Executive Summary
Objective
To assess the effectiveness and safety of low-density lipoprotein (LDL) apheresis performed with the heparin-induced extracorporeal LDL precipitation (HELP) system for the treatment of patients with refractory homozygous (HMZ) and heterozygous (HTZ) familial hypercholesterolemia (FH).
Background on Familial Hypercholesterolemia
Familial hypercholesterolemia is a genetic autosomal dominant disorder that is caused by several mutations in the LDL-receptor gene. The reduced number or absence of functional LDL receptors results in impaired hepatic clearance of circulating low-density lipoprotein cholesterol (LDL-C) particles, which results in extremely high levels of LDL-C in the bloodstream. Familial hypercholesterolemia is characterized by excess LDL-C deposits in tendons and arterial walls, early onset of atherosclerotic disease, and premature cardiac death.
Familial hypercholesterolemia occurs in both HTZ and HMZ forms.
Heterozygous FH is one of the most common monogenic metabolic disorders in the general population, occurring in approximately 1 in 500 individuals1. Nevertheless, HTZ FH is largely undiagnosed and an accurate diagnosis occurs in only about 15% of affected patients in Canada. Thus, it is estimated that there are approximately 3,800 diagnosed and 21,680 undiagnosed cases of HTZ FH in Ontario.
In HTZ FH patients, half of the LDL receptors do not work properly or are absent, resulting in plasma LDL-C levels 2- to 3-fold higher than normal (range 7-15mmol/L or 300-500mg/dL). Most HTZ FH patients are not diagnosed until middle age when either they or one of their siblings present with symptomatic coronary artery disease (CAD). Without lipid-lowering treatment, 50% of males die before the age of 50 and 25% of females die before the age of 60, from myocardial infarction or sudden death.
In contrast to the HTZ form, HMZ FH is rare (occurring in 1 case per million persons) and more severe, with a 6- to 8-fold elevation in plasma LDL-C levels (range 15-25mmol/L or 500-1000mg/dL). Homozygous FH patients are typically diagnosed in infancy, usually due to the presence of cholesterol deposits in the skin and tendons. The main complication of HMZ FH is supravalvular aortic stenosis, which is caused by cholesterol deposits on the aortic valve and in the ascending aorta. The average life expectancy of affected individuals is 23 to 25 years. In Ontario, it is estimated that there are 13 to 15 cases of HMZ FH. An Ontario clinical expert confirmed that 9 HMZ FH patients have been identified to date.
Diagnosis
There are 2 accepted clinical diagnostic criterion for the diagnosis of FH: the Simon Broome FH Register criteria from the United Kingdom and the Dutch Lipid Network criteria from the Netherlands. The criterion supplement cholesterol levels with clinical history, physical signs and family history. DNA-based-mutation-screening methods permit a definitive diagnosis of HTZ FH to be made. However, given that there are over 1000 identified mutations in the LDL receptor gene and that the detection rates of current techniques are low, genetic testing becomes problematic in countries with high genetic heterogeneity, such as Canada.
Treatment
The primary aim of treatment in both HTZ and HMZ FH is to reduce plasma LDL-C levels in order to reduce the risk of developing atherosclerosis and CAD.
The first line of treatment is dietary intervention, however it alone is rarely sufficient for the treatment of FH patients. Patients are frequently treated with lipid-lowering drugs such as resins, fibrates, niacin, statins and cholesterol absorption-inhibiting drugs (ezetimibe). Most HTZ FH patients require a combination of drugs to achieve or approach target cholesterol levels.
A small number of HTZ FH patients are refractory to treatment or intolerant to lipid-lowering medication. According to clinical experts, the prevalence of refractory HTZ FH in Ontario is between 1 to 5%. Using the mean of 3%, it is estimated that there are approximately 765 refractory HTZ FH patients in Ontario, of which 115 are diagnosed and 650 are undiagnosed.
Drug therapy is less effective in HMZ FH patients since the effects of the majority of cholesterol-lowering drugs are mediated by the upregulation of LDL receptors, which are often absent or function poorly in HMZ FH patients. Some HMZ FH patients may still benefit from drug therapy, however this rarely reduces LDL-C levels to targeted levels.
Existing Technology: Plasma Exchange
An option currently available in Ontario for FH patients who do not respond to standard diet and drug therapy is plasma exchange (PE). Patients are treated with this lifelong therapy on a weekly or biweekly basis with concomitant drug therapy.
Plasma exchange is nonspecific and eliminates virtually all plasma proteins such as albumin, immunoglobulins, coagulation factors, fibrinolytic factors and HDL-C, in addition to acutely lowering LDL-C by about 50%. Blood is removed from the patient, plasma is isolated, discarded and replaced with a substitution fluid. The substitution fluid and the remaining cellular components of the blood are then returned to the patient.
The major limitation of PE is its nonspecificity. The removal of HDL-C prevents successful vascular remodeling of the areas stenosed by atherosclerosis. In addition, there is an increased susceptibility to infections, and costs are incurred by the need for replacement fluid. Adverse events can be expected to occur in 12% of procedures.
Other Alternatives
Surgical alternatives for FH patients include portocaval shunt, ileal bypass and liver transplantation. However, these are risky procedures and are associated with a high morbidity rate. Results with gene therapy are not convincing to date.
The Technology Being Reviewed: LDL Apheresis
An alternative to PE is LDL apheresis. Unlike PE, LDL apheresis is a selective treatment that removes LDL-C and other atherogenic lipoproteins from the blood while minimally impacting other plasma components such as HDL-C, total serum protein, albumin and immunoglobulins. As with PE, FH patients require lifelong therapy with LDL apheresis on a weekly/biweekly basis with concomitant drug therapy.
Heparin-Induced Extracorporeal LDL Precipitation
Heparin-induced extracorporeal LDL precipitation (HELP) is one of the most widely used methods of LDL apheresis. It is a continuous closed-loop system that processes blood extracorporeally. It operates on the principle that at a low pH, LDL and lipoprotein (a) [Lp(a)] bind to heparin and fibrinogen to form a precipitate which is then removed by filtration. In general, the total duration of treatment is approximately 2 to 3 hours.
Results from early trials indicate that LDL-C concentration is reduced by 65% to 70% immediately following treatment in both HMZ and HTZ FH and then rapidly begins to rise. Typically patients with HTZ FH are treated every 2 weeks while patients with HMZ FH require weekly therapy. Heparin-induced extracorporeal LDL precipitation also produces small transient decreases in HDL-C, however levels generally return to baseline within 2 days. After several months of therapy, long-term reductions in LDL-C and increases in HDL-C have been reported.
In addition to having an impact on plasma cholesterol concentrations, HELP lowers plasma fibrinogen, a risk factor for atherosclerosis, and reduces concentrations of cellular adhesion molecules, which play a role in early atherogenesis.
In comparison with PE, HELP LDL apheresis does not have major effects on essential plasma proteins and does not require replacement fluid, thus decreasing susceptibility to infections. One study noted that adverse events were documented in 2.9% of LDL apheresis treatments using the HELP system compared with 12% using PE. As per the manufacturer, patients must weigh at least 30kgs to be eligible for treatment with HELP.
Regulatory Status
The H.E.L.P.® System (B.Braun Medizintechnologie GmbH, Germany) has been licensed by Health Canada since December 2000 as a Class 3 medical device (Licence # 26023) for performing LDL apheresis to acutely remove LDL from the plasma of 3 high-risk patient populations for whom diet has been ineffective and maximum drug therapy has either been ineffective or not tolerated. The 3 patient groups are as follows:
Functional hypercholesterolemic homozygotes with LDL-C >500 mg/dL (>13mmol/L);
Functional hypercholesterolemic heterozygotes with LDL-C >300 mg/dL (>7.8mmol/L);
Functional hypercholesterolemic heterozygotes with LDL-C >200 mg/dL (>5.2mmol/L) and documented CAD
No other LDL apheresis system is currently licensed in Canada.
Review Strategy
The Medical Advisory Secretariat systematically reviewed the literature to assess the effectiveness and safety of LDL apheresis performed with the HELP system for the treatment of patients with refractory HMZ and HTZ FH. A standard search methodology was used to retrieve international health technology assessments and English-language journal articles from selected databases.
The GRADE approach was used to systematically and explicitly make judgments about the quality of evidence and strength of recommendations.
Summary of Findings
The search identified 398 articles published from January 1, 1998 to May 30, 2007. Eight studies met the inclusion criteria. Five case series, 2 case series nested within comparative studies, and one retrospective review, were included in the analysis. A health technology assessment conducted by the Alberta Heritage Foundation for Medical Research, and a review by the United States Food and Drug Administration were also included.
Large heterogeneity among the studies was observed. Studies varied in inclusion criteria, baseline patient characteristics and methodology.
Overall, the mean acute1 relative decrease in LDL-C with HELP LDL apheresis ranged from 53 to 77%. The mean acute relative reductions ranged as follows: total cholesterol (TC) 47 to 64%, HDL-C +0.4 to -29%, triglycerides (TG) 33 to 62%, Lp(a) 55 to 68% and fibrinogen 56 to 65%.
The mean chronic2 relative decreases in LDL-C and TC with HELP LDL apheresis ranged from 9 to 46% and 5 to 34%, respectively. Familial hypercholesterolemia patients treated with HELP did not achieve the target LDL-C value set by international guidelines (LDL-C < 2.5mmol/L, 100mg/dL). The chronic mean relative increase in HDL-C ranged from 12 to 27%. The ratio of LDL:HDL and the ratio of TC:HDL are 2 measures that have been shown to be important risk factors for cardiac events. In high-risk patients, the recommended target LDL:HDL ratio is less than or equal to 2, and the target TC:HDL ratio is less than 4. In the studies that reported chronic lipid changes, the LDL:HDL and TC:HDL ratios exceeded targeted values.
Three studies investigated the effects of HELP on coronary outcomes and atherosclerotic changes. One noted that twice as many lesions displayed regression in comparison to those displaying progression. The second study found that there was a decrease in Agatston scores3 and in the volume of coronary calcium. The last study noted that 2 of 5 patients showed regression of coronary atherosclerosis, and 3 of the 5 patients showed no change as assessed by a global change score.
Adverse effects were typically mild and transient, and the majority of events were related to problems with vascular access. Of the 3 studies that provided quantitative information, the proportion of adverse events ranged from 2.9 to 5.1%.
GRADE Quality of Evidence
In general, studies were of low quality, i.e., case series studies (Tables 1-3). No controlled studies were identified and no studies directly compared the effectiveness of the HELP system with PE or with diet and drug therapy. Conducting trials with a sufficiently large control group would not have been feasible or acceptable given that HELP represents a last alternative in these patients who are resistant to conventional therapeutic strategies.
A major limitation is that there is limited evidence on the effectiveness and safety of HELP apheresis in HMZ FH patients. However, it is unlikely that better-quality evidence will become available, given that HMZ FH is rare and LDL apheresis is a last therapeutic option for these patients.
Lastly, there is limited data on the long-term effects of LDL apheresis in FH patients. No studies with HELP were identified that examined long-term outcomes such as survival and cardiovascular events. The absence of this data may be attributed to the rarity of the condition, and the large number of subjects and long duration of follow-up that would be needed to conduct such trials.
Economic Analysis
A budget-impact analysis was conducted to forecast future costs for PE and HELP apheresis in FH patients. All costs are reported in Canadian dollars. Based on epidemiological data of 13 HMZ, 115 diagnosed HTZ and 765 cases of all HTZ patients (diagnosed + undiagnosed), the annual cost of weekly treatment was estimated to be $488,025, $4,332,227 and $24,758,556 respectively for PE. For HELP apheresis, the annual cost of weekly treatment was estimated to be $1,025,338, $9,156,209 and $60,982,579 respectively. Costs for PE and HELP apheresis were halved with a biweekly treatment schedule.
The cost per coronary artery disease death avoided over a 10-year period in HTZ FH-diagnosed patients was also calculated and estimated to be $37.5 million and $18.7 million for weekly and biweekly treatment respectively, when comparing HELP apheresis with PE and with no intervention. Although HELP apheresis costs twice as much as PE, it helped to avoid 12 deaths compared with PE and 22 deaths compared with no intervention, over a period of 10 years.
Ontario Health System Impact Analysis
Low-density lipoprotein apheresis using the HELP system is currently being funded by the provinces of Quebec and Alberta. The program in Quebec has been in operation since 2001 and is limited to the treatment of HMZ FH patients. The Alberta program is relatively new and is currently treating HMZ FH patients, but it is expanding to include refractory HTZ FH patients.
Low-density lipoprotein apheresis is a lifelong treatment and requires considerable commitment on the part of the patient, and the patient’s family and physician. In addition, the management of FH continues to evolve. With the advent of new more powerful cholesterol-lowering drugs, some HTZ patients may be able to sufficiently control their hypercholesterolemia. Nevertheless, according to clinical experts, HMZ patients will likely always require LDL apheresis.
Given the substantial costs associated with LDL apheresis, treatment has been limited to HMZ FH patients. However, LDL apheresis could be applied to a much larger population, which would include HTZ FH patients who are refractory to diet and drug therapy. HTZ FH patients are generally recruited in a more advanced state, demonstrate a longer natural survival than HMZ FH patients and are older.
Conclusions
For HMZ FH patients, the benefits of LDL apheresis clearly outweigh the risks and burdens. According to GRADE, the recommendation would be graded as strong, with low- to very low-quality evidence (Table 4).
In both HMZ and HTZ FH patients, there is evidence of overall clinical benefit of LDL apheresis from case series studies. Low-density lipoprotein apheresis has several advantages over the current treatment of PE, including decreased exposure to blood products, decreased risk of adverse events, conservation of nonatherogenic and athero-protective components, such as HDL-C and lowering of other atherogenic components, such as fibrinogen.
In contrast to HMZ FH patients, there remains a lot of uncertainty in the social/ethical acceptance of this technology for the treatment of refractory HTZ FH patients. In addition to the substantial costs, it is unknown whether the current health care system could cope with the additional demand. There is uncertainty in the estimates of benefits, risks and burdens. According to GRADE, the recommendation would be graded as weak with low- to very-low-quality evidence (Table 5).
GRADE of recommendation: Strong recommendation, low-quality or very-low-quality evidence
Benefits clearly outweigh risk and burdens
Case series study designs
Strong, but may change when higher-quality evidence becomes available
GRADE of recommendation: Weak recommendation, low-quality or very-low-quality evidence
Uncertainty in the estimates of benefits, risks and burden, which these may be closely balanced
Case series study designs
Very weak; other alternatives may be equally reasonable
Objective
To assess the effectiveness and safety of low-density lipoprotein (LDL) apheresis performed with the heparin-induced extracorporeal LDL precipitation (HELP) system for the treatment of patients with refractory homozygous (HMZ) and heterozygous (HTZ) familial hypercholesterolemia (FH).
Background
Clinical Need: Target Population and Condition
Familial hypercholesterolemia is a genetic autosomal dominant disorder that is caused by several mutations in the LDL-receptor gene. Under normal circumstances, cholesterol is removed from the blood by LDL receptors. Hypercholesterolemia is caused by an overproduction of cholesterol or a removal defect, or a combination of both. Familial hypercholesterolemia is caused by a removal defect.
The reduced number or absence of functional LDL receptors results in impaired hepatic clearance of circulating low-density lipoprotein cholesterol (LDL-C) particles, which results in extremely high levels of LDL-C in the bloodstream. (3) Familial hypercholesterolemia is characterized by excess LDL-C deposits in tendons and arterial walls, early onset of atherosclerotic disease, and premature cardiac death. (4;5)
The LDL-receptor gene is located on chromosome 19, and to date, over 1000 mutations have been identified worldwide. (6) Mutations vary from single nucleotide substitutions to large deletions and they have been grouped into 5 different classes based on their phenotypic effects on receptor functioning. (5)
Most mutations are for the LDL-receptor gene; however, in a small number of patients, it is the apolipoprotein B100 (aPOB) ligand for the receptor that is defective. To date, several mutations in aPOB have been identified and patients with these mutations are classified as having familial defective apolipoprotein B100, a condition that is clinically indistinguishable from heterozygous (HTZ) FH. (7;8)
According to the World Health Organization (WHO) classification of hyperlipidemias (modified Fredrickson), FH is classified as type IIa hyperlipidemia (See Table 1). The hallmark of this disease is elevated levels of total cholesterol (TC) and LDL-C well above the 95th percentile for age and sex, with high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels usually in the normal range. (9)
Table 1: Homozygous Familial Hypercholesterolemia - Lipid Outcomes.
| Number of Studies |
Study Design | Quality of Studies | Consistency | Directness | Other Modifying Factors |
Overall Quality of Evidence |
|---|---|---|---|---|---|---|
| 1 | Case series=Low | Low | + | Yes | Sparse data | Very low |
| 1 | Retrospective review=Low |
Table 1: World Health Organization (Modified Fredrickson) Classification of Hyperlipidemias.
| Type | Total cholesterol | LDL cholesterol | Plasma triglycerides | Lipoprotein abnormality | Primary Cause |
Secondary cuse |
|---|---|---|---|---|---|---|
| I | Elevated | Low or normal | Elevated | Excess chylomicrons | Lipoprotein lipase deficiency Apo-C II deficiency | Systemic lupus erythematosus |
| Iia | Elevated or normal | Elevated | Normal | Excess LDL | Familial hypercholesterolemia | Hypothyroidism |
| Iib | Elevated | Elevated | Elevated | Excess LDL and VLDL | Familial combined Hyperlipidemia | Nephrotic syndrome, diabetes, anorexia nervosa |
| III | Elevated | Low or normal | Elevated | Excess chylomicron remnants and IDL | Familial type III Hyperlipoproteinemia | Hypothyroidism, diabetes, obesity |
| IV | Elevated or normal | Normal | Elevated | Excess VLDL | Familial combined Hyperlipidemia, familial Hypertriglyceridemia | Diabetes, chronic renal disease |
| V | Elevated | Normal | Elevated | Excess chylomicrons and VLDL | Familial hypertriglyceridemia Apo-C II deficiency | Alcohol, diuretics, beta blockers, oral |
Apo, apolipoprotein: IDL, intermediate density lipoprotein: LDL, low density lipoprotein: VLDL. very low density lipoprotein.
Reprinted from Progress in Pediatric Cardiology, 17(2), McCrindle BW. Drug therapy of hyperlipidemia, 141-50, Copyright 2003; with permission from Elsevier.
Epidemiology of Familial Hypercholesterolemia
Familial hypercholesterolemia occurs in both HTZ and HMZ forms.
Heterozygous FH is one of the most common monogenic metabolic disorders in the general population. (10) Due to the founder gene effect1, the prevalence of FH is higher in certain population such as French Canadians, Johannesburg Jews, the Christian Lebanese population, and the South African Afrikaner population. (5)
Using the Hardy Weinberg principle of population genetics, which describes the relationship between the frequencies of alleles and the genotype of a population, it is estimated that HTZ FH occurs in approximately 1 in 500 individuals2. (11) Nevertheless, underdiagnosis of FH is a common concern and the estimated percentage of diagnosed HTZ FH patients ranges from less than 1 to 44%. (12) A recent study from the United Kingdom reported that only about 25% of cases predicted on the basis of the estimated gene carrier frequency had been diagnosed. The prevalence of diagnosed HTZ FH was estimated at 0.53 per 1000 (95% confidence interval [CI]: 0.48 – 0.60). (13) The highest prevalence was in men aged 50 to 59 years and in women aged 60 to 69 years. Underdiagnosis was found to be greatest among children and young adults. A WHO report estimated that HTZ FH is accurately diagnosed in only about 15% of affected patients in Canada. (14) Thus, it is estimated that there are approximately 3,800 diagnosed and 21,680 undiagnosed cases of HTZ FH in Ontario.
In HTZ FH patients, 50% of LDL receptors do not work properly or are absent, and the rate of removal of LDL-C decreases substantially resulting in plasma LDL-C levels 2- to 3-fold higher than normal (range 7-15mmol/L or 300-500mg/dL). (3-5;12;15) These levels are well above the 95th percentile for all age and gender categories. (3) Most HTZ FH patients are not diagnosed until middle age when either they or one of their siblings present with symptomatic coronary artery disease (CAD). (12;13) Clinically overt CAD frequently occurs at a mean age of 45 to 48 years in affected males and at 55 to 58 years in females. (5;16) The chance of an HTZ FH male suffering a myocardial infarction (MI) is 5% for those under 30 years of age, 50% by 50 years and 85% by 60 years. In females, who are protected from atherosclerotic disease prior to menopause, the corresponding values are 1% for those under 30 years of age, 15% by 50 years and 50% by 60 years (16;17) Without lipid- lowering treatment, 50% of males die before the age of 50 and 25% of females die before the age of 60, from MI or sudden death. (18)
In contrast to the HTZ form, HMZ FH is rare (occurring in one case per million persons) and more severe, with a 6- to 8-fold elevation in plasma LDL-C levels, and is sometimes even higher (range 15-25mmol/L or 500-1000mg/dL). (3-5;12;19) In HMZ FH patients, LDL receptors are either absent (receptor negative) or only have residual activity (receptor defective). (3;20) In receptor-negative patients, receptor activity is classified as less than 2% of normal activity and in receptor-defective patients, receptor activity is described as minimal activity with 5 to 30% of normal activity. Assessment of receptor activity is through testing of biopsied skin fibroblasts. (20)
Homozygous FH patients are typically diagnosed in infancy, usually due to the presence of physical findings related to cholesterol deposits in the skin and tendons, such as tendinous and tuberous xanthomas, cutaneous xanthelasma, or corneal arcus. (3;5;20) The high levels of LDL-C result in accelerated atherosclerosis. The main complication of HMZ FH is supravalvular aortic stenosis, which is caused by cholesterol deposits on the aortic valve and in the ascending aorta. (21) This eventually requires aortic valve replacement or coronary artery bypass grafting (CABG). Typically, patients develop aortic stenosis and CAD by the age of 20 years and average life expectancy is 23 to 25 years. (3;5;18)
In Ontario, based on estimates of genetic frequency, it is estimated that there are approximately 13 to 15 prevalent cases of HMZ FH. An Ontario clinical expert confirmed that 9 HMZ FH patients have been identified to date.
The clinical phenotype of HTZ FH and, to a lesser extent, HMZ FH, are highly influenced by environmental (22) and metabolic factors, the type of LDL receptor mutation, and the coinheritance of other genetic factors. (23) These factors lead to variations between patients in the degree of LDL-C elevation and the onset and severity of atherosclerotic disease. (24)
Diagnosis
There are 2 accepted clinical diagnostic criterion for the diagnosis of FH: the Simon Broome FH Register criteria from the United Kingdom (Box 1) and the Dutch Lipid Network criteria from the Netherlands (Box 2). (12;25;26) The criterion supplement cholesterol levels with clinical history, physical signs and family history, and also take into account evidence of dominant transmission and the age of onset of CAD in family members. In the Simon Broome Register criteria, cases are classified as either definite or possible. (12;27) The Dutch Lipid Network criteria are similar to the Simon Broome criteria, but add the calculation of a numeric score. If the score is greater than 8 points, the diagnosis is considered certain. A score between 6 and 8 points indicates a probable diagnosis, and a score between 3 and 5 points indicates a possible diagnosis. A diagnosis is not made if the score is less than 3 points. (12) Due to the principles of population genetics, an LDL-C measurement above a key threshold becomes a highly specific marker when a diagnosis of FH has been made in a family member. (25)
Box 1. Simon Broome Register Group definition of FH
A definite diagnosis of FH requires
(a) Total cholesterol level above 7.5 mmol/1 (290 mg/dl) in adults or a total cholesterol level above 6.7 mmol/1 (260 mg/dl) for children under 16,
OR LDL levels above 4.9 mm ol/1 (190 mg/dl) in adults (4.0 mmol/1 in children) (either pretreatment or highest on treatment)
PLUS (b) Tendon xanthomas in patient or relative (parent, child, sibling, grandparent, aunt, uncle)
OR (c) DNA-based evidence of an LDL receptor mutation or familial defective apo B-100 Possible FH is defined as (a) above plus one of (d) or (e):
(c) Family history of myocardial infarction before age 50 in grandparent, aunt, uncle or before age 60 in parent, sibling or child.
(d) Family history of raised cholesterol in parent sibling or child, or level above 7.5 mmol/1 (290 mg/dl) in grandparent, aunt, uncle
Apo B-100 refers to aploliprotein B-100; FH, familial hypercholesterolemia; LDL, low-density lipoprotein.
Reproduced from Atherosclerosis 168(1), Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia, 1-14, Copyright 2003, with permission from Elsevier.
Box 2. Dutch lipid clinic network diagnosis of FH
| Family history | ||||
| A | First degree relative with known premature (< 55 years men; < 60 years women) | 1 | ||
| B | First degree relative with known LDL-cholesterol > 95th percentile and/or 2 | |||
| A | First degree relative with tendon xanthomata and/or arcus cornealis. | 2 | ||
| B | Childern below 18 years with LDL cholesterol > 95th percentile | |||
| Clinical history | ||||
| A | Patient has premature (< 55 years men; < 60 years women) coronary artery disease | 2 | ||
| B | Patient has premature (< 55 years men; < 60 years women) cerebral or peripheral vascular disease | 1 | ||
| Physical Examination | ||||
| A | Tendon xanthomata | 6 | ||
| B | Arcus cornealis below 45 years | 4 | ||
| Laboratory analysis | ||||
| mmol/l | mg/dl | |||
| A | LDL-cholesterol | >8.5 | >330 | 8 |
| B | LDL-cholesterol | 6.5-8.4 | 250-329 | 5 |
| C | LDL-cholesterol | 5.0-6.4 | 190-249 | 3 |
| D | LDL-cholesterol | 4.0-4.9 | 155-198 | 1 |
| (HDL-cholesterol ol and triglycerides are normal) | ||||
| DNA analysis | ||||
| A | Functional mutation in the low-density lipoprotein receptor present | |||
FH refers to familial hypercholesterolemia; LDL, low-density lipoprotein.
Reproduced from Atherosclerosis 168(1), Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia, 1-14, Copyright 2003, with permission from Elsevier.
The efficacy of these 2 diagnostic criterion was recently evaluated in a study of 408 HTZ FH patients in Denmark. Molecular diagnosis (considered the “gold standard”) revealed that the 2 sets of criteria had little difference in terms of sensitivity and specificity. This suggests that either approach would be helpful in clinical diagnosis of HTZ FH. (25) Nevertheless, both methods have low sensitivity for a definite diagnosis, which signifies that their ability to detect true HTZ FH cases is limited and that not all cases are captured. Both methods also demonstrate poor specificity for a possible diagnosis, which indicates that some cases are incorrectly labeled as HTZ FH. Patients with HTZ FH often do not display absolutely predictive traits. Thus, arbitrary decisions must be made for diagnosis, which incurs compromises of either sensitivity or specificity. (16;23)
According to clinical experts, HTZ FH should be suspected when a patient has LDL-C levels above the 95th percentile for age and sex, a family history of premature CAD onset, and suggestive physical findings such as cholesterol deposits.
There are no similar clinical diagnostic criteria for HMZ FH, which is usually diagnosed in children based on initial presentation with xanthomata or incidental findings of grossly elevated TC and LDL-C.
DNA-based mutation screening methods permit a definitive diagnosis of HTZ FH to be made and provide the only unequivocal diagnosis. (12) The characterization of the LDL-receptor mutation has important implications for therapy and prognosis. (20) Genetic testing is relatively easy in countries with only one or a few different mutations causing FH. However, given that there are over 1000 identified mutations in the LDL-receptor gene, current methods become problematic in countries with high genetic heterogeneity, such as Canada. (6;25;27) Detection rates are low, ranging from 30 to 50%. Lee et al. (27) state that only about one-third of adult cases and one-half of pediatric cases with clinical and laboratory documented FH have mutations that are detectable in the LDL-receptor gene. Thus, a molecular diagnosis of FH can be made in 50 to 80% of clinically identified cases. (23) The low detection rates are due both to the insensitivity of the method and the large number of LDL-receptor mutations. (28;29) Different types of mutation require different types of genetic testing technologies. (30) With current technologies, Yuan et al. (25) state that molecular genetic testing for FH cannot yet be routinely considered in Ontario because there are very few recurrent FH mutations among Ontario patients. In contrast, in Quebec, there are only 11 common mutations and this high rate of recurring mutations makes genetic testing a reasonable consideration. It is much more costly to screen a person’s entire LDL receptor gene to detect one of many possible mutations, than to screen for a simple positive or negative result for a few well- characterized receptor mutations.
Screening
Heterozygous FH meets the WHO criteria for systematic screening given that HTZ FH is relatively common, is associated with a high risk of early CAD, and is easily treatable with LDL-C lowering methods. (14) Early detection of FH has the potential to save lives and prevent many CAD-related morbidities. (25)
Given the large number of mutations causing FH and the low detection rates with currently available genetic-testing methods, large scale population-based screening programs for FH are not practical (31). On the other hand, in the absence of systematic screening programs, most cases of FH, particularly HTZ FH, remain undiagnosed and thus untreated. (32) A recent health technology assessment (HTA) by Marks et al. (33) evaluated whether screening for FH was appropriate. The authors concluded that a case-finding strategy amongst relatives of FH was the most cost-effective method and that universal systematic screening was the least cost-effective. Pottle (32) also stated that a case-finding strategy is likely to offer the most effective screening strategy in general practice. The United States Preventive Services Task Force also recommended selective screening strategies as the most appropriate approach to screening for dyslipidemia in children and adolescents. However, the Task Force also commented that several key issues about screening could not be addressed due to a lack of studies. (34) The WHO reported that in Canada, only about 10% of cardiologists and general practitioners screen their patients for HTZ FH. (14)
An international organization, Make Early Diagnosis to Prevent Early Death (MEDPED)(35), focuses on the challenge of case identification and early detection of FH. The organization, which is a collaboration between 40 countries and the WHO, is based on a case-finding approach and family screening. (35) Once a diagnosis of FH is made, each person is included in a confidential registry, and screening is extended to detect and inform affected relatives. (5;17;36) In 1999, there were 2,260 FH patients registered in the MEDPED FH registry in Ontario. (14)
Background on Atherosclerosis and the Development of Coronary Artery Disease
Atherosclerosis occurs when homeostasis between the blood and vessel wall is disturbed. Components of the blood cause recurring injuries to the vessel wall in various target organs and incite a chronic inflammatory fibroproliferative response, which eventually leads to obstruction of the arteries and insufficient blood supply to the involved organs. Damage to the coronary arteries surrounding the heart may lead to MI. Other inflammatory components of the blood such as C-reactive protein (CRP), fibrinogen, tumor necrosis factor, viruses, homocysteine, or mechanical shear forces, are also involved in the atherosclerotic process. (37) Repeated injuries of the endothelial layer of the vessel wall weaken its resistance and result in the transmigration of blood-borne constituents into the artery walls. Lipoproteins, particularly LDL-C and lipoprotein (a) [Lp(a)], can also cause injury to the arterial wall and can initiate and propagate arterial injury. Atherosclerosis begins when LDL-C is deposited in the wall of the artery, the cholesterol in an atherosclerotic plaque being derived from LDL-C particles. Injured endothelial cells, blood-derived monocytes, T-lymphocytes and often platelets respond to this accumulation by generating proinflammatory and chemotactic mediators. Within the arterial wall, LDL becomes oxidized and is taken up by macrophages, which transform into lipid-rich foam cells. These cells eventually die, leaving cholesterol deposited in the arterial wall.
A chronically elevated LDL-C plays a major role in damaging the arterial wall, since LDL-C transports cholesterol and triglycerides from the liver and small intestine to cells and tissues that are involved in cholesterol uptake. Low-density lipoprotein cholesterol also transports cholesterol to the arteries where they can be retained by proteoglycans, resulting in the initiation of the atherosclerotic process and plaque formation. The contribution of LDL-C to atherosclerosis also includes the development of endothelial dysfunction, inflammation, formation of foam cells, and thrombotic sequelae, following unstable plaque rupture in atherosclerotic lesions. (10)
Treatment
The primary aim of treatment in both HTZ and HMZ FH is to reduce plasma LDL-C levels in order to reduce the risk of developing atherosclerosis and CAD. (23;38) It is well established that elevated LDL-C is a major risk factor for CAD. (39) Observational studies show a curvilinear relationship between blood cholesterol level and CAD risk. (40) Therefore, the higher the patient’s LDL-C, the higher the risk of CAD. Many recent clinical trials have shown that LDL-C-lowering therapy in high-risk patients reduces the risk of CAD so that, on average, a reduction of 1 mmol/L in LDL-C maintained over 5 years reduces the incidence of CAD by 25%. (40) In comparison to an adult who develops elevated cholesterol due to poor diet or lifestyle, HTZ FH patients have a higher risk of CAD, since LDL-C levels are elevated from birth.
Many international health organizations have outlined optimal ranges for cholesterol. For example, the United States National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) recommends a conservative target LDL-C level of 100mg/dL (2.5mmol/L) (See Table 2). (41) The European Societies on Coronary Prevention also recommend a conservative LDL-C target value (3mmol/L or 115mg/dL). (41;42) The 2003 recommendations of the Canadian Hypercholesterolemia Working Group include 3 levels of CAD risk based on the Framingham Study equation and 2 treatment targets, one for LDL-C levels and one for the TC:HDL ratioThe targets for patients at high risk for CAD, such as patients with FH, are an LDL-C of less than 100mg/dL (2.5mmol/L) and a TC:HDL ratio of less than 4.0. (43) The updated 2006 recommendations of the Canadian Cardiovascular Society advise an even lower target LDL-C level of 80mg/dL (2.0mmol/L) for high-risk patients (See Table 3). (44) Some suggest that in high-risk patients, the lower the level of LDL-C, the better the outcome. (10) Treatment aims to bring the patient’s LDL-C level as close as possible to target levels set by the guidelines.
Table 2: Heterozygous Familial Hypercholesterolemia - Lipid Outcomes.
| Number of Studies |
Study Design | Quality of Studies | Consistency | Directness | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|
| 7+FDA | Case series=Low | Low | + | Yes | Not applicable | Low |
| 1 | Retrospective review=Low |
Table 3: Heterozygous Familial Hypercholesterolemia - Coronary Artery Disease Outcomes.
| Number of Studies |
Study Design | Quality of Studies | Consistency | Directness | Other Modifying Factors | Overall Quality of Evidence |
|---|---|---|---|---|---|---|
| 2+FDA | Case series=Low | Low | + | Yes | Not applicable | Low |
| 1 | Retrospective review=Low |
Table 2: National Cholesterol Education Program Adult Treatment Panel III Cholesterol Ranges.
| LDL cholesterol | |
| <100 | Optimal |
| 100-129 | Near or above optimal |
| 130-159 | Borderline high |
| 160-189 | High |
| ≥190 | Very high |
| Total cholesterol | |
| <200 | Desirable |
| 200–239 | Borderline high |
| ≥240 | High |
| HDL cholesterol | |
| <40 | Low |
| ≥60 | High |
ATP indicates Adult Treatment Panel; LDL, low-density lipoprotein; and HDL, high-density lipoprotein.
Reproduced from: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285(19): 2486-97
Table 3: Canadian Cardiovascular Society 2006 Lipid Guidelines*.
| Risk Category | 10-year CAD risk (%) | Recommendations |
|---|---|---|
| High | ≥20 | Treatment targets: |
| Primary target: LDL-C <2.0mmol/L | ||
| Secondary target: TC:HDL-C <4.0 | ||
| Moderate | 10 - 19 | Treat when: |
| LDL-C ≥3.5mmol/L or | ||
| TC/HDL-C ≥ 5.0 | ||
| Low | <10 | Treat when: |
| LDL-C ≥5.0mmol/L or | ||
| TC/HDL-C ≥ 6.0 |
LDL-C refers to low-density lipoprotein - cholesterol; HDL-C, high-density lipopoprotein - cholesterol; TC, total cholesterol.
Reproduced with permission, Can J Cardiol 2006;22(11):913-27
High-density lipoprotein cholesterol is a strong independent negative risk factor for CAD and, as opposed to LDL-C, HDL-C has cardioprotective effects. High-density lipoprotein cholesterol mediates reverse cholesterol transport and exhibits numerous beneficial effects on the vasculature, including antioxidant, anti-inflammatory and antithrombotic effects. (37) A high level of HDL-C is associated with protection from CAD while low HDL-C levels increase the risk of CAD. Although raising HDL-C is not a primary aim of treatment in FH, achieving optimal HDL-C levels is beneficial. In terms of recommended range, the NCEP ATP III recommends a target HDL-C level greater than 60mg/dL (1.5mmol/L). (41)
The ratio of LDL-C to HDL-C has been shown to be an important risk factor for acute MI across 52 human populations. (45) Results from the Helsinki Heart Study found that the LDL: HDL ratio was the best single predictor of cardiac events. (46) This ratio in combination with TG level revealed a high-risk subgroup. Subjects with an LDL:HDL ratio greater than 5 and a TG level greater than 2.3 mmol/L had a risk ratio of 3.8 (95% CI: 2.2-6.6) compared with those with an LDL:HDL ratio less than or equal to 5 and a TG level less than or equal to 2.3 mmol/L. Thus, a high LDL:HDL ratio indicates a higher risk of cardiac events. (46) According to international guidelines, the target LDL: HDL ratio for patients with CAD is a ratio of less than or equal to 2. (41)
The TC:HDL ratio is another important predictor of CAD risk. (41) As stated, the Canadian lipid guidelines recommend the use of the TC:HDL ratio as a secondary goal of therapy. This simple ratio was chosen because it is a more sensitive and specific index of cardiovascular risk than TC. (43)
The first line of treatment to reduce LDL-C levels in HTZ FH patients is dietary intervention. (5;16;27;32) Homozygous FH patients respond very poorly to dietary therapy. The key elements of the dietary intervention are to reduce the intake of total and saturated fats and cholesterol and increase the intake of solute fibre and plant sterols. (16) The main difficulty with dietary treatment is that the diet is sometimes considered to be monotonous and problems with compliance may develop. (36) Dietary treatment is a main therapeutic approach in children since until recently, many of the cholesterol-lowering drugs have not been approved for use in children under 10 years of age. (47)
A recent Cochrane review by Poustie and Rutherford (47)examined the effect of dietary intervention in FH. They included 5 randomized controlled trials (RCTs) that examined dietary interventions in HTZ FH children and adults. All of the studies were short term. The authors found that no conclusions could be made about the short- or long-term effectiveness of the cholesterol-lowering diet due to the lack of adequate data. The authors highlighted the need to conduct large RCTs to examine the impact of dietary interventions, since data from long-term dietary studies is sparse. Ose et al. (16) found that depending on the baseline diet and levels of fat restriction that are achieved, a cholesterol-lowering diet may lead to a reduction of between 5 to 20% in TC levels.
Dietary therapy alone is rarely sufficient for the treatment of HTZ FH patients. The American NCEP guidelines suggest consideration of drug treatment from the age of 10 years if LDL-C is greater than or equal to 4.9mmol/L, or is greater than or equal to 4.1mmol/L in the presence of other cardiovascular risk factors, including a family history of premature cardiovascular disease. (3;36) There are several classes of lipid-modifying drugs, including bile acid sequestrants (resin), fibric-acid derivatives (fibrates), nicotinic acid (niacin) and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins). A new class of cholesterol-absorption-inhibiting drugs (ezetimibe) reduces the absorption of dietary and biliary cholesterol by inhibiting its transport through a specific transporter on the surface of intestinal cells.
At present, statins provide the most dramatic reductions in LDL-C with an estimated 20 to 55% reduction in LDL-C levels in comparison with other lipid-lowering drugs (See Table 4). (5;9;12) They are usually the first drug prescribed to adults and are generally well tolerated. Statins block the rate-limiting step of cholesterol sysnthesis in the liver, consequently depleting cholesterol content and upregulating the expression of cell surface LDL receptors, resulting in increased removal of LDL-C from the plasma. (25;32) Since HTZ FH patients have one good LDL receptor allele, this can usually be upregulated by statins, and most HTZ FH patients respond reasonably well to this treatment. Several studies have demonstrated the effectiveness of several types of statins including pravastatin, lovastatin, fluvastatin, simvastatin, and atorvastatin in lowering LDL-C in HTZ FH patients. (5)
Table 4: GRADE Recommendation - Homozygous Patients.
| Benefits | Risks | Burdens |
|---|---|---|
| Overall clinical benefit | ||
| Consistency with social/ethical values | ||
| Affordable | ||
| Health system feasibility |
A high-dose statin in addition to dietary modification is often not enough to reduce LDL-C to appropriate levels. Most HTZ FH patients require a combination of drugs to achieve or approach target cholesterol levels. Common combinations include a statin and a resin, a statin and a fibrate or a statin and ezetimibe. (32) Combination therapy of a statin and a resin can reduce LDL-C by up to 50% depending on the type and dose of statin prescribed. (12) In patients with established CAD, combination therapy with a statin and resins is not used since these patients are already on complicated drug regimens and resins interfere with drug absorption. (12) In this situation, a combination of a statin and fibrate, or a statin and ezetimibe, is used. A recent randomized double-blind crossover study by Geiss et al. (48) investigated the effects of ezetimibe in 20 severely hypercholesterolemic patients (18 of whom suffered from HTZ FH) who were treated by statins and regular LDL apheresis. The addition of ezetimibe to intensive lipid lowering (statins + LDL apheresis) resulted in a further mean decrease of LDL-C by 16%.
Table 4: Comparative Efficacy of Various Lipid-Lowering Drugs in Heterozygous Familial Hypercholesterolemia Patients.
| Drug | Percentage reduction in LDL-C |
|---|---|
| HMG-CoA reductase inhibitors | 20–55a |
| Nicotinic acid | 15–30b |
| Cholestyramine, colestipol | 15–30b |
| Fenofibrate, bezafibrate, ciprofibrate | 18–30 |
LDL-C, low-density lipoprotein cholesterol; HMG-CoA, hydroxy methylglutaryl-coenzyme A.
drug and dose dependent.
dose dependent.
Reproduced from: the International Journal of Cardiology, 89(1), Hopkins PN. Familial hypercholesterolemia--improving treatment and meeting guidelines, 13-2, Copyright 2003, with permission from Elsevier.
The observed variation in response to drug therapy may be explained by the differential response of different LDL-receptor mutation types. (23;24) The level of receptor activity also appears to have important implications for response to therapy. (20) At present, there is no specific test that predicts a HTZ FH patient’s response to drug therapy.
The majority of HTZ FH patients are treated effectively with diet and drug therapy. (12) However, a small number of HTZ FH patients are refractory to treatment or intolerant to the lipid- lowering medication. The prevalence of refractory HTZ FH patients is scantily reported in the literature. Using data from a study in the Czech Republic, it is estimated that approximately 5 to 10% of HTZ patients are refractory. (49) According to clinical experts, the prevalence of refractory HTZ patients in Ontario is 1 to 5%, which may be a more reliable estimate given that the prevalence of FH varies in different populations. Using the mean of 3%, it is estimated that there are approximately 765 refractory HTZ FH patients in Ontario, of which 115 are diagnosed and 650 are undiagnosed.
Drug therapy is less effective in HMZ FH patients compared with HTZ FH patients since the effects of the majority of cholesterol-lowering drugs are mediated by the upregulation of LDL receptors, which are often absent or function poorly in HMZ FH patients. (12;20) Nevertheless, some HMZ FH patients may still benefit from drug therapy. (3;4;20) Once HMZ FH patients reach 10 years of age, consideration is given to initiate drug therapy. (9;12;27) Severity of the disease and level of risk based on LDL-C levels, family history of early cardiovascular disease, and gender are also taken into account. (9) In children, resins are usually the first drug to be prescribed and have long been considered the treatment of choice since they are not absorbed systemically and are safer. (9) However, the use of resins has been limited by their poor palatability and negative side effects (constipation, bloating and heartburn) (23). Also, they are only modestly effective at reducing LDL-C levels (10-20%). (9;23) Some studies have evaluated the efficacy of statins in HTZ FH children and adolescents and have reported an approximate 25% reduction in LDL-C. (18) However, the evidence on the use of statins in children is limited by small sample sizes and short follow-up periods, and the studies do not provide extensive information on growth and development. (23) Combination therapy may also be considered in children with HMZ FH. (3) A recent RCT by Gagné et al. (50) evaluated the efficacy, safety, and tolerability of ezetimibe in HMZ patients receiving atorvastatin or simvastatin. They followed patients for a period of 12 weeks and found that ezetimibe coadministered with a statin produced clinically important reductions in LDL-C of approximately 20%. Nevertheless, as stated elsewhere, HMZ FH patients are more resistant to the effects of drugs and even for those who are responsive to drugs, diet and drug therapy alone rarely reduce LDL-C levels to targeted levels. (18) Differential responsiveness from different LDL receptor mutations and mutation types may explain the observed variation in response to therapy. (23)
Existing Treatments Other Than Technology Being Reviewed
Plasma Exchange
An option currently available in Ontario for FH patients who do not respond to standard diet and drug therapy is plasma exchange (PE) therapy. It was first described by DeGennes in 1967 and first used to treat HMZ FH patients in 1974 at Hammersmith Hospital in London. (51) Plasma exchange is delivered on an outpatient basis by various clinical programs such as nephrology and hematology. Patients are treated with this lifelong therapy on a weekly or biweekly basis according to the severity of their condition and are typically treated simultaneously with diet and drug therapy. (20)
Plasma exchange is a nonspecific therapy and eliminates virtually all plasma proteins such as albumin, immunoglobulins, coagulation factors, fibrinolytic factors and HDL-C, in addition to acutely lowering LDL-C. (52;53) Plasma exchange acutely lowers both LDL-C and HDL-C levels by about 50%, and these levels gradually return to pretreatment values within 1 to 3 weeks. (20) Blood is removed from the patient, plasma is isolated, discarded and replaced with a substitution fluid. The substitution fluid and the remaining cellular components of the blood are then returned to the patient. Blood is separated into cellular and plasma components either by centrifugation or membrane filtration. (49;54) During each treatment, which lasts 2 to 3 hours, an average of 3 to 4 litres of plasma are removed and replaced by roughly the same volume of replacement fluid. (54) The most currently used replacement fluid is 5% human albumin. Fresh-frozen plasma can also be used but is associated with a higher risk of infection. (51;54)
The major limitation of PE is its nonspecificity. The removal of HDL-C prevents successful vascular remodeling of the areas stenosed by atherosclerosis. (55) In addition, there is an increased susceptibility to infections (54;55), and costs are incurred by the need for replacement fluid. (15) There is also a restricted plasma volume that can be exchanged per session. Adverse events can be expected to occur in 12% of procedures, but since most patients receive multiple treatments, 40% of patients will experience some reaction during the course of therapy. (54) Most reactions are minor and transient and include nausea, mild hypertension or hypotension, circumoral paresthesia and hives. (56) Plasma exchange may also cause fatigue, which can last for up to 48 hours after treatment. (52)
Evidence on the clinical utility of PE was first published by Thompson et al. (57) in 1985. Plasma exchange was performed on a biweekly basis in 5 HMZ FH patients in the United Kingdom and the United States. Patients were treated between 1975 and 1984, and the mean duration of follow-up was 8.4 years. Survival was compared between the treated patients and their respective untreated HMZ siblings. The authors found that patients treated with PE had survived an average of 5.5 years longer than their respective untreated HMZ FH siblings (P = 0.03), and 4 of the 5 treated patients survived longer than their respective siblings (See Figure 1). At study completion, all but 1 of the treated patients was still alive, whereas all untreated siblings were deceased. The increase in life expectancy was attributed to the 50% reduction of LDL-C.
Figure 1: Differences in Age (Current or at Death) Between Treated and Untreated Homozygous Familial Hypercholesterolemia Siblings*.
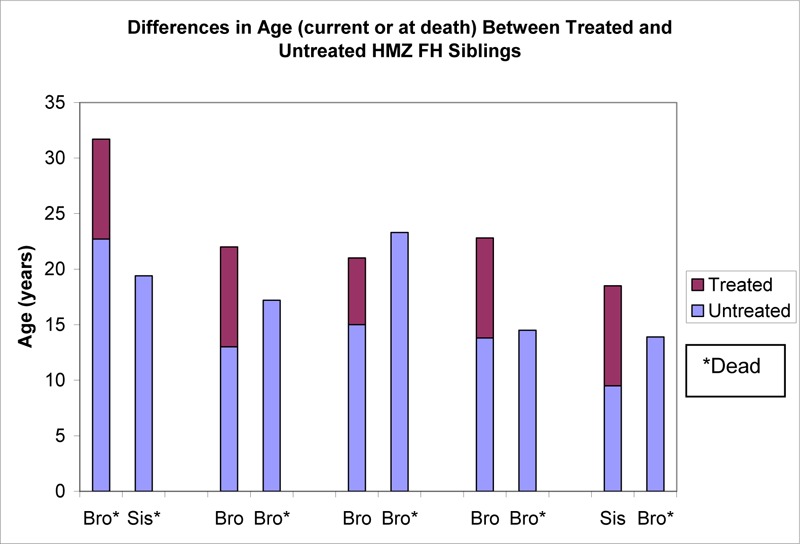
Bro refers to brother; FH, Familial Hypercholesterolemia; HMZ, Homozygous; Sis, sister.
Based on data available in: Thompson GR, Miller JP, Breslow JL. Improved survival of patients with homozygous familial hypercholesterolaemia treated with plasma exchange. Br Med J (Clin Res Ed) 1985; 291(6510): 1671-3
Recently, a group of Ontario clinicians reported on their experience with 10 HMZ FH children, 9 of whom were treated with PE over a period of 20 years. All patients were placed on a cholesterol-lowering diet and received various lipid-lowering medications. These regimens had minimal impact on the levels of LDL-C. Given this inadequate response to diet and drug therapy, PE was initiated at a weekly or biweekly interval depending on the severity of each patient’s condition. Complications were rare and consisted of mild disturbances in serum electrolytes, transient nausea, and problems related to central venous access. All patients except the youngest (age 3.5 years) developed CAD by the end of the observation period. Biweekly PE resulted in a reduction of LDL-C by approximately 50%. (20)
In Ontario, PE has been used for the treatment of FH for about 6 years and the current system for PE is via centrifugation. According to the Canadian Apheresis Group (CAG) (54), of a total 6005 PE procedures performed by 42 participating apheresis units in Canada in 2002, there were 174 procedures performed for hypercholesterolemia (2.9%). Hypercholesterolemia was ranked the eighth most common indication for PE.
Other Alternative Treatments
Surgical alternatives for FH patients include portocaval shunt, ileal bypass and liver transplantation. However, these are risky procedures and are associated with a high morbidity rate. (29;55) In the case of liver transplantation, the inherent risks of organ transplantation and subsequent lifelong treatment with immunosuppressive drugs, make it a technique that is rarely used to treat FH patients. (29)
Gene therapy was once considered a promising area for the treatment of HMZ FH patients, however, results are not convincing to date and further advances must be realized before it can be used for the treatment of FH. (58) (12) Most research has been restricted to animal models and small pilot studies in humans with unfavorable results. Clinical experts estimate that it will be another 10 years before gene therapy becomes a feasible option.
Future advances in drug therapy may also prove to be beneficial in the treatment of FH patients. New and more effective cholesterol-lowering drugs may be developed. A recent dose-escalating study by Cuchel et al. (59) showed some promising results with a microsomal triglyceride transfer protein inhibitor (MTTP), BMS-201038, which regulates the enzyme that assembles lipoprotein particles, including the precursors of LDL. Six HMZ patients were followed for a period of 4 weeks. Although the drug reduced LDL-C levels by at least 50%, some serious adverse effects were observed. The therapy was associated with an increase in liver aminotransferase levels and hepatic fat accumulation. (59) Further research is needed to examine the effects of this approach and its safety.
New Technology Being Reviewed
LDL Apheresis
An alternative to PE is LDL apheresis. Unlike PE, LDL apheresis is a selective treatment that removes LDL-C and other atherogenic lipoproteins from the blood while minimally impacting other plasma components such as HDL-C, total serum protein, albumin and immunoglobulins. (3)
In 1975, Dr. Paul Lupien, a professor of Laval University in Quebec, was the first scientist to develop and clinically apply a more sophisticated method of LDL-C elimination. The method was based on the principle that LDL binds to negatively charged substances such as heparin. Dr. Lupien used a batch adsorption system with heparin agarose beads in a plastic bag to remove LDL-C. (60) This approach was effective in removing LDL-C from the patient’s plasma and the patient’s xanthomas regressed. (61)
Since then, more sophisticated approaches to the selective removal of LDL-C have been developed. All methods are specific and continually remove LDL-C in an automated on-line extracorporeal circulation system. There are several commercially available methods of LDL apheresis, the commonest being immunoadsorption (IMA or IMAL), dextran sulfate adsorption (DSA), LDL hemoperfusion (DALI), membrane differential filtration (MDF) and heparin-induced extracorporeal LDL precipitation (HELP). Each method employs a different principle for the selective removal of LDL-C and has distinct advantages and disadvantages (See Table 5). The main reasons for preference of one method over another include technical advantages, higher effectiveness, the need to account for occasional heparin intolerance, and differences in costs. (18)
Table 5: GRADE Recommendation - Heterozygous Patients.
| Benefits | Risks | Burdens |
|---|---|---|
| Overall clinical benefit | Less affordable | |
| Questionable health system feasibility | ||
| Unknown if consistent with social/ethical values |
Table 5: Characteristics of the Most Commonly Used Low-Density Lipoprotein Apheresis Techniques*.
| Device | Ligand/principle |
|---|---|
| Immunoadsorption (IMAL) | Anti-apoprotein B100-ab immobilized on sepharose which retain LDL and Lp(a) on the columns |
| Dextran Sulfate Adsorption (DSA) | Dextran sulfate-cellulose adsorbs LDL and Lp(a) by virtue of its negative surface charge exploiting the interaction with the positively charged aPOB moiety of lipoproteins |
| Direct Adsorption of Lipoproteins (DALI) LDL Hemoperfusion | Polyacrylate ligands on Eupergit are used to electrostatically adsorb LDL and Lp(a) directly from whole blood Only method without need for prior plasma separation |
| Membarne Differential Filtration (MDF) (Also known as lipidfiltration) | Separation via filtration and based on particle size and geometric properties |
| Heparin-induced Extracorporeal LDL Precipitation (HELP) | Heparin precipitation - At low pH, LDL and Lp (a) bind to heparin and fibrinogen and form a precipitate |
LDL refers to low-density lipoproteins; Lp(a), Lipoprotein (a); pH, probability of hydrogen.
Similar to PE, FH patients require lifelong therapy with LDL apheresis. Low-density lipoprotein apheresis is encouraged to commence as early as possible, usually around 6 or 7 years of age, before the establishment of atherosclerotic lesions. (21;58)
Lipoproteins begin to accumulate as soon as the procedure is completed. The frequency of treatment varies from weekly to biweekly and is dependant on the level of LDL-C and the severity of CAD. (8;21) While substantial variations in the rate of cholesterol biosynthesis have been observed in FH patients, the goal is to keep the time-averaged LDL-C concentration (TAC), which is the average LDL-C concentration before and after LDL apheresis, at or below the patient’s therapeutic goal (60). Typically, HTZ FH patients require treatment every 2 weeks whereas HMZ FH patients require therapy every 7 to 10 days. (52)
The frequency of LDL apheresis is established by measuring the acute reduction in LDL-C as well as the TAC. The acute mean percent reduction in LDL-C is calculated by the difference in pre- and post-treatment values as a percentage of the initial value. The TAC is calculated based on the treatment frequency and the rate of rebound in LDL-C between treatments. Although TAC provides more insight, it is reported less frequently than acute reductions. (62)
As with PE, LDL apheresis is used in conjunction with lipid-lowering medications and dietary therapy. This combined approach helps to further reduce plasma cholesterol levels, slow the post-treatment rebound of cholesterol, and prolong the interval between LDL apheresis sessions. (15;52) Moreover, it allows mean TC and LDL-C levels to be maintained at levels closer to those recommended by the guidelines. (4)
Yamamoto et al. (63) examined whether atorvastatin (a statin) was effective in reducing lipid levels in 9 HMZ FH patients undergoing LDL apheresis therapy with the DSA or MDF methods. Four patients who were LDL receptor-defective and one patient who was LDL receptor-negative responded well to atorvastatin. The remaining 4 patients who did not show a change in LDL-C levels were each receptor-negative. Nevertheless, all patients displayed a considerable increase in HDL-C and a decrease in TG. Thus, the authors stated that statins may increase the efficacy of LDL apheresis given that cholesterol levels rebound quickly after each treatment. Statins may also beneficially impact cost by reducing the frequency of apheresis treatments.
Low-density lipoprotein apheresis is generally well-tolerated, and the occurrence of adverse events is low. Observed events are typical of procedures involving the circulation of blood outside the body. (64) Hypotension and an acute decrease in serum proteins may occur. Further, due to potential anaphylactoid reactions, angiotensin converting enzyme (ACE) inhibitors are contraindicated for patients being treated with the DSA or DALI systems. (8)
Effectiveness
The various LDL apheresis techniques have similar efficacy in reducing LDL-C. Thompson (21) compared the weighted means of the acute decreases in plasma lipoproteins produced by IMAL, DSA, HELP and DALI methods of LDL apheresis (See Table 6). All methods appeared to lower LDL-C to a similar extent, ranging from 60 to 77% (note that results for DALI based on a single study). Dextran sulfate adsorption and DALI methods may acutely decrease HDL-C slightly less than IMAL and HELP. There are also differences in the volume of plasma treated between techniques. (21) Moriarty (8) compared the DSA and HELP systems and commented that although these systems are different in their process, they are generally similar in their ability to reduce lipids. The major difference between DSA and HELP is that HELP acutely reduces fibrinogen levels by 60 to 65% whereas DSA lowers fibrinogen by only 10 to 15%.
Table 6: Average Post-Procedure Decreases in Plasma Lipoproteins Calculated From Comparative Studies* †.
| Procedure | Δ%, weighted mean‡ | ||
|---|---|---|---|
| LDL-C | Lp(a) | HDL-C | |
| IMAL | 61.9 | 53.3 | 16.2 |
| DSA | 63.7 | 51.0 | 13.7 |
| HELP | 59.4 | 67.7 | 16.8 |
| DALI | 77.0 | 63.0 | 13.0 |
DALI refers to direct adsorption of lipoproteins; DSA, dextran sulfate adsorption; IMA, immunoadsorption; HDL-C, high-density lipoprotein - cholesterol; HELP, heparin-induced extracorporeal LDL precipitation; LDL-C, low-density lipoprotein - cholesterol; Lp(a), lipoprotein (a).
Please refer to the original paper to locate specific studies.
Weighted according to number of samples (n) analysed for each variable.
Reproduced from Atherosclerosis, 167(1), Thompson GR. LDL apheresis, pp 1-13, Copyright 2003, with permission from Elsevier.
With regard to the impact of LDL apheresis on angiographic outcomes, some recent studies have demonstrated arrest of progression with stabilization or even regression of coronary atherosclerosis. (55) A 2003 meta-analysis by Thompson (21) evaluated the impact of LDL apheresis versus drug therapy alone or no therapy, on angiographic change in FH patients. The meta-analysis included 8 studies that lasted at least 2 years using any method of LDL apheresis. The weighted mean percentage of patients showing progression of lesions was 46% in the control group, 33% in the drug group and 18% in the LDL apheresis group (P = 0.1). Further, the weighted mean percentage of patients showing no change or regression of lesions was 54% in the control group, 67% in the drug group and 82% in the LDL apheresis group (P = 0.1) (See Figure 2). (21) Although the differences between groups were not statistically significant in this small study, the results are consistent with the idea that LDL apheresis in FH patients, in combination with drug therapy, may be more effective in stopping the progression of CAD than drug therapy alone. As well, these 2 approaches, LDL apheresis and drug therapy and drug therapy alone, are more effective than no treatment.
Figure 2: Frequency of Coronary Angiographic Changes in Familial Hypercholesterolemia Trials According to Treatment Group.
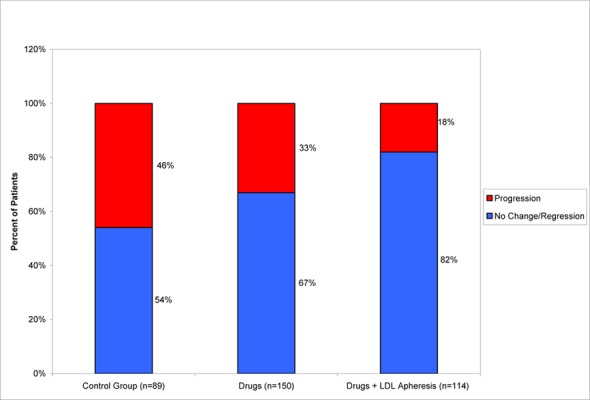
Reproduced from Atherosclerosis, 167(1), Thompson GR. LDL apheresis, pp 1-13, Copyright 2003, with permission from Elsevier.
Questions remain as to whether long-term LDL apheresis prompts an oxidative process. To counteract these continuous oxidative events, some clinicians advocate antioxidant vitamin supplementation. (65) Long-term LDL apheresis can also cause iron deficiency anemia requiring iron supplementation.
Since the late 1980s when statins were introduced, the number of patients who require LDL apheresis has fallen, especially among HTZ FH patients. (61) In addition, with the development of more effective statins, there may be the potential to wean some patients off of LDL apheresis. (66)
Comparison of LDL Apheresis and Plasma Exchange
As stated elsewhere, unlike PE, LDL apheresis is a selective process that does not have major effects on essential plasma proteins and does not require replacement fluid, thus decreasing susceptibility to infections. (53) Schuff-Werner et al. (67) noted that adverse events were documented in 2.9% of LDL apheresis treatments with the HELP system, compared with 12% with PE. Another important difference is that LDL apheresis systems often have weight restrictions, whereas PE does not. This implies that PE may begin at an earlier age.
A study by Berger et al. (68) compared LDL apheresis using DSA with PE in 2 HMZ FH 17 year-old female patients over a period of 8 months. Patients were consecutively given 3 different schedules of LDL apheresis: twice per week, weekly and then biweekly, following which they continued to receive biweekly PE. While both procedures caused an acute reduction in TC and LDL-C levels by approximately 50% to 80%, HDL-C levels were minimally affected by LDL apheresis and dropped by less than 10%. The ratio of LDL to HDL, which has been shown to be an important risk factor for acute MI (45), fell by about 60% (range 48-74%) following LDL apheresis, whereas the ratio fell by only 30% (+2.3 to -47%) with PE. Transient fatigue observed in some patients after PE did not occur after LDL apheresis. Similar results were obtained by Thompson et al. (51) They compared results of PE with those obtained with LDL apheresis using a LDL adsorption column in 4 FH patients (2 HMZ and 2 HTZ). Low-density lipoprotein apheresis was well-tolerated by all 4 patients and patients preferred this method over PE.
According to Thompson (21), although some centers are still using PE to treat severe hypercholesterolemia, it is increasingly being replaced by LDL apheresis.
Heparin-Induced Extracorporeal Low-Density Lipoprotein Precipitation
The Procedure
Heparin-induced extracorporeal LDL precipitation is one of the most widely used methods of LDL apheresis. It was first described in 1982, and the first clinical experience with HELP was in 1984. Heparin-induced extracorporeal LDL precipitation is now offered as a treatment option at approximately 130 medical centers worldwide, including centers in Germany, Austria, Italy, Ireland, and the United States. (69;70) It is also available in 2 centers in Canada (Edmonton and Québec City). To date, over 175000 procedures have been performed in over 900 patients. (70) Heparin-induced extracorporeal LDL precipitation is performed in outpatient settings by a specially trained clinician, and falls under the direction of a specialist in cardiology, internal medicine, or endocrinology.
Heparin-induced extracorporeal LDL precipitation is a continuous closed-loop system that processes blood extracorporeally. After an intravenous line is inserted, blood is withdrawn from the patient and continuously separated into plasma and cellular components by passage through a capillary plasma filter. Cellular components are directly reinfused into the patient, whereas plasma continues on the circuit. A mixture of sodium acetate buffer and heparin are added to the plasma, which causes the lipoprotein complexes to form a precipitate. The precipitate is removed by a filter, which is later discarded. Residual heparin is removed from the LDL-free plasma by a heparin adsorber, physiological pH is corrected by bicarbonate dialysis, and volume is adjusted by ultrafiltration. The cleansed plasma is then returned to the patient (See Figure 3). (37;52;55;71;72) No blood component replacement is required. (70)
Figure 3: The Heparin-Induced Extracorporeal LDL Precipitation System Process.
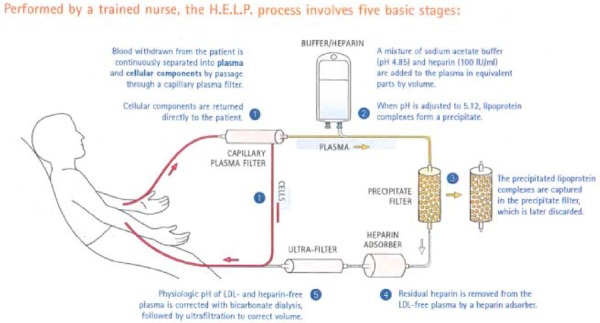
HELP refers to Heparin-induced extracorporeal low-density lipoprotein precipitation; LDL, low-density lipoprotein.
Reproduced from The H.E.L.P. System: a physician guide to LDL apheresis therapy, Copyright 2004, with permission from B. Braun Medical Inc.
In general, the total time for preparation is 1 hour, and delivery of the treatment takes approximately 1 to 2 hours, which corresponds to a total treatment duration of approximately 2 to 3 hours. The duration for each session is dependent on the volume of plasma processed and the rate of blood flow. Approximately 2.5 to 3 litres of plasma are treated per session, and the amount of blood that is extracorporeal never exceeds 300 to 400 ml at any time during the treatment. (37;64;71) In children, the procedure takes 1 to 2 hours and treats between 1 to 1.5 liters of plasma. (73)
The Device
The HELP Secura system is comprised of 3 components:
Plasmat secura: mobile base frame with 4 reusable modules (dialysate, cascade, blood and communication)
HELP filter set: 4 filters through which the blood and plasma flow during treatment, single use only
HELP line set: 9 PVC blood lines that act as interconnectors between patient, filters and pumps, single use only
The system needs to be connected to an AC power source and an external reverse osmosis water supply. In addition, 5 accessory solutions are required for each treatment (See Figure 4).
Figure 4: The HELP System.

Reproduced from The H.E.L.P. System, Copyright 2007, with permission from B. Braun Medical Inc. Available at: http://www.bbraunusa.com/index.cfm?uuid=099D90D3D0B759A1E32BC9906EE13A96 (Accessed November 2007)
Effectiveness
Results from early trials indicate that LDL-C concentration is reduced by 65 to 70% following treatment in both HTZ and HMZ FH, and then rapidly begins to rise. (69) When used in combination with statin therapy, a reduction of 70 to 80% in LDL-C may be achieved. (69) The frequency of therapy depends on the level of rebound, but typically patients with HTZ FH are treated every 2 weeks while patients with HMZ FH require weekly therapy. With regular treatment, long-term reductions in pretreatment and post-treatment LDL-C levels are have been reported. (52) Pretreatment values usually reach a new steady state after 4 to 8 treatments. (69) Heparin-induced extracorporeal LDL precipitation also produces small transient decreases in HDL-C (10-15%) due to a dilution process, however, the levels of HDL-C generally return to baseline within 2 days. After several months of therapy, pretreatment HDL-C levels may actually exceed baseline levels by an average of 15%. (52)
In addition to having an impact on plasma cholesterol concentrations, HELP also has an effect on other plasma components. Unlike other apheresis systems, HELP lowers plasma fibrinogen, a risk factor for atherosclerosis, by approximately 65%. (64) Fibrinogen is involved in coagulation, inflammation and plasma viscosity. (8) HELP has also been shown to reduce concentrations of cellular adhesion molecules (CAM) such as E-selectin, VCAM-1 and ICAM-1, which play a role in early atherogenesis. (74;75)
Ongoing research is focused on the effects of HELP on the vessel wall, including expression of inflammatory markers such as CRP (76) and CAMs. (77)
Additional Information
Patients are treated simultaneously with diet and drug therapy. Unlike other apheresis systems, such as the DALI and DSA systems, HELP is fully compatible with ACE inhibitors and there is no increased risk of developing an anaphylactoid reaction. (37;72;78)
Heparin-induced extracorporeal LDL precipitation is contraindicated in patients for whom the use of heparin would cause excessive or uncontrolled anticoagulation or in whom anticoagulation cannot be safely achieved, such as in patients with hemophilia or those who have had recent surgery. It is also contraindicated in patients with a known hypersensitivity to heparin or ethylene oxide. (64) In addition, according to the manufacturer, it can only be used in patients who weigh 30 kgs or more.
The reported occurrence of adverse events is low (less than 3%) (70) and serious complications have not been observed. (55) Besides hypotension, which occurs in roughly 2% of treatments, the most frequently observed adverse events relate to difficulties with venous access. Venous shunts or fistulas are sometimes required for patients with poor vascular access. (64) Other adverse events include flushing/blotching, chest pain, anemia, abdominal discomfort, hemolysis and arrhythmia (64)
A limitation of the current HELP Secura system is that is requires a reverse osmosis device and external water to dialyze the solution, thus limiting the mobility of the system. A newly upgraded system, the HELP Futura, was introduced in 2001, which does not require a reverse osmosis device and works with sterile dialysis fluid, making the system more flexible. (73;79) In addition, the Futura has new software, a new precipitate filter, a simplified plasma circuit without precipitate recirculation, and improved safety features. Preinstalled filters also make set-up easier, and reduce preparation time. (80) Initial studies have found that the changes in lipid parameters are comparable to the Secura system. (73;79). The new Futura system is now available in the United States.
Recent advances have extended the use of HELP to additional conditions such as: after heart transplantation, cerebral vascular disease, peripheral vascular disease, and acute microcirculatory disturbances such as sudden hearing loss and preeclampsia. (81)
Regulatory Status
Status in Canada
The H.E.L.P.® System (B.Braun Medizintechnologie GmbH, Germany) has been licensed by Health Canada since December 2000 as a Class 3 medical device (Licence # 26023), for performing LDL apheresis to acutely remove LDL from the plasma of 3 high-risk patient populations for whom diet has been ineffective, and maximum drug therapy has either been ineffective or not tolerated. The 3 patient groups are as follows:
Functional hypercholesterolemic homozygotes with LDL-C >500 mg/dL (>13mmol/L);
Functional hypercholesterolemic heterozygotes with LDL-C >300 mg/dL (>7.8mmol/L);
Functional hypercholesterolemic heterozygotes with LDL-C >200 mg/dL (>5.2mmol/L) and documented CAD
No other LDL apheresis system is currently licensed in Canada.
Status in United States
The FDA approved the HELP System in 1997 and it is indicated for “use in performing LDL apheresis to acutely remove LDL-C from the plasma of high-risk patient populations for whom diet and maximum drug therapy has been ineffective or not tolerated.” The Liposorber LA 15® System (Kaneka Pharma America Corporation, New York), a DSA system, is also approved by the FDA. (82)
The FDA has approved LDL-A for 3 categories of patients:
Homozygous FH with LDL-C levels > 500 mg/dL (>13mmol/L)
Heterozygous FH with LDL-C levels ≥ 300 mg/dL (>7.8mmol/L)
Heterozygous FH with LDL-C ≥ 200 mg/dL and documented CAD (>5.2mmol/L)
In each case, the LDL-C levels must be above the stipulated threshold despite 6 months on the American Heart Association Step 2 diet1 and maximum tolerated drug therapy.
Evidence-Based Analysis of Effectiveness
Objective
To assess the effectiveness and safety of LDL apheresis performed with the HELP system for the treatment of patients with refractory HMZ and HTZ FH.
Research Questions
Does LDL apheresis performed with the HELP system improve plasma lipid profiles and CAD status in patients with HMZ and HTZ FH refractory to standard diet and drug therapy?
Is HELP apheresis more effective than PE?
Is HELP apheresis more effective than diet and drug therapy?
Methods
Inclusion Criteria
English language (1998 – present)
HMZ or HTZ FH patients refractory to standard diet and drug therapy
LDL apheresis performed with the HELP Plasmat Secura system
Study design and methods must be clearly described
Exclusion Criteria
Studies that are duplicate publications (outdated by another publication by the same investigators, with the same objective and data)
Non-English articles
Nonsystematic reviews, letters, editorials and case reports
Animal and in-vitro studies
Studies which included patients with several types of hyperlipidemia and not limited to FH patients
Studies using LDL apheresis systems other than the HELP system
Studies that did not examine the outcomes of interest
Outcomes of Interest
Acute and chronic changes in plasma lipid concentrations (LDL-C, TC, HDL-C, TG)
Changes in atherosclerotic lesions
Changes in other markers of coronary atherosclerosis (e.g., coronary calcification)
Fatal and nonfatal cardiovascular events (MI and coronary revascularization procedures)
Survival
Adverse effects of treatment (short-term safety and long-term adverse events)
Method of Review
A search of electronic databases (OVID MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, The Cochrane Library, and the International Agency for Health Technology Assessment [INAHTA] database) was undertaken to identify evidence published from January 1, 1998 to May 30, 2007. The search strategy is detailed in Appendix 1.
Studies meeting the inclusion criteria were selected from the search results. Data on the study characteristics, patient characteristics, primary and secondary treatment outcomes, and adverse events were abstracted. Reference lists of selected articles were also checked for relevant studies.
Assessment of Quality of Evidence
The quality of the evidence was assessed as high, moderate, low or very low according to the GRADE methodology. (1;2) As per GRADE the following definitions apply:
High: Further research is very unlikely to change our confidence in the estimate of effect
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low: Any estimate of effect is very uncertain
Results of Evidence-Based Analysis
The search identified 398 articles published from January 1, 1998 to May 30, 2007. The search did not identify any RCTs that evaluated the impact of LDL apheresis with the HELP system for the treatment of FH patients, or any studies that directly compared HELP apheresis with PE, or HELP apheresis with standard diet and drug therapy, in FH patients. Of the 398 citations identified, 8 met the inclusion criteria (See Table 7). Five case series, 2 case series nested within comparative studies, and 1 retrospective review were included in the review. Table 7 lists the level of evidence and number of studies identified. Study characteristics are detailed in Appendix 3.
Table 7: Quality of Evidence of Included Studies.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT,* systematic reviews of RCT | 1 | 0 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | 0 |
| Small RCT | 2 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 0 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | 0 |
| Case series (multisite) | 4b | 0 |
| Case series (single site) | 4c | 7 |
| Retrospective review, modeling | 4d | 1 |
| Case series presented at international conference | 4(g) | 0 |
RCT refers to randomized controlled trial.
g indicates grey literature.
Summary of Existing Health Technology Assessments
A health technology assessment conducted by the Alberta Heritage Foundation for Medical Research (AHFMR) was included in the review. The AHFMR report reviewed LDL apheresis for the treatment of FH patients using the DSA and HELP systems. In addition, a United States Food and Drug Administration (FDA) review was included, which was conducted as part of its approval process for the HELP system.
United States Food and Drug Administration Report, September 1997
The objective of the FDA report was to assess the safety and effectiveness of the HELP system in 3 different FH patient populations as follows: (83)
HMZ FH with LDL-C levels > 500 mg/dL (>13mmol/L)
HTZ FH with LDL-C levels ≥ 300 mg/dL (>7.8mmol/L)
HTZ FH with LDL-C levels ≥ 200 mg/dL (>5.2mmol/L) and documented CAD
Documented CAD was defined as having at least one prior documented MI, CABG, percutanerous transluminal coronary angioplasty (PTCA) or significant angina with a positive thallium or other heart scanning stress test.
In each case, the LDL-C levels had to be above the stipulated threshold despite 6 months on the American Heart Association Step 2 diet (or equivalent) and maximum tolerated drug therapy. Maximum tolerated combination drug therapy was defined as an adequate trial of drugs from at least 2 separate classes of cholesterol-lowering agents.
Summary of Safety and Effectiveness data
The FDA Report included data from 2 large case series clinical trials in the United States and Germany. Lane et al. (84-86) published the results from the American, clinical investigation, and Schuff-Werner et al.(67) published the results from the German HELP LDL Apheresis Multicentre study. The American clinical study was conducted under an Investigational Device Exemption Application at 4 sites. The German study was conducted at 9 sites in Germany and Italy. Neither of the studies included control groups. The objective in both studies was to evaluate the efficacy and safety of the HELP system in patients suffering from FH.
The inclusion criteria for the American study were FH patients with LDL-C levels greater than 160mg/dL (4.1mmol/L) while on appropriate diet and drug therapy for at least 12 weeks. Patients were first put on weekly HELP treatments for 6 months (25 treatments) and subsequently, those who completed the first 6 months were switched to biweekly treatment for an additional 6 months. The treatment target was one plasma volume per treatment (equivalent to 3,000 ml for the average adult). Patients were maintained on diet and drug therapy during the course of the study period. Changes in lipid levels were measured both acutely (before/after individual treatments) and chronically (across the course of treatment). After one year of follow-up, 2,826 treatments were completed. After completion of one year of HELP therapy, treating physicians prescribed patients to the most appropriate interval of therapy to control their hypercholesterolemia.
The German clinical study enrolled hypercholesterolemic patients with clinically apparent CAD and angiographically proven changes in more than more segment of the coronary artery tree, and LDL-C levels greater than 200mg/dL (5.2mmol/L) despite diet and drug therapy. Patients were treated with weekly HELP sessions while being maintained on their diet and drug regimen. Like the American study, the treatment target was one plasma volume per session. Information was obtained on 21,305 HELP treatments over a 2-year period. Acute and chronic changes in lipid parameters were evaluated. Angiographic studies were performed at baseline and at 2-year follow-up to assess CAD status. Patients were continually evaluated at 6-month intervals during the course of the study.
For the purposes of the analysis, patients were grouped into 3 categories (See Table 8). It is important to note that the LDL-C cutoff value of 160mg/dL in the American study was lower than the minimal cutoff of 200mg/dL (5.2mmol/L) outlined by the FDA criteria. As such, patients with LDL-C levels of 160 to 199 mg/dL (4.1-5.2mmol/L) were categorized as “other” for the purposes of the analysis. The following table summarizes the aggregate number of patients and treatments in both the American and German studies according to patient group.
Table 8: Aggregate Information on Number of Homozygous and Heterozygous Familial Hypercholesterolemia Patients and Treatments in the American and German Studies According to Patient Group*.
| Group | Patients | Treatments |
|---|---|---|
|
Group functional HMZ FH patients with LDL-C levels > 500 mg/dL after 6 months of diet and drug therapy |
4 | 400 |
|
Group B severely affected functional HTZ FH patients with LDL-C levels 300-500mg/dL after 6 months of diet and drug therapy |
32 | 2405 |
|
Group C functional HTZ FH patients with LDL-C levels 200-299 mg/dL after 6 months of diet and drug therapy and CAD |
30 | 2365 |
|
Other patients who did not meet inclusion criteria (LDL-C 160 to 199 mg/dL) |
25 | 1249 |
| TOTAL | 91 | 6419 |
CAD refers to coronary artery disease; HMZ FH, homozygous familial hypercholesterolemia; HTZ FH, heterozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein - cholesterol.
Reproduced from United States Food and Drug Administration. Summary of Safety and Effectiveness Data The H.E.L.P. System. PMA P940016. 1997. Available at: http://www.fda.gov/cdrh/pdf/p940016.pdf
Of the 91 patients that were included in both studies, 77 were treated with HELP for a minimum duration of 6 months and 66 were treated for a minimum duration of 1 year. The mean age was 45.2 years (range 15-66 years), 66% were male and the majority of patients were Caucasian.
A total of 66 patients met criteria outlined by the FDA (Groups A-C), and over 80% of documented treatments were performed in these patients (n=5170). Of these patients, 61% were male and the mean age was less than 47 years in all groups.
Results
Acute Changes in Lipid Parameters
As seen in Table 9 below, in each patient group, acute mean reductions in LDL-C exceeded 50% (range 60- 64%) and acute mean reductions in TC ranged from 49 to 57%. Acute mean reductions in HDL-C ranged from 12 to 17%. Although some patients experienced an increase in HDL-C immediately after treatment, authors noted that these increases were due to the administration of heparin, which is known to produce artificial increases in HDL-C levels. There were also significant reductions in TG (36.0-47.4%), aPOB (52.5-62.7%) and fibrinogen (57.5-64.9%).
Table 9: Acute Mean Reduction in Cholesterol by Homozygous and Heterozygous Familial Hypercholesterolemia Patient Subgroup*.
| Patient Subgroup | LDL-C | Total Cholesterol | HDL-C |
|---|---|---|---|
| Group A | -63.6 | -57.3 | -17.1 |
| (4 patients) | (-78.1 to-24.5) | (-70.1 to -24.5) | (-46.1 to 24.0) |
| n=395 | n=395 | n=395 | |
| Group B | -62.0 | -51.9 | -12.8 |
| (32 patients) | (-95.2 to -5.0) | (-76.2 to -4.1) | (-75.9 to 231.0) |
| n=2349 | n=2362 | n=2351 | |
| Group C | -59.8 | -48.9 | -13.7 |
| (30 patients) | (-91.4 to -8.8) | (-77.7 to -2.0) | (-89.2 to 382.4) |
| N=2266 | n=2331 | n=2318 |
HDL-C refers to high density lipoprotein-cholesterol; LDL-C, low-density lipoprotein - cholesterol; n, number of treatments.
Reproduced from: United States Food and Drug Administration. Summary of Safety and Effectiveness Data The H.E.L.P. System. PMA P940016. 1997. Available at: http://www.fda.gov/cdrh/pdf/p940016.pdf
In the American study, LDL kinetics were examined in a select number of patients who were undergoing biweekly treatment (Group A, n=1, Group B, n=1, Group C, n=2). Samples were taken before/after treatment at 24 hours, 48 hours, 96 hours, 7 days and 14 days post-treatment. The graph below illustrates that although HELP produced an acute reduction in LDL-C, levels began to rebound immediately and returned to 50% of baseline within about 5 days. LDL-C levels also rebounded in a nonlinear fashion, so that the rise in LDL-C was higher during the first 4 days following treatment. If HELP therapy was discontinued, LDL-C levels rebounded to the baseline levels that were achieved with diet and drug therapy. Results for all 3 patient groups were comparable.
Figure 5: Mean Low-Density Lipoprotein Cholesterol Rebound by Homozygous and Heterozygous Familial Hypercholesterolemia Patient Subgroup*.
LDL-C refers to low-density lipoprotein cholesterol.
Reproduced from United States Food and Drug Administration. Summary of Safety and Effectiveness Data The H.E.L.P. System. PMA P940016. 1997. Available at: http://www.fda.gov/cdrh/pdf/p940016.pdf
With regular HELP therapy, TC levels were maintained below baseline levels in all 3 patient groups. As illustrated by the graph below showing TC levels in an HMZ FH patient receiving HELP therapy, TC levels were maintained at lower levels with weekly versus biweekly treatments.
Figure 6: Total Cholesterol Levels in a Homozygous Familial Hypercholesterolemia Patient Receiving HELP Therapy.
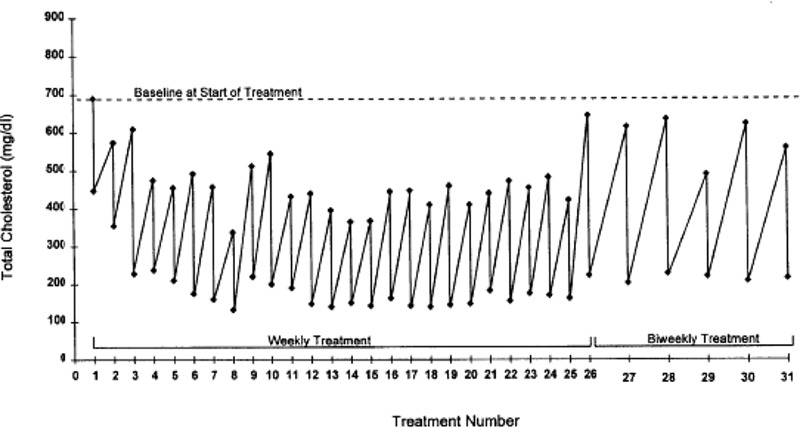
Reproduced from United States Food and Drug Administration. Summary of Safety and Effectiveness Data The H.E.L.P. System. PMA P940016. 1997. Available at: http://www.fda.gov/cdrh/pdf/p940016.pdf
Coronary Angiography Results
In the German study, 33 patients completed 2 years of weekly HELP therapy, and coronary angiography results at baseline and at 2-year follow-up were available. The mean age was 47.2 years, 70% were male, the mean baseline LDL-C was 294 mg/dL, and the mean baseline HDL-C was 44 mg/dL. Weekly HELP therapy acutely reduced LDL-C by 58% in these patients.
An analysis of 187 coronary angiogram segments in the 33 patients revealed that the mean degree of stenosis of all segments decreased by 1.9%, from 32.5% to 30.6% after 2 years (P = .0213). This assumed independence between segments. The stenosis of lesions less than or equal to 30% did not change (n=84) and those greater than 30% (n=103) showed a mean reduction of 4.3% after 2 years (P < 0.001).
Using the standard principle that a difference in the degree of stenosis of greater than or equal to 8% is clinically relevant, 26.7% of lesions regressed (≥8% decrease), 57.8% did not change significantly (<8% change) and progression occurred in 15.5% of segments (≥8% increase). Thus, approximately twice the proportion of lesions showed regression in comparison with the proportion of lesions that displayed progression.
Complications and Adverse Events
The most common adverse events observed in the American and German clinical trials were problems relating to venous access, hypotension, fatigue, chills/shivering, swelling of the face and hands, headache, and nausea. According to the authors, these events are typical of procedures involving extracorporeal circulation.
No nonfatal MIs were recorded during the American study. Nonfatal MIs were not reported in the German study. One fatal MI occurred during the American study and 5 occurred during the German study. None of the deaths were thought to be related to HELP treatment, however this possibility could not be excluded with certainty given the small sample sizes and the lack of a control group.
Study Withdrawal
No patients discontinued treatment in Group A, 8 patients discontinued in Group B (4 patients before 6 months and 4 patients between 6-24 months) and 7 patients discontinued in Group C (1 patient before 6 months and 6 patients between 6-24 months). Reasons for termination of treatment included death, venous access problems and miscellaneous or unknown problems.
Conclusions
The FDA report concluded that the data obtained from the American and German clinical trials supported the safety and effectiveness of the HELP system for the treatment of hypercholesterolemia in the 3 FH patient populations that were previously identified. Nevertheless, the authors noted that both studies were not designed to assess the long-term-clinical impact of lowering LDL-C levels with HELP therapy. Following the report, the FDA approved the HELP System in 1997 and it is currently indicated for “use in performing LDL apheresis to acutely remove LDL-C from the plasma of high-risk patient populations (FH) for whom diet and maximum drug therapy has been ineffective or not tolerated.” In addition, approval of the HELP System was subject to a patient registry for all patients using the device.
The FDA report did not present all of the relevant findings from the Schuff-Werner et al. (67) and Lane et al.(84-86) studies. Additional results from these studies on chronic changes in lipid parameters will be presented in the following section.
Additional Results from Original Studies
Upon further review, additional results were presented in the Lane et al. (84-86) and Schuff-Werner et al. (67) publications which were not included in the original 1997 FDA Report. Table 10 presents characteristics of the original publications. Relevant findings on chronic changes in lipid parameters, lipid kinetics and angiographic changes will be discussed.
Table 10: Characteristics of Studies Included in the United States Food and Drug Administration 1997 Report*.
| Study | N Population |
Patient Characteristics | HELP Treatment Interval | Follow-up | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) | Mean Baseline LDL (mg/dL) |
Acute† | Chronic‡ | ||||
| Lane et al., 1993 § (86) U.S. Multicentre Clinical Trial |
N=33 LDL ≥ 160mg/dL despite diet and drug therapy |
47 | 70 | 289 | weekly | 6 months | lipid parameters | lipid parameters |
| Lane et al., 1995a § (84) U.S. Multicentre Clinical Trial |
N=23 LDL ≥ 160mg/dL despite diet and drug therapy Completed 6 months of weekly therapy 4 HMZ & 19 severe HTZ patients |
45.5 | 70 | 237 | biweekly | 6 months | lipid parameters | lipid parameters |
| Lane et al., 1995b § (85) U.S. Multicentre Clinical Trial |
N=14 LDL > 160mg/dL despite diet and drug therapy Completed 6 months of weekly therapy 10 patients with primary hypercholesterolemia and 4 with combined hyperlipidemia |
44 | 71 | 253 | biweekly | 2 sessions | lipid kinetics (change in 14 day interval between treatments) | |
| Schuff-Werner et al., 1994 (67) HELP LDL Apheresis Multicentre Study |
N=51 Severe CAD and type II hyperlipoproteinemia LDL ≥ 200mg/dL despite diet and drug therapy |
44.4 | 67 | 310 | weekly | 24 months | lipid parameters angiographic outcomes |
|
CAD refers to coronary artery disease; LDL, low-density lipoprotein-cholesterol; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Inclusion criteria for the Lane et al. studies did not meet the lower level of LDL specified by the FDA indications
Additional Results
Lane et al. (84-86) also published additional results to those cited in the FDA Report. In the first study by Lane et al. (86), patients were treated on a weekly basis. Of the 24 patients who completed 25 weekly treatments in the first 6 months, 79% of patients achieved a reduction in LDL-C greater than or equal to 30%. The mean chronic reduction in LDL-C was 39%. In terms of angina symptoms, 3 of the 6 patients who experienced symptoms at baseline no longer had symptoms after 25 weeks of HELP therapy. A total of 44 complications were observed of which 1.7% were device related, 2.8% were user related and 1.9% were patient related. (86) However, not all patients included in the Lane et al. (86) study met Health Canada and FDA criteria for the minimum baseline LDL-C level. The LDL-C inclusion criterion in the Lane et al. study was LDL-C levels greater than 160 mg/dL (4.1mmol/L), whereas the minimal threshold in the Health Canada and FDA indications is 200 mg/dL (5.2mmol/L).
In the second publication in the series by Lane et al. (84), 23 FH patients (4 HMZ, 19 HTZ) who completed the first 6 months of weekly therapy were treated at biweekly intervals. About 98% of patients achieved a reduction in LDL-C greater than or equal to 30%. The mean chronic reduction in LDL-C was 33%. In comparison to weekly therapy, levels of LDL-C before each procedure were higher with biweekly treatment. Nevertheless, these pretreatment levels were lower than the mean levels at baseline. Adverse events occurred in 5.1% of procedures and the most common problems were related to venous access or hypotension. (84)
In both studies of weekly and biweekly treatments, 72% of patients believed that their health was “much better” or “somewhat better” after 6 months of therapy.
In the last publication in the series by Lane et al. (85), the kinetics of LDL apheresis were examined in 14 patients (10 FH patients and 4 patients with combined hyperlipidemia). The HDL-C concentration was acutely decreased by 12% using HELP therapy and was normalized within 2 days, and remained at this concentration until the next treatment. The authors also compared FH patients who were on drug therapy with those without drug therapy and concluded that in order to achieve the maximum therapeutic benefits of HELP apheresis, concomitant lipid-lowering drug therapy should be included in the patient’s treatment plan. (85)
In addition to the results reported in the 1997 FDA report, Schuff-Werner et al. (67) published results on the chronic changes in lipid parameters that were attained with weekly HELP therapy. After 2 years of follow-up, there was a significant long-term decrease of 22.2% in TC and 28.3% in LDL-C (both P < .001), while there was a significant increase of 26.8% in HDL-C (P < .01). Adverse events were only reported in 2.9% of treatments and the reactions were mostly of minor clinical relevance. During the course of treatment, a general improvement in angina symptoms was also observed. At the beginning of the study, 82% of patients reported experiencing angina symptoms compared with 62% of patients after 2 years of regular HELP treatment.
Angiographic follow-up in 33 patients revealed that 16 patients showed regression (48%), 9 patients showed progression (27%) and 8 patients showed no change in CAD status (24%). The mean degree of stenosis per patient decreased by 1.5%, from 34.8% to 33.3% (P = .21). The decrease of 1.9% in the mean degree of stenosis reported by the FDA assumed independence between segments. Over the 2-year follow-up period, the rate of regression was 1.8 times the rate of progression. A statistically relevant correlation was not found between lipid-lowering effects and angiographic outcomes. (67)
Limitations
The trials conducted by Lane et al. (84-86) and Schuff-Werner et al. (67) had several limitations. First, patients in the Schuff-Werner et al. study were not taking concomitant statin therapy since statins were not yet available in Germany at the onset of the study (statins were first introduced in Germany in 1989).
According to current practice, statins are among the first medications prescribed for hypercholesterolemia, especially HTZ FH, thus limiting the external validity of the findings. In the studies reported by Lane et al. (84-86), the inclusion criterion for minimum LDL-C levels was lower than the minimal threshold stipulated in the Health Canada and FDA indications. In addition, details on the HMZ or HTZ status of FH patients were not disclosed in any of the studies, except in the 1995 Lane et al. publication. Results were not presented for HMZ and HTZ FH patients separately in the publications, although some stratified results were presented in the FDA report.
Alberta Heritage Foundation for Medical Research, April 2004
The AHFMR systematically reviewed the literature on LDL apheresis for the treatment of FH. More specifically, the aim of the HTA was to assess the evidence on the safety and effectiveness of LDL apheresis with both the DSC Liposorber and HELP system in lowering the concentration of LDL-C in FH patients. At the time of the review, both the DSC Liposorber and HELP system had market approval from Health Canada. Since the AHFMR review was published, the license for the DSC Liposorber has been cancelled. Currently, the only LDL apheresis system approved by Health Canada is the HELP system. Therefore, only certain studies included in the AHFMR HTA met our inclusion criteria and are applicable to this review.
The authors searched PubMed, EMBASE, Health Star, The Cochrane Library, Science Citation Index and various web sites of HTA agencies, research registers and guidelines. The review included all comparative English language studies published from 1998 to 2004.
No systematic reviews or RCTs met the inclusion criteria. The results on the acute reduction in plasma lipoprotein profile from the multicenter American and German HELP clinical trials were included in the background section of the report.
A total of 8 studies were included in the review. Six controlled studies compared the effectiveness of the DSC Liposorber plus drug therapy with drug therapy alone. Two additional studies included 1 crossover study and 1 comparative study that examined the safety and effectiveness of different LDL apheresis systems. These were the only 2 studies out of the 8 studies included in the review that assessed the HELP system. The remaining 6 studies focused solely on the DSC Liposorber. Appendix 2 summarizes the studies that were included in the AHFMR HTA report.
Findings from the AHFMR review were as follows:
DSC Liposorber in combination with drug therapy lowered LDL-C levels in older patients (>50 years) with severe FH when treated at least once every 2 weeks for a minimum duration of 1 year.
In the 6 controlled studies, most of which were multicenter trials, the number of patients ranged from 18 to 130 patients and the majority of patients were HTZ (308 HTZ, 12 HMZ). The mean percent decrease in LDL-C ranged from 34 to 81%. Low-density lipoprotein apheresis in combination with drug therapy also seemed to reduce the rate of future cardiovascular events such as cardiac death, coronary revascularization procedures (CABG, PTCA), MI or cerebrovascular events in comparison to groups with drug therapy alone.
The results from the crossover study and the comparative study indicated that all apheresis systems were comparatively efficient in the levels of LDL-C reduction.
All of the patients included in the crossover study and the comparative study were HTZ. 34 HTZ FH patients were included in the comparative study, and in the crossover study, 5 HTZ FH patients were included in part A and 6 HTZ FH patients were included in part B. In addition to the HELP and DSA systems, the IMAL and MDF systems were included. The mean percent decrease in LDL-C ranged from 54 to 65%.
Adverse events were only reported in 2 of the 8 studies. The most common adverse events were hypotension, nausea and vomiting, and problems relating to venous access, and all of these were transient effects.
The authors noted that significant heterogeneity existed among the studies included in their HTA in terms of number of patients, baseline lipid concentrations, type and combination of drugs, duration of follow-up and frequency of treatment. The studies also had methodological weaknesses, were conducted primarily by researchers in Japan and Germany (which are where the LDL apheresis systems were developed) and almost all utilized the DSC Liposorber system. No Canadian studies were identified.
The AHFMR acknowledged that at the time of their review, there was only one LDL apheresis program in Canada at the Cliniques des maladies lipidiques at the Centre Hospitalier Universitaire de Québec (CHUQ). The program had been in operation since 2001 and up until January 2004, a total of 436 procedures had been performed in 11 HMZ FH patients. The Quebec clinic chose the HELP system for its efficacy, reliability, tolerability and cost.
Based on these findings, the AHFMR made the following conclusions:
Aggressive lipid-lowering therapy with LDL apheresis combined with drugs is an effective and safe treatment for HMZ FH patients and severely ill HTZ FH patients with CAD.
The safety and efficacy of LDL apheresis has not yet been assessed in special groups such as pregnant or lactating women and young children.
Additional well-designed studies are needed. Studies comparing PE and LDL apheresis would be insightful and for ethical reasons, randomized crossover studies may be the most appropriate design.
There is a need for conducting economic analyses.
Establishing a national registry of FH patients would provide beneficial information on the clinical outcomes of the various treatment options.
The decision to approve and include LDL apheresis has difficult economic and ethical implications.
In addition to including only 2 studies with the HELP System, a very small number of HMZ FH patients were included in the analysis. Only 2 out of the 8 studies included HMZ FH patients for a total of 12 patients, representing 3.4% of all included patients. The 2 comparative studies that included the HELP system will be discussed in more detail in the following sections.
Medical Advisory Secretariat Systematic Review
Eight studies met the inclusion criteria, of which 5 were case series study designs, and 2 were case series nested within comparative studies, which compared the effectiveness of different LDL apheresis systems, one of which was HELP. One study was a retrospective comparison.
Prospective Case Series
Five prospective case series studies were identified. Details of the studies are outlined in Table 11 below.
Table 11: Characteristics of Prospective Case Series Studies *.
| Study | N Population | Patient Characteristics | HELP Treatment Interval | Follow-up | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) |
Mean Baseline LDL (mg/dL) | Acute† | Chronic‡ | ||||
| Moriarty et al., 2001 (87) United States |
N=4 HTZ FH, advanced CAD, refractory to diet and drug therapy |
57.5 | 50 | 275 | biweekly | 6 months | lipid parameters and Creactive protein (CRP) | lipid parameters and CRP |
| Pulawski et al., 2002 (88) Germany |
N=10 HTZ FH, advanced CAD, refractory to diet and drug therapy Undergoing weekly HELP therapy |
52 | 60 | 159 | single session | single session | lipid parameters and soluble adhesion molecules | |
| Hoffmann et al., 2003 (89) Austria |
N=8 HTZ FH and CAD |
46 | 88 | 275 | weekly or biweekly | 29 months | lipid parameters | lipid parameters coronary calcification |
| Moriarty et al., 2004 (29) United States |
N=6 HTZ FH, CVD and LDL > 200mg/dL despite diet and drug therapy All patients successfully completed at least one HELP session prior to enrolment |
58 | 50 | 253 | single session | single session | lipid parameters and blood viscosity | |
| Moriarty et al., 2005 (90) United States |
N=8 HTZ FH, CVD and LDL ≥ 200mg/dL despite diet and drug therapy All patients successfully completed at least one HELP session prior to enrolment |
59 | 50 | 262 | biweekly | 3 months | LDL and Lp-PLA2 | LDL and Lp-PLA2 |
CAD refers to coronary artery disease; CRP, C reactive protein; CVD, cardiovascular disease; HTZ FH, heterozygous familial hypercholesterolemia; LDL, low-density lipoprotein-cholesterol; Lp-PLA2, lipoprotein associated phospholipase 2; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Moriarty et al., 2001
Moriarty et al. (87) conducted a single centre prospective case series to investigate the effect of LDL apheresis with HELP on lipids, fibrinogen and CRP (a marker of inflammation) in 4 refractory HTZ FH patients with advanced CAD. Patients were treated with biweekly HELP apheresis over a period of 6 months.
Over the treatment period, there were no changes in medications, smoking and drinking behavior, and body weight as assessed by a questionnaire. The mean decreases per treatment in lipid levels were as follows: TC 56%, LDL-C 64%, HDL-C 25% and TG 34%. Further, there was a 65% decrease in fibrinogen and a 64% decrease in CRP. After 6 months of treatment, there was a decrease of 5% in TC, 9% in LDL-C, 25% in fibrinogen and 49% in CRP. Unlike lipids, TG and fibrinogen, CRP did not rebound to baseline levels and showed a consistent downward trend over the study period. Chronic increases of 8% and 12% were observed for TG and HDL-C, respectively. No adverse cardiovascular symptoms were reported during the study. All patients tolerated the HELP treatments very well. The authors concluded that LDL apheresis has benefits for FH patients in terms of LDL-C reduction and that the benefits of LDL apheresis may extend to other patient groups given the quick and drastic reduction in serum lipids and inflammatory markers. (87)
Limitations to the study by Moriarty et al. included:
Small sample size
Type of FH was not documented in the published paper, necessitating contact with author for clarification.
Unknown if patients were treated with LDL apheresis prior to study initiation.
No details regarding type and dosage of lipid-lowering medications.
No test of significance was performed for acute or chronic changes in lipids and inflammatory markers (no P values presented). In any event, this sample size would present a high probability of a type 2 error.
Pulawski et al., 2002
Pulawski et al. (88) conducted a study to investigate the influence of a single HELP session on lipid parameters and plasma concentrations of CAMs that are involved in atherosclerosis, such as soluble vascular cellular adhesion molecule-1 (sVCAM-1), soluble intracellular adhesion molecule-1 (sICAM-1) and P-selectin. Ten patients with HTZ FH who were refractory to diet and drug therapy and displayed advanced CAD were enrolled in the study. Prior to study enrolment, patients were undergoing HELP apheresis at weekly intervals and all patients were on concurrent lipid-lowering drug therapy. Detail on the type of drugs was provided.
With a single HELP session, the following was observed: TC was significantly reduced by 49%, LDL-C by 63%, HDL-C by 25% and TG by 62% (all P < .0001). After one week, the lipid levels approximated pretreatment values. Significant reductions in sVCAM-1 (32%, P < .0001), sICAM-1 (18%, P = .0032) and P-selectin (33%, P = .0044) were also observed after a single HELP session. Reductions were due to filtration and depended on the molecular weights of the CAMs. (88)
Pulawski et al. (88) concluded that a single HELP session significantly lowered lipid levels and also favorably impacted concentrations of CAMs. Given that CAMs contribute to atherosclerosis, removal by HELP apheresis may improve the outcome of patients with high atherosclerotic risk, such as FH patients.
Limitations to the study by Pulawski et al. included:
Small sample size
Determination of the clinical utility or effect on disease progression following a single treatment is unlikely.
Mean baseline LDL-C (159 mg/dL) was low in comparison to that of patients included in other studies. Study inclusion criteria stated that patients had to be previously treated with HELP apheresis at weekly intervals, which may have resulted in lower baseline values.
Hoffmann et al., 2003
A prospective study by Hoffmann et al. (89) sought to examine the effects of HELP and oral statin therapy on plasma lipids and coronary calcified plaque in 8 patients with HTZ FH and CAD. (89) Prior to enrolment, all patients were on statin therapy. During the 29 months of follow-up, patients were treated with HELP at weekly or biweekly intervals and received atorvastatin at the highest approved dose (80mg/day). No other drugs influencing lipoprotein metabolism were administered.
During the study period, there were no reports of alterations in patients’ clinical appearance, coronary events, or toxicity due to the treatment. Further, risk factors remained unchanged. Acute mean reductions were as follows: TC 64%, LDL-C 77%, HDL-C 18%, TG 49%, Lp(a) 67% (all P < .01). After 29 months of follow-up, TC was reduced by 29%, LDL-C by 40%, TG by 26% and Lp(a) by 27% (all P < .01). In contrast, long-term HDL-C levels increased by 24% (P < .01). (89)
Imaging results obtained by computed tomography (CT) at baseline and at follow-up demonstrated significant changes in coronary calcium. Coronary calcium is a marker of advanced coronary atheroslcerosis and CAD burden. (52) At baseline, all patients had high Agatston scores1 and were above the 90th percentile for age and sex-adjusted values, indicating a high-risk for MI. An Agatston score greater than 400 signifies extensive evidence of plaque. The median score of 684 at baseline decreased by an average of 26%, to a median score of 497 at follow-up (P < .01). The volume of coronary calcium also decreased in all patients by an average of 23% (median at baseline 622mm3, at follow-up 466mm3, P < .01). The authors noted that the observed reduction in the volume of coronary calcium was higher than the reductions reported in trials with statins alone, which ranged from 7 to 15%. Further, the mean density of coronary calcium increased by 17% (P < .01). Mean plaque density may be used as an indicator of maturity and stability of a plaque although to date, it has not frequently been used to characterize coronary calcium. Additional studies are needed to investigate this hypothesis. (89)
Thus, LDL apheresis combined with statin therapy resulted in significant lipid-lowering effects as well as significant volumetric regression of coronary calcium.
Limitations to the study by Hoffmann et al. included:
Small sample size
The majority of the patients were male (7 out of 8 patients).
Generalizability of the findings was limited since patients were only prescribed statin therapy and were not treated with combination therapy.
The type of LDL apheresis system used was not documented in the published paper, necessitating contact with the author for clarification.
Two different CT imaging modalities were used to assess coronary calcium. At baseline, calcification was assessed by electron beam computed tomography (EBCT), and at follow-up, by multidetector computed tomography (MDCT), due to the unavailability of EBCT. Although this is not ideal, the authors noted that excellent agreement between EBCT and MDCT calcium score measurements have been demonstrated, particularly in patients with high calcium scores.
Moriarty et al., 2004
Six patients with HTZ FH participated in the study by Moriarty et al. (29), which examined the acute effects of a single HELP apheresis session on lipid parameters and blood viscosity. All patients had cardiovascular disease and LDL-C levels greater than 200 mg/dL despite treatment with at least one lipid-lowering drug consisting of a statin, niacin, fibrate, resin or ezetimibe. Prior to study enrolment, all patients had successfully completed at least one HELP session.
After one session, the observed acute mean reductions were as follows: TC 49%, LDL-C 62%, TG 33% and HDL-C 21% (all P < .05). In addition, the single session significantly reduced blood viscosity for all shear rates ranging from 13 to 31% (all P < .01). High blood viscosity levels are associated with cardiovascular events and the early stages of atherosclerosis. (29)
Limitations to the study by Moriarty et al. included:
Small sample size
Determination of the clinical utility or effect on disease progression following a single treatment is unlikely.
The type of FH was not documented in the published paper, necessitating author contact for clarification.
Moriarty et al., 2005
Moriarty et al. (90) conducted a third study in 2005 examining the effect of HELP apheresis on lipid parameters and lipoprotein-associated phospholipase A2 (Lp-PLA2), a biomarker of inflammation that is bound to LDL-C, and has been found to be an important predictor for CAD. Eight patients were included with HTZ FH, cardiovascular disease, and LDL-C levels greater than 200mg/dL (5.2mmol/L), despite treatment with at least 1 lipid-lowering drug (consisting of one of the following: a statin, niacin, fibrate, resin or ezetimibe). As in the 2004 study, all patients had successfully completed at least one HELP session prior to study enrolment. Patients were treated on a biweekly interval over a period of 3 months.
The mean acute reduction in LDL-C achieved was 60% (P < .001) and after 3 months of biweekly therapy, LDL-C was chronically reduced by 14%. Acute reductions in Lp-PLA2 were also observed (22%; P < .003). Reductions in LDL-C and Lp-PLA2 were not significantly correlated (P = .06). The authors concluded by that the reduction in inflammatory markers may be another means by which HELP reduces the risk of cardiovascular events.
Limitations to the study by Moriarty et al. included:
Small sample size
The type of FH was not documented in the published paper, necessitating contact with author for clarification.
Measures of acute and chronic changes were limited to LDL-C and did not include other lipid parameters such as HDL-C and TC, TG.
No test of significance was performed for chronic reduction in LDL-C (P value not provided).
Case Series Nested Within Comparative Studies
Two additional studies were identified that compared HELP with other LDL apheresis techniques (See Table 12). For the purposes of this review, only results presented for patients undergoing HELP therapy were included. Thus, these studies are similar to a case series study design, but one is nested within a comparative study, and one is nested in a crossover study. The studies by Richter et al. (91) and Julius et al.(92) were included in the AHFMR 2004 HTA.
Table 12: Characteristics of Nested Case Series Studies (Only Patients Treated With HELP)*.
| Study | N Population Comparison |
Patient Characteristics | HELP Treatment Interval | Follow- up |
Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) |
Mean Baseline LDL (mg/dL) | Acute† | Chronic‡ | ||||
| Richter et al., 1999 (91) Prospective comparison Germany |
N=8 patients with HELP (total of 34 patients) HTZ FH, CAD, refractory to diet and drug therapy Treated with regular LDL apheresis Compared 3 systems: IMAL, DSA, HELP |
43.6 | 75 | 257 | weekly (majority) | mean 4.6 years (range 1 to 8.6) | lipid parameters | lipid parameters angiographic outcomes |
| Julius et al., 2002 (92) Crossover Germany |
N=6 HTZ FH, CAD, refractory to diet and drug therapy Treated with LDL apheresis ≥ 24 months Part A: Lipidfiltration with Cascadeflo AC-1770 & HELP (n=5) Part B: Lipidfiltration with Lipidfilter EC-50 & HELP (n=6) |
61.5 | 67 | 135 | weekly | 4 sessions (2 in part A and 2 in part B) over 8 weeks | lipid parameters | |
CAD refers to coronary artery disease; DSA, dextran sulfate adsorption; HELP, heparin-induced extracorporeal LDL precipitation; HTZ FH, heterozygous familial hypercholesterolemia; IMAL, immunoadsorption; LDL, low-density lipoprotein-cholesterol; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Richter et al., 1999
A study conducted by Richter et al. (91) compared the efficacy and safety of 3 different LDL apheresis techniques in serum lipoprotein removal, along with the effect on CAD.. A total of 34 HTZ FH patients treated with regular LDL apheresis participated in the study. LDL-C concentrations could not be sufficiently reduced in these patients, who were all on dietary and drug therapy. Patients were treated on a weekly (n=30) or biweekly basis (n=4) with DSA, IMAL, or HELP. Although this was a comparative study with the aim of comparing the 3 different techniques, only results pertaining to patients treated with HELP are pertinent to this review. As such, only results on the 8 HTZ FH patients treated with HELP will be presented.
At study initiation, all lipid-lowering drugs were withdrawn. After 2 years of follow-up, 5 of the 8 patients were administered the maximum tolerable dose of simvastatin. All other patients received variable doses of simvastatin from the commencement of LDL apheresis.
After a mean follow-up of 4.6 years (range 1.0 to 8.6 years), a total of 1,497 HELP sessions were documented in the 8 patients. This study had one of the longest durations of follow-up out of all studies included in this review. Acute mean decreases during one apheresis procedure measured as the mean of the last 5 treatments were as follows: TC 49.3%, LDL-C 58.8%, HDL-C 16.6%, TG 44.4%, Lp(a) 67.7%. Chronically, there was a 45.5% decrease in LDL-C and a 52.2% decrease in Lp(a), whereas HDL-C increased by 20%. Acute and chronic changes in lipid parameters were comparable with all 3 LDL apheresis methods. For all 34 patients, the chronic decrease in LDL-C and simultaneous chronic increase in HDL-C caused the LDL:HDL ratio to favorably decline from 6.5 to 2.6. (91)
In terms of changes in the global coronary score, 5 of the 8 patients treated with HELP were followed for a period greater than 2 years and thus had coronary angiography results. Two patients had a regression of coronary atherosclerosis and the other 3 patients experienced no change. Progression of coronary atherosclerosis was not observed in any of the patients (See Table 13). (91)
Table 13: Global Change Score of Coronary Arteries under LDL Apheresis in HTZ FH Patients*.
| Patient Initials |
Sex/ Age (yr) |
Vessels Affected |
Severity | Duration of Treatment (yr) |
Global Change Score |
||
|---|---|---|---|---|---|---|---|
| HELP | |||||||
| L.H. | F/44 | 3 | IV | 8.6 | 0 | ||
| B.F. | M/38 | 3 | III | 8.5 | 0 | ||
| S.J. | M/47 | 3 | I | 8.3 | −2 | ||
| L.B. | F/55 | 3 | II | 5.4 | −1 | ||
| R.W. | M/50 | 1 | III | 2.2 | 0 | ||
| S.B. | M/49 | 3 | IV | 1.2 | ND | ||
| S.G. | M/35 | 3 | III | 1.8 | ND | ||
| M.F. | M/29 | 3 | III | 1.0 | ND | ||
HELP refers to heparin-induced extracorporeal LDL precipitation; F, female; M, male; yr, year.
For the global change score, 0 refers to no demonstrable change; 1, definitely discernable change; 2, intermediate change; 3, extreme change; ND, not determined. A sequence of films showing progression was given a + sign and films showing regression a –sign.
Reproduced from Metabolism: Clinical & Experimental, 47(7), Richter WO, Donner MG, Hofling B, Schwandt P. Long-term effect of low-density lipoprotein apheresis on plasma lipoproteins and coronary heart disease in native vessels and coronary bypass in severe heterozygous familial hypercholesterolemia, p. 863-8, Copyright 1998, with permission from Elsevier.
Adverse reactions that were observed with all 3 LDL apheresis techniques were usually mild and easily reversible by minor symptomatic treatment. Adverse events specific to HELP were not reported. With all 3 techniques, an adverse clinical event was noted in 1.8% of procedures, and technical pitfalls were encountered in 3.6% of procedures. The majority of adverse events were related to problems with venous access. Further, 2 patients on HELP experienced a sudden cardiac death during the observation period—one patient after 9 months of therapy, and one after 6 years of therapy. The authors reported that these events were not treatment related. (91)
Overall, the results indicated that long-term LDL apheresis was able to halt the progression of coronary lesions in FH patients. All 3 methods were comparably effective in lowering atherogenic lipoproteins and favorably halted the atherosclerotic process. (91)
Limitations to the study by Richter et al. included:
Small sample size
Generalizability of the findings was limited since patients were not treated with other lipid-lowering medications
Results were not always presented separately for each type of LDL apheresis technique (eg. results on adverse events were aggregated)
Results of degree of stenosis were presented on a scale (global change score). It would have been valuable to present additional angiographic information such as degree of stenosis.
No test of significance was performed for acute or chronic changes in lipid levels (no P values presented)
Julius et al., 2002
The prospective study by Julius et al. (92) employed a crossover design to evaluate the efficacy of 2 LDL apheresis techniques, lipidfiltration, also known as MDF, and HELP, in lowering the concentration of plasma lipids and other plasma proteins. Six patients with refractory HTZ FH and CAD were enrolled in the study. Patients were previously treated by long-term LDL apheresis for at least 2 years and were taking lipid-lowering drugs (dosage remained constant during the study period). Patients were treated at weekly intervals with 2 consecutive treatments of either lipidfiltration or HELP in a crossover design, over a period of 8 weeks. Lipidfiltration was evaluated at 2 different stages of technical development. In part A, lipidfiltration with Cascadeflo AC-1770 was compared with HELP (n=5) and in part B, lipidfiltration with the new Lipidfilter EC-50 was compared with HELP (n=6; 5 patients identical to those treated in part A). Patients were randomly assigned to the method of initial LDL apheresis method. Only results for the 2 sessions with HELP in part A and the 2 sessions with HELP in part B will be examined (total of 22 HELP treatments). Results pertaining to the lipidfiltration system will not be discussed as these are not applicable to this review.
In part A, the mean reduction in lipid parameters and plasma proteins of the 2 consecutive HELP treatments were as follows: LDL-C 54.0%, TG 60.7%, Lp(a) 61.5%, fibrinogen 58.0%. HDL-C increased by 0.4%. In part B, the mean reductions observed with HELP were as follows: LDL-C 61.3%, TG 51.6%, Lp(a) 56.3%, fibrinogen 41.5% and HDL-C 1.3%. In addition, the LDL: HDL ratio was favorably reduced from 3.3 to 1.5 in part A and from 3.9 to 1.5 in part B. (92)
Although lipidfiltration and HELP had different modes of action, differences in the changes in lipid parameters between LDL apheresis techniques were not statistically significant. Further, no severe adverse events occurred throughout the observation period. Thus, the authors concluded that regular LDL apheresis with lipidfiltration or HELP resulted in a decrease of TC, LDL-C and TG, as well as an improvement in the LDL:HDL ratio. Pretreatment HDL-C values remained unchanged.(92)
Limitations to the study by Julius et al. included:
Small sample size
Short duration (only 2 sessions per part)
Lack of information on the patients’ type of lipid-lowering drugs
No tests of significance were performed for changes in plasma lipid and protein components exclusively for the HELP system. Only statistical tests that compared the 2 methods of LDL apheresis were performed.
Mean baseline LDL-C (135 mg/dL) was low in comparison to that of patients included in other studies. Study inclusion criteria stated that patients had to be previously treated with LDL apheresis for at least 2 years and this may have resulted in lower baseline values.
Retrospective Study
Krebs et al., 2004
Krebs et al. (18) retrospectively compared 5 different LDL apheresis methods from 5 participating German centers over a period of 15 years, from 1986-2001 (See Table 14). The methods included IMAL, DSA, HELP, DALI and MDF, which are the most commonly used techniques. Of the 20 patients included in the study, 3 patients were HMZ and 17 were HTZ, and all had significant CAD and were prescribed statins once they became available in the early 1990s. Since all patients were retrospectively included and no patient selection took place, it can be inferred that all patients met German indications for LDL apheresis and were thus refractory to diet and drug therapy.
Table 14: Characteristics of Retrospective Study (Only Patients Treated With HELP)*.
| Study | N Population |
Patient Characteristics | HELP Treatment Interval | Follow- up |
Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) |
Mean Baseline LDL (mg/dL) | Acute† | Chronic‡ | ||||
| Krebs et al., 2004 (18) § Retrospective comparison Multisite Germany |
N=10 patients with HELP (total of 20 patients - 3 HMZ and 17 HTZ) FH, CAD, refractory Patients on statins in the early 1990s Compared 5 systems: DALI, HELP, IMAL, MDF, DSA |
47 | 70 | 351 | ? | mean 9 years over a period of 15 years | lipid parameters | Kinetics – calculated average lipid concentrations between aphereses angiographic outcomes |
CAD refers to coronary artery disease; DALI, direct adsorption of lipoproteins; DSA, dextran sulfate adsorption; HELP, heparin-induced extracorporeal LDL precipitation; HMZ FH, homozygous familial hypercholesterolemia; HTZ FH, heterozygous familial hypercholesterolemia; IMAL, immunoadsorption; LDL, low-density lipoprotein-cholesterol; MDF, membrane differential filtration; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Patient characteristics and duration of follow up not available for those specifically treated with HELP, all patients together (N=20)
The effect of the different LDL apheresis techniques on lipid parameters was assessed before and after each apheresis procedure. In order to assess the kinetics of apheresis, the area under the concentration time curve (AUC) between 2 LDL apheresis treatment sessions was derived for each variable. Consequently, the AUC-derived concentration (Cauc) was calculated for some of the key lipid and protein variables by dividing the AUC by the time interval between successive treatments. Coronary outcomes were quantified by coronary angiographies, which were performed every 5 years in most patients. (18)
Over the 15-year study period, the average duration of treatment was 9 ± 4 years, and some patients were treated with more than one LDL apheresis method. Ten patients were treated with HELP, generating results on 2,750 HELP procedures. The acute mean reductions were as follows: TC was 54%, TG was 46%, LDL-C was 62%, HDL-C was 29%, Lp(a) was 55% and fibrinogen was 56%. The Cauc represents the average concentration between 2 LDL apheresis sessions and the authors proposed that this measure allows a better understanding as to whether the patient has achieved target lipid values, as compared with acute measures. With HELP, the Cauc was 4.06mmol/L for LDL-C, 1.84mmol/L for HDL-C, and 6.67mmol/L for TC. Krebs et al. (18) noted that in drug trials, the LDL-C target is often set at 2.6mmol/L, and the time-averaged mean for LDL-C that was observed with HELP in this study exceeded this target. Krebs et al. also noted that the LDL:HDL ratio achieved with HELP was 2.2, which does not greatly surpass the target of 2.0 set by international guidelines.
Cardiovascular outcomes were presented in aggregate form for all LDL apheresis techniques and as such, results were not available separately for HELP patients. It was reported that CAD progressed only in one patient and that most patients were stable or improved. Of the 20 patients, 11 had documented CAD at the start of the analysis, in comparison with 12 patients at the end of analysis. Symptomatic angina pectoris improved in 2 patients whereas heart failure progressed in 2 patients. Progression of atherosclerosis occurred in 65% of patients as measured by sclerosis of the abdominal aorta, carotid artery stenosis, and peripheral vascular disease. Fourteen patients had an MI before treatment was started, and 2 patients had an MI during the treatment period. (18)
Through consultation with specialists and family doctors, there was no evidence regarding life-threatening or fatal events caused by any of the LDL aphereses methods. (18)
Krebs et al. (18) also made some comparisons among the 5 techniques and briefly, there were differences observed between the calculated Cauc for the lipid variables and the differences in reductions in immunoglobulins. The main reasons for changing methods were technical advantages, higher effectiveness, occasional heparin intolerance, and differences in costs.
Overall, the authors concluded that all 5 apheresis methods were safe and suitable for long-term treatment of FH patients. However, calculating the Cauc for LDL-C (3.03-5.59mmol/L) revealed that the target of LDL-C less than 2.6mmol/L was not being achieved in patients regardless of the method of LDL apheresis.
Limitations of the study by Krebs et al. included:
Retrospective review – no patient selection
Small sample size
Some patients were treated with more than one LDL apheresis method over the study period. Therefore, not all results can be directly associated with the HELP system.
Baseline characteristics were only available for all patients combined (n=20) and not just for those treated with HELP (n=10).
Results not presented separately for HMZ and HTZ patients.
Lack of detail on coronary outcomes. It would have been useful to present information on the change in degree of stenosis.
Lack of information on type of concurrent drug therapies
No tests of significance were performed for acute changes in lipid parameters (P values not provided)
Summary of Findings of Systematic Review
Overall, the mean acute relative decrease in LDL-C ranged from 53 to 77% (See Figure 7). The mean acute relative reductions were as follows: TC ranged from 47 to 64%, HDL-C ranged from +0.4 to -29%, TG ranged from 33 to 62%, Lp(a) ranged from 55 to 68%, and fibrinogen ranged from 56 to 65% (See Table 15). In terms of chronic mean relative changes in plasma lipid and protein components, the observed range in LDL-C was from 9 to 46%, and TC ranged from 5 to 34% (See Table 16). As seen in Figure 8 illustrating chronic changes in LDL-C, FH patients treated with HELP LDL apheresis were not achieving the target LDL-C value set by Canadian guidelines. The chronic mean relative increase in HDL-C ranged from 12 to 27%. The mean follow-up ranged from 3 months to 4.6 years.
Figure 7: Acute Decrease in Low-Density Lipoprotein Cholesterol.
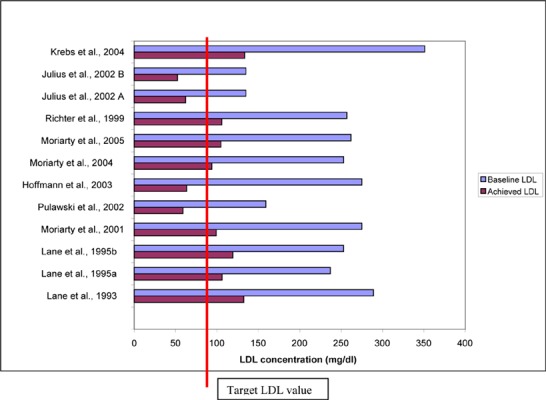
Table 15: Acute Relative Reductions in Plasma Lipid and Protein Components*.
| Study | N | Baseline LDL (mg/dL) |
Achieved LDL (mg/dL) |
LDL | TC (%) | HDL (%) | TG (%) | Lp(a) (%) | Fibrinogen (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lane et al., 1993 ‡ | 33 | 289 | 132 | 54.2 | 47.2 | 14.5 | 49.4 | - | 58.0 |
| Lane et al., 1995a ‡ | 23 | 237 | 106 | 55.2 | 49.1 | 15.0 | - | - | |
| Lane et al., 1995b ‡ | 14 | 253 | 119 | 53.0 | 48.0 | 12.0 | 55.0 | - | - |
| Moriarty et al., 2001 | 4 | 275 | 99 | 64.0 | 56.0 | 25.0 | 34.0 | - | 65.0 |
| Pulawski et al., 2002 | 10 | 159 | 59 | 63.0 | 49.0 | 25.0 | 62.0 | - | - |
| Hoffmann et al., 2003 § | 8 | 275 | 63 | 77.0 | 64.0 | 18.0 | 49.0 | 67.0 | - |
| Moriarty et al., 2004 § | 6 | 253 | 94 | 63.0 | 49.0 | 21.0 | 33.0 | - | - |
| Moriarty et al., 2005 | 8 | 262 | 105 | 60.0 | - | - | - | - | - |
| Richter et al., 1999 | 8 | 257 | 106 | 58.8 | 49.3 | 16.6 | 44.4 | 67.7 | - |
| Julius et al., 2002 A | 5 | 135 | 62 | 54.0 | - | +0.4 | 60.7 | 61.5 | 58.0 |
| Julius et al., 2002 B | 6 | 135 | 52 | 61.3 | - | 1.3 | 51.6 | 56.3 | 62.1 |
| Krebs et al., 2004 | 10 | 351 | 133 | 62.0 | 54.0 | 29.0 | 46.0 | 55.0 | 56.0 |
| RANGE | 53.0-77.0 | 47.2-64.0 | +0.4 to -29.0 | 33.0-62.0 | 55.0-67.7 | 56.0-65.0 |
HDL-C refers to high-density lipoprotein - cholesterol; LDL-C, low-density lipoprotein - cholesterol; Lp(a), lipoprotein (a); N, sample size; TC, total cholesterol; TG, triglycerides,
not all studies measured acute percent changes in the same manner
inclusion criteria for LDL values in the studies by Lane et al. were below LDL minimal values stipulated in the Health Canada indications
mean acute percent changes were not provided in the original publication and values were calculated based on pre and post treatment information
Table 16: Chronic Relative Change in Plasma Lipid and Protein Components.
| Study | N | FU (mths) |
Baseline LDL (mg/dL) |
Achieved LDL (mg/dL) |
LDL (%) |
TC (%) |
HDL (%) |
TG (%) |
Lp(a) (%) | Fibrinogen (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Schuff-Werner et al., 1994 | 39 | 24 | 310 | 222 | 28.3 | 22.2 | + 26.8 | 22.5 | - | - |
| Lane et al., 1993 ‡ | 33 | 6 | 289 | 176 | 39.0 | - | - | - | - | - |
| Lane et al., 1995a ‡ | 23 | 6 | 237 | 158 | 33.2 | - | - | - | - | - |
| Moriarty et al., 2001 | 4 | 6 | 275 | 250 | 9.0 | 5.0 | +12.0 | + 8.0 | - | 25.0 |
| Hoffmann et al., 2003 § | 8 | 29 | 275 | 165 | 40.0 | 29.0 | + 24.0 | 26.0 | 27.0 | - |
| Moriarty et al., 2005 | 8 | 3 | 262 | 225 | 14.0 | - | - | - | - | - |
| Richter et al., 1999 | 8 | 55 | 257 | 140 | 45.5 | - | + 20.0 | - | 52.3 | - |
| RANGE | ↓9.0-45.5 | ↓5.0-29.0 | ↑12.0-26.8 | +8.0 to -26.0 | ↓27.0 -52.3 | ↓25 |
FU refers to follow up duration; HDL-C, high-density lipoprotein - cholesterol; LDL-C, low-density lipoprotein -cholesterol; Lp(a), lipoprotein (a); N, sample size; TC, total cholesterol; TG, triglycerides.
not all studies measured acute percent changes in the same manner
inclusion criteria for LDL values in the studies by Lane et al. were below LDL minimal values stipulated in the Health Canada indications
mean acute percent changes were not provided in the original publication and values were calculated based on pre- and post-treatment information
Figure 8: Chronic Decrease in Low-Density Lipoprotein Cholesterol.
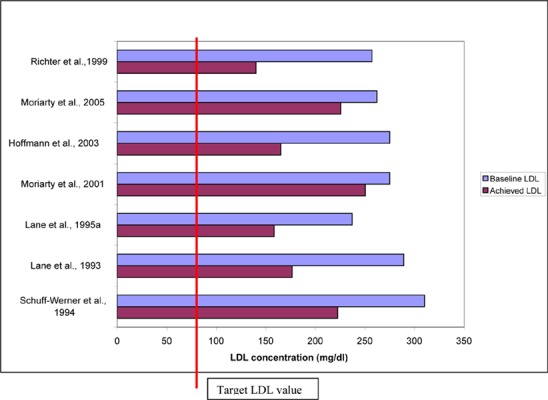
The mean chronic reduction in LDL-C ranged from 25mg/dL to 117 mg/dL. If we apply the principle that a 39mg/dL (1mmol/L) reduction in LDL-C translates into a 25% reduction in risk of cardiac events (40), we can infer that LDL apheresis with HELP reduces the risk of cardiac events by 15 to 70% in HTZ FH patients.
As stated elsewhere, the ratio of LDL:HDL has been shown to be an important risk factor for cardiac events, and the target LDL:HDL ratio for patients with CAD is a ratio less than or equal to 2. (41) Four studies reported on chronic LDL-C and HDL-C changes and as can be seen in the Figure 9a, the LDL:HDL ratios attained with LDL apheresis were above this threshold.
Figure 9a: Ratio of Low-Density Lipoprotein to High Density Lipoprotein.
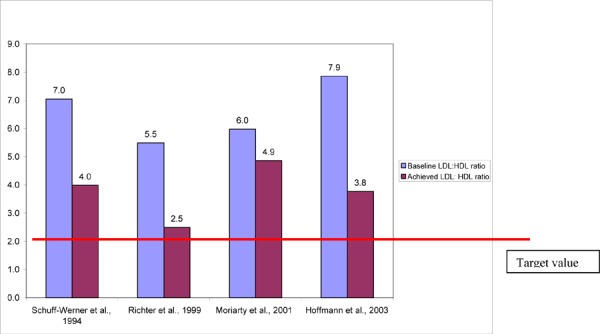
Additionally, the ratio of TC:HDL has also been shown to be an important risk factor for cardiac events. The 2006 Canadian lipid guidelines recommend a target TC:HDL ratio less than 4 in high-risk patients. (44) Three studies reported on chronic TC and HDL-C changes, and as can be seen in Figure 9b, the TC:HDL ratios exceeded the target value.
Figure 9b: Total Cholesterol to HDL Cholesterol Ratio.
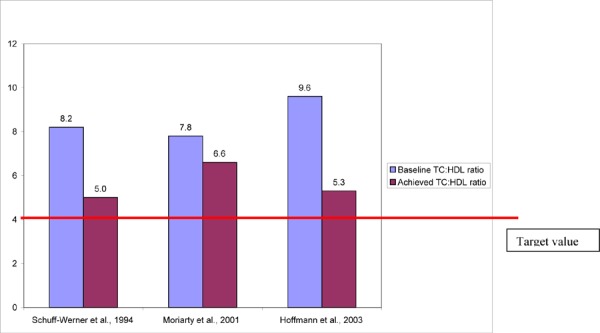
Two studies examined lipid kinetics after a HELP session. Lane et al. (85) reported that LDL-C begins to rebound immediately post-treatment and that after about 5 days, levels return to 50% of baseline values. Low-density lipoprotein-cholesterol also rebounded in a nonlinear fashion such that there was a steeper rise in LDL-C in the first few days following treatment. Krebs et al. (18) calculated that the average concentration in LDL-C between HELP treatments was 4.06mmol/L. This value exceeds the LDL-C target set by international guidelines. Both of these studies yielded information that is applicable to determining the optimal frequency of HELP therapy.
Three studies investigated the effects of HELP on coronary outcomes and atherosclerotic changes. Schuff-Werner et al. (67) noted that twice as many lesions displayed regression in comparison to those displaying progression. Hoffmann et al. (89) found that there was a decrease in Agatston scores and in the volume of coronary calcium. Richter et al. (91) noted that 2 of 5 patients showed regression of coronary atherosclerosis, and 3 of the 5 patients showed no change as assessed by a global change score. Krebs (18) also reported that CAD progressed only in a few patients and that most patients were stable or improved; however, the results were not exclusively for the HELP system. Thus, HELP appears to have a beneficial impact on atherosclerotic changes.
In addition to LDL-C reduction and changes in atherosclerotic lesions, other outcomes that were reported in the studies included decreased blood viscosity, improved blood rheology, decreased levels of oxidized LDL-C, and down regulation of leukocyte and endothelial adhesion molecules.
Adverse events specific to HELP were not recorded in all of the studies. Four studies simply reported than no serious adverse events were noted during the study period. Of the 3 studies that provided quantitative information on adverse events, the proportion of adverse events ranged from 2.9 to 5.1%. Richter et al. (91) also noted that adverse events were observed in 5.4% of treatments, but this data is not exclusively for the HELP system. Overall, adverse events were typically mild and transient, and the majority of events related to problems with vascular access.
Quality of the Evidence
Heterogeneity in Studies
The observed variation in mean acute and chronic changes in plasma lipid, lipoprotein and protein components may be attributed to the large heterogeneity among the studies. Given this heterogeneity, generating summary statistics for changes in plasma lipid, lipoprotein and nonlipid plasma parameters was deemed inappropriate. Studies varied with regard to inclusion criteria, population characteristics and methodology. Variable factors included the number of patients (range 4-51), inclusion criteria such as different minimal LDL-C requirements, and varied past experience with HELP. Specifically, one of the inclusion criteria in the 2004 and 2005 Moriarty et al. (29;90) studies specified that patients had to have successfully completed at least one HELP session before enrolment, whereas Julius et al. (92) stipulated that patients had to have been treated by long-term LDL apheresis for at least 24 months. Further, patients who were treated with HELP prior to study initiation may have had lower baseline lipid values and thus may have experienced a smaller percent reduction in plasma lipid parameters in comparison with patients who had no prior exposure to LDL apheresis. Nevertheless, all studies stipulated that included patients were refractory to diet and drug therapy.
In terms of population characteristics, nearly all patients were refractory HTZ and had established CAD. Homozygous FH patients were included in the studies in the FDA report as well as in the Krebs et al. study. (18;83) The baseline characteristics, such as plasma lipid concentrations, medication history (type and combination of drugs) and experience with HELP before study initiation, varied among the studies. Patients in the Pulawski et al. and Julius et al. studies had baseline LDL-C values that were much lower in comparison to the other studies. (88;92) However, these lower values may be attributed to the inclusion criteria for the studies, which stated that patients had to be previously treated with long-term LDL apheresis. Since no information was presented on the patients’ baseline LDL-C levels before the initiation of LDL apheresis, it can not be determined with certainty that these patients would be eligible for LDL apheresis as specified in the Health Canada indications (LDL>200mg/dL, >5.2mmol/L).
Heterogeneity was also observed in study methodology. The period of follow-up varied largely among studies, and the interval between HELP sessions ranged from weekly to every second week. Further, in most instances, patients were on concomitant drug and diet therapy during the study period, however the exact drugs, combinations and dosages were not always explicitly stated. For instance, in the Hoffmann (89) study, patients were only administered a statin whereas the majority of other studies permitted combination therapy. In addition, studies differed in the laboratory methods employed to analyze plasma components. Laboratory techniques to measure the lipoprotein profile ranged from commercially available kits to enzymatic colorimetric tests to precipitation methods. There was also a lack of consistency in the methods used to calculate acute mean changes in plasma lipids and proteins. Some studies used the mean change across the last 5 treatments to calculate the acute mean reduction, while some used the mean of 2 treatments, and others used the first and last treatment values. This lack of standardization may contribute in part to the observed variation in plasma parameters.
Quality of Evidence
In general, studies were of low quality. Some of the problems associated with the case series studies included the lack of a comparison group, small sample sizes, and lack of sample size calculation with a corresponding lack of statistical power. Several of the authors noted that for ethical reasons, conducting a trial with a sufficiently large control group would not have been feasible or acceptable. Schuff-Werner et al. (67) stated that “HELP represented a last alternative in these patients who were resistant to conventional therapeutic strategies raising the ethical problem of restricting this therapy to randomly selected patients.” Morelli et al. (10) also noted that “randomized comparisons are difficult to conduct when one of the inclusion criteria is failure of conventional therapy.” Therefore, although the quality of studies is low, it is unlikely that better-quality studies will ever be performed for ethical reasons.
An additional quality consideration is that the methodology of the studies was not always clearly defined, and it was sometimes necessary to contact the authors to obtain additional information and/or clarification on details such as the patients’specific type of hyperlipidemia or the method of LDL apheresis employed in the study. Lastly, many of the studies were published by researchers in Germany (which is where the HELP system was developed), which may result in publication bias due to clinical experience or other unmeasured factors.
No controlled studies were identified and no studies directly compared the effectiveness of the HELP system with PE, or with diet and drug therapy. As previously mentioned, conducting randomized trials in this patient population presents considerable ethical challenges since LDL apheresis most often represents a last resort therapy for these patients. The most appropriate strategy, as outlined by the AHFMR HTA, may be to conduct randomized crossover studies.
A major limitation is the limited evidence available on the effectiveness and safety of HELP apheresis in HMZ FH patients. Only the studies included in the FDA report (83) and the study by Krebs et al. (18) included HMZ FH patients, and only the FDA report presented outcomes separately for HMZ FH patients. Studies examining the impact of the HELP system in a large number of HMZ patients may never be conducted for ethical reasons. HMZ FH is rare and LDL apheresis is a last therapeutic option for these patients.
No studies assessed the effectiveness of the HELP system in special groups such as children or pregnant/lactating women. Studies that included a large number of child participants or that included exclusively children would have been more relevant since the large majority of HMZ FH patients are diagnosed in infancy and would be eligible for HELP therapy once they reached the weight limit indicated by the manufacturer. According to clinical experts, children could be treated with PE from diagnosis and then switched to HELP therapy once they reach the 30kg weight target at around 8 to 12 years in boys, and 8 to 14 years in girls. Stefanutti et al. (93) described the technical feasibility, compliance and risks of therapeutic apheresis in children. Eleven children with FH (7 HMZ, 4 HTZ) were treated with LDL apheresis using the DSC Liposorber. The age of patients ranged from 3.5 to 15 years, and weight ranged from 13 to 43 kgs. No serious complications were observed, and the most frequently observed adverse effects were related to problems with venous access (2.0%) and mild hypotension (2.0%). Stefanutti et al. concluded that although selected adaptations may be necessary, therapeutic apheresis can be performed safely and efficiently in low-weight patients. (93) Homozygous FH patients rarely become pregnant, but in this situation, drug therapy is inappropriate but is still necessary to control the patient’s hypercholesterolemia, which is heightened by the hormonal changes. Some case reports have been published indicating the feasibility and safety of performing LDL apheresis during pregnancy. (21)
Another limitation to the currently available evidence is that no studies considered the psychosocial implications of treatment. Low-density lipoprotrin apheresis is a lifelong therapy and patients are required to travel to the hospital on a weekly or biweekly basis for treatment. This schedule can be disruptive to normal activities such as school and work.
Lastly, there is limited data on the long-term effects of LDL apheresis in FH patients. No studies with HELP were identified that examined long-term outcomes such as survival and cardiovascular events. The absence of this data may be attributed to the rarity of the condition along with the larger number of subjects and long duration of follow-up that would be needed to conduct such trials. (4;82)
Indirect Evidence
Given the lack of evidence on the effectiveness of the HELP system in HMZ FH patients, the scope of this review was expanded to include studies that were conducted exclusively in HMZ FH patients using other methods of apheresis. As stated elsewhere, the different methods of LDL apheresis are generally comparable in their ability to reduce LDL-C levels. (38) A retrospective review by Makino et al. (58) examined the effects of LDL apheresis with the DSA system in 8 HMZ FH patients. Although the DSA and HELP systems are different in their process, they are generally similar in their ability to reduce lipids. (8) Patients were treated at different centres for periods ranging between 5 and 22 years. Of the 8 patients, only one patient was free of symptoms. The patient was treated with LDL apheresis for 15 years. The table below illustrates some of the clinical characteristics of the 8 HMZ FH patients. The authors stated that in the majority of HMZ FH patients, LDL apheresis was an effective method for preventing the development of atherosclerosis. However, some patients did show progression toward atherosclerosis in the coronary artery and aortic valve, even with intensive control of LDL-C. Due to the retrospective nature of this study and the lack of quantitative outcomes, this study is of low quality.
Table 17: Clinical Characteristics of Patients with Homozygous Familial Hypercholesterolemia.
| Patient no. | Age span during low-density lipoprotein apheresis treatment |
Cardiovascular findings |
|---|---|---|
| 1(F) | 4–19 years-old | Coronary artery stenosis has not occurred. Aortic wall is smooth and valve is normal. |
| 2(M) | 6–13 years-old | At 5 years, coronary artery and aorta were almost normal. At 12 years, thickening of the aortic wall and supravalvular stenosis (pressure gradient 70 mm Hg). |
| 3(F) | 9–29 years-old | At 10 years, coronary artery stenosis was not observed. At 27 years, 100% stenosis of right coronary artery was apparent. |
| 4(F) | 6–24 years-old | Obstruction of left main trunk was found at age 6. The aortocoronary bypass surgery was carried out at ages 10 and 15.50% stenosis of graft was observed at age 24. |
| 5(M) | 12–27 years-old | Aortic wall became smoother once, but aortic regurgitation worsened. |
| 6(F) | 22–40 years-old | Aortocoronary bypass was carried out at 18 years. |
| 7(M) | 27–29 years | At 29 years, patient died of acute myocardial infarction. |
| 8(F) | 25–32 years | At 31 years, patient had aortic valve stenosis and intact coronary artery. Patient died of myocardial infarction at the age of 32. |
Reproduced with permission from Blackwell Publishing; Makino H, Harada-Shiba M. Long-term effect of low-density lipoprotein apheresis in patients with homozygous familial hypercholesterolemia. Ther Apher Dial 2003; 7(4): 397-401
Given the lack of evidence on long-term outcomes with the HELP system, the scope of this review was expanded to include studies that examined cardiovascular events using other methods of apheresis. Two landmark trials, both published in 1998, reported the impact of LDL apheresis with the DSA method on cardiovascular outcomes. (94;95) As stated elsewhere, the DSA and HELP system comparably impact plasma lipid components. (8)
Gordon et al., 1998 (Liposorber Study Group, United States)
Gordon et al. (94) examined the long-term safety, lipid-lowering capacity, and rate of cardiovascular events with LDL apheresis using the DSC Liposorber in 10 HMZ and 39 HTZ FH patients, over a period of 5 years. Patients had an LDL-C greater than 160mg/dL (4.1mmol/L) despite diet and drug therapy and were treated concurrently with lipid-lowering drug therapy during the study. All 10 HMZ FH patients received LDL apheresis, whereas 9 of the 39 HTZ FH patients were assigned to a diet and drug therapy only control group. Treatment frequency for HMZ FH patients was 11± 5 days, and for HTZ was 14 ± 6 days.
At 4 years, the mean relative acute reduction in LDL-C was 76%, for both HMZ and HTZ FH patients, and the HDL-C reduction was 14% for HMZ and 9% for HTZ. Chronic changes in HMZ patients included a relative decrease in LDL-C by 26.7% (P = .059) and a relative increase in HDL-C by 19.5% (P = .33). Chronic changes in HMZ FH patients included a relative decrease in LDL-C by 4.7% (P = .059) and a relative increase in HDL-C by 2.3% (P = .33). Results on only 3 HTZ FH patients in the control group were available at follow-up. There was a 0.4% increase in LDL-C and a 13.3% decrease in HDL-C. In both HMZ and HTZ FH patients, fibrinogen levels displayed small fluctuations during the 4 year period with a tendency to decrease slightly.
The incidence of cardiovascular events was retrospectively examined in order to explore whether changes in lipid profiles improved clinical outcomes. A before/after comparison was made on the incidence of cardiovascular events, including cardiac death, coronary revascularization, MI or cerebrovascular event. Results were not presented separately for HMZ and HTZ FH patients. A total of 24 cardiovascular events occurred during the 5 years before study initiation in comparison to 7 events during the period of treatment with LDL apheresis. Before study, the rate of events was 6.3 events per 1,000 patient-months of follow-up. The rate during treatment dropped by 44% to 3.5 events per 1,000 patient-months of follow-up (P = .17). Although this difference was not statistically significant, the authors noted that the study was not powered to measure clinical end points.
Twenty of the 49 patients discontinued LDL apheresis during the follow-up period because of financial considerations, inconvenience, relocation and other reasons. Adverse events were observed in 4% of treatments, and hypotension was the most common event reported in 0.9% of treatments.
Results from this study must be interpreted with caution since the before/after study design has many limitations. It is uncertain whether there was a true difference in the incidence of cardiovascular events due to LDL apheresis or whether the observed difference was due to changes over calendar time (supportive therapy, living conditions, nutrition and lifestyles).
Mabuchi et al., 1998 (Hokuriku FH LDL Apheresis Study Group, Japan)
Mabuchi et al (95) examined the long-term safety, efficacy, and incidence of coronary events with LDL apheresis using the DSA system, in HTZ FH patients with CAD over a period of 6 years in a non-RCT. Low-density lipoprotein apheresis combined with drug therapy was compared with drug therapy alone.
Of the 130 patients included in the study, 43 HTZ FH patients whose LDL-C levels remained high despite diet and drug therapy were assigned to the LDL apheresis group and treated on average 2.1 times per month. The remaining 87 HTZ FH patients received intensive drug therapy. Although patients were not randomized, there were no significant differences in baseline characteristics such as gender, age and cardiovascular disease variables, with the exception of smoking status and baseline cholesterol levels. The LDL apheresis group had significantly fewer smokers than the drug treatment group (P = .024). Baseline LDL-C levels were significantly higher in the LDL apheresis group (P = .0002). Overall, LDL apheresis was well-tolerated by patients.
In the LDL apheresis group, the following acute relative decreases were observed: TC by 57%, LDL-C by 66%, TG by 74%, and HDL-C by 22%. The time-averaged concentrations of LDL-C were calculated in order to compare the 2 groups. The LDL-C values were reduced by 58% from baseline in the LDL apheresis group compared with 28% in the drug treatment group (P< .0001). Total cholesterol decreased by 53% with LDL apheresis compared with 25% with drug treatment (P< .0001). Triglyceride and HDL-C values were also significantly lower in the LDL apheresis group in comparison with the drug treatment group.
The incidence of major coronary events, which included coronary death, coronary revascularization or MI, was compared between the 2 treatment groups. The proportion of patients without any coronary events in the LDL apheresis group (90%) was significantly higher than the drug treatment group (64%) by 72% (P = .0088). The proportion of patients without CABG, PTCA, or MI, was higher in the LDL apheresis group than in the drug therapy group, however, these differences were not statistically significant. One death due to MI was observed in each group.
Figure 10: Kaplan-Meier Curves due to all Coronary Events in Heterozygous Familial Hypercholesterolemia Patients Treated With Low-Density Lipoprotein Apheresis and Patients Treated With Drug Therapy.
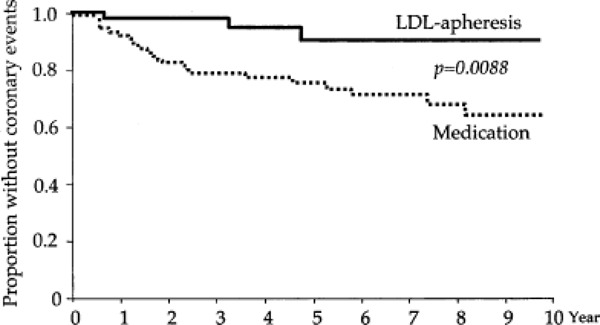
Reproduced from: American Journal of Cardiology, 82(12); Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FH-LDL-Apheresis Study Groug, 1489-95, Copyright 1998, with permission from Elsevier.
Figure 11: Kaplan-Meier Curves for Various End Points in Heterozygous Familial HypercholesterolemiaPatients Treated With Low-Density Lipoprotein Apheresis and Patients Treated with Drug Therapy.
Reproduced from: American Journal of Cardiology, 82(12); Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FH-LDL-Apheresis Study Groug, 1489-95, Copyright 1998, with permission from Elsevier.
There were several limitations of the study by Mabuchi et al. The study design was nonrandomized, and the LDL apheresis group had significantly fewer smokers and a higher baseline LDL-C than the drug therapy-only group. The difference in smoking status between groups introduces substantial bias and therefore weakens the quality of the study. There was also selection bias toward LDL apheresis for more severe cases of FH. Nevertheless, these patients would be expected to have a higher event rate and instead, a greater reduction in events was observed in the LDL apheresis group despite considerably higher pretreatment lipid levels.
The relatively small decrease in LDL-C observed in the drug treatment group (28%) reflected the fact that doses of the statins used in the trial are restricted in Japan. Thus, questions arise as to whether the same outcome would be achieved in countries where statin dosage in not restricted. (21)
GRADE Quality of the Evidence
The quality of the trials was examined according to the GRADE Working Group criteria. (1;2)
Quality refers to criteria such as the adequacy of allocation concealment, blinding, and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there is important, unexplained inconsistency in the results, our confidence in the estimate of effect for that outcome decreases.
Differences in the direction of effect, the size of the differences in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions were used in grading the quality of the evidence.
| High | Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Despite the observed heterogeneity in inclusion criteria, study populations, and methodology, there was a general consensus in findings that LDL apheresis with the HELP system has a beneficial impact on acute and chronic lipid parameters and coronary outcomes in FH patients (See Table 18).
Table 18: Grading System Applied to the Studies on HELP Low-Density Lipoprotein Apheresis in Familial Hypercholesterolemia Patients.
| Homozygous Familial Hypercholesterolemia – Lipid Outcomes | ||||||
|---|---|---|---|---|---|---|
| Number of Studies |
Study Design | Quality of Studies |
Consistency | Directness | Other Modifying Factors |
Overall Quality of Evidence |
| 1 | Case series=Low | Low | + | Yes | Sparse data | Very Low |
| 1 | Retrospective review=Low | |||||
| Heterozygous Familial Hypercholesterolemia Patients – Lipid Outcomes | ||||||
|
Number of Studies |
Study Design |
Quality of Studies |
Consistency | Directness |
Other Modifying Factors |
Overall Quality of Evidence |
| 7+FDA | Case series=Low | Low | + | Yes | Not applicable | Low |
| 1 | Retrospective review=Low | |||||
| Heterozygous Familial Hypercholesterolemia Patients - Coronary Artery Disease Outcomes | ||||||
|
Number of Studies |
Study Design |
Quality of Studies |
Consistency | Directness |
Other Modifying Factors |
Overall Quality of Evidence |
| 2+FDA | Case series=Low | Low | + | Yes | Not applicable | Low |
| 1 | Retrospective review=Low | |||||
Economic Analysis
Economic Literature Review: Summary
A literature review did not identify any economic analyses of LDL apheresis or PE for the treatment of FH.
Notes & Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are 10 or more hospital separations, or one-third or more of hospital separations in the ministry’s data warehouse are for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Where appropriate, costs are adjusted for hospital-specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are used. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum, representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals.
Nonhospital: These include physician services costs obtained from the Provider Services Branch of the Ontario Ministry of Health and Long-Term Care, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment and the Washington Panel of Cost-Effectiveness, respectively.
Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Ontario-Based Economic Analysis
Low-density lipoprotein apheresis is not listed in the Ontario Schedule of Benefits, and there have been no recent Ontario Hospital Insurance Plan requests for out-of-province or out-of-country funding for LDL apheresis in the treatment of FH.
The following table describes the prevalence of HMZ and HTZ FH in the context of Ontario.
Table 19: Prevalence of Homozygous and Heterozygous Familial Hypercholesterolemia*.
| Familial Hypercholesterolemia | Value | Reference |
|---|---|---|
| Homozygous | 0.000001 | Hopkins, P et al. Familial hypercholesterolemia - improving treatment and meeting guidelines. Int J of Cardiol 2003; 89: 12-23 |
| Heterozygous | 0.002 | Hopkins, P et al. Familial hypercholesterolemia - improving treatment and meeting guidelines. Int J of Cardiol 2003; 89: 12-23 |
| Refractory heterozygous patients eligible for treatment | 0.03 | Based on clinical expert opinion from Ontario |
| Estimate 1 - Heterozygous patients diagnosed | 0.15 | Familial hypercholesterolemia - World Health Organization 1999 |
| Estimate 2 - Heterozygous patients undiagnosed | 0.85 | Familial hypercholesterolemia - World Health Organization 1999 |
| ON estimate - Homozygous | 13 | Based on ON population and published estimates |
| ON estimate 1 - Heterozygous diagnosed cases | 115 | Based on ON population, published estimates and expert opinion |
| ON estimate 2 - Heterozygous all cases | 765 | Based on ON population and published estimates |
ON refers to Ontario.
Based on Ontario population statistics(96), a prevalence rate of 0.2% (5), a treatment rate of 3% in Ontario (as per expert opinion), and a diagnosis rate of 15% (14), the prevalence of diagnosed refractory HTZ FH was estimated to be 115 (estimate 1). The prevalence of undiagnosed refractory HTZ FH was estimated to be 650 based on an undiagnosis rate of 85% as per published literature. (14) Thus, the total HTZ FH prevalence was estimated to be 765 cases (estimate 2). The HMZ FH prevalence was estimated to be 13, using the Hardy-Weinberg principle of population genetics. Incidence rates were not identified in the literature and therefore were not incorporated into the analysis. Mortality rates were not included in the analysis. The annual impact was estimated from prevalence only.
Annual Costs of Plasma Exchange and the Low-Density Lipoprotein Apheresis HELP System
Plasma exchange and LDL apheresis can either be administered weekly or biweekly. The annual costs of both the PE and the HELP system reflects both intervals of treatment. Table 20 describes the costs used to calculate the total annual cost for PE. Annual costs are reported in 2007 Canadian dollars.
Table 20. Annual Costs Associated With Plasma Exchange*.
| Parameter | Cost ($ Cdn 2003) | Unit | Annual Cost/patient (2007 Cdn) † Weekly Treatment |
Annual Cost/patient (2007 Cdn) ‡ Biweekly Treatment |
Reference |
|---|---|---|---|---|---|
| Equipment** | $80,000 | acquisition cost | $8,000 | $8,000 | Contacted manufacturer – August 2007 |
| Fluid replacement | $320.00 | per procedure | $18,166 | $9,083 | Rock et al., 2003 |
| Additional supplies** | $200.00 | per procedure | $10,400 | $5,200 | Contacted manufacturer – August 2007 |
| Maintenance** | $4,000 | per year | $4,000 | $4,000 | Contacted manufacturer – August 2007 |
| Medical fees | $38.00 | per procedure | $1,976 | $988.00 | Ontario Schedule of Benefits April 2007 – Code G278 |
| Personnel fee (excluding physician) | $125.00 | per procedure | $7,096 | $3,548 | Rock et al., 2003 |
Training and indirect patient costs were not considered. Equipment and maintenance costs were fixed overtime and were reported on an annual basis irrespective of number of treatments per year.
Bank of Canada calculator was used to convert 2003 costs to 2007 costs - accessed in August 2007.
Costs were reported in $ Cdn 2007.
The manufacturer was contacted in order to ascertain cost information. The cost of the equipment is fixed over time and was reported to be $80,000 over a lifetime period of 10 years. Equipment depreciation was not considered in the calculation of annual costs. Maintenance costs were reported to be $4,000 per year, excluding maintenance during the first year, irrespective of the annual number of treatments. The cost of additional supplies was reported to be $200 per procedure, and the annual cost depended on the number of treatments per year. On average, 3.2L of plasma are exchanged per procedure, and two-thirds is replaced with albumin at a cost of $150/L, yielding a $320 cost per procedure for fluid replacement. (54) Physician medical fees were obtained from the Ontario Schedule of Benefits for 2007, and a cost of $38 per procedure was assumed for more than 5 treatments per year. (97) Nurse’s time was assumed to be $125 per procedure, based on available literature (54). All costs were converted to 2007 Canadian dollars.
Table 21 describes the values used to calculate the total annual cost for LDL apheresis. Annual costs are reported in 2007 Canadian dollars.
Table 21: Annual Costs Associated With the HELP System*.
| Parameter | Unit Cost (2004 CAD) | Unit | Annual Cost/patient (2007 Cdn)* weekly treatment |
Annual Cost/patient (2007 Cdn)* biweekly treatment |
Reference |
|---|---|---|---|---|---|
| Equipment | $39,237 | acquisition cost | $3,924 | $3,924 | AHFMR HTA 2004 |
| Dialysis equipment | $7,847 | acquisition cost | $785 | $785 | AHFMR HTA 2004 |
| Equipment disposables | $1,177 | per procedure | $65,170 | $32,585 | AHFMR HTA 2004 |
| Additional supplies | $98.10 | per procedure | $5,431 | $2,715 | AHFMR HTA 2004 |
| Maintenance | $3,924 | per year | $3,924 | $3,924 | AHFMR HTA 2004 |
| Medical fees | $38.00 | per procedure | $1,976 | $988.00 | Ontario Schedule of Benefits April 2007 -Code G278 |
| Personnel fee (excluding physician)** | $125.00 | per procedure | $7,096 | $3,548 | Rock et al., 2003 |
Training and indirect patient costs were not considered. The manufacturer of the HELP system was contacted for further information but did not respond. Equipment and maintenance costs were fixed overtime and were reported on an annual basis irrespective of number of treatments per year.
Bank of Canada calculator was used to convert 2004 costs to 2007 costs - accessed in August 2007.
Reported in 2003 dollars Cdn.
The cost of the equipment, obtained from literature, was fixed over time and reported to be $39,237 for the HELP system, and $7,847 for the dialysis machine, over a lifetime period of 10 years. (98) Equipment depreciation was not considered in the calculation of annual costs. Maintenance costs were reported to be $3,924 per year, excluding maintenance during the first year, irrespective of annual number of treatments. (98) Additional supplies and disposable equipment costs were reported to be $98.10 and $1177 respectively, per procedure, and the annual costs depended on the number of treatments per year (98). Medical fees were obtained from the Ontario Schedule of Benefits for 2007, and a cost of $38 per procedure was assumed for greater than 5 treatments per year. (97) Nurse’s time was assumed to be $125 per procedure based on literature. (54) All costs were converted to 2007 Canadian dollars.
The annual budget impact based on the HMZ and HTZ FH prevalence reported above was calculated for both weekly and biweekly treatments. Tables 22 and 23 describe the annual budget impact for the PE and HELP systems, respectively. In calculating the annual budget impact, incidence was not included as it was not reported in the literature. An annual impact was reported with no assumptions made in market share changes with competing technologies, and mortality was not factored into the calculation. It was assumed that all HMZ patients met the 30 kg weight limit set by the manufacturer.
Table 22: Annual Budget Impact for Plasma Exchange in Homozygous and Heterozygous Familial Hypercholesterolemia Patients.
| Familial Hypercholesterolemia | Number of Patients | Annual Cost – Year 1 $ Cdn 2007 |
Subsequent Years |
|---|---|---|---|
| Homozygous | |||
| weekly treatments | 13 | $488,025 | $492,025 |
| biweekly treatments | 13 | $248,013 | $252,013 |
| Heterozygous | |||
| Estimate 1 - weekly treatments | 115 | $4,328,227 | $4,332,227 |
| Estimate 1 - biweekly treatments | 115 | $2,168,113 | $2,172,113 |
| Estimate 2 - weekly treatments | 765 | $24,754,556 | $24,758,556 |
| Estimate 2 - biweekly treatments | 765 | $12,381,274 | $12,385,274 |
Table 23: Annual Budget Impact for the HELP System in Homozygous and Heterozygous Familial Hypercholesterolemia Patients.
| Familial Hypercholesterolemia | Number of Patients | Annual Cost – Year 1 $ Cdn 2007 |
Subsequent Years |
|---|---|---|---|
| Homozygous | |||
| weekly treatments | 13 | $1,021,156 | $1,025,338 |
| biweekly treatments | 13 | $513,083 | $517,265 |
| Heterozygous | |||
| Estimate 1 - weekly treatments | 115 | $9,151,196 | $9,156,209 |
| Estimate 1 - biweekly treatments | 115 | $4,578,545 | $4,583,557 |
| Estimate 2 - weekly treatments | 765 | $60,975,898 | $60,982,579 |
| Estimate 2 - biweekly treatments | 765 | $30,491,553 | $30,498,234 |
A major limitation of this analysis was the lack of PE data for HTZ FH patients in the literature that reported relevant chronic risk reductions, such as LDL reduction, which can be projected over time to estimate health outcomes such as CAD events or CAD deaths. Therefore downstream event avoidance and/or cost saving was difficult to calculate by comparing interventions (i.e., HELP versus PE, or HELP versus no intervention). Furthermore, the cost of genetic testing (cost range of $500 - $800 as per expert opinion) was not included in the analysis and may be a requirement for these procedures, adding to the total cost impact. Some assumptions, however, were made to calculate a cost per CAD death avoided, described in the next section.
Cost per Coronary Artery Disease Death Avoided
The budget impact analysis total costs were used to calculate the cost per CAD death avoided over a 10-year period, comparing HELP apheresis versus PE and HELP apheresis versus no intervention in HTZ FH patients. As stated elsewhere, HMZ patients are not treated effectively with diet and drug therapy; hence, LDL apheresis is a last therapeutic alternative for these patients. (5) For this reason, this analysis was limited to refractory HTZ FH patients.
Coronary artery disease deaths in the HTZ FH population for both the HELP versus PE, and HELP versus no intervention groups, were estimated using the Framingham risk equation. (99) The risk equation requires an estimate of the TC: HDL ratio. For the HELP apheresis group, a weighted average of both the TC:HDL ratio and the LDL reduction (based on follow-up duration and sample size) was obtained from 3 cases studies investigating HELP LDL apheresis in HTZ FH patients. (67;87;89) Table 24 describes the 3 case studies.
Table 24. HELP Apheresis Case Studies (Heterozygous Familial Hypercholesterolemia)*.
| Study | N | Follow-up (months) |
TC:HDL Ratio |
LDL Reduction (%) |
|---|---|---|---|---|
| Schuff-Werner et al., 1994 (67) | 51 | 24 | 5 | 28.3% |
| Moriarty et al., 2001 (87) | 4 | 6 | 6.6 | 9% |
| Hoffman et al., 2003 (89) | 8 | 29 | 5.3 | 40% |
| Weighted Averages: | 5.1 | 29.8% |
HDL refers to high-density lipoprotein - cholesterol; HTZ FH, heterozygous familial hypercholesterolemia; LDL, low-density lipoprotein - cholesterol; N, sample size; TC, total cholesterol.
There were no studies identified in the literature investigating HTZ FH patients using PE. It was assumed that the reduction in the LDL level after PE intervention would be comparable to that observed with HELP apheresis (30%). Based on this assumption, a weighted TC:HDL ratio for PE was calculated using the baseline levels from the 3 case studies. The TC:HDL ratio for the no intervention group was calculated from the baseline levels of the 3 case studies, and was assumed to remain constant over time (a conservative estimate, since this refractory patient population has already exhausted statin therapy and therefore their LDL levels would rise over time).
A typical patient profile (i.e., age, gender, blood pressure, etc.) for an HTZ FH patient was obtained from the literature (100) and used in the Framingham risk equation. The same profile was used for all interventions for consistency. The following table describes the estimated CAD death risk over 10 years for each intervention.
Table 25: Coronary Artery Disease Death Risk for HELP, Plasma Exchange and No Intervention Groups (Heterozygous Familial Hypercholesterolemia).
| Intervention | TC:HDL Ratio | CAD Death Risk (10 years) |
|---|---|---|
| HELP | 5.07 | 0.0113775 |
| PE | 7.18 | 0.0225847 |
| No intervention | 8.87 | 0.0326289 |
CAD refers to coronary artery disease; HDL, high-density lipoprotein - cholesterol; HTZ FH, heterozygous familial hypercholesterolemia; LDL, low-density lipoprotein - cholesterol; N, sample size; TC, total cholesterol.
The cost per CAD death avoided was then calculated by comparing HELP apheresis to PE, and HELP apheresis to no intervention. These costs are described in the following table.
Table 26. Cost per Coronary Artery Disease Death Avoided for HELP, Plasma Exchange and No Intervention Groups (Heterozygous Familial hypercholesterolemia)*.
| No Intervention | ||||
| Diagnosed Patients | 10 year Cost ($ Cdn 2007) | Number of Deaths | ||
| weekly treatment | $0 | 33 | ||
| biweekly treatment | $0 | 33 | ||
| PE | ||||
| Diagnosed Patients | 10 year Cost ($ Cdn 2007) | Number of Deaths | ||
| weekly treatment | $377,391,700 | 23 | ||
| biweekly treatment | $189,201,100 | 23 | ||
| HELP | ||||
| Diagnosed Patients | 10 year Cost ($ Cdn 2007) | Number of Deaths | ||
| weekly treatment | $797,651,430 | 11 | ||
| biweekly treatment | $399,278,930 | 11 | ||
| Cost per CAD Death Avoided | ||||
| Cost HELP – | Death HELP – | Cost per | ||
| Diagnosed Patients | Cost PE | Death PE | CAD Death Avoided | |
| weekly treatment | $420,259,730 | -11 | $(37,499,136) | |
| biweekly treatment | $210,077,830 | -11 | $(18,744,925) | |
| Cost HELP - | Death HELP - | Cost per | ||
| Diagnosed Patients | Cost NO Intervention | Death NO Intervention | CAD Death Avoided | |
| weekly treatment | $797,651,430 | -21 | $(37,534,137) | |
| biweekly treatment | $399,278,930 | -21 | $(18,788,395) |
CAD refers to coronary artery disease; HELP, heparin-induced extracorporeal precipitation; PE, plasma exchange.
The cost per CAD death avoided over a 10-year period was $37.5 million and $18.7 million for weekly and biweekly treatments, respectively, when comparing HELP apheresis to PE, as well as when comparing HELP apheresis to no intervention in the diagnosed HTZ FH population. Although HELP apheresis costs twice as much as PE, it helped to avoid 12 deaths compared with PE, and 22 deaths compared with no intervention, over a period of 10 years.
Assumptions
There were several assumptions made to calculate the cost per CAD death avoided:
There were no head-to-head trials comparing HELP to PE, or HELP to no intervention available in the literature; therefore, 3 HELP case studies were used to estimate weighted averages for LDL reduction and the TC:HDL ratio for HELP.
There was no PE data found in the literature; therefore, it was assumed that PE resulted in the same LDL reduction as HELP, and baseline lipid levels from the HELP case studies were used to estimate the chronic TC:HDL ratio for PE.
Baseline lipid data from the HELP case studies was used to estimate the chronic TC:HDL ratio for the no intervention group.
The Framingham risk equation was used to estimate risks for a patient population that has very high baseline LDL levels.
A patient profile from a different study in a different country was used since characteristics required for the risk equation were not reported in the HELP case studies
As a result of these assumptions and due to the limited data available in the literature, uncertainty regarding the estimates of the economic analysis becomes an issue. If and when new evidence is available, these assumptions may change and may better predict health outcomes over time, allowing for a more accurate analysis.
Relevant Guidelines
Several guidelines exist regarding the management of FH patients and include recommendations on the use of LDL apheresis.
Recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention, 1998
Patients with FH will still benefit from lipid lowering on maximum drug therapy even though they have not reached treatment goals. (42)
A combination of a resin and statin or even a triple-drug therapy with a statin, resin and nicotinic acid may be needed to produce satisfactory reductions in LDL-C. (42)
“Rare patients with severe hyperlipidemias, especially homozygous familial hypercholesterolaemia, require specialist evaluation of the need for LDL-apheresis.”(42)
Third Report of the National Cholesterol Education Program (NCEP) Expert Pane on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report, 2002
Therapeutic considerations for HTZ FH patients are as follows: (41)
Initiate LDL-C lowering drugs in young adulthood.
Therapeutic lifestyle change is indicated for all patients.
Statins are the first line of therapy (dietary therapy simultaneously initiated).
Bile acid sequestrants (BAS) can be prescribed in combination with statins if necessary.
If needed, triple drug therapy consisting of a statin, a BAS and nicotinic acid may be considered.
Therapeutic considerations for HMZ FH patients are as follows: (41)
Dietary therapy is not effective.
BAS are not effective.
Nicotinic acid may be mildly effective.
In some patients, statins may be mildly effective.
Ileal exclusion procedure is not effective.
Although liver transplantation is effective, it is not practical.
Low-density lipoprotein apheresis is the currently employed therapy, and concomitant statin therapy may slow down the rebounding hypercholesterolemia in some patients.
Australasian Society of Cardiac and Thoracic Surgeons and the Cardiac Society of Australia and New Zealand, 2006
Therapeutic considerations in the adult population are: (17)
Exercise stress testing and other forms of noninvasive testing are recommended to assist in the early identification of clinically relevant atherosclerosis.
Diet, exercise, and smoking cessation are compulsory.
General measures to protect against vascular events, including the use of aspirin, should be considered.
Cholesterol-lowering treatment such as statins provides effective control of high cholesterol levels.
BAS, cholesterol absorption inhibitors (such as plant sterols), or the recently introduced drug, ezetimibe, may add to the effect of statins.
Many patients with FH can achieve target cholesterol levels.
Therapeutic considerations in the pediatric population are: (17)
Conservative measures relating to diet and avoidance of smoking are safe and effective.
Statin therapy should only be considered in children from the most severely affected families.
American Heart Association Expert Panel on Population and Prevention Science, 2006
Treatment recommendations for HMZ FH children are as follows: (3)
At diagnosis, perform a complete cardiovascular assessment.
Initiate treatment as soon as possible.
The cornerstone of therapy for the majority of patients is weekly or biweekly plasmapheresis, preferably LDL apheresis.
High-dose statin therapy in combination with a cholesterol absorption inhibitor is recommended.
Low-dose anticoagulation may also be needed.
Perform ongoing surveillance for cardiovascular disease.
Treatment recommendations for HTZ FH children are as follows: (3)
In general, routine cardiovascular assessment is not indicated.
Referral to a lipid specialist is recommended.
Focus therapy on the reduction of LDL-C levels through lifestyle interventions.
Consider drug therapy with statins after the age of 10 years in males and after the onset of menses in females.
Bile acid sequestrants (BAS) and cholesterol absorption inhibitors may be used in combination with statins.
National Institute for Health and Clinical Excellence (NICE)
Guidelines for the identification and management of FH patients are in progress and are expected to be published in August 2008. (101)
Ontario Health System Impact Analysis
Status in Other Jurisdictions
Table 27: Survey of Provinces/Territories.
| Province/Territory | Funding Status |
|---|---|
| Newfoundland | No reply to date. |
| New Brunswick | There is no fee code in the New Brunswick Schedule. There has never been a request for out-of-province/country coverage of LDL Column Apheresis. |
| Nova Scotia | Not available |
| Prince Edward Island | Not available |
| Quebec | LDL Apheresis with the H.E.L.P. System is available at the Clinique des maladies lipidiques of the Centre Hospitalier Universitaire de Laval/ Centre Hospitalier Universitaire de Québec (CHUL/CHUQ). The program began in 2001 and over 1000 procedures have been performed to date. The régie de l’assurance maladie du Québec covers the physician fees and it is funded as a special program. Eligible patients must undergo a special application procedure to be admitted to the program. Currently, there are 13 HMZ FH patients ranging in age from 13 to 50 years undergoing treatment every 2nd week. No major adverse events have occurred, although there have been some problems relating to venous access. Due to resource constraints, refractory HTZ patients are not included. |
| Manitoba | No experience in Manitoba with LDL apheresis. |
| Saskatchewan | No reply to date. |
| Alberta | Capital Health, Edmonton, provides LDL apheresis as a clinical pilot project at the University of Alberta Hospital. It has been in operation since January 2006. Three HMZ patients are currently being treated (1 patient treated weekly and 2 patients treated every 2nd week). The program is being extended to include refractory HTZ patients based on strict criteria (compatible with the Health Canada criteria). |
| British Columbia | There have been no requests or approvals for out-of-province LDL column apheresis. There is no fee item in British Columbia’s payment schedule for LDL column apheresis. |
| Yukon | This service is not available in Yukon and no requests have been received for out of-province/country treatment using this technique. |
| Northwest Territories | No reply to date. |
| Nunavut | No reply to date. |
Low-density lipoprotein apheresis with the HELP system is currently being funded in the provinces of Quebec and Alberta and is available in one centre in each province. The program in Quebec has been in operation since 2001 and is only approved for the treatment of HMZ FH patients. The Alberta program is relatively new and is currently treating HMZ FH patients. They are expanding the program to phase in the treatment for refractory HTZ patients. Additional details are found in the above table.
Table 28: Survey of Insurers in United States*.
| Insurer | Funding Status |
|---|---|
| Aetna | Medically necessary for:
|
| Cigna | Covers apheresis for severe, refractory FH as medically necessary for patients who have failed a 6-month treatment plan of diet and maximum tolerated drug therapy (unless contraindicated or not tolerated) when ANY of the following conditions are met:
|
| Excellus | Medically appropriate for severely hypercholesteremic patients:
|
| Regence | Medically necessary for:
|
| Wellmark, Blue Cross Blue Shield | Medically necessary for:
|
CAD refers to coronary artery disease; HMZ FH, homozygous familial hypercholesterolemia; HTZ FH, heterozygous familial hypercholesterolemia; LDL, low-density lipoprotein-cholesterol.
Other Jurisdictions: Germany
Health Canada indications currently limit the use of HELP to HMZ and HTZ FH patients. In the future, Health Canada may want to consider licensing HELP for other high-risk patient groups. One such group is patients with CAD and elevated LDL-C levels despite drug therapy. (21) In Germany, the indications for HELP are much broader in comparison to FDA and Health Canada indications. Besides HMZ and HTZ FH patients, German indications extend the use of HELP to the following indications: patients with CAD and LDL-C greater than 135 mg/dL despite maximal statin treatment (or drug intolerance), patients with transplant-associated CAD and LDL-C levels greater than 135 mg/dL despite statin treatment (or drug intolerance), patients with inoperable ischemic cardiomyopathy, patients with generalized severe atherosclerosis, and patients with CAD and additional serious risk factors (e.g., diabetes, plasma Lp(a) levels >30 mg/dL, chronically high plasma fibrinogen levels >4 g/l). (37) According to Moriarty et al. (29), 6000 FH patients would qualify for LDL apheresis in North America based on FDA indications. If indications were extended to include some of the patient groups outlined in the German recommendations, this estimate would be greatly inflated.
Considerations
Low-density lipoprotein apheresis is a lifelong treatment and requires considerable commitment on the part of the patient, and the patient’s family and physician. At a minimum, patients must undergo HELP therapy twice a month, which involves travel to the hospital, time for the procedure and interruption of normal activities. Thus, not all patients are ideal candidates for this procedure and the decision to initiate therapy must be carefully considered by the patient and their physician.
The management of FH continues to evolve and achievements have been made in the treatment of hyperlipidemia in recent years. With the advent of new, more powerful cholesterol-lowering drugs, some HTZ patients may be able to sufficiently control their hypercholesterolemia and thus the number of FH patients meeting Health Canada’s criteria for HELP will likely decrease. Nevertheless, according to clinical experts, HMZ patients will likely always require this therapeutic option. Recent advances in gene therapy are promising, but additional development is needed in this area before it becomes a viable option for FH patients.
Target Population
Given the substantial costs associated with LDL apheresis, treatment has been limited to HMZ FH patients.
However, LDL apheresis could be applied to a much larger population, which would include HTZ FH patients who are refractory to diet and drug therapy. Blaha et al. (49) raise some additional issues on treating HTZ FH patients, and comment that HTZ patients are generally recruited in a more advanced state, demonstrate a longer natural survival than HMZ FH patients, and are older. Another issue in treating HTZ patients is whether LDL apheresis should be available to all HTZ patients or to just HTZ patients with established CAD. In Germany, LDL apheresis is limited to HTZ patients with established CAD. (40)
Another consideration is that the diagnosis of HTZ FH patients is problematic. Some non-FH patients may be incorrectly diagnosed as having FH due to the low specificity of the diagnostic tools and the low detection rates of the genetic test. However, according to expert opinion, this would rarely occur since the levels of LDL-C in a HTZ patient (>300mg/dL, 7.8mmol/L) greatly exceed the levels of LDL-C in a person with non-FH hypercholesterolemia (which can reach ~200mg/dL, 5.2mmol/L).
Yokoyama et al. (61) noted that several companies have developed LDL apheresis technologies with the expectation that this would become a profitable sector, similar to the statin market. However, their hopes have not been fulfilled and they are forced to stay in the market for ethical reasons since some patients are entirely dependent on LDL apheresis for their survival.
Conclusions
For HMZ FH patients, the benefits of LDL apheresis clearly outweigh the risks and burdens. According to GRADE, the recommendation would be graded as strong with low- to very low-quality evidence (See Table 29).
Table 29: GRADE Recommendation – Homozygous Patients.
| Benefits | Risks | Burdens |
|---|---|---|
| Overall clinical benefit | ||
| Consistency with social/ethical values | ||
| Affordable | ||
| Health system feasibility |
In both HMZ and HTZ FH patients, there is evidence of overall clinical benefit of LDL apheresis from case series studies. In addition, LDL apheresis has several advantages over the current treatment of PE. It permits less exposure to blood products, decreased risk of adverse events, conservation of nonatherogenic and athero-protective components (such as HDL-C), and lowering of other atherogenic components, such as fibrinogen. Further, experiences with HELP in other jurisdictions, including Alberta and Quebec, have demonstrated its clinical utility and safety.
In contrast to HMZ FH patients, there remains uncertainty in the social/ethical acceptance of this technology for the treatment of refractory HTZ FH patients. In addition to the substantial costs, it is unknown whether the current health care system could cope with the additional demand. There is uncertainty in the estimates of benefits, risks and burdens. According to GRADE, the recommendation would be graded as weak with low- to very low-quality evidence (See Table 30).
Table 30: GRADE Recommendation – Heterozygous Patients.
| Benefits | Risks | Burdens |
|---|---|---|
| Overall clinical benefit | Less affordable | |
| Questionable health system feasibility | ||
| Unknown if consistent with social/ethical values |
GRADE of recommendation: Strong recommendation, low-quality or very low-quality evidence
Benefits clearly outweigh risk and burdens
Case series studies
Strong, but may change when higher-quality evidence becomes available
GRADE of recommendation: Weak recommendation, low-quality or very low-quality evidence
Uncertainty in the estimates of benefits, risks and burden; benefits, risk and burden may be closely balanced
Case series studies
Very weak; other alternatives may be equally reasonable
Glossary
- Agatston score
a method used to quantify the amount of calcium in the coronary vessel wall
- Atherosclerosis
A condition in which fatty material collects along the walls of arteries. This fatty material thickens, hardens, and may eventually block the arteries.
- Corneal arcus
a white or gray opaque ring in the corneal margin resulting from cholesterol deposits
- Coronary angiography
a procedure that uses a special dye (contrast material) and x-rays to see how blood flows through your heart
- Coronary artery disease
results from the build-up of fatty material and plaque, a condition called atherosclerosis. As the coronary arteries narrow, the flow of blood to the heart can slow or stop, causing chest pain (stable angina), shortness of breath, heart attack, or other symptoms.
- Founder gene effect
populations that have arisen from a small number of settlers and possess a few mutations which occur at high frequency
- Hardy-Weinberg Principle
is a relationship between the frequencies of alleles and the genotype of a population
- LDL Apheresis
a procedure in which blood is withdrawn from a donor, LDL cholesterol is separated and retained, and the remainder is retransfused into the donor
- Xanthelasma
cholesterol deposits in the eyelids
- Xanthoma
lesions caused by cholesterol rich lipoprotein deposits
Appendix 1 – Search Strategies
Search date: May 30, 2007
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, Cochrane Library, INAHTA/CRD
Database: Ovid MEDLINE(R) <1996 to May Week 3 2007>
Search Strategy:
--------------------------------------------------------------------------------
exp Lipoproteins, LDL/ (16493)
cholesterol/ or cholesterol, ldl/ (30387)
((cholesterol$ or lipoprotein$) adj2 (LDL or low density)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (29694)
or/1-3 (47783)
exp Blood Component Removal/ or exp Hemoperfusion/ (6373)
exp Extracorporeal Circulation/ (16958)
exp Plasmapheresis/ (1966)
exp Precipitation/ (3291)
exp Plasma Exchange/ or exp Adsorption/ (13342)
(heparin adj (induced or mediated)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1737)
or/5-10 (39694)
4 and 11 (666)
((Plasma or plasmat) adj (Futura or secura)).mp. (1)
((lipoprotein$ or cholesterol$ or LDL) adj2 (Aph?eresis or precipitation or heparin$ or adsorption or plasma exchange or plasmapheresis)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (658)
or/12-14 (890)
exp Lipid Metabolism, Inborn Errors/ or exp Hyperlipidemias/ (24894)
exp Atherosclerosis/ or exp Arteriosclerosis/ or exp Coronary Disease/ or arteriosclerosis.mp. or atherosclerosis.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (98578)
(Hypertriglycerid$ or hyperlipid$ or Hypercholesterol$ or hyperlipoprotein$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (27967)
or/16-18 (122836)
15 and 19 (537)
(“H.E.L.P” adj2 (Aph?eresis or precipitation or heparin$ or adsorption or plasma exchange or plasmapheresis or lipoprotein$ or cholesterol$ or LDL or braun)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (115)
20 or 21 (588)
limit 22 to (humans and english language and yr=“1998 - 2007”) (392)
(meta analy$ or metaanaly$ or random$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or medline or embase or data synthesis or data extraction or cochrane).ab. (343431)
23 and 24 (35)
23 (392)
limit 26 to (case reports or comment or editorial or letter or “review”) (150)
26 not 27 (242)
25 or 28 (256)
Database: EMBASE <1980 to 2007 Week 21>
Search Strategy:
--------------------------------------------------------------------------------
exp acetyl low density lipoprotein/ or exp low density lipoprotein/ or exp very low density lipoprotein/ (24921)
exp cholesterol/ or exp low density lipoprotein cholesterol/ or exp very low density lipoprotein cholesterol/ (83073)
((cholesterol$ or lipoprotein$) adj2 (LDL or low density)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (59192)
or/1-3 (109818)
exp apheresis/ or exp plasmapheresis/ (16748)
exp HEMOPERFUSION/ (1244)
exp extracorporeal circulation/ (26738)
exp precipitation/ (47974)
plasma exchange.mp. (3395)
(heparin adj (induced or mediated)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3052)
exp adsorption/ (25121)
or/5-11 (118665)
4 and 12 (1799)
((Plasma or plasmat) adj (Futura or secura)).mp. (1)
((lipoprotein$ or cholesterol$ or LDL) adj2 (Aph?eresis or precipitation or heparin$ or adsorption or plasma exchange or plasmapheresis)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2904)
or/13-15 (3560)
exp Familial Hypercholesterolemia/ (3044)
exp hyperlipidemia/ or exp hypercholesterolemia/ (46523)
exp arteriosclerosis/ or exp atherosclerosis/ or exp Coronary artery disease/ or arteriosclerosis.mp. or atherosclerosis.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (144495)
(Hypertriglycerid$ or hyperlipid$ or Hypercholesterol$ or hyperlipoprotein$).mp. (55865)
or/17-20 (184028)
16 and 21 (1639)
(“H.E.L.P” adj2 (Aph?eresis or precipitation or heparin$ or adsorption or plasma exchange or plasmapheresis or lipoprotein$ or cholesterol$ or LDL or braun)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (186)
22 or 23 (1717)
limit 24 to (human and english language and yr=“1998 - 2007”) (696)
Meta-Analysis/ or (meta analy$ or metaanaly$ or random$).mp. or (systematic$ adj2 review$).mp. or (published studies or medline or embase or data synthesis or data extraction or pooled analysis or cochrane).ab. (421414)
25 and 26 (60)
25 (696)
limit 28 to (editorial or letter or note or “review”) (186)
Case Report/ (938168)
28 not (29 or 30) (403)
27 or 31 (429)
Appendix 2 – Summary of AHFMR Studies
Summary of Controlled Studies Included in the AHFMR HTA, 2004*.
| Study | Sample | Device | Follow-up | Treatment Interval |
Results (Mean Reduction in LDL Cholesterol) |
|---|---|---|---|---|---|
| Matsuzaki et al., 2002 | N=18 | DSC Liposorber LA-15 Kaneka |
1 year | biweekly | C: -4% E: -34% |
| C=7 HTZ E=11 HTZ |
Japan | Chronic | |||
| Nishimura et al., 1999 | N=36 | DSC Liposorber LA-15 Kaneka |
2.3 years | 17 days | C: -34% E: -43% |
| C=11 HTZ E=25 HTZ |
Japan | Chronic? | |||
| Mabuchi et al., 1998 | N=130 | DSC Liposorber LA-15 Kaneka |
6 years | biweekly | C: -28% E: -58% |
| C=87 HTZ E=43 HTZ |
Japan | Acute | |||
| Koga et al., 1999 | N=21 C=10 HTZ E=11 (2 HMZ, 9 HTZ) |
DSA Kaneka Co. | 7.8 years | biweekly (HMZ) biweekly/monthly (HTZ) |
C: -23% E: HMZ -61% HTZ -57% Acute |
| Gordon et al., 1998 | N=64 C=9 HTZ E=55 (10 HMZ, 45 HTZ) |
DSC Liposorber LA-15 Kaneka Pharma America, New York |
5 years | HMZ- 11 days HTZ – 14 days |
C: n/a E: HMZ -81% HTZ -76% Acute |
| Thompson et al., 1995 | N=130 | DSC Liposorber LA-15 Kaneka |
2.1 years | biweekly | C: -44% E: -66% |
| C=19 HTZ E=20 HTZ |
Japan | Acute |
C refers to control group; E, experimental group; HELP, heparin-induced extracorporeal LDL precipitation; HMZ, homozygous; HTZ, heterozygous; LDL, low-density lipoprotein - cholesterol; N, number of patients.
Summary of Comparative Studies Included in the AHFMR HTA, 2004*.
| Study | Sample | Device | Follow-up | Treatment Interval | Results (mean reduction in LDL cholesterol) |
|---|---|---|---|---|---|
| Richter et al., 1999 | N=34 HTZ NA=18 NB=8 NC=8 |
A- Immunoadsorption B- Liposorber C- HELP |
4.6 years | majority weekly | A: 62% B: 65% C: 59% Acute |
| Julius et al., 2000 | Part 1 N=5 HTZ |
A - HELP B – Lipidfltration–Cascadeflo AC-1770 |
1 month | weekly | A: 54% B: 56% Acute |
| Part 2 N=6 HTZ |
A - HELP B – Lipidfltration – Lipidfilter EC-50 |
1 month | weekly | A: 61% B: 61% Acute |
C refers to control group; E, experimental group; HELP, heparin-induced extracorporeal LDL precipitation; HMZ, homozygous; HTZ, heterozygous; LDL, low-density lipoprotein - cholesterol, N, number of patients.
Appendix 3 Study Characteristics
Table 1a: Additional Information on Studies Included in 1997 FDA Report*.
| Study | Sample size and Population | Patient Characteristics | Follow-up and HELP Treatment Interval | Outcomes | Results | Comments | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) | Baseline LDL (mg/dL) | Acute | Chronic | |||||
| Lane et al., 1993 (86) § U.S. Multicentre Clinical Trial |
N=33 LDL ≥ 160mg/dL despite diet and drug therapy HMZ patients had to be ≥ 7 years |
47 | 70 | 289 | 6 months weekly interval |
lipid parameters | lipid parameters | 686 weekly treatments 33 patients were given at least 1 weekly treatment 24 patients completed all 25 weekly treatments Mean treatment volume was 2.657l and average time was 1.7hrs Acute: Significant reduction in TC of 47.2%, LDL 54.2%, TG 49.4%, HDL 14.5%, fibrinogen 58% (all P<0.0001) Chronic: Calculated chronic changes by comparing levels of baseline and mean of last 5 treatments 79% achieved a reduction of >=30% in LDL (n=24 that completed 25 treatments) 39% reduction in LDL Other: 3 of 6 patients with angina before treatment no longer had pain after 25 weeks of therapy No clinical cardiac events occurred Most common complications were with vascular access and hypotension 44 total number of complications (6.4%) and of these 1.7% device related, 2.8% user-related and 1.9% patient-related 72% believed their health to be “much better or somewhat better” after 6 months of therapy |
Inclusion criterion for minimum LDL levels (160 mg/dL) was higher than the threshold stipulated in the Health Canada indications (200 mg/dL) No details on the HMZ or HTZ status of FH patients |
| Lane et al., 1995a (84) § U.S. Multicentre Clinical Trial |
N=23 LDL ≥ 160mg/dL despite diet and drug therapy Completed 6 months of weekly therapy 4 HMZ & 19 severe HTZ patients |
45.5 | 70 | 237 | 6 months biweekly interval |
lipid parameters |
lipid parameters |
Mean duration was 1.7hr Mean treatment volume 2.8l Total number of treatments was 276 Acute: Mean reduction in LDL 55.2%, TC 49.1%, HDL 15% Chronic: LDL and TC levels reduced by 30% or more in 98% of biweekly treatments Long-term LDL reduced by 33.2% Compared to weekly treatment, pretreatment levels of LDL were higher with biweekly treatment However, pretreatment levels were lower than mean levels at study entry. After 6 months, 7 patients were switched back to weekly therapy because their LDL-C levels continued to increase (includes all 4 HMZ) Other: 72% of pts believed their health to be much better or somewhat better Adverse events 5.1%, most common were problems with venous access and hypotension |
Inclusion criterion for minimum LDL levels (160 mg/dL) was higher than the threshold stipulated in the Health Canada indications (200mg/dL) Results not presented separately for HMZ and HTZ patients |
| Lane et al., 1995b (85) § U.S. Multicentre Clinical Trial |
N=14 LDL ≥ 160mg/dL despite diet and drug therapy Completed 6 months of weekly therapy 10 patients with primary hypercholesterolemia and 4 with combined hyperlipidemia |
44 | 71 | 253 | 2 sessions biweekly interval |
lipid kinetics (change in lipid parameters in the 14 day interval between treatments) | Total of 28 sessions (14 patients × 2 sessions each) Acute: TC 48%, TG 55%, LDL 53%, HDL 12% Shows the gradual increase over the 14 days between treatments HDL concentration was normal by 2 days and remained there until next treatment. TC and LDL levels increased gradually over the 14 day period, although the increase was less rapid during the second week than the first week. Conclusion - if maximum therapeutic benefits of LDL-A are to be achieved, concomitant lipid-lowering drug therapy must be included in the treatment regimen. |
Inclusion criterion for minimum LDL levels (160 mg/dL) was higher than the threshold stipulated in the Health Canada indications (200mg/dL) No details on the HMZ or HTZ status of FH patients Included 4 patients without FH |
|
| Schuff-Werner et al., 1994 (67) HELP LDL Apheresis Multicentre Study |
N=51 Severe CAD and type II hyperlipoproteinemia LDL ≥ 200mg/dL despite diet and drug therapy |
44.4 | 67 | 310 | 24 months weekly interval |
lipid parameters angiographic outcomes |
39 patients evaluated after 2 years of regular apheresis Average treatment interval was 7.85 days Each patient treated on average 93 times Time of treatment excluding preparation time was 115 mins Average plasma volume treated per procedure was 2.83l Chronic: Significant long-term decrease of total cholesterol (22.2%, P<.001)), LDL (28.3%, P<.001) and TG (22.5%, P<.05). Significant increase in HDL (26.8%, P<.01). Angiographic Outcomes: Analysis of 187 segments in 33 patients revealed: 16 patients - regression (48%) 9 patients - progression (27%) 8 patients - no change in status (24%) Assuming independence between segments, mean degree of stenosis of all segments decreased from 32.5% to 30.6% (P=0.02). Mean degree of stenosis per patient decreased from 34.8% to 33.3% (P=0.21). Rate of regression was 1.8 times the rate of progression for the 2 year period. Stenoses ≤30% did not change and stenoses >30% showed a mean reduction of stenosis by 4.3% after 2 yrs (P<0.001). Using a difference in % stenosis of ≥8% as a threshold for the evaluation of relevant changes, 26.7% regressed, 57.8% did not change significantly and progression occurred in 15.5% of segments. Approximate 2-fold greater regression than progression. Other: Improvement in angina symptoms. At onset, 82% reported symptoms and at end 62% symptomatic. Adverse effects were reported in only 2.9% of treatments and the reactions were generally of minor clinical relevance. See table 3a for details 3 patients suffered non treatment related sudden cardiac deaths (2 in year 1 and 1 in year 2) Conclusion: Regular treatment with HELP favourably influences the course of progressive CAD. |
No details on the HMZ or HTZ status of FH patients At the beginning of the study, statins were not yet approved for regular use in Germany |
|
CAD refers to coronary artery disease; LDL, low -density lipoprotein-cholesterol; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Inclusion criteria for the Lane et al. studies did not meet the lower level of LDL specified by the FDA indications
Table 2a: Study Characteristics of Prospective Case Series*.
| Study | Sample size and Population | Patient Characteristics | Follow-up and HELP Treatment Interval | Outcomes | Results | Comments | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) |
Mean Baseline LDL (mg/dL) | Acute | Chronic | |||||
| Moriarty et al., 2001 (87) United States |
N=4 HTZ FH, advanced CAD, refractory to diet and drug therapy |
57.5 | 50 | 275 | 6 months biweekly |
lipid parameters and C-reactive protein (CRP) | lipid parameters and CRP | Average treatment duration was 2h Plasma volume treated per procedure was 2.8-3.0l Acute: Mean decrease per treatment: TC 56%, LDL 64%, HDL 25%, TG 34%, Fibrinogen 65%, CRP 64% Chronic: Change from baseline to 6 months: Decrease: TC 5%, LDL 9%, Fibrinogen 25% CRP 49% Increase: TG 8%, HDL 12% Other: Patients did not report any adverse cardiovascular symptoms during the study All patients tolerated the HELP treatments very well |
Not known whether patients were treated with LDL apheresis prior to study initiation |
| Pulawski et al., 2002 (88) Germany |
N=10 HTZ FH, advanced CAD, refractory to diet and drug therapy Undergoing weekly HELP therapy |
52 | 60 | 159 | single session | lipid parameters and soluble adhesion molecul es (sAMs) | Plasma volume treated per procedure was 2.866l Acute: Single session reduced: Total C 49%, LDL 63%, HDL 25%, TG 62% All P<0.0001 Significant reductions in sAMs including soluble vascular cellular adhesion molecule-1 (sVCAM-1) (32%, P<.0001), soluble intracellular adhesion molecule-1 (sICAM-1) (18%, P=.0032) and P-selectin (33%, P=.0044) After 1 week, all lipid parameters increased more or less to their initial values |
Unlikely to determine the clinical utility or effects on disease progression following a single treatment Mean baseline LDL (159 mg/dL) was low in comparison to that of patients included in other studies. Study inclusion criteria stated that patients had to be previously treated with HELP apheresis at weekly intervals, which may have resulted in lower baseline values. |
|
| Hoffmann et al., 2003 (89) Austria |
N=8 HTZ FH and CAD |
46 | 88 | 275 | 29 months weekly or biweekly |
lipid parameters | lipid parameters coronary calcification |
Acute: Decreases in TC 64%, LDL 77%, HDL 18%, TG 49%, Lp(a) 67% Chronic: Decreases in TC 29%, LDL 40%, TG 26%, Lp(a) 27% (P<0.01 for all) HDL increased by 24% (P<0.01) Imaging outcomes: Volume of coronary calcium decreased in all patients by an average of 23% (P<0.01). Agatston scores decreased by an average of 26% (P<0.01). Mean density of coronary calcium increased by 17% (P<0.01) (may serve as an indicator of maturity and stability of calcified plaque) Other: no reports of alterations in patients’ clinical appearance, coronary events or toxicity due to the treatment |
Two different CT imaging modalities were used to assess coronary calcium (EBCT at baseline at by MDCT at follow-up) Patients were only prescribed statin therapy and were not treated with combination therapy |
| Moriarty et al., 2004 (29) United States |
N=6 HTZ FH, CVD and LDL ≥ 200mg/dL despite diet and drug therapy All patients successfully completed at least one HELP session prior to enrolment |
58 | 50 | 253 | single session | lipid parameters and blood viscosity | Approximately 3l of plasma treated per procedure Average time ranged from 1.5 to 2.5hrs Acute: Single session reduced: TC 49%, LDL 63%, HDL 21%, TG 33% All P<0.05 Significantly reduced blood viscosity for all shear rates ranging from 13% to 31% (all P<.01) |
Unlikely to determine the clinical utility or effects on disease progression following a single treatment | |
| Moriarty et al., 2005 (90) United States |
N=8 HTZ FH, CVD and LDL ≥ 200mg/dL despite diet and drug therapy All patients successfully completed at least one HELP session prior to enrolment |
59 | 50 | 262 | 3 months biweekly |
LDL and lipoprotein-associated phospholi-pase A2 (Lp-PLA2) | LDL and Lp-PLA2 | Total of 40 treatments were performed Approximately 3 l of plasma treated per procedure Average time ranged from 1.5 to 2.5hrs Acute: Single session reduced: LDL 60% (P<0.0001) Acute reductions in Lp-PLA2 were also observed (22%; P<.003) Reductions in LDL and Lp-PLA2 were not significantly correlated (P=.06) Chronic: Baseline LDL reduced by 14% from initial session (no P value given) |
Outcomes limited to LDL and did not include other lipid parameters |
CAD refers to coronary artery disease; CRP, C reactive protein; CVD, cardiovascular disease; HTZ FH, heterozygous familial hypercholesterolemia; LDL, low-density lipoprotein-cholesterol; Lp-PLA2, lipoprotein associated phospholipase 2; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Table 3a: Study Characteristics of Nested Case Series Studies*.
| Study | Sample size and Population | Patient Characteristics | Follow-up and HELP Treatment Interval | Outcomes | Results | Comments | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) | Mean Baseline LDL (mg/dL) | Acute | Chronic | |||||
| Richter et al., 1999 (91) Prospective comparison Germany |
N=8 patients with HELP (total of 34 patients) HTZ FH, CAD, refractory to diet and drug therapy Treated with regular LDL apheresis Compared 3 systems: IMAL, DSA, HELP |
43.6 | 75 | 257 | mean 4.6 years (range 1 to 8.6) weekly (majority) |
Lipid parameters | Lipid parameters Angiographic outcomes |
1,497 HELP sessions were documented in the 8 patients Acute: Mean decrease during one apheresis procedure was as follows: TC 49.3%, LDL 58.8%, HDL 16.6%, TG 44.4%, Lp (a) 67.7% Chronic: LDL 45.5% decrease, Lp(a) 52.3% decrease, HDL 20.0% increase Angiographic Outcomes: 5 patients eligible for angiographic assessment; 3 patients not followed for at least 2 years and results not available 2 out of 5: regression of coronary atherosclerosis 3 out of 5: no change in progression of coronary lesions Other: Changes in lipid parameters comparable with all 3 methods Adverse reactions were usually mild and easily reversible by minor symptomatic treatment. (1.8% adverse clinical events and 3.6% technical pitfalls) 3 sudden cardiac deaths during study (2 patients on HELP), not treatment related |
patients were only prescribed simvastatin and were not treated with other lipid lowering medications Angiographic results assessed by global coronary score, no data provided on degree of stenosis |
| Julius et al., 2002 (92) Cross over Germany |
N=6 HTZ FH, CAD, refractory to diet and drug therapy Treated with LDL apheresis ≥ 24 months Part A: Lipidfiltratio n with Cascadeflo AC-1770 & HELP (n=5) Part B:, Lipidfiltration with Lipidfilter EC-50 &HELP (n=6) |
61.5 | 67 | 135 | 4 sessions (2 in part A and 2 in part B) over 8 weeks weekly |
Lipid parameters | Total of 22 HELP sessions in 6 patients Acute: Part A: (n=5); 2 HELP sessions per patient Mean treatment volume 2812ml Decrease: LDL 54.0±5.1%, TG 60.7±9.6%, Lp(a) 61.5±20.2%, fibrinogen 58.0±4.2%. Increase: HDL 0.4±3.5% Part B: (n=6); 2 HELP sessions per patient Mean treatment volume 3013ml Decrease: LDL61.3±8.1%, TG 51.6±19.4%, Lp(a) 56.3±29.1%, fibrinogen 62.1±7.4%, HDL 1.3±7.3% Other: Differences in reduction rates in lipids between both LDL apheresis methods were not statistically significant No severe adverse events |
Unlikely to determine the clinical utility or effects on disease progression following a 2 sessions Mean baseline LDL (135 mg/dL) was low in comparison to that of patients included in other studies. Study inclusion criteria stated that patients had to be previously treated with LDL apheresis for at least 2 years and this may have resulted in lower baseline values. |
|
CAD refers to coronary artery disease; DSA, dextran sulfate adsorption; HELP, heparin-induced extracorporeal LDL precipitation; HTZ FH, heterozygous familial hypercholesterolemia; IMAL, immunoadsorption; LDL, low-density lipoprotein-cholesterol; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Table 4a: Study Characteristics – Retrospective Review*.
| Study | Sample size and Population | Patient Characteristics | Follow-up and HELP Treatment Interval | Outcomes | Results | Comments | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean Age (years) | Males (%) |
Mean Baseline LDL (mg/dL) | Acute | Chronic | |||||
| Krebs et al., 2004 (18) § Retrospective comparison Multisite Germany |
N=10 patients with HELP (total of 20 patients - 3 HMZ and 17 HTZ) FH, CAD, refractory Patients prescribed statins once they became available in the early 1990s Compared 5 systems: DALI, HELP, IMAL, MDF, DSA |
47 | 70 | 351 | mean 9 years over a period of 15 years (1986-2001) Frequency not specified |
Lipid parameters | Lipid kinetics – average lipid concentrations between aphereses | Mean volume was 2832ml Mean duration was 96mins Total of 2750 HELP procedures Acute: Mean reduction of TC 54%, TG 46%, LDL 62%, HDL 29%, Lp(a) 55%, fibrinogen 56% Chronic: Derived CAUC – average concentration between aphereses LDL was 4.06±2.04mmol/L and HDL 1.84±0.85, TC 6.67±2.07, Lp(a) 0.52g/L ±0.53, fibrinogen 2.07g/L±0.22 LDL/HDL ratio was 2.2 Other: HELP did not reduce immunoglobulines (IgG, IgA, IgM) Cardiovascular outcomes available, but not separately for HELP patients. CAD progressed in only a few patients, most were stable or improved. Progression of atherosclerosis occurred in 65% of patients no evidence regarding life-threatening or fatal events caused by any of the LDL aphereses method Overall, all 5 apheresis methods were safe and suitable for long-term treatment of FH patients |
Retrospective review – no patient selection Some patients were treated with more than one LDL apheresis method over the study period. Therefore, not all results can be directly associated with the HELP system |
CAD refers to coronary artery disease; DALI, direct adsorption of lipoproteins; DSA, dextran sulfate adsorption; HELP, heparin-induced extracorporeal LDL precipitation; HMZ FH, homozygous familial hypercholesterolemia; HTZ FH, heterozygous familial hypercholesterolemia; IMAL, immunoadsorption; LDL, low-density lipoprotein-cholesterol; MDF, membrane differential filtration; N, sample size.
Acute is defined as changes in lipid parameter immediately before and after treatment
Chronic is defined as long term changes in lipid parameters from baseline to end of study
Patient characteristics and duration of follow up not available for those specifically treated with HELP, all patients together (N=20)
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Low density lipoprotein aphereis: an evidence-based analysis. Ontario Health Technology Assessment Series 2007; 7(5)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-5404-9 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- ApoB
Apolipoprotein B
- AUC
Area under the curve
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- CAM
Cellular adhesion molecule
- CRP
C-reactive protein
- DALI
Direct adsorption of lipoproteins
- DSC
Dextran sulfate adsorption
- FDA
Food and Drug Administration
- FH
Familial hypercholesterolemia
- HDL-C
High density lipoprotein cholesterol
- HELP
Heparin-induced extracorporeal LDL precipitation
- HMZ
Homozygous
- HTA
Health technology assessment
- HTZ
Heterozygous
- ICAM-1
Intracellular adhesion molecule-1
- IMAL
Immunoadsorption
- LDL-C
Low-density lipoprotein cholesterol
- Lp(a)
Lipoprotein (a)
- MDF
Membrane differential filtration
- MI
Myocardial infarction
- OR
Odds ratio
- PTCA
Percutanerous transluminal coronary angioplasty
- RR
Risk ratio
- SD
Standard deviation
- TC
Total cholesterol
- TG
Triglyceride
- VCAM-1
Vascular cellular adhesion molecule-1
- WHO
World Health Organization
Footnotes
Due to the founder gene effect, the prevalence of FH is higher in certain populations such as French Canadians, Johannesburg Jews, the Christian Lebanese population and the South African Afrikaner population.
Changes immediately before and after treatment
Long-term changes from baseline to end of study
Agatston scores are a method used to quantify the amount of calcium in the coronary vessel wall
Populations that have arisen from a small number of settlers and possess a few mutations which occur at high frequency
Hardy Weinberg principles of population genetics do not apply to populations with a founder gene effect
The Step 2 diet restricts saturated fat to less than 7% of total calories and cholesterol to less than 200 mg/day. It is intended for people already at the Step I goals or for patients with a high-risk cholesterol level (240 mg/dL or higher) or who have had a heart attack. The AHA has recently replaced this terminology with “Therapeutic Lifestyle Changes (TLC)” which is a more intensive life-habit intervention encompassing dietary and lifestyle change.
Agatston scores are a method used to quantify the amount of calcium in the coronary vessel wall
References
- 1.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GRADE Working Group GRADE [Web page] [[updated 2007; cited 2007 Sept. 1]]; Available at: www.gradeworkinggroup.org .
- 3.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–38. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 4.Vella A, Pineda AA, O’Brien T. Low-density lipoprotein apheresis for the treatment of refractory hyperlipidemia. Mayo Clin Proc. 2001;76(10):1039–46. doi: 10.4065/76.10.1039. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins PN. Familial hypercholesterolemia--improving treatment and meeting guidelines. Int J Cardiol. 2003;89(1):13–23. doi: 10.1016/s0167-5273(02)00420-5. [DOI] [PubMed] [Google Scholar]
- 6.Leigh S. UCL Low density lipoprotein receptor database [Web page] [[updated 2007; cited 2007 Sept. 1]];Leiden University Medical Centre. Available at: http://www.ucl.ac.uk/ldlr/LOVDv.1.1.0/
- 7.Whitfield AJ, Barrett PH, van Bockxmeer FM, Burnett JR. Lipid disorders and mutations in the APOB gene. Clin Chem. 2004;50(10):1725–32. doi: 10.1373/clinchem.2004.038026. [DOI] [PubMed] [Google Scholar]
- 8.Moriarty PM. LDL-apheresis therapy: current therapeutic practice and potential future use. Future Lipidol. 2006;1(3):299–308. [Google Scholar]
- 9.McCrindle BW. Drug therapy of hyperlipidemia. Prog Pediatr Cardiol. 2003;17(2):141–50. [Google Scholar]
- 10.Morelli F, Carlier P, Giannini G, De Luigi MC, Dejana A, Ruzzenenti MR. Hypercholesterolemia and LDL apheresis. Int J Artif Organs. 2005;28(10):1025–31. doi: 10.1177/039139880502801010. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolaemia. Scriver CT, Beaudet AL, Sly WS, Valle D, . New York: McGraw-Hill. 1995:p. 1981–2030. In. editors. The metabolic and molecular bases of inherited disease. [Google Scholar]
- 12.Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168(1):1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 13.Neil HA, Hammond T, Huxley R, Matthews DR, Humphries SE. Extent of underdiagnosis of familial hypercholesterolaemia in routine practice: prospective registry study. BMJ. 2000;321(7254):148. doi: 10.1136/bmj.321.7254.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. [[cited 2007 Sept. 1]];Geneva: WHO. 1999 Familial hypercholesterolemia (FH): report of a second WHO consultation [report on the Internet] Available at: http://whqlibdoc.who.int/hq/1999/WHO_HGN_FH_CONS_99.2.pdf .
- 15.Mabuchi H, Higashikata T, Kawashiri MA. Clinical applications of long-term LDL-apheresis on and beyond refractory hypercholesterolemia. Transfus Apher Sci. 2004;30(3):233–43. doi: 10.1016/j.transci.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Ose L. An update on familial hypercholesterolaemia. Ann Med. 1999;31(Suppl 1):13–8. [PubMed] [Google Scholar]
- 17.Sullivan D. Guidelines for the diagnosis and management of familial hypercholesterolaemia. Heart Lung Circ. 2007;16(1):25–7. doi: 10.1016/j.hlc.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Krebs A, Krebs K, Keller F. Retrospective comparison of 5 different methods for long-term LDL-apheresis in 20 patients between 1986 and 2001. Int J Artif Organs. 2004;27(2):137–48. doi: 10.1177/039139880402700209. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1(6):445–66. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 20.Al Shaikh AM, Abdullah MH, Barclay A, Cullen-Dean G, McCrindle BW. Impact of the characteristics of patients and their clinical management on outcomes in children with homozygous familial hypercholesterolemia. Cardiol Young. 2002;12(2):105–12. doi: 10.1017/s1047951102000240. [DOI] [PubMed] [Google Scholar]
- 21.Thompson GR. LDL apheresis. Atherosclerosis. 2003;167(1):1–13. doi: 10.1016/s0021-9150(02)00251-4. [DOI] [PubMed] [Google Scholar]
- 22.Sijbrands EJ, Westendorp RG, Defesche JC, de Meier PH, Smelt AH, Kastelein JJ. Mortality over two centuries in large pedigree with familial hypercholesterolaemia: family tree mortality study. BMJ. 2001;322(7293):1019–23. doi: 10.1136/bmj.322.7293.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aalst-Cohen ES, Jansen AC, de Jongh S, Sauvage Nolting PR, Kastelein JJ. Clinical, diagnostic, and therapeutic aspects of familial hypercholesterolemia. Semin Vasc Med. 2004;4(1):31–41. doi: 10.1055/s-2004-822984. [DOI] [PubMed] [Google Scholar]
- 24.Jansen AC, van Wissen S, Defesche JC, Kastelein JJ. Phenotypic variability in familial hypercholesterolaemia: an update. Curr Opin Lipidol. 2002;13(2):165–71. doi: 10.1097/00041433-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Yuan G, Wang J, Hegele RA. Heterozygous familial hypercholesterolemia: an underrecognized cause of early cardiovascular disease. CMAJ. 2006;174(8):1124–9. doi: 10.1503/cmaj.051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303(6807):893–6. doi: 10.1136/bmj.303.6807.893. Scientific Steering Committee on behalf of the Simon Broome Register Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PJ. The management of familial hypercholesterolaemia in childhood. Current Paediatrics. 2002;12(2):104–9. [Google Scholar]
- 28.Ose L. Familial hypercholesterolemia from children to adults. Cardiovasc Drugs Ther. 2002;16(4):289–93. doi: 10.1023/a:1021773724477. [DOI] [PubMed] [Google Scholar]
- 29.Moriarty PM, Gibson CA, Kensey KR, Hogenauer W. Effect of low-density lipoprotein cholesterol apheresis on blood viscosity. Am J Cardiol. 2004;93(8):1044–6. doi: 10.1016/j.amjcard.2003.12.059. [DOI] [PubMed] [Google Scholar]
- 30.Hegele RA. Genetic susceptibility to heart disease in Canada: lessons from patients with familial hypercholesterolemia. Genome. 2006;49(11):1343–50. doi: 10.1139/g06-147. [DOI] [PubMed] [Google Scholar]
- 31.Defesche JC, Kastelein JJ. Molecular epidemiology of familial hypercholesterolaemia. Lancet. 1998;352(9141):1643–4. doi: 10.1016/S0140-6736(05)61443-2. [DOI] [PubMed] [Google Scholar]
- 32.Pottle A. Familial hypercholesterolaemia: clinical features and management. Nurs Stand. 2005;20(14-16):55–65. doi: 10.7748/ns2005.12.20.14.55.c4029. [DOI] [PubMed] [Google Scholar]
- 33.Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HA. Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2000;4(29):1–123. [PubMed] [Google Scholar]
- 34.Haney EM, Huffman LH, Bougatsos C, Freeman M, Steiner RD, Nelson HD. Screening and treatment for lipid disorders in children and adolescents: systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2007;120(1):e189–e214. doi: 10.1542/peds.2006-1801. [DOI] [PubMed] [Google Scholar]
- 35.MEDPED. [[updated 2007; cited 2007 Aug. 1]];Make early diagnosis to prevent early deaths. [Web page] Available at: www.medped.org .
- 36.Ose L. Diagnostic, clinical, and therapeutic aspects of familial hypercholesterolemia in children. Semin Vasc Med. 2004;4(1):51–7. doi: 10.1055/s-2004-822986. [DOI] [PubMed] [Google Scholar]
- 37.Jaeger BR. Evidence for maximal treatment of atherosclerosis: drastic reduction of cholesterol and fibrinogen restores vascular homeostasis. Ther Apher. 2001;5(3):207–11. [PubMed] [Google Scholar]
- 38.Bambauer R. Is lipoprotein (a)-apheresis useful? Ther Apher Dial. 2005;9(2):142–7. doi: 10.1111/j.1774-9987.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 39.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 40.Stegmayr B, Lalau JD, Johnson O. In face of the increasing efficacy of lipid-lowering therapy, is there still a place for LDL-apheresis? Transfus Apher Sci. 2004;30(3):213–20. doi: 10.1016/j.transci.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. [[cited 2007 Oct. 13]];National Institutes of Health. 2002 [report on the Internet] NIH Publication No. 02-5215. Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143 . [PubMed]
- 42.Prevention of coronary heart disease in clinical practice. Eur Heart J. 1998;19(10):1434–503. doi: 10.1053/euhj.1998.1243. Recommendations of the Second Joint Task Force of European and other Societies on coronary prevention. [DOI] [PubMed] [Google Scholar]
- 43.Genest J, Frohlich J, Fodor G, McPherson R. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. CMAJ. 2003;169(9):921–4. [PMC free article] [PubMed] [Google Scholar]
- 44.McPherson R, Frohlich J, Fodor G, Genest J. Canadian Cardiovascular Society. Can J Cardiol. 2006;22(11):913–27. doi: 10.1016/s0828-282x(06)70310-5. Canadian Cardiovascular Society position statement--recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 46.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. 1992;85(1):37–45. doi: 10.1161/01.cir.85.1.37. Circulation. [DOI] [PubMed] [Google Scholar]
- 47.Poustie VJ, Rutherford P. Dietary treatment for familial hypercholesterolaemia. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001918. Issue 2. Art. No.: CD001918. DOI: 10.1002/14651858.CD001918. [DOI] [PubMed] [Google Scholar]
- 48.Geiss HC, Otto C, Hund-Wissner E, Parhofer KG. Effects of ezetimibe on plasma lipoproteins in severely hypercholesterolemic patients treated with regular LDL-apheresis and statins. Atherosclerosis. 2005;180(1):107–12. doi: 10.1016/j.atherosclerosis.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Blaha M. Extracorporeal LDL-cholesterol elimination in the treatment of severe familial hypercholesterolemia. Acta Medica (Hradec Kralove) 2003;46(1):3–7. [PubMed] [Google Scholar]
- 50.Gagne C, Gaudet D, Bruckert E. Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. 2002;105(21):2469–75. doi: 10.1161/01.cir.0000018744.58460.62. Circulation. [DOI] [PubMed] [Google Scholar]
- 51.Thompson GR, Barbir M, Okabayashi K, Trayner I, Larkin S. Plasmapheresis in familial hypercholesterolemia. Arteriosclerosis. 1989;9((1 Suppl)):I152–I157. [PubMed] [Google Scholar]
- 52.Thompsen J, Thompson PD. A systematic review of LDL apheresis in the treatment of cardiovascular disease. Atherosclerosis. 2006;189(1):31–8. doi: 10.1016/j.atherosclerosis.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 53.Bosch T. Therapeutic apheresis--state of the art in the year 2005. Ther Apher Dial. 2005;9(6):459–68. doi: 10.1111/j.1744-9987.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 54.Rock G, Clark B, Sutton D. The Canadian apheresis registry. Transfus Apher Sci. 2003;29(2):167–77. doi: 10.1016/S1473-0502(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 55.Bambauer R, Schiel R, Latza R. Low-density lipoprotein apheresis: an overview. Ther Apher Dial. 2003;7(4):382–90. doi: 10.1046/j.1526-0968.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 56.Rock GA, Tricklebank GW, Kasaboski CA. Plasma exchange in Canada. The Canadian Apheresis Study Group. 1990;142(6):557–62. CMAJ. [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson GR, Miller JP, Breslow JL. Improved survival of patients with homozygous familial hypercholesterolaemia treated with plasma exchange. Br Med J (Clin Res Ed) 1985;291(6510):1671–3. doi: 10.1136/bmj.291.6510.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makino H, Harada-Shiba M. Long-term effect of low-density lipoprotein apheresis in patients with homozygous familial hypercholesterolemia. Ther Apher Dial. 2003;7(4):397–401. doi: 10.1046/j.1526-0968.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 59.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356(2):148–56. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto A, Harada-Shiba M, Kawaguchi A, Tsushima M. Apheresis technology for prevention and regression of atherosclerosis. Ther Apher. 2001;5(4):221–5. doi: 10.1046/j.1526-0968.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 61.Yokoyama S. Brief history of low-density lipoprotein apheresis. Ther Apher Dial. 2003;7(4):378–81. doi: 10.1046/j.1526-0968.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 62.Kroon AA, van’t Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after LDL-apheresis. Atherosclerosis. 2000;152(2):519–26. doi: 10.1016/s0021-9150(00)00371-3. Kinetics and estimation of mean lipoprotein levels. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto A, Harada-Shiba M, Kawaguchi A, Oi K, Kubo H, Sakai S, et al. The effect of atorvastatin on serum lipids and lipoproteins in patients with homozyous familial hypercholesterolemia undergoing LDL-apheresis therapy. Atherosclerosis. 2000;153(1):89–98. doi: 10.1016/s0021-9150(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 64.Moriarty PM. LDL-apheresis therapy. Curr Treat Options Cardiovasc Med. 2006;8(4):282–8. doi: 10.1007/s11936-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 65.Baricchi R, Pizzala R, Cacciavillani G, Rivasi P, Tomasi A. The effect of selective low-density lipoprotein apheresis on plasma lipoperoxides and antioxidant vitamins in familial hypercholesterolemic patients. Ther Apher. 1998;2(3):218–23. doi: 10.1111/j.1744-9987.1998.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 66.Higashikata T, Mabuchi H. Long-term effect of low-density lipoprotein apheresis in patients with heterozygous familial hypercholesterolemia. Ther Apher Dial. 2003;7(4):402–7. doi: 10.1046/j.1526-0968.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 67.Schuff-Werner P, Gohlke H, Bartmann U, Baggio G, Corti MC, Dinsenbacher A, et al. The HELP-LDL-apheresis multicentre study, an angiographically assessed trial on the role of LDL-apheresis in the secondary prevention of coronary heart disease. Eur J Clin Invest. 1994;24(11):724–32. doi: 10.1111/j.1365-2362.1994.tb01068.x. II. Final evaluation of the effect of regular treatment on LDL-cholesterol plasma concentrations and the course of coronary heart disease. The HELP-Study Group. Heparin-induced extra-corporeal LDL-precipitation. [DOI] [PubMed] [Google Scholar]
- 68.Berger GM, Firth JC, Jacobs P, Wood L, Marais AD, Horak A. Three different schedules of low-density lipoprotein apheresis compared with plasmapheresis in patients with homozygous familial hypercholesterolemia. Am J Med. 1990;88(2):94–100. doi: 10.1016/0002-9343(90)90455-m. [DOI] [PubMed] [Google Scholar]
- 69.Seidel D. The H.E.L.P. system: an efficient and safe method for plasma therapy in the treatment of severe hypercholesterolemia. Isr J Med Sci. 1996;32(6):407–13. [PubMed] [Google Scholar]
- 70.Mellwig K-P. Heparin-induced extracorporeal low-density lipoprotein precipitation. Ther Apher Dial. 2003;7(3):365–9. doi: 10.1046/j.1526-0968.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 71.B.Braun Medical Inc. [[updated 2005 cited 2007 May 4]];B. Braun Medical Inc. LDL apheresis [Web page] Available at: http://www.bbraunusa.com/index.cfm?uuid=7E30D9B5D0B759A1E3EE6AA31A7A0F59 .
- 72.Seidel D. H.E.L.P. apheresis therapy in the treatment of severe hypercholesterolemia: 10 years of clinical experience. Artif Organs. 1996;20(4):303–10. doi: 10.1111/j.1525-1594.1996.tb04449.x. [DOI] [PubMed] [Google Scholar]
- 73.Susca M. Heparin-Induced extracorporeal low-density lipoprotein precipitation futura, a new modification of HELP apheresis: technique and first clinical results. Ther Apher. 2001;5(5):387–93. doi: 10.1046/j.1526-0968.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 74.Empen K, Otto C, Brodl UC, Parhofer KG. The effects of three different LDL-apheresis methods on the plasma concentrations of E-selectin, VCAM-1, and ICAM-1. J Clin Apheresis. 2002;17(1):38–43. doi: 10.1002/jca.10010. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Blessing F, Walli AK, Uberfuhr P, Fraunberger P, Seidel D. Effects of heparin-mediated extracorporeal low-density lipoprotein precipitation beyond lowering proatherogenic lipoproteins--reduction of circulating proinflammatory and procoagulatory markers. Atherosclerosis. 2004;175(1):145–50. doi: 10.1016/j.atherosclerosis.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Wieland E, Schettler V, Armstrong VW. Highly effective reduction of C-reactive protein in patients with coronary heart disease by extracorporeal low density lipoprotein apheresis. Atherosclerosis. 2002;162(1):187–91. doi: 10.1016/s0021-9150(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 77.Mellwig KP, van Buuren F, Schmidt HK, Wielepp P, Burchert W, Horstkotte D. Improved coronary vasodilatatory capacity by H.E.L.P. apheresis: comparing initial and chronic treatment. Ther Apher Dial. 2006;10(6):510–7. doi: 10.1111/j.1744-9987.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 78.Krieter DH, Steinke J, Kerkhoff M, Fink E, Lemke HD, Zingler C, et al. Contact activation in low-density lipoprotein apheresis systems. Artif Organs. 2005;29(1):47–52. doi: 10.1111/j.1525-1594.2004.29007.x. [DOI] [PubMed] [Google Scholar]
- 79.Blessing F, Wang Y, Nagel D, Seidel D. The efficacy and safety of the new heparin-induced extracorporeal low-density lipoprotein precipitation system (Plasmat Futura) in comparison with the currently used system (Plasmat Secura) Ther Apher Dial. 2004;8(1):33–8. doi: 10.1111/j.1526-0968.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 80.Bosch T. Recent advances in therapeutic apheresis. J Artif Organs. 2003;6(1):1–8. doi: 10.1007/s100470300000. [DOI] [PubMed] [Google Scholar]
- 81.Blessing F, Wang Y, Walli AK, Seidel D. Heparin-mediated extracorporeal low-density lipoprotein precipitation: rationale for a specific adjuvant therapy in cardiovascular disease. Transfus Apher Sci. 2004;30(3):255–66. doi: 10.1016/j.transci.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Ziajka P. Role of low-density lipoprotein apheresis. Am J Cardiol. 2005;96(4 SUPPL):67E–9E. doi: 10.1016/j.amjcard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 83.United States Food and Drug Administration. [[cited 2007 Oct. 13]];Summary of safety and effectiveness data. 1997 The H.E.L.P. system [report on the Internet] PMA P940016. Available at: http://www.fda.gov/cdrh/pdf/p940016.pdf .
- 84.Lane DM, McConathy WJ, Laughlin LO, Comp PC, von Albertini B, Bricker LA, et al. Selective removal of plasma low density lipoprotein with the HELP system: biweekly versus weekly therapy. Atherosclerosis. 1995;114(2):203–11. doi: 10.1016/0021-9150(94)05484-z. [DOI] [PubMed] [Google Scholar]
- 85.Lane DM, Alaupovic P, Knight-Gibson C, Dudley VS, Laughlin LO. Changes in plasma lipid and apolipoprotein levels between heparin-induced extracorporeal low-density lipoprotein precipitation (HELP) treatments. Am J Cardiol. 1995;75(16):1124–9. doi: 10.1016/s0002-9149(99)80743-7. [DOI] [PubMed] [Google Scholar]
- 86.Lane DM, McConathy WJ, Laughlin LO, Comp PC, von Albertini B, Gibson SM, et al. Weekly treatment of diet/drug-resistant hypercholesterolemia with the heparin-induced extracorporeal low-density lipoprotein precipitation (HELP) system by selective plasma low-density lipoprotein removal. Am J Cardiol. 1993;71(10):816–22. doi: 10.1016/0002-9149(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 87.Moriarty PM, Gibson CA, Shih J, Matias MS. C-reactive protein and other markers of inflammation among patients undergoing HELP LDL apheresis. Atherosclerosis. 2001;158(2):495–8. doi: 10.1016/s0021-9150(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 88.Pulawski E, Mellwig KP, Brinkmann T, Kleesiek K, Horstkotte D. Influence of single low-density lipoprotein apheresis on the adhesion molecules soluble vascular cellular adhesion molecule-1, soluble intercellular adhesion molecule-1, and P-selectin. Ther Apher. 2002;6(3):229–33. doi: 10.1046/j.1526-0968.2002.00405.x. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann U, Derfler K, Haas M, Stadler A, Brady TJ, Kostner K. Effects of combined low-density lipoprotein apheresis and aggressive statin therapy on coronary calcified plaque as measured by computed tomography. Am J Cardiol. 2003;91(4):461–4. doi: 10.1016/s0002-9149(02)03248-4. [DOI] [PubMed] [Google Scholar]
- 90.Moriarty PM, Gibson CA. Effect of low-density lipoprotein apheresis on lipoprotein-associated phospholipase A2. Am J Cardiol. 2005;95(10):1246–7. doi: 10.1016/j.amjcard.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 91.Richter WO, Donner MG, Schwandt P. Three low density lipoprotein apheresis techniques in treatment of patients with familial hypercholesterolemia: a long-term evaluation. Ther Apher. 1999;3(3):203–8. doi: 10.1046/j.1526-0968.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 92.Julius U, Metzler W, Pietzsch J, Fassbender T, Klingel R. Intraindividual comparison of two extracorporeal LDL apheresis methods: lipidfiltration and HELP. Int J Artif Organs. 2002;25(12):1180–8. doi: 10.1177/039139880202501210. [DOI] [PubMed] [Google Scholar]
- 93.Stefanutti C, Lanti A, Di Giacomo S, Mareri M, De Lorenzo F, Landolfo A, et al. Therapeutic apheresis in low weight patients: technical feasibility, tolerance, compliance, and risks. Transfus Apher Sci. 2004;31(1):3–10. doi: 10.1016/j.transci.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Gordon BR, Kelsey SF, Dau PC, Gotto AM Jr, Graham K, Illingworth DR, et al. Long-term effects of low-density lipoprotein apheresis using an automated dextran sulfate cellulose adsorption system. Liposorber Study Group. Am J Cardiol. 1998;81(4):407–11. doi: 10.1016/s0002-9149(97)00947-8. [DOI] [PubMed] [Google Scholar]
- 95.Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K, et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Am J Cardiol. 1998;82(12):1489–95. doi: 10.1016/s0002-9149(98)00692-4. Hokuriku-FH-LDL-Apheresis Study Group. [DOI] [PubMed] [Google Scholar]
- 96.Statistics Canada. [[updated 2007; cited 2007 Aug. 1]];Population and dwelling counts for Canada provinces and territories 2006 and 2001 censuses - 100% data [Web page] Available at: http://www.census2006.ca/english/census06/data/popdwell/Table.cfm?T=101 .
- 97.Ontario Ministry of Health and Long-Term Care. [[updated 2007; cited 2007 Sept. 1]];Ontario Schedule of Benefits. Available at: www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html .
- 98.Moga C, Harstall C. Low density lipoprotein apheresis for the treatment of familial hypercholesterolemia. [[cited 2007 Oct. 13]];Edmonton, AB: Alberta Heritage Foundation for Medical Research. 2004 [report on the Internet] Available at: http://www.ihe.ca/documents/hta/ip18.pdf .
- 99.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 Pt 2):293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 100.Kawaguchi A, Miyatake K, Yutani C, Beppu S, Tsushima M, Yamamura T, et al. Characteristic cardiovascular manifestation in homozygous and heterozygous familial hypercholesterolemia. Am Heart J. 1999;137(3):410–8. doi: 10.1016/s0002-8703(99)70485-0. [DOI] [PubMed] [Google Scholar]
- 101.National Institute for Health and Clinical Excellence (NICE) [[cited 2007 Jan. 8]];National Institute for Health and Clinical Excellence (NICE) 2007 Guidelines for the identification and management of familial hypercholesterolemia patients [report on the Internet] Available at: http://guidance.nice.org.uk/page.aspx?o=guidelines.inprogress.familialhypercholesterolaemia .


