Abstract
We briefly summarize recent advances regarding brain functional representation of chronic pain, reorganization of resting state brain activity, and of brain anatomy with chronic pain. Based on these observations and recent theoretical advances regarding network architecture properties, we develop a general concept of the dynamic interplay between anatomy and function as the brain progresses into persistent pain, and outline the role of mesolimbic learning mechanisms that are likely involved in maintenance of chronic pain.
Keywords: chronic pain, functional magnetic resonance imaging, network, diffusion tensor imaging, neuroplasticity, learning
Introduction
The progression of pain research has been hindered by the dominating view that pain is a purely nociceptive phenomenon. In contrast, the future of pain research must lie in the expanded understanding of how the brain interacts with pain across time, as a function of different pain conditions.
Animal models have allowed us to induce, exaggerate, inhibit, and prevent experimental acute and chronic pain states. Perhaps the greatest lesson animal models have taught us is encapsulated in the idea of central sensitization, as this concept expanded our focus from peripheral pain processing to the spinal cord circuitry [38]. Central sensitization, defined as the enhanced nociceptive synaptic transmission within the spinal cord following persistent pain states, can explain some of the most perplexing clinical symptoms of chronic pain. These symptoms include the dissociation of pain perception from a noxious stimulus or injury, the expansion of hyperalgesia beyond the site of injury, and the extra-dermatomal hypersensitivity across other somatic and visceral structures. Yet despite 20 years of intensive research, we have a limited understanding of the critical elements that drive the induction and maintenance of central sensitization. Some pain scientists have even questioned whether the standard animal models can allow us to disentangle the principles of centrally-maintained pain [24]. The net result is the assumption that chronic pain is either driven by primary afferent input or sustained by the local reverberating circuitry within the spinal cord and facilitated by descending pain modulation. We have, in essence, decapitated pain by assuming that the brain passively reflects spinal cord properties.
Here, in contrast, we expand on the notion that the brain is actively carving the properties of pain as it evolves from an acute to chronic condition [1–2]. Pavlov taught us that pain is a powerful learning signal, and this idea has shaped research on learning and memory in the behavioral neuroscience field, whereas it has been all but forgotten by pain researchers (until recently, see [18]). Surprisingly, human brain imaging has brought these ideas back into focus. Our recent work emphasizes the importance of brain limbic circuitry in chronic pain, pointing to the underlying learning mechanisms that mediate pain chronification. Here we will briefly describe how the individual chronic pain conditions leave different structural imprints on the brain and are associated with unique patterns of brain activity. These features that distinguish chronic pain conditions provide evidence for the lability of brain anatomy and function that are consequences of these learning processes. In this review, we develop a working model of the central mechanisms of chronic pain, based on the dynamic relationship between the brain structural and functional networks that sustain emotional pain learning. Ultimately, we suspect that the standard view of central sensitization as a peripherally-initiated process must be supplemented with the supraspinal mechanisms of brain reorganization that mediate this learning, in order to account for the long-term maintenance of central sensitization (i.e., what differentiates individuals who go on to transition from the acute to chronic pain state).
A Network View of Brain Function
The initial attempts to capture acute pain with functional magnetic resonance imaging (fMRI) yielded a predictable pattern of regional brain activation, dubbed the “Pain Neuromatrix.” The assumption that the experience of pain is reducible to a collection of regional activations, each with a circumscribed sensory or affective role, has been challenged on two grounds: first, these regions—individually and collectively—participate in a diverse set of perceptual, motor, and cognitive-emotional functions that far surpass pain processing [34]. Secondly, their interactions with the rest of the brain need to be taken into account, in effect necessitating an examination of these brain regions in relation to underlying functional and anatomical properties of the brain neural network. The cortex is a densely connected network of regions (network nodes), with reciprocal information flow between multiple hierarchically organized and functionally distinct groups of cells. “Pain Neuromatrix” brain regions show bidirectional interactions (e.g., [33]), with continuous dialogues of information flow occurring within the order of milliseconds, and this information flow is contingent on excitatory and inhibitory connections with the rest of the brain.
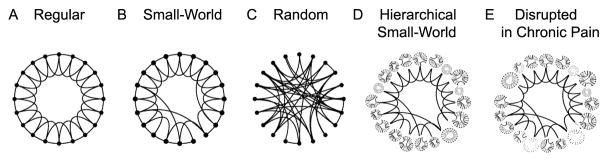
The brain can be conceptualized as a massive network, connected across all scales. Recent advances in our understanding of the architecture of neural networks have revealed how these different hierarchies of networks can coordinate neural processing [23]. In 2005, we provided the first quantitative demonstration that the brain can be characterized as a “small-world” network (also known as “six degrees of separation,” which is logically equivalent to the popular game “six degrees of Kevin Bacon”) [12]. This observation was based on the properties of functional connectivity, which is a measure of the extent of information shared between voxels or regions [39], and were calculated for every voxel in relation to all other voxels in the brain as individuals performed different tasks. The scale invariance of brain functional connectivity (which is conserved whether 2 or 2000 voxels, over 4 scales of magnitude, are functionally correlated, independent of the task being performed) defines it as a small world property. The practical implication is that the brain can efficiently share information across brain regions and it is more resilient to network disruptions (as compared to local or random networks, see Fig. 1) [36]. The function and structure of the groups of brain regions that make up the nodes in this network likely shift with continued nociceptive input from the periphery as it is integrated with ongoing central changes (a hypothetical model is shown in Fig. 2a). The recent popularity of studying the brain in its resting state is one such approach to explore global brain network properties under conditions where the external perturbation is random and thus the external inputs only randomly affect the brain properties [27,30].
Figure 1. Characteristics of the brain as a small-world network, and a conceptual model of its disruption with chronic pain.
Illustrates the seminal theoretical observation that, between Regular (A) and Random (C) connected networks, there is a group of network structures (Small-World Network, B) where a small number of long distance connections increases the efficiency and stability of the network architecture (adapted from [36]). Given the observed properties of the brain connectome [31], it is more accurately represented by an overarching hierarchy of nested, regional small-world networks (Hierarchical Small-World Network, D). We speculate that over time, the presence of chronic pain alters the functional connectivity of these nested regional networks and their crosstalk (through the preferential reduction and/or enhancement of local and but not long-distance node interactions), in a pain condition-specific manner (E).
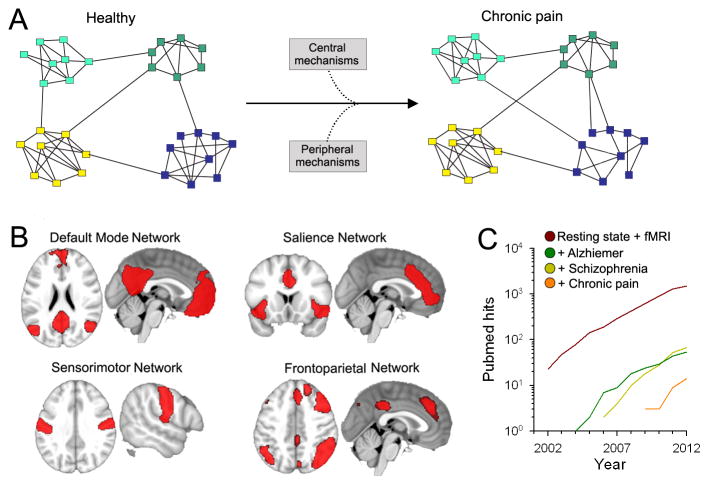
Figure 2. The brain as a network.
(A) The brain can be represented as a network where the nodes correspond to constitutive elements, or nodes (voxels from MRI, neurons, etc.), and these nodes form links based on some type of interaction between nodes. Complex networks also have a tendency to exhibit a modular topology, where links are concentrated within modules. The presence of chronic pain may alter the properties of these modules through changes in inter- or intra-modular interactions, through peripheral and central mechanisms (figure adapted from Bullmore et al. [23]) These may be viewed as subcomponents of the local modules depicted in Fig. 1. (B) Resting state fMRI show that several networks composed of modules (regions) can be identified from the fluctuating patterns of intrinsic activity seen in the human brain. Shown are four major resting state brain networks that might be relevant to pain processes (adapted from [8]). (C) Number of hits in PubMed for search of “Resting state AND fMRI”. The number of studies using resting state analyses exhibits an exponential growth over the last decade. Also shown are resting state studies performed in patients suffering from Alzheimer’s disease and schizophrenia, which are a magnitude higher than the number of studies conducted in chronic pain.
Functional Impact of Chronic Pain
To date, half a dozen studies have looked at resting state networks (RSNs), a measure of the brain’s intrinsic connectivity, across various chronic pain conditions (Fig. 2) [5,10,21,25,32,37]. RSNs consist of brain regions that temporally and spatially share a common pattern of fluctuations, across subjects (usually around 7–10 resting state networks can be distinctly identified [30]). Their spatial distribution and underlying functions have been used to define them. Four major RSNs known as default mode (DMN), salience, sensory/motor engagement, and attention networks are illustrated (Fig. 2b). In general, the representation of chronic pain studies within the RSN literature lags far behind when compared to other neurological and psychiatric conditions (Fig. 2c). The DMN is the most extensively studied RSN and reflects a coherent set of brain regions that are active in the absence of tasks [15,27]. The interpretation of DMN activity is controversial, yet this network is preferentially (and likely distinctly) affected by the presence of chronic pain (see Fig. 3) [5,10,21,25,32,37]. Multiple laboratories have found that functional connectivity between the DMN and the insula is enhanced across pain populations, including chronic back pain, fibromyalgia, and diabetic neuropathy [5,10,25], suggesting that pain disrupts the DMN through increased nociceptive input from the insula.
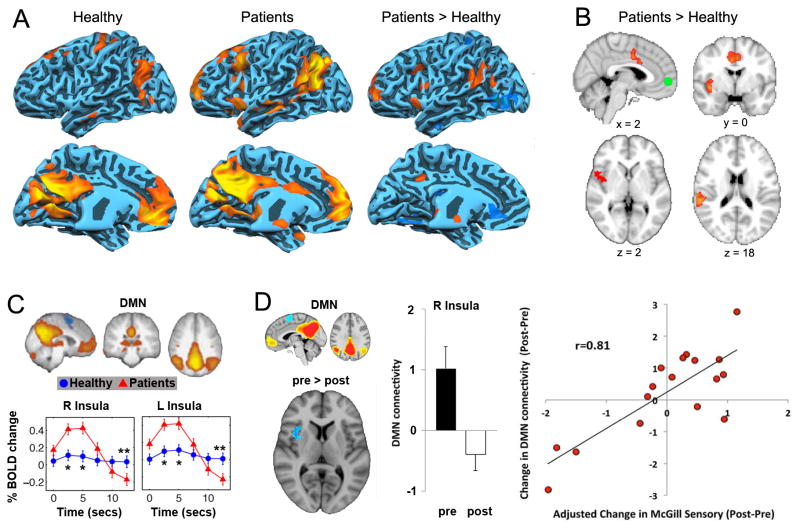
Figure 3. Resting state changes in chronic pain cohorts.
(A) Data adapted from Cauda et al. [10] showing that the default mode network (DMN) shows increased connectivity to the anterior and posterior portions of the insula in patients suffering from diabetic neuropathic pain. (B) Similar results were observed during resting state in chronic back pain patients from our lab, for which the MPFC, a main constituent of the DMN, showed increased correlation to the insula and ACC in patients [4]. (C) Similarly, Tagliazucchi et al. [32] found that the insula is more likely to show increased activity immediately following MPFC activity, only in pain patients but not healthy controls (figure adapted from [32]). (D) Napadow et al. showed that the correlation between DMN-insula activity reduces following treatment in fibromyalgia patients. In addition to these changes, this coupling was related to a change in pain parameters (adapted from [25]). These results collectively suggest that the DMN shows increased coupling with pain-related regions, indicating that chronic pain alters brain dynamics at rest.
Another expression of this intrinsic connectivity is captured by the regional blood oxygen level dependent (BOLD) frequency patterns that characterize the temporal and spatial interrelationships between brain regions. The brain exhibits specific, frequency-dependent organization, with a combination of transient and sustained interactions occurring between different brain regions to optimize the relay of information (see Fig. 4a) [8]. BOLD fluctuations across brain regions (detected at the spectral power distributions < 0.25 Hz) were first described in chronic pain populations by [10,21]. Subsequently we showed that the whole-brain resting state has an anatomically constrained pattern of BOLD oscillations [8], with a shift toward higher frequencies in chronic back pain patients within the DMN that was related to pain perception (Fig. 4c–d) [4]. Thus the presence of pain may interfere with the transmission of information by altering how pain-relevant regional frequencies co-oscillate. Moreover, the DMN frequency shifts directly reflect the presence of ongoing pain, indicating that pain influences intrinsic variations in brain activity.
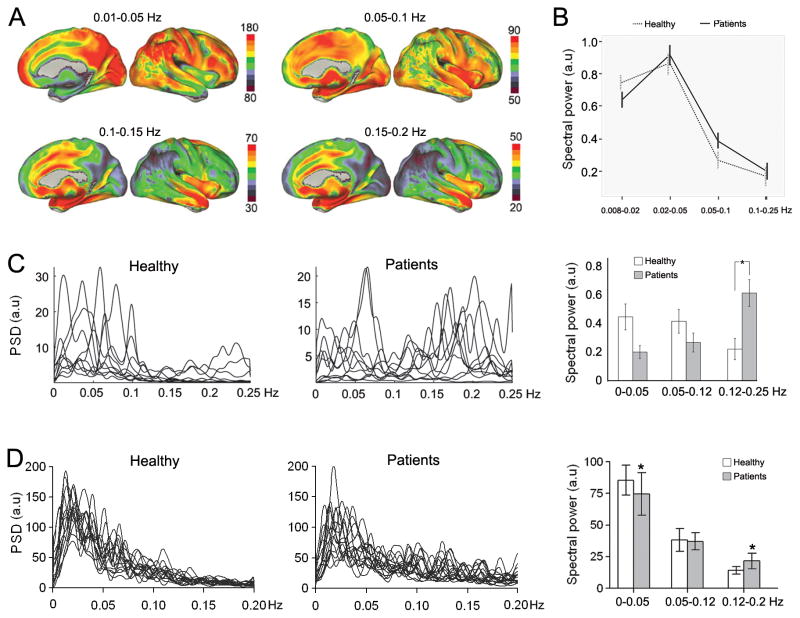
Figure 4. Chronic pain is associated with increased high frequency oscillations in the brain.
(A) The resting state brain shows robust frequency-dependent organization. Brain maps show the spatial distribution of spectral power for four frequency bands (0.01–0.05 Hz, 0.05–0.1 Hz, 0.1–0.15 Hz, and 0.15–0.2 Hz) in healthy subjects. The lowest-frequency band (0.01–0.05 Hz) has the highest power, and is localized mainly in the prefrontal, parietal, and occipital cortices. The higher-frequency bands exhibit less power, and localize more within the cingulate, insula, temporal cortex, and subcortical structures (adapted from [8]). (B) The DMN showed increased power in high frequency BOLD oscillations (0.05–0.1 Hz) in diabetic neuropathic pain patients (adapted from [10]). (C) Chronic pain patients show higher frequency oscillations in the insula. Line plots represent the normalized spectra for insular activity (individual subject data superimposed), separately for healthy controls and patients. Bar graphs show the mean ± SEM spectral power for different frequency bands in healthy and patients (adapted from [22]). (D) MPFC activity in chronic back pain patients, indicating higher frequency oscillations in the MPFC, to a lesser extent than in the insula (C).
The DMN activity is assumed to represent the sum total of activity when individuals are engaged in interoception (i.e., when ruminating), implying that it may be critical in human consciousness. Within this framework, the disruption of DMN with chronic pain can be viewed as the extent to which chronic pain interferes with information processing. It is important to note that DMN properties are usually studied only in low frequency BOLD oscillations (<0.1 Hz), while DMN disruption in chronic pain is seen mainly at high frequencies. Thus, it would be important to test if the DMN would remain disrupted with chronic pain under anesthesia.
Chronic Pain Re-shapes the Brain
In 2004, the first human imaging study of brain grey matter atrophy was reported in chronic back pain patients [3]. A recent PubMed search with “voxel based morphometry AND chronic pain” now yields 35 papers, most of which show brain regional decreases in grey matter. In contrast, only a handful of studies have evaluated the impact of chronic pain on brain white matter (via diffusion tensor imaging, DTI). Given the covariance between brain structure and function [17], the unique patterns of structural reorganization observed across chronic pain conditions likely reflects the long-term consequences of the pain-related changes in brain activity. Patterns of grey matter changes distinguish chronic back pain, osteoarthritis, and complex regional pain syndrome from healthy individuals and are driven by networks of regions, including areas associated with nociceptive and prefrontal regions (Fig. 5) [1,7]. In the presence of chronic pain, the pattern and number of links between brain regions shifts, with local regions increasingly becoming connected to distant regions. The extent of structural reorganization follows distinct trajectories for different types of chronic pain, with the mechanisms driving this long-term restructuring process unfolding over a period of years. The net result is a long-term change in brain architecture, although the precise mechanisms of this restructuring have yet to be identified. Given that a large spectrum of studies of chronic pain populations have shown reductions in grey matter that are not found in healthy cohorts, and that the successful treatment of chronic back pain appears to partially reverse grey matter atrophy [28], this evidence collectively reinforces the notion that the brain’s structure dynamically reflects the clinical pain state (Fig. 6). Although these observations do not preclude the role of predisposing factors in the initiation of pain development, they constitute the strongest evidence of a causal relationship between a biological parameter (i.e., brain anatomy) and the development and resolution of pain. It is tempting to speculate that these structural changes can directly account for the cognitive and emotional disturbances described in the pain literature [20]; however, this too requires future study.
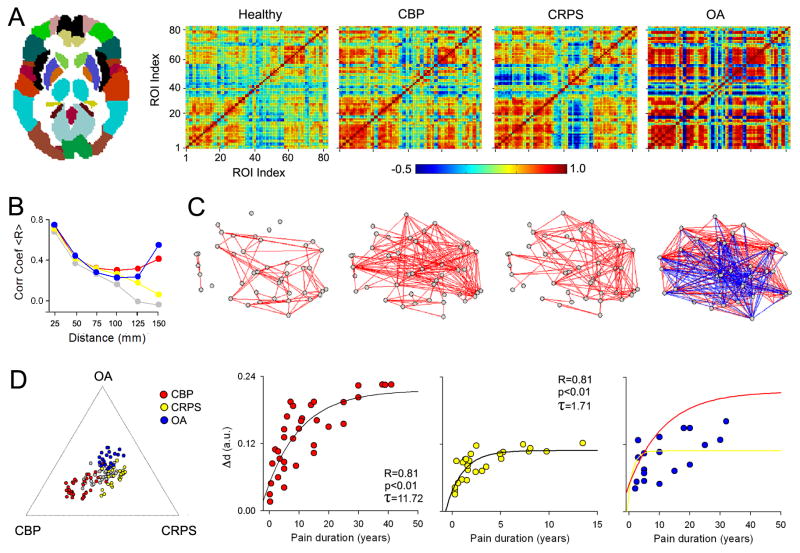
Figure 5. Cortical grey matter interrelationships (structural covariance) are specific for different chronic pain groups.
(A) The relationships between grey matter regions were studied by calculating pair-wise correlations of grey matter density between the 82 Brodmann Area-defined regions (left panel) across subjects, separately for healthy controls, CBP, CRPS, and OA. The resulting correlation matrices show widespread increases in correlation strength (i.e., more blue and red, indicating decreased and increased similiarites in grey matter pairs) in all three patient groups, compared to controls. (B) Line plots show the pair-wise correlations (for 41 regions in left hemisphere) plotted against the mean distance between pairs of regions. Healthy subjects show a linear dependency on distance, indicating that grey matter density in proximal pairs of regions is more similar than density between distal pairs of regions. This relationship is disrupted in unique ways in each chronic pain condition, where patients show higher correlations between pairs of regions that are far apart (for statistical details, see [7]). (C) Graph representation of the data in (A) showing the association between the pairs of regions. Red and blue lines represent positive and negative correlations (links), grey date are the pairs of regions (nodes). (D) The left plot shows relative locations of individual subjects based on the global grey matter structural reorganization that characterizes each condition (indicated by the extremities of the plot, in relation to the center, healthy brains) Right scatter plots show the relationship between global grey matter structural reorganization to pain duration, which follows an exponential growth function, distinct in patients with different pain conditions. All figures adapted from [7].
Figure 6. Partial reversal of brain atrophy with effective treatment.
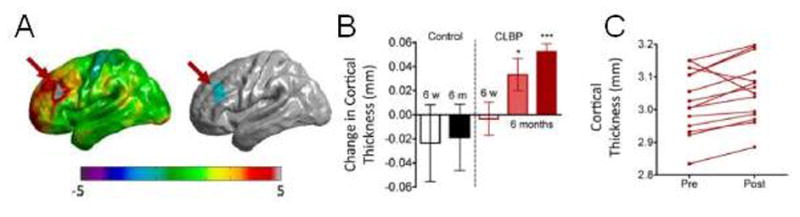
(A) Changes in cortical thickness of the left dorsolateral prefrontal cortex (DLPFC) were observed 6 months following significant pain reduction with spine surgery or facet joint injections in chronic low back pain patients (B, dark red bar). (C) The majority of individual patients showed increases in DLPFC cortical thickness following treatment. Figures adapted from [28].
If brain regions can be conceptualized as nodes of a network, these nodes require pathways of myelinated axons to communicate across short and long distances. As a result, white matter (axonal) properties are essential for understanding local and global changes in brain architecture, and these are quantifiable with DTI. We first described selective alterations in white matter connectivity between atrophied regions (including the ventromedial prefrontal cortex, anterior insula, and nucleus accumbens) in complex regional pain syndrome (CRPS) [14]. Clinical pain characteristics of irritable bowel syndrome also correlate with regional changes in an indirect measure of white matter integrity (fractional anisotropy) in the insula, thalamus, and fornix [11]. Although these findings suggest that white matter changes are localized to regions associated with nociception and valuation, it is unclear whether these changes reflect local and/or global axonal properties. We observed a dissociation between the global elements of brain structure across chronic pain conditions that may clarify the mechanisms underlying the pain-related reorganization of neural networks. In the healthy brain, grey matter density (corrected for age) and fractional anisotropy are positively correlated; in contrast, this correlation is null in CRPS and chronic pelvic pain (Fig. 7) [13,14]. Chronic pain can therefore be conceptualized as an imbalance in the axonal-neuronal relationship. However, a closer inspection of this data shows that, because the white matter properties are roughly equivalent between controls and patients, changes in white matter likely cannot account for the disruption of the grey-white matter relationship. From a network architecture perspective, the local network properties (neurons) rather than the large-scale pathways (axon tracts) have adapted to the presence of pain. Accordingly, the brain in chronic pain exhibits abnormalities in local grey matter and white matter restructuring, thereby degrading local networks but preserving large scale white matter tracks (Fig. 1E).
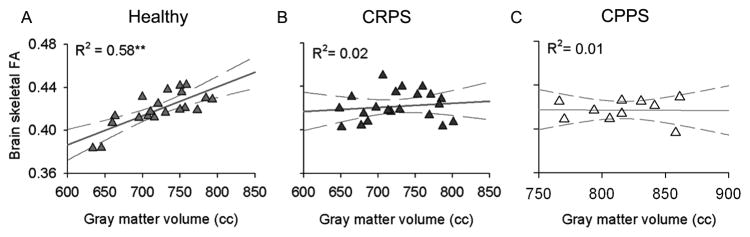
Figure 7. Chronic pain disrupts the global grey-white matter relationship.
(A) The healthy brain is characterized by a positive correlation between grey and white matter integrity (estimated with diffusion tensor imaging using fractional anisotropy, FA) after correcting for age, suggesting that this anatomical interrelationship reflects the brain’s natural organizational properties. In contrast, the brains of individuals with (B) complex regional pain syndrome (CRPS) and (C) male chronic pelvic pain syndrome (CPPS) show a complete dissolution of this grey-white matter relationship. These findings indicate that some chronic pain conditions are associated with massive brain anatomical changes that may dramatically impact how neurons communicate, locally and globally. Figures adapted from [13,14].
Parallels with Neurodegenerative Disease
In general, a convergence between brain structure and function has been demonstrated across both healthy and neurodegenerative states. Some exceptions have been identified, specifically for regional correspondences and mismatches between functional connectivity and white matter properties [e.g., 1,26]. Nevertheless, the interrelation between brain function and structure is a property of the normally developing brain, with the sensorimotor and DMN-related regions emerging before regions that mediate cognitive-emotional and social processes [40]. In adulthood, higher intelligence is correlated with decreased network path length (the number of nodes required to travel from one region to another), suggesting that intelligence is characterized by more efficient network architecture [35]. Therefore the properties of network architecture provide critical information about the efficiency of brain function.
Disruptions of the underlying networks that maintain normal brain function may occur with disease, including chronic pain and brain injury. A network disruption could occur through reduced/strengthened of existing pathways, or through regional shifts in connectivity, leading to the degradation of network coherence. Moreover, it suggests that the primary site of reorganization is limited to local circuitry, rather than alterations in large-scale connectivity. Just as distinct neurodegenerative diseases involve the atrophy and degradation of their corresponding RSNs [26], we have identified chronic pain condition-specific changes in unique patterns of network reorganization and brain functional properties [1].
Chronic Pain as Aversive Learning
The continued experience of chronic pain is essentially a form of emotional learning, with the chronic pain state reflecting a shift from sensory to hedonic brain circuitry (Fig. 7). The integration of pain and hedonic information processing may have far-reaching effects on the appraisal of and interaction with aversion and reward [9,18–19,29]. These neuronal memory traces are forged through functional changes in synapses that underlie the experience of pain, which result in the structural alterations of synapses that define the establishment of a long-term memory. Chronic pain is maintained by these continually reinforced memory traces that strengthen the abnormal processing of pain by spinal cord circuits (i.e., central sensitization), via descending modulation. New learning (i.e., the acquisition of new habits and abilities) is associated with concurrent and diverse regional increases and decreases in grey matter density. These neuronal changes are consistent with targeted regional increases and decreases synaptic strength, reflecting long term potentiation and depression at the single cell level, respectively. The consistent finding of grey matter loss in chronic pain patients suggests our novel working hypothesis that this atrophy is mediated by long-term depression, perhaps through the central endocannabinoid system [16], although multiple alternative explanations for decreased grey matter have been suggested (e.g., water content, vasculature, neuronal cell size changes, glial recruitment, etc.) [22]. Therefore, the exposure of the mesolimbic learning circuitry to repeated pain establishes the pain memory traces, resulting in the reorganized network architecture that we observe in chronic pain (Fig. 5A–C).
Future Directions
Whereas the general clinical expectation is that a single common network underlies all types of chronic pain, which has been speculated to explain pain comorbidity, the structural and functional comparisons across diverse pain conditions have revealed distinct patterns of altered brain anatomy and pain perception representation. For the past 50 years, treatments for pain have been indiscriminately applied across all chronic pain populations, assuming common mechanisms. In contrast, we believe that recent advances in brain imaging are revealing the unique brain imprints of different forms of chronic pain, which will direct the development of treatments tailored to the individual conditions and their respective genetic signatures.
Highlights.
We review literature describing the dynamic interaction between brain networks in chronic pain.
Chronic pain reflects a shift from nociceptive to mesolimbic circuitry over time.
Plasticity of hierarchically-organized networks underlies brain reorganization with persistent pain.
Unique conditions reflect distinct patterns of network reorganization.
Neuroplastic changes associated with emotional pain learning drive changes in brain structure and function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic grey matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–90. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsook D, Becerra L, Carlezon WA, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Cauda F, Sacco K, Duca S, Cocito D, Agata FD, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;25:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 12.EguÍluz VM, Chialvo DR, Cecchi GA, Baliki M, Apkarian AV. Scale-free brain functional networks. Phys Rev Lett. 2005;94:018102. doi: 10.1103/PhysRevLett.94.018102. [DOI] [PubMed] [Google Scholar]
- 13.Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: Abnormal grey-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52:766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 18.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as reward: Hedonic and neural responses to safety from pain. PLoS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–68. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci USA. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Meunier D, Lambiotte R, Bullmore ET. Modular and hierarchically modular organization of brain networks. Front Neurosci. 2010;4:1–11. doi: 10.3389/fnins.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011;10:829–843. doi: 10.1016/S1474-4422(11)70158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GD. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seminowicz DA, Wideman TH, Naso L, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour B, Doherty JPO, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sporns O. The human connectome: A complex network. Ann NY Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 32.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Map. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7:173–181. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- 35.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts DJ, Strogatz SH. Collective dynamics of small-world networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 37.Weissman-Fogel I, Moayedi M, Tenenbaum HC, et al. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 38.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci USA. 2010;107:18191–6. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]