Executive Summary
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease (CAD), an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients suspected of having CAD. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies for the diagnosis of CAD. Evidence-based analyses have been prepared for each of these five imaging modalities: cardiac magnetic resonance imaging, single photon emission computed tomography, 64-slice computed tomographic angiography, stress echocardiography, and stress echocardiography with contrast. For each technology, an economic analysis was also completed (where appropriate). A summary decision analytic model was then developed to encapsulate the data from each of these reports (available on the OHTAC and MAS website).
The Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease series is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas"> www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Single Photon Emission Computed Tomography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography with Contrast for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
64-Slice Computed Tomographic Angiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Cardiac Magnetic Resonance Imaging for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Pease note that two related evidence-based analyses of non-invasive cardiac imaging technologies for the assessment of myocardial viability are also available on the MAS website:
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: an Evidence-Based Analysis
The Toronto Health Economics and Technology Assessment Collaborative has also produced an associated economic report entitled:
The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario [Internet]. Available from: http://theta.utoronto.ca/reports/?id=7
Objective
The objective of the analysis is to determine the diagnostic accuracy of stress echocardiography (ECHO) in the diagnosis of patients with suspected coronary artery disease (CAD) compared to coronary angiography (CA).
Stress Echocardiography
Stress ECHO is a non-invasive technology that images the heart using ultrasound. It is one of the most commonly employed imaging techniques for investigating a variety of cardiac abnormalities in both community and hospital settings. A complete ECHO exam includes M-mode, 2-dimensional (2-D) images and Doppler imaging.
In order to diagnosis CAD and assess whether myocardial ischemia is present, images obtained at rest are compared to those obtained during or immediately after stress. The most commonly used agents used to induce stress are exercise and pharmacological agents such as dobutamine and dipyridamole. The hallmark of stress-induced myocardial ischemia is worsening of wall motion abnormalities or the development of new wall motion abnormalities. A major challenge for stress ECHO is that the interpretation of wall motion contractility and function is subjective. This leads to inter-observer variability and reduced reproducibility. Further, it is estimated that approximately 30% of patients have sub-optimal stress ECHO exams. To overcome this limitation, contrast agents for LV opacification have been developed.
Although stress ECHO is a relatively easy to use technology that poses only a low risk of adverse events compared to other imaging technologies, it may potentially be overused and/or misused in CAD diagnosis. Several recent advances have been made focusing on quantitative methods for assessment, improved image quality and enhanced portability, however, evidence on the effectiveness and clinical utility of these enhancements is limited.
Evidence-Based Analysis
Research Questions
What is the diagnostic accuracy of stress ECHO for the diagnosis of patients with suspected CAD compared to the reference standard of CA?
What is the clinical utility1 of stress ECHO?
Literature Search
A literature search was performed on August 28, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 until August 21, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any relevant studies not identified through the search.
Inclusion Criteria
Systematic reviews, meta-analyses, randomized controlled trials, prospective observational studies, retrospective analyses
Minimum sample size of 20 enrolled patients
Comparison to CA (reference standard)
Definition of CAD specified as either ≥50%, ≥70% or ≥75% coronary artery stenosis on CA
Reporting accuracy data on individual patients (rather than accuracy data stratified by segments of the heart)
English
Human
Exclusion Criteria
Duplicate studies
Non-systematic reviews, case reports
Grey literature (e.g., conference abstracts)
Insufficient data for independent calculation of sensitivity and specificity
Use of ECHO for purposes other than diagnosis of CAD (e.g., arrhythmia, valvular disease, mitral stenosis, pre-operative risk of MI)
Transesophageal ECHO since its primary use is for non-CAD indications such as endocarditis, intracardiac thrombi, valvular disorders
Only resting ECHO performed
Outcomes of Interest
Accuracy outcomes (sensitivity, specificity, positive predictive value, negative predictive value)
Costs
Summary of Findings
Given the vast amount of published literature on stress ECHO, it was decided to focus on the studies contained in the comprehensive 2007 review by Heijenbrok-Kal et al. (1) as a basis for the MAS evidence-based analysis. In applying our inclusion and exclusion criteria, 105 observational studies containing information on 13,035 patients were included. Six studies examined stress ECHO with adenosine, 26 with dipyridamole and 77 with dobutamine, the latter being the most commonly used pharmacological stress ECHO agent in Ontario. A further 18 studies employed exercise as the stressor.2 The prevalence of CAD ranged from 19% to 94% with a mean estimated prevalence of 70%. Based on the results of these studies the following conclusions were made:
Based on the available evidence, stress ECHO is a useful imaging modality for the diagnosis of CAD in patients with suspected disease. The overall pooled sensitivity is 0.80 (95% CI: 0.77 – 0.82) and the pooled specificity is 0.84 (95% CI: 0.82 – 0.87) using CA as the reference standard. The AUC derived from the sROC curve is 0.895 and the DOR is 20.64.
For pharmacological stress, the pooled sensitivity is 0.79 (95% CI: 0.71 – 0.87) and the pooled specificity is 0.85 (95% CI: 0.83 – 0.88). When exercise is employed as the stress agent, the pooled sensitivity is 0.81 (95% CI: 0.76– 0.86) and the pooled specificity is 0.79 (95% CI: 0.71 – 0.87). Although pharmacological stress and exercise stress would be indicated for different patient populations based on ability to exercise there were no significant differences in sensitivity and specificity.
Based on clinical experts, diagnostic accuracy on stress ECHO depends on the patient population, the expertise of the interpreter and the quality of the image.
Background
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease (CAD), an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients suspected of having CAD. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies for the diagnosis of CAD. Evidence-based analyses have been prepared for each of these five imaging modalities: cardiac magnetic resonance imaging, single photon emission computed tomography, 64-slice computed tomographic angiography, stress echocardiography, and stress echocardiography with contrast. For each technology, an economic analysis was also completed (where appropriate). A summary decision analytic model was then developed to encapsulate the data from each of these reports (available on the OHTAC and MAS website).
The Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease series is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Single Photon Emission Computed Tomography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography with Contrast for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
64-Slice Computed Tomographic Angiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Cardiac Magnetic Resonance Imaging for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Pease note that two related evidence-based analyses of non-invasive cardiac imaging technologies for the assessment of myocardial viability are also available on the MAS website:
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: an Evidence-Based Analysis
The Toronto Health Economics and Technology Assessment Collaborative has also produced an associated economic report entitled:
The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario [Internet]. Available from: http://theta.utoronto.ca/reports/?id=7
Objective of Analysis
The objective of the analysis is to determine the diagnostic accuracy of stress echocardiography (stress ECHO) in the diagnosis of patients with suspected coronary artery disease (CAD).
Stress Echocardiography
Stress ECHO is a non-invasive technology that images the heart using ultrasound. It’s one of the most commonly employed imaging techniques for investigating a variety of cardiac abnormalities and its clinical utility extends beyond simple diagnosis. The technology can be used to assess prognosis and risk stratification in patients with established CAD, to perform preoperative risk assessment, to evaluate patients after revascularization, and to evaluate the severity of heart valve stenosis. (2;3)
Due to its portability and relative affordability, stress ECHO is widely used both in community and hospital settings. Results from an ECHO exam are available in real-time and can thus immediately impact further diagnostic work-up, dictate therapeutic decisions, determine response to therapy and predict patient outcome. In most clinical settings, the sonographer performs the ECHO exam and the physician interprets the images. The entire outpatient exam takes approximately one hour. (4)
The technology relies on the differential absorption and reflection of sound waves by body tissues of differing properties. A complete stress ECHO exam includes M-mode, 2-dimensional (2-D) images and Doppler imaging with a stress agent. (3) The M-mode measures left ventricle (LV) cavity size and wall thickness, 2-D imaging quantifies cardiac chamber sizes, wall thickness, ventricular function, valvular anatomy and great vessel size, while Doppler imaging measures blood flow velocities, intracardiac pressures and hemodynamics.
In order to diagnose CAD and assess whether myocardial ischemia is present, images obtained at rest are compared to those obtained during or immediately after stress. The normal cardiac response to stress is an increase in heart rate and myocardial contractility. A normal stress ECHO result is thus defined as normal LV wall motion at rest and with stress. The hallmark of stress-induced myocardial ischemia is a worsening of wall motion abnormalities or the development of new wall motion abnormalities (see Table 1). The interpretation of wall motion contractility and function, however, is subjective, which creates the possibility of inter-reader and inter-institutional variability. For regional wall motion analysis (WMA), there are 16 segments that are evaluated on the basis of their contractility (score of 1-5). This information then goes into calculating a wall motion score index (WMSI). The larger the infarct, the higher the WMSI since wall motion abnormalities become more severe. The WMSI also increases if there is viable myocardium that does not receive sufficient blood flow when under stress (2;3)
Table 1: Interpretation of ECHO Images Obtained at Rest and During Stress.
| Rest | Stress | Diagnosis |
|---|---|---|
| Normal | Normal | Normal |
| Normal | Abnormal | Ischemia |
| Abnormal | Normal (stunned) or Biphasic (subsequent deterioration at peak – hibernating) |
Viability |
| Abnormal | Abnormal | Necrotic |
Table adapted from Sicari et al. (3)
Standardized protocols exist for performing stress ECHO. The most commonly used stressors are exercise, dobutamine and dipyridamole. Exercise ECHO can be performed either using a treadmill or bicycle protocol, whereby images are obtained during the various levels of exercise or immediately following exercise. Interpretation is based on a comparison of images at rest and at peak exercise. A major challenge of exercise stress ECHO is that images need to be obtained rapidly after exercise. Although exercise is the most widely used stress protocol, patients may be unable to exercise, may exercise submaximally, or their results may be uninterpretable. (3) In these patients, a pharmacological agent is used to induce stress. Dobutamine, a vasoconstrictor, is the most commonly employed pharmacological agent in North America, while dipyridamole, a vasodilator, is more commonly used in Europe. (3) Adenosine is another vasodilator but is used much less frequently. These agents induce ischemia through different hemodynamic mechanisms. Dobutamine primarily increases myocardial oxygen demand and dypyridamole and adenosine mainly decrease subendocardial flow supply.
Stress ECHO reports include a baseline and stress assessment of wall motion and systolic function in addition to the stress protocol that was employed, the exercise time or dose of pharmacological agent used, the maximum heart rate achieved, whether the level of stress was adequate, the blood pressure response, the reason for test termination if the results are incomplete, any cardiac symptoms during the test, and electrocardiographic (ECG) changes or significant arrhythmias. (2)
Clear endocardial definition is vital for optimal image interpretation, yet it is estimated that approximately 30% of patients have sub-optimal stress ECHO exams. (5;6) Opacification of the LV cavity and endocardial border detection can be difficult in obese patients and those with lung disease. To overcome this barrier, contrast agents for LV opacification have been developed to enhance endocardial border detection. (5;6) The Medical Advisory Secretariat has undertaken an evidence-based analysis of myocardial contrast ECHO (MCE) and, based on the evidence found, the addition of contrast imaging in patients with suboptimal ECHO results significantly improved interpretability of the results.
The diagnostic accuracy of stress ECHO exams may also be impacted by gender with men and women exhibiting different CAD patterns and responses to cardiac testing. Women are also more likely to have non-obstructive or single-vessel disease when compared to men, which decreases the diagnostic accuracy. (7-9)
There are no known contraindications for stress ECHO. In terms of safety, it does not involve any ionising radiation, which is an important consideration for repeat testing, and serious side effects are uncommon at less than one in 1000 stress ECHO exams. (10) The most common cardiovascular side effects of those that do occur are angina, hypotension and cardiac arrhythmias. (2)
The combination of its relative ease of use and low risk of adverse events have made stress ECHO a popular method of CAD diagnosis; however, there is also the potential for ECHO to be overused or misused. Some patients may not benefit from a stress ECHO test or would have achieved a similar benefit without the addition of the test. Inappropriate use could be costly and may also prompt potentially harmful subsequent testing or treatment such as unnecessary coronary revascularization. (11)
For the future, it is envisioned by experts in the field that there will be more quantitative methods for assessment, improved image quality and enhanced portability. Several recent advancements in stress ECHO have been developed to overcome some of the challenges with the qualitative interpretation of results and image quality. Real-time 3D ECHO has been introduced to enhance endocardial border definition. Initial studies have been encouraging, but the additional value of this technique over traditional WMA remains unknown. (3) Tissue Doppler imaging is another advancement that enables a quantitative and reproducible assessment of myocardial velocity and deformation. Based on the available evidence and expert feedback, this quantitative approach is not yet ready for widespread use. (3) Lastly, hand-carried cardiac ultrasound devices have been developed to diagnose CAD in emergency rooms and at bedsides, but again, evidence on the diagnostic accuracy and clinical utility of these devices is limited. (12)
Alternative Technologies
Stress ECHO is not the only imaging modality that can be used to diagnose CAD. Alternatives to stress ECHO for the diagnosis of CAD currently licensed for use in Canada include single photon emission computed tomography (SPECT), cardiac magnetic resonance imaging (c-MRI) and computed tomography (CT) angiography. All of these technologies are non-invasive and used to make decisions on which patients should go on to CA, which is the only modality that provides a definitive diagnosis based on anatomical information and the degree of coronary artery stenosis, although its invasive nature increases the risk of adverse events. (13)
Regulatory Status
ECHO units are licensed by Health Canada as class III devices. There are currently 8 ECHO systems licensed for sale in Canada, as summarized in Table 2.
Table 2: Echocardiography systems licensed by Health Canada.
| Manufacturer | Device |
|---|---|
| General Electric Medical Systems Israel, Ultrasound | VIVID S6 Ultrasound System VIVID 7 Dimension Ultrasound System VIVID S5 Ultrasound System |
| Siemens Medical Solutions USA, Inc. | Acuson Sequoia C512 Echocardiography System |
| Imacor INC. | Imacor Zura Imaging System |
| St. Jude Medical | View Flex Ultrasound Catheter |
| Dymax Corporation | Site Rite IV Ultrasound System |
| Volcano Corporation | S5 Imaging System |
Evidence-Based Analysis
Research Questions
What is the diagnostic accuracy of stress ECHO in the diagnosis of patients with suspected CAD compared to the reference standard of CA?
What is the clinical utility3 of stress ECHO?
Methods
Literature Search
A literature search was performed on August 28, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 until August 21, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any relevant studies not identified through the search. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established.
Inclusion Criteria
Systematic reviews, meta-analyses, randomized controlled trials, prospective observational studies, retrospective analyses
Minimum sample size of 20 enrolled patients
Comparison to CA (reference standard)
Definition of CAD specified as either ≥50%, ≥70% or ≥75% coronary artery stenosis on CA
Reporting accuracy data on individual patients (rather than stratified by segments of the heart)
English and human studies only
Exclusion Criteria
Non-systematic reviews, case reports
Grey literature (e.g., conference abstracts)
Insufficient data for independent calculation of sensitivity and specificity
Use of ECHO for purposes other than diagnosis of CAD (e.g., arrhythmia or valvular disease)
Trans-esophageal ECHO since its primary use is for non-CAD indications such as endocarditis, intracardiac thrombi, valvular disorders
Only resting ECHO performed
Outcomes of Interest
Accuracy outcomes (sensitivity, specificity, positive predictive value, negative predictive value)
Costs
Statistical Analysis
Pooled estimates of sensitivity, specificity and diagnostic odds ratios (DORs) were calculated using a bivariate, binomial generalized linear mixed model. (14) Statistical significance was defined by P values of less than 0.05, where “false discovery rate” adjustments were made for multiple hypothesis testing. (15) The bivariate regression analyses were performed using SAS version 9.2 (SAS Institute Inc.; Cary, NC, USA). Summary receiver operating characteristic (sROC) curves weighted by inverse variance were produced using Review Manager 5.0.22 (The Nordiac Cochrane Centre, The Cochrane Collaboration, 2008). All other statistics were calculated using STATA version 10.1 (StataCorp; Texas, USA). The area under the sROC curve was estimated by numerical integration with a cubic spline (default option).
Quality of Evidence
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (16) as presented below.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
The search identified 866 articles published from January 1, 2004 to August 21, 2009. Among these was a high quality systematic review by Heijenbrok-Kal et al. (1), which compared the diagnostic performance of stress ECHO, SPECT and electron beam CT for CAD using CA as the reference standard. The authors performed a meta-analysis on 351 patient-series which were reported in 11 meta-analyses.
Given the vast amount of published literature on stress ECHO, it was decided to use the studies contained in the comprehensive review by Heijenbrok-Kal et al. (1) as a basis for the MAS evidence-based analysis. Out of the 11 meta-analyses contained in this review, there were 7 systematic reviews containing information on 226 studies on Stress ECHO compared to CA for the diagnosis of CAD (see Table 3).
Table 3: Characteristics of meta-analyses included in Heijenbrok-Kal et al., 2007 systematic review*.
| Study | Search Dates | Type of Stress ECHO | No of Studies Included | Patient Characteristics | % Diagnosed With CAD on CA | Pooled Sensitivity | Pooled Specificity | Other Technologies Evaluated |
|---|---|---|---|---|---|---|---|---|
| O’Keefe et al., 1995 (17) | Up until Dec 1993 | Ex ECHO Dob ECHO |
12 (913 patients) 14 (1049 patients) |
NR | 72 69 |
81 81 |
89 83 |
Ex SPECT, Ad SPECT |
| Fleischmann et al., 1998 (18) | Jan 1990 - Oct 1997 | Ex ECHO | 24 (2637 patients) | mean age 59 yrs 69% men 20% previous MI |
66 | 85 | 77 | Ex SPECT |
| Picano et al., 2000 (19) | 1985 -1998 | Dip ECHO Dob ECHO |
38 (2856 patients) 59 (5082 patients) |
26% previous MI 26% previous MI |
65 73 |
73 81 |
91 83 |
None |
| de Albuquerque Fonseca et al., 2001 (20) | 1997-2000 | Dip ECHO Ex ECHO |
8 (533 patients) 8 (533 patients) |
NR | 74 74 |
72 79 |
92 82 |
None |
| Kim et al, 2001 (21) | Jan 1997 - June 1999 | Ad ECHO | 6 (516 patients) | mean age 65 yrs 71% men 31% previous MI |
73 | 72 | 91 | Ad SPECT, Dip SPECT, Dob SEPCT, EBCT |
| Dip ECHO | 20 (1835 patients) | mean age 56 yrs 72% men 15% previous MI |
67 | 70 | 93 | |||
| Dob ECHO | 40 (4097 patients) | mean age 59 yrs 66% men 26% previous MI |
70 | 80 | 84 | |||
| Imran et al., 2003 (22) | 1986 - March 2001 | Dip ECHO | 10 (651 patients) | NR | 67 | 70 | 90 | Mix SPECT |
| Noguchi et al., 2005 (23) | 1981 - Dec 2001 | Ex ECHO | 44 (3714 patients) | mean age 57 yrs 73% men 11% previous MI |
70 | 83 | 84 | None |
| Ad ECHO | 11 (678 patients) | mean age 64 yrs 76% men 21% previous MI |
77 | 68 | 81 | |||
| Dob ECHO | 80 (7914 patients) | mean age 59 yrs 70% men 13% previous MI |
69 | 80 | 85 | |||
| Dip ECHO | 40 (3466 patients) | mean age 56 yrs 73% men 9% previous MI |
70 | 71 | 92 |
Ad refers to adenosine; CAD, coronary artery disease; Dip, dipyridamole; Dob, dobutamine; ECHO, echocardiography; Ex, Exercise; Mix, combination of stressors; NR, not reported.
Table adapted from Heijenbrok-Kal et al. (1)
To further refine the analysis, additional inclusion criteria were applied to the 226 studies included in Heijenbrok-Kal et al. (1) systematic review. For feasibility, studies were included if they were published after 1995 and if the sample size was greater than or equal to 20 patients. Applying these additional criteria yielded a total of 105 observational studies containing information on 13, 035 patients, which were used for the MAS evidence-based analysis (see Table 4). In these studies, stress ECHO results were compared to those obtained with CA results in the same patient, thus patients acted as their own controls. Data was abstracted from the original systematic reviews. When information was missing or incomplete, an attempt was made to extract data from the original studies.
Table 4: Quality of evidence of included studies on stress ECHO.
| Study Design | Level of Evidencet |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic review of RCTs | 1 | |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3a | 105 (Patients acted as their own controls) |
| Non-RCT with historical controls | 3b | |
| Non-RCT presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multisite) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modelling | 4d | |
| Case series presented at international conference | 4(g) | |
| Total | 105 |
RCT refers to randomized controlled trial;
Table adapted from Goodman, 1996 (24)
The studies were published between 1995 and 2001, the majority of patients were male (67.6%, n=89 studies) and the mean age of patients was 59 years (n=88 studies). Data on women and the elderly was more limited. The prevalence of CAD ranged from 19% to 94%, with a mean value of 70%. In studies that examined pharmacological stress the mean CAD prevalence was slightly higher than in trials involving exercise stress (71.0% versus 66.5%). Appendix 2 further outlines characteristics of the studies included in the analysis.
Of the 105 studies included in the MAS analysis, the majority of studies examined the diagnostic accuracy of stress ECHO with pharmacological agents. A study was counted twice if data was reported on different stress agents. Six studies used adenosine, 26 used dipyridamole and 77 used dobtamine, which is the most commonly used pharmacological stress ECHO agent in Ontario. A further 18 studies employed exercise (dynamic and/or static) as the stressor.
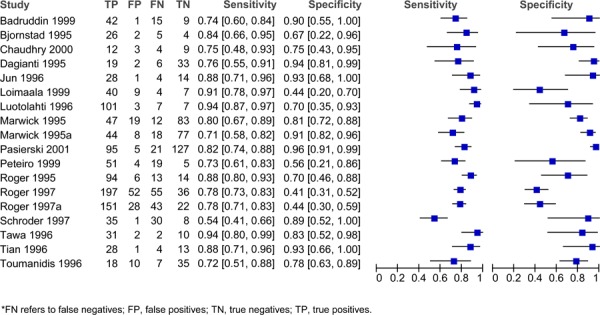
Sensitivity and specificity varied across the different studies and different stress agents. For adenosine, the sensitivity ranged from 0.66 to 0.88 and the specificity ranged from 0.10 to 1.00 (Figure 1). For dipyradamole, the sensitivity ranged from 0.42 to 1.00 and the specificity ranged from 0.14 to 1.00 (Figure 2). For dobutamine, the sensitivity ranged from 0.40 to 1.00 and the specificity ranged from 0.49 to 1.00 (Figure 3). Lastly, for exercise the sensitivity ranged from 0.54 to 0.94 and the specificity ranged from 0.41 to 0.96 (Figure 4).
Figure 1: Estimates of sensitivity and specificity derived from studies comparing adenosine stress ECHO to CA for the diagnosis of CAD.
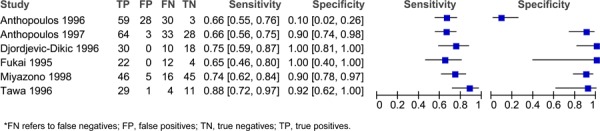
Figure 2: Estimates of sensitivity and specificity derived from studies comparing dipyridamole stress ECHO to CA for the diagnosis of CAD.
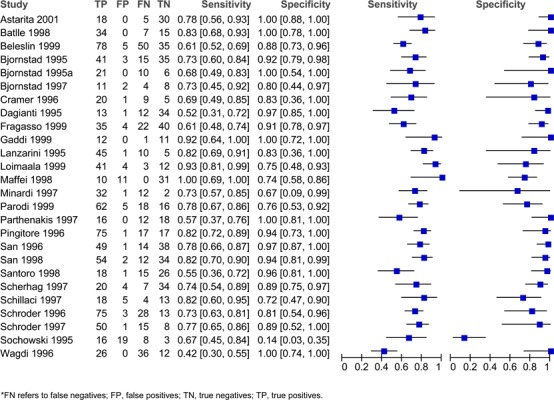
Figure 3: Estimates of sensitivity and specificity derived from studies comparing dobutamine stress ECHO to CA for the Diagnosis of CAD.
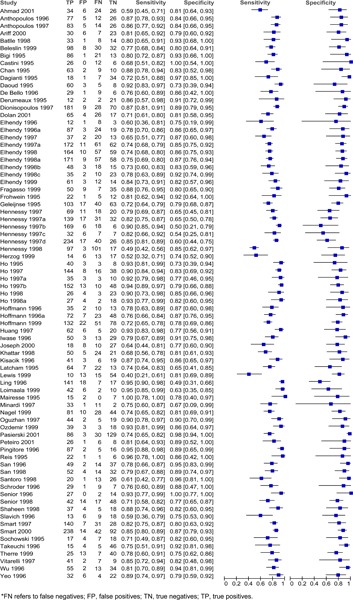
Figure 4: Estimates of sensitivity and specificity derived from studies comparing exercise stress ECHO to CA diagnosis of CAD.
Pooled estimates of sensitivity and specificity were calculated using a bivariate, binomial generalized linear mixed model. (Table 5) When all stress agents were combined, the pooled sensitivity was 0.80 and the pooled specificity was 0.84. There were no significant differences in the estimates of pooled sensitivity and specificity across the different pharmacological stress agents (Table 6). In comparison to pharmacological stress agents, exercise stress had a higher estimate of pooled sensitivity, yet a lower estimated pooled specificity. Nevertheless, the observed differences in sensitivity and specificity between exercise and pharmacological stress were not significant (sensitivity P=0.62, specificity P=0.36). While these differences were not significant, it is important to note that different patient populations would be referred for pharmacological or exercise stress ECHO tests based on ability to exercise.
Table 5: Estimates of pooled sensitivity, pooled specificity and DORs derived from studies comparing stress ECHO to CA for the Diagnosis of CAD.
| Type of Stress | No of Studies (no of patients) |
Pooled Sensitivity (95%CI) |
Pooled Specificity (95%CI) |
DOR (95% CI) |
|
|---|---|---|---|---|---|
| All Stress ECHO | 127*
(13035) |
0.80 (0.77 – 0.82) |
0.84 (0.82 – 0.87) |
20.64 (16.63 – 24.64) |
|
| Subgroups | |||||
| Pharmacological agents: Combined | 109 (11223) |
0.79 (0.71 – 0.87) |
0.85 (0.83 – 0.88) |
21.71 (17.04 – 26.38) |
|
| Adenosine | 6 (501) |
0.79 (0.62 – 0.83) |
0.83 (0.70 – 0.96) |
12.86 (0.02 – 25.71) |
|
| Dobutamine | 26 (1931) |
0.81 (0.79 – 0.83) |
0.84 (0.81 – 0.87) |
22.33 (16.45 – 28.21) |
|
| Dipyridamole | 77 (8791) |
0.74 (0.69 – 0.79) |
0.90 (0.86 – 0.94) |
22.33 (16.45 – 28.21) |
|
| Exercise | 18 (1812) |
0.81 (0.76 – 0.86) |
0.79 (0.71 -0.87) |
16.11 (7.85 – 24.37) |
|
A study was counted twice if data was reported on different stress agents. DOR, refers to diagnostic odds ratio
Table 6: Comparisons of pooled sensitivity and pooled specificity.
| Comparisons | Sensitivity (P-value) |
Specificity (P-Value) |
|---|---|---|
| ECHO Exercise vs. Pharmacologic | 0.62 | 0.36 |
| ECHO Exercise vs. Adenosine | 0.31 | 0.83 |
| ECHO Exercise vs. Dipyridamole | 0.20 | 0.10 |
| ECHO Exercise vs. Dobutamine | 0.99 | 0.47 |
| ECHO Adenosine vs. Dipyridamole | 0.90 | 0.47 |
| ECHO Adenosine vs. Dobutamine | 0.30 | 0.95 |
| ECHO Dipyridamole vs. Dobutamine | 0.09 | 0.10 |
The diagnostic odds ratio (DOR), which is the odds of a positive test in patients with disease compared with the odds of a positive test results in those without the disease, was also calculated. The DOR for all stress sources combined was found to be 20.64, while for pharmacological stress it was 21.71 and for exercise stress it was 16.11.
Summary Receiver Operator Curves (SROC) were generated (Figure 5) and the AUC was calculated. When all stress agents were combined, the AUC for stress ECHO was 0.895 (Table 7). In ranking the AUC of the different stress agents, dobutamine had the highest AUC, followed closely by dipyridamole, adenosine and exercise (Figure 5).
Figure 5: SROC curve for stress ECHO for the Diagnosis of CAD.
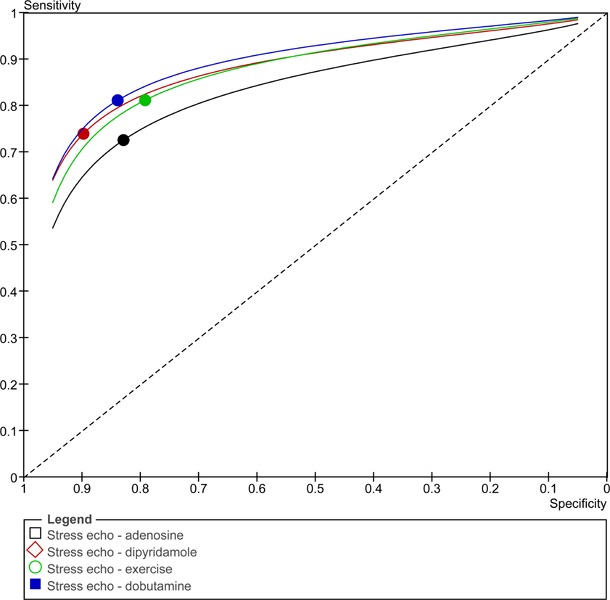
Table 7: AUC for stress ECHO.
| Group | AUC |
|---|---|
| All Stress ECHO | 0.895 |
| Subgroups | |
| Pharmacological agents - combined | 0.898 |
| - Adenosine | 0.893 |
| - Dobutamine | 0.905 |
| - Dipyridamole | 0.899 |
| Exercise | 0.875 |
AUC refers to area under the curve
Given that it was not possible to statistically compare the AUCs for the different stress agents, a subgroup analysis of the DORs was performed. Comparisons of the different pharmacological stressors and between the pharmacological stressors and exercise revealed that there were no significant differences in the pooled DORs between the different stress agents (Table 8).
Table 8: Subgroup analyses of stress agents using DORs for Stress ECHO.
| Subgroup Comparison – Stress Agents | P-Value |
|---|---|
| Exercise vs. Adenosine | 0.87 |
| Exercise vs. Dipyridamole | 0.66 |
| Exercise vs. Dobutamine | 0.66 |
| Adenosine vs. Dipyridamole | 0.66 |
| Adenosine vs. Dobutamine | 0.66 |
| Dipyridamole vs. Dobutamine | 0.87 |
| Exercise vs. Adenosine | 0.87 |
| Exercise vs. All Pharmacologic | 0.66 |
The definition of what qualified as significant CAD, as measured by the degree of coronary artery stenosis, varied across the studies. The majority of the studies used a threshold of 50% stenosis (n=77), but some studies defined significant disease as 70% stenosis (n=18), and one study used a threshold of 75% stenosis. This information was unknown or not reported for 38 studies. According to Jones et al., it may be possible to include studies with differing thresholds in the same meta-analysis. (25) This is due to the fact that even when the reported thresholds are constant between studies, the true thresholds will differ owing to individual reporting styles and variance in image interpretation. A subgroup analysis was performed to ensure that the diagnostic accuracy did not differ by the definition of CAD. Studies that defined significant CAD as greater than 50% coronary artery stenosis (DOR 21.07, 95%CI: 15.21 – 26.94) did not significantly differ in diagnostic accuracy compared to studies that defined significant CAD as greater than 70% stenosis (DOR 19.50, 95%CI: 8.56 – 30.44; P=0.87).
Of the 92 studies that contained information on patient MI history, 66 included patients with a previous MI. The proportion of patients with a previous MI ranged from 8% to 100% (mean= 33.8%). Even though the analysis was intended to focus on patients suspected of CAD and therefore not have a history of previous MI, it was still possible to include these studies in the analysis since the interpreter of the ECHO images was blinded to MI status. In addition, the diagnostic accuracy of studies that included patients with a previous MI (DOR 21.34, 95%CI: 15.05 – 27.63) did not differ from studies that did not include patients with a history of a previous (DOR 20.40, 95%CI: 11.42 – 29.38) (P=0.87).
Results of Evidence-Based Analysis: Additional Systemic Reviews
Two additional systematic reviews published after the Heijenbrook-Kal et al. (1) study were identified, however, these reviews focused on different populations to those previously described in the MAS analysis. The studies included in the MAS review did not limit to specific populations. The first of these, by Geleijnse et al. (9) focused on dobutamine stress ECHO for the detection of CAD in women and the second, by Biagini et al. (26), focused on the accuracy of stress ECHO for the diagnosis of CAD and prediction of cardiac events in patients with left bundle branch block (LBBB). Given that these reviews focused on particular populations, they were not directly incorporated into the MAS analysis. Nevertheless, many of the original studies included in these two existing systematic reviews were included in the Heijenbrook-Kal et al. (1) review and were, therefore, in the MAS analysis. Briefly, Geleijnse et al. (9) included 14 studies (901 patients) that compared the diagnostic accuracy of dobutamine stress ECHO to CA, either solely in women or in both women and men. The pooled sensitivity was 72% and the specificity was 88%. Seven studies compared the diagnostic accuracy of stress ECHO in men and women, achieving a pooled sensitivity of 77% in both women and men and a pooled specificity of 81% in women and 77% in men. The authors concluded that despite some theoretical limitations, the test has reasonable sensitivity and excellent specificity for the detection of CAD in women. These results are comparable to those found in the MAS analysis (the majority of studies included in the Geleijnse et al. review were also included in the MAS analysis).
A second systematic review by Biagini et al. (26) focused on the diagnostic accuracy of stress ECHO in patients with LBBB. In patients with LBBB, mechanical asynchrony between the left and right ventricle results in abnormal septal motion interfering with the interpretation of wall motion abnormalities. They included information on six studies (226 patients), of which five employed a pharmacological stress agent and one used exercise-induced stress. The pooled estimates of sensitivity and specificity were 74.6% and 88.7%, respectively. Although this meta-analysis obtained different estimates of diagnostic accuracy in LBBB patients, they varied only slightly from those obtained in the MAS analysis.
Limitations of Analysis
One of the limitations of the current analysis is that study selection was based on the Heijenbrok-Kal et al. (1) review for feasibility reasons. As a result, the studies were published between 1995 and 2001 and more recent studies were not included in the analysis. According to experts, improvements in ECHO imaging have occurred since then. In order to verify that there have been no meaningful changes in the diagnostic accuracy of stress ECHO over time, a subgroup analysis was performed to compare the sensitivity and specificity of studies published prior to 2000 (n=117) to those published after 2000 (n=10). Over time, there have been no significant changes in the sensitivity (P=0.61) and specificity (P=0.20) of stress ECHO (Table 8).
The MAS evidence-based analysis on stress contrast ECHO also examined five studies that directly compared stress ECHO versus MCE that were published between 2004 and 2007 (for additional information please refer to the EBA on stress contrast ECHO). The estimates of pooled sensitivity for stress ECHO are comparable, though the pooled specificity derived from these newer studies is slightly lower compared to those published prior to 2000 (see Table 9). These results conflict with those from the aforementioned subgroup analysis, however, far fewer newer studies were included. It is possible that a more recent year of publication detrimentally impacts estimates of diagnostic accuracy. Potential explanations include publication bias and selection bias in older studies with strict inclusion and exclusion criteria. (1) Overall, there remains uncertainty on the impact of publication date on estimates of diagnostic accuracy but it appears that the differences over time are not significant.
Table 9: Effect of Publication Date on Diagnostic Accuracy.
| Subgroup Comparison, Year of Publication | Number of studies |
Pooled Sensitivity (95%CI) |
Pooled Specificity (95%CI) |
|---|---|---|---|
| Studies Published Prior to 2000 | 117 | 0.80 (0.78 - 0.82) |
0.84 (0.81 - 0.87) |
| Studies Published After 2000 | 10 | 0.76 (0.69 - 0.84) |
0.90 (0.84 - 0.96) |
| Studies Published 2004-2007 (Data derived from studies included in the direct comparison of Stress ECHO versus Contrast Stress ECHO) |
5 | 0.76 (0.70 - 0.81) |
0.75 (0.67 - 0.81) |
Another limitation of the analysis is that stress ECHO was compared to the reference standard of CA, although these modalities diagnose CAD with different approaches. Diagnosis of CAD with stress ECHO relies on functional information such as wall motion, while diagnosis with CA is based on anatomical information (e.g., the degree of coronary artery stenosis).
GRADE Quality of Evidence
The quality of the body of evidence was assessed according to the GRADE Working Group criteria for diagnostic tests (results in Table 10). Overall, the quality was consistent across the cross-sectional studies that assessed accuracy. In studies with multiple comparisons, the assessors were blinded to data from the other imaging modalities. All studies compared stress ECHO to CA as the reference standard. The majority of studies recruited patients who were referred for CA, which may affect the generalizability of the findings in terms of the pre-test probability of CAD.
Table 10: GRADE Quality Assessment of Diagnostic Accuracy Studies for Stress ECHO Compared to Coronary Angiography for the Diagnosis of CAD.
| Factor | Explanation | GRADE |
|---|---|---|
| Risk of Bias | ||
| Study design | Observational cross-sectional studies (127 studies) | High |
| Limitations | No serious limitations | Unchanged |
| Indirectness | ||
| Outcomes | Diagnostic tests are considered as surrogate outcomes | Reduced by one level → Moderate |
| Patient populations, diagnostic test, comparison test, and indirect comparisons | Some studies included a mix of patients with known and suspected CAD. In a subgroup analysis, there was no significant difference in diagnostic odds ratio between groups. The threshold in defining significant CAD varied across studies (i.e., >50%stenosis or >70% stenosis), however a subgroup analysis revealed there were no significant difference in diagnostic accuracy between groups. The majority of studies recruited patients who were being referred for coronary angiography which may affect the generalizability of the findings. |
Reduced by one level → Low |
| Important inconsistency in study results | No serious inconsistency | Unchanged |
| Imprecise evidence | Some imprecision but confidence intervals not overly wide for estimates of sensitivity and specificity | Unchanged |
| Publication bias | Possible, but not considered sufficient to downgrade quality of evidence | Unchanged |
| Quality of Evidence | Low |
As mentioned, there was heterogeneity in the definition of significant CAD, as defined by the percent stenosis. Some studies also included patients with previous MI, however, assessors were blinded to patient history. Lastly, in addition to the subjective interpretation of ECHO images, there was variability in the definition of a positive test result, which may partly explain the different reported sensitivity and specificity across studies. For example, some studies defined a positive test as a new regional wall motion abnormality, while others defined a positive test as the absence of a hyperkinetic contractile response to exercise.
The overall quality of the evidence was graded as low.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Study Question
The objective of this economic analysis is to determine the cost effectiveness of stress ECHO in the diagnosis of patients with suspected CAD, as compared to contrast ECHO, SPECT, cardiac MRI, and CT angiography. The relative cost-effectiveness of these five non-invasive cardiac imaging technologies was assessed in two patient populations: a) out-patients presenting with stable chest pain; and b) in-patients presenting with acute, unstable chest pain. Note that the term “contrast ECHO” used in the following sections refers to stress echocardiography performed with the availability of contrast medium, if needed, due to poor image quality.
Economic Analysis Overview
For the two patient populations, decision analytic models were developed with two reported outcomes: the cost per accurate diagnosis of CAD (true positives and true negatives) and cost per true positive diagnosis of CAD. The physician and hospital costs for the non-invasive imaging tests were taken from 2009 Ontario Health Insurance Plan (OHIP) and the Ontario Case Costing Initiative (OCCI) administrative databases. (27;28) A budget impact analysis (BIA) was then performed to assess the effect of replacing a certain proportion of stress ECHO tests with other cost-effective, non-invasive modalities. The costs presented in the BIA were estimated from Ontario data sources from 2009; the volumes of tests performed were estimated from data from fiscal years 2002 to 2008.
Economic Literature Review
The purpose of the systematic review of economic literature was to identify, retrieve, and summarize studies evaluating the cost-effectiveness of selected cardiac imaging tests for the diagnosis of CAD. Medline and the National Health Service Economic Evaluation Database (NHSEED) were searched from their inception up to October 2009. Included studies were those full economic evaluations describing both costs and consequences of a) CT angiography, b) Cardiac MRI, c) SPECT, d) stress ECHO, and e) stress contrast ECHO in CAD diagnosis. Article selection was performed by independent pairs of researchers. Target data for extraction included: study first author and year of publication, imaging tests compared, type of economic analysis, reported costs and outcomes, incremental cost-effectiveness ratio (ICER), currency, and patient characteristics (i.e., known or suspected CAD and risk of CAD). The primary outcome of interest was the ICER of each imaging test in relation to another test of interest.
Literature Search Results
A total of 883 non-duplicate citations were found from the two electronic databases after applying the literature search strategy. Based on the content of their abstracts, 147 full-text articles were retrieved for further assessment. Of these, 122 were rejected leaving 25 articles for inclusion. Following the data extraction process, 13 studies were excluded (29-40), with 12 studies being ultimately selected for analysis.(10;41-51) (52)
Characteristics of Included Studies
From the 12 studies included, eight assessed the cost-effectiveness of two of the selected imaging tests (44-47;49-51), three evaluated three concomitant technologies (10;41;48) and one study evaluated five technologies.(42)
Five studies were cost-effectiveness analyses, where the most common outcome was cost per correct/successful CAD diagnosis. (41;42;49-51) The other seven studies were cost-utility analyses using cost per quality adjusted life years (QALYs) as their primary outcome.(10;43-48) The time-horizon used across the included studies ranged from 30 days to lifetime, with five studies having 25 years or more of follow-up. (43-45;47;50) The remaining studies used 18 months (10), 3 months (51), and 30 days of analytical time horizon.(46) Four studies did not report the time-horizon used in their analysis.(41;42;48;49)
All included studies evaluated at least one form of ECHO against one of the other selected tests. (10;41-51) The cost-effectiveness of SPECT was studied in nine studies (10;41;43-45;47;48;50;51), three studies assessed CT angiography in comparison to stress ECHO or MRI (42;46;49), while cardiac MRI was compared to each of the three other selected imaging tests in two studies.(10;42) No full economic analysis between CT angiography and SPECT was found in the published literature.
Literature Results for Stress ECHO
The cost-effectiveness of stress ECHO was assessed against three selected cardiac imaging tests: SPECT, CT angiography and cardiac MRI (see Table 11). Nine comparisons were made against SPECT and in three of these stress ECHO was considered dominant (i.e., lower cost, better outcomes).(41;44;45) In one comparison, stress ECHO produced the same amount of QALYs as SPECT, but with higher costs, thus it was not considered cost-effective.(10) In three other comparisons, the base-case ICER per QALY reported for stress ECHO versus SPECT was above the $50,000 threshold.(43;47;50) In all three analyses, however, stress ECHO showed lower costs and worse outcomes, thus still accepted as cost-effective. Another analysis of stress ECHO versus SPECT estimated an ICER per correct CAD diagnosis of CDN $5,029, but stress ECHO was found to be the alternative with lower costs and worst outcomes.(51) The final comparison did not report an ICER for the analysis, however, it was stated that stress ECHO was cost-effective only when the probability of CAD was lower or equal to 20%.(48)
Table 11: Summary incremental cost-effectiveness ratios across selected studies evaluating stress ECHO.
| Study | Comparator | Outcome of interest | Reported as cost-effective? | ICER |
|---|---|---|---|---|
| Dewey et al., 2007 | CT angio | Cost per successful diagnosis | No | Not reported* |
| Khare et al., 2008 | CT angio | Cost per QALY | No | Dominated |
| Rumberger et al., 1999 | CT angio | Cost per correct diagnosis | No | Not reported† |
| Dewey et al., 2007 | MRI | Cost per successful diagnosis | Yes | Not reported‡ |
| Sharples et al., 2007 | MRI | Cost per QALY | Yes | GBP (2006) £13,200 |
| Bedetti et al., 2008 | SPECT | Cost per correct diagnosis | Yes | Dominant |
| Garber et al., 1999 | SPECT | Cost per QALY | Yes | USD (1996) $78,444** |
| Hayashino et al., 2004 | SPECT | Cost per QALY | Yes | Dominant |
| Hernandez et al., 2007 | SPECT | Cost per QALY | Yes | Dominant |
| Kuntz et al., 1999 | SPECT | Cost per QALY | Yes | USD (1996) $62,800** |
| Lee et al., 2002 | SPECT | Cost per QALY | No | Not reported§ |
| Sharples et al., 2007 | SPECT | Cost per QALY | No | More costly, same QALYs |
| Shaw et al., 2006 | SPECT | Cost per LYS | Yes | USD (2003) $72,187** |
| Tardif et al., 2002 | SPECT | Cost per correct diagnosis | ND | CDN (2000) $5,029 |
Abbreviations: CT angio = CT angiography, ND = Not defined
At a pre-test likelihood of 60%, CT angiography was cost-effective.
For prevalence of disease <=70%, CT angiography was considered cost-effective.
Both not cost effective when compared to CT angiography.
SPECT was cost-effective when the probability of CAD was ≥=30%. Stress ECHO was cost-effective when the probability of CAD was <=20%.
Stress ECHO was the alternative reporting lower cost and worst outcome.
When compared against CT angiography, ECHO was not considered cost-effective in all three analyses.(42;46;49) In one of the comparisons, stress ECHO was dominated (i.e., higher cost, worst outcomes).(46) The remaining studies evaluated the cost per correct/successful diagnosis of CAD, but they did not report an ICER value of stress ECHO versus CT angiography. Both studies reported that under pre-test likelihood or prevalence of CAD greater than 60%, CT angiography was the cost-effective strategy.(42;49)
Two economic evaluations compared stress ECHO to MRI.(10;42) In one analysis, stress ECHO was found to be cost-effective over MRI with a reported base-case ICER per QALY of GBP £13,200.(10) The remaining study did not report an ICER, although it was stated that both stress ECHO and MRI were not considered cost-effective when compared to CT angiography. (42)
Conclusion of Systematic Review
Overall, CT angiography was found to be cost-effective or cost-saving in all four comparisons of that technology, stress ECHO was found cost-effective in eight of the 13 comparisons in which it was evaluated, and SPECT was found cost-effective in three of the nine comparisons. Cardiac MRI was not found to be cost-effective or cost-saving in any of the four comparisons found.
According to the published economic data from the literature, CT angiography is often found to be cost-effective when compared to other technologies. SPECT and stress ECHO were also found to be cost-effective in several of the comparative studies examined, while cardiac MRI was not cost-effective in any study. Limitations to these conclusions apply, such as the analyses found in the literature evaluated other forms of the selected cardiac imaging tests which might change the proposed relative cost-effectiveness.
Decision analytic Cost Effectiveness Analysis
Design
This study was designed as a cost effectiveness analysis, with primary results reported as incremental cost per true positive diagnosis, or incremental cost per accurate diagnosis.
Target Population and Perspective
Two populations were defined for evaluating the cost-effectiveness of an accurate diagnosis (i.e. true positive and true negative diagnoses) of CAD: a) out-patients presenting with stable chest pain; and b) in-patients presenting with acute, unstable chest pain. The first population was defined as persons presenting with stable chest pain, with an intermediate risk of CAD following physical examination and a graded exercise test, as defined by the American College of Cardiology / American Heart Association 2002 Guideline Update for the Management of Patients with Chronic Stable Angina.(53) The second population was defined as persons presenting to emergency for acute, unstable chest pain, and who are admitted to hospital, as defined by the American College of Cardiology / American Heart Association 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction.(54)
The analytic perspective was that of the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Comparators and Parameter Estimates
The imaging technologies that were compared in the current cost-effectiveness analysis included: CT angiography, stress ECHO with and without a contrast medium, cardiac perfusion stress MRI, and attenuation-corrected SPECT. Test characteristic estimates (i.e. specificity, sensitivity, accuracy) for each cardiac imaging technology were obtained from the systematic review and meta-analysis conducted by MAS and the MOHLTC. Table 12 shows a list of the parameters with corresponding 95% confidence intervals used for both the outpatient and inpatient decision-analytic cost-effectiveness models.
Table 12: Summary parameter estimates for stress ECHO tests.
| Pooled Diagnostic Accuracy | Point Estimate | 95% Lower | 95% Upper |
|---|---|---|---|
| CAD diagnosis: Sensitivity | 0.795 | 0.774 | 0.816 |
| CAD diagnosis: Specificity | 0.842 | 0.819 | 0.865 |
| Additional time for test (compared to GXT) | Average | Low | High |
| Inpatient population: Additional days for test | 1.5 | 1.0 | 2.0 |
| Uninterpretable test result | Average | Low | High |
| Outpatient population: % of tests that are uninterpretable | 15% | 10% | 30% |
| Inpatient population: % of tests that are uninterpretable | 20% | 15% | 30% |
Note: Sensitivity and specificity estimates are taken from the effectiveness literature review of stress ECHO. Other estimates are based on consultations with experts in cardiology.
The average wait-time for each cardiac imaging test was measured as the additional days needed to wait for a non-invasive test compared to the average wait time for a typical graded exercise stress test (GXT). The proportion of tests deemed uninterpretable by expert opinion is shown with a corresponding range of high and low values. The probability of receiving pharmacological stress versus exercise stress is not listed, but reported here for completeness: approximate values of 30% for the stable, outpatient population and 80% for the unstable, inpatient population.
Time Horizon & Discounting
The time horizon for both decision-analytic models (i.e. for outpatient and inpatient populations) was the time required to determine an accurate, or true positive diagnosis of CAD. As a result, the actual time taken to determine the CAD status of patients may differ across non-invasive test strategies.
Model Structure and Outcomes
Figure 6 provides a simplified illustration of the decision-analytic model structure used for the outpatient and inpatient populations. The following two simplifying assumptions were made for the models:
Figure 6: Decision analytic model used to evaluate the cost-effectiveness of cardiac imaging technologies for the diagnosis of CAD.
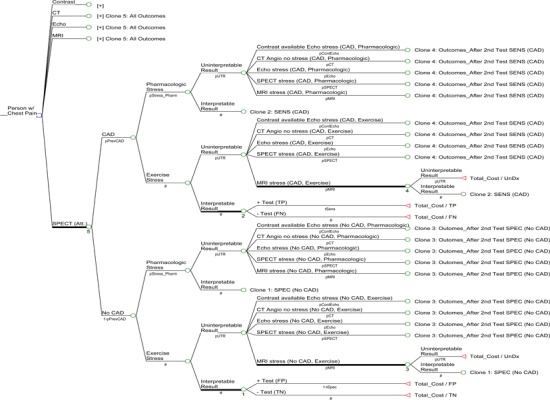
When results of the first cardiac imaging test are un-interpretable, a patient will undergo a second cardiac test. This will be one of the four remaining tests that were not used as the first test.
Should a second test be required, the type of stress (pharmacological or exercise) that a patient receives will be the same type as used in the first.
The short-term outcome presented in this report focuses on an accurate diagnosis of CAD (i.e. true positive and true negative test results). A second outcome of true positive diagnosis was examined for the two models, with results reported in The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario. (52)
Sensitivity Analyses
Various sensitivity analyses were conducted for the outpatient and inpatient populations. First, the prevalence of CAD was varied from 5% to 95% in 5% increments, while all other model estimates were held constant. Willingness-to-pay (WTP) was also varied and a range of results were presented. Second, one-way sensitivity analyses were conducted in which selected estimates were varied over plausible ranges. The varied parameters included sensitivity and specificity estimates, wait times for imaging tests performed in hospital, as well as the costs of CT angiography, ECHO tests, and cardiac MRI. A third series of sensitivity analyses was conducted that specifically addressed the possibility of unavailable imaging technologies.
Resource Use and Costs
Resource use and costs were derived from Ontario data sources: the OHIP and OCCI administrative databases.(27;28) The cost of conducting each cardiac test was calculated as the sum of the test’s respective professional fees and technical fees, as described in the Ontario Schedule of Benefits and listed in Table 13. Note that for ECHO tests with available contrast agent, the cost for the contrast medium was added whenever the contrast was used in the event of uninterpretable ECHO test result. The cost of this contrast medium was estimated as $170 per vial (single use) through consultation with industry experts. Only this cost was added to the base test cost of contrast ECHO. In general, where an imaging test result was uninterpretable, an additional cost of follow-up with the patient (physician fee) was incurred, as well as the cost for conducting another cardiac imaging test. For out-patients presenting with stable chest pain, a consultation professional fee of $30.60 (OHIP code A608 for “partial assessment”) was used after an uninterpretable test result (one time cost).
Table 13: List of cardiac imaging tests and associated OHIP 2009 costs.
| Technology | List of professional fees | Subtotal | List of technical fees | Subtotal | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac CT | Fee code Cost |
×125 $89.20 |
×417 $64.00 |
$153.20 | Imputed $336.52 |
$336.52 |
$489.72 |
||||||
| Cardiac MRI | Fee code | ×441 | ×445 | ×487 | G319 | Imputed | G315 | G174 | |||||
| (dobutamine stress with gadolinium contrast) | Multiplier | 1.0 | 3.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Cost | $75.55 | $37.80 | $37.75 | $62.65 | $289.35 | $463.06 | $33.65 | $37.00 | $533.71 | $823.06 | |||
| Cardiac SPECT (exercise stress) | Fee code | J866 | J811 | J807 | G319 | J866 | J811 | J807 | G315 | ||||
| Cost | $28.70 | $55.30 | $47.00 | $62.65 | $193.65 | $44.60 | $97.55 | $223.15 | $33.65 | $398.95 | $592.60 | ||
| Cardiac SPECT (dobutamine stress) | Fee code | J866 | J811 | J807 | G319 | J866 | J811 | J807 | G315 | G174 | |||
| Cost | $28.70 | $55.30 | $47.00 | $62.65 | $193.65 | $44.60 | $97.55 | $223.15 | $33.65 | $37.00 | $435.95 | $629.60 | |
| Cardiac SPECT (dipyramidole stress) | Fee code | J866 | J811 | J807 | G112 | J866 | J811 | J807 | G111 | ||||
| Cost | $28.70 | $55.30 | $47.00 | $75.00 | $206.00 | $44.60 | $97.55 | $223.15 | $41.10 | $406.40 | $612.40 | ||
| ECHO (exercise stress) | Fee code | G571 | G578 | G575 | G319 | G570 | G577 | G574 | G315 | ||||
| Cost | $74.10 | $36.90 | $17.45 | $62.65 | $191.10 | $76.45 | $45.15 | $16.45 | $33.65 | $171.70 | $362.80 | ||
| ECHO (dobutamine stress) | Fee code | G571 | G578 | G575 | G319 | G570 | G577 | G574 | G315 | G174 | |||
| Cost | $74.10 | $36.90 | $17.45 | $62.65 | $191.10 | $76.45 | $45.15 | $16.45 | $33.65 | $37.00 | $208.70 | $399.80 | |
| ECHO (dipyramidole stress) | Fee code | G571 | G578 | G575 | G112 | G570 | G577 | G574 | G111 | ||||
| Cost | $74.10 | $36.90 | $17.45 | $75.00 | $203.45 | $76.45 | $45.15 | $16.45 | $41.10 | $179.15 | $382.60 | ||
Notes: Fee codes are taken from the 2009 OHIP fee schedule.(28) Imputed technical fees were based on the proportion of average technical fees associated with above ECHO and SPECT fee code combinations. For cardiac SPECT and ECHO stress tests, an average test cost was calculated using dobutamine and dipyramidole fee codes.
In the case of patients presenting with acute, unstable chest pain, costs for inpatient hospitalization were also included in the model. The total cost of hospitalization was calculated based on the average wait time for each cardiac imaging test and a cost per diem for each day spent in hospital (for the stress ECHO wait time, see Table 12). An additional consultation fee was also used only for the inpatient population: $29.20 (OHIP code C602 for “subsequent visit- first five weeks”) was used for each inpatient day spent in hospital.
Willingness-to-pay
The WTP must be determined by the MOHLTC. As such, all reasonable WTP values presented in the Results and Discussion section are interpreted at two WTP ‘anchors’ representing the estimated cost of the most expensive non-invasive test considered in our model (cardiac MRI perfusion, $804) and the estimated cost of a coronary angiography ($1,433). These anchors are intended to guide discussion only.
Note that the following points are useful in determining the WTP:
An “accurate diagnosis” of CAD can be obtained through a coronary angiography for $1,433. It would thus be reasonable to expect the WTP for an accurate diagnosis through a non-invasive test to resemble this amount; however, an accurate diagnosis does not include the value or benefit of providing additional diagnostic or prognostic information from either non-invasive imaging or coronary angiography
The MOHLTC is currently willing to pay up to $804 for a non-invasive test with less-than-perfect diagnostic accuracy. Its willingness to pay for an accurate diagnosis from such a test thus appears to be greater than $804.
While coronary angiography is invasive, the other tests are non-invasive and would presumably be of greater value (i.e., incur a higher premium). These tests do, however, impose risks not applicable to coronary angiography, such as increased radiation exposure and adverse reaction to contrast agents
These tests are not perfectly accurate. An accurate diagnosis from such a test may be valued less than one from a coronary angiography
Results and Discussion
As shown in Tables 14 and 15, stress ECHO was dominated by both CT angiography and contrast ECHO (that is, it had higher costs and was less effective) in both populations for the outcome of interest (i.e., accurate diagnosis of CAD). Sensitivity analysis results changed very little from those of the base-case analysis, with one exception. When both CT angiography and contrast ECHO were removed from the analysis, stress ECHO appeared to be the most cost-effective strategy for stable outpatients for CAD prevalences ranging between 5% and 30%, and possibly the most cost-effective strategy for a CAD prevalence of up to 95%.
Stress ECHO is generally not cost-effective in comparison to other non-invasive strategies for the diagnosis of CAD in either stable outpatients or acute inpatients. Stress ECHO appears cost-effective only in specific situations where other more cost-effective technologies are unavailable.
Table 14: Cost-effectiveness analysis base case results for stable outpatients.
| Technology | Cost (C) | Δ Cost | Effect (E) | Δ Effect | C / E | ICER |
|---|---|---|---|---|---|---|
| Stress ECHO (contrast) | $433.49 | $81.83 | $530 | N/A | ||
| CT angiography | $517.73 | $84.24 | 87.35% | 5.52% | $593 | $1,527 |
| Stress ECHO | $551.58 | 81.06% | $680 | (Dominated) | ||
| Attenuated SPECT | $634.63 | 82.80% | $766 | (Dominated) | ||
| MRI (perfusion) | $835.47 | 85.15% | $981 | (Dominated) |
Table 15: Cost-effectiveness analysis base case results for acute inpatients.
| Technology | Cost (C) | Δ Cost | Effect (E) | Δ Effect | C / E | ICER |
|---|---|---|---|---|---|---|
| Stress ECHO (contrast) | $1,794.58 | 81.47% | $2,203 | 0 | ||
| Attenuated SPECT | $1,982.91 | $188.32 | 85.16% | 3.68% | $2,329 | $5,113 |
| Stress ECHO | $2,550.87 | 81.77% | $3,120 | (Dominated) | ||
| CT angiography | $3,267.39 | $1,284.48 | 91.92% | 6.77% | $3,554 | $18,981 |
| MRI (perfusion) | $4,918.02 | 88.05% | $5,585 | (Dominated) |
Budget Impact Analysis
The budget impact analysis (BIA) was performed taking the perspective of the MOHLTC and includes both physician and hospital (clinic) costs of non-invasive cardiac imaging tests. Volumes of cardiac tests in Ontario were taken from administrative databases (OHIP, DAD, NACRS) for fiscal years 2004 to 2008. (52) The following technologies were considered in the current BIA for the diagnosis of CAD: ECHO (including both stress and stress with contrast agent available), nuclear cardiac imaging (including MPI and SPECT tests), cardiac MRI, and CT angiography.
In the current BIA, the effect of moving a certain proportion of the volume of specific tests to another, substitute technology was assessed for various scenarios. These scenarios are presented irrespective of whether a technology was found to be cost-effective and are reported as general reference tables. To summarize briefly, stress ECHO tests are the least expensive of the compared cardiac imaging modalities. When the volume of stress ECHO tests is shifted to other technologies, all scenarios result in higher projected costs. If 25% of the stress ECHO tests are moved to other imaging technologies, ensuing projected costs would be higher: from a small cost difference of about $166K per year for contrast available stress ECHO testing to a large difference of $10.2M for cardiac MRI testing. The largest possible cost difference corresponds to replacing 50% of stress ECHO tests with cardiac MRI imaging ($20.5M per year); the smallest possible cost difference occurs by replacing 5% of stress ECHO tests with contrast available stress ECHO imaging ($33.2K per year).
Existing Guidelines for ECHO
There are several existing guidelines published by cardiology and echocardiography societies concerning the clinical application of stress ECHO. Some guidelines also focus on appropriateness criteria for stress ECHO. Table 11 below outlines some of the most widely cited guidelines.
Table 11: Guidelines on ECHO.
| Guideline Developers | Publication Date | Title |
|---|---|---|
| ACC/AHA(4) | 2003 | ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) |
| ACC/AHA/ASE(55;56) | 2003 update to 1997 guidelines | ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) |
| Joint CCS and CSE consensus panel (57) | 2004 | Guidelines for the Provision of Echocardiography in Canada |
| ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR (11) | 2007 | ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine |
| ASE (2) | 2007 | American Society of Echocardiography Recommendations for Performance, Interpretation, and Application of ECHO |
| ACC/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR(58) | 2008 | ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for ECHO: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance: endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine |
| EAE (3) | 2009 | Stress Echocardiography Expert Consensus Statement - Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) |
ACC, American College of Cardiology; ACEP, American College of Emergency Physicians; AHA, American Heart Association; ASE, American Society of Echocardiography; ASNC, American Society of Nuclear Cardiology; CCS, Canadian Cardiovascular Society; CSE, Canadian Society of Echocardiography; EAE, European Association of Echocardiography; SCAI, Society for Cardiovascular Angiography and Interventions; SCCM, Society of Critical Care Medicine SCCT, Society of Cardiovascular Computed Tomography; SCMR, Society for Cardiovascular Magnetic Resonance.
Ontario Health System Impact Analysis
Considerations and/or Implications
The Cardiac Imaging Expert Advisory Panel met on Oct 5th and November 25th, 2009. Experts in ECHO were also consulted and the following comments were made about stress ECHO.
There is no limitation as to where or by which physicians ECHO can be provided
There is growth in the number of ECHO tests performed outside of major centers.
It is estimated that greater than 80% of cardiologists practicing in the community have ECHO and stress capabilities situated on-site within their own clinics. Of these, it is estimated that roughly half of cardiologists practicing in the community perform stress ECHO.
Outpatient stress ECHO facilities perform exclusively exercise ECHO.
In-hospital stress ECHO labs employ pharmacological stress agents and exercise as stress in roughly equal amounts.
Dobutamine is the pharmacological stress agent that is most frequently used in Ontario
There are no accessibility issues for ECHO.
Since analysis of ECHO images is subjective in nature, the physician’s degree of expertise and the technologist’s experience largely impacts confidence in test results.
ECHO image quality may have improved over time.
3-Dimensional ECHO is still in developmental phases.
Newer quantitative methods of interpreting ECHO images are not yet routinely used.
The physician billing structure for stress ECHO should be advised to reflect that a minimum ECHO study to perform diagnostic information would include an M-mode, D-dimensional and Doppler component.
There are concerns about inappropriate use of stress ECHO. Emerging appropriateness criteria should be examined and adapted for Ontario. This should include training requirements for the performance and interpretation of stress ECHO.
Conclusion
Given the vast amount of published literature on stress ECHO, it was decided to use the studies contained in the comprehensive review by Heijenbrok-Kal et al. (1) as a basis for the MAS analysis. In applying our inclusion and exclusion criteria, 105 observational studies containing information on 13,102 patients were included in our analysis. Six studies examined stress ECHO with adenosine, 26 with dipyridamole and 77 with dobutamine, which is the most commonly used pharmacological stress ECHO agent in Ontario. A further 18 studies employed exercise as the stressor.4 The prevalence of CAD ranged from 19% to 94% with a mean estimated prevalence of 70%. Based on the results of these studies the following conclusions were made:
Based on the available evidence, stress ECHO is a useful imaging modality for the diagnosis of CAD in patients with suspected disease. The overall pooled sensitivity is 0.80 (95% CI 0.77 – 0.82) and the pooled specificity is 0.84 (95% CI 0.82 – 0.87) using CA as the reference standard. The AUC derived from the sROC curve is 0.895 and the DOR is 20.64.
For pharmacological stress, the pooled sensitivity is 0.79 (95% CI 0.71 – 0.87) and the pooled specificity is 0.85 (95% CI 0.83 – 0.88). When exercise is employed as the stress agent, the pooled sensitivity is 0.81 (95% CI 0.76– 0.86) and the pooled specificity is 0.79 (95% CI 0.71 – 0.87). Although pharmacological stress and exercise would be indicated for different patient populations (based on ability to exercise) there were no significant differences in sensitivity and specificity between the two groups.
Based on the opinions of clinical experts, the diagnostic accuracy of stress ECHO depends on the patient population, the expertise of the interpreter, and the quality of the image.
Appendices
Appendix 1: Literature Search Strategies
Search date: August 28, 2009
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1950 to August Week 3 2009≥
Search Strategy:
exp Myocardial Ischemia/ (301645)
(coronary adj2 arter* disease*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (58420)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or isch?emi* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. (258913)
(myocardi* adj2 hibernat*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (843)
(stenocardia* or angina).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (53364)
heart attack*.mp. (2887)
exp Heart Failure/ (66084)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. (107720)
exp Ventricular Dysfunction, Left/ (14865)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (22713)
or/1-10 (462748)
exp Echocardiography/ (82415)
echocardiogr*.ti,ab. (79353)
13 or 12 (106410)
11 and 14 (39243)
exp Exercise Test/ (41317)
(test* adj2 (exercise or bicycle or treadmill or stress or ergometr* or pharmacolog* or dobutamine or dipyridamole or persantine or adenosine or regadenoson or lexiscan)).ti,ab. (31014)
16 or 17 (56646)
18 and 15 (4497)
limit 19 to (english language and humans and yr=”2007 -Current”) (453)
Database: EMBASE <1980 to 2009 Week 34>
Search Strategy:
exp ischemic heart disease/ (238393)
exp coronary artery disease/ (88415)
exp stunned heart muscle/ (1519)
(coronary adj2 arter* disease*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (71532)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or ischemi* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (275725)
(myocardi* adj2 hibernat*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1057)
(stenocardia* or angina).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (46318)
heart attack*.mp. (2025)
exp heart failure/ (125232)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (107656)
exp heart left ventricle failure/ (9312)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (16093)
or/1-12 (430251)
exp echocardiography/ (93200)
echocardiogra*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (104880)
14 or 15 (104927)
exp exercise test/ (16129)
exp pharmacologic stress testing/ (258)
(test* adj2 (exercise or bicycle or treadmill or stress or ergometr* or pharmacolog* or dobutamine or dipyridamole or persantine or adenosine or regadenoson or lexiscan)).ti,ab. (26337)
or/17-19 (33746)
16 and 20 (4760)
exp exercise electrocardiography/ (2329)
21 or 22 (6823)
13 and 23 (5316)
limit 24 to (human and english language and yr=”2007 -Current”) (651)
Appendix 2: Studies Examining the Diagnostic Accuracy of Stress ECHO for CAD
Table A1: Characteristics of studies comparing the accuracy of Stress ECHO to coronary angiography for the diagnosis of CAD.
| Study, Year | N | % male | meanage | Definition of CAD (% CAS) | With CAD on CA (%) | Included patients with previous MI | Percentage with previous MI | Included patients with unstable angina |
|---|---|---|---|---|---|---|---|---|
| Adenosine Stress | ||||||||
| Anthopoulos, 1996 (59) | 120 | 60 | 75 | 50 | 0.74 | Y | NA | N |
| Anthopoulos, 1997 (60) | 128 | 61 | 73 | 50 | 0.76 | Y | 30.0 | |
| Djordjevic-Dikic, 1996 (61) | 58 | 87.9 | 50 | 50 | 0.69 | Y | 41.4 | N |
| Fukai, 1995 (62) | 38 | 62.8 | 61 | 75 | 0.89 | Y | NA | N |
| Miyazono, 1998 (63) | 112 | 72.3 | 99 | 70 | 0.55 | Y | NA | NA |
| Tawa, 1996 (64) | 45 | 70.1 | 58 | 70 | 0.73 | Y | NA | N |
| Dipyridamole Stress | ||||||||
| Astarita, 2001 (65) | 53 | 54.7 | 58 | 50 | 0.43 | N | N | |
| Batlle, 1998 (66) | 56 | 0.73 | ||||||
| Beleslin, 1999 (67) | 168 | 0.76 | ||||||
| Bjornstad, 1995 (68) | 94 | 34.2 | 59 | 50 | 0.60 | Y | 29.2 | N |
| Bjornstad, 1995 (69) | 37 | 81.1 | 58 | 50 | 0.84 | Y | NA | N |
| Bjornstad, 1997 (70) | 25 | 81 | 58 | 0.60 | Y | 58.0 | ||
| Cramer, 1996 (71) | 35 | 0.83 | ||||||
| Dagianti, 1995 (72) | 60 | 0.42 | ||||||
| Fragasso, 1999 (73) | 101 | 54.5 | 61 | 50 | 0.56 | N | NA | |
| Gaddi, 1999 (74) | 24 | 100 | 55 | 50 | 0.54 | N | N | |
| Lanzarini, 1995 (75) | 61 | 95.1 | 53 | 50 | 0.90 | Y | NA | N |
| Loimaala, 1999 (76) | 60 | 66.7 | 55 | 50 | 0.73 | Y | 15.0 | N |
| Maffei, 1998 (77) | 52 | 0.19 | ||||||
| Minardi, 1997 (78) | 47 | 0.94 | ||||||
| Parodi, 1999 (79) | 101 | 80.2 | 55 | 50 | 0.79 | N | N | |
| Parthenakis, 1997 (80) | 46 | 0.61 | ||||||
| Pingitore, 1996 (81) | 110 | 83.3 | 60 | 50 | 0.84 | Y | NA | N |
| San Roman, 1996 (82) | 102 | 57 | 62 | 50 | 0.62 | N | N | |
| San Roman, 1998 (83) | 102 | 49 | 64 | 50 | 0.65 | N | N | |
| Santoro, 1998 (84) | 60 | NA | 70 | 0.55 | N | N | ||
| Scherhag, 1997 (85) | 65 | 0.42 | ||||||
| Schillaci, 1997(86) | 40 | 0.55 | ||||||
| Schroder, 1996 (87) | 119 | 0.87 | ||||||
| Schroder 1997(88) | 74 | 0.88 | ||||||
| Sochowski, 1995 (89) | 46 | 67.4 | 58 | 70 | 0.52 | N | N | |
| Wagdi, 1996(90) | 74 | 86.5 | 61 | 50 | 0.84 | Y | NA | N |
| Dobutamine Stress | ||||||||
| Ahmad, 2001 (91) | 90 | 46.6 | NA | 50 | 0.64 | Y | NA | N |
| Anthopoulos, 1996 (59) | 120 | 60 | 75 | 50 | 0.74 | Y | NA | N |
| Anthopoulos, 1997 (60) | 128 | 0.76 | ||||||
| Ariff, 2000 (92) | 66 | 68.2 | NA | 70 | 0.56 | NA | ||
| Batlle, 1998 (66) | 56 | 0.73 | ||||||
| Beleslin, 1999 (67) | 168 | 82 | 51 | 50 | 0.76 | Y | 50 | |
| Bigi, 1995 (93) | 121 | 0.88 | ||||||
| Castini, 1995 (94) | 44 | 93.2 | 56 | 70 | 0.86 | Y | NA | Y |
| Chan, 1995 (95) | 84 | 79.4 | 61 | 70 | 0.86 | Y | NA | N |
| Dagianti, 1995 (72) | 60 | 0.42 | ||||||
| Daoud, 1995 (96) | 76 | 57.9 | 60 | 50 | 0.86 | Y | NA | NA |
| De Bello, 1996 (97) | 45 | 73.3 | 53 | 50 | 0.84 | N | N | |
| Derumeaux, 1995 (98) | 37 | 0.38 | ||||||
| Dionisopoulos, 1997 (99) | 288 | 65 | 61 | 50 | 0.73 | N | ||
| Dolan, 2001 (100) | 112 | 61 | 70 | 0.81 | Y | NA | N | |
| Elhendy, 1996 (101) | 24 | 69.4 | 59 | 50 | 0.83 | Y | NA | NA |
| Elhendy, 1996 (102) | 133 | 76.5 | 60 | 50 | 0.83 | Y | NA | N |
| Elhendy, 1997 (103) | 72 | 0.79 | ||||||
| Elhendy, 1997 (104) | 306 | 0.76 | ||||||
| Elhendy, 1998 (105) | 290 | 69.7 | 58 | 50 | 0.76 | Y | NA | N |
| Elhendy, 1998 (106) | 295 | NA | 50 | 0.77 | Y | NA | N | |
| Elhendy, 1998 (107) | 84 | 63.1 | 60 | 50 | 0.79 | Y | NA | N |
| Elhendy, 1998 (108) | 70 | 0 | 58 | 50 | 0.64 | Y | NA | N |
| Elhendy, 1999 (109) | 90 | 80 | 57 | 50 | 0.81 | Y | 100 | NA |
| Fragasso, 1999 (73) | 101 | 54.5 | 61 | 50 | 0.56 | N | NA | |
| Frohwein, 1995 (110) | 40 | 100 | NA | 50 | 0.68 | Y | NA | N |
| Geleijnse, 1995 (111) | 223 | 68.6 | 58 | 50 | 0.64 | N | N | |
| Hennessy, 1997 (112) | 118 | 0.74 | ||||||
| Hennessy, 1997(113) | 219 | 0.78 | ||||||
| Hennessy, 1997 (114) | 199 | 0.94 | ||||||
| Hennessy, 1997(115) | 52 | 71.2 | 56 | 50 | 0.75 | Y | 26.9 | N |
| Hennessy, 1997 (116) | 317 | 72.2 | 60 | 50 | 0.86 | Y | NA | N |
| Hennessy, 1998 (117) | 218 | 72.9 | 62 | 50 | 0.91 | Y | NA | N |
| Herzog, 1999 (118) | 50 | 60 | 51 | 50 | 0.54 | Y | 8 | N |
| Ho, 1995 (119) | 54 | 0.80 | ||||||
| Ho, 1997 (120) | 206 | 100 | 58 | 50 | 0.78 | Y | 42.5 | |
| Ho, 1997 (121) | 51 | 76.5 | 56 | 50 | 0.75 | Y | NA | N |
| Ho, 1997 (122) | 223 | 80.7 | 58 | 50 | 0.73 | Y | NA | N |
| Ho, 1998 (123) | 56 | 73.3 | 60 | 50 | 0.52 | Y | NA | N |
| Ho, 1998 (124) | 51 | 0 | 62 | 50 | 0.57 | Y | NA | N |
| Hoffmann, 1996 (125) | 60 | 0.75 | ||||||
| Hoffmann, 1996 (126) | 150 | 79.5 | 46 | 50 | 0.63 | Y | 9.3 | N |
| Hoffmann, 1999 (127) | 283 | 78.4 | 56 | 50 | 0.65 | Y | 16.6 | N |
| Huang, 1997 (128) | 93 | 0.72 | ||||||
| Iwase, 1996 (129) | 95 | 69.8 | 59 | 70 | 0.66 | Y | NA | N |
| Joseph, 2000 (130) | 63 | 86.8 | 65 | 70 | 0.44 | Y | NA | N |
| Khattar, 1998 (131) | 100 | 70 | 62 | 50 | 0.74 | Y | NA | N |
| Kisacik, 1996 (132) | 69 | 0.68 | ||||||
| Latcham, 1995 (133) | 106 | 60.7 | 63 | 50 | 0.81 | Y | NA | NA |
| Lewis, 1999 (134) | 92 | 0 | 57 | 50 | 0.27 | |||
| Ling, 1996 (135) | 183 | 0.81 | ||||||
| Loimaala, 1999 (76) | 60 | 66.7 | 55 | 50 | 0.73 | Y | 15 | N |
| Mairesse, 1995 (136) | 24 | 75 | 61 | 50 | 0.63 | Y | NA | NA |
| Minardi, 1997 (78) | 47 | 0.94 | ||||||
| Nagel, 1999 (137) | 163 | 70.7 | 60 | 50 | 0.67 | N | N | |
| Oguzhan, 1997 (138) | 70 | 84.3 | 51 | 70 | 0.70 | Y | NA | N |
| Ozdemir, 1999 (139) | 63 | 79.2 | 52 | 50 | 0.67 | Y | NA | N |
| Pasierski, 2001 (140) | 248 | 67 | 53 | 50 | 0.47 | N | NA | |
| Peteiro, 2001 (141) | 41 | 78 | 63 | 50 | 0.78 | Y | NA | NA |
| Pingitore, 1996 (81) | 110 | 83.3 | 60 | 50 | 0.84 | Y | NA | N |
| Reis, 1995 (142) | 30 | 62.9 | 47 | 50 | 0.77 | Y | NA | NA |
| San Roman, 1996 (82) | 102 | 57 | 62 | 50 | 0.62 | N | N | |
| San Roman, 1998 (83) | 102 | 49 | 64 | 50 | 0.65 | N | N | |
| Santoro, 1998 (84) | 60 | NA | 70 | 0.55 | N | N | ||
| Schroder, 1996 (87) | 46 | 0.83 | ||||||
| Senior, 1996 (143) | 43 | 74.4 | 89 | 50 | 0.67 | Y | NA | N |
| Senior, 1998 (144) | 121 | 77 | 62 | 70 | 0.49 | Y | 29 | |
| Shaheen, 1998 (145) | 64 | NA | 70 | 0.66 | NA | NA | NA | |
| Slavich, 1996 (146) | 46 | 0 | 59 | 50 | 0.48 | N | ||
| Smart, 1997 (147) | 206 | 0.83 | ||||||
| Smart, 2000 (148) | 386 | 65.5 | 61 | 50 | 0.73 | Y | NA | N |
| Sochowski, 1995 (89) | 46 | 67.4 | 58 | 70 | 0.52 | N | N | |
| Takeuchi, 1996 (149) | 70 | 0 | 65 | 50 | 0.29 | N | N | |
| Therre, 1999 (150) | 85 | 60 | 50 | 0.38 | Y | NA | N | |
| Vitarelli, 1997 (151) | 59 | 64.4 | 52 | 70 | 0.81 | Y | NA | N |
| Wu, 1996 (152) | 104 | 0.65 | ||||||
| Yeo, 1996 (153) | 64 | 57.8 | 57 | 70 | 0.56 | Y | NA | NA |
| Exercise Stress | ||||||||
| Badruddin, 1999 (154) | 67 | 91.9 | 59 | 50 | 0.85 | Y | NA | N |
| Bjornstad, 1995 (69) | 37 | 64 | 57 | 50 | 0.84 | Y | NA | NA |
| Chaudhry, 2000 (155) | 28 | 100 | 66 | 50 | 0.57 | Y | NA | NA |
| Dagianti, 1995 (72) | 60 | 79 | 54 | 0.42 | Y | 39 | ||
| Jun, 1996 | 47 | 83 | 54 | 0.68 | Y | 17 | ||
| Loimaala, 1999 (76) | 60 | 66.7 | 55 | 50 | 0.73 | Y | 15 | N |
| Luotolahti, 1996 (156) | 118 | 85 | 55 | 0.92 | Y | 48 | ||
| Marwick, 1995 (157) | 161 | 0 | 60 | 0.37 | N | |||
| Marwick, 1995(158) | 147 | 59 | 58 | 0.42 | N | |||
| Pasierski, 2001 (140) | 248 | 67 | 53 | 50 | 0.47 | N | NA | NA |
| Peteiro, 1999 (159) | 79 | 77.5 | 62 | 50 | 0.89 | Y | NA | NA |
| Roger, 1995 (160) | 127 | NA | 50 | 0.84 | NA | |||
| Roger, 1997(161) | 340 | 71.8 | 65 | 50 | 0.74 | N | NA | |
| Roger, 1997 (162) | 244 | 100 | 64 | 0.80 | N | |||
| Schroder, 1997 (88) | 74 | 0.88 | ||||||
| Tawa, 1996 (64) | 45 | 70.1 | 58 | 70 | 0.73 | Y | NA | N |
| Tian, 1996 (163) | 46 | 83 | 54 | 50 | 0.70 | Y | NA | N |
| Toumanidis, 1996 (164) | 70 | 71.4 | 54 | 50 | 0.36 | N | NA | |
Note: Data was obtained from existing systemic reviews and an attempt was made to capture missing study information from original publications when possible.
CA, coronary angiography; CAD, coronary artery disease; CAS, coronary artery stenosis; NA, not available
Table A2: Calculated estimates of diagnostic accuracy from studies comparing stress ECHO to coronary angiography for the diagnosis of CAD.
| Study, Year | TP | FP | FN | TN | Sens (%) |
Spec (%) |
PPV (%) |
NPV (%) |
+LR | -LR | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine Stress | |||||||||||
| Anthopoulos, 1996 (59) | 59 | 28 | 30 | 3 | 66.3 | 9.7 | 67.8 | 9.1 | 0.7 | 3.5 | 51.7 |
| Anthopoulos, 1997 (60) | 64 | 3 | 33 | 28 | 66.0 | 90.3 | 95.5 | 45.9 | 6.8 | 0.4 | 71.9 |
| Djordjevic-Dikic, 1996 (61) | 30 | 0 | 10 | 18 | 75.0 | 97.3 | 98.4 | 64.3 | 27.8 | 0.3 | 82.1 |
| Fukai, 1995 (62) | 22 | 0 | 12 | 4 | 64.7 | 88.9 | 97.8 | 25.0 | 5.8 | 0.4 | 67.5 |
| Miyazono, 1998 (63) | 46 | 5 | 16 | 45 | 74.2 | 90.0 | 90.2 | 73.8 | 7.4 | 0.3 | 81.3 |
| Tawa, 1996 (64) | 29 | 1 | 4 | 11 | 87.9 | 91.7 | 96.7 | 73.3 | 10.5 | 0.1 | 88.9 |
| Dipyridamole Stress | |||||||||||
| Astarita, 2001 (65) | 18 | 0 | 5 | 30 | 78.3 | 98.4 | 97.3 | 85.7 | 47.7 | 0.2 | 89.7 |
| Batlle, 1998 (66) | 34 | 0 | 7 | 15 | 82.9 | 96.8 | 98.6 | 68.2 | 25.7 | 0.2 | 86.7 |
| Beleslin, 1999 (67) | 78 | 5 | 50 | 35 | 60.9 | 87.5 | 94.0 | 41.2 | 4.9 | 0.4 | 67.3 |
| Bjornstad, 1995 (68) | 41 | 3 | 15 | 35 | 73.2 | 92.1 | 93.2 | 70.0 | 9.3 | 0.3 | 80.9 |
| Bjornstad, 1995 (69) | 21 | 0 | 10 | 6 | 67.7 | 92.3 | 97.7 | 37.5 | 8.8 | 0.3 | 72.0 |
| Bjornstad, 1997 (70) | 11 | 2 | 4 | 8 | 73.3 | 80.0 | 84.6 | 66.7 | 3.7 | 0.3 | 76.0 |
| Cramer, 1996 (71) | 20 | 1 | 9 | 5 | 69.0 | 83.3 | 95.2 | 35.7 | 4.1 | 0.4 | 71.4 |
| Dagianti, 1995 (72) | 13 | 1 | 12 | 34 | 52.0 | 97.1 | 92.9 | 73.9 | 18.2 | 0.5 | 78.3 |
| Fragasso, 1999 (73) | 35 | 4 | 22 | 40 | 61.4 | 90.9 | 89.7 | 64.5 | 6.8 | 0.4 | 74.3 |
| Gaddi, 1999 (74) | 12 | 0 | 1 | 11 | 92.3 | 95.7 | 96.0 | 91.7 | 21.2 | 0.1 | 93.9 |
| Lanzarini, 1995 (75) | 45 | 1 | 10 | 5 | 81.8 | 83.3 | 97.8 | 33.3 | 4.9 | 0.2 | 82.0 |
| Loimaala, 1999 (76) | 41 | 4 | 3 | 12 | 93.2 | 75.0 | 91.1 | 80.0 | 3.7 | 0.1 | 88.3 |
| Maffei, 1998 (77) | 10 | 11 | 0 | 31 | 95.2 | 73.8 | 47.6 | 98.4 | 3.6 | 0.1 | 78.1 |
| Minardi, 1997 (78) | 32 | 1 | 12 | 2 | 72.7 | 66.7 | 97.0 | 14.3 | 2.2 | 0.4 | 72.3 |
| Parodi, 1999 (79) | 62 | 5 | 18 | 16 | 77.5 | 76.2 | 92.5 | 47.1 | 3.3 | 0.3 | 77.2 |
| Parthenakis, 1997 (80) | 16 | 0 | 12 | 18 | 57.1 | 97.3 | 97.0 | 60.0 | 21.1 | 0.4 | 73.1 |
| Pingitore, 1996 (81) | 75 | 1 | 17 | 17 | 81.5 | 94.4 | 98.7 | 50.0 | 14.7 | 0.2 | 83.6 |
| San Roman, 1996 (82) | 49 | 1 | 14 | 38 | 77.8 | 97.4 | 98.0 | 73.1 | 30.3 | 0.2 | 85.3 |
| San Roman, 1998 (83) | 54 | 2 | 12 | 34 | 81.8 | 94.4 | 96.4 | 73.9 | 14.7 | 0.2 | 86.3 |
| Santoro, 1998 (84) | 18 | 1 | 15 | 26 | 54.5 | 96.3 | 94.7 | 63.4 | 14.7 | 0.5 | 73.3 |
| Scherhag, 1997 (85) | 20 | 4 | 7 | 34 | 74.1 | 89.5 | 83.3 | 82.9 | 7.0 | 0.3 | 83.1 |
| Schillaci, 1997(86) | 18 | 5 | 4 | 13 | 81.8 | 72.2 | 78.3 | 76.5 | 2.9 | 0.3 | 77.5 |
| Schroder, 1996 (87) | 75 | 3 | 28 | 13 | 72.8 | 81.3 | 96.2 | 31.7 | 3.9 | 0.3 | 73.9 |
| Schroder 1997(88) | 50 | 1 | 15 | 8 | 76.9 | 88.9 | 98.0 | 34.8 | 6.9 | 0.3 | 78.4 |
| Sochowski, 1995 (89) | 16 | 19 | 8 | 3 | 66.7 | 13.6 | 45.7 | 27.3 | 0.8 | 2.4 | 41.3 |
| Wagdi, 1996(90) | 26 | 0 | 36 | 12 | 41.9 | 96.0 | 98.1 | 25.0 | 10.5 | 0.6 | 51.0 |
| Dobutamine Stress | |||||||||||
| Ahmad, 2001 (91) | 34 | 6 | 24 | 26 | 58.6 | 81.3 | 85.0 | 52.0 | 3.1 | 0.5 | 66.7 |
| Anthopoulos, 1996 (59) | 77 | 5 | 12 | 26 | 86.5 | 83.9 | 93.9 | 68.4 | 5.4 | 0.2 | 85.8 |
| Anthopoulos, 1997 (60) | 83 | 5 | 14 | 26 | 85.6 | 83.9 | 94.3 | 65.0 | 5.3 | 0.2 | 85.2 |
| Ariff, 2000 (92) | 30 | 6 | 7 | 23 | 81.1 | 79.3 | 83.3 | 76.7 | 3.9 | 0.2 | 80.3 |
| Batlle, 1998 (66) | 33 | 1 | 8 | 14 | 80.5 | 93.3 | 97.1 | 63.6 | 12.1 | 0.2 | 83.9 |
| Beleslin, 1999 (67) | 98 | 8 | 30 | 32 | 76.6 | 80.0 | 92.5 | 51.6 | 3.8 | 0.3 | 77.4 |
| Bigi, 1995 (93) | 86 | 1 | 21 | 13 | 80.4 | 92.9 | 98.9 | 38.2 | 11.3 | 0.2 | 81.8 |
| Castini, 1995 (94) | 26 | 0 | 12 | 6 | 68.4 | 92.3 | 98.1 | 33.3 | 8.9 | 0.3 | 71.9 |
| Chan, 1995 (95) | 63 | 2 | 9 | 10 | 87.5 | 83.3 | 96.9 | 52.6 | 5.3 | 0.2 | 86.9 |
| Dagianti, 1995 (72) | 18 | 1 | 7 | 34 | 72.0 | 97.1 | 94.7 | 82.9 | 25.2 | 0.3 | 86.7 |
| Daoud, 1995 (96) | 60 | 3 | 5 | 8 | 92.3 | 72.7 | 95.2 | 61.5 | 3.4 | 0.1 | 89.5 |
| De Bello, 1996 (97) | 29 | 1 | 9 | 6 | 76.3 | 85.7 | 96.7 | 40.0 | 5.3 | 0.3 | 77.8 |
| Derumeaux, 1995 (98) | 12 | 2 | 2 | 21 | 85.7 | 91.3 | 85.7 | 91.3 | 9.9 | 0.2 | 89.2 |
| Dionisopoulos, 1997 (99) | 181 | 9 | 28 | 70 | 86.6 | 88.6 | 95.3 | 71.4 | 7.6 | 0.2 | 87.2 |
| Dolan, 2001 (100) | 65 | 4 | 26 | 17 | 71.4 | 81.0 | 94.2 | 39.5 | 3.8 | 0.4 | 73.2 |
| Elhendy, 1996 (101) | 12 | 1 | 8 | 3 | 60.0 | 75.0 | 92.3 | 27.3 | 2.4 | 0.5 | 62.5 |
| Elhendy, 1996 (102) | 87 | 3 | 24 | 19 | 78.4 | 86.4 | 96.7 | 44.2 | 5.7 | 0.3 | 79.7 |
| Elhendy, 1997 (103) | 37 | 2 | 20 | 13 | 64.9 | 86.7 | 94.9 | 39.4 | 4.9 | 0.4 | 69.4 |
| Elhendy, 1997 (104) | 172 | 11 | 61 | 62 | 73.8 | 84.9 | 94.0 | 50.4 | 4.9 | 0.3 | 76.5 |
| Elhendy, 1998 (105) | 164 | 10 | 57 | 59 | 74.2 | 85.5 | 94.3 | 50.9 | 5.1 | 0.3 | 76.9 |
| Elhendy, 1998 (106) | 171 | 9 | 57 | 58 | 75.0 | 86.6 | 95.0 | 50.4 | 5.6 | 0.3 | 77.6 |
| Elhendy, 1998 (107) | 48 | 3 | 18 | 15 | 72.7 | 83.3 | 94.1 | 45.5 | 4.4 | 0.3 | 75.0 |
| Elhendy, 1998 (108) | 35 | 2 | 10 | 23 | 77.8 | 92.0 | 94.6 | 69.7 | 9.7 | 0.2 | 82.9 |
| Elhendy, 1999 (109) | 61 | 3 | 12 | 14 | 83.6 | 82.4 | 95.3 | 53.8 | 4.7 | 0.2 | 83.3 |
| Fragasso, 1999 (73) | 50 | 9 | 7 | 35 | 87.7 | 79.5 | 84.7 | 83.3 | 4.3 | 0.2 | 84.2 |
| Frohwein, 1995 (110) | 22 | 1 | 5 | 12 | 81.5 | 92.3 | 95.7 | 70.6 | 10.6 | 0.2 | 85.0 |
| Geleijnse, 1995 (111) | 103 | 17 | 40 | 63 | 72.0 | 78.8 | 85.8 | 61.2 | 3.4 | 0.4 | 74.4 |
| Hennessy, 1997 (114) | 69 | 11 | 18 | 20 | 79.3 | 64.5 | 86.3 | 52.6 | 2.2 | 0.3 | 75.4 |
| Hennessy, 1997(113) | 139 | 17 | 31 | 32 | 81.8 | 65.3 | 89.1 | 50.8 | 2.4 | 0.3 | 78.1 |
| Hennessy, 1997 (112) | 169 | 6 | 18 | 6 | 90.4 | 50.0 | 96.6 | 25.0 | 1.8 | 0.2 | 87.9 |
| Hennessy, 1997(115) | 32 | 6 | 7 | 7 | 82.1 | 53.8 | 84.2 | 50.0 | 1.8 | 0.3 | 75.0 |
| Hennessy, 1997 (116) | 234 | 17 | 40 | 26 | 85.4 | 60.5 | 93.2 | 39.4 | 2.2 | 0.2 | 82.0 |
| Hennessy, 1998 (117) | 97 | 3 | 101 | 17 | 49.0 | 85.0 | 97.0 | 14.4 | 3.3 | 0.6 | 52.3 |
| Herzog, 1999 (118) | 14 | 6 | 13 | 17 | 51.9 | 73.9 | 70.0 | 56.7 | 2.0 | 0.7 | 62.0 |
| Ho, 1995 (119) | 40 | 3 | 3 | 8 | 93.0 | 72.7 | 93.0 | 72.7 | 3.4 | 0.1 | 88.9 |
| Ho, 1997 (120) | 144 | 8 | 16 | 38 | 90.0 | 82.6 | 94.7 | 70.4 | 5.2 | 0.1 | 88.3 |
| Ho, 1997 (121) | 35 | 3 | 3 | 10 | 92.1 | 76.9 | 92.1 | 76.9 | 4.0 | 0.1 | 88.2 |
| Ho, 1997 (122) | 152 | 13 | 10 | 48 | 93.8 | 78.7 | 92.1 | 82.8 | 4.4 | 0.1 | 89.7 |
| Ho, 1998 (123) | 26 | 4 | 3 | 23 | 89.7 | 85.2 | 86.7 | 88.5 | 6.1 | 0.1 | 87.5 |
| Ho, 1998 (124) | 27 | 4 | 2 | 18 | 93.1 | 81.8 | 87.1 | 90.0 | 5.1 | 0.1 | 88.2 |
| Hoffmann, 1996 (125) | 35 | 2 | 10 | 13 | 77.8 | 86.7 | 94.6 | 56.5 | 5.8 | 0.3 | 80.0 |
| Hoffmann, 1996 (126) | 72 | 7 | 23 | 48 | 75.8 | 87.3 | 91.1 | 67.6 | 6.0 | 0.3 | 80.0 |
| Hoffmann, 1999 (127) | 132 | 22 | 51 | 78 | 72.1 | 78.0 | 85.7 | 60.5 | 3.3 | 0.4 | 74.2 |
| Huang, 1997 (128) | 62 | 6 | 5 | 20 | 92.5 | 76.9 | 91.2 | 80.0 | 4.0 | 0.1 | 88.2 |
| Iwase, 1996 (129) | 50 | 3 | 13 | 29 | 79.4 | 90.6 | 94.3 | 69.0 | 8.5 | 0.2 | 83.2 |
| Joseph, 2000 (130) | 18 | 8 | 10 | 27 | 64.3 | 77.1 | 69.2 | 73.0 | 2.8 | 0.5 | 71.4 |
| Khattar, 1998 (131) | 50 | 5 | 24 | 21 | 67.6 | 80.8 | 90.9 | 46.7 | 3.5 | 0.4 | 71.0 |
| Kisacik, 1996 (132) | 41 | 3 | 6 | 19 | 87.2 | 86.4 | 93.2 | 76.0 | 6.4 | 0.1 | 87.0 |
| Latcham, 1995 (133) | 64 | 7 | 22 | 13 | 74.4 | 65.0 | 90.1 | 37.1 | 2.1 | 0.4 | 72.6 |
| Lewis, 1999 (134) | 10 | 13 | 15 | 54 | 40.0 | 80.6 | 43.5 | 78.3 | 2.1 | 0.7 | 69.6 |
| Ling, 1996 (135) | 141 | 18 | 7 | 17 | 95.3 | 48.6 | 88.7 | 70.8 | 1.9 | 0.1 | 86.3 |
| Loimaala, 1999 (76) | 42 | 6 | 2 | 10 | 95.5 | 62.5 | 87.5 | 83.3 | 2.5 | 0.1 | 86.7 |
| Mairesse, 1995 (136) | 15 | 2 | 0 | 7 | 96.8 | 77.8 | 88.2 | 93.3 | 4.4 | 0.0 | 89.8 |
| Minardi, 1997 (78) | 33 | 1 | 11 | 2 | 75.0 | 66.7 | 97.1 | 15.4 | 2.3 | 0.4 | 74.5 |
| Nagel, 1999 (137) | 81 | 10 | 28 | 44 | 74.3 | 81.5 | 89.0 | 61.1 | 4.0 | 0.3 | 76.7 |
| Oguzhan, 1997 (138) | 44 | 2 | 5 | 19 | 89.8 | 90.5 | 95.7 | 79.2 | 9.4 | 0.1 | 90.0 |
| Ozdemir, 1999 (139) | 39 | 3 | 3 | 18 | 92.9 | 85.7 | 92.9 | 85.7 | 6.5 | 0.1 | 90.5 |
| Pasierski, 2001 (140) | 86 | 3 | 30 | 129 | 74.1 | 97.7 | 96.6 | 81.1 | 32.6 | 0.3 | 86.7 |
| Peteiro, 2001 (141) | 26 | 1 | 6 | 8 | 81.3 | 88.9 | 96.3 | 57.1 | 7.3 | 0.2 | 82.9 |
| Pingitore, 1996 (81) | 87 | 2 | 5 | 16 | 94.6 | 88.9 | 97.8 | 76.2 | 8.5 | 0.1 | 93.6 |
| Reis, 1995 (142) | 22 | 1 | 1 | 6 | 95.7 | 85.7 | 95.7 | 85.7 | 6.7 | 0.1 | 93.3 |
| San Roman, 1996 (82) | 49 | 2 | 14 | 37 | 77.8 | 94.9 | 96.1 | 72.5 | 15.2 | 0.2 | 84.3 |
| San Roman, 1998 (83) | 52 | 4 | 14 | 32 | 78.8 | 88.9 | 92.9 | 69.6 | 7.1 | 0.2 | 82.4 |
| Santoro, 1998 (84) | 20 | 1 | 13 | 26 | 60.6 | 96.3 | 95.2 | 66.7 | 16.4 | 0.4 | 76.7 |
| Schroder, 1996 (87) | 29 | 1 | 9 | 7 | 76.3 | 87.5 | 96.7 | 43.8 | 6.1 | 0.3 | 78.3 |
| Senior, 1996 (143) | 27 | 0 | 2 | 14 | 93.1 | 96.6 | 98.2 | 87.5 | 27.0 | 0.1 | 94.3 |
| Senior, 1998 (144) | 42 | 14 | 17 | 48 | 71.2 | 77.4 | 75.0 | 73.8 | 3.2 | 0.4 | 74.4 |
| Shaheen, 1998 (145) | 37 | 4 | 5 | 18 | 88.1 | 81.8 | 90.2 | 78.3 | 4.8 | 0.1 | 85.9 |
| Slavich, 1996 (146) | 13 | 6 | 9 | 18 | 59.1 | 75.0 | 68.4 | 66.7 | 2.4 | 0.5 | 67.4 |
| Smart, 1997 (147) | 140 | 7 | 31 | 28 | 81.9 | 80.0 | 95.2 | 47.5 | 4.1 | 0.2 | 81.6 |
| Smart, 2000 (148) | 238 | 14 | 42 | 92 | 85.0 | 86.8 | 94.4 | 68.7 | 6.4 | 0.2 | 85.5 |
| Sochowski, 1995 (89) | 17 | 4 | 7 | 18 | 70.8 | 81.8 | 81.0 | 72.0 | 3.9 | 0.4 | 76.1 |
| Takeuchi, 1996 (149) | 15 | 4 | 5 | 46 | 75.0 | 92.0 | 78.9 | 90.2 | 9.4 | 0.3 | 87.1 |
| Therre, 1999 (150) | 25 | 13 | 7 | 40 | 78.1 | 75.5 | 65.8 | 85.1 | 3.2 | 0.3 | 76.5 |
| Vitarelli, 1997 (151) | 41 | 2 | 7 | 9 | 85.4 | 81.8 | 95.3 | 56.3 | 4.7 | 0.2 | 84.7 |
| Wu, 1996 (152) | 55 | 2 | 13 | 34 | 80.9 | 94.4 | 96.5 | 72.3 | 14.6 | 0.2 | 85.6 |
| Yeo, 1996 (153) | 32 | 6 | 4 | 22 | 88.9 | 78.6 | 84.2 | 84.6 | 4.1 | 0.1 | 84.4 |
| Exercise Stress | |||||||||||
| Badruddin, 1999 (154) | 42 | 1 | 15 | 9 | 73.7 | 90.0 | 97.7 | 37.5 | 7.4 | 0.3 | 76.1 |
| Bjornstad, 1995 (69) | 26 | 2 | 5 | 4 | 83.9 | 66.7 | 92.9 | 44.4 | 2.5 | 0.2 | 81.1 |
| Chaudhry, 2000 (155) | 12 | 3 | 4 | 9 | 75.0 | 75.0 | 80.0 | 69.2 | 3.0 | 0.3 | 75.0 |
| Dagianti, 1995 (72) | 19 | 2 | 6 | 33 | 76.0 | 94.3 | 90.5 | 84.6 | 13.3 | 0.3 | 86.7 |
| Jun, 1996 | 28 | 1 | 4 | 14 | 87.5 | 93.3 | 96.6 | 77.8 | 13.1 | 0.1 | 89.4 |
| Loimaala, 1999 (76) | 40 | 9 | 4 | 7 | 90.9 | 43.8 | 81.6 | 63.6 | 1.6 | 0.2 | 78.3 |
| Luotolahti, 1996 (156) | 101 | 3 | 7 | 7 | 93.5 | 70.0 | 97.1 | 50.0 | 3.1 | 0.1 | 91.5 |
| Marwick, 1995 (157) | 47 | 19 | 12 | 83 | 79.7 | 81.4 | 71.2 | 87.4 | 4.3 | 0.2 | 80.7 |
| Marwick, 1995(158) | 44 | 8 | 18 | 77 | 71.0 | 90.6 | 84.6 | 81.1 | 7.5 | 0.3 | 82.3 |
| Pasierski, 2001 (140) | 95 | 5 | 21 | 127 | 81.9 | 96.2 | 95.0 | 85.8 | 21.6 | 0.2 | 89.5 |
| Peteiro, 1999 (159) | 51 | 4 | 19 | 5 | 72.9 | 55.6 | 92.7 | 20.8 | 1.6 | 0.5 | 70.9 |
| Roger, 1995 (160) | 94 | 6 | 13 | 14 | 87.9 | 70.0 | 94.0 | 51.9 | 2.9 | 0.2 | 85.0 |
| Roger, 1997(161) | 197 | 52 | 55 | 36 | 78.2 | 40.9 | 79.1 | 39.6 | 1.3 | 0.5 | 68.5 |
| Roger, 1997 (162) | 151 | 28 | 43 | 22 | 77.8 | 44.0 | 84.4 | 33.8 | 1.4 | 0.5 | 70.9 |
| Schroder, 1997 (88) | 35 | 1 | 30 | 8 | 53.8 | 88.9 | 97.2 | 21.1 | 4.8 | 0.5 | 58.1 |
| Tawa, 1996 (64) | 31 | 2 | 2 | 10 | 93.9 | 83.3 | 93.9 | 83.3 | 5.6 | 0.1 | 91.1 |
| Tian, 1996 (163) | 28 | 1 | 4 | 13 | 87.5 | 92.9 | 96.6 | 76.5 | 12.3 | 0.1 | 89.1 |
| Toumanidis, 1996 (164) | 18 | 10 | 7 | 35 | 72.0 | 77.8 | 64.3 | 83.3 | 3.2 | 0.4 | 75.7 |
FN, false negative; FP, false positive; LR, likelihood ratio; Sens, sensitivity; Spec, specificity; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Stress echocardiography for the diagnosis of coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 June [cited YYYY MM DD]; 10(9) 1-61. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/cardiac_stress_echo_20100528.pdf
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-1965-6 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables
| Table 1: Interpretation of ECHO Images Obtained at Rest and During Stress |
| Table 2: Echocardiography Systems Licensed by Health Canada |
| Table 3: Characteristics of Meta-Analyses Included in Heijenbrok-Kal, 2007 Systematic Review* |
| Table 4: Quality of Evidence of Included Studies on Stress ECHO |
| Table 5: Estimates of Pooled Sensitivity, Pooled Specificity and Diagnostic Odds Ratio Derived From Studies Comparing Stress ECHO to Coronary Angiography for the Diagnosis of CAD |
| Table 6: Comparisons of Pooled Sensitivity and Pooled Specificity |
| Table 7: Area Under the Curve for Stress ECHO |
| Table 8: Subgroup Analyses of Stress Agents Using Diagnostic Odds Ratios for Stress ECHO |
| Table 9: Effect of Publication Date on Diagnostic Accuracy |
| Table 10: GRADE Quality Assessment of Diagnostic Accuracy Studies for Stress ECHO Compared to Coronary Angiography for the Diagnosis of CAD |
| Table 11: Summary incremental cost-effectiveness ratios across selected studies evaluating stress ECHO |
| Table 12: Summary parameter estimates for stress ECHO tests: sensitivity, specificity; additional days needed to wait for specific cardiac tests; proportion of non-invasive tests considered uninterpretable |
| Table 13: List of cardiac imaging tests and associated OHIP 2009 costs |
| Table 14: Cost-effectiveness analysis base case results for stable outpatients |
| Table 15: Cost-effectiveness analysis base case results for acute inpatients |
| Table 11: Guidelines on ECHO |
| Table A1: Characteristics of studies comparing the accuracy of Stress ECHO to coronary angiography for the diagnosis of CAD |
| Table A2: Calculated estimates of diagnostic accuracy from studies comparing stress ECHO to coronary angiography for the diagnosis of CAD |
List of Abbreviations
- AUC
Area under the curve
- CA
Coronary angiography
- CAD
Coronary artery disease
- CI
Confidence interval(s)
- DOR
Diagnostic odds ratio
- ECHO
Echocardiography
- LV
Left ventricle
- MAS
Medical Advisory Secretariat
- MCE
Myocardial contrast echocardiography
- MI
Myocardial infarction
- OHTAC
Ontario Health Technology Advisory Committee
- RCT
Randomized controlled trial
- SD
Standard deviation
- SROC
Summary receiver operating characteristic
- WMA
Wall motion analysis
Footnotes
Clinical utility is defined as a technology that aids in clinical treatment decision-making
A study was counted twice if data was reported on different stress agents.
Clinical utility is defined as a technology that aids in clinical treatment decision-making.
A study was counted twice if data was reported on different stress agents.
References
- 1.Heijenbrok-Kal MH, Fleischmann KE, Hunink MG. Stress echocardiography, stress single-photon-emission computed tomography and electron beam computed tomography for the assessment of coronary artery disease: a meta-analysis of diagnostic performance. Am Heart J. 2007;154(3):415–23. doi: 10.1016/j.ahj.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20(9):1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress Echocardiography Expert Consensus Statement - Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30(3):278–89. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 4.Quinones MA, Douglas PS, Foster E, Gorcsan J, III, Lewis JF, Pearlman AS, et al. American College of Cardiology/American Heart Association clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians--American Society of Internal Medicine Task Force on Clinical Competence. 2003;107(7):1068–89. doi: 10.1161/01.cir.0000061708.42540.47. Circulation. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21(5):409–16. doi: 10.1016/j.echo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, et al. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009;10(2):194–212. doi: 10.1093/ejechocard/jep005. [DOI] [PubMed] [Google Scholar]
- 7.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, et al. Gender differences in the management and clinical outcome of stable angina. 2006;113(4):490–8. doi: 10.1161/CIRCULATIONAHA.105.561647. Circulation. [DOI] [PubMed] [Google Scholar]
- 8.Bigi R, Cortigiani L. Stress testing in women: Sexual discrimination or equal opportunity? Eur Heart J. 2005;26(5):423–5. doi: 10.1093/eurheartj/ehi129. [DOI] [PubMed] [Google Scholar]
- 9.Geleijnse ML, Krenning BJ, Soliman OII, Nemes A, Galema TW, ten Cate FJ. Dobutamine stress echocardiography for the detection of coronary artery disease in women. Am J Cardiol. 2007;99(5):714–7. doi: 10.1016/j.amjcard.2006.09.124. [DOI] [PubMed] [Google Scholar]
- 10.Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess. 2007;11(49):iii–iv. ix–115. doi: 10.3310/hta11490. [DOI] [PubMed] [Google Scholar]
- 11.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Brindis RG, Patel MR, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol. 2007;50(2):187–204. doi: 10.1016/j.jacc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Hirano Y, Uehara H, Nakamura H, Ikuta S, Nakano M, Akiyama S, et al. Efficacy of ultrasound-assisted stress testing using a hand-carried ultrasound device for diagnosis of coronary artery disease. J Am Soc Echocardiogr. 2006;19(5):536–9. doi: 10.1016/j.echo.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Wong JTH, Chan AWK. Investigation of ischaemic chest pain: Which tests to choose? Hong Kong Practitioner. 2006;28(12):530–9. [Google Scholar]
- 14.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006;59(12):1331–2. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 16.Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Keefe, Jr. JH, Barnhart CS, Bateman TM. Comparison of stress echocardiography and stress myocardial perfusion scintigraphy for diagnosing coronary artery disease and assessing its severity. Am J Cardiol. 1995;75(11):25D–34D. [PubMed] [Google Scholar]
- 18.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280(10):913–20. doi: 10.1001/jama.280.10.913. [DOI] [PubMed] [Google Scholar]
- 19.Picano E, Bedetti G, Varga A, Cseh E. The comparable diagnostic accuracies of dobutamine-stress and dipyridamole-stress echocardiographies: a meta-analysis. Coron Artery Dis. 2000;11(2):151–9. doi: 10.1097/00019501-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 20.de Albuquerque FL, Picano E. Comparison of dipyridamole and exercise stress echocardiography for detection of coronary artery disease (a meta-analysis) Am J Cardiol. 2001;87(10):1193–6. doi: 10.1016/s0002-9149(01)01493-x. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J. 2001;142(6):934–44. doi: 10.1067/mhj.2001.119761. [DOI] [PubMed] [Google Scholar]
- 22.Imran MB, Palinkas A, Picano E. Head-to-head comparison of dipyridamole echocardiography and stress perfusion scintigraphy for the detection of coronary artery disease: a meta-analysis. Comparison between stress echo and scintigraphy. Int J Cardiovasc Imaging. 2003;19(1):23–8. doi: 10.1023/a:1021746515555. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi Y, Nagata-Kobayashi S, Stahl JE, Wong JB. A meta-analytic comparison of echocardiographic stressors. Int J Cardiovasc Imaging. 2005;21(2-3):189–207. doi: 10.1007/s10554-004-5808-x. [DOI] [PubMed] [Google Scholar]
- 24.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm Sweden: Swedish Council on Technology Assessment in Health Care. 1996 doi: 10.1017/s0266462300008321. 81 p. SBU Report No. 119E. [DOI] [PubMed] [Google Scholar]
- 25.Jones CM, Athanasiou T. Diagnostic accuracy meta-analysis: review of an important tool in radiological research and decision making. Br J Radiol. 2009;82(978):441–6. doi: 10.1259/bjr/28171084. [DOI] [PubMed] [Google Scholar]
- 26.Biagini E, Shaw LJ, Poldermans D, Schinkel AFL, Rizzello V, Elhendy A, et al. Accuracy of noninvasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: A meta-analysis. Eur J Nucl Med Mol Imaging. 2006;33(12):1442–51. doi: 10.1007/s00259-006-0156-9. [DOI] [PubMed] [Google Scholar]
- 27.Ontario Case Costing Initiative. Costing analysis tool (CAT) [Internet] [updated 2009 cited 2009 Nov 18] Available from: http://www.occp.com/
- 28.Ontario Ministry of Health and Long-Term Care. Ontario health insurance schedule of benefits and fees. [Internet] [updated 2009 cited 2009 Nov 24] Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html .
- 29.Hayashino Y, Shimbo T, Tsujii S, Ishii H, Kondo H, Nakamura T, et al. Cost-effectiveness of coronary artery disease screening in asymptomatic patients with type 2 diabetes and other atherogenic risk factors in Japan: factors influencing on international application of evidence-based guidelines. Int J Cardiol. 2007;118(1):88–96. doi: 10.1016/j.ijcard.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 30.Kreisz FP, Merlin T, Moss J, Atherton J, Hiller JE, Gericke CA. The pre-test risk stratified cost-effectiveness of 64-slice computed tomography coronary angiography in the detection of significant obstructive coronary artery disease in patients otherwise referred to invasive coronary angiography. Heart Lung Circ. 2009;18(3):200–7. doi: 10.1016/j.hlc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, Cutler DM, Weinstein MC, et al. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. Am J Roentgenol. 2008;191(2):455–63. doi: 10.2214/AJR.07.3611. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzoni R, Cortigiani L, Magnani M, Desideri A, Bigi R, Manes C, et al. Cost-effectiveness analysis of noninvasive strategies to evaluate patients with chest pain. J Am Soc Echocardiogr. 2003;16(12):1287–91. doi: 10.1067/j.echo.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Maddahi J, Gambhir SS. Cost-effective selection of patients for coronary angiography. J Nucl Cardiol. 1997;4(2):S141–S151. doi: 10.1016/s1071-3581(97)90093-3. [DOI] [PubMed] [Google Scholar]
- 34.Medical AS. Medical Advisory Secretariat. Positron emission tomography for the assessment of myocardial viability: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2005 October [cited 2010 03 03]; 5(16) 1-167. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_petmyo_100105.pdf . [PMC free article] [PubMed]
- 35.Moir S, Shaw L, Haluska B, Jenkins C, Marwick TH. Left ventricular opacification for the diagnosis of coronary artery disease with stress echocardiography: an angiographic study of incremental benefit and cost-effectiveness. Am Heart J. 2007;154(3):510–8. doi: 10.1016/j.ahj.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. 2004;8(30):iii–iiv. 1–207. doi: 10.3310/hta8300. Health Technol Assess. [DOI] [PubMed] [Google Scholar]
- 37.Paterson DI, Schwartzman K. Strategies incorporating spiral CT for the diagnosis of acute pulmonary embolism: a cost-effectiveness analysis. 2001;119(6):1791–800. doi: 10.1378/chest.119.6.1791. Chest. [DOI] [PubMed] [Google Scholar]
- 38.Shaw LJ, Monaghan MJ, Nihoyannopolous P. Clinical and economic outcomes assessment with myocardial contrast echocardiography. Heart. 1999 doi: 10.1136/hrt.82.2008.iii16. 82 Suppl 3:III16-III21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyrick JJ, Kalvaitis S, McConnell KJ, Rinkevich D, Kaul S, Wei K. Cost-efficiency of myocardial contrast echocardiography in patients presenting to the emergency department with chest pain of suspected cardiac origin and a nondiagnostic electrocardiogram. 2008;102(6):649–52. doi: 10.1016/j.amjcard.2008.05.008. Am J Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong Y, Wu D, Fernandes V, Kopelen HA, Shimoni S, Nagueh SF, et al. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. 2002;89(6):711–8. doi: 10.1016/s0002-9149(01)02344-x. Am J Cardiol. [DOI] [PubMed] [Google Scholar]
- 41.Bedetti G, Pasanisi EM, Pizzi C, Turchetti G, Lore C. Economic analysis including long-term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardiovasc Ultrasound. 2008;6(21) doi: 10.1186/1476-7120-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewey M, Hamm B. Cost effectiveness of coronary angiography and calcium scoring using CT and stress MRI for diagnosis of coronary artery disease. 2007;17(5):1301–9. doi: 10.1007/s00330-006-0439-3. Eur Radiol. [DOI] [PubMed] [Google Scholar]
- 43.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. 1999;130(9):719–28. doi: 10.7326/0003-4819-130-9-199905040-00003. Ann Intern Med. [DOI] [PubMed] [Google Scholar]
- 44.Hayashino Y, Nagata-Kobayashi S, Morimoto T, Maeda K, Shimbo T, Fukui T. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with Type 2 diabetes and additional atherogenic risk factors. 2004;19(12):1181–91. doi: 10.1111/j.1525-1497.2004.40012.x. J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez R, Vale L. The value of myocardial perfusion scintigraphy in the diagnosis and management of angina and myocardial infarction: a probabilistic economic analysis. 2007;27(6):772–88. doi: 10.1177/0272989X07306111. Med Decis Making. [DOI] [PubMed] [Google Scholar]
- 46.Khare RK, Courtney DM, Powell ES, Venkatesh AK, Lee TA. Sixty-four-slice computed tomography of the coronary arteries: cost-effectiveness analysis of patients presenting to the emergency department with low-risk chest pain. 2008;15(7):623–32. doi: 10.1111/j.1553-2712.2008.00161.x. Acad Emerg Med. [DOI] [PubMed] [Google Scholar]
- 47.Kuntz KM, Fleischmann KE, Hunink MG, Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. 1999;130(9):709–18. doi: 10.7326/0003-4819-130-9-199905040-00002. Ann Intern Med. [DOI] [PubMed] [Google Scholar]
- 48.Lee DS, Jang MJ, Cheon GJ, Chung JK, Lee MC. Comparison of the cost-effectiveness of stress myocardial SPECT and stress echocardiography in suspected coronary artery disease considering the prognostic value of false-negative results. 2002;9(5):515–22. doi: 10.1067/mnc.2002.125217. J Nucl Cardiol. [DOI] [PubMed] [Google Scholar]
- 49.Rumberger JA, Behrenbeck T, Breen JF, Sheedy PF. Coronary calcification by electron beam computed tomography and obstructive coronary artery disease: a model for costs and effectiveness of diagnosis as compared with conventional cardiac testing methods. 1999;33(2):453–62. doi: 10.1016/s0735-1097(98)00583-x. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 50.Shaw LJ, Marwick TH, Berman DS, Sawada S, Heller GV, Vasey C, et al. Incremental cost-effectiveness of exercise echocardiography vs. SPECT imaging for the evaluation of stable chest pain. 2006;27(20):2448–58. doi: 10.1093/eurheartj/ehl204. Eur Heart J. [DOI] [PubMed] [Google Scholar]
- 51.Tardif JC, Dore A, Chan KL, Fagan S, Honos G, Marcotte F, et al. Economic impact of contrast stress echocardiography on the diagnosis and initial treatment of patients with suspected coronary artery disease. J Am Soc Echocardiogr. 2002;15(11):1335–45. doi: 10.1067/mje.2002.125287. [DOI] [PubMed] [Google Scholar]
- 52.Toronto Health Economics and Technology Assessment collaborative (THETA) The relative cost-effectiveness of five non-invasive cardiac imaging technologies for diagnosing coronary artery disease in Ontario [Internet] [updated 2010 cited 2010 Mar 9] Available from: http://theta.utoronto.ca/reports/?id=7 . [Google Scholar]
- 53.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107(1):149–58. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 2007. 116(7):e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 55.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation 2003. 108(9):1146–62. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 56.Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography) Developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95(6):1686–744. doi: 10.1161/01.cir.95.6.1686. [DOI] [PubMed] [Google Scholar]
- 57.Recommendations of a Joint Canadian Cardiovascular Society and Canadian Society of Echocardiography Consensus Panel. Guidelines for the provision of echocardiography in Canada [Internet] 2004. Oct 23 [Cited: 2009 Aug 1] Available from: http://www.ccs.ca/download/consensus_conference/consensus_conference_archives/2004_Echo.pdf .
- 58.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance: endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. Circulation 2008. 117(11):1478–97. doi: 10.1161/CIRCULATIONAHA.107.189097. [DOI] [PubMed] [Google Scholar]
- 59.Anthopoulos LP, Bonou MS, Kardaras FG, Sioras EP, Kardara DN, Sideris AM, et al. Stress echocardiography in elderly patients with coronary artery disease: applicability, safety and prognostic value of dobutamine and adenosine echocardiography in elderly patients. 1996;28(1):52–9. doi: 10.1016/0735-1097(96)00127-1. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 60.Anthopoulos LP, Bonou MS, Sioras EP, Kranidis AI, Kardaras FG, Antonellis IP. Echocardiographic detection of the extent of coronary artery disease in the elderly using dobutamine and adenosine infusion. 1997;8(10):633–43. doi: 10.1097/00019501-199710000-00006. Coron Artery Dis. [DOI] [PubMed] [Google Scholar]
- 61.Djordjevic-Dikic AD, Ostojic MC, Beleslin BD, Stepanovic J, Petrasinovic Z, Babic R, et al. High dose adenosine stress echocardiography for noninvasive detection of coronary artery disease. 1996;28(7):1689–95. doi: 10.1016/S0735-1097(96)00374-9. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 62.Fukai T, Koyanagi S, Tashiro H, Ichiki T, Tsutsui H, Matsumoto T, et al. Adenosine triphosphate stress echocardiography in the detection of myocardial ischemia. 1995;9(4):237–44. Am J Card Imaging. [PubMed] [Google Scholar]
- 63.Miyazono Y, Kisanuki A, Toyonaga K, Matsushita R, Otsuji Y, Arima S, et al. Usefulness of adenosine triphosphate-atropine stress echocardiography for detecting coronary artery stenosis. 1998;82(3):290–4. doi: 10.1016/s0002-9149(98)00345-2. Am J Cardiol. [DOI] [PubMed] [Google Scholar]
- 64.Tawa CB, Baker WB, Kleiman NS, Trakhtenbroit A, Desir R, Zoghbi WA. Comparison of adenosine echocardiography, with and without isometric handgrip, to exercise echocardiography in the detection of ischemia in patients with coronary artery disease. 1996;9(1):33–43. doi: 10.1016/s0894-7317(96)90102-9. J Am Soc Echocardiogr. [DOI] [PubMed] [Google Scholar]
- 65.Astarita C, Palinkas A, Nicolai E, Maresca FS, Varga A, Picano E. Dipyridamole-atropine stress echocardiography versus exercise SPECT scintigraphy for detection of coronary artery disease in hypertensives with positive exercise test. 2001;19(3):495–502. doi: 10.1097/00004872-200103000-00018. J Hypertens. [DOI] [PubMed] [Google Scholar]
- 66.Batlle E, Vilacosta I, San Roman JA, Peral V, Hernandez M, Castillo JA, et al. Rev Esp Cardiol. 1998;51(1):35–42. doi: 10.1016/s0300-8932(98)74708-6. [Elective noninvasive test in the diagnosis of coronary disease in the aged] [DOI] [PubMed] [Google Scholar]
- 67.Beleslin BD, Ostojic M, Djordjevic-Dikic A, Babic R, Nedeljkovic M, Stankovic G, et al. Integrated evaluation of relation between coronary lesion features and stress echocardiography results: the importance of coronary lesion morphology. 1999;33(3):717–26. doi: 10.1016/s0735-1097(98)00613-5. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 68.Bjornstad K, Aakhus S, Hatle L. Digital high frame rate stress echocardiography for detection of coronary artery stenosis by high dose dipyridamole stress testing. 1995;11(3):163–70. doi: 10.1007/BF01143105. Int J Card Imaging. [DOI] [PubMed] [Google Scholar]
- 69.Bjornstad K, Aakhus S, Hatle L. Comparison of digital dipyridamole stress echocardiography and upright bicycle stress echocardiography for identification of coronary artery stenosis. Cardiology. 1995;86(6):514–20. doi: 10.1159/000176932. [DOI] [PubMed] [Google Scholar]
- 70.Bjornstad K, Aakhus S, Torp HG. How does computer-assisted digital wall motion analysis influence observer agreement and diagnostic accuracy during stress echocardiography? 1997;13(2):105–14. doi: 10.1023/a:1005752331136. Int J Card Imaging. [DOI] [PubMed] [Google Scholar]
- 71.Cramer MJ, van der Wall EE, Jaarsma W, Verzijlbergen JF, Niemeyer MG, Zwinderman AH, et al. Detection of coronary artery disease: comparison between technetium 99m-labeled sestamibi single-photon emission computed tomography and two-dimensional echocardiography with dipyridamole low-level exercise-stress. 1996;3(5):389–94. doi: 10.1016/s1071-3581(96)90073-2. J Nucl Cardiol. [DOI] [PubMed] [Google Scholar]
- 72.Dagianti A, Penco M, Agati L, Sciomer S, Dagianti A, Rosanio S, et al. Stress echocardiography: comparison of exercise, dipyridamole and dobutamine in detecting and predicting the extent of coronary artery disease. 1995;26(1):18–25. doi: 10.1016/0735-1097(95)00121-f. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 73.Fragasso G, Lu C, Dabrowski P, Pagnotta P, Sheiban I, Chierchia SL. Comparison of stress/rest myocardial perfusion tomography, dipyridamole and dobutamine stress echocardiography for the detection of coronary disease in hypertensive patients with chest pain and positive exercise test. 1999;34(2):441–7. doi: 10.1016/s0735-1097(99)00231-4. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 74.Gaddi O, Tortorella G, Picano E, Pantaleoni M, Manicardi E, Varga A, et al. Diagnostic and prognostic value of vasodilator stress echocardiography in asymptomatic Type 2 diabetic patients with positive exercise thallium scintigraphy: a pilot study. 1999;16(9):762–6. doi: 10.1046/j.1464-5491.1999.00145.x. Diabet Med. [DOI] [PubMed] [Google Scholar]
- 75.Lanzarini L, Fetiveau R, Poli A, Diotallevi P, Barberis P, Previtali M. Results of dipyridamole plus atropine echo stress test for the diagnosis of coronary artery disease. 1995;11(4):233–40. doi: 10.1007/BF01145191. Int J Card Imaging. [DOI] [PubMed] [Google Scholar]
- 76.Loimaala A, Groundstroem K, Pasanen M, Oja P, Vuori I. Comparison of bicycle, heavy isometric, dipyridamole-atropine and dobutamine stress echocardiography for diagnosis of myocardial ischemia. 1999;84(12):1396–400. doi: 10.1016/s0002-9149(99)00583-4. Am J Cardiol. [DOI] [PubMed] [Google Scholar]
- 77.Maffei S, Baroni M, Terrazzi M, Paoli F, Ferrazzi P A, Biagini A. Preoperative assessment of coronary artery disease in aortic stenosis: a dipyridamole echocardiographic study. Ann Thorac Surg. 1998;65(2):397–402. doi: 10.1016/s0003-4975(97)01177-6. [DOI] [PubMed] [Google Scholar]
- 78.Minardi G, Di SM, Manzara CC, Pulignano G, Chiantera A, De SF, et al. Diagnostic and prognostic value of dipyridamole and dobutamine stress echocardiography in patients with Q-wave acute myocardial infarction. Am J Cardiol. 1997;80(7):847–51. doi: 10.1016/s0002-9149(97)00534-1. [DOI] [PubMed] [Google Scholar]
- 79.Parodi G, Picano E, Marcassa C, Sicari R, Marzullo P, Verna E, et al. High dose dipyridamole myocardial imaging: simultaneous sestamibi scintigraphy and two-dimensional echocardiography in the detection and evaluation of coronary artery disease. Italian Group of Nuclear Cardiology. Coron Artery Dis. 1999;10(3):177–84. doi: 10.1097/00019501-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Parthenakis FI, Karidis KS, Zuridakis G, Kanoupakis EM, Chlouverakis GI, Vardas PE. Left ventricular diastolic filling changes during dipyridamole-induced ischaemia. An echo-Doppler study. Coron Artery Dis. 1997;8(7):449–54. doi: 10.1097/00019501-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Pingitore A, Picano E, Colosso MQ, Reisenhofer B, Gigli G, Lucarini AR, et al. The atropine factor in pharmacologic stress echocardiography. Echo Persantine (EPIC) and Echo Dobutamine International Cooperative (EDIC) Study Groups. 1996;27(5):1164–70. doi: 10.1016/0735-1097(95)00586-2. J Am Coll Cardiol. [DOI] [PubMed] [Google Scholar]
- 82.San Roman JA, Vilacosta I, Castillo JA, Rollan MJ, Peral V, Sanchez-Harguindey L, et al. Dipyridamole and dobutamine-atropine stress echocardiography in the diagnosis of coronary artery disease. Comparison with exercise stress test, analysis of agreement, and impact of antianginal treatment. 1996;110(5):1248–54. doi: 10.1378/chest.110.5.1248. Chest. [DOI] [PubMed] [Google Scholar]
- 83.San Roman JA, Vilacosta I, Castillo JA, Rollan MJ, Hernandez M, Peral V, et al. Selection of the optimal stress test for the diagnosis of coronary artery disease. 1998;80(4):370–6. doi: 10.1136/hrt.80.4.370. Heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santoro GM, Sciagra R, Buonamici P, Consoli N, Mazzoni V, Zerauschek F, et al. Head-to-head comparison of exercise stress testing, pharmacologic stress echocardiography, and perfusion tomography as first-line examination for chest pain in patients without history of coronary artery disease. 1998;5(1):19–27. doi: 10.1016/s1071-3581(98)80006-8. J Nucl Cardiol. [DOI] [PubMed] [Google Scholar]
- 85.Scherhag AW, Pfleger S, Schreckenberger AB, Gruttner J, Voelker W, Staedt U, et al. Detection of patients with restenosis after PTCA by dipyridamole-atropine-stress-echocardiography. 1997;13(2):115–23. doi: 10.1023/a:1005745908633. Int J Card Imaging. [DOI] [PubMed] [Google Scholar]
- 86.Schillaci O, Moroni C, Scopinaro F, Tavolaro R, Danieli R, Bossini A, et al. Technetium-99m sestamibi myocardial tomography based on dipyridamole echocardiography testing in hypertensive patients with chest pain. 1997;24(7):774–8. doi: 10.1007/BF00879666. Eur J Nucl Med. [DOI] [PubMed] [Google Scholar]
- 87.Schroder K, Voller H, Dingerkus H, Munzberg H, Dissmann R, Linderer T, et al. Comparison of the diagnostic potential of four echocardiographic stress tests shortly after acute myocardial infarction: submaximal exercise, transesophageal atrial pacing, dipyridamole, and dobutamineatropine. Am J Cardiol. 1996;77(11):909–14. doi: 10.1016/s0002-9149(96)00027-6. [DOI] [PubMed] [Google Scholar]
- 88.Schroder K, Wieckhorst A, Voller H. Comparison of the prognostic value of dipyridamole and dobutamine stress echocardiography in patients with known or suspected coronary artery disease. Am J Cardiol. 1997;79(11):1516–8. doi: 10.1016/s0002-9149(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 89.Sochowski RA, Yvorchuk KJ, Yang Y, Rattes MF, Chan KL. Dobutamine and dipyridamole stress echocardiography in patients with a low incidence of severe coronary artery disease. J Am Soc Echocardiogr. 1995;8(4):482–7. doi: 10.1016/s0894-7317(05)80335-9. [DOI] [PubMed] [Google Scholar]
- 90.Wagdi P, Kaufmann U, Fluri M, Meier B. High dose dipyridamole as a pharmacological stress test during cardiac catheterisation in patients with coronary artery disease. Heart. 1996;75(3):247–51. doi: 10.1136/hrt.75.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad M, Xie T, McCulloch M, Abreo G, Runge M. Real-time three-dimensional dobutamine stress echocardiography in assessment stress echocardiography in assessment of ischemia: comparison with two-dimensional dobutamine stress echocardiography. J Am Coll Cardiol. 2001;37(5):1303–9. doi: 10.1016/s0735-1097(01)01159-7. [DOI] [PubMed] [Google Scholar]
- 92.Ariff B, Thom SA, Foale RA, Mayet J. Stress echocardiography for the diagnosis of ischaemia in hypertensives. J Hum Hypertens. 2000;14(6):399–401. doi: 10.1038/sj.jhh.1001029. [DOI] [PubMed] [Google Scholar]
- 93.Bigi R, Occhi G, Fiorentini C, Partesana N, Bandini P, Sponzilli C, et al. Dobutamine stress echocardiography for the identification of multivessel coronary artery disease after uncomplicated myocardial infarction: the importance of test end-point. Int J Cardiol. 1995;50(1):51–60. doi: 10.1016/0167-5273(95)02326-r. [DOI] [PubMed] [Google Scholar]
- 94.Castini D, Gentile F, Ornaghi M, Montani E, Lippolis A, Mangiarotti E, et al. Dobutamine echocardiography: usefulness of digital image processing. Eur Heart J. 1995;16(10):1420–4. doi: 10.1093/oxfordjournals.eurheartj.a060750. [DOI] [PubMed] [Google Scholar]
- 95.Chan RK, Tonkin AM, Byrgiotis S, Calafiore P. Assessment of coronary artery disease by dobutamine stress echocardiography (DSE) Aust N Z J Med. 1995;25(6):707–15. doi: 10.1111/j.1445-5994.1995.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 96.Daoud EG, Pitt A, Armstrong WF. Electrocardiographic response during dobutamine stress echocardiography. Am Heart J. 1995;129(4):672–7. doi: 10.1016/0002-8703(95)90314-3. [DOI] [PubMed] [Google Scholar]
- 97.De B V, Bellina CR, Molea N, Talarico L, Boni G, Magagnini E, et al. Simultaneous dobutamine stress echocardiography and dobutamine scintigraphy (99mTc-MIBI-SPET) for assessment of coronary artery disease. Int J Card Imaging. 1996;12(3):185–90. doi: 10.1007/BF01806221. [DOI] [PubMed] [Google Scholar]
- 98.Derumeaux G, Redonnet M, Mouton-Schleifer D, Bessou JP, Cribier A, Saoudi N, et al. Dobutamine stress echocardiography in orthotopic heart transplant recipients. VACOMED Research Group. J Am Coll Cardiol. 1995;25(7):1665–72. doi: 10.1016/0735-1097(95)00084-h. [DOI] [PubMed] [Google Scholar]
- 99.Dionisopoulos PN, Collins JD, Smart SC, Knickelbine TA, Sagar KB. The value of dobutamine stress echocardiography for the detection of coronary artery disease in women. J Am Soc Echocardiogr. 1997;10(8):811–7. doi: 10.1016/s0894-7317(97)70040-3. [DOI] [PubMed] [Google Scholar]
- 100.Dolan MS, Riad K, El-Shafei A, Puri S, Tamirisa K, Bierig M, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients Am Heart J. 2001;142(5):908–15. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 101.Elhendy A, Geleijnse L, Salustri A, Van Domburg RT, Cornel JH, Arnese M, et al. T wave normalization during dobutamine stress testing in patients with non-Q wave myocardial infarction. A marker of myocardial ischaemia? Eur Heart J. 1996;17(4):526–31. doi: 10.1093/oxfordjournals.eurheartj.a014904. [DOI] [PubMed] [Google Scholar]
- 102.Elhendy A, yan Domburg RT, Roelandt JR, Geleijnse ML, Cornel JH, el-Said GM, et al. Accuracy of dobutamine stress echocardiography for the diagnosis of coronary artery stenosis in patients with myocardial infarction: the impact of extent and severity of left ventricular dysfunction. Heart. 1996;76(2):123–8. doi: 10.1136/hrt.76.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elhendy A, Geleijnse ML, Roelandt JR, Van Domburg RT, ten Cate FJ, Nierop PR, et al. Comparison of dobutamine stress echocardiography and 99m-technetium sestamibi SPECT myocardial perfusion scintigraphy for predicting extent of coronary artery disease in patients with healed myocardial infarction. Am J Cardiol. 1997;79(1):7–12. doi: 10.1016/s0002-9149(96)00667-4. [DOI] [PubMed] [Google Scholar]
- 104.Elhendy A, Van Domburg RT, Roelandt JR, Geleijnse ML, Ibrahim MM, Fioretti PM. Safety and feasibility of dobutamine-atropine stress testing in hypertensive patients. Hypertension. 1997;29(6):1232–9. doi: 10.1161/01.hyp.29.6.1232. [DOI] [PubMed] [Google Scholar]
- 105.Elhendy A, Van Domburg RT, Bax JJ, Poldermans D, Nierop PR, Kasprzak JD, et al. Optimal criteria for the diagnosis of coronary artery disease by dobutamine stress echocardiography. Am J Cardiol. 1998;82(11):1339–44. doi: 10.1016/s0002-9149(98)00638-9. [DOI] [PubMed] [Google Scholar]
- 106.Elhendy A, Van Domburg RT, Poldermans D, Bax JJ, Nierop PR, Geleijnse ML, et al. Safety and feasibility of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in diabetic patients unable to perform an exercise stress test. Diabetes Care. 1998;21(11):1797–802. doi: 10.2337/diacare.21.11.1797. [DOI] [PubMed] [Google Scholar]
- 107.Elhendy A, Geleijnse ML, Van Domburg RT, Bax JJ, Nierop PR, Beerens SA, et al. Comparison of dobutamine stress echocardiography and technetium-99m sestamibi single-photon emission tomography for the diagnosis of coronary artery disease in hypertensive patients with and without left ventricular hypertrophy. Eur J Nucl Med. 1998;25(1):69–78. doi: 10.1007/s002590050196. [DOI] [PubMed] [Google Scholar]
- 108.Elhendy A, Van Domburg RT, Bax JJ, Nierop PR, Geleijnse ML, Ibrahim MM, et al. Noninvasive diagnosis of coronary artery stenosis in women with limited exercise capacity: comparison of dobutamine stress echocardiography and 99mTc sestamibi single-photon emission CT. Chest. 1998;114(4):1097–104. doi: 10.1378/chest.114.4.1097. [DOI] [PubMed] [Google Scholar]
- 109.Elhendy A, Bax JJ, Van Domburg RT, Valkema R, Cornel JH, Reijs AE, et al. Dobutamine stress thallium-201 single-photon emission tomography versus echocardiography for evaluation of the extent and location of coronary artery disease late after myocardial infarction. Eur J Nucl Med. 1999;26(5):467–73. doi: 10.1007/s002590050412. [DOI] [PubMed] [Google Scholar]
- 110.Frohwein S, Klein JL, Lane A, Taylor WR. Transesophageal dobutamine stress echocardiography in the evaluation of coronary artery disease. J Am Coll Cardiol. 1995;25(4):823–9. doi: 10.1016/0735-1097(94)00464-2. [DOI] [PubMed] [Google Scholar]
- 111.Geleijnse ML, Marwick TH, Boersma E, Deckers JW, Melin JA, Fioretti PM. Optimal pharmacological stress testing for the diagnosis of coronary artery disease: a probabilistic approach. Eur Heart J. 1995;16(Suppl M):3–10. doi: 10.1093/eurheartj/16.suppl_m.3. [DOI] [PubMed] [Google Scholar]
- 112.Hennessy TG, Codd MB, Hennessy MS, Kane G, McCarthy C, McCann HA, et al. Comparison of dobutamine stress echocardiography and treadmill exercise electrocardiography for detection of coronary artery disease. Coron Artery Dis. 1997;8(11-12):689–95. doi: 10.1097/00019501-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 113.Hennessy TG, Codd MB, McCarthy C, Kane G, McCann HA, Sugrue DD. Dobutamine stress echocardiography in the detection of coronary artery disease in a clinical practice setting. Int J Cardiol. 1997;62(1):55–62. doi: 10.1016/s0167-5273(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 114.Hennessy TG, Codd MB, McCarthy C, Kane G, McCann HA, Sugrue DD. Dobutamine stress echocardiography in the detection of coronary artery disease in a clinical practice setting. Int J Cardiol. 1997;62(1):55–62. doi: 10.1016/s0167-5273(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 115.Hennessy TG, Codd MB, Kane G, McCarthy C, McCann HA, Sugrue DD. Evaluation of patients with diabetes mellitus for coronary artery disease using dobutamine stress echocardiography. Coron Artery Dis. 1997;8(3-4):171–4. doi: 10.1097/00019501-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Hennessy TG, Codd MB, Kane G, McCarthy C, McCann HA, Sugrue DD. Dobutamine stress echocardiography in the detection of coronary artery disease: importance of the pretest likelihood of disease. Am Heart J. 1997;134(4):685–92. doi: 10.1016/s0002-8703(97)70052-8. [DOI] [PubMed] [Google Scholar]
- 117.Hennessy TG, Siobhan HM, Codd MB, Kane G, McCarthy C, McCann HA, et al. Detection of coronary artery disease using dobutamine stress echocardiography in patients with an abnormal resting electrocardiograph. Int J Cardiol. 1998;64(3):293–8. doi: 10.1016/s0167-5273(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 118.Herzog CA, Marwick TH, Pheley AM, White CW, Rao VK, Dick CD. Dobutamine stress echocardiography for the detection of significant coronary artery disease in renal transplant candidates. Am J Kidney Dis. 1999;33(6):1080–90. doi: 10.1016/S0272-6386(99)70145-9. [DOI] [PubMed] [Google Scholar]
- 119.Ho FM, Huang PJ, Liau CS, Lee FK, Chieng PU, Su CT, et al. Dobutamine stress echocardiography compared with dipyridamole thallium-201 single-photon emission computed tomography in detecting coronary artery disease. Eur Heart J. 1995;16(4):570–5. doi: 10.1093/oxfordjournals.eurheartj.a060952. [DOI] [PubMed] [Google Scholar]
- 120.Ho YL, Wu CC, Chao CL, Lin LC, Tseng WK, Chen WJ, et al. Localizing individual coronary artery obstructions with the dobutamine stress echocardiography. Cardiology. 1997;88(2):197–202. doi: 10.1159/000177329. [DOI] [PubMed] [Google Scholar]
- 121.Ho YL, Wu CC, Huang PJ, Tseng WK, Lin LC, Chieng PU, et al. Dobutamine stress echocardiography compared with exercise thallium-201 single-photon emission computed tomography in detecting coronary artery disease-effect of exercise level on accuracy. Cardiology. 1997;88(4):379–85. doi: 10.1159/000177363. [DOI] [PubMed] [Google Scholar]
- 122.Ho YL, Wu CC, Lin LC, Liu YB, Chen WJ, Chen MF, et al. Assessment of the functional significance of coronary artery stenosis by dobutamine-atropine stress echocardiography. Cardiology. 1997;88(4):386–92. doi: 10.1159/000177364. [DOI] [PubMed] [Google Scholar]
- 123.Ho YL, Wu CC, Lin LC, Huang CH, Chen WJ, Chen MF, et al. Assessment of the coronary artery disease and systolic dysfunction in hypertensive patients with the dobutamine-atropine stress echocardiography: effect of the left ventricular hypertrophy. Cardiology. 1998;89(1):52–8. doi: 10.1159/000006743. [DOI] [PubMed] [Google Scholar]
- 124.Ho YL, Wu CC, Huang PJ, Lin LC, Chieng PU, Chen WJ, et al. Assessment of coronary artery disease in women by dobutamine stress echocardiography: comparison with stress thallium-201 single-photon emission computed tomography and exercise electrocardiography. Am Heart J. 1998;135(4):655–62. doi: 10.1016/s0002-8703(98)70282-0. [DOI] [PubMed] [Google Scholar]
- 125.Hoffmann R, Lethen H, Falter F, Flachskampf FA, Hanrath P. Dobutamine stress echocardiography after coronary artery bypass grafting. Transthoracic vs biplane transoesophageal imaging. Eur Heart J. 1996;17(2):222–9. doi: 10.1093/oxfordjournals.eurheartj.a014838. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann R, Lethen H, Marwick T, Arnese M, Fioretti P, Pingitore A, et al. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol. 1996;27(2):330–6. doi: 10.1016/0735-1097(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 127.Hoffmann R, Lethen H, Kuhl H, Lepper W, Hanrath P. Extent and severity of test positivity during dobutamine stress echocardiography. Influence on the predictive value for coronary artery disease. Eur Heart J. 1999;20(20):1485–92. doi: 10.1053/euhj.1999.1628. [DOI] [PubMed] [Google Scholar]
- 128.Huang PJ, Ho YL, Wu CC, Chao CL, Chen MF, Chieng PU, et al. Simultaneous dobutamine stress echocardiography and thallium-201 perfusion imaging for the detection of coronary artery disease. Cardiology. 1997;88(6):556–62. doi: 10.1159/000177419. [DOI] [PubMed] [Google Scholar]
- 129.Iwase M, Fukui M, Tamagaki H, Kimura M, Hasegawa K, Matsuyama H, et al. Advantages and disadvantages of dobutamine stress echocardiography compared with treadmill exercise electrocardiography in detecting ischemia. Jpn Circ J. 1996;60(12):954–60. doi: 10.1253/jcj.60.954. [DOI] [PubMed] [Google Scholar]
- 130.Joseph T, Vieillard-Baron A, Chikli F, Goeau-Brissoniere O, Coggia M, Lacombe P, et al. Left ventricular volume analysis for the detection of coronary artery disease during dobutamine stress echocardiography in patients undergoing vascular surgery. Eur J Echocardiogr. 2000;1(4):263–70. doi: 10.1053/euje.2000.0039. [DOI] [PubMed] [Google Scholar]
- 131.Khattar RS, Senior R, Lahiri A. Assessment of myocardial perfusion and contractile function by inotropic stress Tc-99m sestamibi SPECT imaging and echocardiography for optimal detection of multivessel coronary artery disease. Heart. 1998;79(3):274–80. doi: 10.1136/hrt.79.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kisacik HL, Ozdemir K, Altinyay E, Oguzhan A, Kural T, Kir M, et al. Comparison of exercise stress testing with simultaneous dobutamine stress echocardiography and technetium-99m isonitrile single-photon emission computerized tomography for diagnosis of coronary artery disease. Eur Heart J. 1996;17(1):113–9. doi: 10.1093/oxfordjournals.eurheartj.a014669. [DOI] [PubMed] [Google Scholar]
- 133.Latcham AP, Orsinelli DA, Pearson AC. Recognition of the segmental tendency of false-positive dobutamine stress echocardiograms and its effects on test sensitivity and specificity. Am Heart J. 1995;129(5):1047–50. doi: 10.1016/0002-8703(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 134.Lewis JF, Lin L, McGorray S, Pepine CJ, Doyle M, Edmundowicz D, et al. Dobutamine stress echocardiography in women with chest pain. Pilot phase data from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE) J Am Coll Cardiol. 1999;33(6):1462–8. doi: 10.1016/s0735-1097(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 135.Ling LH, Pellikka PA, Mahoney DW, Oh JK, McCully RB, Roger VL, et al. Atropine augmentation in dobutamine stress echocardiography: role and incremental value in a clinical practice setting. J Am Coll Cardiol. 1996;28(3):551–7. doi: 10.1016/0735-1097(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 136.Mairesse GH, Marwick TH, Arnese M, Vanoverschelde JL, Cornel JH, Detry JM, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol. 1995;76(5):321–5. doi: 10.1016/s0002-9149(99)80093-9. [DOI] [PubMed] [Google Scholar]
- 137.Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–70. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 138.Oguzhan A, Kisacik HL, Ozdemir K, Altunkeser BB, Durmaz T, Altinyay E, et al. Comparison of exercise stress testing with dobutamine stress echocardiography and exercise technetium-99m isonitrile single photon emission computerized tomography for diagnosis of coronary artery disease. Jpn Heart J. 1997;38(3):333–44. doi: 10.1536/ihj.38.333. [DOI] [PubMed] [Google Scholar]
- 139.Ozdemir K, Kisacik HL, Oguzhan A, Durmaz T, Altunkeser BB, Altinyay E, et al. Comparison of exercise stress testing with dobutamine stress echocardiography and radionuclide ventriculography for diagnosis of coronary artery disease. Jpn Heart J. 1999;40(6):715–27. doi: 10.1536/jhj.40.715. [DOI] [PubMed] [Google Scholar]
- 140.Pasierski T, Szwed H, Malczewska B, Firek B, Kosmicki M, Rewicki M, et al. Advantages of exercise echocardiography in comparison to dobutamine echocardiography in the diagnosis of coronary artery disease in hypertensive subjects. J Hum Hypertens. 2001;15(11):805–9. doi: 10.1038/sj.jhh.1001265. [DOI] [PubMed] [Google Scholar]
- 141.Peteiro J, Monserrat L, Fabregas R, Manuel VJ, Calvino R, Castro-Beiras A. Comparison of two-dimensional echocardiography and pulsed Doppler tissue imaging during dobutamine-atropine stress testing to detect coronary artery disease. Echocardiography. 2001;18(4):275–84. doi: 10.1046/j.1540-8175.2001.00275.x. [DOI] [PubMed] [Google Scholar]
- 142.Reis G, Marcovitz PA, Leichtman AB, Merion RM, Fay WP, Werns SW, et al. Usefulness of dobutamine stress echocardiography in detecting coronary artery disease in end-stage renal disease. Am J Cardiol. 1995;75(10):707–10. doi: 10.1016/S0002-9149(99)80658-4. [DOI] [PubMed] [Google Scholar]
- 143.Senior R, Basu S, Handler C, Raftery EB, Lahiri A. Diagnostic accuracy of dobutamine stress echocardiography for detection of coronary heart disease in hypertensive patients. Eur Heart J. 1996;17(2):289–95. doi: 10.1093/oxfordjournals.eurheartj.a014847. [DOI] [PubMed] [Google Scholar]
- 144.Senior R, Khattar R, Lahiri A. Value of dobutamine stress echocardiography for the detection of multivessel coronary artery disease. Am J Cardiol. 1998;81(3):298–301. doi: 10.1016/s0002-9149(97)00910-7. [DOI] [PubMed] [Google Scholar]
- 145.Shaheen J, Luria D, Klutstein MW, Rosenmann D, Tzivoni D. Diagnostic value of 12-lead electrocardiogram during dobutamine echocardiographic studies. Am Heart J. 1998;136(6):1061–4. doi: 10.1016/s0002-8703(98)70163-2. [DOI] [PubMed] [Google Scholar]
- 146.Slavich GA, Guerra UP, Morocutti G, Fioretti PM, Fresco C, Orlandi C, et al. Feasibility of simultaneous Tc99m sestamibi and 2D-echo cardiac imaging during dobutamine pharmacologic stress. Preliminary results in a female population. Int J Card Imaging. 1996;12(2):113–8. doi: 10.1007/BF01880742. [DOI] [PubMed] [Google Scholar]
- 147.Smart SC, Knickelbine T, Stoiber TR, Carlos M, Wynsen JC, Sagar KB. Safety and accuracy of dobutamine-atropine stress echocardiography for the detection of residual stenosis of the infarct-related artery and multivessel disease during the first week after acute myocardial infarction. Circulation. 1997;95(6):1394–401. doi: 10.1161/01.cir.95.6.1394. [DOI] [PubMed] [Google Scholar]
- 148.Smart SC, Knickelbine T, Malik F, Sagar KB. Dobutamine-atropine stress echocardiography for the detection of coronary artery disease in patients with left ventricular hypertrophy. Importance of chamber size and systolic wall stress. Circulation. 2000;101(3):258–63. doi: 10.1161/01.cir.101.3.258. [DOI] [PubMed] [Google Scholar]
- 149.Takeuchi M, Sonoda S, Miura Y, Kuroiwa A. Comparative diagnostic value of dobutamine stress echocardiography and stress thallium-201 single-photon-emission computed tomography for detecting coronary artery disease in women. Coron Artery Dis. 1996;7(11):831–5. doi: 10.1097/00019501-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 150.Therre T, Ribal JP, Motreff P, Lusson JR, Espeut JB, Cassagnes J, et al. Assessment of cardiac risk before aortic reconstruction: noninvasive work-up using clinical examination, exercise testing, and dobutamine stress echocardiography versus routine coronary arteriography. Ann Vasc Surg. 1999;13(5):501–8. doi: 10.1007/s100169900290. [DOI] [PubMed] [Google Scholar]
- 151.Vitarelli A, Luzzi MF, Penco M, Fedele F, Dagianti A. On-line quantitative assessment of left ventricular filling during dobutamine stress echocardiography: a useful addition to conventional wall motion scoring. Int J Cardiol. 1997;59(1):57–69. doi: 10.1016/s0167-5273(96)02895-1. [DOI] [PubMed] [Google Scholar]
- 152.Wu CC, Ho YL, Kao SL, Chen WJ, Lee CM, Chen MF, et al. Dobutamine stress echocardiography for detecting coronary artery disease. Cardiology. 1996;87(3):244–9. doi: 10.1159/000177095. [DOI] [PubMed] [Google Scholar]
- 153.Yeo TC, Ling LH, Ng WL, Cheng GK, Lee SS, Yeoh JK, et al. Dobutamine stress echocardiography: angiographic correlates. Ann Acad Med Singapore. 1996;25(2):196–9. [PubMed] [Google Scholar]
- 154.Badruddin SM, Ahmad A, Mickelson J, Abukhalil J, Winters WL, Nagueh SF, et al. Supine bicycle versus post-treadmill exercise echocardiography in the detection of myocardial ischemia: a randomized single-blind crossover trial. J Am Coll Cardiol. 1999;33(6):1485–90. doi: 10.1016/s0735-1097(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 155.Chaudhry FA, Tauke JT, Alessandrini RS, Greenfield SA, Tommaso CL, Bonow RO. Enhanced detection of ischemic myocardium by transesophageal dobutamine stress echocardiography: comparison with simultaneous transthoracic echocardiography. Echocardiography. 2000;17(3):241–53. doi: 10.1111/j.1540-8175.2000.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 156.Luotolahti M, Saraste M, Hartiala J. Exercise echocardiography in the diagnosis of coronary artery disease. Ann Med. 1996;28(1):73–7. doi: 10.3109/07853899608999078. [DOI] [PubMed] [Google Scholar]
- 157.Marwick TH, Anderson T, Williams MJ, Haluska B, Melin JA, Pashkow F, et al. Exercise echocardiography is an accurate and cost-efficient technique for detection of coronary artery disease in women. J Am Coll Cardiol. 1995;26(2):335–41. doi: 10.1016/0735-1097(95)80004-z. [DOI] [PubMed] [Google Scholar]
- 158.Marwick TH, Torelli J, Harjai K, Haluska B, Pashkow FJ, Stewart WJ, et al. Influence of left ventricular hypertrophy on detection of coronary artery disease using exercise echocardiography. J Am Coll Cardiol. 1995;26(5):1180–6. doi: 10.1016/0735-1097(96)81472-0. [DOI] [PubMed] [Google Scholar]
- 159.Peteiro J, Fabregas R, Montserrat L, Alvarez N, Castro-Beiras A. Comparison of treadmill exercise echocardiography before and after exercise in the evaluation of patients with known or suspected coronary artery disease. J Am Soc Echocardiogr. 1999;12(12):1073–9. doi: 10.1016/s0894-7317(99)70104-5. [DOI] [PubMed] [Google Scholar]
- 160.Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc. 1995;70(1):5–15. doi: 10.1016/S0025-6196(11)64659-4. [DOI] [PubMed] [Google Scholar]
- 161.Roger VL, Pellikka PA, Bell MR, Chow CW, Bailey KR, Seward JB. Sex and test verification bias. Impact on the diagnostic value of exercise echocardiography. Circulation. 1997;95(2):405–10. doi: 10.1161/01.cir.95.2.405. [DOI] [PubMed] [Google Scholar]
- 162.Roger VL, Seward JB, Bailey KR, Oh JK, Mullany CJ. Aortic valve resistance in aortic stenosis: Doppler echocardiographic study and surgical correlation. Am Heart J. 1997;134(5 Pt 1):924–9. doi: 10.1016/s0002-8703(97)80016-6. [DOI] [PubMed] [Google Scholar]
- 163.Tian J, Zhang G, Wang X, Cui J, Xiao J. Exercise echocardiography: feasibility and value for detection of coronary artery disease. Chin Med J (Engl) 1996;109(5):381–4. [PubMed] [Google Scholar]
- 164.Toumanidis ST, Pantelia MI, Trika CO, Saridakis NS, Stamatelopoulos SF, Sideris DA, et al. Detection of coronary artery disease in the presence of left ventricular atrophy. Int J Cardiol. 1996;57(3):245–55. doi: 10.1016/s0167-5273(96)02830-6. [DOI] [PubMed] [Google Scholar]


