Executive Summary
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Assessment of Myocardial Viability, an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients undergoing viability assessment. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies that can be used for the assessment of myocardial viability: positron emission tomography, cardiac magnetic resonance imaging, dobutamine echocardiography, and dobutamine echocardiography with contrast, and single photon emission computed tomography.
A 2005 review conducted by MAS determined that positron emission tomography was more sensitivity than dobutamine echocardiography and single photon emission tomography and dominated the other imaging modalities from a cost-effective standpoint. However, there was inadequate evidence to compare positron emission tomography and cardiac magnetic resonance imaging. Thus, this report focuses on this comparison only. For both technologies, an economic analysis was also completed.
The Non-Invasive Cardiac Imaging Technologies for the Assessment of Myocardial Viability is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Objective
The objective of this analysis is to assess the effectiveness and safety of positron emission tomography (PET) imaging using F-18-fluorodeoxyglucose (FDG) for the assessment of myocardial viability. To evaluate the effectiveness of FDG PET viability imaging, the following outcomes are examined:
the diagnostic accuracy of FDG PET for predicting functional recovery;
the impact of PET viability imaging on prognosis (mortality and other patient outcomes); and
the contribution of PET viability imaging to treatment decision making and subsequent patient outcomes.
Clinical Need: Condition and Target Population
Left Ventricular Systolic Dysfunction and Heart Failure
Heart failure is a complex syndrome characterized by the heart’s inability to maintain adequate blood circulation through the body leading to multiorgan abnormalities and, eventually, death. Patients with heart failure experience poor functional capacity, decreased quality of life, and increased risk of morbidity and mortality.
In 2005, more than 71,000 Canadians died from cardiovascular disease, of which, 54% were due to ischemic heart disease. Left ventricular (LV) systolic dysfunction due to coronary artery disease (CAD)1 is the primary cause of heart failure accounting for more than 70% of cases. The prevalence of heart failure was estimated at one percent of the Canadian population in 1989. Since then, the increase in the older population has undoubtedly resulted in a substantial increase in cases. Heart failure is associated with a poor prognosis: one-year mortality rates were 32.9% and 31.1% for men and women, respectively in Ontario between 1996 and 1997.
Treatment Options
In general, there are three options for the treatment of heart failure: medical treatment, heart transplantation, and revascularization for those with CAD as the underlying cause. Concerning medical treatment, despite recent advances, mortality remains high among treated patients, while, heart transplantation is affected by the limited availability of donor hearts and consequently has long waiting lists. The third option, revascularization, is used to restore the flow of blood to the heart via coronary artery bypass grafting (CABG) or through minimally invasive percutaneous coronary interventions (balloon angioplasty and stenting). Both methods, however, are associated with important perioperative risks including mortality, so it is essential to properly select patients for this procedure.
Myocardial Viability
Left ventricular dysfunction may be permanent if a myocardial scar is formed, or it may be reversible after revascularization. Reversible LV dysfunction occurs when the myocardium is viable but dysfunctional (reduced contractility). Since only patients with dysfunctional but viable myocardium benefit from revascularization, the identification and quantification of the extent of myocardial viability is an important part of the work-up of patients with heart failure when determining the most appropriate treatment path. Various non-invasive cardiac imaging modalities can be used to assess patients in whom determination of viability is an important clinical issue, specifically:
dobutamine echocardiography (echo),
stress echo with contrast,
SPECT using either technetium or thallium,
cardiac magnetic resonance imaging (cardiac MRI), and
positron emission tomography (PET).
Dobutamine Echocardiography
Stress echocardiography can be used to detect viable myocardium. During the infusion of low dose dobutamine (5 – 10 μg/kg/min), an improvement of contractility in hypokinetic and akentic segments is indicative of the presence of viable myocardium. Alternatively, a low-high dose dobutamine protocol can be used in which a biphasic response characterized by improved contractile function during the low-dose infusion followed by a deterioration in contractility due to stress induced ischemia during the high dose dobutamine infusion (dobutamine dose up to 40 ug/kg/min) represents viable tissue. Newer techniques including echocardiography using contrast agents, harmonic imaging, and power doppler imaging may help to improve the diagnostic accuracy of echocardiographic assessment of myocardial viability.
Stress Echocardiography with Contrast
Intravenous contrast agents, which are high molecular weight inert gas microbubbles that act like red blood cells in the vascular space, can be used during echocardiography to assess myocardial viability. These agents allow for the assessment of myocardial blood flow (perfusion) and contractile function (as described above), as well as the simultaneous assessment of perfusion to make it possible to distinguish between stunned and hibernating myocardium.
SPECT
SPECT can be performed using thallium-201 (Tl-201), a potassium analogue, or technetium-99 m labelled tracers. When Tl-201 is injected intravenously into a patient, it is taken up by the myocardial cells through regional perfusion, and Tl-201 is retained in the cell due to sodium/potassium ATPase pumps in the myocyte membrane. The stress-redistribution-reinjection protocol involves three sets of images. The first two image sets (taken immediately after stress and then three to four hours after stress) identify perfusion defects that may represent scar tissue or viable tissue that is severely hypoperfused. The third set of images is taken a few minutes after the re-injection of Tl-201 and after the second set of images is completed. These re-injection images identify viable tissue if the defects exhibit significant fill-in (> 10% increase in tracer uptake) on the re-injection images.
The other common Tl-201 viability imaging protocol, rest-redistribution, involves SPECT imaging performed at rest five minutes after Tl-201 is injected and again three to four hours later. Viable tissue is identified if the delayed images exhibit significant fill-in of defects identified in the initial scans (> 10% increase in uptake) or if defects are fixed but the tracer activity is greater than 50%.
There are two technetium-99 m tracers: sestamibi (MIBI) and tetrofosmin. The uptake and retention of these tracers is dependent on regional perfusion and the integrity of cellular membranes. Viability is assessed using one set of images at rest and is defined by segments with tracer activity greater than 50%.
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (cardiac MRI) is a non-invasive, x-ray free technique that uses a powerful magnetic field, radio frequency pulses, and a computer to produce detailed images of the structure and function of the heart. Two types of cardiac MRI are used to assess myocardial viability: dobutamine stress magnetic resonance imaging (DSMR) and delayed contrast-enhanced cardiac MRI (DE-MRI). DE-MRI, the most commonly used technique in Ontario, uses gadolinium-based contrast agents to define the transmural extent of scar, which can be visualized based on the intensity of the image. Hyper-enhanced regions correspond to irreversibly damaged myocardium. As the extent of hyper-enhancement increases, the amount of scar increases, so there is a lower the likelihood of functional recovery.
Cardiac Positron Emission Tomography
Positron emission tomography (PET) is a nuclear medicine technique used to image tissues based on the distinct ways in which normal and abnormal tissues metabolize positron-emitting radionuclides. Radionuclides are radioactive analogs of common physiological substrates such as sugars, amino acids, and free fatty acids that are used by the body. The only licensed radionuclide used in PET imaging for viability assessment is F-18 fluorodeoxyglucose (FDG).
During a PET scan, the radionuclides are injected into the body and as they decay, they emit positively charged particles (positrons) that travel several millimetres into tissue and collide with orbiting electrons. This collision results in annihilation where the combined mass of the positron and electron is converted into energy in the form of two 511 keV gamma rays, which are then emitted in opposite directions (180 degrees) and captured by an external array of detector elements in the PET gantry. Computer software is then used to convert the radiation emission into images. The system is set up so that it only detects coincident gamma rays that arrive at the detectors within a predefined temporal window, while single photons arriving without a pair or outside the temporal window do not active the detector. This allows for increased spatial and contrast resolution.
Evidence-Based Analysis
Research Questions
What is the diagnostic accuracy of PET for detecting myocardial viability?
What is the prognostic value of PET viability imaging (mortality and other clinical outcomes)?
What is the contribution of PET viability imaging to treatment decision making?
What is the safety of PET viability imaging?
Literature Search
A literature search was performed on July 17, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 to July 16, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. In addition, published systematic reviews and health technology assessments were reviewed for relevant studies published before 2004. Reference lists of included studies were also examined for any additional relevant studies not already identified. The quality of the body of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
Inclusion Criteria
Criteria applying to diagnostic accuracy studies, prognosis studies, and physician decision-making studies:
English language full-reports
Health technology assessments, systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies
Patients with chronic, known CAD
PET imaging using FDG for the purpose of detecting viable myocardium
Criteria applying to diagnostic accuracy studies:
Assessment of functional recovery ≥3 months after revascularization
Raw data available to calculate sensitivity and specificity
Gold standard: prediction of global or regional functional recovery
Criteria applying to prognosis studies:
Mortality studies that compare revascularized patients with non-revascularized patients and patients with viable and non-viable myocardium
Exclusion Criteria
Criteria applying to diagnostic accuracy studies, prognosis studies, and physician decision-making studies:
PET perfusion imaging
< 20 patients
< 18 years of age
Patients with non-ischemic heart disease
Animal or phantom studies
Studies focusing on the technical aspects of PET
Studies conducted exclusively in patients with acute myocardial infarction (MI)
Duplicate publications
Criteria applying to diagnostic accuracy studies
Gold standard other than functional recovery (e.g., PET or cardiac MRI)
Assessment of functional recovery occurs before patients are revascularized
Outcomes of Interest
Diagnostic accuracy studies
Sensitivity and specificity
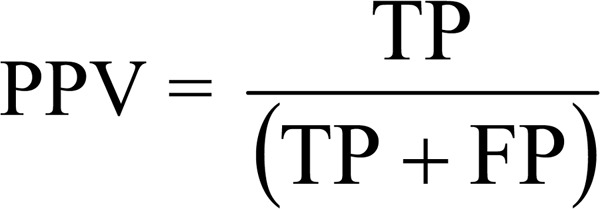
Positive and negative predictive values (PPV and NPV)
Positive and negative likelihood ratios
Diagnostic accuracy
Adverse events
Prognosis studies
Mortality rate
Functional status
Exercise capacity
Quality of Life
Influence on PET viability imaging on physician decision making
Statistical Methods
Pooled estimates of sensitivity and specificity were calculated using a bivariate, binomial generalized linear mixed model. Statistical significance was defined by P values less than 0.05, where “false discovery rate” adjustments were made for multiple hypothesis testing. Using the bivariate model parameters, summary receiver operating characteristic (sROC) curves were produced. The area under the sROC curve was estimated by numerical integration with a cubic spline (default option). Finally, pooled estimates of mortality rates were calculated using weighted means.
Quality of Evidence
The quality of evidence assigned to individual diagnostic studies was determined using the QUADAS tool, a list of 14 questions that address internal and external validity, bias, and generalizibility of diagnostic accuracy studies. Each question is scored as “yes”, “no”, or “unclear”. The quality of the body of evidence was then assessed as high, moderate, low, or very low according to the GRADE Working Group criteria. The following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Summary of Findings
A total of 40 studies met the inclusion criteria and were included in this review: one health technology assessment, two systematic reviews, 22 observational diagnostic accuracy studies, and 16 prognosis studies. The available PET viability imaging literature addresses two questions: 1) what is the diagnostic accuracy of PET imaging for the assessment; and 2) what is the prognostic value of PET viability imaging. The diagnostic accuracy studies use regional or global functional recovery as the reference standard to determine the sensitivity and specificity of the technology. While regional functional recovery was most commonly used in the studies, global functional recovery is more important clinically. Due to differences in reporting and thresholds, however, it was not possible to pool global functional recovery.
Functional recovery, however, is a surrogate reference standard for viability and consequently, the diagnostic accuracy results may underestimate the specificity of PET viability imaging. For example, regional functional recovery may take up to a year after revascularization depending on whether it is stunned or hibernating tissue, while many of the studies looked at regional functional recovery 3 to 6 months after revascularization. In addition, viable tissue may not recover function after revascularization due to graft patency or re-stenosis. Both issues may lead to false positives and underestimate specificity. Given these limitations, the prognostic value of PET viability imaging provides the most direct and clinically useful information. This body of literature provides evidence on the comparative effectiveness of revascularization and medical therapy in patients with viable myocardium and patients without viable myocardium. In addition, the literature compares the impact of PET-guided treatment decision making with SPECT-guided or standard care treatment decision making on survival and cardiac events (including cardiac mortality, MI, hospital stays, unintended revascularization, etc).
The main findings from the diagnostic accuracy and prognosis evidence are:
Based on the available very low quality evidence, PET is a useful imaging modality for the detection of viable myocardium. The pooled estimates of sensitivity and specificity for the prediction of regional functional recovery as a surrogate for viable myocardium are 91.5% (95% CI, 88.2% – 94.9%) and 67.8% (95% CI, 55.8% – 79.7%), respectively.
-
Based the available very low quality of evidence, an indirect comparison of pooled estimates of sensitivity and specificity showed no statistically significant difference in the diagnostic accuracy of PET viability imaging for regional functional recovery using perfusion/metabolism mismatch with FDG PET plus either a PET or SPECT perfusion tracer compared with metabolism imaging with FDG PET alone.
FDG PET + PET perfusion metabolism mismatch: sensitivity, 89.9% (83.5% – 96.4%); specificity, 78.3% (66.3% – 90.2%);
FDG PET + SPECT perfusion metabolism mismatch: sensitivity, 87.2% (78.0% – 96.4%); specificity, 67.1% (48.3% – 85.9%);
FDG PET metabolism: sensitivity, 94.5% (91.0% – 98.0%); specificity, 66.8% (53.2% – 80.3%).
Given these findings, further higher quality studies are required to determine the comparative effectiveness and clinical utility of metabolism and perfusion/metabolism mismatch viability imaging with PET.
Based on very low quality of evidence, patients with viable myocardium who are revascularized have a lower mortality rate than those who are treated with medical therapy. Given the quality of evidence, however, this estimate of effect is uncertain so further higher quality studies in this area should be undertaken to determine the presence and magnitude of the effect.
While revascularization may reduce mortality in patients with viable myocardium, current moderate quality RCT evidence suggests that PET-guided treatment decisions do not result in statistically significant reductions in mortality compared with treatment decisions based on SPECT or standard care protocols. The PARR II trial by Beanlands et al. found a significant reduction in cardiac events (a composite outcome that includes cardiac deaths, MI, or hospital stay for cardiac cause) between the adherence to PET recommendations subgroup and the standard care group (hazard ratio, .62; 95% confidence intervals, 0.42 – 0.93; P = .019); however, this post-hoc sub-group analysis is hypothesis generating and higher quality studies are required to substantiate these findings.
The use of FDG PET plus SPECT to determine perfusion/metabolism mismatch to assess myocardial viability increases the radiation exposure compared with FDG PET imaging alone or FDG PET combined with PET perfusion imaging (total-body effective dose: FDG PET, 7 mSv; FDG PET plus PET perfusion tracer, 7.6 – 7.7 mSV; FDG PET plus SPECT perfusion tracer, 16 – 25 mSv). While the precise risk attributed to this increased exposure is unknown, there is increasing concern regarding lifetime multiple exposures to radiation-based imaging modalities, although the incremental lifetime risk for patients who are older or have a poor prognosis may not be as great as for healthy individuals.
Background
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Assessment of Myocardial Viability, an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients undergoing viability assessment. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies that can be used for the assessment of myocardial viability: positron emission tomography, cardiac magnetic resonance imaging, dobutamine echocardiography, and dobutamine echocardiography with contrast, and single photon emission computed tomography.
A 2005 review conducted by MAS determined that positron emission tomography was more sensitivity than dobutamine echocardiography and single photon emission tomography and dominated the other imaging modalities from a cost-effective standpoint. However, there was inadequate evidence to compare positron emission tomography and cardiac magnetic resonance imaging. Thus, this report focuses on this comparison only. For both technologies, an economic analysis was also completed.
A summary decision analytic model was then developed to encapsulate the data from each of these reports (available on the OHTAC and MAS website).
The Non-Invasive Cardiac Imaging Technologies for the Assessment of Myocardial Viability is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Objective of Analysis
The objective of this analysis is to assess the effectiveness and safety of positron emission tomography (PET) imaging using F-18-fluorodeoxyglucose (FDG) for the assessment of myocardial viability. To evaluate the effectiveness of FDG PET viability imaging, the following outcomes are examined:
the diagnostic accuracy of FDG PET for predicting functional recovery;
the impact of PET viability imaging on prognosis (mortality and other patient outcomes); and
the contribution of PET viability imaging to treatment decision making and subsequent patient outcomes.
Clinical Need and Target Population
Left Ventricular Systolic Dysfunction and Heart Failure
Heart failure is a complex syndrome characterized by the heart’s inability to maintain adequate blood circulation through the body leading to multiorgan abnormalities and, eventually, death. Patients with heart failure experience poor functional capacity, decreased quality of life, and increased risk of morbidity and mortality. (1)
In 2005, more than 71,000 Canadians died from cardiovascular disease, of which 54% were due to ischemic heart disease. (2) Left ventricular (LV) systolic dysfunction due to coronary artery disease (CAD)2 is the primary cause of heart failure accounting for more than 70% of cases. (1;3;4) The prevalence of heart failure was estimated at one percent of the Canadian population in 1989. (5) Since then, the increase in the older population has undoubtedly resulted in a substantial increase in cases. Heart failure is associated with a poor prognosis: one-year mortality rates were 32.9% and 31.1% for men and women, respectively in Ontario between 1996 and 1997. (1)
Treatment Options
In general, there are three options for the treatment of heart failure: medical treatment, heart transplantation, and revascularization for those with CAD as the underlying cause. Despite advances in medical treatment such as the introduction of angiotensin converting enzyme (ACE) inhibitors, angiotensin II inhibitors, β-blockers, spironolactone, and aldosterone antagonists, mortality is still high among patients with heart failure. (4;6;7) While heart transplantation improves long-term prognosis, there are inadequate donor hearts and consequently long waiting lists for transplantation. (4) The third option, revascularization, is a surgical procedure used to restore the flow of blood to the heart. This can be achieved by coronary artery bypass grafting (CABG) or, in some cases, minimally invasive percutaneous coronary interventions (balloon angioplasty and stenting). (1) Both methods, however, are associated with important perioperative risks including mortality, so it is essential to properly select patients for this procedure. (6;7)
Myocardial Viability
Left ventricular dysfunction may be permanent, due to the formation of myocardial scar, or it may be reversible after revascularization. Reversible LV dysfunction occurs when the myocardium is viable but dysfunctional (reduced contractility). There are two types of dysfunctional but viable myocardium: stunned myocardium and hibernating myocardium. Stunned myocardium is characterized by reduced contractile function in the presence of normal (or near normal) resting perfusion. (3) This is caused by short periods of ischemia followed by restoration of perfusion (e.g., after an episode of unstable angina or after ischemia induced by exercise testing). The myocardium may be dysfunctional for several days, but after perfusion returns to normal, function is eventually restored. (7)
Prolonged or repetitive reductions in perfusion may lead to a state of chronically dysfunctional but viable myocardium also known as hibernating myocardium. Hibernating myocardium is characterized by reduced contractile function but maintained cell viability (intact cell membrane and cell metabolism) in areas with reduced perfusion. (3;8) In contrast to stunned myocardium, hibernating myocardium does not recover function spontaneously; it may, however, recover function after restoration of normal blood flow following coronary revascularization. (3;7)
Since patients with dysfunctional but viable myocardium benefit from revascularization, the identification and quantification of the extent of myocardial viability is an important part of the work-up of patients with heart failure to determine the most appropriate treatment path. (9) Various non-invasive cardiac imaging modalities can be used to assess patients in whom determination of viability is an important clinical issue:
dobutamine echocardiography (ECHO),
stress ECHO with contrast,
SPECT using either technetium or thallium,
cardiac magnetic resonance imaging (cardiac MRI), and
positron emission tomography (PET).
Dobutamine Echocardiography
Stress ECHO can be used to detect viable myocardium. Stress can be induced using exercise or pharmacological agents. Since imaging is difficult during exercise, pharmacologic agents, particularly dobutamine, are most commonly used. (7) During the infusion of low dose dobutamine (5 – 10 μg/kg/min), an improvement of contractility in hypokinetic and akentic segments is indicative of the presence of viable myocardium. (3;7;9) Alternatively, a low-high dose dobutamine protocol can be used in which a biphasic response characterized by improved contractile function during the low-dose infusion followed by a deterioration in contractility due to stress induced ischemia during the high dose dobutamine infusion (dobutamine dose up to 40 μg/kg/min) represents viable tissue. (3;7;9;10) Newer techniques including echocardiography using contrast agents, harmonic imaging, and power doppler imaging may help to improve the diagnostic accuracy of echocardiographic assessment of myocardial viability. (3;9;10)
Stress Echocardiography with Contrast
Intravenous contrast agents, which are high molecular weight inert gas microbubbles that act like red blood cells in the vascular space, can be used during echocardiography to assess myocardial viability. (3;9) The contrast agent allows for the assessment of myocardial blood flow (perfusion) as well as the assessment of contractile function (as described above), and the simultaneous assessment of perfusion makes it possible to distinguish between stunned and hibernating myocardium. (3)
SPECT
SPECT can be performed using thallium-201 (Tl-201), a potassium analogue, or technetium-99 m labelled tracers. When Tl-201 is injected intravenously into a patient, it is taken up by the myocardial cells through regional perfusion, and Tl-201 is retained in the cell due to sodium/potassium ATPase pumps in the myocyte membrane. (3;9) The two most common methods of assessing viability using Tl-201 SPECT imaging are stress-redistribution-reinjection and rest-redistribution. The former protocol involves three sets of images. The first two image sets (taken immediately after stress and then three to four hours after stress) identify perfusion defects, which may represent scar tissue or viable tissue that is severely hypoperfused. The third set is taken a few minutes after the re-injection of Tl-201 and after the second set of images is completed. These re-injection images identify viable tissue if the defects exhibit significant fill-in (> 10% increase in tracer uptake) on the re-injection images. (9)
The alternative protocol, rest-redistribution, does not involve stress imaging. Instead, imaging is performed at rest 5 minutes after Tl-201 is injected and again 3 to 4 hours later. Viable tissue is identified if the delayed images exhibit significant fill-in of defects identified in the initial scans (> 10% increase in uptake) or if defects are fixed but the tracer activity is greater than 50%. (9) This protocol provides information on viability only, whereas, the stress-redistribution-reinjection protocol also provides information on stress induced ischemia. (4)
There are two technetium-99 m tracers: sestamibi (MIBI) and tetrofosmin. The uptake and retention of these tracers is dependent on regional perfusion and the integrity of cellular membranes. (3;9) Viability is assessed using one set of images at rest and defined by segments with tracer activity greater than 50%. (9)
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (cardiac MRI) is a non-invasive, x-ray free technique which uses a powerful magnetic field, radio frequency pulses and a computer to produce detailed images of the structure and function of the heart. Two types of cardiac MRI are used to assess myocardial viability: dobutamine stress magnetic resonance imaging (DSMR), and delayed contrast-enhanced cardiac MRI (DE-MRI). DSMR is a technique that determines the contractile reserve of dysfunctional myocardium through the application of pharmacological stress with dobutamine. (11) Contractile reserve will be present in viable myocardium. DE-MRI uses gadolinium-based contrast agents to define the transmural extent of scar, which can be visualized based on the intensity of the image. (11) Hyper-enhanced regions correspond to irreversibly damaged myocardium. (12) As the extent of hyperenhancement increases, the amount of scar increases, so there is a lower the likelihood of functional recovery. (13)
Cardiac Positron Emission Tomography
Positron emission tomography (PET) is a nuclear medicine technique used to image tissues based on the distinct ways in which normal and abnormal tissues metabolize positron-emitting radionuclides. In PET imaging, the radionuclides are injected into the body and, as they decay, they emit positively charged particles (positrons), which travel several millimetres into tissue and collide with orbiting electrons. This collision results in annihilation and releases energy in the form of two 511 keV gamma rays that are emitted in opposite directions (180 degrees) and captured by an external array of detector elements in the PET gantry. (14;15) Computer software is used to convert the radiation emission into images. (1) The system is set up so that it only detects co-incident gamma rays that arrive at the detectors within a predefined temporal window; while single photos that arrive without a pair or outside the temporal window do not active the detector. This allows for increased spatial and contrast resolution. (14;15)
Electrocardiogram- (ECG) gated PET synchronizes the acquisition of PET images to the cardiac cycle and applies computer algorithms for objective quantification of regional and global LV function parameters such as wall thickening, wall motion, LV ejection fraction (LVEF), end-diastolic volume, end systolic volume, stroke volume, and LV mass. Successful application of this technique allows three-dimensional co-registration of ventricular function and metabolic information within a single PET exam. (1) Hybrid PET scanners that combine PET and computed tomography (CT) scanners integrate structure and function scans with attenuation correction without performing separate scans. (1;14)
PET Radionuclides
Radionuclides are radioactive analogs of common physiological substrates such as sugars, amino acids, and free fatty acids that are used by the body. (1) The most commonly used in PET imaging is F-18 fluorodeoxyglucose (FDG). Other radionuclides used in PET scanning include 11C-acetate, 13N-ammonia, 15O-water, and rubidium-82. (1)
F-18-Fluorodeoxyglucose (FDG)
F-18-fluorodeoxyglucose is an analog of glucose that is used to assess viability. Free fatty acids are the preferred source of energy for myocardial cells (myocytes) during periods of fasting, while glucose is preferred during periods of ischemia or after a meal. Under the latter conditions, the degree of utilization of external glucose reflects the metabolic capacity and therefore the viability of the myocardium. (8) In PET imaging, FDG is injected into the body where it is taken up by myocytes in proportion to glucose uptake. In the myocyte, FDG is phosphorated to FDG-6-phosphate which becomes trapped within the cell and is measured through PET imaging.
Viable myocardium can be identified by several methods. The most common method combines results of FDG PET scans with perfusion scans which may be done using PET perfusion tracers (most commonly, rubidium-82 or 13N-ammonia) or SPECT perfusion imaging tracers (technetium or thallium). Based on the combined perfusion and metabolism information, regions are classified into the following patterns:
normal tissue: regions with normal perfusion and normal glucose metabolism;
perfusion/metabolism mismatch: regions with reduced perfusion and maintained glucose metabolism (FDG uptake);
perfusion/metabolism match: regions with reduced perfusion and reduced glucose metabolism;
The first two patterns represent viable myocardium while the latter represents non-viable, scar tissue. (1;8) Other patterns such as perfusion/metabolism reverse mismatch, which is characterized by normal perfusion and reduced glucose metabolism may also occur. (8) Less commonly, viable myocardium may be determined based on metabolism imaging using FDG PET alone.
Regulatory Status
PET scanners are licensed by Health Canada as class II and III devices. (16)
PET Imaging in Ontario
Since October 1, 2009, cardiac PET imaging using FDG for myocardial viability assessment is an insured service in Ontario for patients that:
have severe ischemic LV dysfunction (LVEF < 35%) despite maximal medical therapy; and
are suitable candidates for cardiac revascularization procedure or cardiac transplantation. (17)
Before this, access to PET imaging for viability assessment was available through the Ontario Cardiac FDG PET Registry (CADRE) run by the Ottawa Heart Institute for the same indications. (18)
Methods of Evidence-Based Analysis
Research Questions
What is the diagnostic accuracy of PET for detecting myocardial viability?
What is the prognostic value of PET viability imaging (mortality and other clinical outcomes)?
What is the contribution of PET viability imaging to treatment decision making?
What is the safety of PET viability imaging?
Literature Search
A literature search was performed on July 17, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 to July 16, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. In addition, published systematic reviews and health technology assessments were reviewed for relevant studies published before 2004. Reference lists of included studies were also examined for any additional relevant studies not already identified.
Inclusion Criteria
Criteria applying to diagnostic accuracy studies, prognosis studies, and physician decision-making studies:
English language full-reports
Health technology assessments, systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies
Patients with chronic, known CAD
PET imaging using FDG for the purpose of detecting viable myocardium
Criteria applying to diagnostic accuracy studies
Assessment of functional recovery ≥3 months after revascularization
Raw data available to calculate sensitivity and specificity
Gold standard: prediction of global or regional functional recovery
Criteria applying to prognosis studies
Mortality studies that compare revascularized patients with non-revascularized patients and patients with viable and non-viable myocardium
Exclusion Criteria
Criteria applying to diagnostic accuracy studies, prognosis studies, and physician decision making studies
PET perfusion imaging
< 20 patients
< 18 years of age
Patients with non-ischemic heart disease
Animal or phantom studies
Studies focusing on the technical aspects of PET
Studies conducted exclusively in patients with acute myocardial infarction (MI)
Duplicate publications
Criteria applying to diagnostic accuracy studies
Gold standard other than functional recovery (e.g., PET or cardiac MRI)
Assessment of functional recovery occurs before patients are revascularized
Outcomes of Interest
Diagnostic accuracy studies
Sensitivity and specificity
Positive and negative predictive values (PPV and NPV)
Positive and negative likelihood ratios
Diagnostic accuracy
Adverse events
Prognosis studies
Mortality rate
Functional status
Exercise capacity
Quality of Life
Influence on PET viability imaging on physician decision making
Statistical Analysis
The diagnostic accuracy outcomes are calculated using a two-by-two table and formulas as shown below and in Table 1:
Table 1: Two-by-two table for calculations.
| Outcome (Functional Recovery) | |||
|---|---|---|---|
| Positive | Negative | ||
| Diagnostic Test (PET) | Positive | TP | FP |
| Negative | FN | TN | |
FN refers to false negatives; FP, false positives; PET, positron emission tomography; TN, true negatives; TP, true positives
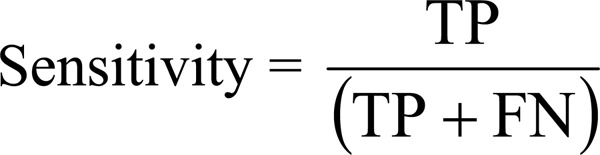

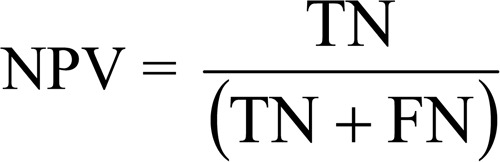


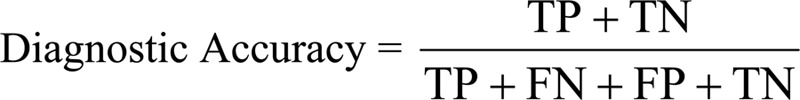
Pooled estimates of sensitivity and specificity were calculated using a bivariate, binomial generalized linear mixed model. (19) Statistical significance was defined by P values less than 0.05, where “false discovery rate” adjustments were made for multiple hypothesis testing. (20) The bivariate regression analyses were performed using SAS version 9.2 (SAS Institute Inc.; Cary, NC, USA). Using the bivariate model parameters, summary receiver operating characteristic (sROC) curves were produced using Review Manager 5.0.22 (The Nordiac Cochrane Centre, The Cochrane Collaboration, 2008). The area under the sROC curve was estimated by numerical integration with a cubic spline (default option) using STATA version 10.1 (StataCorp; Texas, USA). Pooled mortality estimates were calculated using weighted means.
Likelihood ratio (LR) plots were produced using the following guidelines:
Positive LRs greater than ten and negative LRs less than 0.1 generate large and often conclusive changes from pre- to post-test probability (very useful test).
Positive LRs between five and ten and negative LRs between 0.1 and 0.2 generate moderate shifts from pre- to post-test probability (moderately useful test).
Positive LRs between two and five and negative LRs between 0.2 and 0.5 generate small but sometimes important changes from pre- to post-test probability (somewhat useful test).
Positive LRs between one and two and negative likelihood ratios between 0.5 and one alter pre- to post-test probability to a small and rarely important degree (not useful test). (1;21)
Quality of Evidence
The quality of evidence assigned to individual diagnostic studies was determined using the QUADAS tool. The QUADAS tool is a list of 14 questions that address internal and external validity, bias, and generalizibility of diagnostic accuracy studies. Each question is scored as “yes”, “no”, or “unclear”. (22) For systematic reviews, the quality of evidence assigned to reviews was determined using the AMSTAR checklist. The tool consists of 11 questions which are scored as “yes”, “no”, and “can’t answer”. (23) The quality of the body of evidence was then assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (24), which state that:
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
The database search yielded 2,970 citations published between January 1, 2004, and July 16, 2009. Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded in the analysis.
Figure 1: Citation flow chart.
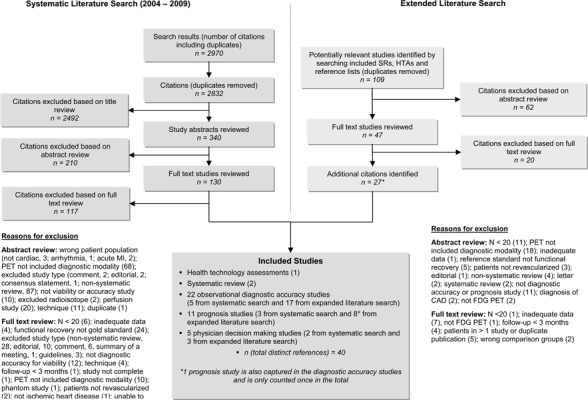
One hundred forty-eight of the identified citations were duplicates (the same article identified by more than 1 database) and excluded from further review. Thirteen studies (one health technology assessment, two systematic reviews, five observational diagnostic accuracy studies, three prognosis studies, and two physician decision making studies) met the inclusion criteria. Given the limited number of studies identified, the review was expanded to include relevant studies from previously published systematic reviews and health technology assessments. The references lists of the included studies were hand searched to identify any additional potentially relevant studies. While all systematic reviews and meta-analyses identified in the older literature were reviewed to identify any additional relevant studies, these reviews are not summarized in this report. A total of 109 citations were identified (duplicates removed), of which 26 met the inclusion criteria.
For each included study, levels of evidence were assigned according to a ranking system based on the hierarchy by Goodman. (25) An additional designation “g” was added for preliminary reports of studies that had been presented to international scientific meetings. Table 2 lists the level of evidence and number of studies identified.
Table 2: Quality of evidence of included studies.
| Number of Eligible Studies | ||||
|---|---|---|---|---|
| Study Design | Level of Evidence† | Diagnostic Accuracy | Prognosis | Physician Decision Making |
| Large RCT, systematic review of RCTs | 1 | 3* | 0 | 2† |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 0 | 0 | 0 |
| Small RCT | 2 | 0 | 0 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 | 0 | 0 |
| Non-RCT with contemporaneous controls | 3a | 21 | 11 | 1 |
| Non-RCT with historical controls | 3b | 0 | 0 | 0 |
| Non-RCT presented at international conference | 3(g) | 0 | 0 | 0 |
| Surveillance (database or register) | 4a | 0 | 0 | 0 |
| Case series (multisite) | 4b | 0 | 0 | 2‡ |
| Case series (single site) | 4c | 0 | 0 | 0 |
| Retrospective review, modelling | 4d | 0 | 0 | 0 |
| Case series presented at international conference | 4(g) | 0 | 0 | 0 |
| Total | 24 | 11 | 5 | |
refers to grey literature; RCT, randomized controlled trial
One health technology assessment and 2 systematic reviews of diagnostic accuracy observational studies
RCTs
Before/after surveys
Health Technology Assessments
One Health Technology Assessment (HTA) was identified that met the inclusion criteria. This HTA, conducted by the Medical Advisory Secretariat in 2005, evaluated the effectiveness, safety, and cost-effectiveness of PET, dobutamine stress echocardiography (echo), SPECT, cardiac MRI, and endocardial electromechanical mapping for the detection of myocardial viability and prediction of long-term outcomes. (1) Based on moderate to low quality evidence, the findings were as follows:
Diagnostic accuracy
PET has a higher sensitivity (median, 90%; range, 71% – 100%) and better negative likelihood ratio (median, 0.16; range, 0.0 – 0.38) for predicting regional functional recovery than other diagnostic imaging modalities;
The specificity of PET (median, 73%; range, 33% – 91%) for predicting regional functional recovery is similar to other radionuclide imaging modalities, but lower than dobutamine echo;
Given its higher sensitivity, PET is able to identify some patients who might benefit from revascularization that other modalities would not identify;
Cardiac MRI is a promising technique for viability assessment, but given the small number of poor quality studies on this area, no conclusion can be drawn on the effectiveness of PET versus cardiac MRI; and
No conclusion can be made comparing the accuracy of PET with other imaging modalities for predicting global functional due to a lack of direct comparisons. (1)
Prognosis
No firm conclusion can be reached about the incremental value of PET over other non-invasive techniques for predicting long-term outcomes due to lack of direct comparison. (1)
Systematic Reviews
Schinkel et al. (26) conducted a systematic review comparing the diagnostic accuracy of five cardiac imaging modalities (PET, dobutamine echo, thallium-201 and technetium-99m scintigraphy, and cardiac MRI) for the evaluation of viable myocardium and assessment of patient outcomes. The SR included 151 studies published from 1980 to January 2007 that assessed at least one of the following patient outcomes: regional functional recovery, global LV functional recovery, improvement in heart failure symptoms and exercise capacity, and long-term prognosis. (26)
As shown in Table 3, when regional functional recovery was used as the gold standard, resting cardiac MRI had the highest sensitivity (95%) followed by PET (92%), while dobutamine cardiac MRI had the highest specificity (82%) followed by dobutamine echo (78%). When global functional recovery was used as the gold standard, thallium and technetium SPECT had the highest sensitivity (84%) followed by PET (83%) and dobutamine echo had the highest specificity (73%) followed by technetium SPECT (68%). (26)
Table 3: Summary of weighted mean sensitivity, specificity, positive predictive value, and negative predictive value for predicting hibernating myocardium from Schinkel et al.*.
| Outcome | No. Studies | N | Sensitivity (%) | Specificity (%) | PPV (%) | NPV(%) |
|---|---|---|---|---|---|---|
| Positron Emission Tomography | ||||||
| Regional Function | 24 | 756 | 92 | 63 | 74 | 87 |
| Global Function | 3 | 253 | 83 | 64 | 68 | 80 |
| Dobutamine Echocardiography | ||||||
| Regional Function | 41 | 1,421 | 80 | 78 | 75 | 83 |
| Global Function | 6 | 287 | 57 | 73 | 63 | 68 |
| SPECT: Thallium-201 | ||||||
| Regional Function | 40 | 1,119 | 87 | 54 | 67 | 79 |
| Global Function | 5 | 235 | 84 | 53 | 76 | 64 |
| SPECT: Technetium-99m | ||||||
| Regional Function | 25 | 721 | 83 | 65 | 74 | 76 |
| Global Function | 2 | 98 | 84 | 68 | 74 | 80 |
| Cardiac MRI: Resting MRI (End-Diastolic Wall thickness) | ||||||
| Regional Function | 3 | 100 | 95 | 41 | 56 | 92 |
| Global Function | ||||||
| Cardiac MRI: Dobutamine MRI | ||||||
| Regional Function | 9 | 272 | 74 | 82 | 78 | 78 |
| Global Function | ||||||
| Cardiac MRI: Contrast Enhanced MRI | ||||||
| Regional Function | 5 | 178 | 84 | 63 | 72 | 78 |
| Global Function | ||||||
Cardiac MRI refers to cardiac magnetic resonance imaging; N, sample size; no., number; NPV, negative predictive value; PPV, positive predictive value; revasc., revascularization; SPECT, single photon emission computed tomography
Source: Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 2007; 32(7):375-410. Mortality rates were compared between patients with viable myocardium who were revascularized or treated with medical therapy and patients without viable myocardium who were revascularized or treated with medical therapy (Table 3). Mortality rates were pooled and annualized for ten studies that used PET for the assessment of viability. Patients with viable myocardium who were revascularized experienced the lowest annualized mortality rate (4%) compared with the highest rate (17%) among patients with viable myocardium treated with medical therapy. Similar trends were observed for the other diagnostic imaging modalities.
Changes in heart failure symptoms and exercise capacity after revascularization were compared for patients with and without viable myocardium based on PET imaging (Table 4). The pooled results showed that heart failure symptoms improved only in patients with viable myocardium after revascularization. While exercise capacity improved in both groups after revascularization, the improvement was larger in the group of patients with viable myocardium.
Table 4: Summary of changes in heart failure symptoms, exercise capacity, and prognosis.
| Outcome | No. Studies | N | Mean NYHA Functional Class | Mean Capacity (METS) | Annualized Mortality Rate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viable Myocardium | Non-Viable Myocardium | Viable Myocardium | Non-Viable Myocardium | Viable Myocardium | Non-Viable Myocardium | |||||||||
| Pre-revasc. | Post-revasc. | Pre-revasc. | Post-revasc. | Pre-revasc. | Post-revasc | Pre-revasc. | Post-revasc. | Revasc. | Medical Therapy | Revasc. | Medical Therapy | |||
| Positron Emission Tomography | ||||||||||||||
| Heart Failure Symptoms | 8† | 423† | 2.9* | 1.6* | 5.1† | 5.9† | ||||||||
| Exercise Capacity | 3 | 122 | 4.4 | 5.7 | 5.1 | 5.9 | ||||||||
| Prognosis | 10 | 1,046 | 4 | 17 | 6 | 8 | ||||||||
| Dobutamine Echocardiography | ||||||||||||||
| Prognosis | 11 | 1,753 | 3 | 12 | 7 | 12 | ||||||||
| SPECT: Thallium-201 | ||||||||||||||
| Prognosis | 9 | 975 | 4 | 7 | 14 | 7 | ||||||||
| SPECT: Technetrium-99m | ||||||||||||||
| Prognosis | 1 | 56 | 3 | 9 | ||||||||||
METS refers to metabolic equivalents; NYHA, New York Heart Association; revsac.; revascularized; SPECT, single photon emission computed tomography
As there were few studies that reported the change in heart failure symptoms, the results for all of the studies were combined, regardless of the diagnostic technology. The weighted mean NYHA functional class results included 4 studies with PET, 1 with dobutamine echo, and 3 with SPECT (Tl-201).
The change in hart failure symptoms before and after revascularisation was not statistically significant.
Source: Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 2007; 32(7):375-410.
The second SR, conducted by Beanlands et al. (27;28), compared PET, multi-detector CT angiography, and cardiac MRI for one or more of the following outcomes: diagnostic accuracy for the detection of CAD, CAD prognostication, diagnostic accuracy of myocardial viability detection, and viability prognostication. This review identified the most recent SR in the literature for each technology and updated it to include studies published until June 2005.
As the topic of this report is the diagnostic accuracy of myocardial viability and viability prognostication, only the results pertaining to these outcomes are summarized here (Table 5). The weighted mean sensitivity for the prediction of regional function recovery was highest for PET (91%) and dobutamine stress cardiac MRI (91%). Both cardiac MRI techniques had higher weighted mean specificities than PET. (27;28)
Table 5: Weighted mean sensitivity and specificity results for diagnostic accuracy of detection of viable myocardium from 2005 Beanlands et al. systematic review*.
| Imaging Technology | No. Studies | N | Weighted Mean Sensitivity (%) | Weighted Mean Specificity (%) |
|---|---|---|---|---|
| Positron Emission Tomography (weighted by no. segments/patients) | 28† | 1,047 | 91/90 | 61/61 |
| Magnetic Resonance Imaging: Dobutamine Stress (weighted by no. patients) | 10 | 401 | 91 | 94 |
| Magnetic Resonance Imaging: Late Gadolinium Enhancement (weighted by no. patients) | 13 | 357 | 81 | 83 |
N refers to sample size; no., number
Eight individual studies and one meta-analysis with 20 studies
Similar to the results from Schinkel et al. (26), the lowest mortality rate was observed in patients with viable myocardium who were revascularized and the highest mortality rate in patients with viable myocardium who were treated with medical therapy (Table 6). The pooled mortality rates must, however, be interpreted with caution as studies were pooled regardless of follow-up duration, which ranged from 12 months to 48 months.
Table 6: Prognosis comparing patients with viable and non-viable myocardium and treatment method.
| Source of Data | No. Studies | N | Mortality Rates (%) | |||
|---|---|---|---|---|---|---|
| Viable Myocardium | Non-Viable Myocardium | |||||
| Revasc. | Medical Therapy |
Revasc. | Medical Therapy |
|||
| Allman meta-analysis | 4 | 1,029 | 6.0 | 21.0 | 7.0 | 8.0 |
| Beanlands meta-analysis | 9* | 933 | 9.4 | 30.9 | 11.8 | 17.7 |
N refers to sample size; no., number; revasc., revascularized
Eight individual studies and one meta-analysis with 20 studies
Based on these findings, the following recommendations were made regarding FDG PET viability imaging (22):
“The interpretation of FDG PET viability imaging should be carried out only by physicians and institutions with adequate training and expertise.
Class I Indications
-
To define myocardial viability in patients with:
ischemic heart disease and severe LV dysfunction, to identify extent of recoverable myocardium and prognosis in patients being considered for revascularization or cardiac transplantation (Level B evidence);
moderate to large fixed perfusion defects or with equivocal results on another viability test (Level B evidence)
Class IIa Indication
Moderate systolic LV dysfunction and IHD to identify the extent of recoverable viable myocardium and prognosis in patients being considered for revascularization or cardiac transplantation (Level B evidence).
Class III (no benefit or harm)
Contraindications to insulin;
Severe untreated hypokalemia;
Contraindications to radiation exposure.”
Sources: a) Beanlands RS, Chow BJ, Dick A, Friedrich MG, Gulenchyn KY, Kiess M et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can J Cardiol 2007; 23(2):107-19. b) Beanlands, R. S., Chow, B. J., Dick, A., Friedrich, M. G., Gulenchyn, K. Y., Kiess, M., Leong-Poi, H., Miller, R. M., Nichol, G., Freeman, M., Bogaty, P., Honos, G., Hudon, G., Wisenberg, G., Van Berkom, J., Williams, K., Yoshinaga, K., and Graham, J. CCS / CAR / CANM / CNCS / Can SCMR joint position statement on advanced non-invasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multi-detector computed tomography angiography in the diagnosis and evaluation of ischemic heart disease. Ottawa, ON: Canadian Cardiovascular Society. 2006 [cited: 2009 Aug 26]. 48 p. Available from: http://www.ccs.ca/download/position_statements/cardiac_imaging_Dec11_appen_tables.pdf
Limitations and Quality Assessment
Both reviews include a systematic review conducted by Bax et al. (29) and use the summary estimates from the Bax review in the calculation of new summary sensitivity, specificity, PPV, and NPV estimates. These estimates were thus impacted by several errors identified in the Bax review such as the inclusion of a duplicate study and data extraction errors. In addition, the summary estimates for PET in the Beanlands review (27;28) includes data from two studies that were not performed using PET (FDG SPECT studies) and so are inappropriate to include in the analysis.
It is also important to note that cardiac MRI results were inconsistent across the two reviews. In Schinkel et al. (26), dobutamine cardiac MRI had a lower mean sensitivity (74%) than contrast-enhanced cardiac MRI (84%). In Beanlands et al. (27;28), however, dobutamine cardiac MRI had a higher mean sensitivity (91%) than contrast-enhanced cardiac MRI (81%). Furthermore, the weighted mean specificity for both contrast-enhanced and dobutamine cardiac MRI was substantially higher in Beanlands et al (27;28) than Schinkel et al. (26).
Full details on quality assessment of the two included systematic reviews using the AMSTAR checklist are provided in Appendix 2. The Schinkel review (26) met two of the 11 components on the AMSTAR checklist, while the Beanlands review (27;28) met three of the 11 components. Thus, the estimates of effect based on these systematic reviews are uncertain and may change with higher quality reviews.
Diagnostic Accuracy of PET to Detect Myocardial Viability
Twenty-two studies that assessed the diagnostic accuracy of PET for detecting myocardial viability were identified. The characteristics of these studies are provided in Table 7. The first five studies in the table were identified through the systematic literature search while the latter studies were identified using the reference lists of previous systematic reviews and health technology assessments on this topic. A description of the threshold used to define viability and functional recovery (regional and/or global recovery) in each study is shown in Table 8.
Table 7: Characteristics of included viability diagnostic accuracy studies.
| Author, Year | Study Design & No. Pts | Technique | Patient Population | Technique, Mean Timing to Assess Functional Recovery | Mean Age ± SD (% Male) | Mean LVEF ± SD (%) | History of MI (%) | Diabetes (%) | HT (%) | 3 Vessel CAD (%) | Mean# stenosed vessels | Mean# Revasc Vessel | CABG/PCI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kuhl et al., 2006 (30) | P n=29*† |
99mTc-sestamibi SPECT/FDG PET | Patients with chronic ischemic heart disease, regional wall motion abnormalities, and LVEF <50% with clinical indication of myocardial viability | ce-CARDIAC MRI, 6 mo post revascularization | 66±9 (72%) |
32±10 | 83 | 34 | 76 | NR | NR | PCI, 1.2±0.4; CABG, 3.2±0.7 | 14/15 |
| Slart et al., 2006 a (31) | P n=47† |
13N-ammonia / FDG gated PET | Patients with chronic CAD and LV dysfunction scheduled to undergo revascularization | MRI, 6 mo post revascularization | 65±9 (87%) |
33±12 | 72 | 21 | NR | NR | 2.4±0.8 | 2.1±1 | 27/20 |
| Slart et al., 2006 b (32) | P n=38† |
FDG gated PET | Patients with chronic CAD and LV dysfunction referred for revascularization | MRI, 6 mo post revascularization | 65±8 Range: 41-80 (87%) |
33±10 | 74 | 26 | NR | NR | 2.5±0.7 | 2.5±0.7 | 23/15 |
| Barrington et al., 2004 (33) | P n=25† |
13N-ammonia / FDG PET | Patients waiting for CABG surgery with LVEF ≤40% | Rest ECHO, mean, 8.1 ± 2.8 mo post revascularization. | 57.8 Range: 36-72 (100%) |
36.2±7.3 | NR | 16 | NR | NR | NR | 3.5±0.8 | 25/0 |
| Schmidt et al., 2004 (34) | P n=40‡ |
FDG PET | Patients with chronic myocardial infarction referred for assessment of myocardial viability | MRI, 4 to 6 mo post revascularization | 57±9 Range: 32-76 (93%) |
42±10 | 100 | NR | NR | 33 | NR | NR | 21/19 |
| Nowak et al., 2003 (35) | NR n=15†§ |
99mTc SPECT / FDG PET | Patients with severe CAD and regional wall motion abnormalities scheduled for FDG PET viability scans | MUGA (7 patients), 6.4±0.7 mo post revascularization and transthoracic ECHO (8 patients), 17.1±4.5 mo post revascularization | 63±11 Range: 40-78 (83%) |
38±13 | 79 | 21 | NR | 48 | NR | NR | 7/8 |
| Bax et al., 2002 (36) | P n=34 |
FDG PET | Patients with ischemic LV dysfunction scheduled for surgical revasc. | MUGA, 4–6 mo post revascularization | 61±9 Range: 36-74 (94%) |
32±9 | 100 | 18 | NR | NR | 2.2±0.8 | NR | 34/0 |
| Lund et al., 2002 (37) | P n=34¶ |
FDG PET | Patients with chronic MI and severe regional LV dysfunction | Coronary angiography, 4.8±2.5 mo post revascularization | 60±9 (91%) |
42±13 | 91 | NR | NR | 47 (multi-vessel disease) | NR | NR | 11/23 |
| Gerber et al., 2001 (38) | P n=178# |
FDG PET | Patients with CAD | 4-6 months post revascularization LVEF Gated angio (73) contrast angio (23), or 2D ECHO (75) Regional Functional Recovery Digitized 2D ECHO (108), multiple gated angio (40), or contrast angio (23) |
58±10 Range: 34-77 (92%) |
38±14 | 81 | 11 | NR | 35 | NR | NR | 140/38 |
| Tani et al., 2001 (39) | NR n=30 |
FDG PET | Patients with history of post infarction angina | ECHO, 5±3 mo post revascularization | 62±11 (97%) |
NR | 100 | NR | NR | NR | NR | NR | 6/24 |
| Wiggers et al., 2000 (40) | P n= 46†|| |
FDG PET | Patients with CAD and reduced EF (<50%) scheduled for CABG | ECHO, 6.2±1.5 mo post revascularization | 62±8 (96%) |
35±7 (range, 19–46) | 93 | 0 | 28 | 80 | NR | NR | 43/2¶ |
| Fath-Ordoubadi et al., 1999 (41) | NR n=18†** |
FDG PET | Patients with CAD | ECHO, 17±2 weeks post-revascularization | 62±10 (94%) |
41±11 | NR | 12.5 | 17 | NR | NR | NR | 0/24 |
| Schoder et al., 1999 (42)§§ | R n=40† †† |
13N-ammonia / FDG PET | Patients with CAD | 2D ECHO, group 1, 156±118 (range: 25–365) days post revasc and group 2, 160±130 days (range: 25–380) | 64±9 Range: 41-87 (88%) |
29±6 (range, 23–43) | 70 | 48 | NR | NR | NR | Mean # grafts, Group 1, 4.2±0.9 Group 2, 4.1±0.9 | 37/3 |
| Zhang et al., 1999 | NR n=34‡‡ |
99mTc-SPECT/FDG PET | Patients with previous MI and LV dysfunction | ECHO, 3–6 mo post revascularization | 54±9 (29–69) (97%) |
44±15 | 100 | 5 | NR | 58 | Pts with viable myocardium, 2.5±0.8; pts without viable myocardium, 2.6±0.5 | NR | 53/7 |
| Pagano et al., 1998 (43) | P n=30 |
FDG PET | Patients with multivessel CAD and stable chronic heart failure (NYHA class ≥ 3) | LVEF MUGA, 6 mo post revascularization Regional Functional Recovery Transthoracic ECHO | 57±7 Range: 41-72 (87%) |
25±7 (range, 10–37) | 100 | 23 | 17 | 83 | NR | mean# grafts, 3 | 30/0 |
| Maes et al., 1997 (44) | P n=23§§ |
13N-ammonia / FDG PET | Patients with CAD, an occlusion or severe stenosis (≥70%) of the LAD, and anterior wall motion abnormalities | MUGA, 3 mo post revascularization | 63±14 (83%) |
46.5±12 | 13 | 0 | NR | NR | NR | NR | 30/0 |
| Baer et al., 1996 (45) | P n=42† || || |
FDG PET | Patients with chronic CAD and regional akinesia or dyskinesia | Transesophageal ECHO, 4–6 mo post revasc. | 59±8 Range: 36-73 (90%) |
40±13 (range, 18–55) | 100 | 0 | NR | 31 | NR | NR | 22/20 |
| Gerber et al., 1996 (46) | NR n=39†¶¶ |
13N-ammonia / FDG PET | Patients with chronic CAD and severe LV dysfunction scheduled for revascularization | Two-dimensional ECHO, 5.0±1.9 mo post revascularization | 60±9 Range: 39-75 (87%) |
33±10 | 59## | 18 | NR | 56 | NR | NR | 31/8 |
| vom Dahl et al., 1996 (47) | P n=52*** |
99mTc SPECT / FDG PET | Patients with CAD and ischemic wall motion abnormalities considered for revascularization | LV angiography, 5±2 (range, 2–20) mo post revasc. | 56±8 (92%) |
47±10 | 76 | NR | NR | 37 | 2.2±0.8 | NR | 56/47 |
| Grandin et al., 1995 | P n=25 |
13N-ammonia / FDG PET | Patients with chronic left anterior wall dysfunction and well-defined coronary anatomy scheduled for revascularization | Contrast left ventriculography, 6 –9 mo post revascularization | 57±12 Range: 30-72 (80%) |
49±11 | 56 | 0 | NR | 4 | NR | NR | 7/18 |
| Carrel et al., 1992 (48) | P n=23 |
82Rb / FDG PET | Patients with advanced chronic CAD and severe LV dysfunction | Two-dimensional ECHO, 3 mo post revascularization | 56 Range: 49-63 (91%) |
34 (range, 19–45) | 100 | NR | NR | NR | NR | NR | 23/0 |
| Marwick et al., 1992 (49) | P n=23 |
Rb-82 / FDG PET | Patients with previous MI with clinical uncertainty about presence of viable myocardium | ECHO, 22±14 wk post revascularization | 58±9 (48%) |
35±14 | 100 | NR | NR | 52††† | NR | NR | 11/12 |
CABG refers to coronary artery bypass graft; CAD, coronary artery disease; ce-CARDIAC MRI, contrast enhanced cardiac magnetic resonance imaging; D, days; ECHO, echocardiography; FDG, F-18-fluorodeoxyglucose; HT, hypertension; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; mo, months; MRI, magnetic resonance imaging; MUGA, multigated acquisition scan (radionuclide ventriculography); MV, multivessel; NR, not reported; P, prospective; PCI, percutaneous coronary intervention; Pts, patients; R, retrospective; revasc., revascularization; SD, standard deviation; yr, years
36 patients were enrolled in the study, but the analysis is limited to the 29 patients that completed follow-up. Patients were excluded for the following reasons: three patients died (two from cardiac causes and one from sepsis); three refused to complete follow-up MRI; and one patient was lost to follow-up. (30)
Consecutive patients
98 consecutive patients studied, but results reported for only 40 patients who were revascularized and had an open infarct related artery at follow-up (34)
42 consecutive patients were enrolled in the study, but only 20 patients were revascularized, of which, only 15 patients received follow-up assessment of functional recovery. While the analysis only included 15 patients, the demographic characteristics, except for the number of patients who received CABG and PCI, are based on all of the 42 patients enrolled in the study.
PET was only performed in 38 of the 46 patients (6 patients did not receive PET for logistic reasons and 2 patients because of claustrophobia)
1 patients received one-vessel minimal invasive surgery
259 patients were enrolled in the study, but only 178 patients completed the follow-up and were included in the final analysis. The reported patient characteristics are restricted to the 178 patients included in the analysis only.
24 consecutive patients were enrolled in the study, but only 18 were included in the final analysis. Patients were excluded for the following reasons: died from ruptured abdominal aortic aneurysm before the procedure (1); refused treatment with PCI (1); more suitable to CABG due to severity of disease (1); PCI failed (3). Patient population characteristics given for the 18 patients included in the final analysis only.
40 patients were divided into 2 groups: group 1 consisted of 19 patients with diabetes and group 2 consisted of 21 patients without diabetes.
60 patients were enrolled in the study, but only 34 patients received a follow-up ECHO to assess functional recovery. The patient population characteristics reported in this table correspond to all 60 patients enrolled in the study.
30 patients were enrolled in the study, but the results are restrict to 23 patients only because PET images could not be acquired due to time constraints for 4 patients and 3 patients refused follow-up measurements of functional recovery.
121 consecutive patients were enrolled in the study, but only 42 (a subgroup who were revascularized, had a coronary angiogram to determine if the revascularization was successful and were successfully revascularized) were included in the analysis. Population characteristics are reported for the included 42 patients only.
6normal subjects were included for the control measurements of absolute myocardial blood flow and glucose uptake.
Anterior Q-wave MI
193 consecutive patients were enrolled in the study, but only 52 patients were used in the final analysis. Reasons for exclusion were: treated conservatively (88) or had a heart transplant (2); no angiographic follow-up (31); and unusable follow-up angiograms (20). Population characteristics are reported for the 52 patients who were included in the analysis except for the CABG vs. PCI which is reported for the 103 patients who were revascularized.
Multi-vessel disease
Table 8: Summary of the thresholds to define viability and functional improvement by study*.
| Author, Year | Viability threshold | Definition of Functional Improvement |
|---|---|---|
| Kuhl et al., 2006 (30) | Regional: Normal perfusion (Tc uptake >70%) or mismatch (Tc uptake ≤70%, FDG uptake >70% and difference FDG-Tc ≥ 20%) | Regional: Difference in wall motion score ≤–1 |
| Slart et al., 2006 a (31) | Regional: 7% difference in relative segmental uptake between metabolism and perfusion (based on ROC curve analysis) | Regional: Improvement in regional wall motion score by ≥1 grade (change dys- to akinesia did not represent improvement) |
| Global: ≥ 2 viable segments (based on ROC curve analysis) | Global: improvement LVEF ≥5% | |
| Slart et al., 2006 b (32) | Regional: segmental FDG uptake ≥50% (based on ROC curve analysis) or 10% wall thickness for gated PET (based on ROC curve analysis) | Regional: improvement in regional wall motion ≥ 1 grade (dyskinesia to akinesia not considered improvement) |
| Global: ≥ 3 viable segments for FDG or wall thickness (based on ROC curve analysis) | Global: improvement LVEF ≥ 5% | |
| Barrington et al., 2004 (33) | Regional: Normal perfusion (ammonia uptake ≥ 70% of maximum uptake) and mismatch (increased FDG uptake in presence of reduced perfusion, from ROC curve, FDG threshold ≥ 68%) | Regional: Improvement in function by ≥ 1 grade in at least 2 adjacent segments within a vascular territory |
| Schmidt et al., 2004 (34) | Regional: Mean segmental FDG uptake ≥ 50% compared to the reference segment (entire infarct region graded viable if FDG uptake ≥50% in ≥50% of infarct related segments) | Regional: For each segment recovery was defined by mean systolic wall thickening at rest ≥2 mm after revasc., and functional recovery of an infarct region was defined as systolic wall thickening ≥2 mm in ≥50% of related segments |
| Nowak et al., 2003 (35) | Regional: Flow-metabolism mismatch: Tc uptake ≤70% and FDG uptake ≥70%, 60%, 50%, or 80% | Regional: Wall motion score was reduced for at least 1 point after revasc. |
| Bax et al., 2002 (36) |
Global: ≥ 3 viable segments (based on ROC curve analysis) Regional: a) absolute MRG: > 0.25 umol/g/min (based on ROC curve) or b) relative MRG: >60% (based on ROC curve) |
Global: improvement LVEF >5% |
| Lund et al., 2002 (37) | Regional: FDG uptake >55% (based on ROC curve analysis) | Regional: wall motion abnormality improved > +1 standard deviation |
| Gerber et al., 2001 (38) | Global: a) ≥45% uptake in ≥ 3 segments, b) ≥ 50% glucose uptake in ≥ 3 segments, or c) ≥60% uptake in ≥2 segments | Global: Improvement in LVEF >5% (graded semi-quantitatively) |
| Tani et al., 2001 (39) | Regional: normal FDG uptake (70-100%) and mildly reduced FDG uptake (50 to ≤ 70%) | Regional: Improvement of >1 grade of wall motion index |
| Wiggers et al., 2000 (40) | Regional: FDG uptake ≥70% of that of the reference segment | Regional: Improvement by ≥ 1 score (graded semi-quantitatively) and on a per patient basis: improvement in ≥ 2 adjacent segments |
| Fath-Ordoubadi et al., 1999 (41) | Regional: MRG ≥ 0.25 umol/g/min (based on ROC curve analysis) | Regional: Improvement in resting wall motion score of 1 grade (assessed visually using both endocardial motion and wall thickness). The wall motion score index was derived for the entire left ventricle and for each vascular territory using the sum of individual scores divided by number of segments |
| Schoder et al., 1999 (42) | Regional: mismatch defined as the relative difference between relative FDG and ammonia concentrations > 2 standard deviations above the normal mean (vascular territory displays match or mismatch pattern only if >15% of that territory was hypoperfused) | Regional: change by ≥1 grade in motion score in a territory was considered significant |
| Zhang et al., 1999 (50) | Regional: regions with perfusion deficit but maintained accumulation of FDG were classified as mismatch regions, but no thresholds were provided (evaluated semi-quantitatively) | Regional: Regional functional recovery not defined |
| Pagano et al., 1998 (43) |
Regional: MRG uptake ≥0.25 umol/min/g (mean myocardial tracer uptake minus 1 standard deviation measured in normally contracting regions) Global: 8 viable segments (based on ROC curve analysis) |
Regional: Recovery if reduction of ≥1 point in wall motion/systolic thickening score (wall thickening used for assessment of septal segments and wall motion score index calculated as sum of scores of LV segments divided by number of segments) Global: LVEF increase > 5% |
| Maes et al., 1997 (44) | Regional: flow index >0.8 or ratio metabolic and flow index > 1.2 | Regional: regional LVEF in anterior wall was 5% higher at 3 months |
| Baer et al., 1996 (45) | Regional Mean segmental FDG uptake was ≥50% of maximal uptake or for infarct regions: ≥ 50% of akinetic or dyskinetic segments related to an infarct region had uptake ≥50% of maximal uptake (quantitative assessment) | Regional: Systolic wall thickening became apparent in segment graded akinetic or dyskinetic at rest before revasc. (score improvement from 3 or 4 to 1 or 2) |
| Gerber et al., 1996 (46) | Regional flow-metabolism mismatch if relative ammonia uptake was <70% and ratio of FDG to ammonia exceeded 1.2 | Regional Wall motion decreased by 1 full grade in any of the 3 segments assigned to the LAD after revasc. |
| vom Dahl et al., 1996 (47) | Regional mismatch: Tc uptake ≤70%, FDG >50% and FDG – Tc uptake >20% | Regional change in regional wall motion ≥ 1 standard deviation |
| Grandin et al., 1995 (51) | Regional flow-metabolism mismatch: segmental FDG to ammonia activity ratio > 1.2 (note, includes patients with normal perfusion (>80% of maximal perfusion) | Regional wall motion score improved by ≥1 full grade and end-systolic volume decreased after revasc. (a change from dyskinesis to akinesis was not considered improvement) |
| Carrel et al., 1992 (48) | Regional flow-metabolism mismatch: areas with reduced blood flow and maintained glucose metabolism | Regional Functional recovery was not defined in the paper |
| Marwick et al., 1992 (49) | Regional: Avid FDG uptake despite hypoperfusion at rest | Regional: Functional recovery was not defined in the paper |
FDG refers to F-18-fluorodeoxyglucose; g, gram; LVEF, left ventricular ejection fraction; MRG, metabolic rate of glucose; revasc., revascularized; ROC, receiver operating characteristics; Tc, Technetium;
Regional (Segmental) Functional Improvement
Functional recovery is the surrogate reference standard that is used to assess viability and can be measured in two ways: regional (segmental) functional recovery and global functional recovery (improvement in LVEF). Regional functional recovery is measured by assessing changes in wall motion (also known as contractile function) before and after revascularization (Figure 2). To assess changes in wall motion, the LV is divided into segments and wall motion is assessed for each segment. If wall motion improves by at least 1 grade after revascularization then the segment is classified as viable.
Figure 2: Steps involved in the assessment of regional functional recovery*.
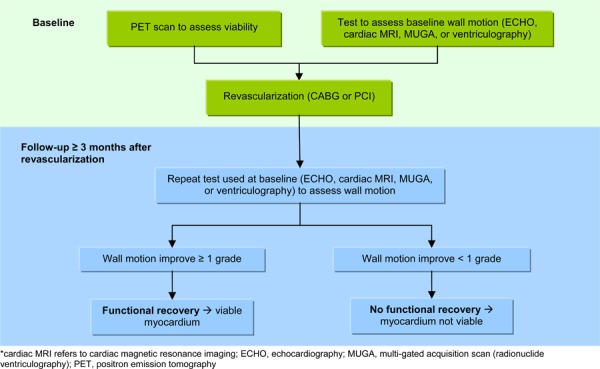
Of note, the number of segments varied between studies depending on what model was used to divide the LV (common examples include the 17-segment American Heart Association model, an 8 segment model, and a 13-segment model). While regional functional recovery is most commonly reported for each segment, it is sometimes reported by vascular territory. When vascular territories are used, the segments are grouped into 3 vascular territories per patient. (33) Alternatively, segments are reported on a per patient basis. There are numerous techniques used for grouping segments per patient including reporting results for only one segment per patient or classifying patients as viable or not viable depending on whether there are several adjacent viable segments or if more than 50% of the segments are viable. (34;40;44)
Regional functional recovery was assessed in 20 studies. The sensitivity, specificity, PPV, NPV, positive LR, negative LR, and diagnostic accuracy of each study are reported in Table 9. As some studies reported these outcomes in several ways (e.g., on a per segment basis and per patient basis) or for several thresholds (e.g. FDG uptake > 50% and > 60%), the option that resulted in the highest sensitivity and specificity combination were chosen for any further analyses (the selected options are identified by italics in Table 8).
Table 9: Study results for diagnostic accuracy of PET in predicting regional functional recovery after revascularization*.
| Author, Year | Viability Threshold | No. Dysfunctional Segments | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kuhl et al., 2006 (30)† | Normal perfusion: Tc uptake >70% Flow/metabolism mismatch: ≤70% uptake of Tc-sestamibi, >70% uptake of FDG, and ≥20% difference between FDG and Tc-sestamibi uptake | 187 ‡ 29 (successfully revasc.) |
86.5 69.2 |
73.6 93.8 |
77.6 90.0 |
83.8 78.9 |
3.28 11.08 |
0.18 0.33 |
80.2 82.8 |
| Slart et al., 2006 a (31) | Flow/metabolism mismatch: a difference of 7% in relative segmental uptake (from ROC curve analysis) | 264 (successfully revasc.) | 90.9 | 86.8 | 89.0 | 89.0 | 6.9 | 0.10 | 89.0 |
| Slart et al., 2006 b (32) | FDG uptake ≥50% 10% WT (gated PET) |
213 (successfully revasc.) 213 (successfully revasc.) |
94.0 89.5 |
85.0 77.5 |
91.2 86.9 |
89.5 81.6 |
6.3 4.0 |
0.07 0.14 |
90.6 85.0 |
| Barrington et al., 2004 (33) | FDG uptake ≥ 68% Normal perfusion or flow-metabolism mismatch |
31 vascular territories 28 vascular territories |
100.0 100.0 |
92.0 86.4 |
75.0 66.7 |
100.0 100.0 |
12.5 7.3 |
0.00 0.00 |
93.5 89.3 |
| Schmidt et al., 2004 (34) | Normalized FDG uptake ≥50% of reference segment uptake | 40 patients (successfully revasc.) | 100.0 | 73.3 | 86.2 | 100.0 | 3.75 | 0.00 | 90.0 |
| Nowak et al., 2003 (35) | Normalized FDG uptake ≥ 70% | 72 (successfully revasc.) | 80.0 | 71.9 | 78.0 | 74.2 | 2.8 | 0.28 | 76.4 |
| Normalized FDG uptake ≥ 60% | 72 (successfully revasc.) | 90.0 | 62.5 | 75.0 | 83.3 | 2.4 | 0.16 | 77.8 | |
| Normalized FDG uptake ≥ 50% | 72 (successfully revasc.) | 95.0 | 21.9 | 60.3 | 77.8 | 1.2 | 0.23 | 62.5 | |
| Normalized FDG uptake ≥ 80% | 72 (successfully revasc.) | 55.0 | 84.4 | 81.5 | 60.0 | 3.5 | 0.53 | 68.1 | |
| Lund et al., 2002 (37) | Normalized FDG uptake > 55% | 34 patients (successfully revasc.) | 88.9 | 68.0 | 50.0 | 94.4 | 2.8 | 0.16 | 73.5 |
| Tani et al., 2001 (39) | FDG uptake ≥ 50% of the maximum uptake | 110 (successfully revasc.) 91 (successfully revasc)§ |
89.9 88.1 |
61.0 62.5 |
79.5 81.3 |
78.1 74.1 |
2.3 2.4 |
0.17 0.19 |
79.1 79.1 |
| Wiggers et al., 2000 (40) | FDG uptake ≥70% of that of the reference region for the patient | 314 (successfully revasc) | 79.2 | 52.1 | 25.1 | 92.5 | 1.7 | 0.40 | 56.7 |
| Fath-Ordoubadi et al., 1999 (41) | MRG ≥ 0.25 umol/g/min | 51 (successfully revasc.) 63 |
96.7 97.0 |
90.5 76.7 |
93.5 82.1 |
95.0 95.8 |
10.2 4.2 |
0.04 0.04 |
94.1 87.3 |
| Schoder et al., 1999 (42) | Relative FDG and ammonia uptake > 2 SD above the normal mean (>15 of the vascular territory must be hypoperfused) | 107 vascular territories (successfully revasc) | 93.0 | 81.3 | 87.0 | 89.7 | 5.0 | 0.09 | 88.0 |
| Zhang et al., 1999 (50) | Flow-metabolism mismatch (semi-quantitative assessment, no threshold reported) | 101 | 75.9 | 86.0 | 88.0 | 72.5 | 5.4 | 0.28 | 80.2 |
| Pagano et al., 1998 (43) | MRG ≥ 0.25 umol/min/g | 336 (successfully revasc.) | 99.0 | 33.3 | 66.4 | 96.0 | 1.5 | 0.03 | 70.8 |
| Maes et al., 1997 (44) | Ratio of metabolic and flow index > 1.2 | 23 patients | 83.3 | 90.9 | 90.9 | 83.3 | 9.2 | 0.18 | 87.0 |
| Baer et al., 1996 (45) | FDG uptake ≥ 50% of the maximal uptake | 371 (successfully revasc,)|| | 92.8 | 66.0 | 72.0 | 90.6 | 2.7 | 0.11 | 79.0 |
| ≥50% of akinetic/dyskinetic segments related to an infarct region had uptake ≥50% of maximal uptake | 42 patients (successfully revasc)|| | 96.2 | 68.8 | 83.3 | 91.7 | 3.1 | 0.06 | 85.7 | |
| Gerber et al., 1996 (46) | Flow-mismatch: ammonia uptake < 70% and ratio of FDG to ammonia > 1.2 | 39 patients (successfully revasc.) | 75.0 | 66.7 | 78.3 | 62.5 | 2.3 | 0.38 | 71.8 |
| vom Dahl et al., 1996 (47) | Flow-metabolism mismatch: Tc uptake ≤ 70%, FDG uptake > 50% and FDG minus Tc uptake > 20% | 48 vascular territories (successfully revasc.) | 92.0 | 34.8 | 60.5 | 80.0 | 1.4 | 0.23 | 64.6 |
| Grandin et al., 1995 (51) | Flow-metabolism mismatch: segmental FDG to ammonia activity ratio > 1.2 | 25 patients | 64.7 | 50.0 | 73.3 | 40.0 | 1.3 | 0.71 | 60.0 |
| Segments with flow <80% of maximal flow and flow-metabolism mismatch: segmental FDG to ammonia activity ratio > 1.2 | 17 patients | 88.9 | 50.0 | 66.7 | 80.0 | 1.8 | 0.22 | 70.6 | |
| Carrel et al., 1992 (48) | Flow-metabolism mismatch (no threshold reported) | 23 patients (successfully revasc.)¶ | 94.1 | 50.0 | 84.2 | 75.0 | 1.9 | 0.12 | 82.6 |
| Marwick et al., 1992 (49) | Flow-metabolism mismatch: avid FDG uptake in presence of hypoperfusion at rest | 73 segments 23 patients |
61.3 86.7 |
83.3 62.5 |
73.1 81.3 |
74.5 71.4 |
3.7 2.3 |
.46 0.21 |
74.0 78.3 |
FDG refers to F-18-fluorodeoxyglucose; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LR, refers to likelihood ratio; MI, myocardial infarction; MRG, rate of metabolic glucose utilization; NPV, negative predictive value; PPV, positive predictive value; revasc., revascularized; Tc, technetium; WT, wall thickness
Sensitivity, specificity, PPV, and NPV values reported in this table vary slightly from those reported in the text of the published report because a different equation was used in the report to calculate these values.
Only segments with severely reduced function (wall motion score ≥ 3) were included in the analysis
Results restricted to only those patients who had a previous MI (excluding acute MI patient population)
Results for dyskinetic/akinetic segments only
Patient defined as viable or not viable based on 1 segment/patient that with abnormal contractility on preoperative ECHO and clearly PET-documented blood-flow metabolism match or mismatch
Figure 3 shows the sROC curve obtained by plotting the sensitivity and specificity. The area under the curve (AUC) is 0.893 which indicates that PET is a good to excellent test for assessing viability. (31)
Figure 3: Diagnostic accuracy of PET for detecting regional functional recovery, sROC Curve.
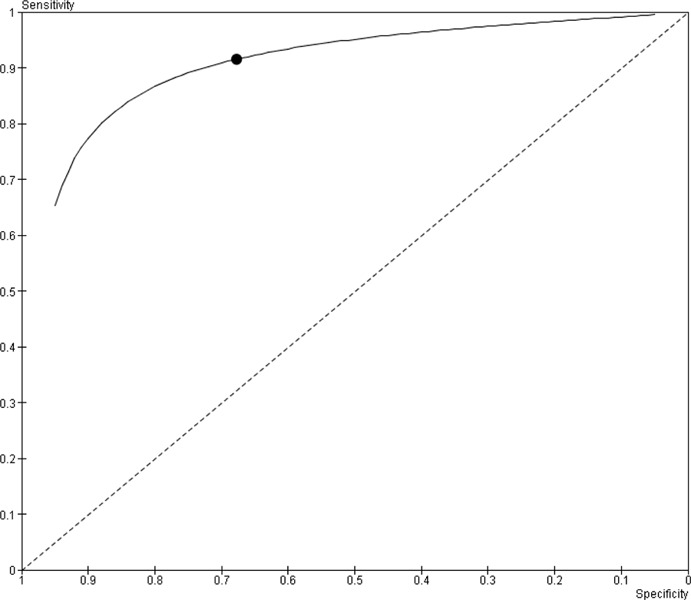
Figure 4 (page 39) shows the sensitivity and specificity forest plots by study. The reported sensitivities ranged from 76% to 100%. The pooled estimate of sensitivity was 91.5% (95% CI, 88.2% – 94.9%). There was substantial heterogeneity in the reported specificity values, which ranged from 33% to 92%. The pooled estimate of specificity was 67.8% (95% CI, 55.8% – 79.7).
Figure 4: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery.
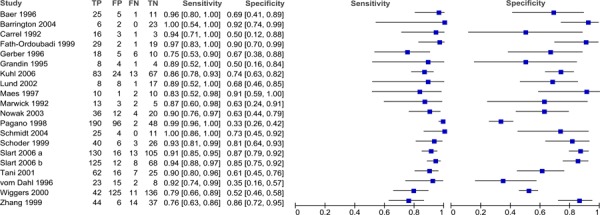
A likelihood ratio plot (Figure 5) was obtained by plotting the negative likelihood ratio by the positive likelihood ratio. Based on the clustering of points in the somewhat useful and moderately useful areas, PET is a potentially useful technique for assessing myocardial viability.
Figure 5: Likelihood ratio plot showing the diagnostic accuracy of PET for predicting regional functional recovery.
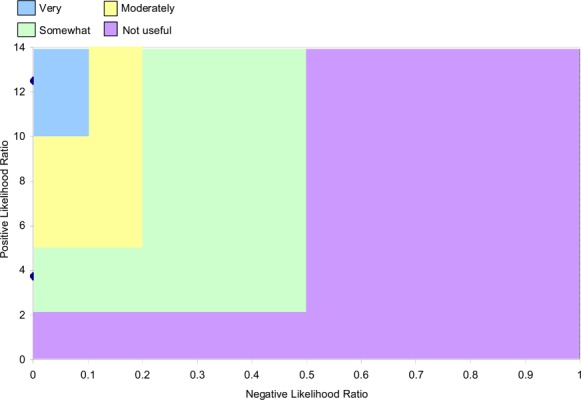
Subgroup Analyses
Wall motion can be measured by ECHO, cardiac MRI, multi-gated acquisition scan (MUGA), and left ventricular angiography. Abnormality detection varies across these technologies as wall motion is assessed semi-quantitatively and detection is dependent on operator skill and subjective interpretation. The method of assessment is a potential confounder that could bias the results of the accuracy studies, and studies were thus grouped based on the method of functional recovery assessment used in each study. (38)
Another common difference between studies that might affect the results is the type of segments that are included in the analysis. Some studies include all identified dysfunctional segments, while other studies include only those segments that were successfully revascularized based on repeat coronary angiography. After revascularization, re-stenosis of the artery may occur making it impossible to differentiate between a viable segment that did not exhibit improved functional recovery because the revascularization was not successful (a false negative) and a non-viable segment.
Three other factors varied across the included studies:
the unit of analysis: segment, vascular territories, and patient;
the radiotracers used to detect viability: FDG PET alone, FDG PET + PET perfusion tracers, or FDG PET + SPECT perfusion tracers; and
the mean pre-revascularization LVEF: < 40% and ≥ 40%.
The breakdown of the included studies by these factors as well as the method of functional recovery assessment and the type of segment examined is shown in Table 10.
Table 10: Stratification variables for regional functional assessment by study*.
| Study | Method of Regional Functional Recovery Assessment | Type of Segment | Unit of Analysis | Type of Radiotracer Tracer | Mean LVEF (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECHO | cardiac MRI | MUGA | LVA | Successfully revasc. segments | All dysfunctional segments | Patient | Vascular Territories | Segment | PET Perfusion Tracer | SPECT Perfusion Tracer | No Perfusion Tracer | < 40 | ≥ 40 | ||
| Barrington 2004 (33) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Baer 1996 (45) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Carrel 1992 (48) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Fath-Ordoubadi 1999 (41) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Grandin 1995 (51) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Gerber 1996 (46) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Kuhl 2006 (30) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lund 2002 (37) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Maes 1997 (44) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Marwick 1992 (49) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Nowak 2003† (35) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Pagano 1998 (43) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Schmidt 2004 (34) | ✓ | ✓ | ✓‡ | ✓ | ✓ | ||||||||||
| Schoder 1999 (42) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Slart 2006 a (31) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Slart 2006 b (32) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Tani 2001§ (39) | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Wiggers 2000 (40) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Zhang 1999 (50) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| vom Dahl 1996 (47) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
Cardiac MRI refers to cardiac magnetic resonance imaging; ECHO, echocardiography; LVA, left ventricular angiography; LVEF, left ventricular ejection fraction; MUGA, multiple gated acquisition scan; PET, positron emission tomography; SPECT, single photon emission computed tomography
In Nowak et al. (35) Functional recovery was assessed using MUGA for 7 patients and ECHO for 8 patients, but it was impossible to separate the results for these 2 methods, so Nowak was excluded from the subgroups related to method of functional recovery assessment
infarct regions (≥50% of infarct related segments were viable)
Tani et al. (39) did not report a mean pre-revascularization LVEF for the patients enrolled in the study, so it was excluded from the subgroup related to mean LVEF
Pooled estimates and ranges for sensitivity and specificity and the area under the curve (AUC) based on the summary receiver operating characteristics curves for each subgroup are shown in Table 11. The sensitivity and specificity forest plots the subgroups are available in Appendix 3. Similar to the combined data results, for most subgroups the reported sensitivities varied less than the reported specificities. While some factors such as the method of functional recovery assessment, the type of radiotracer, the type of segment, and the unit of analysis had an impact on the sensitivity, specificity and/or area under the curve (AUC), none of these differences were statistically significant (Table 12).
Table 11: Pooled estimates of sensitivity and specificity by subgroup.
| Subgroup | Sensitivity (%) | Specificity (%) | AUC | ||
|---|---|---|---|---|---|
| Pooled estimate (95% CI) | Range | Pooled estimate (95% CI) | Range | ||
| Mean preoperative LVEF | |||||
| LVEF < 40% | 92.0 (86.5 – 97.5) | 75 – 100 | 73.0 (59.7 – 86.2) | 33 – 92 | 0.898 |
| LVEF ≥ 40% | 91.4 (87.2 – 95.6) | 76 – 100 | 70.9 (59.9 – 81.8) | 35 – 91 | 0.909 |
| Method of Functional Recovery Assessment | |||||
| ECHO | 91.0 (86.4 – 95.6) | 76 – 100 | 69.8 (58.2 – 81.4) | 33 – 92 | 0.892 |
| cardiac MRI | 92.9 (87.4 – 98.3) | 86 – 100 | 80.9 (67.8 – 94.0) | 73 – 87 | 0.945 |
| MUGA* | 84.5 (57.1 – 100.0) | 83 | 92.1 (72.9 – 100.0) | 91 | Cannot calculate |
| Ventriculography | 91.1 (80.1 – 100.0) | 89 – 92 | 51.3 (24.6 – 78.0) | 35 – 68 | 0.798 |
| Type of Radiotracer | |||||
| PET perfusion tracer | 89.9 (83.5 – 96.4) | 75 – 100 | 78.3 (66.3 – 90.2) | 50 – 92 | 0.926 |
| SPECT perfusion tracer | 87.2 (78.0 – 96.4) | 76 – 92 | 67.1 (48.3 – 85.9) | 35 – 86 | 0.864 |
| FDG alone | 94.5 (91.0 – 98.0) | 79 – 100 | 66.8 (53.2 – 80.3) | 33 – 90 | 0.926 |
| Type of Segment | |||||
| Revascularized segments | 93.0 (89.8 – 96.2) | 69 – 100 | 70.9 (61.2 – 80.5) | 33 – 94 | 0.915 |
| All dysfunctional segments | 88.1 (80.0 – 96.3) | 76 – 100 | 81.0 (69.2 – 92.8) | 50 – 92 | 0.903 |
| Unit of Analysis | |||||
| Patient | 91.1 (84.8 – 97.4) | 75 – 100 | 68.6 (53.3 – 83.9) | 50 – 91 | 0.892 |
| Vascular territories | 93.9 (86.4 – 100.0) | 92 – 100 | 73.2 (52.7 – 93.7) | 35 – 92 | 0.864 |
| Segment | 89.9 (85.5 – 94.2) | 76 – 99 | 72.7 (62.7 – 82.6) | 33 – 90 | 0.886 |
AUC refers to area under the curve; cardiac MRI; cardiac magnetic resonance imaging; ECHO, echocardiography; FDG, F-18-fluorodeoxyglucose; LVEF, left ventricular ejection fraction; MUGA, multigated acquisition scan; PET, positron emission tomography; SPECT, single photon emission computed tomography
There is only 1 study in the MUGA subgroup, so a range is not available.
Table 12: Pairwise comparisons of sensitivity and specificity by subgroup.
| Subgroup | P value | |
|---|---|---|
| Sensitivity | Specificity | |
| Mean preoperative LVEF | ||
| LVEF <40% vs. ≥ 40% | .9204 | .9955 |
| Method of Functional Recovery Assessment | ||
| ECHO vs. cardiac MRI | .8942 | .4573 |
| ECHO vs. MUGA | .8942 | .2570 |
| ECHO vs. Ventriculography | .9868 | .4573 |
| cardiac MRI vs. MUGA | .8942 | .5758 |
| cardiac MRI vs. Ventriculography | .8942 | .2570 |
| MUGA vs. Ventriculography | .8942 | .2280 |
| Type of Radiotracer | ||
| PET perfusion tracer vs. SPECT perfusion tracer | .8942 | .5758 |
| PET perfusion tracer vs. FDG alone | .8942 | .4573 |
| SPECT perfusion tracer vs. FDG alone | .8942 | .9955 |
| Type of Segment | ||
| Revascularized vs. all dysfunctional segments | .8908 | .9970 |
| Unit of Analysis | ||
| Patient vs. vascular territories | .8942 | .9955 |
| Patient vs. segments | .8942 | .9955 |
| Vascular territories vs. segment | .8942 | .9955 |
Cardiac MRI refers to cardiac magnetic resonance imaging; ECHO, echocardiography; FDG, F-18-fluorodeoxyglucose; LVEF, left ventricular ejection fraction; PET, positron emission tomography; SPECT, single photon emission computed tomography
In addition, five studies reported accuracy results for hypokinetic segments and akinetic/dyskinetic segments separately. So, an additional subgroup analysis was performed using these studies. Table 13 shows the sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and diagnostic accuracy results stratified by hypokinetic and akinetic/dyskinetic segments. As some studies reported these outcomes in several ways (e.g., on a per segment basis and per patient basis) or for several thresholds (e.g., wall thickness vs. FDG uptake), the option that resulted in the highest sensitivity and specificity combination were chosen for any further analyses (the selected options are identified by italics in Table 13). The sensitivity and specificity forest plot is available in Appendix 3.
Table 13: Study results for diagnostic accuracy of PET in predicting regional functional recovery after revascularization stratified by hypokinetic and akinetic/dyskinetic segments*.
| Author, Year | Viability Threshold | No. Dysfunctional Segments |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Positive LR |
Negative LR |
Diagnostic Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Hypokinetic segments | |||||||||
| Slart et al., 2006 a (31) | Flow/metabolism mismatch: a difference of 7% in relative segmental uptake | 174 (successfully revasc.) | 90.0 | 86.0 | 91.5 | 83.8 | 6.4 | 0.12 | 88.5 |
| Slart et al., 2006 b (32) |
FDG uptake ≥50% Viability defined as 10% WT |
145 (successfully revasc.) 145 (successfully revasc) |
94.0 91.0 |
86.0 78.0 |
92.6 88.5 |
88.2 83.7 |
6.7 4.1 |
0.07 0.12 |
91.0 86.9 |
| Pagano et al., 1998 (43) | MRG ≥ 0.25 umol/min/g | 180 (successfully revasc) | 100.0 | 19.1 | 55.8 | 100.0 | 1.2 | 0.00 | 60.0 |
| Akinetic/dyskinetic segments | |||||||||
| Slart et al., 2006 a (31) | Flow/metabolism mismatch: a difference of 7% in relative segmental uptake | 90 (successfully revasc.) | 89.0 | 84.0 | 77.5 | 92.0 | 5.6 | 0.13 | 85.6 |
| Slart et al., 2006 b (32) |
FDG uptake ≥50% 10% WT |
68 (successfully revasc,) 68 (successfully revasc.) |
91.0 86.0 |
85.0 79.0 |
81.8 76.5 |
91.4 88.2 |
6.1 4.1 |
0.11 0.18 |
86.8 82.4 |
| Pagano et al., 1998 (43) | MRG ≥ 0.25 umol/min/g | 156 (akinetic segments, successfully revasc) | 98.0 | 54.5 | 79.8 | 93.8 | 2.2 | 0.04 | 82.7 |
| Baer et al., 1996 (45) | FDG uptake > 50% of the maximal uptake ≥50% of akinetic or dyskinetic segments related to an infarct region had uptake ≥50% of maximal uptake |
371 (successfully revasc,) 42 patients (successfully revasc) |
92.8 96.2 |
66.0 68.8 |
72.0 83.3 |
90.6 91.7 |
2.7 3.1 |
0.11 0.06 |
79.0 85.7 |
FDG refers to F-18--fluorodeoxyglucose; LR, likelihood ratio; No, number; NPV, negative predictive value; PPV, positive predictive value; revasc., revascularized; WT, wall thickness
There was no significant difference between the pooled estimates of sensitivity for akinetic/dyskinetic segments compared with hypokinetic segments [akinetic/dyskinetic, 89.2% (95% CI, 80.9% – 97.6%); hypokinetic, 95.9% (95% CI, 92.0% – 99.9%); P = .8942] or between the estimates of specificity for these subgroups [akinetic/dyskinetic, 84.9% (73.1% – 96.7%); hypokinetic, 67.0% (95% CI, 45.7% – 88.4%); P = .4573].
Gated PET
In Slart et al. 2006 a (31) and Slart et al 2006 b (32), gated PET was used to assess regional functional recovery. Using gated PET, functional recovery can be assessed based on wall thickness rather than metabolic and/or flow tracer uptake. In Slart et al. 2006 b, (32) the accuracy of gated PET (viability was defined as a 10% increase in wall thickness) was reported as a sensitivity and specificity of 90% and 78%, respectively (Table 8). In contrast, the reported sensitivity and specificity using FDG uptake were 94% and 85% in this study. (32) This suggests FDG uptake is a more accurate method for predicting regional functional recovery after revascularization than wall thickness, although this comparison must be considered with caution as it is based on only one study.
Limitations
Numerous limitations exist that may reduce the accuracy and reliability of the reported regional functional recovery results. First, since functional recovery was assessed using other imaging modalities, it is possible that segments were misaligned when comparing the ECHO, cardiac MRI, or LV angiography results with the PET results, which could bias the results. (45) Second, studies used a variety of different thresholds to define viability. While the bivariate model that was used to pool sensitivity and specificity helps to reduce any threshold effect that may occur when combining studies using different thresholds, the threshold effect may still bias the results.
Third, six of the studies reported the results for all dysfunctional segments and did not attempt to assess whether revascularization was successful. In these studies, recurrence of stenosis and graft patency may have prevented functional recovery in some viable segments resulting in false positives which could decrease the accuracy of the results. (43)
Fourth, functional recovery is only a surrogate for viability. Since functional recovery is dependent on the interaction between the extent of scar and viable tissue within a segment, it is possible that PET imaging can detect a segment with viable tissue and yet not have that segment recover function. For instance, a segment with an epicardial rim of viable myocardium that is tethered to subendocardial scar may not recover function, but may actually have some viable tissue which the PET imaging picked up. (38) These segments count as false positives in the calculation of sensitivity and specificity, but the PET results would be correct about the identification of viable tissue in that segment.
Finally, depending on the extent of damage and the amount of stunned versus hibernating myocardium, it make take a year or more after revascularization for functional recovery to occur. Most of the included studies, however, only followed patients for three to six months after revascularization, which may be inadequate to properly assess functional recovery and thereby underestimate the accuracy of PET imaging for the assessment of myocardial viability. (43) These limitations which result in false positives may lead to an underestimate of specificity.
A variety of additional limitations that relate to the quality of the included studies exist including small sample sizes with no a priori sample size calculations, lack of blinding in the interpretation of PET results and functional recovery, selection bias, and no details on withdrawals from studies. These and other quality issues have been identified using the QUADAS checklist, the results of which are shown in Appendix 4.
Global Functional Improvement
Global LV function is defined as an improvement in global LVEF by five percent or more after revascularization. (1;26) Regional functional improvement does not necessarily result in an improvement in global function because global functional recovery requires a substantial amount of viable myocardium (35% – 50%). (1) Compared with regional wall motion function, global function is more important clinically because it correlates better with symptoms of heart failure, physical capacity, and survival. (31;38)
Five studies reported the diagnostic accuracy of PET for predicting global functional recovery. Table 7 provides a summary of the study characteristics for these studies. The sensitivity, specificity, and positive and negative likelihood ratios for these studies are presented in Table 14. The sensitivity and specificity ranged from 74% to 100% and 45% to 100%, respectively. As was observed for regional functional recovery, the reported specificities were more heterogeneous than the reported sensitivities.
Table 14: Study results for diagnostic accuracy of PET in predicting global LV functional recovery after revascularization.
| Author, Year | Viability Threshold | Sensitivity (%) | Specificity (%) | Positive LR | Negative LR |
|---|---|---|---|---|---|
| Slart et al., 2006 a (31) | ≥ 2 viable segments | 86 | 100 | -- | 0.14 |
| Slart et al., 2006 b (32) |
≥ 3 viable segments (based on FDG uptake) ≥ 3 viable segments (based on WT) |
87 75 |
85 85 |
5.8 5.0 |
0.15 0.12 |
| Bax et al., 2002 (36) | ≥ 3 viable segments (MRG value >0.25 umol/min/g) ≥ 3 viable segments (relative MRG >60%) |
90 100 |
71 71 |
3.1 3.4 |
0.14 0.00 |
| ≥ 3 dysfunctional segments and ≥ 45% glucose uptake | 79 | 55 | 1.8 | 0.38 | |
| Gerber et al., 2001 (38) | ≥ 3 dysfunctional segments and > 50% glucose uptake | 74 | 58 | 1.8 | 0.45 |
| ≥ 2 dysfunctional segments and > 60% glucose uptake | 87 | 45 | 1.6 | 0.29 | |
| Pagano et al., 1999 (43) | 8 viable segments (glucose uptake > 0.25 umol/min/g) | 88 | 75 | 3.5 | 0.16 |
FDG refers to F-18-fluorodeoxyglucose; LR, likelihood ratio; WT, wall thickness
The likelihood ratio plot (Figure 6) indicates that PET is a potentially useful test for detecting global functional recovery as three of the four points3 cluster in the somewhat useful and moderately useful areas of the graph. (As some studies reported the outcomes using different viability thresholds, the results with the highest sensitivity and specificity combination were chosen for any further analyses and are identified by italics in Table 14).
Figure 6: Diagnostic Accuracy of PET for Predicting Global Functional Recovery Likelihood Ratio Plot.
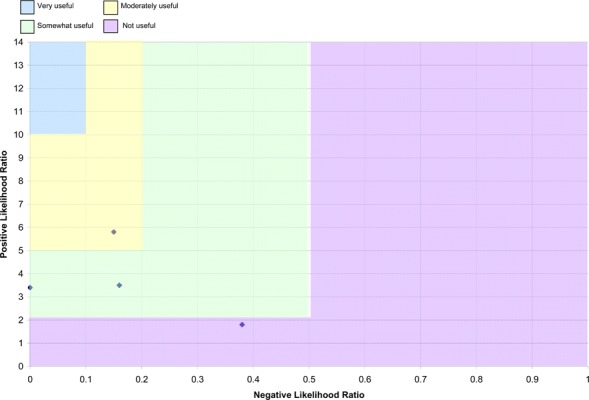
Limitations
It was impossible to pool the sensitivity and specificity results from global functional recovery analyses as raw data on true/false positives and true/false negatives were not provided in the studies. Furthermore, since each study used a different threshold to define viability, it is difficult to draw an overall conclusion regarding diagnostic accuracy for the prediction of global functional recovery based on the results.
There are a variety of additional limitations that relate to the quality of the studies and the most common are summarized here. First, with the exception of Gerber et al. (38), the studies had very small sample sizes (< 40 patients) and no a priori sample size calculations, so they are unlikely to be adequately powered. Second, since these are observational studies, a number of issues related to study design affect the accuracy and reliability of the results including the potential for selection bias due to the lack of randomization, and the lack of blinding for the assessment of viability based on PET scans or functional recovery assessment. Third, many studies do not report withdrawals as the studies either report results for only those patients who completed follow-up or do not report reasons for withdrawals. It is thus not possible to determine whether there were important differences between patients who did and did not complete the study or even whether loss to follow-up is an issue in the study. These limitations and others are detailed in the Quality Assessment using QUADAS Tables available in Appendix 4.
Quality of Evidence: Diagnostic Accuracy of PET
Individual study quality was assessed using the QUADAS checklist (Appendix 4). Similar limitations were observed in the studies that examined regional and global functional recovery. Common study limitations were: non-representative patient populations, not reporting study withdrawals, not reporting uninterpretable or intermediate test results, no or unclear blinding of those interpreting the PET results, and no or unclear blinding of those who interpreted the functional recovery results. In addition, some studies did not adequately describe the inclusion and exclusion criteria. The quality of the overall body of evidence was evaluated using the GRADE system (Tables 15 and 16). For the diagnostic accuracy of PET, quality was found to be very low for the prediction of viable myocardium using either regional or global functional recovery as a surrogate for viability. Thus, any estimate of effect is uncertain.
Table 15: GRADE quality of evidence for the diagnostic accuracy of PET for the detection of viable myocardium based on regional functional recovery in patients with known CAD.
| Factor | Explanation | GRADE |
|---|---|---|
| Risk of Bias | ||
| Study design | Observational cohort studies (20 studies) | High |
| Limitations | Serious limitations* | Reduced by one level → Moderate |
| Indirectness | ||
| Outcomes | Diagnostic tests are considered as surrogate outcomes | Reduced by one level → Low |
| Patient populations, diagnostic test, comparison test, and indirect comparisons | Patient populations generally not representative of that of Ontario | Reduced by one level → Very Low |
| Inconsistency in study results | No serious inconsistencies | Unchanged |
| Imprecise evidence | No serious imprecision | Unchanged |
| Publication bias | No publication bias suspected | Unchanged |
| Quality of Evidence | Very Low | |
Downgraded due to serious limitations in the quality of evidence of the individual studies including: not reporting study withdrawals; not reporting uninterpretable or intermediate test results; not blinding those who interpreted the PET results and those interpreting the functional recovery results.
Table 16: GRADE quality of evidence for the diagnostic accuracy of PET for the detection of myocardial viability based on global functional recovery in patients with known CAD.
| Factor | Explanation | GRADE |
|---|---|---|
| Risk of Bias | ||
| Study design | Observational cohort studies (5 studies) | High |
| Limitations | Serious limitations* | Reduced by one level → Moderate |
| Indirectness | ||
| Outcomes | Diagnostic tests are considered as surrogate outcomes | Reduced by one levels→Low |
| Patient populations, diagnostic test, comparison test, and indirect comparisons | Patient populations are generally not representative of the Ontario population that would receive viability testing | Reduced by one level → Very Low |
| Inconsistency in study results | No serious inconsistencies | Unchanged |
| Imprecise evidence | No serious imprecision | Unchanged |
| Publication bias | No publication bias suspected | Unchanged |
| Quality of Evidence | Very Low | |
Downgraded due to serious limitations in the quality of evidence of the individual studies including: not reporting study withdrawals; not reporting uninterpretable or intermediate test results; not blinding those who interpreted the PET results and those interpreting the functional recovery results.
PET Viability and Prognosis
Mortality
In order to examine the long-term benefit of assessing myocardial viability using PET on patient outcomes, studies that compared the prognosis of patients with viable myocardium with those with non-viable tissue were included. The study characteristics and patient population characteristics of the nine identified studies are presented in Tables 17 and 18. The first three studies in the table were identified in the systematic search of the literature, while the others were identified from reference lists of systematic reviews and health technology assessments on this topic.
Table 17: Study characteristics of prognostic studies.
| Author, Year | Study Design & No. Patients | Patient Population | Mean Follow-up | Technique (tracer) | Outcomes | Viability Criteria |
|---|---|---|---|---|---|---|
| Desideri et al., 2005 (52) | Prospective cohort n=261 |
|
2.1y (median) | 13N- ammonia / FDG |
|
|
| Feola et al., 2008 (53) | Prospective cohort n=93* |
|
342 ± 78 days Range: 110- 434 |
13N-ammonia / FDG |
|
|
| Sawada et al., 2005 (54) | Prospective cohort n=61 |
|
4.3 ± 8.8 years | 13N-ammonia / FDG |
|
|
| Rohatgi et al., 2001 (55) | Retrospective cohort n=99† |
|
25±9 months Range: 9-50 |
13N-ammonia / FDG |
|
|
| Zhang et al., 2001 (56) | Cohort‡ n=123 |
|
26±10 months Range: 1-36 Median: 28 |
Tc-MIBI SPECT / FDG PET |
|
|
| Di Carli et al., 1998 (57) | Cohort‡ n=93 |
|
3.8 years Range: 0-6.2 |
13N-ammonia / FDG |
|
|
| vom Dahl et al., 1997 (58) | Prospective cohort n=161* |
|
29±6 months Range: 22-44 |
99Tc-MIBI SPECT / FDG PET |
|
|
| Lee et al., 1994 (59) | Retrospective cohort n=129§ |
|
17±9 months | Rb-82 / FDG |
|
|
| Eitzman et al., 1992 (60) | Retrospective cohort n=82║ |
|
12 months | 13N-ammonia / FDG or Rb-82 / FDG |
|
|
CAD refers to coronary artery disease; CCS, Canadian Cardiovascular Society; d, days; FDG, F-18-flurodeoxyglucose; LV, left ventricular; LVEF, left ventricular ejection fraction; mo, months; MI, myocardial infarction; NR, not reported; NYHA, New York Heart Association; revasc, revascularization; Rb-82, rubidium-82; yr, years
Consecutive patients
144 patients were eligible for inclusion, but only 99 were included as 46 were lost to follow-up.
Prospective or retrospective not reported in paper.
137 patients were enrolled in the study, but the analysis only includes 129 patients. Reasons for exclusion were: technically inadequate studies (2 patients); cardiac transplantation (4 patients); loss to follow-up (2 patients).
110 patients were eligible for the study, but complete data acquisition was not possible in 23 patients, 3 received heart transplants, and 2 had poor quality PET scans which provided inadequate data for inclusion in the study.
Table 18: Patient characteristics in prognosis studies.
| Author, Year | Patient Characteristics by Group [R = Revascularization Group; MT = Medical Therapy Group] |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean Age (Range) | % Male | LVEF(%) | Previous MI (%) | Hypertension (%) | Diabetes (%) | 3 vessel CAD (%) | |||||||||
| R | MT | R | MT | R | MT | R | MT | R | MT | R | MT | R | MT | R | MT | |
| Desideri et al., 2005 (52) | 94 | 167 | 66 (58–70) |
66 (57–69) |
89 | 89 | 30 | 29 | 79 | 82 | 50 | 50 | 12 | 10 | NR | 47 |
| Feola et al., 2008 (53) | 51 | 42 | 65.7±9.1 | 62.6±9 | 76 | 30.8 ± 7.1 | 29.7 ± 7.7 | 100 | 100 | 65 | 29 | 31 | 35 | |||
| Sawada et al., 2005 (54) | 33 | 28 | 58±9 | 84 | 29±11 | NR | NR | NR | NR | 100 | 100 | NR | 78 | |||
| Rohatgi et al., 2001 (55) | 37 | 62 | V: 63±10 NV: 58±10 | V: 61±12 NV: 58±12 | 84 | 79 | 22±6 | V: 86 NV: 76 | V: 63 NV: 67 | NR | NR | V: 52 NV: 50 | V: 41 NV: 33 | NR | NR | |
| Zhang et al., 2001 (56) | 67 | 56 | V: 53±10 NV: 56±7 | V: 56±9 NV: 56±9 | V: 83 NV: 92 | V: 93 NV: 92 | V: 36±5 NV: 36±6 | V: 35±8 NV:34±6 | 100 | 100 | V: 50 NV: 48 | V: 40 NV: 27 | V: 19 NV: 12 | V: 7 NV: 12 | 55 | |
| Di Carli et al., 1998 (57) | 43 | 50 | median, 68 | Median, 69 | 88 | 80 | 25 | 25 | 72 | 64 | 26 | 12 | 16 | 10 | 37 | 24 |
| vom Dahl et al., 1997 (58)* | 84 | 77 | V: 57±9 NV (scar): 59±7 NV (mild match): 55±9 | V: 55±8 NV (scar): 58±9 NV (mild match): 55±9 | V: 89 NV: 94 | V: 89 NV: 87 | V: 49±10 NV (scar): 41±12 NV (mild match): 47±10 | V: 50±14 NV (scar): 44±13 NV (mild match): 48±1 | 88 | NR | NR | NR | NR | V: 45 NV: 52 | V: 56 NV: 25 | |
| Lee et al., 1994 (59) | 68 | 61 | V: 60±11 NV: 64±9 | V: 62±12 NV: 62±12 | 79 | V: 37±17 NV: 34±12 | V: 39±18 NV: 38±17 | 100 | 100 | NR | NR | NR | NR | V:82‡ NV: 89‡ | V:62‡ NV: | |
| Eitzman et al., 1992 (60) | 40 | 42 | V: 59±11 NV: 56±9 | V: 61±8 NV: 59±10 | 88 | V: 36±13 NV: 37±12 | V: 33±11 NV: 32±16 | NR | NR | NR | NR | NR | NR | NR | NR | |
R refers to revascularization group; MT, medical therapy group; FDG, F-18-fluorodeoxyglucose; LVEF, left ventricular ejection fraction; meds, medical therapy; NV, not viable myocardium; Rb, rubidium; revasc., revascularization; V, viable myocardium
In vom Dahl et al. (58), patients with non-viable myocardium were classified as either scar (marked reduction of technetium ≤ 50% without evidence for a mismatch, FDG minus technetium ≤ 20%) or mild match (mild reduction of technetium uptake of 51% to 70% without evidence for a mismatch, FDG minus technetium ≤ 20%).
Percentage of patients with multi-vessel disease.
The mortality rates for the following four groups were compared:
Patients with viable myocardium who were revascularized,
Patients with viable myocardium who were treated with medical therapy,
Patients without viable myocardium who were revascularized, and
Patients without viable myocardium who were treated with medical therapy.
As shown in Table 19, in all nine studies, the highest mortality rate was observed in patients with viable myocardium who were treated with medical therapy. With the exception of two studies, the second highest mortality rate was observed in patients with non-viable myocardium who were treated with medical therapy. In most studies, the lowest mortality rates were observed in patients either viable or non-viable myocardium who were revascularized. The mortality rates were pooled for studies with similar mean follow-up time periods (Figure 7). Mortality rates increased substantially in all groups between one and four years of follow-up. Overall, patients with viable myocardium who were revascularized had the lowest mortality rate, and patients with viable tissue who were treated with medical therapy had the highest mortality rate.
Table 19: Mortality rate by viability status and treatment.
| Author, Year | Mean Follow-up | Mortality Rate % (No. Deaths/No. Patients) | |||
|---|---|---|---|---|---|
| Viable Myocardium | Not Viable Myocardium | ||||
| Revascularization Group |
Medical Therapy Group |
Revascularization Group |
Medical Therapy Group |
||
| Desideri et al., 2005 (52) | 2.1 yr (median) | 14.5 (8/55) | 28.3 (17/60) | 10.3 (4/39) | 21.5 (23/107) |
| Feola et al., 2008 (53) | 342 ± 78 d (Range: 110 - 434 days) | 5.9 (3/51) | 50.0 (1/2) | 0.0 (0/6) | 15.0 (6/40) |
| Sawada et al., 2005 (54) | 4.3 ± 8.8 years | 47.4 (9/19) | 83.3 (10/12) | 57.1 (8/14) | 43.8 (7/16) |
| Rohatgi et al., 2001 (55) | 25±9 mo (Range: 9 - 50 months) | 0.0 (0/29) | 34.5 (10/29) | 0.0 (0/8) | 15.2 (5/33) |
| Zhang et al., 2001 (56) | 26±10 mo (Range: 1 - 36 months) | 0.0 (0/42) | 26.7 (8/30) | 8.0 (2/25) | 3.8 (1/26) |
| Di Carli et al., 1998 (57) | 3.8 yr (Range: 0 - 6.2 years) | 26.9 (7/26) | 64.7 (11/17) | 29.4 (5/17) | 42.4 (14/33) |
| vom Dahl et al., 1997 (58) | 29±6 mo (Range: 22 - 44 months) | 0.0 (0/36) | 22.2 (2/9) | 8.3 (4/48) | 10.3 (7/68) |
| Lee et al., 1994 (59) | 17±9 months | 8.2 (4/49) | 14.3 (3/21) | 5.3 (1/19) | 12.5 (5/40) |
| Eitzman et al., 1992 (60) | 12 months | 3.8 (1/26) | 33.3 (6/18) | 0.0 (0/14) | 8.3 (2/24) |
| Weighted Mortality Rate | Approximately 1 year (Feola, Lee, and Eitzman) | 6.3 | 24.4 | 2.6 | 12.5 |
| Weighted Mortality Rate | Approximately 2 years (Desideri, Rohatgi, Zhang, and vom Dahl) | 4.9 | 28.9 | 8.3 | 15.4 |
| Weighted Mortality Rate | Approximately 4 years (Sawada and Di Carli) | 35.6 | 72.4 | 41.9 | 42.9 |
Figure 7: Mortality rates in patients with viable and non-viable myocardium based on FDG PET by treatment group.
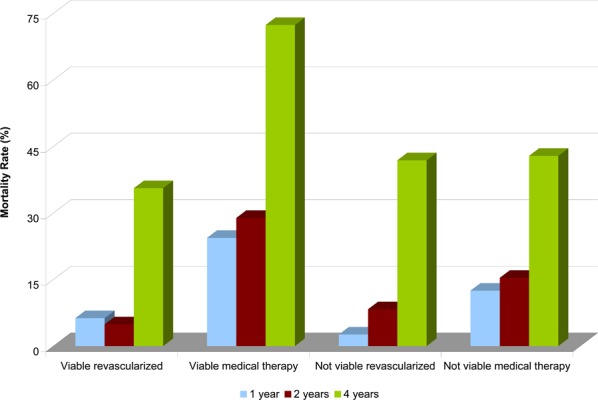
In Di Carli et al. (57), the survival rate in patients with non-viable myocardium who were revascularized was higher than in patients who were treated with medical therapy. This survival benefit, however, applied mostly to patients with severe angina (4-year adjusted survival probably, 100% vs. 60%; P = .085) and was not observed in patients with minimal or no angina symptoms (63% vs. 52%; P = .462). (57) Patients with viable tissue who were revascularized had better survival than those who were treated with medical therapy, regardless of the degree of angina symptoms (Figure 8); although, higher survival was observed in patients with minimal or no angina symptoms. (57)
Figure 8: Kaplan-Meier estimated survival probabilities for patients with viable and non-viable myocardium by method of treatment and degree of angina symptoms.
Reprinted from The Journal of Thoracic and Cardiovascular Surgery, Vol. 116, Issue 6, Di Carli et al. Long-term survival of patients with coronary artery disease and left ventricular dysfunction: implications for the role of myocardial viability assessment in management decisions, p. 997-1004, Copyright 1998, with permission from The American Association for Thoracic Surgery.
Several studies stratified the survival analyses comparing revascularized patients with medical therapy patients by mean pre-operative LVEF. In Di Carli et al. (57), the statistically significant survival benefit observed in revascularized patients was only observed in patients with an LVEF greater than 20% (LVEF < 20%, P = 0.989; LVEF > 20% – 29% %, P = 0.017; LVEF 30% – 39%, P = 0.004). In Lee et al. (59), patients with an LVEF less than 30% had a significantly higher cardiac mortality rate than those with an LVEF greater than 30% (mortality rate, 23% vs. 2%, P < 0.001).
Limitations
In general, the individual study and pooled results show that regardless of how they were treated, people with non-viable myocardium had lower mortality rates than people with viable myocardium who were treated with medical therapy. This finding may be due to the fact that patients with stable scar tissue may have a lower risk of cardiac events than those with ischemic tissue. (61) However, given that these results are based on observational studies, they are subject to numerous biases including selection bias, so these results must be considered with caution.
Other Clinical Outcomes: Exercise Capacity, Functional Status, and Quality of Life
In addition, two studies were identified that examined the impact of viable myocardium as assessed by PET on exercise capacity, functional status, and quality of life and mortality among patients who were revascularized. The characteristics of these studies are summarized in Table 20.
Table 20: Study characteristics of prognostic studies.
| Author, year | Study design | Patient population | Sample size |
Mean follow-up (months) |
Technique (tracer) |
Outcomes | Viability criteria |
|---|---|---|---|---|---|---|---|
| Marwick et al., 1999 (62) |
Prospective observational study | Patients with LV dysfunction who underwent CABG | 63 | 11±11 months (QOL) 6±4 (exercise testing) |
Rb-82 / FDG PET | Exercise capacity, functional class, quality of life, and mortality/MI | Segments that demonstrate ischemic (<15% relative reduction of activity after stress) or hibernating (FDG activity within 2 standard deviations (>70%) of normal within a perfusion defect were defined as viable |
| Marwick et al., 1992 (49) |
Prospective observational study | Patients with previous MI with clinical uncertainty about presence of viable myocardium | 23 | 22±14 wk | Rb-82 / FDG PET | Exercise capacity, functional class, and regional/global functional recovery | Avid FDG uptake despite hypoperfusion at rest |
FDG refers to F-18-fluorodeoxyglucose; LV, left ventricle; MI, myocardial infarction; QOL, quality of life; Rb, rubidium; SPECT, single-photon emission computed tomography; Tl, thallium; wk, week
Two studies by Marwick et al. (49;62) evaluated the change in exercise capacity and functional status before and after revascularization. In both studies, the mean exercise capacity increased in patients with viable myocardium and in those without (Table 21).
Table 21: Impact of revascularization on exercise capacity.
| Author, Year | Mean Exercise Capacity (METS) | |||
|---|---|---|---|---|
| Viable Myocardium | Non-Viable Myocardium | |||
| Pre-revasc. | Post-revasc. | Pre-revasc. | Post-revasc. | |
| Marwick et al., 1999 (62) | 4.6±1.5 | 5.6±1.4 | 5.9±2.8 | 6.3±2.8 |
| Marwick et al., 1992 (49) | 5.6±2.7 | 7.5±1.7 | 6.5±2.8 | 8.2±2.2 |
METS refers to metabolic equivalents; revasc., revascularization
In Marwick et al. 1992 (49), 56% (5 out of 9) of people with viable myocardium experienced an improvement in functional class after revascularization; whereas in the group of patients with non-viable myocardium, only 21% (3 out of 14) improved. In the 1999 paper, changes in functional class were not reported separately for the viable and non-viable patient groups. In both groups combined, the mean functional class improved from 2.6±0.7 to 1.9±0.7 after revascularization. (62)
Marwick et al (62) measured quality of life before and after revascularization using The Nottingham Health Profile. Significant improvements were reported for four categories: energy (32% of patients improved), pain (27%), emotion (29%), and mobility status (21%). (62)
Quality of Evidence: PET Viability Imaging and Prognosis
Overall, the body of evidence regarding PET viability imaging and prognosis is very low quality due to the observational study design as well as a variety of limitations in individual study quality (Table 22). The estimate of effect is thus very uncertain, so definitive conclusions cannot be drawn based on this evidence.
Table 22: GRADE quality of evidence for prognosis studies.
| No. of Studies |
Design | Limitations | Consistency | Directness | Imprecision | Other Modifying Factors |
Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Mortality | |||||||
| 9 | Prospective and retrospective cohorts | Serious limitations* | No serious inconsistency | Serious limitations† | No serious imprecision | None | Very low |
| Outcome: Exercise Capacity, Functional Status | |||||||
| 2 | Prospective cohorts | Serious limitations‡ | No serious inconsistency | No serious indirectness | No serious imprecision | None | Very low |
| Outcome: Quality of Life | |||||||
| 1 | Prospective cohort | Serious limitations‡ | No serious inconsistency | No serious indirectness | Serious limitations§ | None | Very low |
Downgraded due to serious limitations including selection bias, lack of blinding, retrospective analyses, non-consecutive patient enrolment, no a priori sample size calculations, and small sample size.
Directness was downgraded because several of the studies are not representative of the target population including Rohatgi (55) (included patients with scar tissue on thallium scan only) and Zhang (56) (included 41 patients with LV aneurysm).
Downgraded due to serious limitations including selection bias, lack of blinding, non-consecutive patient enrolment, no a priori sample size calculations, and small sample size.
Downgraded due to sparse data (only one study reported this outcome).
Contribution of PET Viability Imaging to Treatment Decision Making
Five studies that examine the contribution of the PET viability imaging to treatment decision making were identified. Study characteristics are summarized in Table 23. The first two studies in the table were identified in the systematic search of the literature, while the others were identified from reference lists of systematic reviews and health technology assessments on this topic.
Table 23: Study characteristics for contribution of PET viability imaging to treatment decision making.
| Author, Year | Study Design |
Objective | N | Male (%) | Mean Age ± SD (years) |
Mean Preoperative LVEF(%) |
PET Technique |
Follow-up Duration (months) |
Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Beanlands et al., 2007 (63) | RCT | To determine if PET-assisted decision-making results in improved clinical outcomes compared to standard care excluding PET management in patients with CAD | 430* | 84 | PET: 63±10 Standard care: 62±10 | PET: 27±7 Standard care: 26±8 | Rb-82 / FDG PET or 13N-ammonia / FDG PET | 12 | First occurrence of cardiac death, MI, or hospital stay for a cardiac cause and time to occurrence |
| Felix et al., 2006 (64) | Before/after survey | To examine the influence of PET viability data on physician’s intended management plan | 31 | 74 | 62.6±10.4 | 29.3±10.7 (range, 11-54) | 99mTc-tetrofosmin SPECT / FDG PET | Range, 2 – 4 mo | Need to undergo revascularization, current symptoms, use of medications, management plan before and after PET data available |
| Siebelink et al., 2001 (65) | RCT | To compare PET- vs. SPECT-guided management (PCI, CABG, or medication) of CAD patients on cardiac-event free survival | 112† | 86 | PET: 62±2 SPECT: 63±1 | 35% of patients had LVEF < 30% | 13N-ammonia / FDG PET | median, 28 | Cardiac event-free survival (cardiac events included cardiac death, MI, and unintended revascularization) |
| Beanlands et al., 1997 (66) | Before/after survey | To examine the influence of PET viability data on physician’s intended management plan (heart transplant, medication, or revascularization) for CAD patients | 87 | 89 | 59 ± 9 | 93% patients had LVEF <50% and 47% < 30% | 99mTc-sestamibi SPECT / FDG PET | NR | Physician’s intended management plan before and after PET results were available |
| Haas et al., 1997 (67) | Retrospective observational study | To compare outcomes in patients who were revascularized based on PET viability imaging results or clinical status and angiographic data | 67‡ | 86 | Non-PET: 60±10 PET: 63±9 | Non-PET: 30±4 PET: 26±4§ | 13N-ammonia / FDG PET | Non-PET: 12±9 (range, 3 - 28) PET: 15±6 (range, 6 – 25) | Perioperative and postoperative complications and mortality |
CABG refers to coronary artery bypass graft; CAD, coronary artery disease; LVEF refers to left ventricular ejection fraction; MI, myocardial infarction; N, sample size; NR, not reported; PCI, percutaneous coronary intervention; PET, positron emission tomography; Tc, technetium; SD, standard deviation; SPECT, single photon emission computed tomography
Twelve patients were lost to follow-up (nine in the PET group and three in the standard care group). (63)
One hundred twelve patients were enrolled in the study, but only 103 patients were randomized to treatment groups (three patients withdrew from the study, one patient died, one had a failed PET scan, and four had progressive disease). In addition, one patient was lost to follow-up. (65)
76 patients were referred for CABG or heart transplant at the study institution, but only 69 patients were revascularized and were included in the results of this study. (67)
Mean contrast angiographic LVEF values were normalized to equilibrium radionuclide LVEF using a regression equation. (67)
In Beanlands et al. 1997 (66), patients’ physicians were surveyed about their intended management plan for the patient at two time points: first before they were provided with the results of the PET viability scan and, second, after they were provided with the results of the PET scan. Overall, management plans were modified based on the PET results for 57% of patients (50 out of 87) as follows:
63% (7 out of 11) of patients were switched from heart transplant to revascularization;
44% (8 out of 18) of patients were switched from medical therapy to revascularization; and
42% (16 out of 38) of patients were switched from revascularization to medical therapy. (66)
Based on these results, the kappa score was 0.182, suggesting that the PET viability results had an important impact on the treatment decisions. The impact of PET results on management was even larger for the subgroup of patients with a mean LVEF less than 30%, in whom the management plans were modified in 71% of cases (29 out of 41) based on PET viability results. (66)
In a similar manner, Felix et al. (64) surveyed the physicians of 31 patients to determine how the results of PET viability scans influenced their planned treatment strategy. The PET viability results changed the physicians’ treatment strategy for 68% of patients (21 out of 31) and for six additional patients the PET results confirmed the physicians’ decision. Therefore, PET contributed to the physicians’ decision making in 87% of patients (27 out of 31). (64) Of note, co-incidence PET imaging in a gamma chamber was used in this study rather than a dedicated PET scanner, so the results of this study may not be generalizable to dedicated PET scanners.
The results from Beanlands et al. 1997 (66) and Felix et al. (64) suggest that PET viability imaging provides physicians with information that is useful for determining the appropriate treatment strategy for CAD patients. These studies do not, however, demonstrate whether the PET-guided treatment decisions result in better long-term outcomes than those made without knowledge of PET viability results. Siebelink et al. (65), Beanlands et al. 2007 (63), and Haas et al. (67) address this issue by comparing PET-guided treatment with alternative decision making strategies.
In Siebelink et al. (65), patients received both 13N-ammonia/FDG PET and stress/rest 99mTc-sestamibi SPECT scans to assess viability. Patients were randomized to either a PET- or SPECT-guided treatment group and then the corresponding scan was sent to be evaluated by a revascularization team (the team consisted of a thoracic surgeon, an invasive cardiologist, and a nuclear cardiologist) for viability, which was defined by at least 20% jeopardized myocardium in a region supplied by a coronary artery with more than 50% stenosis. Based on these results, patients were assigned to revascularization (49 patients) or medical treatment (54 patients). (65)
There was no significant difference between the PET and SPECT groups in terms of the number of first cardiac events (Table 24) or Kaplan-Meier event-free survival curves (Figure 9). Subgroup analyses also showed no significant differences comparing cardiac events between the patients in the PET and SPECT groups assigned to revascularization and those assigned to medical treatment and the PET and SPECT-based management groups for patients with LVEF greater than 30% or less than or equal to 30%. (65)
Table 24: Cardiac events stratified by study group.
| Study Group | Unintended Revascularization | Myocardial Infarction | Cardiac Death |
|---|---|---|---|
| PET group | 5 | 2 | 4 |
| SPECT group | 9 | 3 | 1 |
Figure 9: Kaplan-Meier cardiac-event free survival curves for patients randomized to PET or SPECT-based management.
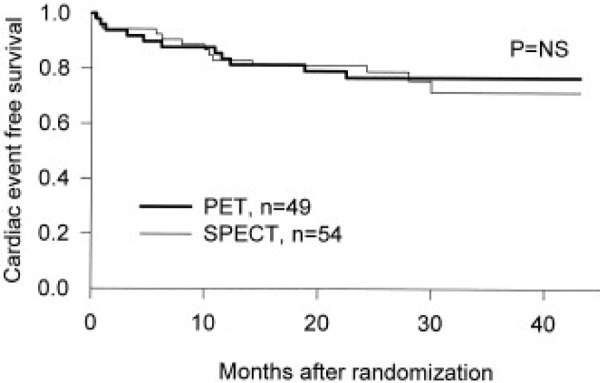
Reprinted from the Journal of the American College of Cardiology, Vol. 37, Issue 1, Siebelink et al. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management: a prospective, randomized comparison of patients with suspicion of jeopardized myocardium, p. 81-8, Copyright 2001, with permission from The American College of Cardiology.
In Beanlands et al. 2007 (63), patients were randomized to the PET-assisted management (218 patients) or standard care (212 patients) arm of the RCT. In the PET-assisted management arm, physicians were provided with the results of PET viability imaging. Revascularization was recommended for patients with significant viable myocardium, but physicians made the treatment decision based on the PET imaging data as well as the individual patient’s condition. In the standard care arm, PET viability imaging was not available for physicians, but other viability tests could be performed (138 patients in the standard care arm had stress or viability testing either in the three months before randomization or after randomization). (63)
Over one year of follow-up, 136 patients experienced cardiac events (first cardiac event breakdown: 29 cardiac deaths; 13 MIs; and 94 cardiac hospital stays). Thirty percent of patients experienced a cardiac event in the PET group compared with 36% of patients in the standard care group [hazard ratio, 0.78; 95% confidence interval (CI), 0.58 – 1.1; P = .15].
There were 19 cardiac deaths in the PET group and 26 in the standard care group (hazard ratio, 0.72; 95% CI, 0.40 – 1.3; P = .25). (63) In the subgroup of patients who had not had a recent angiography before enrolment in the study, the PET group had significantly fewer cardiac deaths compared with the standard care group: seven (7.1%) versus 17 (16.7%) deaths (hazard ratio, 0.4; 95% CI, 0.17 – 0.96; P = .035). (63) This difference was not observed in patients who had a recent coronary angiography.
In 25% of patients in the PET group, physicians did not follow the treatment recommendation for revascularization or no revascularization based on the PET viability imaging. (63) Adherence was significantly lower in the group of patients with moderate amounts of viable myocardium (adherence rate, 66%; P ≤.05 compared with high and low amounts of viable myocardium). A post-hoc analysis that compared those in the PET group that adhered to the PET recommendations to the standard care group found a significant reduction in mortality in the PET group (hazard ratio adjusted for previous CABG, 0.62; 95% CI, 0.42 – 0.93, P = .019). However, a non-intention-to-treat data analysis is subject to bias and therefore must be considered with caution.
A second post-hoc analysis found there was a significant decrease in cardiac death in patients with previous stress or viability testing in the PET group compared with:
patients in the standard care group who had previous testing (hazard ratio adjusted for previous CABG, 0.46; 95% CI, 0.25 - 0.81; P = .007);
patients in the standard care group without previous testing (hazard ratio, 0.44; 95% CI, 0.24 - 0.82; P = .009); and
patients in the PET arm without previous testing (hazard ratio, 0.48; 95% CI, 0.27 - 0.86; P = .013). (63)
Similarly, Haas et al. (67) compared outcomes for patients who were revascularized based on clinical status and angiographic data (standard care group, 35 patients) or PET viability imaging combined with clinical status and angiographic data (PET group, 34 patients). In this retrospective analysis of 69 patients, mortality was significantly lower among those in the PET group than those in the standard care group (30 day mortality rate, 0% vs. 11.4%, P = .04; 1 year mortality rate, 2.9% vs. 14.3%, P = .02). While more patients experienced cardiac arrest in the intensive care unit in the standard care group compared with the PET group, this difference was not significant (14.3% vs. 2.9%, P = .09). Overall, significantly more patients experienced an uncomplicated recovery in the PET group (66.7% vs. 33.3%, P = 0.05). The one year postoperative mean LVEF was not significantly different between the two groups; however, the increase in mean LVEF from the preoperative mean was significant in the PET group (34.8±12.2 vs. 26.2±4.3, P = .003) but not the standard care group (34.3±12.4 vs. 29.8±3.7). (67)
The results from Haas et al. (67) and Beanlands et al. 2007 (63) provide conflicting evidence as to the benefit of PET-guided treatment decision making. One important difference between the studies is that the standard care group in Beanlands et al. 2007 (63) could include viability imaging using techniques other than PET, so the physicians could still access viability information. In contrast, viability information was not included in the standard care group in the study by Haas et al. (67) A second very important difference between the studies is the study design. Beanlands et al. 2007 (63) is a high quality RCT while Haas et al. (67) is a retrospective observational study. Thus, the latter study is subject to biases, especially selection bias due to differences in patient selection between the PET and standard care groups, which could explain the benefit observed in the PET group.
While the lack of significant difference between the study groups in Beanlands et al. 2007 (63) may, therefore, be the result of viability testing using other technologies in the standard care group, the results of this study are more likely to reflect the truth. Based on these findings, PET-guided treatment decisions do not result in significant reductions in cardiac events or cardiac deaths compared with SPECT-guided treatment decisions (65) or standard care treatment decisions, which may include viability testing with other non-invasive cardiac imaging modalities. (63)
Quality of Evidence: The Contribution of PET Viability Imaging to Treatment Decision Making
The body of evidence that assesses the contribution of PET viability imaging to treatment decision making ranged from very low (studies that evaluated the influence of PET of physicians’ planned treatment strategy) to moderate (studies that compared long-term clinical outcomes between PET-guided treatment strategies with alternative decision making strategies) quality (Table 25).
Table 25: GRADE quality of evidence for prognosis studies.
| No. of Studies |
Design | Limitations | Consistency | Directness | Imprecision | Other Modifying Factors |
Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Outcome: Influence of PET on treatment decisions (Beanlands et al. 1997 and Felix et al.) | |||||||
| 2 | Before/after survey | Serious limitations* |
No serious inconsistency |
Serious limitation† |
No serious imprecision |
None | Very low |
| Outcome: Cardiac Death (PET-guided vs. SPECT-guided decision making. Siebelink et al) | |||||||
| 1 | RCT | No serious limitations‡ |
No serious inconsistency |
No serious indirectness |
Serious§ | None | Moderate |
| Outcome: Cardiac Events and Cardiac Death (PET-guided vs. standard care-guided decision making. Beanlands et al 2007) || | |||||||
| 1 | RCT | No serious limitations¶ |
No serious inconsistency |
No serious indirectness |
Serious§ | None | Moderate |
Downgraded due to serious limitations including selection bias, lack of blinding, non-consecutive patient enrolment, no a priori sample size calculations, and small sample size.
Downgraded because Felix et al. (64) uses co-incidence PET scanning in a gamma chamber rather than a dedicated PET scanner.
Study scored three out of five on the Jadad scale, which indicates a high quality study. (68)
Downgraded due to sparse evidence: only one study in this category and the study may be underpowered (small sample size in Siebelink et al. and fewer events than expected in Beanlands et al).
Haas et al. (67) was excluded from the GRADE quality assessment because it is a much lower quality study than Beanlands et al. 2007 (63) and so is unlikely to provide an accurate estimate of the impact of PET viability imaging on patient outcomes.
Study scored five out of five on the Jadad scale, which indicates a high quality study. (68)
Safety
No adverse events were reported in the included studies due to the PET imaging.
PET imaging requires the use of radionuclides, so people undergoing such scans are exposed to some radiation. F-18-fluordeoxyglucose PET scans Furthermore, many PET viability scans involve both FDG PET imaging as well as a perfusion scan thereby introducing increased radiation exposure from either the PET perfusion radionuclide or the SPECT scan. Finally, hybrid PET/CT scanners have become increasingly common, and so patients who undergo viability imaging using a hybrid scanner are exposed to the radiation from both the PET and CT scans. A summary of the approximate radiation doses associated with each combination is shown in Table 26.
Table 26: Summary of radiation exposure dosages associated with PET viability imaging.
| Type of Scan | Total-Body Effective Dose (mSv) |
Combined Total-Body Effective Dose (mSv) |
References | |
|---|---|---|---|---|
| FDG PET scan (10 mCi) | 7 | 7 | (69) | |
| FDG PET scan + PET perfusion scan | ||||
| Rb-82 (20 mCi) | 0.6 | 7.6 | (70) | |
| 13N-ammonia (10 mCi) | 0.74 | 7.74 | (71) | |
| FDG PET + SPECT perfusion scan | ||||
| FDG + 99mTc-sestamibi (30 mCi, single dose) | 9 | 16 | Personal Communication |
|
| FDG + Tl-201 (3 mCi, single dose) | 18 | 25 | Personal Communication |
|
| FDG PET + CT Attenuation Scan | 0.5 | 7.5 | Personal Communication |
|
CT refers to computed tomography; FDG, F-18-fluorodeoxyglucose; mCi, millicuries, mSv, millisievert; N, nitrogen; PET, positron emission tomography; SPECT, single photon emission computed tomography; Rb, rubidium; Tc, technetium; Tl, thallium
Conclusions
Based on the available very low quality evidence, PET is a useful imaging modality for the detection of viable myocardium. The pooled estimates of sensitivity and specificity for the prediction of regional functional recovery as a surrogate for viable myocardium are 91.5% (95% CI, 88.2% – 94.9%) and 67.8% (95% CI, 55.8% – 79.7%), respectively.
-
Based the available very low quality of evidence, an indirect comparison of pooled estimates of sensitivity and specificity showed no statistically significant difference in the diagnostic accuracy of PET viability imaging for regional functional recovery using perfusion/metabolism mismatch with FDG PET plus either a PET or SPECT perfusion tracer compared with metabolism imaging with FDG PET alone.
FDG PET + PET perfusion metabolism mismatch: sensitivity, 89.9% (83.5% – 96.4%); specificity, 78.3% (66.3% – 90.2%);
FDG PET + SPECT perfusion metabolism mismatch: sensitivity, 87.2% (78.0% – 96.4%); specificity, 67.1% (48.3% – 85.9%);
FDG PET metabolism: sensitivity, 94.5% (91.0% – 98.0%); specificity, 66.8% (53.2% – 80.3%).
Given these findings, further higher quality studies are required to determine the comparative effectiveness and clinical utility of metabolism and perfusion/metabolism mismatch viability imaging with PET.
Based on very low quality of evidence, patients with viable myocardium who are revascularized have a lower mortality rate than those who are treated with medical therapy. However, given the quality of evidence this estimate of effect is uncertain so further higher quality studies in this area should be undertaken to determine the presence and magnitude of the effect.
While revascularization may reduce mortality in patients with viable myocardium, current moderate quality RCT evidence suggests that PET-guided treatment decisions do not result in statistically significant reductions in mortality compared with treatment decisions based on SPECT or standard care protocols. The PARR II trial by Beanlands et al. found a significant reduction in cardiac events (a composite outcome that includes cardiac deaths, MI, or hospital stay for cardiac cause) between the adherence to PET recommendations subgroup and the standard care group (hazard ratio, .62; 95% confidence intervals, 0.42 – 0.93; P = .019). However, this post-hoc sub-group analysis is hypothesis generating and higher quality studies are required to substantiate these findings.
The use of FDG PET plus SPECT to determine perfusion/metabolism mismatch to assess myocardial viability increases the radiation exposure compared with FDG PET imaging alone or FDG PET combined with PET perfusion imaging (total-body effective dose: FDG PET, 7 mSv; FDG PET plus PET perfusion tracer, 7.6 – 7.7 mSV; FDG PET plus SPECT perfusion tracer, 16 – 25 mSv). While the precise risk attributed to this increased exposure is not known, there is concern regarding lifetime multiple exposures to radiation-based imaging modalities, although the incremental lifetime risk for older patients or those with a poor prognosis may not be as great as for healthy individuals.
Appendices
Appendix 1: Literature Search Strategies
Search date: July 17, 2009
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley
Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1996 to July Week 2 2009>
Search Strategy
exp Myocardial Ischemia/ (131493)
(coronary adj2 arter* disease*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (37638)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or isch?emi* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. (121821)
(myocardi* adj2 (stun or hibernat*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (610)
(stenocardia* or angina).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (21158)
heart attack*.mp. (1848)
exp Heart Failure/ (33112)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. (58418)
exp Ventricular Dysfunction, Left/ (13473)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (16743)
or/1-10 (220508)
exp Tomography, Emission-Computed/ (39412)
(PET or (Positron Emission adj2 Tomogra*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (33347)
(coincidence adj1 (imaging or detection)).mp. (289)
12 or 13 or 14 (49322)
11 and 15 (5790)
limit 16 to (english language and humans and yr=“2004 -Current”) (2140)
Database: EMBASE <1980 to 2009 Week 28>
Search Strategy
exp ischemic heart disease/ (236154)
exp coronary artery disease/ (87401)
exp stunned heart muscle/ (1511)
(coronary adj2 arter* disease*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (70871)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or ischemi* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (273276)
(myocardi* adj2 (stun or hibernat*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1049)
(stenocardia* or angina).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (45993)
heart attack*.mp. (2007)
exp heart failure/ (123700)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (106492)
exp heart left ventricle failure/ (9206)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (15945)
or/1-12 (426245)
exp positron emission tomography/ (42463)
(PET or (Positron Emission adj2 Tomogra*)).ti,ab. (37810)
15 or 14 (51616)
16 and 13 (3631)
limit 17 to (human and english language and yr=“2004 -Current”) (1224)
Appendix 2: Quality Assessment with AMSTAR Checklist
As detailed in the methods section, the quality of the systematic reviews that were included in this report was assessed with the AMSTAR Checklist. The results of the quality assessment are provided in Table X. Explanations are provided when no, can’t answer, and not applicable was selected for a checklist component.
Table A1: Quality assessment of included systematic reviews with AMSTAR Checklist.
| AMSTAR Checklist | Author, Year | ||
|---|---|---|---|
| Schinkel et al., 2007 (26) | Beanlands et al., 2006 (28) | ||
| 1. | Was an a priori design provided? | No: while the review refers to a previous SR for the inclusion criteria, these criteria only address the diagnostic accuracy analysis and not the prognostication and other symptoms analyses for which no inclusion criteria are specified | Yes |
| 2. | Was there duplicate study selection and data extraction? | Can’t answer: this information was not provided in the review | Can’t answer: this information was not provided in the review |
| 3. | Was a comprehensive literature search performed? | Yes | No: key words and MESH terms not stated and a search strategy was not included |
| 4. | Was the status of publication (i.e. grey literature) used as an inclusion criteria? | Can’t answer: this information was not provided in the review | Can’t answer: this information was not provided in the review. |
| 5. | Was a list of the studies (included and excluded) provided? | No: excluded studies were not listed | No: excluded studies were not listed |
| 6. | Were the characteristics of the included studies provided? | Yes | No: inadequate aggregated information on the characteristics of the participants provided |
| 7. | Was the scientific quality of the included studies assessed and documented? | No: there is no mention of a priori methods to assess the quality of the included studies and only a few general statements regarding study quality were provided. | Yes |
| 8. | Was the scientific quality of the included studies used appropriately in formulating conclusions? | n/a (see question 7) | Yes |
| 9. | Were the methods used to combine the findings of the studies appropriate? | No: tests were not performed to assess the appropriateness of pooling the accuracy or mortality data | No: tests were not performed to assess the appropriateness of pooling the accuracy or mortality data |
| 10. | Was the likelihood of publication bias assessed? | No: the likelihood of publication bias was not addressed | No: the likelihood of publication bias was not addressed |
| 11. | Was the conflict of interest stated? | No: the sources of funding for the included studies in the review and potential conflicts of interest for review authors were not reported | No: the sources of funding for the included studies in the review and potential conflicts of interest (beyond specific funding for the literature search and teleconferencing) for review authors were not reported |
Each component of the checklist is scored as yes, no, can’t answer, or not applicable
Appendix 3: Subgroup Sensitivity and Specificity Forest Plots
Figure A1: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by type of segment.
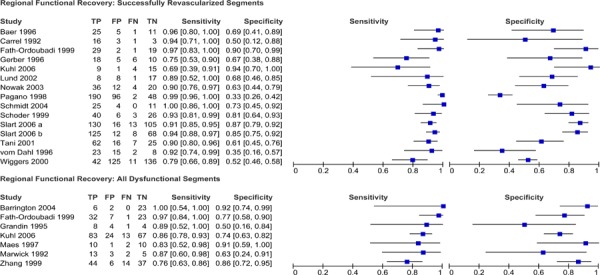
Figure A2: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by method of functional recovery assessment.

Figure A3: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by type of radiotracer.
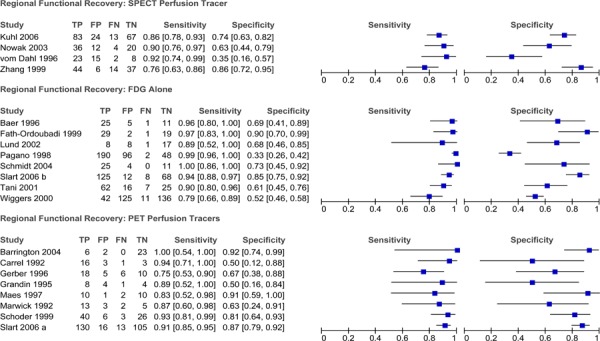
Figure A4: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by mean preoperative LV ejection fraction.
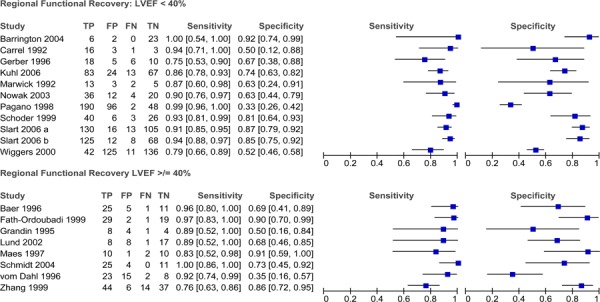
Figure A5: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by unit of analysis.

Figure A6: Sensitivity and specificity Forest plots showing the accuracy of PET for detecting regional functional recovery stratified by hypokinetic segments and akinetic/dyskinetic segments.
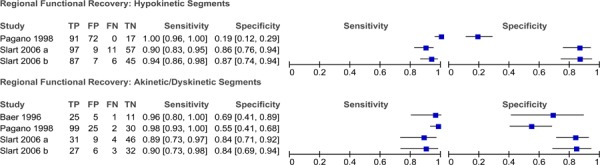
Appendix 4: QUADAS Scoring for Diagnostic Accuracy Studies
Table A2: Quality of studies investigating the accuracy of PET for the detection of regional functional recovery (Part I).
| QUADAS Tool | Author, Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kuhl 2006 | Slart 2006 a | Slart 2006 b | Barring-ton 2004 | Schmidt 2004 | Nowak 2003 | Lund 2002 | Tani 2001 | Wiggers 2000 | Fath-Ordoubad i 1999 | Schoder 1999 | Zhang 1999 | Pagano 1998 | |
| 1. Was the spectrum of patients representative of patients who will receive test?* | Yes | Yes | Yes | No | No | No | No | No | No | No | Yes | No | No |
| 2. Were selection criteria clearly described? | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | No | No | No | Yes |
| 3. Is reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is time period between reference standard and index test short enough to be reasonably sure the target condition did not change between tests? | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 5. Did whole sample or random selection of sample receive verification using reference standard of diagnosis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did patients receive the same reference standard regardless of index test result? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was reference standard independent of index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was execution of index test described in sufficient detail to permit replication of test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was execution of reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Were index test results interpreted without knowledge of results of reference standard? | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
| 11. Were reference standard results interpreted without knowledge of results of index standard? | Unclear | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear | Unclear |
| 12. Were same clinical data available when test results were interpreted as would be available when test is used in practice? | No | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 13. Were uninterpretable / intermediate test results reported? | No | Yes | Yes | Yes | No | No | No | No | Yes | No | n/a | No | Yes |
| 14. Were withdrawals from the study explained? | Yes | n/a | n/a | No | No | No | Yes | Yes | Yes | Yes | n/a | n/a | Yes |
ECHO, echocardiography; LVA, left ventricular angiography; MUGA, multi-gated acquisition scan (radionuclide ventriculography)
Patient characteristics (mean age, percentage male, mean LVEF, percentage with diabetes, percentage with hypertension, and percentage with previous MI) from each study population were compared with the typical patient population undergoing viability testing in Ontario. This typical patient population was defined by the patient population enrolled in the Ontario Cardiac FDG PET Registry (CADRE) study. A study population was considered representative if at least five of the six characteristics were similar (within ± 10) to the reference population. Overall, most study populations had a higher percentage of males, lower percentage of people with diabetes, hypertension, and previous MI than the Ontario reference population.
Table A3: Quality of studies investigating the accuracy of PET for the detection of regional functional recovery (Part II).
| QUADAS Tool | Author, Year | ||||||
|---|---|---|---|---|---|---|---|
| Maes 1997 | Bear 1996 | Gerber 1996 | vom Dahl 1996 | Grandin 1996 | Carrel 1992 | Marwick 1992 | |
| 1. Was the spectrum of patients representative of patients who will receive test?* | No | No | No | Yes | No | Yes | No |
| 2. Were selection criteria clearly described? | No | No | Yes | Yes | No | No | Yes |
| 3. Is reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is time period between reference standard and index test short enough to be reasonably sure the target condition did not change between tests? | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 5. Did whole sample or random selection of sample receive verification using reference standard of diagnosis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did patients receive the same reference standard regardless of index test result? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was reference standard independent of index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was execution of index test described in sufficient detail to permit replication of test? | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 9. Was execution of reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 10. Were index test results interpreted without knowledge of results of reference standard? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes |
| 11. Were reference standard results interpreted without knowledge of results of index standard? | Unclear | Yes | Unclear | Yes | Yes | Unclear | Yes |
| 12. Were same clinical data available when test results were interpreted as would be available when test is used in practice? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | No |
| 13. Were uninterpretable / intermediate test results reported? | No | Yes | No | Yes | No | No | No |
| 14. Were withdrawals from the study explained? | n/a | No | n/a | Yes | Yes | Yes | n/a |
CA refers to coronary angiography; ECHO, echocardiography; LVA, left ventricular angiography; MUGA, multi-gated acquisition scan (radionuclide ventriculography)
Patient characteristics (mean age, percentage male, mean LVEF, percentage with diabetes, percentage with hypertension, and percentage with previous MI) from each study population were compared with the typical patient population undergoing viability testing in Ontario. This typical patient population was defined by the patient population enrolled in the Ontario Cardiac FDG PET Registry (CADRE) study. A study population was considered representative if at least five of the six characteristics were similar (within ± 10) to the reference population. Overall, most study populations had a higher percentage of males, lower percentage of people with diabetes, hypertension, and previous MI than the Ontario reference population.
Table A4: Quality of studies investigating the accuracy of PET for the detection of global functional recovery.
| QUADAS Tool | Author, Year | |||||
|---|---|---|---|---|---|---|
| Slart 2006 a | Slart 2006 b | Bax 2002 | Gerber 2001 | Pagano 1998 | Maes 1997 | |
| 1. Was the spectrum of patients representative of patients who will receive test?* | Yes | Yes | No | No | No | No |
| 2. Were selection criteria clearly described? | Yes | Yes | Yes | No | Yes | No |
| 3. Is reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is time period between reference standard and index test short enough to be reasonably sure the target condition did not change between tests? | n/a | n/a | n/a | n/a | n/a | n/a |
| 5. Did whole sample or random selection of sample receive verification using reference standard of diagnosis? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did patients receive the same reference standard regardless of index test result? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was reference standard independent of index test? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was execution of index test described in sufficient detail to permit replication of test? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was execution of reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | No | Yes | Yes |
| 10. Were index test results interpreted without knowledge of results of reference standard? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 11. Were reference standard results interpreted without knowledge of results of index standard? | Yes | Yes | Yes | Unclear | Yes | Unclear |
| 12. Were same clinical data available when test results were interpreted as would be available when test is used in practice? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 13. Were uninterpretable / intermediate test results reported? | No | No | No | No | No | No |
| 14. Were withdrawals from the study explained? | n/a | n/a | Unclear | Yes | Yes | n/a |
cardiac MRI refers to cardiac magnetic resonance imaging; LVA, left ventricular angiography; MUGA, multigated acquisition scan (radionuclide ventriculography)
Patient characteristics (mean age, percentage male, mean LVEF, percentage with diabetes, percentage with hypertension, and percentage with previous MI) from each study population were compared with the typical patient population undergoing viability testing in Ontario. This typical patient population was defined by the patient population enrolled in the Ontario Cardiac FDG PET Registry (CADRE) study. A study population was considered representative if at least five of the six characteristics were similar (within ± 10) to the reference population. Overall, most study populations had a higher percentage of males, lower percentage of people with diabetes, hypertension, and previous MI than the Ontario reference population.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Positron emission tomography (PET) for the assessment of myocardial viability: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 July [cited YYYY MM DD]; 10(16) 1-80. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/cardiac_viability_PET_20100716.pdf
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN TBA 978-1-4435-1966-3 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables
| Table 1: Two-by-two table for calculations |
| Table 2: Quality of evidence of included studies |
| Table 3: Summary of weighted mean sensitivity, specificity, positive predictive value, and negative predictive value for predicting hibernating myocardium from Schinkel et al.* |
| Table 4: Summary of changes in heart failure symptoms, exercise capacity, and prognosis |
| Table 5: Weighted mean sensitivity and specificity results for diagnostic accuracy of detection of viable myocardium from 2005 Beanlands et al. systematic review* |
| Table 6: Prognosis comparing patients with viable and non-viable myocardium and treatment method |
| Table 7: Characteristics of included viability diagnostic accuracy studies |
| Table 8: Summary of the thresholds to define viability and functional improvement by study* |
| Table 9: Study results for diagnostic accuracy of PET in predicting regional functional recovery after revascularization* |
| Table 10: Stratification variables for regional functional assessment by study* |
| Table 11: Pooled estimates of sensitivity and specificity by subgroup |
| Table 12: Pairwise comparisons of sensitivity and specificity by subgroup |
| Table 13: Study results for diagnostic accuracy of PET in predicting regional functional recovery after revascularization stratified by hypokinetic and akinetic/dyskinetic segments* |
| Table 14: Study results for diagnostic accuracy of PET in predicting global LV functional recovery after revascularization |
| Table 15: GRADE quality of evidence for the diagnostic accuracy of PET for the detection of viable myocardium based on regional functional recovery in patients with known CAD |
| Table 16: GRADE quality of evidence for the diagnostic accuracy of PET for the detection of myocardial viability based on global functional recovery in patients with known CAD |
| Table 17: Study characteristics of prognostic studies |
| Table 18: Patient characteristics in prognosis studies |
| Table 19: Mortality rate by viability status and treatment |
| Table 20: Study characteristics of prognostic studies |
| Table 21: Impact of revascularization on exercise capacity |
| Table 22: GRADE quality of evidence for prognosis studies |
| Table 23: Study characteristics for contribution of PET viability imaging to treatment decision making |
| Table 24: Cardiac events stratified by study group |
| Table 25: GRADE quality of evidence for prognosis studies |
| Table 26: Summary of radiation exposure dosages associated with PET viability imaging |
| Table A1: Quality assessment of included systematic reviews with AMSTAR Checklist |
| Table A2: Quality of studies investigating the accuracy of PET for the detection of regional functional recovery (Part I) |
| Table A3: Quality of studies investigating the accuracy of PET for the detection of regional functional recovery (Part II) |
| Table A4: Quality of studies investigating the accuracy of PET for the detection of global functional recovery |
List of Abbreviations
- AUC
Area under the curve
- CAD
Coronary artery disease
- CI
Confidence interval(s)
- cardiac MRI
Cardiac magnetic resonance imaging
- CT
Computed tomography
- ECHO
echocardiography
- FDG
F-18-Flurodeoxyglucose
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- MRI
Magnetic resonance imaging
- NPV
Negative predictive value
- PET
Positron emission tomography
- PPV
Positive predictive value
- RCT
Randomized controlled trial
- SPECT
Single-photon emission computed tomography
- SR
Systematic review
- SD
Standard deviation
- sROC
Summary receiver operating characteristic
Footnotes
Coronary artery disease (CAD) occurs when plaque builds up in the coronary arteries leading to stenosis and reducing coronary blood flow and oxygen deliver to the myocardium.
Coronary artery disease (CAD) occurs when plaque builds up in the coronary arteries leading to stenosis and reducing coronary blood flow and oxygen deliver to the myocardium.
Only four of the five points could be plotted because the positive likelihood ratio for Slart et al 2006 a (31) was undefined.
References
- 1.Medical Advisory Secretariat and Ontario Ministry of Health and Long-Term Care. Positron emission tomography for the assessment of myocardial viability. 2005. [[cited: 2009 Dec 29]]. [Internet] Available from: http://www.health.gov.on.ca/english/providers/program/mas/mas_mn.html .
- 2.Heart and Stroke Foundation of Ontario. Statistics [Internet]. [updated 2009; cited 2009 Dec 14] Available from: http://www.heartandstroke.on.ca/site/c.pvI3IeNWJwE/b.3581729/k.359A/Statistics.htm#heartdisease .
- 3.Schinkel AF, Bax JJ, Poldermans D. Clinical assessment of myocardial hibernation. Heart. 2005;91(1):111–7. doi: 10.1136/hrt.2004.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bax JJ, van der Wall EE, Harbinson M. Radionuclide techniques for the assessment of myocardial viability and hibernation. Heart. 2004;90(Suppl 5):v26–v33. doi: 10.1136/hrt.2002.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow CM, Donovan L, Manuel D, Johansen H, Tu JV. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005;21(14):1265–71. [PubMed] [Google Scholar]
- 6.Senior R. Diagnostic and imaging considerations: role of viability. Heart Fail Rev. 2006;11(2):125–34. doi: 10.1007/s10741-006-9483-y. [DOI] [PubMed] [Google Scholar]
- 7.Underwood SR, Bax JJ, vom DJ, Henein MY, Knuuti J, van Rossum AC, et al. Imaging techniques for the assessment of myocardial hibernation. Report of a Study Group of the European Society of Cardiology. Eur Heart J. 2004;25(10):815–36. doi: 10.1016/j.ehj.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Lalonde L, Ziadi MC, Beanlands R. Cardiac positron emission tomography: current clinical practice. Cardiol Clin. 2009;27(2):237–55. doi: 10.1016/j.ccl.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Rizzello V, Poldermans D, Bax JJ. Assessment of myocardial viability in chronic ischemic heart disease: current status. Q J Nucl Med Mol Imaging. 2005;49(1):81–96. [PubMed] [Google Scholar]
- 10.Ghesani M, Depuey EG, Rozanski A. Role of F-18 FDG positron emission tomography (PET) in the assessment of myocardial viability. 2005;22(2):165–77. doi: 10.1111/j.0742-2822.2005.04032.x. Echocardiography. [DOI] [PubMed] [Google Scholar]
- 11.Tomlinson DR, Becher H, Selvanayagam JB. Assessment of myocardial viability: comparison of echocardiography versus cardiac magnetic resonance imaging in the current era. Heart Lung Circ. 2008;17(3):173–85. doi: 10.1016/j.hlc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Wu E, Rafael A, Chen E-L, Parker MA, Simonetti OP, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000. pp. 1445–53. [DOI] [PubMed]
- 13.Strzelczyk J, Attili A. Cardiac magnetic resonance evaluation of myocardial viability and ischemia. Semin Roentgenol. 2008;43(3):193–203. doi: 10.1053/j.ro.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. 2007;115(11):1464–80. doi: 10.1161/CIRCULATIONAHA.106.629808. Circulation. [DOI] [PubMed] [Google Scholar]
- 15.Chacko GN. PET imaging in cardiology. Hell J Nucl Med. 2005;8(3):140–4. [PubMed] [Google Scholar]
- 16.Health Canada. Medical devices active licence listing [Internet] [updated 2009 Feb 19; cited 2009 Oct 8] Available from: http://webprod.hc-sc.gc.ca/mdll-limh/dispatch-repartition.do?type=active&lang=eng .
- 17.Ontario Ministry of Health and Long-Term Care. Bulletin 4498 [Internet] [updated 2009 Sep 30; cited 2009 Dec 22] Available from: www.health.gov.on.ca/english/providers/program/ohip/bulletins/4000/bul4498.pdf .
- 18.Beanlands R. Information and contacts: Ontario Cardiac FDG PET Registry (CADRE) [Internet] [updated 2008 Mar 25; cited 2009 Dec 22] Available from: www.ottawaheart.ca/UOHI/doc/CADRE_intro.pdf .
- 19.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006;59(12):1331–2. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 21.Jaeschke R, Guyatt GH, Sackett DL. the Evidence-Based Medicine Working Group. How to use an article about a diagnostic test. [Internet] [updated 2007 Aug 15; cited 2009 Nov 23] Available from: http://www.cche.net/usersguides/diagnosis.asp .
- 22.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(25) doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(10) doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman C. Literature searching and evidence interpretation for assessing health care practices. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care. 1996. [DOI] [PubMed]
- 26.Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32(7):375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Beanlands RS, Chow BJ, Dick A, Friedrich MG, Gulenchyn KY, Kiess M, et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can J Cardiol. 2007;23(2):107–19. doi: 10.1016/s0828-282x(07)70730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beanlands R. S, Chow B. J, Dick A, Friedrich M. G, Gulenchyn K. Y, Kiess M, et al. CCS / CAR / CANM / CNCS / Can SCMR joint position statement on advanced non-invasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multi-detector computed tomography angiography in the diagnosis and evaluation of ischemic heart disease. 2006. [[cited: 2009 Aug 26]]. [Internet]. Ottawa, ON: Canadian Cardiovascular Society. 48 p. Available from: http://www.ccs.ca/download/position_statements/cardiac_imaging_Dec11_appen_tables.pdf . [DOI] [PMC free article] [PubMed]
- 29.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26(2):147–86. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]
- 30.Kuhl HP, Lipke CS, Krombach GA, Katoh M, Battenberg TF, Nowak B, et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27(7):846–53. doi: 10.1093/eurheartj/ehi747. [DOI] [PubMed] [Google Scholar]
- 31.Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Irwan R, Sluiter WJ, et al. Prediction of functional recovery after revascularization in patients with chronic ischaemic left ventricular dysfunction: head-to-head comparison between 99mTc-sestamibi/18F-FDG DISA SPECT and 13N-ammonia/ 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2006;33(6):716–23. doi: 10.1007/s00259-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 32.Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, de BJ, et al. Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol. 2006;13(2):210–9. doi: 10.1007/BF02971245. [DOI] [PubMed] [Google Scholar]
- 33.Barrington SF, Chambers J, Hallett WA, O’Doherty MJ, Roxburgh JC, Nunan TO. Comparison of sestamibi, thallium, echocardiography and PET for the detection of hibernating myocardium. Eur J Nucl Med Mol Imaging. 2004;31(3):355–61. doi: 10.1007/s00259-003-1369-9. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Voth E, Schneider CA, Theissen P, Wagner R, Baer FM, et al. F-18-FDG uptake is a reliable predictory of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22(2):229–36. doi: 10.1016/j.mri.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Nowak B, Schaefer WM, Koch KC, Kaiser HJ, Block S, Knackstedt C, et al. Assessment of myocardial viability in dysfunctional myocardium by resting myocardial blood flow determined with oxygen 15 water PET. J Nucl Cardiol. 2003;10(1):34–45. doi: 10.1067/mnc.2003.128743. [DOI] [PubMed] [Google Scholar]
- 36.Bax JJ, Fath-Ordoubadi F, Boersma E, Wijns W, Camici PG. Accuracy of PET in predicting functional recovery after revascularisation in patients with chronic ischaemic dysfunction: head-to-head comparison between blood flow, glucose utilisation and water-perfusable tissue fraction. Eur J Nucl Med Mol Imaging. 2002;29(6):721–7. doi: 10.1007/s00259-002-0793-6. [DOI] [PubMed] [Google Scholar]
- 37.Lund GK, Freyhoff J, Schwaiger M, Lubeck M, Lund CH, Buchert R, et al. Prediction of left ventricular functional recovery by dobutamine echocardiography, F-18 deoxyglucose or 99mTc sestamibi nuclear imaging in patients with chronic myocardial infarction. Cardiology. 2002;98(4):202–9. doi: 10.1159/000067311. [DOI] [PubMed] [Google Scholar]
- 38.Gerber BL, Ordoubadi FF, Wijns W, Vanoverschelde JL, Knuuti MJ, Janier M, et al. Positron emission tomography using(18)F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Eur Heart J. 2001;22(18):1691–701. doi: 10.1053/euhj.2000.2585. Results from the European Community Concerted Action Multicenter study on use of(18)F-fluoro-deoxyglucose Positron Emission Tomography for the Detection of Myocardial Viability. [DOI] [PubMed] [Google Scholar]
- 39.Tani T, Teragaki M, Watanabe H, Muro T, Yamagishi H, Akioka K, et al. Prediction of functional recovery in patients with myocardial infarction after revascularization--comparison of low-dose dobutamine stress echocardiography with fluorine-18 fluorodeoxyglucose positron emission tomography. Jpn Circ J. 2001;65(3):177–81. doi: 10.1253/jcj.65.177. [DOI] [PubMed] [Google Scholar]
- 40.Wiggers H, Nielsen TT, Bottcher M, Egeblad H, Botker HE. Positron emission tomography and low-dose dobutamine echocardiography in the prediction of postrevascularization improvement in left ventricular function and exercise parameters. Am Heart J. 2000;140(6):928–36. doi: 10.1067/mhj.2000.110766. [DOI] [PubMed] [Google Scholar]
- 41.Fath-Ordoubadi F, Beatt KJ, Spyrou N, Camici PG. Efficacy of coronary angioplasty for the treatment of hibernating myocardium. Heart. 1999;82(2):210–6. doi: 10.1136/hrt.82.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoder H, Campisi R, Ohtake T, Hoh CK, Moon DH, Czernin J, et al. Blood flow-metabolism imaging with positron emission tomography in patients with diabetes mellitus for the assessment of reversible left ventricular contractile dysfunction. J Am Coll Cardiol. 1999;33(5):1328–37. doi: 10.1016/s0735-1097(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 43.Pagano D, Bonser RS, Townend JN, Ordoubadi F, Lorenzoni R, Camici PG. Predictive value of dobutamine echocardiography and positron emission tomography in identifying hibernating myocardium in patients with postischaemic heart failure. Heart. 1998;79(3):281–8. doi: 10.1136/hrt.79.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maes AF, Borgers M, Flameng W, Nuyts JL, van de WF, Ausma JJ, et al. Assessment of myocardial viability in chronic coronary artery disease using technetium-99m sestamibi SPECT. Correlation with histologic and positron emission tomographic studies and functional follow-up. J Am Coll Cardiol. 1997;29(1):62–8. doi: 10.1016/s0735-1097(96)00442-1. [DOI] [PubMed] [Google Scholar]
- 45.Baer FM, Voth E, Deutsch HJ, Schneider CA, Horst M, de Vivie ER, et al. Predictive value of low dose dobutamine transesophageal echocardiography and fluorine-18 fluorodeoxyglucose positron emission tomography for recovery of regional left ventricular function after successful revascularization. J Am Coll Cardiol. 1996;28(1):60–9. doi: 10.1016/0735-1097(96)00106-4. [DOI] [PubMed] [Google Scholar]
- 46.Gerber BL, Vanoverschelde JL, Bol A, Michel C, Labar D, Wijns W, et al. Myocardial blood flow, glucose uptake, and recruitment of inotropic reserve in chronic left ventricular ischemic dysfunction. Implications for the pathophysiology of chronic myocardial hibernation. Circulation. 1996;94(4):651–9. doi: 10.1161/01.cir.94.4.651. [DOI] [PubMed] [Google Scholar]
- 47.vom Dahl J, Altehoefer C, Sheehan FH, Buechin P, Uebis R, Messmer BJ, et al. Recovery of regional left ventricular dysfunction after coronary revascularization. Impact of myocardial viability assessed by nuclear imaging and vessel patency at follow-up angiography. J Am Coll Cardiol. 1996;28(4):948–58. doi: 10.1016/s0735-1097(96)00259-8. [DOI] [PubMed] [Google Scholar]
- 48.Carrel T, Jenni R, Haubold-Reuter S, von SG, Pasic M, Turina M. Improvement of severely reduced left ventricular function after surgical revascularization in patients with preoperative myocardial infarction. Eur J Cardiothorac Surg. 1992;6(9):479–84. doi: 10.1016/1010-7940(92)90244-r. [DOI] [PubMed] [Google Scholar]
- 49.Marwick TH, Nemec JJ, Lafont A, Salcedo EE, MacIntyre WJ. Prediction by postexercise fluoro-18 deoxyglucose positron emission tomography of improvement in exercise capacity after revascularization. Am J Cardiol. 1992;69(9):854–9. doi: 10.1016/0002-9149(92)90782-t. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Liu X, Shi R, Wu Q, Gao R, Liu Y, et al. Evaluation of the clinical value of combination of 99mTc-MIBI myocardial SPECT and 18F-FDG PET in assessing myocardial viability. Radiat Med. 1999;17(3):205–10. [PubMed] [Google Scholar]
- 51.Grandin C, Wijns W, Melin JA, Bol A, Robert AR, Heyndrickx GR, et al. Delineation of myocardial viability with PET. J Nucl Med. 1995;36(9):1543–52. [PubMed] [Google Scholar]
- 52.Desideri A, Cortigiani L, Christen AI, Coscarelli S, Gregori D, Zanco P, et al. The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. 2005;46(7):1264–9. doi: 10.1016/j.jacc.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 53.Feola M, Biggi A, Chauvie S, Vado A, Leonardi G, Rolfo F, et al. Myocardial scar and insulin resistance predict cardiovascular events in severe ischaemic myocardial dysfunction: a perfusion-metabolism positron emission tomography study. Nucl Med Commun. 2008;29(5):448–54. doi: 10.1097/MNM.0b013e3282f5d2bc. [DOI] [PubMed] [Google Scholar]
- 54.Sawada S, Hamoui O, Barclay J, Giger S, Fain R, Foltz J, et al. Usefulness of positron emission tomography in predicting long-term outcome in patients with diabetes mellitus and ischemic left ventricular dysfunction. Am J Cardiol. 2005;96(1):2–8. doi: 10.1016/j.amjcard.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Rohatgi R, Epstein S, Henriquez J, Ababneh AA, Hickey KT, Pinsky D, et al. Utility of positron emission tomography in predicting cardiac events and survival in patients with coronary artery disease and severe left ventricular dysfunction. Am J Cardiol. 2001;87(9):1096–9. doi: 10.1016/s0002-9149(01)01468-0. A6. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Liu XJ, Wu Q, Shi R, Gao R, Liu Y, et al. Clinical outcome of patients with previous myocardial infarction and left ventricular dysfunction assessed with myocardial (99m)Tc-MIBI SPECT and (18)F-FDG PET. J Nucl Med. 2001;42(8):1166–73. [PubMed] [Google Scholar]
- 57.Di Carli MF, Maddahi J, Rokhsar S, Schelbert HR, Bianco-Batlles D, Brunken RC, et al. Long-term survival of patients with coronary artery disease and left ventricular dysfunction: implications for the role of myocardial viability assessment in management decisions. J Thorac Cardiovasc Surg. 1998;116(6):997–1004. doi: 10.1016/S0022-5223(98)70052-2. [DOI] [PubMed] [Google Scholar]
- 58.vom DJ, Altehoefer C, Sheehan FH, Buechin P, Schulz G, Schwarz ER, et al. Effect of myocardial viability assessed by technetium-99m-sestamibi SPECT and fluorine-18-FDG PET on clinical outcome in coronary artery disease. J Nucl Med. 1997;38(5):742–8. [PubMed] [Google Scholar]
- 59.Lee KS, Marwick TH, Cook SA, Go RT, Fix JS, James KB, et al. Prognosis of patients with left ventricular dysfunction, with and without viable myocardium after myocardial infarction. Relative efficacy of medical therapy and revascularization. Circulation. 1994;90(6):2687–94. doi: 10.1161/01.cir.90.6.2687. [DOI] [PubMed] [Google Scholar]
- 60.Eitzman D, al-Aouar Z, Kanter HL, vom DJ, Kirsh M, Deeb GM, et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol. 1992;20(3):559–65. doi: 10.1016/0735-1097(92)90008-b. [DOI] [PubMed] [Google Scholar]
- 61.D’Egidio G, Nichol G, Williams KA, Guo A, Garrard L, deKemp R, et al. Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC Cardiovasc Imaging. 2009;2(9):1060–8. doi: 10.1016/j.jcmg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 62.Marwick TH, Zuchowski C, Lauer MS, Secknus MA, Williams J, Lytle BW. Functional status and quality of life in patients with heart failure undergoing coronary bypass surgery after assessment of myocardial viability. J Am Coll Cardiol. 1999;33(3):750–8. doi: 10.1016/s0735-1097(98)00642-1. [DOI] [PubMed] [Google Scholar]
- 63.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50(20):2002–12. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Martins Felix RC, Correa PL, de Azevedo JC, Da Rocha Dohmann HF, Mesquita ET, Mesquita CT. Clinical impact of positron emission tomography by coincidence system with 18F-FDG on therapeutic decision-making of patients with ischemic cardiomyopathy after myocardial infarction. Arq Bras Cardiol. 2006;86(5):337–45. doi: 10.1590/s0066-782x2006000500003. [DOI] [PubMed] [Google Scholar]
- 65.Siebelink HM, Blanksma PK, Crijns HJ, Bax JJ, van Boven AJ, Kingma T, et al. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management: a prospective, randomized comparison of patients with suspicion of jeopardized myocardium. J Am Coll Cardiol. 2001;37(1):81–8. doi: 10.1016/s0735-1097(00)01087-1. [DOI] [PubMed] [Google Scholar]
- 66.Beanlands RS, deKemp RA, Smith S, Johansen H, Ruddy TD. F-18-fluorodeoxyglucose PET imaging alters clinical decision making in patients with impaired ventricular function. Am J Cardiol. 1997;79(8):1092–5. doi: 10.1016/s0002-9149(97)00054-4. [DOI] [PubMed] [Google Scholar]
- 67.Haas F, Haehnel CJ, Picker W, Nekolla S, Martinoff S, Meisner H, et al. Preoperative positron emission tomographic viability assessment and perioperative and postoperative risk in patients with advanced ischemic heart disease. J Am Coll Cardiol. 1997;30(7):1693–700. doi: 10.1016/s0735-1097(97)00375-6. [DOI] [PubMed] [Google Scholar]
- 68.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 69.Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13(1):19–23. doi: 10.1016/j.nuclcard.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 70.International Commission on Radiological Protection. Recommendations of the ICRP. ICRP publication 103. 2008. Ottawa, ON: Elsevier. [DOI] [PubMed]
- 71.International Commission on Radiological Protection. Radiation dose to patients from radiopharmaceuticals. ICRP publication 80. 1999. Ottawa, ON: Elsevier. [PubMed]


