Abstract
Epstein-Barr virus (EBV) infection and latency has been associated with malignant diseases including nasopharyngeal carcinoma, Hodgkin lymphoma, Burkitt lymphoma, and immune deficiency associated lymphoproliferative diseases. EBV-encoded latent membrane protein 2A (LMP2A) recruits Lyn and Syk kinases via its SH2-domain binding motifs, and modifies their signaling pathways. LMP2A transgenic mice develop hyperproliferative bone marrow B cells and immature peripheral B cells through modulation of Lyn kinase signaling. LMP2A/λ-MYC double transgenic mice develop splenomegaly and cervical lymphomas starting at 8 weeks of age. We reasoned that targeting Lyn in LMP2A-expressing B cells with dasatinib would provide a therapeutic option for EBV-associated malignancies. Here, we show that dasatinib inhibits B cell colony formation by LMP2A transgenic bone marrow cells, and reverses splenomegaly and tumor growth in both a pre-tumor and a syngeneic tumor transfer model of EBV-associated Burkitt lymphoma. Our data support the idea that dasatinib may prove to be an effective therapeutic molecule for the treatment of EBV-associated malignancies.
Keywords: Burkitt lymphoma, dasatinib, Epstein-Barr virus (EBV), latent membrane protein 2A (LMP2A), Lyn, post-transplant lymphoproliferative diseases (PTLD)
1. Introduction
Greater than ninety percent of humans are infected with Epstein-Barr virus (EBV) by adulthood, and the virus establishes a lifelong latent infection in memory B lymphocytes (Cohen, 2000; Thorley-Lawson, 2001; Thorley-Lawson and Gross, 2004). Latent EBV infection causes minimal to no harm to hosts with intact immune systems, but infection is associated with a variety of malignancies including Burkitt Lymphoma and Hodgkin Lymphoma. In humans with decreased T cell immunity, including those with acquired immunodeficiency syndrome (AIDS) and those who are immunosuppressed following hematopoietic stem cell or solid organ transplant, latent EBV infection can result in lymphoproliferative disorders or lymphoid malignancies. Traditional antiviral treatments that target viral mechanisms of acute lytic infection are not effective in latent EBV infection (Cohen, 1991). Currently, first-line treatments include attempts to restore cellular immunity by decreasing immunosuppressive therapy and treatment with cytotoxic chemotherapy, both of which offer substantial risk to patients.
Latent membrane protein 2A (LMP2A) is an EBV-encoded transmembrane protein with an N-terminus intracellular signaling domain that associates with and activates B cell signaling components such as Lyn and Syk kinases (Caldwell et al., 1998; Fruehling and Longnecker, 1997; Fruehling et al., 1998; Merchant et al., 2000; Rickinson and Kieff, 2007). Previous studies have shown that LMP2A is constitutively phosphorylated and alters B lymphocyte growth and differentiation signaling (Merchant et al., 2000; Rickinson and Kieff, 2007). Furthermore, LMP2A is consistently detected in latently infected B lymphocytes and in EBV-associated malignancies, providing a direct link between benign latent infection and malignant transformation (Caldwell et al., 1998; Ong et al., 2009; Tao et al., 1998; Thorley-Lawson and Gross, 2004; Xue et al., 2002). Normally, in the absence of antigen stimulation, the B cell receptor (BCR) delivers a tonic signal that is essential for B lymphocyte survival (Lam et al., 1997). A member of the Src family protein tyrosine kinases Lyn is a key regulator of many downstream signaling events in B lymphocytes, including maturation, apoptosis, proliferation, and tolerance (Chan et al., 1997; Ferry et al., 2005; Hibbs et al., 1995; Meade et al., 2002; Mirnics et al., 2004; Nishizumi et al., 1995). Lyn phosphorylates tyrosine residues within the immunoreceptor tyrosine activation motif (ITAM) of the BCR, to which the non-receptor protein tyrosine kinase Syk is recruited, resulting in a cascade of activating downstream events that promote survival and inhibition of apoptosis in B lymphocytes (Miller et al., 1995; Miller et al., 1994; Thorley-Lawson, 2001). LMP2A contains a similar ITAM motif and is able to mimic the BCR survival signal by sequestering Lyn and Syk from the BCR, resulting in constitutive activation of downstream signaling proteins that enhance B lymphocyte survival and regulate EBV latency (Engels et al., 2001; Fruehling and Longnecker, 1997; Miller et al., 1995). By activating key kinases in the BCR signaling pathway, LMP2A is able to generate anti-apoptotic signals that enhance B lymphocyte survival and may contribute to the development of malignancies.
During B cell genesis, B lymphocytes lacking a baseline survival signal from the BCR undergo apoptosis in the bone marrow. However, in LMP2A transgenic mice, the expression of LMP2A permits the bypass of normal B lymphocyte checkpoints, allowing the escape and accumulation of BCR-negative cells in the periphery (Caldwell et al., 1998). This indicates that LMP2A expression offers a constitutive survival signal in B cells that would otherwise undergo apoptosis (Caldwell et al., 2000; Caldwell et al., 1998). LMP2A transgenic mice have normal spleen size and do not develop lymph node tumors. However, double transgenic mice that express both LMP2A and the MYC oncogene (LMP2A/λ-MYC) develop marked splenomegaly prior to tumor formation, as compared to λ-MYC transgenic mice. Additionally, LMP2A/λ-MYC double transgenic mice demonstrate accelerated development of cervical and axillary lymph node primary tumors compared to λ-MYC transgenic mice (Bieging et al., 2009; Bultema et al., 2009). These findings support the hypothesis that expression of LMP2A allows B lymphocytes to bypass MYC-induced apoptosis, resulting in enhanced B lymphocyte survival.
With significant morbidity and mortality rates associated with the EBV-associated malignancies and the lack of efficient therapies, novel therapeutic approaches exploiting the mechanisms of EBV latency are needed to treat or prevent these malignancies. Targeting and inhibiting the function of signaling proteins modulated by LMP2A may decrease the number of B lymphocytes that depend on the LMP2A survival signal and may have the potential to be used in the treatment of EBV-associated malignancies. Lyn kinase constitutes one such therapeutic target since it preferentially associates with LMP2A, relative to other Src kinases, and is required for the bypass of the normal BCR signal and propagation of the LMP2A survival signal (Rovedo and Longnecker, 2008). Dasatinib is a small molecule inhibitor of Src and Abl kinases and is currently used in the treatment of imatinib-resistant chronic myeloid leukemia (CML) and Philadelphia chromosome-associated acute lymphoblastic leukemia (ALL) (Doggrell, 2005; Guilhot et al., 2007; Jabbour et al., 2011; Kantarjian et al., 2006; Lee et al., 2011; Lindauer and Hochhaus, 2010; Lombardo et al., 2004; Shah et al., 2004). Therefore, dasatinib is a promising drug candidate targeting LMP2A-modulated B cell signaling.
Targeted inhibition of signaling molecules activated by LMP2A, including Lyn, may interrupt LMP2A-mediated survival signals and result in enhanced death of LMP2A-expressing malignant B lymphocytes. In this study, we aimed to test the therapeutic efficacy of dasatinib to target the LMP2A-modulated survival signals in tumors in vitro as well as in vivo in a murine model of EBV-associated lymphoma. Here we report that dasatinib specifically inhibits the ability of bone marrow cells from LMP2A transgenic mice to form colonies in vitro and prevents splenomegaly and tumor development by B cell tumors from LMP2A/λ-MYC double transgenic (Tg6/λ-MYC) mice in vivo.
2. Materials and methods
2.1 Mice
EµLMP2A (TgE and Tg6 strains), MYC (λ-MYC), and LMP2A/λ-MYC double transgenic mice (Tg6/λ-MYC) have been previously described (Bieging et al., 2009; Bultema et al., 2009; Caldwell et al., 2000; Caldwell et al., 1998; Kovalchuk et al., 2000). λ-MYC mice were obtained from the National Cancer Institute (Kovalchuk et al., 2000). Rag1knockout (Rag-1KO) mice (B6.129S7-Rag1tmMom/J, catalog number 002216) were purchased from Jackson Laboratories. All mice were bred and housed at the Northwestern University Center for Comparative Medicine in accordance with Institutional Animal Care and Use Committee guidelines.
2.2 Preparation of dasatinib
Dasatinib was generously provided by Bristol-Myers Squibb. For in vitro experiments, dasatinib was dissolved in dimethyl sulfoxide (DMSO) at 10 mg/ml and stored in aliquots at −20°C. For in vivo experiments, dasatinib was dissolved in DMSO at 60 mg/ml and stored in aliquots at −20°C. On each treatment day, aliquots were thawed and diluted with 5.1% polyethylene glycol (PEG-400; EMD, Fisher) and 5.1% Tween-λ80 (Fisher) immediately before use, as previously described (Cen and Longnecker, 2011). Control mice were treated with an equivalent concentration of DMSO dissolved in vehicle buffer.
2.3 Isolation of bone marrow cells and growth in methylcellulose media with dasatinib
Bone marrow cells were collected from wild-type (wt) or TgE mice (6–12 weeks old) and cultured in methylcellulose media containing IL-7 (MethoCult® M3630, Stemcell Technologies), as previously described (Caldwell et al., 1998; Cooper and Longnecker, 2002; Ikeda and Longnecker, 2005). Specifically, WT cells were seeded at a concentration of 2 to 4 × 106 cells per mL in 0.5 mL of media in 12-well plates. TgE cells were seeded at a concentration of 1×106 cells per mL. The cells were cultured in duplicate with varying concentrations of dasatinib (10 nM-300 nM) or equivalent concentrations of DMSO for 7 days at 37°C in 5% CO2. Each sample well was photographed under the microscope (Nikon SMZ10A, 7.5×), and the colonies were counted manually. The number of colonies in each dasatinib-treated well was normalized as the percent of the number of colonies in DMSO-treated control wells. Dose-response curves were calculated based on this normalization. Data for each concentration point are the average of 2–4 separate experiments..
2.4 Treatment of transgenic mice
Wild-type (6–16 weeks old), TgE (6–10 weeks old), λ-MYC (16–20 weeks old), and Tg6/λ-MYC (5–10 weeks old, in a given experiment, age difference of mice was less than two weeks) mice were treated with dasatinib (30 mg/kg intraperitoneally) or equivalent amount of vehicle alone once daily for 14 days. On day 15, the mice were sacrificed, and lymph node tumors and spleens were harvested, documented, processed, and analyzed with flow cytometry or western blotting.
2.5 Transgenic tumor graft model
Peripheral lymph node tumor cells from λ-MYC and Tg6/λ-MYC mice were harvested, processed into single cell suspensions, and frozen at −80°C. Cells were later thawed, washed, and 0.5–1 × 106 cells from λ-MYC mice or 1–2 × 106 cells from Tg6/λ-MYC mice in 200 µl of PBS were injected subcutaneously into the right flank of anesthetized Rag-1KO mice (10–20 weeks old). For each separate experiment, two-times the number of Tg6/λ-MYC lymphoma cells was used compared to λ-MYC lymphoma cells, as previous experiments had demonstrated that λ-MYC tumors developed more rapidly than Tg6/λ-MYC tumors, in the tumor transfer system, as opposed to the primary tumor development in the transgenic mice, when the same number was injected (O Cen, unpublished data). For each experiment, the same number of λ-MYC or Tg6/λ-MYC cells was injected into each mouse in each cohort. When tumors were evident, mice were treated with dasatinib (30 mg/kg intraperitoneally daily) or vehicle alone for 10–14 days, depending on the health and anticipated survival of the mice. On the day following the last day of treatment, the mice were sacrificed, and tumors and spleens were harvested, documented, and processed for flow cytometry or western blotting (Cen and Longnecker, 2011).
2.6 Flow cytometry analysis
Spleens, lymph nodes, and tumors were processed into single cells. One million cells from each sample were stained with B220 (eBiosciences), CD3, and IgM (BD Biosciences) antibodies and 7AAD (Invitrogen) and acquired with FacsCANTO-II (BD Biosciences). The following successive gating strategy was used in the given order for all samples: live cells (7AAD negative), singlet cells, and lymphocytes. The percentages of B220 and CD3 positive cells were determined within the lymphocyte gate.
2.7 Western Blotting
Tumor cells from primary tumors of transgenic mice (Figure 3) and transgenic tumor grafts following treatment with dasatinib or vehicle (Figure 5) were collected and lysed in modified RIPA buffer (0.1 M Tris, pH 8.2, 0.15 M NaCl, 2% sodium dodecyl sulfate, 1% NP-40, 0.5% NaF, 200 mM Na3VO4, 200 mM phenylmethylsulfonyl fluoride, 10 mM dithiothreitol, 10 units benzonase nuclease, Complete Mini EDTA-free protease inhibitor, and PhosSTOP phosphatase inhibitor cocktail (Roche)). Equal amount of lysate from each sample was separated by SDS-PAGE, transferred to Immobilon-P membrane (Millipore), and then probed with Lyn (sc-15, Santa Cruz Biotechnology), phosphorylated Lyn (Ab-40660, Abcam, Inc.), GAPDH (Abcam Inc.), or anti-LMP2A 14B7 rat monoclonal antibody (Fruehling et al., 1996). Blots were developed with ECL (Amersham) and detected with X-ray films.
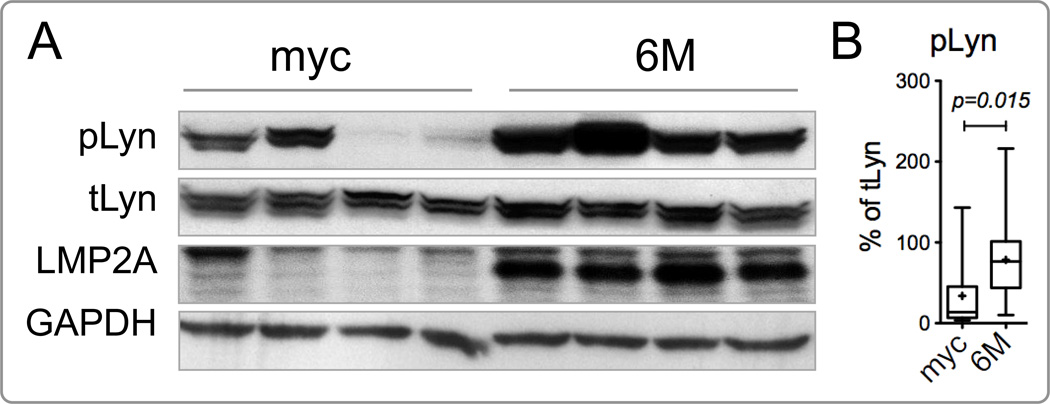
Figure 3.
Lyn is hyperphosphorylated in Tg6/λ-MYC tumors. (A) Representative western blots of cervical tumors for phosphorylated Lyn (pLyn), total Lyn (tLyn), LMP2A, and GAPDH from 4 Tg6/λ-MYC (6M) and 4 λ-MYC (myc) transgenic mice (a non-specific band consistently detected with anti-LMP2A antibody is also seen just above the LMP2A). (B) Densitometric analysis of pLyn normalized to tLyn for each tumor sample. The raw densitometric value for each pLyn band from each sample is divided to that of tLyn from the same sample and multiplied by 100 to find the percent of pLyn for the respective mouse. Data are from 4 independent experiments, totaling 14 Tg6/λ-MYC and 15 λ-MYC transgenic mice. See Material and Methods for the details of data presentation on the graphs.
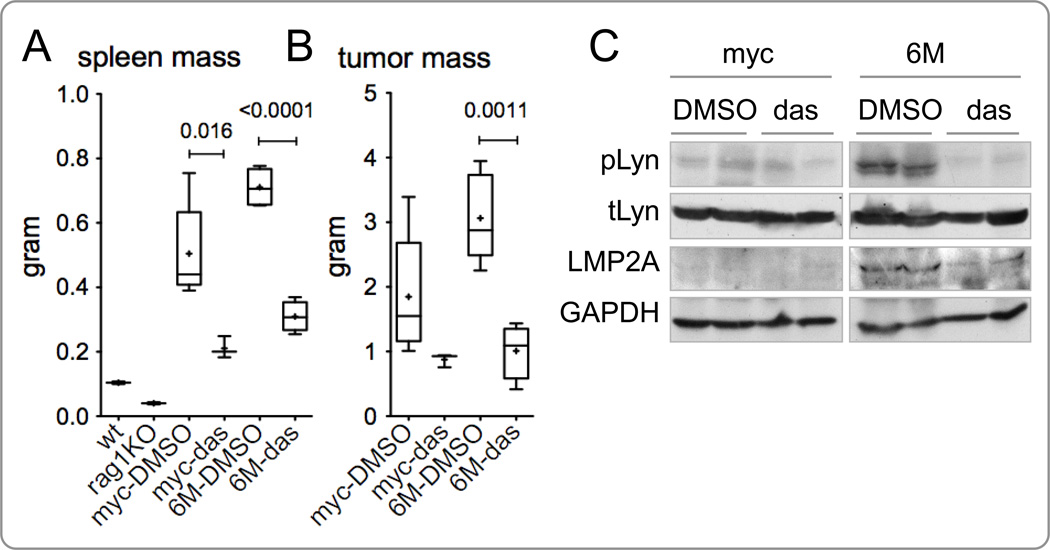
Figure 5.
Dasatinib inhibits splenomegaly and secondary tumor formation in Rag1KO mice implanted with primary tumor from Tg6/λ-MYC mice. Lymph node tumor cells from Tg6/λ-MYC (6M) and λ-MYC (myc) transgenic mice were injected subcutaneously into the flank of Rag-1KO mice. When tumors were palpable, mice were intraperitoneally treated with dasatinib (das) or vehicle (DMSO) alone daily for 10–14 days, depending on the health and anticipated survival of the mice. The masses of spleens (A) and tumors (B) were analyzed on the day following the last day of treatment. Data of one representative experiment of three separate experiments are shown. The spleen masses from untreated wild-type (wt) and Rag-1 knock-out (rag1KO) mice are shown as reference in A. Each data point represents 3 to 6 mice. (C) Representative protein lysates of tumors from two mice in each group from panel B above were analyzed by western blotting for the phosphorylated Lyn (pLyn), total Lyn (tLyn), LMP2A, and GAPDH. Each lane indicates a single tumor of the specified genotype; the data is representative of three different immunoblot analyses. See Material and Methods for the details of data presentation on the graphs.
2.8 Data analysis and presentation
Unpaired Student’s t tests with two-tailed p values were used to analyze the data. Statistical analysis was performed using SPSS (version 18.0) and GraphPad Prism (version 5.0a – GraphPad Software Inc). A p value of 0.05 or less was considered statistically significant. The data were graphed for each figure in the following format: the box plots for each group represents the interquartile range (25th–75th percentiles) and the longer horizontal line in each box represents the median value. The mean is marked as a + sign, which is a short horizontal line when it coincides with a vertical line or absent or a short vertical line when the median and mean correspond. The whiskers indicate minimum and maximum data points. When there are less than 4 data points in a group, a vertical line is shown instead of a box. Only p values of less than or equal to 0.05 are shown as decimal numbers above the connected data points.
3. Results
3.1 Dasatinib specifically inhibits colony formation by bone marrow cells from LMP2A transgenic mice in methyl cellulose culture
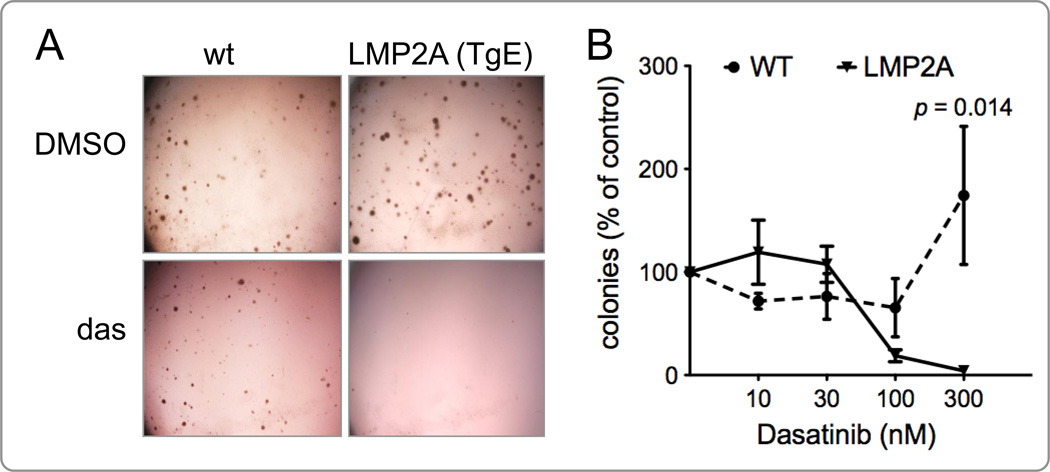
The LMP2A transgenic mice express LMP2A in B cells early in development. Bone marrow cells from the TgE line of LMP2A transgenic mice are hyperproliferative and produce more colonies than bone marrow cells from wild-type C57BL/6 mice (WT) in methylcellulose colony formation assays (Caldwell et al., 1998; Cooper and Longnecker, 2002; Ikeda and Longnecker, 2005). Given the importance of Lyn kinase in the development of B cells and its association with LMP2A, we aimed to test the effect of the Lyn inhibitor dasatinib on the ability of bone marrow cells from TgE mice to form colonies in a methylcellulose culture. Bone marrow cells from TgE or WT mice were cultured in methylcellulose medium containing IL-7 in the absence or presence of varying concentrations of dasatinib for 7 days. Results from four independent experiments show that colony formation by bone marrow cells from TgE mice, but not from WT mice, was significantly inhibited in the presence of dasatinib (Fig. 1). The percent of colonies of TgE bone marrow cells were decreased from 100% in untreated wells to 4.12% in dasatinib treated wells. The values for WT bone marrow cells ranged from 100% to 174.31% (Figure 1B), but this increase is not statistically significant (p > 0.05). In the presence of dasatinib, the difference in the percentage of colonies formed by WT and TgE bone marrow cells is statistically significant (174.31% of untreated WT colonies vs. 4.12% of untreated TgE colonies at 300 nM, p = 0.0143) (Fig. 1B). Although not statistically significant, also at 100 nM concentration of dasatinib, there is a difference in the reduction of colonies formed by WT and TgE bone marrow cells (65.45% colonies in WT vs. 18.77% colonies in TgE cultures at 100 nM, p = 0.073) (Fig. 1B). These data indicate that dasatinib targets LMP2A-induced proliferation and/or survival of B progenitor cells. This also suggests that expression of LMP2A is able to promote B lymphocyte survival and proliferation, which can be inhibited by targeting Lyn and/or c-Abl kinases through dasatinib.
Figure 1.
Dasatinib inhibits colony formation by bone marrow cells from LMP2A transgenic (TgE) mice. (A) Representative micrographs of colonies formed in the methylcellulose culture by bone marrow cells from wild-type (wt) or LMP2A transgenic (TgE line) mice in the presence of dasatinib or DMSO. The micrograph is a representative of 4 different experiments. (B) Macroscopic colonies were manually counted and calculated as explained in Materials and Methods. The normalized percentage of colonies for each dasatinib concentration for wt or TgE bone marrow cells is graphed versus the concentration of dasatinib (n=2–4). Data were analyzed using Student’s t-test. A p value of 0.05 or less was considered statistically significant.
3.2 Dasatinib treatment decreases the spleen size in LMP2A transgenic mice
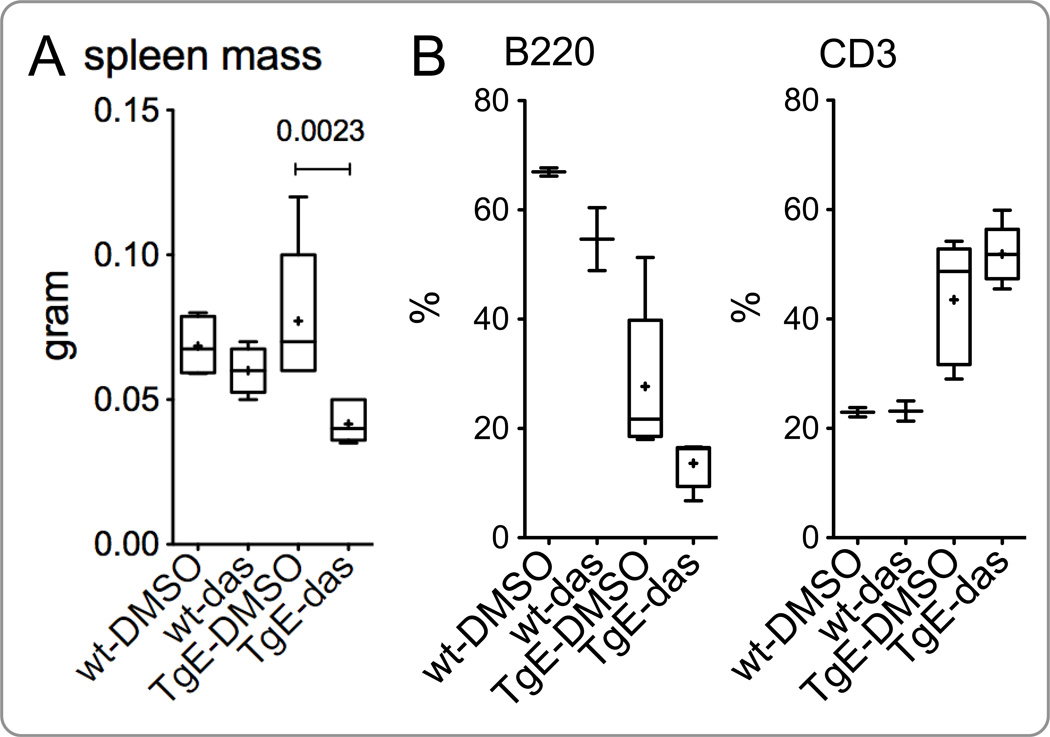
To further determine the in vivo effect of dasatinib in the context of LMP2A, we treated the TgE line of LMP2A transgenic mice with dasatinib or DMSO (vehicle). Results from two independent experiments demonstrate that dasatinib treatment causes a decrease in the spleen mass of TgE mice compared to that of TgE mice treated with vehicle alone (Fig. 2A). In contrast, such a decrease in spleen mass was not observed in WT mice treated with dasatinib (Fig. 2A). The mean spleen mass of TgE mice treated with vehicle or dasatinib alone were 77.14 mg and 41.57 mg, respectively (p = 0.0023); the mean spleen mass of WT mice treated with vehicle or dasatinib alone were 68.50 mg and 60.00 mg, respectively (p = 0.2509). Compared to WT, the TgE mice have a decreased percentage of B cells and increased percentage of T cells in the periphery (Fig. 2B and 2C). With dasatinib treatment, we observed a further considerable, but not statistically significant (p > 0.05), decrease in the percentage of B220 cells and an increase in the percentage of CD3-positive cells (Fig. 2B and 2C, respectively). These data indicate that dasatinib targets LMP2A-modulated signaling in B cells.
Figure 2.
Dasatinib decreases spleen size in LMP2A transgenic mice. LMP2A transgenic (TgE) and wild-type (wt) mice were treated intraperitoneally with dasatinib or vehicle alone daily for 14 days. The spleen mass and cell composition were analyzed on day 15. (A) The spleen mass of wt or TgE mice treated with DMSO or dasatinib (das) is shown. The percent of B220-positive (B) and CD3-positive (C) lymphocytes in the spleen of respective groups was analyzed with flow cytometry as explained in Materials and Methods. Each data point is from 2–6 mice. See Material and Methods for the details of data presentation on the graphs.
3.3 Lyn is hyperphosphorylated in tumors from LMP2A/MYC double transgenic mice
MYC transgenic mice develop B cell lymphoma and leukemia (Adams et al., 1985; Kovalchuk et al., 2000). In double transgenic mice expressing LMP2A and deregulated MYC (Tg6/λ-MYC), MYC-induced lymphoma development is greatly accelerated (Bieging et al., 2009; Bultema et al., 2009). Our lab has previously shown that LMP2A preferentially associates with Lyn (Rovedo and Longnecker, 2008). To examine if LMP2A expression in B cells upregulates Lyn kinase activity that may contribute to accelerated lymphoma development, we analyzed the baseline level of phosphorylated Lyn in the context of LMP2A expression. Autophosphorylation of the kinase domain on tyrosine 396 of Lyn is associated with its kinase activity. To this end, cervical lymph node tumors from four Tg6/λ-MYC and four λ-MYC transgenic mice were harvested and analyzed for the expression and phosphorylation of Lyn kinase by western blotting (Fig. 3). Even though the expression levels of total Lyn in both groups were similar, the phosphorylated Lyn levels varied from moderate to almost no phosphorylation in λ-MYC tumors while these levels were consistently higher in the Tg6/λ-MYC tumors (Fig. 3). The Tg6/λ-MYC primary lymphoma cells had significantly higher phosphorylated Lyn levels than λ-MYC primary lymphoma cells (78.44% of total Lyn expression in Tg6/λ-MYC tumors vs. 33.86% in λ-MYC, p < 0.05) (Fig. 3A and 3B). These results suggest that LMP2A provides a constitutive signal that leads to activation of Lyn in B lymphoma cells in vivo.
3.4 Dasatinib reverses splenomegaly in LMP2A/MYC double transgenic mice
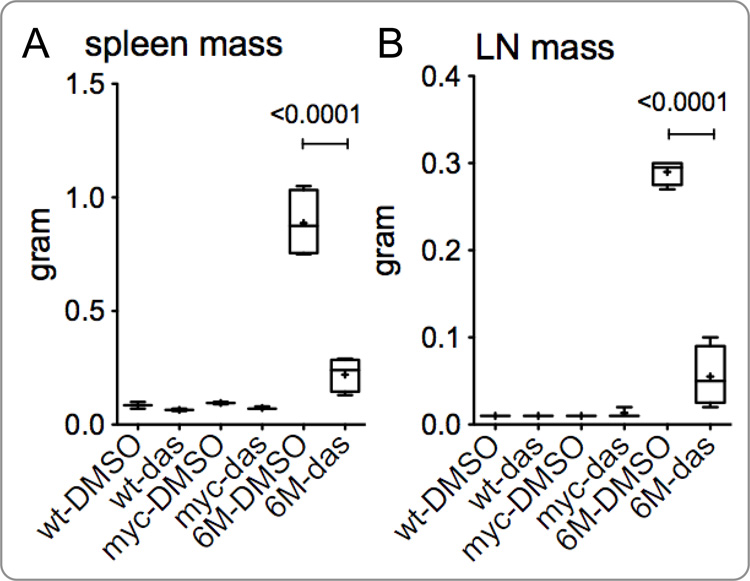
Tg6/λ-MYC mice develop splenomegaly at as young as two weeks of age and develop tumors starting at 8 weeks of age. The λ-MYC mice do not develop splenomegaly until much later, generally after 120 days of age (Bieging et al., 2009; Bultema et al., 2009). While the Tg6/λ-MYC mice rarely live beyond 14 weeks of age, some λ-MYC mice do not develop tumors even after one year of age. Since dasatinib specifically prevented colony formation by LMP2A expressing bone marrow B cells and decreased spleen size in the TgE mice, we anticipated that dasatinib treatment would prevent splenomegaly and tumor development in Tg6/λ-MYC mice. Wild type, λ-MYC, or pre-tumor/early tumor Tg6/λ-MYC transgenic mice were treated with dasatinib or vehicle alone to determine its effect on the sizes of spleen and lymph nodes. Results from three independent experiments demonstrate that the spleen mass is significantly decreased in dasatinib treated Tg6/λ-MYC mice when compared to the control group (Fig. 4A). The mean spleen masses of Tg6/λ-MYC mice treated with either dasatinib or vehicle alone were 220.00 mg and 887.50 mg, respectively (p < 0.0001) (Fig. 4A). No significant differences in spleen masses of WT or λ-MYC transgenic mice were observed with dasatinib treatment. The mean spleen masses of λ-MYC mice treated with dasatinib or vehicle alone were 73.33 mg and 95.00 mg, respectively (p > 0.05) (Fig. 4A). The mean spleen masses of WT mice treated with dasatinλib or vehicle alone were 65.00 mg and 85.00 mg, respectively (p > 0.05) (Fig. 4A).
Figure 4.
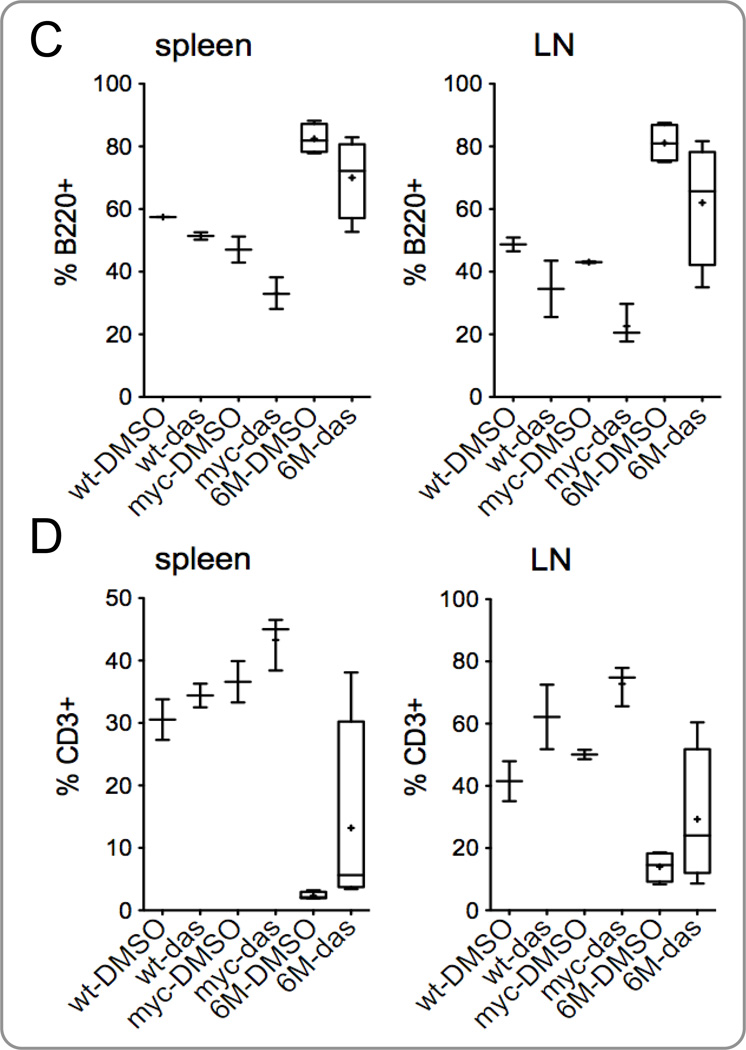
Dasatinib inhibits splenomegaly and lymphadenopathy in Tg6/λ-MYC mice. Tg6/λ-MYC (6M), λ-MYC (myc), and wild-type (wt) mice were intraperitoneally treated with dasatinib (das) or vehicle (DMSO) alone daily for 14 days; the mass of spleens (A) and lymph nodes (B) were analyzed on day 15. The percentage of B220-positive (C) and CD3-positive (D) lymphocytes in the spleens (left panels) and lymph nodes (LN - right panels) of respective groups were analyzed by flow cytometry as indicated in the Materials and Methods section. Each data point is from 2 to 6 mice. See Material and Methods for the details of data presentation on the graphs.
3.5 Dasatinib inhibits lymphadenopathy in LMP2A/MYC double transgenic mice
Similar to inhibiting splenomegaly, dasatinib also inhibited lymphadenopathy in the pretumor or early tumor Tg6/λ-MYC mice. The mean lymph node masses of Tg6/λ-MYC mice treated with dasatinib or vehicle alone were 60.00 mg and 285.00 mg, respectively (p < 0.0001) (Fig. 4B). Similar to our observations with spleen mass, dasatinib did not exert any significant effect on the masses of lymph nodes of WT or λ-MYC transgenic mice. The mean masses of lymph nodes of λ-MYC mice treated with dasatinib or vehicle alone was 13.33 mg and 10.00 mg, respectively (p > 0.05) (Fig. 4B), and those of WT mice treated with dasatinib or vehicle alone were both less than 10.00 mg (Fig. 4B).
We also analyzed the ratios of B and T cells in the spleens and lymph nodes of these mice. Similar to the results we observed in the TgE mice treated with dasatinib (Fig. 2B, 2C), we observed a considerable but not statistically significant (p > 0.05) decrease in the percentage of B220-positive cells (Fig. 4C) and an increase in the percentage of CD3-positive cells (Fig. 4D) in the spleens and lymph nodes of dasatinib treated groups. The decrease in the B220-positive cells and increase in the CD3-positive cells were also observed in WT and λ-MYC groups, albeit less pronounced. The decrease in the B220-positive cells (Fig. 4C) and the increase in the CD3-positive cells (Fig. 4C) in the Tg6/λ-MYC mice after dasatinib treatment were pronounced in some individual mice. However, these changes were not statistically significant. These data indicate that dasatinib is targeting B cells more than T cells.
3.6 Dasatinib reverses splenomegaly in Rag1KO mice engrafted with tumor cells from LMP2A/MYC double transgenic mice
Since dasatinib was able to significantly inhibit splenomegaly and tumor development in the Tg6/λ-MYC mice, we tested whether dasatinib could inhibit tumor cell growth in a syngeneic tumor transfer model developed in our laboratory (Cen and Longnecker, 2011). In this tumor graft model, primary tumor cells from Tg6/λ-MYC or λ-MYC mice are subcutaneously implanted into Rag1-deficient mice (Rag1KO) and mice are monitored until local tumor development is observed, typically within 2 to 3 weeks. The injected tumor cells also metastasize to the spleen and bone marrow of recipient mice, resulting in splenomegaly and peripheral lymphadenopathy. Once the tumors were palpable, the mice were treated intraperitoneally with either dasatinib or vehicle. Results from three independent experiments demonstrate that treatment of tumor recipient mice with dasatinib results in significantly decreased spleen mass, compared to the recipient mice treated with vehicle alone (Figure 5A). Results from one illustrative experiment show mean spleen masses of 309.50 mg and 710.74 mg (p < 0.0001) in mice that had received Tg6/λ-MYC tumors treated with dasatinib or vehicle alone, respectively (Fig. 5A). Dasatinib treatment also decreased the size of spleens in mice that had received the λ-MYC tumor cells, albeit less significantly (p = 0.0169). The mean spleen mass of mice receiving λ-MYC tumor cells was 210.57 mg in the dasatinib treated group and 504.62 mg in the vehicle treated group (p = 0.0169) (Fig. 5A).
3.7 Dasatinib inhibits tumor growth in Rag1KO mice engrafted with tumor cells from LMP2A/MYC double transgenic mice
Similar to the inhibition of splenomegaly, tumor development was also inhibited by dasatinib in the Rag1KO recipient mice. The local tumor mass in mice that had received Tg6/λ-MYC tumors cell and treated with dasatinib was significantly decreased, compared to the control mice treated with vehicle alone (Fig. 5B). Results from one illustrative experiment show mean tumor mass of 1,009 mg and 3,063 mg (p = 0.0011) in mice with Tg6/λ-MYC tumors treated with dasatinib or vehicle alone, respectively (Fig. 5B). Results from the same experiment show mean tumor mass of 875 mg in mice with λ-MYC tumors treated with dasatinib compared to 1,847 mg in those treated with vehicle alone (Fig. 5B). Although there was a considerable decrease in the tumor mass of the mice that had received λ-MYC tumors cells with dasatinib treatment, the difference from that of control group did not reach statistical significance (p = 0.132). These results support the above findings that signaling through Lyn and/or c-Abl is necessary for LMP2A-associated B lymphocyte survival and proliferation, and that inhibition of this signaling is a viable therapeutic target. Furthermore, this signaling pathway is more specific for LMP2A signaling in B lymphoma cells.
3.8 Dasatinib therapy inhibits Lyn phosphorylation in B lymphocyte tumors expressing LMP2A
In order to determine if dasatinib therapy interferes with LMP2A signaling through Lyn, two samples from each of Tg6/λ-MYC and λ-MYC tumor grafts from recipient mice that were treated with either dasatinib or vehicle alone were analyzed with Western Blotting to detect the levels of total Lyn, phosphorylated Lyn, LMP2A, and GAPDH (Fig. 5C). We observed that tumors from Rag1KO mice with Tg6/λ-MYC tumors and treated with vehicle alone showed high levels of phosphorylated Lyn, while the tumors from the Tg6/λ-MYC group that had been treated with dasatinib demonstrated little to no detectable phosphorylated Lyn (Fig. 5C, right panel). In the same experiment, dasatinib treatment did not show a significant effect on the level of Lyn phosphorylation in the tumors from Rag1KO mice with λ-MYC tumors. This may be because the phosphorylation levels of Lyn were already minimal in λ-MYC tumors (Fig. 5C, left panel). These data suggest that LMP2A preferentially signals through Lyn and that dasatinib therapy interferes with LMP2A-induced Lyn signaling.
4. Discussion
There is a considerable clinical need for the development of novel therapeutics that can be used to treat herpesvirus-related diseases in humans. Most members of the herpesvirus family have the ability to cause severe and life-threatening diseases in both immunocompromised and immunocompetent hosts, as a result of either lytic replication or latent infection. Currently available antiviral therapies target lytic replication, but there are few, if any, therapies that are effective against latent herpes virus infections. LMP2A is one of the few EBV transcripts routinely expressed in peripheral B lymphocytes during latent EBV infection (Babcock et al., 1999; Babcock et al., 1998; Babcock et al., 2000; Green et al., 1998; Qu et al., 2000; Tierney et al., 1994). LMP2A is also expressed in all EBV-associated malignancies including nasopharyngeal carcinoma, Burkitt lymphoma, and lymphoproliferative diseases (Bell et al., 2006; Ong et al., 2009; Qu et al., 2000; Rickinson and Kieff, 2007; Tao et al., 1998; Xue et al., 2002). Therefore, therapeutic agents that target LMP2A-modulated cell signaling have the potential to be used for both prevention and treatment of latent EBV infections and EBV-associated malignancies.
In this study, we investigated the therapeutic efficacy of the protein tyrosine kinase inhibitor dasatinib in a murine model of lymphoma expressing Myc and EBV-LMP2A. Our data demonstrate that treatment with dasatinib results in decreased proliferation of LMP2A-expressing B lymphocytes in vitro (Fig 1) and reverses tumor growth and splenomegaly in Tg6/λ-MYC double transgenic mice in vivo (Fig. 4). Dasatinib also inhibits splenomegaly and tumor development by Tg6/λ-MYC tumor cells in a syngeneic tumor transfer model (Fig. 5). This effect of dasatinib appears to be mediated through inhibition of LMP2A-induced Lyn signaling (Figs. 3 and 5C). Interestingly, dasatinib treatment also resulted in decreased splenomegaly in mice with λ-MYC tumor grafts, but this decrease was less pronounced compared to LMP2A/λ-MYC tumor grafts (Fig. 5). It is possible that some λ-MYC tumors have stochastically expressed high levels of active Lyn (Fig. 3A) due to the heterogeneity in tumor development in the λ-MYC mice as described previously (Bieging et al., 2009).
Dasatinib decreased proliferation of LMP2A-expressing but not wild type bone marrow B-lymphocytes in vitro (Fig 1). It is compelling that at higher concentration of dasatinib, even though not statistically significant, there is a tendency towards increased proliferation of wt bone marrow B cells which we consistently observed (Fig 1B). Even though we do not have a clear explanation for this, it may be due to a compensation mechanism, which may have been compromised by the presence of LMP2A. Although this seems like acquisition of an anchorage-independent growth, this is less likely given the fact that the lymphocytes are not dependent on adherence for growth at least in the cell culture.
It is likely that the effect we have observed is through combined inhibition of other dasatinib targets, such as c-Abl and c-Kit. Our data does not exclude the possibility that LMP2A may also modulate c-Abl, a ubiquitously expressed non-receptor protein tyrosine kinase that is also important in B cell signaling, and whose deregulation is associated with the development of leukemias and lymphomas (Engels et al., 2001; Ikeda and Longnecker, 2009; Shishido et al., 2001). Even though the LMP2A has not been reported to directly modulate the Abl activity, it is possible that the activity of Abl is modulated by LMP2A indirectly through consecutive actions of Lyn, Cbl, and Crk (Matskova et al., 2001; Rovedo and Longnecker, 2008). Further studies are required to clarify if LMP2A modulates the Abl signaling. The role of dasatinib to abrogate LMP2A-modulated phosphorylation of Lyn (and possibly of Abl) may provide useful information for designing therapeutic strategies for EBV-associated diseases such as PTLD and BL.
Dasatinib treatment targeted B cell populations of all genotypes, but the effects were pronounced in non-malignant B cells from TgE mice (Fig. 2B and 2C) as well as malignant tumor cells from Tg6/λ-MYC mice (Fig. 4C, and 4D). Even though these effects did not reach to a statistical significance, it is notable that the decrease in the B cell population corresponds to an increase in the T cell population. It is not surprising that dasatinib can produce a larger effect on B cells than on T cells because its target Lyn is absent or at very low level in T cells.
In conclusion, we show that dasatinib effectively and specifically inhibits proliferation of bone marrow B lymphocytes that express the latent EBV protein LMP2A. Our data also show that dasatinib impairs the growth of LMP2A-expressing B cell tumors, likely through inhibition of Src protein tyrosine kinase Lyn. Our data support the previous studies showing that LMP2A modulates Lyn and that Lyn is a viable therapeutic target in EBV-associated abnormalities (Kantarjian et al., 2006; Lindauer and Hochhaus, 2010). Dasatinib is currently clinically available and is being used to treat a number of pediatric and adult malignancies (Jabbour et al., 2011; Lee et al., 2011). Dasatinib is generally well-tolerated with fewer associated toxicities than other cytotoxic chemotherapeutic agents traditionally used to treat post-transplant lymphoproliferative disorders and Burkitt lymphoma. With confirmation of our findings in further preclinical studies, the use of dasatinib to treat EBV-associated diseases has the potential to result in greater treatment efficacy, improved survival, and decreased therapy-associated toxicities
Highlights.
Dasatinib specifically inhibits B-cell colony formation by bone marrow cells transgenic for LMP2A gene of Epstein-Barr virus.
Dasatinib specifically decreases the spleen size of LMP2A-transgenic mice
Dasatinib specifically inhibits Lyn kinase phosphorylation in LMP2A-expressing MYC-induced tumors
Dasatinib specifically inhibits splenomegaly and tumor development in double transgenic mice for EBV-LMP2A and MYC oncogene both in a pre-tumor and a tumor transfer model of Burkitt lymphoma.
Acknowledgments
Dasatinib was generously provided by Bristol-Myers Squibb. We would like to thank the members of the Longnecker laboratory for their help with these studies. R.L. is Dan and Bertha Spear Research Professor. We thank Alex A Vrazo for kindly reading the manuscript prior to submission.
Grant support
R.L. was supported by Public Health Service grants CA133063 and CA73507. K.F. was in part supported by the Viral Replication Training Program (T32-AI060523) and J.D. was supported in part by T32-CA079447 from the National Institutes of Health National Cancer Institute and by the Washington Square Health Foundation. This work was also supported by the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility.
Abbreviations
- BCR
B cell receptor
- Das
dasatinib, a small molecule inhibitor of Src and Abl kinases
- EBV
Epstein-Barr virus
- λ-MYC
transgenic mice expressing MYC under the regulatory DNA sequence of immunoglobulin λ gene
- LMP2A
EBV-encoded latent membrane protein 2A
- PTLD
post-transplant lymphoproliferative disorders
- Rag1 KO
recombinase activating gene-1 knockout mice
- Tg6, TgE
two independently derived transgenic mouse lines expressing LMP2A in B cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no potential conflicts of interest to disclose.
Contributor Information
Jamie L. Dargart, Email: jamie.dargart@promedica.org.
Kamonwan Fish, Email: kamonwanfish2015@u.northwestern.edu.
Leo I. Gordon, Email: l-gordon@northwestern.edu.
Richard Longnecker, Email: r-longnecker@northwestern.edu.
Osman Cen, Email: o-cen@northwestern.edu.
References
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Bell AI, Groves K, Kelly GL, Croom-Carter D, Hui E, Chan AT, Rickinson AB. Analysis of Epstein-Barr virus latent gene expression in endemic Burkitt's lymphoma and nasopharyngeal carcinoma tumour cells by using quantitative real-time PCR assays. J Gen Virol. 2006;87:2885–2890. doi: 10.1099/vir.0.81906-0. [DOI] [PubMed] [Google Scholar]
- Bieging KT, Amick AC, Longnecker R. Epstein-Barr virus LMP2A bypasses p53 inactivation in a MYC model of lymphomagenesis. Proc Natl Acad Sci U S A. 2009;106:17945–17950. doi: 10.1073/pnas.0907994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema R, Longnecker R, Swanson-Mungerson M. Epstein-Barr virus LMP2A accelerates MYC-induced lymphomagenesis. Oncogene. 2009;28:1471–1476. doi: 10.1038/onc.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Cen O, Longnecker R. Rapamycin reverses splenomegaly and inhibits tumor development in a transgenic model of Epstein-Barr virus-related Burkitt's lymphoma. Mol Cancer Ther. 2011;10:679–686. doi: 10.1158/1535-7163.MCT-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Cohen JI. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine. 1991;70:137–160. doi: 10.1097/00005792-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Cohen JI. Epstein-Barr virus infection. New England Journal of Medicine. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- Cooper L, Longnecker R. Inhibition of host kinase activity altered by the LMP2A signalosome-a therapeutic target for Epstein-Barr virus latency and associated disease. Antiviral Research. 2002;56:219–231. doi: 10.1016/s0166-3542(02)00110-9. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. BMS-354825: a novel drug with potential for the treatment of imatinib-resistant chronic myeloid leukaemia. Expert Opin Investig Drugs. 2005;14:89–91. doi: 10.1517/13543784.14.1.89. [DOI] [PubMed] [Google Scholar]
- Engels N, Merchant M, Pappu R, Chan AC, Longnecker R, Wienands J. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J Exp Med. 2001;194:255–264. doi: 10.1084/jem.194.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry H, Crockford TL, Silver K, Rust N, Goodnow CC, Cornall RJ. Analysis of Lyn/CD22 double-deficient B cells in vivo demonstrates Lyn- and CD22-independent pathways affecting BCR regulation and B cell survival. European Journal of Immunology. 2005;35:3655–3663. doi: 10.1002/eji.200535247. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Lee SK, Herrold R, Frech B, Laux G, Kremmer E, Grasser FA, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. Journal of Virology. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Cacciarelli TV, Mazariegos GV, Sigurdsson L, Qu L, Rowe DT, Reyes J. Serial measurement of Epstein-Barr viral load in peripheral blood in pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative disease. Transplantation. 1998;66:1641–1644. doi: 10.1097/00007890-199812270-00012. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, Heim D, Larson RA, Branford S, Muller MC, Agarwal P, Gollerkeri A, Talpaz M. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Longnecker R. Pre-B-cell colony formation assay. Methods in Molecular Biology. 2005;292:279–284. doi: 10.1385/1-59259-848-x:279. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Longnecker R. The c-Cbl proto-oncoprotein downregulates EBV LMP2A signaling. Virology. 2009;385:183–191. doi: 10.1016/j.virol.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour E, Cortes J, Kantarjian H. Long-term outcomes in the second-line treatment of chronic myeloid leukemia: a review of tyrosine kinase inhibitors. Cancer. 2011;117:897–906. doi: 10.1002/cncr.25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P. Dasatinib. Nat Rev Drug Discov. 2006;5:717–718. doi: 10.1038/nrd2135. [DOI] [PubMed] [Google Scholar]
- Kovalchuk AL, Qi CF, Torrey TA, Taddesse-Heath L, Feigenbaum L, Park SS, Gerbitz A, Klobeck G, Hoertnagel K, Polack A, Bornkamm GW, Janz S, Morse HC., 3rd Burkitt lymphoma in the mouse. J Exp Med. 2000;192:1183–1190. doi: 10.1084/jem.192.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 2011;117:1583–1594. doi: 10.1002/cncr.25690. [DOI] [PubMed] [Google Scholar]
- Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2010;184:83–102. doi: 10.1007/978-3-642-01222-8_7. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Matskova L, Ernberg I, Pawson T, Winberg G. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J Virol. 2001;75:10941–10949. doi: 10.1128/JVI.75.22.10941-10949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J, Fernandez C, Turner M. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by over-expression of Bcl-2. European Journal of Immunology. 2002;32:1029–1034. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–9124. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Burkhardt AL, Lee JH, Stealey B, Longnecker R, Bolen JB, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- Miller CL, Lee JH, Kieff E, Burkhardt AL, Bolen JB, Longnecker R. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect Agents Dis. 1994;3:128–136. [PubMed] [Google Scholar]
- Mirnics ZK, Caudell E, Gao Y, Kuwahara K, Sakaguchi N, Kurosaki T, Burnside J, Mirnics K, Corey SJ. Microarray analysis of Lyn-deficient B cells reveals germinal center-associated nuclear protein and other genes associated with the lymphoid germinal center. Journal of Immunology. 2004;172:4133–4141. doi: 10.4049/jimmunol.172.7.4133. [DOI] [PubMed] [Google Scholar]
- Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Ong KW, Teo M, Lee V, Ong D, Lee A, Tan CS, Vathsala A, Toh HC. Expression of EBV latent antigens, mammalian target of rapamycin, and tumor suppression genes in EBV-positive smooth muscle tumors: clinical and therapeutic implications. Clin Cancer Res. 2009;15:5350–5358. doi: 10.1158/1078-0432.CCR-08-2979. [DOI] [PubMed] [Google Scholar]
- Qu L, Green M, Webber S, Reyes J, Ellis D, Rowe D. Epstein-Barr virus gene expression in the peripheral blood of transplant recipients with persistent circulating virus loads. J Infect Dis. 2000;182:1013–1021. doi: 10.1086/315828. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr Virus. In: Knipe DM, PT H, DE G, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5 ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2655–2700. [Google Scholar]
- Rovedo M, Longnecker R. Epstein-Barr virus latent membrane protein 2A preferentially signals through the Src family kinase Lyn. J Virol. 2008;82:8520–8528. doi: 10.1128/JVI.00843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shishido T, Akagi T, Chalmers A, Maeda M, Terada T, Georgescu MM, Hanafusa H. Crk family adaptor proteins trans-activate c-Abl kinase. Genes to Cells. 2001;6:431–440. doi: 10.1046/j.1365-2443.2001.00431.x. [DOI] [PubMed] [Google Scholar]
- Tao Q, Robertson KD, Manns A, Hildesheim A, Ambinder RF. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. New England Journal of Medicine. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue SA, Labrecque LG, Lu QL, Ong SK, Lampert IA, Kazembe P, Molyneux E, Broadhead RL, Borgstein E, Griffin BE. Promiscuous expression of Epstein-Barr virus genes in Burkitt's lymphoma from the central African country Malawi. Int J Cancer. 2002;99:635–643. doi: 10.1002/ijc.10372. [DOI] [PubMed] [Google Scholar]