Abstract
Non-small-cell lung cancer (nsclc) remains the leading cause of cancer-related death globally, with most patients presenting with non-curable disease. Platinum-based doublet chemotherapy has been the cornerstone of treatment for patients with advanced-stage disease and has resulted in a modest increase in overall survival (on the order of an incremental 2 months increased survival per decade) and quality of life. Improved knowledge of the molecular signalling pathways found in nsclc has led to the development of biomarkers with associated targeted therapeutics, thus changing the treatment paradigm for many nsclc patients. In this review, we present a summary of many of the currently investigated nsclc targets, discuss their current clinical trial status, and provide commentary as to the likelihood of their success making a positive impact for nsclc patients.
Keywords: Lung cancer, clinical trials, novel targets, novel therapeutics
1. INTRODUCTION
The global burden of non-small-cell lung carcinoma (nsclc) and the very modest improvement in survival for advanced-stage patients since the early 1990s argues strongly for a paradigm shift in treatment strategies 1,2. With the advent of improved molecular methodologies and, now, next-generation sequencing, it is predicted that during the next 5 years, most patients with nsclc will be found to have either activating mutations, translocations creating fusion proteins, or gene amplifications that will allow for therapy with targeted agents or, at the very least, for the generation of treatment algorithms based on tumour re-classification prognostication 3.
There is little doubt that targeted therapies in nsclc have increased survival in select patient groups. The small-molecule epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) erlotinib and gefitinib now have defined roles in patient treatment.
Two pivotal phase iii studies (ipass and interest) highlighted the role of gefitinib. In chemotherapynaïve patients, ipass compared gefitinib with carboplatin–paclitaxel. In the EGFR-unselected population, the study showed no benefit of gefitinib in overall survival, time to progression, or overall response rate. However, in patients with EGFR mutated tumours, progression-free survival (pfs) was significantly longer [hazard ratio (hr): 0.48; 95% confidence interval (ci): 0.36 to 0.64; p < 0.001] 4. In the phase iii interest trial, single-agent gefitinib was noninferior to docetaxel in second- and third-line treatment. No difference in benefit was seen in patients with EGFR gene amplification; however, a suggestion of benefit in terms of overall response rate and pfs was observed in an unplanned analysis of patients with EGFR mutation 5.
The first phase iii study directly comparing erlotinib with standard chemotherapy in the first-line advanced setting in Chinese patients with an activating EGFR mutation was the optimal trial. That trial showed a pfs of 13.1 months with erlotinib compared with 4.6 months with gemcitabine–carboplatin chemotherapy (hr: 0.16; 95% ci: 0.1 to 0.26; p < 0.001) 6. A second trial called eurtac, the first to involve a Western European population, randomized patients to a platinum-based doublet chemotherapy regimen (docetaxel–gemcitabine) or to erlotinib in patients with an EGFR activating mutation. Patients treated with erlotinib experienced a pfs advantage (9.7 months vs. 5.2 months; hr: 0.37; 95% ci: 0.25 to 0.54) 7. Additionally, the phase iii saturn trial examined erlotinib as maintenance therapy after platinum-based chemotherapy. That trial met the primary endpoint of significantly longer pfs in patients treated with erlotinib (12.3 weeks) than in patients receiving placebo (11.1 weeks; hr: 0.69; 95% ci: 0.58 to 0.82; p < 0.0001) 8.
Similarly, the roles of EML4–ALK gene rearrangements and of targeted Alk tyrosine kinase inhibitors as active agents in nsclc patients have been established. Rearrangements of the ALK gene are felt to be mutually exclusive of EGFR and KRAS mutations and occur in approximately 4% of lung cancers. The ALK mutations are more common in adenocarcinomas and in light smokers or nonsmokers 9. Crizotinib, an oral atp-selective inhibitor of Alk tyrosine kinase, received approval from the U.S. Food and Drug Administration for that setting in 2011. The phase i trial of this agent in advanced ALK-positive nsclc revealed a response rate of 57% (95% ci: 46% to 68%) and an estimated 6-month pfs probability of 72% (95% ci: 61% to 83%) 10. A retrospective review of 82 ALK-positive patients (including patients who had received multiple lines of therapy) treated with crizotinib revealed an impressive 1-year survival of 74% (95% ci: 63% to 82%) and 2-year survival of 54% (95% ci: 40% to 66%) 11.
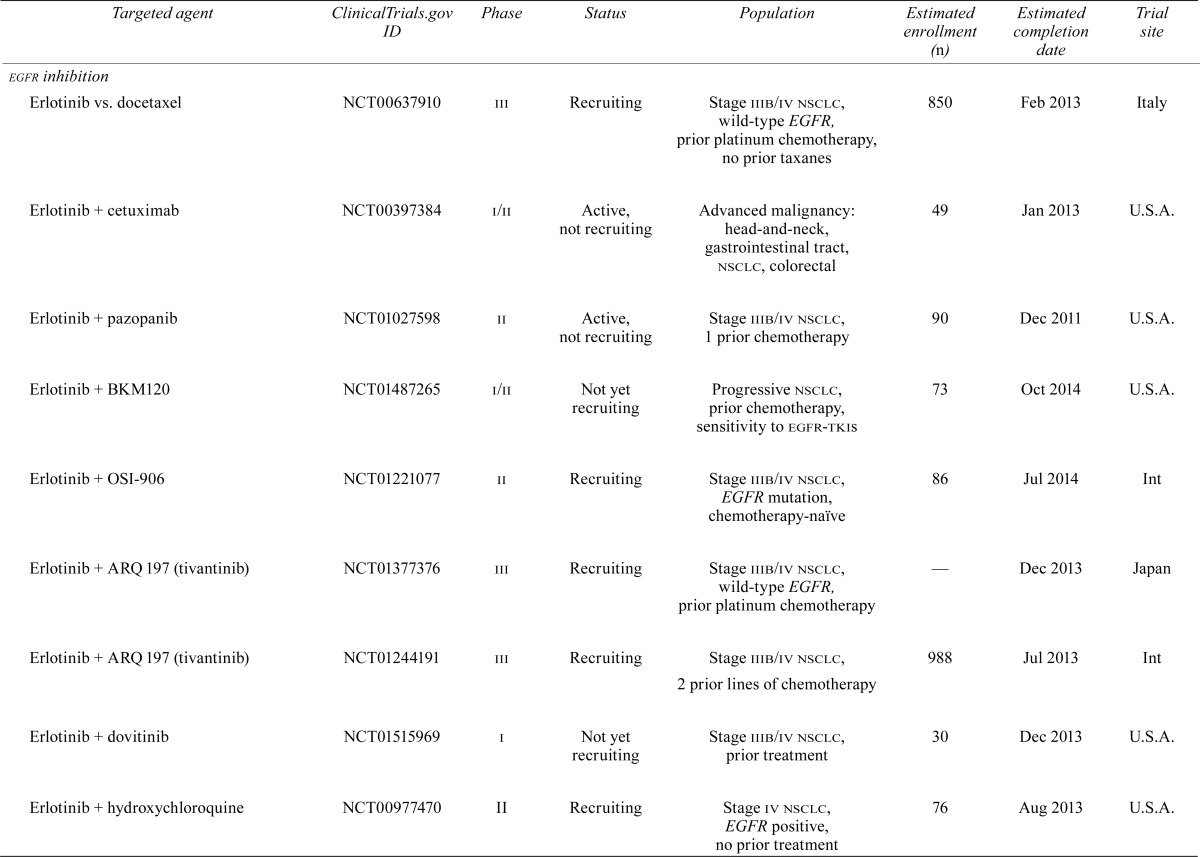
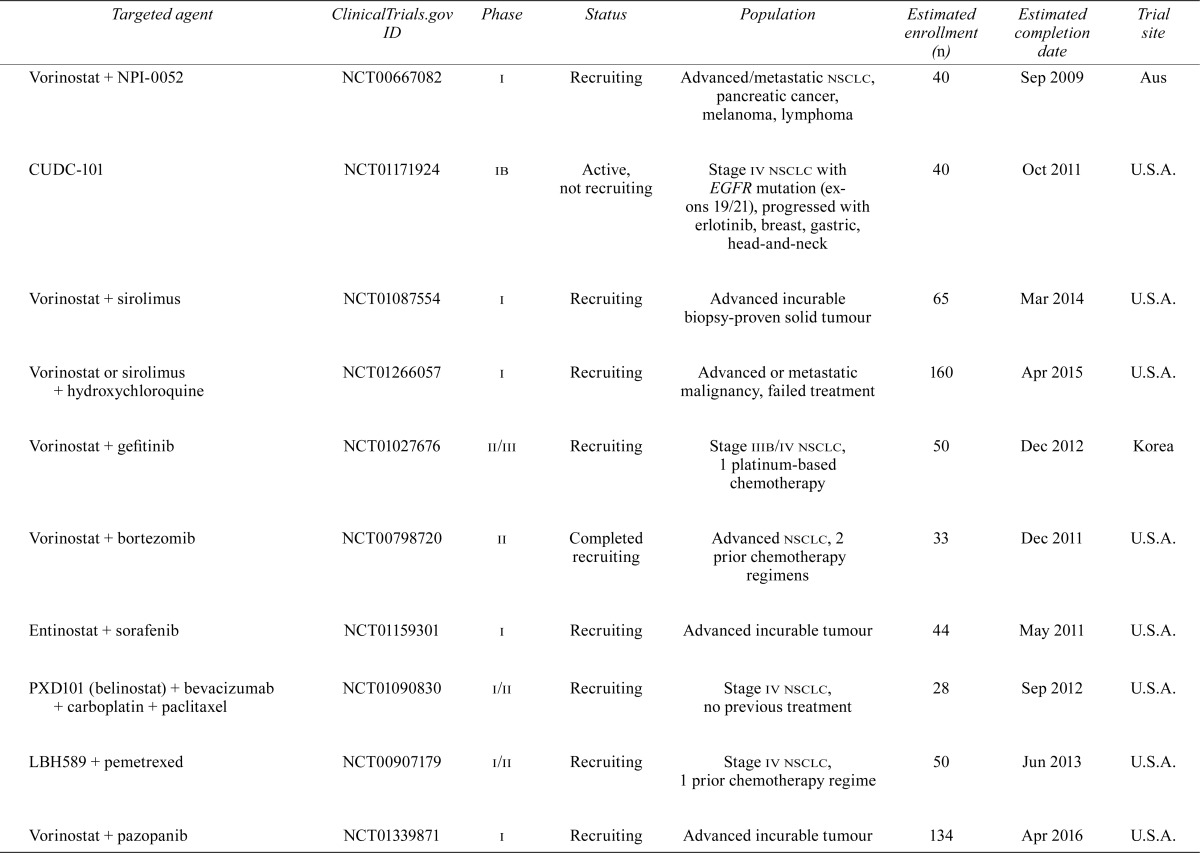
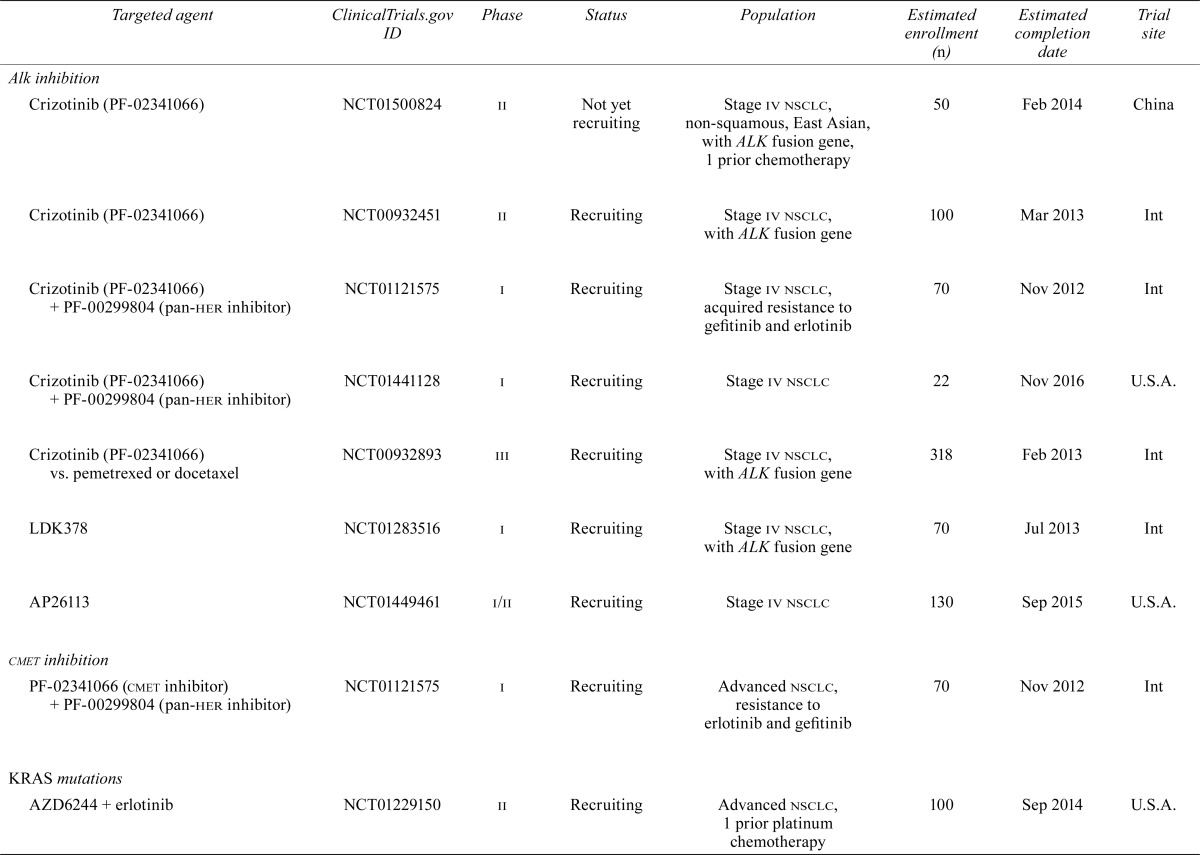
Despite the significant improvement in outcomes for these highly selected patients, treatment failures secondary to resistance are now being seen. Combinations of targeted therapies or dual inhibitors may overcome resistance; however, it is more likely that such strategies will simply delay the inevitable. The subsections that follow review the potential targets that hold promise for nsclc patients and the current or planned trials involving those targets. Table i summarizes what we feel are the relevant trials at the time of writing.
TABLE I.
Current or planned trials involving targeted agents relevant to patients with non-small-cell lung cancer
| Targeted agent | ClinicalTrials.gov ID | Phase | Status | Population | Estimated enrollment (n) | Estimated completion date | Trial site |
|---|---|---|---|---|---|---|---|
| egfr inhibition | |||||||
| Erlotinib vs. docetaxel | NCT00637910 | iii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy, no prior taxanes | 850 | Feb 2013 | Italy |
| Erlotinib + cetuximab | NCT00397384 | i/ii | Active, not recruiting | Advanced malignancy: head-and-neck, gastrointestinal tract, nsclc, colorectal | 49 | Jan 2013 | U.S.A. |
| Erlotinib + pazopanib | NCT01027598 | ii | Active, not recruiting | Stage iiib/iv nsclc, 1 prior chemotherapy | 90 | Dec 2011 | U.S.A. |
| Erlotinib + BKM120 | NCT01487265 | i/ii | Not yet recruiting | Progressive nsclc, prior chemotherapy, sensitivity to egfr-tkis | 73 | Oct 2014 | U.S.A. |
| Erlotinib + OSI-906 | NCT01221077 | ii | Recruiting | Stage iiib/iv nsclc, EGFR mutation, chemotherapy-naïve | 86 | Jul 2014 | Int |
| Erlotinib + ARQ 197 (tivantinib) | NCT01377376 | iii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy | — | Dec 2013 | Japan |
| Erlotinib + ARQ 197 (tivantinib) | NCT01244191 | iii | Recruiting | Stage iiib/iv nsclc, 2 prior lines of chemotherapy | 988 | Jul 2 013 | Int |
| Erlotinib + dovitinib | NCT01515969 | i | Not yet recruiting | Stage iiib/iv nsclc, prior treatment | 30 | Dec 2013 | U.S.A. |
| Erlotinib + hydroxychloroquine | NCT00977470 | ii | Recruiting | Stage iv nsclc, EGFR positive, no prior treatment | 76 | Aug 2013 | U.S.A. |
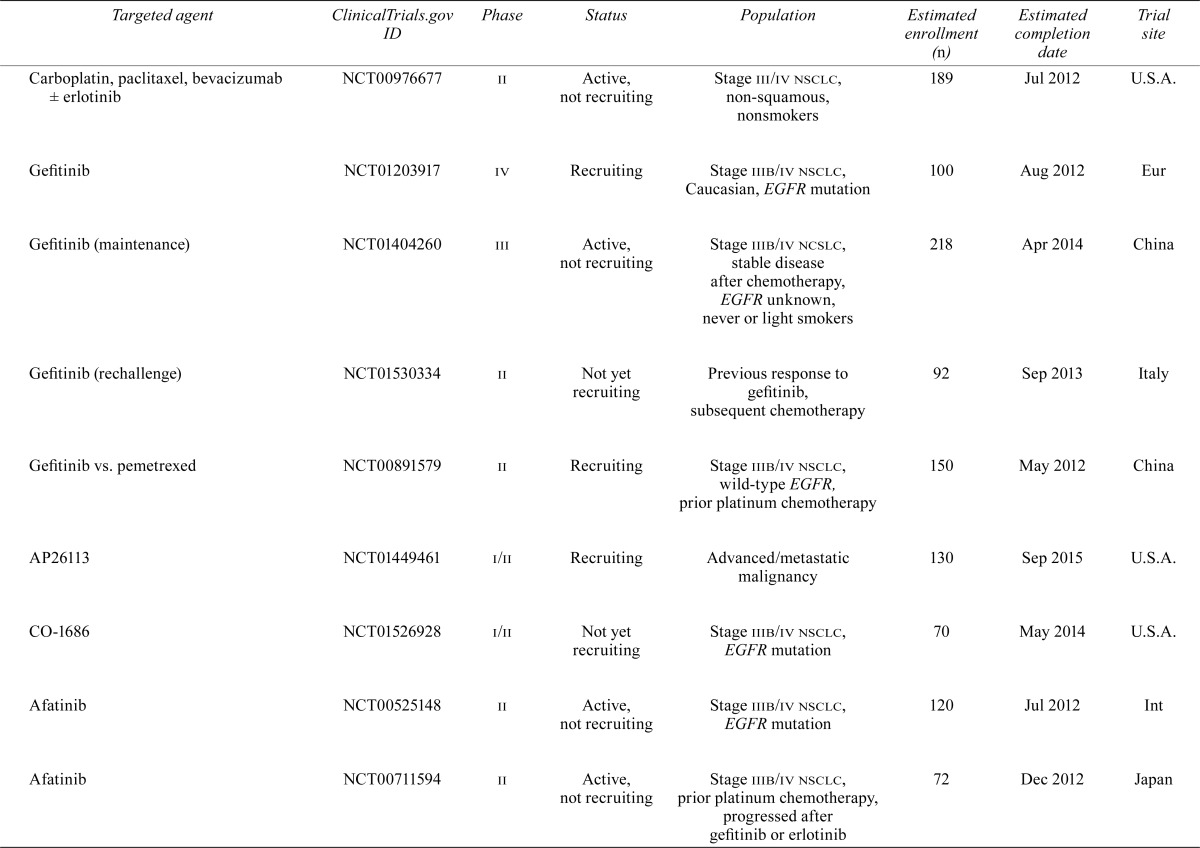
| Carboplatin, paclitaxel, bevacizumab ± erlotinib | NCT00976677 | ii | Active, not recruiting | Stage iii/iv nsclc, non-squamous, nonsmokers | 189 | Jul 2012 | U.S.A. |
| Gefitinib | NCT01203917 | iv | Recruiting | Stage iiib/iv nsclc, Caucasian, EGFR mutation | 100 | Aug 2012 | Eur |
| Gefitinib (maintenance) | NCT01404260 | iii | Active, not recruiting | Stage iiib/iv ncslc, stable disease after chemotherapy, EGFR unknown, never or light smokers | 218 | Apr 2014 | China |
| Gefitinib (rechallenge) | NCT01530334 | ii | Not yet recruiting | Previous response to gefitinib, subsequent chemotherapy | 92 | Sep 2013 | Italy |
| Gefitinib vs. pemetrexed | NCT00891579 | ii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy | 150 | May 2012 | China |
| AP26113 | NCT01449461 | i/ii | Recruiting | Advanced/metastatic malignancy | 130 | Sep 2015 | U.S.A. |
| CO-1686 | NCT01526928 | i/ii | Not yet recruiting | Stage iiib/iv nsclc, EGFR mutation | 70 | May 2014 | U.S.A. |
| Afatinib | NCT00525148 | ii | Active, not recruiting | Stage iiib/iv nsclc, EGFR mutation | 120 | Jul 2012 | Int |
| Afatinib | NCT00711594 | ii | Active, not recruiting | Stage iiib/iv nsclc, prior platinum chemotherapy, progressed after gefitinib or erlotinib | 72 | Dec 2012 | Japan |
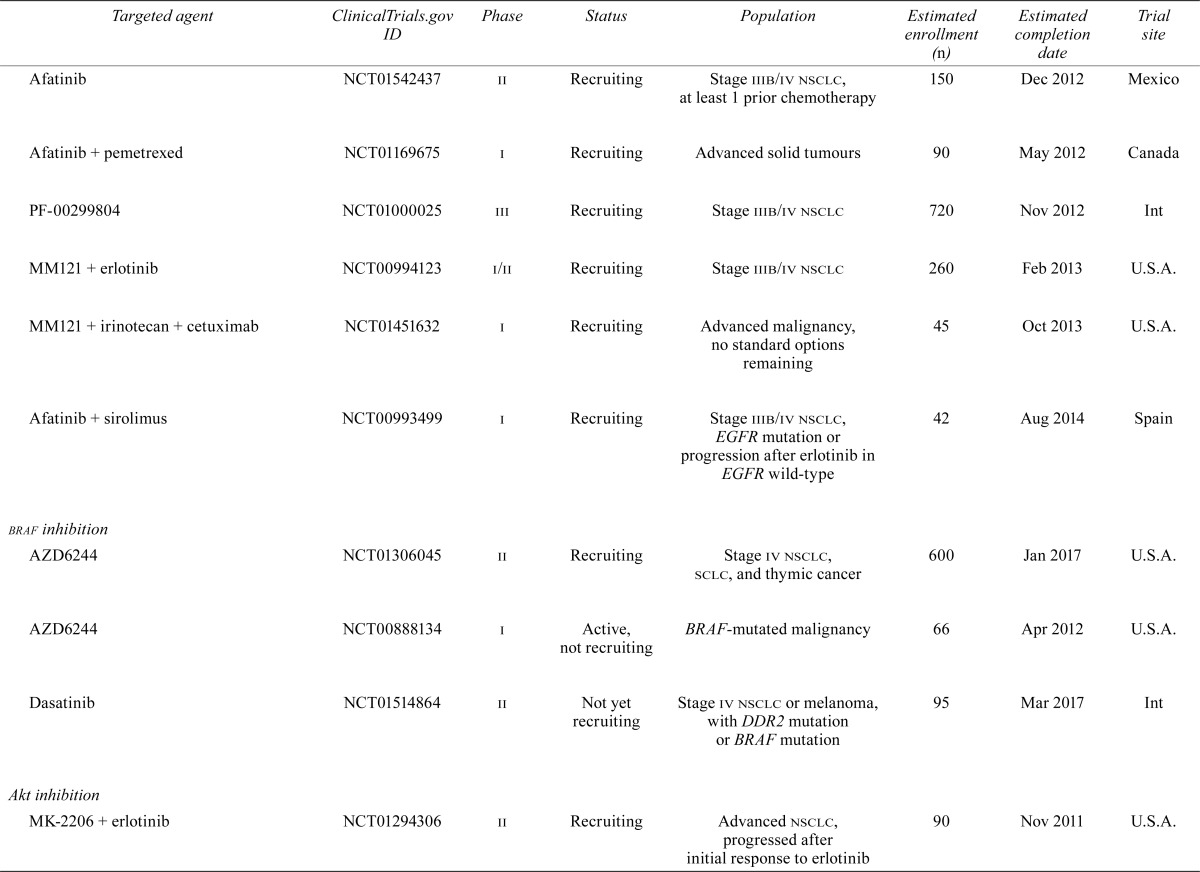
| Afatinib | NCT01542437 | ii | Recruiting | Stage iiib/iv nsclc, at least 1 prior chemotherapy | 150 | Dec 2012 | Mexico |
| Afatinib + pemetrexed | NCT01169675 | i | Recruiting | Advanced solid tumours | 90 | May 2012 | Canada |
| PF-00299804 | NCT01000025 | iii | Recruiting | Stage iiib/iv nsclc | 720 | Nov 2012 | Int |
| MM121 + erlotinib | NCT00994123 | i/ii | Recruiting | Stage iiib/iv nsclc | 260 | Feb 2013 | U.S.A. |
| MM121 + irinotecan + cetuximab | NCT01451632 | i | Recruiting | Advanced malignancy, no standard options remaining | 45 | Oct 2013 | U.S.A. |
| Afatinib + sirolimus | NCT00993499 | i | Recruiting | Stage iiib/iv nsclc, EGFR mutation or progression after erlotinib in EGFR wild-type | 42 | Aug 2014 | Spain |
| braf inhibition | |||||||
| AZD6244 | NCT01306045 | ii | Recruiting | Stage iv nsclc, sclc, and thymic cancer | 600 | Jan 2017 | U.S.A. |
| AZD6244 | NCT00888134 | i | Active, not recruiting | BRAF-mutated malignancy | 66 | Apr 2012 | U.S.A. |
| Dasatinib | NCT01514864 | ii | Not yet recruiting | Stage iv nsclc or melanoma, with DDR2 mutation or BRAF mutation | 95 | Mar 2017 | Int |
| Akt inhibition | |||||||
| MK-2206 + erlotinib | NCT01294306 | ii | Recruiting | Advanced nsclc, progressed after initial response to erlotinib | 90 | Nov 2011 | U.S.A. |
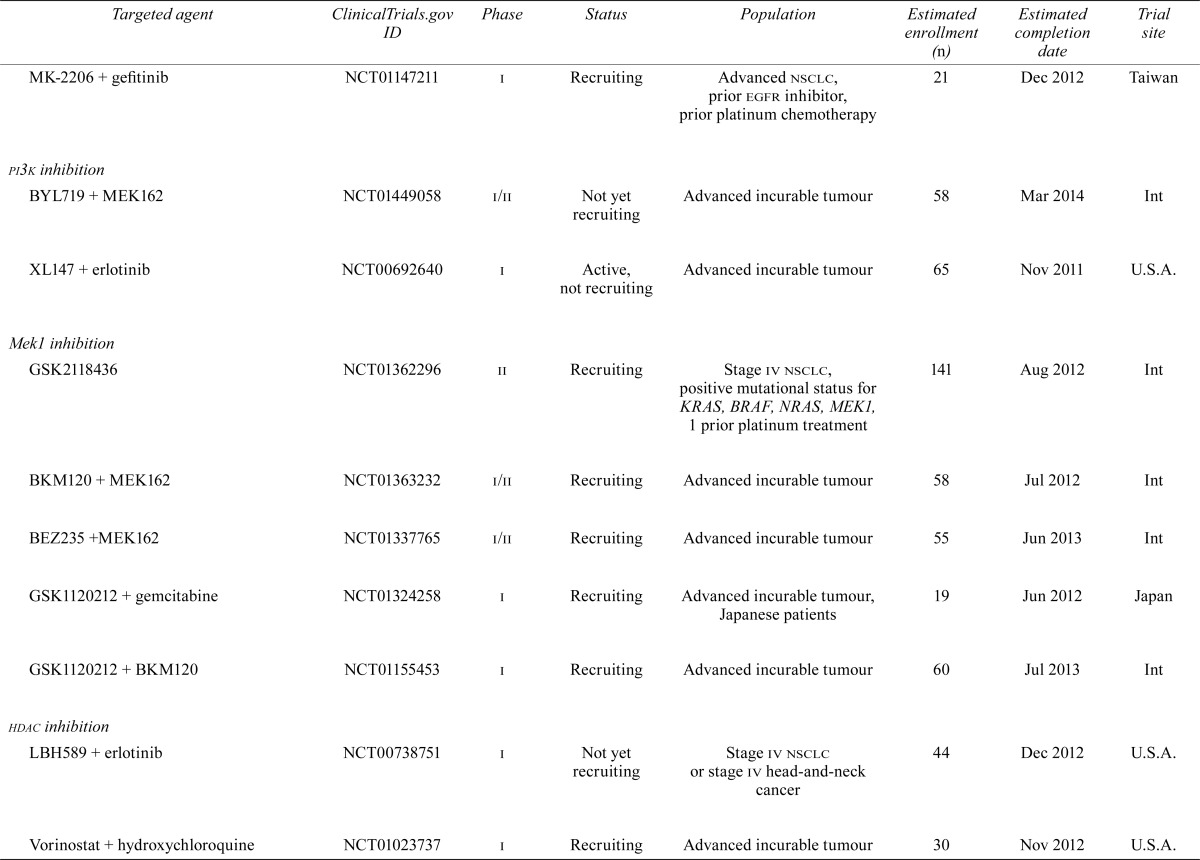
| MK-2206 + gefitinib | NCT01147211 | i | Recruiting | Advanced nsclc, prior egfr inhibitor, prior platinum chemotherapy | 21 | Dec 2012 | Taiwan |
| pi3k inhibition | |||||||
| BYL719 + MEK162 | NCT01449058 | i/ii | Not yet recruiting | Advanced incurable tumour | 58 | Mar 2014 | Int |
| XL147 + erlotinib | NCT00692640 | i | Active, not recruiting | Advanced incurable tumour | 65 | Nov 2011 | U.S.A. |
| Mek1 inhibition | |||||||
| GSK2118436 | NCT01362296 | ii | Recruiting | Stage iv nsclc, positive mutational status for KRAS, BRAF, NRAS, MEK1, 1 prior platinum treatment | 141 | Aug 2012 | Int |
| BKM120 + MEK162 | NCT01363232 | i/ii | Recruiting | Advanced incurable tumour | 58 | Jul 2012 | Int |
| BEZ235 +MEK162 | NCT01337765 | i/ii | Recruiting | Advanced incurable tumour | 55 | Jun 2013 | Int |
| GSK1120212 + gemcitabine | NCT01324258 | i | Recruiting | Advanced incurable tumour, Japanese patients | 19 | Jun 2012 | Japan |
| GSK1120212 + BKM120 | NCT01155453 | i | Recruiting | Advanced incurable tumour | 60 | Jul 2013 | Int |
| hdac inhibition | |||||||
| LBH589 + erlotinib | NCT00738751 | i | Not yet recruiting | Stage iv nsclc or stage iv head-and-neck cancer | 44 | Dec 2012 | U.S.A. |
| Vorinostat + hydroxychloroquine | NCT01023737 | i | Recruiting | Advanced incurable tumour | 30 | Nov 2012 | U.S.A. |
| Vorinostat + NPI-0052 | NCT00667082 | i | Recruiting | Advanced/metastatic nsclc, pancreatic cancer, melanoma, lymphoma | 40 | Sep 2009 | Aus |
| CUDC-101 | NCT01171924 | ib | Active, not recruiting | Stage iv nsclc with EGFR mutation (exons 19/21), progressed with erlotinib, breast, gastric, head-and-neck | 40 | Oct 2011 | U.S.A. |
| Vorinostat + sirolimus | NCT01087554 | i | Recruiting | Advanced incurable biopsy-proven solid tumour | 65 | Mar 2014 | U.S.A. |
| Vorinostat or sirolimus + hydroxychloroquine | NCT01266057 | i | Recruiting | Advanced or metastatic malignancy, failed treatment | 160 | Apr 2015 | U.S.A. |
| Vorinostat + gefitinib | NCT01027676 | ii/iii | Recruiting | Stage iiib/iv nsclc, 1 platinum-based chemotherapy | 50 | Dec 2012 | Korea |
| Vorinostat + bortezomib | NCT00798720 | ii | Completed recruiting | Advanced nsclc, 2 prior chemotherapy regimens | 33 | Dec 2011 | U.S.A. |
| Entinostat + sorafenib | NCT01159301 | i | Recruiting | Advanced incurable tumour | 44 | May 2011 | U.S.A. |
| PXD101 (belinostat) + bevacizumab + carboplatin + paclitaxel | NCT01090830 | i/ii | Recruiting | Stage iv nsclc, no previous treatment | 28 | Sep 2012 | U.S.A. |
| LBH589 + pemetrexed | NCT00907179 | i/ii | Recruiting | Stage iv nsclc, 1 prior chemotherapy regime | 50 | Jun 2013 | U.S.A. |
| Vorinostat + pazopanib | NCT01339871 | i | Recruiting | Advanced incurable tumour | 134 | Apr 2016 | U.S.A. |
| Alkinhibition | |||||||
| Crizotinib (PF-02341066) | NCT01500824 | ii | Not yet recruiting | Stage iv nsclc, non-squamous, East Asian, with ALK fusion gene, 1 prior chemotherapy | 50 | Feb 2014 | China |
| Crizotinib (PF-02341066) | NCT00932451 | ii | Recruiting | Stage iv nsclc, with ALK fusion gene | 100 | Mar 2013 | Int |
| Crizotinib (PF-02341066) + PF-00299804 (pan-her inhibitor) | NCT01121575 | i | Recruiting | Stage iv nsclc, acquired resistance to gefitinib and erlotinib | 70 | Nov 2012 | Int |
| Crizotinib (PF-02341066) + PF-00299804 (pan-her inhibitor) | NCT01441128 | i | Recruiting | Stage iv nsclc | 22 | Nov 2016 | U.S.A. |
| Crizotinib (PF-02341066) vs. pemetrexed or docetaxel | NCT00932893 | iii | Recruiting | Stage iv nsclc, with ALK fusion gene | 318 | Feb 2013 | Int |
| LDK378 | NCT01283516 | i | Recruiting | Stage iv nsclc, with ALK fusion gene | 70 | Jul 2013 | Int |
| AP26113 | NCT01449461 | i/ii | Recruiting | Stage iv nsclc | 130 | Sep 2015 | U.S.A. |
| cmet inhibition | |||||||
| PF-02341066 (cmet inhibitor) + PF-00299804 (pan-her inhibitor) | NCT01121575 | i | Recruiting | Advanced nsclc, resistance to erlotinib and gefitinib | 70 | Nov 2012 | Int |
| KRAS mutations | |||||||
| AZD6244 + erlotinib | NCT01229150 | ii | Recruiting | Advanced nsclc, 1 prior platinum chemotherapy | 100 | Sep 2014 | U.S.A. |
| Erlotinib + ARQ 197 vs. single-agent chemotherapy | NCT01395758 | ii | Recruiting | Stage iv nsclc, with KRAS mutation | 98 | Jun 2012 | U.S.A. |
| GSK1120212 vs. docetaxel | NCT01362296 | ii | Recruiting | Stage iv nsclc, positive mutational status for KRAS, NRAS, BRAF, MEK1 | 141 | Aug 2012 | Int |
nsclc = non-small-cell lung cancer; egfr-tki = epidermal growth factor receptor tyrosine kinase inhibitor; Int = international (more than one continent); Eur = Europe; sclc = small-cell lung cancer; Aus = Australia; her = human epidermal growth factor receptor.
2. EGFR INHIBITION
Dysregulation of egfr is associated with poorer prognosis in nsclc 11. Although many patients with EGFR mutation will benefit from egfr-tkis, it is now appreciated a portion of the population will not benefit and that some will acquire resistance to those agents. Known mechanisms of resistance include an EGFR mutation that reduces the inhibitory ability of gefitinib or erlotinib, and MET amplification with subsequent activation of downstream pathways 12,13.
The discovery of resistance to the egfr-tkis has led to the development of second-generation egfr-tkis. PF 0299804 (dacomitinib) is currently the subject of a phase iii trial that will compare it with placebo in patients in whom standard treatment has failed.
PF 0299804 is an oral irreversible inhibitor of the egfr/her1, her2, and her4 tyrosine kinases. Preclinical data showed activity for PF 0299804 against EGFR mutations and T790M 12,14. Two phase ii studies highlighted the agent’s clinical antitumour effect, both in first-line therapy and in treatment-refractory patients. In the first of the studies, PF 0299804 was compared with erlotinib. That trial enrolled a range of molecular subgroups, including a group of patients with wild-type KRAS. In all subgroups, PF 0299804 showed a pfs advantage (12.4 weeks vs. 8.3 weeks; hr: 0.704; p = 0.030) 15. Another phase ii study of PF 0299804 showed preliminary evidence of tumour shrinkage in patients with EGFR activating mutations, wild-type EGFR, and mutations in exon 20 16.
Afatinib, an irreversible egfr-, her2-, and her4-tki demonstrated preclinical activity against the T790M mutation. The phase iib/iii lux-Lung 1 trial (randomized, double-blind) examined best supportive care plus afatinib or placebo in patients in whom chemotherapy and a reversible egfr inhibitor had failed. No difference in overall survival was observed; however, pfs was significantly improved with afatinib (3.3 months vs. 1.1 months with placebo; hr: 0.38; 95% ci: 0.306 to 0.475; p < 0.001), as were tumour-related symptoms 17 and quality of life.
A number of phase ii trials are currently examining afatinib. NCT00525148 has completed enrolment of patients with activating EGFR mutations in whom first-line chemotherapy has failed. Similarly, NCT00711594, a Japanese trial, has completed accrual; results are awaited from this group of stage iiib/iv nsclc patients who progressed after platinum chemotherapy and either erlotinib or gefitinib. A phase i trial, NCT01169675 will assess afatinib in combination with pemetrexed. The lux-Lung 3 trial has finished accrual of patients with EGFR mutation–positive tumours who are being treated in the first line with afatinib or cisplatin and pemetrexed. Results are to be reported at the 2012 American Society of Clinical Oncology annual meeting.
Targeted therapies in combination may have a synergistic benefit. A number of phase i trials are currently examining newer therapies in combination with erlotinib. Cetuximab, pazopanib, BKM120 (an inhibitor of pi3k), OSI-906 (an inhibitor of insulin-like growth factor 1 receptor), dovitinib (an inhibitor of fibroblast growth factor receptor), and MM121 (anti-ErbB3) are all in trial in combination with erlotinib.
3. Mek INHIBITION
The Raf/Ras/Mek pathway is one of the key signalling pathways involved in the pathogenesis of lung cancer. Activation of Ras and subsequent recruitment of Raf leads to a cascade of events, including phosphorylation of Mek1/Mek2 and the mapks 18. This pathway creates a number of treatment targets, including the Ras family, braf and Mek, with novel therapies already in trial.
K-ras is a member of the Ras family, and KRAS mutations are present in 15%–25% of nsclc tumours 19. The importance of KRAS as a prognostic marker arose from patient treatment failures in trials of egfr inhibitors. A meta-analysis examined the ability of KRAS mutations to predict a lack of response to egfr targeting agents. The sensitivity was low (0.47; 95% ci: 0.43 to 0.52), indicating alternative mechanisms for resistance to egfr inhibitors. However, KRAS was found to have a high specificity, suggesting that patients with a KRAS mutation were unlikely to respond to treatment based on anti-egfr agents 20. That finding has led to stratification and prognostication based on the KRAS mutation status of patients.
Although K-ras is difficult to target, interest in Mek inhibition is increasing. As a downstream effector of the egfr pathway that signals through K-ras, Mek inhibition may play a role in patients who become resistant to egfr inhibitors. A number of trials to examine Mek inhibitors alone or in combination with other targeted treatments are currently recruiting. GSK2118436 is a potent Mek inhibitor that has been shown to have preclinical activity in nsclc and melanoma. A phase ii trial is currently recruiting and will examine GSK2118436 in comparison with docetaxel in advanced nsclc patients with a KRAS mutation. For exploratory purposes, a small population of patients with BRAF, NRAS, and MEK1 mutations will also be included. Patients must have had previous exposure to platinum chemotherapy. The primary outcome will be pfs, and the trial is expected to be completed in late 2012.
A phase i trial of GSK1120212, another potent Mek inhibitor, in combination with gemcitabine is currently recruiting in Japan. MEK162 is a Mek1/2 inhibitor, and a number of phase i trials are currently examining the combination of this Mek inhibitor with pi3k inhibitors.
4. BRAF INHIBITION
The protein kinase braf is a member of the Raf family, kinases that act downstream of Ras and are responsible for further activation of the mapk pathway, controlling cellular proliferation 21. BRAF mutations were first identified in melanoma cells, with 80% of mutations involving the Val600 residue in the kinase domain. By contrast, BRAF mutations account for only 1%–3% of nsclc. These non-Val600Glu mutations include Gly468Ala and Leu596Val 22–24. BRAF mutations result in increased kinase activity and activation of mapk2 and mapk3 24. A number of studies are currently examining the effect of therapeutic agents on BRAF-mutated patients. AZD6244 is a Mek inhibitor that is the focus of two trials specifically examining its effect on BRAF-mutated patients. The NCT00888134 phase ii trial of AZD6244 is enrolling patients with metastatic malignancy and a BRAF mutation. The primary outcome is response rate, with secondary outcomes of pfs and response rate specifically in the nsclc and colon cancer populations.
NCT01514864, although not yet recruiting, is planning to examine the effect of dasatinib in patients with a malignancy harbouring a BRAF mutation. Dasatinib is a Src-tki, and abnormal expression of activated Src has been found in many tumour types, including nsclc 25. High levels of Src expression and activation have been correlated with poor prognosis in human malignancies 26. Src has been shown to interact with many signalling pathways, including egfr, mapk, pi3k/Akt, and vascular endothelial growth factor 27. The role of Src as a therapeutic target in nsclc is supported by preclinical studies 28. NCT01514864 will include patients with nsclc and melanoma with BRAF mutations, squamous cell nsclc with a DDR2 mutation, and any other malignancies with one of those two mutations.
5. Alk INHIBITION
Knowledge about ALK gene rearrangements, including the EML4–ALK fusion, is rapidly growing. That fusion arises from an inversion on the short arm of chromosome 2 that joins exons 1–14 of EML4 with exons 20–29 of ALK 29. Despite having a phenotype similar to that seen in EGFR mutations, ALK mutations occur almost exclusively in the absence of EGFR and KRAS mutations. Preclinical trials have shown that cells habouring ALK mutations are exquisitely sensitive to Alk inhibition 30. Crizotinib was the first of the Alk inhibitors to gain profile. The impressive results of a phase i trial, with a response rate of 57% and an estimated 6-month pfs of 72%, led directly to a phase iii trial and a host of other trials aiming to clarify the role of crizotinib in treatment 31.
The phase iii trial is comparing crizotinib with single-agent chemotherapy after a platinum-based regimen in 318 patients with a mutated ALK gene. The primary outcome is pfs, with secondary outcomes including response rate, overall survival, patient-reported symptoms, and EML4–ALK variants.
The exact position of crizotinib in the treatment paradigm continues to be explored in trials in the first line of treatment that are currently recruiting. Crizotinib pharmacokinetics have already been identified to possibly be different in Asian populations 10, but to date, that hypothesis has not been clarified. The phase ii NCT01500824 trial, which will focus on previously-treated ALK-mutated East Asian patients is not yet open to recruitment. The primary outcome will be response rate.
Investigation of resistance to current egfr inhibitors has highlighted the role of the c-Met/Alk pathway. Resistance can occur through not only the egfr pathway, but also c-Met/Alk. Combinations of egfr and c-Met/Alk inhibitors therefore hold potential for overcoming resistance 32. PF 0299804 is an irreversible pan-her inhibitor. Preclinical data have shown that this agent has activity against EGFR activating mutations and T790M 13,33. Two trials, currently recruiting, will examine the combination of crizotinib and PF 0299804, studying the combination in patients who show resistance to the egfr inhibitor (gefitinib or erlotinib).
New Alk inhibitors are under investigation, with phase i trials of LDK378 and AP26113 currently recruiting. NCT01449461, a phase i trial of AP26113, will be conducted in two parts, with the second part including expansion cohorts. The cohorts include ALK mutations with no previous exposure to Alk inhibitors, ALK mutation with resistance to an Alk inhibitor, EGFR mutation with resistance to egfr inhibitors, and non-lung malignancies with ALK mutations.
6. HDAC INHIBITION
Modification of dna and histones is the most common method of epigenetic gene control. The family of histone deacetylases (hdacs) plays a central role in that process. The hdacs act to tighten the bond between histones and dna, thus inhibiting gene transcription by blocking binding sites on promoters 15. Inhibition of hdacs leads to induction of apoptosis in malignant cells 34. The hdac inhibitors continue to be investigated in nsclc, and preclinical data suggest that they may increase E-cadherin and sensitize malignant cells to egfr inhibition 35. Consequently, in addition to monotherapy trials of new hdac inhibitors, combinations of egfr-tkis with hdac inhibitors are receiving focus.
In this class of therapeutics, vorinostat is currently the furthest along in development. Vorinostat is already known to act synergistically with a variety of chemotherapy agents, suggesting that it works through number of pathways, shifting the balance of apoptotic genes, inducing reactive oxygen species, and inhibiting angiogenesis 36. In preclinical studies, vorinostat combined with platinum or taxanes exhibits synergistic antitumour effects 37,38. A phase ii/iii trial in combination with paclitaxel and carboplatin was unfortunately stopped early because the trial did not meet a pre-specified proof-of-concept criterion.
A number of phase i trials to examine the effect of vorinostat with other targeted treatments are currently recruiting. Those targeted treatments include inhibitors of egfr, vascular endothelial growth factor, the mammalian target of rapamycin (mtor), and a proteasome inhibitor, NP10052. Vorinostat is well tolerated, a clinical advantage when looking for combination therapies.
New hdac inhibitors continue to be investigated. The effects of LBH589 (panobinostat) are currently under examination in both hematologic and solid tumours. Preclinical studies show synergy not only with chemotherapy, but also with proteasome inhibitors and demethylators 39,40. Two phase i trials of LDH589 are combining it with pemetrexed and erlotinib chemotherapy respectively. Belinostat (PXD101), another hdac inhibitor, has shown efficacy for induction of apoptosis in a number of solid malignancies 41. A phase i/ii study, NCT01090830, currently recruiting, will examine belinostat in combination with bevacizumab and platinum chemotherapy in treatment-naïve patients.
7. PI3K/Akt INHIBITION
The pi3k/Akt/mtor pathway is essential for cell proliferation, protein synthesis, and angiogenesis. The pi3k/Akt pathway upregulates mtor in response to stimulation by growth factors 42. The tumour suppressor gene PTEN antagonizes the pi3k/Akt pathway. Loss of inactivating mutations of PTEN results in a gain in function of the PI3KCA gene. Loss of PTEN, resulting in overexpression of phosphorylated Akt, is associated with poorer prognosis in lung cancer 43.
Several small molecules in early-phase clinical trials are currently known to target the mtor pathway. Everolimus, an oral mtor inhibitor, has shown activity in metastatic nsclc. A phase ii study of everolimus examined its use in nsclc patients who had received prior chemotherapy or erlotinib. The median pfs was 2.6 months, and the relative risk, 4.6% 44. Even in the absence of the PIK3CA mutation, the mtor inhibitors may be active, because dysregulation of mtor occurs at several levels. Preclinical trials of pi3k inhibitors have shown efficacy, and research is ongoing 45,46. BYL719 is a selective inhibitor of pi3kα. A phase i/ii trial will see it combined with the Mek inhibitor MEK162. This international multicentre trial is not yet recruiting, but is expected to be completed by 2014.
8. NOVEL THERAPIES
In the current environment of targeted treatments, oncolytic viruses are also attracting increasing attention. Reolysin (Oncolytics Biotech, Calgary, AB) is a clinical gmp strain of reovirus serotype 3–Dearing. It is a double-stranded rna virus that specifically replicates in cells possessing an activated Ras signalling pathway (or up- and downstream elements of that pathway) 47,48. Preclinical data highlight the ability of reovirus to repetitively replicate within cells and demonstrated a synergistic effect with chemotherapy (especially with microtubule-inhibiting agents) and radiation 49,50. There are data to suggest that the anticancer effects of Reolysin may be augmented by combination with chemotherapy directly and by the potential of chemotherapy to reduce immune clearance of the reovirus 51,52.
Reolysin is the focus of a number of clinical trials involving solid tumours. A phase ii study, NCT00861627, is currently recruiting patients. It will examine Reolysin in combination with carboplatin and paclitaxel. The trial aims to enroll 36 patients with stage iiib/iv nsclc having a KRAS or EGFR activating mutation. The patients will be chemonaïve, but may have received treatment with an egfr-tki.
9. SUMMARY
The discovery of new biomarkers for targeted therapies has greatly changed the management and prognosis of many patients with nsclc. Further, knowledge of the molecular pathways and mutational drivers of lung cancer will expand the use of targeted treatments. Hopefully, the identification of new therapeutic targets such as Alk, c-Met, mtor, and pi3k, and investigations into simultaneously inhibiting multiple pathways and overcoming resistance, will provide personalized and precise treatments for lung cancer patients in the near future.
10. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
11. REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Erratum in: CA Cancer J Clin 2011;61:134] [DOI] [PubMed] [Google Scholar]
- 2.NSCLC Meta-analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small cell lung cancer (interest): a randomized phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen C, et al. Efficacy results from the randomized phase iii optimal (ctong 0802) study comparing first line erlotinib versus carboplatin (cbdca) plus gemcitabine (gem) in Chinese advanced non-small cell lung cancer (nsclc) patients (pts) with EGFR activating mutations [abstract LBA13] Ann Oncol. 2010;21(suppl 8):viii6. doi: 10.1093/annonc/mdp507. [DOI] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small cell lung cancer; a multicenter, randomized, placebo controlled phase iii study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Yeap BY, Mino–Kenudson M, et al. Clinical features and outcome of patients with non-small cell lung cancer who harbor EML4–ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch FR, Varella–Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ErbB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–32. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 16.Ramalingam SS, Boyer M, Park K, et al. Randomized phase 2 study of PF299804, an irreversible human epidermal growth factor receptor (egfr) inhibitor, versus (v) erlotinib (e) in patients (pts) with advanced non-small cell lung cancer (nsclc) after chemotherapy (ct) failure: quantitative and qualitative benefits [abstract 365] Ann Oncol. 2010;21(suppl 8):viii122. doi: 10.1093/annonc/mdq518. [DOI] [Google Scholar]
- 17.Mok TSK, Spigel DR, Park K, et al. Efficacy and safety of PF299804 as first-line treatment (tx) of patients (pts) with advanced (adv) nsclc selected for activating mutation (mu) of epidermal growth factor receptor (egfr) [abstract LBA18] Ann Oncol. 2010;21(suppl 8):viii8. [Google Scholar]
- 18.Miller VA, Hirsh V, Cadranel J, et al. Phase iib/iii double-blind randomized trial of afatinib (BIBW 2992, an irreversible inhibitor of egfr/her1 and her2) + best supportive care (bsc) versus placebo + bsc in patients with nsclc failing 1–2 lines of chemotherapy and erlotinib or gefitinib (lux-Lung 1) [abstract LBA1] Ann Oncol. 2010;21(suppl 8):viii1–12. [Google Scholar]
- 19.Avruch J, Khokhlatchev A, Kyriakis JM, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the map kinase cascade. Recent Prog Horm Res. 2001;56:127–55. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Liu L, Liu Z, et al. The status of KRAS mutations in patients with non-small cell lung cancers from mainland China. Oncol Rep. 2009;22:1013–20. doi: 10.3892/or_00000529. [DOI] [PubMed] [Google Scholar]
- 21.Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic K-ras mutations as a mechanism associated with resistance to egfr-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 22.Wellbrock C, Karasarides M, Marais R. The Raf proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 23.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 25.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–3. [PubMed] [Google Scholar]
- 26.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. doi: 10.1023/A:1023772912750. [DOI] [PubMed] [Google Scholar]
- 27.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 28.Brown MT, Cooper JA. Regulation, substrates and functions of Src. Biochim Biophys Acta. 1996;1287:121–49. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 30.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 31.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 32.Ou SI, Salgia R, Clark JW, et al. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J Thorac Oncol. 2010;5(suppl 5):S382. [Google Scholar]
- 33.Spigel D, Ervin T, Ramlau R, et al. Randomized multicenter double-blind placebo controlled phase ii study evaluating MetMAb, an antibody to Met receptor, in combination with erlotinib, in patients with advanced non-small cell lung cancer [abstract LBA15] Ann Oncol. 2010;21(suppl 8):viii7. [Google Scholar]
- 34.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindemann RK, Newbold A, Whitecross KF, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci U S A. 2007;104:8071–6. doi: 10.1073/pnas.0702294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witta SE, Gemmill RM, Hirsch FR, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–50. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 37.Stimson L, La Thangue NB. Biomarkers for predicting clinical responses to hdac inhibitors. Cancer Lett. 2009;280:177–83. doi: 10.1016/j.canlet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA. Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer. 2010;126:743–55. doi: 10.1002/ijc.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanzaki M, Kakinuma H, Kumazawa T, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide, enhance the effects of gemcitabine and docetaxel in hormone refractory prostate cancer cells. Oncol Rep. 2007;17:761–7. [PubMed] [Google Scholar]
- 40.Maiso P, Carvajal–Vergara X, Ocio EM, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66:5781–9. doi: 10.1158/0008-5472.CAN-05-4186. [DOI] [PubMed] [Google Scholar]
- 41.Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (tdac) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–9. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumour. Clin Cancer Res. 2008;14:804–10. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 43.Carnero A, Blanco–Aparicio C, Renner O, Link W, Leal JF. The pten/pi3k/Akt signaling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 44.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:151–91. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced nsclc previously treated with chemotherapy alone or with chemotherapy and egfr inhibitors. Ann Oncol. 2009;20:1674–81. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 46.Ihle NT, Lemos R, Jr, Wipf P, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumour activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–50. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumour activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–72. [PubMed] [Google Scholar]
- 48.Duncan MR, Stanish SM, Cox DC. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol. 1978;28:444–9. doi: 10.1128/jvi.28.2.444-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sei S, Mussio JK, Yang QE, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–23. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 51.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–53. [PubMed] [Google Scholar]
- 52.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]