Abstract
Individuals differ widely in cortisol output over the day, but the etiology of these individual differences remains poorly understood. Twin studies are useful for quantifying genetic and environmental influences on variation in cortisol output, lending insight into underlying influences on the components of Hypothalamic-Pituitary-Adrenal (HPA) axis functioning.
Salivary cortisol was assayed on 446 twin pairs (157 monozygotic, 289 dizygotic; ages 7–8). Parents helped youth collect saliva 30 min after waking, mid-afternoon, and 30 minutes prior to bedtime across 3 consecutive days. We used hierarchical linear modeling to extract predicted cortisol levels and to distinguish cortisol’s diurnal rhythm using a slopes-as-outcome piecewise growth curve model; two slopes captured the morning-to-afternoon and afternoon-to-evening rhythm, respectively. Separate genetic models were then fit to cortisol level at waking, mid-afternoon, and evening as well as the diurnal rhythm across morning-to-afternoon and afternoon-to-evening hours.
Three results from these analyses are striking. First, morning-to-afternoon cortisol level showed the highest additive genetic variance (heritability), consistent with prior research. Second, cortisol’s diurnal rhythm had an additive genetic component, particularly across the morning-to-afternoon hours. In contrast, additive genetic variation did not significantly contribute to variation in afternoon-to-evening slope. Third, the majority of variance in cortisol concentration was associated with shared family environments. In summary, both genetic and environmental factors influence cortisol’s circadian rhythm, and they do so differentially across the day.
Keywords: HPA axis, cortisol, diurnal rhythm, twins, behavior genetics
The Hypothalamic-Pituitary-Adrenal (HPA) axis is both highly sensitive to context and highly responsive to stress; it helps individuals to recalibrate or adapt their physiological activity to meet the demands of a constantly changing environment. Cortisol is the principal end-product steroid hormone produced by the HPA axis. A single measure of cortisol differentially reflects the confluence of momentary, day-to-day, rhythmic and individual difference factors (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Adam, Klimes-Dougan, & Gunnar 2007). Cortisol reactivity to acute challenges has often been investigated (Dickerson & Kemeny, 2004), yet cortisol is also responsive to chronic environmental forces; moreover, cortisol has a stable, trait-like component (Essex, Klein, Cho, & Kalin, 2002; Shirtcliff & Essex, 2008). Individual differences in cortisol have been linked to a broad range of physiological outcomes such as cardiovascular function, metabolism, and neural functions (Lupien et al., 2006; Sapolsky, Romero, & Munck, 2000), as well as to mental and physical health outcomes (Boyce et al., 1995; Pajer, Gardner, Rubin, Perel, & Neal, 2001; Smider, Essex, et al., 2002, 2002; Taylor, Lerner, Sage, Lehman, & Seeman, 2004), rendering HPA functioning clearly important but difficult to attribute to any single process.
Arguably, the strongest influence on cortisol is the time of day when a sample is collected (Vreeburg et al., 2009). An individual’s physiological activity is recalibrated daily to allow intrinsic biological rhythms to adjust to extrinsic environmental signals (Carskadon, Acebo, Richardson, Tate, & Seifer, 1997). Basal HPA activity thus follows a circadian rhythm with the highest activity occurring within the first hour after awakening, after which activity decreases throughout the day (Kirschbaum & Hellhammer, 1989). This rhythm is also an outcome of interest as (a) disruptions in rhythmicity are key components of allostasis and allostatic load (Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011)(Lupien et al., 2006); (b) rhythmicity is a reliable indicator of a broad range of (dys)regulatory processes (Siever & Davis, 1985); (c) large individual differences in cortisol levels exist at all points of the circadian cortisol curve (Smyth et al., 1997); and (d) there are multiple illustrations that high (or low) cortisol levels are associated with negative outcomes differentially depending on the time of day (Ruttle et al., 2011; Shirtcliff & Essex, 2008). Thus, understanding influences on HPA functioning is important because the underlying components may be partly controlled by unique genetic mechanisms (Veen et al., 2011), and these underlying mechanisms may be differentially related to behavioral, emotional and physiological characteristics and persistent risk factors (Shirtcliff & Essex, 2008).
Summary of Twin Study Findings
Twin methodology constitutes a powerful approach for identifying the genetic and environmental (shared and unique) influences on HPA functioning. Nevertheless, few studies have explored the genetic architecture of cortisol in humans. Bartels et al. (2003) reviewed nine published twin studies. Results varied widely, with some studies reporting no twin similarity in cortisol levels and others finding that genetic factors accounted for 45–72% of individual variation. Bartels and colleagues noted that many of these earlier studies suffered from small sample sizes, with samples ranging from as low as 7 twin pairs to a high of only 150 twin pairs. Several studies failed to account for the diurnal rhythm at either the sample collection or analysis phase. In addition, most of the studies reviewed focused on adults. Simultaneously analyzing all available data from twin studies using similar methodologies, without regard to timing of sample collection, Bartels et al reported that 62% of cortisol variation was associated with genetic variation.
Since the Bartels et al (2003a) review, some larger twin studies have been published that avoided many of the problems highlighted in the review. Riese et al. (2009) measured salivary cortisol in adult female twins at four time points – waking, 30, 45, and 60 minutes after waking. Genetic factors contributed to the variance in all measures of early morning cortisol (heritability ranged from 46% to 69%) with the remaining variation was accounted for by non-shared environmental factors. Kupper et al. (2005) also found evidence for genetic influences on early morning cortisol (waking and 30 min after waking) in adult twins. Mid-day to late evening samples, however, did not appear to be affected by familial factors. Turning to studies of children and adolescents, Bartels et al. (2003) measured salivary cortisol in 12 year-old twins at multiple time points from waking to evening across two days. Familial factors, genes or shared environment, influenced cortisol at all times, except for the evening sample. However, only the mid-morning sample was influenced unambiguously by genetic factors (heritability = 56%). Genetic and shared environmental factors could not be disambiguated at other collection times (waking and mid-day). Steptoe et al. (2009) measured afternoon salivary cortisol in 11 year old twins. Genetic factors accounted for 56% of cortisol’s variance. In contrast, Schreiber et al (2006) found that shared family environment accounted for the majority of variation in afternoon cortisol measured in 7–8 year old twins (69%), with the remaining variation accounted for by non-shared environmental factors, and no significant additive genetic influences.
In summary, previous studies consistently find that variation in morning cortisol level appears to be at least partially heritable. This is true regardless of the age of the participants. Evidence for genetic or shared environmental influences on cortisol in mid-afternoon, however, remains mixed. Interestingly, previous studies suggest that variation on cortisol sampled in late evening is largely influenced by individual specific factors in both adults and children, despite the vastly increased time twins spend together in childhood compared to adulthood.
Even within these newer, more rigorous investigations, few studies attempted to obtain stable measures of basal cortisol. Most investigators relied on cortisol measured at a single time point or, at best, within a single day. Studies that did collect multiple cortisol measures typically analyzed the cortisol serially, rather than employing analytic strategies that would help uncover the underlying unique components of cortisol measures (Shirtcliff et al., In press). Importantly, the possibility of an independent genetic contribution to cortisol’s diurnal rhythm has never been assessed although the heritability patterns across the day hint that the diurnal rhythm itself may be partially heritable.
Goals of the Study
We aimed to clarify outstanding issues concerning the quantitative genetic of variation in basal cortisol. We used hierarchical linear modeling (HLM) to extract predicted cortisol levels and to distinguish cortisol’s diurnal rhythm using a slopes-as-outcome piecewise growth curve model (Shirtcliff et al., in press). Using these improved measures of cortisol level and slope, we sought to powerfully test the emerging hypothesis suggested by trends in the literature: a) individual differences in stable measures of morning cortisol will be primarily influenced by the heritable factors, but late day stable measures of cortisol will be influenced to a greater degree by shared environment; b) likewise, morning-to-afternoon slope will exhibit significant heritable influences whereas afternoon-to-evening slope will be influenced primarily by environmental factors.
Materials and Methods
Participants
Participants included a subset of twin pairs (N=452 pairs) recruited from the birth record-based Wisconsin Twin Project; all twins were born between the years 1997–2002. Salivary samples were collected during a follow-up study, when most twins were between the ages of 7–8 years (M=90.4 months, SD=8.5). The sample was 50% female and included approximately equal numbers of monozygotic (MZ; 35%), same-sex dizygotic (DZ; 33%) and opposite-sex DZ (31%) twin pairs. Mothers had an average education of 15.2 years and fathers had an average education of 14.7 years, median family income was $60,000–$70,000, and the majority of twin pairs were Caucasian (98%). Compliance was very high and missing data were minimal, with 873 twins (97% of the total sample) providing at least one saliva sample (N’s per collection time/day ranged from 762 to 820) and 59% percent providing all nine saliva samples. Out of the 7,857 saliva samples requested, the N=7,142 samples obtained were sufficient enough to measure cortisol (90.9%).
Zygosity
Zygosity was classified during the age two assessment using the Zygosity Questionnaire for Young Twins (Goldsmith, 1991), which has demonstrated over 95% agreement with genotypic zygosity determination (Forget-Dubois, et al. 2003). Cases of ambiguous zygosity were resolved via hospital placenta(e) reports (an unambiguous monochorionic placenta indicating monozygosity) and follow-up zygosity questionnaires. If this information was not definitive, photographs, video images, and genotyping were utilized. Five pairs of twins (1.1%) with unknown or ambiguous zygosity who were not genotyped were excluded from genetic analyses.
Procedure
We contacted families for a telephone interview to screen for symptoms of psychopathology when twins were aged 7–8 years. Twins were selected for a follow-up study if they were deemed broadly “at-risk” or “control” (see Lemery-Chalfant, Goldsmith, Schmidt, Arneson, & Van Hulle, 2006 for selection details). We also included all unselected co-twins in the follow-up study. This resulted in 37% children designated at-risk, 34% designated control, and 29% unselected co-twins. Shortly after the screening process (6–10 months), parents completed a series of telephone interviews and mailed questionnaires; there was also a home visit. Families provided saliva samples for assaying as described below. All protocols were approved by the University of Wisconsin IRB, and parents completed a consent form prior to the study. Families were paid for their participation.
Cortisol Collection
Cortisol was measured in saliva because it captures the free unbound fraction of the total hormone that is biologically active and able to pass through cell membranes to influence gene expression directly within the cell nucleus (Bober, Weller, Weller, & Tait, 1988). Nine basal salivary samples were collected from each twin. Prior to the home visit, parents were mailed prelabeled Salivette collection tubes (Starstadt) and instructions. Parents were asked to collect saliva 30 minutes after waking, in the late afternoon (between 3–5 pm), and 30 minutes prior to bedtime on three consecutive days. 95% of morning samples were collected between 6:30 and 9:45 AM (mean=27.6 min post-awakening, SE=48.8 min); 95% of afternoon samples were collected between 2:00 PM and 6:00 PM and 95% of evening samples were collected between 7:30 PM and 10:00 PM. To control for the inherent variation in wake times across individuals, all times were converted to time-since-awakening (TSW). Accordingly, 95% of individuals awoke between 6:00 AM and 9:00 AM on the mornings of saliva collection. Families were instructed not to eat or drink one hour prior to saliva collection and to store samples in the freezer immediately after collection. The research team subsequently collected all samples during the home visit and transferred them back to the laboratory on ice. Samples were then stored at −80°C until assayed. Parents recorded the date and time of collection in addition to waking time, nap schedule (if applicable), medication use, and general health for each twin on each collection day. Only 16% of participants were taking medication during sample collection, <1% were taking any form of corticosteroid. Medication use was not related to raw cortisol concentration.
Cortisol Assays
Saliva samples were thawed, then centrifuged at 5000 rpm for 10 minutes to remove impurities. Cortisol was assessed in duplicate with a salivary enzymeimmunoassay kit (Salimetrics, State College, PA). Two non-blind internal controls were included in each assay. For the low control, the average value was 0.082 μg/dL with inter- and intra-assay Coefficent of Variations (CVs) of 7.2% and 6.1%, respectively. For the high control, the average value was 0.84 μg/dL with inter- and intra-assay CVs of 8.1% and 5.3% respectively. Results were considered acceptable if the CV of the duplicate samples was < 20%. Repeat assays were performed on any samples not meeting this requirement. Families were assayed across one or two batches.
Data Analysis
Forming cortisol composites
Risk status was not related to raw cortisol concentration, therefore all analyses were conducted on the full sample. We used hierarchical linear modeling (HLM; Raudenbush, Bryk, & Congdon, 2004) to extract basal cortisol levels and diurnal rhythm scores. HLM captures the inherent dependency or correlation of repeated samples collected on the same participant. Empirical Bayes estimates extracted only the stable, correlated cortisol component separate from uncorrelated moment-to-moment or day-to-day cortisol fluctuations (Shirtcliff et al., 2005). A second advantage of HLM is that it does not require complete data, but rather uses all the samples provided to calculate basal cortisol and diurnal rhythm scores. A third advantage is that HLM allows for time-varying predictors of cortisol levels, thereby permitting individualized estimation of HPA functioning at the specific times of collection for each individual and for each sample rather than necessitating that all samples were collected at equivalent intervals. This advantage is often important for estimations of the diurnal slope when several hours pass between sample collections.
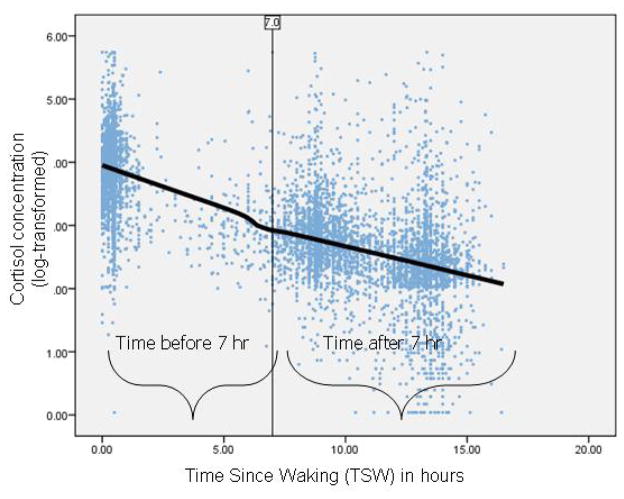
Specifically, a three-level HLM was used to capture within-the-day (level 1), day-to-day (level 2) and between-individual variation (level 3) in cortisol. Our a priori expectation was that the diurnal rhythm was not a single slope, but rather composed of the confluence of intrinsic and extrinsic influences on cortisol levels with a potentially nonlinear curve (Adam, 2006; Shirtcliff et al., in press). The diurnal rhythm was captured using a piece-wise growth curve comprised of 2 linear slopes (Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011; Ranjit, Young, Raghunathan, & Kaplan, 2005). Visual inspection of Figure 1 suggested a natural inflection point in the diurnal rhythm approximately 7 hours after awakening. This interval is advantageous as it (a) naturally divides the daytime hours roughly in half, (b) has many observations at and around that time to avoid extrapolation to determine the slopes, and (c) serendipitously maps onto the “morning” and “evening” hours of a circadian rhythm. Therefore, we included two predictors of cortisol levels at level 1: Morning-to-afternoon Slope was TSW between 0 hours and 7 hours after awakening (π1). Afternoon-to-evening Slope was TSW between 7 hours and 16.5 hours after awakening (π2). TSW was centered on 7 hours after awakening so that the intercept captured predicted cortisol levels at 7 hours after awakening (π0). Residual variation (e) captured within-the-day cortisol fluctuations.
Figure 1.
Scatterplot of all raw cortisol concentrations (log-transformed) plotted against time since waking and lowess curve fit to raw cortisol concentrations.
At level 1, TSW predicted cortisol levels, but thereafter became an outcome of interest using a slopes-as-outcome approach (Snijders & Bosker, 1999). At level 2, residual variation of cortisol level (r0) and morning-to-afternoon slope (r1) captured day-to-day fluctuations in cortisol level and its slope; residual variation for afternoon-to-evening slope was set to be fixed because we did not have enough degrees of freedom to allow it to vary. Finally, at level 3, the residual variation of cortisol level (u00), morning-to-afternoon slope (U10) and afternoon-to-evening slope (U20) captured individual differences in cortisol levels at 7 hours post-awakening and its morning-to-afternoon and afternoon-to-evening slope:
Empirical Bayes scores of cortisol level (β00), morning-to-afternoon slope (β10) and afternoon-to-evening slope (β20) were extracted from level 3 and used as estimates of basal cortisol and its diurnal rhythm. Each individual has a single predicted level at 7 hours post awakening, morning-to-afternoon slope and afternoon-to-evening slope, free from within-the-day and day-to-day fluctuations in cortisol.
Parallel to the models above, we also extracted Empirical Bayes scores of cortisol level (β00) upon awakening to examine whether morning basal cortisol was influenced by genetic factors to a different degree than afternoon cortisol level. TSW was included as a level 1 predictor to control for the influence of the diurnal rhythm (γ100=−.11, p<.0001). To derive evening basal cortisol level, Empirical Bayes scores of basal evening cortisol were extracted (β00) after centering TSW on 13 hours after awakening and controlling for TSW across the first thirteen hours of the day (γ100=−.117, p<.0001), and later between 13 and 16.5 hours after awakening (γ200=−.015, p=.48). Thirteen hours after awakening was selected to avoid extrapolation into the evening hours for individuals who were asleep between 13–16.5 hours after awakening.
Twin biometric modeling
Standard univariate biometric models decomposed observed variance in cortisol measures into variance attributable to unobserved, latent additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E). A refers to the cumulative additive effects of all segregating genes. C represents those environmental experiences shared by individuals that make family members more similar, such as (presumably) socioeconomic status or neighborhood characteristics. E represents experiences that are unique to each individual or that make family members less similar, plus measurement error (Neale & Cardon, 1992). All models were fit to raw data using OpenMx software (http://openmx.psyc.virginia.edu/). For each cortisol measure we calculated a saturated model (i.e. unconstrained variances and covariances) in addition to the biometric ACE model. The fit of the genetic model was compared to the saturated model by means of the log-likelihood ratio test (LRT). The difference in minus two times the log-likelihood (−2LL) between two nested models has a Chi-square distribution with the degrees of freedom (df) equaling the difference in df between the two models. The more constrained ACE model was kept as the most parsimonious and best fitting model if a value of p > .05 was obtained from the chi-square test and the Akaike’s Information Criterion was lowest. Similarly, we tested the significance of familial effects by comparing the full ACE model to either an AE only or CE only model, again using difference in log-likelihoods to determine if dropping A or C resulted in a significant loss in fit (Neale, Boker, Xie, & Maes, 2006).
Results
Estimating Basal Cortisol and the Diurnal Rhythm
The raw data illustrate that cortisol declines across the day (see Table 1) and that the HLM-derived Empirical Bayes estimates capture this underlying change pattern. Specifically, cortisol levels declined sharply across the morning hours, γ100=−.12, p<.0001, and continued to decline across the afternoon and evening hours, γ200=−.098, p<.0001, though not as steeply. After accounting for the diurnal slopes, a significant portion of the total cortisol variance 7 hours after awakening was due to between-individual differences (38.7%–57.2%), ps <.0001. Day-to-day variance was significant, ps <.0001, although it represented a much smaller portion of the total cortisol variance (2.5–8.4%). Finally, within-the-day momentary fluctuations in cortisol accounted for a substantial portion of the total variance in cortisol level across the day (53.0–56.0%). Table 1 presents the intra-class correlations at all three levels of the HLM (that is, the percentage of cortisol variation due to between-individual differences, day-to-day and within-day variation) as well as descriptive statistics for the HLM-based estimates.
Table 1.
Descriptive statistics for raw cortisol measures and HLM-derived Empirical Bayes’ cortisol estimates.
| Morning Level | Afternoon Level | Evening Level | Morning–to-afternoon Slope | Afternoon-to-evening Slope | |
|---|---|---|---|---|---|
| Raw data prior to transformation (μg/dL) | M=0.38 (SD=0.21) | M=0.15 (SD=0.14) | M=0.11 (SD=0.13) | NA | NA |
| HLM predicted cortisol estimates | M=3.9 (SD=0.31) | M=2.3 (SD=0.51) | M=3.0 (SD=0.31) | M=−0.13 (SD=0.04) | M=−0.1 (SD=0.10) |
| Intra-Class Correlation indicates % Cortisol Variation at each hierarchical level | |||||
| Level 1: Within the day | 56.0 | 40.4 | 53.0 | NAa | NAa |
| Level 2: Day-to-day | 5.3 | 2.5 | 8.4 | 50.4 | NAb |
| Level 3: Between-individual differences | 38.7 | 57.2 | 38.6 | 49.6 | NAb |
Notes:
The morning-to-afternoon and afternoon-to-evening slope are derived at level 1 (within the day), so do not have within-the-day %variance estimates.
Residual variation for evening slope was fixed because we did not have enough degrees of freedom to allow it to vary.
Genetic and Environmental Influences on Cortisol
Table 2 shows the twin intraclass correlations. MZ twin correlations estimate the upper limit on the total familial effects, since MZ’s are assumed to share all their genetic and shared environmental influences. Deviations from 1.0 indicate environmental influences unique to each individual and measurement error. Twice the difference between MZ and DZ twin correlations provides a rough estimate of the heritability of a trait (Falconer & MacKay, 1996). Under a purely additive genetic model, DZ correlations should be half the MZ correlations, whereas DZ correlations greater than this indicate shared environmental influences. Both MZ and DZ correlations were strikingly high for all cortisol measures. In particular, DZ correlations were substantially higher than half the MZ correlations, suggesting that shared environment plays a large role in influencing trait-like cortisol throughout the day. These observations of the twin similarity were tested formally using comparative model-fitting.
Table 2.
Intraclass twin correlations and results from univariate model fitting for HLM-derived basal and diurnal cortisol measures.
| Intraclass Correlations
|
Model Fitting Results
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MZ | DZ | Model | −2LL | AIC | πβ2 | df | p | |
| Morning Level | .70 | .56 | Saturated | 186.8 | −1519 | |||
| ACE | 193.9 | −1524 | 7.2a | 6 | .31 | |||
| AE | 212.4 | −1507 | 18.1 | 1 | <.001 | |||
| CE | 203.5 | −1516 | 11.5 | 1 | <.001 | |||
| Afternoon Level | .79 | .70 | Saturated | 91.3 | −1614 | |||
| ACE | 102.0 | −1615 | 10.7a | 6 | .10 | |||
| AE | 157.6 | −1562 | 55.6 | 1 | <.001 | |||
| CE | 108.1 | −1611 | 6.1 | 1 | .01 | |||
| Evening Level | .79 | .72 | Saturated | 909.3 | −796 | |||
| ACE | 918.7 | −799 | 9.4a | 6 | .15 | |||
| AE | 993.7 | −726 | 74.9 | 1 | <.001 | |||
| CE | 919.5 | −859 | .75 | 1 | .39 | |||
| Morning –to-afternoon | ||||||||
| .65 | .46 | Saturated | −4420.9 | −6126 | ||||
| Slope | ||||||||
| ACE | −4408.1 | −6126 | 12.8a | 6 | .05 | |||
| AE | −4399.2 | −6119 | 7.6 | <.001 | ||||
| CE | −4401.3 | −6121 | 7.0 | .01 | ||||
| Afternoon-to-evening Slope | .69 | .61 | Saturated | −2618.1 | −4324 | |||
| ACE | −2605.6 | −4324 | 12.6 | 6 | .05 | |||
| AE | −2572.1 | −4292 | 35.4 | 1 | <.001 | |||
| CE | −2603.4 | −4323 | 3.5 | 1 | .14 | |||
Comparison with an un-constrained, saturated model.
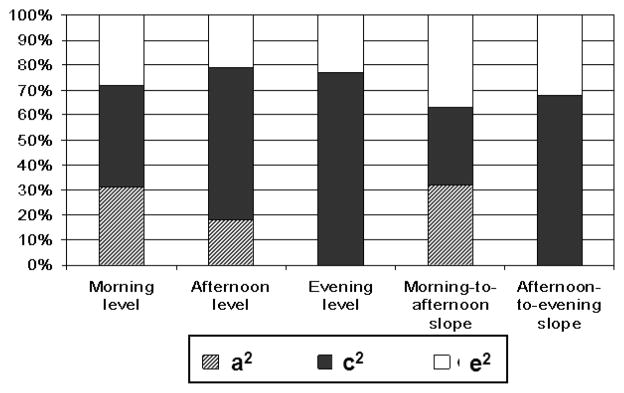
Results from fitting biometric twin models to each cortisol measure separately are also shown in Table 2. In general, the biometric model fit as well as the saturated model. The full ACE model fit the data best for morning level, afternoon level, and morning-to-afternoon slope. For evening level and afternoon-to-evening slope, dropping the additive genetic factor from the model did not worsen the overall fit (Δχ2 = .75, 1df, p=.39 for evening level, Δχ2 = 3.5, 1df, p=.14 for evening slope). The proportions of variation accounted for by A, C, and E are shown in Figure 2. Heritability estimates were highest for morning level (31%) and slope (32%) and dropped to 0% for evening level. In contrast, shared environment estimates increased from 40% for morning level to 71% for evening level. With the exception of morning-to-afternoon slope, shared environment accounted for a greater portion of the overall variation than genetic or non-shared environmental factors.
Figure 2.
Proportion of total variance in HLM based cortisol measures accounted for by genetic (a2), shared environmental (c2), and non-shared environmental (e2) factors derived from best-fitting genetic model.
Discussion
Approximately 40–60% of variation in our cortisol measures reflect stable, between individual differences. These findings are similar to those reported by Kertes & van Dulmen (2012) for a similarly aged sample. Both Kertes & van Dulmen and the current study find that variation accounted for by between-person effects was similar in early-morning and late-evening samples. Interestingly, we found that afternoon cortisol level was most heavily influenced by between-person effects. Since consistently elevated (or consistently low) diurnal cortisol is linked with a wide variety of clinical conditions, distinguishing a true trait-like component of cortisol level and its circadian rhythm provides an opportunity to understand the putative mechanisms underlying individual differences in HPA set-points.
Three results from our genetic analyses are striking. First, morning cortisol level and slope showed the highest amount of variation attributable to additive genetic factors, which is consistent with prior research involving substantially smaller sample sizes. Second, our findings are the first to suggest that cortisol’s circadian rhythm itself has an additive genetic component, particularly across the morning hours. Third, the majority of variance in cortisol was due to shared environmental factors that make family members more similar to each other.
Although morning level and morning-to-afternoon slope were influenced by genetic factors, the heritability was modest. In fact, our estimates of heritability are among the lowest reported, although they are similar in magnitude to at least one other study of children (Bartels, de Geus, et al., 2003). If true, these findings have cautionary implications for genetic research, particularly candidate gene research. A high heritability is desirable when testing for association of specific sequence variants with complex traits (Gottesman & Gould, 2003). Therefore, basal salivary cortisol, especially when measured later in the day, may not be the best measure to use when searching for genes that have a direct influence on HPA regulation. Future research should work to identify a more highly heritable endophenotype to stand as a marker of HPA activity.
Later day cortisol levels, on the other hand, may provide a useful biological index of cumulative family stress. This possibility is supported by the large effect of shared environment on cortisol level and circadian rhythm in the afternoon and evening. It should be noted that our estimate of shared environment, particularly for evening, is quite high compared to previous studies. It is unclear to what extent this is due to our use of trait-like cortisol measures as opposed to measures where trait and state components are confounded. However, this finding suggests that identifying family environmental factors may be key to understanding later-day HPA regulation. In the majority of families, salivary cortisol was collected at the same time for both twins. In many cases twins were engaged in the same activities prior to collection (e.g. watching television or doing homework) Alternatively, prenatal stress influences later HPA regulation (Gutteling, Weerth, & Buitelaar, 2005), and thus twins’ shared intrauterine experience, rather than present day family experiences, may partially explain the high estimated shared environmental influences. However, Schreiber et al. (2006) found that mothers’ and fathers’ afternoon cortisol was found to correlate at the same magnitude as mothers (or fathers) and their offspring, indicating that intrauterine experiences are unlikely to account for all of the shared environmental variance in later day cortisol. Moreover, studies of adult twin samples do not report similar significant shared environmental influences. In the absence of shared influences, non-shared environmental effects play a larger role in variation in cortisol activity.
These results largely support previous findings, but with a more trait-like measure. This is the first study to incorporate such measures in the genetically informative design. The advantage of using measures derived from HLM, rather than an analysis of the raw cortisol concentration, is that by modeling level and slope simultaneously we can account for the fact that subsequent changes in values depends on the initial values. Indeed, while both morning-to-afternoon and afternoon-to-evening slopes are modestly (r=.27) correlated with morning level, the pattern of genetic and environmental influences is quite different for the two slopes.
Limitations
Parents were instructed to obtain the first sample 30 min after waking. This prevented us from measuring and modeling a separate slope for the awakening response (CAR) as other studies have done (Bartels, Van den Berg, et al., 2003; Wust, Federenko, Hellhammer, & Kirschbaum, 2000). In previous studies, the strongest genetic effects occurred 30–45 min after waking, thus, our measure of morning cortisol likely captures the most heritable point in the diurnal rhythm.
Although we modeled several key variables that the literature implicates in cortisol variation, some potentially relevant variables could not be included. Day of the week (weekday vs. weekend) and season (and thus length of day) when the samples were collected varied from family to family as testing continued throughout the year. Given that these types of sample collection issues are constant across the members of twin pairs, any variance that they account for should accrue to the shared environmental (C) component in our biometric models. That is, the overall high proportion of variance attributed to shared environment could be in part explained by timing of sample collection, although this is unlikely to explain the differential impact of shared environment across the day. In addition, we did not randomly distribute twin pairs over different assay batches. While some families were assayed in the same batch others were spread over different batches. In the former case, we would expect an increase in the amount of shared variance, while in the latter we would expect a slight increase in error variance and subsequent reduction in shared variance. In either case, given that laboratory error typically accounts for less than 2% of the total variation in cortisol levels (Shirtcliff, Granger, Booth, & Johnson, 2005), variation due to assay is unlikely to substantially affect the findings.
As with all studies that rely on twin methods, the results may not be generalizable to a population of singleton births, especially because twin births are more likely to involve prenatal or birth complications than singleton births (Åkerman & Fischbein, 1991). However, Kupper et al. (2005) did not find any differences in mean cortisol levels between adult twins and their singleton siblings. Likewise, Schreiber et al (2006) found mean cortisol level was similar between twins aged 7–8 and their non-twin siblings.
Conclusion and Future Directions
The findings illustrate that both genetic and environmental factors influence cortisol’s circadian rhythm differentially across the day in middle childhood. HPA functioning is notoriously sensitive to both transient and stable contextual factors. Delineating the genetic and environmental influences on a trait-like component of HPA activity provides a foundation for examining factors that contribute to the stability and change in HPA functioning across development as well as the complex relationship between genetic and environmental influences HPA function.
Acknowledgments
This work was supported by research grants from the National Institute of Mental Health (R01 MH59785 and R37 MH50560 to Goldsmith) and the Wisconsin Center for Affective Science (P50 MH069315). Infrastructure support was provided by the Waisman Center via a core grant from the National Institute of Child Health and Human Development (P30 HD03352). Salary support was provided by a Career Development Award for Shirtcliff (K01 MH077687).
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Klimes-Dougan B, Gunnar Megan. Human behavior, Learning, and the developing brain: Atypical development. New York, NY: Guilford Press; 2007. Response to Stress in infants, children, and adolescents; pp. 264–304. [Google Scholar]
- Åkerman BA, Fischbein S. Twins: Are they at risk? A longitudinal study of twins and nontwins from birth to 18 years of age. Acta Geneticae Medicae et Gemellologiae: Twin Research. 1991;40(1):29–40. doi: 10.1017/s000156600000670x. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus E, Kirschbaum C, Sluyter F, Boomsma D. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33(4):421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma D, de Geus E. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28(2):121–137. doi: 10.1016/S0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bober J, Weller E, Weller R, Tait A. Correlation of serum and salivary cortisol levels in prepubertal school-aged children. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27(6):748–750. doi: 10.1097/00004583-198811000-00014. [DOI] [PubMed] [Google Scholar]
- Boyce W, Adams S, Tschann J, Cohen F, Wara D, Gunnar M. Adrenocorticol and behavior predictors of immune-responses to starting school. Pediatric Research. 1995;38(6):1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Acebo C, Richardson G, Tate B, Seifer R. An approach to studying circadian rhythms of adolescent humans. Journal of Biological Rhythms. 1997;12(3):278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23(01):85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M, Klein M, Cho E, Kalin N. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52(8):776–784. doi: 10.1016/S0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Gould T. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gutteling B, Weerth C, Buitelaar J. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30(6):541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kertes DA, van Dulmen M. Latent state trait modeling of children’s cortisol at two points of the diurnal cycle. Psychoneuroendocrinology. 2012;37(2):249–255. doi: 10.1016/j.psyneuen.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer D. Salivary cortisol in psychobiological research - An overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus E, van den Berg M, Kirschbaum C, Boomsma D, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30(9):857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Goldsmith HH, Schmidt NL, Arneson CL, Van Hulle CA. Wisconsin Twin Panel: Current Directions and Findings. Twin Research and Human Genetics. 2006;9(6):1030–1037. doi: 10.1375/183242706779462363. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, Pruessner J, et al. Developmental Neuroscience, Developmental Psychopathology. 2. Vol. 2. Hoboken, NJ: John Wily & Sons Inc; 2006. Beyond the stress concept: Allostatic load -- a developmental biological and cognitive perspective; pp. 578–628. Retrieved from http://psycnet.apa.org/psycinfo/2006-03610-014. [Google Scholar]
- Neale M, Boker SM, Xie G, Maes HH. Statistical modeling. 7. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2006. [Google Scholar]
- Pajer K, Gardner W, Rubin R, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young E, Raghunathan T, Kaplan G. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30(7):615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM 6 for Windows (Computer software) Lincolnwood, IL: Scientific Sofware International, Inc; 2004. [Google Scholar]
- Riese H, Rijsdijk F, Rosmalen J, Snieder H, Ormel J. Neuroticism and Morning Cortisol Secretion: Both Heritable, But No Shared Genetic Influences. Journal of Personality. 2009;77(5):1561–1576. doi: 10.1111/j.1467-6494.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- Ruttle P, Shirtcliff E, Serbin L, Fisher D, Stack D, Schwartzman A. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Romero L, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schreiber J, Shirtcliff E, Van Hulle C, Lemery-Chatfant K, Klein M, Kalin N, Essex M, et al. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31(9):1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery M, Kalin N, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and diurnal rhythms from childhood to adolescence. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(01):167–184. doi: 10.1017/S0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff E, Essex M. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L, Davis K. Toward a dysregulation hypothesis of depression - Overview. American Journal of Psychiatry. 1985;142(9):1017–1031. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Skinner M, Shirtcliff E, Haggerty K, Coe C, Catalano R. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011;23:1165–11184. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider N, Essex M, Kalin N, Buss K, Klein M, Davidson R, Goldsmith H. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels M, Gorin AA, Catley D, Porter L, Kirschbaum C, Hellhammer D, et al. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22(2):89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks: Sage Publications; 1999. [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;17:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Veen G, Giltay E, van Vliet I, DeRijk R, Klaassens E, van Pelt J, Zitman F. C-reactive protein polymorphisms are associated with the cortisol awakening response in basal conditions in human subjects. Stress-The International Journal on the Biology of Stress. 2011;14(2):128–135. doi: 10.3109/10253890.2010.515273. [DOI] [PubMed] [Google Scholar]
- Vreeburg S, Kruijtzer B, van Pelt J, van Dyck R, DeRijk R, Hoogendijk W, Smit J, et al. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34(8):1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer D, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]