Abstract
Objective
Deaths related to HIV/AIDS have declined due to improved HIV therapies. However, people with AIDS remain at elevated risk for cancer and cancer deaths. Prior studies evaluated cancer deaths using death certificates, which may be inaccurate. We utilized population attributable risk methods (which do not rely on death certificates) to assess cancer mortality.
Design
Data from a U.S. population-based record linkage study were used to identify incident cancers and deaths in 372,364 people with AIDS (1980–2006) followed for up to 5-years after AIDS onset. We utilized Cox regression to compare mortality in subjects with and without cancer and to calculate cancer-attributable mortality across calendar periods (AIDS onset in 1980–1989, 1990–1995, and 1996–2006).
Results
Mortality declined across calendar periods for all people with AIDS but remained higher among those with cancer vs. those without. During 1996–2006, among individuals with an AIDS-defining cancer (ADC) who died, 88.3% of deaths were attributable to their ADC; likewise, among individuals with a non-AIDS-defining cancer (NADC), 87.1% of deaths were attributable to their NADC. The fraction of all deaths in people with AIDS attributable to ADC (i.e., population attributable risk) decreased significantly from 6.3% (1980–1990) to 3.9% (1996–2006), but NADC population attributable mortality increased significantly over time from 0.5% (1980–1989) to 2.3% (1996–2006).
Conclusions
Among those with AIDS and cancer who subsequently die, most deaths are attributable to their cancer. With declining overall mortality, the proportion of all deaths attributable to NADCs has increased. These results highlight the need for improved cancer prevention and treatment.
Keywords: AIDS, attributable risk, cancer, HIV
Introduction
Following widespread use of highly active antiretroviral therapy (HAART) in 1996, survival improved dramatically among people infected with HIV [1, 2]. These changes in the mortality experience of people with HIV infection are largely due to steep declines in deaths from complications, such as opportunistic infections associated with advanced immunosuppression (i.e., AIDS). Despite improved overall survival, people with HIV infection, and particularly those with AIDS, remain at elevated risk for cancer and cancer-related death [3–5]. There are 3 AIDS-defining cancers (ADCs) that occur at elevated rates in HIV-infected people, each of which is caused by an oncogenic virus: Kaposi sarcoma (KS, caused by KS herpesvirus), non-Hodgkin lymphoma (NHL, where the major AIDS-defining subtypes are due to Epstein Barr virus), and cervical cancer (due to human papillomavirus) [6]. People with HIV/AIDS are also at increased risk for other cancers (i.e., non-AIDS-defining cancers or NADCs), such as Hodgkin lymphoma (caused by Epstein Barr virus infection) and lung cancer (related to tobacco exposure), for which HIV-related immunosuppression or inflammation may also contribute [7].
As deaths among HIV-infected people decline, especially those due to AIDS-related opportunistic infections, a larger fraction of the remaining deaths may be attributable to cancer. Indeed, studies that have reviewed causes of death among HIV-infected people have signaled that the crude fraction of deaths due to cancer (i.e., the number of deaths with cancer as the underlying cause divided by the total number of deaths) may be increasing in the HAART era [8–10]. Notably, however, it can be difficult to determine a single cause of death in people with HIV/AIDS, many of whom have multiple serious medical conditions. Clinicians may inaccurately ascribe death to cancer, when AIDS or another illness is actually responsible, or vice versa.
An alternate approach to assigning a proportion of deaths to cancer is premised on a calculated estimate of cancer-attributable mortality [11, 12]. This method is, in principle, based on a simple comparison of the all-cause mortality experience of people with cancer to people without cancer, i.e., it assumes that the extra deaths in people with cancer are due to the cancer. Because the attributable mortality framework uses only the total number of deaths, an advantage is that it does not require specified causes of death from death certificates, which can be inaccurate. However, no previous study of HIV-infected people has utilized this methodology.
We used population-based data from a large cohort of people with AIDS in the U.S. to determine trends in mortality and applied statistical models to determine the fraction of deaths attributable to cancer over time.
Methods
Study design
The HIV/AIDS Cancer Match Study links registry data on persons with HIV or AIDS diagnosed between 1980 and 2008 to cancer registries in 15 U.S. states and metropolitan regions (http://hivmatch.cancer.gov) [13]. Following linkage, only de-identified data are retained for analyses. Institutional review boards at participating sites approved the study.
Our goal was to determine mortality attributable to incident cancer among an immunosuppressed population. We therefore constructed a cohort of people with AIDS who were initially cancer free. AIDS was defined according to the 1993 CDC surveillance case definition [6]. We required subjects to be cancer-free before and at AIDS diagnosis, because cancer prior to AIDS onset might have artifactually accelerated the development of AIDS, and because 3 cancers are themselves AIDS-defining conditions. Importantly, KS can occur as an initial AIDS-defining event but has lower mortality than other AIDS-defining conditions [14]. Therefore, our exclusion of people with ADCs as their AIDS-defining event precludes the possibility of findings which would suggest ADCs as protective for mortality.
Follow-up for cancer and mortality began 4 months after AIDS onset. Of N=574,242 potentially eligible people with AIDS, we excluded individuals who had any cancer reported to the cancer registry, or an ADC reported to the HIV/AIDS registry, before 4 months after AIDS onset (N=33,374 and N=18,107, respectively), and people whose observation time ended before month 4 (N=128,831). We also excluded those whose follow-up for cancer began after month 4 (N=16,743) in order to make the cohort uniform at baseline. In addition, people diagnosed with AIDS prior to 1980 (N=7) and children aged less than 14 years (N=4,816) were excluded, yielding a cohort of adults and adolescents with AIDS who were cancer-free at the start of month 4 after AIDS onset (N=372,364). The exclusions used in the current study of people with AIDS are the same ones used in a prior analysis of underlying causes of death reported on death certificates,[10] allowing for a direct comparison of findings between the two methods.
Information on invasive cancers was obtained using the linked data from cancer registries, and malignancies were coded according to the 3rd edition of the International Classification for Diseases for Oncology [15]. Cancers were categorized as ADCs and NADCs using a modification of the Surveillance, Epidemiology, and End Results (SEER) program’s “Site recode with Kaposi sarcoma and mesothelioma” [16]. Only first cancers were considered, and vital status was obtained from HIV/AIDS registries at the time of the linkage.
Statistical methods for estimating cancer-attributable mortality
Subjects were classified according to calendar year of AIDS onset: 1980–1989 (no or limited availability of antiretroviral therapy), 1990–1995 (monotherapy and/or dual therapy) and 1996–2006 (HAART). Person-time was measured from month 4 after AIDS onset until last follow-up for cancer from the cancer registry, death, or a maximum follow-up of 5 years, whichever occurred first. Mortality rates among people with AIDS and cancer, and among people with AIDS alone who were cancer-free (the reference group), were calculated, with people contributing person-time to the cancer-free group until they developed cancer, then contributing person-time the group with cancer subsequently.
To determine the proportion of mortality due to cancer over this 5-year (60-month) period following AIDS onset, we calculated (1) the fraction of deaths attributable to cancer among persons with cancer (attributable risk [AR]) and (2) the fraction of deaths attributable to cancer among all persons with AIDS (population attributable risk [PAR]). Analyses were conducted separately to assess mortality attributable to ADC, NADC, and any cancer combined.
The attributable mortality method assigns a proportion of deaths to an exposure or condition (e.g., cancer) based on the relative difference in mortality rates among exposed and unexposed groups. A difficulty in applying standard formulae for AR and PAR is that they evaluate individuals at a single time point, when all subjects can be unambiguously classified with respect to the exposure and outcome. To account for the fact that cancers and deaths both continued to occur throughout 5 years of observation, we divided the person-time into 10 intervals of 6-month duration. Subjects were then classified according to their cancer status at the start of each interval. Next, for each interval, we fitted a Cox regression model to measure the association between presence of cancer on or before the start of the interval with death during the interval, adjusted for gender, race/ethnicity and age group. For each interval, we then calculated AR=(HR−1)/HR and PAR=Pe*(HR−1)/[Pe*(HR−1)+1], where HR was the adjusted hazard ratio and Pe was the proportion of subjects with cancer (i.e., exposed) on or before the start of the interval [11,12]. We derived overall AR and PAR estimates for the entire 60 months of follow-up by multiplying the interval-specific AR and PAR estimates by the interval-specific number of deaths in persons with cancer or the interval-specific total deaths, respectively; summing these interval-specific attributable deaths across the intervals; and expressing the total attributable deaths as a fraction of all deaths in persons with cancer (AR) or all deaths in the cohort (PAR). Variances for AR and PAR estimates were derived using the delta method [17], and pairwise estimates were compared for subjects diagnosed with AIDS during 1980–1989, 1990–1995, and 1996–2006 using a 2-sided t-test. Finally, in addition to these proportional measures, we report cancer-attributable mortality as rates per 1000 person-years. We calculated attributable mortality rates by dividing the number of deaths in people with cancer that were attributable to their cancer by the person-time contributed by people with cancer. Likewise, we calculated population attributable mortality rates by dividing the number of deaths in the entire population attributable to cancer by the person-time contributed by all people regardless of cancer status. These rates are presented by AIDS onset calendar periods.
P-values <0.05 were considered significant. All analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographic characteristics of the 372,364 people with AIDS included in the study are presented in Table 1. Across calendar periods, the proportion of male subjects declined (88.0% in 1980–1989 to 74.7% in 1996–2006) and the median age at AIDS onset increased (35 years in 1980–1989 to 38 years in 1996–2006). The proportion of non-Hispanic whites decreased from 50.4% in 1980–1989 to 28.6% in 1996–2006. The proportion of men reporting male-to-male sexual contact as their mode of HIV exposure declined, while the proportion with injection drug use or heterosexual exposure increased over time (Table 1). During 5 years of follow-up, 19,126 people developed an ADC, 4,137 developed a NADC, and 170,712 died.
Table 1.
Demographic characteristics of people with AIDS in the United States, 1980–2006 (N=372,364)
| Characteristic | Calendar period of AIDS onset
|
||
|---|---|---|---|
| 1980–1990 | 1990–1995 | 1996–2006 | |
| Total No. | 51,575 | 171,932 | 148,857 |
| Sex, n (%) | |||
| Male | 45,395 (88.0) | 13,9166 (80.9) | 11,1128 (74.7) |
| Female | 6180 (12.0) | 32,766 (19.1) | 37,729 (25.3) |
| Age in years at AIDS onset, n (%) | |||
| 15–29 | 10,817 (21.0) | 28,742 (16.7) | 20,718 (13.9) |
| 30–39 | 25,185 (48.8) | 79,819 (46.4) | 62,518 (42.0) |
| 40–49 | 11,304 (22.0) | 46,727 (27.2) | 46,688 (31.4) |
| 50+ | 4269 (8.2) | 16,644 (9.7) | 18,933 (12.7) |
| Median | 35 | 37 | 38 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 26,007 (50.4) | 68,007 (39.6) | 42,571 (28.6) |
| Non-Hispanic black | 16,296 (31.6) | 66,093 (38.4) | 72,329 (48.6) |
| Hispanic | 8764 (17.0) | 35,907 (20.9) | 32,106 (21.6) |
| Other/unknown | 508 (1.0) | 1925 (1.1) | 1851 (1.2) |
| Mode of HIV exposure, n (%)* | |||
| MSM | 28,497 (59.2) | 75,401 (51.3) | 52,810 (52.8) |
| IDU | 14,194 (29.5) | 52,850 (35.9) | 33,423 (33.4) |
| MSM and IDU | 3602 (7.5) | 10,553 (7.2) | 7117 (7.1) |
| Heterosexual | 1826 (3.8) | 8327 (5.7) | 6649 (6.7) |
Abbreviations: MSM, male-to-male sex; IDU, injection drug use.
Column percentages for mode of HIV exposure are reported for the four most common modes of exposure excluding people in the ‘other/unknown’ category (most subjects in the other/unknown category had unknown rather than other specified modes of transmission).
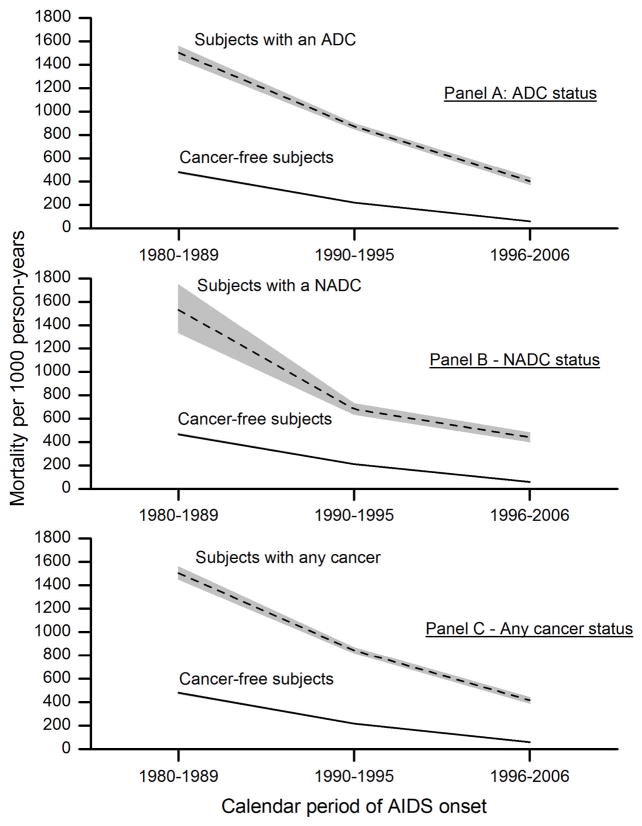
Mortality following an ADC, NADC, or any cancer was highest among those with AIDS onset in 1980–1989 and declined over time (Figure 1). Overall, mortality rates among people who were cancer-free were markedly lower and also declined more steeply with AIDS calendar time. The mortality attributable to cancer (in relative or absolute terms) reflects the excess deaths in people with cancer compared to those without cancer. As a result of the temporal changes in mortality rates, we found that among people with cancer who subsequently died, the proportion of their deaths attributable to the cancer increased over time (i.e., attributable risk, Table 2). For example, the proportion of deaths among people with an ADC that were attributable to their ADC increased significantly across AIDS calendar periods from 68.6% during 1980–1989 to 77.8% during 1990–1995 to 88.3% during 1996–2006. Similar patterns were observed for NADCs and all cancers combined. Nonetheless, the pattern was different when attributable mortality was viewed as a rate, as a result of the overall decline in mortality rates: attributable mortality rates due to ADC, NADC and any cancer overall each exhibited a >60% decrease across the study period (Table 2).
Figure 1. Five-year mortality rates in people with AIDS, by cancer status and calendar period of AIDS onset, 1980–2006.
Panels show 5-year mortality rates per 1000 person-years among people who had cancer (dashed line) and those who remained cancer-free (solid line), according to calendar period of AIDS onset. The shaded area around each line represents the 95% confidence intervals for mortality rates within each group. For the no-cancer group, the confidence intervals are very narrow and not visible in the panels. Panel A shows mortality in subjects with an ADC versus those who were cancer-free. Panel B shows mortality in subjects with a NADC versus those who were cancer-free. Panel C shows mortality in subjects with any cancer (either an ADC or NADC) versus those who were cancer-free. Note that the comparison group of cancer-free people varies slightly across the three panels because it is defined in each instance in relation to the specified cancer outcome. Abbreviations: ADC, AIDS-defining cancer; NADC, non-AIDS-defining cancer.
Table 2.
Five-year cancer-attributable mortality among people with AIDS in the United States, 1980–2006
| Cancer attributable deaths, by cancer type | Calendar period of AIDS onset
|
||
|---|---|---|---|
| 1980–1989 | 1990–1995 | 1996–2006 | |
| AIDS-defining cancer | |||
| Attributable risk, % (95%CI) | 68.6 (67.4–69.7) | 77.8 (77.2–78.3)* | 88.3 (87.5–89.2)* |
| Attributable mortality rate per 1000 person-years | 1030 | 679 | 356 |
| Population attributable risk, % (95%CI) | 6.3 (6.0–6.6) | 6.3 (6.1–6.5) | 3.9 (3.6–4.2)* |
| Population attributable mortality rate per 1000 person-years | 32 | 15 | 2.4 |
| Non-AIDS-defining cancer | |||
| Attributable risk, % (95%CI) | 71.9 (68.1–75.8) | 73.9 (72.3–75.6) | 87.1 (85.9–88.1)* |
| Attributable mortality rate per 1000 person-years | 1156 | 503 | 384 |
| Population attributable risk, % (95%CI) | 0.5 (0.4–0.5) | 0.8 (0.8–0.9)* | 2.3 (2.1–2.5)* |
| Population attributable mortality rate per 1000 person-years | 2.6 | 1.8 | 1.4 |
| Any cancer | |||
| Attributable risk, % (95%CI) | 68.7 (67.6–69.9) | 77.1 (76.5–77.6)* | 87.6 (87.1–88.2)* |
| Attributable mortality rate per 1000 person-years | 1032 | 650 | 365 |
| Population attributable risk, % (95%CI) | 6.7 (6.3–7.0) | 6.9 (6.8–7.1) | 6.0 (5.7–6.4)* |
| Population attributable mortality rate per 1000 person-years | 34 | 16 | 3.8 |
Attributable mortality estimates were derived from formulas presented in the methods section at 5-years of follow-up after AIDS onset. Attributable risk and population attributable risk estimates are expressed as percentages and compared across calendar periods (1990–1995 vs. 1980–1989 and 1996–2006 vs. 1990–1995) via a 2-sided t-test. The * indicates a significant difference at P<.01. Abbreviation: CI, confidence interval.
Table 2 also presents information on deaths among the entire AIDS population attributable to cancer. These results incorporate the population proportion of people with cancer as well as mortality rates. When expressed as a rate, population attributable mortality declined over time. For example, the population mortality rates attributable to any cancer were 34, 16, and 3.8 per 1000 person-years for AIDS onset in 1980–1989, 1990–1995, and 1996–2006, respectively. Similar patterns were seen for ADCs and NADCs separately. Likewise, when expressed as a proportion of all deaths, population mortality attributable to ADCs (i.e., PAR) declined significantly over time, from 6.3% of all deaths in 1980–1989 to 3.9% in 1996–2006. In contrast, however, the population mortality attributable to NADCs increased significantly over time, rising from 0.5% of all deaths in 1980–1989 to 2.3% in 1996–2006. Finally, the decline in deaths attributable to ADCs led to a modest decline in population mortality attributable to any cancer, from a peak of 6.9% of deaths in 1990–1995 to 6.0% in 1996–2006.
Discussion
In this large and nationally representative cohort of people with AIDS in the U.S., we noted significant declines in mortality among those with and without cancer, which we interpret to reflect the impact of increasingly effective antiretroviral therapies in decreasing deaths related to HIV infection. With these improvements in overall mortality, a significantly growing fraction of deaths in people with an ADC or NADC were attributable to their cancer. Furthermore, the fraction of all deaths among people with AIDS that were attributable to a NADC (i.e., population attributable risk) increased significantly in the HAART era.
An important feature of this analysis was our evaluation of the contribution of cancer mortality among people with AIDS on multiple scales. In absolute terms, attributable mortality rates (ADC and NADC) declined over the study period. This increased survival after cancer diagnosis may be related to improvements in cancer treatment. However, on the relative scale, our attributable risk estimates demonstrated the persisting importance of cancer-related mortality among people with AIDS and cancer. For those with any cancer who died, the fraction of deaths attributable to that cancer increased from 68.7% to 87.6% in the HAART era.
These results suggest that, while deaths from non-cancer causes have decreased dramatically, cancer-specific mortality has not improved as rapidly. We therefore emphasize that successful cancer treatment among people with AIDS will become increasingly important and should address both clinical issues (e.g., immunosuppression, comorbid conditions, chemotherapeutic drug interactions with HAART) and social barriers (e.g., limited access to healthcare, societal marginalization and stigma) [18–20]. Further, because a single cancer treatment center is unlikely to see many HIV-infected people with a given cancer type, coordinated multicenter trials are needed to determine optimized therapeutic regimens. It has also been argued that HIV-infected people should be included in cancer treatment trials open to the general population [18].
Our population attributable mortality rate findings demonstrate that ADC-related mortality among people with AIDS has declined dramatically. This population-level decrease is partly due to steep declines in the incidence of these cancers [3, 5], which have resulted from improved HIV therapies and better immune control of cancer-causing viruses (e.g., Epstein Barr virus for NHL). Population attributable mortality rates for NADC also declined, albeit more slowly than for ADCs. In contrast to ADCs, the incidence of NADCs among people with AIDS has remained stable [3, 5, 21]. At the population level, the mortality attributable to NADCs increased from 0.5% of all deaths in 1980–1989 to 2.3% in 1996–2006. This increase in population attributable mortality should be considered in the context of declines in mortality from other causes: as mortality due to opportunistic infections and ADCs decreased over time, a greater fraction of all deaths were attributed to NADCs.
Our findings are consistent with other reports describing cancer-related mortality among people with AIDS. One study among people with AIDS in New York City (1999–2004) found 5% of deaths had cancer listed as the underlying cause [8], similar to our population attributable risk estimate of 6.0% during 1996–2006. An analysis of deaths among HIV-infected patients in Europe found 8% of deaths were due to NADC [22]. This estimate was substantially higher than ours, perhaps because it was based on a population that included people without AIDS, who likely had fewer AIDS-related deaths (and therefore proportionally more cancer-related deaths). A recent collaborative analysis of causes of death among people with HIV in Europe and North America reported a NADC mortality rate of 1.2 per 1000 person-years in 1996–2006, very similar to our population attributable mortality rate of 1.4 per 1000 person-years [23]. The same study showed no decline in NADC mortality in the most recent calendar period (2003–2006 vs. 1996–1997), emphasizing the continuing importance of cancer in this population.
A potential source of heterogeneity in findings across studies is the challenge in accurately determining a single cause of death in patients with multiple medical problems [24]. Based on the underlying cause of death (UCOD) reported on death certificates, we previously reported the fraction of deaths among people with AIDS related to cancer [10]. For NADCs, the results were largely similar to those we report here. However, the results differed for ADCs, especially for people diagnosed with AIDS before the availability of HAART. For example, for people diagnosed with AIDS in the 1980s and who subsequently died, only 1.05% had an ADC listed as their UCOD [10], whereas herein we estimated a PAR of 6.3%. These estimates were more similar for people diagnosed with AIDS in the HAART era (2.47% of deaths due to ADCs based on UCODs vs. 3.9% based on the calculated PAR). We suspect that in the pre-HAART era, when AIDS-related mortality was very high, clinicians were more likely to attribute any death in a person with an ADC to AIDS rather than the cancer, but with improved HIV treatment, this tendency may have reversed. The sensitivity of death certificates for detecting deaths related to cancer (ADCs in particular) may therefore be low and somewhat variable. In an additional analysis, we found that most people included in the current study who died from cancer (according to the UCOD on their death certificate) also had a prior cancer diagnosis in the cancer registry (68% for ADCs and 62% for NADCs during 1980–2006), suggesting UCODs on death certificates have moderate positive predictive value. Nonetheless, differences between the present study and our prior study [10] underscore the difficulties in attributing a single cause when deaths involve multiple disease processes, and they demonstrate how different methods of counting and classifying deaths can lead to different conclusions. Similar challenges in correctly classifying cancer-related deaths from UCODs reported on death certificates arise in the general population [25]. Taken together, these findings support our use of an attributable mortality framework, which is not limited by the accuracy of information included on death certificates and not subject to changes in diagnostic patterns or death certificate coding over time.
Strengths of our study include its large size and representative inclusion of major cities and states affected by the HIV epidemic, making our cancer-attributable death results applicable to the U.S. AIDS population. An additional strength was our novel analytic approach which accurately accounted for incident cancers and deaths occurring throughout the follow-up period. Others have recently proposed a piecewise constant hazards model to estimate population attributable mortality [26], which appears similar to our method, although the variance estimation procedures are more computationally intensive. Of note, the attributable mortality framework is based on a causal relationship between exposure (i.e., cancer) and the outcome of interest (i.e., death), which assumes there are no unmeasured confounders. While our multivariate models adjusted for gender, race/ethnicity and age group, we lacked information on individual-level risk factors, such as tobacco and alcohol use, comorbid conditions, and HAART use. Thus, our mortality estimates reflect the combined effects of both cancer and the unmeasured factors.
We chose to evaluate mortality at 5 years because longer follow-up would require adjustments to account for potential out-migration of subjects with AIDS [5, 27]. In addition, from a clinical standpoint, estimating mortality due to cancer at 5 years after AIDS onset is appropriate for a disease such as AIDS for which survival was previously poor (measured in months) but has subsequently improved (now measured in years). Future studies with longer periods of follow-up and into more recent calendar periods may also be informative as mortality among people with AIDS continues to change over time. Also, cohorts of individuals with HIV but not AIDS should be evaluated, especially as the number of HIV-infected people on HAART increases and AIDS becomes a less common condition.
As the spectrum of HIV/AIDS-related sequelae continues to change in the HAART era, accurate monitoring of cancer-related deaths will remain necessary to inform clinicians treating people with HIV and cancer, epidemiology researchers, and public health officials. To our knowledge, this is the first study to use attributable mortality methods to evaluate cancer deaths among an AIDS cohort. Although mortality due to NADCs has declined in the HAART era, these malignancies account for an increasing proportion of all deaths. Our results highlight the importance of improved cancer screening, prevention, and treatment among people with AIDS.
Acknowledgments
We thank the staff at the HIV/AIDS and cancer registries at the following locations: Colorado; Connecticut; Florida; Illinois; Georgia; Massachusetts; Michigan; New Jersey; New York, New York; Los Angeles, San Diego and San Francisco, California; Seattle, Washington; Texas and Washington, D.C. We also thank Mr. Tim McNeel (Information Management Systems, Rockville, Maryland) for database management. This study was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
Potential Financial Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 5.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 7.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–885. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;19;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet F, Burty C, Lewden C, Costagliola D, May T, Bouteloup V, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009;48:633–639. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 10.Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51:957–962. doi: 10.1086/656416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslow NE, Day NE. The Design and Analysis of Cohort Studies. Lyon: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 12.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;20;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 14.Munoz A, Schrager LK, Bacellar H, Speizer I, Vermund SH, Detels R, et al. Trends in the incidence of outcomes defining acquired immunodeficiency syndrome (AIDS) in the Multicenter AIDS Cohort Study: 1985–1991. Am J Epidemiol. 1993;137:423–438. doi: 10.1093/oxfordjournals.aje.a116691. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 16.National Cancer Institute. SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 17.Lehman EL. Elements of Large-Sample Theory. New York: Springer; 1998. Convergence in Probability and Law: Taylor’s theorem and the delta method. [Google Scholar]
- 18.Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. J Clin Oncol. 2008;26:1027–1032. doi: 10.1200/JCO.2007.14.5532. [DOI] [PubMed] [Google Scholar]
- 19.Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP. Drug interactions between antineoplastic and antiretroviral therapies: Implications and management for clinical practice. Crit Rev Oncol Hematol. 2009;72:10–20. doi: 10.1016/j.critrevonc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117:1089–1096. doi: 10.1002/cncr.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin B, Thiebaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice AC. Commentary: Treated HIV infection is a chronic disease: the case against cause of death analyses. Int J Epidemiol. 2009 doi: 10.1093/ije/dyp342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laaksonen MA, Knekt P, Harkanen T, Virtala E, Oja H. Estimation of the population attributable fraction for mortality in a cohort study using a piecewise constant hazards model. Am J Epidemiol. 2010;171:837–847. doi: 10.1093/aje/kwp457. [DOI] [PubMed] [Google Scholar]
- 27.Buehler JW, Frey RL, Chu SY. The migration of persons with AIDS: data from 12 states, 1985 to 1992. AIDS Mortality Project Group. Am J Public Health. 1995;85:1552–1555. doi: 10.2105/ajph.85.11.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]