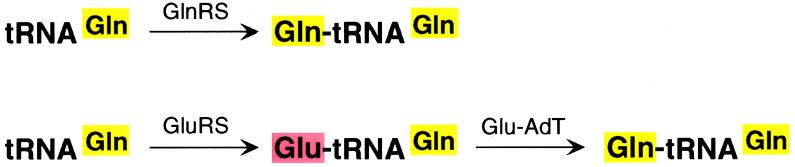
Aminoacyl-tRNA synthetases play a central role in protein synthesis by covalently linking the correct amino acid to the correct tRNA (1). Each aminoacyl-tRNA is then carried to the ribosome where it interacts with the cognate trinucleotide codon on the mRNA and transfers the amino acid onto a growing polypeptide chain. Work on tRNAs and aminoacyl-tRNA synthetases from bacteria, fungi, plants, and mammals led to the general notion of the occurrence of 20 aminoacyl-tRNA synthetases in all organisms, one for each of the 20 amino acids. Early indication of an exception came from the finding that three Gram-positive bacteria lacked the enzyme glutaminyl-tRNA synthetase (GlnRS),† which covalently links glutamine to the glutamine tRNA (tRNAGln) (2). Instead the glutamine tRNA is first aminoacylated with glutamic acid using glutamyl-tRNA synthetase (GluRS) to form Glu-tRNAGln. In a second step, the Glu-tRNAGln is converted to Gln-tRNAGln by an amidotransferase now called Glu-AdT (Fig. 1). The latter reaction requires ATP, which activates the side chain carboxyl group of glutamic acid on the tRNA to form a carboxyphosphate anhydride and glutamine or asparagine, which provides the amino group for transamidation (3). Thus, depending on the organism, there are two pathways for the synthesis of Gln-tRNAGln, a direct pathway involving GlnRS and an indirect pathway involving GluRS and Glu-AdT (Fig. 1). Further work showed that also in archaebacteria (4, 5), cyanobacteria, mitochondria, and chloroplasts (6, 7), Gln-tRNAGln is formed not directly but in a two-step process as outlined above. In archaebacteria, such as halobacteria, Asn-tRNAAsn is also synthesized in an analogous two-step process involving Asp-tRNAAsn as an intermediate (8). The widespread utilization of the Glu-AdT pathway for incorporation of glutamine into proteins and its possible similarity to other glutamine dependent amidotransferase reactions involved in a variety of biochemical reactions (9) has generated much interest in the nature of enzymes involved in this process.
Figure 1.
Alternate pathways in the production of glutaminyl-tRNA for protein synthesis.
The paper by Curnow et al. (10) in this issue of Proceedings describes the cloning, sequence analysis, and molecular characterization of the Glu-AdT gene from Bacillus subtilis. The enzyme is shown to have three subunits, A, B, and C. Homologues to these subunits have been identified in other organisms. The A subunit may have a P-loop-type ATP binding motif, suggesting that this subunit might be involved in activation of the side chain carboxyl group of glutamic acid. The A subunit also has substantial homology to a class of amidases and can convert glutamine to glutamic acid and ammonia in vitro. This reaction provides enzyme bound ammonia as a source of amino group for the amidation of glutamic acid on the tRNA to glutamine. The B subunit might be used to select the correct tRNA substrate. Curnow et al. (10) have identified two different types of B subunits in some archaebacteria, one more closely related to the B. subtilis B subunit than the other. They suggest that one is used for transamidation of Glu-tRNAGln whereas the other is used for transamidation of Asp-tRNAAsn. The role of the C subunit is not clear. It may not be present in all other organisms that use the Glu-AdT pathway or it could have diverged enough not to be recognized as a homologue. The C subunit is necessary for the expression of the B. subtilis A subunit in Escherichia coli suggesting that it could be involved in the modification, folding and/or stabilization of the A subunit.
The genes for the three subunits are organized into an operon, which is transcribed to produce a polycistronic mRNA. The polycistronic nature of the transcript and the close proximity of the stop codons of the preceding ORFs to the start codons of the downstream ORFs raises the possibility of coupled translation to produce stoichiometric amounts of the three subunits and possibility of translational regulation (11). The gene for Glu-AdT is shown to be essential in B. subtilis. Thus, this enzyme provides the only pathway for the formation of Gln-tRNAGln in this organism.
The Glu-AdT belongs to an important class of enzymes that modify amino acids while they are linked to tRNAs. Examples of these are enzymes that convert methionine to formyl methionine (used for initiation of protein synthesis in eubacteria and in mitochondria and chloroplasts; reviewed in ref. 12), serine to selenocysteine (used for insertion of selenocysteine at very specific sites in proteins; reviewed in refs. 13 and 14), and glutamic acid to glutamic α-semialdehyde (used for synthesis of δ-amino levulinic acid on the pathway to chlorophyll biosynthesis in plants, archaebacteria and eubacteria; ref. 15). These enzymes are specific for the amino acid and for the tRNA to which the amino acid is attached (16–18). Like these other enzymes, the Glu-AdT must also act specifically on only one tRNA, Glu-tRNAGln, the glutamine tRNA that is aminoacylated with glutamic acid. The successful reconstitution of the Glu-AdT subunits expressed in E. coli described in this paper and the availability, thereby, of the purified enzyme should now expedite studies on the mechanism of transamidation and the molecular basis of tRNA specificity.
A surprising aspect of the B. subtilis Glu-AdT gene is the lack of any sequence homology to aminoacyl-tRNA synthetases (10). This implies that these two enzymes had quite different origins. It is, therefore, intriguing that the B. subtilis tRNAGln is a substrate for E. coli GlnRS (10). This implies that most of the determinants necessary for recognition by the E. coli GlnRS (19) are present on the B. subtilis tRNAGln. An inspection of the tRNA sequence indicates that this is indeed the case. Because B. subtilis does not have GlnRS, why does the tRNAGln contain determinants for a bacterial GlnRS? One possibility is that these determinants, particularly in the acceptor stem of the tRNA, are needed for aminoacylation of the tRNA by the B. subtilis GluRS (Fig. 1). This is probably not the case because there is virtually no homology in the acceptor stem between B. subtilis tRNAGln and tRNAGlu. Another possibility is that the B. subtilis Glu-AdT uses essentially the same determinants for specific recognition of the tRNA as the E. coli GlnRS.
The use of Glu-tRNAGln as an intermediate in the synthesis of Gln-tRNAGln means that the Glu-tRNAGln must not be transported to the ribosome prior to conversion of the glutamic acid on the tRNA to glutamine. If that were to happen, the Glu-tRNAGln would insert glutamic acid in response to the glutamine codons, CAA and CAG, and cause errors in protein synthesis. Interestingly, the chloroplast elongation factor EF-Tu, which binds to and transports aminoacyl-tRNAs to the ribosome, appears specifically not to bind to the “mischarged” tRNAGln (20). Thus, the miscoding is prevented by blocking EF-Tu from binding to the “mischarged” tRNA. This is somewhat similar to the way in which the initiator tRNA in E. coli is blocked from participating in elongation; by preventing its binding to EF-Tu (21). Not resolved, however, is the question of how the chloroplast EF-Tu binds to the same tRNA when the glutamic acid attached to the tRNA is changed to glutamine. A similar example of elongation factor discrimination against an aminoacyl-tRNA occurs during the conversion of serine attached to a specific tRNA to selenocysteine. The tRNA has special structural features that prevent its binding to the elongation factor EF-Tu and a special elongation factor which binds to the tRNA, only when it carries the amino acid selenocysteine, is used (22). Another example of the transient formation of a mischarged tRNA is found in the case of some aminoacyl-tRNA synthetases, which activate the wrong amino acid, often closely related structurally (for example, isoleucine and valine, valine and threonine), and attach it to the tRNA (23, 24). In these cases, the enzymes have an editing activity, which corrects the mistake by hydrolysis of the amino acid from the mischarged tRNA (25).
As in other areas of biology, as generalities become evident, exceptions emerge. There are now some exceptions to the notion that Glu-AdT is found only in Gram-positive bacteria, archaebacteria, cyanobacteria, mitochondria, and chloroplasts. Rhizobium meliloti, a Gram-negative bacterium, has Glu-AdT and does not have GlnRS (26). Because Rhizobium belongs to the α-subdivision of Gram-negative purple bacteria, this has led to the suggestion that the α-subdivision of purple bacteria in general uses Glu-AdT for synthesis of Gln-tRNAGln. The finding that Rhizobium has Glu-AdT is consistent with the proposed role of the α-subdivision of purple bacteria being the ancestors of mitochondria in the endosymbiotic hypothesis (27).
Recent work has also shown that GlnRS is not absent from all mitochondria. Mitochondria from two trypanosomatids, Leishmania tarentolae and Trypanosoma brucei, contain GlnRS (28). The absence of GlnRS in the α-subdivision of purple bacteria and its presence in trypanosomatid mitochondria raises the question of the origin of GlnRS in the trypanosomatid mitochondria. Is it possible that trypanosomatid mitochondria have an origin different from that of other mitochondria? In other words, could mitochondria have a polyphyletic instead of monophyletic origin? This is not inconceivable, given the many unusual features of trypanosomatid mitochondria, the presence of highly interlocked maxi- and mini-circular DNAs, the phenomenon of extensive RNA editing including multiple additions and deletions of nucleotides onto an RNA transcript, etc. However, another possibility is that the mitochondrial GlnRS in trypanosomatids evolved from the gene for cytoplasmic GlnRS by duplication and the acquisition of a signal sequence that directs the protein into mitochondria. The possibility of a horizontal gene transfer event is also not ruled out (28, 29).
With archaebacteria missing GlnRS and AsnRS, there is a maximum of 18 aminoacyl-tRNA synthetases in these organisms (4, 5, 8). The complete genome sequence of Methanococcus jannaschii indicated that besides GlnRS and AsnRS, the enzymes for cysteine and lysine were also absent. Further studies have shown that the enzyme for lysine is present although its homology to lysyl-tRNA synthetases from other organisms is very low (30). The question of the cysteine enzyme, however, remains unresolved. It is, therefore, conceivable that cysteinyl-tRNA could also be made on the tRNA through transformation of another amino acid, for example serine. If so, this would further reduce the number of aminoacyl-tRNA synthetases in some archaebacteria to 17. Is it possible then that somewhere, in some highly specialized ecological niches, there exists organisms which contain even fewer number of aminoacyl-tRNA synthetases?
The isolation and sequencing of the gene for Glu-AdT and the finding that it has no homology to any of the aminoacyl-tRNA synthetases raises a whole host of interesting questions (10, 26, 29), such as: (i) How did Glu-AdT and GlnRS evolve? (ii) Did the Glu-AdT pathway predate the GlnRS pathway in evolution? (iii) Because an extra molecule of ATP is needed for conversion of Glu-tRNAGln to Gln-tRNAGln, why is the Glu-AdT pathway retained in so many organisms? Is this a consequence of very different glutamic acid and glutamine pools in these organisms?
Finally, because the Glu-AdT pathway is used for a critical step in protein synthesis in Gram-positive bacteria whereas the GlnRS pathway is used in eukaryotic cells, Glu-AdT could be a potential target for antibacterial drugs, assuming that such drugs do not affect mitochondrial Glu-AdT activity. The availability of the Glu-AdT gene and the enzyme also makes studies along these lines possible.
Acknowledgments
Work in our laboratory is supported by Grant R-37GM17151 from the National Institutes of Health.
Footnotes
A three-letter symbol for amino acid is used. The amino acid symbol followed by RS specifies a particular aminoacyl-tRNA synthetase; for example, GlnRS, glutaminyl-tRNA synthetase; or GluRS, glutamyl-tRNA synthetase. The amino acid symbol used as a superscript following tRNA identifies the amino acid specificity of the tRNA; for example, tRNAGln, tRNA specific for glutamine or glutamine tRNA, or tRNAGlu, glutamic acid tRNA. The amino acid symbol preceding tRNA and separated from it by a hyphen indicates that the tRNA is aminoacylated with a particular amino acid; for example, Gln-tRNA, glutaminyl-tRNA, or Glu-tRNA, glutamyl-tRNA.
References
- 1.Berg P. Annu Rev Biochem. 1961;30:293–324. [Google Scholar]
- 2.Wilcox M, Nirenberg M. Proc Natl Acad Sci USA. 1968;61:229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcox M. Eur J Biochem. 1969;11:405–412. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 4.White B N, Bayley S T. Can J Biochem. 1972;50:600–609. doi: 10.1139/o72-082. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R. J Biol Chem. 1984;259:9461–9471. [PubMed] [Google Scholar]
- 6.Martin N, Rabinowitz M, Fukuhara H. Biochemistry. 1977;16:4672–4677. doi: 10.1021/bi00640a022. [DOI] [PubMed] [Google Scholar]
- 7.Schon A, Kannangara C G, Gough S, Söll D. Nature (London) 1988;331:187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- 8.Curnow A W, Ibba M, Söll D. Nature (London) 1996;382:589–590. doi: 10.1038/382589b0. [DOI] [PubMed] [Google Scholar]
- 9.Zalkin H. Adv Enzymol. 1993;66:203–309. doi: 10.1002/9780470123126.ch5. [DOI] [PubMed] [Google Scholar]
- 10.Curnow A W, Hong K-w, Yuan R, Kim S-i, Martins O, Winkler W, Henkin T M, Söll D. Proc Natl Acad Sci USA. 1997;94:11819–11826. doi: 10.1073/pnas.94.22.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura M, Gourse R, Baughman G. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- 12.RajBhandary U L. J Bacteriol. 1994;176:547–552. doi: 10.1128/jb.176.3.547-552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron C, Böck A. In: tRNA: Structure, Biosynthesis and Function. Söll D, RajBhandary U L, editors. Washington, DC: Am. Soc. Microbiol. Press; 1995. pp. 529–544. [Google Scholar]
- 14.Stadtman T C. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 15.Schon A, Krupp G, Gough S, Berry-Lowe S, Kannangara C G, Söll D. Nature (London) 1986;322:281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- 16.Giege R, Ebel J P, Clark B F C. FEBS Lett. 1973;30:291–295. doi: 10.1016/0014-5793(73)80672-6. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Kumar N V, Varshney U, RajBhandary U L. J Biol Chem. 1996;271:1022–1028. doi: 10.1074/jbc.271.2.1022. [DOI] [PubMed] [Google Scholar]
- 18.Marcker K, Sanger F. J Mol Biol. 1964;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- 19.Hayase Y, Jahn M, Rogers M J, Sylvers L A, Koizumi M, Inoue H, Ohtsuka E, Söll D. EMBO J. 1992;11:4159–4165. doi: 10.1002/j.1460-2075.1992.tb05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanzel M, Schon A, Sprinzl M. Eur J Biochem. 1994;219:435–439. doi: 10.1111/j.1432-1033.1994.tb19956.x. [DOI] [PubMed] [Google Scholar]
- 21.Seong B, RajBhandary U L. Proc Natl Acad Sci USA. 1987;84:8859–8863. doi: 10.1073/pnas.84.24.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forchhammer K, Leinfelder W, Böck A. Nature (London) 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- 23.Norris A T, Berg P. Proc Natl Acad Sci USA. 1964;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fersht A R. Biochemistry. 1977;16:1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt E, Schimmel P. Biochemistry. 1995;34:11204–11210. doi: 10.1021/bi00035a028. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon Y, Lacoste L, Champagne N, Lapointe J. J Biol Chem. 1996;271:14856–14863. doi: 10.1074/jbc.271.25.14856. [DOI] [PubMed] [Google Scholar]
- 27.Gray M W. Int Rev Cytol. 1992;141:233–357. doi: 10.1016/s0074-7696(08)62068-9. [DOI] [PubMed] [Google Scholar]
- 28.Nabholz C E, Hauser R, Schneider A. Proc Natl Acad Sci USA. 1997;94:7903–7908. doi: 10.1073/pnas.94.15.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamour V, Quevillon S, Diriong S, N’Guyen V C, Lipinski M, Mirande M. Proc Natl Acad Sci USA. 1994;91:8670–8674. doi: 10.1073/pnas.91.18.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibba, M., Morgan, S., Curnow, A. W., Pridmore, D. R., Vothknecht, U. C. Gardner, W., Lin, W., Woese, C. R. & Söll, D. (1997) Science, in press. [DOI] [PubMed]