Abstract
ABT-737 is a small molecule Bcl-2 homology (BH)-3 domain mimetic that binds to the Bcl-2 family proteins Bcl-2 and Bcl-xL and is currently under investigation in the clinic. In this study, we investigated potential mechanisms of resistance to ABT-737 in leukemia cell lines. Compared with parental cells, cells that have developed acquired resistance to ABT-737 showed increased expression of Mcl-1 in addition to post-translational modifications that facilitated both Mcl-1 stabilization and its interaction with the BH3-only protein Bim. In order to sensitize resistant cells, Mcl-1 was targeted by two pan-Bcl-2 family inhibitors, obatoclax and gossypol. While gossypol was effective only in resistant cells, obatoclax induced cell death in both parental and ABT-737-resistant cells. NOXA levels were increased substantially by treatment with gossypol and its expression was critical for the gossypol response. Mechanistically, the newly generated NOXA interacted with Mcl-1 and displaced Bim from the Mcl-1/Bim complex, freeing Bim to trigger the mitochondrial apoptotic pathway. Together, our findings indicate that NOXA and Mcl-1 are critical determinants for gossypol-mediated cell death in ABT-737-resistant cells. These data therefore reveal novel insight into mechanisms of acquired resistance to ABT-737.
Keywords: Mcl-1, Noxa, ABT-737, gossypol, B-cell tumor
Introduction
The interplay between Bcl-2 family members is essential for controlling the mitochondrial cell death pathway and thereby the survival of most cells, including those of hematopoietic origin. Based on their Bcl-2 homology (BH) domains, the Bcl-2 proteins have been grouped in three classes: anti-apoptotic (containing the BH1–BH4 domains), pro-apoptotic, containing the BH1–BH3 domains, and those with the BH3 domain-only (1,2). The members of this group regulate mitochondrial outer membrane permeabilization (MOMP), monitoring release of cytochrome c and activating downstream effector caspases (3). The imbalance in expression of these partners has been implicated in development of various tumor types and resistance to chemotherapeutic regimens (1). This often results from high-level expression of anti-apoptotic members, such as Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1 that prevent cell death by sequestering BH3-only proteins, such as Bim, Puma, and Noxa, and regulate activation of the pro-apoptotic proteins Bax and Bak. In most of these cases, up-regulation and binding of significant amounts of anti-apoptotic proteins to activator proteins keeps these cells alive (1,2,4,5).
ABT-737 is a small molecule inhibitor that is effective against certain Bcl-2 family members. It has a strong affinity for Bcl-2, Bcl-xL, and Bcl-w that are bound to Bim (6) by releasing Bim from anti-apoptotic Bcl-2 partners, thereby initiating MOMP. The oral derivative of ABT-737, navitoclax (ABT-263) is currently under investigation in several clinical trials in lymphoid malignancies, such as chronic lymphocytic leukemia (CLL), and tumors, such as small cell lung cancer (7–10). Importantly, ABT-737-mediated cell death is Bax/Bak-dependent as Bax/Bak double knock-out mouse fibroblasts are resistant to this treatment (11). However, it is expected that even for the most effective chemotherapeutics acquired resistance to be a serious clinical problem, hence compounds that overcome drug resistance are of special interest in cancer therapy (7,12–15). Studies with solution competition assays have shown that ABT-737 has very weak affinity for Mcl-1 (16). Various in vitro and in vivo studies have shown that sensitivity to ABT-737 is decreased in cells expressing elevated levels of Mcl-1 (5). Moreover, cells initially sensitive to ABT-737 become resistant by up-regulating Mcl-1 levels (7).
To investigate the probable mechanisms of resistance to ABT-737, resistant cell lines were generated from pre-B tumor cells that developed increased levels of Mcl-1 protein that was also post-translationally modified. These Mcl-1-dependent ABT-737-resistant cells (ABT-R) were exquisitely sensitive to the pan-Bcl-2 inhibitor gossypol, but not obatoclax. Knockdown of Mcl-1 or Noxa overcame gossypol sensitivity of ABT-R cells. Gossypol-induced, NOXA-dependent cell death led to release of Bim from Mcl-1 in ABT-R cells. These studies reveal novel insights into regulation and role of Mcl-1 in response to ABT-737 and provide mechanistic approaches for overcoming the acquired resistance to ABT-737 in leukemic cells.
Materials and Methods
Cell lines and reagents
Human B-cell acute lymphoblastic leukemia (ALL) cell lines Nalm-6 and Reh were obtained from ATCC (Manassas, VA). These pre-B cells express CD19 and CD127 surface markers with rearranged immunoglobulin heavy chains. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals), L-glutamine, Antibiotic-antimycotic (Invitrogen). ABT-R cells were cultured in 5% FBS. Cell lines were routinely verified for growth rates, morphological characteristics, and response to stimuli using Trypan blue exclusion or Annexin V/Propidium iodide staining. Cell lines were periodically tested to be mycoplasma free and their passage number did not exceed 20. ABT-737 was provided by Abbott Laboratories (Abott Park, IL). Gossypol, actinomycin D, and cycloheximide were from Sigma-Aldrich and obatoclax from Selleck Chemicals.
Generation of ABT-737-resistant cell lines
Nalm-6 and Reh cells were cultured in increasing concentrations of ABT-737 administered intermittently, with the drug being washed off to allow cells to recover. Gradually, the ABT-737 concentration was increased until cells remained viable when ABT-737 concentrations double to that of their IC50 value was administered continuously. Cells were treated with verapamil (Sigma-Aldrich) to exclude the possibility of acquiring resistance due to increase in expression of drug efflux pumps (7, 17). The ABT-R cells were routinely monitored for resistance to ABT-737; they were cultured without drug for 72 h before performing experiments.
Flow cytometry
Cell death was measured by phosphatidylserine externalization (5), by staining with fluorescein-conjugated Annexin V (BD Biosciences, San Jose, CA) and propidium iodide, and analyzed on a BD FACS Calibur flow cytometer. The raw data obtained was analyzed by CellQuest Version 5.2.1 software. The results were normalized to survival of control cells that have been treated with DMSO or ethanol.
Immunoblotting and immunoprecipitation
Protein lysates were prepared with 1% NP-40 lysis buffer (20 mM Tris-HCl, pH 7.5; 150 mM Nacl; 1 mM EDTA; 1% NP-40) containing protease inhibitors (Roche) and phosphatase inhibitors cocktail 2 and 3 (Sigma). The cells were lysed for 30–45 min at 4°C. 50–60 μg of protein was resolved on 10%–12% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotting was performed with primary antibodies. For immunoprecipitation cells were lysed with CHAPS buffer (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 2% CHAPS, Calbiochem) containing protease and phosphates inhibitors for 1 h. Protein lysates were incubated with primary antibody overnight at 4°C followed by 1 h incubation of protein A agarose beads (eBioscience, Calbiochem) at 4°C. Immunoprecipitates were washed 3 times with CHAPS and eluted with loading buffer. The co-immunoprecipitated proteins were resolved by SDS-PAGE. For Bax activation, cells were lysed with 1% CHAPS lysis buffer for 1 h at 4°C and active Bax was immunoprecipitated with 6A7 (BD Bioscienes) and probed with N20 (Santa Cruz Biotechnology) antibodies. Primary antibodies used were for Mcl-1, Bim (BD-Biosciences), NOXA (Enzo Life Sciences), Bcl-2, Bcl-xL (Santa Cruz Biotechnology), PUMA (ProSci Corporation), USP-9X (Abnova), PARP1 (Cell Signaling), Mcl-1 (Ser-64) (18) (provided by Dr. Gregory J. Gores, Mayo Clinic), Mcl-1 (Thr-163) (Cell Signaling), phospho-Erk (Cell Signaling), and β-actin (Sigma).
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
RNA isolated from parental and ABT-R cells after ABT-737 treatment (500–1000 nM) for 4–24 h was examined by qRT-PCR, using primers for Mcl-1 and NOXA, and normalized for β-actin as described (5). Similar experiments were performed with ABT-R cells followed by gossypol (10 μM) at 12 h. The half-life of Mcl-1 mRNA was determined following actinomycin D treatment and qRT-PCR analysis.
Genetic manipulation of Mcl-1 and NOXA
NOXA knockdown was achieved in ABT-R Nalm-6 and Reh cells by shRNA delivered by lentivirus transduction (5). Single cell clones with stable expression of shNOXA (Santa Cruz; sc-37305-V), shMcl-1 (Sigma-Aldrich: NM_021960.3-3125s1c1, NM_021960.3-1001s1c1) and scrambled shRNA (control; sc-108080) were selected with puromycin (Sigma-Aldrich). NOXA shRNA lentiviral particles were obtained as a pool of concentrated, transduction-ready viral particles containing three target-specific constructs that encode 19–25 nt (plus hairpin). Mcl-1 shRNA lentiviral particles were prepared according to manufacturer’s protocol.
Statistical Analysis
Statistical comparisons between groups were conducted by 2-way ANOVA using Prism software. Standard deviation was calculated from experiments performed in triplicates and indicated as error bars. All experiments were repeated three times independently.
Results
Acquired resistance of B-cells to ABT-737 after prolonged exposure
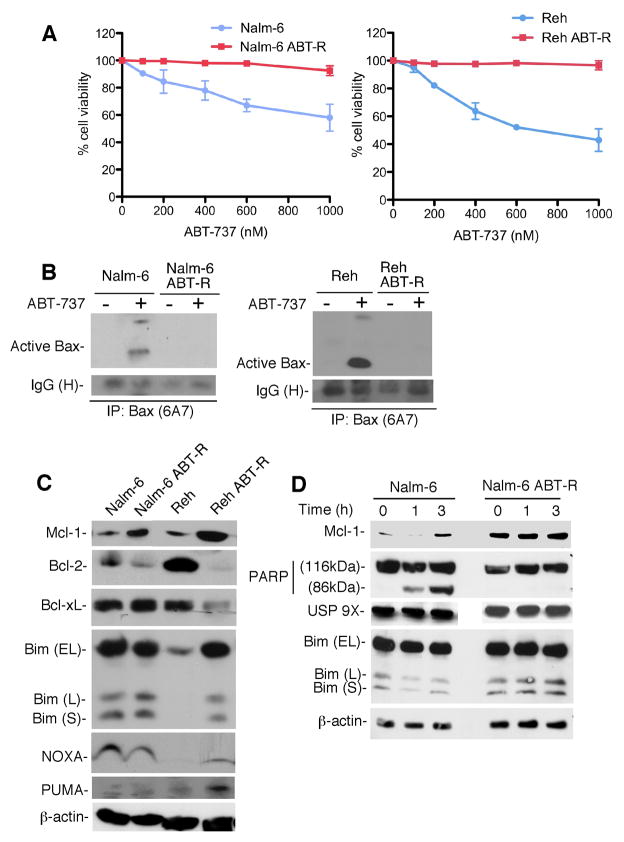
ABT-737 is a BH3-mimetic that binds only to certain Bcl-2 family proteins, such as Bcl-2, Bcl-xL, and Bcl-w, but not Mcl-1. In our previous studies we have shown that patients with increased Mcl-1 levels do not respond to ABT-737 and thereby become resistant to this drug (5). Hence, in order to investigate the mechanism responsible for resistance, we generated ABT-737-resistant (ABT-R) cell lines from initially sensitive pre-B Nalm-6 and Reh cells. These cell lines were selected for their sensitivity to ABT-737, with IC50 values of ~1000 and ~500 nm, respectively. The Nalm-6 ABT-R and Reh ABT-R cells generated could tolerate continuous exposure to ABT-737 at a concentration double to that of their IC50 value (Fig. 1A). It has been known that ABT-737 triggers Bax/Bak-mediated apoptosis (11). ABT-737 treatment led to Bax activation, as indicated by 6A7 antibody reactivity in Nalm-6 and Reh cells, but not in their resistant derivatives (Fig. 1B). These results are in support of how resistant cells could evade Bax-mediated apoptosis caused by ABT-737. Further examination of Bcl-2 family proteins by immunoblotting in parental and ABT-R cells indicated increased Mcl-1 levels in ABT-R cells (Fig. 1C). Bfl-1 levels were below our detection limit. We could also observe decreased Bcl-2 and increased Bim levels in Reh ABT-R cells, which were comparable to those found in Nalm-6 ABT-R cells. Puma and NOXA expression was not markedly altered (Fig. 1C). Mcl-1 protein levels increased in parental but not in ABT-R cells following acute ABT-737 treatment (Fig. 1D). In addition, there was no further change of Mcl-1 levels in ABT-R cells following 18 h ABT-737 exposure (Supplementary Fig. S1C). Parental cells were sensitive to ABT-737 and within 3 h they were committed to cell death, as indicated by cleavage of poly-(ADP-ribose) polymerase 1 (PARP1). Increased Mcl-1 levels were attributed to increased deubiquitination as a result of increased levels of the USP9X deubiquitinating enzyme (19), however, USP9X levels (Fig. 1D) and its association with Mcl-1 (data not shown) did not change in ABT-R cells. Our results show that ABT-737-resistant cells could evade Bax-mediated cell death and develop resistance by elevating Mcl-1 levels.
Figure 1. Acquired resistance development in leukemic cells.
(A) Parental and ABT-737 resistant (ABT-R) Nalm-6 and Reh cells were treated with ABT-737 for 24 h. Cell viability is shown as a percentage of Annexin V-FITC/PI-negative, relative to control cells treated with DMSO, as determined by flow cytometric analyses. Standard deviation (SD) is indicated as error bars (n=3). (B) Cells were treated with ABT-737 for 24 h with 1000 nM (Nalm-6) or 500 nM (Reh) ABT-737, lysed with 1% CHAPS lysis buffer, and 1 mg protein was immunoprecipitated for 6A7-specific Bax and imunoblotted for total Bax. (C) Whole cell lysates were analyzed by immunoblotting for expression of Bcl-2 family proteins and β-actin by immunobloting with the indicated primary antibodies. (D) Parental and ABT-R Nalm-6 cells were treated with ABT-737 (1000 nM for 1–3 h) and expressions of Bim, USP 9X, Mcl-1, PARP1, and β-actin was determined by immunobloting. The results in B-D are representative of three independent experiments.
ABT-737 resistance is associated with increased Mcl-1 protein stability
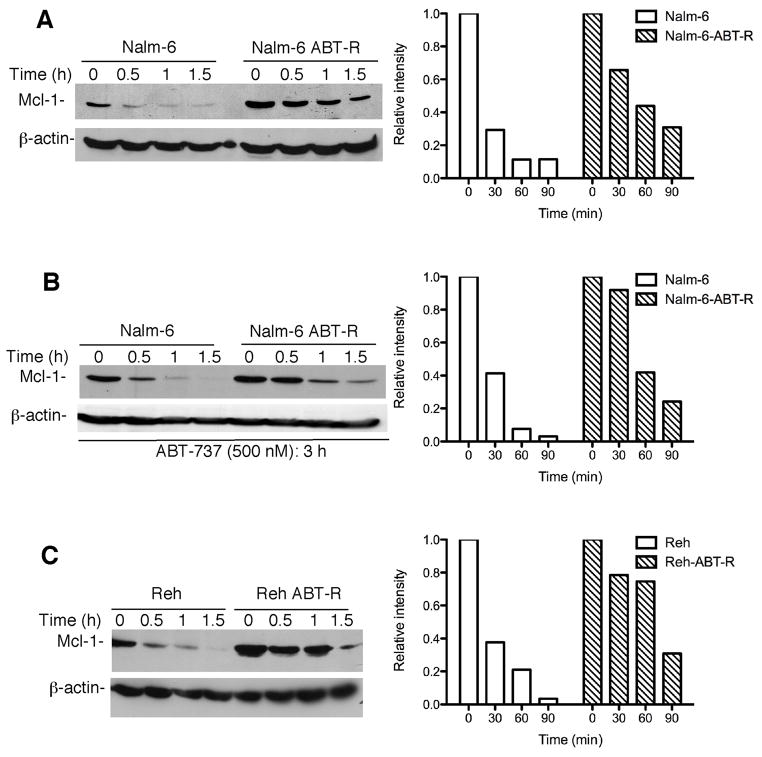
Previous studies of acquired ABT-737-resistance reported that increased expression levels of Mcl-1 in ABT-R cells are a result of increased mRNA levels (7). However, there was no difference in constitutive Mcl-1 and NOXA mRNA levels in resistant compared to parental Nalm-6 and Reh cells, as determined by qRT-PCR (Supplementary Fig. S1A). Similar results were found for Bfl-1 (data not shown). Most importantly, there was no change in Mcl-1 mRNA levels following chronic ABT-737 treatment in Nalm-6 (1 μM) and Reh (500 nM) parental and ABT-R cells (Supplementary Fig. S1B). These results indicate that Mcl-1 was not transcriptionally up-regulated, however do not preclude the possibility of a reduced turnover of the Mcl-1 transcript in ABT-737-resistant cells. ABT-R Nalm-6 and Reh cells examined up to 2 h following treatment with actinomycin D, a known transcriptional inhibitor, indicated that the half-life of Mcl-1 mRNA was not altered (data not shown). Since the Mcl-1 protein has a very short half-life, Mcl-1 protein stability was further evaluated. Immunoblotting following 30–90 min of cycloheximide treatment revealed that the half-life of Mcl-1 protein was increased considerably, to 60–90 min in ABT-R compared to 15–20 min in parental cells (Fig. 2A–C). These results indicate that increased Mcl-1 levels of ABT-R cells were due to its stabilization at the protein and not at mRNA level.
Figure 2. Mcl-1 levels are regulated by protein stabilization.
(A) Mcl-1 protein stability was determined following 10 μg/ml cycloheximide (CHX) treatment (LHS). (B) Nalm-6 and Nalm-6 ABT-R cells were pretreated with ABT-737 (500 nM) for 3 h prior to exposure to CHX to determine Mcl-1 protein stability (LHS). (C) Mcl-1 protein half-life was determined in Reh and Reh ABT-R cells following CHX treatment (LHS). Data in A–C were quantified by Image J software and the relative intensity of each lane was calculated with respect to the sample at 0 h. Results of a representative experiment (n=3) are shown.
Increased Mcl-1 levels sequester Bim following its displacement from Bcl-2 and Bcl-xL complexes in ABT-R cells
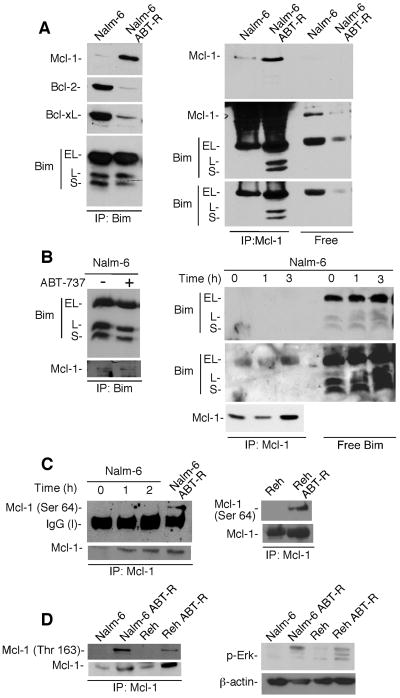
It has been reported that increased Mcl-1 levels in ABT-R cells associate with Bim after its competitive dissociation from Bcl-2/Bcl-xL complexes by ABT-737. Importantly, displacement of Bim from Bcl-2/Bcl-xL complexes by ABT-737 in parental, sensitive cells was shown to be a critical event for committing cells to death (7). Indeed, our co-immunoprecipitation analyses indicated that association of Mcl-1 with Bim in ABT-R cells was more pronounced compared to those in parental cells. On other hand, there was more Bim present that was not bound to Mcl-1 in parental cells, instead was found in the supernatant following immunodepletion of Mcl-1 (Fig. 3A; RHS). These results were confirmed by reciprocal immunoprecipitation-immunoblot analyses with Mcl-1 and Bim, respectively (Fig. 3A; LHS).
Figure 3. Increased Mcl-1 levels are associated with Bim in ABT-R cells.
(A) Bim was immunoprecipitated and immunoblot analyses were performed for Mcl-1, Bcl-2, Bcl-xL, and Bim (LHS). Reciprocal immunoprecipitation-Western blot analysis was performed for Mcl-1 followed by immunoblotting for Bim to determine Mcl-1-bound and free Bim (RHS). (B) Bim was immunoprecipitates from cells treated with ABT-737 (1000 nM) for 3 h and immunoblotted for Mcl-1 and Bim (LHS) to determine the proportion of Mcl-1-bound and Mcl-1-free Bim (RHS). (C) Mcl-1 was immunoprecipitated from ABT-737-treated Nalm-6 or untreated ABT-R cells followed by immunoblot analysis for Ser-64-Mcl-1, Mcl-1 (LHS). Similar analyses were performed with parental and ABT-R Reh cells (RHS). (D) Mcl-1 immunoprecipitates and analyzed by immunoblotting for Thr-163-Mcl-1 (LHS). Whole cell lysates were analyzed by immunoblot for phospho-Erk expression and β-actin (RHS). The results in A-D are representative of 3 independent experiments.
We next investigated the interaction of Bim with Bcl-2/Bcl-xL in parental and ABT-R Nalm-6 cells. There was less association of Bim with Bcl-2/Bcl-xL in ABT-R compared to parental Nalm-6 cells, which was in contrast to binding of Bim to Mcl-1 (Fig. 3A; LHS). Taken altogether, these data suggest that Bim was dissociated from Bcl-2/Bcl-xL complexes by competitive displacement by ABT-737. Moreover, this displaced Bim was bound to Mcl-1 in resistant but not in parental cells, even when ABT-737 was absent. In contrast, in parental cells, even though Mcl-1 levels were increased following acute ABT-737 treatment, Mcl-1 could not bind to Bim (Fig. 3A and B). Similar observations were made in Reh ABT-R cells (data not shown).
We next explored the mechanism responsible for the differences in the ability of Mcl-1 to bind Bim in ABT-R compared to parental cells. It has been shown that Mcl-1 Ser-64 phosphorylation is associated with enhanced binding to proapoptotic proteins, such as Bim, NOXA, and Bak (18). Interestingly, Mcl-1 was phosphorylated on Ser-64 in ABT-R Reh and Nalm-6, but not in parental cells (Fig. 3C). In contrast, while Mcl-1 levels increased, nevertheless Mcl-1 Ser-64 phosphorylation was not detected in Nalm-6 following 1–2 h acute treatment with ABT-737 (Fig. 3C; LHS). These findings suggest that Mcl-1, the levels of which increased following ABT-737 treatment in parental cells, failed to associate with Bim due to lack of Ser-64 Mcl-1 phosphorylation. It has been reported that Mcl-1 phosphorylation also regulates Mcl-1 protein stability, with Thr-163 Mcl-1 phosphorylation by extracellular-signal-regulated kinases (Erk) extending its half-life (20). Indeed, Mcl-1 was phosphorylated on Thr163 in ABT-R Nalm-6 and Reh, but not in parental cells (Fig. 3D; LHS). Erk was also activated, as indicated by p-Erk expression in ABT-R but not the parental cells (Fig. 3D; RHS). These results indicate that phosphorylation of Mcl-1 at specific residues has developed during acquired resistance to ABT-737 treatment. These Mcl-1 postranslational modifications facilitate its interaction with Bim, leading to increased Mcl-1 protein stability in ABT-R cells, as previously suggested (21, 22).
Gossypol sensitizes ABT-737-resistant cells by up-regulating NOXA
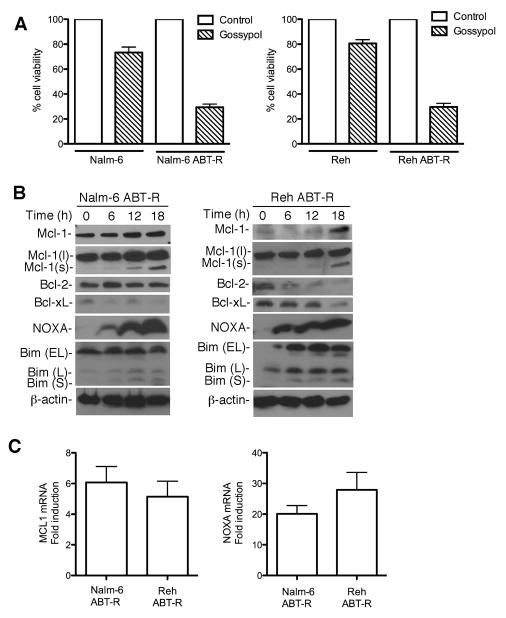
To overcome Mcl-1-dependent acquired resistance, parental and resistant cells were treated with increasing concentrations of gossypol. Annexin V FITC-PI staining (Supplementary Fig. S2A), and trypan blue exclusion (Fig. 4A), indicated that ABT-R cells were exquisitely sensitive to gossypol treatment compared to parental cells. In addition, Cyclin E cleavage, another marker of apoptosis (23,24) was generated in ABT-R cells (data not shown). Time-course experiments with gossypol showed maximum cell death at 24 h. To study the mechanism of its action and avoid consequences of cell death, the 18 h time-point and a 10 μM gossypol dose were chosen, as the minimum concentration at which ABT-R but not parental cells were most sensitive (all other experiments used gossypol at 10 μM for 18 h, unless otherwise stated). Mcl-1 protein stability was not altered substantially following gossypol treatment in ABT-R cells (data not shown). The levels of anti-apoptotic and pro-apoptotic proteins were next examined in ABT-R Nalm-6 (LHS) and Reh cells (RHS) following a 6–18 h exposure to gossypol (Fig. 4B). Interestingly, a low molecular weight Mcl-1 species was present, most likely an alternative splice variant of Mcl-1 (Mcl-1s), which has been reported to function as a pro-apoptotic molecule (25). The expression of NOXA increased greatly in a time-dependent manner (Fig. 4B). Bcl-xL and Bcl-2 levels were downregulated following treatment in Reh ABT-R cells (Fig. 4B; RHS), however PUMA levels did not change significantly (data not shown). There was a modest increase in Mcl-1 levels in Nalm-6 cells following gossypol treatment, and those of NOXA also increased less compared to ABT-R cells (Supplementary Fig. S2B). When NOXA and Mcl-1 mRNA levels were examined at 12 h following gossypol treatment there was a 5–6-fold increase of Mcl-1 mRNA and a robust 20 to 25-fold induction of NOXA levels in ABT-R cells (Fig. 4C). These findings indicate that up-regulation of Mcl-1 and NOXA following gossypol treatment was at mRNA level.
Figure 4. ABT-737-resistant cells are sensitive to gossypol treatment.
(A) Parental and ABT-R cells were treated with gossypol (10 μM) for 18 h. Cells were then examined by trypan blue exclusion for cell viability, shown as a percentage of control cells treated with DMSO. (B) Immunoblot analyses were performed in Nalm-6 ABT-R (LHS) and Reh ABT-R cells (RHS) following gossypol treatment for expression of Mcl-1, Bcl-2, Bcl-xL, NOXA, and Bim. Data are representative of three independent experiments. (C) ABT-R Nalm-6 and Reh cells were treated with RNA levels of Mcl-1 (LHS) and NOXA (RHS) were determined at 12 h following gossypol treatment by qRT-PCR (fold change). SD is indicated in A and C as error bars (n=3). RNA levels of untreated cells were set to 1.
It has been reported that obatoclax (GX15-070), similarly to gossypol, targets Mcl-1 (26) and is predicted to overcome Mcl-1-dependent ABT-737 resistance (27). Indeed, Nalm-6 ABT-R cells were sensitive to obatoclax, however Nalm-6 cells were equally sensitive (IC50: 10 μM). Reh parental cells (IC50: 5 μM) were also more sensitive to obatoclax compared to ABT-R cells as measured by trypan blue exclusion (Supplementary Fig. S3A). Mcl-1 protein levels were downregulated following obataclax treatment. Noxa levels also increased, however these changes did not correlate with cell death (Supplementary Fig. S3B). Hence, gossypol was used for further studies, as it selectively sensitizes ABT-R cells.
NOXA expression mediates gossypol-induced cell death
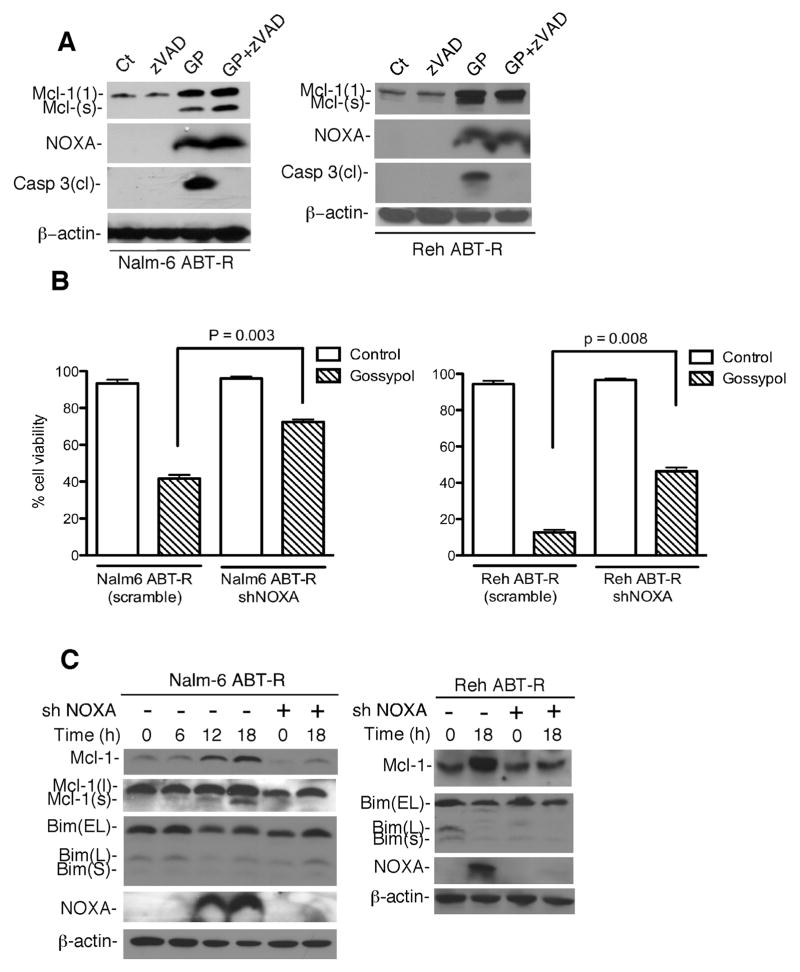
To determine whether NOXA induction and cell death are a consequence of caspase activation, ABT-R Nalm-6 and Reh cells were treated with a pan-caspase inhibitor prior to gossypol treatment. zVAD-fmk addition prevented caspase activation, but not NOXA induction and generation of Mcl-1(s) (Fig. 5A). To directly demonstrate a critical role for NOXA for the gossypol response, NOXA was depleted by shRNA in ABT-R Nalm-6 and Reh cells, under conditions in which Mcl-1 and Bim levels were unaltered. Remarkably, NOXA knockdown (Fig. 5C) prevented significantly gossypol-induced cell death of ABT-R Nalm-6 (p=0.003) and Reh cells (p=0.008) (Fig. 5B), thus demonstrating the critical role played by NOXA in the cytotoxic gossypol response. In addition, shRNA-mediated Mcl-1 knockdown in ABT-R cells (two independent clones) restored resistance to gossypol treatment as measured by trypan blue exclusion (Nalm-6 ABT-R, p=0.001; Reh ABT-R, p=0.007) (Fig. S2D) and PARP1 cleavage (data not shown). These data demonstrate that gossypol is more effective in Mcl-1-dependent ABT-R cells.
Figure 5. Induction of NOXA is critical for gossypol-induced cell death.
(A) Cell were incubated with zVAD-fmk (100 μM) for 1 h prior to gossypol treatment and expression of cleaved caspase-3, NOXA, and Mcl-1 determined by immunoblot analyses. (B) NOXA knockdown was achieved by shRNA and cell viability determined by trypan blue exclusion following gossypol treatment. Cells expressing scrambled shRNA were used as control. Data (A, B) are representative of three independent experiments. (C) Cells expressing scrambled shRNA and shNOXA were immunoblotteted for Mcl-1, Bim, NOXA, and β-actin following gossypol treatment. Error bars for A represent SD from three independent experiments. p value was calculated by 2-way ANOVA.
Association of NOXA with Mcl-1 leads to release of Bim following gossypol treatment
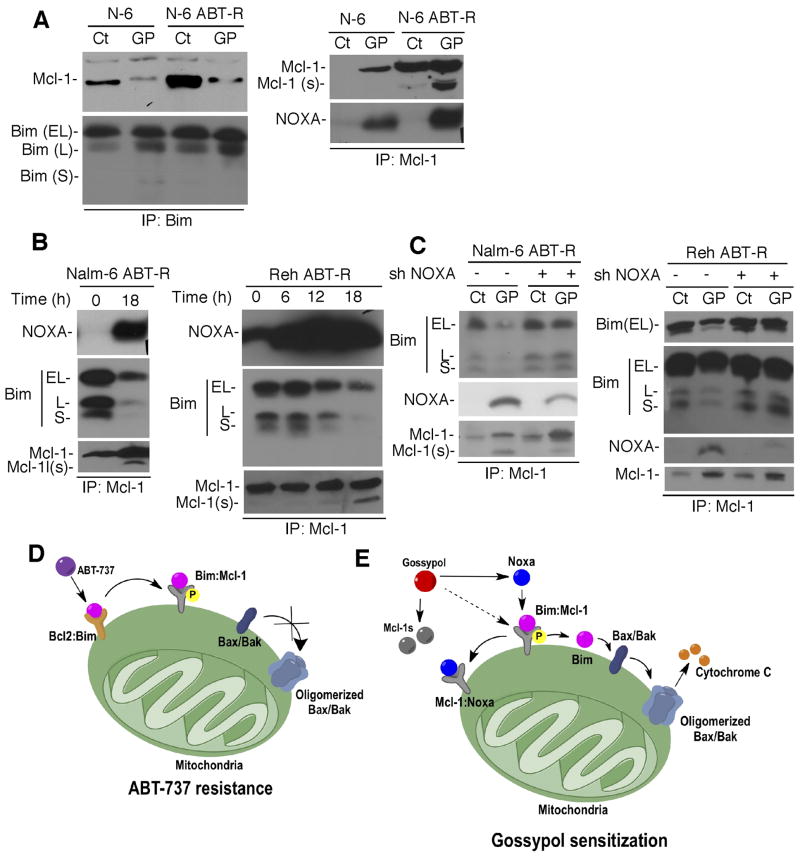
Next, to address the mechanism of sensitization by gossypol, the Mcl-1/Bim association was examined. Bim immunoprecipitation revealed that Mcl-1 was no longer associated with Bim in Nalm-6 ABT-R cells following gossypol treatment, indicating that the Bim/Mcl-1 complex was disrupted (Fig. 6A; LHS). In contrast, Bcl-2 and Bcl-xL were still bound to Bim in these Nalm-6 and Reh ABT-R cells (Supplementary Fig. S2C). Moreover, Mcl-1 pull-down revealed that NOXA interaction with Mcl-1 facilitated Bim release from the Mcl-1/Bim complex in ABT-R cells (Fig. 6B). The released Bim, present in the supernatant (Supplementary Fig. S2E), most likely initiated gossypol-induced mitochondrial cell death. The Bim/Mcl-1 complex was also disrupted by NOXA in parental cells but to a lesser extent and with a slower kinetics compared to ABT-R cells (Fig. 6A). To determine the direct effect of Noxa on the Mcl-1/Bim association after gossypol treatment, NOXA levels were downregulated by shRNA. Interestingly, shRNA-mediated NOXA knockdown not only prevented cell death but also the release of Bim from Mcl-1 and Mcl-1(s) genesis in ABT-R Nalm-6 and Reh cells following gossypol treatment (Fig. 5B, 6C). These results indicate that the association of NOXA with Mcl-1 in ABT-R cells following gossypol treatment lead to release of Bim that was critical for inducing mitochondrial cell death.
Figure 6. Interaction of NOXA with Mcl-1 triggers the release of Bim from Mcl-1 following gossypol treatment.
(A) Bim was immunoprecipitated from gossypol-treated cells followed by immunoblotting to determine Mcl-1 association (LHS). Immunoprecipitation for Mcl-1 was followed by immunoblotting for NOXA (RHS). (B) Association of NOXA and Bim with Mcl-1 were determined by immunoprecipitation-immunoblot analysis in gossypol-treated cells. (C) Mcl-1 immunoprecipitates from cells following NOXA knockdown and gossypol treatment were analyzed by immunoblotting for NOXA, Bim, and Mcl-1. Data shown are representative of three independent experiments. (D) Model for ABT-737 resistance. ABT-737 does not target Mcl-1, which confers resistance by sequestering Bim displaced from Bcl-2. The Mcl-1/Bim association was enhanced by phosphorylation of Mcl-1, thereby preventing Bax activation. (E) Model for overcoming ABT-737 resistance by gossypol. Gossypol induces NOXA that binds to p-Mcl-1 and displaces Bim from the Mcl-1/Bim complex, leading to cell death that is augmented by Mcl-1s (splice variant) generation by gossypol treatment.
Discussion
Acquired resistance is a concern for chemotherapeutic treatments used for lymphoma, CLL, and other malignancies. Although there have been basic advances in understanding the mechanisms for this resistance, few efforts have been made to study the contribution of post-translational regulation of proteins that leads to such resistance. The focus of our current studies was to understand the mechanism responsible for acquired ABT-737 resistance in leukemic cells and how to overcome it selectively. Nuclear magnetic resonance (NMR) studies have shown that ABT-737 binds to Bcl-2, Bcl-xl, and Bcl-w at sub-nM concentrations, but not to Mcl-1. Hence, Mcl-1 levels can determine ABT-737 sensitivity (7). Moreover, inherent increased levels of these proteins can be a frequent cause of resistance. Various drugs, such as flavopiridol, R-roscovitine (Seliciclib), and PHA 767491 that downregulate Mcl-1 at mRNA level are being tested for sensitizing such tumor cells (7,28). Recently, sorafenib (BAY43-9006; Nexavar) was shown to induce apoptosis by downregulation of Mcl-1 at translational rather than post-translational level (29–32). However, due to lack of in vivo studies, except for ABT-737 and gossypol, most of the drugs acting as BH3 mimetic or Bcl-2 antagonists in clinical trials may have potential toxicities (12).
This study has examined the molecular mechanism for acute versus chronic response to ABT-737 in leukemic cells. Recent studies and our previous report indicate that increased levels of Mcl-1 accumulate in ABT-R cells, with Mcl-1 being bound to Bim significantly more in resistant compared to parental cells (5,7). As in our leukemic model of ABT-737 resistance, Mcl-1 was not upregulated at transcriptional level, led us to explore its post-translational regulation. Previous studies have shown that post-translational modifications, such as phosphorylation of Mcl-1 at specific residues are important for it’s binding to BH3-only proteins and its stability (18,33). Here, we have demonstrated that, in ABT-R cells, phosphorylation of Mcl-1 at Ser-64 facilitates association of Bim with Mcl-1. Ser-64 Mcl-1 is not detected in parental cells, not even following acute treatment with ABT-737 and it is, rather, acquired during the course of resistance. Moreover, Mcl-1 was also phosphorylated on Thr-163 in ABT-737-resistant cells through Erk activation. Since Erk pathway was activated, we next studied its role on post-translational regulation that could impact on Mcl-1 turnover. It has been shown that Erk phosphorylates Thr163 on Mcl-1 within its PEST region and stabilizes it (20). It has been suggested that Thr163 is the priming phosphate that initiates Ser159 phosphorylation of Mcl-1 through GSK3β leading to its ubiquitination and degradation(34). Clearly, phosphorylation at Thr163 but not subsequent Ser159 phosphorylation on Mcl-1 in ABT-R cells supports why Mcl-1 was more stable in ABT-R compared to parental cells. Mcl-1 is a short-lived protein and it is a target of E3 ubiquitin ligases that mediate its proteasomal-mediated degradation. Mcl-1 is inaccessible to E3-ubiquitin ligases, FBW7 and β-Trcp when it is bound to BH3-only proteins, such as Bim, as more association of Bim with Mcl-1 prevents their access to Mcl-1. As there is competitive binding between these E3-ubiquitin ligases and BH3-only proteins because they share the same C-terminal-binding region of Mcl-1 (22), most likely this could contribute towards Mcl-1 stability, as more Bim is associated with Mcl-1 in ABT-R cells.
Approaches to overcome resistance towards a single agent rely on targeting cells with another agent to exploit cells’ dependability on the resistance determinant. Response to chronic exposure to ABT-737 leads to displacement of Bim from Bcl-2, Bcl-xl, and Bcl-w, which is the then captured by another pro-survival protein, Mcl-1. In ABT-737-resistant cells levels of Mcl-1, its stability, and affinity towards Bim is increased, making them Mcl-1-dependent. To target this induced Mcl-1 primed with Bim in ABT-R cells two pan-Bcl-2 family antagonists, were examined. Gossypol has a significant antitumor activity against lymphoma, head and neck, and prostate cancer through induction of Noxa and Puma (35–37). Obatoclax is a small molecule that disrupts interaction between Mcl-1 and Bak and hence shows potential to overcome ABT-737 resistance (27). While obatoclax was effective against ABT-R cells, it did not have specificity for ABT-737-resistant cells. In contrast, gossypol emerges from this study as a very potent compound against ABT-737-resistant leukemic cells that could target Mcl-1 indirectly by inducing NOXA. We have previously reported that primary cells from CLL patients with elevated Mcl-1 levels are less responsive to ABT-737 (5). Here we show that gossypol preferentially targets ABT-R with elevated Mcl-1 presumably that is also phosphorylated Mcl-1 found in cells and causes cell death by inducing NOXA. shRNA-mediated downregulation of Mcl-1 in ABT-R cells decreased gossypol sensitivity, indicating that elevated levels of Mcl-1 are the cause of gossypol sensitivity in conjunction with NOXA induction following gossypol treatment. NOXA induction in our study is at the mRNA level. The resulting increased NOXA levels following gossypol treatment then associate with Mcl-1 leading to release of Bim from the Bim/Mcl-1 complex and thereby neutralize the anti-apoptotic function of Mcl-1. Mcl-1-free Bim then initiates Bax/Bak activation (38,39) and triggers the mitochondrial apoptotic pathway. A similar mechanism was reported to be responsible for apoptosis induced by Bortezomib in multiple myeloma (31) and HeLa cells following ER stress and proteasome inhibition (40). Noxa was reported to have the highest binding affinity to Mcl-1 compared to other anti-apoptotic Bcl-2 family members (41–45). Moreover, since following Noxa knockdown Bim is no longer released from the Mcl-1/Bim complex in ABT-R cells, hence Noxa specifically displaces Bim from Mcl-1 in these cells following gossypol treatment. Gossypol-induced cell death was clearly NOXA-dependent as prevention of NOXA expression significantly prevented cell death.
Generation of an alternative, pro-apoptotic splice variant of Mcl-1, Mcl-1(s) has also a significant contribution to gossypol-induced cell death. Mcl-1(s) was not generated by caspase-mediated proteolytic cleavage, as suggested earlier (30–32), since zVAD-fmk addition prevented caspase-3 activation but not the appearance of this faster migrating form of Mcl-1 corresponding to Mcl-1(s). Mcl-1 mRNA induction by gossypol facilitated generation of Mcl-1(s), which can also contribute to mitochondrial cell death pathway. Moreover, gossypol-induced cell death was NOXA-dependent as prevention of NOXA expression significantly prevented cell death. In summary, gossypol-induced cell death is dependent on Mcl-1 expression and its phosphorylation in ABT-R cells that is targeted by NOXA induction and subsequent binding to Mcl-1, thereby displacing Bim, leading to cell death (Fig. 6D and E). Gossypol, a natural product has been shown to be effective in inducing apoptosis as well as autophagy-mediated cell death in various cancer cell lines and xenograft tumor models as pan-Bcl-2 inhibitor (35,36, 46). It is possible that apogosspyol, its semisynthetic derivative with structural modifications that increase its affinity for anti-apoptotic Bcl-2 proteins and reduces its toxic effects could be also effective in ABT-737-resistant patients, as both are under evaluation in phase I/II clinical trials for CLL and several other malignancies, such as prostate, lung, and brain tumors. These findings provide insights into the molecular mechanism of ABT-737 resistance and how it can be overcome with gossypol, which can be considered for clinical treatment of navitoclax-resistant leukemia patients.
Supplementary Material
Acknowledgments
We thank Drs. G.J. Gores (Mayo Clinic) for Mcl-1 antibody and S. Elmore (Abbott Laboratories) for ABT-737. This work was supported by NIH grant CA127264-02/03.
Footnotes
The authors report no conflict of interest related to this work
References
- 1.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkholi R, Floros KV, Chipuk JE. The Role of BH3-Only Proteins in Tumor Cell Development, Signaling, and Treatment. Genes Cancer. 2011;2:523–37. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–33. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–32. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 5.Al-Harbi S, Hill BT, Mazumder S, Singh K, Devecchio J, Choudhary G, et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood. 2011;118:3579–90. doi: 10.1182/blood-2011-03-340364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–39. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–13. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky LD. From mitochondrial biology to magic bullet: navitoclax disarms BCL-2 in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:554–7. doi: 10.1200/JCO.2011.37.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–7. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Zhou Y, Huang HC, Mitchison TJ. Navitoclax (ABT-263) accelerates apoptosis during drug-induced mitotic arrest by antagonizing Bcl-xL. Cancer Res. 2011;71:4518–26. doi: 10.1158/0008-5472.CAN-10-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan N, Malek M, Zha J, Yue P, Kassees R, Berry L, et al. Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin Cancer Res. 2011;17:1394–404. doi: 10.1158/1078-0432.CCR-10-2353. [DOI] [PubMed] [Google Scholar]
- 15.Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther. 2010;9:2046–56. doi: 10.1158/1535-7163.MCT-10-0033. [DOI] [PubMed] [Google Scholar]
- 16.Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, et al. Crystal structure of ABT-737 complexed with Bcl-xL: implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14:1711–3. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- 17.Safa AR, Glover CJ, Sewell JL, Meyers MB, Biedler JL, Felsted RL. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987;262:7884–8. [PubMed] [Google Scholar]
- 18.Kobayashi S, Lee SH, Meng XW, Mott JL, Bronk SF, Werneburg NW, et al. Serine 64 phosphorylation enhances the antiapoptotic function of Mcl-1. J Biol Chem. 2007;282:18407–17. doi: 10.1074/jbc.M610010200. [DOI] [PubMed] [Google Scholar]
- 19.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–7. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 20.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–15. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 21.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–17. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogarty MD. Mcl1 becomes ubiquitin-ous: new opportunities to antagonize a pro-survival protein. Cell Res. 2010;20:391–3. doi: 10.1038/cr.2010.37. [DOI] [PubMed] [Google Scholar]
- 23.Mazumder S, Plesca D, Kinter M, Almasan A. Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis. Mol Cell Biol. 2007;27:3511–20. doi: 10.1128/MCB.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plesca D, Mazumder S, Gama V, Matsuyama S, Almasan A. A C-terminal fragment of Cyclin E, generated by caspase-mediated cleavage, is degraded in the absence of a recognizable phosphodegron. J Biol Chem. 2008;283:30796–803. doi: 10.1074/jbc.M804642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–61. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 26.Cruickshanks N, Hamed H, Bareford MD, Poklepovic A, Fisher PB, Grant S, et al. Lapatinib and Obatoclax kill tumor cells through blockade of ERBB1/3/4 and through inhibition of BCL-XL and MCL-1. Mol Pharmacol. 2012 doi: 10.1124/mol.112.077586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–64. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 28.Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, et al. Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via down-regulation of Mcl-1 in multiple myeloma. Blood. 2005;106:1042–7. doi: 10.1182/blood-2005-01-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber S, Oelsner M, Decker T, zum Buschenfelde CM, Wagner M, Lutzny G, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25:838–47. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 30.Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F, et al. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim–induced apoptosis. Oncogene. 2004;23:7863–73. doi: 10.1038/sj.onc.1208069. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, Trichet V, Robillard N, Philippe M, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67:5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 32.Michels J, O’Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, et al. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23:4818–27. doi: 10.1038/sj.onc.1207648. [DOI] [PubMed] [Google Scholar]
- 33.Liao M, Zhao J, Wang T, Duan J, Zhang Y, Deng X. Role of bile salt in regulating Mcl-1 phosphorylation and chemoresistance in hepatocellular carcinoma cells. Mol Cancer. 2011;10:44. doi: 10.1186/1476-4598-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Balakrishnan K, Wierda WG, Keating MJ, Gandhi V. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volate SR, Kawasaki BT, Hurt EM, Milner JA, Kim YS, White J, et al. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol Cancer Ther. 2010;9:461–70. doi: 10.1158/1535-7163.MCT-09-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (−)–gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–99. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Lopez H, George NM, Liu X, Pang X, Luo X. Selective involvement of BH3-only proteins and differential targets of Noxa in diverse apoptotic pathways. Cell Death Differ. 2011;18:864–73. doi: 10.1038/cdd.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–71. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 43.Ploner C, Kofler R, Villunger A. Noxa: at the tip of the balance between life and death. Oncogene. 2008;27 (Suppl 1):S84–92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol. 2011;194:39–48. doi: 10.1083/jcb.201102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, et al. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–95. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.