Abstract
Increased osteoblast activity in sclerostin-knockout (Sost−/−) mice results in generalized hyperostosis and bones with small bone marrow cavities due to hyperactive mineralizing osteoblast populations. Hematopoietic cell fate decisions are dependent on their local microenvironment, which contains osteoblast and stromal cell populations that support both hematopoietic stem cell quiescence and facilitate B cell development. In this study, we investigated whether high bone mass environments affect B cell development via the utilization of Sost−/− mice, a model of sclerosteosis. We found the bone marrow of Sost−/− mice to be specifically depleted of B cells, due to elevated apoptosis at all B cell developmental stages. In contrast, B cell function in the spleen was normal. Sost expression analysis confirmed that Sost is primarily expressed in osteocytes and is not expressed in any hematopoietic lineage, which indicated that the B cell defects in Sost−/− mice are non-cell autonomous and this was confirmed by transplantation of wildtype (WT) bone marrow into lethally irradiated Sost−/− recipients. WT→Sost−/− chimeras displayed a reduction in B cells, whereas reciprocal Sost−/−→WT chimeras did not, supporting the idea that the Sost−/− bone environment cannot fully support normal B cell development. Expression of the pre-B cell growth stimulating factor, Cxcl12, was significantly lower in bone marrow stromal cells of Sost−/− mice while the Wnt target genes Lef-1 and Ccnd1 remained unchanged in B cells. Taken together, these results demonstrate a novel role for Sost in the regulation of bone marrow environments that support B cells.
Keywords: Osteoblast, Osteocyte, B cell, Sclerostin, Hematopoiesis, Sost, Sclerosteosis, High Bone Mass
INTRODUCTION
It is well appreciated that cellular crosstalk between osteoblasts (OBs) and osteoclasts (OCs) in the adult bone is required for proper bone homeostasis (1) and that disruption of the balanced activity between bone-building OBs and bone-resorbing OCs can result in altered bone metabolism, leading to high or low bone mass, respectively. More recently, the relationship between abnormal bone phenotypes on the development and differentiation of bone marrow (BM) stromal cells and hematopoietic cells has been an active area of investigation (2,3).
Hematopoietic stem cells (HSCs) produce all cells of the blood and immune system. HSC self-renewal and their subsequent differentiation into committed hematopoietic lineages is guided by a combination of cell-to-cell interactions, secreted factors and transcriptional regulation (4) in a physiological unit termed the HSC niche (5,6). Endosteal osteoblasts are often considered the primary “niche cell” for HSCs and have been shown to support HSC self-renewal, as demonstrated by transgenic and knockout mouse models in which OB populations were increased or decreased (6–9). In addition, mesenchymal progenitors, endothelial cells and perivascular cells can also support HSCs, suggesting than other cell types also contribute to the HSC niche in the bone marrow (10,11). Myeloid and lymphoid cell differentiation can also be influenced by OBs (12,13), but the exact mechanisms that OBs utilize to regulate these cell fate decisions is still unclear. Furthermore, how the hematopoiesis-supporting ability of OBs changes as the OB matures from the mesenchymal stem cell (MSC) to an early osteoprogenitor and then to a mature osteocyte is not well-understood. Stages of OB development have been identified using a combination of in vivo studies in transgenic mice and in vitro studies of OB cultures (14). At embryonic day 12, some MSCs begin expressing Runx2, solidifying commitment to the osteoblastic lineage (15). Activation of Wnt signaling and expression of Osterix foster further differentiation to the osteoprogenitor stage (16). Commitment to the mature osteoblast stage is confirmed by the upregulation of mineralization genes (17). Finally, terminal differentiation to the osteocyte requires downregulation of Wnt signaling by Wnt antagonists (16,17).
Canonical and noncanonical Wnt signaling has been implicated in various aspects of hematopoiesis but results from Wnt loss- and gain-of-function models have yielded contradictory results (18,19). For example, activation of canonical Wnt signaling via exogenous Wnt3a ligand has been shown to preserve HSC populations in vitro, while Wnt3a deficiency resulted in a decrease in the number of HSCs and progenitor cells in the fetal liver (FL), as well as a reduced capacity to reconstitute as measured by secondary transplantation (20). In addition, it has been shown that B lymphocyte development in the bone marrow of β-catenin deficient mice is normal (21), whereas B cell development is increased by noncanonical Wnt5a mediated signaling (22). Wnt signaling is also important for osteoblast development, as canonical Wnt3a-signaling inhibited or promoted osteogenesis depending on the Wnt3a concentration and age of the mice examined (23). Haploinsufficiency of the noncanonical Wnt5a gene in mice resulted in loss of bone mass and increased adipogenesis in the bone marrow in vivo (24), but promoted osteogenesis from human mesenchymal stem cells in vitro (25). Taken together, the role of Wnt signaling in hematopoiesis and osteogenesis is clearly influential in preserving bone homeostasis.
Sclerostin (Sost, Entrez GeneID: 50964) antagonizes canonical Wnt signaling by its binding to the Wnt co-receptors LRP4, LRP5, and/or LRP6 (26,27), blocking signaling via Frizzled receptors. SOST is a secreted protein that is primarily expressed by fully mature osteocytes and acts on OBs as a negative regulator of bone growth by inducing OB apoptosis in culture and effectively preventing osteoblast maturation into osteocytes (26). Mice with deletions of the Sost coding region display highly mineralized bones with reduced BM cavity size, due to increased activity of OBs without affecting osteoclast development and activity (28). Van Buchem’s disease in humans has been traced to a 52kb deletion in the Sost regulatory region, which results in deforming increases in bone mass (29). Despite the clear role of SOST in the regulation of Wnt signaling, osteoblast activity and the size of the BM cavity, the function of SOST in the regulation of bone marrow hematopoiesis has not been investigated. Here, we analyzed hematopoietic differentiation and the bone marrow environment in Sost−/− mice to examine whether the lack of Sost in the bone affects hematopoiesis, particularly B cell development.
METHODS
Mice
C57BL/6J and B6.SJL-Ptprca Pepcb/BoyJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Sost−/− mice on the B6 background were generated by Regeneron Pharmaceuticals, Inc. as a Sost-LacZ knock-in as part of the Knockout Mouse Project (KOMP) (http://www.velocigene.com/komp/detail/10069 (30)). of both sexes were analyzed between 3 – 4 months of age. Data were combined from both male and female mice, as no sex-specific differences were observed (Refs. (28,30), and data not shown). All mice were euthanized by CO2 asphyxiation followed by cervical dislocation. All animal procedures were approved by the LLNL and UC Merced Institutional Animal Care and Usage Committees.
Antibodies
Monoclonal antibodies (mAb) were purchased either from eBioscience, Biolegend or BD Biosciences (San Diego, CA). The mAb clone name is listed in parentheses. Purified anti-CD16/32 (93) was used to block Fc receptors. Biotinylated-anti-CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD19 (6D5), CD11b (M1/70), NK1.1 (PK136), Gr-1 (RB6-8C5), and TER-119 (Ter119) were used for lineage depletions. Other antibodies and stains used are listed as follows: anti-c-Kit-eFluor-780 (ACK2), CD34-FITC (RAM34), CD135-PE (A2F10.1), CD16/32-PerCp-Cy5.5 (93), CD25-PerCp-cy5.5 (PC61) Sca-1-APC (D7), IL7Rα-PeCy7 (A7R34), AA4.1-APC (AA4.1), B220-FITC (RA3-6B2), IgD-PerCp-Cy5.5 (11-26c.2a), CD19-APCCy7, -APC, or -PE (6D5), IgM-PE (RMM-1), CD21-FITC (7G6), and CD23-Biotin (B3B4), CD45-FITC (30-F11), NK 1.1-PeCy5 (PK136), CD3ε-APC (145-2C11), TER-119-PeCy7 (Ter119,) Gr-1-PeCy5 (RB6-85C), CD11b-PE (M1/70), CD45.1-FITC (A20), CD45.1-APC (A20), CD45.2-PE (104), CD45.2-APC-Cy7 (104), Annexin V-FITC, and 7-AAD.
Sorting and analysis of hematopoietic progenitor and stromal populations by flow cytometry (FCM)
Bone marrow cells were obtained and counted as described (31). For stromal cells, flushed tibiae and femora were digested in M199+ containing 0.125% (w/v) collagenase D (Roche) and 0.1% DNase (Roche) on an agitator at 37C, in which fresh media was added every 15 minutes, for a total of 75 minutes. Then, 0.125% Neutral Protease (Worthington) was added for 15 minutes and then stromal cells were incubated in a mixture of PBS, 5mM EDTA, 1% FCS, 0.02% NaN3 for 10 minutes to help disrupt cellular fragments.
All cells were incubated with purified anti-CD16/32 to block Fc receptors and MACS depleted as described (31). Live Lineageneg cells were then counted by hemocytometer using Trypan Blue exclusion. All Lineageneg cells were stained with antibodies specific for c-Kit, Sca-1 and IL7Rα for 20 min. at 4C, washed and resuspended in M199+ media with 0.ug/ml of DAPI (Fisher). LSK HSC, MPP, CLP, CMP, MEP/GMP populations were then sorted using a FACS Aria II (BD Biosciences, San Jose, CA). All populations were sorted to 80–90% purity, as verified by post-sort analysis. Analysis of flow cytometric data was performed with FlowJo software (Treestar, Ashland, OR).
Flow cytometric sorting and analysis of committed cell lineage population in the bone marrow and spleen
Bone marrow cells were isolated as described (31), counted, and cells were stained with anti-CD16/32 and then stained with fluorochrome-conjugated Abs specific for CD3, CD19, NK1.1, Ter119, CD11b, and Gr-1. Splenocytes obtained by gentle physical disruption of spleens with the base of a 5ml syringe and resuspended in M199+. Splenocytes were filtered, treated with ACK lysis buffer, incubated with anti-CD16/32, and then either stained with fluorochrome-conjugated Abs specific for CD3, CD19, NK1.1, Ter119, CD11b, and Gr-1, or fluorochrome-conjugated Abs specific for AA1.1, CD19, IgM, CD21, and CD23. Analysis was performed as described above.
RNA Isolation, cDNA synthesis, and PCR
mRNA from cells was collected from collagenase-digested bones, FACS-sorted cells and whole bone, as described (31). Briefly, cells were placed in Trizol (Invitrogen, Grand Island, NY) and RNA was purified using phenol-chloroform extraction. Purified mRNA was then used as a template to synthesize cDNA using oligo-dT primers with the Superscript III kit (Invitrogen). Conventional reverse-transcriptase PCR (RT-PCR) of cDNA was performed using the following thermocycler conditions: 90°C for 5 min., then 35–40 cycles of 95°C for 1min., 55–60°C for 30 sec. and 72°C for 1 min., followed by a 5 min. 72°C extension. PCR products were visualized by electrophoresis on a 1.5% agarose gel.
Quantitative real-time PCR (qPCR) was performed as described (31). All primers used were validated for efficiency using standard curves on control tissues and were used only if the primer efficiencies exceeded 90% and only one PCR product was visualized after gel electrophoresis. All primer sequences are listed in Table S6.
Analysis of apoptosis and cell death in B cells
Bone marrow B cells were enriched as described above and stained with fluorochrome-conjugated antibodies specific for B220, IgM, c-Kit, and CD19. Cells were washed in M199+ one time, and then subsequently washed twice in 100μl of Annexin V Binding Buffer (Biolegend) and then resuspended at a concentration of 106 cells/ml in Annexin Binding Buffer. 5μl of Annexin V (Biolegend) and 10μl of 7-AAD (eBioscience) were then added for 15 minutes and washed with Annexin-V binding buffer and analyzed by FCM.
LacZ staining of bone marrow sections
Tibiae and calvariae from 6-month old and Sost−/− and wild-type littermate control mice were prepared and sectioned as previously described (32). Samples were decalcified, stained with X-gal, paraffin processed, sectioned and counterstained with Nuclear Fast Red. All photos were taken near growth plates and trabecular bone regions at 1000X magnification with oil immersion.
Bone marrow transplantation assay
Sost−/−→WT and WT→ Sost−/− bone marrow chimeras were generated. All recipient mice were lethally irradiated with 1000 rads using a Cesium-137 source (J.L. Shepherd and Associates, San Fernando, CA), and a minimum of 4 hours were allowed to pass before bone marrow reconstitution. For the Sost−/−→WT chimeras, B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+) recipients were transplanted with 5 × 106 Sost−/− CD45.2+ bone marrow cells (BMC) via retro-orbital intravenous injection. Control WT(CD45.2)→WT(CD45.1) chimeras were prepared by transplantation of wildtype C57BL/6J BMC into wildtype or B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+) recipients. For the reciprocal WT→ Sost−/− chimeras, C57BL/6J or Sost−/− recipients (both CD45.2+) were transplanted with B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) BMC, and control WT(CD45.1)→WT(CD45.2) chimeras were prepared as described above. Peripheral blood samples were stained for CD45.1, CD45.2, Gr-1, CD11b, CD3ε, and CD19 and analyzed for the presence of donor chimerism at 3 weeks by FCM. Chimeras were euthanized at 5 weeks post-transplantation for analysis of donor hematopoietic lineages in the bone marrow and spleen.
Statistical analysis
Differences between the means of biological replicates for all samples were calculated using two tailed T-test (GraphPad Prism, La Jolla, CA, USA). The two tailed T-test was justified by the assumption that all samples follow a Gaussian distribution even though sample sizes are small, and are not paired samples. All samples were considered statistically significant if p < 0.05.
RESULTS
Reduction of B cells in the bone marrow of Sost−/− mice
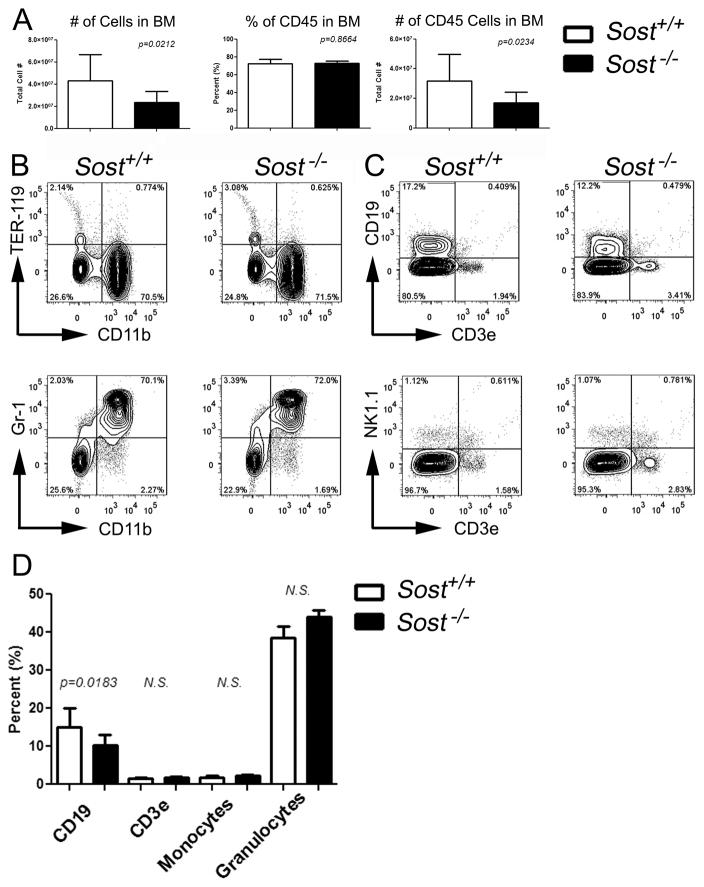
Sost−/− mice were generated using conventional gene targeting methods, in which the Sost open reading frame was replaced with LacZ to generate the null allele (30). Sost−/− mice display a high bone mass phenotype and reduced BM cavity volume in both male and female mice, very similar to the phenotype of the Sost knockout mice generated by Li et al. (28,30). Consistent with this, the total numbers of BM cells and CD45pos (hematopoietic) cells were significantly decreased in Sost−/− mice (Figure 1A). However, no difference in the percentage of CD45pos cells was observed between Sost−/− and wild-type (Sost+/+) controls (Figure 1A). Using established cell surface markers to distinguish HSCs and lineage-committed progenitors (33,34), we analyzed the cellular composition of the BM of Sost−/− mice. Given their documented increase in osteoblast activity and Wnt signaling, we hypothesized that Sost−/− mice would display an increase in HSCs. On the contrary, we observed no differences in the frequency or absolute number of HSCs, common lymphoid progenitors (CLP), common myeloid/megakaryocyte erythroid progenitors (CMP/MEP), or granulocyte/monocyte progenitors (GMP) (Figure S1 and Table S1). Therefore, the loss of Sost was not sufficient to influence changes in Lineageneg Sca-1high c-kithigh (LSK) HSCs or other hematopoietic progenitor populations.
Figure 1. CD19pos B cell populations in the bone marrow are reduced in Sost−/− mice.
(A) Total number of bone marrow cells (left panel), total percentage (middle panel) and total number of CD45pos (right panel) in the bone marrow. (B) FCM plots of myeloid lineages in wildtype (Sost+/+) and Sost−/− mice. (C) FCM plots of lymphoid lineages in wild type and Sost−/− mice. (D) Total percentages of B cells (CD19pos), T cells (CD3εpos), monocytes (CD11bpos Gr1neg) and granulocytes (CD11b pos Gr-1pos). Data are representative of Sost+/+ (n=6) and Sost−/− (n=12) of pooled sexes at 8 to 13 weeks of age. Mean ± SD are shown, and were considered to be statistically significant if p < 0.05, two-tailed Student’s T-test.
We also examined the frequencies of committed lymphoid and myeloid lineages in Sost−/− mice. with the clear reduction in overall BM cellularity, the numbers of cells amongst all lymphoid and myeloid lineages were severely reduced in Sost−/− mice (Table S2). No differences in the frequencies of T lymphocytes (CD3εpos), natural killer cells (NK1.1pos), monocytes (CD11bpos Gr-1neg), granulocytes (CD11bpos Gr-1pos) and erythroid cells (TER-119pos) were observed in the BM (Figures 1B – 1D). However, CD19pos B cells were significantly reduced in both their frequency and cell number in the BM (Figures 1C, 1D and Table S2), indicating a B cell-specific defect due to the absence of Sost.
Elevated apoptosis in B cells in the bone marrow of Sost−/− mice
B cell maturation in the BM proceeds through a series of steps that have been defined by cell surface marker expression. HSCs differentiate into CLP, which then give rise to the early pre/pro-B cell progenitor (also known as Fraction A) identified as negative for CD3ε, CD4, CD8, CD11b, Gr-1, NK1.1, Ter119, CD19, IgM, and c-Kit and positive for B220 (35). Subsequent immunoglobulin heavy chain gene rearrangements ensure commitment and differentiation into pro-B cells (also known as Fraction B/C) that are CD19pos B220low c-kitpos IgMneg (36,37), but negative for other lineage-specific markers. Further rearrangement of light chain genes confers differentiation into the pre-B cell (also known as Fraction D) with subsequent c-Kit down regulation. Functionally immature B cells (CD19low B220low c-kitneg IgMpos) that survive negative selection become mature IgD-expressing B cells, which then migrate out of the BM into the periphery. These mature, recirculating B cells can then be identified by their surface phenotype (CD19high B220high c-kitneg IgMpos) when they return to the BM (35,37).
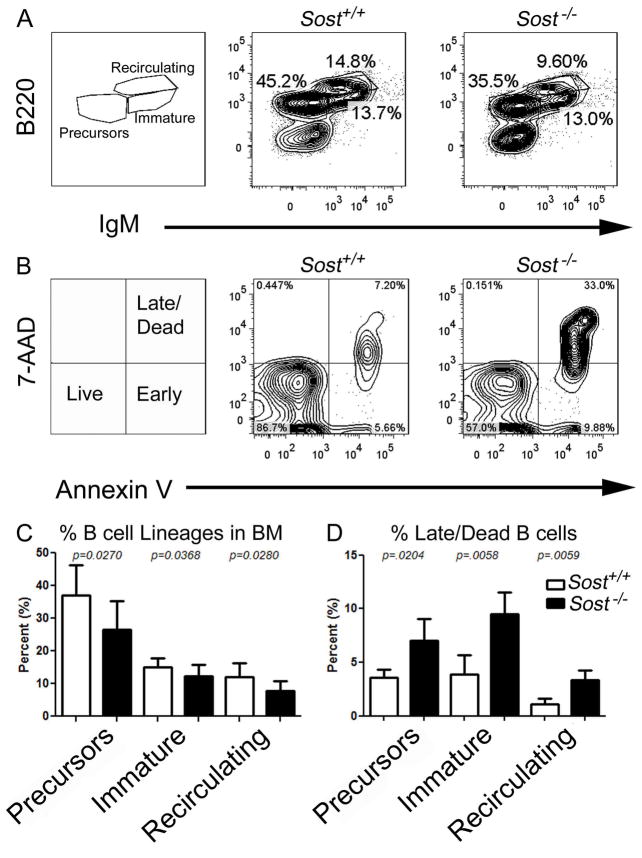
To identify if and where a block in B cell development occurred in Sost−/− mice, we examined the frequencies of the stages of B cell differentiation in the BM in Sost+/+ and Sost−/−mice. In our analysis, we used a staining strategy in which pre/pro-, pro- and pre-B cells are observed as one group (designated “B cell precursors” for simplicity), but immature and recirculating B cells in the BM can be distinguished (36–38). We observed significant decreases in the frequencies of all committed B cell developmental stages (Figures 2A, 2C and Table S3). Additional flow cytometric analysis using the Hardy nomenclature confirmed that block in B cell development occurred very early at the Fraction B (pro-B/pre-B-1) stage, and this block is maintained until the Fraction D (late pre-B) stage in Sost−/− mice (Figure S2). In addition, the number of mature B cells in Fraction F was notably decreased (Figure S2). The decline in B cells directly correlated with increased levels of apoptotic cells at the “precursor”, immature and recirculating stages of B cell development in Sost−/− mice, as measured by co-staining with Annexin V and 7-AAD (Figures 2B and 2D). However, no difference in apoptosis was evident in the Lineageneg CD19neg B220neg IgMneg populations, which contain the HSC and CLP, and stromal populations in the bone marrow (data not shown).
Figure 2. Elevated B cell apoptosis in Sost−/− mice.
(A) FCM plots showing B cell developmental stages from bone marrow. The left panel shows the gating strategy for B cell “precursors” (which includes pre/pro-, pro- and pre-B cells combined), immature and recirculating B cell populations. The middle and the right panels represent staining from the Sost+/+ and Sost−/− mice, respectively. (B) Representative analysis of apoptosis by FCM in “precursor” B cells, as measured by staining with Annexin-V and 7-AAD. Live (Annexin-Vneg, 7-AADneg), early apoptotic (Annexin-Vpos, 7-AADneg) and combined late apoptotic and dead (Annexin-Vpos, 7-AADpos) B cells are discriminated. (C) Total percentages of “precursors” (B220pos, IgMneg) (left panel), immature (B220pos, IgMpos) (middle panel) and recirculating (B220hi, IgMpos) (right panel) B cells in Sost+/+ (n=8) and Sost−/− (n=10) mice. (D) Total percentages of Annexin-Vpos, 7-AADpos ”pre-cursor” (left panel), immature (middle panel) and recirculating (right panel) B cells in Sost+/+ and Sost−/− bone marrow. Data are representative of Sost+/+ (n=4) and Sost−/− (n=3) that are of pooled sexes and 12 to 15 weeks of age. Mean ± SD are shown, and all data were considered to be statistically significant if p < 0.05, two-tailed Student’s T-test.
Interestingly, the observed decrease in B cell populations in the bone marrow did not extend to the spleen, and but we did note an increase in splenic granulocytes of Sost−/− mice (Figures S3 and S4). Splenic B cells in Sost−/− mice were comparable to wildtype mice in frequency and in function when stimulated by lipopolysaccharide (Figures S5 and S6). These data indicate that the reduction of B cells observed in the BM of Sost−/− mice is due to increased apoptosis at all committed B cell developmental stages in the bone marrow, but does not affect survival and antigen response in peripheral lymphoid organs.
Expression of Wnt signaling pathway and target genes in B cells
High expression of Sost mRNA has been reported in osteocytes, with associated diffuse SOST protein staining in osteocytic dendrites and canaliculi (39). To assess whether the B cell phenotype observed in Sost−/− mice is due to a cell-autonomous versus non-cell-autonomous defect in the BM niche, we examined purified “precursor”, immature, and recirculating B cell populations from the bone marrow for expression of Sost by RT-PCR. Sost expression was not observed in any B cell population (Figures 3A and 3B), supporting that the effect of the absence of Sost on B cells is non-cell-autonomous.
Figure 3. Wnt target genes in Sost−/− B cell populations.
(A) B cell precursors, immature and recirculating B cells were examined for the expression of Sost, Lrp4, Lrp5, and Lrp6, and β-actin. Sost+/+ bone was used as the positive control (“+ control”) tissue. (B) LacZ RT-PCR analysis to determine Sost expression in Sost+/+ (left) and Sost−/− (right) B cell subsets. Sost−/−collagenase-digested bone was used as the positive control tissue for LacZ. (C) qRT-PCR for Wnt target genes Lef-1, c-Myc and Ccnd1 in sorted B cell subsets. Rpl-7 was used as the housekeeping gene. Relative gene expression in Sost−/− mice was calculated by normalizing to expression in the Sost+/+ controls. Mean ± SD are shown from three mice of each genotype, and were considered to be statistically significant if p < 0.05, two-tailed Student’s T-test.
All purified B cell populations expressed Lrp5 and Lrp6 but lacked expression of Lrp4 (Figure 3A). In hematopoietic stem cells and progenitors, Lrp4 is not expressed, Lrp5 is differentially expressed, and Lrp6 is universally expressed (Figure S7). We hypothesized that the lack of SOST binding to LRP5 and/or LRP6 on developing B cells could result in hyperactive Wnt signaling, and this could be measured in Sost−/− B cells by the expression of known Wnt target genes, such as Ccnd1 (also known as cyclin-D1), Lef-1, and c-Myc to see if these genes were increased in the absence of Sost (40,41). Amongst “precursor” and immature B cells, no differences in Lef-1, c-Myc or Ccnd1 expression was observed. Expression of c-Myc increased up to two-fold in the recirculating B cells in Sost−/− mice (Figure 5C). These data showed that in the absence of Sost, expression of these Wnt target genes was unchanged in the early stage B cells, but differentially affected in recirculating B cells.
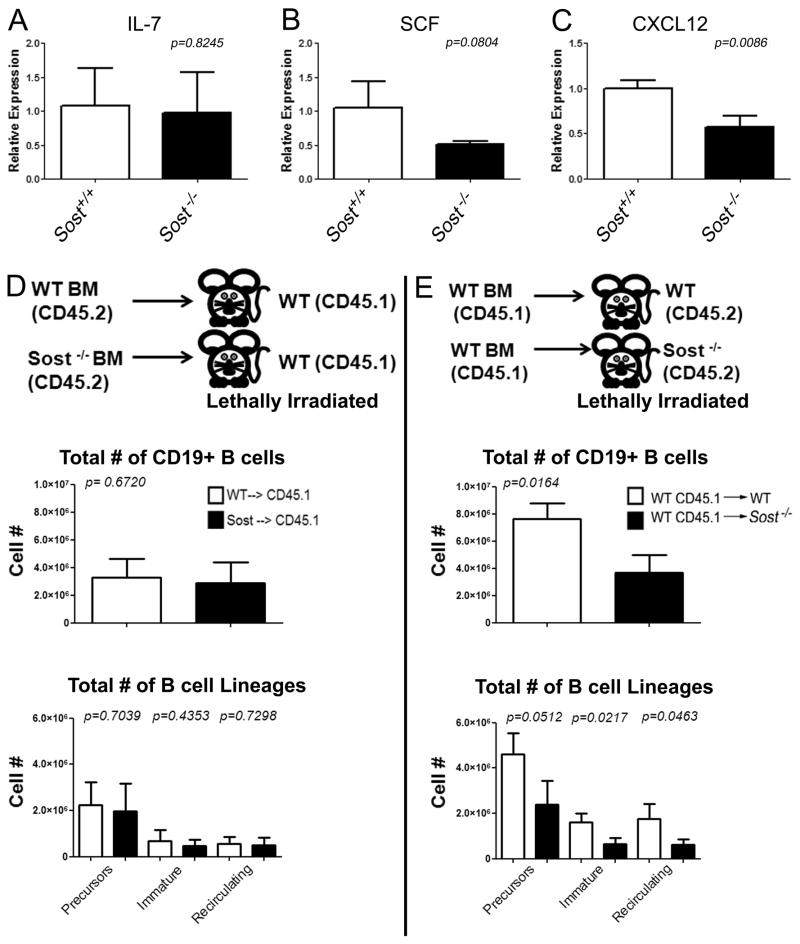
Figure 5. Evidence that the B cell defect in Sost−/− mice is cell-extrinsic.
Expression analysis of Il-7 (A), Scf (B) and Cxc12 (C) by qRT-PCR. mRNA was extracted from digested, bone-marrow flushed bones. Rpl-7 was used as the housekeeping gene. Relative gene expression in Sost−/− mice was calculated by normalizing to expression in the Sost+/+ control. (D) Experimental scheme of bone marrow transplantation of CD45.2+ WT (n=5) or Sost−/− (n=5) bone marrow into CD45.1+ WT recipients (top panel). CD45.2+ (donor) cells were gated for analysis post-transplantation. The total number of donor-derived CD19+ cells in the bone marrow (middle panel), and the total number of B cell precursors, immature and recirculating B cells (bottom panel) in WT(CD45.2)→WT(CD45.1) and Sost−/−→WT chimeras are shown. (E) Scheme of reciprocal bone marrow transplantation of CD45.1+ WT bone marrow into CD45.2+ WT (n=3) or Sost−/− (n=3) mice (top panel). Donor CD45.1+ cells were gated for analysis post-transplantation, and the total number of donor-derived CD19+ cells in the bone marrow (middle panel), and the total number of donor-derived B cell precursors, immature and recirculating B cells (bottom panel) in WT(CD45.1)→WT(CD45.2) and WT→Sost−/− chimeras are shown. Mean ± SD are shown from age and sex matched mice, and were considered to be statistically significant if p < 0.05, two-tailed Student’s T-test.
Sost is not expressed in any hematopoietic lineages in the bone marrow
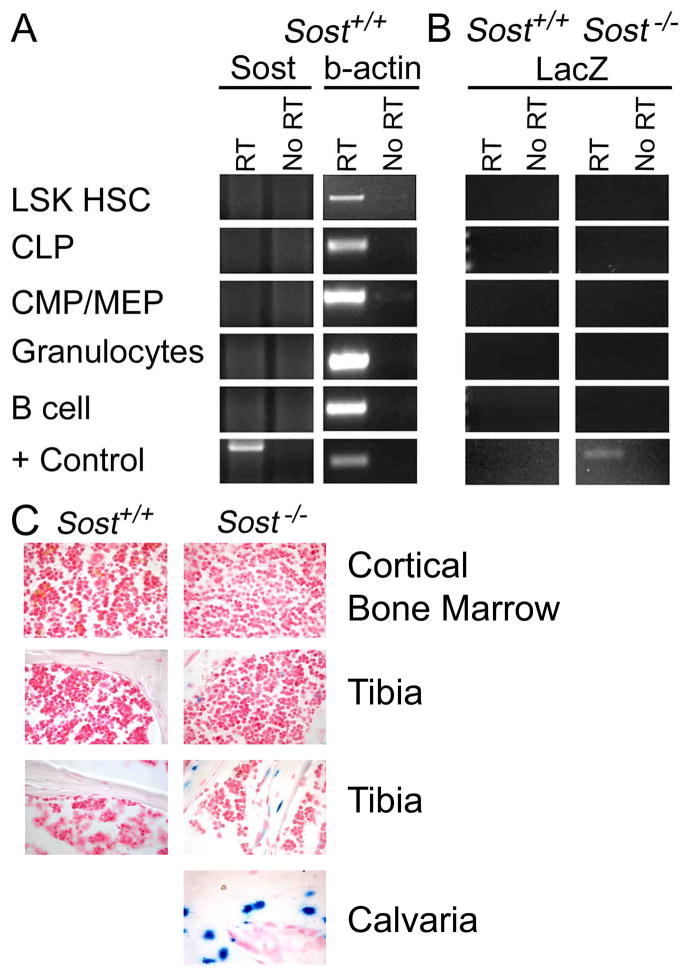
We also examined all hematopoietic progenitors and committed lineages in the BM of Sost+/+ mice for Sost expression by RT-PCR, and did not observe Sost expression in any of these cells (Figure 4A and data not shown). In contrast, Sost was clearly expressed in cells obtained from collagenase-digested bone (Figure 4A). These results were confirmed by RT-PCR for LacZ, which is a knocked-in reporter for endogenous Sost expression in Sost−/− mice (Figure 4B). The RT-PCR results were further validated by histology of whole bone sections (in which osteocytes as well as the BM cavity cells can be observed). Sost expression, as reported by LacZ activity, was clearly observed in the osteocytes in the tibias and calvaria of Sost−/− mice, but not in wildtype mice. In contrast, very low levels of LacZ activity were observed in the BM cavity (Figure 4C).
Figure 4. Sost is restricted to non-hematopoietic lineages.
(A) Sost expression was determined using RT-PCR of mRNA isolated from FCM-sorted LSK HSC, CLP, CMP, granulocytes and B cells from Sost+/+ mice. β-actin was used as a housekeeping gene and internal control. The positive control tissue for Sost expression was collagenase-digested bone. (B) RT-PCR for LacZ in sorted hematopoietic cell lineages in Sost−/− mice. mRNA from collagenase-digested bones from Sost−/− mice was used as the positive control for LacZ. RT-PCR for Sost in the Sost−/− mice was negative in all tissues examined (data not shown). (C) Sost+/+ and Sost−/− cortical bone marrow (top), trabecular (two middle) and calvarial (bottom) 6 μm whole bone sections were stained for LacZ activity using β-gal (blue) and counterstained with Nuclear Fast Red. Sost−/−calvaria sections were used as a positive control for LacZ activity. Representative images from 20 slides prepared from 2 Sost+/+ and 2 Sost−/− mice are shown.
Cxcl12 expression is significantly reduced in bone marrow stromal cells in Sost−/− mice
The lack of Sost expression in hematopoietic cells and its clear expression in the non-hematopoietic cells supported the idea that the B cell defect observed in Sost−/− mice is non-cell autonomous, and implicated the osteoblast, osteocyte or other stromal cell populations in the bone as the source. B cell development, proliferation and survival in the BM rely on the production of interleukin (IL-7), stem cell factor (SCF), and CXCL12 (also known as SDF-1), which are produced by BM stromal cells (35). Examination of Il-7 and Scf levels by quantitative PCR of collagenase-digested bones showed no statistical difference between Sost−/− and wildtype controls, although the data appeared to show a trend towards reduction of Scf levels in Sost−/−mice (Figures 5A and 5B). Cxcl12 is highly expressed in bone marrow stromal cells including osteoblasts, endothelial cells and reticular cells, but is not expressed in hematopoietic cells (35,42). Cxcl12 was significantly reduced in Sost−/− mice, providing an possible explanation for their altered B cell development (Figure 5C).
Bone marrow transplantation assays confirm a non-cell autonomous role of Sost on B cell development
The reduction of Cxcl12 and the lack of Sost expression in hematopoietic cell populations indicated that the reduction of B cells in Sost−/− mice was indeed due to a non-cell autonomous effect. To further test this hypothesis, we performed reciprocal bone marrow transplantation experiments, in which WT →Sost−/− and Sost−/−→WT bone marrow chimeras were prepared. We hypothesized that if the effect of the absence of Sost on bone marrow B cells was cell extrinsic, then transplantation of WT bone marrow into Sost−/− mice would result in a block in B cell development beginning at the “precursor” stage, but Sost−/− bone marrow transplanted into WT recipients would be result in normal B cell development. Sost−/− bone marrow transplanted into WT hosts engrafted and differentiated similarly to WT → WT control chimeras (Figure 5D). In contrast, transplantation of WT bone marrow into Sost−/− recipients resulted in a decrease in CD19+ B cells, as well as a significant decreases in immature and recirculating B cell populations (Figure 5E) in the chimeras, similar to that observed in the Sost−/− mice (Figure 2). These results confirm that the bone microenvironment of the Sost−/− mice is unable to sufficiently support B cell development in the bone marrow, and the effect of Sost on B cell development is non-cell autonomous.
DISCUSSION
Here, we demonstrate that the Wnt antagonist SOST plays an important role in bone marrow B cell development in the BM through a non-cell autonomous mechanism. Substantial reductions in CXCL12 in the stromal cells of Sost−/− mice are likely to be the causative mechanism for reduced B cell numbers in these mice (43). Very recently, it has been shown that activation of Wnt signaling decreases CXCL12 expression in BM stromal cells in vitro (44), which supports our conclusions and provides a feasible link between Sost, Wnt signaling, and B cell development. Conditional ablation of osteoblasts resulted in blocks at the early pre/pro-B, pre-B and/or pro-B cell developmental stages or total loss of B cell development in the BM (7,45,46). We propose a model in which osteocyte-secreted SOST regulates Wnt signaling in BM stromal cells and their production of Cxcl12 at levels that are permissive for the support of B cell differentiation (Figure 6A). According to this model, overactive Wnt signaling in the stromal cells in the absence of Sost results in a reduction of Cxcl12 to levels that are not conducive for B cell survival (Figure 6B). We speculate that this occurs via a set of events in which Sost normally promotes bone homeostasis by blocking osteoblast differentiation directly (39), which in turn, perhaps affects the differentiation or function of early mesenchymal stem cells (MSC) or osteoprogenitor populations. MSC and other BM stromal cells (namely, CXCL12 abundant reticular cells (47) are located in the BM cavity and have been shown to express CXCL12 and produce appropriate B cell microenvironments, reinforcing our ideas. However, the causative link between Sost, changes in osteolineage cells, the reduction in Cxcl12 expression and altered B cell development must still be experimentally verified. Clearly, the elucidation of the exact mechanisms by which SOST indirectly promotes B cell development will benefit from the creation of new osteoprogenitor-specific and BM stromal-cell specific transgenic and knockout mouse strains, and identification of biomarkers that can distinguish between cells at distinct stages of osteogenesis as well as different stromal cell types (48).
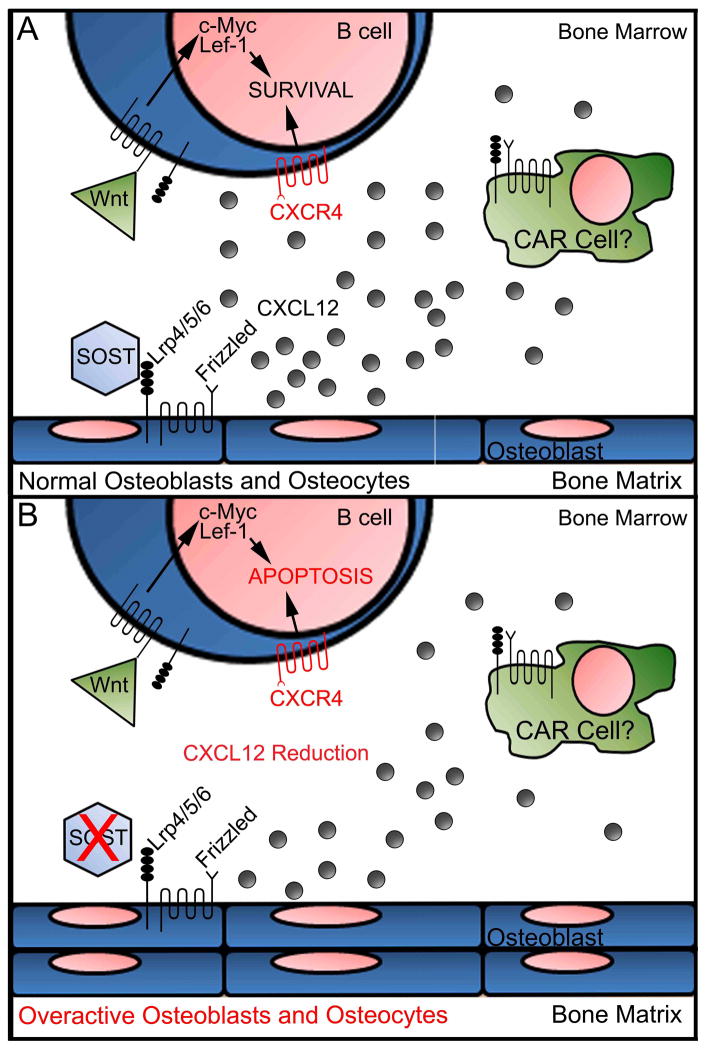
Figure 6. Proposed model for the effect of Sost on B lymphopoiesis.
(A) Under normal circumstances, osteocytes secrete SOST, which binds to LRP4, LRP5 or LRP6 (which are associated with Frizzled receptors) to regulate maturation of osteoblasts into osteocytes. It is unclear whether SOST can directly bind to LRP5/6 receptors on B cells or if SOST can regulate Wnt signaling in other cell types (e.g. CXCL12 abundant reticular (CAR) cells or osteoclasts (not depicted in figure)). In this model, CXCL12 expression (represented by gray circles) by endosteal OBs and CAR cells is activated by downregulation of Wnt signaling via SOST, to promote B cell survival via CXCL12/CXCR4 signaling. (B) Sost deletion results in excessive osteoblast differentiation into osteocytes, resulting in high bone mass. In addition, CXCL12 expression by Sost−/− osteoblasts and/or other stromal cell populations is reduced due to lack of SOST-mediated inhibition of Wnt signaling. In turn, this reduction of CXCL12 results in the induction of apoptosis at all B cell stages in the bone marrow.
The possibility that SOST could directly bind to LRP5 or LRP6 on developing B cells to antagonize Wnt activation is not formally excluded by our results. Lrp5−/− mice display an osteoporotic bone phenotype, but have normal B cell development (C.J.C., unpublished results). Lrp6−/− mice are embryonically lethal (26), hence precluding the study of B cell development in these mice. Our results, which show no difference in the expression of Wnt target genes in B cell precursors and immature stages of B cell development, suggest that direct regulation of Wnt signaling by SOST at these stages is irrelevant for their development and support the idea that the effect of SOST on B cell development is non-cell autonomous. Our bone marrow transplantation studies confirm the hypothesis that the reduction in B cells is due to alterations in the bone microenvironment in Sost−/− mice. increase in c-Myc expression in mature, recirculating B cells and its relationship to apoptosis is unclear, as recirculating B cells in c-Myc-deficient mice do not undergo apoptosis, and no studies have explicitly examined c-Myc overexpression and mature B cell survival together (49,50).
We cannot completely rule out that hematopoiesis and B cell development are simply negatively regulated by a hypermineralized environment or by the size of the BM cavity, independent of SOST. HSC self-renewal, colony-forming ability, and hematopoietic differentiation appears to be negatively affected by osteoblast mineralization in vitro [(51) and C.J.C., unpublished results)]. Several knockout mouse models which display reduced BM cavity size and similar defects in hematopoiesis to that observed in Sost−/− mice exist. For example, the op/op, oc/oc, mi/mi, and Fos−/− mice are models of osteopetrotic disease that present with small BM cavities and defective B lymphopoiesis in the BM. In contrast to the Sost−/− mice, whose high bone mass is caused by overactive osteoblasts that produce high quality bone, the high bone mass in the aforementioned mice are caused by defective or absent osteoclasts, which results in poor quality, fragile bone (52,53). Pharmacological inhibition of osteoclasts by zoledronic acid also adversely affects B cell differentiation by reducing the levels of CXCL12 and IL-7 produced by BM stromal cells (53). In digested bones of Sost−/− mice, we observed a significant reduction of Cxcl12, evidence of reduced Scf, but no changes in Il-7 expression. Interestingly, evidence that B cells are needed for proper bone homeostasis also exists. For example, IL-7R knockout mice lack B cell development past the pre-B cell stage, and present with increased bone mineral density (54). Paradoxically, μMT-knockout mice with a genetic mutation of the mu immunoglobulin heavy-chain constant region, also display a block at the pre-B cell stage but have the opposite bone phenotype (55). In addition, B cells are an important source of RANK ligand that induces osteoclast maturation, promoting bone homeostasis (2). Taken together, our results and these data reinforce the idea that reciprocally beneficial crosstalk exists between cells involved in bone homeostasis and hematopoiesis. Further experimentation is required to investigate the mechanisms by which physical space is detected and interpreted by developing B cells in the BM.
Other Wnt antagonists, such as Dickkopf-1 (DKK1) and SFRP-1, are robustly expressed in osteoblasts and possibly other cell types in the BM, and it is possible that DKK1 could compensate for the loss of SOST (34,56). Dkk1deficiency results in high bone mass phenotypes, while the overexpression of Dkk1 in osteoblasts of Dkk1-transgenic (Tg) mice resulted in reduction of trabecular bone (34). Dkk1-Tg mice did not display any overt phenotype in the frequencies or absolute numbers of any hematopoietic cell lineages, similar to our observations in the Sost−/− mice. We have observed an increase in Dkk1 mRNA expression in Sost−/− mice (data not shown). The observation that no changes in the frequencies of HSC, CLP, CMP/MEP and GMPs in Sost−/− mice lead us to conclude that DKK1 or other bone-derived Wnt antagonists could compensate for the loss of SOST for these cell subsets. The effect of Dkk1 loss-of-function on hematopoiesis is unknown, and it would be interesting to investigate whether different Wnt antagonists render distinct effects on HSC maintenance and differentiation.
The spleen is an alternative site of hematopoiesis that can sometimes compensate for non-ideal BM environments. Spleens of Sost−/− mice are increased in mass, but show no evidence of extramedullary hematopoiesis or an increase in HSCs (data not shown), which supports that the role of Sost in hematopoiesis is limited to B cell development in the bone marrow (43,57). Recirculating B cells in the bone marrow include plasma cells which produce high levels of antigen-specific antibodies after stimulation in secondary lymphoid organs. Plasma cell migration back to the BM is believed to act as an efficient way to release these antibodies into the circulation during infection. The reduction of recirculating B cells in the BM suggests that the BM environment of Sost−/− mice is not conducive for B cell survival or plasma cell maintenance even after they mature in the periphery, and/or that the low levels of CXCL12 in the Sost−/− BM is not sufficient for retention of mature B cells homing from the periphery.
Since inhibition of SOST has been proposed as a pharmacologic target for the anabolic stimulation of bone formation in the treatment for osteoporosis and other bone thinning disorders (58), our findings that B cell survival is impaired in the Sost−/− mice suggest that patients receiving these treatments be closely monitored for alterations in B cell development and their ability to combat infection. Common variable immunodeficiency disease can be diagnosed by antibody deficiency and impaired immune responses to bacterial infections or vaccinations (59). Although LPS-induced proliferation of Sost−/− splenic B cells was normal, it is possible that B cell-mediated immune responses to diverse antigenic challenges may be affected in the absence of SOST. Further detailed analyses of B cell proliferation to T-dependent antigens, cytokine production, isotype class switching and the development and survival of plasma cells and memory B cells are required to identify any contribution of SOST in acquired immunity and susceptibility to infection.
Supplementary Material
Acknowledgments
C.J.C. is a PhD candidate from UC Merced submitting this work as a partial fulfillment in the requirement for the PhD. We are grateful to Summer McCloy, Deepa Murugesh, Roy Hoglund and all the staff members of the UC Merced Laboratory Animal Resource Center and Livermore National Laboratory Animal Care Facility for their excellent mouse care. We also thank Mike Cleary, Jesus Ciriza and Lyn Hsu for their comments on the manuscript.
Grant Support: This work was funded by a UC Merced Graduate Student Fellowship to C.J.C., the University of California, and the California Institute for Regenerative Medicine (RN1-00554-1) to J.O.M. N.M.C. and G.G.L. were supported by NIH DK075730. Lawrence Livermore National Laboratory is operated by Lawrence Livermore National Security, LLC, for the U.S. Department of Energy, National Nuclear Security Administration under Contract DE-AC52-07NA27344.
Footnotes
Supplemental material is included with this manuscript.
DISCLOSURE PAGE
All authors have no conflicts of interest.
C.J.C. designed and performed the majority of the experiments. R.R. and B.M. provided technical assistance in qPCR and flow cytometry. N.M.C. performed the histological analysis of bone sections. G.G.L. provided Sost−/− mice, critical input into experimental design, data analysis and manuscript writing. J.O.M. was responsible for experimental conception and design, financial support, data analysis and interpretation, manuscript writing and approval of the final manuscript.
References
- 1.Nakahama K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67(23):4001–4009. doi: 10.1007/s00018-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz MC, Fretz JA, Lorenzo JA. How B cells influence bone biology in health and disease. Bone. 2010;47(3):472–479. doi: 10.1016/j.bone.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacifici R. The immune system and bone. Arch Biochem Biophys. 2010;503(1):41–53. doi: 10.1016/j.abb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Can A. Haematopoietic stem cells niches: interrelations between structure and function. Transfus Apher Sci. 2008;38(3):261–268. doi: 10.1016/j.transci.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 6.Wu JY, Scadden DT, Kronenberg HM. The Role of the Osteoblast Lineage in the Bone Marrow Hematopoietic Niches. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 8.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 10.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 13.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguila HL, Rowe DW. Skeletal development, bone remodeling, and hematopoiesis. Immunol Rev. 2005;208:7–18. doi: 10.1111/j.0105-2896.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 15.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473(2):98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann C. Transcriptional networks controlling skeletal development. Curr Opin Genet Dev. 2009;19(5):437–443. doi: 10.1016/j.gde.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4(1):27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24(2):171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38(7):1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Q, Quinn WJ, 3rd, Salay T, Crowley JE, Cancro MP, Sen JM. Role of beta-catenin in B cell development and function. J Immunol. 2008;181(6):3777–3783. doi: 10.4049/jimmunol.181.6.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol. 2008;181(6):3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quarto N, Behr B, Longaker MT. Opposite spectrum of activity of canonical Wnt signaling in the osteogenic context of undifferentiated and differentiated mesenchymal cells: implications for tissue engineering. Tissue engineering. 2010;16(10):3185–3197. doi: 10.1089/ten.tea.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nature cell biology. 2007;9(11):1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 25.Baksh D, Tuan RS. Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212(3):817–826. doi: 10.1002/jcp.21080. [DOI] [PubMed] [Google Scholar]
- 26.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 27.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 29.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68 (3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Lowik CW, ten Dijke P. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem. 2010;285(53):41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravano DM, Manilay JO. Inhibition of proteolysis of Delta-like-1 does not promote or reduce T-cell developmental potential. Immunol Cell Biol. 2010;88(7):746–753. doi: 10.1038/icb.2010.30. [DOI] [PubMed] [Google Scholar]
- 32.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22 (12):1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26(6):678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 36.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol. 2003;171(11):5921–5930. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 37.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3(4):399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 39.Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87(2):99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13(1):15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 41.Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(9):3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20 (6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Tamura M, Sato MM, Nashimoto M. Regulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cells. Int J Biochem Cell Biol. 2011;43 (5):760–767. doi: 10.1016/j.biocel.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009;37(1):19–30. doi: 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallespinos M, Fernandez D, Rodriguez L, Alvaro-Blanco J, Baena E, Ortiz M, Dukovska D, Martinez D, Rojas A, Campanero MR, Moreno de Alboran I. B Lymphocyte commitment program is driven by the proto-oncogene c-Myc. J Immunol. 2011;186(12):6726–6736. doi: 10.4049/jimmunol.1002753. [DOI] [PubMed] [Google Scholar]
- 50.de Alboran IM, Baena E, Martinez AC. c-Myc-deficient B lymphocytes are resistant to spontaneous and induced cell death. Cell Death Differ. 2004;11(1):61–68. doi: 10.1038/sj.cdd.4401319. [DOI] [PubMed] [Google Scholar]
- 51.Cheng YH, Chitteti BR, Streicher DA, Morgan JA, Rodriguez-Rodriguez S, Carlesso N, Srour EF, Kacena MA. Impact of osteoblast maturational status on their ability to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res. 2010 doi: 10.1002/jbmr.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagaya H, Kunisada T, Yamazaki H, Yamane T, Tokuhisa T, Wagner EF, Sudo T, Shultz LD, Hayashi SI. Intramedullary and extramedullary B lymphopoiesis in osteopetrotic mice. Blood. 2000;95(11):3363–3370. [PubMed] [Google Scholar]
- 53.Mansour A, Anginot A, Mancini SJ, Schiff C, Carle GF, Wakkach A, Blin-Wakkach C. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011 doi: 10.1038/cr.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M, Suda T. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci U S A. 1997;94(17):9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakajima H, Ito M, Morikawa Y, Komori T, Fukuchi Y, Shibata F, Okamoto S, Kitamura T. Wnt modulators, SFRP-1, and SFRP-2 are expressed in osteoblasts and differentially regulate hematopoietic stem cells. Biochem Biophys Res Commun. 2009;390(1):65–70. doi: 10.1016/j.bbrc.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 57.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200(9):1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papapoulos SE. Targeting sclerostin as potential treatment of osteoporosis. Ann Rheum Dis. 2011;70(Suppl 1):i119–122. doi: 10.1136/ard.2010.141150. [DOI] [PubMed] [Google Scholar]
- 59.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145(6):709–727. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.