Abstract
Epithelial-mesenchymal transition (EMT) has been shown to play a key role in embryogenesis and cancer progression. We previously found that fibronectin (FN) carrying O-GalNAc at a specific site is selectively expressed in cancer and fetal cells/tissues, and termed oncofetal FN (onfFN). Here, we show that (i) a newly-established monoclonal antibody against FN lacking the O-GalNAc, termed normalFN (norFN), is useful for isolation of onfFN, (ii) onfFN, but not norFN, can induce EMT in human lung carcinoma cells, (iii) onfFN has a synergistic effect with transforming growth factor (TGF)β1 in EMT induction.
Keywords: Oncofetal FN, O-GalNAc, EMT, Non-small cell lung cancer, TGFβ1
1. Introduction
Numerous studies have demonstrated that carbohydrates expressed as glycosphingolipids (GSLs) or N- or O-linked glycans of glycoproteins are correlated with cell phenotypes and are involved in various cell functions such as cell adhesion, signal transduction, growth, motility, and invasiveness [1–5]. In our previous studies, we demonstrated the functional role of certain carbohydrate structures in epithelial-mesenchymal transition (EMT) [6–8].
The EMT process, was initially observed during early embryogenesis and organ formation [9,10], and was demonstrated to play an essential role in disease development such as cancer progression [11–14] and fibrosis [15]. During the EMT process in cancer progression, transformed epithelial cells lose their apical-basal polarity and change to an elongated fibroblastic morphology. The cells also display reduced expression of epithelial cell markers such as E-cadherin (Ecad), concomitant with enhanced expression of mesenchymal cell markers such as vimentin, N-cadherin (Ncad), fibronectin (FN), and enhanced cell motility [11–15]. FN is known to exist in multiple forms resulting from alternative splicing and different glycosylation. The role of different FN isoforms in the EMT process remains largely unknown, although the up-regulation of FN has been widely used for the assessment of EMT induction. We previously established mAb FDC6, using FN isolated from a human cell line, HUH7 [16] as the immunogen. FDC6 reacts with FN in fetal and cancer tissue and cells, but not with FN in normal adult tissue and cells. FDC6-positive FN was therefore termed “oncofetal FN” (onfFN) and FDC6-negative FN as “normal FN” (norFN) [16,17]. Subsequent studies revealed that the epitope of FDC6 is based on the addition of an O-glycan (GalNAcα1-O-Ser/Thr or Galβ1-3GalNAcα1-O-Ser/Thr) to the Thr residue of the peptide sequence-Val-Thr-His-Pro-Gly-Tyr-, which is located at the type III homology connective segment (IIICS) domain of FN [17,18]. Our recent study [6] on the role of onfFN in EMT process demonstrated that i) the expression of onfFN is strongly up-regulated during transforming growth factor TGFβ1-induced EMT process in human prostate cell lines and ii) the knock-down of UDP-N-acetylgalactosamine : polypeptide N-acetylgalactosaminyltransferase (GalNAc-T)3 and GalNAc-T6 with siRNA targeted to them, abolishes the enhanced expression of onfFN without any change in total FN (tFN), and also inhibits EMT induced with TGFβ1. These results indicate the functional role of onfFN in EMT induction.
In this study, we isolated onfFN and norFN using mAb FDC6 and a newly established mAb against norFN, and examined their activities to induce EMT and their synergistic effects with TGFβ1 in EMT induction using human non-small cell lung carcinoma (NSCLC) cells.
2. Materials and methods
2.1. Cell culture
NSCLC cell lines, A549 and NCI-H358, were obtained from the American Type Culture Collection (ATCC, Rockville, MD), and cultured in RPMI 1640. Benign human hepatoma HUH7 cells [16], which were donated by Dr. J. Sato (Okayama Univ., Japan), and HUH7/T6 [6] were grown in DMEM. These media were supplemented with 10% FBS, penicillin (100 IU/ml) and streptomycin (100 µg/ml) unless described otherwise. All cells were cultured in a humidified chamber at 37 °C in 5% CO2/95% air.
2.2. Reagents and antibodies
Antibodies used: anti-Ecad (IgG1; BD Biosciences, San Jose, CA), anti-vimentin (IgM; Sigma, St. Louis, MO), anti-GAPDH (IgG1; Sigma, St. Louis, MO), anti-total FN (EP5, IgG1; Santa Cruz Biotechnology, Santa Cruz, CA), mAb FDC6 (IgG1) was established in our laboratory [16], and mAb 2E11 (IgG1) for human GalNAc-T6 was kindly donated by Drs. U. Mandel and H. Clausen (University of Copenhagen, Denmark) [19]. Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG and -mouse IgM were from Southern Biotech (Birmingham, AL). Human plasma FN was from Sigma and TGFβ1 was from BD Biosciences. Other reagents were from Sigma, unless described otherwise.
2.3. Production of mAb against norFN
The peptide, KTPFVTHPGYDTGNTCQC, consisting of 17 amino acid sequence within the IIICS domain of FN and an additional cysteine residue (C), was purchased from GenScript, (Piscataway, NJ). The peptide was chemically linked with the imject maleimide-activated mcKLH (Thermo, Rockford, IL) through the C, according to the manufacturer’s instructions. Balb/c (6–8 weeks old) mice were intraperitoneally injected with 40 µg of the KLH-conjugated peptide emulsified in TiterMax Gold adjuvant (Titermax, Norcross, GA) twice at 2-week intervals. Seven days after the second injection, the antibody tire in serum was determined by ELISA, using the peptide-conjugated with bovine serum albumin (BSA). The mice that showed high antibody titer were given the last boost with 40 µg of FDC6-negative FN without adjuvant. Three days later, splenocytes were collected and fused with SP2/0 mouse myeloma cells and hybridomas were selected, as previously described [20]. Antibody production was screened by ELISA using total FN. After cloning by limiting-dilution, YKH1 clone was established. Its isotype was determined with IsoStrips (Boehringer Mannheim, Indianapolis, IN). All procedures with mice were approved by the Institutional Animal Care and Use Committee (IACUC).
2.4. Isolation of total FN and separation of onfFN and norFN
Total FN was prepared from the culture supernatant of HUH7/T6 cells by gelatin-agarose (Sigma) column chromatography, as described previously [17,18,21] and were further separated into onfFN and norFN using mAbs YKH1 and FDC6, respectively. Each mAb was purified with proteinG resin (GeneScript), according to the vendor’s instructions. Each purified mAb, ~1.5 mg, was covalently bound to 1.5 mL protein A/G PLUS-Agarose slurry (Santa Cruz Biotechnology), using disuccinimidyl suberate (Thermo, Rockford, IL), following the vendor’s instruction. After equilibration with 50 mM Tris buffer, pH 8.0, total FN (1 mg each) was added to the beads. After mixing overnight at 4 °C, unbound FN from FDC6-bound beads and YKH1-bound beads were collected and used as norFN and onfFN, respectively. Thus, onfFN and norFN were enriched at 8- and 1.3-fold, respectively. In some experiments, FN bound to FDC6-beads was eluted with low pH buffer and used as onfFN. Unbound fraction resulting from consecutive absorptions to FDC6 and toYKH1 did not show detectable amount of FN, which was estimated with EP5 by Western blot.
2.5. Analysis of EMT induction
For EMT induction with TGFβ1, A549 cells (1×105/well) and NCI-H358 (3×105/well) were cultured in 6-well plates overnight, and the cells were treated with TGFβ1 (5 ng/mL), and cultured for 72 hr. For EMT induction with FN, 6-well plates were incubated with plasma FN (pFN), total FN, norFN, onfFN or BSA at 50 µg/ml in D-PBS, at 4 °C overnight, and then washed with D-PBS. A549 cells (1×105) and NCI-H358 (3×105) were seeded on the coated well and incubated for 72 hr. After taking photos of these cells, cell lysates were prepared, protein concentration was determined, and 10 µg protein was analyzed by Western blot, as described previously [8,20,22–24]. Cell motility was determined by two procedures: i) Phagokinetic gold sol assay, as previously described [22,23]. ii) Wound assay, as previously described [6]. Briefly, A549 (2×105) and NCI-H358 (5×105) were seeded or treated as described above, and scratches were made. After taking photos of the scratches, the cells were incubated in culture medium for 18 hr.
3. Results and discussion
3.1. Establishing a new hybridoma for norFN and preparation of onfFN and norFN
Our previous study that showed the functional role of onfFN in TGFβ1-induced EMT [6] implicated that onfFN, but not norFN, may have the activity to induce EMT. For efficient isolation of onfFN, we established a hybridoma that produces mAb against norFN, by immunizing KLH-conjugated peptide that contains Val-Thr-His-Pro-Gly-Tyr, whose Thr is O-glycosylated in onfFN.
Only one out of 60 clones positive for the peptide showed selective binding to norFN over onfFN (Fig. 1A). This clone was named YKH1. The protein A/G agarose-bound with YKH1 removed norFN to enrich onfFN, while FDC6 removed onfFN to enrich norFN (Fig. 1B).
Figure 1. Production of mouse mAb specific for norFN and preparation of onfFN and norFN using the mAb.
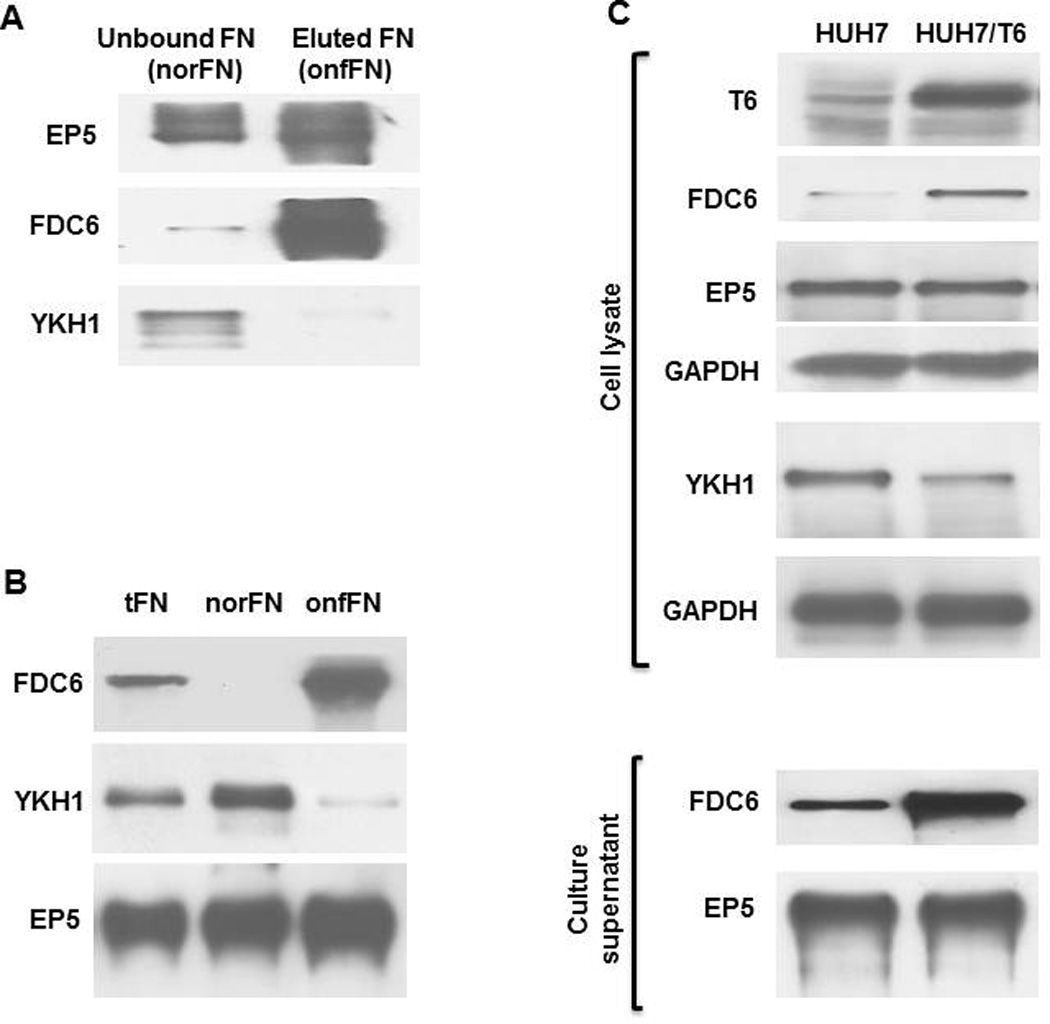
(A) Specificity of mAb YKH1 was determined by Western blot using the unbound fraction (norFN), and the bound and eluted fraction (onfFN) from FDC6-column. Aliquots (0.1 µg protein) of each FN were analyzed with mAbs EP5, FDC6, and YKH1. (B) Separation of norFN and onfFN by immunoaffinity absorption. Aliquots (0.1 µg protein) of tFN, norFN (unbound to FDC6) and onfFN (unbound to YKH1) were analyzed by Western blot using EP5, YKH1 and FDC6. (C) Production and secretion of onfFN in HUH7 cells overexpressing GalNAc-T6 (HUH7/T6). Expression of GalNAc-T6, total FN, norFN and onfFN were detected with mAbs 2E11, EP5, YKH1 and FDC6, respectively, using cell lysates and culture supernatants.
Our previous study [6] indicated that HUH7 cells produce more norFN than onfFN. In order to make the cells secrete more onfFN, we transfected human GalNAc-T6 cDNA and established HUH7/T6 cells, as previously described [6]. HUH7/T6 cells produced more onfFN and less norFN than HUH7 cells, which was determined both with cell lysates and culture supernatants (Fig. 1C). Although accumulating evidence supports the importance of site-specific O-glycosylation [25–27], peptide motifs for O-glycosylation and substrate specificity for each GalNAc-T have not been elucidated. This result supports the previous works showing the involvement of GalNAc-T6 in biosynthesis of onfFN [26, 28].
3.2. Enhanced expression of onfFN during TGFβ1-induced EMT in human lung cell lines, A549 and NCI-H358
Consistent with the previous result with prostate cells [6], the FN expressed in TGFβ1-treated A549 and NCI-H358 cells was mainly onfFN, and norFN which were detected with YKH1 remained at a similar level (Fig. 2). These results suggest that up-regulated FN during TGFβ1-induced EMT belongs mainly to onfFN, although analysis with various other cell lines will need to be performed to generalize the findings.
Figure 2. Characterization of FN up-regulated during TGFβ1-induced EMT in human lung carcinoma cells.
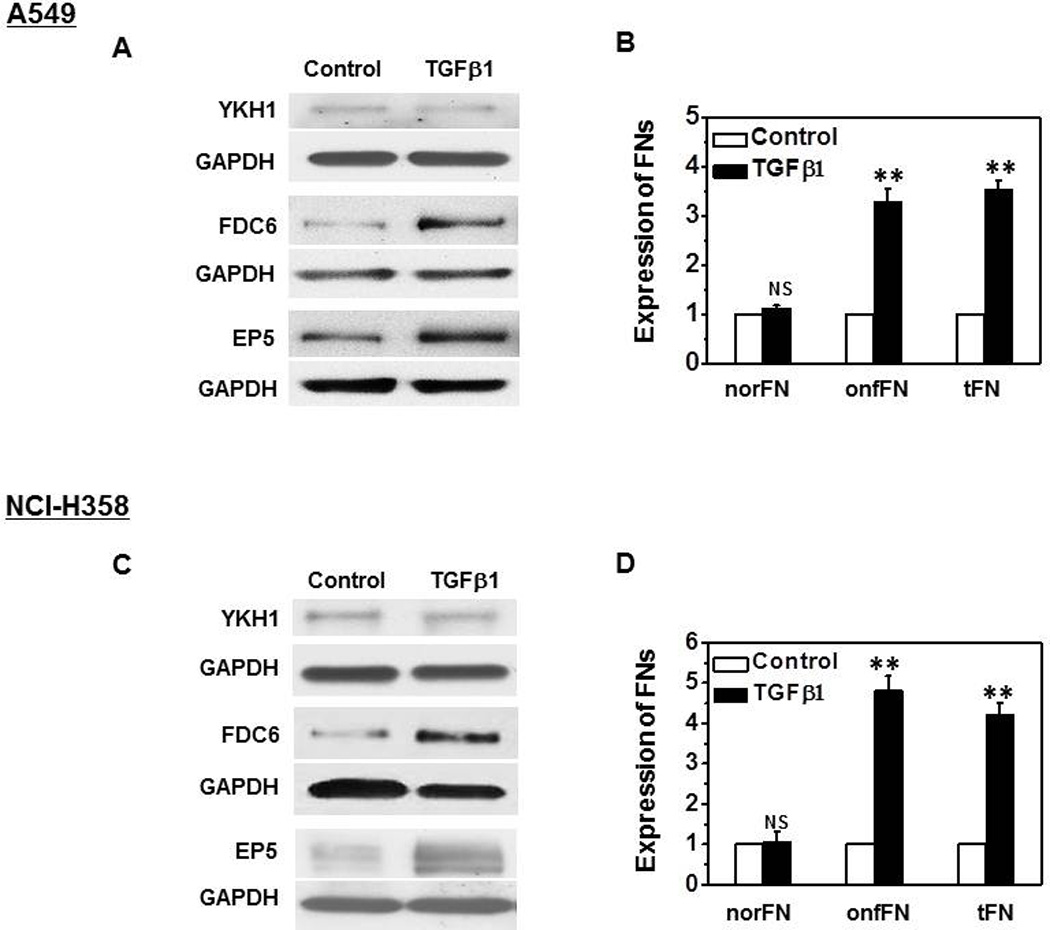
A549 cells (A and B) and NCI-H358 (C and D) were cultured and treated as described in M&M. Cell lysates (10 µg protein) were analyzed by SDS/PAGE and Western blot. Total FN, norFN and onfFN were detected with mAbs EP5, YKH1 and FDC6, respectively; Together with GAPDH used as loading control. Representative results from triplicate experiments are shown (A and C). Signal intensities were normalized, and relative intensities are shown as mean ± SD (B and D). ns: not significant; **P ≤0.005.
3.3. EMT induction with onfFN in A549 and NCI-H358 cells
A functional role of onfFN biosynthesis during EMT was shown in our previous study with prostate cells [6], which implicated that onfFN has the activity to induce EMT. Preliminary studies using total FN showed higher EMT inducing activity with human NSCLCs than with the prostate cells (data not shown), thus further studies were performed with the lung cell lines, A549 and NCI-H358. The cells were seeded on the plates coated with total FN, human pFN or BSA. The cells seeded on the non-coated plates were treated with TGFβ1 and used as a positive control. Significant changes in morphology similar to TGFβ1-treated cells were observed with both cell lines when seeded on total FN, while the cells seeded on pFN or BSA did not show any significant changes (Fig. 3I). EMT induction was also detected by the reduced expression of epithelial cell marker, Ecad, and the enhanced expression of mesenchymal markers, Ncad and vimentin (Fig. 3II); although EMT inducing activity of total FN was lower than that of TGFβ1.
Figure 3. Analysis of EMT induction with total FN.
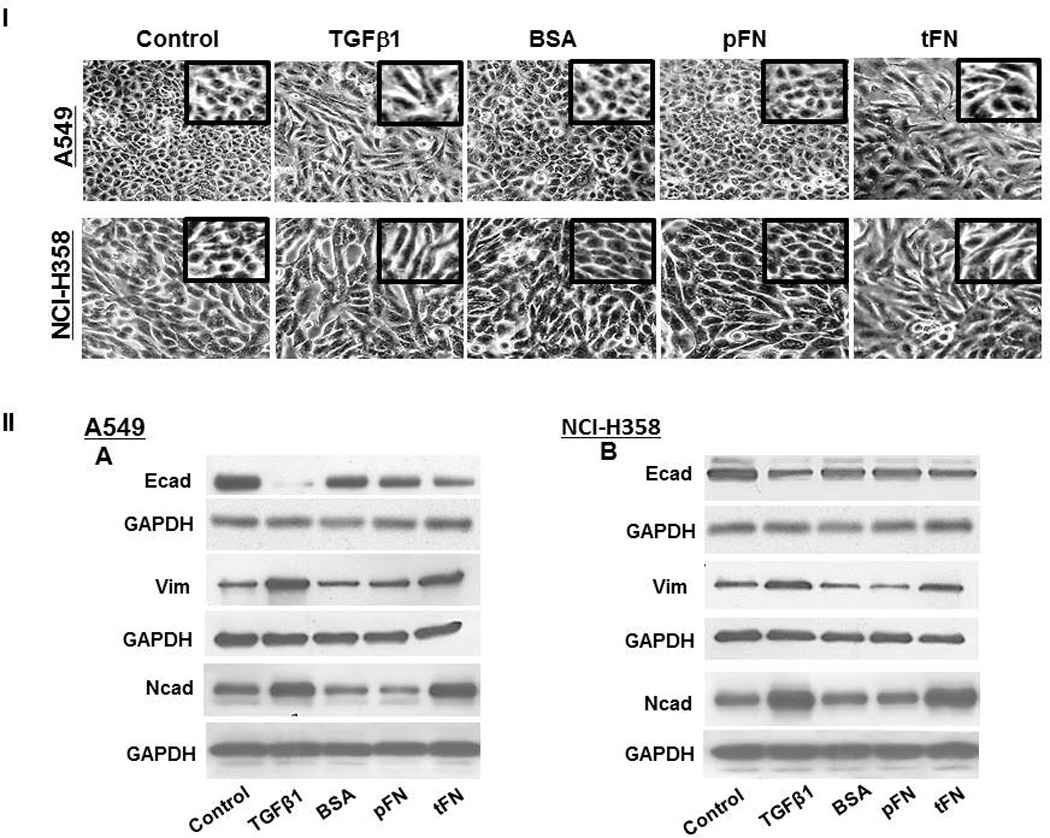
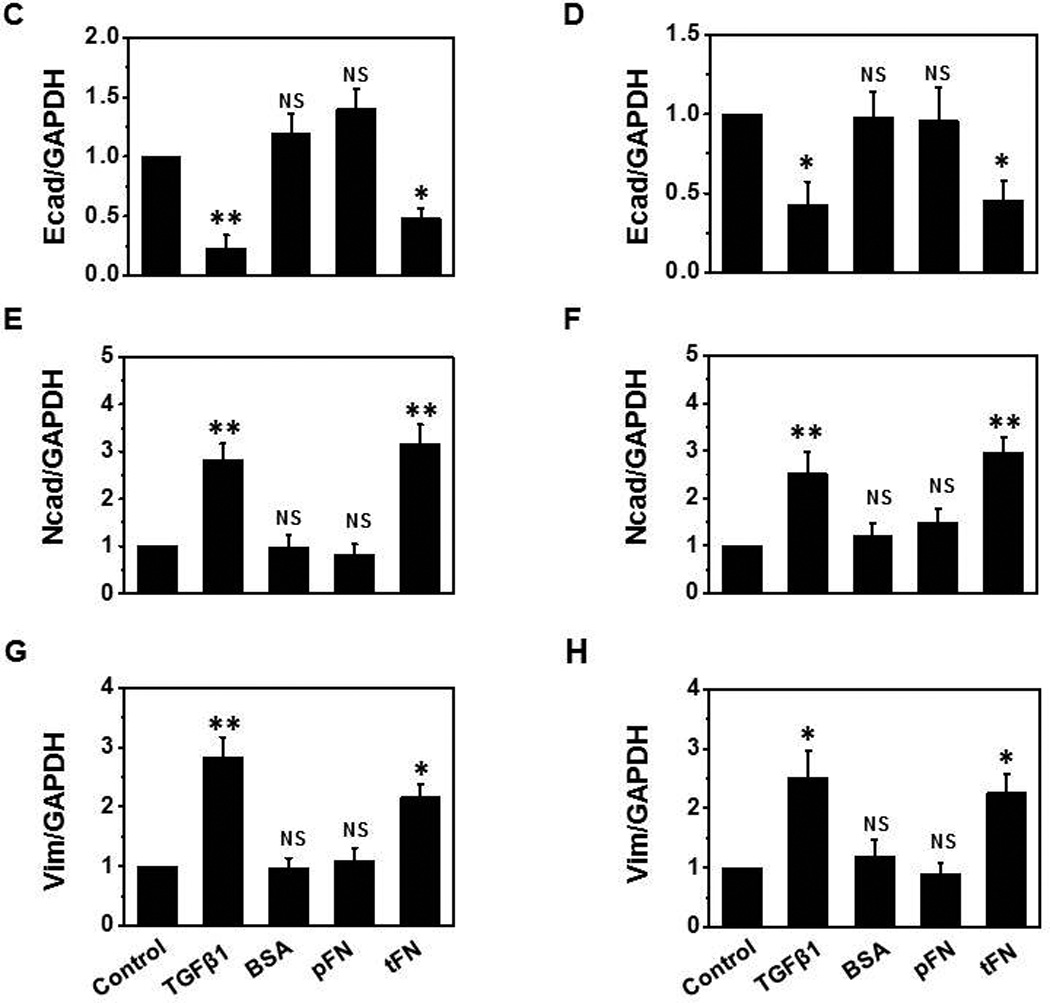
Plates were coated with BSA, plasma FN (pFN), or total FN (tFN), and the cells were seeded on the coated plates as described in M&M. TGFβ1 treatment was used as a positive control. (I) For morphology changes, photos were taken by phase-contrast microscopy (Nikon) at 80X magnification. (II) Expression level of epithelial vs. mesenchymal cell markers were analyzed by Western blot using cell lysates (10 µg protein) of A549 (A, C, E and G) and NCI-H358 cells (B, D, F and H). Representative results from triplicate experiments are shown (A and B). Signal intensities were normalized, and relative intensities are shown as mean ± SD for Ecad (C and D), Ncad (E and F) and vimentin (G and H). ns: not significant; *P ≤ 0.05; **P ≤0.005.
Next, we isolated onfFN and norFN using FDC6 and YKH1 (Fig. 1B), and analyzed their ability to induce EMT. TGFβ1-treated cells and the cells in onfFN-coated wells displayed an elongated shape, and a decrease in cell-cell contact in both cell lines. The cells in norFN-coated wells did not show these changes (Fig. 4I). In addition, onfFN induced the down-regulation of Ecad concomitant with the up-regulation of Ncad and vimentin, similar to TGFβ1, while norFN did not induce detectable changes (Fig. 4II). The different activity of onfFN and norFN is also demonstrated in cell motility, which was assessed by two assays: phagokinetic cell motility assay on gold-sol and wound-scratch assay (Fig. 4III). In addition, the EMT induction with onfFN was blocked by pre-incubation of the onfFN-coated plates with mAb FDC6 (Supplemental Fig. 1).
Figure 4. Analysis of EMT induction with onfFN.
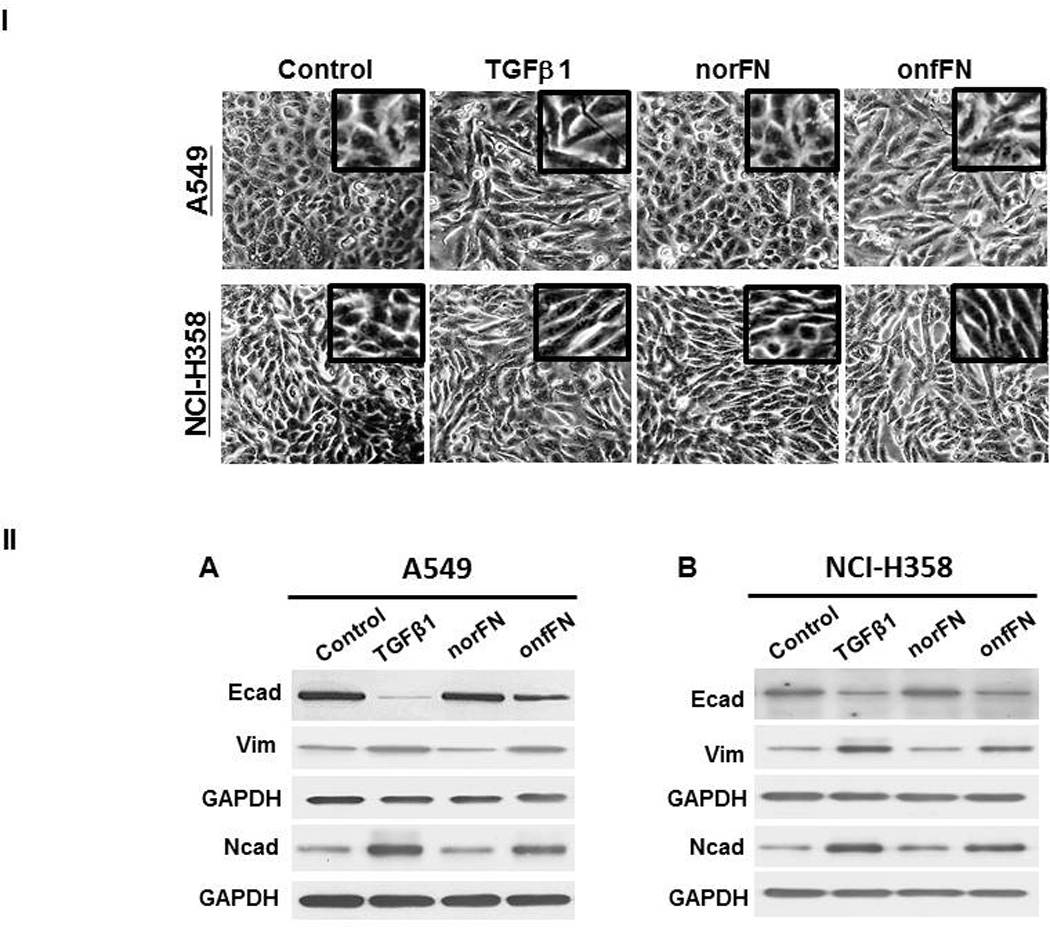
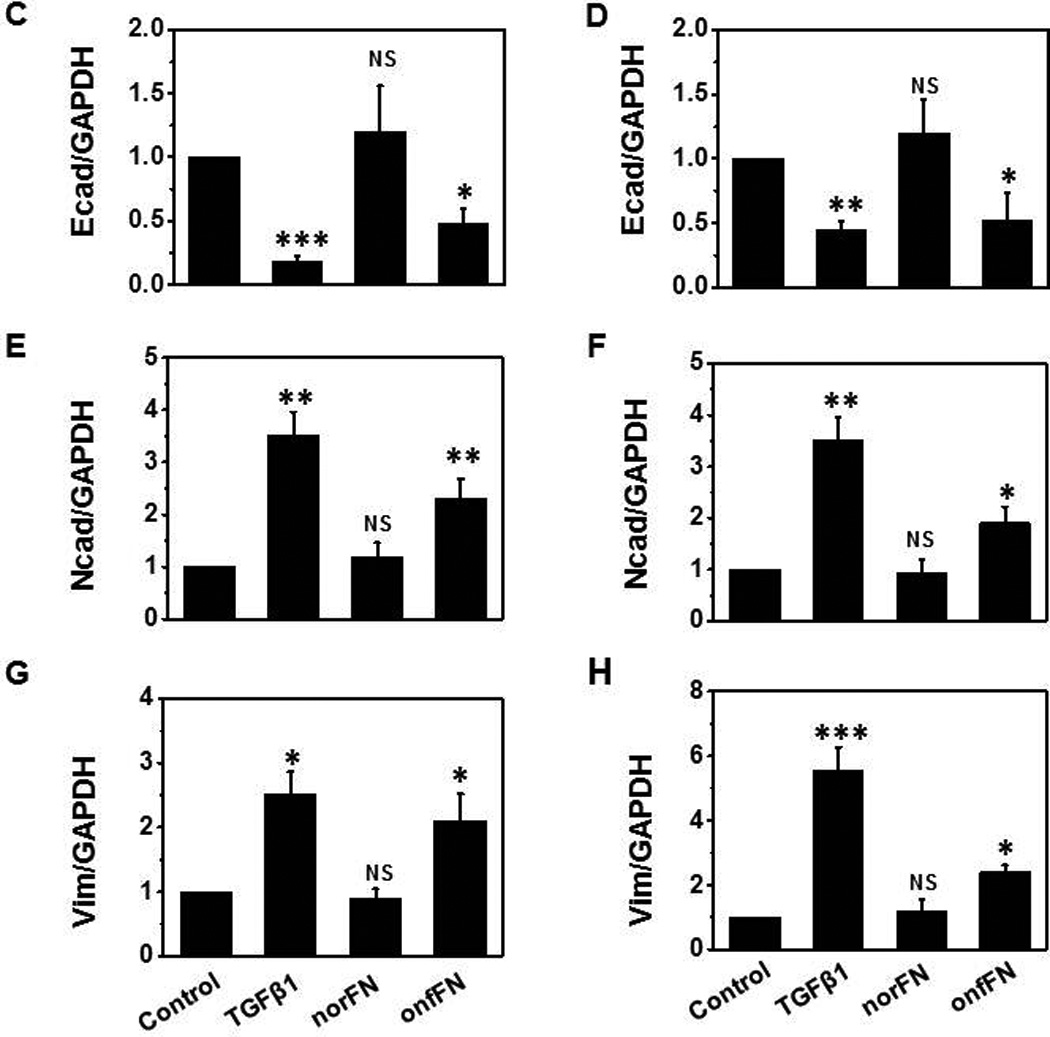
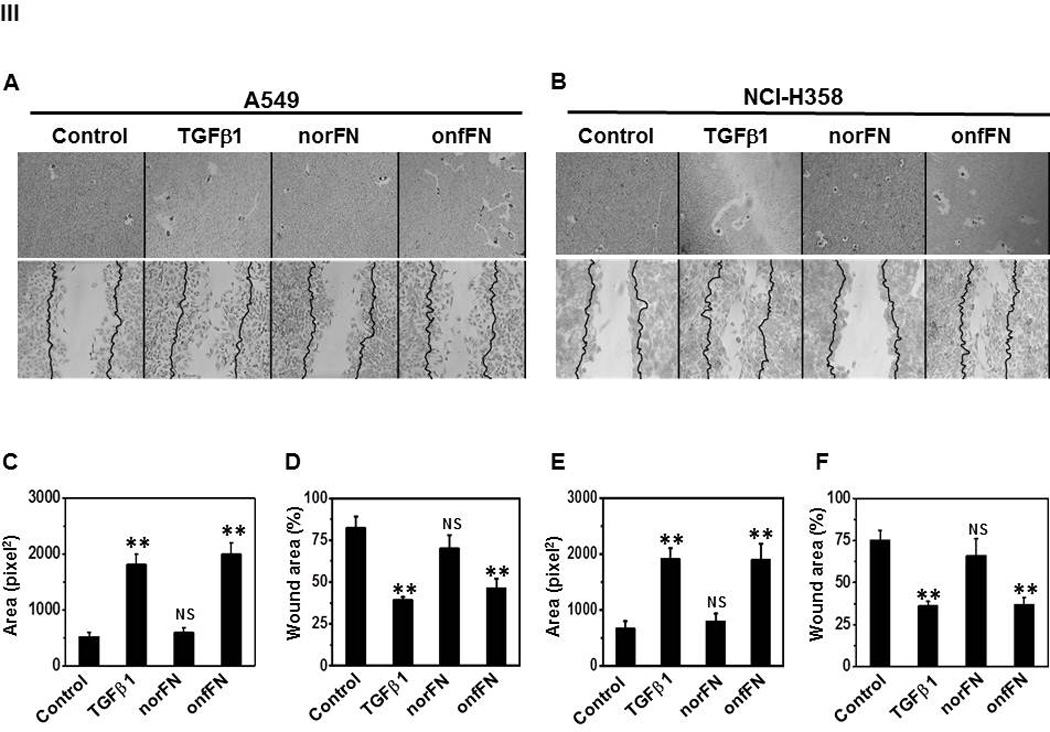
Plates were coated with norFN or onfFN and A549 and NCI-H358 cells were seeded as described in M&M. TGFβ1 treatment was used as a positive control. (I) Cell morphology changes were analyzed as described in Fig. 3. (II) Expression level of epithelial vs. mesenchymal cell markers were analyzed as described in Fig. 3. Representative results from triplicate experiments are shown (A and B). Signal intensities were normalized, and relative intensities are shown as mean ± SD for Ecad (C and D), Ncad (E and F) and vimentin (G and H). ns: not significant; *P ≤ 0.05; **P ≤0.005; ***P ≤ 0.001. (III) Cell motility changes induced with the treatments were assessed by phagokinetic assay (Top of A and B) and wound assay (Bottom of A and B). Phagokinetic assay was performed as described in M&M. Photos of track areas of 30 cells were taken; Representative photos are presented. Cleared areas on gold sol were measured, analyzed using the Scion Image program as squared pixels, and are shown as mean ± SD (C and E). n.s.: not significant; *P ≤ 0.05; **P ≤0.005. The wound assay was performed as described in M&M. Pictures of the wounds at the marked position were taken at 0 hr and after an 18 hr incubation. Bold lines show the original edge of the wounds at 0 hr. Results are expressed as mean ± SD of percent of the initial wound area remaining open, analyzed using the Scion Image program (D and F). ns: not significant; *P ≤ 0.05; **P ≤0.005.
Other types of FN isoforms are known to be produced through alternative splicing; Some are at the IIICS domain [29–31], while others consist of two different complete type III repeats, extra domain (ED)-A and -B [32]. Whether onfFN contains ED-A or ED-B, remains to be studied. Although mAb FDC6 has been used clinically to distinguish fetal and adult cells, the functional role of the O-glycosylation to form onfFN was not fully investigated. As far as we know, this is the first report that shows isolated onfFN and norFN have different biological functions.
Recently, it was reported that O-glycosylation of FN through GalNAc-T6 is associated with EMT-like process in human breast cancer cells [33], although the O-glycosylated FN was not examined for FDC6-binding.
3.4. Synergistic effect of TGFβ1 and onfFN in EMT induction
We tested the possibility that onfFN and TGFβ1 work synergistically in EMT induction. EMT induction with TGFβ1 in these cell lines was dose dependent; clear EMT induction was observed at 5 ng/ml (Fig. 2–4), some at 2 and 1 ng/ml (data not shown), and EMT induction was not clearly detected at 0.5 ng/ml (Fig. 5I, 5II). However, addition of TGFβ1 at 0.5 ng/ml to the cells seeded on onfFN demonstrated synergistic effect in EMT induction, which was assessed by cell morphology change (Fig. 5I), and by the expression of EMT marker molecules (Fig. 5II). Such effect of TGFβ1 was not detected with the cells seeded on norFN, as expected. The synergistic effect between onfFN and TGFβ1 observed here implicates an interesting possibility that onfFN, secreted from the cells that underwent EMT and incorporated into extracellular matrix surrounding the carcinoma cells, facilitates EMT induction with TGFβ1 at low concentration, which is not enough to fully induce EMT alone. Co-regulatory function of FN variants with TGFβ1 has also been reported in phenotype change of mesenchymal/myofibroblasts [34].
Figure 5. Synergistic effect between onfFN and TGFβ1 in EMT induction.
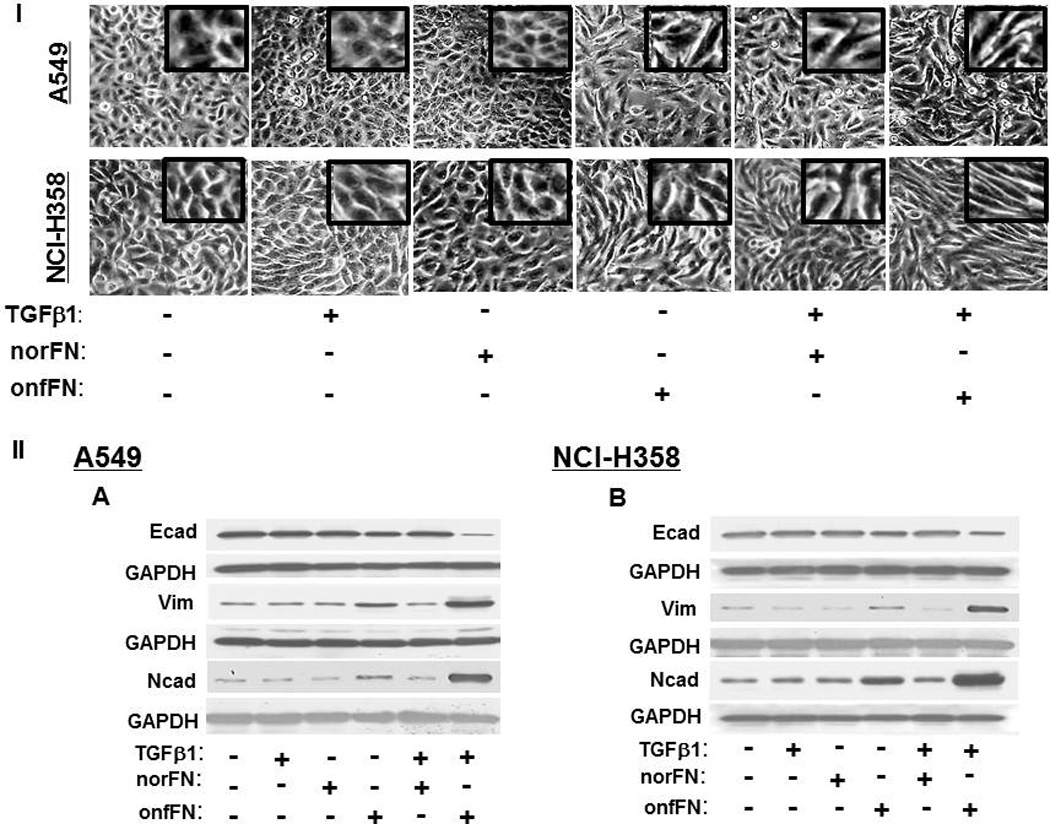
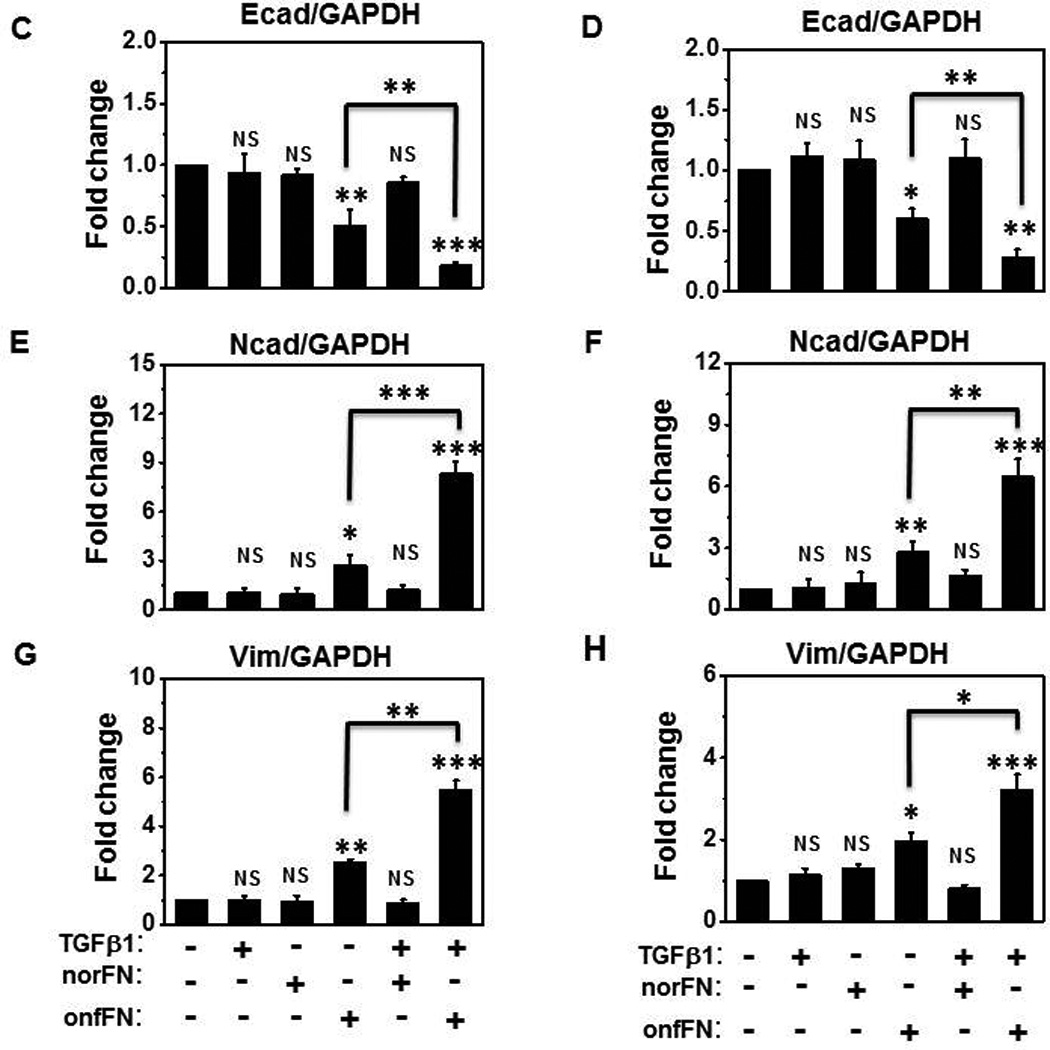
Plates were coated with norFN or onfFN and A549 and NCI-H358 cells were seeded on the plates as described at M&M. After 2 hr incubation, TGFβ1(0.5 ng/ml) were added and the cells were incubated for 70 hr. (I) Cell morphology changes were detected as described in Fig. 3. (II) Expression level of epithelial vs. mesenchymal cell markers were analyzed as described in Fig 3. Representative results from triplicate experiments are shown (A and B). The folds of the change of Ecad (C and D), Ncad (E and F) and vimentin (G and H) expression following the stimulation are shown as mean ± SD. ns: not significant; *P ≤ 0.05; **P ≤0.005; ***P ≤ 0.001.
Supplementary Material
Highlights.
A new hybridoma for an isoform of fibronectin (FN) was established.
FN carrying O-GalNAc, expressed in fetal and cancer cells, was isolated.
FN carrying O-GalNAc could induce epithelial-mesenchymal transition.
Acknowledgements
We thank Drs. Ulla Mandel and Henrik Clausen for mAb 2E11, Marlin Lobaton and Will Price for technical assistance, and Dr. Wai Cheu Lai and Marlin Lobaton for the preparation of the manuscript and figures. This research was supported by NIH grant R01CA080054 and the Biomembrane Institute.
Abbreviations
- FN
fibronectin
- EMT
epithelial-mesenchymal transition
- TGF
transforming growth factor
- Ecad
E-cadherin
- onfFN
oncofetal FN
- norFN
normal FN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 2.Sonnino S, Prinetti A. Gangliosides as regulators of cell membrane organization and functions. Adv. Exp. Med. Biol. 2010;688:165–184. doi: 10.1007/978-1-4419-6741-1_12. [DOI] [PubMed] [Google Scholar]
- 3.Miura Y, Kainuma M, Jiang H, Velasco H, Vogt PK, Hakomori S. Reversion of the Jun-induced oncogenic phenotype by enhanced synthesis of sialosyllactosylceramide (GM3 ganglioside) Proc. Natl. Acad. Sci. U.S.A. 2004;101:16204–16209. doi: 10.1073/pnas.0407297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnino S, Chigorno V, Aureli M, Masilamani AP, Valsecchi M, Loberto N, Prioni S, Mauri L, Prinetti A. Role of gangliosides and plasma membrane-associated sialidase in the process of cell membrane organization. Adv. Exp. Med. Biol. 2011;705:297–316. doi: 10.1007/978-1-4419-7877-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Freire-de-Lima L, Gelfenbeyn K, Ding Y, Mandel U, Clausen H, Handa K, Hakomori S. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17690–17695. doi: 10.1073/pnas.1115191108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan F, Schaffer L, Handa K, Hakomori S. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-β. FASEB J. 2010;24:4889–4903. doi: 10.1096/fj.10-162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan F, Handa K, Hakomori S. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7461–7466. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 11.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuura H, Hakomori S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6517–6521. doi: 10.1073/pnas.82.19.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuura H, Takio K, Titani K, Greene T, Levery SB, Salyan MEK, Hakomori S. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6: Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J. Biol. Chem. 1988;263:3314–3322. [PubMed] [Google Scholar]
- 18.Matsuura H, Greene T, Hakomori S. An a-N-acetylgalactosaminylation at the threonine residue of a defined peptide sequence creates the oncofetal peptide epitope in human fibronectin. J. Biol. Chem. 1989;264:10472–10476. [PubMed] [Google Scholar]
- 19.Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, Geurts van Kessel A, Olofsson S, Clausen H. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J. Biol. Chem. 1999;274:25362–25370. doi: 10.1074/jbc.274.36.25362. [DOI] [PubMed] [Google Scholar]
- 20.Todeschini AR, Dos Santos JN, Handa K, Hakomori S. Ganglioside GM2/GM3 complex affixed on silica nanospheres strongly inhibits cell motility through CD82/cMet-mediated pathway. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1925–1930. doi: 10.1073/pnas.0709619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int. J. Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 22.Todeschini AR, Dos Santos JN, Handa K, Hakomori S. Ganglioside GM2-tetraspanin CD82 complex inhibits Met and its cross-talk with integrins, providing a basis for control of cell motility through glycosynapse. J. Biol. Chem. 2007;282:8123–8133. doi: 10.1074/jbc.M611407200. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuzuka K, Handa K, Satoh M, Arai Y, Hakomori S. A specific microdomain ("glycosynapse 3") controls phenotypic conversion and reversion of bladder cancer cells through GM3-mediated interaction of alpha3beta1 integrin with CD9. J. Biol. Chem. 2005;280:35545–35553. doi: 10.1074/jbc.M505630200. [DOI] [PubMed] [Google Scholar]
- 24.Toledo MS, Suzuki E, Handa K, Hakomori S. Cell growth regulation through GM3-enriched microdomain (glycosynapse) in human lung embryonal fibroblast WI38 and its oncogenic transformant VA13. J. Biol. Chem. 2004;279:34655–34664. doi: 10.1074/jbc.M403857200. [DOI] [PubMed] [Google Scholar]
- 25.Gill DJ, Clausen H, Bard F. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011;21:149–158. doi: 10.1016/j.tcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of Mucin-Type O-Glycosylation - A Classification of the Polypeptide GalNAc-transferase Gene Family. Glycobiology. 2011 doi: 10.1093/glycob/cwr182. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki A, Etzler ME, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2008. pp. 115–127. [PubMed] [Google Scholar]
- 28.Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, Kong Y, Bennett EP, Mandel U, Wandall H, Levery SB, Clausen H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 29.White ES, Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB. Life. 2011;63:538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- 30.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J. Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankov R, Yamada KM. Fibronectin at a glance. J. Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 32.Zardi L, Carnemolla B, Siri A, Petersen TE, Paolella G, Sebastio G, Baralle FE. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987;6:2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, Katagiri T, Chung S, Kijima K, Nakamura Y. Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia. 2011;13:320–326. doi: 10.1593/neo.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


