Abstract
P2X7 receptor (P2X7R) is a purinergic ion-channel receptor. We have previously shown that the activation of P2X7R in alveolar type I cells stimulates surfactant secretion in alveolar type II cells. In this study, we determined whether miR-150 regulates P2X7R-mediated surfactant secretion. The miR-150 expression level in alveolar type II cells was much higher than alveolar type I cells, which was inversely correlated with the P2X7R protein level. An adenovirus expressing miR-150 significantly reduced the P2X7R protein expression in E10 cells, an alveolar type I cell line. Furthermore, pre-treatment of E10 cells with the adenovirus reduced the surfactant secretion induced by E10 cell conditioned medium. Our study demonstrates that miR-150 regulates surfactant secretion through P2X7R.
Keywords: P2X7R, miR-150, surfactant secretion
Introduction
The distal lung epithelium is lined with alveolar epithelial type I and type II cells (AEC I and AEC II). AEC I cover about 95% and AEC II occupy less than 5% of the alveolar surface area [14]. AEC I are large squamous cells responsible for gas exchange, fluid transport and protection against oxidative stress [4;5;11;17]. AEC II are cuboidal and normally located in the corners of alveoli. AEC II also synthesize and secrete pulmonary surfactant, a lipoprotein complex that spreads on the surface of alveoli to form a thin lining layer and reduces surface tension. Insufficient amount of pulmonary surfactant leads to abnormal lung functions [10;22].
The molecular mechanism that regulates surfactant secretion is of great interest. The secretion of surfactant from AEC II can be regulated by many molecules, such as intracellular Ca2+, P2Y2 receptor agonists (ATP and UTP), adenosine, platelet activating factor, and IL-1 etc [1]. Of all these molecules, extracellular ATP is the most effective endogenous regulator. Through binding to P2Y2 receptors on the AEC II plasma membrane, ATP activates phospholipase C, which generates diacylglycerol (DAG) and inositol trisphosphate (IP3), and subsequently activates protein kinase C (PKC) and increases cytoplasmic Ca2+ concentration [1].
P2X7 receptor (P2X7R) belongs to the P2X receptor family which is a ligand-gated ion channel activated by ATP [12]. P2X7R shares many properties with other P2X receptors. The binding of ATP causes P2X receptors to open within millisecond and stimulates cell depolarization by an influx of cationic ions, including Na+, K+ and Ca2+ [8]. P2X7R was initially identified as a unique ATP-gated channel, P2Z due to its low degree of structural homology compared to other family members. P2X7R has a less conserved and unusually long COOH terminus, which forms a large non-selective pore permeable to small molecules such as YO-PRO, Lucifer yellow and ethidium bromide [20]. In comparison with other P2X receptors, the activation of P2X7R needs at least 10-fold higher ATP concentration due to its low affinity for ATP, but the desensitization of P2X7R is much slower. Another unique property of P2X7R is that it mediates a reversible plasma membrane permeabilization. P2X7R activation also enhances the release of endogenous cytokines involved in the immune-response, such as interleukin-1beta (IL-1β) [16]. However, sustained stimulation of P2X7R leads to membrane blebbing and programmed cell death [9;19].
P2X7R is broadly expressed in multiple mammalian cells, including epithelial cells, endothelial cells, fibroblasts, and macrophages. P2X7R is specifically expressed in AEC I [6]. Our previous studies have shown that the activation of P2X7R in AEC I stimulates surfactant secretion in AEC II [15].
MicroRNAs (miRNAs) are naturally existed small non-coding RNAs that either cleave specific mRNAs or inhibit mRNA translation through complementary base pairing and subsequently silence target genes [13]. MiR-150 is a miRNA highly expressed in the tumor cells surrounding the proliferation centers in the bone marrow and lymphoid tissues [23;24]. MiR-150 is also present in mature B and T cells. It blocks B cell development by silencing the expression of c-Myb [23]. miR-150 has been reported to target P2X7R [25].
In the current study, we demonstrated that miR-150 inhibited endogenous P2X7R protein expression in lung cells. Then we characterized miR-150 expression in different organs and lung epithelial cells. Finally, we examined the effect of miR-150 in P2X7R-mediated surfactant secretion in primary cultured AEC II.
Materials and Methods
miRNA viral vector construction
The pENTR/CMV-EGFP-pri-miRNA vector containing the primary transcript of human miRNA was constructed and the miRNA adenoviruses were produced as described previously [3].
Western Blot
The proteins were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane. Dilutions for rabbit anti-P2X7R antibody (Sigma) and mouse anti-β-actin antibody (Bio-Rad) were 1:1,000. Dilution for horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was 1:2,000. The blot was developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Real-time PCR
Total RNAs were isolated using the mirVanaTM miRNA Isolation Kit (Ambion). RNase-freeTM DNase was used to remove genomic DNA contamination. The RNAs were reverse-transcribed into cDNA using oligo dT and random hexamers and M-MLV reverse transcriptase (Invitrogen). The primers used are: P2X7R: forward, 5’-ACCGTGCCAGTGTTGTTCTG-3’, and reverse, 5’-AGCACCTGTGGTTGTTATTTTGT-3’; 18S rRNA: forward, 5’-ATTGCTCAATCTCGGGTGGCTG-3’ and reverse, 5’-CGTTCTTAGTTGGTGGAGCGATTTG-3’.
Real-time PCR was performed as described [21]. 18S rRNA was used as an internal control. Real-time PCR for miR-150 was carried out as described [18]. 5 μg of total RNA was polyadenylated using A-Plus Poly (A) Polymerase tailing kit (Epicentre). The tailed RNA was purified using 1:1 phenol/Chloroform and precipitated with 75% ethonal. One μg poly(A) tailed RNA was reverse-transcribed into cDNA using oligo dT primers by M-MLV reverse transcriptase. The target mature miRNA sequence (5’-CTGGTACAGGCCTGGGGGACAG-3’) was used as the forward PCR primer and a universal primer (5’-GCGAGCACAGAATTAATACGACTCAC-3’) was used as the reverse primer. Real-time PCR was performed as described above. U2 small RNA was used to normalize the data.
Surfactant Secretion
AEC II were isolated from Male Sprague-Dawley rats (180–200g) as previously described [2]. E10 cells were cultured in DMEM supplied with 10% FBS and transduced with 100 Multiplicity of Infection (MOI= ratio of infectious virus particles to cells) adenoviruses for 4 days. The cells were then treated with 25 μM BzATP for 2 hours. The media were collected as conditional media. AEC II (1 X 106) were cultured overnight with [3H] choline (0.6 μCi/106 cells). AEC II were incubated with or without the conditional media for 2 hours. Lipids in media and cells were extracted and surfactant secretion was performed [7]. Surfactant secretion (%) was expressed as (dpm in medium/dpm in medium and cells) x100. A stimulation index was expressed as a ratio of stimulated secretion with a conditioned media to basal secretion without conditioned media.
Results and Discussion
miR-150 reduces P2X7R protein level
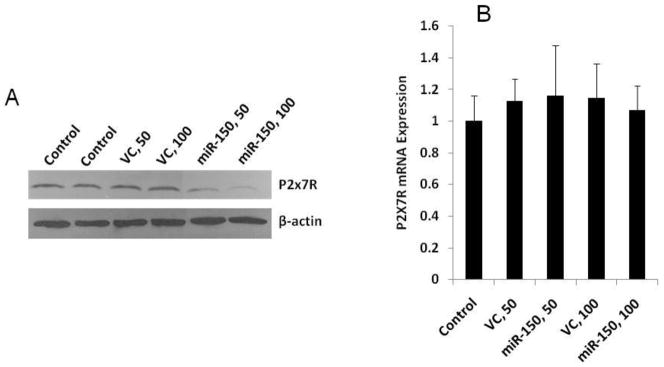
To determine whether miR-150 down-regulates endogenous P2X7R protein expression, we generated an adenovirus to overexpress miR-150. The virus was used to transduce E10 cells for 4 days at a MOI of 50 or 100. As shown in Fig. 1A, the P2X7R protein expression was not affected in the control adenovirus-treated cells. However, a significant decrease was observed in miR-150 expressing adenovirus-transduced cells. This decrease was virus dose-dependent. We also examined the P2X7R mRNA expression using real-time PCR. The control and miR-150 viruses had no effects on the P2X7R mRNA expression (Fig. 1B).
Fig. 1. miR-150 silences the P2X7R protein expression.
E10 cells were treated with different doses of miR-150 overexpressing viruses (MOI, 50 and 100). A virus overexpressing GFP was used as a control. Four days after treatment, protein (A) and mRNA (B) levels were examined using Western blot and Real-time PCR. β-actin were used as an internal control for western blot. 18S rRNA was used as an internal control for real-time PCR. For panel B, data are means ± S.E. (n=3 cell preparations, assayed in duplicate).
Characterization of miR-150 expression
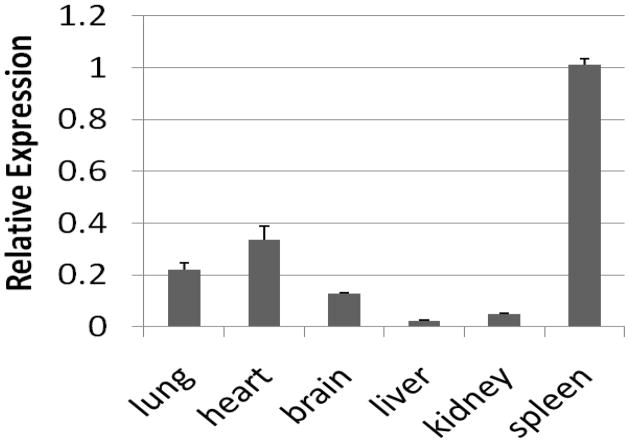
MiR-150 is highly expressed in lymph nodes, spleen, heart, and brain. However, its abundance in the lung is controversial [23;24]. We first examined the expression of miR-150 in various organs by real-time PCR. As previously reported, miR-150 was highly expressed in spleen (Fig. 2). Its expression in the lung was comparable to those in the heart and brain.
Fig. 2. Expression level of miR-150 in organs.
Total RNAs were extracted from various organs, poly (A)-tailed and reversed-transcribed into cDNA. Real-time PCR was used to examine miR-150 levels. The results were normalized to U2. The data were expressed as means ± S.E. (n=3 biological replications, assayed in duplicate).
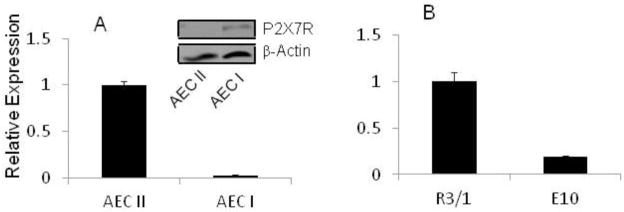
MiR-150 expression level in type II cells was much higher than type I-like cells, which was inversely correlated with the P2X7R protein level (Fig. 3A). We have previously shown that R3/1 cell, a type I cell line , contains little P2X7R while E10 cell, another type I cell line, has a high level of P2X7R [15]. MiR-150 was highly expressed in R3/1 cells and lowly in E10 cells (Fig. 3B).
Fig. 3. miR-150 and P2X7R expression in lung cells.
Total RNA and/or proteins were extracted from AEC II (freshly isolated type II cells) and AEC I-like cells (trans-differentiated type I cells by culturing AEC II on plastic dishes for 5 days) (A), and E10 and R3/1 cells (B). miR-150 level was determined by real-time PCR. U2 was used as an internal reference. The results were expressed as means ± S.E. (n=4–6 cell preparations, all assayed in duplicate). P2X7R protein level was measured by Western blot and β-actin was used a loading control.
MiR-150 reduced P2X7R-mediated surfactant secretion
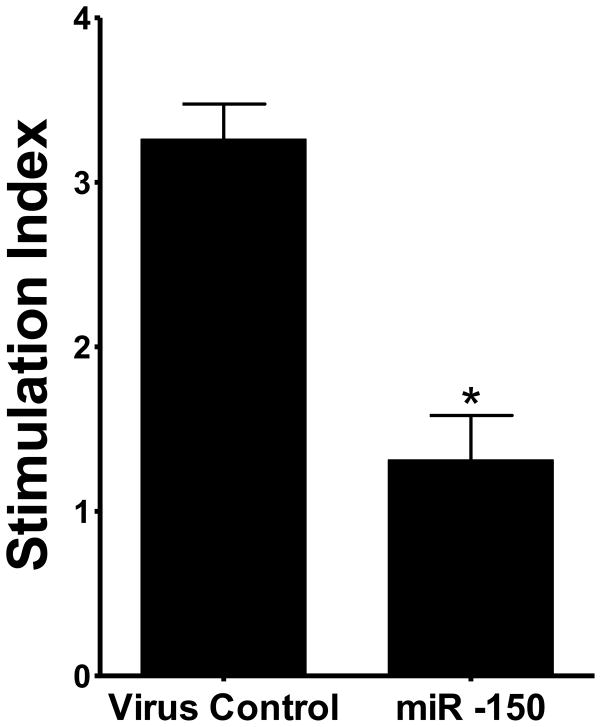
Previously, we have found that BzATP activates P2X7R in AEC I, resulting in an increase in surfactant secretion in AEC II. We therefore examined whether miR-150 affects surfactant secretion. We pre-treated E10 cells with miR-150 overexpression viruses for 4 days and stimulated E10 cells with BzATP for 2 hours. Then the conditioned media were used to stimulate surfactant secretion in AEC II. As shown in Fig. 4, miR-150 significantly decreased the surfactant secretion in AEC II.
Fig. 4. miR-150 inhibits P2X7R-evoked surfactant secretion.
(A) E10 cells were treated with 100 MOI of adenovirus expressing miR-150 for 4 days. Adenovirus only express EGFP was used as a control. E10 cells were stimulated for 2 hours with BzATP (25 μM). The E10 cell conditioned media were used to stimulate AEC II for 2 hours. PC secretion was measured. Data are means ± S.E. (n=3, *P< 0.001 vs. control, student’s test).
In summary, we found that miR-150 had much lower expression in P2X7R-enriched cells, such as type I-like cells and E10 cells. MiR-150 expressing adenovirus dramatically decreased the endogenous P2X7R expression in E10 cells. The conditioned media from miR-150 expressing virus-treated E10 cells had a reduced ability to stimulate surfactant secretion in AEC II. Our data suggests a role of miRNA-150 in surfactant secretion.
Highlights.
miR-150 inhibits surfactant secretion
miR-150 depresses P2X7 receptors
miR-150 is highly expressed in alveolar type I cells
Acknowledgments
We thank Dr. Mary Williams (Boston U) for kindly providing the E10 cells with permission from Dr. Al Malkinson (University of Colorado) and Dr. Roland Koslowski (Dresden U of Technology) for R3/1 cells.
Funding
This work was supported by the National Institutes of Health [HL-052146, HL-087884, HL-095383 (LL)]. TW, YW and YG were supported by American Heart Association predoctoral fellowships [0610143Z], [0810016Z] and [#09PRE2300211].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259–L271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 2.Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the Smad pathway. J Biol Chem. 2007;282:3968–3976. doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaran M, Wang Y, Zhang H, Weng T, Baviskar P, Guo Y, Gou D, Liu L. MicroRNA-127 modulates fetal lung development. Physiol Genomics. 2009;37:268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L599–L608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Chen Z, Chintagari NR, Bhaskaran M, Jin N, Narasaraju T, Liu L. Alveolar type I cells protect rat lung epithelium from oxidative injury. J Physiol. 2006;572:625–638. doi: 10.1113/jphysiol.2005.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Jin N, Narasaraju T, Chen J, McFarland LR, Scott M, Liu L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem Biophys Res Commun. 2004;319:774–780. doi: 10.1016/j.bbrc.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Chintagari NR, Jin N, Wang P, Narasaraju TA, Chen J, Liu L. Effect of cholesterol depletion on exocytosis of alveolar type II cells. Am J Respir Cell Mol Biol. 2006;34:677–687. doi: 10.1165/rcmb.2005-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di VF, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Marcos M, Pochet S, Marino A, Dehaye JP. P2X7 and phospholipid signalling: the search of the "missing link" in epithelial cells. Cell Signal. 2006;18:2098–2104. doi: 10.1016/j.cellsig.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Hutchison AA. Respiratory disorders of the neonate. Curr Opin Pediatr. 1994;6:142–153. doi: 10.1097/00008480-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci U S A. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan SE, Synder JM. Development of Alveoli. In: Harding R, Pinkerton KE, Plopper CG, editors. The lung: development, aging and the environment. Academic Press; 2004. pp. 55–73. [Google Scholar]
- 15.Mishra A, Chintagari NR, Guo Y, Weng T, Su L, Liu L. Purinergic P2X7 receptor regulates lung surfactant secretion in a paracrine manner. J Cell Sci. 2011;124:657–668. doi: 10.1242/jcs.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 17.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 18.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 19.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 20.Tsukimoto M, Harada H, Ikari A, Takagi K. Involvement of chloride in apoptotic cell death induced by activation of ATP-sensitive P2X7 purinoceptor. J Biol Chem. 2005;280:2653–2658. doi: 10.1074/jbc.M411072200. [DOI] [PubMed] [Google Scholar]
- 21.Weng T, Jin N, Liu L. Differentiation between amplicon polymerization and nonspecific products in SYBR green I real-time polymerase chain reaction. Anal Biochem. 2005;342:167–169. doi: 10.1016/j.ab.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136:426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 23.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Qi X, Potashkin JA, Abdul-Karim FW, Gorodeski GI. MicroRNAs miR-186 and miR-150 down-regulate expression of the pro-apoptotic purinergic P2X7 receptor by activation of instability sites at the 3'-untranslated region of the gene that decrease steady- state levels of the transcript. J Biol Chem. 2008;283:28274–28286. doi: 10.1074/jbc.M802663200. [DOI] [PMC free article] [PubMed] [Google Scholar]