Abstract
Many commercial and household products such as lubricants, cosmetics, plastics, and paint contain phthalates, in particular bis-(2-ethyhexyl)- phthalate (DEHP). As a consequence, phthalates have been found in a number of locations and foods (streambeds, household dust, bottled water and dairy products). Epidemiological and animal studies analysing phthalate exposure in males provide evidence of degradation in sperm quality, associated to an increase in the incidence of genital birth defects and testicular cancers. In the testis, spermatogenesis is maintained throughout life by a small number of spermatogonial stem cells (SSCs) that self-renew or differentiate to produce adequate numbers of spermatozoa. Disruption or alteration of SSC self-renewal induce decreased sperm count and sperm quality, or may potentially lead to testicular cancer. GDNF, or glial cell-line-derived neurotrophic factor, is a growth factor that is essential for the self- renewal of SSCs and continuous spermatogenesis. In the present study, the SSC-derived cell line C18-4 was used as a model for preliminary assessment of the effects of mono-(2-ethylhexyl)- phthalate (MEHP, main metabolite of DEHP) on spermatogonial stem cells. Our data demonstrate that MEHP disrupts one of the known GDNF signalling pathways in these cells. MEHP induced a decrease of C18-4 cell viability in a time- and dose-dependent manner, as well as a disruption of ERK1/2 activation but not of SRC signalling. As a result, we observed a decrease of expression of the transcription factor FOS, which is downstream of the GDNF/ERK1/2 axis in these cells. Taken together, our data suggest that MEHP exposure affects SSC proliferation through inhibition of specific signalling molecules.
Keywords: spermatogonial stem cells, phthalates, self-renewal, infertility, GDNF
1. INTRODUCTION
Phthalates such as DEHP (bis-(2-ethylhexyl) phthalate) are chemicals widely used as plasticizers for PVC and other plastics, to which they confer flexibility. In fact they can make up to 40 to 50 percent of the volume of bendable products such as medical tubing. Phthalates are also used in paints and nail polish to create thin and flexible films. Today these compounds are found in virtually everything, from food packaging and insect repellants to bath and teething toys. Unfortunately, phthalates easily leach into the environment because they are not covalently bound to their plastic matrix. Therefore, as a result of their wide use in industrial applications, phthalates are found in water, dust, infant formula, or even in fruit jellies (Latini et al., 2006). The average total exposure to DEHP (food, dermal, and inhalation) has been estimated at 1 mg/kg/day and 2 mg/kg/day for a European adult or child respectively (Wormuth et al., 2006). Several studies have demonstrated a toxic effect of phthalates on liver and other organs, but because these compounds are classified as endocrine disruptors, most studies have focused on their effects on the reproductive tract. For example, urinary concentration of phthalate metabolites can be used as a bio-monitoring tool for reproductive fitness, and studies in young adults demonstrated a link between urinary metabolites and low semen quality (Duty et al., 2004). Major cellular targets of phthalates in the male reproductive organs are Sertoli and Leydig cells of the testis (for a review see: Martino-Andrade and Chahoud, 2010). Within the seminiferous epithelium, increasing expression of FAS ligand by Sertoli cells will activate apoptosis in Fas-positive germ cells such as spermatocytes and round spermatids (Richburg et al, 1999). Reports also indicate that in the rat, early exposure to phthalates in utero (E13.5–E15.5) leads to 30–50% reduction in the number of gonocytes and might affect spermatogonial stem cell proliferation at post-natal day 6 (Ferrara et al, 2006; Jobling et al, 2011). Therefore, because spermatogonial stem cells of the testis, or SSCs, are at the origin of spermatogenesis in the neonate and adult animal, studying their sensitivity to environmental toxicants directly is of paramount importance to gather a complete understanding of the effects of these compounds on testis function. In addition, until now few suitable models for SSC behaviour were available for in vitro investigations. The C18-4 cell line, previously established from stem-progenitor spermatogonia (Hofmann et al., 2005a), allows us now to test the direct effects of reproductive toxicants on these cells at the molecular level.
In the mammalian testis, the seminiferous epithelium is composed of somatic Sertoli cells and germ cells at different stages of maturation. Cells of the germ-line are continuously generated by a small population of spermatogonial stem cells (SSCs) that self-renew, differentiate and ultimately produce spermatozoa. Without a healthy population of SSCs, spermatogenesis would be durably compromised. Homeostasis and fate of SSCs are regulated by a number of signalling molecules, supporting cells and the extracellular microenvironment, which together form the stem cell niche (Shetty and Meistrich, 2007; Xie, 2008). Germ cell differentiation starts when a SSC (or Asingle spermatogonium) divides into 2 daughter cells linked by an intercellular bridge (Apaired spermatogonia). The cells then continue to proliferate while differentiating into chains of Aaligned spermatogonia (De Rooij, 1998). However, recent progress in characterizing SSCs through transplantation assays have indicated that all early type A spermatogonia (Asingle, Apaired and Aaligned) might have stem cell potential, and can be referred as stem-progenitor spermatogonia (Kokkinaki et al., 2009; Orwig et al., 2008). Aaligned spermatogonia further differentiate into A1-A4, B and Intermediate spermatogonia, which become spermatocytes that will undergo meiosis. After meiosis, the haploid spermatids enter a phase of terminal differentiation called spermiogenesis. During spermiogenesis, profound morphologic modifications occur that lead to the shaping of spermatozoa.
The SSC microenvironment, or niche, is composed of factors produced by the Sertoli cells, the basement membrane, interstitial cells between the seminiferous tubules, and the microcirculation. One critical factor produced by Sertoli cells is glial cell line-derived neurotrophic factor (GDNF), which controls maintenance and self-renewal of the SSCs and is essential in maintaining permanent spermatogenesis (Meng et al., 2000). GDNF is a protein belonging to the transforming growth factor beta (TGFβ) family, which binds to a receptor/co-receptor complex formed by RET (REarranged during Transfection) and GFRA1 (GDNF family receptor alpha-1) at the surface of SSCs. Binding of the receptor complex by GDNF activates different cellular responses leading to self-renewal of SSCs and proliferation of undifferentiated spermatogonia (Braydich-Stolle et al., 2005). Mice homozygous for the Gdnf, Gfra1 and Ret targeted mutations lack SSCs in their seminiferous epithelium (Naughton et al., 2006), while Gdnf over-expression leads to the development of germ cell tumours (Sariola and Meng, 2003). GDNF regulates self-renewal through successive phosphorylations of RET and SRC-kinase family proteins (SKFs) (Braydich-Stolle et al., 2007; Oatley et al., 2007). Their activation triggers the phosphorylation of phosphatidylinositol 3-kinase (PI3K), which in turn activates AKT (Lee et al., 2007) and increases the expression of the transcription factor MYCN (Braydich-Stolle et al., 2007). Binding of GDNF to its receptor complex also activates the RAS-ERK pathway, which regulates SSC proliferation through the successive activation of SHC/GRB2 and RAS. This leads to the phosphorylation of ERK, which triggers the transcription of Fos and expression of its protein (He et al., 2008). FOS is a transcription factor that controls the expression of CCNA2 (Cyclin A2) and therefore has a role in the regulation of the cell cycle in premeiotic germ cells.
Studying the effects of chemical toxicants on SSCs is of paramount importance to understand increases in reproductive disorders such as low sperm counts and certain forms of testicular cancers. In a meta-analysis, Carlsen and colleagues (Carlsen et al., 1992; Carlsen et al., 1993) have suggested that the quality of human semen has been decreasing between 1938 and 1991, and additional studies performed by several other laboratories in Europe (Auger et al., 1995) and the United States (Swan et al., 1997) have confirmed this trend. More recent studies showed a relation between location and semen quality, highlighting the effects of environmental factors. In addition, exposure of perinatal or young adult rodents to phthalates, including mono-(2-ethylhexyl)-phthalate (MEHP), a metabolite of DEHP, is able to reduce sperm count (Andrade et al. 2006; Kwack et al., 2009).
Given the importance of GDNF signalling in SSCs, we have hypothesized that alterations of this pathway by an environmental pollutant such as MEHP might ultimately have a negative effect on sperm output. Additionally, the inhibiting effects that MEHP exerts on ERK1/2 activity in Sertoli and liver cells suggest that the GDNF pathway might be a target of MEHP in SSCs (Bhattacharya et al., 2005). Indeed, we report here that MEHP impairs GDNF signalling through inhibition of ERK1/2 phosphorylation, but not SRC, in spermatogonial stem cells. This study is the first to assess the effects of a phthalate ester on SSC behaviour and GDNF signalling.
2. MATERIALS AND METHODS
2.1 Tissue culture
The C18-4 cell line was used as a model of SSCs. The cells are stem-progenitor spermatogonia from BALB/c mice immortalized with the large T antigen (Hofmann et al., 2005a). These cells express known markers for SSCs and are responsive to GDNF stimulation (He et al., 2008; Hofmann et al., 2005a). The cells were cultured using DMEM (Hyclone, Logan, UT) supplemented with 10% FCS (Hyclone, Logan, UT), 2 mM glutamine (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (100x stock, Invitrogen, Carlsbad, CA). The cells were maintained at 33°C and 5% CO2 in 96- or 12-well plates or in 100 mm dishes (Falcon; Fisher Scientific, Pittsburgh, PA). Twenty-four hours prior to the experiments, Nu-serum (BD Biosciences, San Jose, CA) was used to replace FCS in culture in order to maintain a basal environment.
2.2 Dosage of MEHP
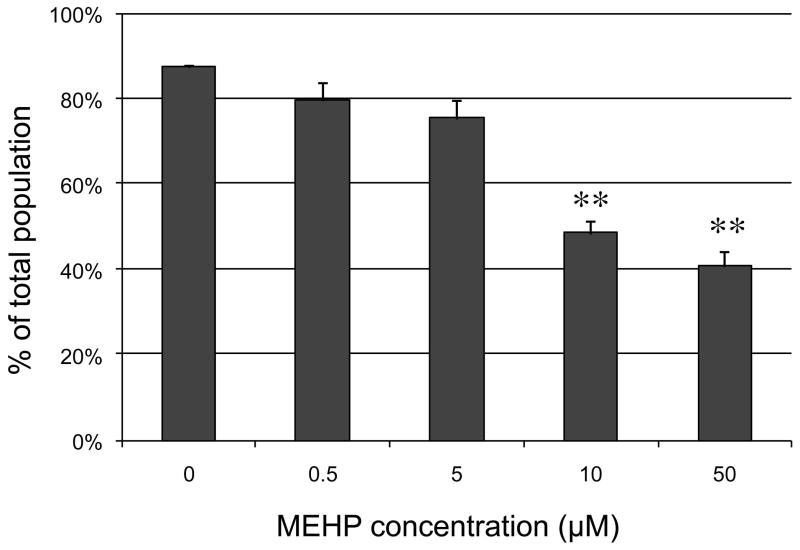
Many studies have measured the concentration of diverse phthalate metabolites in human urine, and they have indicated that MEHP metabolites are frequently detected at concentrations up to the micro-molar (Blount et al., 2000; Tranfo et al., 2011; Wittassek et al., 2007). Fewer studies have measured phthalate concentrations in other fluids. Frederiksen and colleagues assessed phthalate concentrations in serum, seminal plasma and urine of 60 men (Blount et al., 2000; Frederiksen et al., 2010). They detected an average of 110 ng/mL, 10 ng/mL and 1 ng/mL of DEHP and metabolites in urine, serum and seminal plasma respectively. Additionally there was a significant correlation between urine and serum concentrations for MEHP and other metabolites. Other studies have shown that the concentrations of DEHP and its metabolites in semen varied between 0.6 and 3 μg/mL, with average around 1μg/mL (Han et al., 2009). Serum concentrations were reported ranging from 2 μg/mL (Hines et al., 2009) to 5 μg/mL (Durmaz et al., 2010). The concentrations of MEHP used in the present study were determined using a dose response curve. Briefly, C18-4 cells were exposed for 10 hours to increasing concentrations of MEHP (from 0.0 μM to 50 μM, with 1 μM of MEHP equivalent to 0.3 μg/mL). The next day, cells were stained with propidium iodide and dead cells counted with the image-based cytometer TALI™ (Invitrogen, Carlsbad, CA). Figure 1 shows that for this time of exposure, concentrations of MEHP above 5 μM significantly decreased the percentage of live cells in the population, and many cell debris were detected at concentrations of 10 μM and 50 μM (Supplemental Figure S2). Therefore, for most experiments, we chose concentrations of MEHP ranging from 0 to 5 μM. MEHP was graciously provided by Dr J. Flaws, University of Illinois, Urbana, USA. MEHP was synthesized using standard procedures (Kashahara et al., 2002) by Dr. Lalji Gediya, Thomas Jefferson University, Philadelphia, PA, and its purity was > 98% based on NMR and TLC.
Figure 1. Effect of MEHP on C18-4 cells viability.
C18-4 cells were exposed to increased concentrations of MEHP or vehicle control for 10 hours. They were then harvested, stained for 20 min with propidium iodide, and the percentage of live cells evaluated using TALI™ cytometry. Results indicate that for this length of exposure MEHP exerts a dose-dependent decrease of viability that becomes significant at doses ≥10 μM. (** indicates a p-value <0.005).
2.3 MTS assay
Cells were exposed to MEHP at concentrations ranging from from 0 μM to 0.75 μM for 6h to 48h. Viability was assessed using the CellTiter 96 AQueous One Solution according to the manufacturer (Promega Corp., Madison, WI). Cells were seeded in 96-well dishes (5000 cells per well) and grown until reaching a sufficient confluence for the time of exposure as previously described (Braydich-Stolle et al., 2010). For instance, the cells where allowed to reach 80% confluence for a 6h exposure and 50 % confluence for a 48h exposure to MEHP. To ease dispersion, MEHP was first dissolved in DMSO (Sigma-Aldrich, St Louis, MO), then in DMEM-Nu-serum, and increasing concentrations of MEHP were added to the cells, which were incubated for another 6 to 72 hours. For each condition, the final concentration of DMSO was inferior to 0.1%. MTS (3-(4,5,dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium) is a molecule that is reduced by mitochondrial succinate dehydrogenase into a red-coloured dye. According to the manufacturer, the quantity of reduced MTS is strongly related to cell number. Viability was defined as the ratio of optical densities at 490 nm after addition of the MTS reagent (20% in DMEM without phenol red) and incubation at 33°C (until coloration developed, usually 2h to 3h). For each experiment, measurements were done in triplicates and the data shown are the average of 3–4 independent experiments. Data were reported in graphs which each data point representing the averages with SEM.
2.4 ROS assay
Cells were cultured in 12-well plates in DMEM-FCS Nu-Serum until they reached 70% confluence as previously described (Braydich-Stolle et al., 2010). After 10 h incubation with 0.0, 0.5, 5.0, 10 and 50 μM MEHP in DMEM-Nu-serum, the cells were washed with warm PBS, trypsinized and collected by centrifugation for 5 min at 300g at room temperature. Cells were incubated with 25 μM Carboxy-H2DCFDA (Image IT Live Green Reactive Oxygen Species Detection Kit; Molecular Probe/Invitrogen, Carlbad, CA) for 30 min at 33°C. The nonfluorescent 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy- H2DCFDA) enters live cells and is deacetylated by nonspecific intracellular esterases into carboxy-DCFH. In the presence of ROS, the reduced fluorescein compound is oxidized into carboxy-DCF and emits in the green range (520 nm). For each sample, 25 μL of cell suspension was loaded into one Tali™ Cellular Analysis Slide’s chamber (Invitrogen, Carlsbad, CA) and the slide was read in the Tali™ Image-Based Cytometer (Invitrogen, Carlsbad, CA). For each sample, 20 fields were analyzed. Counting parameters were set at 0.6 for sensitivity and 8 for circularity to reduce the background of cell debris. These settings led to an average of 5000 cells analysed per sample. The percentage of cells positive for ROS was expressed over the total counted population. The experiment was repeated three times, and the data are represented as the mean between 3 independent experiments ± SEM.
2.5 Apoptosis/Necrosis
Cells were cultured in 12-well plates (Falcon; BD Biosciences) and exposed to MEHP as above (section 2.4, ROS assay), or to 0.1 μM Staurosporine as positive control. The cells were then washed with ice-cold PBS and collected as above (ROS assay) but cell collection was carried out on ice. The Vybrant Apoptosis Assay Kit #4 (Molecular Probes/Invitrogen, Carlsbad, CA) was used to evaluate cell apoptosis versus necrosis. This kit contains 2 dyes: Yo-Pro, which stains the DNA of apoptotic cells (Idziorek et al., 1995), and propidium iodide (PI), which stains the DNA of cells displaying damaged membranes. The cells were stained for 20 min on ice in cold PBS containing 100 nM of Yo-Pro and 1.5 μM of PI. Again, for each sample 25 μL of cell suspension was loaded into one Tali™Cellular Analysis Slide’s chamber and analysed in the Tali™ Image-Based Cytometer using the above settings (ROS assay). For each experimental condition, 20 fields were analysed and around 5000 cells were counted. The cells were classified into two groups: cells staining for both PI and YoPro (membrane integrity compromised, sign of necrosis), or strong YoPro staining and low PI staining (apoptosis). The percentage of PI-/YoPro+ and PI+/YoPro+ cells was calculated over the total cell population. The experiment was repeated three times, and the data were represented as the mean between three independent experiments ± SEM.
To confirm the results obtained with the Vybrant Apoptosis Assay Kit #4 (Molecular Probes/Invitrogen, Carlsbad, CA), we investigated the occurrence of DNA laddering. After 10 h exposure to the above range of concentrations of MEHP or 0.1 μM Staurosporine, the cells were washed with PBS, the non-adherent cells were collected by centrifugation (300g, 5 minutes) and returned to the corresponding dish in 500 μl of lysis buffer (100 mM HEPES, pH 7.5/8, 10 mM EDTA, 0.2% SDS, 200mM NaCl, 0.5 g/ml Proteinase K). The lysates were collected into 2 mL centrifuge tubes and incubated at 37 °C for 3 h. Then 1.5 mL of 100% ice cold ethanol was added and the samples were incubated overnight at −70 °C to precipitate DNA. The DNA was pelleted by centrifugation at 20,000 g and 4 °C for 30 min, and the supernatant discarded. After a wash with 75% ethanol, the pellet was vacuum-dried for 5 minutes. Finally the DNA was dissolved overnight in 200 μL of TE buffer. The following day, 10 μg of the DNA samples were run in 2% agarose gels containing ethidium bromide in Tris–borate–EDTA buffer. The experiment was repeated three times, and the gels where photographed. A representative gel in presented in the result section 3.1.3.
2.6 Western Blotting
Activity of the GDNF/MYCN pathway was evaluated by Western blotting for SRC and phospho-SRC (Calbiochem/EMD Biosciences, Gibbstown, NJ) as well as for MYCN (Abcam, Cambridge, MA). The activity of the GDNF/FOS pathway was evaluated using antibodies for ERK1/2, phospho-ERK1/2 and FOS (Cell Signaling, Boston, MA). The cells were seeded in 100 mm dishes (Falcon, Fisher Scientific, Pittsburgh, PA) and allowed to reach 70% confluence in DMEM-10% Nu-serum. The cells were then pre-exposed to MEHP (0, 0.5, 5 μM) for 10h. Next, they were stimulated with GDNF and harvested after 20 minutes for phospho-SRC and phospho- ERK1/2 analysis. For MYCN and FOS protein expression analysis, the cells were harvested after 18h. Given the proportion of necrotic cells observed at exposures above 5μM MEHP, we did not investigate higher concentrations. Monolayers were washed with PBS (Hyclone, Logan, UT) and 0.1% Tween (Fisher-Scientific, Rockford, IL), and then lysed using a non-denaturing lysis buffer complemented with 1% PMSF, 1% Halt Phosphatase Inhibitor and 1% Halt Protease Inhibitor (Pierce; Fisher-Scientific, Rockford, IL). The cleared lysates were run on a 10% SDS-PAGE (Biorad, Hercules, CA) gel and transferred onto nitrocellulose membranes (Biorad, Hercules, CA). After blocking the membranes with a 4% BSA solution (Sigma-Aldrich, St Louis, MO) in PBS-0.1% Tween, the membranes were incubated with anti-SRC, anti-phospho-SRC and anti-MYCN antibodies or anti ERK1/2, anti-phospo-ERK1/2 and anti-FOS antibodies. Beta-actin was used as loading control. Bands were revealed using the horseradish peroxidase system with a standard chemiluminescence substrate and chemiluminescent film (Pierce, Rockford, IL). Once developed, the films were scanned and protein band intensities (I) were computed using the Image J software (NIH, Wayne Rasband, Bethesda, MD). The results were expressed using the formula:
Each Western blot intensity value was obtained by averaging three independent experiments ± SEM. Each experiment was done in duplicate.
2.8 Quantitative PCR
The cells were grown in 6-well plates until cell density reached 80%. Quantitative RT-PCR was performed after pre-exposure to MEHP for 10 hours and stimulation with GDNF for 12 h. Next, the RNeasy Mini Kit (Qiagen, Valencia, CA) was used to harvest RNA from the cells. The cells were rinsed with 500μL of ice cold PBS. 350 μL of lysis buffer was added, and the cells were scraped using a clean RNAse-free pipet tip. For each sample, the cell suspension was transferred in a Qiashredder (Qiagen, Valencia, CA) spin column for homogenization, and centrifuged for 2 min at max speed (Eppendorf Centrifuge 5415 C, Hauppauge, NY). One volume of 70% ethanol was added to the flow-through, which was then transferred into the RNeasy spin column. After 15 sec of centrifugation at maximum speed, the flow-through was discarded, and 350 μL of wash buffer was added to wash the column by centrifugation at maximum speed for 15 seconds. After each washing step, the flow-through was discarded. Genomic DNA was digested using RNase-free DNase (Qiagen, Valencia, CA). Ten μL of DNAse and 70 μL of digestion buffer were added to each column, and the columns were incubated for 15 minutes at room temperature. After another 3 washes, the columns were centrifuged for 1 minute, transferred into a clean collection tube and centrifuged for another 2 minutes. Finally, the columns were eluted into clean RNAse-free 1.5 mL tubes by adding 30 μL of RNAse-free water. A minute later the columns were centrifuged at maximum speed for 1 minute. RNA concentration was measured using a nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) and the quality of the RNA assessed by agarose gel electrophoresis.
Complementary DNA was synthesized using Superscript III (Invitrogen, Carlsbad, CA) and 1 μg of RNA as a template. One μg of RNA was mixed with 1μl of random primer, 1μL of DNTP solution and RNAse free water up to 10μL. The mix was incubated at 65°C for 5 minutes to anneal and then placed on ice. Ten μL of cDNA synthesis mix was prepared per reaction (2μL of 10X RT buffer, 4μL of MgCl2 solution, 2μL of DTT solution, 1μL of RnaseOUT, 1μL of SuperScript III RT enzyme) and distributed into the tubes containing the annealed RNA. Complementary DNA was synthesized by incubating the samples for 10 min at 25°C, followed by 50 min at 50°C. The reaction was terminated by incubating for 5 min at 85°C and placed on ice. Remaining RNA was digested by incubating the samples at 37°C for 20 min with 1 μL of RNase H.
Fos and Mycn expression levels were evaluated using Taqman technology (Applied Biosystems, Foster City, CA). For each reaction 2 μL of cDNA was mixed with 8 μL of PCR mix (0.5μL of Taqman assay mix, 5μL of 2X Taqman Gene Expression Master Mix, and 2.5μL of RNAse-free water). The housekeeping gene Rps3 was used as reference. The experiment was independently repeated twice in duplicates and analysis was carried on using the ∂∂Ct method.
2.9 Statistics
Results were graphically presented as averages of 2 to 4 independent experiments in duplicates or triplicates, with error bars representing standard error of the mean. The results form the Tali™ Image-Based Cytometer where analyzed using Excel. The R statistical analysis software was used to perform one-way ANOVA on the results using an alpha of 5%. Whenever the ANOVA’s p-value was <10%, we performed pairwise t tests but we considered them not significant. When the ANOVA’s p-values were < 5%, p-values below 10% were considered borderline significant, and p-values < 5% were considered statistically significant.
3 RESULTS
3.1 Cytotoxicity studies
3.1.1 MEHP affects cell viability and rate of proliferation of the C18-4 cells
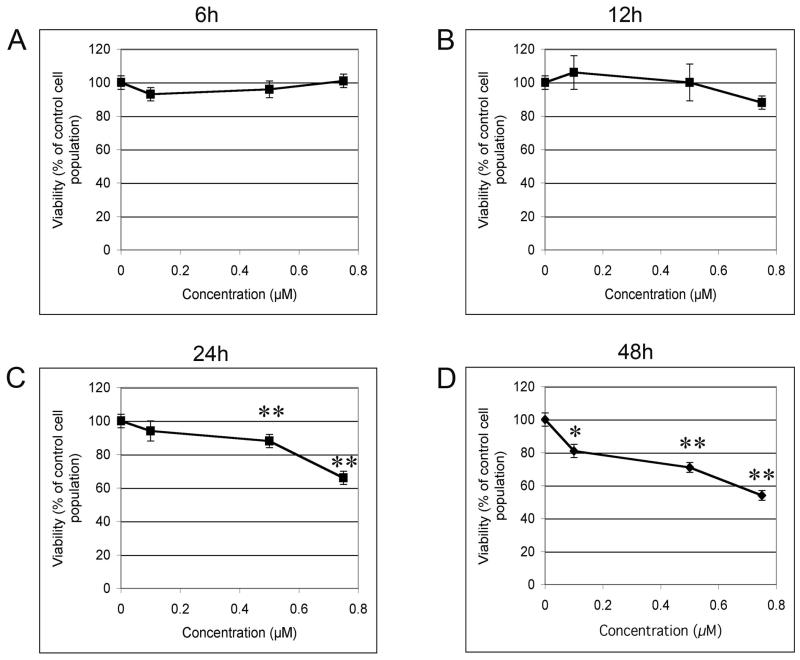
To first evaluate the possibility of a direct effect of MEHP on the C18-4 cells, we exposed the cell line to MEHP for 6 to 48 hours in 96 well plates using DMEM complemented with 10% Nu-Serum IV. We assessed viability as a function of MEHP concentration and time of exposure (rate of proliferation). This assay revealed that MEHP has a dose-dependent and time-dependent effect on the C18-4 spermatogonial stem cell line viability (Figure 2). Both effects were statistically significant for MEHP concentrations higher than 0.5 M and 12h of exposure or more. Figures 2A and 2B show that the lower concentration (0.1 μM) and shortest time (6h) of incubation did not significantly affect C18-4 viability. After 12 hours of exposure, the highest dose produced a slight, but not statistically significant, decrease in cell viability. After 24 hours of exposure, only the 2 highest doses significantly reduced cell viability (p-value<0.005). After 48 hours of exposure, all doses significantly reduced cell viability (p-value<0.005). Figure 3 shows that each concentration decreases viability in a time-dependent manner.
Figure 2. Effects of low doses of MEHP on C18-4 cell viability.
MTS assay (Invitrogen) was performed after exposure to increasing doses of MEHP (0, 0.1, 0.5, 0.75 μM) for different lengths of time (6h to 48h). Results indicated that MEHP decreased C18-4 cells viability in a time- and dose-dependent manner. A and B: Exposure for 6 and 12h to low doses of MEHP did not exerted any significant effects. C: After 24h of exposure the effects were significant for the highest doses (p<0.005). D: After 48h of exposure each dose significantly decreased cell viability. Optical density was standardized over the vehicle control (0 μM MEHP). Data are expressed as the means of 3 to 4 experiments in triplicates, and error bars represent the standard error to the mean. Asterisks represent a significant difference from the vehicle control. * indicates a p-value <0.01, ** indicates a p-value <0.005.
Figure 3. Effects of low doses of MEHP on C18-4 cells rate of proliferation.
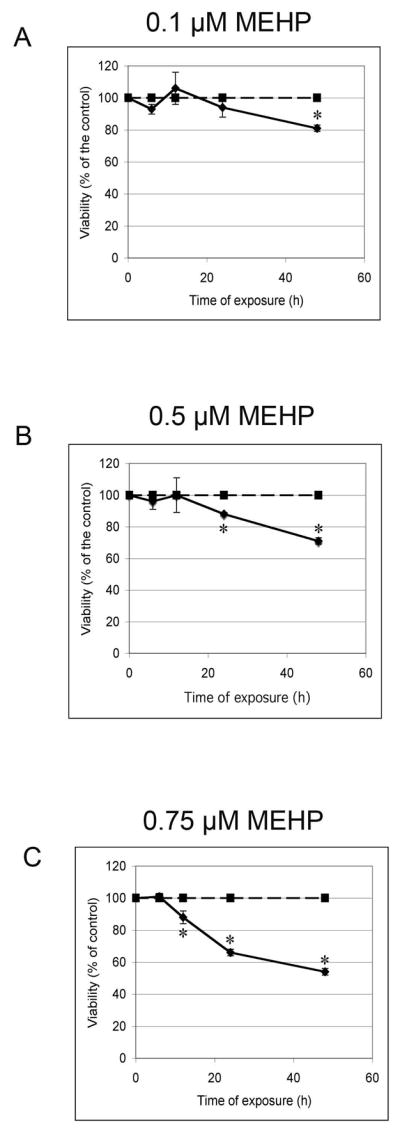
MTS assay (Invitrogen) was performed after exposure to increasing doses of MEHP (0, 0.1, 0.5, 0.75 μM) for different lengths of time (6h to 48h). Results of Figure 2 were here represented as number of viable cells in function of time, showing that MEHP impairs the rate of cell proliferation. Optical density was standardized over the vehicle control (0 μM MEHP). Data are expressed as the means of 3 to 4 experiments, and error bars represent the standard error to the mean. Asterisks represent a significant difference from the vehicle control. A: 0.1 μM MEHP. B: 0.5 μM MEHP. C: 0.75 μM MEHP.
3.1.2 MEHP does not increase ROS production in C18-4 cells
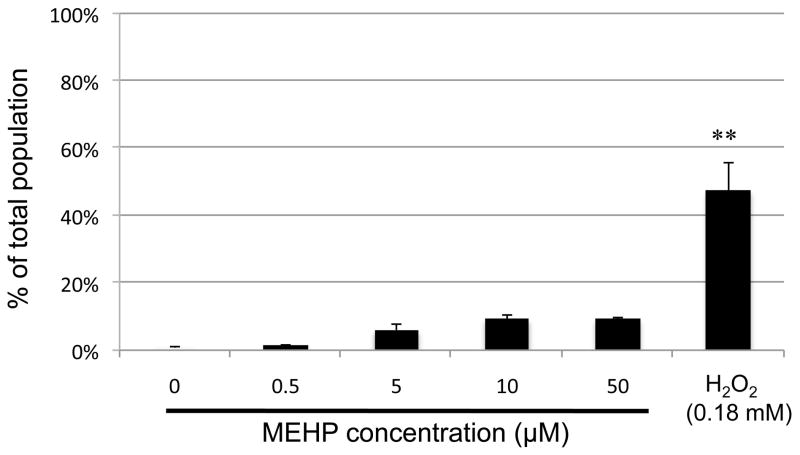
Because recent studies have demonstrated that phthalates can increase ROS production in a number of cell types, including liver cells, prostate adenocarcinoma cells (Erkekoglu et al., 2010) and Leydig cells (Zhang et al., 2007), we assessed whether the decrease of cell viability that we observed with the C18-4 cells was being triggered by a direct effect of MEHP on the cellular oxidative status. We measured ROS production using standard assays, and data obtained are shown in Fig. 4. Interestingly, no increase of ROS was statistically detectable at any dose of MEHP. These results confirm previous data obtained by counting a total of 300 cells with a standard fluorescent microscope (Supplemental Figure 1A). However, for the higher doses of 10 μM and 50 μM MEHP, a reduced number of 500 cells per sample was available for Tali™ image-based cytometry. Additionally, a large number of cell debris was present in these two conditions (Supplemental Figure S2). This suggests that cell death massively occurs at these doses, which was further confirmed by apoptosis/necrosis analysis, as seen below.
Figure 4. Effects of MEHP on ROS production.
ROS assay (Invitrogen) was performed after exposure to increasing doses of MEHP (0, 0.5, 5.0, 10, 50 μM) for 10 h. Following treatment, the cells positively stained for ROS were counted using Tali™ image-based cytometer. Hydrogen peroxide (0.18 mM for 90 minutes) was used as positive control. Results indicated that there was no significant production of ROS induced by MEHP in the C18-4 cells. The counts were standardized over the vehicle control. Data are expressed as the means of 3 experiments in duplicates, and error bars represent the standard error to the mean. ** indicates a p-value <0.005.
3.1.3 MEHP alters membrane integrity at higher doses in the C18-4 cells and does not induce apoptosis
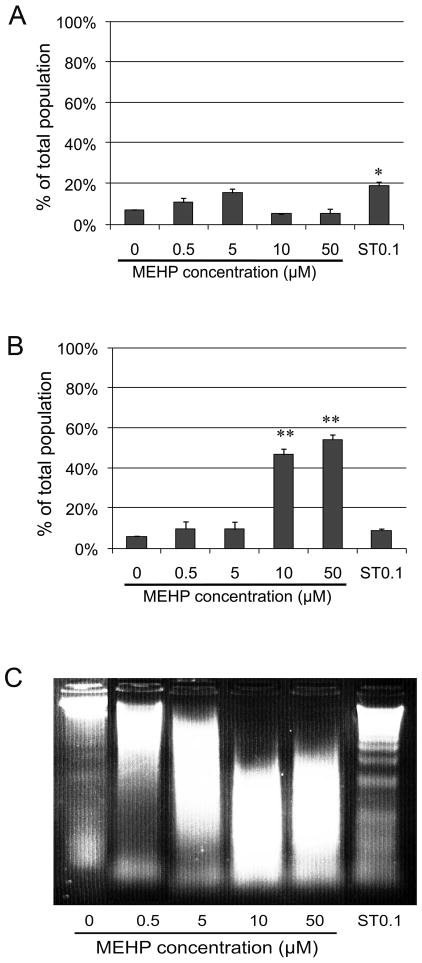
In order to evaluate whether a decrease in cell viability and rate of proliferation is due to apoptosis or necrosis, we used the Vybrant apoptosis kit assay. Apoptotic cells become permeable to Yo-Pro but not to propidium iodide. Apoptosis can be induced in the C18-4 cells by Staurosporine, a non-specific ATP-competitive kinase inhibitor, which we used as a positive control (Figures 5A and 5C). Staurosporine did not induce necrosis since the cell membranes were not permeable to propidium iodide (Figure 5B). None of the MEHP concentrations significantly increased the number of apoptotic cells stained with Yo-Pro (Figure 5A). Also, lower concentrations of MEHP (0.5–5 μM) did not trigger cell necrosis. However, necrosis was significantly induced at MEHP concentrations of 10 and 50 μM, as seen by a 9x-11x increase in the number of Yo-Pro/PI double-stained cells (Figure 5B). Taken together these results suggest that MEHP does not induce apoptosis in the C18-4 cells and that membrane integrity is altered only at higher doses (10 and 50 μM). These results support previous results obtained by counting a total of 300 cells with a standard fluorescent microscope (Supplemental Figure 1B). Similarly to the ROS assay, for the higher doses of 10 μM and 50μM MEHP, a reduced number of 500 cells per sample was available for Tali™ image-based cytometry. Supplemental data S2 indicates that the measured cell diameter was greatly reduced for these higher concentrations of MEHP and the positive control. This suggests that cell death occurs, and what the TALI™ recognises is mostly cell debris, which is compatible with necrosis.
Figure 5. Effects of MEHP on cell death.
Apoptosis/necrosis assay (Invitrogen/Molecular Probes) was performed after exposure to increasing doses of MEHP (0, 0.5, 5, 10, 50 μM) for 10 h or to 0.1 μM Staurospaurine (positive control ST0.1). Following treatment, cells positively stained by Yo-Pro and/or propidium iodide (PI) were counted using Tali™ image based cytometer. Cells positive for Yo-Pro only were apoptotic (A), while PI positive cells were considered necrotic (B). TALI™ counts were standardized over the vehicle control. Data are expressed as the means of 3 experiments in duplicates, and error bars represent the standard error to the mean. * indicates a p-value <0.01, ** indicates a p-value <0.005. C: apoptosis was evaluated by assessing the presence of DNA laddering by gel electrophoresis. Results indicated that MEHP did not significantly trigger an increase of apoptosis, but significantly increased necrosis for the highest doses (10 and 50 μM). Staurosporine (0.1μM) triggered significant apoptosis, which is shown by DNA laddering.
In order to confirm that MEHP does not induce apoptosis, we isolated and purified DNA from the treated cells, then visualized it on a 2% agarose gel to detect the DNA ladder classically observed during apoptosis. Figure 5C shows a typical gel that presents no laddering after exposure to MEHP (0 to 50 μM). In comparison, the positive control (exposure to 0.1 μM Staurosporine) shows a well-defined DNA ladder. This data confirms that MEHP does not induce apoptosis in the C18-4 cells at any concentration used. The DNA smears observed at higher doses are compatible with cell necrosis.
3.2 GDNF signalling studies
Because we observed a decrease in cell viability not fully explained by an increase in ROS or apoptosis, and because we know that GDNF triggers 2 major signalling pathways regulating spermatogonial stem cells in vitro (Braydich-Stolle et al., 2007; He et al.; Kanatsu-Shinohara et al., 2003) and in vivo (Lee et al., 2007; Oatley et al., 2007), we investigated the effects of MEHP on the activity of certain GDNF signalling components. Previous research has shown that GDNF signals though SRC kinase activation and MYCN expression (Braydich-Stolle et al., 2007), and/or through the canonical RAS pathway and FOS induction (He et al.). Therefore we investigated the effects of MEHP on the activation and regulation of the GNDF signalling pathway effectors SRC, MYCN, ERK1/2 and FOS.
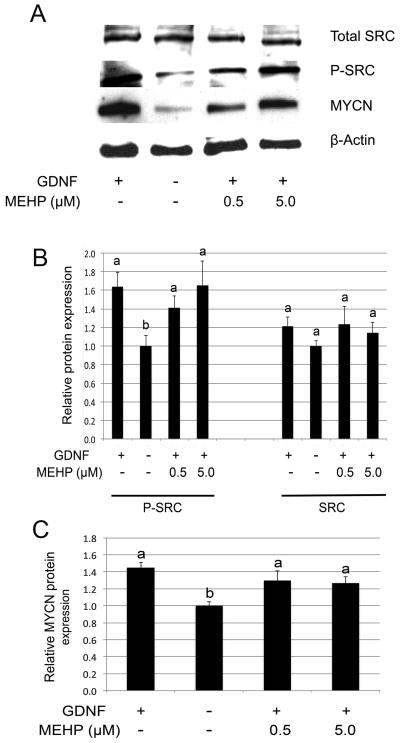
3.2.1 MEHP does not affect SRC phosphorylation or MYCN expression
Previous studies have shown that environmental toxicants may specifically affect SRC family kinases in germ cells (Braydich-Stolle et al., 2010). We therefore assessed if MEHP also alters the GDNF-dependent activation and phosphorylation of SRC. Figures 6A and 6B indicate that MEHP did not significantly affect SRC expression or GDNF-dependent SRC phosphorylation. Further, the GDNF-dependent expression of MYCN was not altered by the presence of MEHP regardless of its concentration (Figure 6A and 6C). We conclude that MEHP does not alter the GDNF-RET-SRC-MYCN axis in the C18-4 spermatogonial stem cell line.
Figure 6. Influence of MEHP on GDNF-SRC signalling.
Western Blot analysis of SRC protein phosphorylation and MYCN protein expression was performed after 10 h exposure to MEHP (0, 0.5 and 5μM), followed by stimulation with GDNF for 20 min (phospho-SRC analysis) or 18 h (MYCN analysis). Beta-actin was used as loading control, and band intensities where standardized over the vehicle control as reference. Figure A represents a typical Western blot, and Figures B and C represent quantification of the band intensities. Neither GDNF-dependent SRC phosphorylation (A and B), nor GDNF-dependent MYCN protein expression (A and C) were significantly impaired by the presence of MEHP.
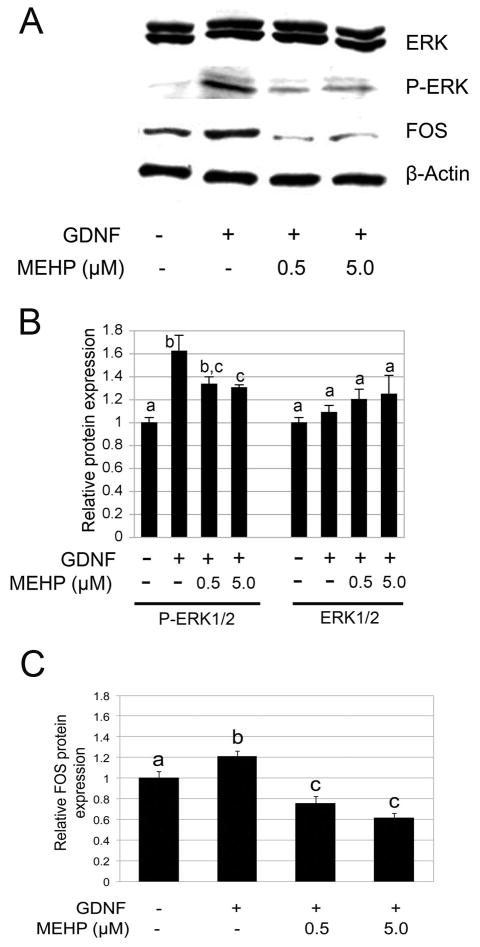
3.2.2 MEHP affects Erk1/2 phosphorylation and FOS protein expression
We then assessed if other critical components of cell signalling in the C18-4 cells were altered. We recently demonstrated that GDNF also triggers an increase of FOS expression through the activation by phosphorylation of ERK1/2 in spermatogonial stem cells (He et al., 2008). Therefore we assessed if MEHP alters ERK1/2 phosphorylation. We observed that GDNF-dependent phosphorylation of ERK1/2 is significantly affected by exposure to MEHP (p-value<0.005, Figures 7A and 7B). Downstream of ERK1/2, expression of the FOS transcription factor is also significantly down-regulated by exposure to MEHP (p-value<0.005, Figures 7A and 7C).
Figure 7. Influence of MEHP on GDNF-ERK1/2 signalling.
Western Blot analysis of ERK protein phosphorylation and FOS protein expression was performed after 10h exposure to MEHP (0, 0.5 and 5μM), followed by stimulation with GDNF for 20 min (phospho-ERK1/2 analysis) or 18 h (FOS analysis). Beta-actin was used as loading control, and band intensities where standardized over the vehicle control as reference. Figure A represents a typical Western blot, and Figures B and C represent quantification of the band intensities. GDNF-dependent ERK1/2 phosphorylation was significantly impaired by MEHP at both concentrations. GDNF-dependent FOS expression is also significantly reduced by MEHP.
3.2.3 MEHP down-regulates Fos but not Mycn mRNA expression
To confirm that FOS expression is down-regulated, but not MYCN, we performed qPCR using the standard Taqman assay and the ∂∂Ct method with Rps3 as housekeeping gene. The results obtained confirmed the protein data. Figure 8 shows that exposure to 0.5 or 5 μM MEHP for 10 h and stimulation with 100 ng of GDNF for 12 h did not affect Mycn mRNA expression, while GDNF dependent Fos expression was significantly reduced in presence of MEHP at both concentrations.
Figure 8. Influence of MEHP on Mycn and Fos mRNA expression.
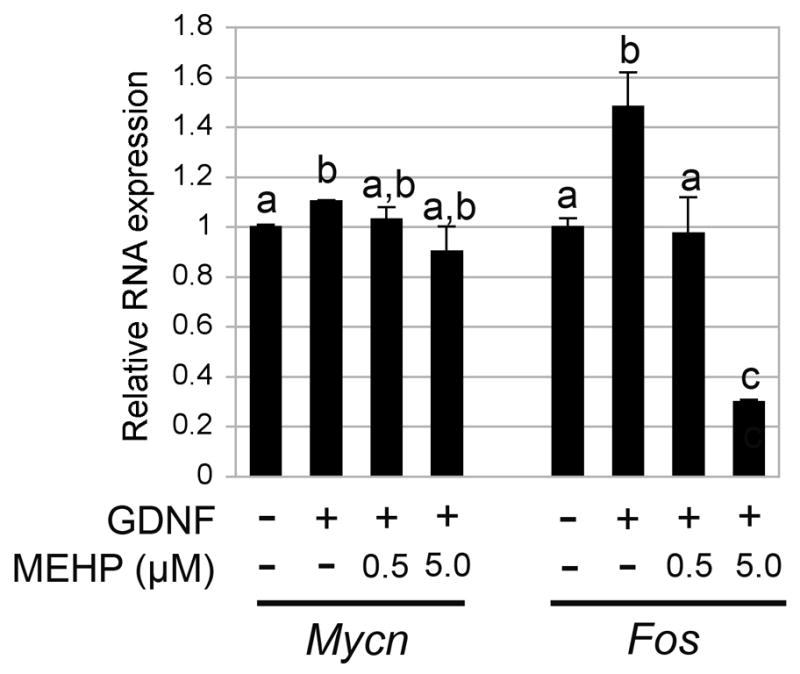
Quantitative PCR analysis of the level of expression of Mycn and Fos was performed after 10 h exposure to MEHP (0, 0.5 and 5μM) followed by stimulation with GDNF for 12 h. Rps3 was used as housekeeping gene, and signals standardized over the vehicle control reference. Results indicate that GDNF-dependent Mycn mRNA expression was not significantly impaired by MEHP. In contrast, GDNF-dependent Fos mRNA expression was significantly reduced by incubation with MEHP.
Taken together these results suggest that MEHP affects spermatogonial stem cells proliferation by down-regulating ERK1/2 activation and Fos expression, but does not affect SRC activation and Mycn expression.
4. DISCUSSION
Numerous monitoring studies have shown the presence of phthalates and their metabolites in virtually every possible environment, such as surface and tap water, household dust, milk, dairy products, infant formula, and cosmetics (Becker et al., 2004; Petersenand Breindahl, 2000). Constant exposure of humans to phthalates is revealed by the presence of related metabolites in their urine, irrespectively of the age of the population studied (Barr et al., 2003; Becker et al., 2004). Phthalates have a variety of effects on different organs: they are known as endocrine disruptors (Parks et al., 2000), peroxisome proliferators (Edlund et al., 1987), and have also been linked to behavioural and sensitizing effects (Bornehagand Nanberg, 2010).
Over the past 15–20 years a number of studies have suggested that sperm counts in man are on the decline (Auger et al., 1995; Carlsen et al.; Swan et al., 2000). These changes are recent and vary with the location, therefore they might reflect adverse effects due to environmental factors rather than genetic changes in susceptibility (Jørgensen et al., 2006). The link between sperm count and environmental toxicants is not straightforward, but past observations made on different organisms have unequivocally shown a relationship between disorders of sex organs development and exposure to certain chemicals such as tributilin (TBT) (Bryan et al., 1986) and diethylstilbestrol (Herbst and Bern, 1981). Furthermore, more recent epidemiological studies established a correlation between the increase of endocrine disruptors within the environment and a rising incidence of infertility in men due to testicular cancer, declining semen quality, undescended testis and hypospadias (Skakkebaek et al., 2001). Phthalates are among the chemicals pointed out by these studies, and experimental exposure of rats and mice to DEHP/MEHP is now an accepted model system to study testicular dysgenesis syndrome (Fisher et al., 2003). Data obtained from adult men have also suggested a direct link between urinary MEHP concentrations and sperm counts (Duty et al., 2004). Additionally, exposure of perinatal or young adult rodents to MEHP and other phthalate esters reduces sperm count (Andrade et al. 2006; Kwack et al., 2009). For instance, a 28-day exposure of young rats to 250 mg/kg of MEHP reduced sperm count by nearly 60% (Kwack et al., 2009).
Many studies investigating the effects of phthalates on testicular cells in vivo and in vitro have reported a direct effect on somatic cells (Li et al., 1998; Richburg and Boekelheide, 1996). Insulin-like 3 (INSL3), which is produced by Leydig cells and is up-regulated by testosterone, influences testicular descent and is a survival factor for germ cells (Anand-Ivell et al., 2009; Zimmermann et al., 1999). Interestingly, MEHP partly inhibits testosterone action on Insl3 expression in a Leydig cell line (MA-10) and primary cultures of rat Leydig cells (Laguë and Tremblay, 2008). This effect could potentially explain occurrences of cryptorchidism after exposure to MEHP in utero (Ge et al., 2007). Other changes induced by phthalates and affecting fetal Leydig cells include a decrease of testosterone production by interfering with the expression of Cyp17a1, P450scc, SR-B1 and StAR, and an increase of their rate of proliferation possibly as a compensation mechanism for the reduced testosterone (Clewell et al., 2010; Parks et al., 2000; Shultz et al., 2001). Abnormal fetal Leydig cells aggregation was also reported (Mahood et al., 2005). In Sertoli cells, MEHP interferes with the synthesis of vimentin filaments, which destroys the cytoskeleton (Richburg and Boekelheide, 1996). MEHP also disrupts junctional complexes, which is seen as the primary cause of premature loss of spermatocytes and spermatids from the seminiferous epithelium (Yao et al., 2010). Sertoli cell injury caused by MEHP leads to increased levels of FASL in vivo, which accounts for induction of apoptosis in spermatocytes and round spermatids through FAS signalling (Richburg et al, 1999; Yao et al., 2007). Finally, MEHP inhibits both basal and FSH-stimulated Sertoli cell proliferation (Li et al., 1998) and inhibits ERK1/2 phosphorylation in these cells after 5 min to 20 minutes exposure. However, the affected signalling pathway is not known (Bhattacharya et al., 2005). Because Leydig and Sertoli cells are of paramount importance for the development of germ cells before birth, and maintenance of spermatogenesis after birth, it is likely that changes in their morphology or physiology will indirectly impair germ cell development, in particular spermatocytes and round spermatids. However, it is also possible that MEHP directly affects spermatogonial stem cells, independently of somatic cells. For example, DEHP metabolites have been found in germ cells, a finding consistent with direct effects (Ono et al, 2004). In addition, in vivo studies have demonstrated that there is a significant decrease in the proliferation index of rat spermatogonia at day 6 after birth (P6) following in utero exposure to phthalates from E13.5 to birth (Ferrara et al., 2006; Jobling et al., 2011). This reduction of the proliferation index (down to 30% of the control) was seen in germ cells located in the basal part of the seminiferous epithelium, which at this age are mostly spermatogonial stem cells. Interestingly, the authors did not observe apoptosis in these cells, and there is so far no published evidence that phthalates significantly alter GDNF expression by Sertoli cells. GDNF expression pattern from a genome-wide microarray performed by Lahousse and colleagues is inconclusive in this respect (Lahousse et al, 2006). Therefore, a decrease of proliferation and number of stem/progenitor spermatogonia could be explained by an alteration of the intrinsic GDNF signaling pathway by MEHP.
For a preliminary assessment of a possible direct effect of MEHP on spermatogonial stem cells, we used the C18-4 cell line as a model. We observed that MEHP decreases cell viability and rate of proliferation in a dose-and time-dependent manner at bioequivalent concentrations (Figures 1–3). These effects could have been due to an increase in ROS production in the germ cells, since Pant and colleagues recently demonstrated that ROS concentration in sperm correlates with phthalate esters concentration in semen (Pant et al., 2008). Several investigators have detected ROS in cultured germ cells after exposures to MEHP ranging from 30 minutes to 24 hours (Kashahara et l, 2002; Onorato et al, 2007). However, the germ cells investigated were in both cases spermatocytes, tested either in primary cultures (Kashahara et al, 2002) or as a cell line (Onorato et al., 2007). Our results did not confirm any increase in ROS production in the C18-4 cells (Figure 4). This feature could be explained by the fact that the C18-4 cells are at a different stage of differentiation, and therefore their behaviour might be different. In addition, the doses used on the spermatocytes were 100 times higher than the doses that we used on the C18-4 cells, which we believe are closer to bioequivalent concentrations.
Since we did not demonstrate an increase in cellular ROS, we tested the possibility that the observed decrease in cell viability might be due to apoptosis. While the mechanisms that regulate normal germ cell apoptosis during development are not completely understood, up-regulation of FASL expression by Sertoli cells after exposure to MEHP, and subsequent germ cell apoptosis through FAS signalling, has been well documented (Lee et al., 1997; Yao et al., 2007). Further, in a FASL knockout model, a single dose of 1 mg/kg of MEHP was unable to significantly increase TUNEL- positive germ cells or caspase cleavage (Lin et al., 2010), confirming that MEHP-induced germ cell apoptosis is dependent of FAS-FASL signalling. Other investigations have indicated that exposure of 30-day-old mice to DEHP promotes apoptosis of germ cells located close to the basement membrane within the seminiferous epithelium, probably type A spermatogonia (Bhartiya et al., 2010). We did not detect a significant increase in apoptosis at any of the MEHP concentrations used (Figure 5A), which is similar to the data obtained by Onorato and colleagues with the GC-2spd cells (Onorato et al., 2007). Since in both in vitro models the germ cells were not associated to Sertoli cells, it is evident that the FAS pathway could not be activated. However, neither the FAS pathway nor the intrinsic apoptosis pathway, which is activated by MEHP in other cell types (Yokoyama et al., 2003), was initiated in the C18-4 cells. This confirms the data of Jobling and colleagues, who observed that decrease of gonocyte and spermatogonial stem cell numbers was not caused by apoptosis in mice exposed to MEHP from E13.5 until birth (Jobling et al, 2011).
The lack of formation of reactive oxygen species and the absence of apoptosis suggests that another mechanism could be responsible for the decrease in C18-4 cell viability and rate of proliferation induced by MEHP. Our group recently reported that spermatogonial stem cell proliferation is impaired by toxicants such as silver nanoparticles. These nanoparticles disrupt GDNF signalling by targeting intracellular kinases like SRC family kinases (SFKs) (Braydich-Stolle et al., 2010). However, in the present study, we could not demonstrate any effect of MEHP on SFKs in spermatogonial stem cells (Figures 6A and 6B). Additionally, we can infer from this observation that, since SRC phosphorylation is not affected, RET phosphorylation or GDNF binding to the receptor/co-receptor complex are not impaired by MEHP either.
Since GDNF also signals through ERK1/2 (He et al., 2008), we investigated a possible effect of MEHP on this signalling pathway. Our results demonstrate a significant decrease of ERK1/2 phosphorylation (Figures 7A and 7B) in the C18-4 cells after treatment with MEHP. The target of MEHP could be ERK1/2 itself, or a protein interacting with ERK1/2, or a protein upstream of ERK1/2. Interestingly, ERK1/2 phosphorylation is also reduced in rat primary Sertoli cells in presence of MEHP, but is increased in liver cells (Bhattacharya et al., 2005). Therefore, the effects of MEHP on signalling pathways might be cell type- and kinase-dependant. Importantly, we also demonstrated a down-regulation of FOS both at the protein (Figures 7A and 7C) and RNA levels (Figure 8). FOS is a transcription factor that is often induced by the activation of ERK1/2, and controls the expression of cell cycle-related proteins by dimerizing with c-JUN to form the AP-1 transcription factor. FOS is essential for the regulation of SSC and undifferentiated germ cell proliferation (He et al., 2008). Therefore, taken together our data imply that MEHP affects the GDNF-ERK1/2-FOS pathway, but not the GDNF-SRC-MYCN pathway in undifferentiated spermatogonia.
GDNF is crucial for self-renewal and maintenance of SSCs (Braydich-Stolle et al., 2005; Meng et al., 2001; Naughton et al., 2006), but might also play a role in the proliferation / differentiation of these cells into Apaired and Aaligned spermatogonia, since the latter also express the receptor complex (Hofmann et al., 2005b; Phillips et al., 2010). Therefore, the two pathways triggered by GDNF might mediate different functions. Since MYCN is linked to maintenance and self-renewal in many stem cell types, (Braydich-Stolle et al., 2007; Knoepfler et al., 2002; Laurenti et al., 2008; Okubo et al., 2005), and has been used to produce induced pluripotent stem cells (iPS cells) (Knoepfler et al., 2008), the GDNF-SRC-MYCN signalling pathway might be mainly responsible for stem cell maintenance and/or their self-renewal. On the other side, the GDNF-ERK1/2-FOS pathway might be used for germ cell proliferation associated with differentiation because FOS expression is ubiquitous and leads to proliferation and differentiation of many cell types (Durchdewald et al., 2009). Also, the cascade ERK1/2/FOS is involved in the proliferation of a model of type B spermatogonia (Sirianni et al., 2008). Taken together, since only the pathway leading to FOS expression is affected by MEHP, this compound might shift the balance between SSC self-renewal and differentiation toward self-renewal. In conclusion our results indicate that MEHP triggers a reduction of stem-progenitor spermatogonia proliferation in vitro by down-regulating ERK1/2 phosphorylation and specifically affecting the GDNF/ERK1/2/FOS signalling pathway.
Supplementary Material
Highlights.
MEHP affects SSC proliferation in a dose- and time- dependent manner
MEHP does not increase apoptosis, necrosis or the production of ROS in SSCs
MEHP reduces the activity of the GDNF/ERK1/2/FOS signalling pathway in SSCs
MEHP does not affect the GDNF/SRC/MYCN signalling pathway in SSCs
Acknowledgments
We would like to thank Dr. Jodi Flaws (University of Illinois at Urbana-Champaign) for providing MEHP, Dr. Sidonie Lavergne (University of Illinois at Urbana-Champaign) for access to the Tali™ image-based cell cytometer, and Dr. Rex Hess for critical reading of the manuscript This work was supported by grant HD054607 from the National Institutes of Health (M.C.H.) and a Lilly Predoctoral Fellowship (B.E.L.).
Footnotes
Conflict of Interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. BIBLIOGRAPHICAL REFERENCES
- Anand-Ivell R, Heng K, Hafen B, Setchell B, Ivell R. Dynamics of INSL3 peptide expression in the rodent testis. Biol Reprod. 2009;81:480–487. doi: 10.1095/biolreprod.109.077552. [DOI] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Gericke C, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology. 2006;228:85–97. doi: 10.1016/j.tox.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Bhartiya D, Kasiviswanathan S, Unni SK, Pethe P, Dhabalia JV, Patwardhan S, Tongaonkar HB. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem. 2010;58:1093–1106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya N, Dufour JM, Vo MN, Okita J, Okita R, Kim KH. Differential effects of phthalates on the testis and the liver. Biol Reprod. 2005;72:745–754. doi: 10.1095/biolreprod.104.031583. [DOI] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int J Androl. 2010;33:333–345. doi: 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle L, Nolan C, Dym M, Hofmann MC. Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann N Y Acad Sci. 2005;1061:94–99. doi: 10.1196/annals.1336.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, Hofmann MC. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci. 2010;116:577–589. doi: 10.1093/toxsci/kfq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan GW, Gibbs PE, Hummerstone LG, Burt GR. The decline of the gastropod Nucella lapillus around southwest England: evidence for the effect of Tributyltin from antifouling paints. J Mar Biol Assoc UK. 1986;66:611–640. [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Skakkabaek NE, Keiding N. Decreasing quality of semen. BMJ (Clinical Research Ed) 1993;306:461–461. doi: 10.1136/bmj.306.6875.461-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell RA, Campbell JL, Ross SM, Gaido KW, Clewell HJ, 3rd, Andersen ME. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol In Vitro. 2010;24:327–334. doi: 10.1016/j.tiv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- De Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchdewald M, Angel P, Hess J. The transcription factor Fos: a Janus-type regulator in health and disease. Histol Histopathol. 2009;24:1451–1461. doi: 10.14670/HH-24.1451. [DOI] [PubMed] [Google Scholar]
- Durmaz E, Ozmert EN, Erkekoglu P, Giray B, Derman O, Hincal F, Yurdakök K. Plasma phthalate levels in pubertal gynecomastia. Pediatrics. 2010;125:122–129. doi: 10.1542/peds.2009-0724. [DOI] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, Overstreet J, Hauser R. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 2004;25:293–302. doi: 10.1002/j.1939-4640.2004.tb02790.x. [DOI] [PubMed] [Google Scholar]
- Edlund C, Ericsson J, Dallner G. Changes in hepatic dolichol and dolichyl monophosphate caused by treatment of rats with inducers of the endoplasmic reticulum and peroxisomes and during ontogeny. Chem Biol Interact. 1987;62:191–208. doi: 10.1016/0009-2797(87)90090-1. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, De Rosa V, Giray B, Favier A, Hincal F. Protective effect of selenium supplementation on the genotoxicity of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate treatment in LNCaP cells. Free Radic Biol Med. 2010;49:559–566. doi: 10.1016/j.freeradbiomed.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Jørgensen N, Andersson A-M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol. 2010;34:400–410. doi: 10.1093/jat/34.7.400. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, Sealfon SC, Bernard DJ, Hardy MP. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J Androl. 2007;28:513–520. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- Han SW, Lee H, Han SY, Lim DS, Jung KK, Kwack SJ, Kim KB, Lee BM. An exposure assessment of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in human semen. J Toxicol Environ Health A. 2009;72:1463–1463. doi: 10.1080/15287390903212972. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL, Bern HA. Developmental effects of diethylstilbestrol (DES) in pregnancy. Thieme-Stratton; New York: 1981. [Google Scholar]
- Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect. 2009;117:86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005a;23:200–210. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005b;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idziorek T, Estaquier J, De Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Jobling MS, Hutchison GR, van den Driesche S, Sharpe RM. Effects of di(n-butyl) phthalate exposure on foetal rat germ-cell number and differentiation: identification of age-specific windows of vulnerability. Int J Androl. 2011;34:e386–396. doi: 10.1111/j.1365-2605.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kashahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS. Why Myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack SJ, Kim KB, Kim HS, Lee BM. Comparative toxicological evaluation of phthalate diesters and metabolites in Sprague-Dawley male rats for risk assessment. J Toxicol Environ Health A. 2009;72:1446–1454. doi: 10.1080/15287390903212923. [DOI] [PubMed] [Google Scholar]
- Laguë E, Tremblay JJ. Antagonistic effects of testosterone and the endocrine disruptor mono-(2-ethylhexyl) phthalate on INSL3 transcription in Leydig cells. Endocrinology. 2008;149:4688–4694. doi: 10.1210/en.2008-0310. [DOI] [PubMed] [Google Scholar]
- Lahousse SA, Wallace DG, Liu D, Gaido KW, Johnson KJ. Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicol Sci. 2006;93:369–381. doi: 10.1093/toxsci/kfl049. [DOI] [PubMed] [Google Scholar]
- Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C. Phthalate exposure and male infertility. Toxicology. 2006;226:90–98. doi: 10.1016/j.tox.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Laurenti E, Varnum-Finney B, Wilson A, Ferrero I, Blanco-Bose WE, Ehninger A, Knoepfler PS, Cheng PF, MacDonald HR, Eisenman RN, Bernstein ID, Trumpp A. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Li LH, Jester WF, Orth JM. Effects of relatively low levels of mono-(2-ethylhexyl) phthalate on cocultured Sertoli cells and gonocytes from neonatal rats. Toxicol Appl Pharmacol. 1998;153:258–265. doi: 10.1006/taap.1998.8550. [DOI] [PubMed] [Google Scholar]
- Lin YC, Yao PL, Richburg JH. FasL gene-deficient mice display a limited disruption in spermatogenesis and inhibition of mono-(2-ethylhexyl) phthalate-induced germ cell apoptosis. Toxicol Sci. 2010;114:335–345. doi: 10.1093/toxsci/kfq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res. 2010;54:148–157. doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- Meng X, de Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic f actor in mouse testis. Cancer research. 2001;61:3267–3271. [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Saito Y, Imai K, Kato M. Subcellular distribution of di- (2-ethylhexyl)phthalate in rat testis. J Toxicol Sci. 2004;29:113–124. doi: 10.2131/jts.29.113. [DOI] [PubMed] [Google Scholar]
- Onorato TM, Brown PW, Morris PL. Mono-(2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. J Androl. 2007;12:12–12. doi: 10.2164/jandrol.107.003335. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Ryu BY, Master SR, Phillips BT, Mack M, Avarbock MR, Chodosh L, Brinster RL. Genes involved in post-transcriptional regulation are overrepresented in stem/progenitor spermatogonia of cryptorchid mouse testes. Stem Cells. 2008;26:927–938. doi: 10.1634/stemcells.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, Saxena DK. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol. 2008;2(31):112–116. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Petersen JH, Breindahl T. Plasticizers in total diet samples, baby food and infant formulae. Food Addit Contam. 2000;17:133–141. doi: 10.1080/026520300283487. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol. 1996;137:42–50. doi: 10.1006/taap.1996.0055. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Nanez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1999;160:271–278. doi: 10.1006/taap.1999.8786. [DOI] [PubMed] [Google Scholar]
- Sariola H, Meng X. GDNF-induced seminomatous tumours in mouse--an experimental model for human seminomas? APMIS. 2003;111:192–196. doi: 10.1034/j.1600-0463.2003.11101231.x. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich ML. The missing niche for spermatogonial stem cells: do blood vessels point the way? Cell Stem Cell. 2007;1:361–363. doi: 10.1016/j.stem.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Shultz VD, Phillips S, Sar M, Foster PMD, Gaido KW. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol Sci. 2001;64:233–242. doi: 10.1093/toxsci/64.2.233. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Ando S, Maggiolini M, Pezzi V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149:5043–5051. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environmental Health Perspectives. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfo G, Caporossi L, Paci E, Aragona C, Romanzi D, De Carolis C, De Rosa M, Capanna S, Papaleo B, Pera A. Urinary phthalate monoesters concentration in couples with infertility problems. Toxicol Lett. 2011 doi: 10.1016/j.toxlet.2011.11.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wittassek M, Wiesmuller GA, Koch HM, Eckard R, Dobler L, Muller J, Angerer J, Schluter C. Internal phthalate exposure over the last two decades--a retrospective human biomonitoring study. Int J Hyg Environ Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in europeans? Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Xie T. Germline stem cell niches. In: Girard L, editor. StemBook [Internet] Harvard Stem Cell Institute; Cambridge, MA: 2008. [PubMed] [Google Scholar]
- Yao P-L, Lin Y-C, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. J Biol Chem. 2007;282:5420–5431. doi: 10.1074/jbc.M609068200. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Okubo T, Kano I, Sato S, Kano K. Induction of apoptosis by mono(2-ethylhexyl)phthalate (MEHP) in U937 cells. Toxicol Lett. 2003;15(144):371–81. doi: 10.1016/s0378-4274(03)00256-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shen H, Ma L, Shen B, Xu Z, Wang X. Differential expression of peroxiredoxin 6 in fetal rat testis following in utero exposure to di(n-butyl) phthalate. Toxicology. 2007;240:86–95. doi: 10.1016/j.tox.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.