Abstract
Aging is an important and critical factor that contributes to the clinical outcome of burn patients. The very young and the elderly are more likely to succumb after major burn as compared to their adult counterparts. With the aging population, improved understanding of the mechanisms underlying age-associated complications after burns becomes even more demanding. It is widely accepted that elderly burn patients have significantly increased morbidity and mortality. Irrespective of the type of burn injury, the aged population shows slower recoveries and suffers more complications. Age-associated immune dysfunction, immunosenescence, may predispose the elderly burn patients to more infections, slower healing and/or to other complications. Furthermore, pre-existing, age-related medical conditions such as, pulmonary/cardiovascular dysfunctions and diabetes in the elderly are other important factors that contribute to their poorer outcomes after major burn. The present review describes the impact of aging on burn patients outcomes.
Keywords: Aging, Burn, Pathophysiology, Immune, Infection
Burn Epidemiology
Burn injury is a significant problem worldwide and represents one of the most devastating forms of trauma. In the United States, more than one million burn injuries occur every year [1]. Burn can be caused by various factors such as heat (flame or scald), freezing, electricity, chemicals, radiation or friction [2]. Different factors affect the morbidity and mortality of burn injury. First, the size of the burn, as determined as a percentage of the total body surface area (TBSA) is directly proportional to survival. The bigger the burn, the greater the risk of subsequent burn wound and systemic infections, which is directly associated with increased morbidity and mortality [3, 4]. Secondly, inhalation injury in combination with burn worsens the clinical outcome of the burn patients to a great extent, despite advances in intensive respiratory support [5, 6]. Thirdly, the age of the patient significantly influences outcomes after burn, as the mortality is higher in very young and elderly patients compared with other age groups [7, 8]. The combination of a burn injury with one or more of the critical contributing factors such as the presence of inhalation injury and extreme age results in a higher number of fatalities [9]. It has been shown repeatedly in clinical studies that mortality was highest in elderly patients who had more severe burns and/or smoke inhalation injury and also had existing underlying diseases [10–14].
Flame burns contribute to 55% of the admissions to the burn centers followed by scald burns with 40%. Flame burns, often associated with inhalation injury and other concomitant injuries; represent the greatest frequency of residential fire and burn mortalities. It has been reported that 12 individuals die in residential fires each day affecting all age groups, but young children and the elderly being the most likely victims. While dependent young children encounter difficulty escaping, the elderly present higher morbidity and mortality because of preexisting diseases and decreased agility [14]. It has been reported that in children, scald burns represent the majority (70%) of the burns, because of contact with hot liquids. In adolescents and young adults, improper handling of fire and flammable liquids causes the majority of the burns. In adults, flame burns are most common, with one third related to work accidents [15]. In the elderly, flame and scald burns or scalds alone are the major causes for burns, most commonly occurring at home, especially in the kitchen and bathroom [16].
While physical and psychological dependency or hyperactivity are major causes of high mortality in young children (under 3 years old), a myriad of factors, such as, collapse, physical disability, diminished alertness, slower response times, impaired mobility and preexisting conditions contribute to higher mortality rates in the elderly (65–79 years). In contrast to the young and the elderly, mortality in adults is related to incidences at work or related to activities outside of the household [17].
The Pathophysiology of the Burn Wound
The nature of the burn wound injury is ultimately the result of a complex process causing local, as well as systemic complications, affecting multiple organ systems distal to the skin [15]. Burn wound progression involves the progressive damage of superficial partial-thickness burns into deep partial-thickness or full-thickness wounds within a sub-acute time frame of 3–5 days [18]. Figure 1 describes three degrees of burn showing different thicknesses of burn wound. Multiple factors, acting via a variety of pathophysiological mechanisms, can advance the burn wound conversion to a more severe state. The burn wound progression is clinically significant, not only because of its relevance to burn wound depth, but also because it affects the long-term morbidity and treatment. An increase in wound advancement indicates the likelihood of hypertrophic scarring, increased excision and grafting requirements, wound infections, sepsis, shock and potentially death [18, 19].
Figure 1.
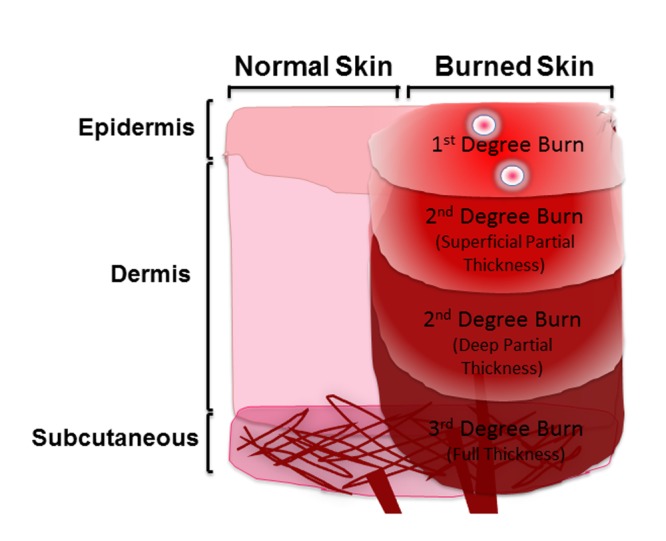
Graphic description of different burn types
Pathogenesis of Burn
The pathogenesis of burn wound progression initiates from the original site of injury. It involves primary tissue loss due to protein denaturation, which further leads to release of toxic pro-inflammatory mediators and platelet activating factors into the circulation [20, 21]. Oxygen radicals and proteases further damage skin and result in an overall immunosuppressive phase commonly known as systemic inflammatory response syndrome (SIRS). Participation of immune cells, such as neutrophils and macrophages, result in production of cytotoxic oxygen free radicals damaging the dermal structures directly or indirectly [22]. The initial pro-inflammatory phase puts the burn patients at the risk for early multiple organ failure (MOF) as a result of the destruction of different vital organs such as heart and lung, whereas in the later phase of immunosuppression, the patients are at the risk of developing MOF due to infection and sepsis [23, 24]. Different local and systemic factors such as, impaired wound perfusion, edema, infection, metabolic derangements and general health status may contribute to wound progression. The elderly patients show increased immunosenescence- the state of dysregulated immune function with aging- and therefore are believed to be at more risks for infections, sepsis and MOF [25, 26].
The Biology of Aging
Aging is a complex, multifactorial phenomenon. Hormones, mitochondrial DNA, genetic material, free radicals, oxidative-inflammation and immunosenescence all play a significant role in the process of aging [27–32]. Moreover, many of the contributing factors seem to interact in a complex way during aging. The aging process occurs at molecular, cellular and immunological levels and is believed to occur as a result of the accumulation of cellular damage overtime [26, 33–35]. When cells undergo a stress response because of exogenous damaging pathogens or endogenous injuries such as burn, free radicals are released. The oxidative stress response causes damage to the macromolecular component of the cell. DNA damage, which occurs continuously in cells, gets repaired most of the times by inherent repair mechanisms. The repair machinery, however, cannot correct the damage as fast as it occurs and the damage accumulates over time with age. It is speculated that lifelong accumulated damage leads to mitochondrial dysfunction and genetic mutations. These age-associated genetic impairments trigger cells and organs to deteriorate and to stop functioning. Therefore, the vulnerability due to aging not only depends on the lifelong exposure to exogenous pathogenic burden and endogenous damaging agents, but also on the genetic integrity of the body’s cells [34, 36, 37].
All cells of the body go through a process of cellular aging. Immunosenescence is the age-related decline in the immune functions and affects various cell types such as neutrophils, monocytes, macrophages, natural killer (NK) cells and dendritic cells (DC) from the innate arm and B-cells and T-cells from the adaptive arm of the immune system [38]. Although aging alters both branches of the immune system, more severe detrimental changes occur in the adaptive immune system [39]. The immunosenescence-mediated decline in cell-mediated immune functions, as well as reduced humoral immune responses, result in a chronic inflammatory state, which has been termed as “inflamm-aging” [40]. Various studies infer that there is a marked increase in the systemic levels of IL-1β IL-6, IL-8 and TNF-α in the aged population [35, 41–47]. In older adults, increased levels of IL-6 are related to loss of mobility and development of disabilities [48]. Moreover, TNF-α, which seems to have specific biological effects, correlates with increased mortality in the very elderly [44]. This age-related increase in cytokines and other components of inflammation is partially responsible for the increased prevalence and severity of infections, vaccine failure and possibly to autoimmune disorders and cancer in the elderly [41, 49–51]. This pro-inflammatory response of elderly patients is also believed to be responsible for the increased risk for developing complications following injury or infection [25]. Of particular interest, many of the markers of inflammation associated with aging (IL-1β, IL-6, TNF-α) are also central in the inflammatory response after burn. These age-related dysfunctions pose significant clinical challenges.
Immunological Responses
Aging: Burn Injury and Inflammatory Response
The elderly and the very young are most likely to succumb to severe burns [52]. The elderly burn patients demonstrate greater mortality and the aged survivors show slower recoveries, increased length of hospital stay and suffer more complications than their younger counterparts [53]. A retrospective study by Le et al. was performed to compare burn patients from two age groups: one younger than 2 years (16%) and the other older than 70 years (8%) of age [54]. The study demonstrated the prevalence of scald burn in younger children (77%) and flame burn in aged patients (79%). With moderate size of burn there was only one death in the younger group versus 51% mortality reported for the aged group. With TBSA greater than 40%, all patients died. There were three times more complications found in older patients than their counterparts. In another study performed in 1981, burns covering 20% TBSA were associated with a mortality of only 20% in healthy young adult patients, while elderly patients with the same burn size showed 75% mortality [55]. Today, with advancements in medical care and burn management, the mortality rate for adult burn patients has gone down to 5.5%; however, the survival rate of elderly injured subjects has not improved as dramatically because of their age-associated immunosenescence and other pre-existing co-morbidities [56, 57].
To better understand the effect of aging on systemic and cellular immune/inflammatory responses animal models, mimicking the moderate sized scald/flame injury, have been developed. Experimental data using animal burn models further corroborates the aged human clinical data and shows greater mortality in aged rodents after burn. In a mouse model of scald burn with 15% TBSA, young mice exhibited a 100% survival and demonstrated no changes in their ability to eating or drinking, whereas only 33% of the aged mice survived a similar injury [58].
Major burn induces a state of immunosuppression that predisposes the burn patients to wound healing and infectious complications. The host defense against these problems requires an intact and healthy local inflammatory response. However, the overwhelming immune response initiated after the injury causes systemic inflammatory response syndrome to prevail, resulting in significant cellular and end organ damage. In general, immunological response to major burn initially initiates both a pro-inflammatory and an anti-inflammatory phase, concurrently or in sequence in efforts to maintain the homeostasis and normal physiology. Both of these responses are mediated by the cytokines and cellular responses. Immunosuppression in severely injured patients is associated with Th1/Th2 shift [59–61]. While the levels of Th1 cytokines, for example, IL-2 and IFN-γ are reduced markedly, the production of Th2 type of cytokines such as, IL-4 and IL-10 are increased significantly with advancing age and are also augmented by injury [61]. Exogenous IGF-I/IGFBP-3 (a mediator with growth hormone effects) treatment partially reversed this predominance of Th2 phenotype [62]. Therefore, impaired immune functions and elevated pro-inflammatory cytokines, which are associated with natural aging even in healthy subjects, may contribute to the increased lethality in elderly burn patients.
Aging: Burn and Hypermetabolic Response
Burn size also determines hypermetabolic response after injury [63]. A severe burn results in hypermetabolism with protein degradation and catabolism. The hypermetabolic state is characterized by proteolysis, lipolysis and futile protein utilization. This hypermetabolic state results in loss of bone density, muscle weakness and poor wound healing [64]. This response is associated with a marked acute phase response that results in inflammation and altered cytokine responses [65]. Perturbations in cytokine expression give rise to altered immune function, end organ damage (MOF) and subsequent death. The severity of the hypermetabolic response following burns correlates with age and may also be a major contributor to the higher morbidity and mortality rates in adult burn patients compared with pediatric burn patients [65]. A study comparing the cytokine levels, and thereby the hypermetabolic response and associated inflammation, in 24 children versus 24 adults showed that following flame burns covering 20% TBSA, the cytokine profiles in young children were different from those of adult burn patients [65]. Due to the relationship between the hypermetabolic and inflammatory responses, the authors compared a multitude of plasma cytokine profiles. Significant changes in both pro- and anti-inflammatory cytokine levels were seen in young versus adult patient groups. Since the cytokine alterations precede the metabolic changes, it may be possible to modulate the injury-associated hypermetabolic response by altering the cytokine responses, potentially improving the age-related clinical outcomes such as morbidity and mortality [65–67].
The post-burn hypermetabolic state is also associated with hyperglycemia, insulin resistance and diabetes [68]. Recent studies evaluated the glucose control and clinical outcomes in diabetic burn patients. The studies showed that age and size of the burn significantly contributed to the mortality. However, the burn patients with pre-existing diabetes were not associated with any worsened clinical outcomes [69, 70]. Major burn and other trauma such as hemorrhage and sepsis induce insulin resistance, which was strongly influenced by the advanced age. Insulin, which is an effective anabolic and anti-catabolic hormone, also regulates the inflammatory and immune responses. Insulin decreases the pro-inflammatory and increases the anti-inflammatory response, thereby restoring aspects of the systemic inflammation and homeostasis after injury [71]. A retrospective review studying 265 aged burn patients documented no definite correlation between the elderly and intensive insulin therapy regimen and clinical outcomes [53]. Nonetheless, it can be speculated that the conclusions of this study are related to the complexity of the problem, rather than the lack of an effect.
Aging: Burn and Wound Healing
Wound healing is a critical component of the immune system orchestrated to restore tissue integrity after burn [72]. Impaired wound healing in the aged burn patients present a major clinical and economical challenge. Normal skin represents the integral part of the wound healing process and undergoes significant characteristic changes as aging. Moreover, the wound healing process itself is altered with aging. Clinical studies and laboratory investigations have yielded a wealth of information describing different aspects of aging and wound healing ranging from cellular, biochemical, immunological to clinical level. It has been shown by various research groups working on animal models of wound healing in the aged that there is a 20–60% delay in the rate of wound healing as compared to young animals [73].
Skin provides essential protection for the internal organs of the body. It protects it against the invading microorganisms, ultraviolet (UV) radiation, regular water loss and assists in thermoregulation [74]. Skin undergoes the process of both extrinsic and intrinsic aging [75]. Extrinsic aging is defined as the changes happening as a result of life-long environmental exposures such as UV-light from the sun. Intrinsic aging includes the changes in skin that occur in sun-protected regions, independent of environmental insults. Life-long progression of extrinsic and intrinsic aging leads to gradual loss of function, susceptibility to the environment and decreased homeostatic potentials [75]. Skin is composed of epidermis - the outer layer and dermis - the lower layer. While, with age, the thickness of the epidermis remains fairly constant, the epidermal-dermal junctions flatten, giving the appearance of atrophy [76]. Skin constitutes a critical component of the immune system and anchors different immune cells, such as fibroblasts, mast cells, macrophages and antigen-presenting cells (mast cells and skin dendritic cells - the Langerhans cells) that impact wound healing [77]. These cells represent the cellular component of the dermis and are decreased with aging [38, 78, 79]. The protein content of the dermis, primarily collagen, is decreased with age as well. This is the result of both decreased production and increased degradation [80]. The microcirculation and thereby the blood flow of the dermis also decreases with age making it more susceptible to injury and temperature fluctuations [81]. Aging also decreases the dermal lymphatic drainage, diminishing the ability to clear the wound of pathogens and also inhibiting wound contraction [82, 83].
Aging leads to interruptions, aberrations or prolongation in the wound healing phases and can lead to delayed or non-healing chronic wounds [72]. Different wound healing phases are hemostasis, inflammation, proliferation and tissue remodeling [75]. After the incidence of burn, skin gets exposed and causes platelet aggregation at the injured endothelium [84]. Platelet adherence is enhanced with age, affecting chemokine levels and vascular permeability [85, 86]. Changes in cellular and immune functions by different cells, such as neutrophils, macrophages and T-cells have been found to be critical to age-associated wound healing defects [38, 77, 79]. Moreover, wound repair in aged mice is accelerated with adoptive transfer of macrophages from the younger mice, but not from old mice [87]. Different growth factors produced by macrophages are also decreased with age [88]. Keratinocytes, fibroblasts, and vascular endothelial cells show a reduced proliferative response in aged animals [89]. Reepithelialization and collagen synthesis also display an age-related delay [88]. Neovascularization, an essential component of the wound healing, is impaired in aged animals, which is due to impaired bone marrow derived dendritic cells (BMDC) mobilization and homing to burn wound tissue [90]. Angiogenesis, which is responsible for neovascularization, is decreased with age [90–93]. All these factors lead to an age-related impairment and delay in wound closure in animal models as well as in human wounds. Wound studies have shown decreased rates of epithelialization and contraction both in older animals and humans [94]. Collagen production as well as deposition is decreased with age, causing lower turnover and remodeling of the collagen and thereby resulting in delayed wound resolution [80]. Impaired and delayed wound healing represents a major clinical problem. Therefore, better understanding the cellular and molecular mechanisms by which aging impairs the wound healing process is essential.
Aging: Burn Wound Infection
While advancement in overall burn care therapy has increased the survival outcome, infectious complications still remain a significant challenge after burn [95]. Among burn patients, the elderly have clearly been found to have both increased and more severe infections. Major burns covering more than 30% TBSA are found to be more susceptible to infections, in part because of the larger surface area involved for bacterial colonization [96]. Among the different kinds of infections harboring the wound, bacterial infections are the most prevalent ones [97]. Other common infections found in major burn wounds are fungal and viral [97, 98]. Bacterial infections are not only found in the wounds directly, but also cause bacterial pneumonia, bloodstream infections and sepsis increasing mortality [97]. With increased bacterial growth, chances for invasive infection and incidences of septicemia also increase [99]. For example, pneumococcal infections are more common in the first two years of life and diminish during adulthood and are again increased significantly in aged individuals over age 75 with mortality approaching 80% in those over age 85 [100].
Dysregulated immune functions with age play a significant role in wound infections and contribute to increased morbidity and mortality in aged burn patients. Both arms of the immune system are impacted by aging; however, the adaptive immunity seems to be more challenged during the aging process [39]. A mouse study with tuberculosis infection by Orme et al. has shown that the aged animals have delayed recruitment of CD4+ T-cells and also produce less IFN-γ leading to more bacterial dissemination and less containment [101]. The study also showed that most of these changes are reversed in the aged animals if adoptively transferred with T-cells from younger mice [101]. Various animal and human studies infer that there is an increased production of pro-inflammatory cytokines, such as IL-1β IL-6, IL-8, TNF-α in the aged population [35, 41–43]. Therefore, the elderly appear to be more sensitive to bacterial endotoxins and thus are more susceptible to end organ inflammation [102]. An animal study comparing the effect of age on the response to injury and infection showed that the aged mice demonstrated an increased susceptibility to infection and sepsis with subsequently increased mortality [103]. The older mice also exhibited a strong positive IL-6 correlation with mortality. This increased cytokines and inflammation with aging, which contributes to immunosenescence, seems to be partially responsible for the increased prevalence and severity of infections and sepsis after injury [103, 104].
Aging: Burn, Pulmonary, Cardiovascular and Renal Dysfunction
Major burn not only affects the site of injury, but also has systemic effects involving the pulmonary, cardiovascular, gastrointestinal, hematopoietic and renal systems. Complications in these systems may lead to acute lung injury (ALI), acute respiratory distress syndrome (ARDS), myocardial depression and renal failure, profoundly impacting the clinical outcome of the burn patients. The pulmonary system is often the first one to fail after major burn [105]. The morbidity and mortality of burn patients increase by 20% when associated with smoke inhalation, an injury that occurs in 10–30% of thermally injured patients [1, 6, 106] and is further increased in the elderly [107, 108]. Recently, animal burn models of young and aged mice demonstrated an increase in the pulmonary neutrophils with an increase in their chemokine levels in aged mice compared with the young mice [109].
It is evident that the advanced age is the primary determinant of cardiovascular disease [110]. The cardiovascular system wanes progressively with aging and becomes more susceptible to stress, injury and burn trauma. Different studies corroborate independently that major burn can lead to age-associated compromised myocardial function due to diminished cardiac contractile response after burn [111–113]. Burn injury covering more than 45% TBSA can produce intrinsic contractile defects [2, 110].
Renal dysfunction is another age-dependent alteration that greatly impacts the elderly and contributes to the adverse clinical outcome of burn patients [114]. Acute kidney injury (AKI) is prevalent in severely burn patients and significantly contributes to early multiple organ failure and higher morbidity and mortality [114, 115]. However, while renal dysfunction is an age-associated setback and major burn may lead to acute kidney injury, there is no evidence for the correlation of AKI in the elderly burn patients. The severity of the renal dysfunction following burns correlates with aging and may be one of the major contributors to the higher morbidity and mortality rates in elderly burn patients. Therefore, it becomes even more meaningful to study this particular problem in aged burn patients.
Summary
Aging, after burn size and concomitant inhalation injury, is one of the major factors that affect the clinical outcome of burn patients. Immune dysfunction also plays an important role and burn in the elderly is associated with greater levels of inflammation. Inflammation also contributes to the post-burn hypermetabolic state. Natural age advancement impairs wound healing significantly and makes the aged burn patients more prone to infections and associated complications and worsens the clinical outcome. Moreover, age-associated co-morbidities such as, pulmonary, cardiovascular, renal dysfunction and diabetes in the burn patients are also good indicators for possible complications, but do not appear at this time to be predictive of mortality. Taken together, it is reasonable to conclude that there are significant differences in a wide range of factors with age that impact outcomes after burn.
Acknowledgments
This work was supported by the grant GM-79122 from the National Institute of Health (NIH). We thank Ms. Teresa Craig for her assistance in the preparation of this manuscript.
References
- [1].Enkhbaatar P. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clinical science (London, England : 1979) 2004;107(2):137–43. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- [2].Keck M, et al. Pathophysiology of burns. Wiener medizinische Wochenschrift. 2009;159:327–36. doi: 10.1007/s10354-009-0651-2. [DOI] [PubMed] [Google Scholar]
- [3].Santaniello JM, et al. Ten year experience of burn, trauma, and combined burn/trauma injuries comparing outcomes. The Journal of trauma. 2004;57:696–700. doi: 10.1097/01.ta.0000140480.50079.a8. [DOI] [PubMed] [Google Scholar]
- [4].Sheridan RL. Evaluating and managing burn wounds. Dermatology nursing / Dermatology Nurses’ Association. 2000;12(1):17–8. [PubMed] [Google Scholar]
- [5].Soejima K, et al. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. American journal of physiology. Lung cellular and molecular physiology. 2001;280:L1233–41. doi: 10.1152/ajplung.2001.280.6.L1233. [DOI] [PubMed] [Google Scholar]
- [6].Sterner JB. Inflammatory mediators in smoke inhalation injury. Inflammation & allergy drug targets. 2009;8:63–9. doi: 10.2174/187152809787582471. [DOI] [PubMed] [Google Scholar]
- [7].Scott JR, et al. Pediatric palm contact burns: a ten-year review. Journal of burn care & research : official publication of the American Burn Association. 2008;29:614–8. doi: 10.1097/BCR.0b013e31817db8f2. [DOI] [PubMed] [Google Scholar]
- [8].Pham TN, et al. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. Journal of burn care & research. 2009;30:30–6. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lundgren RS, et al. Influence of comorbidities and age on outcome following burn injury in older adults. Journal of burn care & research. 2009;30:307–14. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McGill V, Kowal-Vern A, Gamelli RL. Outcome for older burn patients. Archives of surgery. 2000;135(3):320–5. doi: 10.1001/archsurg.135.3.320. [DOI] [PubMed] [Google Scholar]
- [11].Church D, et al. Burn wound infections. Clinical microbiology reviews. 2006;19:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hunt JL, Purdue GF. The elderly burn patient. American journal of surgery. 1992;164:472–6. doi: 10.1016/s0002-9610(05)81183-3. [DOI] [PubMed] [Google Scholar]
- [13].2002. National Safe Kids Campaign: Burn Injury fact sheet.
- [14].Pruitt B. In: Epidemiological, demographic and outcome characteristics of burn injury, in Total Burn Care. Herndon DN, editor. Saunders WB; New York: 2007. pp. 14–32. [Google Scholar]
- [15].Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Experimental dermatology. 2010;19:777–83. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- [16].Keck M, et al. Burn treatment in the elderly Burns. journal of the International Society for Burn Injuries. 2009;35:1071–9. doi: 10.1016/j.burns.2009.03.004. [DOI] [PubMed] [Google Scholar]
- [17].Redlick F, et al. A survey of risk factors for burns in the elderly and prevention strategies. The Journal of burn care & rehabilitation. 2002;23:351–6. doi: 10.1097/00004630-200209000-00009. [DOI] [PubMed] [Google Scholar]
- [18].Johnson RM, Richard R. Partial-thickness burns: identification and management. Advances in skin & wound care. 2003;16:178–87. doi: 10.1097/00129334-200307000-00010. [DOI] [PubMed] [Google Scholar]
- [19].Gibran NS. Cutaneous wound healing. Journal of burn care & research : official publication of the American Burn Association. 2007;28:577–9. doi: 10.1097/BCR.0B013E318093E44C. [DOI] [PubMed] [Google Scholar]
- [20].Piccolo MT, et al. Chemotactic mediator requirements in lung injury following skin burns in rats. Experimental and molecular pathology. 1999;66:220–6. doi: 10.1006/exmp.1999.2263. [DOI] [PubMed] [Google Scholar]
- [21].Piccolo MT, et al. Role of chemotactic factors in neutrophil activation after thermal injury in rats. Inflammation. 1999;23:371–85. doi: 10.1023/a:1020213717336. [DOI] [PubMed] [Google Scholar]
- [22].Singh V. The pathogenesis of burn wound conversion. Annals of plastic surgery. 2007;59:109–15. doi: 10.1097/01.sap.0000252065.90759.e6. [DOI] [PubMed] [Google Scholar]
- [23].Moore FA, et al. Postinjury multiple organ failure: a bimodal phenomenon. The Journal of trauma. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- [24].Fitzwater J, et al. The risk factors and time course of sepsis and organ dysfunction after burn trauma. The Journal of trauma. 2003;54:959–66. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- [25].Nomellini V, et al. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Current immunology reviews. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rozing MP, et al. Familial longevity is associated with decreased thyroid function. The Journal of clinical endocrinology and metabolism. 2010;95:4979–84. doi: 10.1210/jc.2010-0875. [DOI] [PubMed] [Google Scholar]
- [28].Wijsman CA, et al. Familial longevity is marked by enhanced insulin sensitivity. Aging cell. 2011;10:114–21. doi: 10.1111/j.1474-9726.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- [29].Wagner GR, Payne RM. Mitochondrial acetylation and diseases of aging. Journal of aging research. 2011;2011:234875. doi: 10.4061/2011/234875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Afanas’ev I. Reactive oxygen species and age-related genes p66shc, Sirtuin, FOX03 and Klotho in senescence. Oxidative medicine and cellular longevity. 2010;3:77–85. doi: 10.4161/oxim.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transplant international. 2009;22:1041–50. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- [32].Jin K. Modern Biological Theories of Aging. Aging and Disease. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- [33].Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer letters. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [34].Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- [35].Kovacs EJ, et al. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends in immunology. 2009;30:319–24. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cournil A, Kirkwood TB. If you would live long, choose your parents well. Trends in genetics. TIG. 2001;17(5):233–5. doi: 10.1016/s0168-9525(01)02306-x. [DOI] [PubMed] [Google Scholar]
- [37].Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [38].Swift ME, et al. Age-related alterations in the inflammatory response to dermal injury. The Journal of investigative dermatology. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- [39].Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–20. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- [40].Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- [41].Pawelec G. Immunosenescence: impact in the young as well as the old? Mechanisms of ageing and development. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- [42].Burns EA, Goodwin JS. Immunodeficiency of aging. Drugs & aging. 1997;11:374–97. doi: 10.2165/00002512-199711050-00005. [DOI] [PubMed] [Google Scholar]
- [43].Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual review of medicine. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- [44].Pedersen M, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mechanisms of ageing and development. 2003;124:495–502. doi: 10.1016/s0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- [45].Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunology and allergy clinics of North America. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- [46].Bruunsgaard H, et al. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clinical and experimental immunology. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bruunsgaard H, et al. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. The American journal of medicine. 2003;115:278–83. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- [48].Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- [49].Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–9. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- [50].Caruso C, et al. Aging, longevity, inflammation, and cancer. Vol. 1028. Annals of the New York Academy of Sciences; 2004. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- [51].Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmunity reviews. 2006;5:136–9. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [52].Pereira CT, et al. Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. Journal of the American College of Surgeons. 2006;203:536–48. doi: 10.1016/j.jamcollsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [53].Lumenta DB, et al. Mortality and morbidity among elderly people with burns--evaluation of data on admission. Burns : journal of the International Society for Burn Injuries. 2008;34:965–74. doi: 10.1016/j.burns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [54].Le HQ, et al. Burns in patients under 2 and over 70 years of age. Annals of plastic surgery. 1986;17:39–44. doi: 10.1097/00000637-198607000-00008. [DOI] [PubMed] [Google Scholar]
- [55].Griffiths RW, Laing JE. A burn formula in clinical practice. Annals of the Royal College of Surgeons of England. 1981;63:50–3. [PMC free article] [PubMed] [Google Scholar]
- [56].National Burn Repository: American Burn Association. 2002 Report
- [57].Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Experimental gerontology. 2005;40:549–55. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [58].Kovacs E, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J Amer Aging Assoc. 2002;25:3–10. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kovacs EJ, Duffner LA, Plackett TP. Immunosuppression after injury in aged mice is associated with a TH1-TH2 shift, which can be restored by estrogen treatment. Mechanisms of ageing and development. 2004;125:121–3. doi: 10.1016/j.mad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [60].Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266–74. doi: 10.1006/cyto.2001.1003. [DOI] [PubMed] [Google Scholar]
- [61].Plackett TP, et al. Aging enhances lymphocyte cytokine defects after injury. The FASEB journal. 2003;17:688–9. doi: 10.1096/fj.02-0452fje. [DOI] [PubMed] [Google Scholar]
- [62].Wolf SE, et al. Insulin-like growth factor-I/insulin-like growth factor binding protein-3 alters lymphocyte responsiveness following severe burn. The Journal of surgical research. 2004;117:255–61. doi: 10.1016/S0022-4804(03)00305-6. [DOI] [PubMed] [Google Scholar]
- [63].Jeschke MG, et al. Burn size determines the inflammatory and hypermetabolic response. Critical care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jeschke MG, et al. Pathophysiologic response to severe burn injury. Annals of surgery. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Finnerty CC, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Molecular medicine. 2008;14:553–60. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Finnerty CC, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- [67].Finnerty CC, et al. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–5. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gore DC, et al. Association of hyperglycemia with increased mortality after severe burn injury. The Journal of trauma. 2001;51:540–4. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- [69].Dahagam CK, et al. Diabetes does not influence selected clinical outcomes in critically ill burn patients. Journal of burn care & research. 2011;32:256–62. doi: 10.1097/BCR.0b013e31820aaf68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Maghsoudi H, Aghamohammadzadeh N, Khalili N. Burns in diabetic patients. International journal of diabetes in developing countries. 2008;28:19–25. doi: 10.4103/0973-3930.41982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Deng HP, Chai J. The effects and mechanisms of insulin on systemic inflammatory response and immune cells in severe trauma, burn injury, and sepsis. International immunopharmacology. 2009;9:1251–9. doi: 10.1016/j.intimp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- [72].Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Quirinia A, Viidik A. The influence of age on the healing of normal and ischemic incisional skin wounds. Mechanisms of ageing and development. 1991;58:221–32. doi: 10.1016/0047-6374(91)90094-g. [DOI] [PubMed] [Google Scholar]
- [74].Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clinics in dermatology. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- [75].Gosain A, DiPietro LA. Aging and wound healing. World journal of surgery. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- [76].Karimipour DJ, et al. Molecular analysis of aggressive microdermabrasion in photoaged skin. Archives of dermatology. 2009;145:1114–22. doi: 10.1001/archdermatol.2009.231. [DOI] [PubMed] [Google Scholar]
- [77].Park JE. Understanding the role of immune regulation in wound healing. American journal of surgery. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- [78].Jiang J, Fisher EM, Murasko EM. CD8 T cell responses to influenza virus infection in aged mice. Ageing research reviews. 2011 doi: 10.1016/j.arr.2011.02.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Alonso-Fernandez P, De la Fuente M. Role of the Immune System in Aging and Longevity. Current aging science. 2011 doi: 10.2174/1874609811104020078. in press. [DOI] [PubMed] [Google Scholar]
- [80].Sorensen LT. Effect of lifestyle, gender and age on collagen formation and degradation. Hernia : the journal of hernias and abdominal wall surgery. 2006;10:456–61. doi: 10.1007/s10029-006-0143-x. [DOI] [PubMed] [Google Scholar]
- [81].Chang E, et al. Aging and survival of cutaneous microvasculature. The Journal of investigative dermatology. 2002;118:752–8. doi: 10.1046/j.1523-1747.2002.01714.x. [DOI] [PubMed] [Google Scholar]
- [82].Gniadecka M. Effects of ageing on dermal echogenicity. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2001;7:204–7. doi: 10.1034/j.1600-0846.2001.70310.x. [DOI] [PubMed] [Google Scholar]
- [83].Gniadecka M, Serup J, Sondergaard J. Age-related diurnal changes of dermal oedema: evaluation by high-frequency ultrasound. The British journal of dermatology. 1994;131:849–55. doi: 10.1111/j.1365-2133.1994.tb08588.x. [DOI] [PubMed] [Google Scholar]
- [84].Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy/wound management. 2007;53:16–31. [PubMed] [Google Scholar]
- [85].Doria G, Frasca D. Regulation of cytokine production in aging mice. Annals of the New York Academy of Sciences. 1994;741:299–304. doi: 10.1111/j.1749-6632.1994.tb23113.x. [DOI] [PubMed] [Google Scholar]
- [86].Grigorova-Borsos AM, et al. Aging and diabetes increase the aggregating potency of rat skin collagen towards normal platelets. Thrombosis and haemostasis. 1988;60:75–8. [PubMed] [Google Scholar]
- [87].Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2018–20. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Laboratory investigation; a journal of technical methods and pathology. 1999;79:1479–87. [PubMed] [Google Scholar]
- [89].Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mechanisms of ageing and development. 2001;122:1203–20. doi: 10.1016/s0047-6374(01)00260-3. [DOI] [PubMed] [Google Scholar]
- [90].Zhang X, et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. Journal of molecular medicine. 2011 doi: 10.1007/s00109-011-0754-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vascular and endovascular surgery. 2005;2005;39(4):293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- [92].Ryan T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron. 2004;35:161–71. doi: 10.1016/j.micron.2003.11.010. [DOI] [PubMed] [Google Scholar]
- [93].Strigini L, Ryan T. Wound healing in elderly human skin. Clinics in dermatology. 1996;14:197–206. doi: 10.1016/0738-081x(95)00155-9. [DOI] [PubMed] [Google Scholar]
- [94].Holt DR, et al. Effect of age on wound healing in healthy human beings. Surgery. 1992;112:293–7. [PubMed] [Google Scholar]
- [95].Evans J. Massive tissue loss: burns, in Acute and chronic wounds: Current management concepts, N.D. Bryant RA. Mosby Elsevier; 2007. pp. 361–390. [Google Scholar]
- [96].Greenhalgh DG, et al. American Burn Association consensus conference to define sepsis and infection in burns. Journal of burn care & research. 2007;28:776–90. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- [97].D’Avignon LC, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns : journal of the International Society for Burn Injuries. 2010;36:773–9. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [98].Murray CK, et al. Incidence of systemic fungal infection and related mortality following severe burns. Burns : journal of the International Society for Burn Injuries. 2008;34:1108–12. doi: 10.1016/j.burns.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [99].Edwards-Jones V, Greenwood JE. What’s new in burn microbiology? James Laing Memorial Prize Essay 2000 Burns : journal of the International Society for Burn Injuries. 2003;29:15–24. doi: 10.1016/s0305-4179(02)00203-6. [DOI] [PubMed] [Google Scholar]
- [100].Castle SC. Impact of age-related immune dysfunction on risk of infections. Zeitschrift fur Gerontologie und Geriatrie: Organ der Deutschen Gesellschaft fur Gerontologie und Geriatrie. 2000;33:341–9. doi: 10.1007/s003910070030. [DOI] [PubMed] [Google Scholar]
- [101].Orme I. Mechanisms underlying the increased susceptibility of aged mice to tuberculosis. Nutrition reviews. 1995;53:S35–8. doi: 10.1111/j.1753-4887.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- [102].Butler JC, et al. Effectiveness of pneumococcal vaccine. Lancet. 1998;1351:1961. doi: 10.1016/S0140-6736(05)78647-5. [DOI] [PubMed] [Google Scholar]
- [103].Saito H, et al. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mechanisms of ageing and development. 2003;124:1047–58. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [104].Castle SC, et al. Host resistance and immune responses in advanced age. Clinics in geriatric medicine. 2007;23:463–79. doi: 10.1016/j.cger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hollingsed TC, et al. Etiology and consequences of respiratory failure in thermally injured patients. American journal of surgery. 1993;166:592–6. doi: 10.1016/s0002-9610(05)80662-2. [DOI] [PubMed] [Google Scholar]
- [106].Mizutani A. Pulmonary changes in a mouse model of combined burn and smoke inhalation-induced injury. Journal of applied physiology. 2008;105:678–84. doi: 10.1152/japplphysiol.00232.2007. [DOI] [PubMed] [Google Scholar]
- [107].Clayton MC, Solem LD, Ahrenholz DH. Pulmonary failure in geriatric patients with burns: the need for a diagnosis-related group modifier. The Journal of burn care & rehabilitation. 1995;16:451–4. doi: 10.1097/00004630-199507000-00013. [DOI] [PubMed] [Google Scholar]
- [108].Ely EW, et al. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Annals of internal medicine. 2002;136:25–36. [PubMed] [Google Scholar]
- [109].Nomellini V, et al. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. Journal of leukocyte biology. 2008;83:1493–501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Forman DE, et al. Cardiac care for older adults time for a new paradigm. Journal of the American College of Cardiology. 2011;57:1801–10. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Horton JW, et al. Cardiac contractile and calcium transport function after burn injury in adult and aged guinea pigs. The Journal of surgical research. 1993;55:87–96. doi: 10.1006/jsre.1993.1113. [DOI] [PubMed] [Google Scholar]
- [112].Horton JW, White DJ. Diminished cardiac contractile response to burn injury in aged guinea pigs. The Journal of trauma. 1993;34:429–36. doi: 10.1097/00005373-199303000-00021. [DOI] [PubMed] [Google Scholar]
- [113].Adams HR, Baxter CR, Izenberg SD. Decreased contractility and compliance of the left ventricle as complications of thermal trauma. American heart journal. 1984;108:1477–87. doi: 10.1016/0002-8703(84)90695-1. [DOI] [PubMed] [Google Scholar]
- [114].Brusselaers N, et al. Outcome of acute kidney injury in severe burns: a systematic review and meta-analysis. Intensive care medicine. 2010;36:915–25. doi: 10.1007/s00134-010-1861-1. [DOI] [PubMed] [Google Scholar]
- [115].Mosier MJ, et al. Early acute kidney injury predicts progressive renal dysfunction and higher mortality in severely burned adults. Journal of burn care & research : official publication of the American Burn Association. 2010;31:83–92. doi: 10.1097/BCR.0b013e3181cb8c87. [DOI] [PMC free article] [PubMed] [Google Scholar]


