community care may also be a viable option for disease management due to access pressures and time constraints on primary care physicians to manage diabetes.
Several systematic reviews and meta-analyses have examined multidisciplinary care programs, but these have either been limited to a specific components of multidisciplinary care (e.g. intensified education programs), or were conducted as part of a broader disease management program, of which not all teams were multidisciplinary in nature. Furthermore, most reviews are qualitatively reported due to substantial clinical heterogeneity in the interventions being delivered and do not clearly define the intervention of interest, making results difficult to interpret.
Evidence-Based Analysis of Effectiveness
Research Questions
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a registered nurse, registered dietician, and a physician (primary care and/or diabetes specialist) for the management of type 2 diabetes compared to usual care? [Model 1]
What is the evidence of efficacy of specialized multidisciplinary community care provided by at least a pharmacist and a primary care physician for the management of type 2 diabetes compared to usual care? [Model 2]
Inclusion Criteria
English-language full-reports
Randomized controlled trials (RCTs), systematic reviews or meta-analyses
Published between January 1, 2000 to September 28, 2008
Patients with diabetes, where the majority (i.e., ≥ 80%) of the study population has type 2 diabetes
Adults ≥ 18 years of age
Total sample size of ≥ 30
Studies must describe a specialized multidisciplinary community care intervention, defined as:
Multidisciplinary (two or more health care disciplines)
At least one provider is a specialist in diabetes management
Ambulatory-based health care service provision
Integrated communication and care provision between health care providers
Comparator is usual care, defined as health care provision by non-specialist(s) in diabetes (such as primary care providers) and may include usual referral to other health care professionals or services as necessary
Report clinical outcome measures of glycosylated hemoglobin and/or blood pressure
Studies with a minimum follow-up of 6 months
Exclusion Criteria
Studies where discrete results on diabetes cannot be abstracted
Studies without a clearly defined multidisciplinary specialized community-based intervention
Predominantly home-based interventions
Inpatient-based interventions
Outcomes of Interest
Primary outcomes: glycosylated hemoglobin (HbA1c) levels and systolic blood pressure (SBP).
Search Strategy
A literature search was performed on September 28, 2008 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published between January 1, 2000 and September 28, 2008. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established.
Given the high clinical heterogeneity of the articles that met the inclusion criteria, specific models of specialized multidisciplinary community care were examined based on what was reported in the literature, models of care that are currently supported in Ontario, as well as suggestions from an Expert Advisory Panel Meeting held on January 21, 2009. The inclusion criteria were revised to examine specific models of care, as described in the research questions.
Statistical Analyses
Data on study population and intervention characteristics (including multidisciplinary team composition), clinical outcomes of glycemic control and blood pressure, and study design were extracted. Results for studies that reported baseline and final HbA1c (or within-group changes) and/or SBP values were meta-analyzed using a random-effects model.
Meta-analysis of pre-post continuous measurements values (such as HbA1c) presents statistical challenges as studies quite often report only baseline (pre) and final values (post) for intervention and control groups, without reporting between-group changes from baseline to final values. While the absolute difference between pre- and post- can be calculated (final value minus baseline value), the standard deviation of this intra-group difference, necessary for meta-analysis, is often lacking.
In order to account for this discrepancy, baseline values for HbA1c and SBP were meta-analyzed to determine if there were any differences in study populations at baseline. Next, both final values and the change from baseline to follow-up within the intervention and control groups were meta-analyzed. Standard deviations for the change from baseline to final values were generated by imputing varying correlation coefficients (0.25, 0.50, and 0.75) and observing their effect on summary estimates and statistical heterogeneity. The range of correlation coefficients used ensured a wide range of potential correlation coefficients for sensitivity testing. Smaller correlation coefficients (closer to 0) yield more conservative estimates, resulting in an increased standard deviation. This, in turn, generates wider confidence intervals around individual trial effect sizes and results in a slight decrease in the summary of effect size. Using smaller correlation coefficients also decreases statistical heterogeneity by widening confidence intervals. Imputation techniques have been historically shown to have little effect on the summary estimates and conclusions of a meta-analysis. (8) Therefore, all meta-analyses are reported using a correlation coefficient of 0.50.
Assessment of Quality of Evidence
The quality of evidence assigned to individual studies was determined using a modified CONSORT Statement Checklist for Randomized Controlled Trials. (9) The CONSORT Statement was adapted to include three additional quality measures: the adequacy of control group description, significant differential loss to follow-up between groups, and ≥30% study attrition. Individual study quality was defined based on total scores according to the CONSORT Statement checklist: very low (0 to < 40%), low (≥40 to < 60%), moderate (≥60 to < 80%), and high (≥80 to 100%).
The quality of the trials was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (10;11) and is presented in Table 3.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
Table 3: GRADE Quality Assessment for Specialized Multidisciplinary Community Care for Management of Type 2 Diabetes.
| Intervention* | # of Studies |
Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Effect (Mean Difference [95% CI])* |
Quality | ||||||||
| Design | Quality | Consistency | Directness | Other | Int* | Control | ||||
| Outcome: Glycosylated Hemoglobin (HbA1c) | ||||||||||
|
At least a RN, RD and MD |
4 |
RCT High |
Serious limitations† Moderate |
Consistent Moderate |
Direct Moderate |
None Moderate |
341 |
313 |
-1.00 [-1.27, -0.73] |
Moderate |
|
At least a pharmacist and PCP |
2 |
RCT High |
No serious limitations High |
Consistent High |
Direct High |
None‡ High |
148 |
134 |
-1.05 [-1.57, -0.52] |
High |
| Outcome: Systolic Blood Pressure | ||||||||||
|
At least a RN, RD and MD |
2 |
RCT High |
Serious limitations§ Moderate |
Unexplained heterogeneity Low |
Direct Low |
Imprecise or sparse data Unlikely publication bias Very-low |
133 |
197 |
-2.04 [-13.80, 9.74] |
Very-low |
|
At least a pharmacist and PCP |
RCT High |
Serious limitations¥ Moderate |
Consistent Moderate |
Direct Moderate |
None Moderate |
214 |
214 |
-7.13 [-11.78, -2.48] |
Moderate |
|
MD, primary care physician and/or diabetes specialist; PCP, primary care physician; RD, registered dietician; RN, registered nurse; CI, confidence interval; Int, intervention; RCT, randomized controlled trial.
Unclear allocation concealment in 2 studies (12;13); potential for control group contamination in 1 study, where the same physician provided care to intervention and control groups (14); > 30% loss to follow-up in 1 study (12); not analyzed using intention-to-treat in 2 studies (12;15); frequency of testing of HbA1c amongst controls may have effected improvement in glycemic control in 1 study. (14)
Studies were powered to detect a change in HbA1c.
Unclear allocation concealment in 1 study (16); not analyzed using intention-to-treat in 1 study (15); not powered to detect a change in blood pressure in both studies (15;16); no description of methods for obtaining blood pressure measurement in 1 study. (16)
All blood pressure outcome assessment were obtained by automated blood pressure monitors; however, blinding of outcome assessor in only 1 study (17); description of frequency and methods for obtaining blood pressure measurement in only 1 study (18), where an average of 5 measurements were taken 1 minute apart
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
Based on a systematic literature search of six electronic databases, 2,116 unique citations were identified (published between January 2000 and October 2008). Following the title and abstract review, 325 full-text articles were retrieved and reviewed for more detailed evaluation of study objectives and methodology to determine inclusion. Of these, 295 articles were excluded (154 for inappropriate intervention or control group, 128 because of study design or type of report, seven for inappropriate population, three for inadequate follow-up, and three for inappropriate outcomes). Of the remaining full-text studies reviewed, 22 RCTs were eligible for inclusion based on having at least two health care disciplines in the multidisciplinary team. Upon closer examination, however, only five RCTs involved specialized multidisciplinary community care provided by at least a registered nurse, registered dietician, and a physician (primary care and/or specialist), while three RCTs involved specialized multidisciplinary community care provided by at least a pharmacist and primary care physician.
Nine systematic literature reviews were finally identified (eight through systematic search and one through manual searching) that focused on concepts relating to multidisciplinary diabetes care. All the identified RCTs were categorized as Level 1 evidence (Table 4).
Table 4: Quality of Evidence of Included Studies*.
| Study Design | Level of Evidence |
Number of Eligible Studies | ||
|---|---|---|---|---|
| Any Multidisciplinary Team |
At least a Nurse, Dietician, and Physician |
At least a Pharmacist and Primary Care Physician |
||
| Large RCT, systematic review of RCTs | 1 | 31 | 5 | 3 |
| Large RCT unpublished but reported to an international scientific meeting |
1(g) | 0 | 0 | 0 |
| Small RCT | 2 | 0 | 0 | 0 |
| Small RCT unpublished but reported to an international scientific meeting |
2(g) | 0 | 0 | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 | 0 | 0 |
| Non-RCT with historical controls | 3b | 0 | 0 | 0 |
| Non-RCT presented at international conference | 3(g) | 0 | 0 | 0 |
| Surveillance (database or register) | 4a | 0 | 0 | 0 |
| Case series (multisite) | 4b | 0 | 0 | 0 |
| Case series (single site) | 4c | 0 | 0 | 0 |
| Retrospective review, modeling | 4d | 0 | 0 | 0 |
| Case series presented at international conference | 4(g) | 0 | 0 | 0 |
For each included study, levels of evidence were assigned according to a ranking system based on a hierarchy proposed by Goodman. (19) An additional designation “g” was added for preliminary reports of studies that have been presented at international scientific meetings. Non-RCT, clinical trial that is not randomized, e.g. a cohort study; RCT, randomized controlled trial.
A diagram of the literature search flow is presented in Appendix 2.
Summary of Existing Evidence: Systematic Reviews and Meta-Analyses
Nine systematic reviews were identified (eight through systematic literature search and one through manual-searching) that focused on concepts relating to multidisciplinary programs for diabetes care. Of these, seven were narrative systematic reviews, one was a meta-analysis, and one was a meta-regression analysis. A summary of the systematic reviews, including the search years, number of trials included, objective, and applicability to the present research questions is presented in Appendix 3. The majority of these reviews were not applicable to the present analysis as they were part of a broader disease management program, quality improvement strategies in diabetes management, case management, did not report a clinical outcome of glycosylated hemoglobin or systolic blood pressure, or did not restrict inclusion criteria to interventions that were specialized and multidisciplinary in nature. No systematic reviews were identified that examined specialized multidisciplinary care provided by at least a registered nurse, registered dietician and physician and none had the same inclusion and exclusion criteria as the current review.
A meta-analysis of 24 studies published between 1987 and 2001 was conducted by Knight and colleagues, which focused on broader disease management programs for diabetes. (20) Inclusion was not restricted to programs that were multidisciplinary in nature. Upon pooling the study results, diabetes disease management programs resulted in a 0.49% reduction in HbA1c (95% CI, -0.56 to -0.41%). The results of this meta-analysis are, however, not interpretable as there was significant clinical and statistical heterogeneity (p < 0.001 for test for homogeneity) amongst the disease management programs, with no attempt made for exploratory subgroup analysis.
A meta-regression of 66 studies published between 1966 and 2006 was conducted by Shojania et al., focusing on quality improvement strategies for the management of diabetes. (21) Most quality improvement strategies examined resulted in small to modest improvements in glycemic control. Two of the strategies that may have involved specialized multidisciplinary care resulted in more robust improvements in HbA1c. Specifically, team changes resulted in a reduction in HbA1c of -0.67% (95% CI, -0.91% to -0.43%) and case management in a reduction in HbA1c of -0.52% (95% CI, -0.73% to -0.31%). Yet although team changes included interventions involving the addition of a team member (i.e., shared care), or the use of multidisciplinary teams, interventions involving expansion or revision of professional roles were also included, such as nurses or pharmacists who played a more active role in medication management. The results of the team changes analysis may, therefore, not be directly applicable to the current review.
Summary of Findings
Of the 2,116 citations reviewed, 22 RCTs were eligible for inclusion based on having at least two health care disciplines in the multidisciplinary team (Table 6). Overall, there was substantial clinical heterogeneity across the 22 RCTs with respect to patient populations, the composition of the health care team, the various components of care being provided, and the outcomes reported. As such, two specific models of care were focused on in this analysis:
Table 6: Specialized Multidisciplinary Teams Identified through Broad Literature Search.
| Study | Health Care Team* | Model of Care Examined |
|---|---|---|
| California Medi-Cal Diabetes Study Group, 2004 (14) | RN, RD, E, PCP, CDE | At least RN, RD, MD |
| Choe, et al, 2005 (22) | Pharm, PCP | At least Pharm, PCP |
| Gaede, et al, 2001 (13) | RN, RD, MD | At least RN, RD, MD |
| Gabbay, et al, 2006 (23) | dRN, PCP | NA |
| Gary, et al, 2003 (24) | RN (CDE in training), CHW, MD | NA |
| Groeneveld, et al, 2001 (16) | dRN (CDE), RD, PCP | At least RN, RD, MD |
| Hiss, et al, 2007 (25) | dRN, PCP | NA |
| Johansen, et al, 2007 (15) | dRN, RD, D, Physio | At least RN, RD, MD |
| Krein, et al, 2004 (26) | NP, PCP | NA |
| Litaker, et al, 2003 (27) | NP, PCP | NA |
| Maislos, et al, 2004 (12) | dRN (CDE), RD, D | At least RN, RD, MD |
| McLean, et al, 2008 (18) | Pharm, PCP, dRN | At least Pharm, PCP |
| McMurray, et al, 2002 (28) | dRD, MD (N, PCP, I, or E) | NA |
| O’Hare, et al, 2004 (29) | dRN, CHW, usual care | NA |
| Piette, et al, 2001 (30) | RN, PCP | NA |
| Rothman, et al, 2005 (17) | Pharm (CDE), PCP | At least Pharm, PCP |
| Shea, et al (31) | dRN, D, PCP | NA |
| Shibayama, et al, 2007 (32) | dRN (CDE), MD | NA |
| Smith, et al, 2004 (33) | dRN, PCP | NA |
| Soja, et al, 2007 (34) | RNs, MDs (I, C) | NA |
| Taylor, et al, 2003 (35) | dRN, MD | NA |
| Wolf, et al, 2004 (36) | RD, PCP | NA |
C, cardiologist; CDE, certified diabetic educator; CHW, community health worker (non-health care professional); D, diabetologist; dRN, diabetes specialist nurse; dRD, diabetes specialist dietician; E, endocrinologist; I, internist; MD, physician (unspecified specialty); N, nephrologist; NP, nurse practitioner; PCP, primary care physician; Pharm, pharmacist; Physio, physiotherapist; RD, registered dietician; RN, registered nurse; NA, not applicable.
Model 1: care provided by a registered nurse, registered dietician and physician (primary care and/or specialist).
Model 2: care provided by a pharmacist and primary care physician.
Summaries of selected studies are presented in Table 7 (pages 19-20) and Table 8 (page 21), highlighting their patient demographic and design details as they relate to the two alternate models of care.
Table 7: Summary of Study Characteristics: Model 1.
| Study, Design (N), Country |
Inclusion Criteria† |
Intervention Group | Setting | Control¶ | Outcomes# | Length of FU (Freq. of FU)** |
Study Quality |
||
|---|---|---|---|---|---|---|---|---|---|
| Care provider |
Types of Interventions Delivered | Method of Care Delivery |
|||||||
| MODEL OF CARE: 1. At least a registered nurse, registered dietician and physician (primary care and/or specialist) | |||||||||
|
California Medi-Cal Diabetes Study Group, 2004 (14) RCT (N = 335) USA |
T2DM (>1y duration), age ≥18 y, HbA1c ≥7.5% | RN, RD, E, PCP, CDE |
|
Clinic visits (Individual) | Community outpatient clinics (n=3) | Usual care (by PCP) | Primary: HbA1c | 36 months (every 6 months) | High |
|
Gaede, et al, 2001 (13) RCT (N = 149) Denmark |
T2DM, age 45-65y | RN, RD, MD |
|
Clinic visits (Individual & Group) | Hospital outpatient clinic | Usual care (by PCP) | †† HbA1c, total-and HDL-cholesterol, triglycerides, body weight, current smokers, daily dietary intake, exercise, use of glucose- or lipid-lowering drugs | Mean: 3.8 years (every 3 months) | Moderate |
|
Groeneveld, et al, 2001 (16) Cluster RCT N = 246 Netherlands |
T2DM, age <76y, treated by a PCP | dRN (CDE), RD, PCP |
|
Clinic visits (Individual) | Primary carepractices (n=15) | Usual care (by PCP) | †† HbA1c, FBG, lipids, BP, weight | 12 months (every 3 months) | Moderate |
|
Johansen, et al, 2007 (15) RCT N = 106 Norway |
T2DM, age 18-75 y, Caucasian, ≥ 1 CV risk factor | dRN, RD, D, Physio |
|
Clinic visits (Individual & Group) | Hospital outpatient clinic | Usual care (by PCP) | †† HbA1c; BP; FBG; total, HDL and LDL cholesterol; triglycerides; microalbumin-uria; leisure-time activity; HRQoL | 24 months (every 3 months) | High |
|
Maislos, et al, 2004 (12) Cluster RCT N = 82 Israel |
T2DM, poorly controlled HbA1c (≥10%) | dRN (CDE), RD, D |
|
Clinic visits (Individual) | Primary carepractices (n=2) | Usual care (by PCP, RN) | †† HbA1c, compliance in attending clinic | 6 months (as needed) | Low |
RCT, randomized controlled trial
BP, blood pressure; CV, cardiovascular; HbA1c, glycosylated hemoglobin; mo, months; PCP, primary care physician; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; y, years
NA, not available (contacted study author; data unavailable); NR, not reported
CDE, certified diabetic educator; D, diabetologist; dRN, diabetes specialist nurse; E, endocrinologist; HCP, health care professional; MD, physician (unspecified specialty); PCP, primary care physician; Pharm, pharmacist; Physio, physiotherapist; RD, registered dietician; RN, registered nurse
PCP, primary care physician
MD, physician; PCP, primary care physician; Pharm, pharmacist; RN, nurse
blood pressure; FBG, fasting blood glucose; HDL, high-density lipoprotein; HRQoL, health-related quality of life; LDL, low-density lipoprotein cholesterol
FU, follow-up
No specification of primary or secondary outcomes
Table 8: Summary of Study Characteristics: Model 2.
| Study, Design (N), Country |
Inclusion Criteria† |
Intervention Group | Setting | Control¶ | Outcomes# | Length of FU (Freq. of FU)** | Study Quality |
||
|---|---|---|---|---|---|---|---|---|---|
| Care provider |
Types of Interventions Delivered | Method of Care Delivery |
|||||||
| MODEL OF CARE: 2. At least a pharmacist and primary care physician | |||||||||
|
Choe, et al, 2005 (22) RCT (N = 80) USA |
T2DM, HbA1c ≥8.0%, age ≤70 y [excluded patients with severe co-morbidity) | Pharm, PCP |
|
Clinicvisits, telephone follow-up (Individual) | Primary care clinic | Usual care (by PCP) |
Primary: HbA1c Secondary: process measures (LDL, retinal exam, urine microalbumin-uria screening, monofilament testing for neuropathy) |
12 months (monthly) | High |
|
McLean, et al, 2008 (18) RCT (N = 227) Canada |
Diabetes, adults, BP >130/80 mm Hg on 2 screening visits 2 weeks apart | Pharm, PCP, dRN |
|
Clinic visits (Individual) | Community | Usual care (by RN or Pharm) + minimal education |
Primary: BP Secondary: BPtargets (≤130/80 mm Hg), anti-hypertensive drug therapy, angiotensin-converting enzyme inhibitor |
6 months (every 6 weeks) | High |
|
Rothman, et al, 2005 (17) RCT (N = 217) USA |
T2DM, age ≥ 18y, HbA1c ≥8%, English-speaking, life expectancy 〉 6 months | Pharm (CDE), PCP |
|
Clinic visits, telephone or in-person follow-up (Individual) | Primary care clinic | Usual care (by PCP) |
Primary: BP, HbA1c, aspirin use at 6 and 12 months Secondary: diabetes knowledge, satisfaction, use of clinical services, adverse events |
12 months (every 2-4 weeks) | High |
RCT, randomized controlled trial;
BP, blood pressure; CV, cardiovascular; HbA1c, glycosylated hemoglobin; mo, months; PCP, primary care physician; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; y, years;
NA, not available (contacted study author; data unavailable); NR, not reported;
CDE, certified diabetic educator; D, diabetologist; dRN, diabetes specialist nurse; E, endocrinologist; HCP, health care professional; MD, physician (unspecified specialty); PCP, primary care physician; Pharm, pharmacist; Physio, physiotherapist; RD, registered dietician; RN, registered nurse; PCP, primary care physician; MD, physician; PCP, primary care physician; Pharm, pharmacist; RN, nurse; blood pressure; FBG, fasting blood glucose; HDL, high-density lipoprotein; HRQoL, health-related quality of life; LDL, low-density lipoprotein cholesterol;
FU, follow-up;
No specification of primary or secondary outcomes.
Summary of Multidisciplinary Community Care Model 1
Summary of Participant Demographics across studies
A total of 918 study participants with type 2 diabetes were randomized and analyzed across the three studies examining specialized multidisciplinary community care provided by at least a nurse, dietician and physician (range: 82 to 335). Study demographics reported were:
Age range of study participants: 55 to 63 years
Percentage of female participants: reported in four studies with a mean of 54% and a range of 23% to 42.6%
BMI: reported in three studies with a range of 28.6 to 33.1 kg/m2
Baseline HbA1c: reported in four studies with a mean range of 7.5% to 12.9%
SBP: reported in three studies with a mean range of 130 to 149 mm Hg
Duration of diabetes: reported only four studies with a range of 3 to 12 years.
Total cholesterol: reported in four studies with a range of 5.0 to 6.2 mmol/L. Three studies also reported HDL cholesterol values, which ranged from 1.01 to 1.3 mmol/L.
Ethnicity: reported in one study in which more than 50% of the population was an ethnic minority (i.e. African American, Hispanic, or other).
Smoking status: reported in three studies in which 8% to 38% were self-reported current smokers.
The study demographics are summarized in Table 9 (pages 22-23)
Table 9: Summary of Participant Demographic Characteristics of Included Studies.
| Study, N | Sex: Female (%) | Age (years)* | Ethnicity (%) | Duration of Diabetes (years)* | BMI (kg/m2)* | Baseline HbA1c (%)* | Baseline SBP (mm Hg)* | Total Cholesterol* | HDL Cholesterol* | Smoker Status (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| MODEL OF CARE: 1. At least a registered nurse, registered dietician and physician (primary care and/or specialist) | ||||||||||
|
California Medi-Cal Diabetes Study Group, 2004 (14) N = 335 |
Intervention: 72.6 Control: 70.9 |
Intervention: 57.0 ± 0.9 Control: 56.9 ± 1.0 |
Intervention: African-Am:16.1 Hispanic: 39.2 Caucasian: 34.9 Other: 9.7 Control: African-Am: 15.7 Hispanic: 38.4 Caucasian: 36.0 Other: 9.9 |
Intervention: 10.3±0.8 Control: 12.0±0.8 |
Intervention: 33.1±0.8 Control: 31.5±0.8 |
Mean ± SE: Intervention:9.6±0.1 Control:9.7±0.1 |
Mean ± SE: Intervention: 136 ± 2 Control: 134 ± 1 |
Mean ± SE: Intervention: 210.0±3.3 mg/dl Control: 212.1±3.7 mg/dl |
Mean ± SE: Intervention:41.9 ± 1.0 mg/dl Control:43.0 ± 1.1 mg/dl |
Intervention: 14.8 Control: 13.0 |
|
Gaede, et al, 2001 (13) N = 149 |
NR | 55.1 ± 7.2 | NR | Median (IQR): 6 (4-10) | NR | Intervention: 8.4±1.5 Control: 8.8±1.7 |
NR | Intervention: 5.4±1.0 mmol/L Control: 5.8±1.3 mmol/L |
Intervention: 1.03±0.2 mmol/L Control: 1.01±0.3 mmol/L |
Intervention: 38.4 Control: 34.2 |
|
Groeneveld, et al, 2001 (16) N = 246 |
Intervention: 65.9 Control: 53.5 |
Intervention: 62.7 ± 11 Control: 62.3 ± 10 |
NR | Intervention: 4.1 ± 3.7 Control: 4.6 ± 4.0 |
NR | NA | Intervention: 137±27 Control: 149±24 |
Intervention:6.2 ± 1.2 mmol/L Control:6.2 ± 1.3 mmol/L |
NR | NR |
|
Johansen, et al, 2007 (15) N = 106 |
Intervention: 28 Control: 23 |
Intervention: 59 ± 9 Control: 58 ± 11 |
NR | Median (IQR): Intervention: 4 (1-10) Control: 3 (1-12) |
Median (min, max): Intervention: 30.6 (22.6, 484) Control: 28.6 (16.1, 42.3) |
Intervention:7.5± 1.5 Control:7.6± 1.6 |
Intervention: 136 ± 16 Control: 130 ± 13 |
Intervention:5.0 ± 1.0 mmol/L Control:5.0 ± 0.9 mmol/L |
Intervention:1.3 ± 0.4 mmol/L Control:1.3 ± 0.4 mmol/L |
Intervention: 8 Control: 15 |
|
Maislos, et al, 2004 (12) N = 82 |
Intervention: 50 Control: 65 |
Intervention: 58 ± 14 Control: 63 ± 9 |
NR | NR | Intervention: 30.8 ± 3.6 Control: 30.8 ± 3.0 |
Intervention: 12.9 ± 3.4 Control: 12.6 ± 2.9 |
NR | NR | NR | NR |
|
Choe, et al, 2005 (22) N = 80 |
Intervention: 51.2 Control: 53.9 |
Intervention: 52.2 ± 11.2 Control: 51.0 ± 9.0 |
Intervention: African-Am: 17.1 Caucasian: 80.5 Other: 2.4 Control: African-Am: 12.8 Caucasian: 71.8 Other: 5.1 Unknown: 10.3 |
NR | NR | Intervention:10.1± 1.8 Control:10.2± 1.7 |
NR | NR | NR | NR |
|
McLean, et al, 2008 (18) N = 227 |
Intervention: 34.8 Control: 65.5 |
Intervention: 66.2 ± 11.3 Control: 63.7 ± 12.7 |
NR | NR | Intervention: 31.7 ± 6.0 Control: 31.6 ± 7.9 |
NR | Intervention: 142.5 ± 15.5 Control: 139.9 ± 11.9 |
NR | NR | Intervention: 9.6 Control: 10.77 |
|
Rothman, et al, 2005 (17) N = 217 |
Intervention: 56 Control: 56 |
Intervention: 54 ± 13 Control: 57 ± 11 |
Intervention: African-Am: 70 Control: African-Am: 59 |
Intervention:8 ± 9 Control: 9 ± 9 |
Intervention: 35 ± 9 Control: 34 ± 8 |
Intervention: 10.8 ± 2.1 Control: 10.7 ± 2.5 |
Intervention: 141.2 ± 21.8 Control: 137.4 ± 21.2 |
Intervention:217.0± 86.5 mg/dl Control:207.1± 64.1 mg/dl |
NR | NR |
All values summarized as mean ± SD unless otherwise specified
Study Characteristics and Setting
All studies included for examination were RCTs published between 2001 and 2007 with two being cluster-RCTs (Table 8). The studies were conducted in a variety of geographical locations, including Europe (three studies), the USA (one study), and Israel (one study); no Canadian studies were identified. The quality of the individual studies varied, with two studies being of high quality, two of moderate quality, and one of low quality. Differences in quality were predominantly attributable to inadequate descriptions of the randomization process, lack of allocation concealment, sample size calculations, and/or a lack of intention-to-treat analyses. All studies were conducted in a community outpatient setting, where two were specifically conducted in outpatient hospital clinics and two in primary care practices.
Intervention Characteristics of Diabetes Programs
Specialized Multidisciplinary Health Care Professional Team
The composition of the specialized multidisciplinary team varied across studies, differing in which professional was termed the ‘diabetes specialist’ and what allied health care providers complemented the core diabetes care team. Two studies involved a primary care physician as part of the multidisciplinary team. Three studies involved a certified diabetic educator (which was a diabetes specialist registered nurse in two instances). Three studies involved diabetes specialist physicians (two diabetologists, one endocrinologist), while one study supplemented the core diabetes health care team with a physiotherapist.
Interventional Characteristics Delivered within Diabetes Programs
All programs were multifaceted in nature, involving at least four interventional components in various combinations. At a minimum, all programs included diabetes education, diet counselling, and pharmacotherapy advice and management. In four of the diabetes programs, exercise advice and training was also provided by the multidisciplinary team. Three studies involved a structured education program, promotion of self-care, behaviour modification or problem-solving skills, and integration of the multidisciplinary team with primary care. Other components included in some programs were smoking cessation counselling, case management, and psychosocial counselling.
Method of Care Delivery and Length and Frequency of Follow-up
All studies involved delivery via patient clinic visits and two studies also used group care or education sessions as an adjunct to clinic visits. Length of follow-up ranged from 6 months to 3.8 years with three studies having a follow-up every 3 months and two having follow-ups as needed and every 6 months.
Comparator Groups
All studies involved comparing specialized multidisciplinary teams (at least a nurse, dietician and physician) compared to usual care provided by a primary care physician. In one trial, usual care was provided by both primary care physicians and nurses. In this instance, however, neither the nurses nor primary care physician were specialty trained in diabetes management (Table 8). (12)
Outcomes
All studies used HbA1c as an outcome, but only one study did so as their primary outcome of interest. Two studies reported SBP as a study outcome (other outcomes were displayed previously in Table 8).
Results: HbA1c
Four of the five studies were eligible for inclusion in the meta-analyses of HbA1c results (baseline HbA1c values were not reported in Groeneveld, et al. 2001). (16) Among these, there was no significant difference in the mean baseline HbA1c values [-0.03% (95% CI, -0.36, 0.29)], as shown in Figure 1a. All five trials did, however, report final mean HbA1c values (including Groeneveld, et al. 2001), as shown in Figure 1b. Based on the reported values, there was a significant reduction in HbA1c associated with care Model 1 compared to usual care [-0.94% (95% CI, -1.32, -0.56)] with moderate statistical heterogeneity (I2=65%). Figure 1c presents the mean change in HbA1c from baseline to follow-up between groups for Model 1 compared to usual care. Overall, care Model 1 resulted in a reduction in HbA1c of 1.0% (95% CI, -1.27, -0.73) compared to usual care, which is considered to be both statistically and clinically significant. Furthermore the statistical heterogeneity associated with this comparison was minimal (I2=4%). The estimate of effect did not vary greatly based on subgroup analysis of moderate-to-high-quality evidence, with Model 1 resulting in an overall reduction in HbA1c of 0.91 % (95% CI, -1.19, -0.62) (Figure 1d).
Figure 1a: Multidisciplinary Care Model 1: Baseline HbA1c (%).
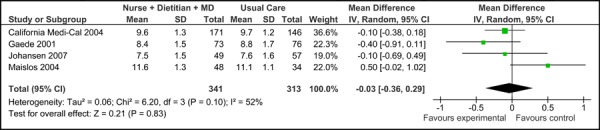
Figure 1b: Multidisciplinary Care Model 1: Final HbA1c (%).
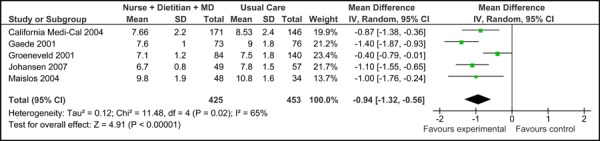
Figure 1c: Multidisciplinary Care Model 1: Mean Change in HbA1c from Baseline to Follow-up between Groups (%).
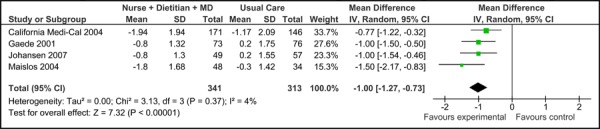
Figure 1d: Multidisciplinary Care Model 1: Subgroup Analysis of Moderate-to-High Quality Evidence of Mean Change in HbA1c from Baseline to Follow-up between Groups* (%).
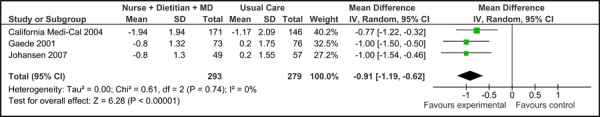
excluded 1 low-quality study (12)
Results: Systolic Blood Pressure
Two of the five studies were eligible for inclusion in the meta-analyses of SBP. The mean baseline SBP values for these two studies are presented in Figure 2a and the final mean SBP values are presented in Figure 2b.
Figure 2a: Multidisciplinary Care Model 1: Baseline Systolic Blood Pressure (mm Hg).
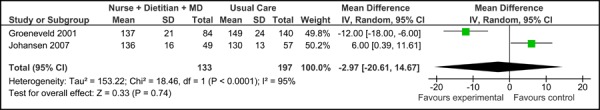
Figure 2b: Multidisciplinary Care Model 1: Final Systolic Blood Pressure (mm Hg).
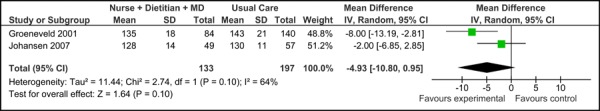
Overall, there was no significant difference in the baseline SBP values between the studies [-2.97 mm Hg (95% CI, -20.61, 14.67)]. Based on the reported values, there was also no difference in SBP associated with care Mode 1 compared to usual care [-4.93 mm Hg (95% CI, -10.80, 0.95)], with moderate statistical heterogeneity associated with the results (I2=64%).
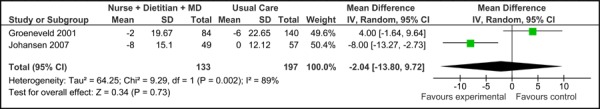
Figure 2c presents the mean change in SBP from baseline to follow-up between groups for care Model 1 compared to usual care. Overall, the model had no effect on the mean change in SBP between groups (-2.04 mm Hg [95% CI, -13.80, 9.72]. However, because this is based on very low quality evidence (according to GRADE, Table 3), the estimate of effect is uncertain; further, there was high statistical heterogeneity associated with this comparison (I2=89%).
Figure 2c: Multidisciplinary Care Model 1: Mean Change in Systolic Blood Pressure from Baseline to Follow-up between Groups (mm Hg).
Summary of Multidisciplinary Community Care Model 2
Summary of Participant Demographics across studies
A total of 524 study participants with type 2 diabetes were randomized and analyzed across three studies examining specialized multidisciplinary community care provided by at least a pharmacist and primary care physician (range: 80 to 227). Study demographics reported were:
Age range of study participants: mean of 51 to 63.7 years
Percentage of female participants: mean of 53% and a range of 35% to 66%
BMI: reported in two studies with a range of 31.6 to 35.0 kg/m2
Baseline HbA1c: reported in two studies with a mean range of 10% to 11%
SBP: reported in two studies with a mean range of 137.4 to 142.5 mm Hg
Duration of diabetes: reported only in one study with a mean of approximately 8 years.
Total cholesterol: reported in one study with a mean of 207.1 to 217.0 mg/dL (no trials reported HDL cholesterol values)
Ethnicity: reported in two studies with Caucasians making up 80% of study participants in one study and African Americans making up more than 50% of study participants in the second
Smoking status: reported in one study with 9 to 11% of patients being self-reported current smokers.
The study demographics were summarized previously in Table 9 (pages 22-23).
Study Characteristics and Setting
The included studies were all RCTs conducted in North America and published between 2005 and 2008; one was a Canadian study. All three studies were also conducted in the community outpatient setting, with two specifically conducted in primary care practices and one in community pharmacies. According to the CONSORT Statement for Randomized Controlled Trials, the studies were all of high quality.
Intervention Characteristics of Diabetes Programs
Specialized Multidisciplinary Health Care Professional Team
In each of the studies, the composition of the specialized multidisciplinary team was fairly homogeneous, with each involving a core team consisting of a pharmacist and primary care physician. In one trial, two pharmacists were involved in the team, one of whom was a certified diabetic educator. (17) Another study involved a diabetes specialist registered nurse in addition to the core team. (18)
Interventional Characteristics Delivered within Diabetes Programs
All programs were multifaceted in nature, involving at least five interventional components in various combinations (see Table 8, page 21). All programs included diabetes education and counselling, integration of the pharmacist with primary care, pharmacotherapy advice and management, and the promotion of self-care, behaviour modification or problem-solving skills. Two studies involved a structured education program, case management, or a diabetes care coordinator. Other components of care included cardiovascular disease risk counselling and a tracking registry of patient outcomes.
Method of Care Delivery and Length and Frequency of Follow-up
All studies involved care delivered by individual patient clinic visits. In addition to clinic visits, two studies involved telephone follow-up. Length of follow-up ranged from 6 to 12 months, while the frequency of follow-up ranged from 2 to 6 weeks.
Comparator Groups
Two studies involved comparing specialized multidisciplinary community care provided by at least a pharmacist and primary care physician to usual care provided by a primary care physician. In the third study, usual care was provided by a registered nurse or pharmacist, plus minimal diabetes education in pamphlet form. (18) Neither the nurse nor pharmacist in this study had specialty training in diabetes management, nor did they work together to provide care as a multidisciplinary team.
Outcomes
Two studies used HbA1c as a primary outcome (17;22), while two used SBP (Rothman et al., 2005 used both metrics). (17;18) Other outcomes included diabetes process measures, achievement of blood pressure targets, aspirin and drug utilization, diabetes knowledge, diabetes satisfaction, use of clinical services, and adverse events.
Results: HbA1c
Two of the three studies were eligible for inclusion in the meta-analyses of HbA1c. As shown in Figure 3a, there was no significant difference in the mean baseline Hba1c values between studies [0.10% (95% CI, -0.40, 0.60)] for care Model 2. Following diabetes management with the model, patients achieved a significant reduction in HbA1c compared to usual care [-0.95% (95% CI, -1.43, -0.46)] with no statistical heterogeneity (I2=0%) (Figure 3b).
Figure 3a: Multidisciplinary Care Model 2: Baseline HbA1c (%).
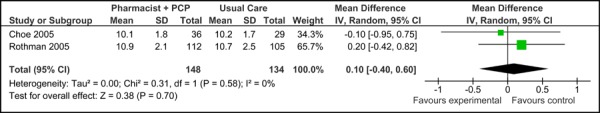
Figure 3b: Multidisciplinary Care Model 2: Final HbA1c (%).
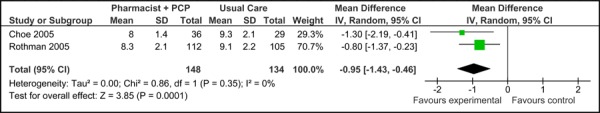
Figure 3c presents the mean change in HbA1c from baseline to follow-up between groups for studies comparing care Model 2 to usual care. Overall, the model resulted in a reduction in mean HbA1c of 1.05% (95% CI, -1.57, -0.52) compared to usual care, which is considered to be both statistically and clinically meaningful. No statistical heterogeneity associated with this comparison (I2=0%).
Figure 3c: Multidisciplinary Care Model 2: Mean Change in HbA1c from Baseline to Follow-up between Groups (%).
Results: Systolic Blood Pressure
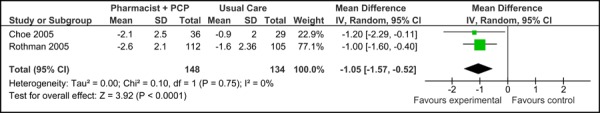
Two of the three studies examined were eligible for inclusion in the meta-analysis of SBP. Figure 4a presents the baseline SBP values for studies applying care Model 2. Overall, there was no significant difference in the mean baseline SBP values between studies [2.96 mm Hg (95% CI, -0.17, 6.09)]. Figure 4b presents the final mean SBP values for both trials, which reported a significant reduction in SBP associated with care Model 2 compared to usual care [-6.10 mm Hg (95% CI, -11.41, -0.79)] with no statistical heterogeneity (I2=0.02%).
Figure 4a: Multidisciplinary Care Model 2: Baseline Systolic Blood Pressure (mm Hg).
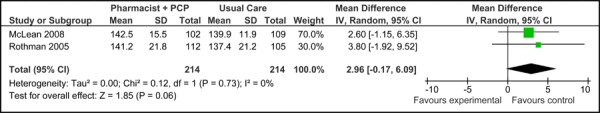
Figure 4b: Multidisciplinary Care Model 2: Final Systolic Blood Pressure (mm Hg).
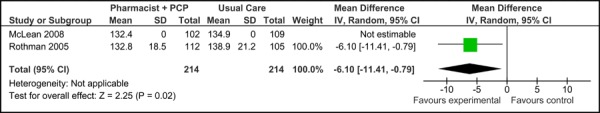
Figure 4c presents the mean change in SBP from baseline to follow-up between groups for care Model 2 and usual care. Overall, the model resulted in a significant and clinically meaningful reduction in mean SBP of 7.13 mm Hg (95% CI, -11.78, -2.48). Moderate statistical heterogeneity was associated with this comparison (I2=46%).
Figure 4c: Multidisciplinary Care Model 2: Mean Change in Systolic Blood Pressure from Baseline to Follow-up between Groups (mm Hg).
Conclusions
-
Model 1: Specialized multidisciplinary community care provided by at least a registered nurse, registered dietician and physician (primary care and/or specialist) for the management of type 2 diabetes:
- Has demonstrated a statistically and clinically significant reduction in HbA1c compared to usual care based on moderate quality evidence.
- Has demonstrated an uncertain estimate of effect on SBP compared to usual care based on very-low quality evidence.
-
Model 2: Specialized multidisciplinary community care provided by at least a pharmacist and primary care for the management of type 2 diabetes:
- Has demonstrated a statistically and clinically significant reduction in HbA1c compared to usual care based on high quality evidence.
- Has demonstrated a statistically and clinically significant reduction in SBP compared to usual care based on moderate quality evidence.
For both models, the evidence does not suggest a preferred setting for care delivery (i.e. primary care vs. hospital outpatient clinic vs. community clinic).
Appendices
Appendix 1: Search Strategies
Search date: September 28, 2008
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library, CINAHL, INAHTA/CRD
Database: Ovid MEDLINE ® <1996 to September Week 3 2008>
exp Intermediate Care Facilities/ (223)
(intermedia* adj2 care).ti,ab. (514)
exp ambulatory care/ (15683)
exp Ambulatory Care Facilities/ (14875)
exp Outpatients/ (3629)
((outpatient* or ambulatory) adj2 (care* or service* or clinic* or facility or facilities)).ti,ab. (15858)
exp Patient Care Team/ (22124)
exp Nursing, Team/ (624)
exp Cooperative Behavior/ (12319)
exp Interprofessional Relations/ (20749)
exp “Delivery of Health Care, Integrated”/ (5240)
team*.ti,ab. (33586)
(multidisciplin$ or multi-disciplin$ or interdisciplin$ or inter-disciplin$ or collaborat$ or cooperat$ or co-operat$ or multi?special$).ti,ab. (92458)
(integrat$ or share or shared or sharing).ti,ab. (167984)
exp Community Health Services/ (181030)
exp Program Evaluation/ (30015)
exp “episode of care”/ (910)
exp Professional Role/ (35965)
exp Primary Health Care/ (34098)
exp “Continuity of Patient Care”/ (6191)
exp Disease Management/ (6014)
disease management program*.ti,ab. (794)
(patient care adj2 manage$).ti,ab. (245)
exp Case Management/ or exp Subacute Care/ (6515)
(care adj2 model*).ti,ab. (2957)
exp Program Development/ (11519)
or/1-26 (564400)
limit 27 to yr=“2000 - 2008” (423967)
limit 28 to (english language and humans) (318172)
limit 29 to (controlled clinical trial or meta analysis or randomized controlled trial) (14433)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (34042)
(health technology adj2 assess$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (617)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ab. (64322)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (367054)
exp Double-Blind Method/ (52682)
exp Control Groups/ (696)
exp Placebos/ (9167)
(RCT or placebo? or sham?).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (93164)
or/30-38 (473324)
29 and 39 (38646)
(diabet* adj2 (program* or clinic* or center* or centre*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3130)
limit 41 to (english language and humans and yr=“2000 - 2008”) (2165)
42 and 39 (414)
exp Diabetes Mellitus, Type 2/ (37974)
((ketosis resistant or adult onset or slow onset or maturity onset or non?insulin dependent or stable or type 2 or type II) adj2 (diabet$ or DM)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (46955)
(t2dm or niddm).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (4237)
46 or 45 or 44 (47508)
40 and 47 (783)
43 or 48 (1100)
Database: EMBASE <1980 to 2008 Week 39>
(intermedia* adj2 care).ti,ab. (631)
exp ambulatory care/ (12187)
exp Outpatient Department/ (9466)
exp outpatient care/ (12499)
((outpatient* or ambulatory) adj2 (care* or service* or clinic* or facility or facilities)).ti,ab. (20467)
exp TEAM NURSING/ (6)
exp Cooperation/ (13299)
exp TEAMWORK/ or team*.ti,ab. (41041)
exp Integrated Health Care System/ (231)
(multidisciplin$ or multi-disciplin$ or interdisciplin$ or inter-disciplin$ or collaborat$ or cooperat$ or co-operat$ or multi?special$).ti,ab. (116921)
(integrat$ or share or shared or sharing).ti,ab. (208598)
exp Case Management/ (454)
exp Rehabilitation Care/ (2739)
exp community care/ (23465)
exp Social Care/ (34975)
exp ambulatory care nursing/ (5)
exp primary health care/ (41469)
*Disease Management/ (254)
disease management program*.ti,ab. (869)
(patient care adj2 manage$).ti,ab. (196)
exp Program Development/ (753)
(care adj2 model*).ti,ab. (2336)
exp Health Program/ (53182)
or/1-23 (511612)
limit 24 to (human and english language and yr=“2000 - 2009”) (194121)
Randomized Controlled Trial/ (162835)
exp Randomization/ (26273)
exp RANDOM SAMPLE/ (1261)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (292930)
(health technology adj2 assess$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (645)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (61896)
Double Blind Procedure/ (70620)
exp Triple Blind Procedure/ (12)
exp Control Group/ (2245)
exp PLACEBO/ or placebo$.mp. or sham$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (207387)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (420855)
(control$ adj2 clinical trial$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (279987)
or/26-37 (778561)
38 and 25 (36604)
exp Non Insulin Dependent Diabetes Mellitus/ (54933)
((ketosis resistant or adult onset or slow onset or maturity onset or non?insulin dependent or stable or type 2 or type II) adj2 (diabet$ or DM)).ti,ab. (38625)
(t2dm or niddm).ti,ab. (7266)
42 or 40 or 41 (62672)
39 and 43 (841)
(diabet* adj2 (program* or clinic* or center* or centre*)).ti,ab. (4401)
limit 45 to (human and english language and yr=“2000 - 2009”) (2068)
38 and 46 (567)
44 or 47 (1307)
Database: CINAHL/Pre-CINAHL
# Query Limiters/Expanders Last Run Via Results
S45 (S44 or S39) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 387
S44 (S43 and S37) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 191
S43 (S42 or S41 or S40) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 13141
S42 (type 2 N2 diabet*) or (type II N2 diabet*) or t2dm or NIDDM Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 8239
S41 (diabet* N2 ketosis resistant) or (diabet* N2 adult onset) or (diabet* N2 slow onset) or (diabet* N2 maturity onset) or (diabet* N2 non?insulin dependent) or (diabetes N2 stable) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 128
S40 (MH “Diabetes Mellitus, Non-Insulin-Dependent”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 11286
S39 (S38 and S36) Limiters - Published Date from: 200001-200912; Language: English
Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 224
S38 diabet* N2 center* or diabet* N2 centre* or diabet* N2 program* or diabet* N2 clinic* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 1980
S37 (S36 and S23) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 10438
S36 (S35 or S34) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 104806
S35 (S33 or S32 or S31 or S30 or S29) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 43639
S34 S28 or S27 or S26 or S25 or S24 Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 88261
S33 control* N2 clinical trial* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 2023
S32 (MH “Control (Research)+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 2444
S31 (MH “Placebos”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 4709
S30 (MH “Double-Blind Studies”) or (MH “Single-Blind Studies”) or (MH “Triple-Blind Studies”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 15190
S29 meta analy* or metaanaly* or pooled analysis or (systematic* N2 review*) or published studies or medline or embase or data synthesis or data extraction or cochrane Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 26178
S28 (MH “Cochrane Library”) or (MH “Systematic Review”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 6070
S27 (MH “Meta Analysis”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 6967
S26 health technology N2 assess* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 167
S25 random* or sham* or RCT* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 80324
S24 (MH “Random Assignment”) or (MH “Random Sample+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 35755
S23 (S22 or S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1) Limiters - Published Date from: 200001-200912; Language: English Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 87017
S22 multidisciplin* or multi-disciplin* or interdisciplin* or inter-disciplin* or collaborat* or cooperat* or co-operat* or multi-special* or multispecial* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 49990
S21 (MH “Nurse-Managed Centers”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL 1427
S20 team* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S19 care N2 model* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S18 (MH “Professional Role+”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S17 (MH “Subacute Care”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S16 (MH “Case Management”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S15 disease management program* Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S14 (MH “Disease Management”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S13 (MH “Continuity of Patient Care”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S12 (MH “Primary Health Care”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S11 (MH “Community Health Services”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S10 (MH “Health Care Delivery, Integrated”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S9 (MH “Teamwork”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S8 (MH “Interprofessional Relations+”) or (MH “Collaboration”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S7 (MH “Cooperative Behavior”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S6 (MH “Multidisciplinary Care Team+”) or (MH “Team Nursing”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S5 outpatient* care* or outpatient* service* or outpatient* clinic* or outpatient* facility or outpatient* facilities Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S4 ambulatory care* or ambulatory service* or ambulatory clinic* or ambulatory facility or ambulatory facilities Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S3 (MH “Outpatients”) or (MH “Outpatient Service”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S2 (MH “Ambulatory Care”) or (MH “Ambulatory Care Facilities+”) or (MH “Ambulatory Care Nursing”) Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
S1 intermedia* N2 care Search modes - Boolean/Phrase Interface - EBSCOhost
Search Screen - Advanced Search
Database - CINAHL;Pre-CINAHL Display
Appendix 2: Literature Search Flow Diagram
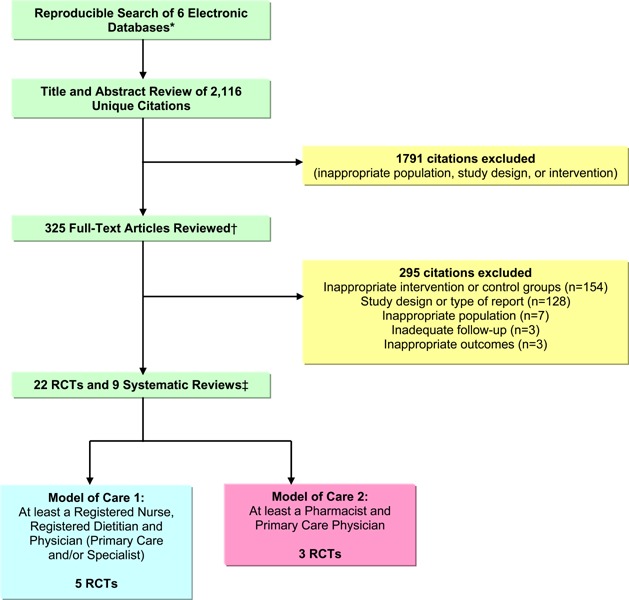
MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library, Ebsco CINAHL, & INAHTA/CRD
Articles that were determined as unknown eligibility were reviewed by a second reviewer and consensus was established
1 systematic review identified by manual searching
Appendix 3: Summary of the systematic reviews analyzed
Table A1: Summary of Existing Evidence on Specialized Multidisciplinary Community Care for the Management of Type 2 Diabetes (n=9).
| Study (type, search years)* | No. of trials | Objective | Applicability to MAS analysis |
|---|---|---|---|
| Glazier, et al, 2006 (37) (SR, 1986-2004) | 17 | To determine the effectiveness of patient, provider and health system interventions to improve diabetes care among socially disadvantaged populations. | Did not restrict to articles that were specialized or multidisciplinary in nature, restricted to populations with low socioeconomic status |
| Knight, et al, 2005 (20) (MA, 1987-2001) | 24 | To determine the effect of disease management programs for patients with diabetes on processes and outcomes of care | Not all studies involved specialized multidisciplinary care; meta-analysis had significant clinical and statistical heterogeneity and no attempt of subgroup analysis |
| Norris, et al, 2002 (38) (SR, 1966-2000) | 42 | To determine the effectiveness and economic efficiency of disease management and case management for people with diabetes. | Not all included articles involved specialized multi-disciplinary care; did not report HbA1c or SBP outcomes |
| O’Reilly, et al, 2006 (39) (SR, 1993-2005) | 24 | To determine the efficacy/effectiveness of multidisciplinary primary care interventions and diabetes programs to improve the management of patients with type 2 diabetes in a variety of delivery settings | Relevant review on multidisciplinary care for diabetes management; However, do not describe inclusion criteria of the intervention (i.e. characteristics of the diabetes programs) |
| Renders, et al, 2000 (40) (SR, 1966-2000) | 41 | To determine the effectiveness of interventions targeted at health care professionals and/or the structure of care to improve the management of diabetes in primary care, outpatient and community settings. | Although some interventions involved multidisciplinary teams, not all included involved interventions that were multidisciplinary |
| Shojania, et al, 2006 (21) (MR, 1966-2006) | 66 | To assess the impact of 11 distinct strategies for quality improvement in adults with type 2 diabetes (audit and feedback, case management, team changes, electronic patient registry, clinician education, clinician reminders, facilitated relay of clinical information to clinicians, patient education, promotion of self-management, patient reminder systems and continuous quality improvement) | Not all team changes or case management involved specialized multidisciplinary care |
| van Bruggen, et al, 2007 (41) (SR, 1990-2005)) | 22 | To determine if shared care and allocated care tasks lead to improved quality in diabetes care and a reduction in the cardiovascular risks in diabetes patients. | Different inclusion/exclusion criteria; not all were multidisciplinary care; included delegated care (action being allocated to someone with a lower level of training) |
| Whittemore, et al, 2007 (42) (SR, 1990-2006) | 11 | To describe interventional components and efficacy (clinical outcomes, behavioural outcomes, knowledge) of multifaceted, culturally competent interventions aimed at improving outcomes in Hispanic adults with type 2 diabetes; to describe cultural strategies of the interventions; and to examine factors associated with attendance and attrition | Focus on Hispanic adult diabetic population; not all included studies involved multidisciplinary care |
| Wubben, et al, 2008 (43) (SR, 1937-2007) | 21 | To assess the impact of diabetes quality improvement strategies that used pharmacists in outpatient settings on improvement of glycemic control and other direct outcomes for diabetic adults. | Focused on integration of a pharmacist specifically into team; however, not all studies were multidisciplinary |
SR, Systematic review; MA, meta-analysis; MR, meta-regression
HbA1c, glycosylated hemoglobin; SBP, systolic blood pressure;
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Community-based care for the management of type 2 diabetes: an evidence-based analysis. Ontario Health Technology Assessment Series 2009;9(10).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-9433-5 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
Abbreviations
- BMI
Body Mass Index
- CI
Confidence interval(s)
- HbA1c
Glycosylated hemoglobin
- HDL
High-Density Lipoprotein
- LDL
Low-Density Lipoprotein
- MAS
Medical Advisory Secretariat
- ODD
Ontario Diabetes Database
- OR
Odds ratio
- OHTAC
Ontario Health Technology Advisory Committee
- QALY
Quality adjusted life year
- QoL
Quality of life
- RCT
Randomized controlled trial
- RR
Relative risk
- SBP
Systolic blood pressure
- SD
Standard deviation
- UKPDS
United Kingdom Prospective Diabetes Study
References
- 1.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32((Suppl 1)):S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet. 2007;369(9563):750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–53. UK Prospective Diabetes Study (UKPDS) Group. [PubMed] [Google Scholar]
- 4.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–13. UK Prospective Diabetes Study Group. [PMC free article] [PubMed] [Google Scholar]
- 5.Shah BR, Hux JE, Laupacis A, Mdcm BZ, Austin PC, van WC. Diabetic patients with prior specialist care have better glycaemic control than those with prior primary care. J Eval Clin Pract. 2005;11(6):568–75. doi: 10.1111/j.1365-2753.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 6.McDonald KM, Sundaram V, Bravata DM, Lewis R, Lin N, Kraft S, et al. Care Coordination. Vol 7 of: Shojania KG, McDonald KM, Wachter RM, Owens DK, editors. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Technical Review 9 (Prepared by the Stanford University-UCSF Evidence-based Practice Center under contract 290-02-0017) [Internet]. Rockville, MD: Agency for Healthcare Research and Quality. 2007 June. [[cited: 2008 Oct 23]. 158 p]; AHRQ Publication No. 04(07)-0051-7. Available from: http://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf . [Google Scholar]
- 7.O’Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux J, Tarride JE, et al. Long-term cost-utility analysis of a multidisciplinary primary care diabetes management program in Ontario. Can J Diabetes. 2007;31(3):205–14. [Google Scholar]
- 8.Thiessen PH, Barrowman N, Garg AX. Imputing variance estimates do not alter the conclusions of a meta-analysis with continuous outcomes: a case study of changes in renal function after living kidney donation. J Clin Epidemiol. 2007;60(3):228–40. doi: 10.1016/j.jclinepi.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 9.CONSORT Group. The CONSORT statement [Internet] [updated 2007 Oct 22; cited 2009 Feb 13] Available from: http://www.consort-statement.org/?o=1011 .
- 10.GRADE Working Group. GRADE [Internet] [updated 2006; cited 2009 Feb 2] Available from: http://www.gradeworkinggroup.org/index.htm . [Google Scholar]
- 11.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maislos M, Weisman D. Multidisciplinary approach to patients with poorly controlled type 2 diabetes mellitus: a prospective, randomized study. Acta Diabetol. 2004;41(2):44–8. doi: 10.1007/s00592-004-0143-1. [DOI] [PubMed] [Google Scholar]
- 13.Gaede P, Beck M, Vedel P, Pedersen O. Limited impact of lifestyle education in patients with Type 2 diabetes mellitus and microalbuminuria: results from a randomized intervention study. Diabet Med. 2001;18(2):104–8. doi: 10.1046/j.1464-5491.2001.00444.x. [DOI] [PubMed] [Google Scholar]
- 14.Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations: the California Medi-Cal type 2 diabetes study. Diabetes Care. 2004;27(1):95–103. doi: 10.2337/diacare.27.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Johansen OE, Gullestad L, Blaasaas KG, Orvik E, Birkeland KI. Effects of structured hospital-based care compared with standard care for Type 2 diabetes-The Asker and Baerum Cardiovascular Diabetes Study, a randomized trial. Diabet Med. 2007;24(9):1019–27. doi: 10.1111/j.1464-5491.2007.02198.x. [DOI] [PubMed] [Google Scholar]
- 16.Groeneveld Y, Petri H, Hermans J, Springer M. An assessment of structured care assistance in the management of patients with type 2 diabetes in general practice. Scand J Prim Health Care. 2001;19(1):25–30. doi: 10.1080/028134301300034585. [DOI] [PubMed] [Google Scholar]
- 17.Rothman RL, Malone R, Bryant B, Shintani AK, Crigler B, Dewalt DA, et al. A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118(3):276–84. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 18.McLean DL, McAlister FA, Johnson JA, King KM, Makowsky MJ, Jones CA, et al. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists-hypertension (SCRIP-HTN) Arch Intern Med. 2008;168(21):2355–61. doi: 10.1001/archinte.168.21.2355. [DOI] [PubMed] [Google Scholar]
- 19.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: The Swedish Council on Technology Assessment in Health Care. 1993 doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 20.Knight K, Badamgarav E, Henning JM, Hasselblad V, Gano AD Jr, Ofman JJ, et al. A systematic review of diabetes disease management programs. Am J Manage Care. 2005;11(4):242–50. [PubMed] [Google Scholar]
- 21.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–40. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 22.Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S. Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am J Manage Care. 2005;11(4):253–60. [PubMed] [Google Scholar]
- 23.Gabbay RA, Lendel I, Saleem TM, Shaeffer G, Adelman AM, Mauger DT, et al. Nurse case management improves blood pressure, emotional distress and diabetes complication screening. Diabetes Res Clin Pract. 2006;71(1):28–35. doi: 10.1016/j.diabres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Gary TL, Bone LR, Hill MN, Levine DM, McGuire M, Saudek C, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban African Americans. Prev Med. 2003;37(1):23–32. doi: 10.1016/s0091-7435(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 25.Hiss RG, Armbruster BA, Gillard ML, McClure LA. Nurse care manager collaboration with community-based physicians providing diabetes care: a randomized controlled trial. Diabetes Educ. 2007;33(3):493–502. doi: 10.1177/0145721707301349. [DOI] [PubMed] [Google Scholar]
- 26.Krein SL, Klamerus ML, Vijan S, Lee JL, Fitzgerald JT, Pawlow A, et al. Case management for patients with poorly controlled diabetes: a randomized trial. Am J Med. 2004;116(11):732–9. doi: 10.1016/j.amjmed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Litaker D, Mion LC, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician-nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients’ perception of care. J Interprof Care. 2003;17(3):223–37. doi: 10.1080/1356182031000122852. [DOI] [PubMed] [Google Scholar]
- 28.McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40(3):566–75. doi: 10.1053/ajkd.2002.34915. [DOI] [PubMed] [Google Scholar]
- 29.O’Hare JP, Raymond NT, Mughal S, Dodd L, Hanif W, Ahmad Y, et al. Evaluation of delivery of enhanced diabetes care to patients of South Asian ethnicity: the United Kingdom Asian Diabetes Study (UKADS) Diabet Med. 2004;21(12):1357–65. doi: 10.1111/j.1464-5491.2004.01373.x. [DOI] [PubMed] [Google Scholar]
- 30.Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care. 2001;24(2):202–8. doi: 10.2337/diacare.24.2.202. [DOI] [PubMed] [Google Scholar]
- 31.Shea S, Weinstock RS, Starren J, Teresi J, Palmas W, Field L, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibayama T, Kobayashi K, Takano A, Kadowaki T, Kazuma K. Effectiveness of lifestyle counseling by certified expert nurse of Japan for non-insulin-treated diabetic outpatients: a 1-year randomized controlled trial. Diabetes Res Clin Pract. 2007;76(2):265–8. doi: 10.1016/j.diabres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Bury G, O’Leary M, Shannon W, Tynan A, Staines A, et al. The North Dublin randomized controlled trial of structured diabetes shared care. Fam Pract. 2004;21(1):39–45. doi: 10.1093/fampra/cmh109. [DOI] [PubMed] [Google Scholar]
- 34.Soja AM, Zwisler AD, Frederiksen M, Melchior T, Hommel E, Torp-Pedersen C, et al. Use of intensified comprehensive cardiac rehabilitation to improve risk factor control in patients with type 2 diabetes mellitus or impaired glucose tolerance--the randomized DANish StUdy of impaired glucose metabolism in the settings of cardiac rehabilitation (DANSUK) study. Am Heart J. 2007;153(4):621–8. doi: 10.1016/j.ahj.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CB, Miller NH, Reilly KR, Greenwald G, Cunning D, Deeter A, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care. 2003;26(4):1058–63. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- 36.Wolf AM, Conaway MR, Crowther JQ, Hazen KY, Nadler JL, Oneida B, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27(7):1570–6. doi: 10.2337/diacare.27.7.1570. [DOI] [PubMed] [Google Scholar]
- 37.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29(7):1675–88. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 38.Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. Am J Prev Med. 2002;22(4:Suppl):Suppl-38. doi: 10.1016/s0749-3797(02)00423-3. A systematic review. [DOI] [PubMed] [Google Scholar]
- 39.O’Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux J, Guan J, et al. Development of an Ontario Diabetes Economic Model (ODEM) and application to a multidisciplinary primary care diabetes management program. [[cited: 2009 Oct 22]];Hamilton, Ontario: Programs for Assessment of Technology in Health, McMaster University. 2006 Nov 2. Available from: http://www.path-hta.ca/diabetes.pdf . [Google Scholar]
- 40.Renders CM, Valk GD, Griffin S, Wagner EH, van Eijk JTM, Assendelft WJJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev 2000;(4) doi: 10.1002/14651858.CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Bruggen JA, Gorter KJ, Stolk RP, Rutten GE. Shared and delegated systems are not quick remedies for improving diabetes care: a systematic review. Primary care diabetes. 2007;1(2):59–68. doi: 10.1016/j.pcd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Whittemore R. Culturally competent interventions for Hispanic adults with type 2 diabetes: a systematic review. J Transcult Nurs. 2007;18(2):157–66. doi: 10.1177/1043659606298615. [DOI] [PubMed] [Google Scholar]
- 43.Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: A systematic review. Pharmacotherapy. 2008;28(4):421–36. doi: 10.1592/phco.28.4.421. [DOI] [PubMed] [Google Scholar]


