Abstract
Targeting of the epidermal growth factor receptor (egfr) pathway has become routine practice in the treatment of lung carcinoma. As more health authorities approve targeted compounds in a variety of treatment lines, use of this approach is expected only to increase.
Gefitinib, an oral tyrosine kinase inhibitor (tki), is approved by Health Canada in the first-line setting of advanced non-small-cell lung carcinoma (nsclc) for tumours that harbour the EGFR gene mutation. Erlotinib, another tki, is currently approved in advanced nsclc in the second- and third-line settings.
The side-effect profile of this class of drugs is unique. Hematologic toxicity is seldom seen. The most frequent side effects are rash and diarrhea. Although no randomized trials have addressed treatment of the side effects of this class of drugs, some basic principles of management have been agreed on and can likely improve patient compliance and decrease inappropriate dose reduction. The prognostic and predictive implications of side effects are also evolving.
Finally, the ALK fusion mutation is being recognized as a mutation driver. The use of crizotinib (again, a tki) in this setting awaits approval. The side-effect profile of crizotinib is interesting and is also reviewed here.
Keywords: Lung cancer, side effects, targeted therapy, rash, diarrhea
1. INTRODUCTION
Tyrosine kinase inhibitors (tkis) that target the epidermal growth factor receptor (egfr) are small molecules given orally. By blocking the intracellular adenosine triphosphate binding site, they prevent the completion of phosphorylation and inhibit the signalling cascade that activates growth and proliferation factors 1.
In 2004, erlotinib received approval from the U.S. Food and Drug Administration for patients with advanced non-small-cell lung cancer (nsclc) in whom at least 1 prior chemotherapy regimen has failed. The approval was based on results of a pivotal phase iii trial (br.21) in which erlotinib prolonged median survival by 42.5% over best supportive care (6.7 months vs. 4.7 months, p < 0.001) in patients after 1 or 2 prior chemotherapy regimens 2.
The side effects reported in br.21 included diarrhea (50%) and rash (75%). Typically, the rash developed about 7–10 days after the start of treatment and affected skin areas above the waist. Spontaneous resolution of the rash without treatment has been reported. In most patients, the rash is mild (grade 1 or 2). Only 8% of patients experienced grade 3 rash, and fewer than 1% experienced grade 4 rash 2. For grades 3 and 4 rashes, the study protocol recommended stopping the drug for 7–10 days and then applying a 30% dose reduction. Subsequent analysis suggested a positive correlation between rash and response and survival 3.
In 2009, gefitinib was approved by Health Canada for patients with advanced nsclc whose tumours test positive for EGFR gene mutation (EGFR M+). Approval followed the reporting of ipass (the Iressa Pan-Asia Study), which randomized patients in Asia to either chemotherapy or to gefitinib in the first-line setting 4. That landmark trial changed the standard of care in that setting. The rates of diarrhea (46%) and rash (66%) were similar to those seen in the br.21 trial mentioned earlier. However, the rates of grade 3 or 4 diarrhea and rash (3.8% and 3.1% respectively) were less. Those findings mimic the experience of most clinicians treating patients outside a clinical trial, where the side effects of gefitinib seem to be less severe than the side effects of erlotinib.
2. PATHOPHYSIOLOGY OF EGFR INHIBITORS
Epidermal growth factor receptor is expressed in the basal layer of the epidermis. Its roles include stimulation of epidermal growth, inhibition of differentiation, and acceleration of wound healing. Effects of egfr inhibition include impaired growth and migration of keratinocytes and chemokine expression by those keratinocytes. Those effects lead to inflammatory cell recruitment and subsequent cutaneous injury, which accounts for most of the rash symptoms, including tenderness, papulopustules, and periungual inflammation. Histologic specimens reveal a mixed inflammatory infiltrate surrounding the upper areas of the dermis, follicular rupture, and epithelial acantholysis. Direct immunofluorescence studies show a nonspecific pattern of staining. Inhibition of Mek (mitogen-activated protein kinase kinase), a downstream effector in the egfr pathway, also leads to rash, indicating a mechanism-based effect 5.
2.1. Rash and Epidermis-Derived Organ Changes
The effects of egfr inhibitors on epidermal-derived tissue include facial and scalp acneiform eruption, truncal rash, dry skin, pruritus, nail changes, trichomegaly, and alopecia.
Common terms used to describe the rash are “acneiform skin reaction,” “acneform rash,” “acneiform follicular rash,” “acne-like rash,” “maculopapular skin rash,” and “monomorphic pustular lesions.” The rash develops in phases:
Week 1: sensory disturbance, erythema, and edema
Week 2: papulopustular eruption
Week 4: crusting
Weeks 4–6: if the rash is successfully treated, a background of erythema and dry skin appears in areas previously affected by the papulopustular eruption
Other events such as pruritus, erythema, and paronychial inflammation associated with the lateral nail folds of the toes and fingers can occur. Paronychia may occur after a longer period of treatment.
2.1.1. Treatment of Rash
In the randomized trials in which the primary endpoints were survival and response, rash treatment was often vague and not well-documented. Interpreting the randomized trials can also be difficult because rash was graded according to the U.S. National Cancer Institute criteria and may not accurately reflect the clinical situation.
The specific treatment algorithms for rash caused by egfr inhibitors vary widely between the expert centers that use those agents in their clinics. Nonetheless, some basic principles apply:
Patients should be educated on the importance of taking oral tkis on an empty stomach.
Patients should be instructed to use an alcoholfree emollient cream applied twice daily, preferably to the entire body.
Sun exposure can make the rash worse. A physical sunscreen should be applied to sun-exposed areas twice daily.
Skin reactions can be classified as mild, moderate, or severe:
Mild rash is generally localized, with few symptoms and no signs of infection. Treatment options include observation alone or a topical steroid such as hydrocortisone 1% or 2.5%. Addition of clindamycin 1% gel to hydrocortisone 1% is an option. The egfr inhibitor should be continued.
Moderate rash is generalized, with increased symptoms, but like mild rash, does not affect activities of daily living. Recommended treatment is a hydrocortisone 1% or 2.5% cream with or without clindamycin 1% gel, and a 4-week course of a twice-daily oral tetracycline antibiotic such as doxycycline 100 mg or minocycline 100 mg.
Severe rash is generalized and accompanied by major symptoms affecting activities of daily living and intolerable to the individual involved. A temporary 7- to 10-day discontinuation of the tki is recommended, with subsequent reintroduction at the dose reduction specified in the product monograph. Treatment with both a steroid cream and oral tetracycline as described for moderate rash is encouraged.
Rash may be successfully treated using the foregoing basic principles, but patients can often develop lesions and plaques on the scalp under hair. Scalp lesions can be treated with topical clindamycin 2% and triamcinolone acetonide 0.1% in equal parts of propylene glycol and water until resolution.
Dry skin over the trunk and extremities is very common. Creams and ointments are preferred over lotions, which may contain alcohol. Suitable emollient creams should be perfume-free. Aggressive twice-daily moisturizing can give the patient marked relief.
Nail changes are usually mild, but as with rash, may also be severe and symptomatic. Oral tetracycline is often effective. In truly resistant cases in which patients are receiving a marked cancer benefit, removal of the nail bed may be beneficial.
Eyelash growth can be seen when egfr inhibitors are given for a prolonged period of time. Intermittently cutting the eyelashes to a shorter length can add to patient comfort.
The hope is that, with proper education of both patient and physician, rash and other side effects caused by egfr inhibitors can be successfully treated in the early stages so that severe rashes do not occur and dose reductions can be avoided. Mild rash can disappear with just 50 mg minocycline daily.
Table i presents a treatment algorithm for rash.
TABLE I.
Treatment algorithm
| Grade of toxicity | Symptoms | Management | |
|---|---|---|---|
| 1 | Mild | Macular or papular eruption or erythema with no associated symptoms | Maintain dose level. Consider clindamycin 2% and hydrocortisone 1% in a lotion to be applied topically twice daily as needed. |
| 2 | Moderate | Macular or popular eruption or erythema with pruritus or other symptoms that are tolerable or that interfere with daily life | Maintain dose level. Clindamycin 2% and hydrocortisone 1% in a lotion to be applied topically twice daily as needed. Oral minocycline 100 mg twice daily for 4 weeks (or longer as needed). |
| 3 | Severe | Severe generalized erythroderma or macular, papular, or vesicular eruption | Withhold infusion for 2–4 weeks. Upon improvement to grade 2 or less, continue at 50% of original dose. If toxicities do not worsen, escalate. If no improvement, discontinue. Clindamycin 2% and hydrocortisone 1% in a lotion to be applied topically twice daily as needed. Oral minocycline 100 mg twice daily for 4 weeks (or longer as needed). |
2.1.2. Prognostic and Predictive Implications
As already mentioned, the most common side effect of egfr inhibitors is rash. Multiple studies with this class of drugs, both small-molecule and antibody, suggest that the incidence of rash may parallel both response and survival in an inverse fashion. That is, patients who experience the worst rash may benefit the most. All studies revealing such a relationship were retrospective.
The foregoing finding is difficult to understand and explain. Is it just a correlative relationship indicating a good host immune system? Or is there a direct relationship between egfr in the host’s skin and the tumour? If the latter, is prophylactic treatment, with rash incidence being delayed and decreased, safe? Is efficacy being sacrificed?
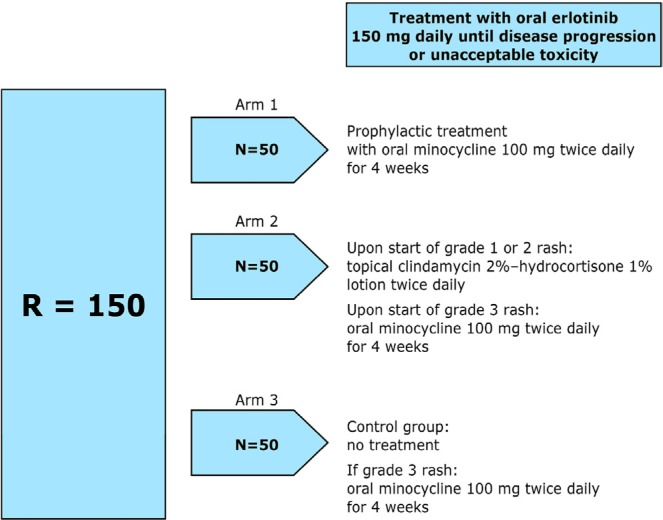
Results are expected soon from the Pan-Canadian Trial, in which patients starting erlotinib were randomized to one of three arms: prophylactic minocycline for 4 weeks, rash treatment according to grade, or no treatment unless rash is severe. This 150-patient trial is closed and is currently being analyzed. For the study schema, see Figure 1.
FIGURE 1.
Pan-Canadian rash trial design. Randomization will occur before treatment with erlotinib is initiated.
2.2. Diarrhea Induced by EGFR-TKIs
2.2.1. Diarrhea Incidence and Causes
The incidence of diarrhea with egfr-tkis varies from 27% to 87% in phase iii clinical trials, with up to 25% of patients experiencing severe reactions (grade 3 or higher). The incidence is increased with second-generation tkis, and so it is extremely important to monitor, provide education about, and treat this side effect (Table i). Patients should be advised to immediately discuss any symptoms of diarrhea with their health care team.
Diarrhea induced by egfr-tkis is thought to be a result of excess chloride secretion, causing a secretory form of diarrhea. Severe diarrhea can result in fluid and electrolyte losses, which may lead to dehydration, electrolyte imbalances, and renal insufficiency.
2.2.2. Assessment
The first step in the assessment of diarrhea potentially induced by egfr-tkis is to rule out other possible causes. Causes of diarrhea include medications such as laxatives, stool softeners, antacids, or antibiotics, and dietary factors such as excess consumption of fibre or lactose. Laboratory investigations are also useful to rule out other causes of diarrhea. Recommended laboratory investigations include a complete blood count and differential to rule out neutropenia, blood tests to assess renal function and to determine the presence of electrolyte abnormalities, and stool culture or a Clostridium difficile toxin screen to detect the presence of bacterial pathogens. Other investigations may include abdominal radiography, endoscopy, or biopsy to rule out coexisting disorders such as bowel obstruction or perforation.
2.2.3. Management
Although egfr-tki–induced diarrhea is usually mild to moderate, early management is essential to prevent dose reduction or discontinuation of anticancer therapies. Management is identical to that for chemotherapy-induced diarrhea. Daily fluid intake of approximately 3–4 L is recommended to avoid dehydration from volume loss. At least some of the fluids should contain sugar or salt to avoid hyponatremia and hypokalemia caused by electrolyte loss. The pharmacologic management of diarrhea is usually limited to treatment with over-the-counter loperamide. Patients should begin taking loperamide at the first sign of diarrhea, starting with 4 mg (2 tablets) and then 2 mg (1 tablet) every 4 hours or after each loose stool, to a maximum dose of 20 mg (10 tablets) daily. If diarrhea persists for more than 24 hours, the dose of loperamide may be increased to 4 mg at the start, followed by 2 mg every 2 hours. After 12 hours have passed with no episodes of diarrhea, pharmacologic treatment can be stopped, and the patient’s diet can be expanded as tolerated. If patients present with grade 3 or 4 diarrhea, dose reduction or discontinuation of the egfr-tki may be necessary. Once severe diarrhea has subsided, the egfr-tki may be restarted at a lower dose
3. OTHER TARGETED AGENTS
Chromosomal rearrangements involving the tyrosine kinase anaplastic lymphoma kinase (ALK) occur in 3%–5% of nsclc cases. The activation of Alk signaling leads to marked sensitivity to Alk inhibitors such as crizotinib (another tki).
Crizotinib was studied in 255 patients with locally advanced or metastatic ALK-positive nsclc across two multicentre single-arm studies. Response rates of more than 60% led to the U.S. Food and Drug Administration approving crizotinib in patients with advanced nsclc harboring an ALK fusion mutation. Health Canada approval is expected.
Crizotinib was well tolerated. The most common adverse reactions are vision disorder, nausea, diarrhea, vomiting, edema, and constipation. Grade 3 and 4 adverse reactions were rare (4%). When present, they included neutropenia and increased levels of transaminase, especially alanine aminotransferase.
The visual disorders were unexpected. Impairments included photopsia, blurred vision, vitreous floaters, photophobia, and diplopia. These disorders were common and were reported in 62% of patients in clinical trials. The events generally started within 2 weeks of drug administration. The causes of the visual side effects are not yet understood. The effects are not permanent and not threatening to vision. Nonetheless, ophthalmologic evaluation should be considered, particularly if patients experience photopsia or experience new or increased vitreous floaters 6.
4. SUMMARY
Currently, lung cancer treatment targets the egfr pathway using oral tkis. These agents have become standard treatment in lung carcinoma in multiple lines. The side effects shared by this class of drugs are skin rash and other epidermal changes. The inverse relationship between benefit and the incidence of side effects is extremely interesting. Prospective trials are needed to help fully understand that relationship. The use of prophylactic treatment to curb side effects should await the results of those trials. Rash management is a key factor in patient tolerance and compliance. Severe rash and dose reductions can be avoided with proper education for patient and physician alike.
The side effects seen with inhibition of ALK fusion are unique. An increase in liver transaminases might be seen and must be followed. Visual disorders are common, and the causes remain unknown.
As cancer treatment moves into the world of targeted therapy, a wle new range of side effects, much different than those seen with traditional chemotherapy, are emerging. It is an exciting but challenging time in the world of thoracic oncology.
5. CONFLICT OF INTEREST DISCLOSURES
BM has received honoraria from Roche, Lilly, and Boehringer Ingelheim Pharmaceuticals.
6. REFERENCES
- 1.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor–related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-I. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. on behalf of the National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase iii studies. Clin Cancer Res. 2007;13:3913–21. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Lacouture ME. Mechanisms of cutaneous toxicities to egfr inhibitors. Nat Rev Cancer. 2006;6:803–12. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Yeap BY, Mino–Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4–ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]