Abstract
Aging is associated with reorganization of brain in both structure and function. In recent years, graph theoretical analysis of brain organization has drawn increasing attention, and reorganization of brain in aging has been investigated in terms of connectivity and networks in topology such as modular organization, global and local efficiency, and small-worldness. Beyond studying on abnormity in local brain regions, connectivity quantifies alternations of correlation between two regions that may be spatially far separated, and graph theoretical analysis of brain network examines the complex interactions among multiple regions. This article reviewed complex brain networks of human in normal aging or with age-related diseases such as stroke and Alzheimer’s disease after a technical introduction of brain networks and graph theoretical analysis. We further discussed the relationship between the functional and the structural brain networks of subjects in aging or with age-related diseases. Finally, we proposed several interesting topics for future research in this field.
Keywords: Aging, Brain network, Connectivity, Functional, Neuroimaging, Small-world, Structural
Brain is organized with two fundamental principles, i.e., segregation and integration [1]. According to these two principles, the connectivity in local regions is important for specialized functions, the global interactions among multiple spatially separated regions play important roles in advance cognitive function. Brain connectivity has been extensively investigated at various temporal and spatial scales for decades, providing new insights of human brain and evidences and implications for neurological diseases [2]. Since the introduction of small-world network and scale-free network [3, 4], investigation on human brain has gone beyond the scope of pair-wise relation between regions, and therefore advanced into the concept of networks [5, 6].
The word “network” in literature has different meanings. In graph theory and complex networks, “network” explicitly implies a set of nodes and pair-wise edges that connect the nodes. Graph theoretical analysis of brain networks refers to this sense. In neuroimaging field, “network” may denote: 1) a group of voxels or regions of interest (ROIs), which have similar activity at resting state or under certain cognitive task [7, 8]; 2) the component map derived by independent component analysis or principal component analysis [8–10]; or 3) the seed correlation map formed with voxels, which have high correlation with the time course of a selected seed ROI [8, 11]. Both component map and seed correlation map could demonstrate spatial features of correlated fMRI voxels, but they also have inherent limitations. For the method of component map, voxels within component map only share variance to some extent, and the meaning of each component map and the interactions among the component maps of the same subject are not explicit. In addition, comparison of component maps from multiple cohorts (e.g., adults and elderly subjects) would be much computationally complicated and with no explicit meaning. For the method of seed correlation map, a map should be generated for each selected seed region, and a single or several seed maps cannot give a full view of all interactions among ROIs. In the following content, we mainly review brain networks with graph theoretical analysis, and “network” therefore mainly refers to a collection of nodes and edges.
Clustering coefficient and characteristic path length are two basic metrics of network. Clustering coefficient measures how densely nodes in local regions connected to each other, and can be considered as an index of segregation. Characteristic path length generally quantifies how long one node is connected to other nodes in topology, that is, the average number of the edges in the shortest paths that connect nodes over the whole networks, and could reflect the integration among multiple brain regions [3, 5]. Compared to matched random networks, the network with small characteristic path length and large clustering coefficient is called small-world network [3]. Various studies have reported that both structural and functional brain networks are small-world networks [5, 6]. Small-world brain network is an optimal organization in the aspect of information transmission in both local and global efficiency [5, 12]. For example, graph theoretical analysis to structural brain networks of healthy young adults showed that high intelligence group has higher global efficiency than the general intelligence group [13]. In addition, the network measures have significant correlations with Intelligence quotient scores across all subjects. High global efficiency of structural brain network may imply that parallel information transfers more efficiently over brain network, and thus the corresponding subjects are with high intelligence [13].
Brain Network Construction
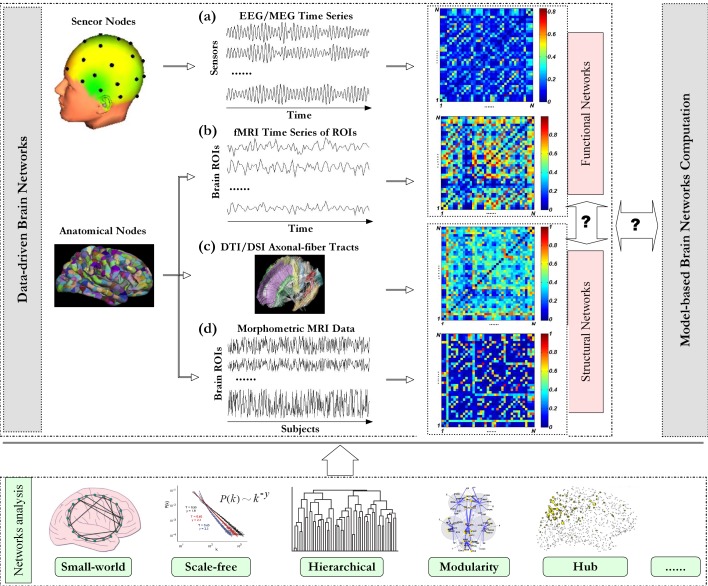
Nodes of neural networks can be defined in multiple scales: single neuron (micro-scale), neuronal ensembles (meso-scale), sub-cortex (macro-scale), regions of interest (ROIs), and edges are defined as functional or structural connectivity in brain units [5, 6]. There are two ways to define nodes for brain network at macro-scale: 1) brain regions where sensors located are defined as nodes; 2) the whole brain cortex is divided into a set of ROIs according to predefined atlas templates and each ROI is defined as a node (Figure 1) [6, 13–15]. There are three different types of brain networks, i.e., functional brain network, effective brain network, and structural brain network, which are constructed with different types of connectivity.
Figure 1.
Schematic paradigm of graph theoretical analysis of brain networks. Investigation on brain networks includes data-driven brain network analysis [5, 6] and model-based brain network computation [42, 43]. With neuroimaging data, different types of data-driven brain networks can be constructed. (a) For EEG/MEG, multichannel time series are measured with an array of sensors, and the scalp region where each sensor located is defined as one node respectively. Pair-wise association between nodes can be quantified with time series at the corresponding locations [44]. For fMRI and DTI/DSI data, regions of interesting (ROIs) are first defined with predefined anatomical templates [6, 14]. (b) The representative fMRI time series of each ROI is extracted from all the voxels in that ROI via a certain way (e.g., by averaging). Pair-wise association between ROIs can be estimated from the representative time series. (c) For DTI/DSI data, pair-wise association can be traced with axonal-fiber tracts [29]. (d) Morphometric variables can be estimated for each ROI from individual structural MRI images, and then multivariate morphometric (e.g., cortical thickness) time series over subjects can be obtained. Pair-wise association between these multivariate time series can be used as a measure of anatomical connectivity [18]. With association matrix constituted by connectivity in all possible node pairs, graph theoretical analysis can be performed to complex brain network. This figure is organized with our data and others [16, 21, 45–47].
Functional brain networks can be constructed with electroencephalography (EEG), magnetoencephalography (MEG), or functional magnetic resonance imaging (fMRI) data measured at resting state or under specific cognitive task. There are two different ways to construct functional brain network: 1) the first way is based on multichannel EEG/MEG, where pair-wise association between the time series measured by sensors is considered as the respective connectivity (Figure 1a); 2) the second way is based on fMRI, where a representative time series for each ROI defined according to atlas templates is extracted via a certain way, and the association between the representative time series is defined as the connectivity of that node pair (Figure 1b). Association matrix can be constituted by associations of all possible node pairs. With a selected threshold, association matrix could be converted into a binary adjacent matrix by setting the cells to be 1 when their association strength is bigger than the threshold or otherwise 0. With adjacent matrix, various measures developed in complex networks can be applied to investigate the properties of brain network such as small-worldness [3, 16], scale-free degree distribution [16], hubs [17], modularity [18, 19], and hierarchical structure [20, 21]. Association strength can be set as the weight of corresponding edge, and then brain network can be investigated in the aspect of weighted complex network [22].
Effective brain network is constructed with effective connectivity, which reveals not only association strength but also influence direction in nodes, that is, how one node exerts influence to the other node. Various methods such as Granger causality [23–25], structural equation modeling [26], dynamic causal modeling [27] have been proposed to infer effective connectivity. Since effective connectivity is estimated from functional brain signals such as EEG, MEG, and fMRI signals, effective brain network can be considered as a specific case of functional brain network with directed edges [5, 6]. At present, studies of brain networks are mainly based on binary or weighted networks and less directed networks. This may be due to well established tools for undirected binary/weighted networks while tools for directed networks are still under development.
Generally, there are two ways to construct structural brain network. The first type of structural brain network is based on diffusion tensor imaging (DTI) or diffusion spectrum imaging (DSI). Structural connectivity is determined by axonal fiber tracts or probabilistic tractography in ROIs [28–31]. Axonal-fiber tracts are traced by the deterministic “streamline” tractography which could infer the continuity of axonal fiber bundles in brain units (Figure 1c) [29]. In contrast, the probabilistic tractography computes the connectivity probabilities in brain units [30]. The second type of structural brain network is based on structural MRI.
Interregional co-variation of morphometric measurements across subjects is taken as structural connectivity (Figure 1d) [20, 32]. The rationale behind is that mutually trophic effects on the development of connected brain regions could result in strong correlations in morphometric measurements of those regions [33]. In addition, connectivity suggested by interregional co-variation of gray matter in structural MRI is highly consistent with that revealed by tractographic analysis of DTI or DWI [34]. Note that for the first type of structural brain network, one network is constructed for each subject; while for the second type of network, only one structural network is generated for a group of subjects.
With increasing average age of human, brain aging and age-related diseases are drawing increasing attention from basic research, clinical research, and rehabilitation engineering. Various modern biological theories of aging have been proposed, and can be classified into two main categories, i.e., programmed theories and error theories. However, none of these theories could fully explain the mechanisms of aging, and no consensus on this issue has been reached yet [35, 36]. These theories may work together to reveal the mysteries of aging. In this paper, we mainly review the studies on brain networks of aging subjects or patients with age-related diseases. More information on methodology aspects of complex networks can be found in [37–39], and general topics of brain networks have been reviewed in [5, 6, 40, 41].
Development of Human Brain Networks
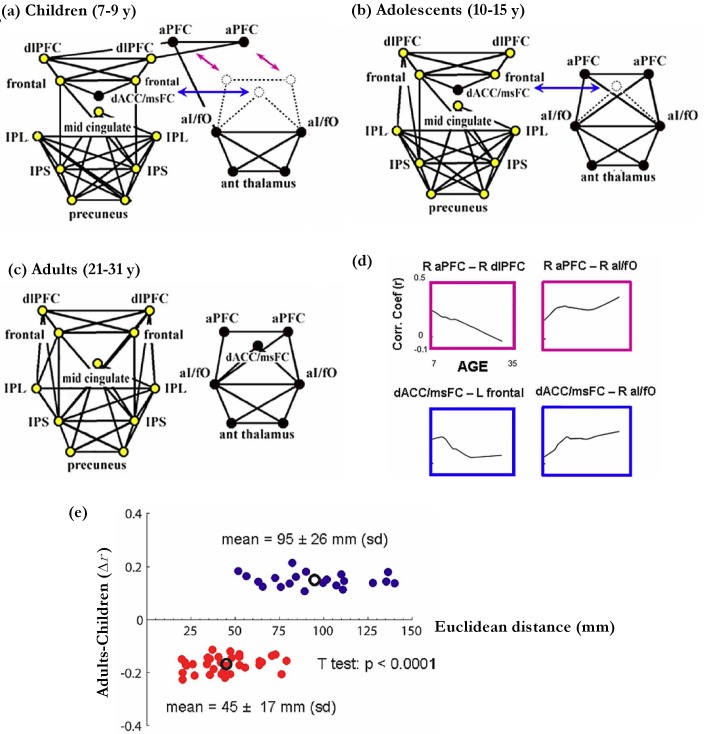
Children and adults differ both in behavior and brain networks during perform tasks [48, 49]. Recently, the development of task control network, constituted with 39 ROIs, was investigated with resting state fMRI measured from subjects of different ages [49]. It has been reported that the task control network can be divided into two distinct networks with different functions. One is labeled as fronto-parietal (FP) network, which plays an important role in initiating and adjusting control on a rapid time scale. The other is termed as cingulo-opercular (CO) network, which acts to maintain brain state in cognitive tasks over a longer time scale (Figures 2 and 4) [49–52]. For Children, the task control network was a connected network with the node of dorsal anterior cingulate/medial superior frontal cortex (dACC/msFC) embedded within the FP network and the node of bilateral anterior prefrontal cortex (aPFC) linking the FP and the CO networks (Figure 2a). For adolescents, as the link between FP and CO decreased, the 39 ROIs organized as two separate networks with the dACC/msFC node still in FP (Figure 2b). For adults, the 39 ROIs appeared as two distinct networks with the dACC/msFC node shifted into CO (Figure 2c). The connectivity strength of aPFC-dlPFC and dACC/msFC-frontal decreased, while the connectivity strength of aPFC-aI/fO and dACC/msFC-aI/fO increased during brain development (Figure 2d). In addition, the strength of connectivity linking regions with short spatial distance was larger in children and tended to decrease over development. While strength of connectivity with long spatial distance was smaller in children and tended to increase over development (Figure 2e). This trend of connectivity strength is consistent with results reported by others [53, 54].
Figure 2.
Development of task control networks. (a) 7–9 year-Children, (b) 10–15 year-adolescents, and (c) 21–31 year-adults. In each group, only the 75 strongest correlations were chosen for network analysis. The correlation coefficients were combined across matched subjects with the Schmidt-Hunter method [55, 56]. The nodes in the fronto-parietal and the cingulo-opercular networks are colored in yellow and black, respectively. IPS: intraparietal sulcus; dF: dorsal frontal; IPL: inferior parietal lobule; dlPFC: dorsolateral prefrontal cortex; aI/fO: anterior insula/frontal operculum; dACC/msFC: dorsal anterior cingulate cortex/medial superior frontal cortex; aPFC: anterior prefrontal cortex; aT: anterior thalamus; TPJ: temporoparietal junction; vmPFC: ventromedial prefrontal cortex. (d) The fit LOWESS curves of connectivity strength versus age. (e) The significant changes (Δr) of connectivity strength between the adults group and the children group are plotted with respect to the Euclidean distance (millimeters) in the corresponding ROIs. The connectivity with significantly increased/decreased strength across development is displayed in blue/red dots respectively. The mean of strength differences and Euclidean distance for each group are plotted as black circles. Adapted from Fair and et al. [49].
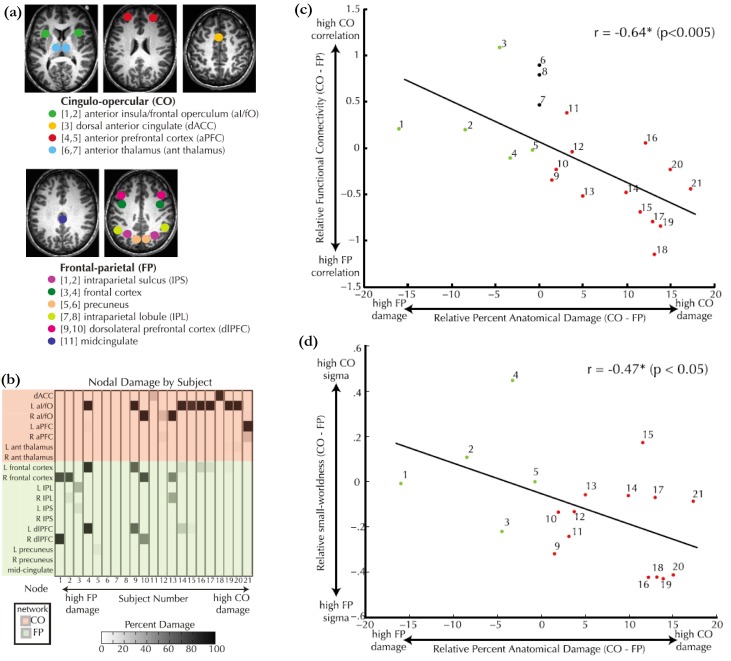
Figure 4.
Alternation of dual cognitive control networks with focal brain lesions. (a) The selected ROIs in the FP and the CO networks [50, 51]. (b) The damaged ROIs of each patient. Subjects 5–8 had no lesions in both the FP and the CO networks but had lesions elsewhere. (c) Relative functional connectivity (i.e., the difference of average connectivity strength between the CO and the FP networks) versus relative anatomical lesion in the CO and the FP networks. (d) Relative small-worldness of within-ROI networks (i.e., the difference of the average within-ROI small-worldness between the CO and the FP networks) versus relative anatomical lesion in the CO and the FP networks. Adapted from Nomura and et al. [52].
Brain Networks of Normal Aging Subjects
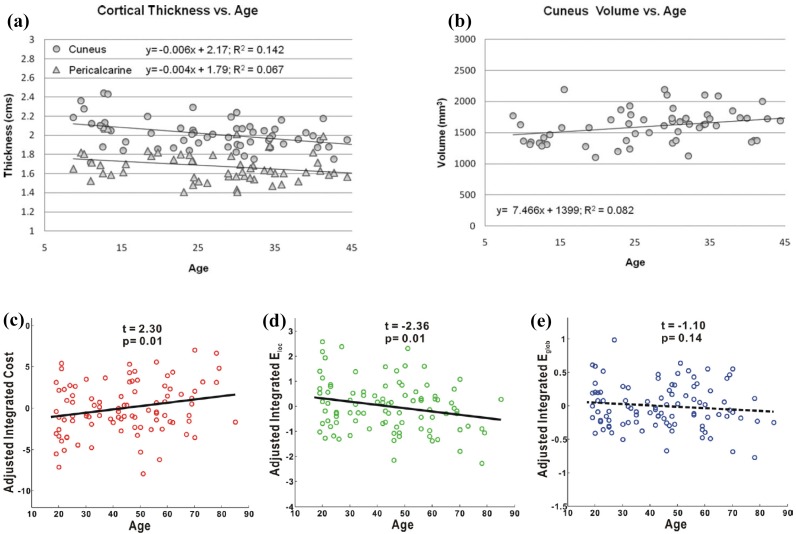
During brain development and aging, both gray and white matter changes in structure, and function [57]. Human brain reaches maximum weight at the age of 20 years, and begins to decline at the age of 55 years [58], while white matter volume increases until the mid-40s [59]. Comparison with healthy adults and children showes that age demonstrates negative correlations with cortical thickness of the cuneus and pericalcarine areas, and positive correlation with cuneus volume (Figures 3a and 3b) [60]. To investigate the mechanisms of brain aging, many age-related studies on both functional [7, 19, 61] and structural [18, 31, 60, 62, 63] brain networks have been performed from various aspects, including regional connectivity [64], modular organization [18, 19], global and local efficiency [61, 62, 65, 66], and etc. [8, 11, 67–74].
Figure 3.
Correlation of aging with respect morphometric variables and network metrics of brain. (a) Correlations between age and cortical thickness of cuneus (p<0.005) and pericalcarine (p= 0.06). (b) Correlation between age and cuneus volume (p <0.05). (c) Correlation between integrated network cost and age. (d) Correlation between integrated local efficiency and age. (e) Correlation between integrated global efficiency and age. In (c), (d), and (e), the results were obtained from structural brain networks, in which, the integrals of network metrics over the range of sparsity* 8–27% were used as accumulative metrics for network topology. In each subfigures, one symbol (circle or triangle) denotes the result of one subject. (a) and (b) are adapted from Gaetz and et al. [60], and (c), (d), and (e) are adapted from Gong and et al. [31]. *Sparsity is defined as the ratio between the number of existed edges and the number of all possible edges that fully connect all nodes.
The large-scale brain networks of subjects in normal aging have been examined from various aspects [31, 61, 62, 66]. In a study of normal subjects with diffusion MRI, the connectivity between 78 ROIs was quantified by probabilistic tractography [31]. Graph theoretical analysis to these networks showed that the structural brain networks have small-world topology over the sparsity range of 8–27%. Age showed significant positive correlation to the integrated cost (Figure 3c) but significant negative correlation to the integrated local efficiency (Figure 3d), while showed no significant correlation to the integrated global efficiency (Figure 3e) [31]. These results imply that structural brain networks become less economical in aging. The less optimal organization of structural brain network, as well as degeneration of white matter in aging brains [75, 76], may impair brain function. Similar small-world characteristic has also been observed in other studies on both structural and functional brain networks of aging subjects [61, 62, 66]. In another study of structural brain networks, structural MRI (T1-weighted) of 1483 normal aging subjects was collected. These data were assigned to the young adult group (18–50 years), the middle age adults (41–60 years), and the old age group (61–80 years), and 350 subjects were random selected from them for each group. One structural brain network was constructed for each group with the Pearson correlation coefficient between gray matter volumes of 90 ROIs [14] across 350 subjects in that group. Structural brain networks of all three age groups demonstrated small-world properties. In particular, the integrated local efficiency of the middle age group was smaller than those of both the young age group and the old age group. In contrast, the integrated global efficiency showed a inverse alternation trend of integrated local efficiency with respect to three groups [62]. Changes of functional brain networks with respect to age have been examined with fMRI measured from younger and older subjects during memory encoding and recognition. Results showed that the older adults had longer characteristic path length compared with the younger adults. In addition, the older adults showed reduced regional centrality in frontal areas, but the younger subjects demonstrated increased regional centrality in several default-mode regions [61].
Modular organization of brain networks also changes during aging [18, 19, 62, 77]. In a study with structural MRI of healthy young adults and normal older adults, structural brain networks were constructed with 78 ROIs and the absolute Pearson correlation coefficients between regional cortical thicknesses across subjects [18]. Graph theoretical analysis revealed that the structural networks of two cohorts possessed modularity at the executive and auditory/language processing regions. But compared to young adults, the older subjects exhibited reduction of modularity and decrease of intra-/inter-module connectivity related to both executive and default mode networks. These results may imply that age-related alternations in large-scale structural brain networks are the substrates of degeneration of brain cognitive function [18]. Changes of modularity in functional brain networks have also been reported. Functional brain networks of healthy young and older subjects have been compared with fMRI [19]. Both young and older subjects showed significantly non-random modularity, with reduced inter-modular connections to frontal modular regions and increased number of connector nodes in posterior and central modules. The brain networks of two groups showed no significant difference in maximal modularity, which implied that modular organization was retained but with changes in the composition and topological roles of modules. It has been argued that modular organization had “the advantage of allowing evolutionary or developmental adaptation”, with no too much risk of function loss in one module while other modules were damaged [19, 78].
In summary, during development and normal aging, brain experiences changes in both structure and fucntion. The organization of structural brain network demonstrates alternations in both global and local efficiency. Correspondingly, functional connectivity and functional network also change. The strength of connectivity linking regions of short/long Euclidean distance decreases/increases respectively during development. And modular organization or sub-networks show different patterns during development and aging. Generally, the local efficiency of structural brain networks decreases during development, and the global efficiency of both structural and fucntional brain networks decreases in aging. But some non-consistent results of brain networks in aging have also been reported, and further studies are needed.
Brain Networks of Stroke Patients
In previous section, we reviewed changes of the FP and the CO networks over brain development. Here, we provided strong evidence for the existence of the functionally dissociable FP and CO networks by a study on functional brain networks of patients with focal brain lesions and healthy subjects (Figure 4) [52]. The strength of connectivity within the FP and within the CO networks was significantly higher than the strength of connectivity between the FP and the CO networks for both patients and healthy controls. The relative strength of connectivity within FP or CO was negatively correlated with the degree of damage in that network (Figure 4c). The damage in one network of FP or CO only affected the connectivity within itself and not influenced the connectivity within the other undamaged one. The local changes due to focal lesions were examined by analyzing within-ROI network, which was constructed with voxels within ROI as nodes and the correlation between the voxels as connectivity. The relative average small-worldness of these within-ROI networks was negatively correlated with the amount of within network damage, but showed no significant correlation to the amount of damage in the other network (Figure 4d). With these results, it was concluded that “anatomical damage to one network has a specific detrimental effect on the remaining undamaged nodes in that network but no effect on the other network” [52].
Plasticity, the changes of synaptic connection induced by stimulus or alteration of synaptic activity, is the main mechanism for brain function recovery after stroke [79]. Therefore, revealing how to engage optimal reorganization of surviving neural networks will provide encouraging treatment strategies for function recovery after stroke. A mass of studies have provided evidence that abnormality of connectivity between local or remote cortical regions were correlated with function recovery after stroke [80–84]. For examples, study with resting state fMRI of stroke patients showed that the strength of inter-hemispheric functional connectivity was significantly correlated with patients’ performance in detection of visual stimuli, while alternations of intra-hemispheric connectivity were not correlated with patients’ motor performance [81]. Another study based on positron emission tomography examined the relationship between functional connectivity and language performance of patients with aphasic stroke, and showed that preservation of inter-hemispheric connectivity was associated with better performance in language tasks [82]. Stroke patients with neglect have also been examined, and results showed that the strength of functional connectivity between the frontal and parietal cortices was positively correlated with degree of neglect [84]. Alternations of effective connectivity for stroke patients have been reported extensively as well [85–87]. More information on reorganization of connectivity of stroke patients can be found in reference [80].
Recently, a study on reorganization of functional brain network of stroke patients with motor deficits gave new insights for brain network plasticity after stroke. Longitudinal fMRI were measured at five time points from 1 week to 1 year after stroke [22]. The motor execution network of each subject was constructed with 21 selected ROIs for each follow-up time point. Graph theoretical analysis showed that clustering coefficient significantly decreased, whereas the characteristic path length showed no significant change during the recovery process after stroke. This implied that the motor execution network became less segregated in function. The topology of brain networks shifted towards a random mode during function recovery, which may be due to random outgrowth of new connections [88]. In addition, connectivity with gradually increase in strength over time was mainly between ipsilateral primary cortex and contralateral key motor areas, while connectivity with gradually decrease in strength mainly between ipsilateral subcortical areas and cerebellum. Correlation analysis showed that clustering coefficient and connectivity between some ROIs were significantly correlated with different clinical scores of patients, which may be used to predict function recovery after stroke [89]. Studies of the dynamics of brain networks after stroke are rare. More research based on longitudinal fMRI or EEG/MEG data are needed to provide more evidences and implications for treament of stroke patients.
Brain Networks of Patients with Alzheimer’s Disease
Alzheimer’s disease (AD) is age-related disease. Most studies on AD examined atrophy or other abnormality in isolated brain regions using structural MRI, but neglected the fact that brain not only functioned in local cortex (i.e., segregation) but also as an integrated network of multiple spatially separated regions. Therefore, investigation on the connectivity or network abnormality may give new insights on functional impairment of AD patients [7, 8, 11, 17, 73, 90–96].
AD patients and normal controls have been compared via structural MRI analysis [97]. Structural brain networks were constructed by co-variation of regional cortical thickness across subjects. Compared to the controls, AD patients showed abnormal small-world architecture and altered nodal centrality in some regions [97]. In another study, structural brain networks of AD patients and healthy controls were constructed by DTI tractography, and graph theoretical analysis revealed that both structural brain networks of AD patients and controls had a small-world topology. Compared with control subjects, AD patients had larger shortest path length, reduced nodal efficiency in the frontal regions. In addition, several network metrics were significantly correlated with the memory-related performance scores of AD patients [89].
The alternations in functional brain network of AD patients have been reported as well [98, 99]. AD patients were compared to healthy control subjects based on functional brain networks constructed with pair-wise synchronization likelihood of beta waves of multichannel EEG signals. Graph theoretical analysis demonstrated that AD patients had a significant longer characteristic path length than control subjects over a wide range of thresholds [98], which is consistent with that revealed by the study on structural brain networks of AD patients [89]. However, another study on functional brain networks of AD patients demonstrated different results [99]. In that study, functional brain networks of mild AD patients and healthy control subjects were constructed with resting state fMRI. Results showed that characteristic path length of AD patients was significantly smaller than that of healthy controls for a wide range of sparsity. The topology of AD patient networks shifted towards the matched random networks, while cluster coefficient of AD patients demonstrated no significant differences from control subjects [99]. These results may imply that alternations in topology of networks are related to functional impairment of AD patients. However, further investigation is still needed to get more reliable and consistent results for clinical applications.
Brain networks of other neuropsychiatric disorders such as schizophrenia [20, 100, 101], epilepsy [102], depression [103, 104], and mild cognitive impairment [105] have also been investigated by graph theoretical analysis. The topology of functional brain network shifted towards matched random networks has been observed in patients with other brain related diseases including brain tumors [106] and traumatic brain injury [107]. Alternations of brain nentworks in spectific aspects such as modular organization, and global and local efficciency may be different for different brain related diseases. Thus, we should take brain related diseases case by case, and examine their specific organization of brain networks, so as to reveal network-based biomakers which are consistent across subjects with the same disease, but specific and sensitive across different brain related diseases.
Discussion and Future Direction
In summary, both functional and structural brain networks were small-world networks, and alternations occurred in brain networks of subjects during aging or with age-related diseases. In particular, organization of brain networks during aging or with age-related diseases shifted towards that of matched random networks in topology, and showed changes in modular organization and regional centrality at specific brain regions. In addition, some changes in brain network organization have been demonstrated to be significantly correlated to clinical scores of age-related diseases, which implies that metrics of brain networks are with potential to be used as biomarkers.
We believe that several topics on brain networks of subjects in aging or with age-related diseases are needed further exploration as follows:
Relationship between functional and structural brain networks at multiple temporal and spatial scales. As introduced previously, almost all studies on age-related brain networks were based on one single modality of neuroimaging, and only one type of functional or structural brain networks were investigated. Presently, the relationship between functional and structural brain networks is mainly compared with results obtained from different aging cohorts. Therefore, direct comparison of functional and structural brain networks with multiple modality neuroimaging of same subjects may provide new information on brain networks in aging. Study has demonstrated varying relationship between functional and structural brain networks at multiple time scales [108]. So exploring the relationship between functional and structural brain networks at multiple temporal and spatial scales may reveal new insights.
Joint study of brain networks by data-driven brain network analysis and model-based network computation. There are two different strategies in brain network investigation (Figure 1). One is data-driven brain network analysis, and the other is model-based network computation. Presently, only few joint studies with these two strategies have been reported [42, 43, 109]. Model-based network computation can help to explore the mechanisms of neural networks at multiple scales where data-driven brain network analysis cannot treat with. New insights are expected to be revealed if real neuroimaging is taken into consideration of model-based network computation. However, no joint study of brain networks in aging has been reported yet.
Network-based biomarkers for normal aging or age-related diseases. Some alternations of connectivity or brain network metrics have been demonstrated to be significantly correlated to clinical scores of age-related diseases [89], which implies that specific metrics of brain networks may be used as biomarkers, but more studies are needed before they can be applied clinically.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 60901025), National Basic Research Program of China (973 Program) (No. 2011CB013304), and Med-X Research Fund of Shanghai Jiao Tong University (No. YG2010MS86).
References
- [1].Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci U S A. 1994;91(11):5033–7. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- [3].Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- [4].Barabasi AL, Albert R. Emergence of scaling in random networks. Science. 1999;286(5439):509–12. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- [5].Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- [6].Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- [7].Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Front Syst Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104(47):18760–5. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(5):1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3(2):e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5(5):e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- [15].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [16].van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43(3):528–39. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- [17].Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen ZJ, He Y, Rosa-Neto P, Gong G, Evans AC. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. NeuroImage. 2011;56(1):235–45. doi: 10.1016/j.neuroimage.2011.01.010. [DOI] [PubMed] [Google Scholar]
- [19].Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44(3):715–23. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- [20].Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28(37):9239–48. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou C, Lucia Z, Zamora G, Hilgetag CC, Kurths J. Hierarchical Organization Unveiled by Functional Connectivity in Complex Brain Networks. Phys Rev Lett. 2006;97(23):238103. doi: 10.1103/PhysRevLett.97.238103. [DOI] [PubMed] [Google Scholar]
- [22].Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133(4):1224–38. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- [23].Kaminski M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85(2):145–57. doi: 10.1007/s004220000235. [DOI] [PubMed] [Google Scholar]
- [24].Sato JR, Fujita A, Cardoso EF, Thomaz CE, Brammer MJ, Amaro E., Jr Analyzing the connectivity between regions of interest: An approach based on cluster Granger causality for fMRI data analysis. Neuroimage. 2010;52(4):1444–55. doi: 10.1016/j.neuroimage.2010.05.022. [DOI] [PubMed] [Google Scholar]
- [25].Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37(3):424–38. [Google Scholar]
- [26].Astolfi L, Cincotti F, Babiloni C, Carducci F, Basilisco A, Rossini PM, Salinari S, Mattia D, Cerutti S, Dayan DB, Ding L, Ni Y, He B, Babiloni F. Estimation of the cortical connectivity by high-resolution EEG and structural equation modeling: simulations and application to finger tapping data. IEEE Trans Biomed Eng. 2005;52(5):757–68. doi: 10.1109/TBME.2005.845371. [DOI] [PubMed] [Google Scholar]
- [27].Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- [28].Yan C, Gong G, Wang J, Wang D, Liu D, Zhu C, Chen ZJ, Evans A, Zang Y, He Y. Sex- and brain size-related small-world structural cortical networks in young adults: a DTI tractography study. Cereb Cortex. 2011;21(2):449–58. doi: 10.1093/cercor/bhq111. [DOI] [PubMed] [Google Scholar]
- [29].Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7–8):468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- [30].Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- [31].Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;29(50):15684–93. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17(10):2407–19. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- [33].Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25(36):8303–10. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- [35].Jin K. Modern Biological Theories of Aging. Aging Dis. 2010;1(2):72–74. [PMC free article] [PubMed] [Google Scholar]
- [36].Buga A-M, Vintilescu R, Pop OT, Popa-Wagner A. Brain aging and regeneration after injuries: an organismal approach. Aging Dis. 2011;2(1):64–79. [PMC free article] [PubMed] [Google Scholar]
- [37].Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang DU. Complex networks: Structure and dynamics. Physics Reports. 2006;424(4–5):175–308. [Google Scholar]
- [38].Albert R, Barabasi AL. Statistical mechanics of complex networks. Reviews of Modern Physics. 2002;74(1):47–97. [Google Scholar]
- [39].Newman MEJ. The Structure and Function of Complex Networks. SIAM Review. 2003;45(2):167–256. [Google Scholar]
- [40].Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- [41].Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–48. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ponten SC, Daffertshofer A, Hillebrand A, Stam CJ. The relationship between structural and functional connectivity: Graph theoretical analysis of an EEG neural mass model. NeuroImage. 2010;52(3):985–94. doi: 10.1016/j.neuroimage.2009.10.049. [DOI] [PubMed] [Google Scholar]
- [43].Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sun J, Small M. Unified framework for detecting phase synchronization in coupled time series. Phys Rev E. 2009;80:046219. doi: 10.1103/PhysRevE.80.046219. [DOI] [PubMed] [Google Scholar]
- [45].Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43(3):447–57. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gerloff C, Hallett M. Big news from small world networks after stroke. Brain. 2010;133:952–955. doi: 10.1093/brain/awq062. [DOI] [PubMed] [Google Scholar]
- [48].Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31(6):852–62. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D’Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci U S A. 2010;107(26):12017–22. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15(9):1332–42. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- [56].Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. 2001;6(2):161–80. doi: 10.1037/1082-989x.6.2.161. [DOI] [PubMed] [Google Scholar]
- [57].Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- [58].Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4(4):345–56. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- [59].Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- [60].Gaetz W, Roberts TP, Singh KD, Muthukumaraswamy SD. Hum Brain Mapp. 2011. Functional and structural correlates of the aging brain: Relating visual cortex (V1) gamma band responses to age-related structural change. published online in advance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang L, Li Y, Metzak P, He Y, Woodward TS. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 50(3):862–72. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
- [62].Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp. doi: 10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage. 2010;49(2):1750–9. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52(2):643–57. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Micheloyannis S, Vourkas M, Tsirka V, Karakonstantaki E, Kanatsouli K, Stam CJ. The influence of ageing on complex brain networks: a graph theoretical analysis. Hum Brain Mapp. 2009;30(1):200–8. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhu W, Wen W, He Y, Xia A, Anstey KJ, Sachdev P. Neurobiol Aging. 2011. Changing topological patterns in normal aging using large-scale structural networks. published online in advance. [DOI] [PubMed] [Google Scholar]
- [67].Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- [68].Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39(4–5):1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- [70].Zhu C, Guo X, Jin Z, Sun J, Qiu Y, Zhu Y, Tong S. Influences of brain development and ageing on cortical interactive networks. Clin Neurophysiol. 2011;122(2):278–83. doi: 10.1016/j.clinph.2010.06.016. [DOI] [PubMed] [Google Scholar]
- [71].Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–51. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- [72].Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- [73].Wen W, He Y, Sachdev P. Structural brain networks and neuropsychiatric disorders. Curr Opin Psychiatry. 2011;24(3):219–25. doi: 10.1097/YCO.0b013e32834591f8. [DOI] [PubMed] [Google Scholar]
- [74].Goh JO. Functional Dedifferentiation and Altered Connectivity in Older Adults: Neural Accounts of Cognitive Aging. Aging Dis. 2011;2(1):30–48. [PMC free article] [PubMed] [Google Scholar]
- [75].Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26(3):891–9. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- [76].Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26(8):1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- [77].Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex. 2008;18(10):2374–81. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kashtan N, Alon U. Spontaneous evolution of modularity and network motifs. Proc Natl Acad Sci U S A. 2005;102(39):13773–8. doi: 10.1073/pnas.0503610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- [80].Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134(5):1264–76. doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132(12):3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wu W, Sun J, Jin Z, Guo X, Qiu Y, Zhu Y, Tong S. Impaired neuronal synchrony after focal ischemic stroke in elderly patients. Clin Neurophysiol. 2011;122(1):21–6. doi: 10.1016/j.clinph.2010.06.003. [DOI] [PubMed] [Google Scholar]
- [84].He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- [85].Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage. 2008;41(4):1382–94. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- [86].Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236–46. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- [87].Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009;66(5):604–16. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16(3):258–64. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [89].Lo CY, Wang PN, Chou KH, Wang J, He Y, Lin CP. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J Neurosci. 2010;30(50):16876–85. doi: 10.1523/JNEUROSCI.4136-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Neurobiol Aging. 2011. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. published online in advance. [DOI] [PubMed] [Google Scholar]
- [92].Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain. 2009;132(1):213–24. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- [94].He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci. 2008;28(18):4756–66. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Stam CJ. Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci. 2010;289(1–2):128–34. doi: 10.1016/j.jns.2009.08.028. [DOI] [PubMed] [Google Scholar]
- [96].Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [97].Evans A, He Y, Chen Z. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s Disease. Journal of Neuroscience. 2008;28(18):4756–66. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17(1):92–9. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- [99].Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SA, Maris E, Barkhof F, Scheltens P, Stam CJ. Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5(11):e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97(1–3):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [102].Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol. 2007;118(4):918–27. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [103].Fingelkurts AA, Fingelkurts AA, Rytsala H, Suominen K, Isometsa E, Kahkonen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007;28(3):247–61. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T. Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Comput Biol. 2010;6(11):e1001006. doi: 10.1371/journal.pcbi.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bartolomei F, Bosma I, Klein M, Baayen JC, Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de Jongh A, Cover KS, Stam CJ. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006;117(9):2039–49. doi: 10.1016/j.clinph.2006.05.018. [DOI] [PubMed] [Google Scholar]
- [107].Nakamura T, Hillary FG, Biswal BB. Resting network plasticity following brain injury. PLoS One. 2009;4(12):e8220. doi: 10.1371/journal.pone.0008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104(24):10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Breakspear M, Jirsa V, Deco G. Computational models of the brain: From structure to function. Neuroimage. 2010;52(3):727–30. doi: 10.1016/j.neuroimage.2010.05.061. [DOI] [PubMed] [Google Scholar]