Abstract
Total disc replacement (TDR) is expected to provide a more physiologic alternative to fusion. However, long-term clinical data proving the efficacy of the implants is lacking. Limited clinical data suggest somewhat of a disagreement between the in vitro biomechanical studies and in vivo assessments. This conceptual paper presents the potential biomechanical challenges affecting the TDR that should be addressed with a hope to improve the clinical outcomes and our understanding of the devices. Appropriate literature and our own research findings comparing the biomechanics of different disc designs are presented to highlight the need for additional investigations. The biomechanical effects of various surgical procedures are analyzed, reiterating the importance of parameters like preserving uncinate processes, disc placement and its orientation within the cervical spine. Moreover, the need for a 360° dynamic system for disc recipients who may experience whiplash injuries is explored. Probabilistic studies as performed already in the lumbar spine may explore high risk combinations of different parameters and explain the differences between “standard” biomechanical investigations and clinical studies. Development of a patient specific optimized finite element model that takes muscle forces into consideration may help resolve the discrepancies between biomechanics of TDR and the clinical studies. Factors affecting long-term performance such as bone remodeling, subsidence, and wear are elaborated. In vivo assessment of segmental spine motion has been, and continues to be, a challenge. In general, clinical studies while reporting the data have placed lesser emphasis on kinematics following intervertebral disc replacements. Evaluation of in vivo kinematics following TDR to analyze the quality and quantity of motion using stereoradiogrammetric technique may be needed.
Keywords: Biomechanics, Total disc replacement, Finite element technique, Kinematics, Patient specific biomechanical models
Introduction
The intervertebral disc is a major load bearing component of the spine. The mechanical loading of the disc is one of the main causes of its degeneration [1]. For chronic patients, the treatment regimen may range from conservative approach to surgical procedures, including fusion with and without instrumentation.
The fusion has proven successful in reducing the segmental motion. However, a majority of patients after a fusion procedure may still experience pain. The procedure may also lead to adjacent level degeneration [2–5], pseudarthrosis [6, 7], and donor site pain. These shortcomings have led the researchers to develop alternative approaches such as the total disc arthroplasty which is expected to preserve motion and relieve pain [8].
Thus, it is essential to evaluate the clinical outcomes in terms of an artificial disc’s ability to provide normal kinematics following cervical total disc replacements (TDR). Several parameters like center of rotation (COR/quality), range of motion [ROM = elastic zone (EZ) + neutral zone (NZ)/quantity], stiffness (quality) and hysteresis (quantity) are used to assess the quality and the quantity of motion in a spinal segment. However, the notion of reproducing “normal” motion following TDR surgery presents several challenges.
The clinical experience of more than 50 years in the area of lower extremity joint replacements suggests that the long-term success of any total arthroplasty system is a function of three primary factors: implant design parameters (geometry, material, kinetics, ROM in all 6 degrees of freedom), patient related parameters (weight, kinematics, age) and surgeon related factors such as surgical procedures. One expects that similar parameters would influence the long-term success of the cervical TDR. For instance, several designs of cervical artificial discs are proposed that utilize different materials, articulations and surgical placement procedures. Such variations in design may result in different contact forces, different ranges of motion and wear debris of the prosthesis itself. There is evidence in the literature that this may be the case for the cervical disc arthroplasty as well [9–29].
Thus, this conceptual paper presents the potential biomechanical challenges such as effects of implant design variations, surgical procedures and whiplash. The long-term consequences of TDR like bone remodeling, subsidence, facet joint loading, wear and in vivo kinematics are also included. For these reasons, the manuscript somewhat takes a leap into the future. The pertinent biomechanical studies, including from our group, are presented in the following paragraphs to illustrate the points. Due to the conceptual nature of the manuscript, authors’ understanding of the research is presented without adequate literature support with the hope that it will encourage the readers to conduct additional investigations to support/refute the concepts.
Effects of implant related parameters
Disc design variations
Rousseau et al. [30] analyzed the behavior of functional spinal unit (FSU) with the variation of position of the center and the size of the radius of a cervical ball-and-socket design. The ROM was similar for all the cases with variations within 30% of the intact. The large radius of curvature was associated with the partial unloading of the facet joints.
Both the cadaver and finite element model data suggest that the basic shape of ball and socket (BT-Prestige® and BS-Prodisc®, Fig. 1a) does not make that much of a difference in the kinematics of the implanted segment, Faizan [31]. These authors subsequently simulated other variations of ball and socket designs such as combinations of superior (S) and inferior (I) components with spherical (SPH) and oval (OVL) shaped ball designs respectively at the C5–C6 level in the C-spine FE model, Fig. 1b.
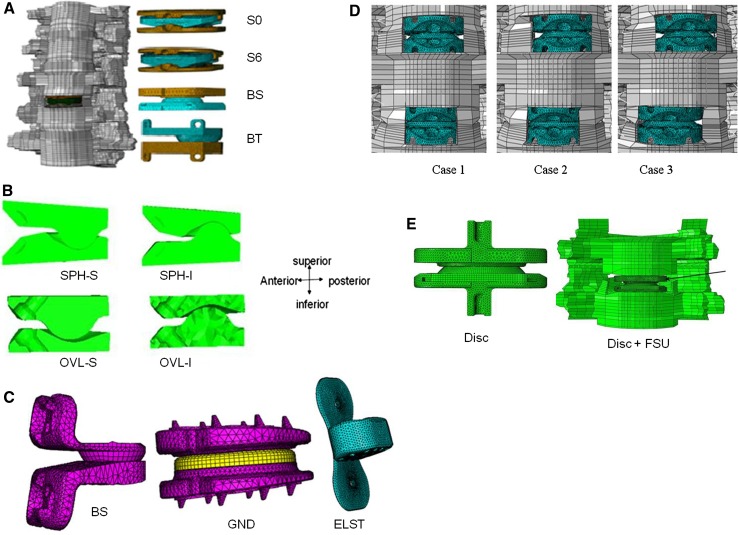
Fig. 1.
An experimentally validated intact ligamentous cervical spine finite element model was modified to simulate various cases. a Synergy 0 degrees (S0), synergy 6 degrees (S6), ball and socket (BS), ball and trough BT) at C5–C6 level; b cross sectional view of four different disc designs: SPH-S; SPH-I; OVL-S; and OVL-I. In the spherical designs (SPH-I and SPH-S), small radius ball component articulated with socket component. In the oval designs (OVL-I and OVL-S), large radius ball component articulated with a non conforming socket component; c Three basic concepts simulated at C4–5 level: the Ball and socket (BS); Sandwich design (SND)—the artificial disc consisted of three components. A polyethylene core was sandwiched between two metallic endplates, and the elastomeric (ELST) artificial disc was made of silicon with a flap attached to it. The flap was the replacement of anterior longitudinal d Bi-level disc implants at C4–C5 and C5–C6 levels. Compared to Case1, discs in others are moved slightly to the left or right and e models of Disc alone and Disc placed in a cervical segment (Disc + FSU) for wear analysis
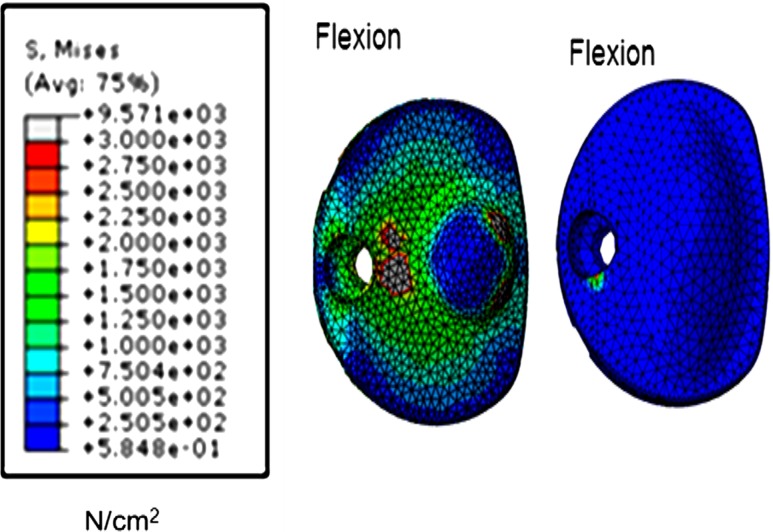
No major kinematic differences were observed among the disc designs tested. For both spherical and oval designs, the facet loads in extension were lower for the designs with an inferior ball component (67 vs. 91 N in extension, 48 vs. 43 N in left lateral bending and 32 vs. 31 N in right axial rotation), compared to intact facet loads. However, the stresses in the spinal structures, a parameter important for the long-term performance of the device, like its wear characteristics, differed with shape, Fig. 2. The implant stresses were substantially higher in the spherical designs when compared to the oval designs. Overall, the oval design with an inferior ball component produced motion, facet loads, implant stresses and capsule ligament strains closest to the intact spine, which may be key to long-term implant survival.
Fig. 2.
von Mises stress contours on the artificial disc under hybrid loading conditions. The stress contours are shown for the spherical shaped inferior ball component (SPH-I) and oval inferior ball component (OVL-I) designs of the artificial discs as shown in Fig. 1b
Faizan et al. [32] simulated three distinct philosophies of designs in the finite element model, Fig. 1c. These were as follows:
BS design The design consisted of two metallic components (superior ball and inferior socket-BS) and placed such that the BS articulation was towards the posterior region of the disc space.
Sandwich design (SND) The sandwich design possessed three components where the core polyethylene ball was sandwiched between the superior and inferior metallic components. The size of the ball component was larger in the sandwich design when compared to the BS design.
Elastomer design with ALL replacement (ELST) The single piece type artificial disc design (Abbott Spine, Houston, TX) used an elastomeric cervical disc replacement implant. The disc implant replaced the intervertebral disc and the anterior longitudinal ligament (ALL). The flap substituting ALL was TwillWeave™ longitudinal. The load-displacement characteristics of the flap were very close to cervical ALL.
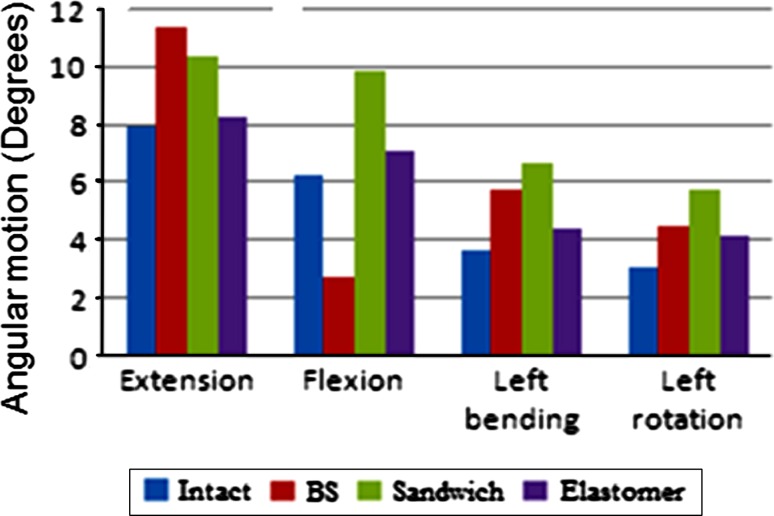
The sandwich design and the elastomeric design produced motions higher than intact (57 and 13% greater than intact, respectively), Fig. 3. The total facet loads at the C4–C5 level during extension were similar for BS and elastomeric designs. The facet load for sandwich design was lower than intact. With the sandwich design, the large radius ball of the artificial disc imposed kinematics which resulted in the loading of the facets during flexion motion. Among the three disc designs, the facet loads with the elastomeric design were closer to the intact facet loads. Overall, the elastomeric disc reproduced intact segment behavior better than the other two designs.
Fig. 3.
Finite element model based C4–C5 level motion for the intact spine and the spines implanted with the three disc designs of artificial discs under 75 N compression + 1.5 Nm moment
Sagittal balance following TDR
In Johnson et al. [33] study patients who underwent two level Bryan cervical artificial discs showed no significant change in lordosis, but there was a 4.7° mean reduction in lordosis for those who underwent single level disc replacement. Kim et al. [34] found that only 36% of patients were able to maintain lordosis following Bryan artificial disc surgery. However, the overall sagittal alignment of the cervical spine was preserved in 86% of cases at the final follow up. Interestingly, preoperatively kyphotic FSU resulted in lordotic FSU in 13% of patients during the late follow up, and preoperatively kyphotic overall cervical alignment resulted in lordosis in 33% of the patients postoperatively. Kulkarni et al. [35, 36] performed a FE study to evaluate the effects of four artificial discs (Synergy 0 degree, S0; Synergy 6 degrees, S6; ball and socket disc, BS; ball and trough disc, BT) on sagittal balance in neutral posture (NP), Fig. 1a. Neutral posture (NP) loading, simulated as follower type preload, resulted in less than 1° of extension for intact, S0 and S6, extension around 3° for BS and flexion over 1° for BT.
Quality and quantity of motion after cervical TDR
In the cervical region, the in vitro and in vivo motion studies [37–40], suggest that the range of motion differs with level, gender and subject (e.g., 9° at C2–C3 and 23° at C4–C5, [38]). The motion also changes as a function of degeneration. Should the TDR be gender, level and subject specific? Should we restore the motion (quantity and quality) to a normal person or to the adjacent degenerated level motion of that patient?
Coupling, an association of translation/rotation in one plane with the translation/rotation in the other plane, is another motion parameter of import [41]. The cervical spine exhibits a significant degree of coupled motion. Should TDR restore coupled motion behavior? To examine this aspect, Puttlitz et al. [42] conducted an in vitro study using ProDisc-C (Synthes Spine, West Chester, PA, USA). There were no significant differences in coupling between the intact and implanted conditions. However, a similar study undertaken by our group [35] revealed that the coupling following TDR simulations differed among different disc designs, compared to intact values, although these discs provided similar flexion–extension motions. Thus the coupling following TDR seems to be design-dependent.
The above analyses clearly suggest that kinematics of the involved segments do not change that much, especially when we do not know the desired optimal value with design variations, but the stress distributions and load sharing among various components are quite different. These variations may have long-term implications, as explained later.
Effects of surgical procedures
Hybrid constructs—fusion and discs
Our group used the finite element model of C3–C7 cervical spine segment to study adjacent level effects of fusion (C4–C5) plus disc replacement (C5–C6), and bi-level disc replacement at C4–C5 and C5–C6 levels [43]. The bi-level fusion models required more than two times the moment of the intact model to produce intact motion. In contrast, the bi-level disc replacement model required moments lower than the intact model. Fusion plus the disc replacement model required moments 10–25% more than intact, except in extension. Overall, fusion plus the disc replacement model had less severe effects on adjacent levels when compared to bi-level fusion model. Bi-level disc replacement model did not have any biomechanically harmful effects on adjacent levels [44]. These computer model based predictions are in agreement with the clinical observations of Shin et al. [45].
FE simulation and experimental study of uncinatectomies during TDR surgery
The uncinate processes may or may not be preserved during TDR surgery. Faizan et al. [46] simulated both scenarios using a BS type disc in a cervical spine finite element model. Unilateral and bilateral uncinatectomies increased the implant level motions by 90% following bilateral uncinatectomy, as compared to no uncinatectomy case, facet loads (maximum increase was in extension, 110 vs. 64 N) and the ligament strains by 100% following uncinatectomy in flexion and axial rotation. In a cadaver study, the uncinatectomies increased the sagittal plane ROM values of TDR significantly compared to the intact (8.4° ± 3.5°), with the maximum increase in bilateral uncinatectomy (14.1° ± 3.0°), followed by unilateral (13.3° ± 2.6°) and unilateral partial (12.8° ± 2.6°) uncinatectomy procedures, (Snyder et al. [47]). Thus, it will be beneficial to preserve uncinate processes.
FE simulation of disc placement in various orientations
The effect of placement orientation of the artificial disc was also studied by our group [36]. In the first configuration, the endplates at C5–C6 level were horizontal (H). Two other configurations of disc placements were created by giving a posterior downward tilt (PDT) and a posterior upward tilt of 6° (PUT) to the disc. Tilting the disc altered the motion, especially during lateral bending and axial rotation. The facet loading increased only in rotation in all three cases. The stresses were highest with the H configuration (~150 MPa), however, the stress values were much lower than the yield and fatigue strength of titanium (~300 MPa).
FE simulation of variations in bi-level disc placement locations
The FE model was modified to study the effect of variations in the disc placement by accommodating two ball and socket type discs (DMT, Inc, Boca Raton, FL, USA) at the C4–C5 and C5–C6 levels [48]. In the first model (case 1), both the disc implants were symmetrically simulated in the center of the respective disc spaces (Fig. 1d). In the second model (case 2), the C4–C5 disc implant was shifted laterally towards the right by 2.5 mm from its symmetric position while the C5–C6 disc was symmetric. In the third model (case 3), the C5–C6 disc implant was also shifted laterally towards the left (opposite to the C4–C5 disc) from its symmetrical position. The extension increased with the artificial discs (case 1: 30%, case 2: 7%, case 3: 20% increase, as compared to intact) but the increases were lower than in bending and rotation. Similarly, flexion motion also increased following TDR with the increase being approximately ~15%, as compared to intact. The percentage increase in motion was highest during lateral bending and axial rotation (~50% greater than intact); however, no major inter configuration differences were found in these loading modes.
The implant level total facet loads were larger than intact facet loads following TDR. During extension, the total facet loads at the C5–C6 level increased by 25% (Case 1), 40% (Case 2) and 45% (Case 3), as compared to intact. During lateral bending, the only major increase following TDR was found for Case 2 configuration (45% increase). The facet loads during axial rotation also increased with all three configurations (~50% for each configuration).
Specific surgical considerations must be given to cervical TDR in making the transition from one procedure to the other. An example is excision of PLL, which not only ensures that a complete decompression has been achieved but also ensures that the disc space is mobilized and facilitates parallel distraction, restoration of the intervertebral height, and mobility of the segment. Also, cervical TDR will not function as designed and allow restoration of motion without proper midline identification and placement of the device.
Cervical spine biomechanics following TDR and whiplash
Demetropoulos et al. [49] studied the biomechanics of the cervical artificial disc in cadavers during low speed rear end impacts. The tests indicated that immediate post implantation stability of the Prodisc® was sufficient to sustain low speed rear-end impact. However, with high speed whiplash, the remaining posterior structures may get ruptured. How will the implanted segment perform under these conditions?
Faizan et al. [50] tested ligamentous C4–T1 spine specimens sequentially for the following cases: intact, artificial disc (Discover™, DePuy Spine, Raynham, MA, USA) implanted at C5–C6 level—pre-injury case, the posterior ligaments (interspinous and facet capsule) transected at the C5–C6 level—post injury case, and finally a cervical posterior dynamic system (C-PDS) consisting of bilateral screws and a rubber band across the screws. (The load-displacement behavior for the rubber band was determined experimentally.) The effect of a whiplash type injury on a disc implanted cervical spine was also simulated using the previously described FE model. In the FE simulations, a ball and socket type disc implant (Prodisc®, Synthes Spine, West Chester, PA, USA) was used to simulate the TDR at the C5–C6 level (pre-injury model). The implanted model was modified to simulate the following additional cases:
Transection of ligaments (ISL, SSL and facet capsules, unilateral and bilateral) progressively to study the effect of sacrificing various ligaments (post-injury model).
A posterior dynamic stabilization (C-PDS) using screws and a cord.
The predicted FE motion data was similar to in vitro results. The C-PDS was able to restore the stability. Motions were lower than those in the pre-injury cases after stabilization. During extension, the maximum stress in the disc in the pre-injury, the post-injury, the rigid system stabilized and the C-PDS stabilized case were 30, 34, 12 and 28 MPa, respectively. Thus, one could make a case that like the lumbar disc arthroplasties, we may need a 360° dynamic system for the cervical spine as well, to cover the whiplash effects.
Development of patient specific models
The development of in vivo patient specific models may bridge the gap between the in vitro biomechanical results and clinical observations. In addition to that, such data would provide an opportunity to evaluate the biomechanical effects on the spine post surgery. A number of groups have pursued studies along these lines in the lumbar region [51–59]. However, at present a comprehensive model is not available in the literature. Extending such investigations into the cervical spine region would definitely provide valuable data.
Long-term effects of cervical TDR
Biomechanical and clinical studies suggest a reduction in adjacent level effects after cervical TDR, compared with fusion [2–4, 60–62]. However, some of the long-term potential complications like subsidence, and wear debris related issues might arise.
Bone remodeling
Bone is a highly vascular connective tissue, and adapts itself to a variety of mechanical situations. Our group performed several studies by applying bone remodeling theory in conjunction with the finite element model based stress and strain predictions in the lumbar region [63, 64]. The studies showed that the external shape of a vertebra is optimal. The internal remodeling showed a heterogeneous distribution of Young’s modulus in the cancellous region of the vertebral bodies, in agreement with experimental and clinical data. The density of the vertebral cortex approached that of a cortical bone, while the thickness of the anterior cortex exceeded that of the posterior shell and either endplate. Such studies need to be undertaken with implants in the model, like the cervical total disc replacement. Different disc designs have different geometries which could influence the loading environment at the bone-implant interface and the adaptive response of the bone.
Subsidence
Subsidence is a frequently reported problem with an occurrence rate of 3–10%, depending on the type of prosthesis used [65]. According to Bertagnoli et al. [66] the subsidence is most often due to inadequate pre-op assessment of bone quality. However, it may also be due to the improper disc design affecting the endplate preparation during surgery and load distribution. Lin et al. [67] performed a FE study in which they analyzed and compared segmental motion and bone-implant interface stresses at C5–C6 level for Bryan, Prestige LP, and ProDisc-C. Over all, the von Mises stresses on the C6 superior surface were higher in the models with Prestige LP and ProDisc-C discs, compared to the model with Bryan disc. They concluded that the lower stresses with Bryan disc were a result of its disc end plate, which has no fixators on it, and the polymeric nucleus. Anderson and Rouleau [65] reported that the structural integrity of the end plates should be assessed and the prostheses should have as large a footprint as possible to dissipate the load evenly rather than in concentrated areas. Hence, the artificial disc design and development must be done in such a way that the subsidence is minimized.
Facet joint loading
In order to protect the facet joints from abnormal stresses, cervical arthroplasty devices should have an axis of rotation that mimics the kinematics of the normal spine. They should also restore the physiologic range of motion, be able to restore disc height, and transmit axial loading forces from the superior vertebral body to the one inferior [68]. Chang et al. [69] found that following TDR, the facet loads increased at the index level in all the bending modes, with maximum in extension. Metzger et al. [70] in a cadaver study found that facet forces were sensitive to the device placement location. In contrast, a similar study conducted by Steiber et al. [71] using ovine spines demonstrated no significant increase in the facet loading following TDR. Data from Faizan et al. [30] suggests that adjacent level facet degeneration may not be a major concern following disc replacement, and under hybrid loading conditions TDR maintains total facet loads similar to the intact facet loads (with lower facet loads for the SPH-I and OVL-I designs). Long-term clinical follow ups of the disc implant recipients may provide insight into the issue.
Wear
Most of the cervical total disc replacements are of articulating type. These have potential for wear, and may further lead to the altered biomechanics and implant loosening over time. Experimental evaluation of wear is expensive, labor intensive and time consuming (3+ months per test). The protocols follow ASTM/ISO loading profiles. However, the clinical relevance of these profiles is being debated. In vitro studies are unable to address this issue, while finite element-FE wear models may.
One wear study developed a chain of simulations which included geometric modeling, implant motion, load estimation, contact stress, velocity and wear prediction using a linear wear model [72]. The simulation’s wear volume (using the ISO test protocol inputs and the initial wear rate for the whole life), was 1.18 mm3 per million cycles. A percentage weight loss of 1.9% was predicted. Likewise, Vicars et al. [73] demonstrated the agreement between an experimental and a computational simulation for the wear of a fixed bearing total disc replacement. Our group undertook a FE study to evaluate the hypothesis that the wear pattern differs in simulations of device alone and device in situ for a given loading profile [74].
A predictive finite element wear model of the disc alone (Disc) was developed along the lines proposed in the literature and then was incorporated into a ligamentous C5–C6 finite element model (Disc + FSU), Fig. 1e. Both of the models were subjected to a motion (rotations about the three axes) profile along with a varying preload of 50–150 N at 1 Hz as per ISO 18192. A subroutine based on Archard’s law simulated the abrasive wear on the polymeric core up to 10 million cycles. The predicted wear patterns in the isolated disc and in FSU were completely different. The Disc + FSU model showed localized wear in certain regions in comparison to uniformly distributed wear pattern of the Disc only model (Fig. 4a). The cumulative volumetric wear for the Disc only model was three times that of the Disc + FSU model (Fig. 4b). The Disc + FSU model revealed separation at the articulating interface during extension and lateral bending. The proposed model approach (Disc + FSU) will enable the scientists to pursue effects of clinical and other parameters (like surgical variables, different loading profiles, different disc designs and bone quality) on wear of the artificial disc.
Fig. 4.
a The linear wear contour for Disc Only and Disc + FSU at 1,3,5,7 and 10 million cycles as per ISO18192 (Flexion/Extension (Flex/Ext) of ±7.5°, lateral bending (LB) of ±6°, and axial rotation (AR) of ±4° and a compressive-follower load of 50–150 N). Wear contours clearly indicate the circumferential wear pattern for the Disc only test case, while an uneven, one-sided wear pattern is shown for the Disc + FSU test case. Black denotes maximum wear, while orange or red means minimal wear. In the inset, FV1 stands for magnitude of linear wear (mm). b Cumulative volumetric wear (mm3) at the end of each million cycles for Disc only and Disc + FSU model as per ISO18192 (Flexion/Extension (Flex/Ext)) of ±7.5°; lateral bending (LB) of ±6°, axial rotation (AR) of ±4° and a compressive-follower load of 50–150 N)
Finally, another beneficial approach to analyze interplay among parameters described above is the use of the probabilistic approach, as adapted for the lumbar spine biomechanics [75]. Such an approach may explore high risk combinations of different parameters and explain the differences between “standard” biomechanical studies and clinical studies.
In vivo kinematics—stereoradiogrammetric (SR) technique
Assessment of in vivo segmental spine motion has been, and continues to be, a difficult clinical problem. The most commonly used modality is routine lateral and antero-posterior radiographs. The inaccuracy of the measurement from radiographs and the inability to measure the out-of-plane motions are the two significant sources of error in these studies [76–78].
Several studies have reported segmental motion of the cervical spine in both surgical and non-surgical groups [77–79]. Pearcy et al. [80] have used bi-planar radiographs, where subjects were filmed from two directions simultaneously, to determine three-dimensional motions based on the same “identifiable” anatomic landmarks in both views. A three-dimensional CT scans imaging technique using cadaver-cervical was developed by Lim et al. [81]. Cargill et al. [82] attempted motion analysis of MRI scans of spinal segments and Conrad et al. [83] attempted to analyze the segmental motion of the spine in vivo by modifying the fluoroscopy based motion analysis system. These studies have also documented a measurement error (1°–2° in rotation, 1–2 mm in translation) in the absence of reliable identifiable markers needed to reconstruct the motion segment [84, 85].
Stereoradiogrammetric analysis (SRA) is an accurate 3D in vivo measurement technique for motion measurement [86, 87] However, in this case the tantalum beads are placed in each of the vertebra of the segment of interest at surgery to track vertebra displacements. This makes the technique a bit less practical to use by a surgeon. Using this technique, Ordway et al. [88] conducted a clinical trial with 12 patients to examine the kinematics following ProDisc-L. Kinematic analysis of the intervertebral motions was accomplished using SRA postoperatively at 1.5, 3, 6, and 12 months. They found that the sagittal ROM following total disc replacement averaged 2.5° at 1.5 months, 5.6° at 3 months, 6.6° at 6 months, and 6.3° at 12 months. Coupled motions observed in lateral bending and translational motions did not correspond to pathologic motion in this group of patients. Robertson et al. [89], Rabin et al. [90], Yang et al. [91] and Pickett et al. [92] investigated the in vivo cervical spine kinematics postoperatively in patients with Prestige-1 at 4 years, Bryan at 1 year, Bryan at 5.2 months and Bryan at 1 year, respectively. They concluded that the ROM was maintained in Prestige-1, a mean of 8.4°, 7.8° and 4.8° sagittal motion was observed at their respective follow up periods. The kinematic data from the following clinical studies indicate the segmental motion preservation after TDR.
The above studies highlight the advantages of SRA in the evaluation of spine ROM. Thus, by far: it is the only method capable of adequately measuring 3D ROM of the spine. Such studies along with the patient specific models eluded to earlier may provide us with an in-depth understanding of the issues pertaining to the design and development of an optimal artificial disc.
Discussion
Disc implants abut against the two endplates. The endplate preparation may vary from patient to patient. The orientation of a disc implant with respect to these prepared endplates can potentially affect the post-op biomechanics. Likewise, the use of TDR as an adjunct to fusion is recommended to address the iatrogenic adjacent segment effects of the fusion surgery alone. Several studies, both in the lumbar and cervical regions, do provide evidence that the parameters under the control of a surgeon can alter the spinal biomechanics. These biomechanical effects of the surgical procedure, if any, are important to understand. For example, the importance of intact ligaments in providing stability following TDR needs investigation. How does the resection of important structures such as uncinate processes affect the biomechanics of the spine? In addition to this, lumbar arthroplasty studies indicate that the variations in the placement of the disc may influence the long-term success of the implant [93]. Long term effects such as bone remodeling, subsidence, facet joint loading, wear and in vivo measurements are crucial parameters to be considered in TDR procedure. The comparative studies on the wear of the cervical TDR have the potential to help in understanding the appropriate simulation parameters to match clinical wear rates. The above mentioned protocols on wear also lay emphasis on the implant retrieval analyses, whose access would provide with the appropriateness of the current state of wear standards. All these experimental and computational studies have proved that wear is a major factor that needs to be addressed and the design of wear resistant cervical disc implants are highly recommended. Are newer implant materials like nano (10−9) particle based coating of Ti alloys being pursued? Probabilistic studies as performed already at the lumbar spine may explore high risk combinations of different parameters and explain the differences between “standard” biomechanical studies and clinical studies [75].
Correlation of the kinematics evaluated from the clinical studies of total disc replacements and the biomechanical literature is somewhat inconsistent, as described earlier. Part of the reason could be the parameters, enumerated above, that alter the spine biomechanics following TDR. The lack of muscles in the in vitro experimental and finite element studies may be another contributing factor.
A large number of TDR concepts, and an equally large number of other parameters that influence their biomechanical characteristics make it impractical to understand the interplay among various factors. For example, there is a large scatter in the cadaver based kinematics, due to the quality of the specimens, lack of muscles, and variation in the test parameters. Consequently, cadaver data suggests that most of the disc designs restore motion at the involved and adjacent levels to the intact data. However, finite element studies do show variations in motion. Several parameters like center of rotation, range of motion (elastic zone, EZ + Neutral zone, NZ), stiffness, and hysteresis are used to assess the quality and the quantity of motion in a spinal segment. Compared to metal on metal and metal on polymer disc designs, elastomeric disc concepts better reproduce intact segment behavior. More importantly, these predictions reveal that the load sharing among structures, including TDR devices, and stresses in the disc components are functions of designs, surgical procedures and other variables. Since coupling phenomena in the cervical region is quite significant, the need for disc designs that mimic coupling behavior (axial rotation and lateral bending) may be important. Overall, fusion plus disc replacement model had less severe effects on adjacent levels when compared to bi-level fusion model. These have long-term consequences in terms of subsidence, and wear of the articulating surfaces, for example. Also, the clinical implications of the disc arthroplasty induced increase in the facet loads indicate a need for more in vivo studies. Since the subjects with cervical TDR may experience whiplash injuries at the operated level, biomechanical studies suggest that, like the lumbar disc arthroplasties, we may need a 360° motion preservation system for cervical spine as well. Discord between the biomechanical data and clinical observations may be resolved by blending muscle forces with appropriate ligamentous finite element models. In vivo kinematics evaluations using techniques like the SRA will help in predicting the long-term effects of TDR on the spine.
In summary, there is disconnect between the clinical outcomes following TDR and the biomechanical studies, unlike the clinical outcomes for fusion and corresponding biomechanical understanding. A number of biomechanical parameters may contribute to this disagreement, besides the biology. We have presented the following issues: effects of implant design variations, surgical procedures, whiplash, the long-term consequences of TDR like bone remodeling, subsidence, facet joint loading, wear and in vivo kinematics. It is our hope that this conceptual paper will encourage the readers for additional investigations to support/refute the concepts.
Acknowledgments
Work supported in part by grants from DePuy Spine, Medtronic Inc, Abbott Spine, OrthoKinetic Technologies, LLC, DMT Inc, and Ohio Research Scholar Program.
Conflict of interest
None.
References
- 1.Morishita Y, Hida S, Miyazaki M, Hong SW, Zou J, Wei F, Naito M, Wang JC. The effects of the degenerative changes in the functional spinal unit on the kinematics of the cervical spine. Spine J. 2008;33(6):E178–E182. doi: 10.1097/BRS.0b013e318166f059. [DOI] [PubMed] [Google Scholar]
- 2.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine J. 1993;18(15):2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80(7):941–951. doi: 10.2106/00004623-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Gore DR, Gardner GM, Sepic SB, Murray MP. Roentgenographic findings following anterior cervical fusion. Skeletal Radiol. 1986;15(7):556–559. doi: 10.1007/BF00361055. [DOI] [PubMed] [Google Scholar]
- 5.Terai T, Faizan A, Sairyo K, Goel VK (2010) Operated and adjacent segment motions for fusion vs. cervical arthroplasty: a pilot study. In: Current concepts in cervical spine surgery (Drs. Alberto Di Martino & Vincenzo Denaro, Guest Editors). CORR. (Epub ahead of print). http://www.ncbi.nlm.nih.gov/pubmed/21053112
- 6.Vaccaro AR, Falatyn SP, Scuderi GJ, Eismont FJ, McGuire RA, Singh K, Garfin SR. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11(5):410–415. doi: 10.1097/00002517-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, McDonough PW, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25(1):41–45. doi: 10.1097/00007632-200001010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Goel VK, Faizan A, Felon L, Biyani A, McGowan D, Wang ST. Biomechanical aspects of the spine motion preservation systems. In: Innovations in spinal reconstruction: clinical examples of basic science, biomechanics, and engineering. New York: Taylor and Francis; 2006. [Google Scholar]
- 9.Mummaneni PV, Robinson JC, Haid RW., Jr Cervical arthroplasty with the PRESTIGE LP cervical disc. Neurosurgery. 2007;60(4 Suppl 2):310–314. doi: 10.1227/01.NEU.0000255376.42099.13. [DOI] [PubMed] [Google Scholar]
- 10.Nabhan A, Ahlhelm F, Shariat K, Pitzen T, Steimer O, Steudel WI, Pape D. The ProDisc-C prosthesis: clinical and radiological experience 1 year after surgery. Spine. 2007;32(18):1935–1941. doi: 10.1097/BRS.0b013e31813162d8. [DOI] [PubMed] [Google Scholar]
- 11.Amit A, Dorward N. Bryan cervical disc prosthesis: 12-month clinical outcome. Br J Neurosurg. 2007;21(5):478–484. doi: 10.1080/02688690701474937. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PA, Rouleau JP, Bryan VE, Carlson CS. Wear analysis of the Bryan Cervical Disc prosthesis. Spine. 2003;28(20):S186–S194. doi: 10.1097/01.BRS.0000092212.42388.79. [DOI] [PubMed] [Google Scholar]
- 13.Bartels RH, Donk R. Fusion around cervical disc prosthesis: case report. Neurosurgery. 2005;57(1):194. doi: 10.1227/01.NEU.0000163419.59635.78. [DOI] [PubMed] [Google Scholar]
- 14.Bertagnoli R, Yue JJ, Pfeiffer F, Fenk-Mayer A, Lawrence JP, Kershaw T, Nanieva R. Early results after ProDisc-C cervical disc replacement. J Neurosurg Spine. 2005;2(4):403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 15.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Range of motion change after cervical arthroplasty with ProDisc-C and prestige artificial discs compared with anterior cervical discectomy and fusion. J Neurosurg Spine. 2007;7(1):40–46. doi: 10.3171/SPI-07/07/040. [DOI] [PubMed] [Google Scholar]
- 16.Chi JH, Ames CP, Tay B. General considerations for cervical arthroplasty with technique for ProDisc-C. Neurosurg Clin N Am. 2005;16(4):609–619. doi: 10.1016/j.nec.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Coric D, Finger F, Boltes P. Prospective randomized controlled study of the Bryan Cervical Disc: early clinical results from a single investigational site. J Neurosurg Spine. 2006;4(1):31–35. doi: 10.3171/spi.2006.4.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Gay E, Palombi O, Ashraf A, Chirossel JP. The Bryan cervical disc prosthesis. Preliminary clinical experience with nine implants. Neurochirurgie. 2004;50(6):624–629. doi: 10.1016/S0028-3770(04)98453-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim SW, Shin JH, Arbatin JJ, Park MS, Chung YK, McAfee PC. Effects of cervical disc prosthesis on maintaining sagittal alignment of the functional spinal unit and overall sagittal balance of the cervical spine. Eur Spine J. 2007;17(1):20–29. doi: 10.1007/s00586-007-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malloy KM, Hilibrand AS (2002) Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res 394:27–38 [DOI] [PubMed]
- 21.Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige l disc in an end-stage indication: 4-year results from a pilot study. Neurosurg Focus. 2004;17(3):10. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau MA, Cottin P, Levante S, Nogier A, Lazennec JY, Skalli W. In vivo kinematics of two types of ball-and-socket cervical disc replacements in the sagittal plane: cranial versus caudal geometric center. Spine. 2008;33(1):E6–E9. doi: 10.1097/BRS.0b013e31815e5dce. [DOI] [PubMed] [Google Scholar]
- 23.Shim CS, Lee SH, Park HJ, Kang HS, Hwang JH. Early clinical and radiologic outcomes of cervical arthroplasty with Bryan Cervical Disc prosthesis. J Spinal Disord Tech. 2006;19(7):465–470. doi: 10.1097/01.bsd.0000211235.76093.6b. [DOI] [PubMed] [Google Scholar]
- 24.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6(3):198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 25.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. Spine. 2007;32(26):2933–2940. doi: 10.1097/BRS.0b013e31815d0034. [DOI] [PubMed] [Google Scholar]
- 26.Pimenta L, McAfee PC, Cappuccino A, Cunningham BW, Diaz R, Coutinho E. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine. 2007;32(12):1337–1344. doi: 10.1097/BRS.0b013e318059af12. [DOI] [PubMed] [Google Scholar]
- 27.Siepe CJ, Mayer HM, Wiechert K, Korge A. Clinical results of total lumbar disc replacement with ProDisc II: three-year results for different indications. Spine. 2006;31(17):1923–1932. doi: 10.1097/01.brs.0000228780.06569.e8. [DOI] [PubMed] [Google Scholar]
- 28.Shim CS, Lee SH, Shin HD, Kang HS, Choi WC, Jung B, Choi G, Ahn Y, Lee S, Lee HY. CHARITE versus ProDisc: a comparative study of a minimum 3-year follow-up. Spine. 2007;32(9):1012–1018. doi: 10.1097/01.brs.0000260795.57798.a0. [DOI] [PubMed] [Google Scholar]
- 29.Beaurain J, Bernard P, Dufour T, Fuentes JM, Hovorka I, Huppert J, Steib JP, Vital JM, Aubourg L, Vila T. Intermediate clinical and radiological results of cervical TDR (Mobi-C®) with up to 2 years of follow-up. Eur Spine. 2009;J18:841–850. doi: 10.1007/s00586-009-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rousseau MA, Bonnet X, Skalli W. Influence of the geometry of a ball-and-socket intervertebral prosthesis at the cervical spine a finite element study. Spine J. 2008;33(1):E10–E14. doi: 10.1097/BRS.0b013e31815e62ea. [DOI] [PubMed] [Google Scholar]
- 31.Faizan A (2008) Investigation into cervical spine biomechanics following total disc replacement. Dissertation, University of Toledo, Toledo
- 32.Faizan A, Goel VK, Bergeron B. The anterior longitudinal ligament is essential to restore disc biomechanics following artificial disc replacement. 52nd Annual Meeting. Chicago: Orthopedic Research Society; 2006. [Google Scholar]
- 33.Johnson PJ, Lauryssen C, Helen OC, Robert P, Regan J, Anand N, Robert B (2004) Sagittal alignment and the Bryan cervical artificial disc. Neurosurg Focus 17(6):E14 [DOI] [PubMed]
- 34.Seok WK, Jae HS, Jose JA, Moon SP, Yung KC, Paul CM (2008) Effects of a cervical disc prosthesis on maintaining sagittal alignment of the functional spinal unit and overall sagittal balance of the cervical spine. Eur Spine J 17:20–29 [DOI] [PMC free article] [PubMed]
- 35.Kulkarni N, Goel VK, Kodigudla M, Ferrara L, Chikka A (2009) Effects of design variables on cervical spinal kinematics, neutral posture and quality of motion as demonstrated by four different artificial discs—a finite element analysis study. In: 55th annual meeting of the Orthopaedic Research Society, Las Vegas, NV
- 36.Crawford NR, Arnett JD, Butters JA, Ferrara LA, Kulkarni N, Goel VK, Duggal N. Biomechanics of a posture-controlling cervical artificial disc: mechanical, in vitro, and finite-element analysis. J Neurosurg Neurosurg Focus. 2010;28(6):11. doi: 10.3171/2010.3.FOCUS1063. [DOI] [PubMed] [Google Scholar]
- 37.Aho A, Vartiainen O, Salo O. Segmentary antero-posterior mobility of the cervical spine. Ann Med Intern Fenn. 1955;44(4):287–299. [PubMed] [Google Scholar]
- 38.Bhalla SK, Simmons EH. Normal ranges of intervertebral-joint motion of the cervical spine. Can J Surg. 1969;12(2):181–187. [PubMed] [Google Scholar]
- 39.Dvorak J, Froehlich D, Penning L, Baumgartner H, Panjabi MM. Functional radiographic diagnosis of the cervical spine: flexion/extension. Spine. 1988;13(7):748–755. doi: 10.1097/00007632-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Lind B, Sihlbom H, Nordwall A, Malchau H. Normal range of motion of the cervical spine. Arch Phys Med Rehabil. 1989;70(9):692–695. [PubMed] [Google Scholar]
- 41.Charles R. Clark ECB, Bradford LC (eds) (2004) The cervical spine, 4th edn. Lippincott Williams & Wilkins, Philadelphia
- 42.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Intervertebral disc replacement maintains cervical spine kinetics. Spine. 2004;29(24):2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 43.Faizan A, Goel VK, Kulkarni N, Biyani A, Garfin S, Bono C, Maguire P, Serhan H (2008) Biomechanical comparison following bilevel fusion, bilevel total disc replacement and fusion plus total disc replacement at adjacent levels in cervical spine. In: 54th annual meeting of the Orthopaedic Research Society, San Francisco, CA
- 44.Faizan A, Goel VK, Garfin SR, Bono CM, Serhan H, Biyani A, Elgafy H, Krishna M, Friesem T (2009) Do design variations in the artificial disc influence cervical spine biomechanics? A finite element investigation. Eur Spine J. doi:10.1007/s00586-009-1211-6 [DOI] [PMC free article] [PubMed]
- 45.Shin DA, Yi S, Yoon do H, Kim KN, Shin HC. Artificial disc replacement combined with fusion versus two-level fusion in cervical two-level disc disease. Spine J. 2009;34(11):1153–1159. doi: 10.1097/BRS.0b013e31819c9d39. [DOI] [PubMed] [Google Scholar]
- 46.Faizan A, Goel VK, Krishna M, Friesem T. Effects of removal of uncinate process in the cervical disc replacement model: a finite element study. San Diego, CA: 53rd annual meeting of Orthopedic Research Society; 2007. [Google Scholar]
- 47.Snyder JT, Tzermiadianos MN, Ghanayem AJ, Voronov LI, Rinella A, Dooris A, Carandang G, Renner SM, Havey RM, Patwardhan AG. Effect of uncovertebral joint excision on the motion response of the cervical spine after total disc replacement. Spine J. 2007;32(26):2965–2969. doi: 10.1097/BRS.0b013e31815cd482. [DOI] [PubMed] [Google Scholar]
- 48.Faizan A, Goel VK, Krishna M, Friesem T. Placement of artificial disc affects the biomechanics of the cervical spine: a finite element investigation. Miami: Spine Arthroplasty Society; 2008. [Google Scholar]
- 49.Demetropoulos CK, Srinivasan S, Bilkhu SK, Hardy WN, Yang KH, Bishop J, Abjornson C, Bey MJ, Herkowitz HN, Bartol SW. Consequences of whiplash injury following ProDisc-C disc replacement: evaluation of cervical kinematics during low speed rear-end impact. Miami: Spine Arthroplasty Society; 2008. [Google Scholar]
- 50.Faizan A, Goel VK, Elgafy H, Kodigudla M, Chikka A (2009) Effects of whiplash injury to the mechanics of a cervical specimen implanted with disc—an in vitro study SAS09, ninth annual global symposium on motion preservation technology, London, England; April 28–May 01
- 51.Rohlmann A, Bergmann G, Graichen F. Loads on an internal spinal fixation device during walking. J Biomech. 1997;30(1):41–47. doi: 10.1016/S0021-9290(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 52.Rohlmann A, Bergmann G, Graichen F. Loads on internal spinal fixators measured in different body positions. Eur Spine J. 1999;8(5):354–359. doi: 10.1007/s005860050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohlmann A, Bergmann G, Graichen F, Mayer HM. Telemeterized load measurement using instrumented spinal internal fixators in a patient with degenerative instability. Spine. 1995;20(24):2683–2689. doi: 10.1097/00007632-199512150-00009. [DOI] [PubMed] [Google Scholar]
- 54.Rohlmann A, Bergmann G, Graichen F, Weber U. In vivo measurement of implant loads in a patient with a fractured vertebral body. Eur Spine J. 1995;4(6):347–353. doi: 10.1007/BF00300295. [DOI] [PubMed] [Google Scholar]
- 55.Rohlmann A, Graichen F, Weber U, Bergmann G. Monitoring in vivo implant loads with a telemeterized internal spinal fixation device. Spine. 2000;25(23):2981–2986. doi: 10.1097/00007632-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 56.Rohlmann A, Bergmann G, Graichen F, Mayer HM. Influence of muscle forces on loads in internal spinal fixation devices. Spine. 1998;23(5):537–542. doi: 10.1097/00007632-199803010-00005. [DOI] [PubMed] [Google Scholar]
- 57.Matyas A, Goel V, Vadapalli S, Khandha A, Navarro R, Biyani A, Cameron B (2004) Motion characteristics of a metal-on-polymer disc and a telemeterized natural motion elastomer disc (TNMED)—a finite element study. In: 31st annual meeting of International Society for the Study of Lumbar Spine, Porto, Portugal, May 31–June 5
- 58.Kong WZ, Goel VK, Gilbertson LG, Weinstein JN. Effects of muscle dysfunction on lumbar spine mechanics. A finite element study based on a two motion segments model. Spine. 1996;21(19):2197–2206. doi: 10.1097/00007632-199610010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Marras WS, Knapik G, Gabriel J (2007) The development of a personalized hybrid emg-assisted/finite element biomechanical model to assess surgical options, Chap 88. In: Yue JJ, Bertagnoli R, McAfee P, An H (eds) Motion preservation surgery of the spine: advanced techniques and controversies. PM Gordon Associates, Philadelphia, PA, pp 687–694
- 60.Phillips FM, Garfin SR. Cervical disc replacement. Spine. 2005;30(17 Suppl):S27–S33. doi: 10.1097/01.brs.0000175192.55139.69. [DOI] [PubMed] [Google Scholar]
- 61.Durbhakula M, Ghiselli G. Cervical total disc replacement, part i: rationale, biomechanics, and implant types. Orthop Clin North Am. 2005;36(3):349–354. doi: 10.1016/j.ocl.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Phillips FM, Tzermiadianos MN, Voronov LI, Havey RM, Carandang G, Dooris A, Patwardhan AG (2009) Effect of two-level total disc replacement on cervical spine kinematics. Spine J 34(2):E794–E799 [DOI] [PubMed]
- 63.Goel VK, Ramirez SA, Kong W, Gilbertson LG. Cancellous bone Young’s modulus variation within the vertebral body of a ligamentous lumbar spine-application of bone adaptive remodeling concepts. J Biomech Eng. 1995;117(3):266–271. doi: 10.1115/1.2794180. [DOI] [PubMed] [Google Scholar]
- 64.Grosland NM, Goel VK. Vertebral endplate morphology follows bone remodeling principles. Spine. 2007;32(23):E667–E673. doi: 10.1097/BRS.0b013e318158cfaf. [DOI] [PubMed] [Google Scholar]
- 65.Anderson PA, Rouleau JP. Intervertebral disc arthroplasty. Spine. 2004;29(23):2779–2786. doi: 10.1097/01.brs.0000146460.11591.8a. [DOI] [PubMed] [Google Scholar]
- 66.Bertagnoli R, Zigler J, Karg A, Voigt S. Complications and strategies for revision surgery in total disc replacement. Orthop Clin North Am. 2005;36(3):389–395. doi: 10.1016/j.ocl.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Lin CY, Kang H, Rouleau JP, Hollister SJ, Marca FL. Stress analysis of the interface between cervical vertebrae end plates and the Bryan, Prestige LP, and ProDisc-C cervical disc prostheses: an in vivo image-based finite element study. Spine. 2009;34(15):1554–1560. doi: 10.1097/BRS.0b013e3181aa643b. [DOI] [PubMed] [Google Scholar]
- 68.Sekhon LH, Ball JR. Artificial cervical disc replacement: principles, types and techniques. Neurol India. 2005;53(4):445–450. doi: 10.4103/0028-3886.22611. [DOI] [PubMed] [Google Scholar]
- 69.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7(1):33–39. doi: 10.3171/SPI-07/07/033. [DOI] [PubMed] [Google Scholar]
- 70.Metzger MF, Acosta FL, Buckley JM, O’Reilly OM, Lotz JL (2008) Facet forces sensitive to total disc replacement device position. Orthopedics Research Society, San Francisco, CA
- 71.Stieber J, Quirno M, Kang M, Valdevit A, Errico TJ. The facet joint loading profile of a cervical intervertebral disc replacement. San Francisco: Orthopedic Research Society; 2008. [DOI] [PubMed] [Google Scholar]
- 72.Jongh CU, Basson AH, Scheffer C. Predictive modelling of cervical disc implant wear. J Biomech. 2008;41(15):3177–3183. doi: 10.1016/j.jbiomech.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Vicars R, Brown T, Fisher J, Hall R (2009) Agreement between novel physical and computational wear simulations for total disc replacement. In: 55th annual meeting of Orthopedic Research Society, Las Vegas, NV
- 74.Bhattacharya S, Liu X, Kiapour A, Goel VK, Serhan H (2010) Models that incorporate spinal structures predict better wear performance of cervical artificial discs [DOI] [PubMed]
- 75.Rohlmann A, Boustani HN, Bergmann G, Zander T (2010) A probabilistic finite element analysis of the stresses in the augmented vertebral body after vertebroplasty. Eur Spine J [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 76.Herrmann AM, Geisler FH. Geometric results of anterior cervical plate stabilization in degenerative disease. Spine. 2004;29(11):1226–1234. doi: 10.1097/00007632-200406010-00012. [DOI] [PubMed] [Google Scholar]
- 77.Ochia RS, Inoue N, Takatori R, Andersson GB, An HS. In vivo measurements of lumbar segmental motion during axial rotation in asymptomatic and chronic low back pain male subjects. Spine. 2007;32(13):1394–1399. doi: 10.1097/BRS.0b013e318060122b. [DOI] [PubMed] [Google Scholar]
- 78.Bono CM, Khandha A, Vadapalli S, Holekamp S, Goel VK, Garfin SR. Residual sagittal motion after lumbar fusion: a finite element analysis with implications on radiographic flexion-extension criteria. Spine. 2007;32(4):417–422. doi: 10.1097/01.brs.0000255201.74795.20. [DOI] [PubMed] [Google Scholar]
- 79.Cargill SC, Pearcy M, Barry MD. Three-dimensional lumbar spine postures measured by magnetic resonance imaging reconstruction. Spine. 2007;32(11):1242–1248. doi: 10.1097/01.brs.0000263404.66369.a5. [DOI] [PubMed] [Google Scholar]
- 80.Ochia RS, Inoue N, Renner SM, Lorenz EP, Lim TH, Andersson GB, An HS. Three-dimensional in vivo measurement of lumbar spine segmental motion. Spine. 2006;31(18):2073–2078. doi: 10.1097/01.brs.0000231435.55842.9e. [DOI] [PubMed] [Google Scholar]
- 81.Pearcy MJ, Whittle MW. Movements of the lumbar spine measured by three-dimensional X-ray analysis. J Biomed Eng. 1982;4(2):107–112. doi: 10.1016/0141-5425(82)90070-X. [DOI] [PubMed] [Google Scholar]
- 82.Lim TH, Eck JC, An HS, McGrady LM, Harris GF, Haughton VM. A noninvasive, three-dimensional spinal motion analysis method. Spine. 1997;22(17):1996–2000. doi: 10.1097/00007632-199709010-00011. [DOI] [PubMed] [Google Scholar]
- 83.Conrad B, Jacob P, Banks S (2007) Accurate measurement of cervical disc replacement kinematics using single plane fluoroscopy. In: 7th annual meeting of the global symposium on motion preservation technology, Berlin, Germany
- 84.Rogers BP, Haughton VM, Arfanakis K, Meyerand ME. Application of image registration to measurement of intervertebral rotation in the lumbar spine. Magn Reson Med. 2002;48(6):1072–1075. doi: 10.1002/mrm.10319. [DOI] [PubMed] [Google Scholar]
- 85.Haughton VM, Rogers B, Meyerand ME, Meyerand ME. Measuring the axial rotation of lumbar vertebrae in vivo with MR imaging. AJNR Am J Neuroradiol. 2002;23(7):1110–1116. [PMC free article] [PubMed] [Google Scholar]
- 86.Gunnarsson G, Axelsson P, Johnsson R, Strömqvist B. A method to evaluate the in vivo behaviour of lumbar spine implants. Eur Spine J. 2000;9(3):230–234. doi: 10.1007/s005860000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Axelsson P, Karlsson BS. Intervertebral mobility in the progressive degenerative process. A radiostereometric analysis. Eur Spine J. 2004;13(6):567–572. doi: 10.1007/s00586-004-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nathaniel RO, Amir HF et al (2008) Twelve-month follow-up of lumbar spine range of motion following intervertebral disc replacement using radiostereometric analysis. Spine Arthroplasty Soc 2(1):9–15 [DOI] [PMC free article] [PubMed]
- 89.Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results from a pilot study. Neurosurg Focus. 2004;17(3):E10. doi: 10.3171/foc.2004.17.3.10. [DOI] [PubMed] [Google Scholar]
- 90.Rabin D, Pickett GE, Bisnaire L, Duggal N (2007) The kinematics of anterior cervical discectomy and fusion versus artificial cervical disc: a pilot study. Neurosurgery (3 Suppl):100–104; discussion 104–105 [DOI] [PubMed]
- 91.Yang S, Hu Y, Zhao J, He X, Liu Y, Xu W, Du J, Fu D (2007) Follow-up study on the motion range after treatment of degenerative disc disease with the bryan cervical disc prosthesis. J Huazhong Univ Sci Technol 27(2):176–178 [DOI] [PubMed]
- 92.Pickett GE, Rouleau JP, Duggal N et al (2007) Kinematic analysis of the cervical spine following implantation of an artificial cervical disc. Spine J 30(17):1949–1954 [DOI] [PubMed]
- 93.Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine. 2001;26(6):E122–E129. doi: 10.1097/00007632-200103150-00004. [DOI] [PubMed] [Google Scholar]