Abstract
It has been postulated that mucus stasis is central to the pathogenesis of obstructive lung diseases. In Scnn1b-transgenic (Scnn1b-Tg+) mice, airway-targeted overexpression of the epithelial Na+ channel β subunit causes airway surface dehydration, which results in mucus stasis and inflammation. Bronchoalveolar lavage from neonatal Scnn1b-Tg+ mice, but not wild-type littermates, contained increased mucus, bacteria, and neutrophils, which declined with age. Scnn1b-Tg+ mice lung bacterial flora included environmental and oropharyngeal species, suggesting inhalation and/or aspiration as routes of entry. Genetic deletion of the Toll/Interleukin-1 receptor adapter molecule MyD88 in Scnn1b-Tg+ mice did not modify airway mucus obstruction, but caused defective neutrophil recruitment and increased bacterial infection, which persisted into adulthood. Scnn1b-Tg+ mice derived into germ-free conditions exhibited mucus obstruction similar to conventional Scnn1b-Tg+ mice and sterile inflammation. Collectively, these data suggest that dehydration-induced mucus stasis promotes infection, compounds defects in other immune mechanisms, and alone is sufficient to trigger airway inflammation.
INTRODUCTION
Respiratory health is maintained by an integrated network of innate and adaptive defense mechanisms. At the airway surface, mechanical mucus clearance is largely responsible for efficient removal of noxious stimuli, and mucus stasis has been suggested to produce airway lung diseases, including cystic fibrosis (CF), primary ciliary dyskinesia (PCD), and chronic obstructive pulmonary disease (COPD). Effective mucus clearance relies upon multiple processes, e.g., epithelial ion and water transport, mucin secretion, ciliary beat, phasic airway motion, and cough 1. Experimentally, mucus stasis has been achieved in mice by airway-targeted overexpression of the epithelial Na+ channel β subunit (βENaC, encoded by the Scnn1b gene) 2. In Scnn1b-transgenic (Scnn1b-Tg+) mice, airway epithelial Na+ hyperabsorption causes a depletion of airway surface liquid and an increase in mucus concentration 2, which is predicted to impair mucus clearance. Indeed, Scnn1b-Tg+ mice exhibit mucus stasis and airway inflammation, which emerges in the neonatal period and produces a pathology resembling human CF/chronic bronchitis 2,3.
Although Scnn1b-Tg+ mice exhibit delayed clearance of instilled bacteria 2 and chronic neutrophilic inflammation 3, the initial studies failed to detect spontaneous bacterial infection, a hallmark of CF lung disease 4 and an important component of COPD exacerbations 5. This observation suggested that mucus stasis could produce inflammation without infection, i.e., sterile inflammation. However, the original microbiological studies were conducted only in adult mice and did not take into account the role of normal developmental processes, including maturation of innate and adaptive immunity 6,7 and transient abundance of mucous secretory cells 8,9, which likely play a role in the evolution of lung pathology in Scnn1b-Tg+ mice. Moreover, static airway mucus creates a microaerophilic/hypoxic environment 10, which may favor the growth of bacterial species not detectable in the conventional aerobic bacterial cultures performed in the original studies of Scnn1b-Tg+ mice.
Accordingly, we initiated a series of longitudinal studies designed to explore the interactions between mucus clearance and host defense in Scnn1b-Tg+ mice. First, we tested the hypothesis that neonatal Scnn1b-Tg+ mice are more susceptible to spontaneous bacterial infection than adult mice, due to the combination of defective mucus clearance, likely compounded by the transient increase in mucin production that occurs in the early postnatal period 8, and immature immune defenses. Thus, we measured bacterial colony forming units (CFUs) in bronchoalveolar lavage (BAL) as a function of age utilizing bacterial culture conditions that permitted detection of non-fastidious aerobes and microaerophilic bacteria. Second, we hypothesized that mechanical mucus clearance interacts with other developmentally regulated cellular defense mechanisms, e.g., Toll-like receptors (TLRs) 11,12, which could trigger airway inflammation in response to noxious stimuli accumulating in static mucus. Thus, we longitudinally evaluated whether components of Scnn1b-Tg+ lung pathogenesis were dependent on Toll/Interleukin-1 receptor domain adapter protein myeloid differentiation factor 88 (MyD88), which mediates signaling through several TLRs 13 and is critical for early post-natal immunity 14,15. Finally, to test whether live bacteria were necessary to trigger pulmonary inflammation due to airway surface dehydration and mucus stasis, we generated germ-free Scnn1b-Tg+ mice and characterized their lung phenotype.
RESULTS
Scnn1b-Tg+ mice routinely exhibit spontaneous bacterial infection during the neonatal period but are inflamed throughout life
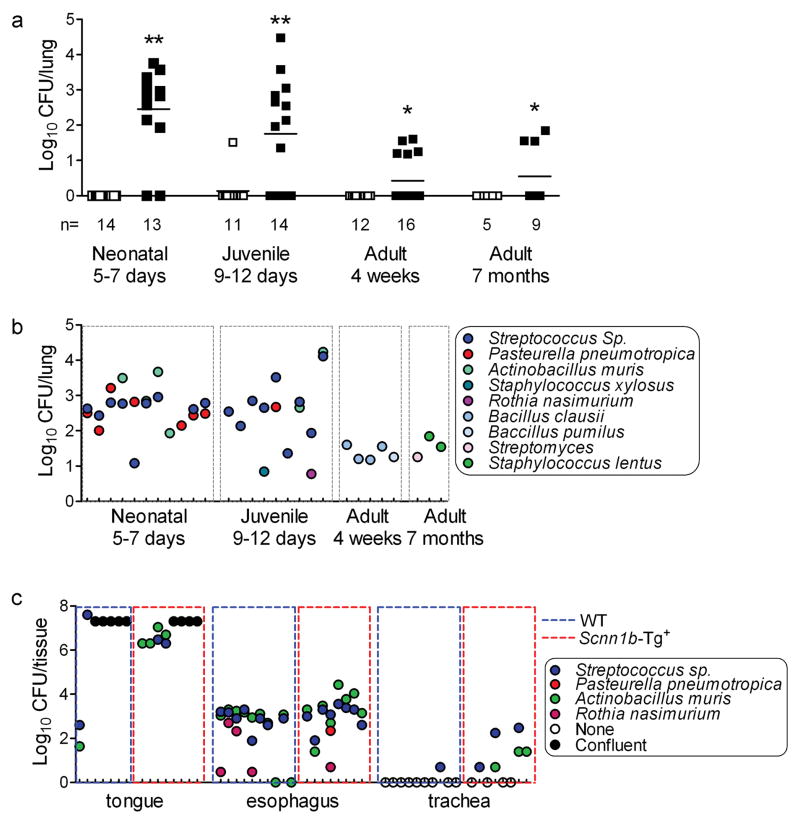
The first goal of this study was to determine whether airway surface dehydration and mucus stasis promoted spontaneous bacterial infection in Scnn1b-Tg+ mice. Longitudinal analysis of BAL from congenic C57BL6/N Scnn1b-Tg+ and wild-type (WT) littermates revealed no culturable bacteria in WT mice, whereas the majority of Scnn1b-Tg+ pups harbored intrapulmonary bacteria (Figure 1a). As Scnn1b-Tg+ mice aged, both the proportion of infected mice and the number of bacterial colony forming units (CFU) decreased.
Figure 1. Spontaneous bacterial colonization in C57BL/6N Scnn1b-Tg+ mice.
(a) Timecourse analysis of total colony forming units (CFU) in BAL samples from Scnn1b-Tg+ mice (■) and WT littermates (□). (Log10+1)-transformed data. n= number of mice/group. T test ** p<0.005, * p<0.05 vs. WT littermates. (b) Individual CFUs and bacterial species isolated from C57BL/6N Scnn1b-Tg+ mice. Each tick on the x axis represents an individual mouse. (c) Bacteria isolated from tongue, esophagus, trachea, and lung tissue homogenates of PND 5 C57BL/6N Scnn1b-Tg+ mice and WT littermates. (Log10+1)-transformed data. Each tick on the x axis represents an individual mouse. CFUs in confluent plates could not be enumerated and were arbitrarily set at 2×107 CFU/tissue.
Morphologically distinct bacterial colonies recovered from Scnn1b-Tg+ mice were identified by ribosomal 16S gene sequencing (Figure 1b). Scnn1b-Tg+ neonatal lung microflora included both Gram positive (mainly Streptococcus species and to lesser extent Staphylococcus xylosus, and Rothia nasimurium) and Gram negative bacteria (mainly Pasturella pneumotropica and Actinobacillus muris). Similar bacterial species were present in tissue homogenates of tongue and esophagus from 5 day-old mice, with no clear distinction between WT or Scnn1b-Tg+ mice (Figure 1c, tongue and esophagus), whereas these bacteria were frequently detected in tracheal homogenates from 5 day-old Scnn1b-Tg+ mice as compared to WT littermates (Figure 1c, trachea). The composition of the lung microflora in adult Scnn1b-Tg+ mice differed from younger mice, featuring low levels of Gram positive bacteria, e.g., Bacillus clausii and Bacillus pumilus at 1 month and Streptomyces and Staphylococcus lentus at 7 months of age (Figure 1b), suggesting a process of selection/adaptation.
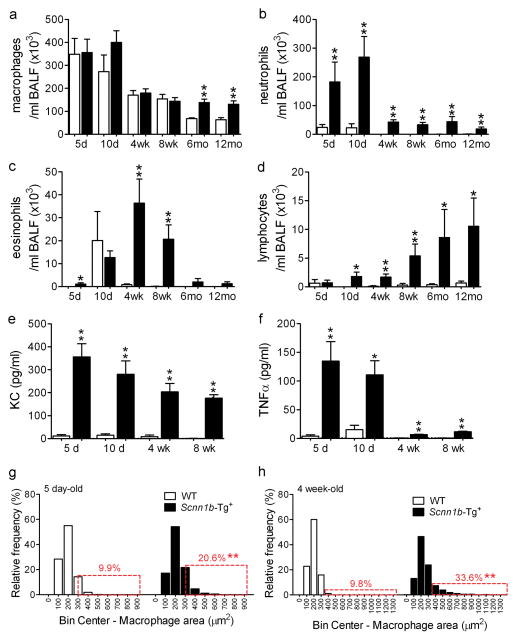
Longitudinal analysis of BAL inflammatory markers indicated that Scnn1b-Tg+ mice exhibited an increased inflammatory infiltrate, predominantly neutrophilic, as compared to WT littermates at all ages (Figure 2). The relative increases in BAL neutrophils, KC and TNFα were more robust in neonatal mice (Figure 2b,e,f) when bacterial burden was highest. In contrast, the number of BAL macrophages and lymphocytes in the Scnn1b-Tg+ mice increased with age (Figure 2a,d), and eosinophils peaked at 4–8 wks (Figure 2c). Macrophages were morphologically activated in both neonatal (5 day-old) and adult (4 week-old) Scnn1b-Tg+ mice, as indicated by higher incidence of enlarged macrophages compared to WT littermates (Figure 2g,h).
Figure 2. Developmental profile of lung inflammation in C57BL/6N Scnn1b-Tg+ mice.
(a-d). Longitudinal BAL cell counts in Scnn1b-Tg+ mice (■) and WT littermates (□). n= 6 and 8 at 5 days (5d); n= 7 and 12 at 10 days (10d); n=12 and 13 at 4 weeks (4wk); n=10 and 8 at 8 weeks (8wk); n=4 and 4 at 6 months (6mo); n=14 and 6 at 12 months (12mo), for WT and Scnn1b-Tg+, respectively. (e-f) Longitudinal KC and TNFα levels in BAL fluid. n= 4 WT and 6 Scnn1b-Tg+ mice. T test ** p<0.005, * p<0.05 vs. WT littermates. (g–h) Macrophage size distribution in 5 day and 4 week-old C57BL/6N Scnn1b-Tg+ mice and WT littermates. n= 6 WT and 8 Scnn1b-Tg+ mice at PND 5; 10 WT and 13 Scnn1b-Tg+ mice at PND 28. Boxed regions highlight the percentage of total macrophages larger than the 90th percentile in WT mice. T test ** p<0.005, * p<0.05 vs. WT littermates.
Ablation of MyD88 signaling in Scnn1b-Tg+ mice causes defective neutrophil recruitment and higher lung bacterial burden, but does not affect airway mucus obstruction
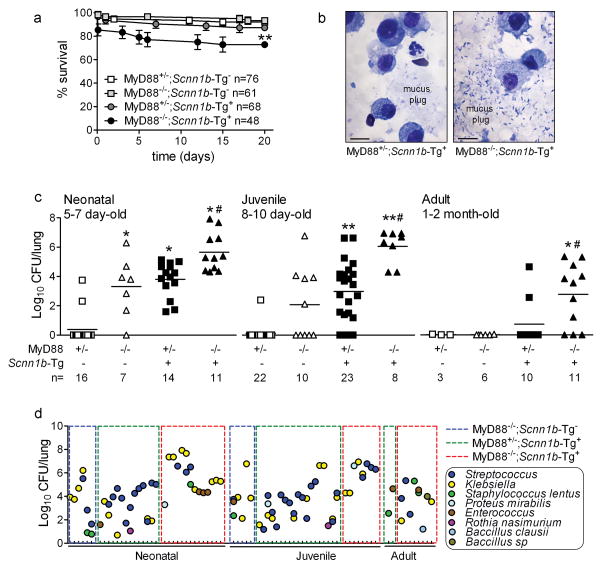
The temporal pattern of infection in Scnn1b-Tg+ mice suggests the interaction of airway mucus clearance with other developmentally regulated innate immune components, e.g., MyD88-dependent TLR signaling. To test this hypothesis, we generated Scnn1b-Tg+ and WT littermates (for this cross, referred to as Scnn1b-Tg-) that were either MyD88-sufficient (MyD88+/−) or MyD88-deficient (MyD88−/−). When raised under controlled husbandry conditions (see Methods), survival of all Scnn1b-Tg+ mice ranged between 75–100% (Figure 3a), reflecting the favorable effect of the C57Bl/6N genetic background 8 compared to the original C3H:C57 background, which had ~ 50% survival 2. Importantly, survival of MyD88−/−;Scnn1b-Tg+ mice was significantly lower than MyD88+/−;Scnn1b-Tg+ or the other genotypes (ANOVA, p = 0.004), indicating that combined Scnn1b transgene overexpression and MyD88 homozygous deletion had a detrimental effect.
Figure 3. Genetic deletion of MyD88 decreases neonatal survival and increases lung bacterial burden in WT and Scnn1b-Tg+ mice.
(a) Survival curves. Absence of MyD88 in Scnn1b-Tg+ mice lowers survival. ANOVA * p = 0.004 vs. littermates. (b) Representative photomicrograph of BAL cytospin preparations from neonatal MyD88-sufficient and -deficient Scnn1b-Tg+ mice, illustrating mucus-associated bacteria in MyD88−/−;Scnn1b-Tg+ mice. Scale bar = 10 μm. (c) Bacterial CFU in mice from the MyD88−/− × Scnn1b-Tg+ cross at the ages indicated, (Log10+1)-transformed data. n= number of mice/group. ANOVA ** p<0.005, * p<0.05 vs. MyD88+/−;Scnn1b-Tg− mice. # p<0.05 vs. MyD88+/−;Scnn1b-Tg+ mice. (d) Lung microflora in mice from the MyD88−/− × Scnn1b-Tg+ cross. Each tick on the x axis represents an individual mouse.
Microscopic analysis of BAL cytospin preparations revealed rod- and cocci-shaped bacteria in association with mucus plugs in neonatal MyD88−/−;Scnn1b-Tg+ mice, which were never observed in MyD88+/−;Scnn1b-Tg+ mice, despite similar mucus plugs (Figure 3b). Quantification of bacterial burden revealed that MyD88−/−;Scnn1b-Tg+ mice harbored significantly more bacteria than MyD88+/−;Scnn1b-Tg+ mice at all time points, and infection persisted into adulthood, although its severity decreased with age (Figure 3c). In particular, heterozygosity for MyD88 was sufficient to protect mice not carrying the Scnn1b transgene (MyD88+/−;Scnn1b-Tg-) from spontaneous bacterial infection. Conversely, complete MyD88 deficiency (MyD88−/−;Scnn1b-Tg-) led to significant bacterial burden in neonatal mice, which decreased at post-natal day (PND) 8–10 and was undetectable in adult mice. In agreement with the data presented in Figure 1a, bacteria were present in BAL of all neonatal MyD88+/−;Scnn1b-Tg+ mice, and were sporadically detected in adult mice.
The lung microflora of colonized mice from the MyD88−/− × Scnn1b-Tg+ cross (Figure 3d) was also more diverse than in C57BL/6N Scnn1b-Tg+ mice (Figure 1b). Streptococcus and Klebsiella species were prevalent, and Staphylococcus, Proteus, Enterococcus, and Rothia species were frequently detected but at lower density (Figure 3d). Of note, Streptococcus, Klebsiella, Staphylococcus and Enterococcus species persisted in MyD88−/−;Scnn1b-Tg+ mice throughout adulthood.
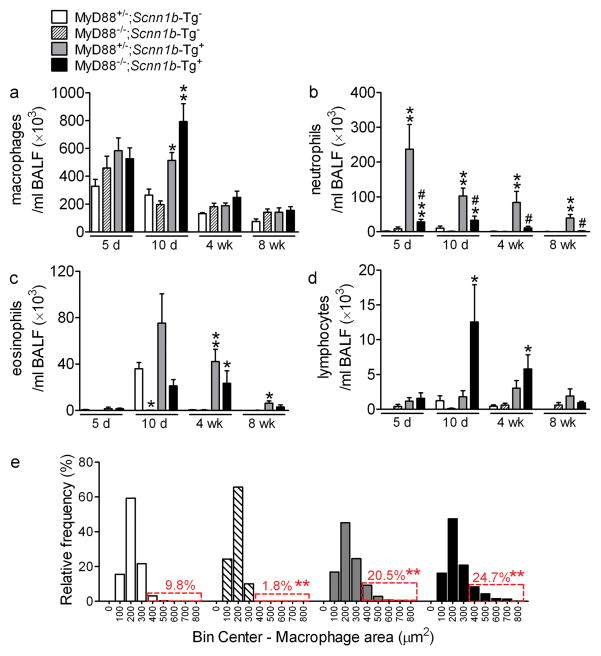
A longitudinal analysis of BAL cell counts and inflammatory mediators was performed to assess the contribution of MyD88 signaling to lung inflammation. Notably, BAL neutrophil counts were lower in MyD88−/−;Scnn1b-Tg+ versus MyD88+/−;Scnn1b-Tg+ mice at all time points (Figure 4b). Macrophages constituted the predominant cell population in MyD88+/− and MyD88−/− mice not carrying the Scnn1b transgene (Figure 4a). Except for PND 10, when macrophages were increased, Myd88−/−;Scnn1b-Tg+ mice exhibited macrophage numbers (Figure 4a) and morphological activation (Figure 4e) similar to Myd88+/−;Scnn1b-Tg+ mice. Absence of MyD88 also reduced BAL eosinophils in 10 day-old Scnn1b-Tg+ mice and prevented the transient developmental eosinophilia in Scnn1b-Tg− mice 8 (Figure 4c). Finally, BAL lymphocytes were sharply increased in Myd88−/−;Scnn1b-Tg+ mice at PND 10, but waned afterwards (Figure 4d).
Figure 4. Genetic deletion of MyD88 reduces lung neutrophilia in Scnn1b-Tg+ mice, but does not blunt macrophage activation.
(a–d) Differential BAL cell counts. n= 8, 9, 8, 8 at 5 days (5d), n= 14, 9, 12, 12 at 10 days (10d), n= 5, 5, 5, 9 at 4 weeks (4wk), and n= 3, 6, 7, 8 at 8 weeks (8wk) for MyD88+/−;Scnn1b-Tg-, MyD88−/−;Scnn1b-Tg-, MyD88+/−;Scnn1b-Tg+, and MyD88−/−;Scnn1b-Tg+ mice, respectively. ANOVA ** p<0.005, * p<0.05 vs. MyD88+/−;Scnn1b-Tg− mice. # p<0.05 vs. MyD88+/−;Scnn1b-Tg+ mice. (e) Macrophage size distribution in 4 week-old mice from the MyD88−/− × Scnn1b-Tg+ cross. Boxed regions highlight the percentage of total macrophages larger than the 90th percentile in MyD88+/−;Scnn1b-Tg− mice. n=5 MyD88+/−;Scnn1b-Tg-, 4 MyD88−/−;Scnn1b-Tg-, 5 MyD88+/−;Scnn1b-Tg+, and 8 MyD88−/−;Scnn1b-Tg+. ANOVA ** p<0.005, * p<0.05 vs. MyD88+/−;Scnn1b-Tg-littermates.
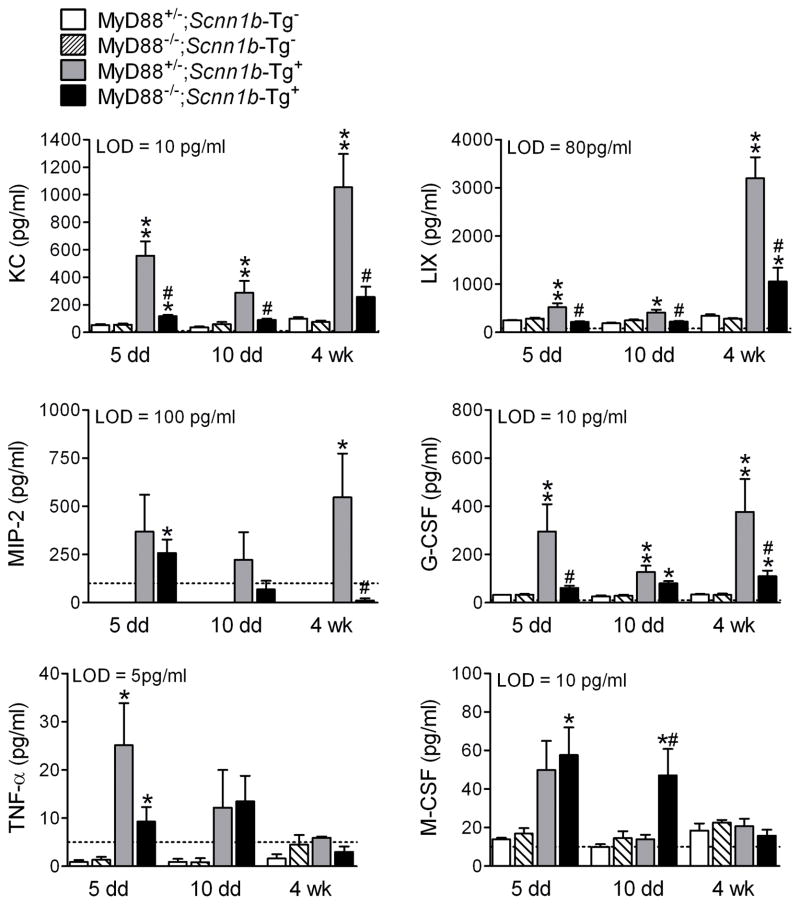
The inflammatory mediator profile of cell-free BAL (Figure 5) mirrored the changes in BAL cell composition. Myd88−/−;Scnn1b-Tg+ mice exhibited reduced neutrophil chemoattractants (KC, LIX, MIP-2), granulocyte differentiation factors (G-CSF), and TNFα at PND 5 compared to Myd88+/−;Scnn1b-Tg+ mice. Macrophage colony stimulating factor (M-CSF) was higher at PND 10 in Myd88−/−;Scnn1b-Tg+ mice, paralleling higher BAL macrophages at this time point. MIP-1α, IL-6, GM-CSF, IL-1α, IL-1β, and MCP-1 were either at or below the lower limit of detection, and differences among groups failed to be significant or exhibit consistent trends (data not shown).
Figure 5. MyD88 deletion in Scnn1b-Tg+ mice alters BAL neutrophil- and macrophage-related inflammatory mediators.
BAL cytokines in 5 day-, 10 day- and 4 week-old mice. The dotted line represents the assay lower detection limit (LOD). n = 4 for WT mice and n=6 for Scnn1b-Tg+ mice. ANOVA ** p<0.005, * p<0.05 vs. age-matched MyD88+/−;Scnn1b-Tg− mice. # p<0.05 vs. MyD88+/−;Scnn1b-Tg+ mice.
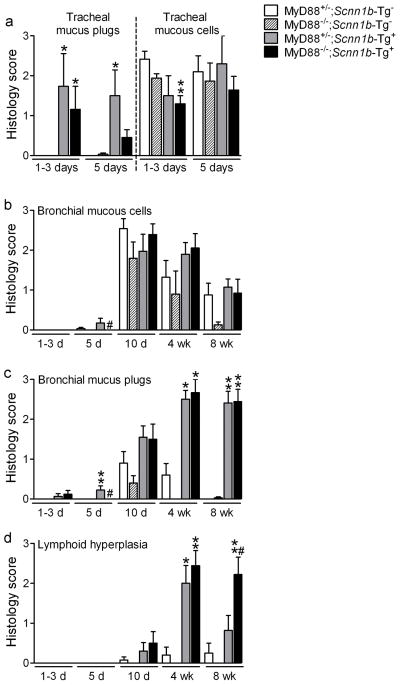
A longitudinal histological analysis was performed to test whether MyD88 deficiency modified the lung pathology in Scnn1b-Tg+ mice (Figure 6). Absence of MyD88 did not alter the transient abundance of tracheal and bronchial mucous secretory cells normally observed postnatally 8,9 (Figure 6a,b). Myd88+/−;Scnn1b-Tg+ and Myd88−/−;Scnn1b-Tg+ mice exhibited comparable tracheal mucus plugging and mucous secretory cells in neonates (Figure 6a), a similar progression of mucus obstruction (from trachea to bronchi, Figure 6a,c), and equivalent air space enlargement (data not shown). However, 8 week-old Myd88−/−;Scnn1b-Tg+ mice had significantly more lymphoid aggregates than Myd88+/−;Scnn1b-Tg+ mice (Figure 6d), suggesting a response to a greater bacterial burden and/or alteration of adaptive immune responses.
Figure 6. MyD88 deletion does not modify mucus plugging, but promotes development of lymphoid aggregates in Scnn1b-Tg+ mice.
(a-c)Semi-quantitative histopathology scores for mucus plugs and mucous secretory cells (AB-PAS positive) in (a) neonatal trachea and (b-c) left lobe intrapulmonary main stem bronchus, at different time points. (d) Semi-quantitative histopathology scores for lymphoid aggregates (BALT). n= 6, 8, 3, 5 at 1–3 days (1–3d), n= 5, 6, 4, 8 at 5 days (5d), n= 13, 5, 10, 12 at 10 days (10d), n= 5, 4, 5, 9 at 4 weeks (4wk), and n= 8, 8, 11, 9 at 8 weeks (8wk) for MyD88+/−;Scnn1b-Tg-; MyD88−/−;Scnn1b-Tg-; MyD88+/−;Scnn1b-Tg+; and MyD88−/−;Scnn1b-Tg+ mice, respectively. ANOVA ** p<0.005, * p<0.05 vs. MyD88+/−;Scnn1b-Tg− mice. # p<0.05 vs. MyD88+/−;Scnn1b-Tg+ mice.
Germ-free Scnn1b-Tg+ mice are devoid of bacteria, but still develop mucus obstructive lung disease
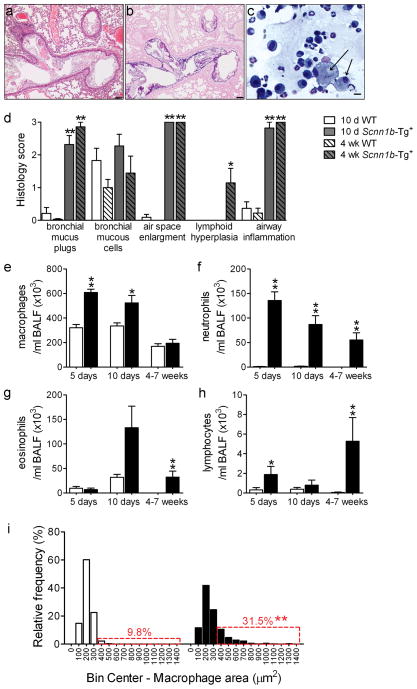
To define the relative contributions of dehydrated mucus vs. neonatal bacterial infection to the development of obstructive airways disease, we derived Scnn1b-Tg+ mice in a germ-free (GF) environment. As expected, BAL from GF Scnn1b-Tg+ mice harvested at PND 5, when both incidence of infection and bacterial load are maximal in SPF Scnn1b-Tg+ mice, was sterile (n=5). Adult GF Scnn1b-Tg+ mice exhibited overt airway mucus obstruction and, strikingly, persistent inflammation (Figure 7a–c). Indeed, the overall lung pathology, as assessed by semi-quantitative histology score, was comparable in GF and SPF Scnn1b-Tg+ mice, at both 10 days and 4 weeks of age (Figure 7d).
Figure 7. Germ-free (GF) Scnn1b-Tg+ mice develop lung inflammation similar to Scnn1b-Tg+ mice raised in conventional SPF conditions.
(a, b) Representative photomicrographs of left lobe main stem bronchus from 6 week-old GF Scnn1b-Tg+ mice, illustrating alveolar space enlargement, mucus obstruction, and airway inflammation. H&E (a) and AB-PAS (b) stain. Scale bar 100 μm. (c) Representative photomicrograph of BAL cytospin preparation from GF Scnn1b-Tg+ mice, illustrating mucus plugs (light blue), granulocytes and large/foamy macrophages (arrows). Giemsa stain, scale bar = 20 μm. (d) Semi-quantitative histopathology scores for 10 day old (10 d, open bars) and 4 week-old (4 wk, hatched bars) Scnn1b-Tg+ mice (gray) and WT littermates (white) raised in GF conditions, n= 11 Scnn1b-Tg+ and 11 WT littermates at 10 days, n=7 Scnn1b-Tg+ and 9 WT littermates at 4 weeks of age. T test ** p<0.005, * p<0.05 vs. WT littermates. (e-h) Longitudinal differential BAL cell counts for GF Scnn1b-Tg+ mice (■) and WT littermates (□). n= 11 and 11 at 5 days, n= 18 and 8 at 10 days, n= 8 and 7 at 4–7 weeks, for GF WT and GF Scnn1b-Tg+ mice, respectively. (i) Macrophage size distribution in 4–7 week-old GF Scnn1b-Tg+ mice (■, n=7) and WT littermates (□, n=8). Boxed regions highlight the percentage of total macrophages larger than the 90th percentile in WT mice. T test ** p<0.005 vs. WT littermates.
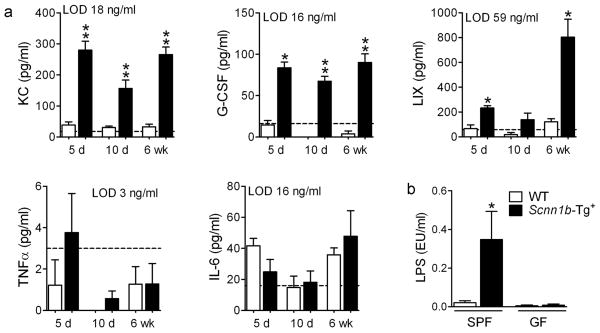
BAL longitudinal analysis revealed that 5–10 day-old GF Scnn1b-Tg+ mice had more macrophages than their WT littermates (Figure 7e), which was not a feature of SPF Scnn1b-Tg+ mice (Figure 2a). Although macrophage number in adult GF Scnn1b-Tg+ mice normalized towards WT levels, GF Scnn1b-Tg+ macrophages remained morphologically activated (Figure 7i). GF Scnn1b-Tg+ mice had higher neutrophil counts compared to WT littermates at all time points (Figure 7f) and more eosinophils and lymphocytes in adult mice (Figure 7g-h). Of note, BAL neutrophil counts were lower in neonatal GF Scnn1b-Tg+ mice compared to SPF Scnn1b-Tg+ mice (87 ± 18×103 vs. 269 ± 71×103 for GF and SPF 10 day-old mice, respectively, p<0.05), but did not differ in adult mice. As in the SPF environment, the BAL cytokine profile of GF Scnn1b-Tg+ mice was characterized by elevated neutrophil-related mediators (KC, LIX, and G-CSF), whereas, unlike SPF Scnn1b-Tg+ mice, TNFα was not elevated compared to WT littermates (Figure 8a).
Figure 8. BAL cytokine profile and BAL LPS content in germ-free Scnn1b-Tg+ mice and WT littermates.
(a) BAL cytokines in 5 day-, 10 day- and 6 week-old mice. The dotted line represents the assay lower detection limit (LOD). n = 6 for WT and 8 for Scnn1b-Tg+ mice. ANOVA ** p<0.005, * p<0.05 vs. WT littermates. (b) LPS content in BAL isolated from SPF or GF Scnn1b-Tg+ mice (■) and WT littermates (□). n= 14 and 11 for SPF WT and Scnn1b-Tg+ mice, respectively; n= 4 and 5 for GF WT and Scnn1b-Tg+ mice, respectively. T test * p<0.05 vs. WT littermates.
Because sterilized feed and bedding in GF conditions are not free of bacterial products, we tested whether environmental LPS, trapped after inhalation by static airway mucus, could be responsible for stimulating inflammation in GF Scnn1b-Tg+ mice. As expected, the level of LPS in unfractionated BAL from 5 day-old SPF Scnn1b-Tg+ was significantly higher than in WT littermates, but LPS was barely detectable and equivalent in GF Scnn1b-Tg+ mice and WT littermates (Figure 8b). Thus, the inflammation observed in GF Scnn1b-Tg+ mice appears to be independent of both live bacteria and at least one major bacterial product (LPS).
DISCUSSION
In the respiratory tract, effective host defense depends primarily upon rapid mechanical clearance of inhaled noxious agents 16. The paradigm that defective mucus clearance favors bacterial infections of the airways is widely accepted 1, but has never been rigorously tested, nor has the interplay between mucus clearance and other innate defense mechanisms been explored. We used an in vivo model of abnormal mucus clearance, i.e., the Scnn1b-Tg+ mouse, to explore the mechanisms involved in acquisition versus protection from airway bacterial infections.
Spontaneous bacterial infections are rarely reported in mouse models and usually occur in immunocompromized strains 17–19. Our longitudinal studies indicate that defective mucus clearance caused a breach in host defense resulting in an age-dependent presence of bacteria in the lungs (Figure 1a). This presence likely constitutes an “infection”, as indicated by higher neutrophils and TNFα in BAL from SPF Scnn1b-Tg+ mice (Figure 2b,f) compared to GF Scnn1b-Tg+ mice which have similar mucus obstruction but no bacteria (Figures 7f and 8a). As occurs in CF 10, the infection in Scnn1b-Tg+ mice appears to be centered on the airways (Figure 1c, trachea, and Figure 3b, airway mucus). We speculate that the bacterial density (103/lung) likely reprises that observed in human muco-obstructive airway infections. Specifically, we estimated that the mucus volume in neonatal Scnn1b-Tg+ mice is ~ 1 μl (see Methods), producing a density of 106 bacteria/ml, a value typical for sputum samples in CF subjects 20.
The decrease in lung bacterial burden as Scnn1b-Tg+ mice aged is likely due to the postnatal maturation of innate 21 and adaptive 7 immunity, including changes in pattern recognition receptor expression 11,12, neutrophils 22 and antigen presenting cell functions 23, as well as changes in airway mucous secretory cell number/secretions 8,9,24,25. Moreover, in mouse airways the density of airway ciliated cells increases with age 26 and maximal mucus transport rates are generated after PND 9 27, partially offsetting the clearance defect due to mucus dehydration in Scnn1b-Tg+ mice.
The bacterial species most prevalent in the lungs of Scnn1b-Tg+ mice during the neonatal period also populated the mouse tongue and esophagus (Figure 1c), suggesting the oropharynx as the portal of entry. We speculate that while aspiration occurred in both WT and Scnn1b-Tg+ mice, infection selectively affected Scnn1b-Tg+ mice due to the poor clearance of aspirated contents. CF patients also exhibit concordant bacterial genotypes in the upper and lower airways 28–30, indicating aspiration as a likely mechanism of spread in human diseases associated with pulmonary mucus stasis.
The shift in bacterial species between neonatal and adult Scnn1b-Tg+ mice may indicate that, after clearing the initial infection, Scnn1b-Tg+ mice became susceptible to intermittent infection with species already present in the cage and/or oral environment. Alternatively, bacterial competition in the mucus niche may have favored the species recovered in older mice. Regardless, these data highlight a feature of this model which should be helpful for studying the longitudinal pathogenesis of chronic lung diseases characterized by persistent/recurrent bacterial infection 31.
Certain caveats apply to our microbiologic studies. First, mice housed in SPF facilities are not exposed to highly infectious murine pathogens, which may be more successful in establishing persistent infection in Scnn1b-Tg+ mice. Second, BAL sampling might have not harvested all bacteria, but it was preferred over lung homogenization which was found to inhibit growth of the isolated species. Third, bacterial detection was limited to species capable of growth under the specific culture conditions used, which were not permissive for strict anaerobes and fastidious aerobes. Future studies should focus on exposure to selected pathogens and more sophisticated culture-independent methods suitable to detect small numbers of bacteria, i.e., emulsion PCR and 454 pyrosequencing.
Our studies also demonstrated that other mucosal defense mechanisms modulate the response to airway bacterial infection due to defective mucus clearance. Studies designed to identify ancillary protective mechanisms revealed that MyD88-dependent innate immunity is key in determining the severity of airway bacterial infections in Scnn1b-Tg mice. In neonatal mice, MyD88 deficiency in the absence of mucus stasis (MyD88−/−;Scnn1b-Tg− mice) produced bacterial burdens comparable to those caused by mucus obstruction alone (MyD88+/−;Scnn1b-Tg+ mice) (Figure 3c), whereas the presence of both MyD88 deficiency and mucus stasis (MyD88−/−;Scnn1b-Tg+ mice) produced an additive effect, suggesting that the two defense mechanisms operate independently. Of note, the greater bacterial burden in neonatal MyD88−/−;Scnn1b-Tg+ mice compared to MyD88+/−;Scnn1b-Tg+ mice was not associated with increased mucus obstruction or mucous secretory cells (Figure 6a–c), indicating that MyD88 deletion predominantly affected the immune functions of inflammatory 32 and epithelial cells 33.
The sensitivity of MyD88−/− mice to bacterial infection differs depending on the microorganism studied 34, but is generally regarded as “broad”. MyD88 deletion prevents signaling through TLR 1, 2, 4, 5, 7, and 9 and blunts immune responses by impairing neutrophil chemotaxis (Figure 4b) and secretion of inflammatory mediators, such as KC, MIP-2, CXCL5/LIX (Figure 5). However, both MyD88−/−;Scnn1b-Tg− mice and MyD88−/−;Scnn1b-Tg+ mice exhibited reduced bacterial burdens as a function of age (Figure 3c), indicating the maturation of MyD88-independent protective mechanisms 13,16,35–37. Nonetheless, the higher and persistent bacterial burden in adult MyD88−/−;Scnn1b-Tg+ mice suggests that the MyD88-independent mechanisms were insufficient to completely overcome two additive defects in mucosal defense.
The studies described above highlight the complex interplay between mucus obstruction, infection, and inflammation in our model. The persistence of lung neutrophilia in adult Scnn1b-Tg+ mice (Figure 2b) could reflect the presence of non-culturable bacteria. Alternatively, airway mucus stasis per se could contribute to the inflammatory response observed in muco-obstructive lung diseases by impairing clearance of noxious stimuli. To distinguish between these two hypotheses, we generated GF Scnn1b-Tg+ mice, which were reared in a low-endotoxin environment (Figure 8b). Notably, adult GF Scnn1b-Tg+ mice exhibited lung inflammation and histopathology comparable to SPF Scnn1b-Tg+ mice (Figure 7), supporting the hypothesis that mucus stasis per se can lead to inflammation by trapping non-infectious, noxious materials.
As LPS levels were minimal in the GF environment (Figure 8b), we speculate that other stimuli contributed to macrophage activation (Figure 7i) and neutrophil recruitment (Figure 7f) in GF Scnn1b-Tg+ mice. Beside inhaled noxious stimuli, necrotic bronchial epithelial cells transiently present in 3 day-old Scnn1b-Tg+ mice 3 could also contribute to the sterile inflammation observed in Scnn1b-Tg+ mice. We speculate that once initiated by poor clearance of toxic materials, the inflammatory process is perpetuated by the retention of stimuli causing macrophage activation and the sequelae of neutrophil recruitment, including secondary necrosis 38 and release of other chemoattractants, e.g., high-mobility group box 1 and PGP 39.
Additional features of mice reared in the GF environment were noted. GF mice still exhibit the transient neonatal surge in mucous secretory cells and eosinophils described for SPF mice 8 (Figure 7d,g). Furthermore, neonatal GF WT mice had fewer neutrophils (Figure 7f) than neonatal SPF WT mice (Figure 2b), consistent with the notion that normal airways may not be completely sterile and intrapulmonary bacteria help guide immune development 40. Indeed, the absence of environmental bacteria might affect the overall immune response to airway mucus stasis, as suggested by the higher number of macrophages recovered in neonatal GF vs. SPF Scnn1b-Tg+ mice (Figures 7e and 2a). Finally, both SPF and GF Scnn1b-Tg+ mice develop bronchial-associated lymphoid tissue (BALT) (Figure 7d and 8), which is usually thought to result from TLR stimulation 41 and whose incidence correlates with worsening airflow obstruction in COPD patients 42. The observation that both MyD88−/−; Scnn1b-Tg+ mice and GF Scnn1b-Tg+ mice exhibit lymphoid aggregates suggests that neither MyD88 signaling nor bacterial stimulation is required for this response in the context of airway mucus stasis.
The hypothesis that CF is characterized by intrinsic lung inflammation in the absence of bacterial infection is controversial 43,44, and novel animal models have been generated to better understand the critical phases of disease onset and progression 45,46. Although we did not perform BAL studies on prenatal Scnn1b-Tg+ mice, whose airways are not exposed to bacteria or bacterial products, previous studies reported that the concentration of KC and MIP-2 in lung homogenates from newborn (PND 1) Scnn1b-Tg+ mice was not different from WT littermates 2, whereas an inflammatory infiltrate was clearly present in Scnn1b-Tg+ mice just a few days later (PND3), in conjunction with tracheal mucus plugging 3,47, suggesting that the inflammatory response develops as a consequence of airway mucus obstruction. Collectively, our data from the GF and SPF Scnn1b-Tg+ mice suggest that mucus stasis per se produces airway inflammation and that mucus stasis results in susceptibility to infection by bacteria aspirated from the oropharynx. Due to its unique phenotype and the amenability to complex genetic and environmental manipulations, the Scnn1b-Tg+ mouse model has allowed us to probe the interactions of abnormal mucus clearance and other layers of lung defense, e.g., MyD88-dependent pathways. Our data suggest that stagnant mucus can initiate both the inflammatory and infectious components of obstructive lung diseases and thus, is a primary therapeutic target. As such, therapies aimed at promoting mucus clearance would provide three interconnected benefits, namely to alleviate inflammation, eradicate infection, and restore immune homeostasis.
METHODS
Mice
Mice were housed in a specific pathogen free (SPF) facility at the University of North Carolina at Chapel Hill. Congenic C57Bl6/N Scnn1b-Tg+ mice were generated by backcrossing C3:B6 Scnn1b-Tg+ mice line 6608 2 with C57Bl/6N mice (Taconic, Hudson, NY) for more than 12 generations. Mice were housed in hot-washed, individually ventilated micro-isolator cages with corn cob bedding, on a 12-hour day/night cycle, fed regular chow and given water ad libitum. MyD88−/− mice [B6.129P2-Myd88tm1Aki 48, kindly provided by Dr. Shizuo Akira, Osaka University, through Dr. Jonathan Serody, University of North Carolina at Chapel Hill] were bred with C57Bl6 Scnn1b-Tg+ mice to obtain experimental animals of four predicted genotypes with expected Mendelian distribution of 25% each: MyD88+/−;Scnn1b-Tg-, MyD88−/−;Scnn1b-Tg-, MyD88+/−;Scnn1b-Tg+, and MyD88−/−;Scnn1b-Tg+. This breeding strategy generated control mice heterozygous for MyD88, which were not expected to be different from MyD88+/+ mice, and gave the advantage that experimental animals of all four genotypes were littermates and shared an identical environment. Our first attempt to establish this colony and monitor survival by early toe excision (PND 2–3) was unsuccessful as MyD88−/− mice rapidly died, likely due to susceptibility to infection after early toeing. To increase survival, breeder pairs were housed in “cleaner conditions”, i.e., cages with autoclaved TEK-FRESH bedding (Harlan) changed weekly, and were given autoclaved food and antibiotic-supplemented water (sulfamethoxazole 0.64 mg/ml and trimethoprim, 0.13 mg/ml) until the dams gave birth. Pups were toed for identification and genotyping at PND 5. Germ-free Scnn1b-Tg+ mice were generated in the National Gnotobiotic Rodent Resource Center at UNC. Germ-free rodents are axenic, with no detectable bacteria, yeast, molds, parasites or viruses (except retroviruses). To monitor the sterility of the isolator containing the GF Scnn1b-Tg+ mouse colony, samples of fresh feces, mouth/paws/cages swabs, and drinking water were collected from breeders and holding mice every time the port was open. Collected samples were tested for the presence of contaminating bacteria by plating onto sheep blood agar, growth in fluid thioglycollate medium (FTG), and Gram staining. Regardless of port opening, all isolators were periodically monitored (every other month) through Gram staining and 16S PCR on sampled feces. The isolator containing the GF Scnn1b-Tg+ mice colony never suffered a breach in germ-free status, and we studied mice from different litters at different ages, over a several months period. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill and performed according to the principles outlined by the Animal Welfare and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Bronchoalveolar lavage (BAL), differential cell counts, lung histology, and macrophage size
For 10 day-old or older mice, we obtained both BAL and lung histology from each animal, as previously described 8. Due to their small size, 5 day-old pups were subject to either whole lung BAL or tissue harvesting. Macrophage size was determined by measuring the surface area of 60–80 macrophages/mouse. Cytospin preparations were photographed with a Leica DMIRB inverted microscope interfaced with a Micro Publisher 3.3 color camera (Q-Imaging, Surrey, BC, Canada) at 20× magnification and macrophage area was determined using the calibrated freehand selection tool of Image J analysis software (NIH, Bethesda). Histology specimens were scored by an investigator blinded to genotype using a semi-quantitative scoring system, as described previously 8.
Cytokine profile
Mouse TNFα, KC, MIP-2, MIP-1α, M-CSF, MCP-1, LIX, IL-6, IL-1α, IL-1β, GM-CSF, and G-CSF were measured in cell-free BAL using a Luminex-based assay (EMD Millipore, Billerica, MA), according to the manufacturer instructions.
Bacteriology
BAL was performed aseptically, inserting the cannula by tracheotomy in the lower portion of the trachea, to avoid oropharyngeal bacteria. Whole lungs were lavaged 4 times, using a weight-based formula 8 and fractions pooled. Serial BAL dilutions were plated onto Columbia anaerobe sheep blood agar (Becton Dickinson, NJ) and incubated in a candle jar to facilitate the growth of microaerophilic bacteria. Plates were incubated at 37°C for 24 hours, and colony forming units (CFU) were enumerated and classified based on their morphology. Individual colonies representative of each morphologic group were expanded and processed for molecular identification. For detection of bacteria in tissue homogenates, tongue, esophagus, trachea and lungs were dissected from 5 day-old mice and homogenized in 1 ml of sterile Dulbecco’s phosphate-buffered saline (D-PBS, Sigma, MO) using a Tissue Tearor (Biospec products, OK) on ice. Serial dilutions were plated and processed as described above.
Molecular identification of bacterial species
Bacterial DNA was extracted using the FastDNA SPIN Kit, lysing Matrix B protocol (MP Biomedicals, OH), according to manufacturer’s instructions. The ribosomal 16S gene was amplified by PCR using HPLC-purified primers (forward 5′ AGAGTTTGATC(A + C)TGGCTCAG 3′, reverse 5′ TACGG(C+T)TACCTTGTTACGACTT 3′) and the following PCR conditions: denaturing 95°C for 5 min; 35 cycles of amplification (95°C for 20 sec, 51°C for 20, 72°C for 1 min and 10 sec); 72°C for 8 min. Primers and dNTPs were removed from the PCR product by digestion with exonuclease I and shrimp alkaline phosphatase (ExoSAP-IT, USB Corp., OH). The PCR product was sequenced using both forward and reverse primers (Genewiz, NJ). Bacterial identities were inferred from the degree of alignment of the ribosomal 16S gene sequences with existing databases (NCBI Nucleotide collection, excluding “models” and “uncultured/environmental sample sequences”), using the NCBI Basic Local Alignment Search Tool (BLAST).
LPS assay
Limulus amebocyte lysate assay was performed on unfractionated BAL from 5 day-old WT and Scnn1b-Tg+ mice using the Pyrochrome kinetic method, according to manufacturer instructions (Associates of Cape Cod, Inc. MA). The assay was performed under conditions that allowed cumulative detection of both LPS and β-glucan.
Estimate of mucus volume
Mucus volume was calculated from the adult mouse ASL volume of 2.27 μl 49 and the adult/pup scaling factor of 2, obtained by dividing the average tracheal length or diameter in 20 g vs. 3 g mice. Measurements were courtesy of Dr. Barbara Grubb, UNC Chapel Hill.
Statistical analyses
Statistical analyses were performed using SigmaStat 3.1 or JMP 8.0.2. Survival curves were compared using Kaplan-Meier log rank analysis and Holm-Sidak multiple comparison. All numeric values were log10 transformed with an offset of +1 before inferential statistical analyses. Comparisons between measurements from 2 groups with significant difference in variances were performed using Student t test assuming non-equal variance, or non-parametric Wilcoxon rank-sum test. Comparison between multiple groups were performed using one-way analysis of variance (ANOVA) and differences among the group means were assessed by Tukey-Kramer post-hoc test for multiple test correction. For inferential statistics, p<0.05 was considered statistically significant and “n” represents the number of animals in each experimental group. Data presented in plots with error bars are expressed as mean ± SEM. Distribution of macrophage sizes between control (WT or MyD88+/−;Scnn1b-Tg-) and test groups were performed by determining the 90% threshold in the control group, and comparing the proportion of cells in the test group beyond the threshold. Statistical significance between the proportions of cells that passed the threshold in the test group vs. control group (10%) was evaluated using χ2 test.
Acknowledgments
The authors thank: The authors thank: Nanette B. Fulcher for advice in microbiology techniques and data analysis; Kimberly Burns, Donald Joyner and Tracy Eldred for technical assistance with histology; Kristy Terrell and Kimberly Brassard for assistance with bacterial species identification; Rodney Gilmore for assistance with mouse genotyping; the UNC Michael Hooker Microscopy Facility, funded by an anonymous private donor; the Clinical Proteomics Laboratory at the UNC Thurston Arthritis Research Center and the Immunotechnology Core at the UNC Center for Gastrointestinal Biology and Disease for Luminex assays; Maureen A. Bower, Kathy Mohr, and Jamison D. Cameron in the UNC Center for Gastrointestinal Biology and Disease Gnotobiotic Core directed by Dr. B. Sartor and supported by NIH grant P30 DK34987 for generating and maintaining the germ-free Scnn1b-Tg mouse colony. The studies were supported by grant RANDEL07P0 awarded to S.H. Randell by the Cystic Fibrosis Foundation, and by the Cystic Fibrosis Research Development Program grant RDP R026, and National Institute of Health P30 DK065988 and P50 HL060280 to W.K. O’Neal and R.C. Boucher.
Footnotes
Conflict of interest: The authors have no conflicting financial interests.
LITERATURE CITED
- 1.Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35:20–8. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–93. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 3.Mall MA, et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–42. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev. 2011;24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35:1209–15. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 6.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 7.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi A, et al. Airway and lung pathology due to mucosal surface dehydration in {beta}-epithelial Na+ channel-overexpressing mice: role of TNF-{alpha} and IL-4R{alpha} signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol. 2009;182:4357–67. doi: 10.4049/jimmunol.0802557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy MG, et al. Mucin Production During Pre- and Post-Natal Mouse Lung Development. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadeghi K, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med. 2007;30:E44–53. doi: 10.25011/cim.v30i2.979. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 14.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–6. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousfiha A, et al. Primary immunodeficiencies of protective immunity to primary infections. Clin Immunol. 2010;135:204–9. doi: 10.1016/j.clim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–11. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozalo AS, et al. Spontaneous Staphylococcus xylosus Infection in Mice Deficient in NADPH Oxidase and Comparison with Other Laboratory Mouse Strains. J Am Assoc Lab Anim Sci. 49:480–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Forlow SB, Foley PL, Ley K. Severely reduced neutrophil adhesion and impaired host defense against fecal and commensal bacteria in CD18−/−P-selectin−/− double null mice. FASEB J. 2002;16:1488–96. doi: 10.1096/fj.02-0230com. [DOI] [PubMed] [Google Scholar]
- 19.Ostanin DV, Barlow S, Shukla D, Grisham MB. NADPH oxidase but not myeloperoxidase protects lymphopenic mice from spontaneous infections. Biochem Biophys Res Commun. 2007;355:801–6. doi: 10.1016/j.bbrc.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Tunney MM, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 21.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 22.Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation. 1996;20:499–512. doi: 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 23.Garvy BA, Qureshi MH. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J Immunol. 2000;165:6480–6. doi: 10.4049/jimmunol.165.11.6480. [DOI] [PubMed] [Google Scholar]
- 24.Van Winkle LS, et al. Epithelial cell distribution and abundance in rhesus monkey airways during postnatal lung growth and development. J Appl Physiol. 2004;97:2355–63. doi: 10.1152/japplphysiol.00470.2004. discussion 2354. [DOI] [PubMed] [Google Scholar]
- 25.Lamb D, Reid L. Acidic glycoproteins produced by the mucous cells of the bronchial submucosal glands in the fetus and child: a histochemical autoradiographic study. Br J Dis Chest. 1972;66:248–53. doi: 10.1016/0007-0971(72)90043-5. [DOI] [PubMed] [Google Scholar]
- 26.Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. Temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L454–459. doi: 10.1152/ajplung.00036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis RJ, et al. Initiation and maturation of cilia-generated flow in newborn and postnatal mouse airway. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1067–75. doi: 10.1152/ajplung.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlebach MS, et al. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis? Pediatr Pulmonol. 2006;41:445–51. doi: 10.1002/ppul.20396. [DOI] [PubMed] [Google Scholar]
- 29.Bonestroo HJ, de Winter-de Groot KM, van der Ent CK, Arets HG. Upper and lower airway cultures in children with cystic fibrosis: do not neglect the upper airways. J Cyst Fibros. 9:130–4. doi: 10.1016/j.jcf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Mainz JG, et al. Concordant genotype of upper and lower airways P aeruginosa and S aureus isolates in cystic fibrosis. Thorax. 2009;64:535–40. doi: 10.1136/thx.2008.104711. [DOI] [PubMed] [Google Scholar]
- 31.Didierlaurent A, Goulding J, Hussell T. The impact of successive infections on the lung microenvironment. Immunology. 2007;122:457–65. doi: 10.1111/j.1365-2567.2007.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–75. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 72:413–35. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balamayooran T, Balamayooran G, Jeyaseelan S. Review: Toll-like receptors and NOD-like receptors in pulmonary antibacterial immunity. Innate Immun. 16:201–10. doi: 10.1177/1753425910366058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 11:701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 36.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 11:785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zola TA, Lysenko ES, Weiser JN. Natural antibody to conserved targets of Haemophilus influenzae limits colonization of the murine nasopharynx. Infect Immun. 2009;77:3458–65. doi: 10.1128/IAI.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva MT, do Vale A, dos Santos NM. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–82. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe SM, et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178:822–31. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilty M, et al. Disordered microbial communities in asthmatic airways. PLoS One. 5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moghaddam SJ, et al. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–38. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogg JC, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 43.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–30. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 44.Bruscia EM, et al. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 120:3149–60. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wielputz MO, et al. In vivo monitoring of cystic fibrosis-like lung disease in mice by volumetric computed tomography. Eur Respir J. 38:1060–70. doi: 10.1183/09031936.00149810. [DOI] [PubMed] [Google Scholar]
- 48.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 49.Durairaj L, et al. Safety assessment of inhaled xylitol in mice and healthy volunteers. Respir Res. 2004;5:13. doi: 10.1186/1465-9921-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]