Executive Summary
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease (CAD), an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients suspected of having CAD. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies for the diagnosis of CAD. Evidence-based analyses have been prepared for each of these five imaging modalities: cardiac magnetic resonance imaging, single photon emission computed tomography, 64-slice computed tomographic angiography, stress echocardiography, and stress echocardiography with contrast. For each technology, an economic analysis was also completed (where appropriate). A summary decision analytic model was then developed to encapsulate the data from each of these reports (available on the OHTAC and MAS website).
The Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease series is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Single Photon Emission Computed Tomography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography with Contrast for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
64-Slice Computed Tomographic Angiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Cardiac Magnetic Resonance Imaging for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Pease note that two related evidence-based analyses of non-invasive cardiac imaging technologies for the assessment of myocardial viability are also available on the MAS website:
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: an Evidence-Based Analysis
The Toronto Health Economics and Technology Assessment Collaborative has also produced an associated economic report entitled:
The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario [Internet]. Available from: http://theta.utoronto.ca/reports/?id=7
Objective
The objective of this report is to compare echocardiography (ECHO) performed with microsphere contrast agents (contrast echocardiography) to ECHO performed without contrast and to single photon emission computed tomography (SPECT).
Contrast ECHO
Contrast agents for ECHO have been available since the technology was first introduced in the 1990s. Composed of tiny ‘microbubbles’ of an inert gas encapsulated within a lipid, protein, or polymer coat, these agents act to scatter incident ultrasound waves at the gas/liquid interface to increase the strength of a returning ECHO signal. When injected into a patient’s arm, they are transported throughout even the smallest capillaries to greatly enhance the blood pool signal, which would otherwise appear black on conventional two dimensional ECHO. The enhanced signal then helps cardiologists to determine what parts of the patient’s heart muscle are poorly perfused.
The first commercially available microsphere contrast agent was Albunex, which received approval by the Food and Drug Administration in the United States in 1994. This original microsphere agent was limited by its rapid gas volume loss which caused a decline in the ultrasound signal. It worked well in the right chambers of the heart, but dissolved when passing through the pulmonary capillaries and so was unable to provide contrast in the left side. Second generation agents employed different gases that prolonged the life of the microbubbles within the circulation and increased the reproducibility of results.
Today, the most common use for contrast ECHO is to enhance the definition of the left ventricular (LV) endocardial border for cases of LV opacification. The aim of contrast ECHO is to provide better quantification of LV volume and assessment of LV wall motion than ECHO alone. The newest area of development in the research of contrast ECHO is myocardial perfusion assessment, also known as myocardial contrast ECHO. Theoretically, since myocardial ischemia and infarction affect both perfusion and contractility (wall motion), contrast ECHO could be an ideal non-invasive imaging test as it could assess both perfusion and contractility, simultaneously and in real time.
Notably, critically ill patients on ventilators and those with lung problems are more likely to generate poor or ‘suboptimal’ echocardiograms than other patients, as are obese patients and those who’ve undergone recent chest operations. Contrast agents can potentially be used in 10% to 15% of all studies and in approximately 33% of stress tests due to from such suboptimal echocardiograms. Stress can be induced either pharmaceutically (e.g., through dobutamine, dipyrimidamole, adenosine) or with exercise. Generally, contrast agents are used more in pharmaceutical stress echocardiograms than in exercise stress echocardiograms.
Evidence-Based Analysis
This MAS analysis sought to address the following research questions:
Is contrast ECHO more effective than 99-technetium SPECT in terms its ability to detect CAD?
What is the effectiveness of contrast ECHO in assessing patients with suboptimal echocardiograms?
Is contrast ECHO safe compared to other cardiac imaging modalities?
Is contrast ECHO cost-effective compared to other cardiac imaging modalities?
Literature Search
Literature searches were performed on June 22, 2009 and July 27, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 until June 30, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria; full-text articles were obtained. Reference lists were also examined for any relevant studies not identified through the search.
Inclusion Criteria
Systematic reviews, meta-analyses, randomized controlled trials, prospective observational studies, retrospective analyses
Minimum sample size of 20 enrolled patients (human only)
The contrast agent used in the study must be licensed by Health Canada
Comparison to reference standard (coronary angiography for the diagnosis of coronary artery disease)
Reporting accuracy data on individual patients (rather than accuracy data stratified by segments of the heart)
English language
Exclusion Criteria
Non-systematic reviews, case reports
Grey literature (e.g. conference abstracts)
Outcomes of Interest
Accuracy outcomes (sensitivity, specificity, positive predictive value, negative predictive value)
Adverse events
Costs
Summary of Findings
Twenty-three observational studies were identified that assessed the diagnostic accuracy of contrast ECHO for the diagnosis of CAD. All of these studies used stress ECHO with contrast. In addition, nine retrospective chart reviews were identified, which assessed the safety of contrast ECHO at rest or stress. Based on the results of these studies the following conclusions were made:
Stress ECHO with contrast has a higher diagnostic accuracy in the diagnosis of CAD than stress ECHO (without contrast).
Stress ECHO with contrast seems to have a similar diagnostic accuracy to 99 technetium SPECT.
The addition of contrast to ECHO in patients with suboptimal ECHO results significantly improves interpretability of the results.
There is not a statistically significantly higher mortality rate in patients who receive contrast compared to those who do not.
Background
In July 2009, the Medical Advisory Secretariat (MAS) began work on Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease (CAD), an evidence-based review of the literature surrounding different cardiac imaging modalities to ensure that appropriate technologies are accessed by patients suspected of having CAD. This project came about when the Health Services Branch at the Ministry of Health and Long-Term Care asked MAS to provide an evidentiary platform on effectiveness and cost-effectiveness of non-invasive cardiac imaging modalities.
After an initial review of the strategy and consultation with experts, MAS identified five key non-invasive cardiac imaging technologies for the diagnosis of CAD. Evidence-based analyses have been prepared for each of these five imaging modalities: cardiac magnetic resonance imaging, single photon emission computed tomography, 64-slice computed tomographic angiography, stress echocardiography, and stress echocardiography with contrast. For each technology, an economic analysis was also completed (where appropriate). A summary decision analytic model was then developed to encapsulate the data from each of these reports (available on the OHTAC and MAS website).
The Non-Invasive Cardiac Imaging Technologies for the Diagnosis of Coronary Artery Disease series is made up of the following reports, which can be publicly accessed at the MAS website at: www.health.gov.on.ca/mas or at www.health.gov.on.ca/english/providers/program/mas/mas_about.html
Single Photon Emission Computed Tomography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Stress Echocardiography with Contrast for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
64-Slice Computed Tomographic Angiography for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Cardiac Magnetic Resonance Imaging for the Diagnosis of Coronary Artery Disease: An Evidence-Based Analysis
Pease note that two related evidence-based analyses of non-invasive cardiac imaging technologies for the assessment of myocardial viability are also available on the MAS website:
Positron Emission Tomography for the Assessment of Myocardial Viability: An Evidence-Based Analysis
Magnetic Resonance Imaging for the Assessment of Myocardial Viability: an Evidence-Based Analysis
The Toronto Health Economics and Technology Assessment Collaborative has also produced an associated economic report entitled:
The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario [Internet]. Available from: http://theta.utoronto.ca/reports/?id=7
Contrast Echocardiography
The addition of contrast enhancing agents to echocardiography (ECHO) has been available since ECHO was first introduced. Contrast enhancement using agitation was first developed in the 1960s, but the challenge for such early methods was that the air bubbles formed by agitation were unstable and could lead to severe adverse events, including embolisms. In the 1980s, the concept of encapsulating air bubbles in a protective shell was realized. These shell-encapsulated bubbles, now referred to as microbubbles or microspheres, preserve the gas to increase the duration of opacification.
The first commercially available microsphere contrast agent was Albunex, which received approval by the Food and Drug Administration (FDA) in the United States in 1994. (1) This original microsphere was limited by a rapid loss of gas volume that caused a decline in ultrasound signal. (1) It worked well in the right chambers of the heart, but dissolved when passing through the pulmonary capillaries and so was unable to provide contrast in the left side. The microsphere technology comprises a unique class of contrast agents other than dyes, chemical compounds or radioisotopes. The microsphere contrast agents have been developed in conjunction with updated ultrasound imaging techniques to maximize the contrast agents’ capabilities. The microspheres are typically 4- to 5-µm in diameter and thus able to pass through the microcirculation. (2) These new agents have also allowed for the expansion of the clinical applications of contrast ECHO. It is widely cited that contrast ECHO can be used to identify Doppler signal enhancement, evaluation of non-compaction cardiomyopathy, thrombus detection, assessment of global and regional wall motion and to enhance the endocardial border. (2)
Endocardial Border Enhancement
The most common use for contrast ECHO is to enhance the endocardial border. Left ventricular opacification with contrast ECHO has the potential to improve the definition of the LV border and may improve quantification of LV volume and the assessment of LV wall motion analysis than ECHO alone. (2) In critically ill patients, for instance, LV opacification is used to assess LV contractility and ejection fraction (personal communication, August 2009).
Myocardial Perfusion Assessment
The newest area of development in the research of contrast ECHO is myocardial perfusion assessment, also known as myocardial contrast ECHO. Theoretically, as myocardial ischemia and infarction affect both perfusion and contractility (wall motion), contrast ECHO could be an ideal non-invasive imaging test since it can assess both perfusion and contractility simultaneously and in real time. (2)
Perfusion requires that the echocardiograph is set to a high mechanical index (MI), which is a standardized measure of peak acoustic intensity. At a high MI, the microbubbles burst, thus new microspheres replenish the myocardium. Perfusion is assessed by measuring how quickly the microspheres are replenished within the myocardium. If the microspheres are replenished in the myocardium within 5 to 7 cardiac cycles (about 5 seconds), the myocardium is considered normal. If the microspheres are not replenished within this time frame, the myocardium perfusion has decreased. (2-5) Myocardial perfusion assessment with contrast ECHO is still considered mostly a research technique and not routinely used in most ECHO laboratories.
Suboptimal ECHO
Some patients are more likely to have poor echocardiograms than others, including critically ill patients on ventilators, those with lung problems, obese patients, and those who’ve recently undergone chest operations (personal Communication, August 2009). (6) Contrast agents could potentially be used in 10% to 15% of all studies and in approximately 33% of stress tests that have produced such suboptimal results. (7;8) Stress can be induced pharmaceutically (dobutamine, dipyrimidamole, adenosine) or with exercise but, generally, contrast agents are more commonly used with pharmaceutical stress. (2) The American Society of ECHO guidelines stated that 75%-90% of suboptimal echo results can yield interpretable results with the use of contrast agents. (9)
Regulatory Status
There is only one contrast agent for ECHO fully licensed by Health Canada, the Definity, manufactured by Lantheus Medical Imaging (North Billerica, MA, United States). There are several other contrast agents for ECHO that have received Notice of Compliance approval from Health Canada, but are not yet marketed in Canada. Notice of Compliance indicates that the contrast agent has been approved for its safety and effectiveness, but the marketing and labelling of the packaging has not been approved for distribution. For the purposes of this review, any study using a contrast agent with at least a Notice of Compliance from Health Canada was included. The Definity itself is indicated for “contrast-enhanced ultrasound imaging of cardiac structures (ventricular chambers and endocardial borders) and function (regional wall motion) in adult patients with suboptimal echocardiograms. The safety and efficacy of Definity with exercise stress or pharmacologic stress testing (e.g.: I.V. dipyridamole) have not been established” (Product Monograph for Definity, September 22, 2008). It is important to note that using the contrast agents with stress ECHO is an off-label use of the agent.
Evidence-Based Analysis
The Health Services Branch submitted an application for the microsphere contrast enhancing agents for cardiac ultrasound imaging (ECHO). ECHO is an alternative to technetium (Tc99m) for cardiac testing as set out by the Ontario Isotope Working Group (OIWG) report developed by MAS with clinical experts in December 2008. The OIWG report provided a tiered response to disruption in Tc99m supply and is currently being implemented by the Emergency Management Unit. At the time when the current disruption in the Tc99m declared itself, the ministry received a request to add contrast to ECHO to improve the accuracy of cardiac function testing making it more comparable to Tc99m than ECHO alone.
Research Questions
Is contrast ECHO more effective than 99-technetium SPECT in terms its ability to detect CAD?
What is the effectiveness of contrast ECHO in assessing patients with suboptimal echocardiograms?
Is contrast ECHO safe compared to other cardiac imaging modalities?
Is contrast ECHO cost-effective compared to other cardiac imaging modalities?
Methods
Literature Search
Literature searches were performed on June 22, 2009 and July 27, 2009 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2004 until June 30, 2009. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria; full-text articles were obtained. Reference lists were also examined for any relevant studies not identified through the search. The full search strategy is described in Appendix 1.
Inclusion Criteria
Systematic reviews, meta-analyses, randomized controlled trials (RCTs), prospective observational studies, retrospective analyses
Minimum sample size of 20 enrolled patients (human only)
Contrast agent used in the study must be licensed by Health Canada (at least Notice of Compliance)
Comparison to coronary angiography for the diagnosis of CAD (reference standard)
Definition of CAD: clearly defined degree of coronary artery stenosis on coronary angiogram
Reported accuracy data on individual patients (rather than stratified by heart segment)
English language
Exclusion Criteria
Non-systematic reviews, case reports
Grey literature (e.g. conference abstracts)
Outcomes of Interest
Accuracy outcomes (sensitivity, specificity, positive predictive value, negative predictive value)
Adverse events
Costs
Statistical Analysis
Pooled estimates of sensitivity and specificity were calculated using Meta-Disc version 1.4 (Madrid, Spain) which calculates weighted averages using the sample size of each study as its weight. Summary receiver operating characteristic (sROC) curves weighted by inverse variance were produced using Review Manager 5.0.22 (The Nordiac Cochrane Centre, The Cochrane Collaboration, 2008). All other statistics were calculated using STATA version 10.1 (StataCorp; Texas, USA). The area under the sROC curve was estimated by numerical integration with a cubic spline (default option). Diagnostic odds ratios (DOR) were calculated with a random effects model using the metan command and a meta-regression was used to compare the diagnostic odds ratios for each subgroup. (10) Statistical significance was defined by P values of less than 0.05.
Literature Search Results
Twenty-three observational studies were identified that assessed the diagnostic accuracy of contrast ECHO for the diagnosis of CAD. All of these studies used stress ECHO with contrast agents. In addition, nine retrospective chart reviews were identified, which assessed the safety of contrast ECHO at rest or stress. Table 1 lists the number and type of studies identified for this report.
Table 1: Quality of evidence of included studies.
| Study Design | Level of Evidence† | Number of Eligible Studies | |
|---|---|---|---|
| Diagnostic Accuracy | Safety | ||
| Large RCT, systematic review of RCTs | 1 | 0 | 0 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 0 | 0 |
| Small RCT | 2 | 0 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 | 0 |
| Non-RCT with contemporaneous controls | 3a | 23 | 0 |
| Non-RCT with historical controls | 3b | 0 | 0 |
| Non-RCT presented at international conference | 3(g) | 0 | 0 |
| Surveillance (database or register) | 4a | 0 | 0 |
| Case series (multisite) | 4b | 0 | 0 |
| Case series (single site) | 4c | 0 | 0 |
| Retrospective review, modelling | 4d | 0 | 9 (chart reviews) |
| Case series presented at international conference | 4(g) | 0 | 0 |
| Total | 23 | 9 | |
RCT refers to randomized controlled trial.
g indicates grey literature.
Quality of Evidence
The quality of the evidence was analysed on a study by study basis by QUADAS (11), and then for overall quality by GRADE Working Group Criteria. (12) The QUADAS tool (11) is a 14-item questionnaire specifically designed to assess the quality of diagnostic studies. Overall, the quality is consistent across the studies. In all studies the observers were blinded to data from other imaging modalities. All studies compared stress contrast ECHO to coronary angiography as the reference standard as established in the inclusion criteria. A consistent weakness across all the studies was that none of the studies were designed to specifically investigate the use of contrast in patients with previous suboptimal ECHO results. In clinical practice, this is the intent of the contrast agents—to be primarily used in patients whose standard ECHO results are not interpretable. A full listing of the 14-item questionnaire and the results from the studies included in this analysis are in Appendix 2.
The GRADE developers have specifically developed strategies for assessing the overall quality of diagnostic tests using GRADE. (12) Tables 2 and 3 describe GRADE for the diagnosis of CAD using myocardial contrast ECHO. Table 4 describes GRADE for the studies which included patients with suspected CAD while Table 5 includes patients with both suspected and known CAD.
Table 2: GRADE quality of evidence: stress contrast ECHO vs. coronary angiography for the diagnosis of CAD (patients with suspected CAD) – Diagnostic test as a surrogate for patient outcome measures.
| Factor | Explanation | GRADE |
|---|---|---|
| Risk of Bias | ||
| Study design | Observational cohort studies (11 studies) | High |
| Limitations | No serious limitations | Unchanged |
| Indirectness | ||
| Outcomes | Diagnostic tests are considered as surrogate outcomes | Reduced by one level→ Moderate |
| Patient populations, diagnostic test, comparison test, and indirect comparisons | Patients in studies may or may not have had previous suboptimal echo results, even though the contrast agents are specifically indicated for patients with suboptimal echo results | Reduced by one level→ Low |
| Important inconsistency in study results | No inconsistency | Unchanged |
| Imprecise evidence | Sufficient consistent evidence | Unchanged |
| Publication bias | No publication bias suspected | Unchanged |
| Overall Quality of Evidence | Low | |
Table 3: GRADE quality of evidence: stress contrast ECHO vs. coronary angiography for the diagnosis of CAD (known or suspected) – Diagnostic test as a surrogate for patient outcome measures.
| Factor | Explanation | GRADE |
|---|---|---|
| Risk of Bias | ||
| Study design | Observational cohort studies (12 studies) | High |
| Limitations | Included patients with known CAD | Reduced by one level → Moderate |
| Indirectness | ||
| Outcomes | Diagnostic tests are considered as surrogate outcomes | Reduced by one level → Low |
| Patient populations, diagnostic test, comparison test, and indirect comparisons | Patients in studies may or may not have had previous suboptimal echo results—even though the contrast agents are specifically indicated for patients with suboptimal echo results | Reduced by one level → Very low |
| Important inconsistency in study results | No inconsistency | Unchanged |
| Imprecise evidence | Sufficient consistent evidence | Unchanged |
| Publication bias | No publication bias suspected | Unchanged |
| Overall Quality of Evidence | Very low | |
Table 4: Studies comparing the accuracy of stress contrast ECHO vs. coronary angiography for the detection of CAD.
| Study | Patient population | Age (% male) | LVEF ±SD | Medical history | Contrast agent | Type of stress | Time between stress contrast echo and CA | Definition of CAD | Other imaging modalities included in study |
|---|---|---|---|---|---|---|---|---|---|
| Hayat et al. 2008 (3) | 63 (all LBBB) |
71 ±10 (63) |
42 ±14 |
48% HPN 30% DM |
Sonovue | Stress and rest (dipyridamole) | Unclear | > 50% CAS | Technetium SPECT |
| Aggeli et al. 2007 (4) | 50 | 67 ±5 (68) |
NR | 100% HPN | Sonovue | Adenosine | Within 1 month | > 50% CAS | Thallium SPECT |
| Miszalski-Jamka 2007 (18; 19) | 44 (2 excluded) |
57.3 ±10 (71) |
60.4 ±7.7 | 57% HPN 7% DM |
Sonovue | Supine bicycle | Within 15 days | > 50% CAS | Stress echo |
| Moir et al. 2007 (16) | 135 | 57 ±10 (79) |
NR | 52% HPN 31% DM |
Definity | Exercise or dobutamine | Unclear | > 50% CAS | Stress echo |
| Osorio et al. 2007 (20) | 71 (results for 56) |
58 ±11 (36) |
65 ±4 | 86% HPN 66% DM |
PESDA | Adenosine | Unclear | > 50% CAS | None |
| Karavidas et al. 2006 (21) | 55 ±6 (62) |
NR | 37% HPN 12% DM |
Levovist | Adenosine | Within 1 week | > 50% CAS | Thallium SPECT | |
| Moir et al. 2005 1 (17) | 57 ±11 (80) |
NR | 52% HPN 32% DM |
Definity | Dipyridamole + exercise stress | NR | > 50% CAS | Stress echo | |
| Moir et al. 2005 2 (14) | 47 (all LBBB) |
56 ±11 (72) |
NR | 75% HPN 55% DM |
Definity | Dipyridamole + exercise stress | NR | > 50% CAS | Stress echo |
| Senior et al. 2005 (22) | 90 | 63 ±12 (67) |
35 ±13 | 48% HPN 35% DM |
Optison (21 patients) Sonovue (31 patients) |
Rest + stress (dipyrimidamole) | Within time of hosp admission | > 50% CAS | None |
| Moir et al. 2004 (15) | 85 (70 had CA) |
57 ±11 (87) |
NR | 47% HPN 20% DM |
Definity | Dipyrida-mole + exercise stress | Unclear | > 50% CAS | Stress echo |
| Peltier et al. 2004 (13)* | 35 | 62 ±10 (71) |
“normal” | 66% HPN 17% DM |
Sonovue | Dipyridamole | NR | > 70% CAS | Technetium SPECT |
Note: CA, coronary angiography; CAS, coronary artery stenosis; DM, diabetes mellitus; HF, heart failure; HPN, hypertension; LVEF, left ventricular ejection fraction; NR, not reported; SD, standard deviation; stress echo, stress echocardiography
The study by Peltier et al (13) was excluded from the analysis because this study used a threshold of >70% coronary artery stenosis to define CAD, while all the other studies used > 50% coronary artery stenosis to define CAD.
Table 5: Diagnostic accuracy of stress contrast ECHO in patients with suspected CAD.
| Study | CAD on CA | Type of analysis | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # With | # Without | |||||||||||||
| Hayat et al. 2008 (3) | 25 | 38 | MPA + WMA | 23 | 2 | 2 | 36 | 92% | 95% | 92% | 95% | 17.5 | 0.084 | 94% |
| Aggeli et al. 2007 (4) | 32 | 18 | MPA | 28 | 2 | 4 | 16 | 88% | 89% | 93% | 80% | 7.88 | 0.141 | 88% |
| Miszalski-Jamka et al. 2007 (18;19) | 25 | 17 | MPA + WMA | 23 | 3 | 2 | 14 | 92% | 82% | 88% | 88% | 5.21 | 0.097 | 88% |
| Moir et al. 2007 (16) | 75 | 60 | WMA | 68 | 14 | 7 | 46 | 91% | 77% | 83% | 87% | 3.89 | 0.122 | 84% |
| Osorio et al. 2007 (20) | 25 | 31 | MPA | 16 | 2 | 9 | 29 | 64% | 93% | 89% | 76% | 9.92 | 0.384 | 80% |
| Karavidas et al. 2006 (21) | 11 | 36 | MPA + WMA | 10 | 3 | 1 | 33 | 91% | 92% | 77% | 97% | 10.9 | 0.099 | 91% |
| Moir et al. 2005—1 (17) | 40 | 39 | MPA + WMA | 37 | 14 | 3 | 25 | 93% | 65% | 73% | 89% | 2.58 | 0.117 | 78% |
| Moir et al. 2005—2 (14) | 28 | 55 | MPA + WMA | 23 | 6 | 5 | 49 | 82% | 89% | 79% | 91% | 7.53 | 0.200 | 87% |
| Senior et al. 2005 (22) | 22 | 30 | MPA | 18 | 1 | 4 | 29 | 82% | 97% | 95% | 88% | 24.5 | 0.188 | 90% |
| Moir et al. 2004 (15) | 43 | 27 | MPA + WMA | 39 | 8 | 4 | 19 | 91% | 70% | 83% | 83% | 3.06 | 0.132 | 83% |
| Peltier et al. 2004 (13)* | 22 | 13 | MPA + WMA | 19 | 4 | 3 | 9 | 86% | 69% | 83% | 75% | 2.81 | 0.197 | 80% |
Note: CA, coronary angiography; CAD, coronary artery disease; FN, false negative; FP, false positive; LR, likelihood ratio; MPA, myocardial perfusion analysis; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive; WMA, wall motion analysis
The study by Peltier et al (13) was excluded from the analysis because this study used a threshold of ≥70% coronary artery stenosis to define CAD, while all the other studies used ≥50% coronary artery stenosis to define CAD.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of the Evidence-Based Analysis
Diagnostic Accuracy of Contrast ECHO
The studies assessing diagnostic accuracy of contrast ECHO were split into two groups, studies that included patients with suspected CAD only and studies that included patients with suspected or known CAD. All of the studies used contrast in stress ECHO (none used rest ECHO with contrast to establish CAD diagnosis). As mentioned in the introduction, contrast ECHO is typically assessed through regional wall motion analysis (WMA). More recently, researchers have investigated the ability of ECHO with contrast to assess myocardial perfusion (MPA). Some studies report both means of assessment, while others use only one mode of analysis. Essentially, WMA measures left ventricular opacification and MPA measures how quickly the contrast agent replenishes in the myocardium.
Studies with patients with suspected CAD only
Eleven studies assessed the diagnostic accuracy of stress contrast ECHO in patients with suspected CAD. Coronary angiography was the reference standard in all of the studies and, with the exception of the study by Peltier et al (13), CAD was defined as ≥50% coronary artery stenosis on coronary angiography. The threshold in the Peltier et al study was ≥70%. The Peltier study was excluded for the analysis because of the difference in the threshold. The heterogeneity of the studies is evident when examining the characteristics of the studies in Table 4. For instance, different contrast agents are used, different stress inducers (exercise, various pharmaceuticals) are used and 2 of the studies included only patients with left bundle branch block. The type of analysis performed by each study also varied. Most incorporated WMA and MPA into their analysis and four of these were published by Moir et al. (14-17) MAS contacted the authors of these studies to establish if these were distinct studies, or whether there was overlap in the patient population. The authors informed MAS that there was approximately a 10 to 15 patient overlap between studies (personal communication, August 2009). Table 5 lists the accuracy data for the studies and Figure 1 presents the forest plots for the sensitivities and specificities reported in the studies investigating the role of stress contrast ECHO in patients with suspected CAD.
Figure 1: Sensitivity and specificity of stress contrast ECHO in the diagnosis of CAD.
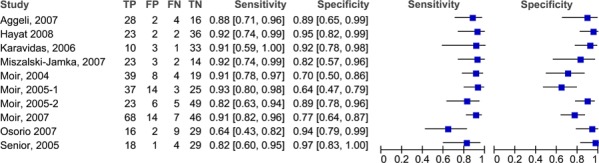
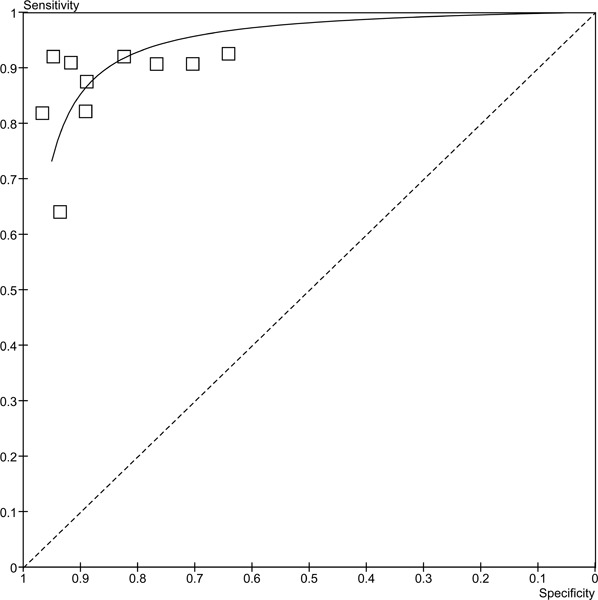
From these plots it appears that although the sensitivities are consistent across the studies (with the exception of the study by Osorio et al (20)), specificity is more variable. In is unclear why the study by Osorio et al (20) has such a lower sensitivity for contrast ECHO than the rest of the studies. The major difference between this study and the others is that Osorio et al. used a non-commercial contrast agent (PESDA), while the others used various commercially available agents. The pooled sensitivity is 87.3% (95% CI 83.2%-90.8%) and the pooled specificity is 86.0% (95% CI 82.0%-89.4%). The ROC curve for these studies is in Figure 2. The area under the curve (AUC) is 0.944.
Figure 2: ROC curve for stress contrast ECHO for the diagnosis of CAD.
Studies with patients with suspected or known CAD
Twelve studies were identified that investigated the diagnostic accuracy of stress contrast ECHO for the diagnosis of CAD in patients with suspected or known CAD. Like the studies which included only patients with suspected CAD, there was a lot of heterogeneity among these studies (Table 6). Two of the studies identified did not use the ≥50% coronary artery stenosis threshold for defining CAD on coronary angiography. The study by Plana et al. (23) used ≥70% as the cut-off and Korosoglou et al. (5) used ≥75% as the cut-off. For this reason, both of these studies were excluded from the analysis. Elhendy et al. (24) included only patients with diabetes, while Tsutsui et al (25) included only elderly patients (≥70 years) and Hu et al. (26) limited their inclusion criteria to overweight and obese patients. The patient population was thus variable across the studies.
Table 6: Studies comparing the accuracy of stress contrast ECHO vs. coronary angiography for the detection of CAD.
| Study | Patient population | Age ±SD (% male) | LVEF ±SD | Medical history | Contrast agent | Type of stress | Time between stress contrast echo and CA | Definition of CAD | Other imaging modalities in study |
|---|---|---|---|---|---|---|---|---|---|
| Lonnebakken et al. 2009 (28) | 37 | 59 ±8 (78) |
61 ±9 | 51% HPN 8% DM 16% previous MI |
Sonovue | Dobutamine | CA done before stress contrast echo | ≥50% CAS | None |
| Aggeli et al. 2008 (27) | 532 | 65 ±11 (71) |
NR | 62% HPN 40% DM 36% previous MI |
Definity | Dobutamine-atropine | NR | ≥50% CAS | None |
| Lipiec et al. 2008 (29;30) | 103 | 58 ±9 (63) |
NR | 76% HPN 16% DM 61% previous MI |
Optison | Dipyrimidamole | Within 14 days | ≥50% CAS | 99 Tc SPECT |
| Plana et al. 2008* (23) | 108 | 60 ±9 (74) |
56 ±7% | 75% HPN 40% DM 28% previous MI |
Definity | Dobutamine | Within 30 days of study enrolment | ≥70% CAS | Stress echo |
| Tsutsui et al. 2008 (25) | 399 (only 60 had CA) All ≥70 yrs |
78 ±5 (48) |
9% LVEF <50% |
72% HPN 33% DM 27% previous MI |
Optison | Dobutamine | Within 1 month | ≥50% CAS | None |
| Hu et al. 2007 (26) | 62 (overweight or obese patients) | 69 ±8 (71) |
NR | 87% HPN 45% DM 24% previous MI |
Optison or Sonovue | Dobutamine | Within 1 month | ≥50% CAS | Stress echo |
| Korosoglou et al. 2006* (5) | 120 (only 89 had CA) |
64 ±9 (61) |
NR | 59% HPN 28% DM 25% previous MI |
Optison | Dipyrimidamole | Within 3 months | ≥75% CAS | 99 Tc SPECT |
| Lin et al. 2006 (31) | 72 | 56 ±10 (74) |
NR | 59% HPN 31% DM 10% previous MI |
PESDA | Dipyrimidamole | Within 2 weeks | ≥50% CAS | Thallium and 99 Tc SPECT |
| Elhendy et al. 2005 (24) | 128 (all diabetics) |
NR | 55±12% | 100% DM | Optison or Definity | Dobutamine and rest | Within 1 month | ≥50% CAS | None |
| Tsutsui et al. 2005 (32) | 158 (61 had CA) |
61 ±13 (51) |
58 ±11 | 73% HPN 11% DM 28% previous MI |
Optison or Definity | Dobutamine-atropine | Within 1 month | ≥50% CAS | None |
| Chiou et al. 2004 (33) | 140 (132 in analysis) |
67 ±11 (75) |
NR | 59% HPN 31% DM |
PESDA | Dobutamine | Within 7 days | ≥50% CAS | None |
| Elhendy et al. 2004 (34) | 170 | 60 ±12 (58) |
60±14% | 73% HPN 14% DM |
Definity or Optison | Dobutamine-atropine | Within 1 month | ≥50% CAS | None |
Note: CA, coronary angiography; CAS, coronary artery stenosis; DM, diabetes mellitus; HF, heart failure; HPN, hypertension; LVEF, left ventricular ejection fraction; NR, not reported; SD, standard deviation; stress echo, stress echocardiography
All studies were prospective observational studies, with the exception of Aggeli et al (27), which was a retrospective chart review primarily investigating the adverse events associated with contrast agents. This study also reported the diagnostic accuracy of stress contrast ECHO compared to coronary angiography in a proportion of the patients. Table 7 lists the accuracy data for these studies.
Table 7: Diagnostic accuracy of stress contrast ECHO in patients with suspected or known CAD.
| Study | CAD on CA | Type of analysis | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # With | # Without | |||||||||||||
| Lonnebakken et al. 2009 (28) | 37 | 0 | MPA WMA |
34 21 |
N/A N/A |
3 16 |
N/A N/A |
92% 42% |
N/A N/A |
N/A N/A |
N/A N/A |
N/A N/A |
N/A N/A |
N/A N/A |
| Aggeli et al. 2008 (27) | 413 | 119 | MPA + WMA | 378 | 46 | 35 | 73 | 92% | 61% | 89% | 68% | 2.37 | 1.38 | 85% |
| Lipiec et al. 2008 (29;30) | 89 | 14 | MPA* WMA WMA or MPA |
60 68 78 |
2 3 5 |
29 21 11 |
12 11 9 |
67% 76% 88% |
86% 79% 64% |
97% 96% 94% |
29% 34% 45% |
4.72 3.57 2.45 |
0.380 0.300 0.192 |
70% 77% 84% |
| Plana et al. 2008 (23)† | 52 | 35 | WMA | 42 | 16 | 10 | 19 | 80% | 55% | 72% | 66% | 1.77 | 0.354 | 70% |
| Tsutsui et al. 2008 (25) | 50 | 10 | MPA WMA |
48 37 |
4 3 |
2 13 |
6 7 |
96% 74% |
60% 70% |
92% 93% |
75% 35% |
2.40 2.47 |
0.067 0.371 |
90% 73% |
| Hu et al. 2007 (26) | 44 | 18 | WMA | 36 | 4 | 8 | 14 | 82% | 78% | 90% | 64% | 3.68 | 0.234 | 81% |
| Korosoglou et al. 2006 (5)† | 62 | 27 | MPA WMA |
52 51 |
2 6 |
10 11 |
25 21 |
84% 82% |
93% 78% |
96% 89% |
71% 66% |
11.3 3.70 |
0.174 0.228 |
87% 81% |
| Lin et al. 2006 (31) | 25 | 15 | MPA WMA MPA+WMA |
19 19 21 |
1 2 1 |
6 6 4 |
14 13 14 |
76% 76% 84% |
93% 87% 935 |
95% 90% 95% |
70% 68% 78% |
11.4 5.70 12.6 |
0.257 0.277 0.171 |
83% 80% 88% |
| Elhendy et al. 2005 (24) | 101 | 27 | MPA | 90 | 13 | 11 | 14 | 89% | 52% | 87% | 56% | 1.85 | 0.210 | 81% |
| Tsutsui et al. 2005 (32) | 48 | 13 | MPA WMA |
44 30 |
3 2 |
4 18 |
10 11 |
92% 62% |
77% 85% |
94% 94% |
71% 38% |
3.97 4.06 |
0.108 0.443 |
89% 67% |
| Chiou et al. 2004 (33) | 85 | 47 | MPA WMA |
69 71 |
11 9 |
16 14 |
36 38 |
81% 84% |
77% 81% |
86% 88% |
69% 73% |
3.47 4.36 |
0.246 0.203 |
80% 83% |
| Elhendy et al. 2004 (34) | 127 | 43 | MPA WMA |
116 89 |
21 11 |
11 38 |
22 32 |
91% 70% |
51% 74% |
85% 89% |
67% 46% |
1.87 2.74 |
0.169 0.402 |
81% 71% |
Note: CA, coronary angiography; CAD, coronary artery disease; FN, false negative; FP, false positive; LR, likelihood ratio; MPA, myocardial perfusion analysis; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive; WMA, wall motion analysis
The study by Tsutsui et al published in 2005 reported the accuracy of contrast echo compared to coronary angiography and reported 3-year follow-up of cardiac events (25 cardiac events among the 131 patients who completed follow-up). They reported that the incidence of cardiac events was higher in patients with positive MPA or WMA results compared to those with negative MPA or WMA results. The predictors of cardiac events included male gender, resting LVEF, previous PCI, use of nitrates, positive WMA and positive MPA. Three-year event-free survival was 87% in patients with negative WMA and MPA results, and 49% in patients with positive MPA and WMA results.
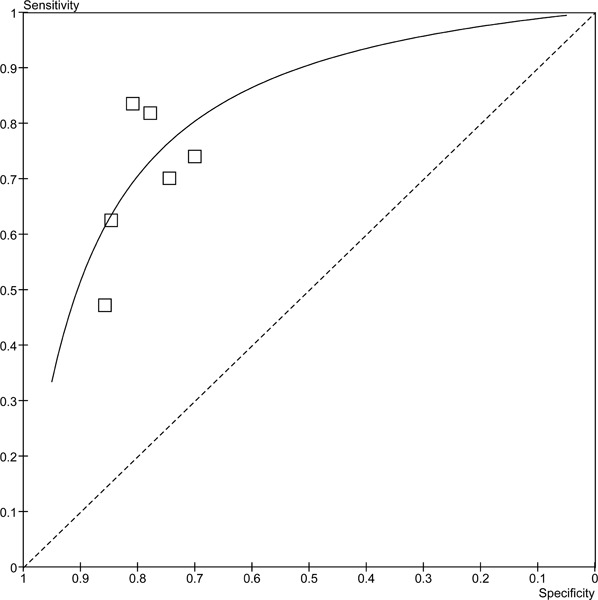
Since not all of the studies reported MPA or WMA, the results have been stratified by the type of analysis reported. The forest plots in figure 3 outlines the sensitivities and specificities in the studies reporting MPA. There is a high degree of variability in both the sensitivity (range 67% to 96%) and specificity (51% to 86%). There may be more variability because the patients included in these studies had either suspected or known CAD. The pooled sensitivity is 87.8% (95% CI: 83.5% to 89.9%) and the pooled specificity is 64.9% (95% CI: 59.1% to 70.4%). The studies in the previous section included only patients with suspected CAD, and the range of sensitivities and specificities were much narrower. A similar trend for the sensitivities (range 47%-84%) is observed in the studies that reported wall motion analyses (Figure 4), yet for the specificities the range is much narrower and consistent across the studies (range 70%-86%). The pooled sensitivity is 69.2% (95% CI 64.8% to 73.4%) and the pooled specificity is 79.4% (95% CI 72.3% to 85.4%). Figures 5 and 6 are the ROC curves for the diagnostic accuracy of stress contrast ECHO for MPA and WMA, respectively. The AUC for the studies using MPA is 0.865, and the AUC for studies using WMA is 0.867. It is important to note that currently contrast agents are routinely being used to assess wall motion; perfusion is still considered in a research context.
Figure 3: Sensitivity and specificity of stress contrast ECHO using MPA for CAD Diagnosis.
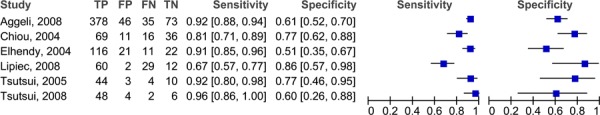
Figure 4: Sensitivity and specificity of stress contrast ECHO using WMA for CAD Diagnosis.
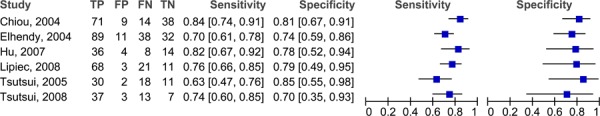
Figure 5: ROC Curve of stress contrast ECHO using MPA for CAD Diagnosis.
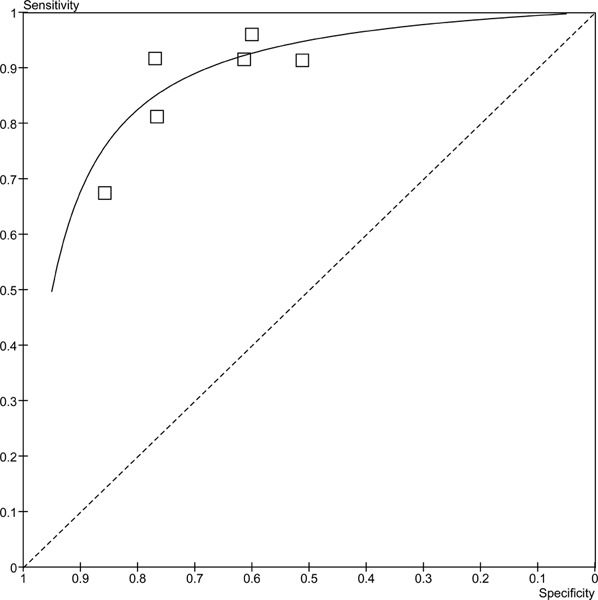
Figure 6: ROC curve of stress contrast ECHO using WMA for CAD Diagnosis.
Stress Contrast ECHO versus Technetium-99m SPECT
Dijkmans et al (35) published a non-systematic review in 2006 reviewing contrast ECHO. As part of their review they included a meta-analysis of the sensitivity of stress contrast ECHO versus SPECT. The meta-analysis included 8 studies. The studies were heterogeneous in terms of the type of contrast agent used, including 1 study that used the contrast agent, Sonazoid, which is not licensed in Canada. Some studies used exercise stress while others used pharmacological stress (dobutamine). They reported that the sensitivity of stress contrast ECHO was significantly higher than the sensitivity of SPECT. (P<.001). However, these results need to be interpreted with caution due to the heterogeneity previously mentioned and because the 8 studies were crossover studies and all of the patients were double-counted. That is, they were included in the stress contrast ECHO analysis and then in the SPECT analysis. In addition, there was variation across the studies in terms of which isotope patients were given for the SPECT studies—either thallium or technetium.
MAS identified 5 studies published since 2004 that compared stress contrast ECHO to 99Tc SPECT. These studies are quite heterogeneous. The characteristics of the studies are outlined in Table 8. The study by Hayat et al (3) includes only patients with left bundle branch block, while none of the other studies did. In the study by Korosoglou et al (5) the threshold for establishing CAD on coronary angiography was ≥75% coronary artery stenosis. Peltier et al (13) set their threshold at ≥70% coronary artery stenosis, and the other 3 studies used ≥50% coronary artery stenosis as their cut-off for diagnosis CAD on coronary angiography. The studies that did not use the ≥50% cut-off were excluded from the analysis. The study by Lin et al (31) used a non-commercial contrast agent. In other words, they used a contrast agent that they made in their laboratory called perfluoropropane-exposed sonicated dextrose albumin (PESDA). PESDA is an unregulated contrast agent. Also, 3 of the studies (Lipiec et al (29;30), Korosoglou et al (5)and Lin et al (31)) included patients with both suspected and known CAD, while the other 2 studies limited their inclusion criteria to those with suspected CAD only.
Table 8: Studies comparing stress contrast ECHO vs. Technetium 99m SPECT for CAD detection using coronary angiography as the reference standard.
| Study | Patient population | Age ±SD (% male) | LVEF ±SD | Medical history | Contrast agent | Type of stress | Time between stress contrast echo and CA | Definition of CAD | Suspected or Suspected/Known CAD |
|---|---|---|---|---|---|---|---|---|---|
| Hayat et al. 2008 (3) | 63 | 71 ±10 (63) |
42 ±14 | 48% hypertension 30% diabetes |
Sonovue | Stress and rest (dipyridamine) | Unclear | ≥ 50% CAS | Suspected |
| Lipiec et al. 2008 (29;30) | 103 | 58 ±9 (63) |
NR | 76% HPN 16% DM 61% previous MI |
Optison | Dipyrimidamole | Within 14 days | ≥50% CAS | Suspected or known CAD |
| Korosoglou, et al. 2006 (5)* | 120 (only 89 had CA) |
64 ±9 (61) |
NR | 59% HPN 28% DM 25% previous MI |
Optison | Dipyrimidamole | Within 3 months | ≥75% CAS | Suspected or known CAD |
| Lin et al. 2006 (31) | 72 | 56 ±10 (74) |
NR | 59% HPN 31% DM 10% previous MI |
PESDA | Dipyrimidamole | Within 2 weeks | ≥50% CAS | Suspected or known CAD |
| Peltier et al. 2004 (13)* | 35 | 62 ±10 (71) |
‘normal’ | 66% HPN 17% DM |
Sonovue | Dipyridamole | Unclear | ≥ 70% CAS | Suspected |
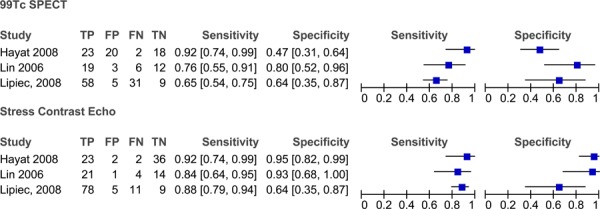
Table 9 lists the diagnostic accuracy for the studies comparing stress contrast ECHO to 99Tc SPECT. In some studies the stress contrast ECHO results were based on wall motion analysis (WMA) and in others the stress contrast ECHO results relied on the myocardial perfusion analysis (MPA) to diagnose CAD. Two of the studies used a combination of WMA and MPA to detect CAD. The sensitivities and specificities of stress contrast ECHO and 99Tc SPECT are presented in forest plots in Figure 7. There is variability among the 3 studies in the analysis for both the diagnostic accuracy of stress contrast ECHO and for 99Tc SPECT. In studies reporting various results for WMA, MPA and combinations of the two, the results with the highest sensitivity and specificity were used in the analysis.
Table 9: Diagnostic accuracy of stress contrast ECHO vs. Technetium 99m SPECT.
| Study | CAD on CA | Type of analysis | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # With | # Without | |||||||||||||
| Hayat et al. 2008 (3) | 25 | 38 | MPA + WMA 99-Tc SPECT |
23 23 |
2 20 |
2 2 |
36 18 |
92% 92% |
95% 47% |
92% 53% |
95% 90% |
17.5 1.75 |
0.084 0.169 |
94% 65% |
| Lipiec et al. 2008 (29;30) | 89 | 14 | MPA* WMA WMA or MPA 99-Tc SPECT |
60 68 78 58 |
2 3 5 5 |
29 21 11 31 |
12 11 9 9 |
67% 76% 88% 65% |
86% 79% 64% 64% |
97% 96% 94% 92% |
29% 34% 45% 23% |
4.72 3.57 2.45 1.82 |
0.380 0.300 0.192 0.542 |
70% 77% 84% 65% |
| Korosoglou et al. 2006 (5)† | 62 | 27 | MPA WMA 99-Tc SPECT |
52 51 48 |
2 6 13 |
10 11 14 |
25 21 14 |
84% 82% 77% |
93% 78% 52% |
96% 89% 79% |
71% 66% 50% |
11.3 3.70 1.61 |
0.174 0.228 0.435 |
87% 81% 70% |
| Lin et al. 2006 (31) | 25 | 15 | MPA WMA MPA + WMA 99-Tc SPECT |
19 19 21 19 |
1 2 1 3 |
6 6 4 6 |
14 13 14 12 |
76% 76% 84% 76% |
93% 87% 93% 80% |
95% 90% 95% 86% |
70% 68% 78% 67% |
11.4 5.70 12.6 3.80 |
0.257 0.277 0.171 0.300 |
83% 80% 88% 78% |
| Peltier et al. 2004 (13)† | 22 | 13 | MPA + WMA 99-Tc SPECT |
19 18 |
4 2 |
3 4 |
9 11 |
86% 82% |
69% 85% |
83% 90% |
75% 73% |
2.81 5.32 |
0.197 0.215 |
80% 83% |
Note: CA, coronary angiography; CAD, coronary artery disease; FN, false negative; FP, false positive; LR, likelihood ratio; MPA, myocardial perfusion analysis; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive; WMA, wall motion analysis
Figure 7: Forest plots of the sensitivity and specificity of the studies comparing 99Tc SPECT to stress contrast ECHO for the diagnosis of CAD.
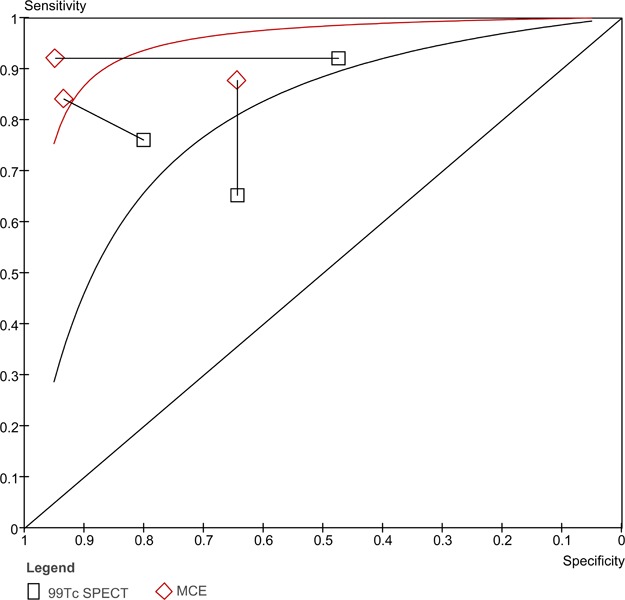
When ROC curves are created for both stress contrast ECHO and 99Tc SPECT, the curve for stress contrast ECHO is higher than the curve for 99Tc SPECT for the 3 studies in this analysis (Figure 8). The AUC for stress contrast ECHO is 0.902 and the AUC for 99Tc SPECT is 0.792. Due to the limitations outlined above, these results need to be interpreted with caution.
Figure 8: ROC curve comparing stress contrast ECHO to 99Tc SPECT for the diagnosis of CAD.
Stress Contrast ECHO versus Thallium SPECT
Two studies were identified that compared stress contrast ECHO to SPECT using the radiotracer 201 Thallium (their characteristics are listed in Table 10). These two studies had similar sample sizes (47 and 50 patients), used adenosine to induce stress in the patients, and both patient populations were limited to those with suspected CAD only. There were no patients with known CAD included in the trials. The study by Aggeli et al. (4) included only patients with hypertension, while the study by Karavidas et al. (21) did not limit to hypertension status, however, they did limit to patients with left bundle branch block. Patients in the Karavidas et al. study (21) were also younger than those in Aggeli et al. (4)
Table 10: Studies comparing stress contrast ECHO vs. Thallium SPECT for CAD detection using coronary angiography as the reference standard.
| Study | N | Age ±SD (% male) | LVEF | Medical history | Contrast agent | Type of stress | Time between stress contrast echo and CA | Definition of CAD | Suspected CAD or Suspected + Known CAD |
|---|---|---|---|---|---|---|---|---|---|
| Aggeli et al. 2007 (4) | 50 | 67 ±5 (68) |
NR | 100% HPN | Sonovue | Adenosine | Within 1 month | ≥50% CAS | Suspected |
| Karavidas et al. 2006 (21) | 47 | 55 ±6 (62) |
NR | 37% HPN 12% DM |
Levovist | Adenosine | Within 1 week | ≥50% CAS | Suspected |
The results for stress contrast ECHO (interpreted using both wall motion analysis and myocardial perfusion analysis) were consistent between the two studies with sensitivities of 88% in Aggeli et al. and 91% in Karavidas et al. and specificities of 89% and 92%, respectively. The results for SPECT were not as consistent, particularly with regards to specificity. Aggeli et al. reported a sensitivity and specificity of 80% and 94%, while Karavidas et al. reported much lower sensitivity and specificity for SPECT at 73% and 72% (see Table 11).
Table 11: Diagnostic accuracy of stress contrast ECHO vs. Thallium SPECT.
| Study | CAD on CA | Type of analysis | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # With | # Without | |||||||||||||
| Aggeli et al. 2007 (4) | 32 | 18 | MPA + WMA | 28 | 2 | 4 | 16 | 88% | 89% | 93% | 80% | 7.88 | 0.141 | 88% |
| 201 Tl SPECT | 26 | 1 | 6 | 17 | 80% | 94% | 96% | 74% | 14.6 | 0.199 | 85% | |||
| Karavidas et al. 2006 (21) | 11 | 36 | MPA + WMA | 10 | 3 | 1 | 33 | 91% | 92% | 77% | 97% | 10.9 | 0.099 | 91% |
| 201 Tl SPECT | 8 | 10 | 3 | 26 | 73% | 72% | 44% | 90% | 2.62 | 0.378 | 72% | |||
Note: CA, coronary angiography; CAD, coronary artery disease; FN, false negative; FP, false positive; LR, likelihood ratio; MPA, myocardial perfusion analysis; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; TN, true negative; TP, true positive; WMA, wall motion analysis
Stress Contrast ECHO versus Stress ECHO without contrast
Six studies comparing stress ECHO without contrast to stress ECHO with contrast were identified (Table 12). Three of these assessed WMA and the other three assessed WMA and MPA. Five of the studies defined the diagnosis of CAD as ≥50% coronary artery stenosis on coronary angiography, while the study by Plana et al (23) used a threshold of ≥70% coronary artery stenosis to define CAD. For this reason, the study by Plana et al (23) was excluded from the analysis. There were three distinct studies by Moir et al included in the analysis. (15-17) The authors of these studies were contacted to establish if there was overlap in the patients between the studies, which was found to be between 10 and 15 patients (personal communication, August 2009). Four of the studies included in the analysis included only patients with suspected CAD, while two studies [Plana et al (23) and Hu et al (26)] included patients with both suspected and known CAD (Table 13).
Table 12: Studies comparing stress contrast ECHO vs. stress ECHO for the detection of CAD using coronary angiography as the reference standard.
| Study | N | Age ±SD (% male) | LVEF ±SD | Medical history | Contrast agent | Type of stress | Time between stress contrast echo and CA | Definition of CAD | Suspected CAD or Suspected + Known CAD |
|---|---|---|---|---|---|---|---|---|---|
| Plana et al. 2008 (23)* | 108 | 60 ±9 (74) |
56 ±7% | 75% HPN 40% DM 28% previous Ml |
Definity | Dobutamine | Within 30 days of study enrolment | ≥ 70% CAS | Suspected or known CAD |
| Hu et al. 2007 (26) | 62 (overweight or obese patients) |
69 ±8 (71) |
NR | 87% HPN 45% DM 24% previous Ml |
Optison or Sonovue | Dobutamine | Within 1 month | ≥ 50% CAS | Suspected or known CAD |
| Miszalski-Jamka et al. 2007 (18;19) | 44 (2 excluded) |
57 ±10.2 (71) |
60.4 ±7.7 (range 41-74) |
57% HPN 7% DM |
Sonovue | Supine bicycle | Within 15 days | ≥ 50% CAS | Suspected |
| Moir et al. 2007 (16) | 135 | 57±10 (79) |
NR | 52% HPN 31% DM |
Definity | Exercise or dobutamine | Unclear | ≥ 50% CAS | Suspected |
| Moir et al. 2005—1 (17) | 90 | 57 ±11 (80) |
NR | 52% HPN 32% DM |
Definity | Dipyridamole + exercise stress | Unclear | ≥ 50% CAS | Suspected |
| Moir et al. 2004 (15) | 85 (only 70 had CA) |
57 ±11 (87) |
NR | 47% HPN 20% DM | Definity | Dipyridamole + exercise stress | Unclear | ≥ 50% CAS | Suspected |
Note: CA, coronary angiography; CAS, coronary artery stenosis; DM, diabetes mellitus; HF, heart failure; HPN, hypertension; LVEF, left ventricular ejection fraction; NR, not reported; SD, standard deviation; stress echo, stress echocardiography
The study by Plana et al was excluded from the analysis because they defined CAD as ≥70% coronary artery stenosis, while the other studies used a threshold of ≥50%.
Table 13: Diagnostic accuracy of stress contrast ECHO vs. stress ECHO.
| Study | CAD on CA | Type of analysis | TP | FP | FN | TN | Sensitivity | Specificity | PPV | NPV | +LR | -LR | Diagnostic Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # With | # Without | |||||||||||||
| Plana et al. 2008 (23)* | 52 | 35 | WMA Stress ECHO |
42 39 |
16 17 |
10 13 |
19 18 |
80% 75% |
55% 51% |
72% 70% |
66% 58% |
1.77 1.54 |
0.354 0.486 |
70% 66% |
| Hu et al. 2007 (26) | 44 | 18 | WMA Stress ECHO |
36 31 |
4 6 |
8 13 |
14 12 |
82% 70% |
78% 67% |
90% 84% |
64% 46% |
3.68 2.11 |
0.234 0.443 |
81% 68% |
| Miszalski-Jamka et al. 2007 (18; 19) | 25 | 17 | MPA + WMA Stress ECHO |
23 19 |
3 3 |
2 6 |
14 14 |
92% 76% |
82% 82% |
88% 86% |
88% 70% |
5.21 4.31 |
0.097 0.291 |
88% 79% |
| Moir et al. 2007 (16) | 75 | 60 | WMA Stress ECHO |
68 60 |
14 17 |
7 15 |
46 43 |
91% 80% |
77% 72% |
83% 78% |
87% 74% |
3.89 2.82 |
0.122 0.279 |
84% 76% |
| Moir et al. 2005-1 (14) | 40 | 39 | MPA + WMA Stress ECHO |
37 30 |
14 10 |
3 10 |
25 29 |
93% 75% |
65% 74% |
73% 75% |
89% 74% |
2.58 2.93 |
0.117 0.336 |
78% 75% |
| Moir et al. 2004 (15) | 43 | 27 | MPA + WMA Stress ECHO |
39 32 |
8 5 |
4 11 |
19 22 |
91% 74% |
70% 81% |
83% 86% |
83% 67% |
3.06 4.02 |
0.132 0.314 |
83% 77% |
Note: CA, coronary angiography; CAD, coronary artery disease; FN, false negative; FP, false positive; LR, likelihood ratio; MPA, myocardial perfusion analysis; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive; WMA, wall motion analysis.
The study by Plana et al was excluded from the analysis because they defined CAD as ≥70% coronary artery stenosis, while the other studies used a threshold of ≥50%.
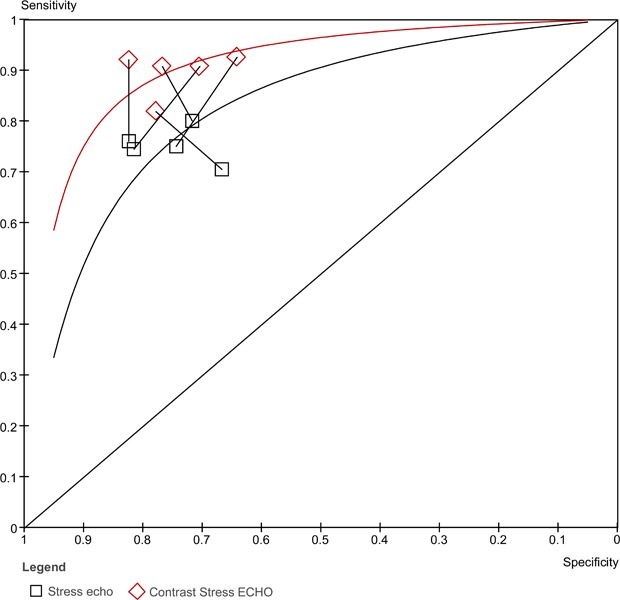
The sensitivities and specificities of stress contrast ECHO and stress ECHO (without contrast) are compared in Figure 9. The sensitivity range was generally consistent for both stress ECHO without contrast (range 70% to 80%) and stress ECHO with contrast (82% to 93%). The specificities for both with and without contrast are less consistent. The range for specificity for stress ECHO without contrast was 67% to 81%, and the range for stress ECHO with contrast was 64% to 82%. Figure 10 displays the ROC curves for both stress ECHO with and without contrast. The curve for stress ECHO with contrast is higher than the curve without contrast. The AUC for stress ECHO with contrast is 0.885 and the AUC for stress ECHO without contrast is 0.842.
Figure 9: Stress contrast ECHO using myocardial perfusion analysis versus stress ECHO (without contrast) for the diagnosis of CAD.
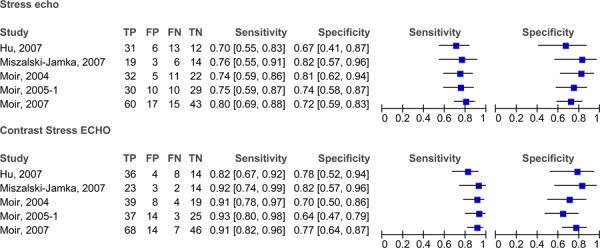
Figure 10: ROC curves comparing stress ECHO with and without contrast.
Contrast ECHO in Patients with Suboptimal Echocardiograms
One of the limitations of the evidence on the use of contrast ECHO in CAD diagnosis was the method with which patients were selected for inclusion to the studies. In a clinical setting, contrast ECHO is meant to be reserved for patients who have suboptimal echocardiograms. From the literature, 5% to 15% of resting ECHOs and up to 30% of stress ECHOs are suboptimal. (7;8) In the majority of the contrast ECHO studies, patients were given contrast ECHO regardless of previous non-contrast ECHO outcomes. This is considered a limitation because when measuring the sensitivity and specificity of the technology, the estimate of accuracy may be imprecise as the contrast is not being used exclusively in. those with suboptimal echocardiograms.
Kurt et al. (36) prospectively enrolled 632 patients with “technically difficult” ECHO results into their study. They stratified their results by the type and location of the patient with subgroups of: inpatients (non-intensive care), medical intensive care unit patients, surgical intensive care unit patients, and outpatients. As previously mentioned, without contrast the ECHO studies were technically difficult or could not be interpreted. After contrast administration, 89.9% of the ECHO studies were considered “adequate” for interpretation by 1 of 6 experienced echocardiographers (see Table 14). Based on their results, it appears that patients in surgical intensive care units benefit from contrast administration, but not to a lesser degree than other subgroups. It is important to note, however, that contrast administration still resulted in a significant improvement in all subgroups.
Table 14: Subgroup results reported after contrast ECHO from Kurt et al. (36).
| Contrast ECHO Result | Inpatients (non-ICU) | Medical ICU | Surgical ICU | Outpatient | Total |
|---|---|---|---|---|---|
| Adequate | 90.1% | 96.2% | 77.4% | 96.6% | 89.9% |
| Technically difficult / could not be interpreted | 9.9% | 3.8% | 22.6% | 3.4% | 10.1% |
Note: ICU, intensive care unit
In 2008, Plana et al. (23) published the results of the OPTIMIZE trial, which randomized patients to undergo stress ECHO with dobutamine (non-contrast DSE) and stress contrast ECHO with dobutamine (contrast DSE). The patients (N=108) underwent both contrast and non-contrast DSE within a 24 hour period. The results were split into three groups: patients with >2 segments not visualized (i.e., suboptimal ECHO), patients with 1 or 2 segments not visualized, and patients with all segments visualized (see Table 15). There was no difference between the diagnostic accuracy of ECHO, with or without contrast, for the diagnosis of CAD when a minimum of 1 or 2 segments were not visualized. There was a significant difference in the diagnostic accuracy contrast versus no contrast when patients had suboptimal echocardiograms without contrast (i.e., >2 segments not visualized). This study highlights the importance of contrast in suboptimal echocardiograms, but indicates that it is unnecessary in patients with interpretable echocardiograms without contrast.
Table 15: Results of Plana et al. (23) comparing suboptimal ECHO vs. interpretable ECHO, with and without contrast.
| Diagnostic accuracy | >2 segments no visualized (suboptimal) |
1-2 segments not visualized | All segments visualized |
|---|---|---|---|
| No contrast | 28% | 67% | 69% |
| Contrast | 59% | 72% | 70% |
| P-value | 0.005 | NS | NS |
Note: NS, not significant
In 2001, Dolan et al. (37) reported the results of a non-random study comparing diagnostic accuracy in patients receiving contrast due to previously suboptimal echocardiograms (n=117) to patients with interpretable non-contrast ECHO (n=112). The reference standard for establishing a true diagnosis of CAD was coronary angiography. All patients under went stress ECHO using dobutamine. The authors reported a sensitivity of 78% and a specificity of 73% for the contrast group, and 71% and 62%, respectively, for interpretable ECHO group. At baseline (prior to contrast administration) endocardial border visualization (EBV) was 74% for the contrast group and 88% for the non-contrast group (P=0.01). At rest, however, EBV was 88% for contrast and 84% for non-contrast (P=NS), while at peak stress EBV it was 88% and 87% (P=NS), respectively. Thus, the results of this study also conclude that, in patients with suboptimal echocardiograms, contrast administration makes the images comparable to interpretable non-contrast echocardiograms.
In the 2000 RCT by Kitzman et al. (38), 211 patients with suboptimal echocardiograms were randomized to undergo contrast ECHO with contrast (Definity) or placebo (saline). At baseline, 47% of segments were visible (without contrast), and after the contrast injection 81% of segments were visible with the contrast agent compared to 49% with the placebo (P<.01). The mean duration that contrast was “clinically useful” for was 99 seconds (SD 60 seconds).
Safety of Contrast ECHO
In May 2008, Lantheus Medical Imaging, the manufacturer of the licensed contrast agent, sent a letter to Health Canada indicating that: “serious cardiopulmonary reactions, including fatalities” had occurred worldwide during administration of the agent, within 30 minutes of receiving the agent and within days of receiving the agent. As of March 31, 2008, they reported one fatality in Canada after an adverse reaction following administration of the agent.
The indications and contraindications of the microbubble contrast agent, as written in product monograph, are listed below. (39)
Indications
The contrast agent is “indicated for contrast-enhanced ultrasound imaging of cardiac structures and function in adult patients with suboptimal echocardiograms. The safety and efficacy of [the contrast agent] with exercise stress or pharmacologic stress testing have not been established.”
Contraindications
Do not administer [the contrast agent] to patients with known:
Hypersensitivity to [the contrast agent] or its components
Right-to-left, bi-directional, or transient right-to-left cardiac shunts
[The contrast agent] should not be injected by direct intra-arterial injection.
Gas contrast agents, for use in diagnostic ultrasound examinations, should not be administered within 24 hours prior to extracorporeal shock wave lithotripsy.
Safety studies
Nine large retrospective studies investigating the safety of contrast agents were identified in the literature search (see Table 16). The studies included assessed safety and adverse effects in patients undergoing rest or stress ECHO with or without contrast. The follow-up periods varied across the studies from 1 day to 30 days.
Table 16: Studies investigating the safety of microsphere contrast agents for ECHO.
| Study | Type of study | N | Mean age (% male) | Contrast agent | Stress or rest | Time period observing for AE | Deaths |
|---|---|---|---|---|---|---|---|
| Anantharam et al. 2009 (42) | Retrospective analysis | 3,704 contrast | 63±12 (53) |
Luminity or Sonovue | Stress (DSE or exercise) |
Unclear | None |
| Dolan et al. 2009 (41) | Retrospective analysis | 42,408 contrast | Unclear | Definity or Optison | Rest: 23,659 pts Stress: 18,749 pts (DSE or exercise) |
- Within 30 minutes - Within 24 hours - Within 30 days |
1 death within 24 hours |
| Gabriel et al. 2008 (43) | Retrospective analysis | 4,786 contrast; 5,012 non-contrast |
61 ±12 (64) |
Definity or Optison | Stress (dobutamine or exercise) | - Within 24 hours - Within 30 days |
Contrast: No fatalities within 24 hours, 10 deaths within 30 days (0.2%) Non-contrast: 2 deaths within 24 hours, 16 deaths within 30 days (0.8%) |
| Kusnetzky et al. 2008 (40) | Retrospective analysis | 12,475 non-contrast 6,196 contrast | 66±15 (64) | Definity | Unclear | -Within 1 hour - Within 24 hours |
Contrast: - 26 patients died within 24 hours (0.42%) Non-contrast: - 46 patients died within 24 hours (0.37%) |
| Main et al. 2008 (44) | Retrospective analysis | 58,254 contrast; 4,242,712 non-contrast | 66 ±14 (61) | Definity | Rest | - Within 1 day | Mortality rate: -1.08% non-contrast -1.06% contrast |
| Shaikh et al. 2008 (45) | Retrospective analysis | 2,914 contrast; 2,155 non-contrast | 61 (53-70) (53) | Optison or Definity | Stress (dobutamine, exercise) | - During test | None |
| Aggeli et al. 2007 (4) | Retrospective analysis | 5,250 contrast | 64.5 ±10.5 (71) | Sonovue | Stress (dobutamine and atropine) | - During DSE - Within 24h of DSE |
None |
| Timperley et al. 2005 (46) | Retrospective analysis | 751 (332 non-contrast, 419 contrast) | 64 ±12 (59) | Sonovue or Optison | Stress (dobutamine) | Unclear | None |
| Tsutsui et al. 2005 (47) | Retrospective analysis | 1,486 contrast | 62 ±14 | Optison or Definity | Stress (dobutamine) | Unclear | None |
Note: AE, adverse event; DSE, dobutamine stress echocardiography; VT, ventricular tachycardia
The study by Kusnetzky et al. (40) retrospectively compared mortality data of patients undergoing contrast ECHO to patients undergoing non-contrast ECHO. They included over 18,000 records in their analysis. When they randomly selected 403 patient records to review, they found that the patients undergoing ECHO with contrast were less healthy than those who underwent ECHO without contrast. In the contrast group, significantly more patients were diabetics (36% versus 18%, P<.001), had hypertension (86% versus 59%, P<.001), had chronic obstructive lung disease (24% versus 11%, P<.001), and had known CAD (71% versus 32%, P<.001). The patients in the contrast group also had a lower mean left ventricular ejection fraction (48% versus 59%, P<.001). Despite the poorer overall health of the patients in the contrast group compared to those in the non-contrast group, there was not a significant difference in the mortality rate within 24 hours of the ECHO (0.42% for contrast versus 0.37% for non-contrast).
There was 1 death (N=42,408) reported within 24 hours of dobutamine stress ECHO with contrast in the study by Dolan et al. (41) The patient who died had been in hospital for 11 days prior to the stress echo with “frequent runs of ventricular tachycardia requiring intravenous antiarrhythmic therapy.” The stress echo was performed to assess myocardial viability and without any complications, but the patient developed recurring VT and died 22 hours after the stress ECHO.
Five of the studies reported adverse events in addition to mortality data (see Table 17).
Table 17: Adverse events reported in safety studies.
| Adverse Event | Frequency (%) | ||||
|---|---|---|---|---|---|
| Anantharam et al. 2009 (42) | Gabriel et al. 2008 (43) | Shaikh et al. 2008 (45) | Aggeli et al. 2008 (27) | Tsutsui et al. 2005 (47) | |
| Allergic reaction/hypersensitivity | 0.1% | 0 | 0.03% | Grade 1: 0.29% Grade 2: 0.15% |
NR |
| Cardiac arrhythmia | 0.9% | 2.0% | 3.7% | Grade 1/2: 6.0% Grade3: 0.29% Grade 4: 0.03% |
4.2% (sustained arrhythmias) |
| Cardiac troponin I elevation | NR | NR | NR | Grade 3: 0.04% | NR |
| Hypertension | 0 | NR | 3.9% | Grade 1/2: 2.1% | 1.8% |
| Fatigue | NR | NR | 2.9% | Grade 1/2: 1.3% | NR |
| Xerostomia (dry mouth) | NR | NR | NR | Grade 1: 19.8% | NR |
| Confusion | NR | NR | NR | Grade 1/2: 0.2% | NR |
| Dizziness | NR | NR | NR | Grade 1: 7.4% | NR |
| Memory impairment | NR | NR | NR | Grade 2: 0.04% | NR |
| Tremor | NR | NR | NR | Grade 1: 2.4% | NR |
| Headache | NR | NR | 3.3% | Grade 1/2: 5.3% Grade 3: 1.0% |
NR |
| Back pain | NR | 0.3% | 0.6% | Grade 1/2: 1.4% | NR |
| Dyspnea (shortness of breath) | NR | 4.6% | 9.7% | Grade 1: 0.9% | 1.7% |
| Urinary retention | NR | NR | NR | Grade 1: 0.8% | NR |
| Hypotension | NR | NR | 1.9% | NR | NR |
| Nausea/vomiting | NR | 3.1% | 2.6% | NR | NR |
| Leg pain | NR | NR | 4.5% | NR | NR |
| Palpitations | NR | NR | 5.4% | NR | NR |
| Chest pain | NR | 3.9% | 11.0% | NR | 7.1% |
| Diaphoresis (flushing) | NR | 1.1% | NR | NR | NR |
Note: NR, not reported
Conclusions
Based on the results of all studies included in the systematic review the following conclusions were made:
Stress ECHO with contrast has a higher diagnostic accuracy in the diagnosis of CAD than stress ECHO (without contrast).
Stress ECHO with contrast seems to have a similar diagnostic accuracy to 99 technetium SPECT.
The addition of contrast to ECHO in patients with previous suboptimal ECHO results significantly improves interpretability of the results.
Statistically, the addition of contrast agents to stress ECO tests does not significantly improve patient mortality rates.
Existing Guidelines for Contrast ECHO
The American Society of Echocardiography published a consensus statement in 2008 on the use of contrast agents in ECHO. (9) They recommended using contrast agents in the following situations:
-
In patients presenting for rest ECHO with reduced image quality
To enable improved endocardial visualization when ≥2 contiguous segments are not seen on non-contrast images
-
In patients presenting for stress ECHO with reduced image quality
To obtain diagnostic assessment of wall motion and thickening at rest and stress
In all patients presenting for rest ECHO for the assessment of LV systolic function, to reduce variability and increase interpreter confidence in LV volume measurements
-
To confirm or exclude the following LV structural abnormalities, when non-enhanced images are suboptimal for definitive diagnosis:
Apical variant of hypertrophic cardiomyopathy
Ventricular non-compaction
Apical thrombus
Complications of MI (e.g. LV aneurysm, pseudoaneurysm, myocardial rupture)
To assist in detection and classification of intracardiac masses
For use in the intensive care unit when standard ECHO is inadequate
To enhance Doppler signals when profiles are suboptimal, in the assessment of diastolic and/or valvular function
To increase confidence of interpretation
In 2009, the European Association of ECHO also made recommendations on the use of contrast agents in ECHO. (8) They made similar recommendations to the American Society of ECHO, primarily using contrast in patients at rest or stress with suboptimal standard ECHO images (when ≥2 contiguous segments are not seen on non-contrast images)
The Canadian Cardiovascular Society and the Canadian Society of ECHO published a position paper in 2007 regarding the use of contrast ECHO. (48) The recommendations made were similar to those for the American and European Societies. They concluded that the addition of contrast ECHO can limit the use of other cardiac imaging technologies which are not as readily available as ECHO.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Study Question
The objective of this economic analysis is to determine the cost effectiveness of stress contrast ECHO for the diagnosis of patients with suspected CAD, when compared to the following cardiac imaging modalities: stress ECHO (without contrast), SPECT, cardiac MRI, and CT angiography. The relative cost-effectiveness of these five non-invasive cardiac imaging technologies was assessed in two patient populations: a) out-patients presenting with stable chest pain; and b) in-patients presenting with acute, unstable chest pain. Note that the term “contrast ECHO” used in the following sections refers to stress ECHO performed with the availability of contrast medium if needed, due to poor image quality.
Economic Analysis Overview
For the two patient populations, decision-analytic cost-effectiveness analyses were conducted to evaluate the relative cost-effectiveness of the five imaging technologies. Two decision analytic models were developed for these patient populations with two reported outcomes: the cost per accurate diagnosis of CAD and the cost per true positive diagnosis of CAD. The physician and hospital costs for the were taken from 2009 Ontario Health Insurance Plan (OHIP) and the Ontario Case Costing Initiative (OCCI) administrative databases.(49;50) A budget impact analysis (BIA) was them performed to assess the effect of replacing a certain proportion of stress contrast ECHO tests with other cost-effective, non-invasive modalities. The costs presented in this BIA were estimated from Ontario data sources from 2009; the volumes of tests performed were estimated from data from fiscal years 2002 to 2008.
Economic Literature Review
The purpose of the systematic review of economic literature was to identify, retrieve, and summarize studies evaluating the cost-effectiveness of selected cardiac imaging tests for the diagnosis of CAD. Medline and the National Health Service Economic Evaluation Database (NHSEED) were searched from their inception up to October 2009. Included studies were those full economic evaluations describing both costs and consequences of a) CT angiography, b) Cardiac MRI, c) SPECT, d) stress ECHO, and e) stress contrast ECHO in CAD diagnosis. Article selection was performed by independent pairs of researchers. Target data for extraction included: study first author and year of publication, imaging tests compared, type of economic analysis, reported costs and outcomes, incremental cost-effectiveness ratio (ICER), currency, and patient characteristics (i.e. known or suspected CAD and risk of CAD). The primary outcome of interest was the ICER of each imaging test in relation to another test of interest.
Literature Search Results
A total of 883 non-duplicate citations were found from the two electronic databases after applying the literature search strategy. Based on the content of their abstracts, 147 full-text articles were retrieved for further assessment of their inclusion/exclusion. Of these, 122 were rejected, leaving 25 articles for inclusion. Following the data extraction process, 13 studies were excluded (16;51-61), with 12 studies being ultimately selected for analysis. (62-73) (74)
Characteristics of Included Studies
From the 12 studies included, eight studies assessed the cost-effectiveness of two of the selected imaging tests (65-68;70;72;73), three evaluated three concomitant technologies (62;69;71), and one study evaluated five technologies.(63)
Five studies were cost-effectiveness analyses, where the most common outcome was cost per correct/successful CAD diagnosis.(62;63;70;72;73) The other seven studies were cost-utility analyses using cost per quality adjusted life years (QALYs) as their primary outcome.(64-69;71) The time-horizon used across the included studies ranged from 30 days to lifetime, with five studies having 25 years or more of follow-up.(64-66;68;72) The remaining studies used 18 months (71), 3 months (73), and 30 days of analytical time horizon.(67) Four studies did not report the time-horizon used in their analysis.(62;63;69;70)
All included studies evaluated at least one form of ECHO against one of the other remaining selected imaging tests.(62-73) The cost-effectiveness of SPECT was studied in nine studies.(62;64-66;68;69;71-73), three studies assessed CT angiography in comparison to stress ECHO or MRI (63;67;70), while cardiac MRI was compared to each of the three other selected imaging tests in two studies.(63;71) No full economic analysis between CT angiography and SPECT was found in the published literature.
Cost-effectiveness results for strategies involving stress contrast ECHO were not found in the systematic literature review performed in this report. Comparative cost-effectiveness was rather evaluated for stress ECHO alone without contrast agents. As a consequence, no further results are presented here for contrast ECHO technology.
Conclusion of systematic review
Overall, CT angiography was found to be cost-effective or cost-saving in all four of the comparisons for that technology; stress ECHO was found cost-effective in eight of the 13 comparisons in which it was evaluated; and SPECT was found cost-effective in three of the nine comparisons. Cardiac MRI was not found to be cost-effective or cost-saving in any of the four comparisons found.
According to the published economic data, CT angiography is often found to be cost-effective when compared to other technologies. SPECT and stress ECHO were also found to be cost-effective in several of the comparative studies examined, while cardiac MRI was not cost-effective in any study. Limitations to these conclusions apply, such as the analyses found in the literature evaluated other forms of the selected cardiac imaging tests which might change the proposed relative cost-effectiveness.
Decision analytic Cost Effectiveness Analysis
Design
This study was designed as a cost effectiveness analysis, with primary results reported as incremental cost per true positive diagnosis, or incremental cost per accurate diagnosis.
Target Population and Perspective
Two populations were defined for evaluating the cost-effectiveness of an accurate diagnosis (i.e. true positive and true negative diagnoses) of CAD: a) out-patients presenting with stable chest pain; and b) in-patients presenting with acute, unstable chest pain. The first population was defined as persons presenting with stable chest pain, with an intermediate risk of CAD following physical examination and a graded exercise test, as defined by the American College of Cardiology / American Heart Association 2002 Guideline Update for the Management of Patients with Chronic Stable Angina.(75) The second population was defined as persons presenting to emergency for acute, unstable chest pain, and who are admitted to hospital, as defined by the American College of Cardiology / American Heart Association 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction.(76)
The analytic perspective was that of the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Comparators and Parameter Estimates
The imaging technologies that were compared in the current cost-effectiveness analysis included: CT angiography, stress ECHO (with and without contrast mediums, cardiac perfusion stress MRI, and attenuation-corrected SPECT. Test characteristic estimates (i.e., specificity, sensitivity, accuracy) for each cardiac imaging technology were obtained from the systematic review and meta-analysis conducted by MAS and the MOHLTC. Table 18 shows a list of the parameters with corresponding 95% confidence intervals used for both the outpatient and inpatient decision-analytic cost-effectiveness models.
Table 18: Summary parameter estimates for contrast ECHO tests.
| Pooled Diagnostic Accuracy | Point Estimate | 95% Lower | 95% Upper |
|---|---|---|---|
| CAD diagnosis: Sensitivity CAD diagnosis: Specificity |
0.844 0.800 |
0.792 0.725 |
0.896 0.874 |
| Additional time for test (compared to GXT) | Average | Low | High |
| Inpatient population: Additional days for test | 1.5 | 1.0 | 2.0 |
| Uninterpretable test result | Average | Low | High |
| Outpatient population: % of tests that are uninterpretable Inpatient population: % of tests that are uninterpretable |
4.3% 4.0% |
1.0% 1.0% |
5.0% 5.0% |
Note: Sensitivity and specificity estimates are taken from the effectiveness literature review of stress contrast ECHO. Other estimates are based on consultations with experts in cardiology.
The average wait-time for each cardiac imaging test was measured as the additional days needed to wait for a non-invasive test compared to the average wait time for a typical graded exercise stress test (GXT). The proportion of tests deemed uninterpretable by expert opinion is shown with a corresponding range of high and low values. The probability of receiving pharmacological stress versus exercise stress is not listed, but reported here for completeness: approximate values of 30% for the stable, outpatient population and 80% for the unstable, inpatient population.
Time Horizon & Discounting
The time horizon for both decision-analytic models (i.e. for outpatient and inpatient populations) was the time required to determine an accurate, or true positive diagnosis of CAD. As a result, the actual time taken to determine the CAD status of patients may differ across non-invasive test strategies.
Model Structure and Outcomes
Figure 11 provides a simplified illustration of the decision-analytic model structure used for the outpatient and inpatient populations. The following two simplifying assumptions were made for the models:
Figure 11: Decision analytic model used to evaluate the cost-effectiveness of cardiac imaging technologies for the diagnosis of CAD.
When results of the first cardiac imaging test are un-interpretable, a patient will undergo a second cardiac test, This will be one of the four remaining tests that were not used as the first test.
Should a second test be required, the type of stress (pharmacological or exercise) that a patient receives will be the same type of stress as used in the first test.
The short-term outcome presented in this report focuses on an accurate diagnosis of CAD (i.e. true positive and true negative test results). A second outcome of true positive diagnosis was examined for the two models, with results reported in The Relative Cost-effectiveness of Five Non-invasive Cardiac Imaging Technologies for Diagnosing Coronary Artery Disease in Ontario. (74)
Sensitivity Analyses
Various sensitivity analyses were conducted for the outpatient and inpatient populations. First, the prevalence of CAD was varied from 5% to 95% in 5% increments, while all other model estimates were held constant. Willingness-to-pay (WTP) was also varied and a range of results were presented. Second, one-way sensitivity analyses were conducted in which selected estimates were varied over plausible ranges. The varied parameters included sensitivity and specificity estimates, wait times for imaging tests performed in hospital, as well as the costs of CT angiography, ECHO tests, and cardiac MRI. A third series of sensitivity analyses was conducted that specifically addressed the possibility of unavailable imaging technologies.
Resource Use and Costs
Resource use and costs were derived from Ontario data sources: the OHIP and OCCI administrative databases.(49;50) The cost of conducting each cardiac test was calculated as the sum of the test’s respective professional fees and technical fees, as described in the Ontario Schedule of Benefits and listed in Table 19.
Table 19: List of cardiac imaging tests and associated OHIP 2009 costs.
| Technology | List of professional fees | Subtotal | List of technical fees | Subtotal | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac CT | Fee code Cost |
X125 $89.20 |
X417 $64.00 |
$153.20 |
Imputed $336.52 |
$336.52 | $489.72 | ||||||
| Cardiac MRI (dobutamine stress with gadolinium contrast) | Fee code Multiplier Cost |
X441 1.0 $75.55 |
X445 3.0 $37.80 |
X487 1.0 $37.75 |
G319 1.0 $62.65 |
$289.35 | Imputed 1.0 $463.06 |
G315 1.0 $33.65 |
G174 1.0 $37.00 |
$533.71 | $823.06 | ||
| Cardiac SPECT (exercise stress) | Fee code Cost |
J866 $28.70 |
J811 $55.30 |
J807 $47.00 |
G319 $62.65 |
$193.65 | J866 $44.60 |
J811 $97.55 |
J807 $223.15 |
G315 $33.65 |
$398.95 | $592.60 | |
| Cardiac SPECT (dobutamine stress) | Fee code Cost |
J866 $28.70 |
J811 $55.30 |
J807 $47.00 |
G319 $62.65 |
$193.65 | J866 $44.60 |
J811 $97.55 |
J807 $223.15 |
G315 $33.65 |
G174 $37.00 |
$435.95 | $629.60 |
| Cardiac SPECT (dipyramidole stress) | Fee code Cost |
J866 $28.70 |
J811 $55.30 |
J807 $47.00 |
G112 $75.00 |
$206.00 | J866 $44.60 |
J811 $97.55 |
J807 $223.15 |
G111 $41.10 |
$406.40 | $612.40 | |
| ECHO (exercise stress) | Fee code Cost |
G571 $74.10 |
G578 $36.90 |
G575 $17.45 |
G319 $62.65 |
$191.10 | G570 $76.45 |
G577 $45.15 |
G574 $16.45 |
G315 $33.65 |
$171.70 | $362.80 | |
| ECHO (dobutamine stress) | Fee code Cost |
G571 $74.10 |
G578 $36.90 |
G575 $17.45 |
G319 $62.65 |
$191.10 | G570 $76.45 |
G577 $45.15 |
G574 $16.45 |
G315 $33.65 |
G174 $37.00 |
$208.70 | $399.80 |
| ECHO (dipyramidole stress) | Fee code Cost |
G571 $74.10 |
G578 $36.90 |
G575 $17.45 |
G112 $75.00 |
$203.45 | G570 $76.45 |
G577 $45.15 |
G574 $16.45 |
G111 $41.10 |
$179.15 | $382.60 | |
Notes: Fee codes are taken from the 2009 OHIP fee schedule.(50) Imputed technical fees were based on the proportion of average technical fees associated with above ECHO and SPECT fee code combinations. For cardiac SPECT and ECHO stress tests, an average test cost was calculated using dobutamine and dipyramidole fee codes.
Note that for ECHO tests with available contrast agent, the cost for the contrast medium was added whenever the contrast was used in the event of uninterpretable ECHO test result. The cost of this contrast medium was estimated as $170 per vial (single use) through consultation with industry experts. Only this cost was added to the base test cost of contrast ECHO. In general, where an imaging test result was uninterpretable, an additional cost of follow-up with the patient (physician fee) was incurred, as well as the cost for conducting another cardiac imaging test. For out-patients presenting with stable chest pain, a consultation professional fee of $30.60 (OHIP code A608 for “partial assessment”) was used after an uninterpretable test result (one time cost).
In the case of patients presenting with acute, unstable chest pain, costs for inpatient hospitalization were also included in the model. The total cost of hospitalization was calculated based on the average wait time for each cardiac imaging test and a cost per diem for each day spent in hospital. An additional consultation fee was also used only for the inpatient population: $29.20 (OHIP code C602 for “subsequent visit- first five weeks”) was used for each inpatient day spent in hospital.
Willingness-To-Pay
The WTP must be determined by the MOHLTC. As such, all reasonable WTP values presented in the Results and Discussion section are interpreted at two WTP ‘anchors’ representing the estimated cost of the most expensive non-invasive test considered in our model (cardiac MRI perfusion, $804) and the estimated cost of a coronary angiography ($1,433). These anchors are only intended to guide discussion.
Note that the following points might be useful in determining the WTP:
An “accurate diagnosis” of CAD can be obtained through a coronary angiography for $1,433. It would thus be reasonable to expect the WTP for an accurate diagnosis through a non-invasive test to resemble this amount; however, an accurate diagnosis does not include the value or benefit of providing additional diagnostic or prognostic information from either non-invasive imaging or coronary angiography
The MOHLTC is currently willing to pay up to $804 for a non-invasive test with less-than-perfect diagnostic accuracy. Its willingness to pay for an accurate diagnosis from such a test thus appears to be greater than $804.
While coronary angiography is invasive, the other tests are non-invasive and would presumably be of greater value (i.e., incur a higher premium). These tests do, however, impose risks not applicable to coronary angiography, such as increased radiation exposure and adverse reaction to contrast agents
These tests are not perfectly accurate. An accurate diagnosis from such a test may be valued less than one from a coronary angiography
Results and Discussion
As shown in Tables 20 and 21, stress contrast ECHO was the least costly strategy in both stable outpatients and acute inpatients. In stable outpatients, however, CT angiography showed greater effectiveness and appeared to dominate all strategies, other than contrast ECHO. Whether contrast ECHO or CT angiography is cost-effective for such patients thus depends on the willingness-to-pay (WTP) for an accurate diagnosis of CAD, which we have considered at two anchors: $804 and $1,433 per accurate diagnosis. Since CT angiography has an ICER of $1,527 per accurate diagnosis compared to contrast ECHO, contrast ECHO appears to be more cost-effective than CT angiography at either anchor. In acute inpatients, CT angiography did not appear cost-effective in the base case analysis due to its assumed longer hospital wait time. As such, contrast ECHO appeared clearly cost-effective at both anchors.
Table 20: Cost-effectiveness analysis base case results for stable outpatients.
| Technology | Cost (C) | Δ Cost | Effect (E) | Δ Effect | C / E | ICER |
|---|---|---|---|---|---|---|
| Stress contrast ECHO | $433.49 | 81.83% | $530 | N/A | ||
| CT angiography | $517.73 | $84.24 | 87.35% | 5.52% | $593 | $1,527 |
| Stress ECHO | $551.58 | 81.06% | $680 | (Dominated) | ||
| SPECT | $634.63 | 82.80% | $766 | (Dominated) | ||
| Cardiac MRI | $835.47 | 85.15% | $981 | (Dominated) |
Table 21: Cost-effectiveness analysis base case results for acute inpatients.
| Technology | Cost (C) | Δ Cost | Effect (E) | Δ Effect | C / E | ICER |
|---|---|---|---|---|---|---|
| Stress contrast ECHO | $1,794.58 | 81.94% | $2,190 | N/A | ||
| SPECT | $1,982.91 | $188.32 | 83.92% | 1.99% | $2,363 | $9,489 |
| Stress ECHO | $2,550.87 | 81.53% | $3,129 | (Dominated) | ||
| CT angiography | $3,267.39 | $1,284.48 | 87.49% | 3.56% | $3,735 | $36,055 |
| Cardiac MRI | $4,918.02 | 85.55% | $5,749 | (Dominated) |
The analysis of the prevalence of CAD revealed that, in stable outpatients, contrast ECHO was more cost-effective at lower prevalence rates of CAD. At the two WTP anchors of $804 and $1433 per accurate diagnosis, contrast ECHO was considered cost-effective in the stable outpatient population when the prevalence of CAD was less than 70% or 50% respectively, with CT angiography appearing cost-effective otherwise. In acute inpatients, contrast ECHO appeared cost-effective at both WTP anchors at any prevalence of CAD. When the hospital wait times were assumed to be normalized across all tests, contrast ECHO still appeared cost-effective, with the sole exception that CT angiography appeared cost-effective only at the higher WTP anchor and when the prevalence of CAD was greater than 80%.
Contrast ECHO was found to be generally cost-effective in both stable outpatients and acute inpatients. In the stable outpatient population, contrast ECHO was more cost-effective at lower prevalence rates of CAD, while in the acute inpatient population stress ECHO appeared to be the most cost-effective strategy at any prevalence of CAD.
Budget Impact Analysis
The budget impact analysis (BIA) was performed taking the perspective of the MOHLTC and includes both physician and hospital (clinic) costs of non-invasive cardiac imaging tests. Volumes of cardiac tests in Ontario were taken from administrative databases (OHIP, DAD, NACRS) for fiscal years 2004 to 2008. (74) The following technologies were considered in the current BIA for the diagnosis of CAD: ECHO (including both stress and stress with contrast agent available), nuclear cardiac imaging (including MPI and SPECT tests), cardiac MRI, and CT angiography.
In the current BIA, the effect of moving a certain proportion of the volume of specific tests to another, substitute technology was assessed for various scenarios. These scenarios are presented irrespective of whether a technology was found to be cost-effective and are reported as general reference tables. To summarize briefly, stress contrast ECHO tests are the second least expensive of the compared cardiac imaging modalities; stress ECHO without contrast is the least expensive. When the volume of contrast stress ECHO tests is shifted to other technologies, all scenarios result in higher projected costs, except for standard stress ECHO tests without contrast agent available. If 25% of the contrast stress ECHO tests is moved to other imaging technologies, ensuing projected costs would be higher (excluding standard stress ECHO): from a small cost difference of about $14.6K per year for CT angiography testing to a large difference of $95.3K for cardiac MRI testing. The largest possible cost difference corresponds to replacing 50% of contrast stress ECHO tests with cardiac MRI imaging ($190.7K per year); the smallest cost difference occurs for replacing 5% of contrast stress ECHO tests with CT angiography ($2.9K per year), excluding standard stress ECHO.
Appendices
Appendix 1: Literature Search Strategies
Search 1.
Search date: June 18, 2009
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1950 to June Week 2 2009>
Search Strategy
exp Ventricular Dysfunction, Left/ (14322)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).ti,ab. (12423)
exp Heart Failure/ or exp Myocardial Infarction/ (181961)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).ti,ab. (84862)
heart attack.mp. (2167)
((myocardi* or heart or cardiac) adj2 infarct*).ti,ab. (113525)
or/1-6 (257911)
exp Contrast Media/ (69972)
(contrast adj (enhancement or dye* or medium* or agent* or media or material*)).ti,ab. (37005)
exp Microbubbles/ (926)
exp microspheres/ (17710)
(microsphere* or microbubble*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (27675)
exp Fluorocarbons/ (5813)
(fluorocarbon* or perflutren or perfluoropropane or octafluoropropane or aerosomes).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (7752)
(Luminity or albunex or Cardiosphere or definity or Optison or levovist or SonoVue or imagify).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1514)
or/8-15 (117239)
7 and 16 (3287)
limit 17 to (english language and humans) (1592)
limit 18 to (case reports or comment or editorial or letter) (235)
18 not 19 (1357)
Database: EMBASE <1980 to 2009 Week 24>
Search Strategy
exp Heart Failure/ or exp Heart Infarction/ (224790)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).ti,ab. (11388)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).ti,ab. (73359)
((myocardi* or heart or cardiac) adj2 infarct*).ti,ab. (88382)
heart attack.mp. (1584)
or/1-5 (256589)
exp Contrast Medium/ (55404)
exp contrast enhancement/ (35101)
(contrast adj (enhancement or dye* or medium* or agent* or media or material*)).ti,ab. (30042)
exp Microbubble/ (803)
exp Microsphere/ (9644)
(microsphere* or microbubble*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (20092)
exp Perflutren/ (423)
(fluorocarbon* or perflutren or perfluoropropane or octafluoropropane or aerosomes).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (4069)
(Cardiosphere or definity or Optison or levovist or SonoVue or imagify or luminity or albunex).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2614)
or/7-15 (105550)
6 and 16 (4812)
limit 17 to (human and english language) (2776)
limit 18 to (editorial or letter or note) (210)
Case Report/ (1040274)
18 not (19 or 20) (2083)
Search 2.
Search date: July 27, 2009
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1950 to July Week 3 2009>
Search Strategy
exp Myocardial Ischemia/ (300035)
(coronary adj2 arter* disease*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (57864)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or isch?emi* or calci* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. (260088)
(myocardi* adj2 hibernat*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (840)
(stenocardia* or angina).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (53177)
heart attack*.mp. (2838)
exp Heart Failure/ (65605)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. (106901)
exp Ventricular Dysfunction, Left/ (14642)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (22471)
or/1-10 (461388)
exp Contrast Media/ (70603)
(contrast adj (enhancement or dye* or medium* or agent* or media or material*)).ti,ab. (37348)
exp Microbubbles/ (961)
exp microspheres/ (17851)
(microsphere* or microbubble*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (27902)
exp Fluorocarbons/ (5863)
(fluorocarbon* or perflutren or perfluoropropane or octafluoropropane or aerosomes).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (7814)
(Luminity or albunex or Cardiosphere or definity or Optison or levovist or SonoVue or imagify).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1531)
or/12-19 (118240)
11 and 20 (7586)
limit 21 to (english language and humans and yr=“2004 -Current”) (1566)
limit 22 to (case reports or comment or editorial or letter) (283)
22 not 23 (1283)
Database: EMBASE <1980 to 2009 Week 30>
Search Strategy
exp ischemic heart disease/ (236752)
exp coronary artery disease/ (87656)
exp stunned heart muscle/ (1511)
(coronary adj2 arter* disease*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (71044)
((myocardi* or heart or cardiac or coronary) adj2 (viable or viability or perfusion or function or ischemi* or atheroscleros* or arterioscleros* or infarct* or occlu* or stenos* or thrombosis)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (273922)
(myocardi* adj2 hibernat*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1050)
(stenocardia* or angina).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (46078)
heart attack*.mp. (2015)
exp heart failure/ (124113)
((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (106828)
exp heart left ventricle failure/ (9231)
(left adj2 ventric* adj2 (dysfunction* or failure or insufficienc*)).mp. (15978)
or/1-12 (427322)
exp Contrast Medium/ (56006)
exp contrast enhancement/ (35600)
(contrast adj (enhancement or dye* or medium* or agent* or media or material*)).ti,ab. (30274)
exp Microbubble/ (838)
exp Microsphere/ (9740)
(microsphere* or microbubble*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (20251)
exp Perflutren/ (435)
(fluorocarbon* or perflutren or perfluoropropane or octafluoropropane or aerosomes).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (4093)
(Cardiosphere or definity or Optison or levovist or SonoVue or imagify or luminity or albunex).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2643)
or/14-22 (106611)
13 and 23 (9575)
limit 24 to (human and english language and yr=“2004 -Current”) (3133)
limit 25 to (editorial or letter or note) (266)
case report/ (1046017)
25 not (26 or 27) (2322)
Appendix 2: QUADAS Assessing Quality of Studies in the Analysis
Table A1: Quality of studies investigating the role of contrast ECHO in patients with suspected CAD.
| QUADAS Tool | Hayat, 2008 | Aggeli, 2007 | Miszalski-Jamka, 2007 | Moir, 2007 | Osorio, 2007 | Karavidas, 2006 | Moir, 2005 - 1 | Moir, 2005 - 2 | Senior, 2005 | Moir, 2004 | Peltier, 2004 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the spectrum of patients representative of those who will receive test?* | No | No | No | No | No | No | No | No | No | No | No |
| 2. Were selection criteria clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Is the reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is time period between the reference standard and index test short enough to be reasonably sure the target condition did not change between tests? | Unclear | Yes (1 month) |
Yes (15 days) |
Unclear | Unclear | Yes (1 week) |
Unclear | Unclear | Yes (within hosp adm) |
Unclear | Unclear |
| 5. Did whole sample or random selection of sample receive verification using the reference standard of dx? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did patients receive the same reference standard regardless of index test result? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the reference standard independent of index test (index test did not form part of the reference standard)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was execution of index test described in sufficient detail to permit replication of test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was execution of the reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Were index test results interpreted without knowledge of results of the reference standard? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. Were the reference standard results interpreted without knowledge of results of index standard? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were same clinical data available when test results were interpreted as would be available when test is used in practice? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13. Were uninterpretable/ intermediate test results reported? | N/A | N/A | Yes | N/A | Yes | N/A | Yes | N/A | N/A | Yes | Yes |
| 14. Were withdrawals from study explained? | N/A | N/A | Yes | N/A | Yes | N/A | Yes | N/A | N/A | Yes | Yes |
Patients with suboptimal ECHO results are candidates for contrast echo. In the majority of studies, patients were given contrast echo, regardless of standard ECHO results.
Table A2: Quality of studies investigating the role of contrast ECHO in patients with suspected or known CAD.
| QUADAS Tool | Lonnebakken, 2009 | Aggeli, 2008 | Lipiec, 2008 | Plana, 2008 | Tsutsui, 2008 | Hu, 2007 | Korosoglou, 2006 | Lin 2006 | Elhendy, 2005 | Tsutsui, 2005 | Chiou, 2004 | Elhendy, 2004 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the spectrum of patients representative of patients who will receive test?* | No | No | No | No | No | No | No | No | No | No | No | No |
| 2. Were selection criteria clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Is the reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Is time period between the reference standard and index test short enough to be reasonably sure the target condition did not change between tests? | Unclear | Yes (1 month) |
Yes (2 weeks) |
Yes (1 month) |
Yes (1 month) |
Yes (1 month) |
Yes (3 months) |
Yes (2 weeks) |
Yes (1 month) |
Yes (1 month) |
Yes (1 week) |
Yes (1 month) |
| 5. Did whole sample or random selection of sample receive verification using the reference standard of diagnosis? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did patients receive the same reference standard regardless of index test result? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the reference standard independent of index test (index test did not form part of the reference standard)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was execution of index test described in sufficient detail to permit replication of test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was execution of the reference standard described in sufficient detail to permit its replication? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Were index test results interpreted without knowledge of results of the reference standard? | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. Were the reference standard results interpreted without knowledge of results of index standard? | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12. Were same clinical data available when test results were interpreted as would be available when test is used in practice? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 13. Were uninterpretable / intermediate test results reported? | Yes | N/A | Yes | Unclear | N/A | N/A | No | Yes | N/A | N/A | Unclear | No |
| 14. Were withdrawals from study explained? | Yes | N/A | N/A | No | N/A | N/A | N/A | N/A | N/A | N/A | Yes | Yes |
Patients with suboptimal ECHO results are candidates for using contrast echo. In the majority of the studies all patients were given contrast echo, regardless of standard ECHO results.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Stress echocardiography with contrast for the diagnosis of coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 June [cited YYYY MM DD]; 10(10) 1-59. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/cardiac_contrast_echo_20100528.pdf
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit
http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-1964-9 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Tables
| Table 1: Quality of evidence of included studies |
| Table 2: GRADE quality of evidence: stress contrast ECHO vs. coronary angiography for the diagnosis of CAD (patients with suspected CAD) – Diagnostic test as a surrogate for patient outcome measures |
| Table 3: GRADE quality of evidence: stress contrast ECHO vs. coronary angiography for the diagnosis of CAD (known or suspected) – Diagnostic test as a surrogate for patient outcome measures |
| Table 4. Studies comparing the accuracy of stress contrast ECHO vs. coronary angiography for the detection of CAD |
| Table 5: Diagnostic accuracy of stress contrast ECHO in patients with suspected CAD |
| Table 6: Studies comparing the accuracy of stress contrast ECHO vs. coronary angiography for the detection of CAD |
| Table 7: Diagnostic accuracy of stress contrast ECHO in patients with suspected or known CAD |
| Table 8: Studies comparing stress contrast ECHO vs. Technetium 99m SPECT for CAD detection using coronary angiography as the reference standard |
| Table 9: Diagnostic accuracy of stress contrast ECHO vs. Technetium 99m SPECT |
| Table 10: Studies comparing stress contrast ECHO vs. Thallium SPECT for CAD detection using coronary angiography as the reference standard |
| Table 11: Diagnostic accuracy of stress contrast ECHO vs. Thallium SPECT |
| Table 12: Studies comparing stress contrast ECHO vs. stress ECHO for the detection of CAD using coronary angiography as the reference standard |
| Table 13: Diagnostic accuracy of stress contrast ECHO vs. stress ECHO |
| Table 14: Subgroup results reported after contrast ECHO from Kurt et al. (35). |
| Table 15: Results of Plana et al. (22) comparing suboptimal ECHO vs. interpretable ECHO, with and without contrast |
| Table 16: Studies investigating the safety of microsphere contrast agents for ECHO |
| Table 17: Adverse events reported in safety studies |
| Table 18: Summary parameter estimates for contrast ECHO tests |
| Table 19: List of cardiac imaging tests and associated OHIP 2009 costs |
| Table 20: Cost-effectiveness analysis base case results for stable outpatients |
| Table 21: Cost-effectiveness analysis base case results for acute inpatients |
| Table A1: Quality of studies investigating the role of contrast ECHO in patients with suspected CAD |
| Table A2: Quality of studies investigating the role of contrast ECHO in patients with suspected or known CAD |
List of Abbreviations
- AUC
Area under the curve
- BIA
Budget Impact Analysis
- CA
Coronary angiography
- CAD
Coronary artery disease
- CCT
Cardiac computed tomography (CT angiography)
- CI
Confidence interval(s)
- CMG
Case Mix Group
- ECHO
Echocardiography
- GXT
Graded exercise test
- ICER
Incremental cost-Effectiveness ratio
- LR
Likelihood ratio
- LYS
Life years saved
- MAS
Medical Advisory Secretariat
- MOHLTC
Ministry of Health and Long-Term Care
- MPA
Myocardial perfusion analysis
- MPI
Myocardial Perfusion Imaging
- OCCI
Ontario Case Costing Initiative
- OR
Odds ratio
- OHIP
Ontario Health Insurance Plan
- OHTAC
Ontario Health Technology Advisory Committee
- QALY
Quality-Adjusted Life Years
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SPECT
Single-photon emission computed tomography
- SROC
Summary receiver operating characteristic
- USD
United States dollars
- WMA
Wall motion analysis
- WTP
Willingness-to-pay
References
- 1.Lindner JR, Sklenar J. Placing faith in numbers: Quantification of perfusion with myocardial contrast echocardiography. J Am Coll Cardiol. 2004;43(10):1814–6. doi: 10.1016/j.jacc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Oh J.K, Seward J.B, Tajik A.J. Contrast echocardiography. Philadelphia: Lippincott Williams & Wilkins. 2006:245–250. In: The Echo Manual. [Google Scholar]
- 3.Hayat SA, Dwivedi G, Jacobsen A, Lim TK, Kinsey C, Senior R. Effects of left bundle-branch block on cardiac structure, function, perfusion, and perfusion reserve: implications for myocardial contrast echocardiography versus radionuclide perfusion imaging for the detection of coronary artery disease. Circulation. 2008;117:1832–41. doi: 10.1161/CIRCULATIONAHA.107.726711. [DOI] [PubMed] [Google Scholar]
- 4.Aggeli C, Christoforatou E, Giannopoulos G, Roussakis G, Kokkinakis C, Barbetseas J, et al. The diagnostic value of adenosine stress-contrast echocardiography for diagnosis of coronary artery disease in hypertensive patients: comparison to Tl-201 single-photon emission computed tomography. Am J Hypertens. 2007;20(5):533–8. doi: 10.1016/j.amjhyper.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Korosoglou G, Dubart A-E, DaSilva GC, Labadze N, Hardt S, Hansen A, et al. Real-time myocardial perfusion imaging for pharmacologic stress testing: added value to single photon emission computed tomography. Am Heart J. 2006;151(1):131–8. doi: 10.1016/j.ahj.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 6.Chelliah RK, Senior R. Contrast echocardiography: an update. Curr Cardiol Rep. 2009;11:216–24. doi: 10.1007/s11886-009-0031-y. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21(5):409–16. doi: 10.1016/j.echo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, et al. Contrast echocardiography: Evidence-based recommendations by European Association of Echocardiography. Eur J Echocardiogr. 2009;10(2):194–212. doi: 10.1093/ejechocard/jep005. [DOI] [PubMed] [Google Scholar]
- 9.Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig M, et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr. 2008;21(11):1179–201. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Pai M, McCulloch M, Colford J. [[updated 2004; cited 2009 Nov 23].]. STATA meta-analysis commands version 6 [Internet] Available from: http://www.medepi.net/meta/software/STATA_Metaanalysis_commands_V6_March2004.pdf .
- 11.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:1–13. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schunemann AHJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. GRADE: grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltier M, Vancraeynest D, Pasquet A, Ay T, Roelants V, D’hondt A-M, et al. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography. J Am Coll Cardiol. 2004;43(2):257–64. doi: 10.1016/j.jacc.2003.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Moir S, Haluska B, Jenkins C, McNab D, Marwick TH. Comparison of specificity of quantitative myocardial contrast echocardiography for diagnosis of coronary artery disease in patients with versus without diabetes mellitus. Am J Cardiol. 2005;96(2):187–92. doi: 10.1016/j.amjcard.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Moir S, Haluska BA, Jenkins C, Fathi R, Marwick TH. Incremental benefit of myocardial contrast to combined dipyridamole- exercise stress echocardiography for the assessment of coronary artery disease. Circulation. 2004;110(9):1108–13. doi: 10.1161/01.CIR.0000139905.47128.9F. [DOI] [PubMed] [Google Scholar]
- 16.Moir S, Shaw L, Haluska B, Jenkins C, Marwick TH. Left ventricular opacification for the diagnosis of coronary artery disease with stress echocardiography: an angiographic study of incremental benefit and cost-effectiveness. Am Heart J. 2007;154(3):510–8. doi: 10.1016/j.ahj.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Moir S, Haluska BA, Jenkins C, McNab D, Marwick TH. Myocardial blood volume and perfusion reserve responses to combined dipyridamole and exercise stress: a quantitative approach to contrast stress echocardiography. J Am Soc Echocardiogr. 2005;18:1187–93. doi: 10.1016/j.echo.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Miszalski-Jamka T, Kuntz-Hehner S, Schmidt H, Jost P, Luderitz B, Omran H. Diagnosis of ischaemic heart disease by myocardial contrast echocardiography during supine bicycle stress. Kardiol Pol. 2006;64(4):355–61. [PubMed] [Google Scholar]
- 19.Miszalski-Jamka T, Kuntz-Hehner S, Schmidt H, Hammerstingl C, Tiemann K, Ghanem A, et al. Real time myocardial contrast echocardiography during supine bicycle stress and continuous infusion of contrast agent. Cutoff values for myocardial contrast replenishment discriminating abnormal myocardial perfusion. Echocardiography. 2007;24(6):638–48. doi: 10.1111/j.1540-8175.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 20.Osorio AFF, Tsutsui JM, Kowatsch I, Guerra VC, Ramires JAF, Lemos PA, et al. Evaluation of Blood Flow Reserve in Left Anterior Descending Coronary Artery Territory by Quantitative Myocardial Contrast and Doppler Echocardiography. J Am Soc Echocardiogr. 2007;20(6):709–16. doi: 10.1016/j.echo.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Karavidas AI, Matsakas EP, Lazaros GA, Brestas PS, Avramidis DA, Zacharoulis AA, et al. Comparison of myocardial contrast echocardiography with SPECT in the evaluation of coronary artery disease in asymptomatic patients with LBBB. Int J Cardiol. 2006;112:334–40. doi: 10.1016/j.ijcard.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Senior R, Janardhanan R, Jeetley P, Burden L. Myocardial contrast echocardiography for distinguishing ischemic from nonischemic first-onset acute heart failure. Circulation. 2005;112:1587–93. doi: 10.1161/CIRCULATIONAHA.104.530089. [DOI] [PubMed] [Google Scholar]
- 23.Plana JC, Mikati IA, Dokainish H, Lakkis N, Abukhalil J, Davis R, et al. A randomized crossover study for evaluation of the effect of image optimization with contrast on the diagnostic accuracy of dobutamine echocardiography in coronary artery disease. JACC Cardiovasc Imaging. 2008;1(2):145–52. doi: 10.1016/j.jcmg.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Elhendy A, Tsutsui JM, O’Leary EL, Xie F, McGrain AC, Porter TR. Noninvasive diagnosis of coronary artery disease in patients with diabetes by dobutamine stress real-time myocardial contrast perfusion imaging. Diabetes Care. 2005;28(7):1662–7. doi: 10.2337/diacare.28.7.1662. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsui JM, Xie F, Cloutier D, Kalvaitis S, Elhendy A, Porter TR. Real-time dobutamine stress myocardial perfusion echocardiography predicts outcome in the elderly. Eur Heart J. 2008;29:377–85. doi: 10.1093/eurheartj/ehm445. [DOI] [PubMed] [Google Scholar]
- 26.Hu S-J, Liu S-X, Katus HA, Luedde M. The value of contrast dobutamine stress echocardiography in detecting coronary artery disease in overweight and obese patients. Can J Cardiol. 2007;23(11):885–9. doi: 10.1016/s0828-282x(07)70844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggeli C, Giannopoulos G, Roussakis G, Christoforatou E, Marinos G, Toli C, et al. Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for the detection of ischaemia in 5250 studies. Heart. 2008;94:1571–7. doi: 10.1136/hrt.2007.135145. [DOI] [PubMed] [Google Scholar]
- 28.Lonnebakken MT, Bleie O, Strand E, Staal EM, Nygard OK, Gerdts E. Myocardial contrast echocardiography in assessment of stable coronary artery disease at intermediate dobutamine-induced stress level. Echocardiography. 2009;26(1):52–60. doi: 10.1111/j.1540-8175.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 29.Lipiec P, Wejner-mik P, Krzeminska-pakula M, Kusmierek J, Plachcinska A, Szuminski R, et al. Accelerated stress real-time myocardial contrast echocardiography for the detection of coronary artery disease: comparison with 99mTc single photon emission computed tomography. J Am Soc Echocardiogr. 2008;21(8):941–7. doi: 10.1016/j.echo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Lipiec P, Wejner-mik P, Krzeminska-pakula M, Kusmierek J, Plachcinska A, Szuminski R, et al. Detection of single-vessel coronary artery disease by dipyridamole stress echocardiography: No longer a problem? Clin Physiol Funct Imaging. 2009;29(2):151–7. doi: 10.1111/j.1475-097x.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin SL, Chiou K-R, Huang W-C, Peng NJ, Tsay D-G, Liu C-P. Detection of coronary artery disease using real-time myocardial contrast echocardiography: a comparison with dual isotope resting thallium-201/stress technetium-99m sestamibi single-photon emission computed tomography. Heart Vessels. 2006;21:226–35. doi: 10.1007/s00380-005-0890-0. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui JM, Xie F, O’Leary EL, Elhendy A, Anderson JR, McGrain AC, et al. Diagnostic accuracy and prognostic value of dobutamin stress myocardial contrast echocardiography in patients with suspected acute coronary syndromes. Echocardiography. 2005;22(6):487–95. doi: 10.1111/j.1540-8175.2005.40037.x. [DOI] [PubMed] [Google Scholar]
- 33.Chiou K-R, Huang W-C, Lin S-L, Hsieh P-L, Liu C-P, Tsay D-G, et al. Real-time dobutamine stress myocardial contrast echocardiography for detecting coronary artery disease: Correlating abnormal wall motion and disturbed perfusion. Can J Cardiol. 2004;20(12):1237–43. [PubMed] [Google Scholar]
- 34.Elhendy A, O’Leary EL, Xie F, McGrain AC, Anderson JR, Porter TR. Comparative accuracy of real-time myocardial contrast perfusion imaging and wall motion analysis during dobutamine stress echocardiography for the diagnosis of coronary artery disease. J Am Coll Cardiol. 2004;44(11):2185–91. doi: 10.1016/j.jacc.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 35.Dijkmans PA, Senior R, Becher H, Porter TR, Wei K, Visser CA, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol. 2006;48(11):2168–77. doi: 10.1016/j.jacc.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 36.Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53(9):802–10. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Dolan MS, Riad K, El-Shafei A, Puri S, Tamirisa K, Bierig M, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001;142(5):908–15. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 38.Kitzman DW, Goldman ME, Gillam LD, Cohen JL, Aurigemma GP, Gottdiener JS. Efficacy and safety of the novel ultrasound contrast agent perflutren (Definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am J Cardiol. 2000;86:669–74. doi: 10.1016/s0002-9149(00)01050-x. [DOI] [PubMed] [Google Scholar]
- 39.Lantheus Medical Imaging. Definity. [[updated 2009; cited 2010 Mar 4]]. [Internet] Available from: http://www.definityimaging.com/main.html?#indication .
- 40.Kusnetzky LL, Khalid A, Khumri TM, Moe TG, Jones PG, Main ML. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent. J Am Coll Cardiol. 2008;51(17):704–6. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Dolan MS, Gala SS, Dodla S, Abdelmoneim SS, Xie F, Cloutier D, et al. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography. J Am Coll Cardiol. 2009;53(1):32–8. doi: 10.1016/j.jacc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 42.Anantharam B, Chahal N, Chelliah R, Ramzy I, Gani F, Senior R. Safety of contrast in stress echocardiography in stable patients and in patients with suspected acute coronary syndrome but negative 12-hour troponin. Am J Cardiol. 2009;104(1):14–8. doi: 10.1016/j.amjcard.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Gabriel RS, Smyth YM, Menon V, Klein AL, Grimm RA, Thomas JD, et al. Safety of ultrasound contrast agents in stress echocardiography. Am J Cardiol. 2008;102:1269–72. doi: 10.1016/j.amjcard.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 44.Main ML, Ryan AC, Davis TE, Albano MP, Kusnetzky LL, Hibberd M. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients) Am J Cardiol. 2008;102:1742–6. doi: 10.1016/j.amjcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Shaikh K, Chang SM, Peterson L, Rosendahl-Garcia K, Quinones MA, Nagueh SF, et al. Safety of contrast administration for endocardial enhancement during stress echocardiography compared with noncontrast stress. Am J Cardiol. 2008;102:1444–50. doi: 10.1016/j.amjcard.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Timperley J, Mitchell ARJ, Thibault H, Mirza IH, Becher H. Safety of contrast dobutamine stress echocardiography: a single center experience. J Am Soc Echocardiogr. 2005;18(2):163–7. doi: 10.1016/j.echo.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui JM, Elhendy A, Xie F, O’Leary EL, McGrain AC, Porter TR. Safety of dobutamine stress real-time myocardial contrast echocardiography. J Am Coll Cardiol. 2005;45(8):1235–42. doi: 10.1016/j.jacc.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 48.Honos G, Amyot R, Choy J, Leong-Poi H, Schnell G, Yu E. Contrast echocardiography in Canada: Canadian Cardiovascular Society/Canadian Society of Echocardiography position paper. Can J Cardiol. 2007;23(5):351–6. doi: 10.1016/s0828-282x(07)70767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ontario Case Costing Initiative. Costing analysis tool (CAT) [[updated 2009; cited 2009 Nov 18]]. [Internet] Available from: http://www.occp.com/
- 50.Ontario Ministry of Health and Long-Term Care. Ontario health insurance schedule of benefits and fees. [[updated 2009; cited 2009 Nov 24]]. [Internet] Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html .
- 51.Hayashino Y, Shimbo T, Tsujii S, Ishii H, Kondo H, Nakamura T, et al. Cost-effectiveness of coronary artery disease screening in asymptomatic patients with type 2 diabetes and other atherogenic risk factors in Japan: factors influencing on international application of evidence-based guidelines. Int J Cardiol. 2007;118(1):88–96. doi: 10.1016/j.ijcard.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 52.Kreisz FP, Merlin T, Moss J, Atherton J, Hiller JE, Gericke CA. The pre-test risk stratified cost-effectiveness of 64-slice computed tomography coronary angiography in the detection of significant obstructive coronary artery disease in patients otherwise referred to invasive coronary angiography. Heart Lung Circ. 2009;18(3):200–7. doi: 10.1016/j.hlc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, Cutler DM, Weinstein MC, et al. Cost-effectiveness of coronary MDCT in the triage of patients with acute chest pain. Am J Roentgenol. 2008;191(2):455–63. doi: 10.2214/AJR.07.3611. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzoni R, Cortigiani L, Magnani M, Desideri A, Bigi R, Manes C, et al. Cost-effectiveness analysis of noninvasive strategies to evaluate patients with chest pain. J Am Soc Echocardiogr. 2003;16(12):1287–91. doi: 10.1067/j.echo.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Maddahi J, Gambhir SS. Cost-effective selection of patients for coronary angiography. J Nucl Cardiol. 1997;4(2):S141–S151. doi: 10.1016/s1071-3581(97)90093-3. [DOI] [PubMed] [Google Scholar]
- 56.Medical Advisory Secretariat. Positron emission tomography for the assessment of myocardial viability: an evidence-based analysis. [October [cited 2010 03 03]];Ont Health Technol Assess Ser. 2005 5(16):1–167. [Internet] Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_petmyo_100105.pdf . [PMC free article] [PubMed] [Google Scholar]
- 57.Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess. 2004;8(30):iii–iiv. 1–207. doi: 10.3310/hta8300. [DOI] [PubMed] [Google Scholar]
- 58.Paterson DI, Schwartzman K. Strategies incorporating spiral CT for the diagnosis of acute pulmonary embolism: a cost-effectiveness analysis. Chest. 2001;119(6):1791–800. doi: 10.1378/chest.119.6.1791. [DOI] [PubMed] [Google Scholar]
- 59.Shaw LJ, Monaghan MJ, Nihoyannopolous P. Clinical and economic outcomes assessment with myocardial contrast echocardiography. Heart. 1999;82:III16–III21. doi: 10.1136/hrt.82.2008.iii16. Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyrick JJ, Kalvaitis S, McConnell KJ, Rinkevich D, Kaul S, Wei K. Cost-efficiency of myocardial contrast echocardiography in patients presenting to the emergency department with chest pain of suspected cardiac origin and a nondiagnostic electrocardiogram. Am J Cardiol. 2008;102(6):649–52. doi: 10.1016/j.amjcard.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yong Y, Wu D, Fernandes V, Kopelen HA, Shimoni S, Nagueh SF, et al. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. Am J Cardiol. 2002;89(6):711–8. doi: 10.1016/s0002-9149(01)02344-x. [DOI] [PubMed] [Google Scholar]
- 62.Bedetti G, Pasanisi EM, Pizzi C, Turchetti G, Lore C. Economic analysis including long-term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardiovascular Ultrasound. 2008;6:21. doi: 10.1186/1476-7120-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewey M, Hamm B. Cost effectiveness of coronary angiography and calcium scoring using CT and stress MRI for diagnosis of coronary artery disease. Eur Radiol. 2007;17(5):1301–9. doi: 10.1007/s00330-006-0439-3. [DOI] [PubMed] [Google Scholar]
- 64.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130(9):719–28. doi: 10.7326/0003-4819-130-9-199905040-00003. [DOI] [PubMed] [Google Scholar]
- 65.Hayashino Y, Nagata-Kobayashi S, Morimoto T, Maeda K, Shimbo T, Fukui T. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with Type 2 diabetes and additional atherogenic risk factors. Gen Intern Med. 2004;19(12):1181–91. doi: 10.1111/j.1525-1497.2004.40012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez R, Vale L. The value of myocardial perfusion scintigraphy in the diagnosis and management of angina and myocardial infarction: a probabilistic economic analysis. Med Decis Making. 2007;27(6):772–88. doi: 10.1177/0272989X07306111. [DOI] [PubMed] [Google Scholar]
- 67.Khare RK, Courtney DM, Powell ES, Venkatesh AK, Lee TA. Sixty-four-slice computed tomography of the coronary arteries: cost-effectiveness analysis of patients presenting to the emergency department with low-risk chest pain. Acad Emerg Med. 2008;15(7):623–32. doi: 10.1111/j.1553-2712.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- 68.Kuntz KM, Fleischmann KE, Hunink MG, Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130(9):709–18. doi: 10.7326/0003-4819-130-9-199905040-00002. [DOI] [PubMed] [Google Scholar]
- 69.Lee DS, Jang MJ, Cheon GJ, Chung JK, Lee MC. Comparison of the cost-effectiveness of stress myocardial SPECT and stress echocardiography in suspected coronary artery disease considering the prognostic value of false-negative results. J Nucl Cardiol. 2002;9(5):515–22. doi: 10.1067/mnc.2002.125217. [DOI] [PubMed] [Google Scholar]
- 70.Rumberger JA, Behrenbeck T, Breen JF, Sheedy PF. Coronary calcification by electron beam computed tomography and obstructive coronary artery disease: a model for costs and effectiveness of diagnosis as compared with conventional cardiac testing methods. J Am Coll Cardiol. 1999;33(2):453–62. doi: 10.1016/s0735-1097(98)00583-x. [DOI] [PubMed] [Google Scholar]
- 71.Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess. 2007;11(49):iii–iv. ix–115. doi: 10.3310/hta11490. [DOI] [PubMed] [Google Scholar]
- 72.Shaw LJ, Marwick TH, Berman DS, Sawada S, Heller GV, Vasey C, et al. Incremental cost-effectiveness of exercise echocardiography vs. SPECT imaging for the evaluation of stable chest pain. Eur Heart J. 2006;27(20):2448–58. doi: 10.1093/eurheartj/ehl204. [DOI] [PubMed] [Google Scholar]
- 73.Tardif JC, Dore A, Chan KL, Fagan S, Honos G, Marcotte F, et al. Economic impact of contrast stress echocardiography on the diagnosis and initial treatment of patients with suspected coronary artery disease. J Am Soc Echocardiogr. 2002;15(11):1335–45. doi: 10.1067/mje.2002.125287. [DOI] [PubMed] [Google Scholar]
- 74.Toronto Health Economics and Technology Assessment collaborative (THETA) The relative cost-effectiveness of five non-invasive cardiac imaging technologies for diagnosing coronary artery disease in Ontario. [[updated 2010; cited 2010 Mar 9]]. [Internet] Available from: http://theta.utoronto.ca/reports/?id=7 .
- 75.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107(1):149–58. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 76.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116(7):e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]


