Abstract
Background
Clopidogrel is a prodrug that undergoes in vivo bioactivation to show its antiplatelet effects. Recent studies have shown that cytochrome P450 (CYP), ATP-binding cassette transporters (ABCB1), and paraoxonase-1 (PON1) play crucial roles in clopidogrel bioactivation. Here, we aim to determine the effects of genetic polymorphisms of CYP (CYP 2C19*2, CYP 2C19*3, and CYP 2C19*17), ABCB1 (ABCB1 3435C>T, ABCB1 129T>C, and ABCB1 2677G>T/A), and PON1 (PON1 Q192R, PON1 L55M, and PON1 108C>T) on the development of stent thrombosis (ST) in patients receiving clopidogrel after percutaneous coronary intervention (PCI).
Methods and Results
We evaluated the incidence of ST (0.64%) in 4964 patients who were recruited in the CAPTAIN registry (Cardiovascular Atherosclerosis and Percutaneous TrAnsluminal INterventions). The presence of genetic polymorphisms was assessed in 20 subjects who developed ST after aspirin and clopidogrel therapy and in 40 age- and sex-matched control subjects who did not develop ST, which was documented after 9 months of angiographic follow-up. ST was acute in 5 subjects, subacute in 7, late in 7, and very late in 1. The presence of CYP 2C19*2 allele was significantly associated with ST (adjusted odds ratio [ORadj]: 4.20, 95% confidence interval [CI], 1.263–9.544; P = 0.031). However, genetic variations in PON1 and ABCB1 showed no significant association with ST.
Conclusion
We conclude that in a Taiwanese population, PON1 Q192R genotype is not associated with ST development after PCI. However, the presence of CYP 2C19*2 allele is a risk factor for ST development after PCI.
Introduction
Dual antiplatelet therapy with aspirin and clopidogrel, an adenosine diphosphate (ADP)-P2Y12 receptor antagonist, has become the standard treatment for patients with coronary artery disease (CAD) who undergo percutaneous coronary intervention (PCI) with stenting [1], [2]. However, one of the shortcomings of clopidogrel therapy is high interindividual variability of its antiplatelet response [3], [4], [5]. Inadequate platelet inhibition may result in stent thrombosis (ST) and increase the frequency of cardiovascular events [6], [7], [8]. Clopidogrel is a prodrug that requires bioactivation, i.e., in vivo conversion into an active metabolite, to show its antiplatelet effects. Pharmacokinetic and pharmacodynamic studies have shown that the bioconversion of clopidogrel is a 2-step process and is mediated by different enzymes such as ATP-binding cassette transporters (ABCB1), hepatic cytochrome P450s (CYPs), and esterase paraoxonase-1 (PON1) [9].
Previous studies in young healthy volunteers receiving clopidogrel have shown that a loss-of-function mutation in CYP, which yields the CYP 2C19*2 allele, is associated with a marked decrease in platelet responsiveness to clopidogrel [10]. Other studies have shown that the presence of CYP 2C19*2 is significantly associated with a low rate of clopidogrel bioactivation [11], [12], [13]. Clinical studies conducted in a large group of patients with cardiovascular conditions who underwent PCIs have also confirmed that CYP 2C19*2 is associated with diminished clopidogrel responsiveness and increased frequency of major adverse cardiovascular events, such as recurrent myocardial infarction, ST, and long-term mortality [14], [15], [16], [17], [18].
However, the CYP 2C19 polymorphisms are only partly responsible for the low rates of clopidogrel bioactivation and its role in cardiovascular outcome is still controversial [19]. Previous studies showed that genetic variations in CYP 3A4-encoding CYP enzymes, which contribute to clopidogrel bioactivation [20], ABCB1, which modulates clopidogrel absorption [21], and PON1, may play major roles in clopidogrel metabolism.
PON1 is an arylesterase found in the liver and is involved in cell-mediated oxidation of high- and low-density lipoproteins (HDL and LDL) and inhibition of atherosclerotic processes. Previous studies revealed a controversial associated between PON1 polymorphism and coronary artery disease [22], [23]. A recent study showed that a common PON1 polymorphism, Q192R, is associated with clopidogrel bioactivation [24]. Bouman et al. showed that PON1 is the rate-limiting enzyme in the second step of clopidogrel bioactivation, namely, hydrolytic cleavage of 2-oxo-clopidogrel to form the active thiol metabolite. They performed a case-cohort study of 112 individuals and showed that the PON1 Q192R polymorphism, rather than CYP 2C19, is the major determinant of clopidogrel bioactivation.
Recent studies do not associate platelet responsiveness and the risk of ST development with PON1 Q192R polymorphism [25], [26], [27], [28]. The reason for the different results obtained in various studies on the influence of PON1 in clopidogrel bioactivation is unclear. We hypothesize that this could be because of the differences in the ethnicity and genetic background of the study subjects. There might be some differences in PON1 or CYP genotype distribution between Asian and Caucasian populations [29], [30]. Because different genotype prevalences can lead to different clinical effects, we investigated the effects of gene polymorphisms of PON1 (PON1 Q192R, PON1 L55M, and PON1 108C>T), ABCB1 (ABCB1 3435C>T, ABCB1 129T>C, and ABCB1 2677G>T/A), and CYP (CYP 2C19*2, CYP 2C19*3, and CYP 2C19*17) on the development of ST in Taiwanese patients receiving clopidogrel after PCI.
Materials and Methods
Ethics Statement
Written informed consent was obtained from 26 patients with ST and from 40 patients who served as controls. The study was approved by the Chang Gung Medical Foundation Institutional Review Board and conforms to the ethical guidelines of the Helsinki declaration.
Study Population and Study Principle
Since November 1995, we have been registering CAD patients in the CAPTAIN registry (Cardiovascular Atherosclerosis and Percutaneous TrAnsluminal INterventions). To date, 4964 patients have been enrolled in the registry. We enroll only those patients who have undergone PCI with stenting and have been followed up regularly at the outpatient clinic. The overall follow-up rate is 72%. Long-term follow-up data up to June 2011 were obtained from the outpatient clinics.
For this study, we screened the data of ST patients from the CAPTAIN registry. These definition of ST complied with the consensus criteria definition by the Academic Research Consortium (ARC), and the cases of ST were further classified as acute (within 24 h after stent implantation), subacute (1–30 days), late (>30 days to 1 year) and very late (>1 year) [31]. We also screened and analyzed the data of age-, gender-, and risk factor-matched subjects without ST from the registry. PCI and post-PCI treatment procedures complied with current standard guidelines [1].
The patients in the normal control Han Chinese and Caucasian groups were randomly selected from the Cell and Genome Bank in Taiwan [32].
Genotyping
Blood sampling was performed after PCI. DNA was extracted from 5 mL of blood using DNeasy blood kit (Qiagen) according to the manufacturer’s instructions. Sequencing of PON1 Q129R (rs662), CYP 2C19*2 (rs4244285), CYP 2C19 *3 (rs4986893), CYP 2C19*17 (rs12248560), and ABCB1 C3435T (rs1045642) was performed with a TaqMan assay by using an ABI Prism Sequence Detector 7000 (Applied Biosystems) according to the manufacturer’s protocols. Hardy–Weinberg equilibrium within each ethnic group was tested and was found to be nonsignificant for all gene polymorphisms (P>0.05). The genotyping results were reconfirmed by performing polymerase chain reaction (PCR) analysis and direct sequencing. The overall error rate was found to be less than 1%.
Statistical Analysis
All variables are presented as mean ± standard deviation (SD) values and counts (in percentages). Categorical variables were compared using the χ2 test. The Kolmogorov–Smirnov test was used to check for normal distribution of continuous data. Continuous variables were evaluated using the Student’s t-test or one-way analysis of variance (ANOVA), as appropriate. Binary and polychotomous variables were examined using Fisher’s exact and χ2 tests. A multiple logistic regression model was used to test whether gene polymorphisms of PON1 and ABCB1 and CYP 2C19*2, CYP 2C19*3, and CYP 2C19*17 were independent predictors of ST. In addition to PON1 Q192R and CYP 2C19*2, the other polymorphisms were also considered in the multivariable model and their details were entered as the number of risk alleles identified in the patients (0, 1, or 2) and by assuming a codominant model for the allele effect; all variables that differed (P<0.10) between ST and control subjects were also included in the multivariable model. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed with Statistical Package for Social Sciences for Macintosh (SPSS for Mac; version 18; SPSS Institute).
Results
Subjects with Stent Thrombosis in the CAPTAIN Registry
In the CAPTAIN registry, we enrolled 4964 patients who underwent PCI with stenting from November 1995 to June 2011. The overall follow-up rate was 72%, and the incidence of ST was 0.64%. There were 32 cases of definite ST as defined by the ARC criteria among patients in the registry. Among these 32 patients, 6 refused to participate in the study, and 6 died before we could include them in the study. Thus, 20 ST subjects were enrolled as the ST group. For the control or non-ST group, we enrolled 40 age- and sex-matched subjects from the registry who had undergone PCI but did not develop ST, which was angiographically confirmed during a follow-up period of 9 months. All the 20 ST patients and 40 control patients received clopidogrel treatment after stent implantation for 9 months without discontinuation. Other P2Y12 antagonists were not used in the treatment. In 1 patient, ST occurred very late, i.e., 3 months after the physician recommended the discontinuation of clopidogrel after the 9-month treatment.
The baseline characteristics of patients of both the ST and non-ST groups are listed in Table 1. Clinical variables such as age, sex, hypertension and smoking history were well balanced between the 2 groups.
Table 1. Baseline clinical characteristics.
| *ST group | Non-ST group | P value | |
| Variable | (n = 20) | (n = 40) | |
| Age (mean), y | 60.3±8.9 | 59.0±7.5 | 0.564 |
| Male, n (%) | 17 (85%) | 30 (75%) | 0.384 |
| Hypertension, n (%) | 13 (65%) | 18 (45%) | 0.149 |
| Diabetes mellitus, n (%) | 9 (45%) | 12 (30%) | 0.132 |
| Smoking, n (%) | 11 (55%) | 17 (42.5%) | 0.369 |
| High sensitive † CRP (mg/L) | 14.2±18.9 | 18.9±37.1 | 0.597 |
| Total cholesterol (mg/dL) | 166.2±37.2 | 183.2±35.2 | 0.089 |
| Triglyceride (mg/dL) | 157.1±115.2 | 147.6±60.6 | 0.676 |
| Uric acid (mg/dL) | 5.7±1.3 | 6.2±1.7 | 0.243 |
| Ejection fraction (%) | 60.9±12.4 | 59.3±11.9 | 0.631 |
ST, stent thrombosis.
CRP, C-reactive protein.
Baseline clinical characteristics of the stent thrombosis and non-stent thrombosis groups.
Among the 20 ST patients, 5 (25%) received BMS and 15 (75%) received DES. Among the 40 control patients, 10 (25%) received BMS and 30 (75%) received DES. There was no angiographic coronary dissection in these patients.
Correlation between the Risk of ST Development and Polymorphisms of PON1, CYP, and ABCB1
Among the 60 patients included in this study, 27 (45%) were PON1 RR192 homozygous, 25 (41.7%) were QR192 heterozygous, and 8 (13.3%) were QQ192 homozygous carriers (Table 2). Among the 20 ST patients, 17 (85%) had at least 1 mutant allele of PON1 Q192R, of which 10 were RR192 and 7 were QR192 carriers. Among the 40 non-ST subjects, 35 (87.5%) were carriers of at least 1 PON1 mutant allele (17 were RR192 and 18 were QR192 carriers). There was no significant correlation between the PON1 Q192R genotype and the risk of ST (Odds ratio [OR], 0.86, 95% confidence interval [CI], 0.372–2.821; P = 0.597). No significant associations were observed between ST development and the PON1 L55M and PON1 108C>T polymorphisms (Tables 3).
Table 2. Genotype distributions in stent thrombosis (ST) and non-ST groups.
| *ST group | Non-ST group | ||||
| Genotypes | (n = 20) | (n = 40) | Total | P value | |
| CYP 2C19 * 2 | *2/*2 (AA) | 4 (20%) | 4 (10%) | 8 (13.3%) | 0.038 |
| wt/*2 (GA) | 11 (55%) | 12 (30%) | 23 (38.3%) | ||
| wt/wt (GG) | 5 (25%) | 24 (60%) | 29 (48.4%) | ||
| CYP 2C19 * 3 | AA | 0 | 0 | 0 | 0.591 |
| AG | 2 (10%) | 6 (15%) | 8 (13.3%) | ||
| GG | 18 (90%) | 34 (85%) | 52 (86.7%) | ||
| CYP 2C19 * 17 | TT | 0 | 0 | 0 | - |
| CT | 0 | 0 | 0 | ||
| CC | 20 (100%) | 40 (100%) | 60 (100%) | ||
| PON1 Q192R | RR192 | 10 (50%) | 17 (42.5%) | 27 (45%) | 0.760 |
| QR192 | 7 (35%) | 18 (45%) | 25 (41.7%) | ||
| QQ192 | 3 (15%) | 5 (12.5%) | 8 (13.3%) | ||
| PON1 L55M | AA | 0 | 0 | 0 | 0.309 |
| AT | 1 (5%) | 2 (5%) | 3 (5%) | ||
| TT | 19 (95%) | 38 (95%) | 57 (95%) | ||
| PON1 108C>T | TT | 5 (25%) | 8 (20%) | 13 (21.7%) | 0.873 |
| TC | 10 (50%) | 20 (50%) | 30 (50%) | ||
| CC | 5 (25%) | 12 (30%) | 17 (28.3%) | ||
| ABCB1 C3435T | TT | 6 (30%) | 5 (12.5%) | 11 (18.3%) | 0.188 |
| TC | 9 (45%) | 18 (45%) | 27 (45%) | ||
| CC | 5 (25%) | 17 (42.5%) | 22 (36.7%) | ||
| ABCB1 T129C | CC | 0 | 0 | 0 | 0.509 |
| CT | 1 (5%) | 4 (10%) | 5 (8.3%) | ||
| TT | 19 (95%) | 36 (90%) | 55 (91.7%) | ||
| ABCB1 G2677T | TT | 5 (25%) | 6 (15%) | 11 (18.3%) | 0.544 |
| TA | 3 (15%) | 4 (10%) | 7 (11.7%) | ||
| TG | 6 (30%) | 13 (32.5%) | 19 (31.7%) | ||
| GA | 2 (10%) | 10 (25%) | 12 (20%) | ||
| AA | 0 | 2 (5%) | 2 (3.3%) | ||
| GG | 4 (20%) | 5 (12.5%) | 9 (15%) | ||
ST, stent thrombosis.
The genotype frequencies of PON1, CYP, and ABCB1 polymorphisms in the stent thrombosis (ST) and non-ST groups. A significant difference (P = 0.038) in genotype distribution between the ST and non-ST groups is seen only for CYP 2C19*2 and not for PON1, CYP 2C19*3, CYP 2C19*17, and ABCB1 polymorphisms.
Table 3. Results of multivariable logistic regression for genotype carriers in predicting stent thrombosis.
| Carrier Odds Ratio* | P value | |
| Variable | (95% †CI) | |
| PON1 Q129R rs662 | 0.86 (0.372–2.821) | 0.597 |
| PON1 L55M rs854560 | 1 (0.167–5.985) | 0.423 |
| PON1 108 rs705379 | 0.78 (0.23–2.627) | 0.686 |
| CYP 2C19 * 2 rs4244285 | 4.20 (1.263–9.544) | 0.031 |
| CYP 2C19 * 3 rs4986893 | 0.83 (0.315–4.451) | 0.577 |
| CYP 2C19 * 17 rs12248560 | – | – |
| ABCB1 C3435T rs1045642 | 2.32 (0.853–7.183) | 0.112 |
| ABCB1 129 rs3213619 | 1.541 (0.15–15.830) | 0.341 |
| ABCB1 2677 rs2032582 | 0.529 (0.14–2.008) | 0.763 |
Unadjusted odds ratios (ORs): CYP 2C19*2, OR, 4.50 (1.363–14.844), P = 0.028; PON1 Q129R, OR, 0.74 (0.252–2.171), P = 0.697; ABCB1 C3435T rs1045642, OR, 2.22 (0.674–7.293), P = 0.212.
CI, confidence interval.
Regarding the CYP 2C19*2 genotype, 8 of the 60 patients (13.3%) were *2/*2 homozygous, 23 (38.3%) were wt/*2 heterozygous, and 29 (48.4%) were wt/wt homozygous carriers. Among the 20 ST patients, 15 (75%) were carriers of at least 1 CYP 2C19*2 mutant allele (4 *2/*2 carriers and 11 wt/*2 carriers). However, only 16 (40%) of the 40 non-ST patients were carriers of at least 1 CYP 2C19*2 mutant allele (4 *2/*2 carriers and 12 wt/*2 carriers). There was a significant correlation between the presence of CYP 2C19*2 variants and risk of ST development (OR, 4.2 [95% CI, 1.263–9.544]; P = 0.031).
Of the 20 ST patients, 5 had acute ST, 7 had subacute ST, 7 had late ST, and 1 had very late ST. The PON1 Q192R mutant allele carrier rate was 80% (2 RR192 and 2 QR192 carriers) in 5 acute ST patients, 100% (4 RR192 and 3 QR192 carriers) in 7 subacute ST patients, 71.4% (3 RR192 and 2 QR192 carriers) in 7 late ST patients, and 100% (1 RR192 carrier) in 1 very late ST patient (Table 4). The CYP2C19 mutant allele carrier rate was 80% (2 *2/*2 and 2 wt/*2 carriers) in 5 acute ST patients, 57.1% (4 wt/*2 carriers) in 7 subacute ST patients, 85.7% (2 *2/*2 and 4 wt/*2 carriers) in 7 late ST patients, and 100% (1 wt/*2 carrier) in 1 very late ST patient. There was no difference in genotype distribution of PON1 Q192R and CYP2C19*2 between acute, subacute, late, and very late ST, with P values of 0.549 and 0.747, respectively.
Table 4. Genotype frequencies of acute, subacute, late, and very late stent thrombosis.
| Acute | Subacute | Late | Very late | P value | ||
| Genotypes | (n = 5) | (n = 7) | (n = 7) | (n = 1) | ||
| CYP 2C19*2 | *2/*2 (AA) | 2 (40%) | 0 | 2 (28.6%) | 0 | 0.549 |
| wt/*2 (GA) | 2 (40%) | 4 (57.1%) | 4 (57.1%) | 1(100%) | ||
| wt/wt (GG) | 1 (20%) | 3 (42.9%) | 1 (14.3%) | 0 | ||
| PON1 Q192R | RR192 | 2 (40%) | 4 (57.1%) | 3 (42.8%) | 1 (100%) | 0.747 |
| QR192 | 2 (40%) | 3 (42.9%) | 2 (28.6%) | 0 | ||
| QQ192 | 1 (20%) | 0 | 2 (28.6%) | 0 | ||
There was no difference in the genotype distribution of PON1 Q192R and CYP2C19 between acute, subacute, late, and very late ST, with a P value of 0.549 and 0.747, respectively.
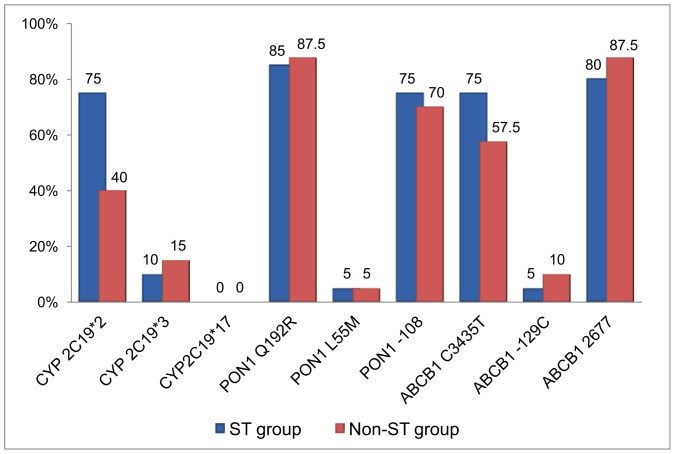
There were no significant correlations between the risk of ST development and the presence of CYP 2C19*3, CYP 2C19*17, PON1 L55M, PON1 108C>T, ABCB1 C3435T, ABCB1 129T>C, and ABCB1 2677G>T (Fig. 1). The mutant allele carrier rate for CYP 2C19*3 was 10% in the ST group and 15% in the non-ST group, with an OR of 0.83 (95% CI, 0.315–4.451; P = 0.577) for stent thrombosis. The carrier rate for ABCB1 C3435T was 75% in the ST group and 57.5% in the non-ST group, with an OR of 2.32 (95% CI, 0.853–7.183; P = 0.112) for ST. The CYP 2C19*17, PON1 L55M, ABCB1 129T>C mutant carrier rates in the study population were less than 5%; therefore, a larger study population is required to detect their effects on clopidogrel bioactivation.
Figure 1. Percentage of mutant allele carriers of CYP 2C19 and PON1 and ABCB1 polymorphisms.
Graph showing the percentage of mutant allele carriers for cytochrome P450 (CYP) and paraoxonase-1 (PON1) and ATP-binding cassette transporter (ABCB1) polymorphisms in stent thrombosis (ST) group (blue) and non-ST group (red). The carrier percentage for the CYP 2C19*2 mutant allele is significantly different between the ST and non-ST groups (75% vs. 40%; carrier odds ratio [OR 4.2; 95% confidence interval [CI], 1.263–9.544; P = 0.031), with a high carrier percentage in the ST group.
Discussion
In this study, we analyzed the effects of gene polymorphisms of CYP (CYP 2C19*2, CYP 2C19*3, and CYP 2C19*17), ABCB1 (ABCB1 3435C>T, ABCB1 129T>C, and ABCB1 2677G>T/A), and PON1 (PON1 Q192R, PON1 L55M, and PON1 108C>T) on the development of ST in patients receiving clopidogrel after PCI (Fig. 1). Our study shows that the PON1 Q192R genotype is not associated with ST development after PCI in Taiwanese population. However, carriers of the CYP 2C19*2 allele showed an increased risk of ST development after PCI.
Clinical studies conducted in a large group of patients, mainly Caucasians, have confirmed that CYP 2C19*2 is associated with diminished clopidogrel responsiveness and increased frequency of key adverse cardiovascular events, such as recurrent myocardial infarction, ST, and long-term mortality [14], [15], [16], [17], [18]. The same result was reported by Luo et al. in a Chinese population [33]. Hence, our results were similar to those of previously reported studies.
Several recently published studies have failed to find the relationship between PON1 Q192R polymorphism and antiplatelet responsiveness of clopidogrel [25], [26], [27], [28], [34]. Moreover, the relationships between PON1 Q192R polymorphism and ST are still controversial [24], [26]. Our study showed that the PON1 Q192R genotype is not associated with ST development after PCI in a Taiwanese population. This finding is in concordance with the results of a recent study by Sibbing et al. [26]; they studied a large cohort of 1524 patients and concluded that the PON1 Q192R genotype is not associated with the risk of ST after coronary stenting by comparing 127 ST patients with 1439 controls in the same registry.
However, these results are different from the results of a recent study by Bouman et al. [24]. With in vitro metabolomics-profiling techniques, they identified PON1 as a key enzyme in the transformation of 2-oxo-clopidogrel to the active thiol metabolite. In this study, they compared PON1 Q192R genotype frequencies in 41 ST patients and 71 controls and found significant association between PON1 Q192R and ST.
The precise reasons for this discrepancy are still unclear. Most of the patients in the study by Bouman et al. and Sibbing et al. were Caucasians. Some researchers suggest that the differences in populations or study design may account for these differences in outcome [34]. Our study showed a different PON1 Q192R genotype distribution in the control group (42.5% for RR192, 45% for QR192, and 12.5% for QQ192) as compared with the control groups reported by Sibbing et al. (8% for RR192, 39% for QR192, and 53% for QQ192) and Bouman et al. (18% for RR192, 47% for QR192, and 35% for QQ192). We have observed that the Taiwanese patients exhibit a high carrier rate for the PON1 Q192R mutant allele.
Our study among the Han Chinese population showed no association between PON1 Q192R and ST development. Further, both PON1 L55M and PON1 108C>T polymorphisms showed no association with ST development in our study. Therefore, our study results support the fact that PON1 polymorphisms do not contribute to ST development in populations with a different ethnic or genetic background.
Although we did not detect a significant association between PON1 gene polymorphisms and ST development, we found differences in the genotype distribution of PON1 and CYP 2C19*2 between Asian and Caucasian populations (Table 5). We performed the genotyping of 92 Han Chinese and 92 Caucasian people without CAD. The Han Chinese population showed an 80.1% carrier rate for the PON1 Q192R mutant allele (44.5% for RR192 and 35.9% for QR192) whereas the Caucasian population showed a 35.9% carrier rate for the same polymorphism (4.4% for RR192 and 31.5% for QR192) (Table 4). The Han Chinese population also showed higher CYP 2C19*2 mutant allele carrier rate (12% for *2/*2 and 39.1% for wt/*2) than that of the Caucasian population (2.2% for *2/*2 and 22.8% for wt/*2). These findings showing significantly high carrier rates for PON1 Q129R and CYP 2C19*2 in the Han Chinese population may have an important clinical impact [29], [30], [35], [36], [37]. The high PON1 Q129R carrier rate in the Han Chinese population did not contribute to the rate of ST development. In contrast, the high carrier rate of CYP 2C19*2 in the Han Chinese population might have influenced the clopidogrel responses and cardiovascular outcome and warrant future investigation.
Table 5. Genotype frequencies in Asian and Caucasian populations.
| Normal Han Chinese population | Normal Caucasian population | ||
| Genotypes | (n = 92) | (n = 92) | |
| CYP 2C19*2 | *2/*2 (AA) | 11 (12.0%) | 2 (2.2%) |
| wt/*2 (GA) | 36 (39.1%) | 21 (22.8%) | |
| wt/wt (GG) | 45 (48.9%) | 69 (75.0%) | |
| CYP 2C19*3 | AA | 0 | 0 |
| AG | 9 (10%) | 0 (0%) | |
| GG | 83 (90%) | 92 (100%) | |
| PON1 Q192R | RR192 (GG) | 41 (44.5%) | 4 (4.4%) |
| QR192 (AG) | 33 (35.9%) | 29 (31.5%) | |
| QQ192 (AA) | 18 (19.6%) | 59 (64.1%) | |
| ABCB1 C3435T | AA | 13 (14.1%) | 30 (32.6%) |
| AG | 45 (48.9%) | 42 (45.7%) | |
| GG | 34 (37.0%) | 20 (31.7%) | |
The genotype frequencies of CYP, PON1 Q192R, and ABCB1 C3435T in normal Han Chinese population (n = 92) and normal Caucasian population (n = 92) are shown. Normal Han Chinese population has a higher CYP 2C19*2 mutant allele carrier rate (12% for *2/*2 and 19% for wt/*2) than that of the Caucasian population (2.2% for *2/*2 and 22.8% for wt/*2). The Han Chinese population also showed higher PON1 Q192R mutant allele carrier rate (80.1%; 44.5% for RR192 and 35.9% for QR192) than that in Caucasian population (35.9%; 4.4% for RR192 and 31.5% for QR192).
Limitations
Our study has some limitations. We had a limited sample size (n = 20 cases of ST) combined with the limited strength of the case-control design. Therefore, the identified ST cases in our study may not represent all ST cases, and we may have introduced a possible bias when selecting the control group. We did not use intravascular ultrasound (IVUS) during stent implantation. IVUS is a useful tool for detecting reduction in stent size and coronary dissection, which are powerful predictors of ST.
Conclusion
In conclusion, we found that the PON1 Q192R polymorphism was not associated with the risk of ST development after PCI in a Taiwanese population. However, the CYP 2C19*2 polymorphism remained a key risk factor for ST development in patients who had undergone PCI.
Acknowledgments
We thank Mei-Hsu Lin, Yu-Jung Hu and Hu-Chiu Chen for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants to Chao-Yung Wang from the National Health Research Institute (NHRI-EX100-9925SC), the National Science Council (98-2314-B-182A-082-MY3) and the Chang Gung Memorial Hospital (CMRPG391861) and to Ming-Shien Wen from NRPB (101TM1033) and the Chang Gung Memorial Hospital (CMRPG3A1071). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 3.O’Donoghue M, Wiviott SD. Clopidogrel response variability and future therapies: clopidogrel: does one size fit all? Circulation. 2006;114:e600–606. doi: 10.1161/CIRCULATIONAHA.106.643171. [DOI] [PubMed] [Google Scholar]
- 4.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, et al. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 5.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 6.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, et al. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154:221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Mangiacapra F, Barbato E. Residual platelet reactivity: predicting short- and long-term clinical outcome in patients undergoing percutaneous coronary revascularization. Biomark Med. 2010;4:421–434. doi: 10.2217/bmm.10.56. [DOI] [PubMed] [Google Scholar]
- 8.Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, et al. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. Thromb Haemost. 2010;103:841–848. doi: 10.1160/TH09-06-0418. [DOI] [PubMed] [Google Scholar]
- 9.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 10.Hulot JS. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 12.Fontana P, Hulot JS, De Moerloose P, Gaussem P. Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J Thromb Haemost. 2007;5:2153–2155. doi: 10.1111/j.1538-7836.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 13.Umemura K, Furuta T, Kondo K. The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics in an active metabolite of clopidogrel in healthy subjects. J Thromb Haemost. 2008;6:1439–1441. doi: 10.1111/j.1538-7836.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 14.Frere C, Cuisset T, Morange PE, Quilici J, Camoin Jau L, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol. 2008;101:1088–1093. doi: 10.1016/j.amjcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 15.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, et al. Relation of Cytochrome P450 2C19 Loss-of-Function Polymorphism to Occurrence of Drug-Eluting Coronary Stent Thrombosis. The American Journal of Cardiology. 2009;103:806–811. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 17.Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 18.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 19.Zabalza M, Subirana I, Sala J, Lluis Ganella C, Lucas G, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012;98:100–108. doi: 10.1136/hrt.2011.227652. [DOI] [PubMed] [Google Scholar]
- 20.Angiolillo DJ, Fernandez Ortiz A, Bernardo E, Ramirez C, Cavallari U, et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler Thromb Vasc Biol. 2006;26:1895–1900. doi: 10.1161/01.ATV.0000223867.25324.1a. [DOI] [PubMed] [Google Scholar]
- 21.Simon T, Verstuyft C, Mary Krause M, Quteineh L, Drouet E, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 22.Aynacioglu AS, Kepekci Y. The human paraoxonase Gln-Argl92 (Q/R) polymorphism in turkish patients with coronary artery disease. Int J Cardiol. 2000;74:33–37. doi: 10.1016/s0167-5273(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira SA, Mansur AP, Ribeiro CC, Ramires JA, Annichino Bizzacchi JM. PON1 M/L55 mutation protects high-risk patients against coronary artery disease. Int J Cardiol. 2004;94:73–77. doi: 10.1016/j.ijcard.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nature Medicine. 2010;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 25.Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, et al. Paraoxonase-1 Q192R Polymorphism and Antiplatelet Effects of Clopidogrel in Patients Undergoing Elective Coronary Stent Placement. Circ Cardiovasc Genet. 2011. [DOI] [PubMed]
- 26.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. European Heart Journal. 2011;32:1605–1613. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Steg PG, Becquemont L, Verstuyft C, Kotti S, et al. Effect of Paraoxonase-1 Polymorphism on Clinical Outcomes in Patients Treated With Clopidogrel After an Acute Myocardial Infarction. Clinical Pharmacology & Therapeutics. 2011;90:561–567. doi: 10.1038/clpt.2011.193. [DOI] [PubMed] [Google Scholar]
- 28.Hulot JS, Collet JP, Cayla G, Silvain J, Allanic F, et al. CYP2C19 But Not PON1 Genetic Variants Influence Clopidogrel Pharmacokinetics, Pharmacodynamics, and Clinical Efficacy in Post-Myocardial Infarction Patients. Circulation: Cardiovascular Interventions. 2011;4:422–428. doi: 10.1161/CIRCINTERVENTIONS.111.963025. [DOI] [PubMed] [Google Scholar]
- 29.Li WF, Pan MH, Chung MC, Ho CK, Chuang HY. Lead exposure is associated with decreased serum paraoxonase 1 (PON1) activity and genotypes. Environ Health Perspect. 2006;114:1233–1236. doi: 10.1289/ehp.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X. Extensive Association Analysis Between Polymorphisms of PON Gene Cluster With Coronary Heart Disease in Chinese Han Population. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;23:328–334. doi: 10.1161/01.atv.0000051702.38086.c1. [DOI] [PubMed] [Google Scholar]
- 31.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, et al. Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 32.Pan WH, Fann CS, Wu JY, Hung YT, Ho MS, et al. Han Chinese cell and genome bank in Taiwan: purpose, design and ethical considerations. Hum Hered. 2006;61:27–30. doi: 10.1159/000091834. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Zhao YT, Verdo A, Qi WG, Zhang DF, et al. Relationship between cytochrome P450 2C19*2 polymorphism and stent thrombosis following percutaneous coronary intervention in Chinese patients receiving clopidogrel. J Int Med Res. 2011;39:2012–2019. doi: 10.1177/147323001103900548. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JP, Fisch AS, Ryan K, O’Connell JR, Gibson Q, et al. Paraoxonase 1 (PON1) Gene Variants Are Not Associated With Clopidogrel Response. Clinical Pharmacology & Therapeutics. 2011;90:568–574. doi: 10.1038/clpt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin BS. Paraoxonase Gene Polymorphism in South-western Korean Population. Journal of Korean Medical Science. 2009;24:561. doi: 10.3346/jkms.2009.24.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang HH, Liao YW, Chiang HL, Wu JY, Chen YT. Novel DNA sequence variations of cytochrome P450 genes in the Han Chinese population. Pharmacogenomics. 2009;10:359–374. doi: 10.2217/14622416.10.3.359. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Xiang P, Li L, Shen M. Analysis of 50 SNPs in CYP2D6, CYP2C19, CYP2C9, CYP3A4 and CYP1A2 by MALDI-TOF mass spectrometry in Chinese Han population. Forensic Science International. 2011;207:183–187. doi: 10.1016/j.forsciint.2010.10.004. [DOI] [PubMed] [Google Scholar]