Executive Summary
Objective
To determine the effectiveness and cost-effectiveness of continuous glucose monitoring combined with self-monitoring of blood glucose compared with self-monitoring of blood glucose alone in the management of diabetes.
Clinical Need: Condition and Target Population
Diabetes is a chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. In 2005, an estimated 816,000 Ontarians had diabetes representing 8.8% of the province’s population.
Type 1 or juvenile onset diabetes is a life-long disorder that commonly manifests in children and adolescents. It represents about 10% of the total diabetes population and involves immune-mediated destruction of insulin producing cells in the pancreas. The loss of these cells necessitates insulin therapy.
Type 2 or “adult-onset” diabetes represents about 90% of the total diabetes population and is marked by a resistance to insulin or insufficient insulin secretion. The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. Approximately 30% of patients with type 2 diabetes eventually require insulin therapy.
Technology
Continuous glucose monitors (CGM) measure glucose levels in the interstitial fluid surrounding skin cells. These measurements supplement conventional self monitoring of blood glucose (SMBG) by monitoring the glucose fluctuations continuously over a stipulated period of time, thereby identifying fluctuations that would not be identified with SMBG alone.
To use a CGM, a sensor is inserted under the skin to measure glucose in the interstitial fluid. The sensor is wired to a transmitter. The device requires calibration using a capillary blood glucose measurement. Each sensor continuously measures glucose every 5-10 seconds averaging these values every 5 minutes and storing this data in the monitors memory. Depending on the device used, the algorithm in the device can measure glucose over a 3 or 6 day period using one sensor. After the 3 or 6 day period, a new sensor is required. The device is equipped with alarms which warn the patient of impending hypo-or hyperglycemia.
Two types of CGM are available:
Systems that is stored in a monitor and can be downloaded later.
Real time systems that continuously provide the actual glucose concentration on a display.
Research Questions
What is the effectiveness and cost-effectiveness of CGM combined with SMBG compared with SMBG alone in the management of diabetes?
Research Methods
Literature Search
Search Strategy
A literature search was performed on September 15, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2002 until September 15, 2010. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist, then a group of epidemiologists until consensus was established. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
Inclusion Criteria
English language
Randomized controlled trials (N>30 patients)
Adults or pediatric patients with insulin dependent diabetes (type 1 or 2 or gestational)
Studies comparing CGM plus SMBG versus SMBG alone
Exclusion Criteria
Case studies
Studies that did not compare CGM plus SMBG versus SMBG alone
Studies that did not report statistical analysis of outcomes or data was unextractable
Outcomes of Interest
Change in glycosylated hemoglobin (HbA1c)
Frequency or duration of hypo-or hyperglycemic episodes or euglycemia
Adverse effects
Summary of Findings
Moderate quality evidence that CGM + SMBG:
is not more effective than self monitoring of blood glucose (SMBG) alone in the reduction of HbA1c using insulin infusion pumps for Type 1 diabetes.
is not more effective than SMBG alone in the reduction of hypoglycemic or severe hypoglycemic events using insulin infusion pumps for Type 1 diabetes.
Background
Objective of Analysis
The objective of the systematic review is to determine the effectiveness and cost-effectiveness of continuous glucose monitoring combined with self-monitoring of blood glucose compared with self-monitoring of blood glucose alone in the management of diabetes.
Clinical Need and Target Population
Diabetes is a chronic metabolic disorder that interferes with the body’s ability to produce or effectively use insulin. In 2005, an estimated 816,000 Ontarians had diabetes representing 8.8% of the province’s population.
Insulin therapy is an important component of the treatment of many people with diabetes. Type 1 or juvenile onset diabetes is a life-long disorder that commonly manifests in children and adolescents. It represents about 10% of the total diabetes population and involves immune-mediated destruction of insulin producing cells in the pancreas. (1) The loss of these cells necessitates insulin therapy.
Type 2 or “adult-onset” diabetes represents about 90% of the total diabetes population and is marked by a resistance to insulin or insufficient insulin secretion. (1) The risk of developing type 2 diabetes increases with age, obesity and lack of physical activity. The condition tends to develop gradually and may remain undiagnosed for many years. Approximately 30% of patients with type 2 diabetes eventually require insulin therapy. (1)
An evidence based analysis (1) of five technologies for the management of diabetes was undertaken by the Medical Advisory Secretariat in 2009 including:
Continuous subcutaneous insulin (CSII) infusion pumps for types 1 and 2 adult diabetes
Bariatric surgery for morbidly obese people with diabetes
Community-based care for the management of type 2 diabetes
Behavioural interventions for type 2 diabetes
Home telemonitoring for type 2 diabetes
Based on the results of these evidence based reviews, the Ontario Health Technology Advisory Committee (OHTAC) made recommendations which are available at: http://www.health.gov.on.ca/english/providers/program/ohtac/tech/recommend/rec_diabetes_20091020.pdf
Technology
Conventional self-monitoring of blood glucose (SMBG) is performed by finger capillary blood sample, where the blood glucose is usually measured using a small handheld device. (2) This provides a value of the blood glucose at the moment when the blood was sampled. Although this method has been found to provide an accurate estimate of the glucose level, marked fluctuations in blood glucose can be missed hindering optimal glycemic control. (2)
Continuous glucose monitors (CGM) measure glucose levels in the interstitial fluid surrounding skin cells. These measurements supplement conventional SMBG by monitoring the glucose fluctuations continuously over a stipulated period of time, thereby identifying fluctuations that would not be identified with SMBG alone. (2)
To use a CGM, a sensor is inserted under the skin to measure glucose in the interstitial fluid. The sensor is wired to a transmitter. The device requires calibration using a capillary blood glucose measurement. Each sensor continuously measures glucose every 5-10 seconds averaging these values every 5 minutes and storing this data in the monitors memory. Depending on the device used, the algorithm in the device can measure glucose over a 3 or 6 day period using one sensor. After the 3 or 6 day period, a new sensor is required. The device is equipped with alarms which warn the patient of impending hypo-or hyperglycemia.
Two types of CGM are available (2):
3. Systems that measure glucose concentrations during a certain time span; the information is stored in a monitor and can be downloaded later.
4. Real time systems that continuously provide the actual glucose concentration on a display.
CGM may be useful for children (to reduce the often high number of finger punctures in this group), for patients with poorly controlled diabetes, for pregnant women in whom tight glucose control is essential with respect to the outcome of pregnancy, and for patients with hypoglycaemia unawareness (to prevent episodes of hypoglycemia). (2)
Regulatory Status
Health Canada licenses 4 Class 3 CGMs indicated for the continuous or periodic monitoring of glucose levels in the fluid under the skin from people with diabetes. CGMs are not licensed to replace SMBG.
CGMS IPRO System®
CGM only and used by a clinician as a holter-style recorder providing retrospective CGM data.
CGM has capacity to record up to 1 week of data that can be downloaded by the clinician and reviewed for patient/disease management.
Patient and physician can see the cause and effect of actions on blood glucose (e.g., eating habits, skipping drug, exercise)
The patient can be on an insulin pump or not.
Guardian Realtime®
Provides real-time glucose levels
Personal stand alone CGM
Paradigm Realtime®
Provides real-time glucose levels
CGM sold separately from insulin delivery component
Sensor can be worn up to 3 days
Paradigm Veo®
Provides real-time glucose levels
CGM integrated into the insulin infusion pump
Sensor can be worn up to 6 days
Status in Ontario
Glucose Monitors
Glucose monitoring equipment and related supplies for insulin users who do not have private coverage are covered under the Ontario Assistive Devices Program (ADP).
The ADP funds blood glucose monitors through the Monitoring for Health Program which is a Transfer Payment Agency of the ADP and is administered by the Ontario Diabetes Association. Patients send applications for general blood glucose monitors directly to the Monitoring for Health Program and from there they are approved or denied.
Under this program, clients receive 75% of the purchase price for a general blood glucose monitor up to a maximum of $75.00 or 75% of the purchase price for a talking general blood glucose monitor up to a maximum of $300.00.
Insulin Infusion Pumps
Insulin infusion pumps approved for funding assistance by the ADP do have CGM capabilities, however ADP funding is for the insulin infusion pump only (the fact that the pump has a glucose monitor is an add-on for which the client would pay).
For insulin infusion pumps, ADP pays 100% of the approved price ($6300) to individuals with Type 1 diabetes who meet the ADP eligibility criteria. The replacement period for an ADP insulin infusion pump is 5 years. ADP will consider early replacement however if there is a change in the patient’s medical condition which makes their previously funded pump ineffective.
ADP provides an annual grant of $2400 to patients with insulin pumps to help pay for supplies required to use their pumps.
Evidence-Based Analysis
Research Question
What is the effectiveness and cost-effectiveness of CGM combined with SMBG compared with SMBG alone in the management of diabetes?
Research Methods
Literature Search
Search Strategy
A literature search was performed on September 15, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2002 to September 15, 2010. The literature search strategy is shown in Appendix 1.
Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established.
Inclusion Criteria
English language
Randomized controlled trials (N>30 patients)
Adults or pediatric patients with insulin dependent diabetes (type 1 or 2 or gestational)
Studies comparing CGM plus SMBG versus SMBG alone
Exclusion Criteria
Case studies
Studies that did not compare CGM plus SMBG versus SMBG alone
Studies that did not report statistical analysis of outcomes or data was unextractable
Outcomes of Interest
Change in glycosylated hemoglobin (HbA1c)
Frequency or duration of hypo-or hyperglycemic episodes or euglycemia
Adverse effects
Quality of Evidence
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (3) as presented below.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
The body of evidence is shown in Table 3. Overall, there were 3 large RCTs (N>100) and 1 small RCT (N<100). Details of the study characteristics are shown in Appendix 2.
Table 3: Body of evidence examined according to study design.
| Study Design | Number of Eligible Studies | |
|---|---|---|
| Type 1 | Type 2 | |
| RCT Studies | ||
| Systematic review of RCTs | - | - |
| Large RCT | 2 RCTs comparing combined pump and real time CGM to pump and SMBG. (4;5) Adults and children | - |
| Small RCT | - | - |
| Total | 2 | - |
RCT refers to randomized controlled trial.
The Continuous Glucose Monitoring System® (retrospective CGM data recorder) is not currently licensed by Health Canada, however, no studies were identified that met the inclusion criteria and used the iPro® (retrospective holter-style professional CGM) which is currently licensed by Health Canada. The systematic review by Golicki et al. (6), and two Australia and New Zealand Horizon Scanning Network reports (7;8) were excluded from the MAS EBA since they included studies that examined the retrospective Continuous Glucose Monitoring System®.
Results for the technology brief by the Canadian Agency for Drugs and Technologies in Health are shown in Table 4. The 2007 report concluded that “based on limited amount of research published to date, the impact of the combined pump and CGM on long-term glycemic control, prevention of diabetic complications or quality of life is unclear.” (9)
Table 4: Results of the Technology Brief by the Canadian Agency for the Drugs and Technologies in Health.
| Study/Year | Population | Results | Comment |
| Canadian Agency for Drugs and Technologies in Health 2007 (9) | Combined insulin pump and CGM in patients with Type 1 diabetes | Based on limited amount of research published to date, the impact of the combined pump and CGM on long-term glycemic control, prevention of diabetic complications or quality of life is unclear. | 1 published randomized study (N=16) and 3 abstracts were included in the Technology Brief. |
| Review focused on the combined pump/CGM Paradigm® Real-Time system. |
Real Time CGM Combined With an Insulin Pump
Pump and CGM Versus Pump and SMBG
Two studies were included in this section. (4;5)
Raccah et al. (4) conducted a 6 month RCT (N=132; n=81 adults; n=51 children) of patients with type 1 diabetes, HbA1c ≥8%, and treated with MDI. Patients assigned to the CGM group agreed to wear the sensor during at least 70% of the study period. The primary objective of the study was to determine the change in HbA1c between the CGM and SMBG group.
There was no significant difference in the change in HbA1c between the CGM (−0.81%) and SMBG (−0.57%) group (P=0.087).
Secondary outcomes are shown in Table 6. There was a significantly greater reduction in blood glucose concentration, hours per day of hyperglycemia >190 mg/dL and hyperglycemia AUC in the CGM group compared to the SMBG group (P≤0.05). However, there was no significant difference in hyperglycemic episodes per day or for any of the hypoglycemia outcomes between the study groups. There was a significant increase in daily insulin doses for the CGM compared to the SMBG group.
Table 6: Results for Secondary Outcomes in Raccah et al. (4).
| Outcome | Pump + CGM (n=46) | Pump + SMBG (n=54) |
|---|---|---|
| Δ blood glucose (mg/dL) | -30.6±54.0 * | -10.8±39.6 |
| Δ hyperglycemia >190 mg/dL (h/day) | -3.5±4.8 * | -0.7±3.8 |
| Δ hyperglycemia AUC (mg/dL/day) | -17.1±31.7† | -5.8±26.7 |
| Δ hyperglycemia (episodes/day) | -0.2±0.7 | -0.2±0.7 |
| Δ hypoglycemia <70 mg/dL (h/day) | 0.3±1.4 | 0±1.2 |
| Δ hypoglycemia AUC (mg/dL/day) | 0.4±1.3 | 0.0±1.8 |
| Δ hypoglycemia (episodes/day) | 0.1±0.9 | 0.1±0.7 |
| Δ daily insulin doses (units/day) | 6.8±17.3† | 1.5±9.1 |
SD refers to standard deviation, * P≤0.005 vs. Pump+SMBG, P≤0.05 vs. Pump+SMBG
Ten serious adverse events were reported: 3 in the CGM group and 7 in the SMBG group (Table 7). Four events in the SMBG group were determined by the authors to be unrelated to the device or study protocol.
Table 7: Adverse Events in Raccah et al. (4).
| Pump + CGM (n=46) | Pump + SMBG (n=54) |
| Total=3 | Total=7 |
| 2 ketoacidosis (patients failed to react to device’s hyperglycemic alarms) | 3 ketoacidosis |
| 1 severe hypoglycemia with loss of consciousness(device improperly calibrated and acute alcohol intoxication) | 4 events that were unrelated to the study devices or protocol. |
Limitations to the study by Raccah et al. (4) included:
23 patients in the CGM group failed to wear the sensors at least 70% of the time.
High attrition rate - a total of 20 patients abandoned the study: 14 from the CGM group and 6 from the SMBG group.
It is unclear whether there were lifestyle differences/modifications between the study groups.
Hirsch et al. (5) conducted a 6 month RCT (N=138) of patients aged 12 to 72 years with type 1 diabetes and initial HbA1c levels ≥7.5%. All patients were previously treated with an insulin infusion pump for at least 6 months. The primary objective of the study was to determine the average change in HbA1c between the combined pump and CGM group (n=72 patients) compared to the pump and SMBG group (n=66 patients).
Change in HbA1c
Change in HbA1c from baseline was significant for both groups (P<0.001), but there was no significant difference between the study groups (P=0.3706) (Table 8). Results for adult and pediatric patients showed similar changes in HbA1c.
Table 8: Results for Change in HbA1c in the study by Hirsch et al. (5).
| Pump + CGM | Pump + SMBG | |||||
| Baseline (n=72) | 6 months (n=72) | Change | Baseline (n=66) | 6 months (n=66) | Change | |
| Mean (SD) | 8.39 (0.64) | 7.84 (0.81) | -0.56 (0.72) | 8.49 (0.76) | 7.77 (0.92) | -0.71 (0.71) |
| Median | 8.3 | 7.8 | -0.7 | 8.4 | 7.8 | -0.7 |
| P value (intragroup) | <0.001 | <0.001 | ||||
| P value (intergroup) | 0.3706 |
SD refers to standard deviation
Target 7% HbA1c
A 6 months, 16(24.2%) patients in the SMBG group achieved the target HbA1c compared to 12(19.4%) in the CGM group (no significant difference, P value not reported). Inter-group comparisons were similarly non-significant for adults and children when analyzed separately.
Hyperglycemia AUC (>180 mg/dL)
There was no significant difference in change from baseline between the study groups (P=0.2913).
Hyperglycemia Incidence
There was no significant difference in the mean number of hyperglycemic events between the study groups (P value not reported).
Hypoglycemia AUC (<70 mg/dL)
The change from baseline between the groups was significant (P<0.0002)
Hypoglycemia Incidence
There was no significant difference in hypoglycemic events between the study groups (P=0.0707).
Severe Hypoglycemia
There were 14 events of severe hypoglycemia, of which 11 occurred in the CGM group within 8 patients.
(Severe hypoglycemia was defined as clinical episode of hypoglycemia resulting in seizure or coma requiring hospitalization or intravenous glucose or glucagon or any hypoglycemia that required assistance from another person; these episodes were reported by patients in their workbooks). Comparison between the groups was statistically significant (P=0.04).
Six of the events were deemed to be not related or unlikely to be related to the device (i.e., not wearing the device). In the remaining 5 instances, The Safety Review Board determined that patients ignored alerts, injected multiple boluses of insulin without using the “Bolus Wizard”, or based treatment decisions on sensor reading only, without confirming with a blood glucose test.
Safety
There were 17 serious adverse events. One person in the SMBG group experienced 2 skin abcesses at the insulin infusion site and 1 person in the CGM group had diabetic ketoacidosis. The remaining adverse events were severe hypoglycemic events.
Limitations to the study by Hirsch et al. (5) included:
The primary endpoint did not specify whether the change in HbA1c was for within or between study groups.
No sample size calculation or justification was reported in the study
Results were not calculated using the intent to treat principle.
Lack of confirmation of severe hypoglycaemic episodes (based on patients workbooks).
Hypoglycemic range was measured over a 6 day period at the beginning and the end of the study.
Meta-Analysis of Studies Comparing Pump and CGM Versus Pump and SMBG
When the mean difference in HbA1c data from the studies by Raccah et al. (4) and Hirsch et al. (5) were pooled, there was no significant difference between CGM + SMBG compared to SMBG alone (Figure 1).
Figure 1: Pooled Mean Difference for Change in HbA1c in Studies Comparing Pump and CGM Versus Pump and SMBG.
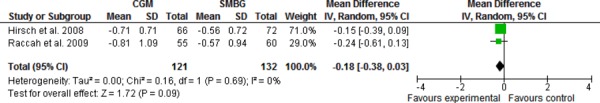
GRADE Quality of the Evidence
The quality of evidence was examined using the GRADE Working Group criteria for interventions (Table 9). Overall, the GRADE quality was moderate.
Table 9: GRADE Quality Assessment of Studies.
| No. of Studies | Design | Quality/Limitations | Indirectness | Inconsistency | Publication Bias | Results | Quality |
| Pump and CGM Versus Pump and SMBG | |||||||
| 2 | RCTs | Compliance issue in Raccah et al. - 35% patients in CGM group failed to wear the sensors ≥% of the time. | No serious inconsistency | No serious inconsistency | Unlikely |
Raccah et al. ΔHbA1c |
Moderate |
| Raccah N=132 6 months |
Possible, but not considered sufficient to downgrade quality of evidence. | CGM (-0.81%) vs. SMBG (-0.57%) P=0.087 Δ Hypoglycemia CGM 0.1±0.9 vs. SMBG 0.1±0.7 episodes per day, P=NS |
|||||
| Hirsch N=146 6 months |
Both studies - no details about changes in patient self management (CMG patients may engage in more lifestyle modifications by being able to see real time glucose trend information) | ||||||
| High | Hirsch et al. ΔHbA1c | ||||||
| Allocation not reported in both studies. | CGM Mean(SD) -0.56 (0.72) SMBG Mean(SD) -0.71(0.71), P=0.37 | ||||||
| No sample size calculation in Hirsch et al. Possible Type 2 error. | Hypoglycemia Incidence # events between CGM and SMBG, P = 0.07 | ||||||
| Severe Hypoglycemia 14 events total, 11 in CGM within 8 patients, P=0.04 | |||||||
| High → Moderate | |||||||
| BUT: 6 events patient not wearing device. In 5 remaining instances, patients ignored alerts or based treatment decision on sensor only. | |||||||
CGM, continuous glucose monitor; RCT, randomized controlled trial; SMBG, self monitoring blood glucose;
Conclusion
There is moderate quality evidence that CGM + SMBG:
is not more effective than self monitoring of blood glucose (SMBG) alone in the reduction of HbA1c using insulin infusion pumps for Type 1 diabetes.
is not more effective than SMBG alone in the reduction of hypoglycemic or severe hypoglycemic events using insulin infusion pumps for Type 1 diabetes
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Study Question
The objective of this economic analysis was to report costs associated with continuous monitoring of blood glucose in Type 1 diabetics in Ontario.
Economic Literature Review
A literature search was performed on March 16th, 2011 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, and Centre for Reviews and Dissemination/International Agency for Health Technology Assessment for studies published from 1948 to March week 1, 2011 for MEDLINE; and from 1980 to week 10, 2011 for EMBASE (Appendix 1). Included studies were those with full economic evaluations describing both costs and consequences of continuous glucose monitoring (CGM), real-time monitoring and certain trade names of CGM systems currently available in Ontario; the same set of search keywords was used as for the effectiveness systematic review.
According to the systematic review performed above, there were no health economic evaluations found comparing the relative cost-effectiveness of continuous monitoring of blood glucose. Several studies have examined the relative effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion when compared to multiple daily injections of insulin, however, none have evaluated the continuous monitoring of blood glucose levels compared to standard self-monitoring.(10-12) These studies have also stated the difficulty of modelling diabeties-related outcomes and incorporating long-term costs and quality-of-life in a cost-effectiveness analysis. In the current analysis, only the incremental costs associated with providing CGM in addition to SMBG are examined below.
Ontario-Based Cost Impact Analysis
The incremental costs associated with providing personal CGM systems to Type 1 diabetics in Ontario involve the costs of purchasing (and replacing) both insulin pump transmitters and blood glucose sensors. Through consultations with CGM system manufacturers, it was estimated that transmitters, which transfer the interstitial blood glucose information from the glucose sensor to the insulin pump, should be replaced twice every five years with an average cost of about $500 per transmitter. According to industry consultants, the blood glucose sensors should be replaced every 6 days for a total of about 61 replacements per year with a cost of about $60 per sensor; or an annual sensor cost of about $$3,652.
In Ontario, the incidence of diabetes was estimated as being 0.82% in 2003 and the prevalence of diabetes was estimated at 8.8% in 2005.(13) According to a previous review performed by MAS, the number of Type 1 diabetics was estimated as being approximately 10% of the incident and prevalent populations.(1) The total volume of Type 1 diabetics was estimated for the current cost impact analysis by using the above proportions in combination with the Ministry of Finance’s Ontario population projections for fiscal years 2011 to 2015.(14) For example, in Ontario in fiscal year 2011, it was estimated that approximately 10,967 new Type 1 patients were among a prevalent population of about 117,690.
Table 10 shows the anticipated additional (incremental) costs of CGM transmitters and glucose sensors over the next five years in Ontario. Note that the incremental costs of prevalent and incident Type 1 diabetics repeat in certain years specifically to replace CGM transmitters (i.e. replacement every two-and-a-half years on average). As a result, over the next five years, the impact of providing personal CGM systems (transmitters and sensors) to Type 1 diabetics was estimated as being approximately $160 million annually.
Table 10: Annual incremental costs of CGM (i.e. costs in addition to SMBG).
| Description | FY2011a | FY2012b | FY2013a,b | FY2014b | FY2015a,b | 5-yr Total | Annualized |
| CGM transmitter (every 2.5 years) | $58.8M | $5.5M | $64.5M | $11.2M | $64.6M | $204.7M | $40.9M |
| Blood glucose sensor (every 6 days) | $429.9M | $40.5M | $41.0M | $41.5M | $42.0M | $594.9M | $119.0M |
| Total incremental cost | $488.7M | $46.1M | $105.5M | $52.7M | $106.6M | $799.6M | $159.9M |
Note: aIncremental costs for prevalent Type 1 diabetics; bIncremental costs for incident Type 1 diabetics
Appendices
Appendix I: Literature Search Strategies
Search date: September 15, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1950 to September Week 1 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Diabetes Mellitus/ (256497)
(diabet* or niddm or iddm or mody or t1dm or t2dm).ti,ab. (303034)
1 or 2 (347536)
exp Monitoring, Physiologic/ (104514)
continuous.mp. (201133)
4 and 5 (9436)
((continuous adj1 glucose monitor*) or cgm or cgms).ti,ab. (962)
(IPRO or (Paradigm* adj (realtime or real-time)) or Paradigm Veo or (guardian adj (realtime or real-time))).ti,ab. (42)
or/6-8 (9851)
3 and 9 (1040)
limit 10 to (english language and humans and yr="2002 -Current") (729)
limit 11 to (controlled clinical trial or meta analysis or randomized controlled trial) (112)
exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ (47142)
(health technology adj2 assess$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (1007)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (98605)
exp Random Allocation/ or random$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (649345)
exp Double-Blind Method/ (108624)
exp Control Groups/ (1237)
exp Placebos/ (29293)
(RCT or placebo? or sham?).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (188273)
or/12-20 (845343)
11 and 21 (163)
Database: EMBASE <1980 to 2010 Week 36>
Search Strategy:
--------------------------------------------------------------------------------
exp diabetes mellitus/ (395793)
(diabet* or niddm or iddm or mody or t1dm or t2dm).ti,ab. (370020)
1 or 2 (463313)
exp blood glucose monitoring/ (8095)
continuous.mp. (246345)
4 and 5 (1030)
(continuous glucose monitor* or cgm or cgms or IPRO or Paradigm* or (guardian adj real?time)).ti,ab. (67179)
(IPRO or (Paradigm* adj (realtime or real-time)) or Paradigm Veo or (guardian adj (realtime or real-time))).ti,ab. (71)
or/6-8 (67660)
3 and 9 (2471)
limit 10 to (human and english language and yr="2002 -Current") (1538)
Randomized Controlled Trial/ (280313)
exp Randomization/ (52313)
exp RANDOM SAMPLE/ (2478)
exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ (459450)
(health technology adj2 assess$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] (1301)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$) or published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (112252)
Double Blind Procedure/ (98937)
exp Triple Blind Procedure/ (19)
exp Control Group/ (16065)
exp PLACEBO/ or placebo$.mp. or sham$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] (292868)
(random$ or RCT).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] (689216)
(control$ adj2 clinical trial$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] (414522)
or/12-23 (1193197)
11 and 24 (349)
CINAHL
| S16 | S11 and S14 | 76 |
| Limiters - Published Date from: 20020101-20101231; English Language; Search modes Boolean/Phrase | ||
| S15 | S11 and S14 | 81 |
| S14 | S12 or S13 | 133005 |
| S13 | random* or sham*or rct* or health technology N2 assess* or meta analy* or metaanaly* or pooled analysis or (systematic* N2 review*) or published studies or medline or embase or data synthesis or data extraction or cochrane or control* N2 clinical trial* | 126032 |
| S12 | (MH "Random Assignment") or (MH "Random Sample+") or (MH "Meta Analysis") or (MH "Systematic Review") or (MH "Double-Blind Studies") or (MH "Single-Blind Studies") or (MH "Triple-Blind Studies") or (MH "Placebos") or (MH "Control (Research)") | 71858 |
| S11 | S3 and S10 | 336 |
| S10 | S8 or S9 | 402 |
| S9 | S5 or S6 or S8 | 402 |
| S8 | (S4 and S7) | 223 |
| S7 | continuous | 15536 |
| S6 | IPRO or Paradigm* N1 realtime or Paradigm* N1 real-time or Paradigm Veo or guardian N1 realtime or guardian N1 real-time or cgm or cgms | 179 |
| S5 | continuous N1 glucose monitor* | 327 |
| S4 | (MH "Blood Glucose Monitoring+") | 2348 |
| S3 | S1 or S2 | 60705 |
| S2 | diabet* or niddm or iddm or mody or t1dm or t2dm | 60553 |
| S1 | (MH "Diabetic Patients") OR (MH "Diabetes Mellitus+") | 48341 |
Search date: March 16, 2011
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE(R) <1948 to March Week 1 2011>
Search Strategy:
--------------------------------------------------------------------------------
exp Diabetes Mellitus/ (259584)
(diabet* or niddm or iddm or mody or t1dm or t2dm).ti,ab. (307734)
1 or 2 (352779)
exp Monitoring, Physiologic/ (106102)
continuous.mp. (203629)
4 and 5 (9613)
((continuous adj1 glucose monitor*) or cgm or cgms).ti,ab. (1049)
(IPRO or (Paradigm* adj (realtime or real-time)) or Paradigm Veo or (guardian adj (realtime or real-time))).ti,ab. (45)
or/6-8 (10055)
3 and 9 (1120)
limit 10 to (english language and yr="2002 -Current") (840)
exp Economics/ (432407)
exp Models, Economic/ (7660)
exp Resource Allocation/ (13551)
exp "Value of Life"/ or exp "Quality of Life"/ (92145)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (193297)
ec.fs. (279805)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or "value of life" or "willingness to pay").ti,ab. (66914)
or/12-18 (742539)
11 and 19 (64)
***************************
Database: EMBASE <1980 to 2011 Week 10>
Search Strategy:
--------------------------------------------------------------------------------
exp diabetes mellitus/ (418250)
(diabet* or niddm or iddm or mody or t1dm or t2dm).ti,ab. (391038)
1 or 2 (488988)
exp blood glucose monitoring/ (8774)
continuous.mp. (256861)
4 and 5 (1186)
(continuous glucose monitor* or cgm or cgms or IPRO or Paradigm* or (guardian adj real?time)).ti,ab. (71128)
(IPRO or (Paradigm* adj (realtime or real-time)) or Paradigm Veo or (guardian adj (realtime or real-time))).ti,ab. (85)
or/6-8 (71676)
3 and 9 (2886)
limit 10 to (english language and yr="2002 -Current") (2323)
exp "Health Care Cost"/ (158213)
exp Health Economics/ (490753)
exp Resource Management/ (22924)
exp Economic Aspect/ or exp Economics/ or exp Quality Adjusted Life Year/ or exp Socioeconomics/ or exp Statistical Model/ or exp "Quality of Life"/ (1087892)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (224312)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or "value of life" or "willingness to pay").ti,ab. (87069)
or/12-17 (1240634)
11 and 18 (319)
CINAHL
| S13 | S10 and S11 | 171 |
| Limiters - Published Date from: 20020101-20111231 | ||
| S12 | S10 and S11 | 183 |
| S11 | (MH "Economics+") or (MH "Resource Allocation+") or MW ec or (MH "Quality of Life+") or (econom* or cost* or budget* or pharmacoeconomic* or pharmaco-economic* or valu*) or ((cost* N1 benefit*) or costbenefit* or (cost N1 S11 effective*) or costeffective* or econometric* or life value or quality-adjusted life year* or quality adjusted life year* or 514324 quality-adjusted life expectanc* or quality adjusted life expectanc* or sensitivity analys* or "value of life" or "willingness to pay") | 514324 |
| S10 | S3 AND S9 | 370 |
| S9 | S5 OR S6 OR S8 | 437 |
| S8 | S4 and S7 | 234 |
| S7 | continuous | 16226 |
| S6 | IPRO or Paradigm* N1 realtime or Paradigm* N1 real-time or Paradigm Veo or guardian N1 realtime or guardian N1 real-time or cgm or cgms | 201 |
| S5 | continuous N1 glucose monitor* | 357 |
| S4 | (MH "Blood Glucose Monitoring+") | 2415 |
| S3 | S1 OR S2 | 63599 |
| S2 | diabet* or niddm or iddm or mody or t1dm or t2dm | 63439 |
| S1 | (MH "Diabetic Patients") OR (MH "Diabetes Mellitus+") | 50818 |
Appendix 2: Characteristics of Studies Included in the Evidence-Based Review
| Study/Year/Country | Sample size/Duration | Adults or Children | Diabetes Status | Treatment & Control/Primary Endpoint | Frequency of CGM use | Frequency of SMBG use | Results | Limitations/Comments |
| Real Time CGM - Pump and CGM Versus Pump and SMBG | ||||||||
| RCT Raccah et al. (4) 2009 France |
N=132 6 months |
Adults (n=81) Children (n=51) |
Type 1 diabetes HbA1c ≥8% |
Pump and CGM Vs. Pump and SMBG Primary outcome to determine change in HbA1c between CGM and SMBG. |
Patients agreed to wear CGM ≥70% of study period. | At least 3 readings daily | No significant difference in ΔHbA1c CGM (-0.81%) and SMBG (-0.57%) (P=0.087). No significant difference in Δ hyperglycemia CGM -0.2±0.7 vs. SMBG -0.2±0.7 or Δ hypoglycemia CGM 0.1±0.9 vs. SMBG 0.1±0.7 episodes per day between the study groups. Adverse Events - CGM 2 ketoacidosis 1 severe hypoglycemia Adverse Events - SMBG 3 ketoacidosis |
All patients previously treated with MDI. 23 patients in the CGM group failed to wear the sensors at least 70% of the time. High attrition rate - a total of 20 patients abandoned the study: 14 from the CGM group and 6 from the SMBG group. It is unclear whether there were lifestyle differences/modifications between study groups. |
| RCT Hirsch et al. (5) 2008 United States |
N=138 6 months |
Adults (n=98) Children (n=40) |
Type 1 diabetes HbA1c ≥7.5% |
Pump and CGM Vs. Pump and SMBG Primary outcome to determine average change in HbA1c between CGM vs. SMBG. |
2 sensors per week (2 3-day periods) | Not reported. | No significant difference in ΔHbA1c between study groups. (P=0.37). CGM Mean (SD) ΔHbA1c -0.56 (0.72) SMBG Mean (SD) ΔHbA1c -0.71(0.71) |
The primary endpoint did not specify whether the change in HbA1c was for within or between study groups. |
|
Target 7% HbA1c No significant difference (P value not reported) CGM: 12(19.4%) patients SMBG: 16 (24.2%) patients |
No sample size calculation or justification was reported in the study Results were not calculated using the intent to treat principle. |
|||||||
|
Hyperglycemia Incidence No significant difference in # events between CGM and SMBG (P value not reported) |
Lack of confirmation of severe hypoglycaemic episodes (based on patients workbooks). | |||||||
|
Hypoglycemia Incidence No significant difference between CGM and SMBG (P = 0.07). |
Hypoglycemic range was measured over a 6 day period at the beginning and the end of the study. | |||||||
|
Severe hypoglycemia 14 events of which 11 occurred in CGM group within 8 patients. Comparison between the groups was statistically significant (P=0.04). 6 events deemed not related or unlikely to be related to the device (i.e., not wearing the device). In remaining 5 instances, it was determined that patients ignored alerts, injected multiple boluses of insulin without using the “Bolus Wizard”, or based treatment decisions on sensor reading only, without confirming with a blood glucose test. 17 serious adverse events. 1 person in SMBG group experienced 2 skin abcesses at the insulin infusion site and 1 person in CGM group had diabetic ketoacidosis. Remaining adverse events were severe hypoglycemic events. |
||||||||
CSII refers to continuous subcutaneous intensive insulin infusion; MDI, multiple daily injections; SMBG, self-monitoring of blood glucose
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Continuous glucose monitoring for patients with diabetes: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2011 July [cited YYYY MM DD]; 11(4) 1-29. Available from:
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-6963-7 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is considered for the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Abbreviations
- AUC
Area under the curve
- CGM
Continuous glucose monitor
- CI
Confidence interval(s)
- HbA1c
Glycosylated haemoglobin
- MAS
Medical Advisory Secretariat
- OR
Odds ratio
- OHTAC
Ontario Health Technology Advisory Committee
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SMBG
Self-monitoring of blood glucose
References
- 1.Medical Advisory Secretariat. Diabetes strategy evidence platform: a summary of evidence-based analyses. Ont Health Technol Assess Ser. 2009;9(19):1–43. [Internet] Oct [cited 2009 Dec 23] Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_diabetes_sum_ 20091020.pdf . [PMC free article] [PubMed] [Google Scholar]
- 2.Langendam MW, Hooft L, De VH, Wentholt IM, Mudde AH, Burt AL, et al. Langendam MW, Hooft L, De Vries H, Wentholt IM, Mudde AH, Burt AL, Scholten RJPM. Continuous glucose monitoring systems for type 1 diabetes mellitus (Protocol) Cochrane Database Syst Rev. 2009;(Issue 4) doi: 10.1002/14651858.CD008101.pub2. Art. No.: CD008101. DOI: 10.1002/14651858.CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J. 2006;328(7454):1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32(12):2245–50. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–83. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 6.Golicki DT, Golicka D, Groele L, Pankowska E. Continuous Glucose Monitoring System in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2008;51(2):233–40. doi: 10.1007/s00125-007-0884-9. [DOI] [PubMed] [Google Scholar]
- 7.Australia and New Zealand Horizon Scanning Network. Continuous glucose monitoring devices. Christchurch: ANZHSN. 2006. [[cited: 2011 Jun 25]]. [Internet] 45 p. Available from: http://nzhta.chmeds.ac.nz/publications/finalcgmd.pdf .
- 8.Australia and New Zealand Horizon Scanning Network. Continuous glucose monitoring in pregnant women with diabetes. Christchurch, NZ: ANZHSN. 2009. [[cited: 2011 Jun 27]]. [Internet] 6 p. Available from: http://www.health.gov.au/internet/horizon/publishing.nsf/Content/68B1F63984E68993CA2575A D0080F3E2/$File/PS_Continous%20Glucose%20Monitoring%20in%20Pregnant%20Women%2 0with%20Diabetes.pdf .
- 9.Pohar SL. Subcutaneous open-loop insulin delivery for type 1 diabetes: Paradigm Real-Time System. Issues Emerg Health Techno. 2007;(105):1–6. [PubMed] [Google Scholar]
- 10.Cohen N, Minshall ME, Sharon-Nash L, Zakrzewska K, Valentine WJ, Palmer AJ. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent Type 1 diabetes mellitus in Australia. Pharmacoeconomics. 2007;25(10):881–97. doi: 10.2165/00019053-200725100-00006. [DOI] [PubMed] [Google Scholar]
- 11.Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess. 2004;8(43):iii–95. doi: 10.3310/hta8430. [DOI] [PubMed] [Google Scholar]
- 12.Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med. 2005;22(9):1239–45. doi: 10.1111/j.1464-5491.2005.01576.x. [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet. 2007;369(9563):750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 14.Ontario Ministry of Finance. Ontario Population Projections Update (Table 8) [Internet] [updated 2011; cited 2011 Apr 11] Available from: http://www.fin.gov.on.ca/en/economy/demographics/projections/


