Abstract
Dysregulation of cell signaling by electrophiles such as 4-hydroxynonenal (4-HNE) is a key component in the pathogenesis of chronic inflammatory liver disease. Another consequence of inflammation is the perpetuation of oxidative damage by the production of reactive oxidative species such as hydrogen peroxide. Previously, we have demonstrated Akt2 as a direct target of 4-HNE in hepatocellular carcinoma cells. In the present study, we used the hepatocellular carcinoma cell line HepG2 as model to understand the combinatorial effects of 4-HNE and hydrogen peroxide. We demonstrate that 4-HNE inhibits hydrogen peroxide mediated phosphorylation of Akt1 but not Akt2. Pretreatment of HepG2 cells with 4-HNE prevented hydrogen peroxide stimulation of Akt-dependent phosphorylation of downstream targets and intracellular Akt activity compared with untreated control cells. Using biotin hydrazide capture, it was confirmed that 4-HNE treatment resulted in carbonylation of Akt1, which was not observed in untreated control cells. Using a synthetic GSK3α/β peptide as a substrate, treatment of recombinant human myristoylated Akt1 (rAkt1) with 20μM or 40μM 4-HNE inhibited rAkt1 activity by 29% and 60%, respectively. We further demonstrate that 4-HNE activates Erk via a PI3 kinase and PP2A-dependent mechanism leading to increased Jnk phosphorylation. At higher concentrations, 4-HNE decreased both cell survival and proliferation as evidenced by MTT assays and EdU incorporation as well as decreased expression of cyclin D1 and β-catenin, an effect only moderately increased by the addition of hydrogen peroxide. The ability of 4-HNE to exert combinatorial effects on Erk, Jnk and Akt-dependent cell survival pathways provides additional insight into the mechanisms of cellular damage associated with chronic inflammation.
Introduction
Oxidative stress has been implicated in a wide range of chronic inflammatory diseases in the liver including Hepatitis C, primary biliary cirrhosis and alcoholic liver disease (ALD) [1-4]. Under conditions of chronic inflammation, reactive species such as hydrogen peroxide (H2O2) and 4-hydroxy-2-nonenal (4-HNE) are produced within the cell. 4-HNE is a primary marker for measuring increased oxidative stress in cells and is increased in ALD [3]. In experimental models of steatohepatitis and liver fibrosis, influx of proinflammatory cells stimulates production of reactive oxidative species (ROS) and formation of lipid peroxides such as 4-HNE, leading to increased cell death via either apoptosis or necrosis [5-7]. 4-HNE is a potent electrophile that will react with nucleophilic amino acids such as Cys, Lys and His [8]. A number of signaling proteins have been identified to be modified by 4-HNE within cells, including the lipid phosphatase PTEN and protein kinases such as Akt2 and LKB1 [9-11]. In addition, using RKO cells treated with 100μM 4-HNE, Codreanu et. al., identified over 1500 proteins using biotin hydrazide capture followed by LC/MS proteomic analysis [12].
Under conditions of enhanced oxidative stress, a major cellular response is the activation of the Akt pathway. Hydrogen peroxide has been shown to induce activation of Akt by several mechanisms including inactivation of PTEN and activation of the PI3K pathway [13, 14]. Although H2O2 is a known activator of Akt, very little is known concerning the specific isoform of Akt activated. In a recent study, knockdown of Akt1 led to increased resistance to low micromolar concentrations of H2O2 in human lens epithelial cells via upregulation of Akt2 [15]. In other studies, using mouse embryonic fibroblasts, a deficiency in both Akt1 and Akt2 led to increased resistance to H2O2 mediated apoptosis at concentrations up to 1mM [16]. Combined, these observations suggest cell type specific regulation of H2O2 resistance via different Akt isoforms.
Previously, 4-HNE has been shown to decrease cellular proliferation in several cell types including prostate and breast cancer cells [17, 18]. One of the major proteins involved in cellular proliferation is the protein kinase Akt. Protein kinases such as Akt regulate the cell cycle and proliferation via phosphorylation of multiple proteins including glycogen synthase kinases 3β (inactivation leading to increased stability of cyclin D1) and ubiquitin ligase mouse double minute 2 (MDM2) (inhibition of p53 degradation) [19, 20]. 4-HNE has been shown to inhibit insulin signaling via direct modification of Akt2 leading to a decrease in phosphorylation of both GSK3β and MDM2, both downstream targets of Akt [11]. It is well known that under oxidative stress and inflammatory conditions, 4-HNE is not the only reactive intermediate produced. Therefore, examining combinatorial effects of different reactive species may provide greater insight into the pathogenesis of inflammation. In this study, we report that pre-incubation HepG2 cells with 4-HNE inhibits H2O2 mediated activation of the Akt pathway in leading to decreased cell proliferation and decreased expression of cyclin D1.
Materials and Methods
Treatment of HepG2 cells
HepG2 cells were maintained at 50-80% confluence in RPMI supplemented with 10% Fetal Bovine Serum, 100mM Hepes, 100 IU/ml Penicillin, 100g/ml Streptomycin. Cells were plated into 6 well plates at a density of 1×106 cells per well. The following day, the cells were washed twice in serum-free RPMI and treated with indicated concentrations of 4-HNE in serum free media. Where indicated, Ly294002 (Calbiochem/EMD Biosciences, Philadelphia, PA) (50μM/30 min), okadaic acid (Calbiochem) (100nM/30 min), U0126 (Calbiochem) (10μM/30 min) or H2O2 (1.0 mM/5 min) (Sigma Aldrich, St. Louis, MO) was added as described.
Western blotting
Following treatment, cells were lysed for 5 min in 50mM HEPES, 100mM NaCl, 1% triton X-100, 2mM EDTA (pH 7.7) plus protease and phosphatase inhibitors Sigma (St. Louis, MO), followed by sonication for 3X10 s. For each gel, 10 μg of whole cell lysates was loaded per well on 7% SDS PAGE gels, electroblotted to PVDF, blocked in Tris buffered saline with 1% Tween (TBST) and 5% nonfat dry milk for 1 h and incubated overnight in primary antibody. The rabbit polyclonal 4-HNE antibody was used at 1:1000 as previously described [21]. The following secondary antibodies were used at 1:5000 dilution in TBST/5% NFDM: HRP conjugated goat polyclonal anti-rabbit (Jackson Immunoresearch laboratories, West Grove, PA), HRP conjugated donkey polyclonal anti-mouse (Jackson). Blots were subsequently processed and developed using chemiluminescence as previously described [9]. All Western blots were quantified using NIH freeware Image J.
Primary antibodies
The following primary antibodies were used at 1:1000 dilution in TBST/5% BSA: rabbit polyclonal: pAkt (Ser473), pAkt (Thr308), pAkt (Thr450), pMDM2 (Ser166), pGSK3β (Ser9), GSK3β (#’s 4060, 4691, 3521, 9323, 9315. Cell Signaling, Danvers, MA), mouse monoclonal: actin 1:5000 (Sigma #A5441), Akt2 (1:1000#5329 Cell Signaling), Akt1 (1:1000 #2967 Cell Signaling), total MDM2 (ABCAM AB-10567).
Preparation of recombinant activated Akt1
The N-terminal myristoylation sequence from Src tyrosine kinase was added to Full-length Akt1 by PCR from Flag Ha tagged Akt1 PCDNA3 (Addgene #9021) using the following oligonucleotides: -5’ (5’- ccc aag gac ccc agc cag cgc atg agc gac gtg gct att gtg -3’ sense), 5’-cat atg cat ggg gag cag caa gag caa gcc caa gga ccc cag c -3’) for the N-terminus and (5’- ggt acc tca ggc cgt gcc gct ggc -3’ antisense). All oligonucleotides were purchased from IDT (Coralville, IA.). Following amplification, the each fragment was TOPO-cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA), transformed into TOP-10 cells and grown overnight on LB-ampicillin plates (100 μg/mL) according to the manufacturer’s instructions. Colonies were picked and placed into 3-mL LB cultures for 16 h with 100 μg/mL of ampicillin. DNA was subsequently purified using Qiagen minipreps (Qiagen, Valencia, CA) and sequences were verified at the University of Colorado Cancer Center core facility. myrAkt1 pCR2.1 was digested for 1h with Nde1/Kpn1 (New England Biolabs, Ipswich, MA), and ligated overnight into pACGHLTA ΔGST using T4 DNA ligase (New England Biolabs). MyrAkt1 pACGHLTA ΔGST was subsequently co-transfected with linearized Baculogold DNA (BD-Pharmingen, San Diego, CA) into SF-9 cells using lipofectamine by the UCDenver Cancer Core Protein Center. Virus was amplified for 5 days, viral titer determined and SF-9 cells were infected for 48 hours. Recombinant protein was purified as previously described [11].
Akt activity assays
Akt activity was determined by immunoprecipitation of p-Ser473 Akt from 150mg of total protein lysates and in vitro phosphorylation of a GST-GSK3α/β fusion protein (1μg/assay). Detection of phosphorylation was via Western blotting using anti-phospho GSK3α/β polyclonal antibodies as previously described [11].
Determination of intracellular carbonylation of Akt1
Akt1 protein carbonylation was determined using 150μg of total cellular protein as previously described [9].
Assessment of cellular proliferation by EdU incorporation
Cell proliferation was assessed using a Click-iT EdU incorporation kit (Invitrogen/Molecular Probes) and confocal microscopy. In these studies, the HepG2 cells were seeded at 0.5×106 cells in triplicate per well onto coverslips in 12-well plates and allowed to attach for 16 h. Medium was removed by aspiration, and the HepG2 cells were either pretreated with 4-HNE (12.5-100 μM) or untreated and then treated with in H2O2 (1mM) for 5 min serum free medium for 24 h. At 20h, 10μM ethynyl-2-deoxyuridine was added and the cells were allowed to incubate for an addition 4 h. The medium was removed, cells were fixed for 30 min 3.7% paraformaldehyde and coverslips were processed according to the manufacturer’s instructions. Confocal images were taken with a 40X oil immersion objective on a Nikon Eclipse TE2000-E instrument, with identical instrument laser and contrast settings used within each group. Images were acquired using EZ-C1 software (Nikon, Melville, NY). Cell proliferation was determined by a comparison of the total number of cells with total nuclei with EdU incorporation counting a minimum of 10 fields with 100 nuclei per condition. EdU was visualized using Alexa flour 488 (excitation 485nm/ emission 565 nm), nuclei were stained with Hoechst 33342 (excitation 355 nm/ emission 465 nm).
Assessment of cell survival by MTT assay
Growth inhibition/cell survival in the human liver HepG2 cell line was measured using the MTT colorimetric assay [22]. In these studies, the HepG2 cells were seeded at 2×103 cells per well in 96-well plates, in triplicate, and allowed to attach for 16 h. Medium was removed by aspiration, and the HepG2 cells were either pretreated with 4-HNE (12.5-100 μM) or untreated and then treated with in H2O2 (1mM) for 5 min serum free medium for 4 and 24 h time periods. The medium was removed, and MTT (50 μg) in complete medium (50 μL) was added to each well and incubated for a further 4 h. Inhibition of cell growth was determined by measuring the cellular reduction of MTT to the crystalline formazan product, dissolved by the addition of DMSO (100 μL). Optical density was determined at 550 nm using a Molecular Devices Thermomax microplate reader.
Statistical analysis
Relative densitometry of Western blots was quantified using ImageJ (http://rsb.info.nih.gov/ij/). All data and statistical analysis was performed using one-way analysis of variance and Prism 4 for Windows (GraphPad Software, San Diego, CA). All data are expressed as mean +/- S.E. and p values <0.05 were considered significant.
Results
In many cell types H2O2 is a known activator of Akt; however, little is known concerning the specific isoforms activated in hepatocarcinoma cells. In order to evaluate the specific isoform(s) of Akt involved in H2O2 mediated signaling, total activated Akt (pAkt Ser473) was immunoprecipitated from H2O2 stimulated HepG2 cells and the specific Akt isoform activated identified using isoform specific antibodies. From the Western blot (Figure 1A), in control cells, only phosphorylation of Akt2 was detectable. Following H2O2 treatment, relative levels of phospho Ser473/4 of Akt1 increase 10-fold and Akt2 increase 30-fold indicating activation (Figure 1B). These data indicate an activation of both isoforms of Akt by H2O2 stimulation in HepG2 cells, with Akt1<Akt2. Previously, it has been demonstrated that 4-HNE activates Akt2 but not Akt1 [11]. Surprisingly, when cells are stimulated with H2O2 following pretreatment with 4-HNE, phosphorylation of Akt1 is reduced to the level of untreated cells whereas phosphorylation of Akt2 is decreased 3-fold compared to H2O2 alone. The level of Akt2 phosphorylation is still 6 fold greater than untreated cells and significantly greater than 4-HNE alone. This suggests 4-HNE inhibits H2O2 induced phosphorylation of Akt1 but does not completely inhibit phosphorylation of Akt2.
Figure 1. Hydrogen peroxide activates both Akt1 and Akt2 in HepG2 cells.
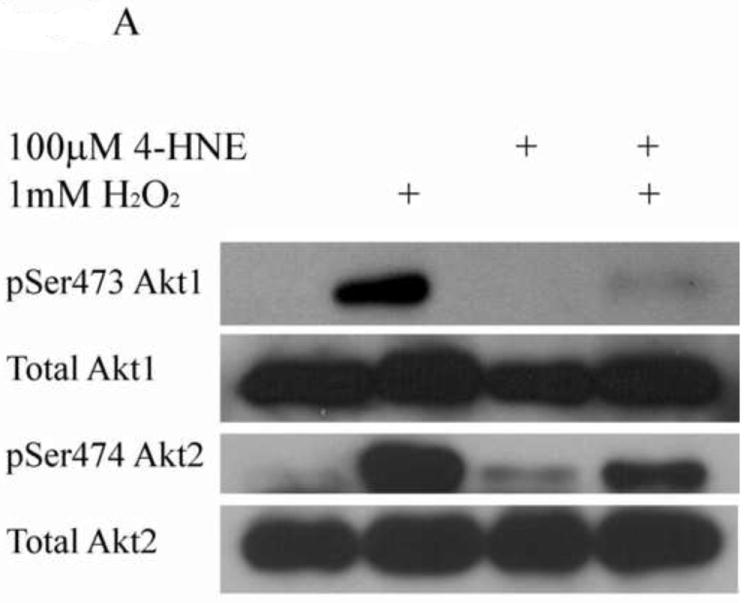
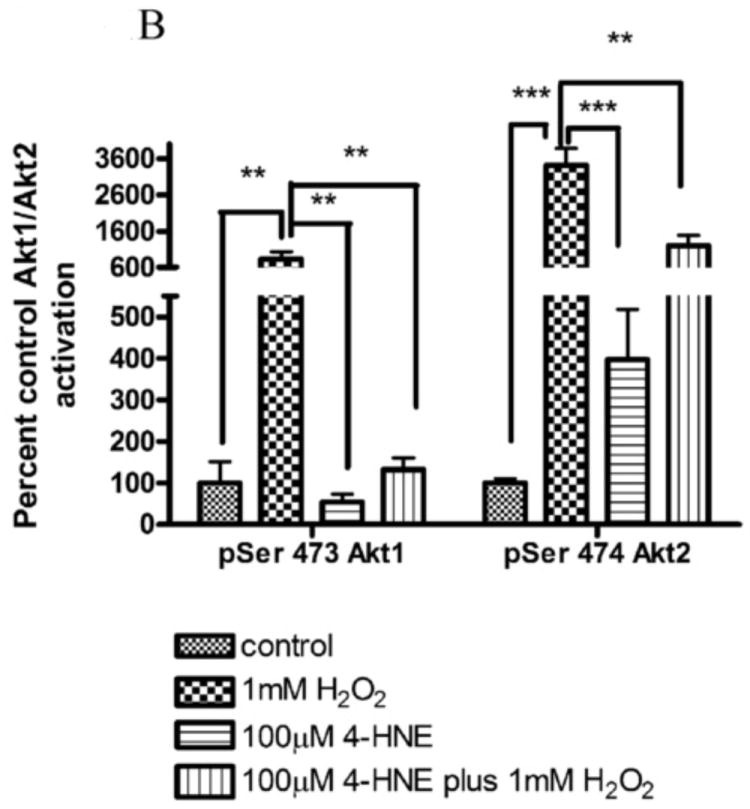
(A) HepG2 cells were treated with 1mM hydrogen peroxide (5 min), 100nM insulin (15min) or 100μM 4-HNE (60min). Cells were lysed and 150μg of total lysate immunoprecipitated using p-Ser473/4 Akt polyclonal antibodies. Immunoprecipitates (IP) were run on an 8% SDS PAGE, blotted and probed for Akt1, Akt2. (B) Quantification of hydrogen peroxide-mediated phosphorylation of Akt1 and Akt2 on Ser473/4. Experiment was performed in at least triplicate and subjected to 1-way ANOVA with Tukey’s multiple comparison test *p<.05, ***p<0.001
From Figure 1, there is no evidence that 4-HNE inhibits the ability of H2O2 to produce intracellular ROS. To verify these results, HepG2 cells were incubated with 4-HNE, H2O2 or 4-HNE plus H2O2 in the presence of an intracellular indicator of ROS (DCFDA). From Supplemental figure 1, 4-HNE did not significantly affect the ability of H2O2 to produce intracellular ROS.
4-HNE significantly reduced both hydrogen peroxide mediated Akt1 and Akt2 phosphorylation. Therefore the capacity of 4-HNE to inhibit H2O2 stimulation of Akt signaling was examined. From Figure 2A, at concentrations of 100μM, 4-HNE did not inhibit H2O2 mediated total Akt phosphorylation. Statistical analysis of data for total phosphorylation of Ser473/4 on Akt indicates a decrease compared to cells treated with H2O2 alone (Figure 2A, B) which was not significantly different. Compared to untreated controls, H2O2 led to a 27-fold increase in phospho-Thr308/9 Akt (Figure 2A, C). 4-HNE alone increased Thr308/9 phosphorylation by 5-fold. Preincubation with 4-HNE followed by H2O2 significantly decreased phospho-Thr308 to the level of 4-HNE alone. Phosphorylation of Akt at Thr450 by C-jun-N-terminal kinases (JNKs) has been reported under conditions of hypoxia [23]. 4-HNE is a known to activate Jnks [24, 25]. In this system neither hydrogen peroxide nor 4-HNE had a significant effect on Akt Thr450 phosphorylation (Figure 2A, Supplemental Figure 2). Previously, 4-HNE has been demonstrated to lead to a decrease in PTEN phosphorylation at Ser380, in this system, phosphorylation of PTEN was significantly decreased by 4-HNE alone and 4-HNE plus H2O2 (Figure 2A, Supplemental Figure 2) [9]. Based on the phosphorylation status of Akt, we hypothesized that there should be an increased in phosphorylation of downstream substrates of Akt. From the Western blot, downstream phosphorylation of the Akt targets MDM2 and GSK3β are clearly suppressed following 4-HNE pre-incubation. Densitometric analysis indicate that GSK3β phosphorylation is decreased by 6-fold (p<0.001) and MDM2 phosphorylation is decreased by greater than 7-fold (p=0.012) compared to H2O2 alone (Figure 2A, D, E). In summary, these data indicate 4-HNE-mediated inhibition of Akt downstream signaling by H2O2 in HepG2 cells.
Figure 2. Inhibition of hydrogen peroxide-mediated Akt signaling by 4-HNE.
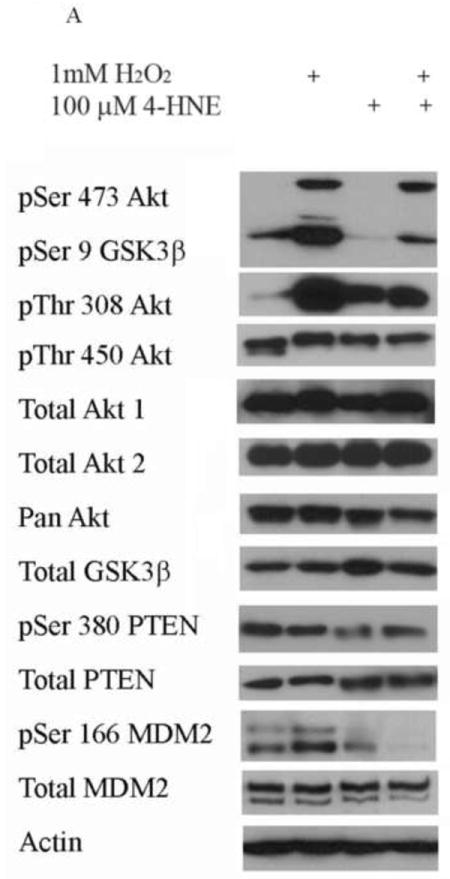
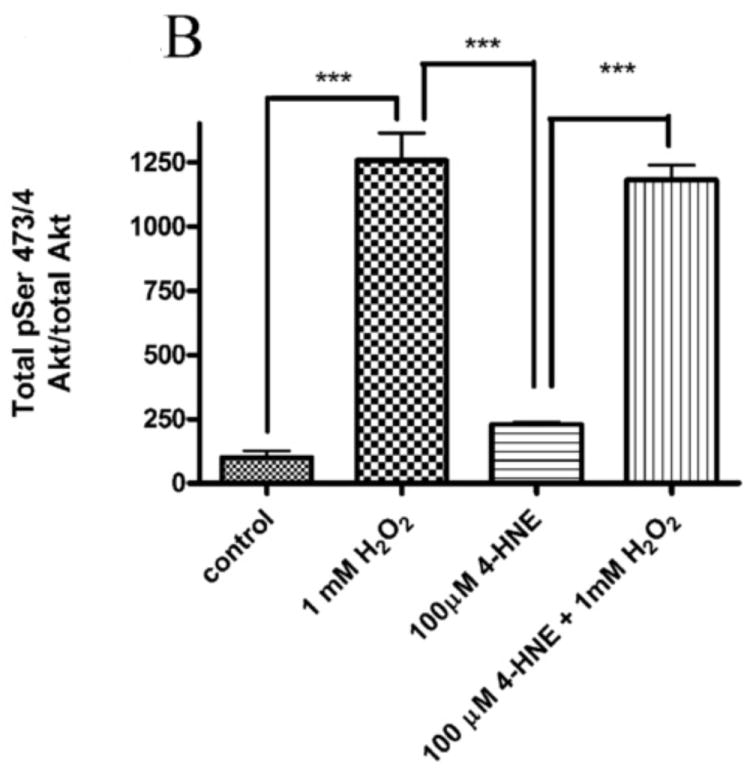
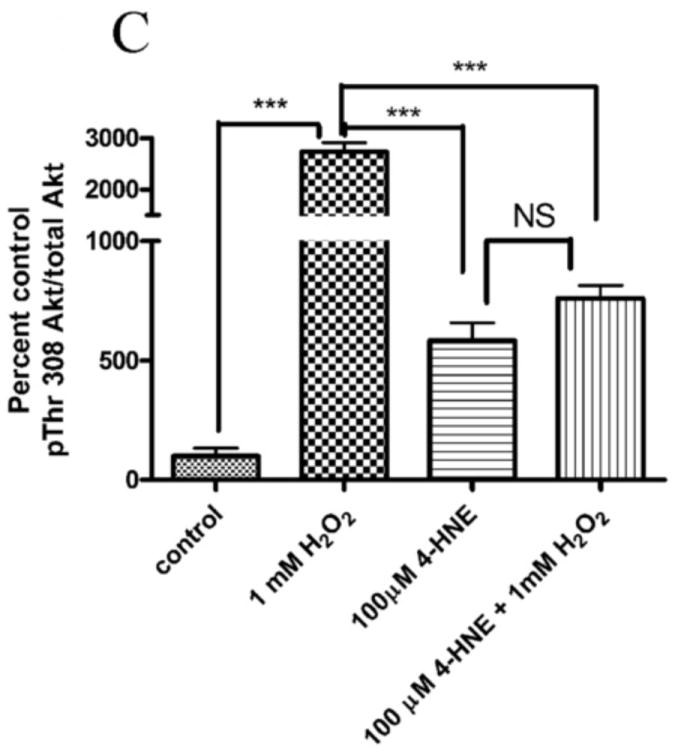
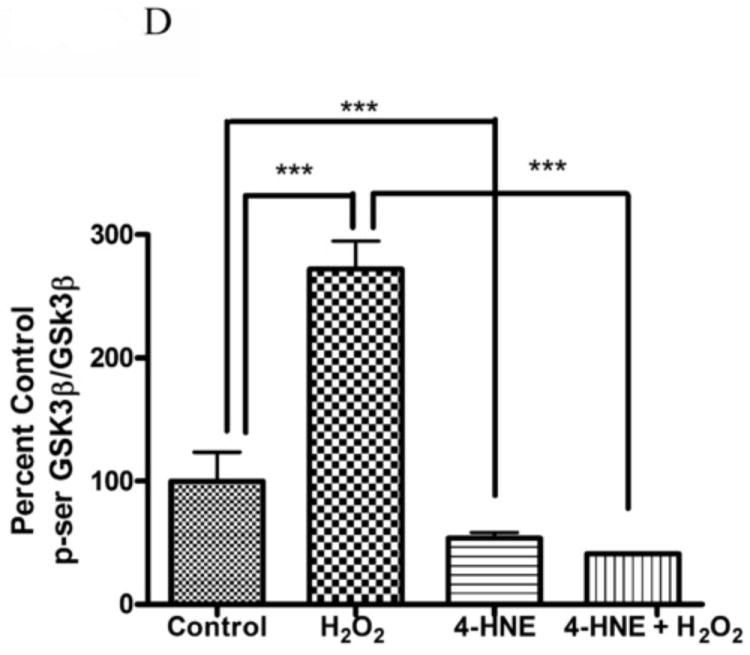
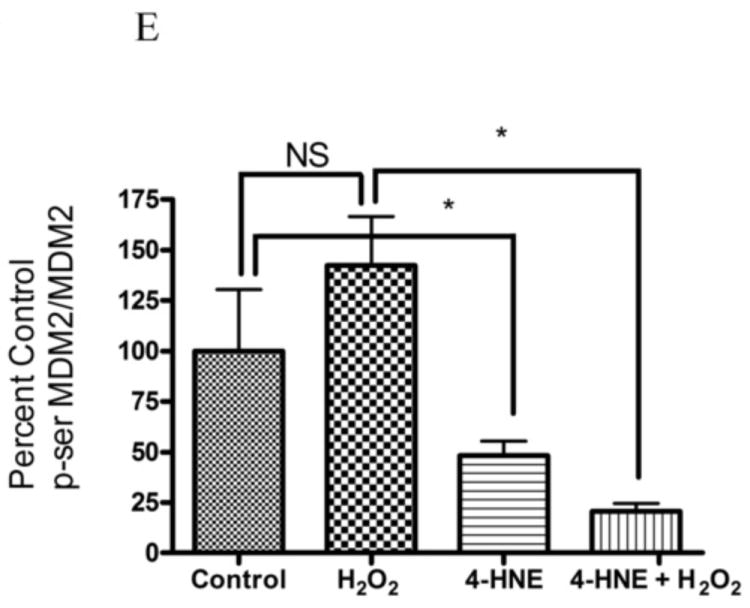
Cells were treated in serum free media with 100μM 4-HNE or control for 60 min, washed in serum free media and stimulated with 1mM hydrogen peroxide for 5 minutes. Cells were lysed and processed as stated in methods. (A) Western blot using antibodies for the following proteins: Akt (Ser473/4), Akt (Thr 308/9), Akt (Thr 450), Akt1, Akt2, PTEN, PTEN (pSer380), p-GSK3β (Ser9), GSK3β, p-MDM2 (Ser166), MDM2 and actin (Note: Film exposures for pSer473 Akt are less than 2 seconds). Each blot is representative of 3 independent experiments, for MDM2 samples were run on a 5% SDS PAGE gel, all other samples were run on a 7% SDS PAGE. (B) Quantification of pSer473/4 Akt. (C) Quantification of pThr 308/9 Akt. (D) Quantification of pSer 9 GSK3b. (E) Quantification of pSer 166 MDM2. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test *p<0.05, ***p<0.001.
Previous reports have demonstrated 4-HNE-mediated increases in p-Ser473 Akt and PtdIns (3,4,5)P3 at the plasma membrane [9]. Although 4-HNE inhibited H2O2-dependent Akt signaling, inhibition could be due to prevention of H2O2 mediated intracellular Akt localization. To determine the effects of 4-HNE on H2O2 stimulated cellular localization of Akt1, confocal microscopy using transiently transfected eGFP or eGFP-tagged Akt1 was performed. Localization of eGFP did not change under any of the conditions examined (data not shown). From Supplemental Figure 3, in the serum-free control cells, eGFP-Akt1 localizes to both the cytoplasm and the nucleus with very little plasma membrane association. Compared to control cells, the addition of 4-HNE led to an increase in nuclear accumulation of GFP-Akt1 with a mild increase in plasma membrane localization. However, treatment with H2O2 led to an increase in both nuclear and plasma membrane association. Pretreatment of 100μM 4-HNE did not affect H2O2 stimulated localization of eGFP-Akt1 to the plasma membrane and nucleus. Combined, these data indicate that 4-HNE does not affect H2O2 stimulated localization of Akt1 within the cell.
To determine if cellular total Akt activity is inhibited by 4-HNE, Akt activity assays were performed using lysates obtained from HepG2 cells pretreated with increasing concentrations of 4-HNE (0-100μM) ± H2O2. From densitometric analysis of the Western blot in Figure 3, compared to hydrogen peroxide stimulation, 4-HNE alone significantly reduces Akt activity in a concentration dependent manner.
Figure 3. Inhibition of hydrogen peroxide stimulation of Akt activity by 4-HNE in HepG2 cells.
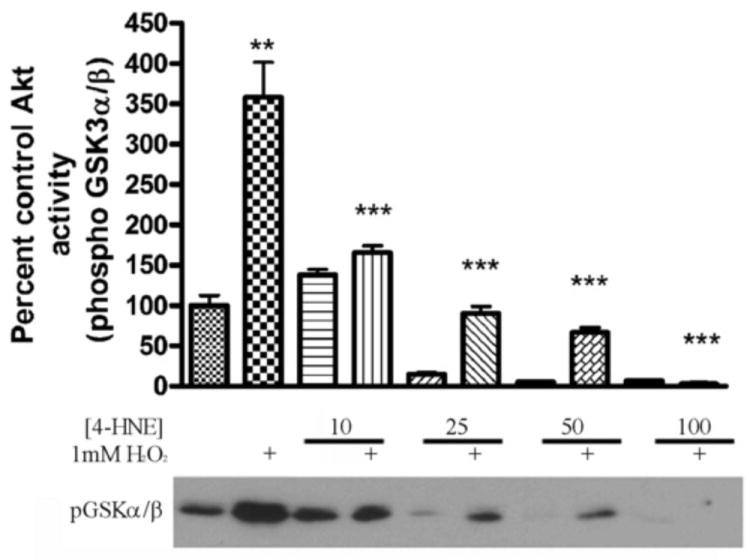
Western blot and quantification of in vitro Akt kinase activity assay using a synthetic GSK3αβ peptide and increasing concentrations of 4-HNE (0, 10, 25, 50, 100μM) ± 1mM hydrogen peroxide. All samples performed in triplicate. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test **p<0.01, ***p<0.001.
Previously, Akt2 was determined to be a direct target of 4-HNE in HepG2 cells. Treatment of HepG2 cells with 4-HNE led to an increase in carbonylation of both total Akt and Akt2 [11]. To determine if carbonylation of cellular Akt1 occurs following 4-HNE, a method of biotin hydrazide modification of protein bound reactive aldehydes was utilized [9]. From the Western blot in Figure 4A, a substantial increase in carbonylated Akt1 is evident. Subsequent densitometric analysis of the Western blots demonstrated a greater than 30-fold increase in Akt1 carbonylation following 4-HNE treatment (Figure 4B). This clearly indicates 4-HNE-mediated modification of Akt1 in HepG2 cells. Combined with previous data, the data presented in Figures 4 and 5 demonstrate increased carbonylation and inhibition of both Akt1 and Akt2 activity in HepG2 cells.
Figure 4. Akt1 is modified by reactive aldehydes following 4-HNE treatment in HepG2 cells.
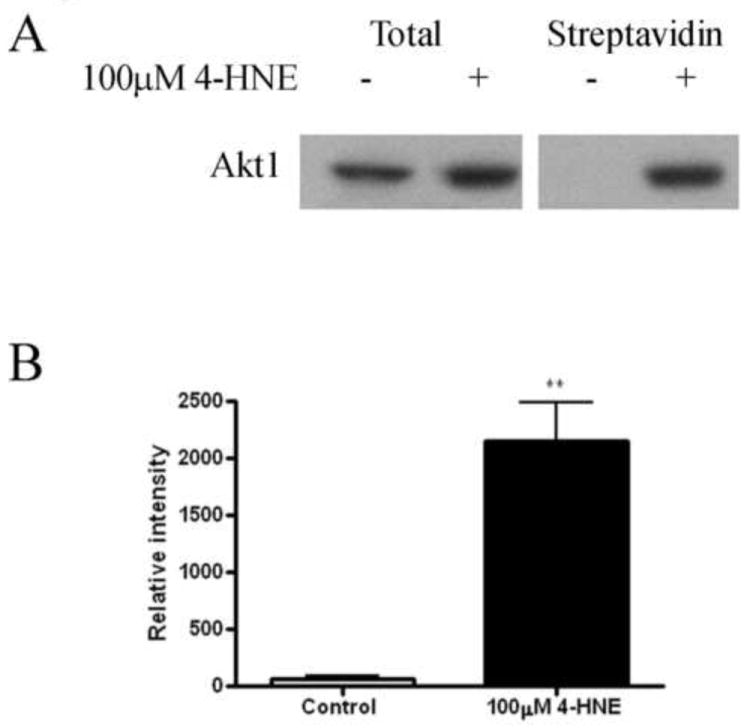
(A) Streptavidin (S.A.) pulldown of 4-HNE modified Akt1 from HepG2 cells. Protein (125 μg) from 4-HNE treated (100μM/60 min in serum free media) or untreated cells was incubated for 2 h with 2.5 mM biotin hydrazide, dialyzed and purified using streptavidin beads. Samples were subsequently analyzed using SDS PAGE/Western blotting with rabbit polyclonal anti-Akt1 antibody. All samples were performed in at least triplicate. (B) Densitometric analysis and quantification of Western blots. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test **p<0.01
Figure 5. Effects of 4-HNE on recombinant myrAkt1.
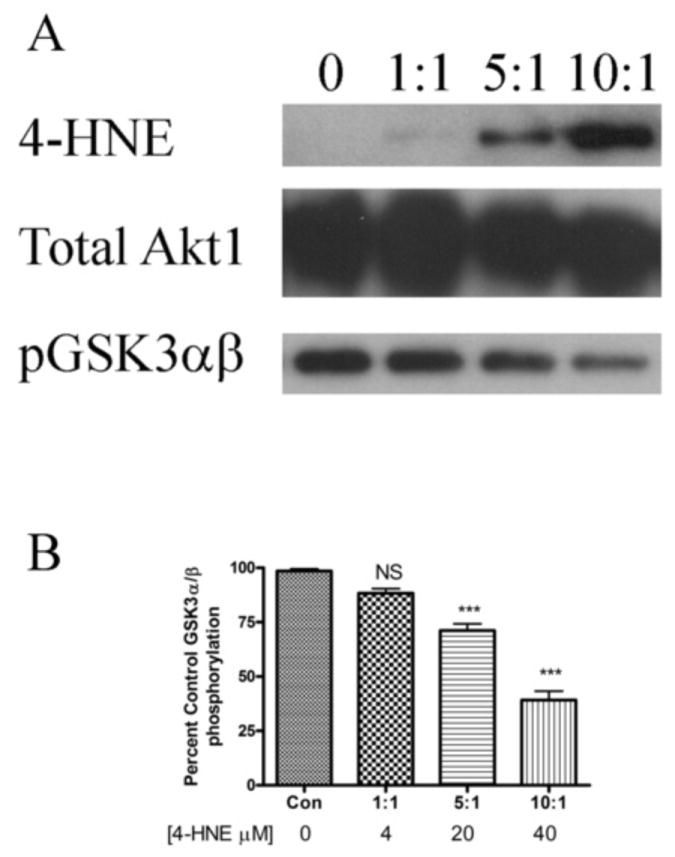
(A) Western blotting of rAkt1 treated with 4-HNE. Purified rAkt1 was incubated with increasing molar ratios of 4-HNE for 30 min at room temperature. Samples were boiled in 5X SDS loading buffer, run on an 8% SDS PAGE gel, blotted and probed for 4-HNE using anti-4-HNE polyclonal antibodies followed by stripping and re-probing for Akt1. (B) Inhibition of rAkt1 by 4-HNE. Purified rAkt1 was incubated with increasing ratios of 4-HNE and phosphorylation assays performed using a GST fusion GSK3α/β synthetic substrate as per methods. All samples were performed in at least triplicate. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test ***p<0.001.
Based on the increase in Akt1 carbonylation an effort was made to identify intracellular sites of modification using immunoprecipitations of total Akt from 2mg of total protein and a ThermoFisher LTQ Orbitrap mass spectrometer. Surprisingly, although Akt1/2 was detectable in the Western blot, no detectable peptides from Akt were identified (data not shown). This suggests that although Akt is identifiable as carbonylated by Western blot, total protein levels are not sufficient for detection by LC/MS.
Although it does not occur naturally in cells, the addition of Src myristoylation sequence on the N-terminus of Akt leads to a constitutively active form of the protein and enables the ability to examine the effects of 4-HNE on an active form of Akt1. To verify the direct effects of 4-HNE on Akt1, purified recombinant myristoylated Akt1 (rmyrAkt1) was treated with increasing concentrations of 4-HNE followed by Western blotting for 4-HNE modification. The data presented in Figure 5A clearly indicate a concentration-dependent increase in 4-HNE modification of rAkt1. To determine if 4-HNE will directly inhibit Akt1 activity, rmyrAkt1 was incubated with increasing concentrations of 4-HNE followed by an Akt activity assay. From Figure 5B, rmyrAkt1 activity is significantly inhibited by 29% and 60% following incubation with 20 and 40μM 4-HNE respectively, corresponding to molar ratios of 5:1 and 10:1 (4-HNE:rmyrAkt1).
The addition of 4-HNE has been shown to decrease cellular proliferation in HepG2 cells [26]. To ascertain the effects of 4-HNE by itself or in combination with H2O2 on cell proliferation, cells were pretreated with 4-HNE (12.5-100μM) followed by 1mM H2O2 and compared with either 4-HNE alone or H2O2 alone. Proliferation was then assessed using incorporation of 5-ethynyl-2-deoxyuridine into DNA 24 hours post treatment. As demonstrated in Figure 6, treatment with H2O2 alone led to a 27% decrease (p<0.001) in EdU incorporation compared to untreated cells. When examining the effects of 4-HNE, a concentration-dependent decrease in EdU incorporation was observed. At 100μM 4-HNE, no incorporation of EdU was evident. Combined these data indicate a concentration-dependent inhibition of cell proliferation by 4-HNE.
Figure 6. Effect of 4-HNE pretreatment and H2O2 on cell proliferation in HepG2 cells.
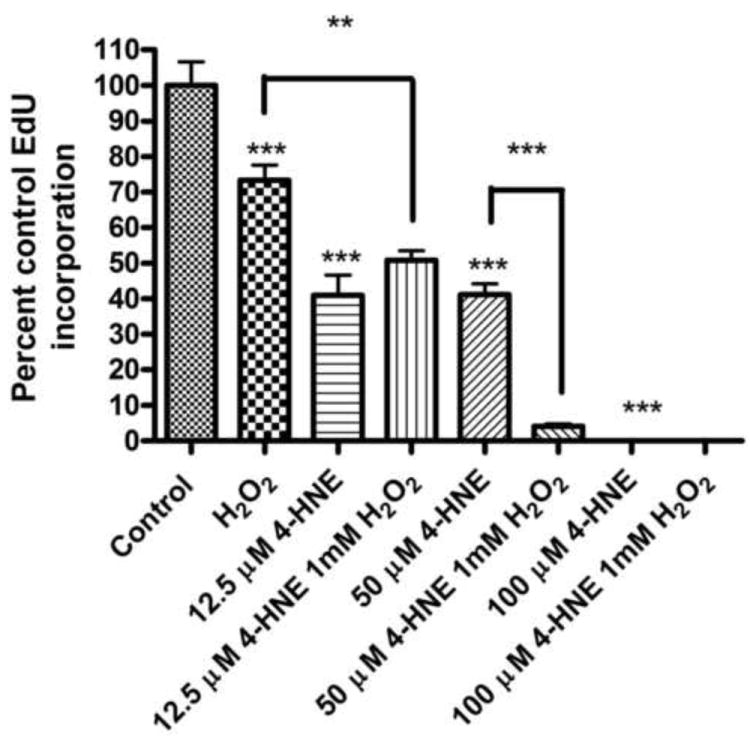
Cells were pretreated with 4-HNE (12.5-100μM) (60 min) and then treated with 1mM H2O2 for 5 min or treated with 4-HNE (12.5-100μM) alone. Cells were then incubated for 24 h in media containing serum. Effects of 4-HNE on cellular proliferation was measured by the incorporation of EdU over the final 4 h. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test (n = 8); **p<0.01, ***p<0.001.
The addition of 4-HNE has also been demonstrated to effect cell survival. To assess the effects of 4-HNE on overall cell survival, MTT assays were performed under at 4 and 24 h post-treatment. There was no appreciable decrease in cell survival in cells treated with 1mM H2O2 alone (Data not shown). From Figure 7, at 4 h and 24 h, treatment using increasing concentrations of 4-HNE for 24 h led to a concentration-dependent decrease in cell survival (12.5μM 4-HNE-94% survival, 100μM 4-HNE-67% survival). The combination of H2O2 followed by 100μM 4-HNE treatment for 24h (59% survival) resulted in a statistically significant decrease in cell survival compared with 100μM 4-HNE alone (67% survival). Combined with the data presented in Figure 6, 4-HNE directly affects both proliferation and survival in HepG2 cells.
Figure 7. Effect of 4-HNE pretreatment and H2O2 on cell survival in HepG2 cells.
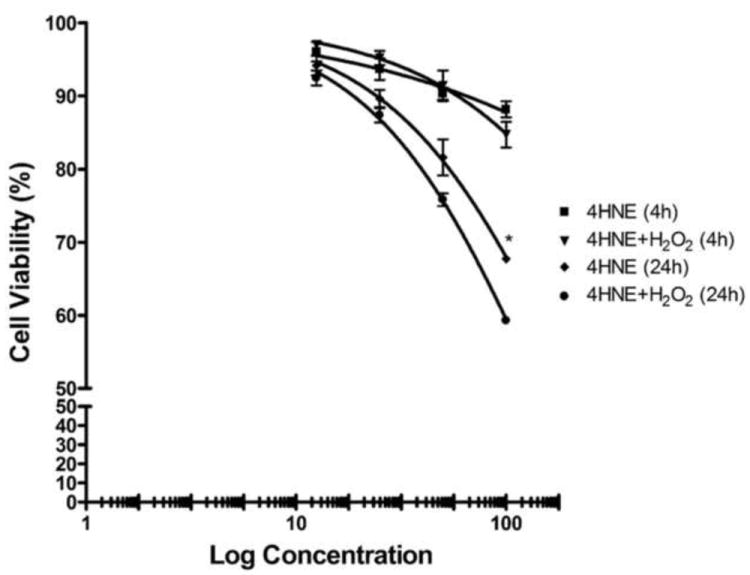
Cells were pretreated with 4-HNE (12.5-100μM) (60 min) and then treated with 1mM H2O2 for 5 min or treated with 4-HNE (12.5-100μM) alone. Cell survival was measured by MTT analysis at 4 and 24 h post- treatment. Points, mean (n = 3); bars, S.D, *p<0.05.
Both β-catenin and cyclin D1 are implicated in cellular proliferation [27, 28]. To evaluate the effects of 4-HNE on the expression of cyclin D1 and β-catenin, cells were treated with H2O2, 4-HNE, or 4-HNE followed by stimulation with H2O2. Following exposure, cells were incubated for an additional 4 or 24h, lysed and Western blotted for cyclin D1 and β-catenin. From the Western blots presented in Figure 8A-D, 1mM H2O2 did not induce a decrease in cyclin D1 or β-catenin expression at 4 or 24 h. Likewise, at 4 h, 12.5μM 4-HNE did not have a significant effect on cyclin D1 expression (Figure 8A). At 24 h 12.5μM 4-HNE led to a 40% decrease in cyclin D1 expression compared to untreated cells (Figure 8B); however, this decrease was not significant. At 4 and 24 h, treatment with 100μM 4-HNE or 100μM 4-HNE plus 1mM H2O2 led to a significant decrease in cyclin D1 (Figure 8C, D). β-catenin expression did not significantly decrease until 100μM 4-HNE was added and cells had incubated for 24 h (Figure 8D). Furthermore, the addition of 1mM H2O2 following 4-HNE led to a significant decrease in β-catenin expression compared to 4-HNE alone. Taken together, the data in Figures 6,7 and 8 indicate the 4-HNE decreases both β-catenin and cyclin D1 levels resulting in a decrease in cell proliferation and cell survival.
Figure 8. 4-HNE decreases both cyclin D1 and β-catenin expression in HepG2 cells.
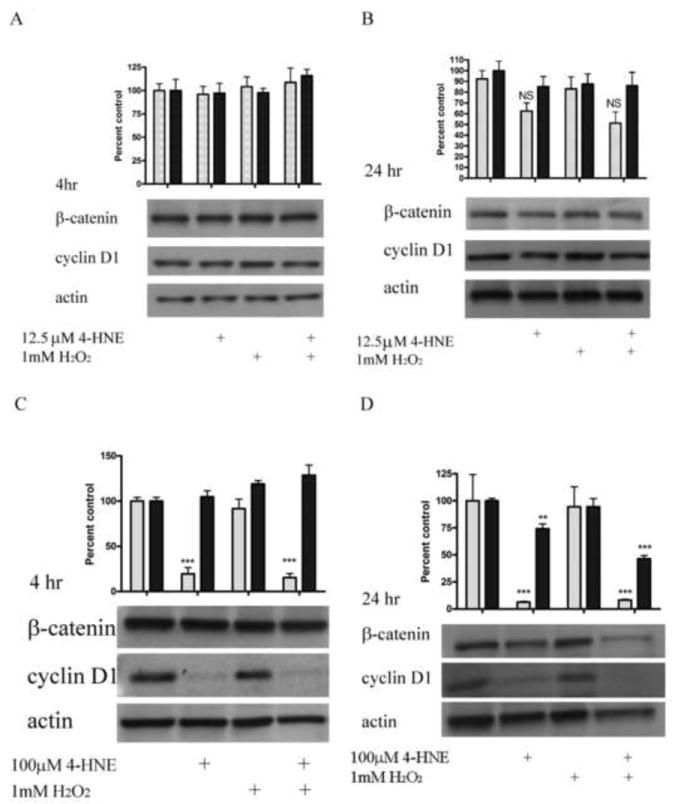
Cells were treated in serum free media with 4-HNE (12.5, 100μM) or control for 60 min, washed in serum free media and stimulated with 1mM H2O2 for 5 minutes. Cells were then washed 2X in RPMI and incubated for an additional 4 or 24 h in RPMI plus serum, lysed and western blotted for β-catenin or cyclin D1. (A) Effects of 12.5μM 4-HNE and H2O2 on β-catenin/cyclin D1 expression (4h). (B) Effects of 12.5μM 4-HNE and H2O2 on β-catenin/cyclin D1 expression (24h). (C) Effects of 100μM 4-HNE and H2O2 on β-catenin/cyclin D1 expression (4h). (D) Effects of 100μM 4-HNE and H2O2 on β-catenin/cyclin D1 expression (24h). Blots were quantified using ImageJ and plotted as described in methods (cyclin D1 (gray), β-catenin (black)). Each blot is representative of 3 independent experiments, Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test *p<0.05, **p<0.01, ***p<0.001.
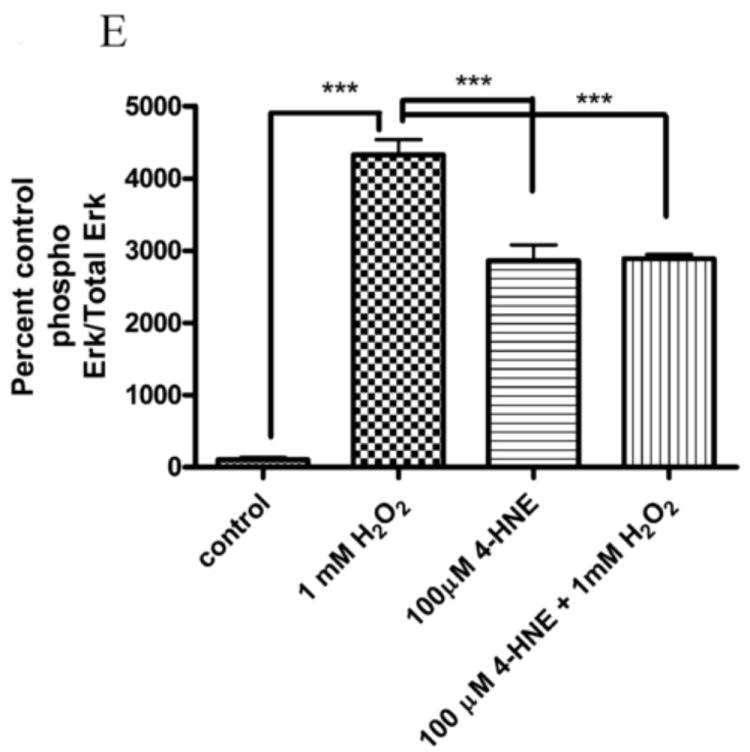
In addition to the Akt pathway, expression of cyclin D1 and β-catenin can be regulated by activation of the c-Jun-N-Terminal Kinase (JNK) and extracellular-signal-related kinase (Erk) pathways [29-31]. To understand the relative contribution of Erk to 4-HNE-mediated decreases in cell proliferation cells were treated as with either 1mM hydrogen peroxide or 100μM 4-HNE in the presence of an Erk inhibitor (U0126). Phosphorylation and expression of p42/44 Erk was examined. From Figure 9A, both hydrogen peroxide and 4-HNE led to an increase in Erk phosphorylation that was abrogated by U0126. Erk has previously been demonstrated to be regulated by PI3 kinases and by protein phosphatase 2A (PP2A) [31-33]. To determine the relative role PI3 kinase and PP2A play in 4-HNE mediated Erk activation were treated with 4-HNE in the presence of a PI3 kinase inhibitor (LY294002) (Figure 9B) or a PP2A inhibitor (okadaic acid) (Figure 9C). From the data in Figure 9B, 4-HNE-mediated Erk phosphorylation was increased in the presence of the inhibitor indicating PI3 kinases play a role in 4-HNE-mediated activation of Erk. Inhibition of PP2A also led to an increase in 4-HNE-mediated Erk phosphorylation (Figure 9C). Combined, these data indicate Erk activation by 4-HNE is mediated by both PI3 kinase and PP2A.
Figure 9. 4-HNE leads to increased Erk activation via a PI3K/PP2A dependent mechanism.
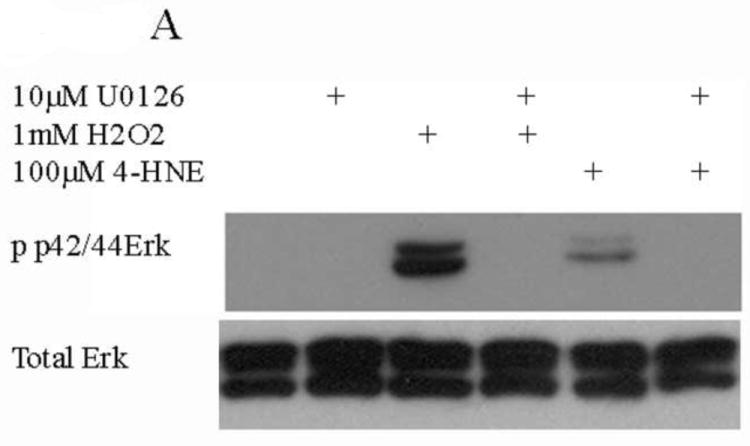
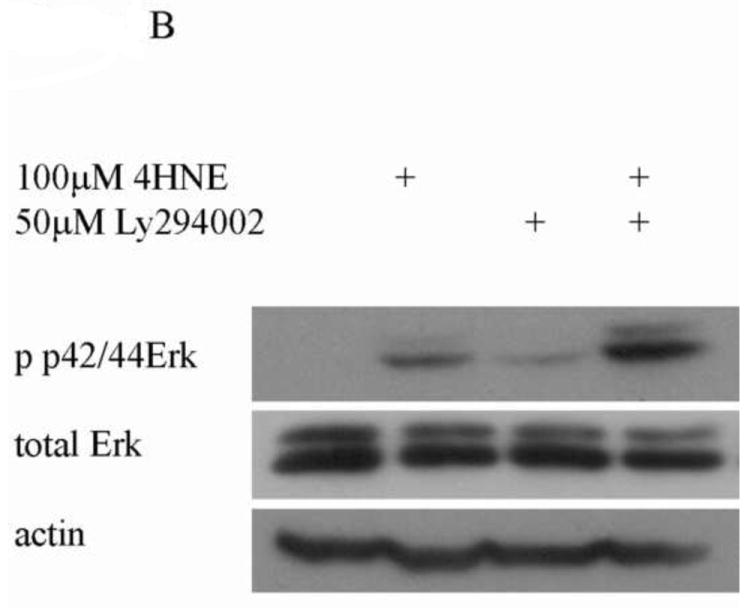
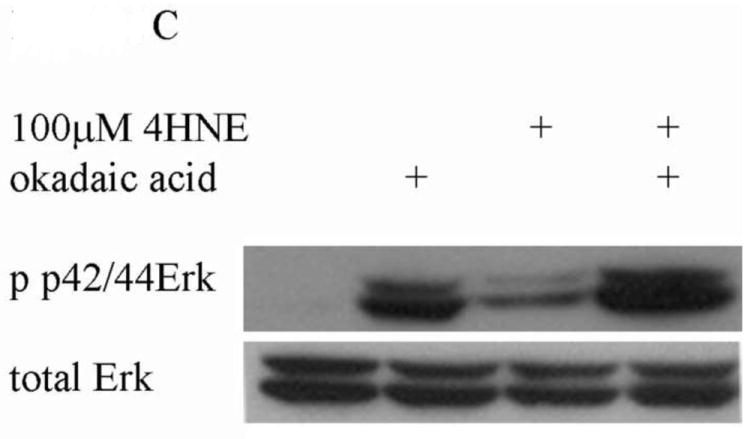
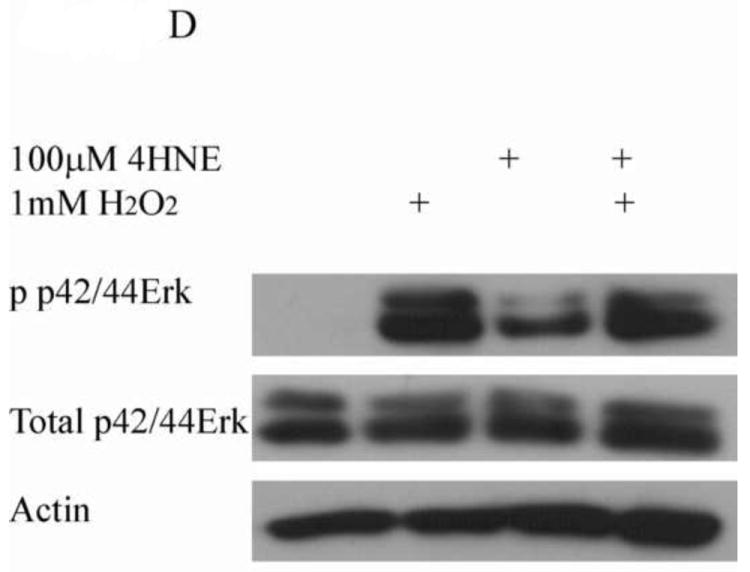
Cells were treated in serum free media with 100μM 4-HNE or control for 60 min following preincubation with (A) U0126 (10μM 30 min), (B) Ly294002 (50μM 60 min) or (C) Okadaic acid (100nM 30 min). (D) Cells were treated in serum free media with 100μM 4-HNE or control for 60 min, washed in serum free media and stimulated with 1mM H2O2 for 5 minutes. After treatment, cells were lysed and Western blotted for phosphorylation of p42/p44 Erk. (E) Quantification of blot depicted in Figure 8d. Statistical analysis was via 1-way analysis of variance with Tukey’s multiple comparison test *p<0.05, **p<0.01, ***p<0.001.
From Figure 6, the pretreatment of 4-HNE followed by hydrogen peroxide stimulation led to a significant decrease in cell proliferation and survival. To determine the role of Erk and Jnk on 4-HNE-mediated decreases in proliferation, cells were treated as in Figure 2 and western blotted for Erk and Jnk phosphorylation. Both hydrogen peroxide and 4-HNE led to an increase in Erk phosphorylation (Figure 9D and 9E). 4-HNE pretreatment did not significantly affect hydrogen peroxide stimulated phosphorylation of Erks. From Figure 10A, B, and C, compared to untreated cells, hydrogen peroxide alone led to a mild increase in p46/p54 Jnk phosphorylation, 4-HNE however significantly increased p46/p54 Jnk phosphorylation. This increase was not significantly affected by the addition of hydrogen peroxide. Combined these data suggest that 4-HNE activates both the Erk and the Jnk pathways in HepG2 cells.
Figure 10. Effects of 4-HNE and hydrogen peroxide on Jnk phosphorylation.
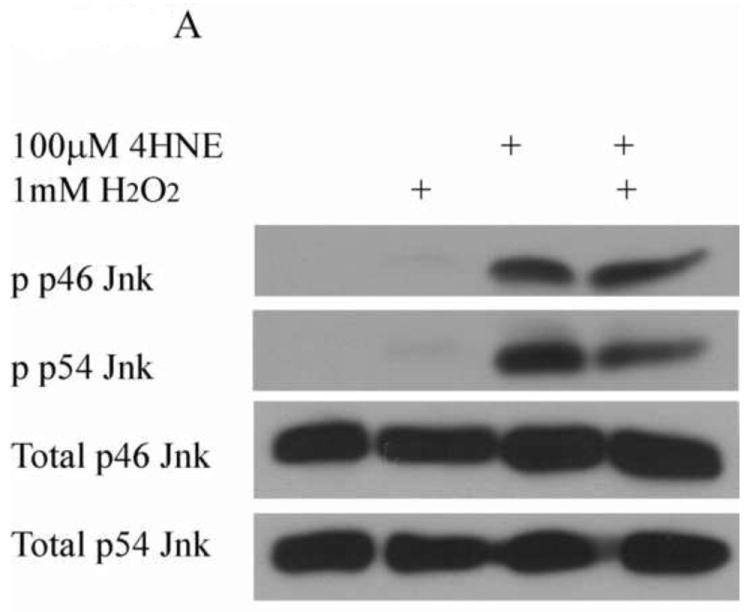
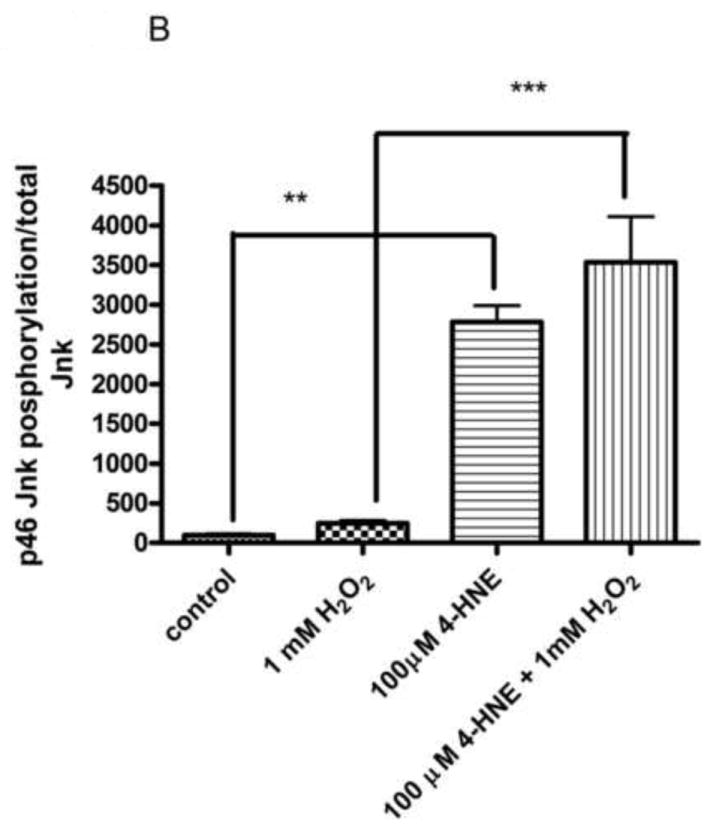
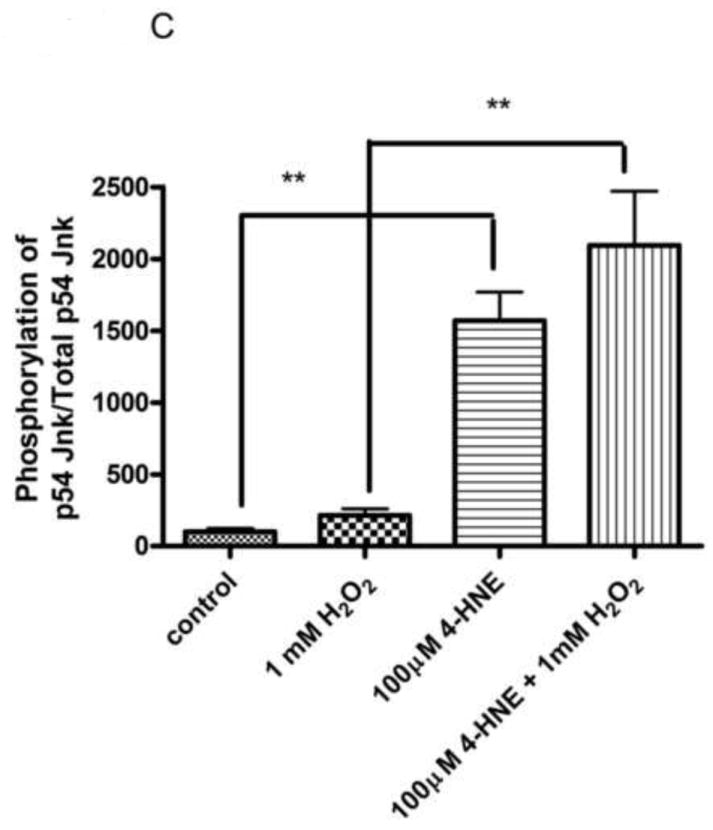
Cells were treated in serum free media with 100μM 4-HNE or control for 60 min, washed in serum free media and stimulated with 1mM H2O2 for 5 minutes. (A) Western blot of p46/54 Jnk phosphorylation. Quantification of p46 (B) and p54 Jnk (C) phosphorylation Western blots were quantified using ImageJ (NIH Freeware) and analyzed using 1-way-ANOVA and GraphPad. **p<0.01, ***p<.001.
Discussion
4-HNE is a product of lipid peroxidation formed under conditions of oxidative stress. Exposure of cells to elevated concentrations of 4-HNE can have pleiotropic effects depending on the concentration and the cell type. For instance, 4-HNE can bind and activate growth factor receptors such as EGFR leading stimulating cell proliferation and atherogenesis [34-36]. Conversely, in Jurkat T-cells, 4-HNE promotes apoptosis via p53 induction [37]. In HepG2 cells, 4-HNE led to a decrease in cell viability, but was not cytotoxic as determined by LDH release or total cellular DNA content [26]. Previous studies have demonstrated that 4-HNE activates JNK signaling leading to an increase in apoptosis [24, 25]. Although the effects of 4-HNE on cell signaling have been extensively studied, most models do not examine the effects of 4-HNE in combination with ROS that are produced concurrently under conditions of chronic inflammation. In the present study, we demonstrate that 4-HNE inhibits H2O2-mediated activation of the Akt pathway by modulating phosphorylation status and via direct modification of Akt1. We further show that in addition to Erk and Jnk activation, total Akt activity is downregulated leading to a decrease in cell viability.
Although activation of the Akt pathway promotes an increase in cell survival and proliferation, the precise isoform of Akt has yet to be determined and could vary from cell type to cell type. Akt1 has been linked to cell proliferation, as demonstrated by the report that deletion of Akt1 led to a decrease in proliferation of cardiomyocytes [38]. Deletion of Akt2 decreased glucose metabolism and sensitized cardiomyocytes to ischemic injury [39]. Ablation of Akt2 however, promotes a decrease in cell proliferation and survival in breast cancer cells [40]. In the present study, we report an increase in both Akt1 and Akt2 phosphorylation and activation following H2O2 treatment. Activated Akt1 levels increased by 10-fold and activated Akt2 levels increased by 30-fold, suggesting both proteins play a role in H2O2-mediated cellular responses. Surprisingly, p-Ser473 Akt1 was decreased to the level of untreated cells in the 4-HNE plus H2O2 treatment, whereas p-Ser474 Akt2 remained 10-Fold higher than untreated cells. This suggests differential regulation of Akt1 and Akt2 by 4-HNE. With respect to p-Thr450 Akt, we find no change in phosphorylation with 4-HNE or H2O2 treatment. Yet we find 4-HNe mediated increases in both Erk and Jnk phosphorylation. This suggests that in HepG2 cells, Thr450 on Akt may not be a target for Jnk.
Hydrogen peroxide has previously been shown to upregulate both the Akt/GSK3β pathway and MDM2 pathway [41, 42]. Treatment of cardiomyocytes with H2O2 increased MDM2 expression and phosphorylation leading to increased protection against H2O2 mediated oxidative stress [43]. This in turn leads to increased degradation of p53 due to its ubiquitination. In human retinal pigment epithelial cells, 1mM H2O2 increased phosphorylation of Akt at Ser473 and GSK3β at Ser9 [42]. In prostate epithelial cells, depletion of mitochondrial DNA led to Akt2 activation, MDM2 phosphorylation and glucose uptake [41]. Previous experiments have indicated that 24h treatment of HepG2 cells with H2O2 leads to a decrease in cell viability and an increase in apoptosis [44]. In the present study, treatment with 1mM H2O2 increased both GSK3β and MDM2 phosphorylation by over 2-fold (Figure 2), and led to a decrease in cell proliferation (Figure 6) but not a decrease in cell survival (Figure 7) or an increase in apoptosis (data not shown). A possible reason for the discrepancy is that Lin et al. treated with 1mM H2O2 for 24h, whereas in our study, cells were treated for 5 min followed by 24h incubation without H2O2. Following 4-HNE pretreatment phosphorylation of both was significantly decreased below levels of untreated cells. Examination of Akt activity in these cells clearly indicates 4-HNE mediated inhibition of H2O2 stimulation of Akt signaling (Figure 3). In summary, preincubation of 4-HNE inhibits H2O2 stimulation of the Akt pathway in HepG2 cells.
β-catenin and Cyclin D1 are key regulators of cell proliferation in HepG2 cells [45]. Both are known to be regulated by the Akt pathway as well as by Erk and Jnk signaling. Cyclin D1 is an important regulator of the G1 to S phase transition and is elevated during cell division and proliferation [20]. In HepG2 cells, Akt, Jnk and Erk all have an effect on levels proliferation [29, 46]. Treatment of HepG2 cells with resveratrol led to decreased proliferation, decreased Akt activation and an increase in Erk phosphorylation [46]. In another study, Inhibition of Jnk signaling protected against adiponectin induced apoptosis and caspase activation [29]. We find that both Jnk and Erk phosphorylation is increased following 4-HNE. Upregulation of the Akt/GSK3β/cyclin D1 signaling pathway promotes cell proliferation [20]. SiRNA knockdown of β-catenin decreases cell growth [47]. Cyclin D1 and β-catenin have also been shown to be regulated by oxidative stress. Increased oxidative stress has been linked to a decrease in cyclin D1 in various lung, prostate and colon cancer cell lines [48]. In previous reports, β-catenin levels increase following 4-HNE exposure in cultured retinal epithelial cells and in MCF-7 cells [17, 49]. Hepatocytes have high concentrations of glutathione providing significant protection against oxidative stress [50]. Treatment of HepG2 cells with H2O2 at concentrations less than 1mM did not lead to an increase in apoptosis, whereas treatment of other cell types such as A549 lung cancer cells with 50-200μM H2O2 led to a decrease in cyclin D1 expression and cell survival [44, 48]. Activation of Akt pathways promote increases in cell growth, proliferation and survival [51]. In our model, H2O2 led to a decrease in proliferation but not cell survival or expression levels of β-catenin and cyclin D1 (Figures 6, 7, 8). In addition, caspase 3 activation was not detected (data not shown). Preincubation with 4-HNE led to a concentration-dependent decrease in both proliferation and the expression of β-catenin and cyclin D1. Examining cell survival, a combinatorial effect between 4-HNE and H2O2 was not evident until concentrations of 4-HNE approached 50-100μM. This effect however was small. In summary, by itself, 4-HNE leads to decreased proliferation as evidenced by downregulation of both cyclin D1 and β-catenin. The addition of H2O2 decreases cell proliferation but does not have a significant effect on cell survival except when in combination with concentrations of 4-HNE greater than 50μM.
Currently, very little is known concerning the isoform of Akt responsible for modulation of cyclin D1 and β-catenin. Recent evidence suggests a role for Akt1 in cyclin D1 expression and ablation of Akt1 but not Akt2 led to a decrease in tumor induction and cyclin D1 expression in mammary epithelia [52]. Although the exact isoform was not examined, treatment of MCF-7 cells with 4-HNE increased both Akt and GSK3β phosphorylation as well as increased β-catenin expression [17]. Treatment of bovine adrenal chromaffin cells with LiCl decreased Akt1 levels and increased β-catenin suggesting a role for Akt1 in β-catenin regulation [53]. In addition, phosphorylation of Akt by the protein kinase CK2 leads to hyperactivation of Akt and upregulation of β-catenin [54]. In a recent report, decreased GSK3β phosphorylation promoted GSK3β activation and degradation of β-catenin [55]. In this system, although Akt is phosphorylated, GSK3β phosphorylation is significantly decreased providing a plausible mechanism for the discrepancy between the studies [11].
In the present study, we demonstrate inhibition of total Akt activity, decreased phosphorylation of Akt1 and inhibition of recombinant myrAkt1 in vitro by 4-HNE. This suggests that in HepG2 cells, as described in Scheme 1, preincubation with 4-HNE is sufficient to inhibit H2O2-mediated stimulation of Akt signaling via both Akt1 and Akt2. The mechanism, however, is subtly different in that 4-HNE directly modifies Akt1 (leading to inhibition of activity) as well as preventing phosphorylation of Akt1 at Ser473 leading to reduced activation of Akt1. PTEN is inhibited and Akt2 is still phosphorylated at Ser474 following 4-HNE pretreatment [9]. Based on previous data, enzymatic activity of Akt2 is inhibited by direct modification of Akt2 by 4-HNE [11]. The inhibition of both Akt isoforms in combination with Erk and Jnk activation leads to a decrease in cyclin D1 expression and β-catenin expression and a decrease in cell proliferation. Furthermore, the inhibition of Akt kinases by 4-HNE during conditions of chronic inflammation and oxidative stress could contribute to decreased cell survival and cell death observed in chronic inflammatory diseases such as ALD and steatohepatitis. How these process are reflected in actual in vivo models of chronic inflammation remains to be elucidated.
Scheme 1. 4-HNE inhibits hydrogen peroxide mediated stimulation of Akt1/2 leading to decreases in cell proliferation.
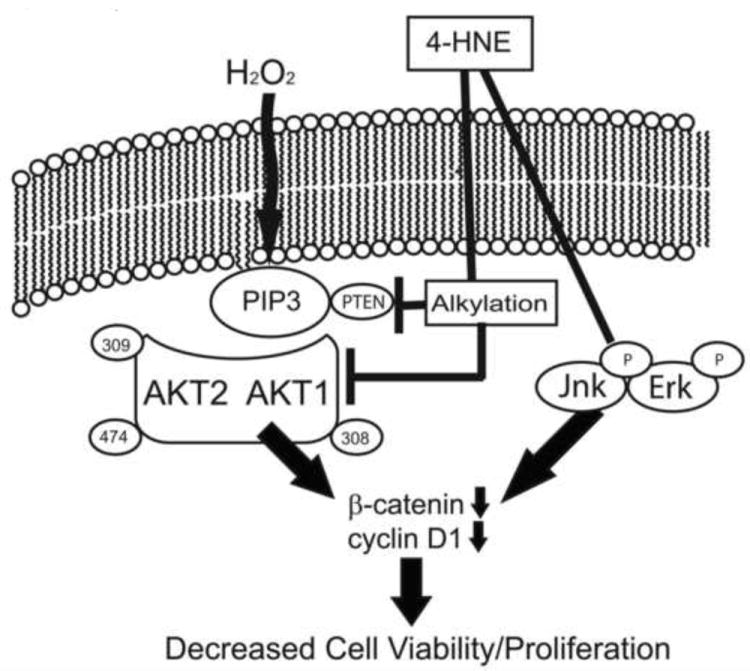
Under normal conditions, H2O2 increases Akt1/2 phosphorylation stimulating cell survival pathways. Under conditions of chronic inflammation, phosphorylation of Akt1 is on Ser473 is abrogated and phosphorylation of Ser474 Akt2 is reduced. Carbonylation of PTEN contributes to increased Akt1 and Akt2 phosphorylation. Both Akt1 and Akt2 are also carbonylated by 4-HNE inhibiting enzymatic activity. 4-HNE-mediated Akt inhibition in combination with Erk and Jnk phosphorylation leading to reduced levels of both cyclin D1 and β-catenin and subsequent reduced cellular proliferation.
Supplementary Material
Highlights.
4-hydroxynonenal inhibits H2O2 mediated Akt activation
Inhibition is due to 4-HNE directly modifying Akt1
4-HNE exerts a combinatorial effect on Erk, Jnk and Akt signaling.
This effect by 4-HNE decreases proliferation and survival in HepG2 cells
Acknowledgments
Funding Sources:
This work was supported by NIH 5F32 AA018613-02 (C.T.S.), 5R37 AA009300-16 (D.R.P.), 5RO1 DK074487-04 (D.R.P.)
Abbreviations
- ALD
alcoholic liver disease
- Erk
extracellular-signal-related kinase
- JNK
c-Jun-N-Terminal Kinase
- GSK3β
glycogen synthase kinase 3 beta
- 4-HNE
4-hydroxy-2-nonenal
- MDM2
ubiquitin ligase murine double minute oncogene 2
- NASH
non-alcoholic steatohepatitis
- PDK-1
phosphoinositide-dependent kinase 1
- PI3K
phosphatidylinositol 3-kinase
- PtdIns (3,4,5) P3
phosphatidylinositol 3,4,5 tris-phosphate
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- ROS
reactive oxidative species
Footnotes
Author Disclosure Statement: No competing financial interests exist for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135–142. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- 2.Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Charlotte F, Piton A, Opolon P, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation in chronic hepatitis C: correlation with pathological features. J Clin Pathol. 1997;50:401–406. doi: 10.1136/jcp.50.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roede JR, Orlicky DJ, Fisher AB, Petersen DR. Overexpression of peroxiredoxin 6 does not prevent ethanol-mediated oxidative stress and may play a role in hepatic lipid accumulation. J Pharmacol Exp Ther. 2009;330:79–88. doi: 10.1124/jpet.109.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roede JR, Stewart BJ, Petersen DR. Decreased expression of peroxiredoxin 6 in a mouse model of ethanol consumption. Free Radic Biol Med. 2008;45:1551–1558. doi: 10.1016/j.freeradbiomed.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol. 2011;251:59–69. doi: 10.1016/j.taap.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 7.Fourcot A, Couchie D, Chobert MN, Zafrani ES, Mavier P, Laperche Y, Brouillet A. Gas6 Deficiency Prevents Liver Inflammation, Steatohepatitis and Fibrosis in Mice. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00311.2010. [DOI] [PubMed] [Google Scholar]
- 8.Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem Res Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N, Petersen DR. PTEN Inhibition by 4-Hydroxynonenal Leads to Increased Akt Activation in Hepatocytes. Mol Pharmacol. 2011 doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner TM, Mullally JE, Fitzpatrick FA. Reactive lipid species from cyclooxygenase-2 inactivate tumor suppressor LKB1/STK11: cyclopentenone prostaglandins and 4-hydroxy-2-nonenal covalently modify and inhibit the AMP-kinase kinase that modulates cellular energy homeostasis and protein translation. J Biol Chem. 2006;281:2598–2604. doi: 10.1074/jbc.M509723200. [DOI] [PubMed] [Google Scholar]
- 11.Shearn CT, Fritz KS, Reigan P, Petersen DR. Modification of Akt2 by 4-Hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011 doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 12.Codreanu SG, Zhang B, Sobecki SM, Billheimer DD, Liebler DC. Global analysis of protein damage by the lipid electrophile 4-hydroxy-2-nonenal. Mol Cell Proteomics. 2009;8:670–680. doi: 10.1074/mcp.M800070-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung SM, Kornelson JC, Al-Alwan M, Marshall AJ. Regulation of phosphoinositide 3-kinase signaling by oxidants: Hydrogen peroxide selectively enhances immunoreceptor-induced recruitment of phosphatidylinositol (3,4) bisphosphate-binding PH domain proteins. Cell Signal. 2007;19:902–912. doi: 10.1016/j.cellsig.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Sun S, Zhou J, Liu J, Lv JH, Yu XQ, Li C, Gong L, Yan Q, Deng M, Xiao L, Ma H, Liu JP, Peng YL, Wang D, Liao GP, Zou LJ, Liu WB, Xiao YM, Li DW. Knockdown of Akt1 Promotes Akt2 Upregulation and Resistance to Oxidative Stress-Induced Apoptosis through Control of Multiple Signaling Pathways Including MDM2-p53-Bak, GSK3beta-MCL-1 and FOXO3A-Bim. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covey TM, Edes K, Coombs GS, Virshup DM, Fitzpatrick FA. Alkylation of the tumor suppressor PTEN activates Akt and beta-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS One. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettazzoni P, Pizzimenti S, Toaldo C, Sotomayor P, Tagliavacca L, Liu S, Wang D, Minelli R, Ellis L, Atadja P, Ciamporcero E, Dianzani MU, Barrera G, Pili R. Induction of cell cycle arrest and DNA damage by the HDAC inhibitor panobinostat (LBH589) and the lipid peroxidation end product 4-hydroxynonenal in prostate cancer cells. Free Radic Biol Med. 2011;50:313–322. doi: 10.1016/j.freeradbiomed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, Kang MB, Jang SH, Qian T, Kim HJ, Kim CH, Kim Y, Kong G. Id-1 activates Akt-mediated Wnt signaling and p27(Kip1) phosphorylation through PTEN inhibition. Oncogene. 2009;28:824–831. doi: 10.1038/onc.2008.451. [DOI] [PubMed] [Google Scholar]
- 21.Stewart BJ, Doorn JA, Petersen DR. Residue-specific adduction of tubulin by 4-hydroxynonenal and 4-oxononenal causes cross-linking and inhibits polymerization. Chem Res Toxicol. 2007;20:1111–1119. doi: 10.1021/tx700106v. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 24.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart BJ, Roede JR, Doorn JA, Petersen DR. Lipid aldehyde-mediated cross-linking of apolipoprotein B-100 inhibits secretion from HepG2 cells. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Z, Fan X, Jiao Y, Ban K. Mammalian target of rapamycin regulates expression of beta-catenin in hepatocellular carcinoma. Hum Pathol. 2010;42:659–668. doi: 10.1016/j.humpath.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Witzel II, Koh LF, Perkins ND. Regulation of cyclin D1 gene expression. Biochem Soc Trans. 38:217–222. doi: 10.1042/BST0380217. [DOI] [PubMed] [Google Scholar]
- 29.Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762–1773. 1773 e1761–1765. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 31.Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol. 2009;34:1749–1757. doi: 10.3892/ijo_00000306. [DOI] [PubMed] [Google Scholar]
- 32.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 34.Auge N, Garcia V, Maupas-Schwalm F, Levade T, Salvayre R, Negre-Salvayre A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler Thromb Vasc Biol. 2002;22:1990–1995. doi: 10.1161/01.atv.0000043453.21629.3b. [DOI] [PubMed] [Google Scholar]
- 35.Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol Aspects Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 36.Akiba S, Kumazawa S, Yamaguchi H, Hontani N, Matsumoto T, Ikeda T, Oka M, Sato T. Acceleration of matrix metalloproteinase-1 production and activation of platelet-derived growth factor receptor beta in human coronary smooth muscle cells by oxidized LDL and 4-hydroxynonenal. Biochim Biophys Acta. 2006;1763:797–804. doi: 10.1016/j.bbamcr.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang Z, Zhang Q, Feng Q, Xu J, Teng T, Luan Q, Shan C, Hu Y, Hemmings BA, Gao X, Yang Z. Deletion of Akt1 causes heart defects and abnormal cardiomyocyte proliferation. Dev Biol. 2010;347:384–391. doi: 10.1016/j.ydbio.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 39.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santi SA, Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One. 2011;6:e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro L, Arbini AA, Yao JL, di Sant’Agnese PA, Marra E, Greco M. Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3K/Akt2. Cell Death Differ. 2009;16:571–583. doi: 10.1038/cdd.2008.178. [DOI] [PubMed] [Google Scholar]
- 42.Yang P, Peairs JJ, Tano R, Jaffe GJ. Oxidant-mediated Akt activation in human RPE cells. Invest Ophthalmol Vis Sci. 2006;47:4598–4606. doi: 10.1167/iovs.06-0140. [DOI] [PubMed] [Google Scholar]
- 43.Pikkarainen S, Kennedy RA, Marshall AK, Tham el L, Lay K, Kriz TA, Handa BS, Clerk A, Sugden PH. Regulation of expression of the rat orthologue of mouse double minute 2 (MDM2) by H(2)O(2)-induced oxidative stress in neonatal rat cardiac myocytes. J Biol Chem. 2009;284:27195–27210. doi: 10.1074/jbc.M109.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CJ, Ho HY, Cheng ML, You TH, Yu JS, Chiu DT. Impaired dephosphorylation renders G6PD-knockdown HepG2 cells more susceptible to H(2)O(2)-induced apoptosis. Free Radic Biol Med. 2010;49:361–373. doi: 10.1016/j.freeradbiomed.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Lee TK, Man K, Ho JW, Sun CK, Ng KT, Wang XH, Wong YC, Ng IO, Xu R, Fan ST. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis. 2004;25:2397–2405. doi: 10.1093/carcin/bgh250. [DOI] [PubMed] [Google Scholar]
- 46.Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp Toxicol Pathol. 2011;63:167–173. doi: 10.1016/j.etp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Wang XH, Meng XW, Sun X, Liu BR, Han MZ, Du YJ, Song YY, Xu W. Wnt/beta-catenin signaling regulates MAPK and Akt1 expression and growth of hepatocellular carcinoma cells. Neoplasma. 2011;58:239–244. doi: 10.4149/neo_2011_03_239. [DOI] [PubMed] [Google Scholar]
- 48.Lim JH, Lee YM, Chun YS, Park JW. Reactive oxygen species-mediated cyclin D1 degradation mediates tumor growth retardation in hypoxia, independently of p21cip1 and hypoxia-inducible factor. Cancer Sci. 2008;99:1798–1805. doi: 10.1111/j.1349-7006.2008.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou T, Zhou KK, Lee K, Gao G, Lyons TJ, Kowluru R, Ma JX. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia. 2011;54:459–468. doi: 10.1007/s00125-010-1943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunati AM, Pagano MA, Bindoli A, Rigobello MP. Thiol redox systems and protein kinases in hepatic stellate cell regulatory processes. Free Radic Res. 2010;44:363–378. doi: 10.3109/10715760903555836. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 52.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 53.Nemoto T, Kanai T, Yanagita T, Satoh S, Maruta T, Yoshikawa N, Kobayashi H, Wada A. Regulation of Akt mRNA and protein levels by glycogen synthase kinase-3beta in adrenal chromaffin cells: effects of LiCl and SB216763. Eur J Pharmacol. 2008;586:82–89. doi: 10.1016/j.ejphar.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 54.Ponce DP, Maturana JL, Cabello P, Yefi R, Niechi I, Silva E, Armisen R, Galindo M, Antonelli M, Tapia JC. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of beta-catenin transcriptional activity. J Cell Physiol. 2010;226:1953–1959. doi: 10.1002/jcp.22527. [DOI] [PubMed] [Google Scholar]
- 55.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


