Abstract
Our previous studies had revealed that the dysregulation of manganese superoxide dismutase (SOD2) expression was a frequent event in tongue squamous cell carcinoma (TSCC) and may be associated with enhanced metastatic potential. To further evaluate the mechanism of SOD2-mediated metastasis in TSCC, TSCC cell lines with different metastatic potential (i.e., the highly metastatic UM1 line and the UM2 line, which displays fewer metastases) were used. Compared to UM2 cells, UM1 cells exhibited significantly higher SOD2 activity and intracellular H2O2, higher protein levels of Snail, MMP-1 and pERK1/2, lower protein levels of E-cadtherin, and not difference of catalase activity. Upon knockdown of SOD2 by RNA interference, UM1 cells displayed significantly reduced migration and invasion abilities, reduced activities of SOD2, lower intracellular H2O2, decreased protein levels of Snail, MMP-1 and pERK1/2, and increased protein levels of E-cadtherin. Migration and invasion ability of UM2 and SOD2 shRNA-transfected UM1 cells were enhanced by H2O2 treatment and accompanied by increased protein levels of Snail, MMP-1 and pERK1/2, and decreased protein levels of E-cadtherin. Moreover the migration and invasion ability of UM1 cells were decreased after catalase treatment. Thus, we conclude that the SOD2-dependent production of H2O2 contributes to both the migration and invasion of TSCC via the Snail signaling pathway through increased Snail, MMP-1 and pERK1/2 protein levels, and the repression of the E-cadtherin protein.
Keywords: SOD2, tongue squamous cell carcinoma, metastasis, Snail, H2O2
Introduction
Despite the tremendous improvements in surgery, radiotherapy and chemotherapy over the last decade, the prognosis for patients with tongue squamous cell carcinoma (TSCC) has remained relatively unchanged for the past 3 decades[1]. This is because patients continue to succumb to metastatic disease at regional and distant sites. Improving patient survival requires an increased understanding of tumor metastasis to allow for early disease detection and the development of targeted therapies. In our previous study, we identified that manganese superoxide dismutase (SOD2) protein level was significantly increased in TSCC with lymph node metastasis[2, 3]. These results suggested that elevated SOD2 levels may be associated with lymph node metastasis in TSCC and may provide predictive values for diagnosing metastasis.
Superoxide dismutases (SODs) are a family of antioxidant enzymes responsible for the detoxification of superoxide anion free radicals, such as H2O2, that will be further removed by catalases or glutathione peroxidases[4]. Of the 3 major forms of SODs, manganese superoxide dismutase (SOD2, or Mn-SOD, which is located in the mitochondria) is crucial for cellular survival because the mitochondria are the major producer of superoxide[5]. The role of SOD2 in carcinogenesis has been widely studied[2-4, 6-9]. Indeed, many studies identified that increased SOD2 levels are related to several cancer types and are associated with metastasis and poor prognosis[2-4, 8, 9]. Although intracellular SOD activity was demonstrated to be involved in cancer metastasis and invasion, the mechanistic rationale for the increased metastatic capacity of tumor cells overexpressing SOD2 is still ambiguous and needs to be further investigated[4].
Reactive oxygen species (ROS, including the superoxide, H2O2) are proposed to be involved in tumor metastasis, which is a complicated processes that includes epithelial-to-mesenchymal transition, migration, invasion of the tumor cells and angiogenesis around the tumor lesion[10]. Under normal physiological conditions, the steady-state concentrations of H2O2 are well within the buffering capacity of the mitochondrial glutathione redox system. However, when SOD2 levels increase in cancer cells, the glutathione buffering capacity of the mitochondria may be overwhelmed by H2O2. Accumulating evidence indicates that the intracellular redox state plays an important role in both cellular signaling transduction and gene expression[4]. Several studies found that the SOD2-dependent production of H2O2 leads to increased expression of MMP family members and that there is a strong correlation between increased MMP levels and enhanced metastasis[10-12]. Additional studies will be needed to fully understand the role(s) of the redox state and SOD2 in TSCC.
Members of the Snail family (e.g., Snai1 and Snai2) play important roles in cancer progression[13]. Researchers discovered that Snail promotes invasion in many types of cancers, including breast cancer[14], pancreatic cancer[15], salivary adenoid cystic carcinoma[16], gastric cancer[17] and oral cancer[9]. Our recent study, using two independent TSCC patient cohorts, confirmed that overexpression of Snai2 is a frequent event in TSCC and is associated with lymph node metastasis and reduced overall survival. As further confirmation, knockdown of Snai2 suppressed cell migration and invasion in vitro[18]. Although Snail expression was associated with invasion and lymph node metastasis in tongue cancer[18], the relationship between Snail and SOD2-dependent production of H2O2 in TSCC metastasis has not been reported.
In this study, we first tested whether the SOD2-dependent production of H2O2 leads to increased migration and invasion of TSCC in vitro. We then investigated the role of Snail family members in SOD2-mediated metastasis and TSCC progression. Our findings suggest that increases in mitochondrial H2O2 by SOD2 can enhance the invasive and migratory properties of TSCC and that Snail signaling may be involved in this process.
Materials and Methods
Cell culture and reagents
Human TSCC cell lines (UM1, UM2, and Tca8113) were maintained in DMEM/F12 containing 10% fetal bovine serum (FBS), 1,000 units/mL penicillin and 500 μg/mL streptomycin in a 37°C incubator with 5% CO2. UM1 and UM2 are paired cell lines from a single TSCC patient, which exhibit different metastatic potential. Indeed, UM1 is more aggressive than UM2 in terms of cell invasion[19]. Another pair of different metastatic potential salivary adenoid cystic carcinoma (SACC) cell lines SACC-83 and SACC-LM was used in this study. SACC-LM cell line with higher lung-metastatic rate was generated from SACC-83[20].
Plasmid construction and transient transfection
The pGPU6/GFP/Neo shRNA expression vector (GenePharma, Shanghai, China) containing an eGFP sequence was used to generate the plasmid vector, pSOD2shRNA. The SOD2 shRNA contains a complement nucleotide sequence (agttcaatggtggtggtcatatcaa, GenBank NM_000636) that is separated by a 9-nucleotide, non-complementary spacer (ttcaagaga). A control vector (non gene-targeting) was constructed in the same way using a nucleotide sequence (ttctccgaacgtgtcacgt). These sequences were confirmed using nucleotide BLAST to ensure that there was no homology with any other known human gene. The annealed sequences were ligated into the pGPU6/GFP/Neo backbone after digestion with BamHI and BbsI. All vector constructs were confirmed by DNA sequencing.
The constructed plasmids were transiently transfected into UM1 cells using Lipofectamine Plus reagent (Invitrogen, CA, USA), according to the manufacturer's instructions[21]. Transfected cells were subsequently used in the following experiments.
SOD Activity
SOD2 activity was determined by measuring the ability of SOD to inhibit xanthine/xanthine oxidase-induced cytochrome c reduction in the presence of 5 mmol/L potassium cyanide (KCN), which inhibits SOD1 and SOD3 activities[22]. One unit of SOD2 activity was defined as the amount of SOD2 needed to exhibit 50% dismutation of the produced superoxide radical at 25°C. The final enzyme activity was calculated by normalizing the results to the total protein concentration of the whole protein extract, as determined by the Bio-Rad protein assay (Richmond, CA, USA).
Catalase activity
Catalase activity was detected using a catalase analysis kit (Beyotime Biotechnology, China) according to the manufacturer's instructions. Briefly, the cell lysates were treated with excess hydrogen peroxide for an indicated time, and then the remaining hydrogen peroxide (not decomposed by catalase) was coupled with a substrate that on treatment with peroxidase produced N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinonemonoimine, which has an absorption maximum at 520 nm and was quantified spectrophotometrically. Catalase activity was then calculated from the assay results.
Measurement of intracellular H2O2
Measurement of intracellular H2O2 were performed as described previously[23]. The H2O2 concentration was determined using the PeroXOquant Quantitative Peroxide Assay Kit (Pierce, IL, USA), according to the manufacturer's instructions. H2O2 oxidation causes the formation of a purple-colored complex that can be read on a spectrophotometer at 560 nm.
Wound healing assay
Wound healing assay were performed as described previously[12], Briefly, cells were grown to confluence and “wounded” by dragging a 1 mL pipette tip through the monolayer. Cells were allowed to migrate for 24 h. Images were taken at time points 0 and 24 h post-wounding using a Nikon Diaphot TMD inverted microscope (4×). The relative distance traveled by the leading edge from 0 to 24 h was assessed using Photoshop 7.0 software (n = 6).
Transwell invasion assay
Transwell invasion assay were performed as described previously[12], Briefly, Biocoat Matrigel invasion chamber inserts (BD Biosciences, NJ, USA) were equilibrated for 2 h at 37°C in serum-free medium. Cells were seeded in serum-free medium in the upper chamber and allowed to invade through the Matrigel to the lower chamber for 24 h. Cells were then fixed with a solution of 3% formaldehyde/PBS for 15 min. Cells on the bottom surface of the filter were rinsed and permeabilized with 1% Triton X-100 in PBS for 20 min, stained with DAPI in the dark and visualized under a fluorescent microscope. Three random fields were captured at 10× magnification (n = 3). The number of cells on the bottom surface was compared between groups.
Cell proliferation assays
Proliferation was measured using an microculture tetrazolium assay (MTT) assay, as described previously[24]. Briefly, cells were seeded in quadruplicate replicates in 96-well plates at a density of 5×103 cells per well. Cell proliferation was analyzed at 24 hr or 48 hr by incubating the cells with 1 mg/ml tetrazolium salt MTT (Sigma). Absorbance (A) at 570 nm was measured and cell inhibition rate was calculated as (1-Atreated/Acontrol) ×100%.
Western Blot Analysis
Western blots were performed as described previously[21] using antibodies specific to SOD2, Catalase, ERK1/2, p-ERK1/2, Snail family members (Snai1 and Snai2), E-cadtherin, MMP1 (Cell Signaling Technology, Beverly, MA, USA) and beta-actin (Sigma-Aldrich, MO, USA).
Statistics
Data were analyzed using either the Student's t-test to determine significance between two variables or by a one-way ANOVA to calculate significance when there were more than two variables. A P-value of less than 0.05 was considered to be significant.
Results
SOD2 overexpression is related to the migration and invasion of tongue squamous cell carcinoma
To evaluate the relationship between SOD2 activity and migration and invasion, Three TSCC cell lines were used: including a pair of different metastatic potential cell lins (UM1 and UM2), and Tca8113 cell line. The migration and invasion abilities of UM1 were significantly higher than those of UM2 and Tca8113, as detected by wound healing and transwell invasion assays (Figs. 1A and 1B). The protein levels and activity of SOD2 in UM1 cells were significantly higher than that of UM2 and Tca8113 cells (Fig. 1C). Not obviously difference of protein level and activity of catalase was found between UM1 and UM2 cells (Fig. 1D). The UM1 cells displayed an approximate 2.2-fold increase in H2O2 production compared to UM2 cells (Fig.1E). Moreover, the cell proliferation rate of UM1 was higher than that of UM2 (Fig. 1F). Similar results were observed in another pair of different metastatic potential cell line (SACC-83/SACC-LM), in which higher metastatic potential SACC-LM cells has higher protein levels of SOD2, intracellular H2O2, and migration and invasion ability than lower metastatic potential SACC-83 cells, not difference was found in the catalase activity between this two cell lins (Figure S1).These results implicate elevated SOD2 expression, concomitant with an increase in H2O2 production, to the aggressiveness of TSCC.
Fig. 1. SOD2 overexpression is related to the migration and invasion of TSCC.
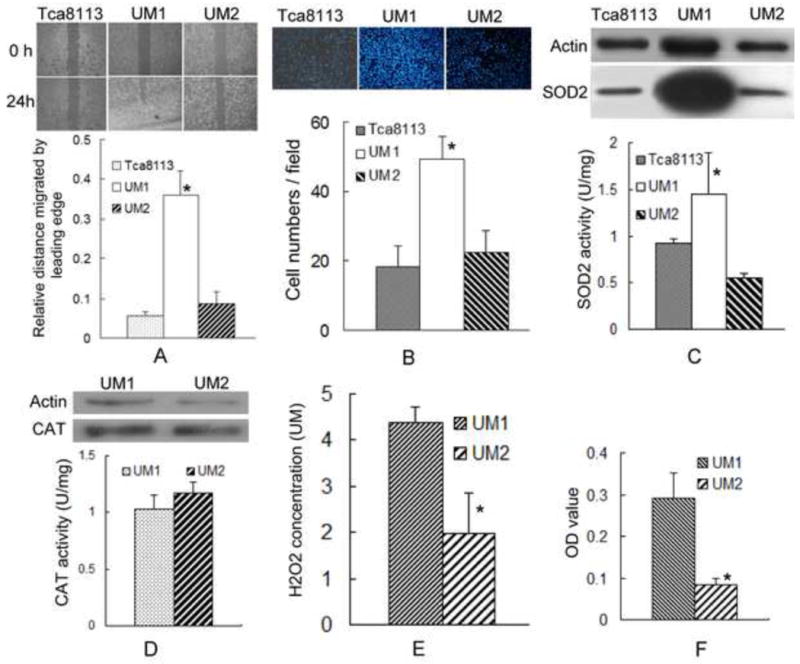
(A) The migration ability of TSCC cells was assessed using a wound healing assay. The confluent monolayer was “wounded” and allowed to migrate for 24 h. Quantification of distance migrated from time 0 to 24 h was calculated (n = 6). UM1 cells displayed significantly more migration than UM2 or Tca8113. *: P< 0.05.
(B) The invasion ability of TSCC cells was assessed by a transwell invasion assay. Cells were seeded in a BD Biocoat Matrigel invasion chamber and allowed to invade through the Matrigel toward the lower chamber for 24 h. The number of cells invading through the chamber was quantified. UM1 cells displayed a significantly higher invasion rate than UM2 or Tca8113. *: P< 0.05.
(C) Levels of SOD2 protein and activity were assessed by western blot and SOD activity assay, respectively. Significant increases in SOD2 protein levels and activities were observed in UM1 cells compared to UM2 or Tca8113 cells. *: P< 0.05.
(D) Levels of CAT (catalase) protein and activity were assessed by western blot and CAT activity assay, respectively. CAT protein levels and activities were not difference between UM1 and UM2 cells.
(E) H2O2 concentrations were measured as described in the Materials and Methods section. UM1 cells displayed significantly higher H2O2 production compared to UM2 cells. *: P< 0.05.
(F) Cell proliferation was measured using an MTT assay. The cell proliferation rate of UM1 was significantly higher than UM2. *: P< 0.05.
SOD2 knockdown inhibits the migration and invasion abilities of tongue squamous cell carcinoma
To further characterize the role of SOD2 in aiding metastasis, we knockdown the expression of SOD2 by RNA interference. Both the activity and protein level of SOD2 were significantly decreased in UM1 cells after transfecting with the SOD2 shRNA (Fig. 2A). UM1 cells transfected with SOD2 shRNA displayed decreased migration and invasion abilities compared to the control vector transfected cells (Figs. 2B and 2C). Furthermore, SOD2 knockdown resulted in reduced H2O2 production in UM1 cells (Fig. 2D). We also found that UM1 cells transfected with SOD2 shRNA displayed a reduced cell proliferation rate (Fig. 2E). These results implicate SOD2 knockdown, concomitant with a decrease in H2O2 production, with a reduction in the aggressiveness of TSCC.
Fig. 2. SOD2 knockdown inhibits both the migration and invasion abilities of TSCC.
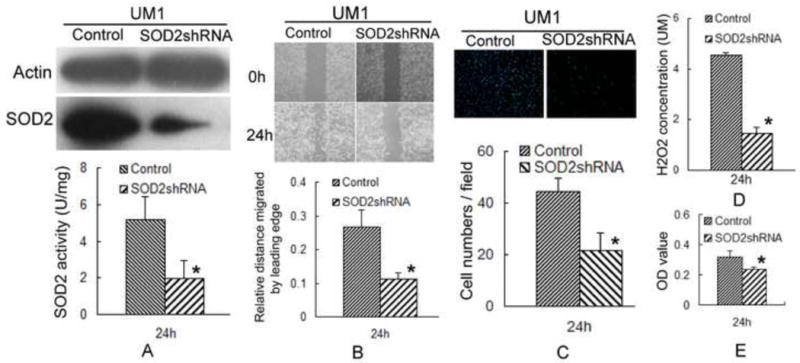
To characterize the role of SOD2 in aiding metastasis, the plasmid containing SOD2 shRNA was transiently transfected into UM1 cells using Lipofectamine Plus reagent. Cells were tested 24 h post-transfection.
(A) Significant reduction of SOD2 protein levels and activities were observed in the SOD2 shRNA-transfected UM1 cells compared to the vector control transfected cells. *: P< 0.05.
(B, C) SOD2 knockdown inhibited the migration and invasion of UM1 cells. *: P< 0.05.
(D) UM1 cells displayed a significant decrease in H2O2 production after transfecting with the SOD2 shRNA. *:p< 0.05.
(E) The cell proliferation rate of the UM1 line was significantly inhibited after transfecting with the SOD2 shRNA. *: P< 0.05.
SOD2-dependent production of H2O2-induced migration and invasion of tongue squamous cell carcinoma
To further confirm that SOD2-dependent H2O2 production induces migration and invasion of TSCC, UM2 cells and SOD2 shRNA-transfected UM1 cells, which display both decreased SOD2 activity and H2O2 production versus UM1 cells, were treated with H2O2. After treating with 100 μmol/L H2O2, both migration and invasion were significantly increased in UM2 cells and SOD2 shRNA-transfected UM1 cells (Figs. 3A and 3B). Moreover, UM2 cells and SOD2 shRNA-transfected UM1 cells displayed increased the cell proliferation rates after treatment with H2O2 (Fig. 3C). Similar results were observed in SACC-83 cells, both migration and invasion were significantly increased in SACC-83 cells after treating with 100 μmol/L H2O2 (Figure S2).
Fig. 3. SOD2-dependent production of H2O2 increases both the migration and invasion of TSCC.
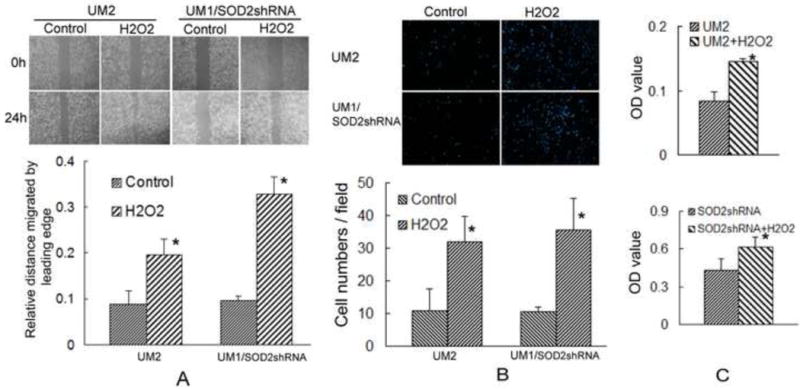
To confirm that H2O2 production induces both the migration and invasion abilities of TSCC, UM2 cells and SOD2 shRNA-transfected UM1 cells (24 h) were treated with H2O2 for 24 h.
(A, B) The migration and invasion abilities of UM2 cells and SOD2 shRNA-transfected UM1 cells were significantly increased after treating with 100 μM H2O2 for 24 h. *: P< 0.05.
(C) The cellular proliferation rates of UM2 cells and SOD2 shRNA-transfected UM1 cells were significantly increased after treating with 100 μM H2O2 for 24 h. *: P< 0.05.
To gain more insight into the role of H2O2, UM1 cells were treated with catalase, which can removal of H2O2. The migration and invasion ability of UM1 were significantly inhibited after treated with 600U catalase(Fig. 4). Similar results were found in SACC-LM cells(Fig. 2S). These results suggest that H2O2 production may be required for the migration and invasion effects induced by SOD2.
Fig. 4. Catalase inhibits both the migration and invasion of TSCC.
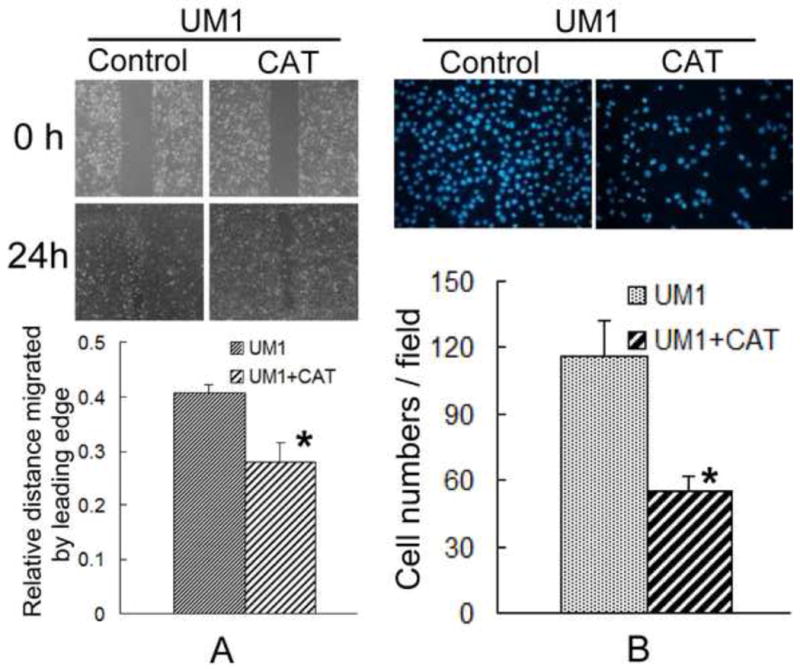
To gain more insight into the role of H2O2, UM1 cells were treated with catalase, which can removal of H2O2. The migration and invasion ability of UM1 were significantly inhibited after treated with 600U catalase.
Snail signaling contributes to SOD2-induced migration and invasion of tongue squamous cell carcinoma
Increased expression and activity of SOD2 was reported to enhance the expression of several metastasis-related genes[10, 25]. UM1 cells, which exhibit higher SOD2 activity, displayed an increase in Snai1, Snai2, MMP-1, ERK1/2 and pERK1/2 protein levels and a decrease in E-cadtherin protein levels compared to UM2 cells (Fig. 5A). However, after knockdown the expression of SOD2 in UM1 cells, the protein levels of snai1, snai2, MMP-1, ERK1/2 and pERK1/2 were significantly decreased, and the protein levels of E-cadtherin were significantly increased (Fig. 5B).
Fig. 5. Snail signaling contributes to SOD2-induced migration and invasion of TSCC.
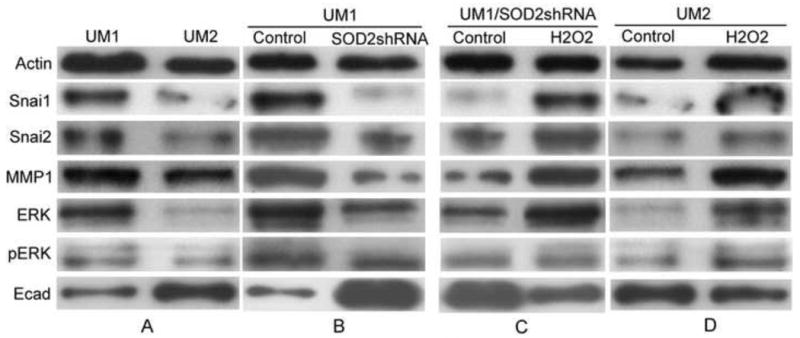
To characterize the role of Snail signaling in SOD2-induced metastasis of TSCC, western blot analysis was used with actin as the loading control. Plasmids containing SOD2 shRNA were transiently transfected into UM1 cells using Lipofectamine Plus reagent. Cells were tested 24 h post-transfection. The UM2 cells or SOD2 shRNA-transfected UM1 cells were treated with 100μM H2O2 for 24 h.
(A) UM1 cells displayed an increase in Snai1, Snai2, MMP-1, ERK1/2 and pERK1/2 protein levels, and decreased protein levels of E-cadtherin compared to UM2 cells.
(B) UM1 cells displayed decreased snai1, snai2, MMP-1, ERK1/2 and pERK1/2 protein levels and increased E-cadtherin protein levels upon SOD2 knockdown.
(C, D) The addition of H2O2 increased the protein levels of Snai1, Snai2, MMP-1, ERK1/2 and pERK1/2 and decreased protein levels of E-cadtherin in both UM2 cells and the SOD2 shRNA-transfected UMl cells.
To evaluate whether an increase in H2O2 production in response to enhanced SOD2 was responsible for the change of Snail signaling-related genes, UM2 cells and SOD2 shRNA-transfected UM1 cells were treated with H2O2. The addition of H2O2 led to a corresponding increase in Snai1, Snai2, MMP-1, ERK1/2 and pERK1/2 protein levels, and a decrease in E-cadtherin protein levels in both the UM2 cells and the SOD2 shRNA-transfected UM1 cells (Figs. 5C and 5D). These results indicated that elevated SOD2 levels can modulate Snail signaling-related genes in an H2O2-dependent fashion.
Discussion
Clinically significant elevations in SOD2 expression are associated with increased tumor invasion and metastasis in certain cancer types[2, 3, 26, 27]. Malafa et al. determined that SOD2 expression is increased in 93% of metastatic versus 44% of nonmetastatic gastric tumors[26]. We previously determined that SOD2 expression is consistently elevated in tongue cancer specimens and that SOD2 expression is significantly higher in lymph node metastases compared to their paired primary tumors[2, 3]. Elevated levels of SOD also correlated with increased metastasis in in vitro studies[12, 28]. In two bladder cancer cell lines, the highly metastatic bladder tumor cell line (253J B-V) displayed significantly higher SOD2 protein and activity levels compared to the parental (253J) cell line, which displayed far fewer metastases[28]. Similarly, in this study we found that UM1 cells displayed significantly higher migration and invasion abilities and both higher SOD2 protein and activity levels, compared to the UM2 and Tca8113 cell line; however, the migration and invasion abilities were inhibited in UM1 cells upon SOD2 knockdown. These findings suggest that increased SOD2 activity contributed to both the invasive and migratory capacity of TSCC.
Several researchers demonstrated that SOD2 overexpression can promote metastasis, which may be dependent on the ability of the cell to detoxify H2O2[10, 28, 29]. These studies identified that SOD2-overexpressing cell lines increased the steady-state concentration of H2O2 and increased the migration and invasion abilities of cancer cells[10, 28, 29]. In agreement with these published results, we found that the highly metastatic UM1 cells displayed both higher SOD2 activity and intracellular H2O2 than the UM2 cells and that the SOD2 activity, intracellular H2O2 and migration and invasion abilities were significantly reduced in UM1 cells upon SOD2 knockdown. Furthermore, the migration and invasion abilities were increased when either UM2 cells or SOD2 shRNA-transfected UM1 cells were treated with H2O2. In this study we also found that the catalase protein lavel and activity, which can removal of H2O2, were not difference between UM1 and UM2 cells. After treated with catalase the migration and invasion ability of UM1 were significantly inhibited. These results implicate that SOD2-mediated metastasis of TSCC may be dependent on H2O2. the accumulated intracellular H2O2 must be related to higher SOD2 activity, but not catalase.
Elevated levels of SOD2 correlate with invasion and metastasis of carcinomas; however, the mechanistic rationale requires further investigation. Researchers revealed that SOD2-dependent increases in the steady-state levels of H2O2 led to extracellular signal-regulated kinase (ERK1/2) activation and subsequent downstream transcriptional increases in matrix metalloproteinases (MMPs)[10, 25], which play an important role in tumor progression and metastasis[30]. In the present study, we determined that the protein expression levels of ERK1/2, pERK1/2 and MMP1 were higher in UM1 cells (which has higher SOD2 activity and intracellular H2O2) than in UM2 cells (which display lower SOD2 activity and intracellular H2O2), but these protein levels were decreased in UM1 cells after transfecting with SOD2 shRNA (decreased SOD2 activity and intracellular H2O2). After treating with H2O2, the expression levels of ERK1/2, pERK1/2 and MMP1 were increased in both the UM2 cells and the SOD2 shRNA-transfected UM1 cells. These findings indicate that SOD2-dependent H2O2 production contributes to the activation of ERK1/2-MMP-1 signaling and increases both the invasive and metastatic potential of TSCC.
Members of the Snail family (including snai1 and snai2) play important roles in cancer invasion in many types of cancer[9, 14-17, 31, 32]. Snail was previously shown to be involved in ERK1/2-MMP signaling. Indeed, there was a reciprocal relationship between the MMPs and Snail, such that Snail can induce MMPs and MMPs can promote Snail expression[31]. Silencing Snai1 induces a decrease in MMP9 concomitant with reduced invasive behavior of MDA-MB-231 cells and oral cancer cells in vitro[9, 33]. Snail was also found to modulate the expression of E-cadherin, which is an adhesion molecule associated with tumor invasion and metastasis[31, 34, 35]. Snail bind to E-boxes in the E-cadtherin promoter to repress gene transcription and induce cell migration and invasion[32]. In many human cancers, there is an inverse relationship between E-cadherin and Snail expression[9, 13, 18, 36-39]. Although Snail was associated with invasion and lymph node metastasis in many human cancers; the role of Snail in SOD2-induced TSCC invasion had not been evaluated. Here, we found both Snai1 and Snai2 were highly expressed in metastatic UM1 cells (higher SOD2 activity and intracellular H2O2) compared with UM2 cells. Upon SOD2 knockdown in UM1 cells, the intracellular H2O2 levels were decreased, and the expression of both Snai1 and Snai2 were inhibited; however, after adding H2O2 to either the UM2 cells or the SOD2 shRNA-transfected UM1 cells, the expression levels of both Snai1 and Snai2 were significantly increased. Furthermore, the protein levels of Snail were positively correlated to the protein levels of MMP-1 and ERK1/2, pERK1/2, and inversely correlated to E-cadherin. These results indicated that SOD2-dependent H2O2 production may activate Snail signaling and increase the invasive and metastatic capacity of TSCC.
In summary, our study confirmed that the SOD2-dependent production of H2O2 contributes to both the migration and invasion abilities of TSCC. This involves Snail signaling, including the increased expression of Snail, MMP-1 and ERK1/2, and the repression of E-cadtherin expression (Fig. 6).
Fig. 6. Potential mechanisms for SOD2-mediated TSCC metastasis in association with Snail signaling.
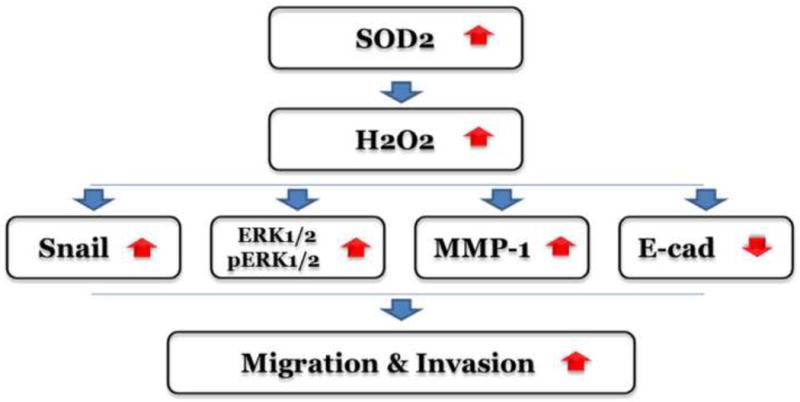
Sod2 is activated by a variety of stimuli leading to an increase in the steady-state production of H2O2. H2O2 activates the Snail-ERK1/2-MMP1 signaling pathway resulting in TSCC metastasis.
Supplementary Material
Highlights.
Higher metastatic cells exhibited higher SOD2 activity and intracellular H2O2.
Knockdown SOD2 inhibited migration and invasion and reduced intracellular H2O2.
H2O2 treatment increased migration and invasion ability of TSCC.
TSCC exhibited not different CAT which can inhibit metastasis of TSCC.
SOD2-dependent production of H2O2 induced metastasis via Snail signaling.
Acknowledgments
This work was supported in part by grants from National Nature Science Foundation of China (NSFC81072228), Guangdong Natural Science Foundation (10151008901000093, S2011020002325), International Cooperative Project of Science and Technology of Guangdong Province (1011420600001), and NIH PHS grants (CA139596 and CA139592).
Abbreviations
- SOD2
manganese superoxide dismutase
- CAT
catalase
- TSCC
tongue squamous cell carcinoma
- RNA
Ribonucleic Acid
- MMPs
matrix metalloproteinases
- ERK
extracellular signal-regulated kinase
- pERK
phosphorylated extracellular signal-regulated kinase
- shRNA
short hairpin RNA
- H2O2
Hydrogen peroxide
- ROS
reactive oxygen species
- SACC
salivary adenoid cystic carcinoma
Footnotes
Conflict of interest statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kokemueller H, Rana M, Rublack J, Eckardt A, Tavassol F, Schumann P, Lindhorst D, Ruecker M, Gellrich NC. The Hannover experience: surgical treatment of tongue cancer--a clinical retrospective evaluation over a 30 years period. Head Neck Oncol. 2011;3:27. doi: 10.1186/1758-3284-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye H, Wang A, Lee BS, Yu T, Sheng S, Peng T, Hu S, Crowe DL, Zhou X. Proteomic based identification of manganese superoxide dismutase 2 (SOD2) as a metastasis marker for oral squamous cell carcinoma. Cancer Genomics Proteomics. 2008;5(2):85–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Wang A, Lo Muzio L, Kolokythas A, Sheng S, Rubini C, Ye H, Shi F, Yu T, Crowe DL, Zhou X. Deregulation of manganese superoxide dismutase (SOD2) expression and lymph node metastasis in tongue squamous cell carcinoma. BMC Cancer. 2010;10:365. doi: 10.1186/1471-2407-10-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer Agents Med Chem. 2011;11(2):191–201. doi: 10.2174/187152011795255911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quiros I, Sainz RM, Hevia D, Garcia-Suarez O, Astudillo A, Rivas M, Mayo JC. Upregulation of manganese superoxide dismutase (SOD2) is a common pathway for neuroendocrine differentiation in prostate cancer cells. Int J Cancer. 2009;125(7):1497–504. doi: 10.1002/ijc.24501. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu H, Kogawa K, Tanaka M, Okumura K, Nishihori Y, Koike K, Kuga T, Niitsu Y. Superoxide dismutase in SAS human tongue carcinoma cell line is a factor defining invasiveness and cell motility. Cancer Res. 1995;55(24):6210–4. [PubMed] [Google Scholar]
- 7.Ough M, Lewis A, Zhang Y, Hinkhouse MM, Ritchie JM, Oberley LW, Cullen JJ. Inhibition of cell growth by overexpression of manganese superoxide dismutase (MnSOD) in human pancreatic carcinoma. Free Radic Res. 2004;38(11):1223–33. doi: 10.1080/10715760400017376. [DOI] [PubMed] [Google Scholar]
- 8.Sun GG, Wang YD, Yu XR, Cheng YJ, Bai S, Liu Q, Zhang J, Yang XR, Wan X. Expression of MnSOD mRNA and protein in esophageal squamous cell carcinoma and its clinical significance. Zhonghua Zhong Liu Za Zhi. 2010;32(11):834–7. [PubMed] [Google Scholar]
- 9.Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, Munshi HG. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res. 2008;6(1):10–20. doi: 10.1158/1541-7786.MCR-07-0208. [DOI] [PubMed] [Google Scholar]
- 10.Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Huang TT, Epstein CJ, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. J Biol Chem. 2001;276(17):14264–70. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- 11.Nagareddy PR, Chow FL, Hao L, Wang X, Nishimura T, MacLeod KM, McNeill JH, Fernandez-Patron C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc Res. 2009;84(3):368–77. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 12.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007;67(21):10260–7. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn AH, Lai CK, Shintaku IP, Fishbein MC, Brugman K, Elashoff DA, Abemayor E, Dubinett SM, St John MA. Snail as a novel marker for regional metastasis in head and neck squamous cell carcinoma. Am J Otolaryngol. 2012;33(1):6–13. doi: 10.1016/j.amjoto.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittal MK, Singh K, Misra S, Chaudhuri G. SLUG-induced elevation of D1 cyclin in breast cancer cells through the inhibition of its ubiquitination. J Biol Chem. 2011;286(1):469–79. doi: 10.1074/jbc.M110.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Chen D, Jiao X, Zhang S, Liu X, Cao J, Wu L, Wang D. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest. 2011;91(3):426–38. doi: 10.1038/labinvest.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Tang Y, Liang X, Zheng M, Zhu Z, Zhu G, Yang J, Chen Y. Expression of c-kit and Slug correlates with invasion and metastasis of salivary adenoid cystic carcinoma. Oral Oncol. 2010;46(4):311–6. doi: 10.1016/j.oraloncology.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Uchikado Y, Okumura H, Ishigami S, Setoyama T, Matsumoto M, Owaki T, Kita Y, Natsugoe S. Increased Slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer. 2011;14(1):41–9. doi: 10.1007/s10120-011-0004-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Liu X, Huang H, Ma H, Cai W, Hou J, Huang L, Dai Y, Yu T, Zhou X. Deregulation of Snai2 is associated with metastasis and poor prognosis in tongue squamous cell carcinoma. Int J Cancer. 2011 Jun 6; doi: 10.1002/ijc.26226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama S, Sasaki A, Mese H, Alcalde RE, Matsumura T. Establishment of high and low metastasis cell lines derived from a human tongue squamous cell carcinoma. Invasion Metastasis. 1998;18(5-6):219–28. doi: 10.1159/000024515. [DOI] [PubMed] [Google Scholar]
- 20.Hu K, Li SL, Gan YH, Wang CY, Yu GY. Epiregulin promotes migration and invasion of salivary adenoid cystic carcinoma cell line SACC-83 through activation of ERK and Akt. Oral Oncol. 2009;45:156–163. doi: 10.1016/j.oraloncology.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Wang J, Pan C. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer. 2008;8:182. doi: 10.1186/1471-2407-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James BP, Staatz WD, Wilkinson ST, Meuillet E, Powis G. Superoxide dismutase is regulated by LAMMER kinase in Drosophila and human cells. Free Radic Biol Med. 2009;46(6):821–7. doi: 10.1016/j.freeradbiomed.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q, Velalar CN, Ruan R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic Biol Med. 2008;44(6):1032–41. doi: 10.1016/j.freeradbiomed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Xu JH, Wang AX, Huang HZ, Wang JG, Pan CB, Zhang B. Survivin shRNA induces caspase-3-dependent apoptosis and enhances cisplatin sensitivity in squamous cell carcinoma of the tongue. Oncol Res. 2010;18(8):377–85. doi: 10.3727/096504010x12644422320663. [DOI] [PubMed] [Google Scholar]
- 25.Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, Pumiglia K, Bennett JA, Melendez JA. Elevated sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin Cancer Res. 2003;9(1):424–32. [PubMed] [Google Scholar]
- 26.Malafa M, Margenthaler J, Webb B, Neitzel L, Christophersen M. MnSOD expression is increased in metastatic gastric cancer. J Surg Res. 2000;88(2):130–4. doi: 10.1006/jsre.1999.5773. [DOI] [PubMed] [Google Scholar]
- 27.Janssen AM, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res. 2000;6(8):3183–92. [PubMed] [Google Scholar]
- 28.Hempel N, Ye H, Abessi B, Mian B, Melendez JA. Altered redox status accompanies progression to metastatic human bladder cancer. Free Radic Biol Med. 2009;46(1):42–50. doi: 10.1016/j.freeradbiomed.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung-man Ho J, Zheng S, Comhair SA, Farver C, Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61(23):8578–85. [PubMed] [Google Scholar]
- 30.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph MJ, Dangi-Garimella S, Shields MA, Diamond ME, Sun L, Koblinski JE, Munshi HG. Slug is a downstream mediator of transforming growth factor-beta1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cells. J Cell Biochem. 2009;108(3):726–36. doi: 10.1002/jcb.22309. [DOI] [PubMed] [Google Scholar]
- 32.Hardy RG, Vicente-Duenas C, Gonzalez-Herrero I, Anderson C, Flores T, Hughes S, Tselepis C, Ross JA, Sanchez-Garcia I. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol. 2007;171(3):1037–46. doi: 10.2353/ajpath.2007.061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 2007;67(24):11721–31. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- 34.Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94(5):661–71. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CJ, Hsu CC, Chang CH, Tsai LL, Chang YC, Lu SW, Yu CH, Huang HS, Wang JJ, Tsai CH, Chou MY, Yu CC, Hu FW. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011;26(4):1003–10. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 36.Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12(18):5395–402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 37.Al Saleh S, Sharaf LH, Luqmani YA. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition (Review) Int J Oncol. 2011;38(5):1197–217. doi: 10.3892/ijo.2011.942. [DOI] [PubMed] [Google Scholar]
- 38.Reinhold WC, Reimers MA, Lorenzi P, Ho J, Shankavaram UT, Ziegler MS, Bussey KJ, Nishizuka S, Ikediobi O, Pommier YG, Weinstein JN. Multifactorial regulation of E-cadherin expression: an integrative study. Mol Cancer Ther. 2010;9(1):1–16. doi: 10.1158/1535-7163.MCT-09-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saad AA, Awed NM, Abd Elkerim NN, El-Shennawy D, Alfons MA, Elserafy ME, Darwish YW, Barakat EM, Ezz-Elarab SS. Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol. 2010;17(11):3059–67. doi: 10.1245/s10434-010-1151-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


