Abstract
Exposure to environmental neurotoxic metals, pesticides and other chemicals is increasingly recognized as a key risk factor in the pathogenesis of chronic neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases. Oxidative stress and apoptosis have been actively investigated as neurotoxic mechanisms over the past two decades, resulting in a greater understanding of neurotoxic processes. Nevertheless, emerging evidence indicates that epigenetic changes, protein aggregation and autophagy are important cellular and molecular correlates of neurodegenerative diseases resulting from chronic neurotoxic chemical exposure. During the Joint Conference of the 13th International Neurotoxicology Association and the 11th International Symposium on Neurobehavioral Methods and Effects in Occupational and Environmental Health, the recent progress made toward understanding epigenetic mechanisms, protein aggregation, autophagy, and deregulated kinase activation following neurotoxic chemical exposure and the relevance to neurodegenerative conditions were one of the themes of the symposium. Dr. Anumantha G. Kanthasamy described the role of acetylation of histones and non-histone proteins in neurotoxicant-induced neurodegenerative processes in the nigral dopaminergic neuronal system. Dr. Arthi Kanthasamy illustrated the role of autophagy as a key determinant in cell death events during neurotoxic insults. Dr. Ajay Rana provided evidence for posttranslational modification of α-synuclein protein by the Mixed Linage Kinase (MLK) group of kinases to initiate protein aggregation in cell culture and animal models of Parkinson’s disease. These presentations outlined emerging cutting edge mechanisms that might set the stage for future mechanistic investigations into new frontiers of molecular neurotoxicology. This report summarizes the views of symposium participants, with emphasis on future directions for study of environmentally and occupationally linked chronic neurodegenerative diseases.
Keywords: Neurodegenerative disease, histone modification, acetylation, epigenetics, autophagy, protein aggregation, environmental chemicals, pesticides, metals, methamphetamine, neurotoxicity, MLK3, alpha-synuclein
1. Epigenetic mechanisms of pesticide-induced dopaminergic degeneration in a Parkinson’s disease model (AGK)
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, affecting several million people worldwide. Patients with PD experience substantial motor disability such as rigidity, bradykinesia, impaired balance, and resting tremor. Pathologically, the disease is characterized by the significant degeneration of dopaminergic neurons, predominantly in the substantia nigra pars compacta (SNpc), and the associated dopamine depletion in the striatum. Its neuropathological hallmarks also include the presence of cytosolic inclusions known as Lewy bodies in the surviving pigmented neurons. The cause of PD remains elusive, but environmental chemical exposures along with susceptible gene mutations have been postulated to be involved in the etiology of PD (Obeso et al., 2010).
The association of agricultural chemical exposure with PD development has been studied extensively in order to understand the etiopathogenesis of PD and to develop a therapy for the disease. Specifically, several epidemiological studies have shown that exposure to pesticides, in particular the widely used organochlorines and the bipyridyl herbicide paraquat, is associated with increased risk of developing PD (Bove et al., 2005, Kanthasamy et al., 2005, Dinis-Oliveira et al., 2006). More importantly, post-mortem analysis revealed the presence of elevated levels of organochlorine pesticides in the brains of PD patients (Fleming et al., 1994, Corrigan et al., 2000, Kanthasamy et al., 2005, Brown et al., 2006). Consistent with these findings, toxicological evidence obtained using experimental models suggests that exposure to pesticides can cause dopaminergic neuronal degeneration in both cell culture and animal models, induce Parkinsonian-like symptoms in animals, and promote α-synuclein-positive cellular inclusions similar to Lewy bodies (Sanchez-Ramos et al., 1998, Brooks et al., 1999, Betarbet et al., 2000, Kitazawa et al., 2001, McCormack et al., 2002, Kitazawa et al., 2003, Richardson et al., 2006, Kanthasamy et al., 2008). To date, several hypotheses have been proposed, which attempt to clarify the pathogenic mechanisms underlying the pesticides-induced dopaminergic neurotoxicity; these include increased oxidative stress, generation of reactive oxygen species (ROS), impairment of the ubiquitin-proteasome system and others (Cory-Slechta et al., 2005, Sun et al., 2005, Wang et al., 2005, Sun et al., 2006, Sun et al., 2007, Yang and Tiffany-Castiglioni, 2007). However, the exact molecular pathways leading to pesticides-induced dopaminergic neurodegeneration remain elusive.
Epigenetic modifications, particularly acetylation and deacetylation of the histone-tail, play a pivotal role in the epigenetic regulation of gene expression and many other cellular events, including growth, differentiation, development, learning and memory, and apoptosis (Abel and Zukin, 2008). Histone acetylations catalyzed by histone acetyltransferases (HATs) promote a more relaxed chromatin structure and allow various transcription factors access to the promoter of target genes; in contrast, deacetylation by histone deacetylase (HDACs) results in a condensed state of chromatin and consequent transcriptional repression (Saha and Pahan, 2006, Yang and Seto, 2007). Thus, aberrant histone acetylation modifications can produce an altered gene expression profile that might lead to disease states. Indeed, disrupted histone acetylation signaling has been proposed to be a common contributor to a variety of brain disorders that involve significant neuronal loss and dysfunction, especially stroke, fragile X tremor ataxia syndrome, and Huntington’s and Alzheimer’s diseases (Chuang et al., 2009). However, modified histone acetylation, as a potential mechanism linking environmental pesticide exposure to PD pathogenesis, has not yet been explored. Our recent studies have characterized a critical role for disrupted histone acetylation homeostasis in the neurotoxic pesticides-induced dopaminergic cell death in cell culture models of PD. In the rat mesencephalic dopaminergic N27 neuronal cell model, organochlorine pesticide dieldrin (100 μM) exposure induced a time-dependent increase in the acetylation of core histones H3 and H4 (Song et al., 2010). The dieldrin-induced histone hyperacetylation occurred as early as 5 min following dieldrin exposure, suggesting that histone acetylation is an early event in dieldrin neurotoxicity (Song et al., 2010). Similarly, exposure to paraquat also increased acetylated histone H3, whereas acetylation of histone H4 remain unaltered (Song et al., 2011). Fig. 1 provides a representative example of dieldrin-induced histone acetylation in N27 dopaminergic neuronal cells (Song et al., 2010). Furthermore, we explored the molecular basis of hyperacetylation of histones by dieldrin. Our results suggest that hyperacetylation contributed to dieldrin-induced proteasomal dysfunction, resulting in accumulation of a key histone acetyltransferase, CBP. In support of this finding, administration of the HAT inhibitor anacardic acid or specific knockdown of CBP protein by siRNA both effectively attenuated the dieldrin-induced histone acetylation (Song et al., 2010). Intriguingly, a distinct mechanism involving reduction of specific HDAC levels and associated HDAC activity, without affecting HAT activity, was observed in paraquat-induced histone H3 hyperacetylation, suggesting that HAT- and/or HDAC-dependent signaling pathways may play a role in mediating the histone hyperacetylation induced by various neurotoxicants. Using the HAT inhibitor anacardic acid, we also demonstrated that HAT inhibition can significantly attenuate the dieldrin and paraquat activated major events of the apoptotic pathway, including caspase-3 activation, PKCδ proteolytic activation and DNA fragmentation in N27 cells (Song et al., 2010, Song et al., 2011), suggesting a neuroprotective effect of HAT inhibitors in protecting dopaminergic neurons against pesticide-induced apoptotic cell death. In addition, anacardic acid was neuroprotective against dieldrin-induced dopaminergic neuronal degeneration in primary mesencephalic neuronal cultures, further suggesting the possibility of a novel neuroprotective strategy targeting HAT activation. Collectively, for the first time, our results demonstrate that neurotoxic pesticide exposure induces acetylation of core histones and that hyperacetylation plays a key role in dopaminergic neuronal degeneration following neurotoxic insults.
Fig. 1. Dieldrin induced a rapid histone hyperacetylation in dopaminergic neuronal N27 cells.
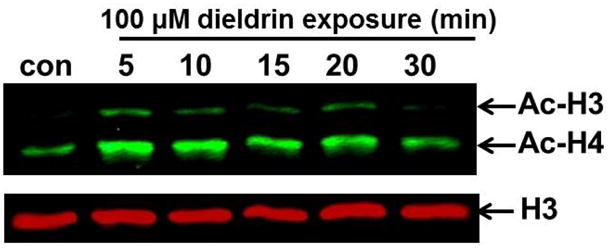
N27 dopaminergic cells were exposed to dieldrin (100 μM) for 5–30 min, and the acetylation of histones H3 and H4 was subsequently detected by Western blot analysis using anti-acetyl-lysine antibody. Native H3 was used as an internal control. A time-dependent increase in histone acetylation was observed.
2. Signaling cross-talk between ubiquitin-proteasome dysfunction, mitochondrial dysfunction and autophagy in a neurotoxicity model of dopaminergic neuronal degeneration (AK)
In addition to epigenetic modifications, several other pathogenic mechanisms, including oxidative stress, mitochondrial dysfunction, impairment of the ubiquitin proteasome system (UPS) and deregulated autophagy, have long been proposed to be involved with development of PD (Mattson, 2000, Chung et al., 2001, Vila and Przedborski, 2003, Abou-Sleiman et al., 2006, Olanow, 2007, Tansey et al., 2007, Burke, 2008, Cheung and Ip, 2009, Levy et al., 2009). The current belief is that dopaminergic neuronal degeneration in PD is likely to result from a combination of multiple interlinking signaling pathways rather than from a single unifying mechanism (Sulzer, 2007, Obeso et al., 2010). However, the functional significance of the aforementioned pathogenic mechanisms and their potential cross talk in the mechanism of dopaminergic neurodegeneration is still unclear.
Methamphetamine (MA) is a powerful psychostimulant drug of abuse that has been shown to markedly affect the central nervous system and neurobehavior in both animal models and humans (Ricaurte et al., 1982, Wilson et al., 1996). Exposure to MA, in particular high doses or exposure over long periods, produces significant damage to the nigrostriatal dopaminergic pathway in a pattern similar to that seen in PD patients, including long-term reduction in striatal dopamine levels, reduced tyrosine hydroxylase activity, and pronounced loss of dopaminergic neurons (Nakayama et al., 1993, Miller and O’Callaghan, 1994, O’Callaghan and Miller, 1994, McCann et al., 2008, Morrow et al., 2011). The epidemiological data demonstrating a higher incidence of PD in MA-addicted users further strengthen the putative association of MA with the etiology of PD (Callaghan et al., 2010). Accumulating evidence suggests that MA-induced neurotoxicity probably results from enhanced oxidative stress involving elevated reactive oxygen species (ROS) generation, but the precise molecular mechanisms accounting for the neurotoxic action of MA on dopaminergic neurons await more detailed investigation.
We used MA as a model toxicant to characterize the autophagic mechanism during neurotoxic insults as well as to evaluate potential cross-talk between autophagy with impaired UPS and mitochondrial dysfunction in MA neurotoxicity. In our recent study, we showed that rat mesencephalic dopaminergic neuronal cells, referred to as N27 cells, are an excellent cell culture model to study the mechanism of methamphetamine (MA)-induced neurotoxicity (Kanthasamy et al., 2011). We also demonstrated that exposure of N27 dopaminergic neuronal cells to MA resulted in the appearance of cytoplasmic vacuolar structures indicative of autophagic vacuoles (Kanthasamy et al., 2006). The induction of autophagy was further confirmed by LAMP-2 lysosomal-associated membrane protein 2) and LC3 (microtubule associated protein 1 light chain 3) staining (Kanthasamy et al., 2006). The MA-induced autophagy is an early event during MA treatment, which was evidenced as early as 3 h after the start of MA treatment in N27 cells. Importantly, when autophagy was inhibited using a pharmacological inhibitor (3-methyladenine) or genetic knockdown by an siRNA specific for LC3, the neuronal cells became sensitized to MA-induced apoptosis, suggesting a neuroprotective role for autophagy in MA-induced neurotoxicity (Lin et al., 2012). The overexpression of LC3 partially conferred resistance against MA-induced neurotoxicity, further supporting the protective role of autophagy in MA neurotoxicity. Intriguingly, further analysis demonstrated that MA-induced autophagy was preceded by reduction in proteasomal function and concomitant dissipation of mitochondrial membrane potential (MMP), which was subsequently followed by significant increases in PKCδ proteolytic activation and caspase-3 activation, as well as accumulation of ubiquitin positive aggregates and elevated LC3-II (microtubule associated light chain-3 II) levels. These results suggest that MA-induced autophagy serves as an adaptive strategy for inhibiting mitochondrial mediated apoptotic cell death and degradation of aggregated proteins. Fig. 2 shows the representative electron microscopic analysis of autophagosomes with damaged mitochondria in MA-treated dopaminergic N27 cells within 12 h of exposure (Lin et al., 2012). To further explore the mechanisms of observed cross-talk between autophagy and impaired UPS and mitochondrial dysfunction, we examined the role of PKCδ by using a genetic approach (PKCδ siRNA knockdown and PKCδ cleavage-resistant mutant). Our laboratory previously reported that the kinase, PKCδ, functions as an oxidative stress-sensitive kinase, and that its persistent activation by caspase-3-mediated proteolytic cleavage has a promotional role in multiple models of PD-associated dopaminergic neurodegeneration (Anantharam et al., 2002, Kaul et al., 2003, Kitazawa et al., 2003, Kaul et al., 2005, Latchoumycandane et al., 2005, Jin et al., 2011a, Jin et al., 2011b). Interestingly, siRNA-mediated knockdown of PKCδ or overexpression of cleavage resistant mutant of PKCδ dramatically reduced MA-induced autophagy, proteasomal function, and associated accumulation of ubiquitinated protein aggregates, which closely paralleled cell survival. Our findings suggest that proteolytic activation of PKCδ has pivotal roles in apoptotic cell death mechanisms associated with UPS dysfunction, mitochondrial impairment and autophagy during neurotoxic insults (Sun et al., 2008, Saminathan et al., 2011). Collectively, these data demonstrate a considerable cross-talk between autophagy and apoptosis dependent cell death signaling events during neurotoxic insults in dopaminergic neurons.
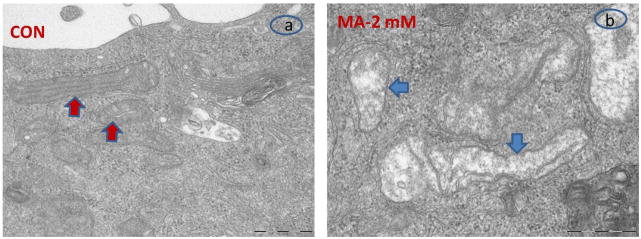
Fig. 2. Electron microscopic (EM) analysis of MA-induced mitochondrial damage in N27 dopaminergic cells.
N27 dopaminergic cells were treated with PBS (CON; Panel a) or MA (2 mM; Panel b) for 12 h and examined by EM. EM analysis revealed swollen mitochondria with disorganized cristae in MA treated N27 dopaminergic cells (blue arrows), whereas control cells revealed tubular mitochondria (red arrows).
3. Alpha-synuclein and Mixed Lineage Kinases (MLK): partners of offense in Parkinson’s disease (AR)
Defects in the ubiquitin proteasome system and autophagy lead to the accumulation and aggregation of toxic proteins, which may eventually result in neurodegeneration. In fact, the presence of insoluble proteinaceous aggregates or inclusion bodies is a common pathological feature in human neurodegenerative disorders including PD. The presence of cytosolic proteinaceous inclusions known as Lewy bodies (LBs) and Lewy neurites (LN) is a key pathological feature of PD. The LBs are primarily made from an unfolded protein, α-synuclein. In addition, several kinases including MLK, are colocalized in the LBs (Nakamura et al., 1997, Zhu et al., 2006, Nagao and Hayashi, 2009). α-Synuclein proteins in LBs are highly phosphorylated as well as ubiquitinated, and these post-translational modifications of α-synuclein are thought to promote neurotoxicity. Specifically, phosphorylation of α-synuclein at S129 is believed to play an important role in α-synuclein metabolism and PD pathogenesis (Fujiwara et al., 2002, Anderson et al., 2006). Recently, Polo Like Kinases (PLKs) have been shown to phosphorylate α-synuclein at S129 (Inglis et al., 2009, Mbefo et al., 2010), however the roles of PLKs in α-synuclein aggregation or PD pathogenesis is not fully understood.
We recently identified a kinase that phosphorylates α-synuclein and promotes its aggregation. Our observed that MLK3, a member of the Mixed Linage Kinase (MLK) family, is localized within LBs in close association with α-synuclein in the human PD brain (Fig. 3, unpublished observations). Alpha-synuclein protein was also directly phosphorylated by MLK3, a kinase that has recently been implicated in neurodegeneration in the mouse model of PD (Saporito et al., 1999, Wang et al., 2004). We also identified the specific phosphorylation sites and their roles in α-synuclein aggregation. Our preliminary results suggested that the phosphorylation-site deficient α-synuclein proteins were not aggregated and did not cause neurotoxicity in the cellular model of PD. In N27 dopaminergic cells, the Parkinsonian toxicant MPP+ caused MLK3 activation and aggregation of α-synuclein protein, whereas the MLKs inhibitor prevented the MPP+-induced α-synuclein aggregation. Interestingly, the pan-MLKs inhibitor, CEP-1347 was shown to prevent dopaminergic neuronal loss in pre-clinical animal models of PD (Kaneko et al., 1997) and thus went on for clinical trials (up to Phase III), which was later abandoned due to several issues related to pharmacology of the pan-inhibitor and types/stages of PD patients recruited for the PRECEPT trial (Marras et al., 2009, Yacoubian and Standaert, 2009). Nevertheless, these results clearly suggested that MLK group of kinases are viable targets of neurodegenerative diseases. Our recent observation with α-synuclein and MLK3 knock out animals suggest that MLKs inhibitor could prove beneficial to halt the progression of the disease by preventing the activation of MLK3 and thus aggregation of α-synuclein protein. Furthermore, there is an urgent need to develop better inhibitors of MLKs group of kinase to treat neurodegenerative diseases.
Fig. 3. Co-localization of MLK3 and α-synuclein in SNpc from the human PD brain.
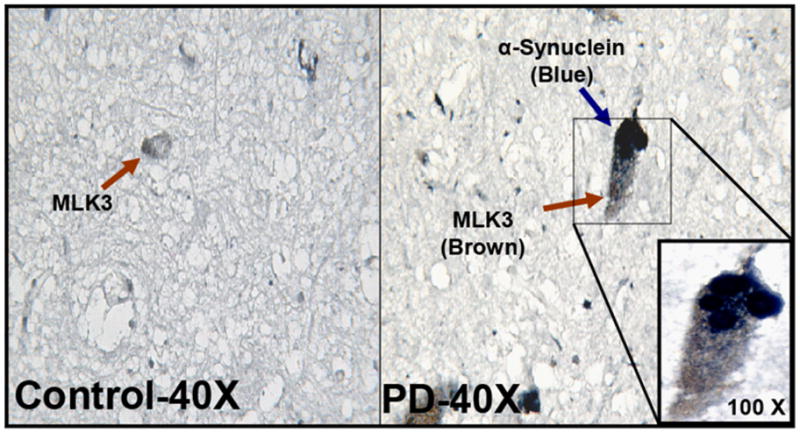
MLK3 (DAB-brown stain) and α-synuclein (Blue-Vector) were co-localized in the SNpc region of human PD and control brain samples.
4. Summary
Epigenetics is emerging as an important and novel mechanism in neurodegenerative diseases. Our data presented in the review demonstrate that alterations of epigenetic patterns, more precisely histone modifications, may play a critical role linking environmental neurotoxins to the pathogenesis of PD. However, there are still several unanswered questions regarding the precise mechanism by which epigenetics contributes to neurodegenerative diseases. Future studies elucidating genome-wide histone modification and DNA methylation profiles will provide an integrated understanding of the development of human neurodegenerative diseases. Furthermore, we suggest a significant mechanistic link between autophagy, mitochondrial dysfunction, impaired UPS and apoptosis during exposure to neurotoxic insults. In addition, we also identified that phosphorylation of α-synuclein via MLK3 kinase signaling may represent a key molecular mechanism in PD pathogenesis. Collectively, these data illustrate the need for a cooperative approach to resolve the complexity underlying PD pathogenesis and highlight the need for developing integrative mechanism-based therapeutic strategies for the treatment of neurodegenerative diseases.
Acknowledgments
The authors thank the organizers of the Joint Conference of the 13th International Neurotoxicology Association meeting and the 11th International Symposium on Neurobehavioral Methods and Effects in Occupational and Environmental Health for their tremendous efforts and support. This work was supported by National Institutes of Health (NIH) Grants ES10586, ES19276, NS074443 (AGK), NS65167 (AK), and GM55835 (AR). The W. Eugene and Linda Lloyd Endowed Chair to A.G.K. is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Current opinion in pharmacology. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nature reviews. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. The Journal of biological chemistry. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature neuroscience. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain research. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environmental health perspectives. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Programmed cell death and new discoveries in the genetics of parkinsonism. Journal of neurochemistry. 2008;104:875–890. doi: 10.1111/j.1471-4159.2007.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Ip NY. The emerging role of autophagy in Parkinson’s disease. Molecular brain. 2009;2:29. doi: 10.1186/1756-6606-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends in neurosciences. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Dawson VL, Dawson TM. The role of the ubiquitin-proteasomal pathway in Parkinson’s disease and other neurodegenerative disorders. Trends in neurosciences. 2001;24:S7–14. doi: 10.1016/s0166-2236(00)01998-6. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Thiruchelvam M, Barlow BK, Richfield EK. Developmental pesticide models of the Parkinson disease phenotype. Environ Health Perspect. 2005;113:1263–1270. doi: 10.1289/ehp.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006 doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nature cell biology. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. The Journal of biological chemistry. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Anantharam V, Rana A, Kanthasamy AG. Transcriptional regulation of pro-apoptotic protein kinase Cdelta: implications for oxidative stress-induced neuronal cell death. The Journal of biological chemistry. 2011a;286:19840–19859. doi: 10.1074/jbc.M110.203687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Ghosh A, Yang Y, Anantharam V, Kanthasamy AG. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J Neurosci. 2011b;31:2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Saito Y, Saito H, Matsumoto T, Matsuda Y, Vaught JL, Dionne CA, Angeles TS, Glicksman MA, Neff NT, Rotella DP, Kauer JC, Mallamo JP, Hudkins RL, Murakata C. Neurotrophic 3,9-bis[(alkylthio)methyl]-and-bis(alkoxymethyl)-K-252a derivatives. Journal of medicinal chemistry. 1997;40:1863–1869. doi: 10.1021/jm970031d. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A, Anantharam V, Ali SF, Kanthasamy AG. Methamphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin-D in methamphetamine-induced apoptotic cell death. Annals of the New York Academy of Sciences. 2006;1074:234–244. doi: 10.1196/annals.1369.022. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Yang Y, Anantharam V, Kanthasamy A. Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson’s disease. Molecular brain. 2008;1:12. doi: 10.1186/1756-6606-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy K, Gordon R, Jin H, Anantharam V, Ali S, Kanthasamy AG, Kanthasamy A. Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Current neuropharmacology. 2011;9:49–53. doi: 10.2174/157015911795017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. The Journal of biological chemistry. 2005;280:28721–28730. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. The European journal of neuroscience. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free radical biology & medicine. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. The Journal of pharmacology and experimental therapeutics. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chandramani Shivalingappa P, Jin H, Anantharam V, Ali S, Kanthasamy AG, Kanthasamy A. Methamphetamine-induced neurotoxicity linked to UPS dysfunction and autophagy related changes that can be modulated by PKCδ in dopaminergic neuronal cells. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.004. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, Lang AE, Eberly SW, Oakes D, Fahn S, Schwid SR, Hyson C, Shoulson I. A comparison of treatment thresholds in two large Parkinson’s disease clinical trial cohorts. Mov Disord. 2009;24:2370–2378. doi: 10.1002/mds.22828. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, Kahle PJ, Hirling H, Lashuel HA. Phosphorylation of synucleins by members of the Polo-like kinase family. The Journal of biological chemistry. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiology of disease. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. The Journal of pharmacology and experimental therapeutics. 1994;270:752–760. [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson’s disease. Neuroscience. 2011;189:277–285. doi: 10.1016/j.neuroscience.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Hayashi H. Mixed lineage kinase 2 and hippocalcin are localized in Lewy bodies of Parkinson’s disease. Journal of the neurological sciences. 2009;281:51–54. doi: 10.1016/j.jns.2009.02.375. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamoto Y, Nakano S, Akiguchi I, Kimura J. p35nck5a and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson’s disease. Acta neuropathologica. 1997;94:153–157. doi: 10.1007/s004010050687. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Koyama T, Yamashita I. Long-lasting decrease in dopamine uptake sites following repeated administration of methamphetamine in the rat striatum. Brain research. 1993;601:209–212. doi: 10.1016/0006-8993(93)91712-2. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. The Journal of pharmacology and experimental therapeutics. 1994;270:741–751. [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nature medicine. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Olanow CW. The pathogenesis of cell death in Parkinson’s disease--2007. Mov Disord. 2007;22 (Suppl 17):S335–342. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain research. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell death and differentiation. 2006;13:539–550. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saminathan H, Asaithambi A, Anantharam V, Kanthasamy AG, Kanthasamy A. Environmental neurotoxic pesticide dieldrin activates a non receptor tyrosine kinase to promote pkcdelta-mediated dopaminergic apoptosis in a dopaminergic neuronal cell model. Neurotoxicology. 2011;32:567–577. doi: 10.1016/j.neuro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Experimental neurology. 1998;150:263–271. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. The Journal of pharmacology and experimental therapeutics. 1999;288:421–427. [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Molecular pharmacology. 2010;77:621–632. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends in neurosciences. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–815. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Anantharam V, Kanthasamy AG. Environmental neurotoxic chemicals-induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacology & therapeutics. 2007;114:327–344. doi: 10.1016/j.pharmthera.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. Journal of cellular and molecular medicine. 2008;12:2467–2481. doi: 10.1111/j.1582-4934.2008.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Experimental neurology. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nature reviews. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, Troncoso J, Dawson VL, Dawson TM, Lim KL. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum Mol Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- Wang LH, Besirli CG, Johnson EM., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annual review of pharmacology and toxicology. 2004;44:451–474. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. Biochimica et biophysica acta. 2009;1792:676–687. doi: 10.1016/j.bbadis.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. J Toxicol Environ Health A. 2007;70:1849–1857. doi: 10.1080/15287390701459262. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- Zhu X, Siedlak SL, Smith MA, Perry G, Chen SG. LRRK2 protein is a component of Lewy bodies. Annals of neurology. 2006;60:617–618. doi: 10.1002/ana.20928. author reply 618–619. [DOI] [PubMed] [Google Scholar]