Abstract
Study Design
Experimental measurement and normalization of in vitro disc torsion mechanics and collagen content for several animal species used in intervertebral disc research and comparing these to the human disc.
Objective
To aid in the selection of appropriate animal models for disc research by measuring torsional mechanical properties and collagen content.
Summary of Background Data
There is lack of data and variability in testing protocols for comparing animal and human disc torsion mechanics and collagen content.
Methods
Intervertebral disc torsion mechanics were measured and normalized by disc height and polar moment of inertia for 11 disc types in 8 mammalian species: the calf, pig, baboon, goat, sheep, rabbit, rat, and mouse lumbar, and cow, rat, and mouse caudal. Collagen content was measured and normalized by dry weight for the same discs except the rat and mouse. Collagen fiber stretch in torsion was calculated using an analytical model.
Results
Measured torsion parameters varied by several orders of magnitude across the different species. After geometric normalization, only the sheep and pig discs were statistically different from human. Fiber stretch was found to be highly dependent on the assumed initial fiber angle. The collagen content of the discs was similar, especially in the outer annulus where only the calf and goat discs were statistically different from human. Disc collagen content did not correlate with torsion mechanics.
Conclusion
Disc torsion mechanics are comparable to human lumbar discs in 9 of 11 disc types after normalization by geometry. The normalized torsion mechanics and collagen content of the multiple animal discs presented is useful for selecting and interpreting results for animal models of the disc. Structural composition of the disc, such as initial fiber angle, may explain the differences that were noted between species after geometric normalization.
Keywords: Intervertebral disc, animal model, biomechanics, torsion, collagen
Introduction
In vivo studies of disc degeneration mechanisms and potential therapeutics require use of animal models. Selection of an appropriate animal model includes consideration of availability, size and cost, but also requires knowledge of the similarities and differences in biomechanical and biochemical factors between the model and human discs. Animal models have been widely used, and many of them include some comparison data with human discs.1-6 However, few studies have comprehensively compared the biomechanical or biochemical disc properties over a broad range of animal species using the same protocol to guide the research community in selection of an animal model. Recent studies in our laboratory have compared disc and nucleus pulposus geometry, water content, glycosaminoglycan content, and axial compression mechanics of lumbar discs in several animal species.3, 7 A key finding of those studies was that although experimental compressive stiffness varied widely, after normalizing by disc geometry the compressive mechanical properties among species were very similar.
The importance of torsional disc mechanics has been debated in the literature. Some state that the amount of torsion experienced by the lumbar disc is insufficient to cause damage.8-10 Researchers utilizing CT or MR imaging to measure in vivo axial rotation confirmed that while the average lumbar disc rotation is low (1-2°), the maximum rotation can be as high as 4.5-5.7°.11-13 Other studies have found that healthy disc anulus fibers resist more torsional motion than the facets and that torsional motion can cause injury to the disc at low magnitudes, even if failure is at much higher rotations.14-16 These findings support the importance studying disc torsion mechanics.
Collagen fibers play a substantial role in disc torsion mechanics, especially in the outer annulus fibrosus (AF), because they are the main tensile-load bearing component of the disc.17 Collagen content in animal discs has not been widely reported and may explain some of the similarities and differences in torsional mechanics across species. Furthermore, a fiber stretch model presented by Hickey and Hukins may be used to interpret the variation in torsional properties.18
The objective of the current study was to measure the torsional mechanical properties of calf, pig, baboon, sheep, goat, rabbit, rat, and mouse lumbar discs and of the cow, rat, and mouse caudal discs and compare these to the human lumbar disc. Furthermore, a normalization method is presented that utilizes disc geometry to facilitate the comparison of torsion mechanics across species. In addition, collagen content was measured for the same discs excluding the mouse and rat samples. Knowledge of the biomechanical and biochemical properties of different animal discs is important for design, analysis, and interpretation in studies of disc degeneration and therapy.
Materials and Methods
Intervertebral disc torsional stiffness and torque range were evaluated for the non-degenerate human disc and for 11 disc types in 8 mammalian species (Table 1), selected based on their use in experimental models of lumbar disc degeneration. Throughout the paper, species from which discs were examined from both the lower back and the tail are identified with L for lumbar or C for caudal to denote the specific disc type. A majority of the samples tested in this study are the same samples tested in axial compression in the previous study7 and all tissues were obtained under approved IACUC protocols. All species were skeletally mature, with the exception of the calf. Magnetic resonance grading of the human discs was performed to verify that they were non-degenerate and categorized as Pfirrman Grade I or II.19
Table 1.
The human and animal specimens used in the study. All animals were skeletally mature, except for the calf spines.
| n | Age | Level | Strain | Acquisition | |
|---|---|---|---|---|---|
| Human | 3 | 22, 29, 42 yrs | L3-L4 | - | Tissue banks |
| Calf | 5 | 4-6 mo | L1-L2 | Holstein | Experiment unrelated to the spine |
| Pig | 5 | 2 yrs | L1-L2 | Landrace cross | Experiment unrelated to the spine |
| Baboon | 4 | 6.5-7 yrs | L2-L3, L3-L4 | Male papio anubis (olive) | Southwest National Primate Research Center and Oklahoma Health Sciences Center |
| Goat | 6 | 2.5-3.5 yrs | L4-L5 | Male large frame (Nubian crosses) | Experiment unrelated to the spine |
| Sheep | 5 | 3.5-5 yrs | L3-L4 | Female Rambouillet-Columbia | Department of Clinical Sciences at Colorado State University |
| Rabbit | 5 | 6-8 mo | L4-L5 | Female New Zealand white | Experiment unrelated to the spine |
| Rat – L | 4 | 12 mo | L3-L4 | Sprague Dawley Retired male breeder | Harlan, Inc. |
| Mouse – L | 3 | 9-12 mo | L3-L4 | C57BL/6 Retired male breeder | Jackson Laboratory |
| Cow – C | 5 | 1.5-2.5 yrs | C1-C2 | - | Local abattoir |
| Rat – C | 4 | 12 mo | C9-C10 | Sprague Dawley Retired male breeder | Harlan, Inc. |
| Mouse – C | 3 | 9-12 mo | C8-C9 | C57BL/6 Retired male breeder | Jackson Laboratory |
Each sample was prepared as previously described.7 Briefly, a bone-disc-bone segment sample was prepared by making a parallel cut through the lumbar vertebrae, transverse to the long axis of the spine. The facet joints were removed. The sample was then potted in polymethylmethacrylate bone cement and Kirschner wires were placed through the bone cement and the vertebral body. Rat and mouse segment samples were gripped directly using custom microvises.20 Disc geometry was measured as follows: height from pre-test lateral fluoroscopic images, and lateral width, anteroposterior width, and cross sectional area of the disc and the NP from post-test axial sections and digital camera images.3, 7 Geometry measurements for the majority of the samples in this study were previously reported (See Table 2 of Beckstein et al., 2008).7 New species geometry are found in Table 2 for goat and mouse caudal disc. Note that the nucleus geometry of the mouse disc was not measured but was estimated using values from a previous study.6 For the quadrupeds, the disc “height” is more accurately described as the craniocaudal width; however, bipedal terminology will be used throughout this study.
Table 2.
Mean (Standard deviation) for geometric, axial mechanical properties, and biochemical data for the goat and mouse samples that were reported previously for the other animal models. Previously reported human parameters are included for comparison.7 Shaded boxes in the mechanical and biochemical properties are significantly different than the human, Dunnett's Multiple Comparison Test
| Geometry | ||||||||
|---|---|---|---|---|---|---|---|---|
| Whole Disc | Nucleus Pulposus | |||||||
| Height (mm) | Lat Width (mm) | AP Width (mm) | Area (mm2) | Lat Width (mm) | AP Width (mm) | Area (mm2) | Axial Force (N) | |
| Human | 10.91 (0.83) | 55.38 (2.01) | 37.67 (2.02) | 1925 (184) | 36.54 (2.00) | 20.81 (0.73) | 598 (46) | 750 |
| Goat | 4.28 (0.73) | 35.9 (2.1) | 21.2 (1.6) | 670 (71) | 9.12 (1.6) | 20.8 (2.5) | 173 (25) | 250 |
| Mouse - C | 0.54 (0.04) | 1.19 (0.14) | 1.51 (0.09) | 1.08 (0.19) | - | - | - | 0.5 |
| Tension/Compression Mechanical Parameters | ||||||
|---|---|---|---|---|---|---|
| Compressive Stiffness (N/mm) | Compressive Range of Motion (mm) | Creep Displacement (mm) | Normalized Stiffness (MPa) | Normalized ROM | Normalized Creep Displacement | |
| Human | 1734 (446) | 1.21 (0.18) | 0.55 (0.03) | 9.95 (3.24) | 0.11 (0.01) | 0.05 (0.002) |
| Goat | 1109** (127) | 0.36*** (0.03) | 0.59 (0.08) | 7.20 (1.92) | 0.09 (0.02) | 0.14 (0.03) |
| Mouse - C | 43.4*** (16.4) | 0.21*** (0.08) | 0.30 (0.05) | 23.2*** (10.7) | 0.40*** (0.13) | 0.59*** (0.09) |
| Glycosaminoglycan and Water Content | ||||||
|---|---|---|---|---|---|---|
| GAG per Dry Weight (μg GAG/mg DW) | Water Content (%) | |||||
| NP | IAF | OAF | NP | IAF | OAF | |
| Human | 466 (205) | 377 (185) | 161 (31.9) | 80 (3) | 81 (2) | 72 (3) |
| Goat | 335 (51) | 164** (39) | 25* (20) | 84 (3) | 78 (6) | 66 (3) |
p < 0.05
p < 0.01
p < 0.001
Mechanical Testing
Following preparation, specimens were stored in a -20 °C freezer until the day of testing. Samples were thawed and hydrated in a refrigerated 4 °C phosphate buffered saline (PBS) solution for 18 hours prior to testing. Mechanical testing was conducted at room temperature in a PBS bath. Testing was completed using an Instron 8874 servohydraulic testing system (Instron, Norwood, MA). The sample was placed in custom designed fixtures for axial and torsional loading. Each sample was first tested under cyclic axial loading and a 1 hour compressive creep. The creep loading served to equilibrate the disc axial load and place the AF into circumferential tension before performing cyclic torsion testing. The axial compressive stress used during torsion was 0.48 MPa, corresponding to human 750 N (approximate body weight) divided by 1560 mm2 disc area. The applied axial load for each species was previously reported7 except for goat (250 N) and mouse caudal (0.5 N). Torsional cyclic loading, performed immediately following the creep test, consisted of 20 sinusoidal cycles from 0° to 6° at 0.5 Hz, executed to both the left and right side of the disc. The rat and mouse samples were tested using a custom-built torsion attachment on an Instron 5542 testing system (Instron, Norwood, MA).6, 21, 22 Torsional frequency was 0.05 Hz due to machine capability.
Data Analysis
Data from the 20th torsional loading cycle was analyzed to obtain values for torque range (TR) and torsional stiffness (K). Experimental findings from preliminary investigation indicate that steady-state is reached within 20 cycles of torsional preconditioning and that the testing protocol did not damage the disc. Torque range was defined as the difference in torque between 0° and 6° during the 20th cycle, averaging values for clockwise and counter-clockwise rotations. Torsional stiffness was calculated as the slope of a linear regression through the data points between 4.5° and 5.5° disc rotation. These values were selected based on preliminary testing as the most linear portion of the torque-angular displacement response among all species. Clockwise and counter-clockwise stiffness values were averaged to calculate the disc torsional stiffness, K.
Normalized torsional parameters were calculated using disc dimensions to facilitate comparison between species. First, the polar moment of inertia of the disc was determined by assuming that only the annulus resists torsional motion and is shaped as a hollow ellipse. Specifically, the polar moment of inertia (J, mm4) was calculated using the equation
| (1) |
where WAP and WL are the anteroposterior and lateral widths of the outer disc, and NAP and NL are the anteroposterior and lateral widths of the NP.6 Normalized torque range (MPa) was calculated using the equation TR*h/J from the measured torque range (TR, N·m), disc height (h, mm) and the polar moment of inertia (J, mm4). Normalized torsional stiffness (MPa/°) was calculated as K*h/J from the measured torsional stiffness (K, N·m/°), disc height (h, mm) and polar moment of inertia (J, mm4), as calculated above.
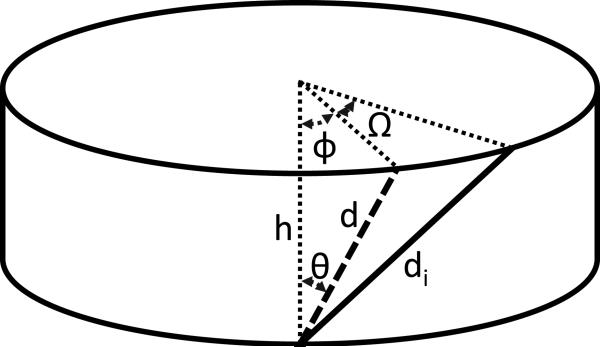
An additional analysis was performed to investigate the role of the outer annular fibers on torsion mechanics based on a study performed by Hickey and Hukins,18 which examined the increase in fiber length of the outer annular fibers with application of torsional rotation. The analysis included the calculation of the stretched fiber length (di) and this equation was used in the current study to calculate the stretched fiber length throughout the 0° to 6° of rotation. Equation 6 from Hickey and Hukins is presented below (note a correction to the original equation, φ4, in the numerator of the first term):
| (2) |
where Vi is the volume of the disc, n is the disc eccentricity, d is initial fiber length, di is the fiber length after rotation, Ω is the amount of disc rotation, θ is the initial fiber angle, and φ is defined as shown in Figure 1. Fiber strain was calculated as (di – d)/d using the initial fiber length at 0° rotation and the fiber length at 6° rotation.
Figure 1.
Definition of terms used in fiber strain equation,20 where d is initial fiber length, di is stretched fiber length, h is disc height, θ is the initial fiber angle from the vertical disc axis, φ is the initial torsion angle, and Ω is the amount of axial rotation.
Biochemical Analysis
Following mechanical testing and geometry measurement the sample was placed in a -20 °C freezer for preservation prior to biochemical analysis, including collagen content.7 The disc was separated from the vertebral bodies and, while frozen, tissue samples from the NP, inner AF, and outer AF were harvested using either a 1.5 mm or 3.0 mm dermal biopsy punch (Miltex Instrument Comp., Bethpage, NY) depending on the size of the disc. The sample was dehydrated at 65 °C for 24 hours after which tissue dry weight was acquired. Dried samples were digested with proteinase-K at 65 °C for 16 hours. A 50 μL aliquot of the digest was used to measure collagen content with the orthohydroxyproline (OHP) colorimetric assay and normalized by dry weight.23 This process was not performed on the mouse and rat discs because of their small size.
Statistical Analysis
One-way ANOVA and Dunnett's Multiple Comparison Test were used to compare the torsion mechanical parameters and collagen content of each of the animal discs to the human disc. Statistical significance set at p < 0.05. Pearson's correlation coefficients were calculated for normalized torsional properties and annulus collagen content.
Results
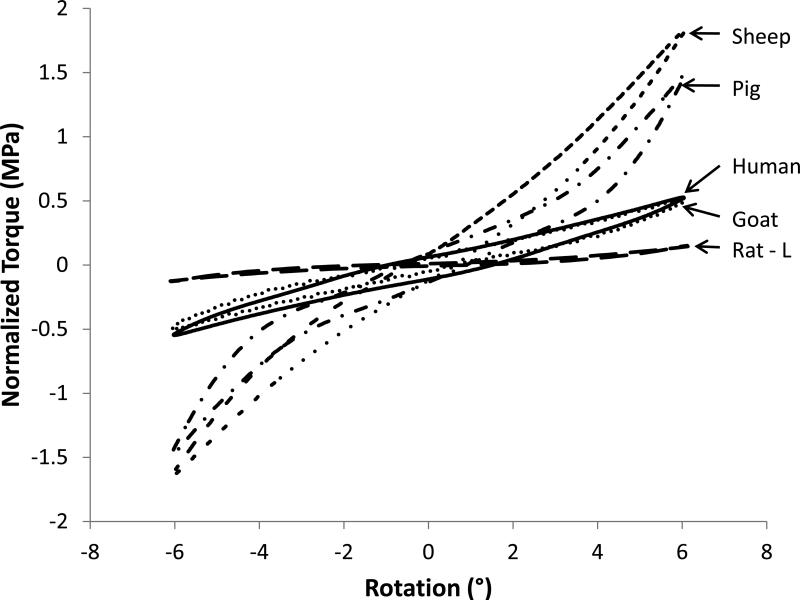
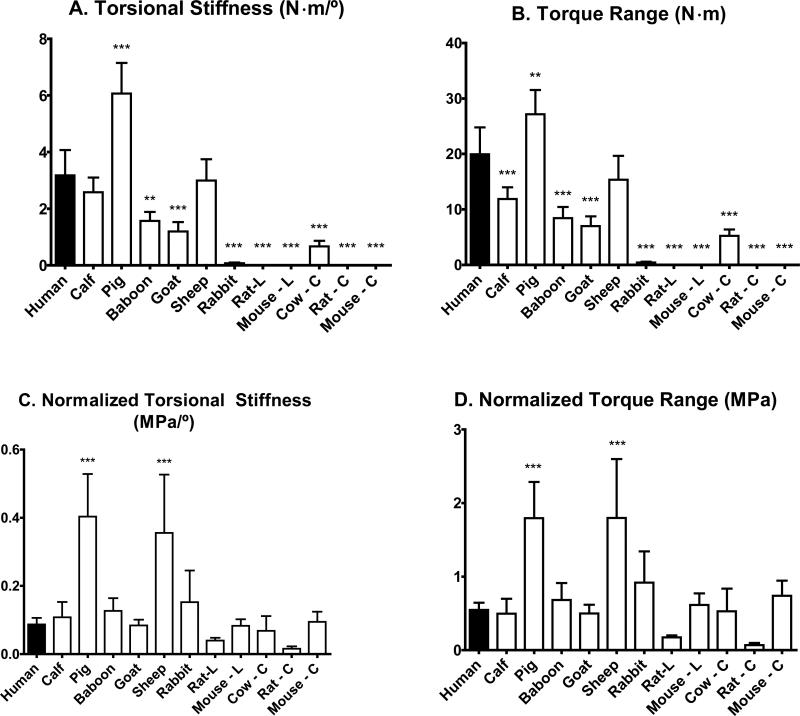
The torsional response of the human disc was fairly linear (Figure 2). After normalization, 9 of the 11 disc types exhibited similar torsional responses to human. However, the pig and sheep samples were notably stiffer than the human and had a more nonlinear response. Torque range and torsional stiffness were calculated for each species (Table 3) and differences were observed for both the measured data and normalized values. The sheep had 6% lower torsional stiffness than human, the pig 91% higher stiffness, and the mouse and rat were less stiff by several orders of magnitude (Figure 3A). Torque range results were similar, where the sheep had a 23% lower torque range compared with human, pig had a range 36% higher, and mouse and rat were several orders of magnitude lower (Figure 3B).
Figure 2.
Representative normalized torsion responses for human discs (solid) and for several species.
Table 3.
Mean (Standard deviation) for torsion mechanical parameter values for each species. Shaded boxes are significantly different than the human, Dunnett's Multiple Comparison Test.
| Torsional Stiffness (N·m/°) | Torque Range (N·m) | Normalized Torsional Stiffness (MPa/°) | Normalized Torque Range (MPa) | |
|---|---|---|---|---|
| Human | 3.18 (0.89) | 20.0 (4.8) | 0.087 (0.019) | 0.548 (0.099) |
| Calf | 2.58 (0.52) | 11.9*** (2.1) | 0.108 (0.045) | 0.498 (0.202) |
| Pig | 6.06*** (1.08) | 27.2** (4.4) | 0.403*** (0.125) | 1.80*** (0.49) |
| Baboon | 1.57** (0.31) | 8.43*** (2.01) | 0.127 (0.037) | 0.683 (0.231) |
| Goat | 1.19*** (0.33) | 7.01*** (1.74) | 0.084 (0.017) | 0.500 (0.118) |
| Sheep | 2.99 (0.75) | 15.4 (4.3) | 0.356*** (0.171) | 1.80*** (0.80) |
| Rabbit | 0.07*** (0.03) | 0.42*** (0.14) | 0.152 (0.093) | 0.919 (0.423) |
| Rat – L | 1.33 E -3*** (3.28 E -4) | 5.84 E -3*** (1.02 E -3) | 0.040 (0.008) | 0.176 (0.026) |
| Mouse – L | 1.10 E -4*** (1.83 E -5) | 8.21 E -4*** (1.54 E -4) | 0.083 (0.020) | 0.614 (0.159) |
| Cow – C | 0.66*** (0.20) | 5.22*** (1.15) | 0.068 (0.043) | 0.530 (0.306) |
| Rat – C | 9.74 E -4*** (3.50 E -4) | 4.33 E -3*** (1.18 E -3) | 0.015 (0.007) | 0.070 (0.029) |
| Mouse – C | 5.88 E -5*** (6.04 E -6) | 4.68 E -4*** (8.25 E -5) | 0.095 (0.030) | 0.741 (0.204) |
*p < 0.05
p < 0.01
p < 0.001
Figure 3.
Mean and standard deviations of measured and normalized properties for mechanical parameters: A) torsional stiffness B) torsional range of motion C) normalized torsional stiffness D) normalized torsional range of motion. L and C represent lumbar and caudal, respectively. Significantly different than the human, Dunnett's Multiple Comparison Test, *p < 0.05, **p < 0.01, ***p < 0.001
The normalized torsional stiffness values for many species were similar to the human, however the pig and sheep values were 363% and 308% higher than human (Figure 3C). Likewise, the normalized torque range of motion for both the pig and sheep was three times higher than the human (Figure 3D), with no statistically significant difference between the other species and human.
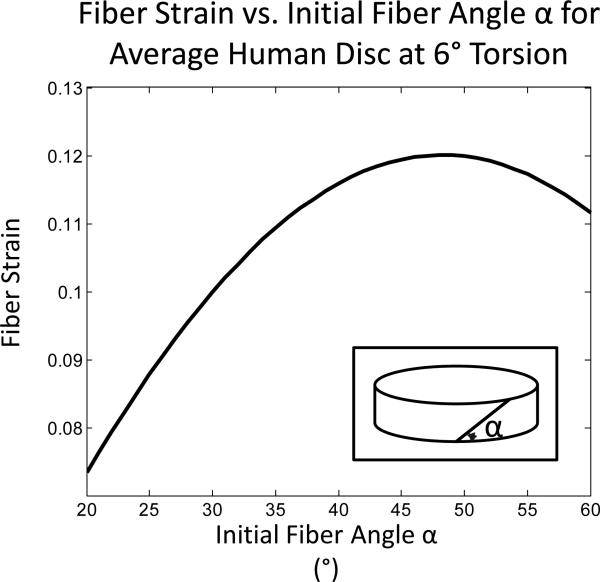
The fibers in the outermost boundary of the AF are likely to provide the primary resistance to torsion.17 Therefore, a fiber stretch model presented by Hickey and Hukins was used to interpret the variation in torsional properties.18 Assuming the initial fiber angle to be 25° from horizontal, the average fiber strain for the human samples in the outer annulus of a disc rotated 6° was calculated to be 0.088 ± 0.005. Fiber strain was found to be highly dependent on the assumed initial fiber angle, with increases in the calculated strain corresponding to increasing fiber angle until it reached a peak of 0.12 at an angle of 48° (Figure 4). This trend was even more pronounced in species with high lateral width to height ratios, such as sheep.
Figure 4.
Influence of initial fiber angle on the calculated fiber strain at six degrees torsion in a human disc using a geometric model.20 The initial fiber angle α in this figure is measured from horizontal (inset).
Total collagen content was calculated for the NP, inner AF, and outer AF disc regions as μg collagen/mg dry weight using the OHP assay (Table 4). Within the NP, the baboon, goat, and sheep discs had similar collagen content as the human. In contrast, pig discs had less than half of the collagen as human, whereas the calf and cow tail discs had 3-6 times more. In the inner AF, the baboon discs were most similar to human with just 4.6% less collagen. Pig discs had the highest collagen content, with 127% more than human, and goat had the lowest, with 44.9% less than human. For the outer AF, the majority of samples had collagen content similar to humans. However, the goat samples had 48.6% less and calf had 44.8% more than human.
Table 4.
Mean (Standard deviation) for total collagen content normalized by dry weight (DW) for each species. Shaded boxes are significantly different than the human, Dunnett's Multiple Comparison Test.
| NP (μg/mg DW) | Inner AF (μg/mg DW) | Outer AF (μg/mg DW) | |
|---|---|---|---|
| Human | 15.6 (4.0) | 47.9 (3.0) | 102.6 (18.9) |
| Calf | 60.3*** (18.5) | 92.9** (22.6) | 148.6* (20.5) |
| Pig | 5.8 (2.9) | 108.7*** (6.4) | 122.4 (22.8) |
| Baboon | 18.9 (6.7) | 45.7 (21.2) | 110.4 (41) |
| Goat | 18.5 (5.8) | 26.4 (15.7) | 52.7* (13.9) |
| Sheep | 19.2 (10.6) | 66.8 (11.1) | 106.9 (18.4) |
| Rabbit | N/A | 34.0 (17.2) | 77.9 (19.2) |
| Cow – C | 43.4** (18) | 70.7 (15.8) | 106.9 (23.4) |
p < 0.05
p < 0.01
p < 0.001
Collagen content of the outer AF did not correlate with the normalized torsional properties. Specifically, the Pearson's correlation coefficient for the outer AF collagen content and the normalized torsional stiffness is 0.21 and for the collagen content and normalized torque range is 0.16.
In addition to the torsion properties and collagen content reported in this paper, the geometry, tension/compression and creep properties, and GAG and water content were previously published for the majority of the samples.7 However, these results had not been published for the goat and mouse caudal samples and, except for mouse biochemical measurements, are presented in Table 2. Normalized compressive stiffness of goat samples was 27.6% less than human and mouse caudal was 133% higher than human. The goat samples had consistently lower GAG content than human. However, the % water content of the goat discs was at most 6% higher or lower than human in all three regions.
Discussion
This study compared the intervertebral disc torsion mechanical properties and collagen content across several different species. The torsional properties of these discs varied by orders of magnitude, but after normalizing by geometry, torsional properties were only statistically different from human for 2 of 11 disc types. These results suggest that several animal models are appropriate for studying the human disc torsion response when disc geometry is accounted for. In particular, the goat, mouse lumbar, and mouse caudal discs exhibited normalized torsional stiffness values within 10% of human, while calf lumbar and bovine caudal discs exhibited values within 25% of human properties. However, the sheep and pig samples were notably stiffer than human. This study of disc torsion mechanics is complementary to previous studies that have examined disc geometry and axial compression.3, 7
Previous studies have compared the mechanical response of various animal discs to human discs.6, 24, 25 Although many of our tested values are similar to those in the literature, variation in observed mechanical properties is expected because of the variability in test protocols. For example, compressive loads are often included to load the annulus in tension,26 but the magnitude of the applied load varies between studies. The current study has the distinct advantage of performing all tests in a uniform manner across species.
The outer annulus experiences the most significant stresses during torsional loading.17 With fiber alignment of intervertebral discs, it is likely that these outermost AF fibers play a large mechanical role in torsion. Thus, calculating the stretched fiber length may provide greater insight into torsion properties. It was determined that the initial fiber angle contributes greatly to the amount of fiber strain (Figure 4). Given the marked increase in fiber strain due to initial fiber angle, measurement of fiber angle in future studies may build upon this finding to determine the actual amount of fiber strain in these species under torsion. This in turn may explain some of the differences observed in the torsional properties.
As with any study, this work has a few limitations to be aware of. The machine capabilities for testing the rat and mouse discs necessitate a slower testing rate, which may have slightly affected the mechanical properties that were measured. Also, the mechanical testing fixed the axis of rotation of the disc to be in the disc's geometric center, which is different from the in vivo axis of rotation due to the facet joints. However, the center of rotation has been calculated to be within the disc at low loads even accounting for facet forces.27 Furthermore, because the axis was fixed in the same location for all of the species, the experimental data obtained from these tests can still be compared. A biochemical limitation of this work is that the OHP assay only determined collagen content within the disc, but did not distinguish between collagen types. Although collagen content provided useful information, it would be of interest to distinguish between different collagen types, primarily Types I and II. Also, collagen content for the rodent discs was not measured due to the small size of the discs. Finally, while the geometrical model is an excellent estimate of fiber strain,18 it was limited by assuming initial fiber angle and may overestimate fiber strain because it does not take into account change in height or change in area that occurs during the creep test or during the torsion test to maintain a constant load. Further differences in torsion response may be explained by looking at other disc constituents such as elastin.28
In conclusion, axial torsional properties of 11 disc types from 8 mammalian species were compared with human disc. In addition, collagen content was measured of 7 disc types from 6 species. In general, the collagen content of the discs is similar across species. While the measured torsion data varied widely, normalization by geometry greatly reduced the variability between species. The goat and mouse discs had the most similar normalized torsional properties while the pig and sheep discs normalized properties were least similar to the human. Unlike a compression study in which geometric normalization accounted for the majority of the variation between species,7 in torsion loading structural arrangement of the disc's components are important to consider.
Key Points.
Disc torsion mechanics were measured for the calf, pig, baboon, goat, sheep, rabbit, rat, and mouse lumbar and cow, rat, and mouse caudal discs and compared to human.
Torsion mechanics for all discs except sheep and pig were similar to human lumbar after normalization by polar moment of inertia and disc height, despite differences of orders of magnitude in direct measurements.
Collagen content was measured for the calf, pig, baboon, goat, sheep, and rabbit lumbar and cow caudal discs and compared to human.
Collagen fiber stretch is highly dependent on initial fiber angle, which may explain variation in torsion mechanics across species not accounted for by geometry normalization.
These results and normalization process facilitate selection of animal model for disc torsion studies and interpretation of study results.
Acknowledgements
We would like to thank Joseph Chiaro for performing some of the biochemical assays.
Brent Showalter received NSF Graduate Research Fellowship grant number DGE-0822 and Dawn Elliott received NIH R01 AR-050052 and RC1 AR-058450 which funded this work.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s). Grant funds were received in support of this work. One or more of the author(s) has/have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this manuscript: e.g., honoraria, gifts, consultancies, royalties, stocks, stock options, decision making position
Conflicts of Interest and Sources of Funding:
None of the authors have a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boszczyk BM, Boszczyk AA, Putz R. Comparative and functional anatomy of the mammalian lumbar spine. Anatomical Record. 2001;264:157–68. doi: 10.1002/ar.1156. [DOI] [PubMed] [Google Scholar]
- 2.Ledet EH, Tymeson MP, DiRisio DJ, et al. Direct real-time measurement of in vivo forces in the lumbar spine. The Spine Journal. 2005;5:85–94. doi: 10.1016/j.spinee.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–33. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 4.Wilke HJ, Kettler A, Wenger KH, et al. Anatomy of the sheep spine and its comparison to the human spine. The Anatomical Record. 1997;247:542–55. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–9. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 6.Elliott DM, Sarver JJ. Young investigator award winner: Validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–22. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- 7.Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine. 2008;33:E166–E73. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 8.Adams MA, Hutton WC. The relevance of torsion to the mechanical derangement of the lumbar spine. Spine. 1981;6:241–8. doi: 10.1097/00007632-198105000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Liu YK, Goel VK, Dejong A, et al. Torsional fatigue of the lumbar intervertebral joints. Spine. 1985;10:894–900. doi: 10.1097/00007632-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Shirazi-Adi A. Nonlinear stress analysis of the whole lumbar spine in torsion --mechanics of facet articulation. Journal of Biomechanics. 1994;27:289–99. doi: 10.1016/0021-9290(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 11.Ochia RS, Inoue N, Takatori R, et al. In vivo measurements of lumbar segmental motion during axial rotation in asymptomatic and chronic low back pain male subjects. Spine. 2007;32:1394–9. doi: 10.1097/BRS.0b013e318060122b. [DOI] [PubMed] [Google Scholar]
- 12.Blankenbaker DG, Haughton VM, Rogers BP, et al. Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: A preliminary study. American Journal of Roentgenology. 2006;186:795–9. doi: 10.2214/AJR.04.1629. [DOI] [PubMed] [Google Scholar]
- 13.Haughton VM, Rogers B, Meyerand ME, et al. Measuring the axial rotation of lumbar vertebrae in vivo with MR imaging. American Journal of Neuroradiology. 2002;23:1110–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Farfan HF, Cossette JW, Robertson GH, et al. The effects of torsion on the lumbar intervertebral joints: The role of torsion in the production of disc degeneration. Journal of Bone and Joint Surgery-American Volume. A. 1970;52:468–97. [PubMed] [Google Scholar]
- 15.Farfan HF. Effects of torsion on the intervertebral joints. The Canadian Journal of Surgery. 1969;12:336–41. [PubMed] [Google Scholar]
- 16.Krismer M, Haid C, Rabl W. The contribution of anulus fibers to torque resistance. Spine. 1996;21:2551–7. doi: 10.1097/00007632-199611150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Jensen GM. Biomechanics of the lumbar intervertebral disk: A review. Physical Therapy. 1980;60:765–73. doi: 10.1093/ptj/60.6.765. [DOI] [PubMed] [Google Scholar]
- 18.Hickey DS, Hukins DWL. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:106–16. doi: 10.1097/00007632-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Pfirrmann CWA, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Boxberger JI, Sen S, Yerramalli CS, et al. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. Journal of Orthopaedic Research. 2006;24:1906–15. doi: 10.1002/jor.20221. [DOI] [PubMed] [Google Scholar]
- 21.Boxberger JI, Auerbach JD, Sen S, et al. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine. 2008;33:146–54. doi: 10.1097/BRS.0b013e31816054f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinoza Orías AA, Malhotra NR, Elliott DM. Rat disc torsional mechanics: Effect of lumbar and caudal levels and axial compression load. The Spine Journal. 2009;9:204–9. doi: 10.1016/j.spinee.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stegemann H, Stalder K. Determination of hydroxyproline. Clinica Chimica Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 24.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–74. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Chan SCW, Ferguson SJ, Wuertz K, et al. Biological response of the intervertebral disc to repetitive short term cyclic torsion. Spine. 2011 doi: 10.1097/BRS.0b013e318203aea5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Gardner-Morse MG, Stokes IA. Physiological axial compressive preloads increase motion segment stiffness, linearity and hysteresis in all six degrees of freedom for small displacements about the neutral posture. Journal of Orthopaedic Research. 2003;21:547–52. doi: 10.1016/S0736-0266(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt H, Heuer F, Claes L, et al. The relation between the instantaneous center of rotation and facet joint forces - a finite element analysis. Clinical Biomechanics. 2008;23:270–8. doi: 10.1016/j.clinbiomech.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Barbir A, Godburn KE, Michalek AJ, et al. Effects of torions on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine. 2011;36:607–14. doi: 10.1097/BRS.0b013e3181d9b58b. [DOI] [PMC free article] [PubMed] [Google Scholar]