Abstract
Engineering is the art of taking what we know and using it to solve problems. As engineers, we build tool chests of approaches; we attempt to learn as much as possible about the problem at hand, and then we design, build, and test our approaches to see how they impact the system. The challenge of applying this approach to the central nervous system (CNS) is that we often do not know the details of what is needed from the biological side. New therapeutic options for treating the CNS range from new biomaterials to make scaffolds, to novel drug-delivery techniques, to functional electrical stimulation. However, the reality is that translating these new therapies and making them widely available to patients requires collaborations between scientists, engineers, clinicians, and patients to have the greatest chance of success. Here we discuss a variety of new treatment strategies and explore the pragmatic challenges involved with engineering therapies in the CNS.
Keywords: CNS, translation, biomaterials, drug-delivery, scaffold, engineering
How Engineers Approach Problems
Engineering is the art of taking what we know and using it to solve problems. As engineers, we build tool chests of approaches; we attempt to learn as much as possible about the problem at hand, and then we design, build, and test our approaches to see how they impact the system. Through the process of needs assessment, brainstorming, building, testing, and outcome assessment, we work to develop solutions to problems.
The challenge of applying this approach to the central nervous system (CNS) is that we often do not know the details of what is needed from the biological side. The complexity of the healthy CNS coupled with the complexity of the injuries to, and diseases of, the CNS is an intimidating matrix. However, engineering approaches have the potential to augment our understanding of the CNS and in some cases have made impressive strides towards providing new therapeutic options for treating the CNS.
The reality is that developing new therapies requires collaborations between scientists, engineers, clinicians, and patients to have the greatest chance of success and impact on injuries to and diseases of the CNS.
Features that Engineers have Mimicked
Structures of the CNS and Scaffold architecture
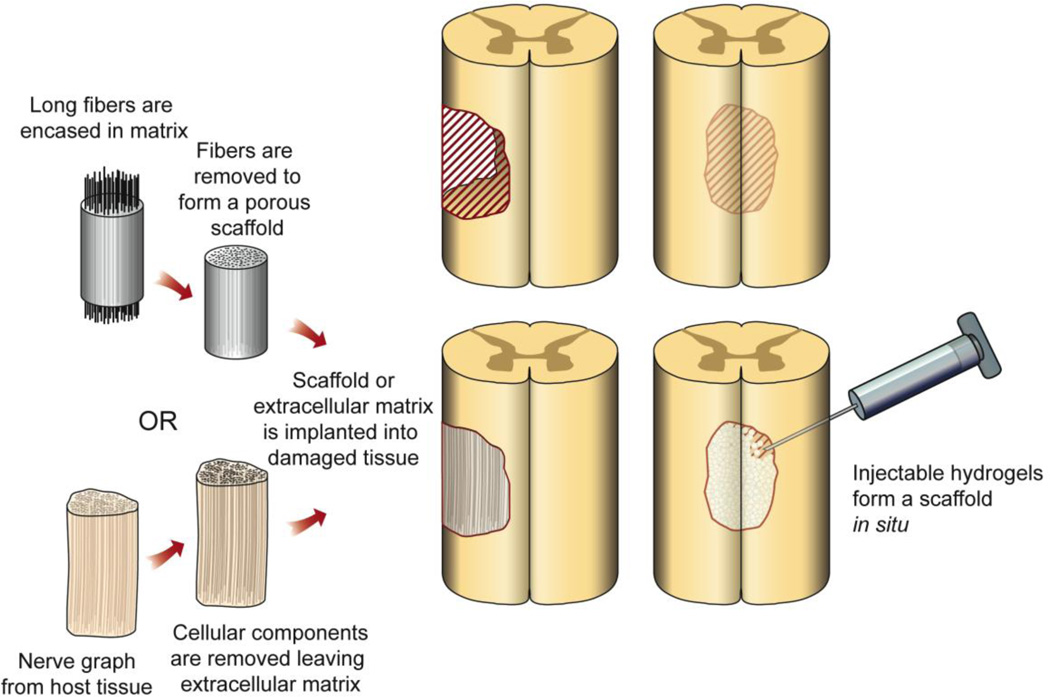
One of the approaches engineers have studied in the CNS is the use of scaffolds to direct the growth or regrowth of tissue (Figure 1). A majority of the focus has been on promoting neurite extension and axonal guidance. Cells grown in vitro tend to align with scratches or grooves in the dishes. A number of groups have taken this observation and engineered surfaces with a range of topographical features to identify those which have the greatest impact on neurite extension [31, 32, 40, 64]. Both the depth and width of grooves seems to affect neurite outgrowth in two dimensions. In three dimensions, the curvature of features also plays a role [49, 111, 112, 127]. Topographical cues play a major role in development, which are recapitulated in the process of tissue repair [49].
Figure 1. Repairing neural damage using implanted or injected materials.
Examples of methods being tested for stimulating regrowth of neurons across damaged parts of the CNS. Left column illustrates engineered (top) and biologically-derived (bottom) materials that are surgically implanted into the damaged part of the CNS to form a porous scaffold upon which axons can regrow. Right column illustrates how various hydrogels can be injected into the damaged area to form a scaffold in situ as well as deliver drugs and therapies locally.
In light of the lessons learned in guiding neural outgrowth in a dish, engineers have worked to create scaffolds that leverage these features to produce or support axonal outgrowth and regeneration. Channels or pores in scaffolds can be created in a variety of ways including casting of the scaffold around fibers, which are then removed after fabrication and electrospinning with parallel-oriented fibers. With each of these methods, controlling fiber orientation and size repeatability are the leading challenges. Pawar et al developed a unique method of capillary channel formation using an ionically-driven self-assembling method with alginate [93]. They were able to reproducibly make scaffolds with varying channel diameters from 18–80 microns and test them in a dorsal root ganglion outgrowth assay. Interestingly, they found that 77 microns produced the largest magnitude outgrowth but at the cost of orientation. Therefore, they determined 25 microns was the optimal channel size balancing outgrowth magnitude and orientation. Being able to direct the orientation of neurons and their neurites in vitro has important applications in building in vitro diagnostic systems and assay systems to understand the mechanisms associated with neural behavior. One question that arises is whether these same features that direct neurons in vitro can be translated in vivo to direct repair.
The application of architectural cues in vivo has been a fascinating road. Implantation of scaffolds in defects in the CNS has increased neural sprouting as well as reduced glial scar formation and the invasion of immune cells. It is particularly striking that these observations have been seen across a broad range of scaffolds based on different materials and different architectural features.
Some of the scaffolds pursued to promote neural regeneration in the nervous system have included collagen scaffolds fabricated with oriented pores to guide axons [75], Matrigel, an extracellular matrix (ECM) substitute derived from mouse sarcoma, in poly(acrylonitrile): poly(vinylchloride) (PAN/PVC) tubes to support the organization of Schwann cells to promote neural regeneration [33, 36, 37, 100, 135–138], scaffolds with oriented pores constructed from poly(lactic-co-glycolic acid) (PLGA) [19, 139], the degradable polyester used in degradable sutures, and carbon filaments [58].
Growth into the scaffolds was seen with all of these approaches, but none of them led to robust regeneration or repair. In light of this, a number of groups have looked at a more holistic approach to building environments that stimulate repair based on trying to more completely engineer the ECM. The motivation for this approach is based on observations including those that decellularized nerve grafts lead to robust ingrowth [45, 128], and progenitor cells respond to a range of ECM-associated molecular cues [71, 78, 109]. These observations motivate the desire to engineer an ECM analogue that would permit either the ability to promote repair, or potentially be a foundation for exploring molecular interactions in the nervous system in three dimensions, bridging the gap between cell culture and in vivo preparations in a controllable system.
Molecular makeup of the ECM of the CNS
The obvious place to start in engineering the ECM is with the ECM provided by nature. The engineering challenges involve finding a reproducible source for the ECM and removing the cellular components from the tissue to reduce the immune response to the material.
Starting with natural ECM (Acellular Grafts)
Acellular grafts are produced by taking a donor peripheral nerve and washing it with detergents and/or treating it with heat to remove the cells, leaving behind the ECM component which comprises a tubular graft which can then be implanted to bridge a nerve gap. These have the advantage of containing the natural components normally found in the nervous system and therefore match mechanically, but have the disadvantage of being difficult to produce in a robust repeatable manner and still require a donor source. Furthermore, they may still contain residual antigens that could cause an immune response upon implantation [86]. It is very challenging to remove all of the cellular components without damaging the ECM. Ultimately, one must make tradeoffs between how much damage to the ECM one finds acceptable and how much of the cellular material one can remove.
Engineering ECM
While the majority of the ECM in the body is composed of collagen, the CNS is a notable exception. The ECM of the CNS is based on a hyaluronic acid (HA) network functionalized with a range of proteoglycan [77] [43] [125] [39]. HA is a glycosaminoglycan or long, unbranched carbohydrate, and as carbohydrates go, it is one of the more simple systems to chemically modify with the note that carbohydrate chemistry is a vast and challenging area with the potential for many side reactions. Saying that carbohydrate chemistry is simple is a bit like saying that one need only drink a small vial to make it down a rabbit hole. It is highly negatively charged and HAs of different molecular weights have different biological functions from potentially creating spaces for cells to migrate in the brain to conferring elasticity to tissues [98].
The proteoglycans are critical for cell attachment to the HA-based networks. Neural progenitors make proteoglycans while their differentiated progeny down regulate production [56]. Therefore, neural progenitors can stick to unmodified HA but their progeny do not. This provides an interesting method for separating progenitors from their progeny.
As neural progenitors differentiate in these materials, expressing more mature markers, the mature cells do not attach unless the HA gels are modified with other molecules to promote attachment [89]. Therefore, other materials are often combined with HA to provide site for differentiated cell interactions. Molecules based on other ECM components such as gelatin and polypeptides have been incorporated [39, 43, 107, 125].
A number of groups have done beautiful work in vitro and in vivo with HA-based gels in the CNS [7, 44, 89, 108, 116, 117], the cost of HA coupled with the challenges of working with it chemically have motivated the development of ECM analogues that present components that interact with cells without the HA-backbone.
Perhaps this is the moment to take a step back as engineers, and ask what we really need from an ECM analogue material for repair. The ideal material would be one that could be implanted in a minimally invasive manner, promote neurogenesis, concomitant angiogenesis, and allow axonal growth through the glial scar to allow functional connection at the distal end. This material would not be inflammatory, and would have identical mechanical properties to the spinal cord. In addition, the material could be easily processed into various sizes and morphologies and be scalable in production.
If we are to develop materials that are suitable for therapy, they must fit with the needs, requirements, and concerns of neurosurgeons and patients. We can engineer materials with beautiful architectures based on a vast range of molecules, but if it cannot be administered in a minimally invasive manner, how do we expect that it could be delivered to the CNS? After all, it is not logical to imagine opening a large path to a stroke cavity or spinal cord cyst for treatment; rather, we need to design the materials appropriately.
The HA-based materials and their ECM analogue counterparts are typically hydrogels, water-soluble polymers that can be crosslinked to form networks. Likewise, the majority of other injectable materials from fibrin-based systems [42, 104, 123] to molecularly engineered self-assembling nanogel systems [110, 124] are hydrogels that allow for minimally invasive administration. These minimally invasive systems have been shown to reduce glial scarring and promote neural ingrowth in a number of models. Tysseling-Mattiace et al. have shown that administration of a hydrogel based on self assembling peptides one day post injury in a spinal cord compression model reduces glial scarring and increases neurite outgrowth and sprouting [124]. Wolery et al saw a similar outcome with a collagen gel based system [130]. Both groups reported better functional outcomes with the administration of their respective polymers.
It may be that we do not need to engineer architecture at all to facilitate outcomes, but the caveat with these findings is that they involve interventions in the acute phase of injury. If we do need architectural control to guide cells and repair, is it possible to achieve in an injectable system? The answer is ‘yes’ in some cases. There are methods to achieve architectural control even in injectable systems. In the case of the hydrogel nanofiber work based on polypeptides, the polymers organize as a function of secondary bonding interactions such as hydrophilicity and hydrophobicity and Van der Waals interactions to form nanofibers [110, 124]. Blends of polymers and block copolymer systems also can achieve some architectural control based on secondary interactions and microphase separation [9, 13, 76], so it is possible to get some aspects of both worlds, the injectable and architectural control.
Modulus of ECM
All gels, and all polymers can be dissimilar even when composed of the same basic building blocks. One of the critical characteristics of materials is their stiffness. The stiffness of a material is crucial to cell behavior. The stiffness, or more specifically from a materials property point of view, the modulus, impacts cell migration [30, 46, 59, 95, 105, 114], differentiation [25, 26, 34, 50, 59, 66, 73, 91, 103], and neurite extension [5, 74, 106] all of which are critical for promoting repair.
While the findings vary, moduli between 2000 and 5000 Pa have been associated with the greatest neurite extension in a number of studies [74, 106] as well as neural differentiation of progenitors [28] suggesting this may be a good starting point for designing materials to promote repair. This literature on the development of materials for neural electrical recording and stimulation follows a similar trend, where it has been realized that the electrode’s modulus of elasticity plays a major role in the inflammatory process [79]. Those being too stiff cause a greater inflammatory response and become encapsulated to a greater extent. This has led to the development of novel materials that change from being stiff during implantation, but become soft once hydrated in the body to facilitate delivery of the material and the neural response [11, 17].
Temporal presentation of growth factors
A major component of building an ECM environment lies with the presentation of soluble molecules. While the tools of drug delivery are well suited to facilitate the spatial and temporal patterning of molecules in an environment, there are engineering challenges that need to be addressed. For example, there are challenges associated with needing to present multiple molecules or present them with very specific temporal and spatially defined patterns, not the least of which is that large proteins such as growth factors have very short half lives and can be denatured easily. These, coupled with the challenges of getting molecules across the blood-brain barrier motivate the work in local, sustained delivery. To this end, molecules including growth factors have been delivered from a number of materials.
A number of growth factors have been studied with regards to sustained delivery to promote neural protection and repair in the CNS. Gels and materials delivering neurotrophin-3 (NT-3) have been shown to promote more robust sprouting and axonal elongation in a number of models coupled with improved functional outcomes in some of the work [96, 119]. Likewise, delivery of brain derived neurotrophic factor (BDNF) from a number of polymers has also led to improved outcomes [51, 90, 92, 115]. Glial derived neurotrophic factor (GDNF) has also been correlated with more outgrowth [48]. The delivery of a range of other growth factors has been studied for protection and repair in the CNS [4, 6, 14, 22, 35, 41, 52, 54, 55, 63, 68, 83, 118, 120, 129, 131, 132].
This list, though incomplete, represents a range of models which suggest that the combination of appropriate materials and delivery paradigms for growth factors lead to better outcomes than simple bolus delivery. There are limits, though, on how long molecules can be delivered from polymers (typically days to months to, in a few cases, years) which motivates the use of strategies to engineer cells either through delivery of DNA or siRNA in vivo [3, 24, 47, 53, 61, 84, 141] or ex vivo followed by transplantation of the cells [47, 62, 72, 87, 126].
There are a lot of options. With all of these options to pursue one could spend hundreds of lifetimes doing tests even using high throughput options available for screening. If our goal is to develop a potential therapy to help outcomes, can we use these parameters to focus our search? How does this impact the possibilities?
If we think about what is clinically feasible, we know that a viable drug has to be easily administered. From a regulatory point of view, it helps if it builds on materials and molecules used in other approved therapies. From a freedom to operate point of view, it helps if we are looking at the delivery of molecules that are off patent so we are not tied up in trying to negotiate licensing agreements.
Methylprednisolone (MP) is an FDA-approved drug for the treatment of spinal cord injury (SCI). It has been used as the standard of care in a large number of centers and is administered at high doses systemically to try to reduce secondary degeneration following SCI and improve outcomes. While the initial clinical trials were promising, as the drug has been used more extensively, it is clear that the efficacy is limited, and there are substantial side effects. However, if one could deliver this drug locally, one might be able to limit or eliminate the systemic side effects and maintain or improve the efficacy. The Bellamkonda group encapsulated MP in PLGA nanoparticles and compared the administration of the MP-nanoparticles to systemic MP, local administration of MP or blank nanoparticles that do not release MP. The MP delivering nanoparticles only delivered MP for three days, but this was enough to show a reduction in lesion volume following a contusion SCI as well as a reduction in a number of markers for apoptosis and inflammation [20, 65]. Excitingly, such an approach, if successful, is designed from the start in a manner that would be more amenable to application in the clinic. It involves minimally invasive administration of the particles.
Signaling: electrical and optical stimulation
So far, we have looked at engineering approaches based on trying to protect and repair the nervous system. While there are some exciting findings, especially for chronic injuries, there are not obvious solutions to date. However, in some cases, we may be able to engineer solutions to augment remaining neural systems either temporarily or permanently.
Functional electrical stimulation (FES) is the use of electric current to activate existing neurons that are no longer functioning properly due to SCI, stroke, or disease. FES can be used to restore functional movements after SCI or stroke by applying appropriate patterns of current to the nerves innervating different paralyzed muscles via surface, percutaneous, or fully implantable systems [18, 60, 94, 97]. There are a multitude of FES applications in the nervous system (Table 1). Some of the systems are designed to be used temporarily during physical therapy to improve therapeutic outcomes, and some are long-term systems to permanently replace lost function. After stroke, therapeutic FES can encourage beneficial plasticity by reactivating and strengthening normal neuromuscular activation patterns [10, 23].
Table 1.
Uses of electrical stimulation in the nervous system
| Deep brain stimulation for movement disorders |
| Retinal or cortical implants to restore vision |
| Cochlear implants to restore hearing |
| Restoration of swallowing after stroke |
| Eliminating shoulder subluxation after stroke |
| Cough assist |
| Cardiac pacemakers |
| Reducing chronic pain |
| Restoring arm function |
| Diaphragm pacing to restore breathing |
| Improving wound healing |
| Trunk support |
| Restoring hand grasp |
| Restoring sexual function |
| Bowel/bladder control after injury and for incontinence |
| Preventing pressure sores by increasing muscle mass |
| Restoring standing and facilitating transfers |
| Restoring walking and locomotion |
| Eliminating foot drop after stroke |
The basic principle of FES relies on providing electrical stimulation to the nerves or neural circuits that enervate the muscles of interest to stimulate those muscles. Therefore, for the approach to work, one must have enough innervated muscles available for stimulation. This need drives the potential coupling of regenerative and reparative therapies with electrical stimulation to have more sites for stimulation. Beyond this, there is evidence that electrical stimulation may be coupled with other therapies such as cell based therapies to promote better outcomes than either alone [8, 113].
FES works and can restore function in patients with few therapeutic options. However, its deployment requires extensive clinician training in the implantation procedures. The reimbursement for FES is also not commensurate with the costs. For large scale deployment, the costs and the clinician training must be addressed. In the meantime, there is early clinical evidence (n=1) that stimulation can be used to reprogram pattern generators for SCI patients. One patient has received a spinal cord stimulator that sits epidurally on the spinal cord and is used to modulate pain. However, in this case, the stimulator is used in conjunction with physical therapy. The hypothesis is that this engages the pattern generators and while physical therapy alone led to no improvements based on the ASIA scale, the coupling of stimulation and therapy has led to the patient regaining standing, stepping on a treadmill, and some bladder and bowel function [27, 38]. There is significant experience in the clinic with deploying the spinal cord stimulators that could help with clinical deployment if the data continue to look promising with the recruitment of more patients.
The most widely clinically deployed electrical stimulation system in the CNS is the deep brain stimulation system (DBS) for Parkinson’s disease. DBS of the subthalamic nucleus reduces the tremor, rigidity, and bradykinesia associated with Parkinson’s. While the mechanism by which DBS modulates tremors is unclear [15, 80, 82, 134], DBS can have a dramatic effect on patient quality of life and DBS is being studied for a variety of conditions [15]. There are a host of engineering issues associated with DBS from the design of the next generation systems [122] to developing methods for electrode placement for optimal outcomes [81] and optimizing the stimulation parameters to give each patient the best outcome over time in the most efficient clinical environment [16].
While there is a tremendous amount that can be achieved with electrical stimulation, selectively activating specific types of neurons is not one of the strong suits of the field. Optical stimulation has drawn a great deal of interest both as an investigational tool and, potentially, as a therapeutic approach in part because it achieves significant selectivity by a combination of the delivery of light coupled with the placement of light sensitive ion channels in the neurons. The best known and most studied of the light sensitive molecules are the channel rhodopsins [140]. This area, optogenetics, has opened the door to be able to understand the nervous system in new ways. It is hard not to look at light sensitive molecules like channel rhodopsins and not think about whether one might be able to engineer their delivery to the eye for patients with photoreceptor degeneration. A number of groups have looked at this and have seen light sensitivity in a number of animal models, but the intensity of light needs to be reasonably high for the effect [69, 121]. Therefore, there has been interest in developing light sensitive molecules that require fewer photons for stimulation such as photoswitches [67].
Building the Team: Clinicians, Scientists, Engineers
One of the striking realities about the majority of the work moving towards or in the clinic is that it has involved strong interdisciplinary teams of clinicians, scientists, and engineers. The mythology of the lone scientist or passionate clinician who develops a new therapy does a disservice to the field. We need the expertise of engineers along with the insights of neuroscientists and the perspective of clinicians to develop therapies that have a potential to get to patients and give them beneficial outcomes. It requires strong teams who are willing to have conversations outside of their comfort zones.
Two examples of interdisciplinary collaborations that have led to therapies are the development of functional electrical stimulation for grasping, the Freehand system [85], and the Gliadel ® wafer for glioblastoma multiforme [12]. Both therapies, radically different in their applications, technologies, and goals were the result of collaborations between teams of engineers, scientists, and clinicians. These successes are based on long term collaborations of teams of people with different expertise and a certain amount of courage not only to work together but to push through the inevitable challenges that come with taking on tremendously difficult problems. The challenges are not solved by the perfect, lone person in the lab but great teams of people who are undaunted.
The Challenges that Lie Ahead
If our goal as a community is to develop new therapies for the CNS, we need to think practically about what will work and what is plausible to scale up, move through the regulatory process and deliver to patients.
Population size and needs
Disorders of the CNS have dramatic impacts on the quality of life of patients and their caregivers and incur significant health care costs. Annually, there are approximately 235,000 people hospitalized due to traumatic brain injuries (TBIs) with is 90,000 per year exhibiting permanent deficits [70]. There are 12,000 new spinal cord injuries (SCIs) each year in the U.S. [1]. The human impact of CNS injuries is enormous and the health care costs associated with the injury are some of the highest in the U.S. [2, 133]. The incidence for multiple sclerosis, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and, the incidence is roughly 70, 11, and 2 per 100,000 individuals respectively [88]. Likewise, the health care and associated costs are tremendous as are the impact on the quality of life of the patient and caregivers.
While these numbers are striking, they are greatly surpassed by the highly prevalent cardiovascular disease (prevalence 80.7 million [102]) or diabetes (prevalence 25.8 million [99]). The FDA designates any rare disease or condition that affects fewer than 200,000 people to an “orphan” population. A substantial number of the disorders of the CNS fall under the definition of orphan conditions. While being an orphan condition can limit some of the regulatory hurdles, the small population means that therapies targeted at these diseases may be challenging to develop economically, at least using traditional models in the pharmaceutical and device industries.
This is not to suggest that one should not pursue therapies for conditions that affect relatively small numbers of people, but one must think about the financial models to support the development and distribution of these therapies so that one can make sure the patients benefit. One possible mechanism for funding a product for a small population is to hedge development costs by constraining efforts to a platform technology, or a technology that has multiple applications. However, with the highly specialized, complex CNS, treatments for repair are also often highly specialized and complex. A second mechanism could be to leverage foundation support and share technology and intellectual property. A number of groups are pursuing this route. Ultimately, though, to get a technology deployed on a large scale to patients, one has to come up with a workable financial model balancing costs and reimbursement.
Scale up
Scale up to production volumes of technology is often an overlooked challenge, but if one cannot make a drug, device, or biologic at scale, the reality is that one will not have enough for testing and therapeutic applications. Simple, reproducible systems are critical if one is going to make large quantities of the technology. The reality is that if one struggles to make reproducible, small amounts of a system at the bench, it is not likely things will get easier on the route to scaling up the system.
Regulatory considerations
As noted above, the FDA designates any rare disease or condition that affects fewer than 200,000 people to an “orphan” population. Drugs and treatments for an orphan population are eligible for various exemptions and incentives, including tax credits, to help mitigate the costs of the translation process. However, to qualify for this designation, the product cannot have indication for use in anything other than a rare disease or condition, contradicting the drive toward platform technologies[29]. Furthermore, these incentives are drops in a bucket when considering the amount of investment that is required to research and commercialize a medical product. Estimates for the cost of medical device development can easily exceed $10–20 million in the preclinical phase, with an overall average price tag of $1.3–1.7 billion to bring the product to market [21, 57].
Engineering and Neuroscience: Where we go from here
Engineering is a discipline that seeks to solve; science seeks to explain [101]. Whereas the formula for science is to observe, hypothesize and test, the formula for engineering is to specify, design, and verify [101]. This is not to suggest one is superior over the other. On the contrary, each is less potent without the other.
There are huge challenges involving discovery of potential new therapies and between discovery and deployment. On the discovery side, engineers bring a host of new tools to build and modulate the nervous system. Through collaboration and our different perspectives, we may be able to gain new insights into mechanisms and therapeutic targets associated with insults to and diseases of the CNS.
If our goal is to develop new approaches for people, we need to move in two directions at once, no small feat. On the one side, we need to work together at the bench to understand and develop potential interventions. On the other side, we need to start at the end and figure out for the condition we hope to treat exactly what is and is not possible from the patient’s and physician’s perspectives. We also need to be cognizant of how the FDA might receive the technology and to that end, many officials at the FDA advocate talking to them early and often if one thinks one’s technology might have clinical value.
One of the fundamental challenges of tissue engineering approaches is that they are often multicomponent systems with polymers, cells, and drugs involved. Such combination systems have been more challenging to get approved because of their complexity and the number of branches of the FDA that are likely to be involved. They also scare potential investors, which limits one’s options for raising the tremendous capital needed to pursue new therapies. The ideal system is a simple one, but the reality is that for a number of complex neurological conditions, successful treatment is likely to involve a combination system. How these might be balanced to optimize efficacy and simplicity is still unfolding.
Nonetheless, there are significant inroads being made into developing and testing new therapies based on single component systems in the nervous system whether they are cellular, pharmacological, electrical, or scaffold-based systems. We can learn from everything that has been done in the clinic and target our research to the most translatable technologies with the greatest chance of success. It takes a team of excellent, collaborative researchers and a certain degree of courage to struggle through the challenges, but the potential to develop therapies should engender a willingness to work together and step beyond our comfortable places.
Highlights.
We examine the pragmatic challenges associated with engineering therapies to CNS disorders.
We discuss the benefits and challenges of various scaffolds for CNS repair.
We discuss the benefits and challenges of drug delivery to the CNS.
We discuss functional electrical stimulation’s role in CNS therapy and rehabilitation.
Simple solutions lend themselves to translation, but the complex CNS may not be amenable to simple.
Acknowledgments
The authors would like to acknowledge support from the NIH Director’s New Innovator Award number DP20D007338.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew J. Shoffstall, Email: ajs215@case.edu.
Dawn M. Taylor, Email: taylord8@ccf.org.
References
- 1.N.S.C.I.S. Center, editor. The 2010 Annual Statistical Report: for the Spinal Cord Injury Model Systems. Birmingam, Alabama: University of Alabama at Birmingham; 2010. pp. 1–76. [Google Scholar]
- 2.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. Journal of Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jamal KT, Gherardini L, Bardi G, Nunes A, Guo C, Bussy C, Herrero MA, Bianco A, Prato M, Kostarelos K, Pizzorusso T. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 108:10952–10957. doi: 10.1073/pnas.1100930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, 2nd, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 18:179–190. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balgude A, Yu X, Szymanski A, Bellamkonda R. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 6.Batchelor PE, Wills TE, Hewa AP, Porritt MJ, Howells DW. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 2008;1209:49–56. doi: 10.1016/j.brainres.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 7.Baumann MD, Kang CE, Tator CH, Shoichet MS. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials. 2010;31:7631–7639. doi: 10.1016/j.biomaterials.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Becker D, Gary DS, Rosenzweig ES, Grill WM, McDonald JW. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp Neurol. 2010;222:211–218. doi: 10.1016/j.expneurol.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behravesh E, Jo S, Zygourakis K, Mikos AG. Synthesis of in situ cross-linkable macroporous biodegradable poly(propylene fumarate-co-ethylene glycol) hydrogels. Biomacromolecules. 2002;3:374–381. doi: 10.1021/bm010158r. [DOI] [PubMed] [Google Scholar]
- 10.Belda-Lois JM, Mena-Del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, Iosa M, Molinari M, Tamburella F, Ramos A, Caria A, Solis-Escalante T, Brunner C, Rea M. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil. 2011;8:66. doi: 10.1186/1743-0003-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamkonda RV. Biomimetic materials: marine inspiration. Nat Mater. 2008;7:347–348. doi: 10.1038/nmat2176. [DOI] [PubMed] [Google Scholar]
- 12.Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33:441–449. doi: 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branco MC, Pochan DJ, Wagner NJ, Schneider JP. The effect of protein structure on their controlled release from an injectable peptide hydrogel. Biomaterials. 2010;31:9527–9534. doi: 10.1016/j.biomaterials.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brock JH, Rosenzweig ES, Blesch A, Moseanko R, Havton LA, Edgerton VR, Tuszynski MH. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 30:9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butson CR, Noecker AM, Maks CB, McIntyre CC. StimExplorer: deep brain stimulation parameter selection software system. Acta Neurochir Suppl. 2007;97:569–574. doi: 10.1007/978-3-211-33081-4_66. [DOI] [PubMed] [Google Scholar]
- 17.Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science. 2008;319:1370–1374. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]
- 18.Chae J, Sheffler L, Knutson J. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Top Stroke Rehabil. 2008;15:412–426. doi: 10.1310/tsr1505-412. [DOI] [PubMed] [Google Scholar]
- 19.Chen BK, Knight AM, de Ruiter GC, Spinner RJ, Yaszemski MJ, Currier BL, Windebank AJ. Axon regeneration through scaffold into distal spinal cord after transection. J Neurotrauma. 2009;26:1759–1771. doi: 10.1089/neu.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier R. Rapidly rising clinical trial costs worry researchers. CMAJ. 2009;180:277–278. doi: 10.1503/cmaj.082041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke MJ, Wang Y, Morshead CM, Shoichet MS. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials. 32:5688–5697. doi: 10.1016/j.biomaterials.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Daly JJ, Ruff RL. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. ScientificWorldJournal. 2007;7:2031–2045. doi: 10.1100/tsw.2007.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 26.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 27.Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother. 2011;11:1351–1353. doi: 10.1586/ern.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 29.F.a.D. Administration, editor. FDA, PART 316--ORPHAN DRUGS. Vol. 316. US GOV, Code of Federal Regulations (CFR); 1992. 21. [Google Scholar]
- 30.Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol. 2009;19:260–265. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fozdar DY, Lee JY, Schmidt CE, Chen S. Hippocampal neurons respond uniquely to topographies of various sizes and shapes. Biofabrication. 2010;2:035005. doi: 10.1088/1758-5082/2/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fozdar DY, Lee JY, Schmidt CE, Chen S. Selective axonal growth of embryonic hippocampal neurons according to topographic features of various sizes and shapes. Int J Nanomedicine. 2011;6:45–57. doi: 10.2147/IJN.S12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier SE, Oudega M, Fragoso M, Chapon P, Plant GW, Bunge MB, Parel J, M Poly(α-hydroxyacids) for Application in the Spinal Cord: Resorbability and Biocompatability with Adult Rat Schwann Cells and Spinal Cord. Journal of Biomedical Materials Research. 1998;42:642–654. doi: 10.1002/(sici)1097-4636(19981215)42:4<642::aid-jbm22>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 35.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, Weiss D, Robert Taylor W, Guldberg RE. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol Heart Circ Physiol. 298:H1959–H1965. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guest JD, Hesse D, Schnell L, Schwab ME, Bunge MB, Bunge RP. Influence of IN-1 Antibody and Acidic FGF-Fibrin Glue on the Response of Injured Corticospinal Tract Axons to Human Schwann Cell Grafts. Journal of Neuroscience Research. 1997;50:888–905. doi: 10.1002/(SICI)1097-4547(19971201)50:5<888::AID-JNR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 37.Guest JD, Rao A, Olson L, Bunge MB, Bunge RP. The Ability of Human Schwann Cell Grafts to Promote Regeneration in the Transected Nude Rat Spinal Cord. Experimental Neurology. 1997;148:502–522. doi: 10.1006/exnr.1997.6693. [DOI] [PubMed] [Google Scholar]
- 38.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heris HK, Rahmat M, Mongeau L. Characterization of a Hierarchical Network of Hyaluronic Acid/Gelatin Composite for use as a Smart Injectable Biomaterial. Macromol Biosci. doi: 10.1002/mabi.201100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollis ER, 2nd, Lu P, Blesch A, Tuszynski MH. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol. 2009;215:53–59. doi: 10.1016/j.expneurol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn EM, Beaumont M, Shu XZ, Harvey A, Prestwich GD, Horn KM, Gibson AR, Preul MC, Panitch A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. Journal of Neurosurgery-Spine. 2007;6:133–140. doi: 10.3171/spi.2007.6.2.133. [DOI] [PubMed] [Google Scholar]
- 43.Hou S, Xu Q, Tian W, Cui F, Cai Q, Ma J, Lee IS. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J Neurosci Methods. 2005;148:60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Hou SP, Xu QY, Tian WM, Cui FZ, Cai Q, Ma J, Lee IS. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. Journal of Neuroscience Methods. 2005;148:60–70. doi: 10.1016/j.jneumeth.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 46.Hurley JR, Balaji S, Narmoneva DA. Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol. 2010;299:C444–C453. doi: 10.1152/ajpcell.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang DH, Jeong SR, Kim BG. Gene transfer mediated by stem cell grafts to treat CNS injury. Expert Opin Biol Ther. 11:1599–1610. doi: 10.1517/14712598.2011.631908. [DOI] [PubMed] [Google Scholar]
- 48.Iannotti C, Li HY, Yan P, Lu XB, Wirthlin L, Xu XM. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Experimental Neurology. 2003;183:379–393. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 49.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255–266. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 50.Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 51.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Jain A, McKeon RJ, Brady-Kalnay SM, Bellamkonda RV. Sustained delivery of activated Rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PLoS One. 6:e16135. doi: 10.1371/journal.pone.0016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jesuraj NJ, Nguyen PK, Wood MD, Moore AM, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Differential gene expression in motor and sensory Schwann cells in the rat femoral nerve. J Neurosci Res. 90:96–104. doi: 10.1002/jnr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin-based tissue engineering scaffolds enhances neural fiber sprouting following subacute spinal cord injury. Biotechnol Bioeng. 2009;104:1207–1214. doi: 10.1002/bit.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 19:89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kabos P, Matundan H, Zandian M, Bertolotto C, Robinson ML, Davy BE, Yu JS, Krueger RC. Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem. Biophys. Res. Commun. 2004;318:955–963. doi: 10.1016/j.bbrc.2004.04.114. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan AV, Baim DS, Smith JJ, Feigal DA, Simons M, Jefferys D, Fogarty TJ, Kuntz RE, Leon MB. Medical device development: from prototype to regulatory approval. Circulation. 2004;109:3068–3072. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- 58.Khan T, Dauzvardis M, Sayers S. Carbon Filament Implants Promote Axonal Growth Across the Transected Rat Spinal Cord. Brain Research. 1991;541:139–145. doi: 10.1016/0006-8993(91)91087-h. [DOI] [PubMed] [Google Scholar]
- 59.Khatiwala C, Peyton S, Putnam A. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Physiol, Cell Physiol. 2006;290:C1640–C1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- 60.Kilgore KL, Peckham P, Keith MW. Twenty year experience with implanted neuroprostheses. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:7212–7215. doi: 10.1109/IEMBS.2009.5335272. [DOI] [PubMed] [Google Scholar]
- 61.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nature Biotechnology. 1999;17:979–983. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 62.Kim H, Cooke MJ, Shoichet MS. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. doi: 10.1016/j.tibtech.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009;4:e4987. doi: 10.1371/journal.pone.0004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Szele FG. Activation of subventricular zone stem cells after neuronal injury. Cell and Tissue Research. 2008;331:337–345. doi: 10.1007/s00441-007-0451-1. [DOI] [PubMed] [Google Scholar]
- 65.Kim YT, Caldwell JM, Bellamkonda RV. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials. 2009;30:2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kramer RH, Fortin DL, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19:544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyhn MV, Klassen H, Johansson UE, Warfvinge K, Lavik E, Kiilgaard JF, Prause JU, Scherfig E, Young M, la Cour M. Delayed administration of glial cell line-derived neurotrophic factor (GDNF) protects retinal ganglion cells in a pig model of acute retinal ischemia. Exp Eye Res. 2009;89:1012–1020. doi: 10.1016/j.exer.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 70.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, Ffrench-Constant C. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. Journal of Comparative Neurology. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 72.Lee KH, Yoon DH, Park YG, Lee BH. Effects of glial transplantation on functional recovery following acute spinal cord injury. J Neurotrauma. 2005;22:575–589. doi: 10.1089/neu.2005.22.575. [DOI] [PubMed] [Google Scholar]
- 73.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P. Neurite Outgrowth in Fibrin Gels Is Regulated by Substrate Stiffness. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- 75.Marchand R, Woerly S. Transected Spinal Cords Grafted With In Situ Self-Assembled Collagen Matrices. Neuroscientist. 1990;36:45–60. doi: 10.1016/0306-4522(90)90350-d. [DOI] [PubMed] [Google Scholar]
- 76.Martin BC, Minner EJ, Wiseman SL, Klank RL, Gilbert RJ. Agarose and methylcellulose hydrogel blends for nerve regeneration applications. J Neural Eng. 2008;5:221–231. doi: 10.1088/1741-2560/5/2/013. [DOI] [PubMed] [Google Scholar]
- 77.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarty JH. Cell adhesion and signaling networks in brain neurovascular units. Curr Opin Hematol. 2009;16:209–214. doi: 10.1097/MOH.0b013e32832a07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McConnell GC, Rees HD, Levey AI, Gutekunst CA, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 80.McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. Journal of Clinical Neurophysiology. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97:561–567. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- 82.Modolo J, Beuter A. Linking brain dynamics, neural mechanisms, and deep brain stimulation in Parkinson's disease: an integrated perspective. Med Eng Phys. 2009;31:615–623. doi: 10.1016/j.medengphy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Moore AM, Wood MD, Chenard K, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE, Borschel GH. Controlled delivery of glial cell line-derived neurotrophic factor enhances motor nerve regeneration. J Hand Surg Am. 35:2008–2017. doi: 10.1016/j.jhsa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 84.Moore N, Sakiyama-Elbert S. Analysis of Cell Binding and Internalization of Multivalent PEG-Based Gene Delivery Vehicles. IEEE Trans Nanobioscience. doi: 10.1109/TNB.2011.2179555. [DOI] [PubMed] [Google Scholar]
- 85.Mulcahey MJ, Betz RR, Kozin SH, Smith BT, Hutchinson D, Lutz C. Implantation of the Freehand System during initial rehabilitation using minimally invasive techniques. Spinal Cord. 2004;42:146–155. doi: 10.1038/sj.sc.3101573. [DOI] [PubMed] [Google Scholar]
- 86.Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27:3982–3994. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng TF, Lavik E, Keino H, Taylor AW, Langer RS, Young MJ. Creating an immune-privileged site using retinal progenitor cells and biodegradable polymers. Stem Cells. 2007;25:1552–1559. doi: 10.1634/stemcells.2006-0780. [DOI] [PubMed] [Google Scholar]
- 88.Noonan CW, Williamson DM, Henry JP, Indian R, Lynch SG, Neuberger JS, Schiffer R, Trottier J, Wagner L, Marrie RA. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 7:A12. [PMC free article] [PubMed] [Google Scholar]
- 89.Pan L, Ren Y, Cui F, Xu Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J Neurosci Res. 2009;87:3207–3220. doi: 10.1002/jnr.22142. [DOI] [PubMed] [Google Scholar]
- 90.Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- 91.Pashuck ET, Cui H, Stupp SI. Tuning supramolecular rigidity of peptide fibers through molecular structure. J Am Chem Soc. 2010;132:6041–6046. doi: 10.1021/ja908560n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25:1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 93.Pawar K, Mueller R, Caioni M, Prang P, Bogdahn U, Kunz W, Weidner N. Increasing capillary diameter and the incorporation of gelatin enhance axon outgrowth in alginate-based anisotropic hydrogels. Acta Biomater. 2011;7:2826–2834. doi: 10.1016/j.actbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 95.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cellular Physiology. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 96.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Experimental Neurology. 2006;201:359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 97.Popovic DB, Popovic MB. Advances in the use of electrical stimulation for the recovery of motor function. Prog Brain Res. 2011;194:215–225. doi: 10.1016/B978-0-444-53815-4.00005-4. [DOI] [PubMed] [Google Scholar]
- 98.Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–1179. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.C.f.D.C.a. Prevention, editor. U.S. Department of Health and Human Services. Atlanta, GA: Centers for Disease Control and Prevention; 2011. C.f.D.C.a. Prevention, National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. [Google Scholar]
- 100.Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional Recovery of Paraplegic Rats and Motor Axon Regeneration in Their Spinal Cords by Olfactory Ensheathing Glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 101.Robinson J. Engineering Thinking and Rhetoric. Journal of Engineering Education July. 1998:227–229. [Google Scholar]
- 102.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 103.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarig-Nadir O, Seliktar D. The role of matrix metalloproteinases in regulating neuronal and nonneuronal cell invasion into PEGylated fibrinogen hydrogels. Biomaterials. 2010;31:6411–6416. doi: 10.1016/j.biomaterials.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 105.Saunders RL, Hammer DA. Assembly of Human Umbilical Vein Endothelial Cells on Compliant Hydrogels. Cell Mol Bioeng. 2010;3:60–67. doi: 10.1007/s12195-010-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott R, Marquardt L, Willits RK. Characterization of poly(ethylene glycol) gels with added collagen for neural tissue engineering. J Biomed Mater Res A. 2010;93:817–823. doi: 10.1002/jbm.a.32775. [DOI] [PubMed] [Google Scholar]
- 107.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater. 7:2401–2409. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 108.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 109.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 111.Smeal RM, Rabbitt R, Biran R, Tresco PA. Substrate curvature influences the direction of nerve outgrowth. Ann Biomed Eng. 2005;33:376–382. doi: 10.1007/s10439-005-1740-z. [DOI] [PubMed] [Google Scholar]
- 112.Smeal RM, Tresco PA. The influence of substrate curvature on neurite outgrowth is cell type dependent. Exp Neurol. 2008;213:281–292. doi: 10.1016/j.expneurol.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 113.Song BN, Li YX, Han DM. Delayed electrical stimulation and BDNF application following induced deafness in rats. Acta Otolaryngol. 2009;129:142–154. doi: 10.1080/00016480802043949. [DOI] [PubMed] [Google Scholar]
- 114.Song S, Kim M, Shin JH. Upstream mechanotaxis behavior of endothelial cells. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2106–2110. doi: 10.1109/IEMBS.2009.5334307. [DOI] [PubMed] [Google Scholar]
- 115.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–451. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 116.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 117.Suri S, Schmidt CE. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 2009;5:2385–2397. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 118.Talbott JF, Cao Q, Bertram J, Nkansah M, Benton RL, Lavik E, Whittemore SR. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Exp Neurol. 2007;204:485–489. doi: 10.1016/j.expneurol.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor SJ, McDonald JW, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. Journal of Controlled Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 120.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 38:225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomita H, Sugano E, Isago H, Hiroi T, Wang Z, Ohta E, Tamai M. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp Eye Res. 2010;90:429–436. doi: 10.1016/j.exer.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 122.Tresco PA, Winslow BD. The challenge of integrating devices into the central nervous system. Crit Rev Biomed Eng. 2011;39:29–44. doi: 10.1615/critrevbiomedeng.v39.i1.30. [DOI] [PubMed] [Google Scholar]
- 123.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. Journal of Neurotrauma. 2004;21:789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- 124.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. Journal of Neuroscience. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Wei YT, Zu ZH, Ju RK, Guo MY, Wang XM, Xu QY, Cui FZ. Combination of hyaluronic acid hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharm Res. 28:1406–1414. doi: 10.1007/s11095-011-0452-3. [DOI] [PubMed] [Google Scholar]
- 126.Webber DJ, Bradbury EJ, McMahon SB, Minger SL. Transplanted neural progenitor cells survive and differentiate but achieve limited functional recovery in the lesioned adult rat spinal cord. Regen Med. 2007;2:929–945. doi: 10.2217/17460751.2.6.929. [DOI] [PubMed] [Google Scholar]
- 127.Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res A. 2006;76:626–637. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- 128.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 129.Willerth SM, Rader A, Sakiyama-Elbert SE. The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res. 2008;1:205–218. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Woerly S, Doan VD, Evans-Martin F, Paramore CG, Peduzzi JD. Spinal cord reconstruction using NeuroGel implants and functional recovery after chronic injury. J Neurosci Res. 2001;66:1187–1197. doi: 10.1002/jnr.1255. [DOI] [PubMed] [Google Scholar]
- 131.Wood MD, Hunter D, Mackinnon SE, Sakiyama-Elbert SE. Heparin-binding-affinity-based delivery systems releasing nerve growth factor enhance sciatic nerve regeneration. J Biomater Sci Polym Ed. 21:771–787. doi: 10.1163/156856209X445285. [DOI] [PubMed] [Google Scholar]
- 132.Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, Moran DW, Borschel GH, Sakiyama-Elbert SE. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 106:970–979. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 133.Wood-Dauphinee S, Exner G. Quality of life in patients with spinal cord injury - basic issues, assessment, and recommendations - Results of a consensus meeting. Restorative Neurology and Neuroscience. 2002;20:135–149. [PubMed] [Google Scholar]
- 134.Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci. 2008;28:11916–11924. doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu X, Zhang S-X, Li H, Aebischer P, Bunge M. Regrowth of Axons into the Distal Spinal Cord Through a Schwann-Cell-Seeded Mini-Channel Implanted into Hemisected Adult Rat Spinal Cord. European Journal of Neuroscience. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- 136.Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann Cell Transplants Promote Axonal Regeneration From Both the Rostral and Caudal Stumps of the Transected Adult Rat Spinal Cord. Journal of Neurocytology. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- 137.Xu XM, Guénard V, Kleitman N, Aebischer P, Bunge MB. A Combination of BDNF and NT-3 Promotes Supraspinal Axonal Regeneration Into Schwann Cell Grafts in Adult Rat Thoracic Spinal Cord. Experimental Neurology. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- 138.Xu XM, Guénard V, Kleitman N, Bunge MB. Axonal Regeneration Into Schwann Cell-Seeded Guidance Channels Grafted Into Transected Adult Rat Spinal Cord. Journal of Comparative Neurology. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 139.Yucel D, Kose GT, Hasirci V. Polyester based nerve guidance conduit design. Biomaterials. 2010;31:1596–1603. doi: 10.1016/j.biomaterials.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 140.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 141.Zhang XQ, Tang H, Hoshi R, De Laporte L, Qiu H, Xu X, Shea LD, Ameer GA. Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials. 2009;30:2632–2641. doi: 10.1016/j.biomaterials.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]