Abstract
Melatonin is a pleiotropically acting regulator molecule, which influences numerous physiological functions. Its secretion by the pineal gland progressively declines by age. Strong reductions of circulating melatonin are also observed in numerous disorders and diseases, including Alzheimer’s disease, various other neurological and stressful conditions, pain, cardiovascular diseases, cases of cancer, endocrine and metabolic disorders, in particular diabetes type 2. The significance of melatonergic signaling is also evident from melatonin receptor polymorphisms associated with several of these pathologies. The article outlines the mutual relationship between circadian oscillators and melatonin secretion, the possibilities for readjustment of rhythms by melatonin and its synthetic analogs, the consequences for circadian rhythm-dependent disorders concerning sleep and mood, and limits of treatment. The necessity of distinguishing between short-acting melatonergic effects, which are successful in sleep initiation and phase adjustments, and attempts of replacement strategies is emphasized. Properties of approved and some investigational melatonergic agonists are compared.
Keywords: Alzheimer’s Disease, Circadian Rhythms, Diabetes, Melatonin, Mood Disorders, Parkinson’s Disease, Sleep
The indoleamine melatonin (N-acetyl-5-metho-xytryptamine) is usually known as the hormone of the pineal gland. This role is of particular importance in a chronobiological context, especially with regard to its effects on the hypothalamic circadian pacemaker, the suprachiasmatic nucleus (SCN). However, its spectrum of functions is considerably broader, in terms of sites of both biosynthesis and action [1–5]. Melatonin is formed in numerous organs and cells, such as the gastrointestinal tract (GIT), bone marrow, several leukocytes, membranous cochlea and, presumably, skin and other regions of the central nervous system. It is frequently overlooked that, in quantitative terms, extrapineal melatonin exceeds by far that found in the pineal and in the circulation. Owing to the size of the organ, the amounts of melatonin present in the GIT are several hundred-fold higher than those in the pineal [6, 7].
Extrapineal melatonin is either poorly released to the circulation or for short periods of time. Relatively high amounts have been reported to enter the blood from the GIT in response to nutritional factors, as a post-prandial response of short duration [7–9]. These pulses of melatonin are of minor importance to the circadian system, not so much because of its brevity, but rather as a consequence of shape and phase position of the so-called phase response curve (PRC). The PRC describes the resetting of a rhythm by entraining signals in dependence of the phase (i.e., the time point within the circadian cycle) of administration of the signal. Usually, a PRC contains phases in which the rhythm is delayed, others in which it is advanced and also a silent zone in which the rhythm is poorly affected. In humans, the PRC for melatonin has been determined, which mainly reflects the resetting in the SCN [10, 11]. A post-prandial release of melatonin during the day mostly occurs in the silent zone, whereas much stronger effects are observed in phases of pineal melatonin secretion in which the hormone is capable of readjusting the rhythmicity of the SCN. In mammals, pineal melatonin biosynthesis and release are, in turn, under the control of the SCN and largely confined to the night. From the pineal gland, melatonin is not only secreted to the circulation, but also, via the pineal recess, to the third ventricle [12–14].
Collectively, all these findings indicate that melatonin serves numerous functions in various organs and that effects at the SCN constitute an important, but by far not the exclusive function. The awareness of this multiplicity of roles and actions gains increasing importance, because melatonin and synthetic melatonergic drugs come more and more into use, e.g., for treating sleep difficulties and mood disorders. These compounds should not be simply regarded as sleeping pills or antidepressants, which might be easily compared with classic drugs for the respective indications. They strongly differ in their mode of action, but, additionally, they exert numerous other effects beyond the reason for treatment. This insight can be of great practical relevance, especially concerning the immunological role of melatonin. Again, the actions are diverse. They comprise antiinflammatory, but also immunoenhancing effects [2–5]. This latter property can be highly undesired in cases of autoimmune diseases and should be regarded as a contraindication for melatonin and melatonergic drugs in these patients. Despite some controversies on this issue and the clearly antiinflammatory actions of melatonin in another context, the methoxyindole obviously aggravates symptoms of rheumatoid arthritis (RA) via stimulation of proinflammatory cytokines [15–17]. Moreover, blood melatonin levels were enhanced in RA patients and the circadian peak of the hormone was advanced [16]. For caveats concerning other diseases, but also adolescents and pregnant women see refs. [18, 19].
The remarkable pleiotropy of melatonin unavoidably leads to a plethora of effects if the hormone or synthetic melatonergic drugs are administered. Many of these actions can be beneficial, but not necessarily all of them. However, the other side of the coin is that a pathological decrease in melatonin formation and secretion has also numerous consequences on the functioning of a body, as will be outlined in this article.
Biosynthesis, Metabolism and Signal Transduction Mechanisms
For the better understanding of several aspects to be discussed, the biosynthetic, catabolic and signaling pathways are briefly described. Melatonin is synthesized from serotonin in two steps, N-acetylation to N-acetylserotonin (NAS) followed by O-methylation (Figure 1). The reverse sequence of these steps is possible, but remains in vertebrates physiologically irrelevant. Sufficiently high amounts of the precursor serotonin are usually available, but an exception was described for a defect mutation of the sepiapterin reductase gene [20]. The product of this enzyme, tetrahydrobiopterin (BH4), is required by aromatic amino acid hydroxylases. BH4 deficiency causes, beside other effects, poor synthesis of 5-hydroxytryptophan and, thus, serotonin, with the further consequence of a flattened melatonin rhythm [20].
Figure 1:
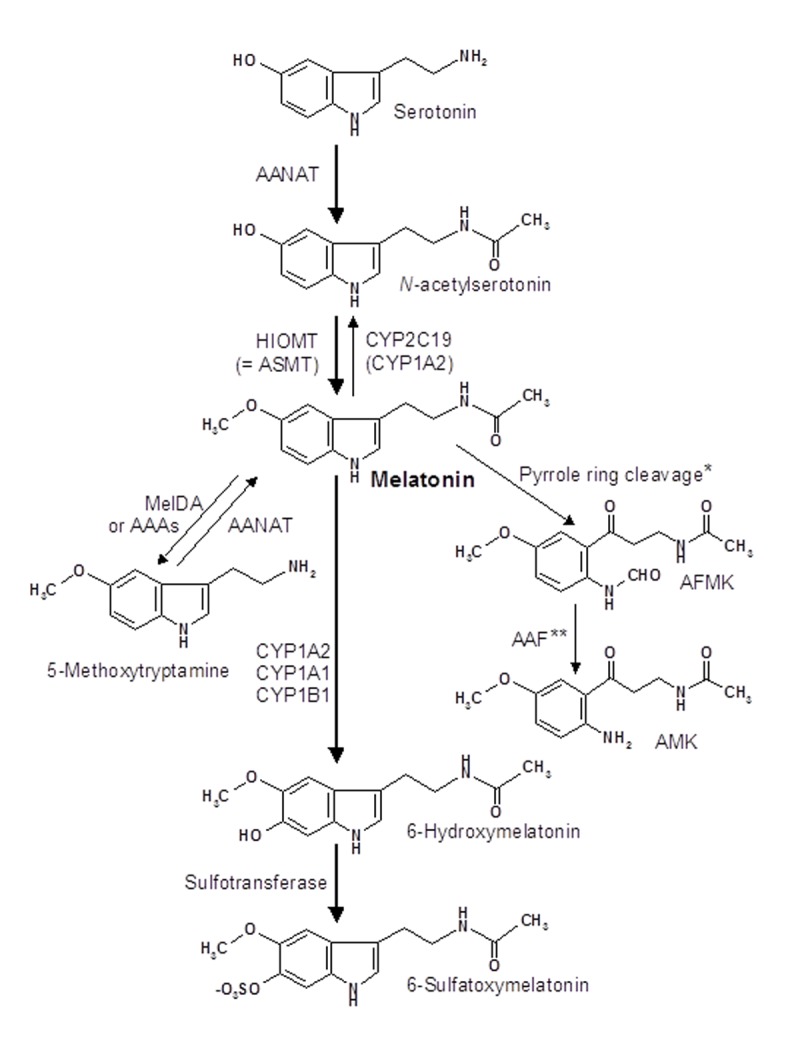
Biosynthesis and catabolism of melatonin. The main pathway is indicated by bold arrows. Abbreviations: AAAs, aryl acylamidases; AAF, arylamine formamidase; AANAT, arylalkylamine N-acetyltransferase; AFMK, N1-acetyl-N2-formyl-5-methoxykynuramine; AMK, N1-acetyl-5-methoxykynuramine; ASMT, acetylserotonin methyltransferase; CYP, cytochrome P450 monooxygenase or dealkylase; HIOMT, hydroxyindole O-methyl-transferase; MelDA, melatonin deacetylase. * Pyrrole ring cleavage is catalysed by various enzymes, such as indoleamine 2,3-dioxygenase, myeloperoxidase, other peroxidases, and by several reactive oxygen species. ** Alternately, AFMK can be deformylated by hemoperoxidases and photochemical mechanisms. For further metabolites see ref. [3].
In the pineal gland and several other sites, N-acetylation of serotonin is catalysed by arylalkylamine N-acetyltransferase (AANAT) [21] and O-methylation of NAS by hydroxyindole O-methyltransferase (HIOMT, alias acetylserotonin methyltransferase, ASMT). AANAT is usually regarded as the rate-limiting enzyme of melatonin biosynthesis, but this conclusion has been disputed for maximal nocturnal values in the rat pineal, in which a limitation by HIOMT has been reported [22]. The situation may be different in some extrapineal sites of melatonin synthesis. Preliminary data indicate that the steps of N-acetylation and O-methylation may sometimes be catalysed by alternate acetyl and methyltransferases [4]. The participation of another arylamine N-acetyltransferase, NAT-1, was assumed to be involved in cutaneous melatonin synthesis [23, 24]. NAS formation was observed in the skin of C57BL/6 mice [25], which are partially deficient in pineal AANAT because of a point mutation, which leads to a splice variant containing a pseudoexon with a stop codon [26]. However, functionally active splice variants of AANAT were found in leukocytes and bone marrow cells of two pineal melatonin-deficient strains and, to a certain extent, also in their pineals [27]. Therefore, the additional absence of an active HIOMT may strongly contribute to melatonin deficiency in the pineals of the respective mouse strains. The extrapineal cells also contained substantial amounts of melatonin, which was released to some extent into the circulation [27]. Moreover, alternate, cell-specific splicing mechanisms seem to allow extrapineal melatonin synthesis even in mice carrying the mutation described. The important message from these findings is that melatonin can be formed in peripheral organs and cells even if the pineal gland does not produce substantial amounts of its hormone.
The major pathway of melatonin catabolism consists of 6-hydroxylation by hepatic monooxygenases, CYP1A2, CYP1A2 and CYP1B1, which allows conjugation with sulfate to give the easily excretable metabolite, 6-sulfatoxymelatonin [2] (Figure 1). For this reason, levels and rhythm of melatonin can be indirectly followed by measuring this urinary product. Surprisingly, formation of 6-sulfatoxymelatonin was also detected in the brain, although the conjugation should disfavor its release from the CNS (for discussion see ref. [28]). In the tissues, alternate pathways of melatonin catabolism exist, but are of minor quantitative importance. Melatonin can be demethylated to NAS by CYP2C19, with eventual contributions by CYP1A2 and CYP1A1 [28]. Deacetylation to 5-methoxytryptamine by a specific melatonin deacetylase or less specific aryl acylamidases is also possible [28, 29]. An entirely different pathway consists of oxidative pyrrole ring cleavage, which leads to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK). This process can be catalyzed by remarkably many agents and includes various enzymes, especially dioxygenases and peroxidases, and several pseudoenzymatic, free radical-mediated and photochemical reactions (summarized in ref. [30]). AFMK was originally believed to be a major brain metabolite, since it was detected in large amounts after injection of melatonin into the cisterna magna of rats [31]. Although AFMK was reported to be also formed by several cell types, such as macrophages and keratinocytes, as discussed in ref. [28], no substantial amounts of this metabolite were detected in human urine and in various mouse tissues [32]. However, the same study described a new metabolite that might have been formed from AFMK, a finding that may be in accordance with the assumed transitory nature of methoxylated kynuramines (cf. ref. [30]). Nevertheless, AFMK has been detected in the human cerebrospinal fluid of patients with viral meningitis [33]. A negative correlation became apparent to the levels of IL-8 and IL-1β, which were lower in CSF samples containing over 50 nM AFMK, compared to those between 10 and 50 nM. Notably, these AFMK concentrations are by orders of magnitude higher than nocturnal plasma concentrations of melatonin. In conjunction with antiinflammatory and neuroprotective properties ascribed to AFMK [30, 31, 34], this metabolite remains to be of pathophysiological interest. The same may be valid for several secondary products that derive from AFMK (cf. ref. [30]). Another metabolite formed from melatonin by free radical-mediated reactions is cyclic 3-hydroxymelatonin (c3OHM), which is strongly elevated under conditions of oxidative stress, such as exposure to ionizing radiation [35].
Detailed information about signal transduction pathways is available for the G protein-coupled membrane receptors of melatonin. In mammals, two receptor subtypes are known, MT1 (alias Mel1a, MTNR1A) [36] and MT2 (alias Mel1b, MTNR1B) [37]. The classic effect of both receptors is transmitted by pertussis toxin-sensitive Gi proteins. In these case, the α subforms are usually αi2 and αi3, which mediate decreases in cAMP and, thereby, in protein kinase A activity and CREB phosphorylation. Meanwhile, additional pathways and regulatory interconnections have been identified, which involve αq and βγ subunits, activation of phospholipase Cβ, protein kinase C, MAP and CaM kinases, upregulation of phosphoinositide 3-kinase (PI3K) and Akt (protein kinase B), regulation of Ca2+ and K+ channels, and modulation of cGMP (summarized in ref. [38]). MT1 was shown to be modulated by the PDZ domain protein MUPP1 (multi-PDZ domain protein 1; PDZ = PSD-95/Drosophila disc large/ZO-1 homology) and the melatonin receptor homolog, GPR50, which has no affinity to melatonin, and, perhaps, by heterodimerization with MT2 [38]. Additional regulatory interconnections seem to exist, in which either circadian oscillator proteins are involved or factors controlling energy metabolism [39]. MT1 and MT2 display overlapping, but not identical actions. In a number of cases, they were shown to mutually substitute for each other, but, with regard to other functions, they can behave in an antagonistic way. For instance, MT1 activation leads to vasoconstriction, but MT2, present in other parts of the vasculature, to vasodilation [40].
The membrane receptors MT1 and/or MT2 have been shown to be present in numerous human tissues, such as duodenal enterocytes, colon, caecum and appendix, gallbladder epithelium, parotid gland, exocrine pancreas, β cells of endocrine pancreas, skin, breast epithelium, myometrium, placenta, granulosa and luteal cells, fetal kidney, cardiac ventricular wall, aorta, coronary and cerebral arteries and other parts of peripheral vasculature, brown and white adipose tissues, platelets, different types of immune cells, and various parts of the CNS and associated tissues (for further details, functions and extensive literature see ref. [5]). This remarkable multiplicity of targets unavoidably leads to highly pleiotropic effects exerted in numerous parts of the body, when melatonin or melatonergic drugs are pharmacologically administered, a fact that is usually not emphasized by vendors of respective pills designed for improving sleep or attenuating depressive symptoms. The highest density of melatonin membrane receptors is found in the SCN, the structure that is responsible for the chronobiotic, i.e., phase shifting effects of melatonin and also for important actions in sleep initiation, as will be discussed in the next section.
Numerous other binding sites of melatonin have been described, in addition to the membrane receptors. However, their physiological relevance in humans is either unknown or controversial. A protein transiently believed to represent a third melatonin receptor and found in the literature under the name “MT3“ has turned out to be an enzyme of xenobiotic metabolism, quinone reductase 2 (QR2) [41–43]. Melatonin also binds to transcription factors belonging to the retinoic acid receptor superfamily, in particular, splice variants of RORα (retinoic acid receptor-related orphan receptor-α; human gene ID: 6095), designated as RORα isoform a (alias RORα1), RORα isoform b (alias RORα2) and RORα isoform d (formerly called RZRα), and the product of another gene, RORβ (alias RZRβ; human gene ID: 6096) [5, 44–46]. RORα isoforms are almost ubiquitously expressed in the body, whereas RORβ is mainly found in the nervous system. Although many investigators agree about regulation of gene expression by melatonin via ROR transcription factors, this issue has remained controversial. RORs are chrono-biologically important, because they also interact with circadian core oscillators and thereby influence phasing, resetting and period lengths of circadian rhythms. However, it remains to be clarified whether or not these are melatonin-independent actions [39]. Moreover, some RORs seem to have other or additional ligands.
Even less is known about the role of melatonin binding to calmodulin, calreticulin and some other proteins with partial homology to calreticulin, and on binding to the mitochondrial complex I [38]. However, these findings may have the potential of further expanding the pleiotropy of melatonin. Finally, it should be mentioned that melatonin and some of its metabolites are potent free radical scavengers [47–51]. In humans, these actions which do not require receptors and signaling mechanisms are, in quantitative terms, presumably only relevant at high pharmacological doses, whereas receptor-mediated circadian, antiexcitatory, antiinflammatory and mitochondrial effects that reduce radical formation and enhance the expression of antioxidant enzymes physiologically contribute to antioxidative protection [50].
Melatonin and the Suprachiasmatic Nucleus: Output and Input
The SCN receives, in addition to inputs from other brain areas, such as the intergeniculate leaflet, its main information from the eye. The relevant photoreceptors are blue-absorbing melanopsin-containing retinal ganglion cells and green-absorbing cones [52–54]. The photic information entrains the circadian master oscillator system, which is composed of numerous cellular clocks. Within the SCN, groups of cells constitute several, internally coupled oscillatory subsets, which can differ in their resetting by photic and non-photic time cues [55, 56]. Moreover, the left and the right SCN may behave differently in respective experimental protocols and can lead to temporal splitting of rhythmic output functions such as locomotor activity [57]. In other, forced desynchronization protocols, differences between the ventrolateral and dorsomedial SCN zones have been observed in rats [58].
In mammals, the information about the circadian phase is transmitted to the pineal gland through a neuronal connection, via the paraventricular nucleus, a sympathetic connection from the intermediolateral cell column of the upper thoracic cord to the superior cervical ganglion, whose fibers innervate the pineal [59]. Melatonin synthesis is stimulated by β1-adrenergic upregulation of cAMP and α1B-adrenergic activation of phospholipase Cβ that leads to rises in Ca2+, protein kinase C and CaM kinases. These processes are modulated by several peptidergic and glutamatergic mechanisms [3].
The rhythm of melatonin synthesized in and released from the pineal gland is characterized by a prominent nocturnal peak, which also contains temporal information that had originated in the SCN [60]. Insofar, melatonin release represents an output function of the SCN. However, melatonin also acts as an input to the SCN by feeding back to this structure [61]. In the SCN, it mainly exerts two effects. One consists in a suppression of neuronal firing that can be attributed to MT1-dependent decreases in cAMP and, presumably, conductivity changes of cation channels. The other one is of chronobiotic nature, i.e., the capability of readjusting the circadian phase in the oscillator system of the SCN, in accordance with the PRC for melatonin. In many mammals, the chronobiotic actions are mediated by MT2 receptors, but their poor expression in the human SCN may indicate an additional function of MT1, as discussed elsewhere [5]. The question of why such a feedback is necessary, although the photic information from the SCN steers the activity of the pineal gland, may find its answer in another effect of light, namely the acute suppression of melatonin biosynthesis [60]. This latter action is more immediate and rapid than a light-induced phase shift of the temporally more inert circadian oscillator that is based on rhythmic gene expression and transcriptional inhibition. Therefore, melatonin contributes to the effective clock resetting by the phase of its rise. However, in modern civilizations, individuals can be exposed to nocturnal light, for reasons of rotatory shift-work or life style. Under these conditions, the acute suppression of melatonin leads to both rhythm perturbations – in addition to those directly induced in the SCN – and melatonin deficiency in circadian phases in which the hormone is required for optimal functioning of the body [39, 62]. The importance of such a lack of melatonin is immediately evident, as soon as one becomes aware of the highly pleiotropic role of the hormone that controls and orchestrates numerous physiological processes [2–5, 39].
A specific effect of melatonin at the SCN concerns sleep initiation. MT1-mediated actions favor the onset of sleep via the hypothalamic sleep switch, a structure characterized by typical on-off responses. On the basis of mutual inhibition, it alternately activates either wake-related neuronal downstream pathways that involve locus coeruleus, dorsal raphe nucleus and tuberomammillary nucleus or, under the influence of melatonin, sleep-related pathways via the ventrolateral preoptic nucleus [63, 64]. The MT1-dependent suppression of firing by SCN neurons seems to be decisive for the activation of the sleep-promoting circuits. However, the sleep-inducing actions of melatonin are more complex and comprise thalamic effects that include thalamocortical interplay and are detectable in the promotion of sleep spindles [18, 65, 66]. Thus, the feedback by melatonin to the SCN and its additional effects in the CNS are important for the onset of sleep, a physiological process that is disturbed in various disorders. A role of melatonin in sleep maintenance may exist as well, but this is not easily demonstrated at physiological concentrations of the hormone, for reasons to be discussed.
Melatonin and the Circadian Multioscillator System
In the literature, chronobiological effects of melatonin have been mostly discussed in relation to the circadian master clock, the SCN. However, the earlier view of a single clockwork exclusively ticking in this central tissue has changed. In fact, numerous peripheral oscillators have meanwhile been identified, which are either directly coordinated by the SCN [67] or may be, sometimes, only loosely coupled to or relatively independent of the master clock [39]. In fact, the existence of peripheral oscillators is not entirely new, since their discovery dates back to 1958 [68], but this and other comparable findings did not seem to be compatible with concepts developed after the identification of the SCN as a circadian pacemaker. To date, extrasuprachiasmatic oscillators have been identified in various mammalian tissues, e.g., intestine, liver, heart, adrenal cortex, pars tuberalis, retina, other CNS areas, and also in cultured cells such as fibroblasts (reviewed in ref. [39]). Circadian oscillations are primarily generated at the cellular level, whereas the coupling of a number of oscillating cells can result in a more stable collective rhythm. With regard to this cellular origin and the expression of clock proteins in many tissues [69, 70], the existence of peripheral clocks can be expected in numerous if not all parts of the body.
The complexity of the circadian oscillator system is even higher, since parallel oscillators are also acting in a single tissue. These are operating on the basis of the alternate use of orthologs or paralogs of core oscillator proteins. For instance, the clock protein PER1 may be replaced by PER2, CRY1 by CRY2, or CLOCK by NPAS2. The consequences can be differences in output functions. Moreover, the expression of a host of additional proteins associated with the core oscillator components can vary between cells, with divergent consequences of feedbacks to the primary clocks [71].
Melatonin has been shown to also influence peripheral oscillators and, moreover, to be important for the phasing and phase coupling of parallel oscillators within a single tissue. Examples for a role in peripheral oscillators have been found in the murine adrenal cortex and retina. In the adrenal cortex of the melatonin-proficient mouse strain C3H, the core oscillator proteins PER1, CRY2 and BMAL1 oscillate with robust amplitudes, whereas only weak fluctuations and reduced average expression levels are observed in the melatonin-deficient strain, C57BL [72]. In the retinal oscillator, C57BL mice did not show significant rhythms in PER1 and CRY2 levels, whereas robust rhythms were detected in C3H mice [73]. In human adrenal explants, inhibitory effects of melatonin on adrenal ACTH-induced responses of Per1 mRNA, BMAL1, StAR and 3β-HSD protein levels as well as cortisol and progesterone production have been demonstrated [74].
In cultured murine striatal neurons, melatonin caused marked decreases in the expression of Per1 and Clock and elevations of NPAS2, effects which were abolished in MT1 knockouts [75]. Effects of melatonin on phase coupling of parallel oscillators were observed in the rat SCN. In pinealectomized animals, the maxima of Per1 and Per2 mRNAs showed an unusual temporal phase difference, but became, again, more tightly coupled to approximately normal when these rats were treated with melatonin [76].
Two important messages can be deduced from these findings. (i) Decreases in melatonin, whether caused by aging or diseases, should not only affect a single master oscillator, but also oscillatory subsets within the SCN, and peripheral oscillators as well. (ii) Treatment of patients with melatonin or synthetic melatonergic drugs can exert effects on the internal coupling of rhythms within the SCN and between SCN and peripheral oscillators. Therefore, the pleiotropic actions of melatonin and its synthetic analogs do not only directly up- or downregulate peripheral physiological functions, but also affect their time structure in a complex chronobiological manner.
Reduced Melatonin Secretion During Aging and in Various Disorders and Diseases
In the course of aging, the nocturnal melatonin peak is usually decreasing, though with considerable interindividual variability [77–80]. In several aged individuals, the nighttime values are almost indistinguishable from those obtained during daytime, whereas others maintain a fairly well pronounced rhythm with only moderate reductions of nocturnal values. In individuals with strongly reduced melatonin, daytime values are often decreased, too. Age-dependent impairments of melatonin formation are not only detected in plasma concentrations, but also in human pineal glands [81], saliva [82], cerebrospinal fluid [83, 84], and in urinary amounts of the main metabolite, 6-sulfatoxymelatonin [82, 85, 86]. The high interindividual variability of the decrease in pineal function is also observed in urinary 6-sulfatoxymelatonin levels, which can vary in apparently healthy subjects by a factor of 20 [86]. As long as a melatonin rhythm is detectable, the nocturnal peak of plasma melatonin is frequently phase-advanced in the elderly relative to young individuals [79]. Age-related reductions of melatonin can have different causes, a progressive deterioration (i) of the SCN or (ii) of the neuronal transmission to the pineal, reminiscent of changes observed in neurodegenerative disorders [79, 87–89], or (iii) pineal calcification [90, 91].
In several neurodegenerative disorders, especially Alzheimer’s disease (AD) and other types of senile dementia, levels of melatonin are frequently more strongly decreased than in age-matched controls [79, 81, 84, 87–89, 92–95] (Table 1). In many of these patients, the melatonin rhythm is practically abolished. These declines seem to frequently result from SCN degeneration. Reduced melatonin secretion as a result of tissue destruction in the SCN has been also observed in young individuals diagnosed with hypothalamic hamartomas, which may cause precocious puberty [96], or with craniopharyngiomas [97–99].
Table 1:
Diseases and disorders associated with reduced melatonin secretion in humans
| Disease/disorder | Comments | References |
|---|---|---|
| Alzheimer’s disease | Stage dependent decreases down to complete loss of melatonin rhythm | [79, 81, 84, 88, 89, 92–95] |
| Pick’s disease | Two cases only | [92] |
| Autism spectrum disorders | Decreases in melatonin or urinary 6-sulfatoxy-melatonin frequent, but not generally observed | [100–106] |
| Schizophrenia | Only in a subpopulation | [107, 108] |
| Multiple sclerosis with major depression | Not observed in major depression alone | [109] |
| Primary obsessive compulsive disorder | [110] | |
| Menière’s disease | Possibly related to stress by tinnitus and vertigo | [111] |
| Macular degeneration | [112] | |
| Cases of severe epilepsy | High interindividual variation. However, increases during seizures |
[113, 114] [113, 115] |
| Coronary heart disease, myocardial infarction, cardiac syndrome X | [116–122] | |
| Fibromyalgia | Decreases observed in women Uncertainties concerning levels Pain reduced by melatonin |
[123] [124] [124–128] |
| Neuralgia | [123] | |
| Migraine | Pain reduced by melatonin | [129, 130] [128] |
| Bulimia | [123] | |
| Critical illness | [131–133] | |
| Postoperative stress | Decreases in patients without complications, but strong increases in those with delirium | [134] |
| Hypothalamic hamartoma | [96] | |
| Craniophapharyngioma | [97–99] | |
| Endometrial cancer | [135] | |
| Non-small cell lung cancer | In part caused by pain? | [136] |
| Acute intermittent porphyria | Further decreased by seizures | [137, 138] |
| Hypergonadotrophic hypogonadism | [139, 140] | |
| Diabetes type 2 | [141, 142] |
Cases of degeneration of the SCN, the pineal or their neuronal connections provide an immediately plausible interpretation of reductions in melatonin secretion. However, there are surprisingly many other diseases and disorders in which the pineal hormone is also decreased. These include various neurological and stressful conditions, pain, cardiovascular diseases, cases of cancer, endocrine and metabolic disorders, in particular diabetes type 2 and acute intermittent porphyria [100–142] (Table 1). Further details are discussed in refs. [5] and [39].
A major question arising from these findings concerns the alternative of cause or consequence. In some cases, such as acute pain and stress, a decrease in melatonin is likely induced by these events. The same may occur if a disease is associated with oxidative stress and the easily oxidizable melatonin is destroyed by high amounts of free radicals. Under conditions of neurodegenerative changes, reduced melatonin secretion may favor the development of other diseases. However, the decision is not always that easy and, moreover, both possibilities may exist simultaneously or sequentially, in that an otherwise initiated decline of melatonin further aggravates a disease, e.g., because of a reduction in immunological and antioxidative protection mechanisms. A contribution of low melatonin to disease development or progression may be deduced, with due caution, from the association of various pathologies with gene polymorphisms related to melatonin. Differences in either rates of melatonin synthesis or signal transduction can lead to the same consequence as an otherwise induced decrease of the hormone. A list of respective gene polymorphisms [103, 143–183] is presented in Table 2. These polymorphisms have been detected in the genes of the melatonin membrane receptors, MT1 and MT2, of the enzymes of melatonin biosynthesis, AANAT and HIOMT, and also of the orphan receptor GPR50. This protein, which does not bind melatonin, has been identified as a mammalian ortholog of the non-mammalian Mel1c receptor [184]. It inhibits MT1 by heterodimerization and prevents G protein coupling [185]. Its precise role in melatonin signaling and the conditions under which GPR50 is upregulated await further clarification. Various metabolic changes have been observed in GPR50 knockout mice [186]. However, GPR50 has obviously additional functions beyond melatonin signaling, since it was found to also interact with the neurite outgrow inhibitor NOGO-A [187] and with TIP60, a coactivator of glucocorticoid receptor signaling and histone acetyltransferase [188].
Table 2:
Diseases, disorders and metabolic changes associated with gene polymorphisms of melatonin membrane receptors, a receptor homolog and enzymes of melatonin biosynthesis
| Disease/disorder | Gene | Refs. |
|---|---|---|
| Autism spectrum disorders | HIOMT (= ASMT) | [103, 143, 144] |
| ADHD (attention-deficit and hyperactivity disorder) |
MT1 (= MTNR1A) HIOMT (= ASMT) |
[145] [145] |
| Schizophrenia | MT1 (= MTNR1A) | [146] |
| Major depression | AANAT | [147] |
| Recurrent depression | HIOMT (= ASMT) | [148] |
| BP (bipolar disorder) | GPR50 | [149, 150] |
| SAD (seasonal affective disorder) | GPR50 | [151] |
| Rheumatoid arthritis | MT2 (= MTNR1B) | [152] |
| Adolescent idiopathic scoliosis (perhaps only in combination with other risk factors) | MT2 (= MTNR1B) | [153] [154] |
| Coronary artery disease | MT1 (= MTNR1A) | [155] |
| Diabetes type 2, prediabetes | MT2 (= MTNR1B) | [156–180] |
| Elevated fasting triglycerides | GPR50 | [181] |
| Elevated fasting glucose | MT2 (= MTNR1B) | [157–160, 162, 167–170, 177–179] |
| Elevated cholesterol | MT2 (= MTNR1B) | [180] |
| Polycystic ovary syndrome |
MT1 (= MTNR1A) MT2 (= MTNR1B) |
[182] [183] |
Notably, there is a remarkable overlap of pathologies listed in Tables 1 and 2. Further connections between melatonin and diseases may exist, inasmuch as polymorphisms of clock genes are concerned, which may alter the melatonin rhythm and, moreover, may be differently influenced by melatonin. This might be of particular interest with regard to cancer, as recently discussed [39]. However, it is important to remain aware of the very meaning of associations between diseases and polymorphisms, which mostly do not represent anything else but risk factors. They often become effective in combination with others in multifactorial etiologies and are sometimes only demonstrable in some but not all populations. Nevertheless, the coincident observations of reduced melatonin and unfavorable gene variants in the same disease or disorder are indicative of possible causal relationship that is worth further investigation.
Weakening of Clock Functions, Dysphased Melatonin Rhythm and Resetting
Impairments of melatonergic activity have always to be seen in the context of clock functions. This may not only concern the SCN as a master clock, but also peripheral oscillators, although the actual knowledge of chronobiological melatonin effects in the periphery are still insufficient. The need for detailed analyses of peripheral signaling pathways and their metabolic links has been recently addressed [39].
Lowered or dysphased melatonin rhythms can result from functional impairments in the SCN or its input and output connections. On the other hand, reduced melatonin secretion can lead to a poor feedback to the SCN and, thus, failure of dark-induced phase resetting. In either case, a crucial question is that of whether exogenous melatonin or administration of synthetic melatonergic drugs may still be capable of readjusting rhythms under these conditions, with regard to both phase and amplitude.
A weakening of clock functions may have different reasons. Impairments of the visual input should be distinguished from changes in the SCN or output pathways, especially with regard to differences in the chances for a successful treatment. Reductions in the unconscious circadian photoreception can occur in aged people because if pupillary miosis or impaired crystalline lens transmission, specially concerning blue light that is perceived by melanopsin-containing retinal ganglion cells. These changes may already promote circadian disruption, which can lead to sleep problems, contribute to the development of affective disorders, metabolic syndrome, an other systemic diseases [189]. In visually blind people, circadian photoreception can persist if intact melanopsin-containing retinal ganglion cells and the connection to the SCN are retained. In other blind subjects, circadian rhythms including that of melatonin may either poorly couple to or uncouple from external time cues. This results, in the first case, in a so-called relative coordination, in which the rhythms are gradually attracted for several days by an external synchronizer, followed by a sequence of days during which the rhythm is more strongly shifted, or, in the second case, in free-running rhythmicity [190–195]. Failure of entrainment by external time cues is also known under the term ’free-running disorder’ (FRD). As long as the neuronal connections between SCN and the pineal gland are functionally active, the rhythmicity of the SCN widely determines the rhythm of melatonin formation and release. However, an additional effect of potentially high significance can strongly modify the melatonin rhythm in sighted subjects, namely, an acute suppression of pineal melatonin by nocturnal light, which has to be distinguished from the circadian effects [196–199]. This is of particular importance in rotating shift work. Both effects of nocturnal light, circadian disruption and acute suppression of melatonin formation, seem to contribute to health problems observed in shift workers. Rotating shift work as a risk factor for various diseases or disorders, including some types of cancer, cardiovascular diseases, peptic ulcers, obesity and metabolic syndrome, as well as the epidemiological limits for these conclusions have been recently reviewed [39].
The interconnections between the SCN and the pineal gland always have to be seen from two sides. On the one hand, reduced photoreception, SCN dysfunction or impaired pineal innervation can be the cause of dysphased or flattened melatonin rhythms. On the other hand, reduced nocturnal melatonin levels lead to an insufficient feedback to the SCN, in other words, to a poorer resetting by the dark signal. The two-sided relationship between SCN and pineal can gain some complexity under conditions of genetic dispositions for extremely short or long spontaneous circadian periods that lead to difficulties in the proper entrainment to external cycles [39, 200]. In a number of individuals, but not in every case, it is possible to favor synchronization with the external 24-h cycle by enhancing the strength of the resetting signals. This may be bright light, especially in the morning. Alternately, to reinforce the signal darkness, administration of melatonin or synthetic melatonergic agonists in the evening can be effective. From a theoretical point of view, stable synchronization by light or by melatonin is impossible if a 24-h cycle is outside the range of entrainment of the deviatant individual cycle. An additional aspect, which deserves future detailed investigation, concerns optimal phase relationships of parallel oscillations in the circadian multioscillator system [39]. Circadian disruption and low nocturnal melatonin seem to promote uncoupling or relative coordination within the multioscillator system. Internal desynchronization of rhythms has, in fact, been observed and discussed as a cause or an indicator of illnesses [39, 201–203].
The pathophysiological deviations related to malfunctioning of the circadian oscillator system seem to be associated with numerous disorders or diseases [5, 39]. However, the probability of developing relevant symptoms may strongly vary, depending on the respective pathology and on differences within populations owing to unequal combinations of risk factors. Among the most frequently observed difficulties, problems of sleep initiation and/or maintenance and mood disorders are of particular interest. Again, it should be emphasized that these disorders, as far as they are related to circadian dysfunction, can either result from deteriorations of oscillators and their neuronal connections, without a primary contribution of changes in melatonin, or, alternately, from changes in melatonin secretion, with secondary consequences to the clocks. In these two situations, the chances for successfully treating these disorders with melatonin or its analogs may not be identical, but in either case a chance does exist, as long as functional melatonin receptors are expressed in the SCN and other relevant central nervous target areas.
Insomnia represents a complex of diverse disorders, which is experienced in the one or other form by most people during their lives. About 10% of the population are affected chronically and, in this case, the treatment is often challenging [204, 205]. Insomnia is characterized by one or more of the following symptoms: difficulty of falling asleep, numerous nocturnal awakenings, early morning awakenings, reduced total amount of sleep or restorative sleep, with consequences of daytime somnolence, fatigue, irritability, difficulty of concentrating and performing everyday tasks. Importantly, insomnia is associated as a comorbid symptom with other illnesses and disorders. Very frequently, this is observed in mood disorders, but it also occurs in cardiovascular diseases, weight gain and glucose intolerance.
With regard to the diversity of causes of insomnia, subtypes related to circadian dysfunction [85, 206–209] have to be distinguished from sleep difficulties of other etiology. The so-called circadian rhythm sleep disorders (CRSDs) can, again, result from different causes. One of these possibilities is an innate or acquired deviation from an easily entrainable spontaneous period, as present in familial advanced sleep phase syndrome (FASPS) and delayed sleep phase syndrome (DSPS). Polymorphisms in the core oscillator genes Per2 and Per3 (Period 2 and 3) have been identified as being causative of some CRSDs [210, 211], but mutations in other clock genes may also lead to this type of disorder. As discussed above, insufficient entrainment may also exist in some blind subjects or because of an otherwise impaired light input pathway. Consequently, free-running or relatively coordinated rhythms lead to sleep difficulties on those days in which a daytime circadian phase is reached at night. Moreover, irregular sleep-wake patterns are associated with low circadian amplitudes [209], especially in elderly patients, in which deteriorations of the SCN [212–214] or decreases in nocturnal melatonin [78, 215] contribute to insomnia or may even be of causal relevance.
The regularly observed association of mood disorders with sleep disturbances has led to numerous assumptions concerning a mechanistic relationship. Some investigators have considered insomnia symptoms as a predictor of a depressive disorder (discussed in ref. [216]). This has been supported by findings showing sleep disturbances or changes in sleep architecture as prodromal symptoms occurring several weeks prior to the recurrence of a depressive episode [217–219]. Although insomnia is a comorbid symptom of most mood disorders, an etiologic relevance of circadian malfunction is only demonstrable or likely in some subtypes of this highly diverse complex of disorders.
Deviations in the circadian system are likely present in seasonal affective and bipolar disorders, as indicated by polymorphisms in core oscillator genes, such as Per2, Cry2, Bmal1 (= Arntl) and Npas2 in winter depression [220–223], Per3, Cry2, Bmal1 (Arntl), Bmal2 (Arntl2), Clock, Dbp, Tim, CsnK1ε and NR1D1 in bipolar disorder [224–231]. Moreover, DSPS, i.e., a CRSD, was found to be associated with seasonal affective disorder [232]. Since both bipolar and seasonal affective disorders display characteristics of long-period rhythmicity, which can be interpreted in terms of poor coupling of circadian oscillations to external and/or internal rhythms, this is not the case in major depressive disorder (MDD), in which the situation is less clear. On the one hand, it has been concluded that no convincing evidence exists for an involvement of the circadian system [233], but, on the other hand, Cry1 and Npas2 polymorphisms were found to be associated with MDD [234]. With regard to the heterogeneity of MDD, a role of circadian rhythmicity cannot be excluded in some of its subtypes.
While the significance of the circadian system for disturbances of sleep and some affective disorders is obvious, the genetic evidence for a role of the melatonergic system is relatively poor. A few hints may exist in findings mentioned in Table 2. Moreover, bipolar disorder was reported to be associated with a polymorphism of the RORB gene [235]. This gene encodes the transcription factor RORβ, which is considered as a nuclear melatonin receptor. However, it has remained unclear as to whether the action of RORβ reflects a melatonin-dependent or melatonin-independent input into the circadian clock. Beyond the genetic aspect, there is, however, good reason to conclude that reduced levels of melatonin or impaired melatonergic signaling contribute to low amplitudes and poor coupling of circadian oscillations. Therefore, melatonin and synthetic melatonergic drugs are an option for reversing circadian malfunction, as long as the SCN is functionally intact. This will presumably be of future importance in the treatment of CRSDs and, especially, cyclically occurring affective disorders.
Melatonin and Alzheimer’s Disease
Several reasons have given rise to the assumption that melatonin might be beneficial in AD patients. Mainly, these include melatonin deficiency in AD, the malfunctioning of the circadian master clock, and the antioxidant and antiinflammatory actions of melatonin, with regard to oxidative stress and atypical inflammatory processes observed as accompanying symptoms with a presumed contribution to disease progression [236]. Some experimental data in transgenic mice or in vitro had indicated a possibly beneficial role. In the transgenic mouse model, melatonin treatment starting relatively early in life not only lead to reductions in oxidative damage and in amyloid accumulation but also to an increase in survival [237]. In another study, reductions in neuronal apoptosis and damage to the cholinergic system were reported, which might indicate a support of cognitive functions, and corresponding behavioral improvements [238]. Moreover, melatonin was shown to possess antifibrillogenic properties [239]. However, no substantial benefits were demonstable after a later onset of treatment [240]. This latter finding is important to humans, as AD is usually diagnosed relatively late in life. Therefore, clinical improvements by melatonin cannot be expected in terms of delay of disease progression or life extension.
With regard to the poor efficacy of other approved treatments in AD, such as cholinergic or memantine therapies [241], and to the unclear early etiology of AD, the findings on melatonin mainly demonstrate that a late onset of therapy is not promising. It remains to be clarified whether new ionophore strategies based on the reduction of metal toxicity and prevention of intracellular metal depletion will be more successful [241–243]. In such a case, melatonin may be re-considered as an adjunctive therapy to improve chronobiological and sleep parameters, as far as they are not accessible to ionophore treatment and remain impaired.
Although a clinical value of melatonin in preventing or delaying disease initiation and progression appears to be questionable, beneficial effects are not generally excluded and have, in fact, been described. These concern AD-associated sleep disorders, behavioral changes, in particular, “sundowning” agitation, and cognitive impairments. However, the findings of several studies are highly divergent. In part, this may be seen on the background of a high interindividual variability among AD patients. In groups with a similar degree of cognitive impairment, degeneration of brain structures related to circadian rhythms and sleep may have progressed to a different extent. As long as the SCN and downstream structures controling wakefulness and sleep are operating, at least to a certain extent, there remains a chance for improvements of sleep and behavioral functions associated with circadian time patterns. Several smaller studies of variable length, from a few weeks to two or three years, reported improvements in sleep onset, sometimes also in number of awakenings, sleep quality or reduction of daytime somnolence [244–250], in one study using a combined therapy of melatonin in the evening and bright light in the morning [251]. Importantly, sundowning was found to be reduced, in some individuals markedly, by melatonin [244, 246–248, 250], an effect of particular importance with regard to the caregiver’s burden. However, the largest clinical trial [252] did not reveal statistically significant differences in objective measures of sleep. An increase in nocturnal total sleep time and decreased wake after sleep onset, as determined on an actigraphic basis, were only apparent as trends in melatonin-treated cohorts, although melatonin facilitated sleep in a certain number of patients. On subjective measures, however, caregivers’ ratings of sleep quality showed a significant improvement in a sustained-release melatonin group relative to placebo. The outcome of this study underlines that the combination of individuals differing in extent and regional distribution of neurodegeneration results in a statistical heterogeneity problem [236]. What is still helpful in one patient may already be unsuccessful in another one.
Melatonin and Parkinson’s Disease
The usefulness of melatonin in Parkinson’s disease (PD) is even more controversial than in AD. A particular problem results from the investigators’ focus on the stage at which the most severe Parkinsonian symptoms appear, namely, the advanced damage to the nigrostriatum. In numerous preclinical studies, animal models based on the administration of neurotoxins such as 6-hydroxydopamine, 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP), sometimes also rotenone, maneb and paraquat, have been applied with the intention of mimicking the nigrostriatal degeneration. In short-term studies of this type, melatonin consistently reduces the damage caused by these compounds [253–262], which are also oxido- and mitochondrial toxins. Since melatonin is a powerful multifunctional antioxidant, antinitrosant, antinitratant and also mitochondrial modulator [47, 48, 50, 263–271], these effects are not surprising, but rather reflect the counteraction against reactive oxygen and nitrogen species including the reduction of their formation. In more extended investigations, the outcome was contradictory. One study reported protective effects in a chronic model [272], whereas two others did not reveal improvements [273, 274]. Regardless of whether nigrostriatal degeneration can be gradually antagonized in the respective models, these approaches do not consider the etiology of PD, which does not start in the nigrostriatum, but rather in the brain stem or even the spinal cord of subjects which remain asymptomatic for a long period of time [275–277]. Therefore, the prodromal extranigral degenerative changes are neglected in the animal models, although the appearance of Lewy bodies can be traced back to much earlier stages.
With regard to the use of melatonin in PD patients, a potentially serious reason of concern has been advanced by Willis, who interprets PD as a disease of melatonin-dopamine imbalance or, in another term of his, as a ”melatonin hyperplasia” disorder [278]. He also reported that melatonin antagonists are beneficial in PD [279]. This conclusion is not in good accordance with findings on reduced MT1 and MT2 expression in the striatum and other brain regions such as the amygdala in PD [280]. No enhanced melatonin secretion was observed in the majority of patients tested [281, 282], but rather, sometimes, reduced amplitudes of the melatonin rhythm [283]. Circadian phase advances of the melatonin rhythm were attributed to the treatment with l-DOPA, but not seen in “de novo“ patients [283]. Therefore, the assumption of a pathologic melatonin overproduction is not supported by available data. Findings on the use of bright light to suppress melatonin, which was also reported to be beneficial [284], may be alternately interpreted in terms of a strengthening of the circadian system, apart from the fact that the approach disregards the enhanced “rebound secretion” of melatonin following the transient suppression [282]. With regard to PD etiology, it remains to be demonstrated whether the concept of a melatonin-dopamine imbalance would be applicable to the early stages of the disease, in which the nigrostriatum is not yet affected. To date, it seems that a caveat concerning the treatment with melatonin or melatonergic agonists may be deduced from the reported beneficial effects of melatonin antagonists [279].
Despite the reservations discussed, melatonin and synthetic melatonergic agonists have been considered for treating sleep problems in PD and depressive symptoms that are frequently associated with this disease [282]. Some improvements of sleep have been demonstrated, but usually they remain relatively modest. With regard to depressive symptoms in PD, effects of agomelatine would include non-melatonergic actions [282, 285].
Melatonin, Metabolic Syndrome, Insulin Resistance and Diabetes Type 2
This complex of subclinical and clinical disorders and diseases is an emerging field in melatonin research. In part, the interest in the role of melatonin in this area has been newly stimulated by the identification of MT2 polymorphism as a risk factor for diabetes type 2, as mentioned above. Moreover, melatonin was shown to modulate insulin secretion in various experimental models [286–291]. Concerning the relevance of various signaling pathways see discussion in ref. [39]. The presence of an endogenous circadian oscillator in the islets of Langerhans [292] underlines the importance of chronobiological interpretations in sugar, fat and energy metabolism, in which melatonin may play a role. In perifused rat pancreatic islets, melatonin was shown to phase shift the rhythm of insulin secretion and to increase its amplitude [293].
With regard to the changes in blood glucose, the mutual paracrine interactions of insulin and glucagon within the pancreatic islet have to be considered. Under conditions of insulin resistance, glucagon secretion is no longer sufficiently inhibited by insulin. Conversely, glucagon stimulates insulin release. In human islets, the activation of glucagon secretion by melatonin via MT1 can override an insulin-depressing, MT2-dependent effect at the β-cell and, thus, leads to enhanced insulin levels [294]. The stimulatory effect of melatonin on glucagon secretion was confirmed in a pancreatic α-cell line [295]. In this context, it is of greatest importance to distinguish between the actions of melatonin in night-active rodents and in humans, and to take notice of the potentially misleading limits of the animal models. Melatonin peaks at night in both nocturnal and diurnal mammals, but unlike rodents, which are active and mainly feed at night, humans are at rest and fasting in this part of the circadian cycle. In humans, glucemia is regulated primarily in the night by gluconeogenesis and reduced glucose utilization. These effects can be induced by nocturnal melatonin which, through stimulation of glucagon secretion, ensures an adequate energy source to the brain [39]. Thus, the well-established suppression of insulin by melatonin observed in rodents is not applicable to humans [39, 294]. From a chronobiological point of view, this is not at all surprising, since the meaning of melatonin for the availability and metabolism of nutrients has to be different in nocturnal rodents and humans. This reservation has also to be made in many other aspects of energy metabolism.
The involvement of melatonin exceeds the effects on insulin and glucagon secretion and extends to prodromal metabolic syndromes, with additional consequences for hypertension, insulin resistance and, perhaps, also obesity. Melatonin’s role in metabolic syndrome has been recently summarized [296]. Persistent insulin resistance was induced in rats by pinealectomy [297–299]. In pharmacological settings, melatonin and other melatonergic drugs were shown to antagonize insulin resistance in rats [19, 300]. Correspondingly, knockout of the melatonin MT1 receptor gene was reported to induce insulin resistance in mice [301]. However, the situation in humans is much less clear. Apart from missing pharmacological support for a relationship between melatonin and insulin sensitivity, the studies on MT2 polymorphisms (cf. Table 2), which largely agree with regard to elevated fasting glucose in the respective variants [157–160, 162, 167–170, 177–179], mostly do not provide data indicating insulin resistance. Some studies, however conducted in young individuals, explicitly state the absence of an association with insulin resistance [164, 175, 179]. Instead, reduced glucose-stimulated insulin release has been repeatedly described in subjects carrying these gene variants [157, 159, 165, 302]. A possible indication for an association of an MT2 variant with insulin resistance has been reported in polycystic ovary syndrome [183]. It seems that additional studies on melatonin receptor variants on subjects of advanced age are required before a final judgment should be made.
The situation is similarly controversial concerning the role of melatonin in obesity. Although melatonin is clearly negatively correlated with the amounts of adipose tissue in nocturnal rodents and can reduce visceral fat masses in these species under various conditions (summarized in ref. [5]), this has not been clearly demonstrated in humans. In some studies, melatonin levels did not substantially differ between obese and normal-weight subjects [303–306], whereas, in others, increased melatonin was reported for some obese individuals [306–308]. Short-term fasting was reported to decrease melatonin [305], which might be in accordance with the latter observations. However, in obese post-menopausal women, melatonin was found to be decreased [309]. Thus, more data on humans appear to be required, and a particular attention should be paid to age and aging. In this context, circadian deviations and chronodisruption have to be also considered, which occur, e.g., in night-eating syndrome, and as far as possible distinguished from obesity alone.
Properties of Melatonin and Synthetic Melatonergic Drugs
The structures of several selected melatonergic agonists are shown in Figure 2. Except for TIK-301 (= β-methyl-6-chloromelatonin), all these compounds represent non-indolic structures. Ramelteon (Rozerem®; TAK-375), developed by Takeda, Japan, has been approved in the USA by the FDA for the treatment of insomnia. The ramelteon metabolite M-II is also depicted, because it has melatonergic properties and contributes substantially to the overall activity of the parent compound. Agomelatine (Valdoxan®; S20098), developed by Servier, France has been licensed by EMEA for the treatment of major depressive episodes (MDE) in adults in Europe. In addition to these synthetic drugs, a melatonin controlled-release tablet (Circadin®), developed by Neurim, Israel and UK and also provided by Lundbeck and Nycomed, has been approved by EMEA for the treatment of insomnia in patients aged 55 years and over. All other compounds are to date investigational drugs, which differ concerning the number and outcome of preclinical and clinical studies.
Figure 2:
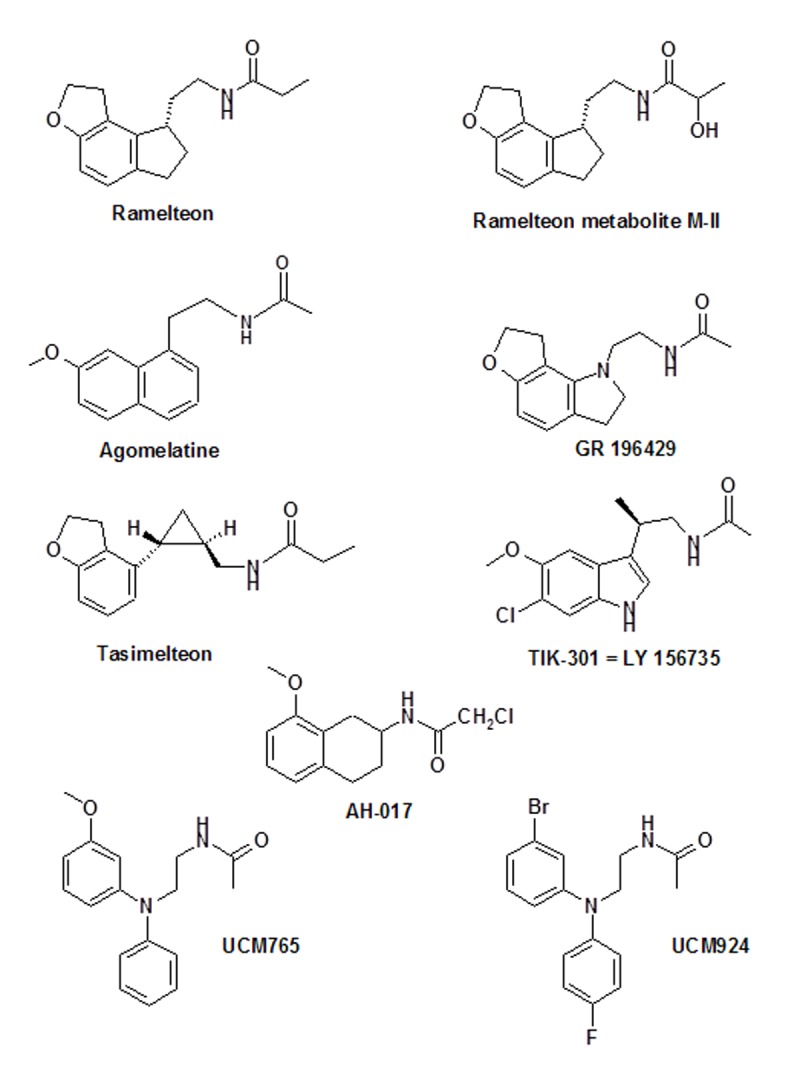
A selection of several approved or investigational melatonergic agonists. For various other agonists see ref. [19].
The affinities of the agonists towards human MT1 and MT2 receptors [19, 310–317] are summarized in Table 3. The natural hormone has a somewhat higher affinity to MT1 than to MT2. This is also the case with ramelteon and agomelatine, but not so with several other compounds. Even TIK-301, which is most closely related to melatonin, has a slightly higher affinity to MT2. This property is even more expressed in AH-017, UCM765 and UCM924. None of the compounds is strictly selective for one of the two melatonin membrane receptors, but the relative preference for MT2 is already very pronounced in UCM765 and UCM924, in which the affinities to the receptors differ by almost or even more than two orders of magnitude, respectively. Whether or not this property may turn out to be of value, e.g. in a possible future treatment of disorders and diseases related to MT2 signaling, remains to be studied.
Table 3:
Affinities of melatonin and a few selected melatonergic drugs towards human MT1 and MT2 receptors.
| Compound | pKi at MT1 | pKi at MT2 | References |
|---|---|---|---|
| Melatonin* | 10.09 | 9.42 | [310] |
| Ramelteon | 10.85 | 9.95 | [310] |
| Agomelatine | 10.21 | 9.57 | [311] |
| GR 196429 | 9.85 | 9.79 | [312,313] |
| Tasimelteon | 9.45 | 9.80 | [314] |
| TIK-301 | 10.09 | 10.38 | [315,316] |
| AH-017 | 8.27 | 9.16 | [313] |
| UCM765 | 8.38 | 10.17 | [316,317] |
| UCM924 | 6.75 | 9.27 | [317] |
Other, moderately deviating values from different laboratories are summarized in ref. [19]. pKi values were either directly obtained from respective publications or calculated from published Ki values.
A major obstacle for the use of melatonin as a clinically efficient drug results from its extremely short half-life in the circulation, which is mostly in the range of 20 - 30 min, sometimes even less, but maximally about 45 min [18, 318]. As a solution to this problem, controlled-release formulations of the natural hormone, such as Circadin®, have been developed or, alternately, synthetic drugs with a substantially longer half-life. Ramelteon is rapidly absorbed by the gastrointestinal tract (absorption rate: about 84%) and the circulating drug has a half-life in the range of 1 - 2 h [319]. Among the melatonergic agonists, ramelteon is the only one with considerably higher affinities to both receptor subtypes (cf. Table 3). Its metabolism differs profoundly from that of melatonin, although it is also substrate of hepatic cytochrome P450 monooxygenases (mainly CYP1A2, CYP2C and CYP3A). Among the metabolites, M-II (cf. Figure 2) is of particular interest, since it also acts as an agonist at MT1 and MT2 receptors, with an approximate potency of 10% compared to the parent compound. Despite its lower affinities, M-II is functionally relevant, since its circulating levels are 20 - 100 fold higher than those of ramelteon after systemic exposure. Moreover, the half-life of M-II is by 2 - 5 h longer than that of the parent compound [319].
The half-life of agomelatine, which has receptor affinities slightly above those of melatonin (Table 3), is also in the range of 1- 2 h. Contrary to melatonin and ramelteon, it displays the additional property as an antagonist of the serotonin receptor 5-HT2C. The inhibition of 5-HT2C signaling has been interpreted as the cause of agomelatine’s direct antidepressant actions [216, 321]. These have to be distinguished from indirect melatonergic actions related to adjustments of circadian rhythms, which are effective in subtypes of depression with an etiology of circadian dysfunction. However, a synergistic interaction of melatonergic and 5-HT2C signaling has been recently assumed to explain the antidepressant action of agomelatine [322].
The combination of properties as a melatonergic agonist and serotonergic antagonist has been recently described for TIK-301. This drug was reported to be an even more potent inhibitor of 5-HT2C and 5-HT2B receptors than agomelatine [19, 323]. Therefore, TIK-301 may also possess direct antidepressive properties, comparable to agomelatine, an assumption which would require further clinical substantiation. To date, studies have mainly focussed on soporific effects. The chlorine at ring atom 6 prevents hydroxylation by CYP isoenzymes in this position, i.e., the major catabolic pathway of the otherwise similar melatonin. However, the half-life of TIK-301 has been reported to be in the range of 1 h [315] and is, thus, only moderately extended. TIK-301 has received an orphan drug designation by FDA and can be used for treatment of sleep disorders in blind individuals.
Tasimelteon is another melatonergic drug that has been clinically tested [314], primarily with regard to sleep promotion and, in exploratory and pre-clinical trials, for antidepressive effects. However, no effects on 5-HT2C receptors are known for this compound. The half-life of tasimelteon seems to be longer than that of melatonin; in monkeys and rats, values around 2 h (between 1 and 3 hours) have been reported. For other pharmacokinetic data see ref. [324].
Pharmacokinetic and, especially, clinical data are poorly available for the other investigational agonists, whereas pre-clinical data on sleep do exist (cf. ref. [19]). GR 196429 and AH-017 were reported to increase the amplitude of the melatonin in rats. Deviations in the phase resetting properties of GR 196429 compared to other melatonergic drugs would require mechanistic explanations [19]. The functional importance and possible value of preferential binding to MT2 receptors described for AH-017 and, even more, UCM765 and UCM924 await further exploration. This aspect has been recently addressed in mice, in which UCM765 was shown to stimulate firing of reticular thalamic neurons in an MT2-dependent manner, thereby increasing non-REM sleep [325]. These compounds may turn out to be of interest for the treatment of disorders and diseases associated with impaired MT2 signaling. Finally, it should be noted that the agonist UCM924 was designed to extent the half-life by blocking a preferred hydroxylation site of UCM765 by attaching a fluorine atom, and by preventing O-demethylation by substituting the methoxy group by its bioisostere, bromine [317]. Another compound, NEU-P1, has received some attention because it was reported to inhibit weight gain and to improve insulin sensitivity in high-fat/high-sucrose-fed rats [300]. Additional data summarized elsewhere [19] have been published at abstract level and include effects on sleep. Chemical and pharmacokinetic data remain to be disclosed. According to high doses administered to rats, receptor affinities are presumably below those of compounds listed in Table 3.
Options for Treatment Based on Short-term Actions
Instead of repeating the numerous clinical data, especially on sleep parameters, which have been frequently reviewed [18, 19, 127, 128, 216, 236, 245, 246, 282, 285, 326–342], a general outline will be given on the strategies of treatment and their chronobiological rationale.
It is important to distinguish between disorders in which only short actions of melatonin are required and others in which a substitution therapy aims to replace insufficient nocturnal levels of the hormone throughout the night. In the first case, the short-lived natural hormone may suffice for treatment. A short action is sufficient in the case of sleep onset difficulties. Melatonin reduces sleep onset latency, frequently determined as LNA (latency to non-awake) or, by polysomnography, as LPS (latency to persistent sleep), already at low doses of 0.1 – 0.3 mg/d of an immediate release formulation [326]. However, the effects on sleep maintenance remain negligibly low, at these doses. A significant reduction of sleep onset latency has been observed with all synthetic melatonergic agonists so-far tested [18, 19, 314, 326–330, 333, 334, 341, 342]. However, the recommended doses of these drugs are considerably higher, such as 4 or 8 mg/d for ramelteon, despite its higher receptor affinity compared to melatonin, or 25 mg for agomelatine. Therefore, the synthetic drugs are not of advantage, as long as only improvements of sleep onset are intended. Without any doubt, the natural hormone has a preferable profile concerning tolerability and physiological metabolism. Moreover, MT1/MT2-independent effects are presumably absent in the synthetic drugs. However, the significance of these actions is still poorly understood.
Short actions are also sufficient if chronobiotic, i.e., phase shifting properties of melatonin are decisive. Resetting of the circadian oscillators is required in cases of rhythm perturbations. These may have been induced either (i) externally by light at night or transmeridian flights, (ii) by clocks poorly coupled to the environmental cycle or (iii) in cases of dysphasing or desynchronization within the multioscillator system. Insufficient coupling may result from flattened oscillations, especially under conditions of reduced melatonin secretion due to age or disease. For this reason, agents capable of enhancing rhythm amplitudes may become of interest. Phenomena such as relative coordination, internal desynchronization and abnormal phase relationships to external synchronizers or between parallel oscillators have been poorly investigated on a systematic basis, but they seem to be involved in impairments of physical and mental fitness as well as bipolar and seasonal affective disorders [230, 233, 343–347].
As far as circadian malfunctioning is implicated in these latter types of mood disorders, melatonin can be effective in readjusting rhythms and, thereby, improving symptoms. It is important to not confuse such effects with direct antidepressive actions, which are obviously also exerted by agomelatine and TIK-301. Treatments with synthetic melatonergic drugs can be expected to be beneficial on a circadian basis, but neither a higher receptor affinity nor a longer half-life are reasons for assuming a superior efficacy compared to melatonin. A short-acting chronobiotic such as melatonin is capable of inducing phase adjustments, because circadian oscillators are largely sensitive to a so-called non-parametric resetting [348], i.e., by stimuli in which the relative change is decisive rather than the absolute level of the synchronizer.
Concerning phase resetting, the treatment has to consider some fundamental chronobiological rules. Resetting signals are acting according to the respective PRC, as outlined above. The time of melatonin administration according to the human PRC [10, 11] is of utmost importance. Readjustment of rhythms by melatonin will only be achieved if it is given in an appropriate, sufficiently sensitive phase within the circadian cycle. If the rhythm is dysphased because of poor coupling to synchronizers, it may take several days more until the oscillation has attained the desired phase. Disregard of these chronobiological fundaments can lead to false conclusions on inefficacy.
Melatonin or synthetic melatonergic drugs are not the only means by which circadian rhythms are reset. As mentioned above, this is also possible by light, as long as light perception in the blue range and neuronal connections to the SCN are not impaired. Light therapy is, thus, an option in these cases. In some individuals, a combination of light and melatonin in the different, respective phases may be also suitable or of advantage. However, under conditions of poor accessibility of the SCN to light signals, melatonin may be preferred.
Limits of Substitution Therapies
The short-term melatonergic actions have to be clearly distinguished from a replacement therapy. This would be desired in aged individuals or patients suffering from the various diseases associated with decreases in melatonin levels (cf. Table 1). Because of the short half-life, immediate-release formulations of melatonin cannot afford a satisfactory substitution.
Therefore, synthetic agonists or a controlled-release melatonin formulation such as Circadin® should be assumed to be superior. With regard to melatonin’s exceptionally good tolerability, the pineal hormone may be tested first [18]. Among the synthetic drugs, ramelteon may be the choice in the USA, because of its approval by the FDA. Agomelatine, which is licensed in Europe, may give comparably good results. However, one should take notice of the restricted approvals of the tablets. Circadin® and ramelteon are only licensed for the treatment of insomnia, in the case of the controlled-release melatonin only for individuals of at least 55 years, agomelatine for the treatment of major depressive episodes in adults and TIK-301 for the use in blind people. Therefore, the full spectrum of possible applications is not covered by the approvals. This is particularly valid for agomelatine, which displays all the sleep-inducing and chronobiotic effects known from melatonin [216, 332, 333, 349]. From this point of view, it seems similarly suitable as ramelteon or melatonin.
In terms of efficacy, all these drugs have been reported to be beneficial concerning sleep maintenance or sleep quality. Although statistical measures have frequently reached significance, the extent of the improvements has remained relatively moderate. In elderly patients with primary chronic insomnia, the efficacy of ramelteon on sleep maintenance was recently found to be highly variable [350]. Despite some statistically demonstrable increases in sleep duration or sleep efficiency, these findings do not imply complete restoration of persistent sleep throughout the night [350–352]. The same can be concluded for the other melatonergic drugs tested for sleep maintenance [314, 316, 324, 340–342, 349, 353, 354]. Therefore, a convincing replacement therapy in melatonin deficiency has not yet been achieved with any of the melatonergic drugs, although they may have a moderate value in sleep efficiency and a good outcome concerning sleep initiation. Whether a replacement therapy will be possible by using much higher doses of melatonin, such as 50 or 100 mg/d, as recently suggested [342], remains to be tested. The standard dose of Circadin® is only 2 mg/d. In terms of tolerability and non-toxicity, doses of melatonin can be increased with less concern than in the case of synthetic drugs. Even 300 mg/d enterally have been administered for up to 2 years to ALS patients and found to be safe [355].
Concerning the use of agomelatine, a distinction between types of depression has to be made, as to whether they are based on circadian dysfunction or on other reasons. In the first case, short actions are only required for phase adjustments and, in chronobiological terms, the efficacy of agomelatine cannot be distinguished from those of other melatonergic agonists of similar receptor affinity. In major depressive disorders, symptoms are not primarily of circadian nature and their improvement has been attributed to the inhibition of 5-HT2C receptors or, perhaps, an interaction between MT1/MT2 and 5-HT2C signaling [322]. However, these properties are not comparable to effects by classic antidepressants. For this reason, some authors have considered the efficacy of agomelatine to be insufficient in major depressive disorder [356–358]. Another criticism concerned biased publication on the efficacy of this drug [359]. However, it seems necessary to clearly distinguish between modes of action. The advantage of agomelatine does not consist in a superior antidepressive effect, but rather in the combination of antidepressive benefits with sleep improvements. This dual action is important because sleep disturbances are often induced by classic antidepressants [285, 330, 332, 333].
Some other limits for melatonergic treatment can arise from drug interactions. This concerns mainly the synthetic agonists, which may attain undesired high concentrations in the presence of other drugs that inhibit CYP isoforms. For instance, ramelteon is mainly metabolized by CYP1A2, CYP2C9 and CYP3A4, agomelatine by CYP1A1, CYP1A2, and CYP2C9, tasimelteon by CYP1A1, CYP1A2, CYP2D6, and CYP2C9 [18]. Therefore, combined treatment with drugs such as fluvoxamine, ciprofloxacin, mexiletine, norfloxacin, azileuton, fluconazole or ketoconazole has to be avoided. In the case of melatonin, elevations caused by CYP inhibition are presumably relatively harmless, with regard to high doses of the methoxyindole applied in patients, volunteers and numerous pre-clinical experiments, without serious adverse effects. Nevertheless, caution is due with all melatonergic agonists including the natural hormone in autoimmune diseases, because of melatonin’s immune modulatory role, in Parkinson’s disease, as long as the controversy concerning interpretations by Willis [278, 279] has not been definitely settled, and with regard to reproductive function, especially in juveniles and during pregnancy. Melatonin has been applied in children and pregnant women under some conditions, but this requires a thorough weighing of benefits and possible risks. Concerning the approved synthetic agonists, hepatic and renal impairment, consumption of alcohol and high fat have been also listed as contraindications [326, 330].
Although all clinically tested melatonergic agonists were well tolerable and usually showed side effects in the placebo range during treatments of several weeks or months, the possibility of long-term toxicity seems to require further attention. Several recent papers indicate long-term safety of ramelteon in their titles [360–362], but they mainly address mild adverse events such as nausea and headache, some hepatological parameters, absence of residual effects, rebound insomnia, withdrawal symptoms and dependence for periods of six or twelve months, but do not exclude mutagenic or carcinogenic actions over extended periods of treatment. Studies on hepatotoxicity, micronuclei formation and mutagenicity have to consider not only properties of the parent compound, but also of the main metabolite M-II, which attains, e.g., concentrations in the range of one third of the no-effect level for tumor induction of ramelteon [326, 330]. Toxicological concerns may also exist in the case of agomelatine, which is well-tolerated during short-term treatment, but, being a naphthalenic compound, would require thorough studies on long-term toxicity, including CYP-related hepatic effects and, perhaps, carcinogenicity [18, 19, 330]. With regard to CYP-dependent metabolism, this is less a matter of drug interactions, which have be avoided or minimized in any case, but that of a possible formation of toxic metabolites, which is a frequent, fundamental problem of naphthalenic substances. The relatively high recommended dose of 25 mg/d should be taken into account. Risks of hepatotoxicity were recently re-addressed and it was criticized that this issue had not been prominently documented in the published studies [357, 359]. Moreover, oncogenicity of very high doses observed in animals experiments should be taken as a caveat [357]. The criteria of long-term safety, including properties of metabolites, have to apply correspondingly to the investigational melatonergic drugs. Their suitability and eventual superiority (i) will less depend on higher receptor affinities, since those of ramelteon may no be easily surpassed, (ii) may be associated with extended half-lifes, (iii) might emerge from effects in addition to melatonergic actions, but (iv) requires non-toxicity over extended periods of time.
Conclusion
Progressive reductions of melatonin secretion are one of the hallmarks of aging. Moreover, decreases in circulating melatonin and/or dysfunction of melatonergic signaling are associated with a remarkable number of age-related and other diseases. With regard to the dependence of the mammalian pineal gland on SCN functioning, to the feedback of melatonin to this central pacemaker and to actions of melatonin at peripheral oscillators, many but, presumably, not all effects of this hormone have to be understood on a chronobiological basis. This is particularly evident in CRSDs, in which melatonin or synthetic melatonergic agonists can be used for circadian phase shifting and, thus, resynchronizing oscillators to favorable phase relationships with external and among internal rhythms, thereby reducing sleep difficulties. Moreover, melatonin is capable of stimulating sleep initiation, via SCN and the hypothalamic sleep switch and, additionally, by acting on other brain areas such as the thalamus. The support of sleep maintenance by the natural hormone or by synthetic melatonergic agonists is also demonstrable but rather limited.
The chronobiotic effects of melatonin and its synthetic analogs seem to be decisive for treating mood disorders with an etiology of circadian dysfunction, such as seasonal affective and bipolar disorders. Agonists that only act via MT1 and MT2 receptors may have no or only marginal direct antidepressive effects. Conclusions on anxiolytic or antidepressive actions drawn from experiments in animal models using nocturnally active rodents should be regarded with caution, because melatonin is associated in these animals, contrary to humans, with enhanced neuronal, physical and metabolic activity, and also because sedating effects observed at high doses may be confused with anxiolysis or an antidepressive potential.
However, direct antidepressive effects are demonstrable in melatonergic agonists with additional properties as antagonists of the serotonergic receptor 5-HT2C, such as agomelatine and TIK-301. Whether a synergistic interaction between melatonergic signaling and 5-HT2C inhibition is of importance in these cases, as recently discussed [322], remains to be confirmed in more detailed studies. These drugs offer options for treating types of major depressive disorder, which are not successfully tractable with other melatonergic agonists. They also have the advantage of combining antidepressive actions with sleep-promoting effects, which is important because of sleep disturbances caused by other antidepressants. However, the entirely different mode of actions of these combined melatonergic agonists/serotonergic antagonists does not lead to effects as strong as observed with traditional antidepressants. Therefore, they may not be sufficient for treating severe cases.
Metabolic regulation by melatonin seems to become an important field of future research. Melatonin is obviously involved in the entire complex of obesity, metabolic syndrome, with consequences for cardiovascular diseases, insulin resistance, prediabetic changes and diabetes type II, as shown by deviations of melatonin secretion and signaling and receptor polymorphisms. This includes a network of interconnected signaling pathways, in which melatonin interacts, e.g., with metabolic sensors such as AMP-activated protein kinase (AMPK) and its downstream factors [5, 39], mechanisms that are also interrelated with circadian oscillators [39]. Moreover, mitochondrial effects of melatonin have to be taken into account, although the role of MT1/MT2 signaling is still poorly understood in this area. To date, neither the cell biological basis of these connections nor the clinical experience suffice for recommendations for a use of melatonin or its agonists in this field. Nevertheless, research in this direction seems to be promising.
Although an immense body of knowledge exists on neuroprotection by melatonin in animal and in vitro models, which includes, again, numerous effects such as antiexcitatory, antiinflammatory, direct and indirect antioxidative actions, the application of the pineal hormone or its analogs to humans has remained marginal. In AD, which is also characterized by deteriorations of melatonin secretion and circadian oscillators, some moderate and, sometimes, inconsistent effects have been described concerning sleep. Reductions of sundowning were observed [363] which can be of value especially for caregivers. However, these effects vanish during disease progression, and even the experience with animal models indicates that a late onset of melatonin treatment does not result in substantial benefits. A similarly pessimistic judgment, except for some sleep improvements, has to be given for other neurodegenerative diseases.
However, benefits of melatonin or, perhaps, synthetic melatonergic agonists, may be expected in the area of healthy aging. This should not be confused with life prolongation, which has been demonstrably achieved, in vertebrates, only in senescence-accelerated mice [364], but not to a convincing extent in wildtype animals [365]. Nevertheless, the experience with melatonin-treated rodents indicates an obvious healthier aging [4, 365]. It should be noted that, also in the context of aging, numerous metabolic cross-connections between melatonin and other signaling pathways exist, which include energy sensing, mitochondrial modulation and proliferation, redox sensing and actions of aging suppressor genes [269, 366]. Despite numerous publications that relate melatonin to aging and age-dependent dysfunctions in animal models, the application to humans has, in this field, more or less remained at a stage of discussion or a suggestion [367, 368].
References
- [1].Tan D-X, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–8. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- [2].Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin – Nature’s most versatile biological signal? FEBS J. 2006;273:2813–38. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- [3].Hardeland R. Melatonin, hormone of darkness and more – occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci. 2008;65:2001–18. doi: 10.1007/s00018-008-8001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hardeland R, Poeggeler B. Melatonin beyond its classical functions. Open Physiol J. 2008;1:1–23. [Google Scholar]
- [5].Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin – A pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- [6].Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665–70. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- [7].Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–48. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- [8].Huether G, Poeggeler B, Reimer A, George A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:945–53. doi: 10.1016/0024-3205(92)90402-b. [DOI] [PubMed] [Google Scholar]
- [9].Huether G. Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann NY Acad Sci. 1994;719:146–58. doi: 10.1111/j.1749-6632.1994.tb56826.x. [DOI] [PubMed] [Google Scholar]
- [10].Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- [11].Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586:639–47. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tricoire H, Locatelli A, Chemineau P, Malpaux B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology. 2002;143:84–90. doi: 10.1210/endo.143.1.8585. [DOI] [PubMed] [Google Scholar]
- [13].Tricoire H, Malpaux B, Møller M. Cellular lining of the sheep pineal recess studied by light-, transmission-, and scanning electron microscopy: morphologic indications for a direct secretion of melatonin from the pineal gland to the cerebrospinal fluid. J Comp Neurol. 2003;456:9–47. doi: 10.1002/cne.10477. [DOI] [PubMed] [Google Scholar]
- [14].Tricoire H, Møller M, Chemineau P, Malpaux B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod Suppl. 2003;61:311–21. [PubMed] [Google Scholar]
- [15].Maestroni GJ, Cardinali DP, Esquifino AI, Pandi-Perumal SR. Does melatonin play a disease-promoting role in rheumatoid arthritis? J Neuroimmunol. 2005;158:106–11. doi: 10.1016/j.jneuroim.2004.08.015. [DOI] [PubMed] [Google Scholar]
- [16].Cutolo M, Maestroni GJ. The melatonin-cytokine connection in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1109–11. doi: 10.1136/ard.2005.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Forrest CM, Mackay GM, Stoy N, Stone TW, Darlington LG. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br J Clin Pharmacol. 2007;64:517–26. doi: 10.1111/j.1365-2125.2007.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hardeland R. New approaches in the management of insomnia: weighing the advantages of prolonged release melatonin and synthetic melatoninergic agonists. Neuropsychiatr Dis Treat. 2009;5:341–54. doi: 10.2147/ndt.s4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hardeland R. Investigational melatonin receptor agonists. Expert Opin Investig Drugs. 2010;19:747–64. doi: 10.1517/13543784.2010.482926. [DOI] [PubMed] [Google Scholar]
- [20].Leu-Semenescu S, Arnulf I, Decaix C, Moussa F, Clot F, Boniol C, Touitou Y, Lévy R, Vidailhet M, Roze E. Sleep and rhythm consequences of a genetically induced loss of serotonin. Sleep. 2010;33:307–14. doi: 10.1093/sleep/33.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klein DC. Arylalkylamine N-acetyltransferase: “the timezyme”. J Biol Chem. 2007;282:423–7. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- [22].Liu T, Borjigin J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J Pineal Res. 2005;39:91–6. doi: 10.1111/j.1600-079X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- [23].Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboodiri MA. Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J Invest Dermatol. 1993;101:660–5. doi: 10.1111/1523-1747.ep12371672. [DOI] [PubMed] [Google Scholar]
- [24].Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–48. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270:3335–44. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- [26].Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–97. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- [27].Gómez-Corvera A, Cerrillo I, Molinero P, Naranjo MC, Lardone PJ, Sanchez-Hidalgo M, Carrascosa-Salmoral MP, Medrano-Campillo P, Guerrero JM, Rubio A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J Pineal Res. 2009;47:15–22. doi: 10.1111/j.1600-079X.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- [28].Hardeland R. Melatonin metabolism in the central nervous system. Curr Neuropharmacol. 2010;8:168–81. doi: 10.2174/157015910792246244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hardeland R, Poeggeler B. Actions of melatonin, its structural and functional analogs in the central nervous system and the significance of metabolism. Cent Nerv Syst Agents Med Chem. 2007;7:289–303. [Google Scholar]
- [30].Hardeland R, Tan D-X, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–26. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- [31].Hirata F, Hayaishi O, Tokuyama O, Senoh S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974;249:1311–3. [PubMed] [Google Scholar]
- [32].Niu S, Li F, Tan D-X, Zhang L, Idle JR, Gonzalez FJ, Ma X. Analysis of N1-acetyl-N2-formyl-5-methoxykynuramine/N1-acetyl-5-methoxy-kynuramine formation from melatonin in mice. J Pineal Res. 2010;49:106–14. doi: 10.1111/j.1600-079X.2010.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silva SO, Ximenes VF, Livramento JA, Catalani LH, Campa A. High concentrations of the melatonin metabolite, N1-acetyl-N2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. J Pineal Res. 2005;39:302–6. doi: 10.1111/j.1600-079X.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- [34].Tan D-X, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo Y-S, Hardeland R, Reiter RJ. N1-Acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–6. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- [35].Tan DX, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST, Vijayalaxmi, Shepherd AM. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–20. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- [36].Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–85. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- [37].Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA. 1995;92:8734–8. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hardeland R. Melatonin: Signaling mechanisms of a pleiotropic agent. BioFactors. 2009;35:183–92. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- [39].Hardeland R, Madrid JA, Tan D-X, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–66. doi: 10.1111/j.1600-079X.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- [40].Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- [41].Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin JM, Lefoulon F, Fauchère JL, Delagrange P, Canet E, Boutin JA. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- [42].Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol. 2001;61:1369–79. doi: 10.1016/s0006-2952(01)00615-3. [DOI] [PubMed] [Google Scholar]
- [43].Mailliet F, Ferry G, Vella F, Berger S, Cogé F, Chomarat P, Mallet C, Guenin SP, Guillaumet G, Viaud-Massuard MC, Yous S, Delagrange P, Boutin JA. Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem Pharmacol. 2005;71:74–88. doi: 10.1016/j.bcp.2005.09.030. [DOI] [PubMed] [Google Scholar]
- [44].Carlberg C, Wiesenberg I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. J Pineal Res. 1995;18:171–8. doi: 10.1111/j.1600-079x.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- [45].Carlberg C. Gene regulation by melatonin. Ann NY Acad Sci. 2000;917:387–96. doi: 10.1111/j.1749-6632.2000.tb05403.x. [DOI] [PubMed] [Google Scholar]
- [46].Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- [47].Tan D-X, Chen L-D, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- [48].Tan D-X, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- [49].Ressmeyer A-R, Mayo JC, Zelosko V, Sáinz RM, Tan D-X, Poeggeler B, Antolín I, Zsizsik BK, Reiter RJ, Hardeland R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–13. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- [50].Hardeland R. Antioxidative protection by melatonin – Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–30. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- [51].Rosen J, Than NN, Koch D, Poeggeler B, Laatsch H, Hardeland R. Interactions of melatonin and its metabolites with the ABTS cation radical: extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J Pineal Res. 2006;41:374–81. doi: 10.1111/j.1600-079X.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- [52].Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- [53].Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Güler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–28. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Silver R, Schwartz WJ. The suprachiasmatic nucleus is a functionally heterogeneous timekeeping organ. Methods Enzymol. 2005;393:451–65. doi: 10.1016/S0076-6879(05)93022-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–4. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- [57].de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- [58].de la Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- [59].Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pévet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci. 2003;17:221–8. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- [60].Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–64. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- [61].Stehle JH, von Gall C, Korf HW. Melatonin: a clock-output, a clock-input. J Neuroendocrinol. 2003;15:383–9. doi: 10.1046/j.1365-2826.2003.01001.x. [DOI] [PubMed] [Google Scholar]
- [62].Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13:303–28. doi: 10.1615/critrevoncog.v13.i4.30. [DOI] [PubMed] [Google Scholar]
- [63].Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- [64].Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–93. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- [65].Dijk DJ, Roth C, Landolt HP, Werth E, Aeppli M, Achermann P, Borbély AA. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett. 1995;201:13–6. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- [66].Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res. 2009;46:1–7. doi: 10.1111/j.1600-079X.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- [67].Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- [68].Bünning E. Das Weiterlaufen der „physiologischen Uhr“ im Säugerdarm ohne zentrale Steuerung. Naturwissenschaften. 1958;45:68. [Google Scholar]
- [69].Vansteensel MJ, Michel S, Meijer JH. Organization of cell and tissue circadian pacemakers: a comparison among species. Brain Res Rev. 2008;58:18–47. doi: 10.1016/j.brainresrev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- [70].Schibler U. The 2008 Pittendrigh/Aschoff lecture: Peripheral phase coordination in the mammalian circadian timing system. J Biol Rhythms. 2009;24:3–15. doi: 10.1177/0748730408329383. [DOI] [PubMed] [Google Scholar]
- [71].Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–76. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- [72].Torres-Farfan C, Serón-Ferré M, Dinet V, Korf HW. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2006;40:64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- [73].Dinet V, Ansari N, Torres-Farfan C, Korf HW. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2007;42:83–91. doi: 10.1111/j.1600-079X.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- [74].Campino C, Valenzuela FJ, Torres-Farfan C, Reynolds HE, Abarzua-Catalan L, Arteaga E, Trucco C, Guzmán S, Valenzuela GJ, Seron-Ferre M. Melatonin exerts direct inhibitory actions on ACTH responses in the human adrenal gland. Horm Metab Res. 2011;43:337–42. doi: 10.1055/s-0031-1271693. [DOI] [PubMed] [Google Scholar]
- [75].Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N, Manev H, Uz T. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal ‘clock’ gene expression in striatal neurons in vitro. J Pineal Res. 2009;46:87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- [76].Agez L, Laurent V, Guerrero HY, Pévet P, Masson-Pévet M, Gauer F. Endogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the rat. J Pineal Res. 2009;46:95–105. doi: 10.1111/j.1600-079X.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- [77].Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. Human melatonin production decreases with age. J Pineal Res. 1986;3:379–88. doi: 10.1111/j.1600-079x.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- [78].Karasek M, Reiter RJ. Melatonin and aging. Neuroendocrinol Lett. 2002;23(Suppl 1):14–6. [PubMed] [Google Scholar]
- [79].Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- [80].Cardinali DP, Esquifino AI, Srinivasan V, Pandi-Perumal SR. Melatonin and the immune system in aging. Neuroimmunomodulation. 2008;15:272–8. doi: 10.1159/000156470. [DOI] [PubMed] [Google Scholar]
- [81].Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pévet P, Ravid D, Swaab DF. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res. 1990;528:170–4. doi: 10.1016/0006-8993(90)90214-v. [DOI] [PubMed] [Google Scholar]
- [82].Kripke DF, Youngstedt SD, Elliott JA, Tuunainen A, Rex KM, Hauger RL, Marler MR. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- [83].Brown GM, Young SN, Gauthier S, Tsui H, Grota LJ. Melatonin in human cerebrospinal fluid in daytime; its origin and variation with age. Life Sci. 1979;25:929–36. doi: 10.1016/0024-3205(79)90498-3. [DOI] [PubMed] [Google Scholar]
- [84].Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-ε4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–7. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- [85].Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001;31:264–72. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- [86].Mahlberg R, Tilmann A, Salewski L, Kunz D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 2006;31:634–41. doi: 10.1016/j.psyneuen.2006.01.009. [DOI] [PubMed] [Google Scholar]
- [87].Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- [88].Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–6. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- [89].Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8:623–36. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- [90].Schmid HA. Decreased melatonin biosynthesis, calcium flux, pineal gland calcification and aging: a hypothetical framework. Gerontology. 1993;39:189–99. doi: 10.1159/000213533. [DOI] [PubMed] [Google Scholar]
- [91].Kunz D, Schmitz S, Mahlberg R, Mohr A, Stoter C, Wolf KJ, Herrmann WM. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacology. 1999;21:765–72. doi: 10.1016/S0893-133X(99)00069-X. [DOI] [PubMed] [Google Scholar]
- [92].Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717:154–9. doi: 10.1016/0006-8993(96)00086-8. [DOI] [PubMed] [Google Scholar]
- [93].Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–21. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- [94].Ohashi Y, Okamoto N, Uchida K, Iyo M, Mori N, Morita Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol Psychiatry. 1999;45:1646–52. doi: 10.1016/s0006-3223(98)00255-8. [DOI] [PubMed] [Google Scholar]
- [95].Ferrari E, Fioravanti M, Magri F, Solerte SB. Variability of interactions between neuroendocrine and immunological functions in physiological aging and dementia of the Alzheimer’s type. Ann NY Acad Sci. 2000;917:582–96. doi: 10.1111/j.1749-6632.2000.tb05424.x. [DOI] [PubMed] [Google Scholar]
- [96].Commentz JC, Helmke K. Precocious puberty and decreased melatonin secretion due to a hypothalamic hamartoma. Horm Res. 1995;44:271–5. doi: 10.1159/000184640. [DOI] [PubMed] [Google Scholar]
- [97].Müller HL, Handwerker G, Wollny B, Faldum A, Sörensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab. 2002;87:3993–6. doi: 10.1210/jcem.87.8.8751. [DOI] [PubMed] [Google Scholar]
- [98].Müller HL, Handwerker G, Gebhardt U, Faldum A, Emser A, Kolb R, Sörensen N. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control. 2006;17:583–9. doi: 10.1007/s10552-005-9012-7. [DOI] [PubMed] [Google Scholar]
- [99].Lipton J, Megerian JT, Kothare SV, Cho YJ, Shanahan T, Chart H, Ferber R, Adler-Golden L, Cohen LE, Czeisler CA, Pomeroy SL. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology. 2009;73:323–5. doi: 10.1212/WNL.0b013e3181af78a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Miyamoto A, Oki J, Takahashi S, Okuno A. Serum melatonin kinetics and long-term melatonin treatment for sleep disorders in Rett syndrome. Brain Dev. 1999;21:59–62. doi: 10.1016/s0387-7604(98)00072-2. [DOI] [PubMed] [Google Scholar]
- [101].Yamashita Y, Matsuishi T, Murakami Y, Kato H. Sleep disorder in Rett syndrome and melatonin treatment. Brain Dev. 1999;21:570. doi: 10.1016/s0387-7604(99)00071-6. [DOI] [PubMed] [Google Scholar]
- [102].Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol Psychiatry. 2005;57:134–8. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [103].Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsäter H, Rastam M, Ståhlberg O, Gillberg IC, Delorme R, Chabane N, Mouren-Simeoni MC, Fauchereau F, Durand CM, Chevalier F, Drouot X, Collet C, Launay JM, Leboyer M, Gillberg C, Bourgeron T. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13:90–8. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Leu RM, Beyderman L, Botzolakis EJ, Surdyka K, Wang L, Malow BA. Relation of melatonin to sleep architecture in children with autism. J Autism Dev Disord. 2011;41:427–33. doi: 10.1007/s10803-010-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783–92. doi: 10.1111/j.1469-8749.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- [107].Monteleone P, Maj M, Fusco M, Kemali D, Reiter RJ. Depressed nocturnal plasma melatonin levels in drug-free paranoid schizophrenics. Schizophr Res. 1992;7:77–84. doi: 10.1016/0920-9964(92)90077-i. [DOI] [PubMed] [Google Scholar]
- [108].Viganò D, Lissoni P, Rovelli F, Roselli MG, Malugani F, Gavazzeni C, Conti A, Maestroni G. A study of light/dark rhythm of melatonin in relation to cortisol and prolactin secretion in schizophrenia. Neuroendocrinol Lett. 2001;22:137–41. [PubMed] [Google Scholar]
- [109].Akpinar Z, Tokgöz S, Gökbel H, Okudan N, Uğuz F, Yilmaz G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res. 2008;161:253–7. doi: 10.1016/j.psychres.2007.11.022. [DOI] [PubMed] [Google Scholar]
- [110].Catapano F, Monteleone P, Fuschino A, Maj M, Kemali D. Melatonin and cortisol secretion in patients with primary obsessive-compulsive disorder. Psychiatry Res. 1992;44:217–25. doi: 10.1016/0165-1781(92)90025-x. [DOI] [PubMed] [Google Scholar]
- [111].Aoki M, Yokota Y, Hayashi T, Kuze B, Murai M, Mizuta K, Ito Y. Disorder of the saliva melatonin circadian rhythm in patients with Meniere’s disease. Acta Neurol Scand. 2006;113:256–61. doi: 10.1111/j.1600-0404.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- [112].Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, Tone P, McCormick SA, Walsh J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–9. [PMC free article] [PubMed] [Google Scholar]
- [113].Bazil CW, Short D, Crispin D, Zheng W. Patients with intractable epilepsy have low melatonin, which increases following seizures. Neurology. 2000;55:1746–8. doi: 10.1212/wnl.55.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Uberos J, Augustin-Morales MC, Molina Carballo A, Florido J, Narbona E, Muñoz-Hoyos A. Normalization of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J Pineal Res. 2011;50:192–6. doi: 10.1111/j.1600-079X.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- [115].Molina-Carballo A, Muñoz-Hoyos A, Sánchez-Forte M, Uberos-Fernández J, Moreno-Madrid F, Acuña-Castroviejo D. Melatonin increases following convulsive seizures may be related to its anticonvulsant properties at physiological concentrations. Neuropediatrics. 2007;38:122–5. doi: 10.1055/s-2007-985138. [DOI] [PubMed] [Google Scholar]
- [116].Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408. doi: 10.1016/s0140-6736(95)92600-3. [DOI] [PubMed] [Google Scholar]
- [117].Girotti L, Lago M, Ianovsky O, Carbajales J, Elizari MV, Brusco LI, Cardinali DP. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J Pineal Res. 2000;29:138–42. doi: 10.1034/j.1600-079x.2000.290302.x. [DOI] [PubMed] [Google Scholar]
- [118].Sewerynek E. Melatonin and the cardiovascular system. Neuroendocrinol Lett. 2002;23(Suppl 1):79–83. [PubMed] [Google Scholar]
- [119].Altun A, Yaprak M, Aktoz M, Vardar A, Betul UA, Ozbay G. Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neurosci Lett. 2002;327:143–5. doi: 10.1016/s0304-3940(02)00368-3. [DOI] [PubMed] [Google Scholar]
- [120].Yaprak M, Altun A, Vardar A, Aktoz M, Ciftci S, Ozbay G. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int J Cardiol. 2003;89:103–7. doi: 10.1016/s0167-5273(02)00461-8. [DOI] [PubMed] [Google Scholar]
- [121].Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Samimi-Fard S, Kaski JC, Reiter RJ. Light/dark patterns of soluble vascular cell adhesion molecule-1 in relation to melatonin in patients with ST-segment elevation myocardial infarction. J Pineal Res. 2008;44:65–9. doi: 10.1111/j.1600-079X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- [122].Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Clinical aspects of melatonin in the acute coronary syndrome. Curr Vasc Pharmacol. 2009;7:367–73. doi: 10.2174/157016109788340749. [DOI] [PubMed] [Google Scholar]
- [123].Rohr UD, Herold J. Melatonin deficiencies in women. Maturitas. 2002;41(Suppl 1):S85–104. doi: 10.1016/s0378-5122(02)00017-8. [DOI] [PubMed] [Google Scholar]
- [124].Acuña-Castroviejo D, Escames G, Reiter RJ. Melatonin therapy in fibromyalgia. J Pineal Res. 2006;40:98–9. doi: 10.1111/j.1600-079X.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- [125].Citera G, Arias MA, Maldonado-Cocco JA, Lázaro MA, Rosemffet MG, Brusco LI, Scheines EJ, Cardinali DP. The effect of melatonin in patients with fibromyalgia: A pilot study. Clin Rheumatol. 2000;19:9–13. doi: 10.1007/s100670050003. [DOI] [PubMed] [Google Scholar]
- [126].Reiter RJ, Acuña-Castroviejo D, Tan D-X. Melatonin therapy in fibromyalgia. Curr Pain Headache Rep. 2007;11:339–42. doi: 10.1007/s11916-007-0215-3. [DOI] [PubMed] [Google Scholar]
- [127].Sánchez-Barceló EJ, Mediavilla MD, Tan D-X, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–95. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- [128].Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011;51:270–7. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- [129].Claustrat B, Loisy C, Brun J, Beorchia S, Arnaud JL, Chazot G. Nocturnal plasma melatonin levels in migraine: a preliminary report. Headache. 1989;29:242–5. doi: 10.1111/j.1526-4610.1989.hed22904242.x. [DOI] [PubMed] [Google Scholar]
- [130].Claustrat B, Brun J, Geoffriau M, Zaidan R, Mallo C, Chazot G. Nocturnal plasma melatonin profile and melatonin kinetics during infusion in status migrainosus. Cephalalgia. 1997;17:511–7. doi: 10.1046/j.1468-2982.1997.1704511.x. [DOI] [PubMed] [Google Scholar]
- [131].Perras B, Kurowski V, Dodt C. Nocturnal melatonin concentration is correlated with illness severity in patients with septic disease. Intensive Care Med. 2006;32:624–5. doi: 10.1007/s00134-006-0069-x. [DOI] [PubMed] [Google Scholar]
- [132].Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33:1954–8. doi: 10.1007/s00134-007-0769-x. [DOI] [PubMed] [Google Scholar]
- [133].Srinivasan V, Pandi-Perumal SR, Spence DW, Kato H, Cardinali DP. Melatonin in septic shock: Some recent concepts. J Crit Care. 2010;25:656.e1–656.e6. doi: 10.1016/j.jcrc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [134].Shigeta H, Yasui A, Nimura Y, Machida N, Kageyama M, Miura M, Menjo M, Ikeda K. Postoperative delirium and melatonin levels in elderly patients. Am J Surg. 2001;182:449–54. doi: 10.1016/s0002-9610(01)00761-9. [DOI] [PubMed] [Google Scholar]
- [135].Grin W, Grünberger W. A significant correlation between melatonin deficiency and endometrial cancer. Gynecol Obstet Invest. 1998;45:62–5. doi: 10.1159/000009926. [DOI] [PubMed] [Google Scholar]
- [136].Hu S, Shen G, Yin S, Xu W, Hu B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv Ther. 2009;26:886–92. doi: 10.1007/s12325-009-0068-8. [DOI] [PubMed] [Google Scholar]
- [137].Puy H, Deybach JC, Baudry P, Callebert J, Touitou Y, Nordmann Y. Decreased nocturnal plasma melatonin levels in patients with recurrent acute intermittent porphyria attacks. Life Sci. 1993;53:621–7. doi: 10.1016/0024-3205(93)90271-4. [DOI] [PubMed] [Google Scholar]
- [138].Bylesjö I, Forsgren L, Wetterberg L. Melatonin and epileptic seizures in patients with acute intermittent porphyria. Epileptic Disord. 2000;2:203–8. [PubMed] [Google Scholar]
- [139].Luboshitzky R, Wagner O, Lavi S, Herer P, Lavie P. Decreased nocturnal melatonin secretion in patients with Klinefelter’s syndrome. Clin Endocrinol (Oxf) 1996;45:749–54. doi: 10.1046/j.1365-2265.1996.8710881.x. [DOI] [PubMed] [Google Scholar]
- [140].Luboshitzky R, Wagner O, Lavi S, Herer P, Lavie P. Abnormal melatonin secretion in hypogonadal men: the effect of testosterone treatment. Clin Endocrinol (Oxf) 1997;47:463–9. doi: 10.1046/j.1365-2265.1997.2881089.x. [DOI] [PubMed] [Google Scholar]
- [141].O’Brien IA, Lewin IG, O’Hare JP, Arendt J, Corrall RJ. Abnormal circadian rhythm of melatonin in diabetic autonomic neuropathy. Clin Endocrinol (Oxf) 1986;24:359–64. doi: 10.1111/j.1365-2265.1986.tb01639.x. [DOI] [PubMed] [Google Scholar]
- [142].Peschke E, Stumpf I, Bazwinsky I, Litvak L, Dralle H, Mühlbauer E. Melatonin and type 2 diabetes - a possible link? J Pineal Res. 2007;42:350–8. doi: 10.1111/j.1600-079X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- [143].Toma C, Rossi M, Sousa I, Blasi F, Bacchelli E, Alen R, Vanhala R, Monaco AP, Järvelä I, Maestrini E, International Molecular Genetic Study of Autism Consortium Is ASMT a susceptibility gene for autism spectrum disorders? A replication study in European populations. Mol Psychiatry. 2007;12:977–99. doi: 10.1038/sj.mp.4002069. [DOI] [PubMed] [Google Scholar]
- [144].Jonsson L, Ljunggren E, Bremer A, Pedersen C, Landén M, Thuresson K, Giacobini M, Melke J. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med Genomics. 2010;3:10. doi: 10.1186/1755-8794-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chaste P, Clement N, Botros HG, Guillaume JL, Konyukh M, Pagan C, Scheid I, Nygren G, Anckarsäter H, Rastam M, Ståhlberg O, Gillberg IC, Melke J, Delorme R, Leblond C, Toro R, Huguet G, Fauchereau F, Durand C, Boudarene L, Serrano E, Lemière N, Launay JM, Leboyer M, Jockers R, Gillberg C, Bourgeron T. Genetic variations of the melatonin pathway in patients with attention-deficit and hyperactivity disorders. J Pineal Res. 2011;51:394–9. doi: 10.1111/j.1600-079X.2011.00902.x. [DOI] [PubMed] [Google Scholar]
- [146].Park HJ, Park JK, Kim SK, Cho AR, Kim JW, Yim SV, Chung JH. Association of polymorphism in the promoter of the melatonin receptor 1A gene with schizophrenia and with insomnia symptoms in schizophrenia patients. J Mol Neurosci. 2011;45:304–8. doi: 10.1007/s12031-011-9522-6. [DOI] [PubMed] [Google Scholar]
- [147].Soria V, Martínez-Amorós E, Escaramís G, Valero J, Crespo JM, Gutiérrez-Zotes A, Bayés M, Martorell L, Vilella E, Estivill X, Menchón JM, Gratacòs M, Urretavizcaya M. Resequencing and association analysis of arylalkylamine N-acetyltransferase (AANAT) gene and its contribution to major depression susceptibility. J Pineal Res. 2010;49:35–44. doi: 10.1111/j.1600-079X.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- [148].Gałecki P, Szemraj J, Bartosz G, Bieńkiewicz M, Gałecka E, Florkowski A, Lewiński A, Karbownik-Lewińska M. Single-nucleotide polymorphisms and mRNA expression for melatonin synthesis rate-limiting enzyme in recurrent depressive disorder. J Pineal Res. 2010;48:311–7. doi: 10.1111/j.1600-079X.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- [149].Thomson PA, Wray NR, Thomson AM, Dunbar DR, Grassie MA, Condie A, Walker MT, Smith DJ, Pulford DJ, Muir W, Blackwood DH, Porteous DJ. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10:470–8. doi: 10.1038/sj.mp.4001593. [DOI] [PubMed] [Google Scholar]
- [150].Macintyre DJ, McGhee KA, Maclean AW, Afzal M, Briffa K, Henry B, Thomson PA, Muir WJ, Blackwood DH. Association of GPR50, an X-linked orphan G protein-coupled receptor, and affective disorder in an independent sample of the Scottish population. Neurosci Lett. 2010;475:169–73. doi: 10.1016/j.neulet.2010.03.072. [DOI] [PubMed] [Google Scholar]
- [151].Delavest M, Even C, Benjemaa N, Poirier MF, Jockers R, Krebs MO. Association of the intronic rs2072621 polymorphism of the X-linked GPR50 gene with affective disorder with seasonal pattern. Eur Psychiatry. 2011 2011 May 10; doi: 10.1016/j.eurpsy.2011.02.011. [DOI] [PubMed] [Google Scholar]
- [152].Ha E, Choe BK, Jung KH, Yoon SH, Park HJ, Park HK, Yim SV, Chung JH, Bae HS, Nam M, Baik HH, Hong SJ. Positive relationship between melatonin receptor type 1B polymorphism and rheumatoid factor in rheumatoid arthritis patients in the Korean population. J Pineal Res. 2005;39:201–5. doi: 10.1111/j.1600-079X.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- [153].Qiu XS, Tang NL, Yeung HY, Lee KM, Hung VW, Ng BK, Ma SL, Kwok RH, Qin L, Qiu Y, Cheng JC. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2007;32:1748–53. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- [154].Mórocz M, Czibula A, Grózer ZB, Szécsényi A, Almos PZ, Raskó I, Illés T. Association study of BMP4, IL6, Leptin, MMP3, and MTNR1B gene promoter polymorphisms and adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:E123–30. doi: 10.1097/BRS.0b013e318a511b0e. [DOI] [PubMed] [Google Scholar]
- [155].Samimi-Fard S, Abreu-Gonzalez P, Dominguez-Rodriguez A, Jimenez-Sosa A. JPI 51 A case–control study of melatonin receptor type 1A polymorphism and acute myocardial infarction in a Spanish population. J Pineal Res. 2011;51:400–4. doi: 10.1111/j.1600-079X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- [156].Staiger H, Machicao F, Schäfer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Häring HU, Fritsche A. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine β-cell function. PLoS One. 2008;3:e3962. doi: 10.1371/journal.pone.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chèvre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jørgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Lévy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- [159].Sparsø T, Bonnefond A, Andersson E, Bouatia-Naji N, Holmkvist J, Wegner L, Grarup N, Gjesing AP, Banasik K, Cavalcanti-Proença C, Marchand M, Vaxillaire M, Charpentier G, Jarvelin MR, Tichet J, Balkau B, Marre M, Lévy-Marchal C, Faerch K, Borch-Johnsen K, Jørgensen T, Madsbad S, Poulsen P, Vaag A, Dina C, Hansen T, Pedersen O, Froguel P. G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes. 2009;58:1450–6. doi: 10.2337/db08-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Rönn T, Wen J, Yang Z, Lu B, Du Y, Groop L, Hu R, Ling C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009;52:830–3. doi: 10.1007/s00125-009-1297-8. [DOI] [PubMed] [Google Scholar]
- [161].Stančáková A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, Boehnke M, Kuusisto J, Laakso M. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. 2009;58:2129–36. doi: 10.2337/db09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Reiling E, van ‘t Riet E, Groenewoud MJ, Welschen LM, van Hove EC, Nijpels G, Maassen JA, Dekker JM, ‘t Hart LM. Combined effects of single-nucleotide polymorphisms in GCK, GCKR, G6PC2 and MTNR1B on fasting plasma glucose and type 2 diabetes risk. Diabetologia. 2009;52:1866–70. doi: 10.1007/s00125-009-1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Chambers JC, Zhang W, Zabaneh D, Sehmi J, Jain P, McCarthy MI, Froguel P, Ruokonen A, Balding D, Jarvelin MR, Scott J, Elliott P, Kooner JS. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes. 2009;58:2703–8. doi: 10.2337/db08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kelliny C, Ekelund U, Andersen LB, Brage S, Loos RJ, Wareham NJ, Langenberg C. Common genetic determinants of glucose homeostasis in healthy children: the European Youth Heart Study. Diabetes. 2009;58:2939–45. doi: 10.2337/db09-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].‘t Hart LM, Simonis-Bik AM, Nijpels G, van Haeften TW, Schäfer SA, Houwing-Duistermaat JJ, Boomsma DI, Groenewoud MJ, Reiling E, van Hove EC, Diamant M, Kramer MH, Heine RJ, Maassen JA, Kirchhoff K, Machicao F, Häring HU, Slagboom PE, Willemsen G, Eekhoff EM, de Geus EJ, Dekker JM, Fritsche A. Combined risk allele score of eight type 2 diabetes genes is associated with reduced first-phase glucose-stimulated insulin secretion during hyperglycemic clamps. Diabetes. 2010;59:287–92. doi: 10.2337/db09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhao J, Bradfield JP, Zhang H, Annaiah K, Wang K, Kim CE, Glessner JT, Frackelton EC, Otieno FG, Doran J, Thomas KA, Garris M, Hou C, Chiavacci RM, Li M, Berkowitz RI, Hakonarson H, Grant SF. Examination of all type 2 diabetes GWAS loci reveals HHEX-IDE as a locus influencing pediatric BMI. Diabetes. 2010;59:751–5. doi: 10.2337/db09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Takeuchi F, Katsuya T, Chakrewarthy S, Yamamoto K, Fujioka A, Serizawa M, Fujisawa T, Nakashima E, Ohnaka K, Ikegami H, Sugiyama T, Nabika T, Kasturiratne A, Yamaguchi S, Kono S, Takayanagi R, Yamori Y, Kobayashi S, Ogihara T, de Silva A, Wickremasinghe R, Kato N. Common variants at the GCK, GCKR, G6PC2-ABCB11 and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia. 2010;53:299–308. doi: 10.1007/s00125-009-1595-1. [DOI] [PubMed] [Google Scholar]
- [168].Liu C, Wu Y, Li H, Langenberg C, Loos RJ, Lin X. MTNR1B rs10830963 is associated with fasting plasma glucose, HbA1C and impaired β-cell function in Chinese Hans from Shanghai. BMC Med Genet. 2010;11:59. doi: 10.1186/1471-2350-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kan MY, Zhou DZ, Zhang D, Zhang Z, Chen Z, Yang YF, Guo XZ, Xu H, He L, Liu Y. Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet Med. 2010;2010;27:598–602. doi: 10.1111/j.1464-5491.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- [170].Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, Martin M, So WY, Ma RC, Chan JC, Ng MC. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired β-cell function in Chinese subjects. PLoS One. 2010;5:e11428. doi: 10.1371/journal.pone.0011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hu C, Zhang R, Wang C, Yu W, Lu J, Ma X, Wang J, Jiang F, Tang S, Bao Y, Xiang K, Jia W. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS One. 2010;5:e11761. doi: 10.1371/journal.pone.0011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Simonis-Bik AM, Nijpels G, van Haeften TW, Houwing-Duistermaat JJ, Boomsma DI, Reiling E, van Hove EC, Diamant M, Kramer MH, Heine RJ, Maassen JA, Slagboom PE, Willemsen G, Dekker JM, Eekhoff EM, de Geus EJ, ‘t Hart LM. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic β-cell function. Diabetes. 2010;59:293–301. doi: 10.2337/db09-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Müssig K, Staiger H, Machicao F, Häring HU, Fritsche A. Genetic variants in MTNR1B affecting insulin secretion. Ann Med. 2010;42:387–93. doi: 10.3109/07853890.2010.502125. [DOI] [PubMed] [Google Scholar]
- [174].Renström F, Shungin D, Johansson I, MAGIC Investigators. Florez JC, Hallmans G, Hu FB, Franks PW. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes. 2011;60:345–54. doi: 10.2337/db10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Holzapfel C, Siegrist M, Rank M, Langhof H, Grallert H, Baumert J, Irimie C, Klopp N, Wolfarth B, Illig T, Hauner H, Halle M. Association of a MTNR1B gene variant with fasting glucose and HOMA-B in children and adolescents with high BMI-SDS. Eur J Endocrinol. 2011;164:205–12. doi: 10.1530/EJE-10-0588. [DOI] [PubMed] [Google Scholar]
- [176].Olsson L, Pettersen E, Ahlbom A, Carlsson S, Midthjell K, Grill V. No effect by the common gene variant rs10830963 of the melatonin receptor 1B on the association between sleep disturbances and type 2 diabetes: results from the Nord-Trøndelag Health Study. Diabetologia. 2011;54:1375–8. doi: 10.1007/s00125-011-2106-8. [DOI] [PubMed] [Google Scholar]
- [177].Barker A, Sharp SJ, Timpson NJ, Bouatia-Naji N, Warrington NM, Kanoni S, Beilin LJ, Brage S, Deloukas P, Evans DM, Grontved A, Hassanali N, Lawlor DA, Lecoeur C, Loos RJ, Lye SJ, McCarthy MI, Mori TA, Ndiaye NC, Newnham JP, Ntalla I, Pennell CE, St Pourcain B, Prokopenko I, Ring SM, Sattar N, Visvikis-Siest S, Dedoussis GV, Palmer LJ, Froguel P, Smith GD, Ekelund U, Wareham NJ, Langenberg C. Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes. 2011;60:1805–12. doi: 10.2337/db10-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Song JY, Wang HJ, Ma J, Xu ZY, Hinney A, Hebebrand J, Wang Y. Association of the rs10830963 polymorphism in MTNR1B with fasting glucose levels in Chinese children and adolescents. Obes Facts. 2011;4:197–203. doi: 10.1159/000329306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Reinehr T, Scherag A, Wang HJ, Roth CL, Kleber M, Scherag S, Boes T, Vogel C, Hebebrand J, Hinney A. Relationship between MTNR1B (melatonin receptor 1B gene) polymorphism rs10830963 and glucose levels in overweight children and adolescents. Pediatr Diabetes. 2011;12:435–41. doi: 10.1111/j.1399-5448.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- [180].Ling Y, Li X, Gu Q, Chen H, Lu D, Gao X. A common polymorphism rs3781637 in MTNR1B is associated with type 2 diabetes and lipids levels in Han Chinese individuals. Cardiovasc Diabetol. 2011;10:27. doi: 10.1186/1475-2840-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Bhattacharyya S, Luan J, Challis B, Keogh J, Montague C, Brennand J, Morten J, Lowenbeim S, Jenkins S, Farooqi IS, Wareham NJ, O’Rahilly S. Sequence variants in the melatonin-related receptor gene (GPR50) associate with circulating triglyceride and HDL levels. J Lipid Res. 2006;47:761–6. doi: 10.1194/jlr.M500338-JLR200. [DOI] [PubMed] [Google Scholar]
- [182].Li C, Shi Y, You L, Wang L, Chen ZJ. Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol Obstet Invest. 2011;72:130–4. doi: 10.1159/000323542. [DOI] [PubMed] [Google Scholar]
- [183].Li C, Shi Y, You L, Wang L, Chen ZJ. Association of rs10830963 and rs10830962 SNPs in the melatonin receptor (MTNR1B) gene among Han Chinese women with polycystic ovary syndrome. Mol Hum Reprod. 2011;17:193–8. doi: 10.1093/molehr/gaq087. [DOI] [PubMed] [Google Scholar]
- [184].Dufourny L, Levasseur A, Migaud M, Callebaut I, Pontarotti P, Malpaux B, Monget P. GPR50 is the mammalian ortholog of Mel1c: evidence of rapid evolution in mammals. BMC Evol Biol. 2008;8:105. doi: 10.1186/1471-2148-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006;25:3012–23. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ivanova EA, Bechtold DA, Dupre SM, Brennand J, Barrett P, Luckman SM, Loudon AS. Altered metabolism in the melatonin-related receptor (GPR50) knockout mouse. Am J Physiol Endocrinol Metab. 2008;294:E176–82. doi: 10.1152/ajpendo.00199.2007. [DOI] [PubMed] [Google Scholar]
- [187].Grünewald E, Kinnell HL, Porteous DJ, Thomson PA. GPR50 interacts with neuronal NOGO-A and affects neurite outgrowth. Mol Cell Neurosci. 2009;42:363–71. doi: 10.1016/j.mcn.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [188].Li J, Hand LE, Meng QJ, Loudon AS, Bechtold DA. GPR50 interacts with TIP60 to modulate glucocorticoid receptor signalling. PLoS One. 2011;2011;6:e23725. doi: 10.1371/journal.pone.0023725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92:1439–44. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sack RL, Lewy AJ, Blood ML, Stevenson J, Keith LD. Melatonin administration to blind people: phase advances and entrainment. J Biol Rhythms. 1991;6:249–61. doi: 10.1177/074873049100600305. [DOI] [PubMed] [Google Scholar]
- [191].Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–34. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- [192].Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- [193].Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- [194].Emens JS, Lewy AJ, Lefler BJ, Sack RL. Relative coordination to unknown “weak zeitgebers” in free-running blind individuals. J Biol Rhythms. 2005;20:159–67. doi: 10.1177/0748730404273294. [DOI] [PubMed] [Google Scholar]
- [195].Emens J, Lewy AJ, Laurie AL, Songer JB. Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods. J Biol Rhythms. 2010;25:381–4. doi: 10.1177/0748730410379080. [DOI] [PubMed] [Google Scholar]
- [196].Reiter RJ, Richardson BA. Some perturbations that disturb the circadian melatonin rhythm. Chronobiol Int. 1992;9:314–21. doi: 10.3109/07420529209064541. [DOI] [PubMed] [Google Scholar]
- [197].Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- [198].Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res. 2009;46:245–7. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- [199].Arendt J. Shift work: coping with the biological clock. Occup Med (Lond) 2010;60:10–20. doi: 10.1093/occmed/kqp162. [DOI] [PubMed] [Google Scholar]
- [200].Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Reinberg AE, Ashkenazi I, Smolensky MH. Euchronism, allochronism, and dyschronism: is internal desynchronization of human circadian rhythms a sign of illness? Chronobiol Int. 2007;24:553–88. doi: 10.1080/07420520701534624. [DOI] [PubMed] [Google Scholar]
- [202].Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–34. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- [203].Garaulet M, Ordovás JM, Madrid JA. The chronobiology, etiology and pathophysiology of obesity. Int J Obes (London) 2010;34:1667–83. doi: 10.1038/ijo.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: An overview. Depress Anxiety. 2003;18:163–76. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- [205].National Institutes of Health State of the Science Conference Statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- [206].Lu BS, Zee PC. Circadian rhythm sleep disorders. Chest. 2006;130:1915–23. doi: 10.1378/chest.130.6.1915. [DOI] [PubMed] [Google Scholar]
- [207].Ebisawa T. Circadian rhythms in the CNS and peripheral clock disorders: Human sleep disorders and clock genes. J Pharmacol Sci. 2007;103:150–4. doi: 10.1254/jphs.fmj06003x5. [DOI] [PubMed] [Google Scholar]
- [208].Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr, Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R, Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68:1136–40. doi: 10.1212/01.wnl.0000258672.52836.30. [DOI] [PubMed] [Google Scholar]
- [210].Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV, American Academy of Sleep Medicine Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Archer SN, Carpen JD, Gibson M, Lim GH, Johnston JD, Skene DJ, von Schantz M. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010;33:695–701. doi: 10.1093/sleep/33.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Hofman MA. The human circadian clock and aging. Chronobiol Int. 2000;17:245–59. doi: 10.1081/cbi-100101047. [DOI] [PubMed] [Google Scholar]
- [213].Hofman MA, Swaab DF. Living by the clock: The circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [214].Zisapel N. Sleep and sleep disturbances: Biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–86. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–9. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- [216].Srinivasan V, Pandi-Perumal SR, Trakht I, Spence DW, Hardeland R, Poeggeler B, Cardinali DP. Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Res. 2009;165:201–14. doi: 10.1016/j.psychres.2007.11.020. [DOI] [PubMed] [Google Scholar]
- [217].Schulz H, Trojan B. A comparison of the eye movement density in normal subjects and in depressed patients before and after remission. Sleep Res. 1979;8:49. [Google Scholar]
- [218].Kupfer DJ, Spiker DG, Coble PA, Neil JF, Ulrich R, Shaw DH. Sleep and treatment prediction in endogenous depression. Am J Psychiatry. 1981;138:429–34. doi: 10.1176/ajp.138.4.429. [DOI] [PubMed] [Google Scholar]
- [219].Perlis ML, Giles DE, Buysse DJ, Tu S, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- [220].Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppä T, Lichtermann D, Praschak-Rieder N, Neumeister A, Nilsson LG, Kasper S, Peltonen L, Adolfsson R, Schalling M, Partonen T. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–9. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- [221].Partonen T, Treutlein J, Alpman A, Alpman A, Frank J, Johansson C, Depner M, Aron L, Rietschel M, Wellek S, Soronen P, Paunio T, Koch A, Chen P, Lathrop M, Adolfsson R, Persson ML, Kasper S, Schalling M, Peltonen L, Schumann G. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–38. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- [222].Lavebratt C, Sjöholm LK, Partonen T, Schalling M, Forsell Y. PER2 variation is associated with depression vulnerability. Am J Med Genet Neuropsychiatr Genet. 2010;153B:570–81. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- [223].Lavebratt C, Sjöholm LK, Soronen P, Paunio T, Vawter MP, Bunney WE, Adolfsson R, Forsell Y, Wu JC, Kelsoe JR, Partonen T, Schalling M. CRY2 is associated with depression. PLoS One. 2010;5:e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Schork NJ, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet Neuropsychiatr Genet. 2006;141B:234–41. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–7. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- [226].Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett. 2008;445:184–7. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [227].Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL, McMahon FJ, Gershon ES, Liu C. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet Neuropsychiatr Genet. 2008;147B:1047–55. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kripke DF, Nievergelt CM, Joo EJ, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Le-Niculescu H, Patel SD, Bhat M, Kuczenski R, Faraone SV, Tsuang MT, McMahon FJ, Schork NJ, Nurnberger JI, Jr, Niculescu AB., 3rd Convergent functional genomics of genome-wide association data for bipolar disorder: comprehensive identification of candidate genes, pathways and mechanisms. Am J Med Genet Neuropsychiatr Genet. 2009;150B:155–81. doi: 10.1002/ajmg.b.30887. [DOI] [PubMed] [Google Scholar]
- [230].Sjöholm LK, Backlund L, Cheteh EH, Ek IR, Frisén L, Schalling M, Osby U, Lavebratt C, Nikamo P. CRY2 is associated with rapid cycling in bipolar disorder patients. PLoS One. 2010;5:e12632. doi: 10.1371/journal.pone.0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Dallaspezia S, Lorenzi C, Pirovano A, Colombo C, Smeraldi E, Benedetti F. Circadian clock gene Per3 variants influence the postpartum onset of bipolar disorder. Eur Psychiatry. 2011;26:138–40. doi: 10.1016/j.eurpsy.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [232].Lee HJ, Rex KM, Nievergelt CM, Kelsoe JR, Kripke DF. Delayed sleep phase syndrome is related to seasonal affective disorder. J Affect Disord. 2011;133:573–9. doi: 10.1016/j.jad.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9:333–42. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Soria V, Martínez-Amorós E, Escaramís G, Valero J, Pérez-Egea R, García C, Gutiérrez-Zotes A, Puigdemont D, Bayés M, Crespo JM, Martorell L, Vilella E, Labad A, Vallejo J, Pérez V, Menchón JM, Estivill X, Gratacòs M, Urretavizcaya M. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35:1279–89. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].McGrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, Biederman J, Mick E, Faraone SV, Niculescu AB, Tsuang MT. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, Hardeland R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav Brain Funct. 2006;2006;2:15. doi: 10.1186/1744-9081-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–8. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- [238].Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, Zhang JT. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res. 2004;37:129–36. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- [239].Poeggeler B, Miravalle L, Zagorski MG, Wisniewski T, Chyan YJ, Zhang Y, Shao H, Bryant-Thomas T, Vidal R, Frangione B, Ghiso J, Pappolla MA. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Aβ peptide. Biochemistry. 2001;40:14995–5001. doi: 10.1021/bi0114269. [DOI] [PubMed] [Google Scholar]
- [240].Quinn J, Kulhanek D, Nowlin J, Jones R, Pratico D, Rokach J, Stackman R. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res. 2005;1037:209–13. doi: 10.1016/j.brainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- [241].Hardeland R. Cognitive enhancers in moderate to severe Alzheimer’s disease. Clin Med Insights Ther. 2011;3:459–76. [Google Scholar]
- [242].Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- [243].Crouch PJ, Savva MS, Hung LW, Donnelly PS, Mot AI, Parker SJ, Greenough MA, Volitakis I, Adlard PA, Cherny RA, Masters CL, Bush AI, Barnham KJ, White AR. The Alzheimer’s therapeutic PBT2 promotes amyloid-β degradation and GSK3 phosphorylation via a metal chaperone activity. J Neurochem. 2011;119:220–30. doi: 10.1111/j.1471-4159.2011.07402.x. [DOI] [PubMed] [Google Scholar]
- [244].Fainstein I, Bonetto A, Brusco LI, Cardinali DP. Effects of melatonin in elderly patients with sleep disturbance. A pilot study. Curr Ther Res. 1997;58:990–1000. [Google Scholar]
- [245].Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol Int. 2000;17:419–32. doi: 10.1081/cbi-100101055. [DOI] [PubMed] [Google Scholar]
- [246].Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly patients with dementia – a preliminary study. Arch Gerontol Geriatr. 2000;31:65–76. doi: 10.1016/s0167-4943(00)00068-6. [DOI] [PubMed] [Google Scholar]
- [247].Brusco LI, Marquez M, Cardinali DP. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuroendocrinol Lett. 2000;21:39–42. [PubMed] [Google Scholar]
- [248].Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer’s disease. Neuroendocrinol Lett. 2002;23(Suppl 1):20–3. [PubMed] [Google Scholar]
- [249].Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer’s type dementia. J Nippon Med Sch. 2003;70:334–41. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- [250].Mahlberg R, Kunz D, Sutej I, Kuhl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer’s disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol. 2004;24:456–9. doi: 10.1097/01.jcp.0000132443.12607.fd. [DOI] [PubMed] [Google Scholar]
- [251].Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:239–46. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, Thomas R, Thal LJ, Alzheimer’s Disease Cooperative Study A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Acuña-Castroviejo D, Coto-Montes A, Gaia MM, Ortiz GG, Reiter RJ. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. 1997;60:L23–9. doi: 10.1016/s0024-3205(96)00606-6. [DOI] [PubMed] [Google Scholar]
- [254].Mayo JC, Sainz RM, Uria H, Antolin I, Esteban MM, Rodriguez C. Melatonin prevents apoptosis induced by 6-hydroxydopamine in neuronal cells: implications for Parkinson’s disease. J Pineal Res. 1998;24:179–92. doi: 10.1111/j.1600-079x.1998.tb00531.x. [DOI] [PubMed] [Google Scholar]
- [255].Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 2001;15:164–70. doi: 10.1096/fj.00-0129com. [DOI] [PubMed] [Google Scholar]
- [256].Antolín I, Mayo JC, Sainz RM, del Brío Mde L, Herrera F, Martín V, Rodríguez C. Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res. 2002;943:163–73. doi: 10.1016/s0006-8993(02)02551-9. [DOI] [PubMed] [Google Scholar]
- [257].Thomas B, Mohanakumar KP. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J Pineal Res. 2004;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- [258].Mayo JC, Sainz RM, Tan DX, Antolín I, Rodríguez C, Reiter RJ. Melatonin and Parkinson’s disease. Endocrine. 2005;27:169–78. doi: 10.1385/ENDO:27:2:169. [DOI] [PubMed] [Google Scholar]
- [259].Sharma R, McMillan CR, Tenn CC, Niles LP. Physiological neuroprotection by melatonin in a 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2006;1068:230–6. doi: 10.1016/j.brainres.2005.10.084. [DOI] [PubMed] [Google Scholar]
- [260].Saravanan KS, Sindhu KM, Mohanakumar KP. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res. 2007;42:247–53. doi: 10.1111/j.1600-079X.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- [261].Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, Entrena A, Gallo MA, Espinosa A, Acuña-Castroviejo D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res. 2009;87:3002–10. doi: 10.1002/jnr.22123. [DOI] [PubMed] [Google Scholar]
- [262].Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res. 2011;50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- [263].Reiter RJ, Garcia JJ, Pie J. Oxidative toxicity in models of neurodegeneration: responses to melatonin. Restor Neurol Neurosci. 1998;12:135–42. [PubMed] [Google Scholar]
- [264].Reiter RJ, Tan D-X, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev. 2002;123:1007–19. doi: 10.1016/s0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- [265].Reiter RJ, Tan D-X, Manchester LC, El Sawi MR. Melatonin reduces oxidant damage and promotes mitochondrial respiration: implications for aging. Ann NY Acad Sci. 2002;959:238–50. doi: 10.1111/j.1749-6632.2002.tb02096.x. [DOI] [PubMed] [Google Scholar]
- [266].Reiter RJ, Tan D-X, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–46. [PubMed] [Google Scholar]
- [267].Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan D-X, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–90. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [268].Hardeland R. Melatonin, mitochondrial electron flux and leakage: recent findings and resolution of contradictory results. Adv Stud Biol. 2009;1:207–30. [Google Scholar]
- [269].Hardeland R. Neuroprotection by radical avoidance: search for suitable agents. Molecules. 2009;14:5054–102. doi: 10.3390/molecules14125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Acuña-Castroviejo D, López LC, Escames G, López A, García JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–40. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- [271].Hardeland R. Melatonin and its metabolites as anti-nitrosating and anti-nitrating agents. J Exp Integ Med. 2011;1:67–81. [Google Scholar]
- [272].Antolín I, Mayo JC, Sainz RM, del Brío Mde L, Herrera F, Martín V, Rodríguez C. Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res. 2002;943:163–73. doi: 10.1016/s0006-8993(02)02551-9. [DOI] [PubMed] [Google Scholar]
- [273].van der Schyf CJ, Castagnoli K, Palmer S, Hazelwood L, Castagnoli N., Jr Melatonin fails to protect against long-term MPTP-induced dopamine depletion in mouse striatum. Neurotox Res. 2000;1:261–9. doi: 10.1007/BF03033256. [DOI] [PubMed] [Google Scholar]
- [274].Morgan WW, Nelson JF. Chronic administration of pharmacological levels of melatonin does not ameliorate the MPTP-induced degeneration of the nigrostriatal pathway. Brain Res. 2001;921:115–21. doi: 10.1016/s0006-8993(01)03106-7. [DOI] [PubMed] [Google Scholar]
- [275].Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- [276].Grinberg LT, Rueb U, Alho AT, Heinsen H. Brainstem pathology and non-motor symptoms in PD. J Neurol Sci. 2010;289:81–8. doi: 10.1016/j.jns.2009.08.021. [DOI] [PubMed] [Google Scholar]
- [277].Knaryan VH, Samantaray S, Le Gal C, Ray SK, Banik NL. Tracking extranigral degeneration in animal models of Parkinson’s disease: quest for effective therapeutic strategies. J Neurochem. 2011;118:326–38. doi: 10.1111/j.1471-4159.2011.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Willis GL. Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19:245–316. doi: 10.1515/revneuro.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- [279].Willis GL. The role of ML-23 and other melatonin analogues in the treatment and management of Parkinson’s disease. Drug News Perspect. 2005;18:437–44. doi: 10.1358/dnp.2005.18.7.939349. [DOI] [PubMed] [Google Scholar]
- [280].Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S. Melatonin MT1 and MT2 receptor expression in Parkinson’s disease. Med Sci Monit. 2010;16:BR61–7. [PubMed] [Google Scholar]
- [281].Sandyk R. The accelerated aging hypothesis of Parkinson’s disease is not supported by the pattern of circadian melatonin secretion. Int J Neurosci. 1997;90:271–5. doi: 10.3109/00207459709000643. [DOI] [PubMed] [Google Scholar]
- [282].Srinivasan V, Cardinali DP, Srinivasan US, Kaur C, Brown GM, Spence DW, Hardeland R, Pandi-Perumal SR. Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Ther Adv Neurol Disord. 2011;4:297–317. doi: 10.1177/1756285611406166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, Destée A. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol. 2003;26:65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- [284].Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–37. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- [285].Pandi-Perumal SR, Srinivasan V, Cardinali DP, Monti MJ. Could agomelatine be the ideal antidepressant? Expert Rev Neurother. 2006;6:1595–608. doi: 10.1586/14737175.6.11.1595. [DOI] [PubMed] [Google Scholar]
- [286].Peschke E, Mühlbauer E, Musshoff U, Csernus VJ, Chankiewitz E, Peschke D. Receptor (MT1) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res. 2002;33:63–71. doi: 10.1034/j.1600-079x.2002.02919.x. [DOI] [PubMed] [Google Scholar]
- [287].Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- [288].Stumpf I, Mühlbauer E, Peschke E. Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic β-cells. J Pineal Res. 2008;45:318–27. doi: 10.1111/j.1600-079X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- [289].Bazwinsky-Wutschke I, Mühlbauer E, Wolgast, Mühlbauer E, Peschke E. Transcripts of calcium/calmodulin-dependent kinases are changed after forskolin- or IBMX-induced insulin secretion due to melatonin treatment of rat insulinoma beta-cells (INS-1) Horm Metab Res. 2009;41:805–13. doi: 10.1055/s-0029-1225631. [DOI] [PubMed] [Google Scholar]
- [290].Stumpf I, Bazwinsky I, Peschke E. Modulation of the cGMP signaling pathway by melatonin in pancreatic β-cells. J Pineal Res. 2009;46:140–147. doi: 10.1111/j.1600-079X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- [291].Peschke E, Schucht H, Mühlbauer E. Long-term enteral administration of melatonin reduces plasma insulin and increases expression of pineal insulin receptors in both Wistar and type 2-diabetic Goto-Kakizaki rats. J Pineal Res. 2010;49:373–81. doi: 10.1111/j.1600-079X.2010.00804.x. [DOI] [PubMed] [Google Scholar]
- [292].Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–6. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- [293].Peschke E, Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41:1085–92. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- [294].Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ. Function and expression of melatonin receptors on human pancreatic islets. J Pineal Res. 2008;44:273–9. doi: 10.1111/j.1600-079X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- [295].Bähr I, Mühlbauer E, Schucht H, Peschke E. Melatonin stimulates glucagon secretion in vitro and in vivo. J Pineal Res. 2011;50:336–44. doi: 10.1111/j.1600-079X.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- [296].Cardinali DP, Cano P, Jiménez-Ortega V, Esquifino AI. Melatonin and the metabolic syndrome: physiopathologic and therapeutical implications. Neuroendocrinology. 2011;93:133–42. doi: 10.1159/000324699. [DOI] [PubMed] [Google Scholar]
- [297].Zanquetta MM, Seraphim PM, Sumida DH, Cipolla-Neto J, Machado UF. Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J Pineal Res. 2003;35:141–8. doi: 10.1034/j.1600-079x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- [298].Alonso-Vale MI, Borges-Silva CN, Anhê GF, Andreotti S, Machado MA, Cipolla-Neto J, Lima FB. Light/dark cycle-dependent metabolic changes in adipose tissue of pinealectomized rats. Horm Metab Res. 2004;36:474–9. doi: 10.1055/s-2004-825723. [DOI] [PubMed] [Google Scholar]
- [299].Nogueira TC, Lellis-Santos C, Jesus DS, Taneda M, Rodrigues SC, Amaral FG, Lopes AM, Cipolla-Neto J, Bordin S, Anhê GF. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology. 2011;152:1253–63. doi: 10.1210/en.2010-1088. [DOI] [PubMed] [Google Scholar]
- [300].She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D, Hu X, Luo Y, Shen Q, Su Z, Yin W. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 2009;59:248–53. doi: 10.1016/j.phrs.2009.01.005. [DOI] [PubMed] [Google Scholar]
- [301].Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring) 2010;18:1861–3. doi: 10.1038/oby.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Langenberg C, Pascoe L, Mari A, Tura A, Laakso M, Frayling TM, Barroso I, Loos RJ, Wareham NJ, Walker M, RISC Consortium Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia. 2009;52:1537–42. doi: 10.1007/s00125-009-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Tamarkin L, Abastillas P, Chen HC, McNemar A, Sidbury JB. The daily profile of plasma melatonin in obese and Prader-Willis syndrome children. J Clin Endocrinol Metab. 1982;55:491–5. doi: 10.1210/jcem-55-3-491. [DOI] [PubMed] [Google Scholar]
- [304].Röjdmark S, Berg A, Rössner S, Wetterberg L. Nocturnal melatonin secretion in thyroid disease and in obesity. Clin Endocrinol (Oxf) 1991;35:61–5. doi: 10.1111/j.1365-2265.1991.tb03497.x. [DOI] [PubMed] [Google Scholar]
- [305].Bylesjö EI, Boman K, Wetterberg L. Obesity treated with phototherapy: four case studies. Int J Eat Disord. 1996;20:443–46. doi: 10.1002/(SICI)1098-108X(199612)20:4<443::AID-EAT14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [306].Fideleff HL, Boquete H, Fideleff G, Albornoz L, Pérez Lloret S, Suarez M, Esquifino AI, Honfi M, Cardinali DP. Gender-related differences in urinary 6-sulfatoxymelatonin levels in obese pubertal individuals. J Pineal Res. 2006;40:214–8. doi: 10.1111/j.1600-079X.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- [307].Ostrowska Z, Swietochowska E, Zwirska-Korczala K, Buntner B, Marek B, Pardela M, Drozdz M. Assessment of the relationship between collagen metabolism and selected hormonal factors in extremely obese women before and after jejunoileostomy. Endocr Regul. 1997;31:55–62. [PubMed] [Google Scholar]
- [308].Shafii M, MacMillan DR, Key MP, Kaufman N, Nahinsky ID. Case study: melatonin in severe obesity. J Am Acad Child Adolesc Psychiatry. 1997;36:412–6. doi: 10.1097/00004583-199703000-00021. [DOI] [PubMed] [Google Scholar]
- [309].Blaicher W, Speck E, Imhof MH, Gruber DM, Schneeberger C, Sator MO, Huber JC. Melatonin in postmenopausal females. Arch Gynecol Obstet. 2000;263:116–8. doi: 10.1007/s004040050008. [DOI] [PubMed] [Google Scholar]
- [310].Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [311].Bourin M, Mocaër E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci. 2004;29:126–33. [PMC free article] [PubMed] [Google Scholar]
- [312].Beresford IJM, Browning C, Starkey SJ, Brown J, Foord SM, Coughlan J, North PC, Dubocovich ML, Hagan RM. GR196429: a nonindolic agonist at high-affinity melatonin receptors. J Pharmacol Exp Ther. 1998;285:1239–45. [PubMed] [Google Scholar]
- [313].Drijfhout WJ, de Vries JB, Homan EJ, Brons HF, Copinga S, Gruppen G, Beresford IJ, Hagan RM, Grol CJ, Westerink BH. Novel non-indolic melatonin receptor agonists differentially entrain endogenous melatonin rhythm and increase its amplitude. Eur J Pharmacol. 1999;382:157–66. doi: 10.1016/s0014-2999(99)00619-6. [DOI] [PubMed] [Google Scholar]
- [314].Rajaratnam SMW, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, Klerman EB. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: Two randomised controlled multicentre trials. Lancet. 2009;373:482–91. doi: 10.1016/S0140-6736(08)61812-7. Receptor affinities in web appendix by Klerman EB, available at www.thelancet.com. [DOI] [PubMed] [Google Scholar]
- [315].Mulchahey JJ, Goldwater DR, Zemlan FP. A single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog β-methyl-6-chloromelatonin. Life Sci. 2004;75:1843–56. doi: 10.1016/j.lfs.2004.03.023. [DOI] [PubMed] [Google Scholar]
- [316].Rivara S, Mor M, Bedini A, Spadoni G, Tarzia G. Melatonin receptor agonists: SAR and applications to the treatment of sleep-wake disorders. Curr Top Med Chem. 2008;8:954–68. doi: 10.2174/156802608784936719. [DOI] [PubMed] [Google Scholar]
- [317].Rivara S, Vacondio F, Fioni A, Silva C, Carmi C, Mor M, Lucini V, Pannacci M, Caronno A, Scaglione F, Gobbi G, Spadoni G, Bedini A, Orlando P, Lucarini S, Tarzia G. N-(Anilinoethyl)amides: design and synthesis of metabolically stable, selective melatonin receptor ligands. ChemMedChem. 2009;4:1746–55. doi: 10.1002/cmdc.200900240. [DOI] [PubMed] [Google Scholar]
- [318].Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- [319].Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high affinity MT1 and MT2 melatonin receptor agonist indicated for the treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- [320].Owen RT. Agomelatine: a novel pharmacological approach to treating depression. Drugs Today (Barc) 2009;45:599–608. [PubMed] [Google Scholar]
- [321].Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306:954–64. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- [322].Racagni G, Riva MA, Molteni R, Musazzi L, Calabrese F, Popoli M, Tardito D. Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J Biol Psychiatry. 2011;12:574–87. doi: 10.3109/15622975.2011.595823. [DOI] [PubMed] [Google Scholar]
- [323].Landolt H-P, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. 2009;29:1795–809. doi: 10.1111/j.1460-9568.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- [324].Hardeland R. Tasimelteon, a melatonin agonist for the treatment of insomnia and circadian rhythm sleep disorders. Curr Opin Investig Drugs. 2009;10:691–701. [PubMed] [Google Scholar]
- [325].Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, Mor M, Tarzia G, Descarries L, Gobbi G. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP. Drug Insight: the use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat Clin Pract Neurol. 2007;3:221–8. doi: 10.1038/ncpneuro0467. [DOI] [PubMed] [Google Scholar]
- [327].Reynoldson JN, Elliott E, Sr, Nelson LA. Ramelteon: a novel approach in the treatment of insomnia. Ann Pharmacother. 2008;42:1262–71. doi: 10.1345/aph.1K676. [DOI] [PubMed] [Google Scholar]
- [328].Simpson D, Curran MP. Ramelteon: a review of its use in insomnia. Drugs. 2008;68:1901–19. doi: 10.2165/00003495-200868130-00011. [DOI] [PubMed] [Google Scholar]
- [329].Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4:69–79. doi: 10.2147/ndt.s483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Hardeland R, Poeggeler B, Srinivasan V, Trakht I, Pandi-Perumal SR, Cardinali DP. Melatonergic drugs in clinical practice. Arzneimittelforschung. 2008;58:1–10. doi: 10.1055/s-0031-1296459. [DOI] [PubMed] [Google Scholar]
- [331].Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol. 2008;4:436–47. doi: 10.1038/ncpneuro0847. [DOI] [PubMed] [Google Scholar]
- [332].Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Poeggeler B, Hardeland R, Cardinali DP. The effect of melatonergic and non-melatonergic antidepressants on sleep: weighing the alternatives. World J Biol Psychiatry. 2009;10:342–54. doi: 10.1080/15622970701625600. [DOI] [PubMed] [Google Scholar]
- [333].Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, Hardeland R, Cardinali DP. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119:821–46. doi: 10.1080/00207450802328607. [DOI] [PubMed] [Google Scholar]
- [334].Pandi-Perumal SR, Srinivasan V, Spence DW, Moscovitch A, Hardeland R, Brown GM, Cardinali DP. Ramelteon: a review of its therapeutic potential in sleep disorders. Adv Ther. 2009;26:613–26. doi: 10.1007/s12325-009-0041-6. [DOI] [PubMed] [Google Scholar]
- [335].Fornaro M, Prestia D, Colicchio S, Perugi G. A systematic, updated review on the antidepressant agomelatine focusing on its melatonergic modulation. Curr Neuropharmacol. 2010;8:287–304. doi: 10.2174/157015910792246227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27:796–813. doi: 10.1007/s12325-010-0065-y. [DOI] [PubMed] [Google Scholar]
- [337].De Berardis D, Di Iorio G, Acciavatti T, Conti C, Serroni N, Olivieri L, Cavuto M, Martinotti G, Janiri L, Moschetta FS, Conti P, Di Giannantonio M. The emerging role of melatonin agonists in the treatment of major depression: focus on agomelatine. CNS Neurol Disord Drug Targets. 2011;10:119–32. doi: 10.2174/187152711794488674. [DOI] [PubMed] [Google Scholar]
- [338].Green B. Focus on agomelatine. Curr Med Res Opin. 2011;27:745–9. doi: 10.1185/03007995.2011.554534. [DOI] [PubMed] [Google Scholar]
- [339].Quera Salva MA, Hartley S, Barbot F, Alvarez JC, Lofaso F, Guilleminault C. Circadian rhythms, melatonin and depression. Curr Pharm Des. 2011;17:1459–70. doi: 10.2174/138161211796197188. [DOI] [PubMed] [Google Scholar]
- [340].Carney RM, Shelton RC. Agomelatine for the treatment of major depressive disorder. Expert Opin Pharmacother. 2011;12:2411–9. doi: 10.1517/14656566.2011.607812. [DOI] [PubMed] [Google Scholar]
- [341].Srinivasan V, Brzezinski A, Pandi-Perumal SR, Spence DW, Cardinali DP, Brown GM. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:913–23. doi: 10.1016/j.pnpbp.2011.03.013. [DOI] [PubMed] [Google Scholar]
- [342].Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res. 2011 doi: 10.1111/j.1600-079X.2011.00962.x. in press. [DOI] [PubMed] [Google Scholar]
- [343].Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Parry B, Cardinali DP. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7:138–51. doi: 10.1080/15622970600571822. [DOI] [PubMed] [Google Scholar]
- [344].McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–32. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS. The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci. 2007;9:291–300. doi: 10.31887/DCNS.2007.9.3/alewy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Westrin A, Lam RW. Seasonal affective disorder: A clinical update. Ann Clin Psychiatry. 2007;19:239–46. doi: 10.1080/10401230701653476. [DOI] [PubMed] [Google Scholar]
- [347].Lewy AJ. Melatonin and human chronobiology. Cold Spring Harbor Symp Quant Biol. 2007;72:623–36. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- [348].Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. 4 Entrainment: pacemaker as clock. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- [349].Pandi-Perumal SR, Moscovitch A, Srinivasan V, Spence DW, Cardinali DP, Brown GM. Bidirectional communication between sleep and circadian rhythms and its implications for depression: lessons from agomelatine. Prog Neurobiol. 2009;88:264–71. doi: 10.1016/j.pneurobio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [350].Pandi-Perumal SR, Spence DW, Verster JC, Srinivasan V, Brown GM, Cardinali DP, Hardeland R. Pharmacotherapy of insomnia with ramelteon: Safety, efficacy and clinical applications. J Cent Nerv Syst Dis. 2011;3:51–65. doi: 10.4137/JCNSD.S1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [352].Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–18. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [353].Zemlan FP, Mulchahey JJ, Scharf MB, Mayleben DW, Rosenberg R, Lankford A. The efficacy and safety of the melatonin agonist β-methyl-6-chloromelatonin in primary insomnia: a randomized, placebo-controlled, crossover clinical trial. J Clin Psychiatry. 2005;66:384–90. doi: 10.4088/jcp.v66n0316. [DOI] [PubMed] [Google Scholar]
- [354].Quera-Salva MA, Hajak G, Philip P, Montplaisir J, Keufer-Le Gall S, Laredo J, Guilleminault C. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. Int Clin Psychopharmacol. 2011;26:252–62. doi: 10.1097/YIC.0b013e328349b117. [DOI] [PubMed] [Google Scholar]
- [355].Weishaupt JH, Bartels C, Pölking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Hüther G, Schneider A, Bach A, Sirén AL, Hardeland R, Bähr M, Nave KA, Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006;41:313–23. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- [356].Howland RH. Critical appraisal and update on the clinical utility of agomelatine, a melatonergic agonist, for the treatment of major depressive disease in adults. Neuropsychiatr Dis Treat. 2009;5:563–76. doi: 10.2147/ndt.s5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Anonymous Agomelatine: new drug. Adverse effects and no proven efficacy. Prescrire Int. 2009;18:241–5. [PubMed] [Google Scholar]
- [358].Howland RH. A benefit-risk assessment of agomelatine in the treatment of major depression. Drug Saf. 2011;34:709–31. doi: 10.2165/11593960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [359].Howland RH. Publication bias and outcome reporting bias: agomelatine as a case example. J Psychosoc Nurs Ment Health Serv. 2011;49:11–4. doi: 10.3928/02793695-20110809-01. [DOI] [PubMed] [Google Scholar]
- [360].Richardson GS, Zammit G, Wang-Weigand S, Zhang J. Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry. 2009;70:467–76. doi: 10.4088/jcp.07m03834. [DOI] [PubMed] [Google Scholar]
- [361].Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–60. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Uchiyama M, Hamamura M, Kuwano T, Nagata H, Hashimoto T, Ogawa A, Uchimura N. Long-term safety and efficacy of ramelteon in Japanese patients with chronic insomnia. Sleep Med. 2011;12:127–33. doi: 10.1016/j.sleep.2010.10.006. [DOI] [PubMed] [Google Scholar]
- [363].Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol. 2010;8:218–27. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Sánchez V, Romeu M, Acuña-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol. 2008;43:749–56. doi: 10.1016/j.exger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [365].Poeggeler B. Melatonin, aging, and age-related diseases: perspectives for prevention, intervention, and therapy. Endocrine. 2005;27:201–12. doi: 10.1385/ENDO:27:2:201. [DOI] [PubMed] [Google Scholar]
- [366].Hardeland R, Coto-Montes A. New vistas on oxidative damage and aging. Open Biol J. 2010;3:39–52. [Google Scholar]
- [367].Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan D-X. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol. 2011;62:13–9. [PubMed] [Google Scholar]


