Abstract
Loss of intercellular adhesion by E-cadherin is a fundamental change that occurs during the progression of cancer to invasive disease as strong cell-cell interaction represents a major barrier to cancer cell mobility. However, some aggressive carcinomas retain characteristics of differentiated epithelial cells including E-cadherin expression. Emerging evidence indicates that proteolysis of E-cadherin generates fragments that promote tumor growth, survival, and motility, suggesting that E-cadherin cleavage converts this tumor suppressor into an oncogenic factor. In this review we discuss the emerging roles of cleaved E-cadherin fragments as modulators of cancer progression and explore the translational and clinical implications of this research.
Keywords: Signal transduction, Tumor promotion and progression, Cell adhesion, Cell-cell interactions, Growth factors: structure and function, Receptors: structure and function, Cell adhesion/cell-cell interactions in apoptosis, Survival factors, Cell motility and migration, Tumor markers and detection of metastasis
Introduction
The overwhelming majority of cancers (>90%) are classified as carcinomas which are derived from the epithelial cells that line the exterior surfaces and internal cavities of the body. These highly specialized cells exhibit unique morphological characteristics that include extensive intercellular junctional complexes, stable cell-cell and cell-matrix adhesions, and distinct apical and basolateral plasma membrane domains. The properties of a well-differentiated epithelium allow individual cells to function collectively as a single organized unit.
A pivotal hallmark of cancer is the ability of cancer cells to break free from the primary tumor and migrate to distant sites within the body to form new tumors. This metastatic capability is generally thought to arise through the progressive loss of epithelial characteristics as cancer cells adopt a more mesenchymal phenotype in a process termed epithelial-to-mesenchymal transition (EMT) (1). In contrast to the well-differentiated epithelium, mesenchymal cells exhibit an elongated morphology with leading edge-trailing edge asymmetry, form only transient adhesions to neighboring cells and the extracellular matrix (ECM), and produce a variety of matrix degrading enzymes. These alterations in cell phenotype simultaneously break up the integrity of the tissue and support increased motility and invasion as the tumor cells seek to penetrate into the vasculature for hematogenous dissemination.
Modulation of the levels of cell-cell adhesion molecules is crucial for the development of aggressive carcinomas, and chief among these are alterations in E-cadherin expression. This protein forms cell-cell adhesions via calcium-dependent homophilic interactions, and downregulation of its expression permits the separation of individual cells from the primary cell mass. Although downregulation of E-cadherin was once thought to be a necessity, several reports have documented invasive and aggressive tumors that nonetheless maintain E-cadherin expression (1). In this review we focus on the proteolytic cleavage and release of E-cadherin fragments from the plasma membrane as a mechanism that enhances tumor growth, survival, and motility. We discuss several reports that have demonstrated the oncogenic functions of these E-cadherin fragments in human cancer, and then explore the wider clinical implications of this research.
E-cadherin Structure and Functions
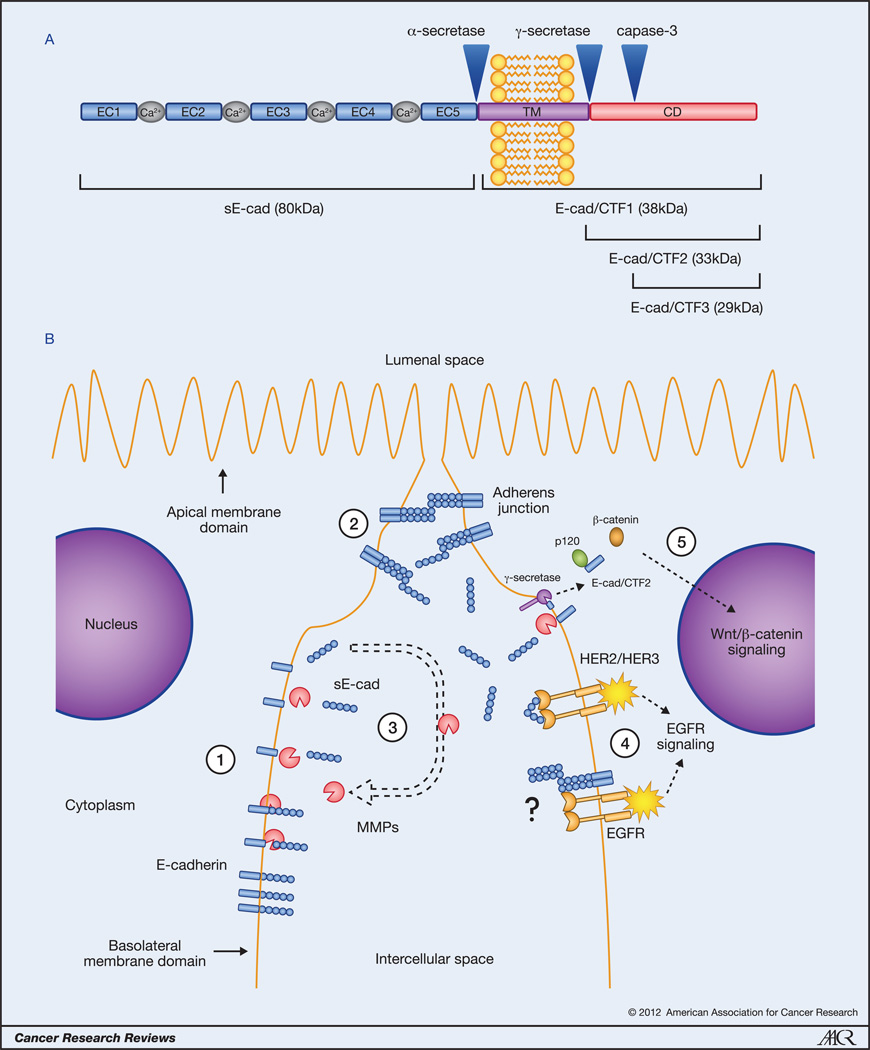
E-cadherin is a single-pass type-I transmembrane glycoprotein that is localized to the adherens junction (AJ) and basolateral membrane in epithelial cells and consists of a large extracellular domain, a transmembrane segment, and a conserved cytoplasmic domain. The extracellular portion contains five extracellular cadherin (EC) domain repeats that bind calcium ions to form a stiffened linear molecule (Fig. 1A). Homophilic interactions between cadherin molecules occur first between adjacent cells (trans-interaction) and then within the same cell by lateral association (cis-interaction) to form a zipper-like structure that strengthens the adhesion between the neighboring cells. The cytoplasmic domain interacts with the catenins and a variety of actin-binding proteins in order to anchor the cadherin-catenin complex to the actin cytoskeleton (2).
Figure 1.
Generation of E-cadherin fragments and their functions. A, cleavage of the full-length E-cadherin by α-secretase, γ-secretase, and caspase-3 generates the sE-cad and E-cad/CTF1, E-cad/CTF2, and E-cad/CTF3 fragments, respectively. B, oncogenic functions of the sE-cad and E-cad/CTF2 fragments. Generation of sE-cad by E-cadherin cleavage reduces the amount of full-length E-cadherin on the plasma membrane (1), disrupts existing adherens junctions (2), activates the expression of MMPs to augment ectodomain shedding (3), and activates EGFR pathway signaling by different mechanisms (4). Intracellular cleavage of E-cadherin activates Wnt/β-catenin pathway signaling (5). CD, cytoplasmic domain; TM, transmembrane domain.
The adhesive function of E-cadherin plays a vital role in epithelial physiology. The fully formed cadherin-catenin complex with its associated actin filaments forms the core of the AJ which brings together two apposed plasma membranes with an intercellular gap of only about 25nm (2). This extremely close association halts the movement of individual epithelial cells (known as contact inhibition of motility) and permits their organization into tightly bound layers with functionally distinct apical and basolateral plasma membrane domains. E-cadherin-mediated AJs are also essential for the formation of the more apical tight junction, a crucial epithelial structure that forms the permeability barrier that blocks the passive diffusion of most solutes between the luminal and interstitial spaces (2). In addition to immobilizing epithelial cells within their respective tissue layers, E-cadherin can also block growth factor-mediated proliferation signaling (contact inhibition of growth), thereby maintaining tissue integrity and preserving tissue function (3). Furthermore, E-cadherin can influence various diverse processes that occur during embryonic development, including cell sorting, transitions between the epithelium and mesenchyme, and even maintenance of embryonic stem cell pluripotency (2, 4).
While E-cadherin is expressed along the length of the basolateral plasma membrane of epithelial cells, it is unlikely that molecules below the AJ can participate in trans-homophilic interaction since the intercellular space is larger between adjacent cells in these regions. The potential role of these non-AJ-associated molecules remains to be investigated.
E-cadherin Expression in Cancer
Loss of E-cadherin
In stark contrast to the epithelial cells from which they are derived, carcinoma cells typically do not exhibit contact inhibition of either motility or growth. Given its prominence in promoting contact inhibition, E-cadherin represents a formidable molecular barrier that must be overcome if tumor cells are to proliferate, detach, and spread. The functional loss of E-cadherin is a frequent and well characterized molecular alteration that occurs during tumor progression and occurs through a variety of mechanisms, including transcriptional repression, epigenetic silencing, inactivating mutation, enhanced degradation, endocytosis, and proteolytic cleavage (5). Downregulation of E-cadherin is a standard feature of developmental EMT, and its loss in cancer plays a causal role in the transition to a malignant phenotype (2, 6). E-cadherin loss can also weaken intercellular adhesions sufficiently to permit the passive dispersion of tumor cells in response to minimal shear forces (1). Finally, reduced expression of E-cadherin is indicative of unfavorable clinical outcome in several malignancies (7).
Retained E-cadherin Expression in Cancer
Although the prevailing dogma is that induction of EMT and loss of E-cadherin is a prerequisite for progression to metastatic disease, tumors are actually remarkably heterogeneous and E-cadherin loss does not always correlate with invasion. EMT is a multifaceted process and cancer cells may undergo only a partial transition that enhances invasion while still retaining certain epithelial characteristics such as E-cadherin (1). In support of this notion, in vitro studies have demonstrated that forced expression of E-cadherin fails to inhibit motility and invasion in certain carcinoma cell lines (8, 9). Moreover, analysis of patient tumor samples from multiple cancers revealed an unexpectedly high frequency of E-cadherin positive tumors that had invaded into the surrounding tissue (1). Surprisingly, high E-cadherin expression was actually associated with aggressive growth in prostate cancer (10) and with growth, invasion, and unfavorable outcome in a rare form of glioblastoma (11). E-cadherin is also highly overexpressed in inflammatory breast cancer (IBC), the most aggressive form of breast cancer, and blocking its function inhibits IBC cell invasion (12). Likewise, E-cadherin is detected in early stage ovarian carcinoma (13) and enhances proliferation and survival by inducing ligand-independent activation of the receptor tyrosine kinase (RTK) epidermal growth factor receptor (EGFR) (14).
In both IBC and EOC, E-cadherin promotes the formation of multicellular aggregates that are able to metastasize collectively (15, 16). This so-called cohort migration occurs as a normal developmental process, but tumors can also utilize this method of migration to permit the movement of carcinoma cells that have not lost their well-differentiated epithelial morphology. Additional tumor types have also been reported to spread by cohort migration, including breast and colorectal tumor cells (1).
Cleaved E-cadherin Fragments in Cancer
Proteolysis of E-cadherin
Given the occurrence of E-cadherin positive yet invasive tumors, it stands to reason that there are additional means whereby E-cadherin contributes to tumor development. Proteolytic processing and release of membrane protein fragments, termed ectodomain shedding, is a common way in which cells modify the functional properties of membrane proteins. It is now known that cleavage of E-cadherin generates protein fragments that possess distinct oncogenic properties. An α-secretase cleavage occurs on the extracellular face of the plasma membrane and is catalyzed by several proteases, including matrix metalloproteinases (MMP-3, MMP-7, MMP-9, MT1-MMP), a disintegrin and metalloproteinases (ADAM10, ADAM15), plasmin, and kallikrein 7 (Fig. 1A) (5, 17). This converts the mature 120kDa E-cadherin into an extracellular N-terminal 80kDa fragment and an intracellular C-terminal 38kDa fragment. The ectodomain fragment, termed soluble E-cadherin (sE-cad), is released from the plasma membrane and diffuses into the extracellular environment and even the bloodstream to serve as a paracrine/autocrine signaling molecule. In contrast, the intracellular C-terminal fragment (E-cad/CTF1) remains embedded within the plasma membrane until cleavage occurs at the intracellular face by a γ-secretase (presenilin-1/2). This disassembles the AJ and releases an intracellular 33-kDa fragment (E-cad/CTF2) into the cytosol that functions in intracellular signaling (Fig. 1A) (18, 19). This fragment can also be further proteolyzed by caspase-3 to yield a 29kDa fragment (E-cad/CTF3) of unknown function (20).
An important question to consider is whether the AJ-associated E-cadherin is cleaved or whether molecules located along the basolateral plasma membrane are preferentially cleaved. We speculate that the non-AJ-associated E-cadherin is the predominant source of sE-cad, as the larger intercellular distances below the AJ may facilitate E-cadherin cleavage by soluble proteases. This supposition remains to be addressed experimentally.
sE-cad Enhances Motility and Invasion
Shedding of the ectodomain fragment of E-cadherin was first discovered in the conditioned media of MCF-7 mammary carcinoma cells (21). Several subsequent studies reported significantly elevated levels of sE-cad in cancer patients' sera from a variety of tumor types and that elevated sE-cad was associated with invasive disease and/or poor prognosis (5). The first function attributed to sE-cad was the disruption of cell-cell adhesion as treatment of cells with sE-cad in vitro decreased cell aggregation and increased migration and invasion (13, 17, 21–26). The mechanism whereby sE-cad can effect these changes occurs in at least four ways (Fig. 1B). First, the cleavage of E-cadherin degrades the full-length molecule, reducing the available pool of adhesion-competent E-cadherin in the plasma membrane. Second, because sE-cad retains the ability to form homophilic bonds, it may obstruct full-length E-cadherin homodimers between adjacent cells. Third, sE-cad displays chemotactic properties and may become trapped within the ECM to serve as anchoring points for full-lengt h E-cadherin molecules on migrating cells (5, 24). Lastly, sE-cad causes upregulation of major MMPs (MMP-2, MMP-9, and MT1-MMP) that degrade the basement membrane to allow tumor invasion into the stroma (26). Notably, since MMP-9 has also been shown to cleave E-cadherin (13), it is tempting to speculate that protease induction by sE-cad also constitutes a positive feedback loop to continuously generate sE-cad.
sE-cad Induces EGFR-dependent Growth and Survival Signaling
It is well known that E-cadherin regulates cell signaling. RTK activation occurs through receptor dimerization and autophosphorylation, and the clustering of E-cadherin molecules in cis following intercellular homophilic interaction can promote ligand-independent dimerization and activation of EGFR. (13, 27, 28). Because the extracellular domain of E-cadherin physically interacts with EGFR (29), cleavage and shedding of this domain into the extracellular environment converts this molecule into a soluble growth factor.
This novel function of sE-cad was first demonstrated in breast cancer cells. The EGFR (ErbB) family of RTKs consists of four members; EGFR (HER1, ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). These receptors can both homo- and heterodimerize in response to ligand binding. Najy et al. observed that sE-cad could complex with both HER2 and HER3, stabilized their interaction, and activate signaling to enhance proliferation and migration (Fig. 1B) (25). Because HER2 overexpression is a relatively common event in breast cancer that portends poor prognosis (30), the proteolytic release of sE-cad may serve to stimulate this pathway to promote the aggressive growth and spread of these tumors.
Signaling by sE-cad can also interfere with apoptosis. Our laboratory demonstrated that treatment of Madin-Darby canine kidney (MDCK) cells with 10µg/mL sE-cad inhibited apoptosis in response to serum withdrawal without altering cell-cell adhesion (31). We further showed that sE-cad prevented the formation of multicellular hollow cysts when these cells were cultured in a 3-D collagen matrix. Cyst formation requires the apoptosis of the centrally located cells, and we found that sE-cad blocked cyst hollowing via ligand-independent activation of EGFR (Fig. 1B) (31). Our studies collectively demonstrated that sE-cad acts as an anti-apoptotic factor which suggests that it may play a critical role in the survival and initiation of a preneoplastic condition in epithelial tissues.
While both of these studies reported the activation of EGFR signaling by sE-cad, they differed in the requirement for full-length E-cadherin expression. Najy et al. showed direct binding of sE-cad to HER2 and HER3 and subsequent signaling in the absence of full-length E-cadherin (25) whereas in our study interaction between sE-cad and full-length E-cadherin was essential to mediate survival signaling (Fig 1B) (31). The binding of sE-cad to cell surface E-cadherin may mimic the intercellular homophilic binding between neighboring cells that results in downstream signaling (13, 27, 28). However, cell-cell adhesion was unaffected in our study suggesting that sE-cad interacts with non-AJ-associated E-cadherin molecules that are expressed along the length of the basolateral plasma membrane.
The link between sE-cad and EGFR also seems to be a reciprocal relationship in which EGFR activation promotes MMP- and ADAM-dependent generation of sE-cad (32, 33). This suggests that a feedback mechanism exists whereby EGFR activation produces sE-cad which can then further activate EGFR signaling to continuously promote oncogenic proliferation and invasion (Fig. 1B).
E-cadherin Cleavage Activates Wnt/β-catenin Pathway Signaling
A central component of the cadherin-catenin complex is β-catenin which also functions as the primary mediator of the Wnt signaling pathway. In the classical Wnt pathway the binding of Wnt ligand triggers the accumulation of β-catenin which translocates into the nucleus and binds to the transcription factor T-cell factor/lymphocyte enhancer factor-1 (TCF/LEF-1) to activate growth stimulatory target genes (34). By complexing with β-catenin, E-cadherin effectively sequesters this protein at the plasma membrane and prevents its transcriptional activities (35). However, cleavage of E-cadherin disassembles this complex and releases β-catenin to potentiate oncogenic Wnt pathway signaling (Fig. 1B) (17–19). This pathway is also enhanced directly by the intracellular E-cad/CTF2 fragment. In addition to freeing β-catenin, E-cadherin cleavage also liberates p120 catenin which translocates into the nucleus and binds to the transcriptional repressor Kaiso to relieve its repression of various Wnt/β-catenin pathway gene targets (36). Importantly, the E-cad/CTF2 fragment can remain bound to p120 to enhance its inhibitory effects upon Kaiso-mediated transcriptional repression (Fig. 1B) (37).
These studies suggest a dual mechanism whereby E-cadherin cleavage facilitates Wnt/β-catenin pathway signaling. The resulting aberrant activation of the Wnt/β-catenin pathway may serve to support rapid tumor cell proliferation and also augment tumor cell survival, as the expression of nuclear E-cad/CTF2 has also been shown to suppress the induction of apoptosis (37). Additionally, E-cadherin cleavage may promote the acquisition of a cancer stem cell phenotype since Wnt/β-catenin pathway signaling is involved in stem cell regeneration (34). Furthermore, since MMP-7 is a Wnt/β-catenin target (38) that has been shown to cleave full-length E-cadherin (23), activation of this gene may establish a positive-feedback loop of continuous E-cadherin cleavage to ensure sustained oncogenic signaling.
Clinical Implications and Future Directions
Preclinical Studies of sE-cad Function
The presence of sE-cad in the sera of healthy patients indicates that it may play a role in normal physiological processes. Given its ability to enhance motility (promoting epithelial cell movement) and simultaneously block apoptosis (to prevent anoikis of detatched epithelial cells), sE-cad production may be a key feature in normal wound healing. Tantalizing pieces of evidence point to this possibility as calcium influx is a critical event in both wound healing and E-cadherin cleavage (17, 18). Additionally, sE-cad may play a role in somatic cell turnover by disrupting cell-cell adhesion to promote the expulsion and replacement of older cells in continuously renewing tissues.
Whether circulating sE-cad contributes directly to tumor growth, survival, and motility in vivo remains to be confirmed experimentally. The wealth of in vitro experimental data and clinical association of invasive disease with high serum sE-cad suggest that sE-cad plays a pathological role in vivo. For instance, circulating sE-cad may aid in the preparation of metastatic sites by disrupting epithelial junctions at distant locations to ease tumor cell invasion and growth into these secondary sites. Furthermore, the induction of lumen filling in 3-D culture may translate in vivo; lumen filling in epithelial tissues adjacent to a tumor mass may transform these neighboring tissues into pre-neoplastic cells that secrete tumor-promoting factors into the local microenvironment. By blocking the normal apoptotic removal of genetically damaged cells, sE-cad-induced lumen filling may also permit the propagation and even transformation of mutated cells within these adjacent tissues into separate tumors. Future investigations will be necessary to expand our understanding of the consequences of E-cadherin ectodomain shedding in cancer progression in vivo.
Pathological Analysis of E-cadherin in Tumor Tissues
It is now widely accepted that EMT in cancer is not an "all or none" phenomenon. Although E-cadherin loss clearly does play a pivotal role in cancer-associated EMT, there still exist numerous cases in which invasive carcinomas have retained differentiated epithelial markers such as E-cadherin (1). Furthermore, immunohistochemical (IHC) studies of tumor samples have likely overestimated the proportion of E-cadherin loss since many of these studies employed antibodies that cannot detect the shedding of ectodomain fragments. This was documented in an analysis of ovarian carcinoma in which focal areas of high MMP-9 staining were found to colocalize with low or absent E-cadherin staining in otherwise E-cadherin-positive tumors indicating active ectodomain shedding in these focal areas (13). The evident heterogeneity of human tumors suggests that accurate profiling of E-cadherin expression requires more sophisticated means. First, IHC analysis should be used to compare the staining of extracellular- versus intracellular-specific antibodies from the same sample. Intracellular staining without concurrent membrane staining may indicate ectodomain shedding in the tumor. This could be further validated by comparing E-cadherin mRNA levels between the tumor and matched normal tissue to confirm that alterations in the IHC staining are not due to transcriptional repression of E-cadherin. Laser-capture microdissection has made it practical to isolate pure populations of tumor cells for RNA extraction, and this technology is also compatible with various tissues, staining methods, and preservation techniques to allow its use for fresh and stored specimens alike. Future studies utilizing these methods may provide a more precise assessment of E-cadherin loss and sE-cad production in human cancer and provide additional insights into the pathophysiological relevance of this molecule.
sE-cad as a Clinical Biomarker for Diagnosis and Treatment
The clinical detection of elevated serum sE-cad in a wide variety of cancer types coupled with its frequent association with invasion and poor prognosis may designate sE-cad as a general diagnostic and prognostic biomarker for invasive and/or metastatic disease. For example, initial measurement of serum sE-cad at clinical presentation may aid in the discrimination of slow growing tumors that do not necessitate immediate intervention versus those that are actively progressing and require more urgent treatment. Continuous monitoring of sE-cad levels may also be a relatively inexpensive method for tracking therapeutic outcome, as treatment efficacy was associated with decreasing sE-cad levels in separate studies of gastric and lung cancer (39, 40). In the future sE-cad may become useful in guiding the decision to continue a given treatment or replace it early in favor of another option. Furthermore, the newly ascribed roles for cleaved E-cadherin fragments in cellular signaling suggest that elevated sE-cad is indicative of EGFR and/or Wnt/β-catenin pathway activation and may predict sensitivity to inhibitors of these pathways.
Validation of sE-cad as a pathologically significant molecule in cancer progression raises the question as to whether it can be targeted to slow cancer growth and invasion. Inhibition of relevant proteases may prevent sE-cad generation, however inhibitors of these enzymes have yet to yield satisfying clinical results (41). Improved specificity and targeted delivery of such inhibitors is an area of ongoing research and may become viable in the future. An alternative target is the EGFR signaling pathway which promotes the shedding of sE-cad (32, 33). Blockade of this pathway may reduce ectodomain shedding and diminish the pathologic effects of sE-cad, however sE-cad production independent of EGFR signaling will necessitate the targeting of other pathways to obtain clinical benefit. It may also be feasible to develop agents that remove sE-cad from the blood and/or tumor microenvironment such as neutralizing antibodies or small molecules that bind specifically to sE-cad and clear it from the body.
Conclusions
It is apparent from multiple investigations that E-cadherin plays a much more complex role in cancer biology than solely that of a tumor suppressive cell adhesion molecule. The existence of invasive yet E-cadherin-expressing tumors presents an apparent paradox that can be reconciled if one considers that maintaining E-cadherin allows for the production of oncogenic sE-cad and E-cad/CTF2 fragments. Because these fragments promote tumor cell growth, survival, and motility, the cleavage and release of these molecules represents a novel method of subverting contact inhibition imposed by full-length E-cadherin. Although further investigation into the pathophysiological consequences of E-cadherin cleavage remains to be done, a better understanding of its diverse functions may yield important advances in the diagnosis and management of numerous cancers.
Acknowledgements and Grant Support
We apologize to the authors whose work was not cited or cited indirectly due to space limitations. This work was supported by funding from NIH grants DK56216 and from the Nemours Foundation.
Footnotes
Conflicts of Interest: None
References
- 1.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6(8):622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 3.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12(7):720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wever O, Derycke L, Hendrix A, De Meerleer G, Godeau F, Depypere H, et al. Soluble cadherins as cancer biomarkers. Clin Exp Metastasis. 2007;24(8):685–697. doi: 10.1007/s10585-007-9104-8. [DOI] [PubMed] [Google Scholar]
- 6.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8(5):629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 8.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147(3):631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr, Bander NH, et al. Na, K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell. 2001;12(2):279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktaş M, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol. 2011;179(1):400–410. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong HM, Liu G, Hou YF, Wu J, Lu JS, Luo JM, et al. Dominant-negative E-cadherin inhibits the invasiveness of inflammatory breast cancer cells in vitro. J Cancer Res Clin Oncol. 2007;133(2):83–92. doi: 10.1007/s00432-006-0140-6. [DOI] [PubMed] [Google Scholar]
- 13.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67(5):2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, et al. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol. 2005;19(10):2564–2578. doi: 10.1210/me.2004-0342. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61(13):5231–5241. [PubMed] [Google Scholar]
- 16.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5(5):355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 17.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102(26):9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18(50):7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- 19.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21(8):1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276(7):4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- 21.Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- 22.Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987;34(3):187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- 23.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114(Pt 1):111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Chunthapong J, Seftor EA, Khalkhali-Ellis Z, Seftor RE, Amir S, Lubaroff DM, et al. Dual roles of E-cadherin in prostate cancer invasion. J Cell Biochem. 2004;91(4):649–661. doi: 10.1002/jcb.20032. [DOI] [PubMed] [Google Scholar]
- 25.Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283(26):18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawrocki-Raby B, Gilles C, Polette M, Bruyneel E, Laronze JY, Bonnet N, et al. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer. 2003;105(6):790–795. doi: 10.1002/ijc.11168. [DOI] [PubMed] [Google Scholar]
- 27.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274(27):19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 28.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275(52):41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 29.Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes. 2003;10(2):105–118. [PubMed] [Google Scholar]
- 30.Yasmeen A, Bismar TA, Al Moustafa AE. ErbB receptors and epithelial-cadherin-catenin complex in human carcinomas. Future Oncol. 2006;2(6):765–781. doi: 10.2217/14796694.2.6.765. [DOI] [PubMed] [Google Scholar]
- 31.Inge LJ, Barwe SP, D'Ambrosio J, Gopal J, Lu K, Ryazantsev S, et al. Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res. 2011;317(6):838–848. doi: 10.1016/j.yexcr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112(9):2508–2517. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 33.Grabowska MM, Sandhu B, Day ML. EGF promotes the shedding of soluble E-cadherin in an ADAM10-dependent manner in prostate epithelial cells. Cell Signal. 2012;24(2):532–538. doi: 10.1016/j.cellsig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 35.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents betacatenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 36.Spring CM, Kelly KF, O'Kelly I, Graham M, Crawford HC, Daniel JM. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp Cell Res. 2005;305(2):253–265. doi: 10.1016/j.yexcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, et al. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem. 2008;283(19):12691–12700. doi: 10.1074/jbc.M708887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155(4):1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Ran J, Tang C, Wu J, Honghua L, Xingwen L, et al. Effect of celecoxib on E-cadherin, VEGF, microvessel density and apoptosis in gastric cancer. Cancer Biol Ther. 2007;6:269–275. doi: 10.4161/cbt.6.2.3629. [DOI] [PubMed] [Google Scholar]
- 40.Reckamp KL, Gardner BK, Figlin RA, Elashoff D, Krysan K, Dohadwala M, et al. Tumor response to combination celecoxib and erlotinib therapy in non-small cell lung cancer is associated with a low baseline matrix metalloproteinase-9 and a decline in serum-soluble E-cadherin. J Thorac Oncol. 2008;3(2):117–124. doi: 10.1097/JTO.0b013e3181622bef. [DOI] [PubMed] [Google Scholar]
- 41.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]