Abstract
Purpose
To investigate whether high-dose thoracic radiation given twice daily during cisplatin–etoposide chemotherapy for limited small cell lung cancer (LSCLC) improves survival, acute esophagitis, and local control rates relative to findings from Intergroup trial 0096 (47%, 27%, and 64%)..
Patients and Methods
Patients were accrued over a 3-year period from 22 U.S. and Canadian institutions. Patients with LSCLC and good performance status were given thoracic radiation to 61.2 Gy over 5 weeks (daily 1.8-Gy fractions on days 1-22, then twice-daily 1.8-Gy fractions on days 23-33). Cisplatin (60 mg/m2 IV) was given on day 1 and etoposide (120 mg/m2 IV) on days 1-3 and days 22-24, followed by 2 cycles of cisplatin+etoposide alone. Patients who achieved complete response were offered prophylactic cranial irradiation. Endpoints included overall and progression-free survival; severe esophagitis (CTC v 2.0) and treatment-related fatalities; response (RECIST); and local control.
Results
Seventy-two patients were accrued from June 2003 through May 2006; 71 were evaluable (median age 63; 52% female; 58% Zubrod 0). Median survival time was 19 months; at 2 years, overall survival rate was 36.6% (95% CI 25.6%-47.7%), and progression-free survival 19.7% (95% CI 11.4%-29.6%). Thirteen patients (18%) experienced severe acute esophagitis and 2 (3%) died of treatment-related causes; 41% achieved complete response, 39% partial response, 10% stable disease, and 6% progressive disease. The local control rate was 73%. Forty-three patients (61%) received prophylactic cranial irradiation.
Conclusions
The overall survival rate did not reach the projected goal; however, rates of esophagitis were lower, and local control higher, than projected. This treatment strategy is now one of three arms of a prospective trial of chemoradiation for LSCLC (RTOG 0538/CALGB 30610).
Keywords: concomitant boost thoracic radiation; limited stage small cell lung cancer; cisplatin, etoposide
INTRODUCTION
Of the estimated 222,520 cases of lung cancer diagnosed in the United States in 2010, approximately 14% are of small-cell histology.1 Small-cell lung cancer (SCLC) differs from non-small-cell carcinoma by its early dissemination, relatively greater initial chemosensitivity, and radiosensitivity.2 About 25% of patients present with limited-stage disease, i.e., disease that is clinically confined to one side of the chest and can be encompassed by a “safe” radiation portal.
The mainstay of treatment for limited SCLC is combined chemotherapy and radiotherapy, with 5-year survival rates of 20% to 25% in clinical trials.3 Cisplatin plus etoposide is the preferred regimen because of its manageable toxicity when combined with radiotherapy.3 Two meta-analyses4,5 have established the combination of chemotherapy and thoracic radiotherapy as the standard of care for limited SCLC.
The best method of integrating thoracic radiation with chemotherapy remains controversial. The optimal timing of concurrent radiation during chemotherapy is generally agreed to be during the first or second cycle.6-8 Twice-daily radiation schedules have been investigated with the goal of better controlling this rapidly proliferating tumor. Turrisi et al.9 found that twice-daily dosing to 45 Gy over 3 weeks produced superior survival over once-daily dosing to 45 Gy over 5 weeks. The Cancer and Leukemia Group B evaluated several once-daily and twice-daily regimens and found 45 Gy in 30 (twice-daily) fractions over 3 weeks and 70 Gy in 35 (daily) fractions over 7 weeks to be feasible when given with platinum and etoposide after 3 cycles of induction therapy.10 Another phase I trial, RTOG 97-12, evaluated dose-escalated thoracic radiation given concurrently with cisplatin and etoposide.11 In that study, radiation was given as 1.8-Gy fractions daily to the clinical target volume (CTV) for the first two cycles followed by twice daily to the gross tumor volume (GTV) for 3, 5, 7, 9, or 11 days (total dose 50.4-64.8 Gy). The maximum tolerated dose was 61.2 Gy; esophagitis was the only dose-limiting toxicity. The severe (grade ≥3) esophagitis rate in that study was 21% (18% for those receiving ≤61.2 Gy). The current phase II study by the Radiation Therapy Oncology Group (RTOG) was undertaken to investigate the efficacy and feasibility of delivering 61.2 Gy in an accelerated schedule involving first once-daily and then twice-daily dosing during cisplatin–etoposide chemotherapy.
PATIENTS AND METHODS
Patients in this prospective, multi-institutional, single-arm trial had pathologically confirmed, limited-stage SCLC (stage I-IIIB, i.e., confined to one hemithorax, but excluding T4 tumors based on malignant pleural effusion or N3 disease based on contralateral hilar or contralateral supraclavicular involvement). Other eligibility stipulations were age ≥18 years, good performance status (Zubrod score 0-1), and adequate hematologic, hepatic, and renal function (absolute granulocyte count ≥1500/μL; platelet count ≥150,000/μL; bilirubin level ≤1.5 mg/dL; and serum creatinine level ≤1.5 mg/dL). Exclusion criteria included serious intercurrent illness (e.g., symptomatic heart disease, myocardial infarction within 6 months before study entry, chronic obstructive pulmonary disease with a forced expiratory volume in 1 second (FEV1) ≤0.8 L, or uncontrolled bronchospasm). All participating institutions were required to obtain approval from their respective institutional review boards, and all patients were required to sign a study-specific consent form, approved by the RTOG, before study entry.
Radiotherapy
Radiotherapy was delivered by photon beams (6-18 MV) to 61.2 Gy over 5 weeks as follows. For the first 16 fractions, radiation was given in large fields at 1.8 Gy/fraction, 5 days/week for a total dose of 28.8 Gy. Beginning on day 23 (second day of week 4), radiation was given twice daily, once in the morning (1.8 Gy) followed by a smaller off-cord boost field (1.8 Gy) at least 6 hours later that same day, on days 23-26. On the final 5 days of the 5-week cycle (days 29-33), patients were given twice-daily off-cord boosts (1.8 Gy/fraction). Boost fields were intended to reduce the amount of esophagus within the treatment field and were designed on the basis of repeat CT scans obtained after delivery of the first 12 radiation fractions.
The radiation fields were defined as follows. Large fields were intended to cover both the primary tumor and regional involved lymph nodes; the smaller boost fields were defined in terms of the smaller GTV and CTV after repeat CT scanning. The GTV encompassed known disease determined by physical examination and computed tomography (CT). The CTV consisted of the GTV plus a 1.0-cm margin for the large fields and the GTV plus a 0.5-cm margin for the boost fields. Ipsilateral supraclavicular irradiation was allowed when necessary for primary tumor coverage, but contralateral hilar or supraclavicular treatment was not allowed. For tumors located in the upper or middle lobes, the lower border of the large fields was extended to 3.0 cm below the carina to cover potentially involved nodes; for the boost fields, that border was reduced to 1.0 cm. If the subcarinal nodes were grossly involved, the margin used was 1.0 cm beyond the gross involvement (GTV) for both the large and boost fields. Any mediastinal node that appeared larger than 1.5 cm on CT was included with at least a 1-cm margin. The planning target volume (PTV) was defined as the CTV plus a 0.5- to 1.5-cm margin to account for variations in treatment delivery, including variations in setup between treatments, patient motion during treatment, and movement or change of size of tissues containing the CTV.
Chemotherapy
Chemotherapy consisted of cisplatin (60 mg/m2 IV, given in 500 mL normal saline plus 12.5 g mannitol over 2 hours) plus etoposide (120 mg/m2 IV over 1 hour) on day 1, followed by etoposide (240 mg/m2 PO/d or 120 mg/m2 IV/d) on days 1, 2, and 3. This 3-day cycle was repeated on days 22-24 during the radiotherapy, followed by 2 cycles of etoposide–cisplatin alone, beginning on days 43 and 64. Etoposide was given IV if patients could not take it orally. Hydration was given before and after chemotherapy, and antiemetics were given before chemotherapy at the treating physician’s discretion.
Hematopoietic support (granulocyte colony-stimulating factor [G-CSF]) was given subcutaneously or IV at 5 μg/kg/d to prevent new episodes of febrile neutropenia during chemotherapy cycles 3-4 for patients who had experienced that complication. G-CSF was discontinued when the absolute granulocyte count recovered to >1000/μL; subsequent chemotherapy could not be restarted sooner than 48 hours after discontinuation of G-CSF.
Baseline and Follow-up Evaluations
Baseline tests included a history and physical examination; assessment of performance status; measurement of body weight and tumor dimensions (on CT, magnetic resonance imaging [MRI], or x-ray); complete blood count; electrolytes and magnesium levels; chest/upper abdomen x-ray or CT with contrast; urinalysis; bronchoscopy; pulmonary function tests; electrocardiography; brain imaging (MRI or CT); and bone scanning.
Toxicity was assessed and complete blood counts obtained weekly during the 5-week radiotherapy period. Performance status, body weight, blood counts, blood chemistry, and urinalysis were assessed and chest x-rays obtained before each course of chemotherapy.
Follow-up evaluations were done at the end of the 5-week treatment period and every 3 months from the beginning of treatment for the first year, every 6 months for the next 2 years, and annually thereafter. These evaluations included physical examination, performance status and body weight assessment, tumor measurements, lab tests, pulmonary function tests (at 6 and 12 months), chest x-ray, thoracic CT (every 6 months), and electrocardiography; bone scans and either brain MRI or CT were obtained as clinically indicated.
Response Assessments
Response was evaluated at 5 weeks and after the completion of 4 cycles of chemotherapy according to the criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee.12 The largest diameter (unidimensional measurement) of the tumor lesions was used. The sum of the longest diameter (LD) for all target lesions was used as a reference for objective tumor response. A complete response (CR) was defined as disappearance of all target lesions as measured by MRI or CT; partial response (PR) as ≥30% decrease in the sum of the LD of target lesions; progressive disease (PD) as ≥20% increase in the sum of the LD of target lesions (the reference being the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions); and stable disease (SD) as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Patients achieving complete response were offered prophylactic cranial irradiation (PCI).
Toxicity
Chemotherapy toxicities and acute radiotherapy toxicities were graded according to the Common Toxicity Criteria v2.0. Late radiotherapy toxicities were graded according to the RTOG/European Organisation for Research and Treatment of Cancer [EORTC] Late Radiation Morbidity Scoring system.
Grade 2 esophagitis is defined in both the acute and the late toxicity criteria as dysphagia requiring predominantly pureed, soft, or liquid diet; grade 3 esophagitis as dysphagia requiring intravenous hydration (acute) or dilatation (late); and grade 4 esophagitis as complete obstruction (cannot swallow saliva) requiring enteral or parenteral nutritional support; perforation; or fistula.
Endpoints
The primary endpoint was overall survival (OS) at 2 years. Secondary endpoints included median survival time, progression-free survival (PFS) rate; rates of severe (grade 3-4) acute treatment-related esophagitis; rate of treatment-related fatalities; and response rates (CR, PR, PD, or SD).
Statistical Analyses
This study was designed to detect an improvement in the 2-year OS rate from 47% (achieved in the twice-daily arm of INT 00969) to 60%. Using a one-group χ2 test with a one-sided significance level of 0.10, a sample of 67 patients was deemed sufficient to detect the difference between the null hypothesis (Ho: p ≤0.47) and the alternative hypothesis (Ha: p ≥0.60) with 80% power. Assuming that 5% of the enrolled patients would be ineligible or not evaluable, 72 patients were required. The two hypotheses were tested with a Fleming single-stage phase II procedure.13
Because RTOG 97-12 did not rule out a severe esophagitis rate >30% or a treatment-related fatality rate >5%, three interim toxicity analyses evaluating severe esophagitis and treatment-related fatalities were planned after accrual of 25%, 50%, and 75% of the total number of evaluable patients. The following early stopping rules would reject the null hypothesis that the proportion of severe esophagitis was ≤30% with an overall significance level of 0.0513: 12 or more cases of severe esophagitis among the first 17 evaluable patients, 17 or more cases of severe esophagitis among the first 34 evaluable patients, or 22 or more cases of severe esophagitis among the first 51 evaluable patients. The final analysis would test the same hypothesis using a rejection rule of 27 or more cases of severe esophagitis among the total sample of 67 evaluable patients, to ensure an overall significance level of 0.05 for the final conclusion. Similarly, the following early stopping rules would reject the null hypothesis that the proportion of treatment-related fatalities was ≤5% with an overall significance level of 0.20: 3 or more fatalities among the first 17 evaluable patients, 4 or more fatalities among the first 34 evaluable patients, or 5 or more fatalities among the first 51 evaluable patients. The final analysis tests the same null hypothesis using the rejection rule of 6 or more treatment-related fatalities among the total sample of 67 evaluable patients, ensuring an overall significance level of 0.20 for the final conclusion.
An event in assessing OS was defined as death from any cause, and corresponding survival times were measured from the date of study registration until the date of death or last follow-up. An event for PFS included any of the following: local failure, regional failure, development of distant metastasis, development of a second primary, or death from any cause; PFS was measured from the date of study registration until the date of the first event or last follow-up if no event occurred. OS and PFS rates were estimated with the Kaplan-Meier method.14 Severe esophagitis was defined as the occurrence of grade 3 or 4 esophagitis at any time, and treatment-related fatalities were any grade 5 toxicity attributable to protocol treatment.
RESULTS
RTOG 0239 opened to accrual June 20, 2003, and closed May 23, 2006 after accruing 72 patients from 22 institutions in the United States and Canada. This analysis was based on all data received at RTOG Headquarters through January 10, 2011. The median follow-up time was 19.0 months (range 0.4-71.4) for all patients and 54.6 (range 45.4-71.4) months for patients alive at the time of analysis. One patient was excluded from the analysis because no radiotherapy plans could be made that met the protocol criteria, leaving 71 evaluable patients. Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics (n=71)
| Age, years | |
| Median | 63 |
| Range | 43–84 |
| n | % | |
|---|---|---|
| Sex | ||
| Male | 34 | 48 |
| Female | 37 | 52 |
| Zubrod score | ||
| 0 | 41 | 58 |
| 1 | 30 | 42 |
| Disease stage | ||
| IA | 2 | 3 |
| IIA | 4 | 6 |
| IIB | 5 | 7 |
| IIIA | 40 | 56 |
| IIIB | 20 | 28 |
| Race | ||
| White | 69 | 97 |
| Black | 2 | 3 |
| Ethnicity | ||
| Hispanic | 1 | 1 |
| Non-Hispanic | 65 | 92 |
| Unknown | 5 | 7 |
Survival
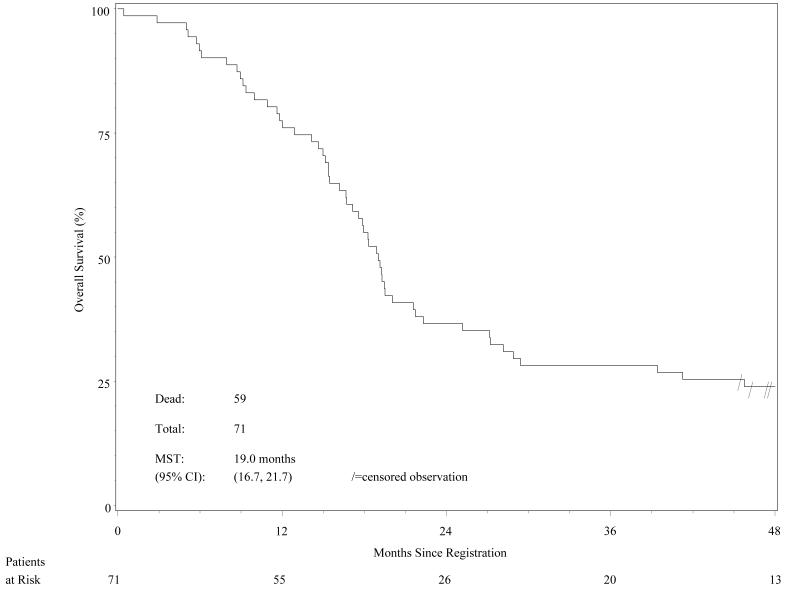
The 2-year OS rate was 36.6% (95% CI 25.6%-47.7%). Because the point estimate was 0.366 (i.e., less than the pre-established upper bound of 0.54815), statistically this new treatment did not improve the 2-year OS rate over that found in the INT 0096 trial (47%), nor did it meet the projected goal of 60%. The Kaplan-Meier OS curve is shown in Figure 1.
Figure 1.
Overall survival (n=71 patients).
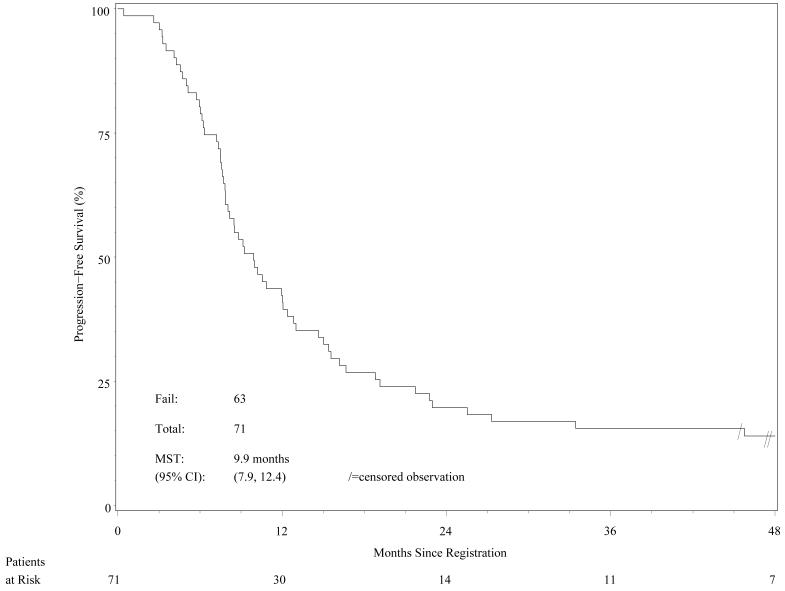
Regarding the secondary endpoints, the median survival time for all patients was 19.0 months (95% CI 16.7-21.7 months; range 0.4–71.4 months) The median PFS time was 9.9 months (95% CI 7.9–12.4 months); and the estimated 2-year PFS rate was 19.7% (95% CI 11.4%-29.6%). The Kaplan-Meier PFS curve is shown in Figure 2.
Figure 2.
Progression-free survival (n=71 patients).
Toxicity
No stopping rules were crossed during any of the three planned interim toxicity analyses. Acute toxicities related to chemotherapy and radiotherapy, and late toxicities related to radiation, are shown in Table 2. Thirteen (18%) of the patients experienced acute grade 3/4 esophagitis; this was lower than the stated endpoint of 30%. Two patients experienced late grade 4 effects, one cardiac (myocardial infarction) and one pulmonary (reduction of diffusion capacity). Two patients (3%) died of treatment-related causes, one of infection/febrile neutropenia (69 days after the end of treatment) and one of pneumonia (33 days after the end of treatment).
Table 2.
Acute and Late Treatment-Related Toxicity
| Grade | |||||
|---|---|---|---|---|---|
| Category | 1 | 2 | 3 | 4 | 5 |
| Acute Chemotherapy- and Radiation-Related Toxicity* (n=71) | |||||
| Allergy/immunology | 2 | 1 | 1 | 0 | 0 |
| Auditory/hearing | 3 | 1 | 1 | 0 | 0 |
| Blood/bone marrow | 4 | 2 | 15 | 50 | 0 |
| Cardiovascular (arrhythmia) | 2 | 0 | 1 | 0 | 0 |
| Cardiovascular (general) | 5 | 9 | 4 | 3 | 0 |
| Coagulation | 1 | 0 | 2 | 0 | 0 |
| Constitutional symptoms | 19 | 31 | 9 | 1 | 0 |
| Dermatology/skin | 16 | 22 | 1 | 0 | 0 |
| Endocrine | 0 | 1 | 1 | 0 | 0 |
| Gastrointestinal | 9 | 34 | 21 | 3 | 0 |
| Esophagitis/dysphagia | 15 | 29 | 12 | 1 | 0 |
| Hemorrhage | 5 | 2 | 0 | 0 | 0 |
| Hepatic | 15 | 6 | 0 | 1 | 0 |
| Metabolic/laboratory | 23 | 4 | 12 | 5 | 0 |
| Musculoskeletal | 3 | 3 | 0 | 0 | 0 |
| Neurology | 13 | 17 | 6 | 0 | 0 |
| Ocular/visual | 2 | 1 | 2 | 0 | 0 |
| Pain | 10 | 16 | 9 | 0 | 0 |
| Pulmonary | 14 | 16 | 7 | 1 | 1 |
| Renal/genitourinary | 7 | 11 | 3 | 1 | 0 |
| Worst non-hematologic | 2 (3%) |
15 (21%) |
40 (56%) |
12 (17%) |
2 (3%) |
| Worst overall | 1 (1%) |
3 (4%) |
14 (20%) |
51 (72%) |
2 (3%) |
| Late Radiation-Related Toxicity (n=69)† | |||||
| Bone | 0 | 1 | 0 | 0 | 0 |
| Esophagus | 6 | 5 | 1 | 0 | 0 |
| Heart | 1 | 0 | 0 | 1 | 0 |
| Lung | 16 | 14 | 7 | 1 | 0 |
| Skin (within the irradiated field) |
4 | 2 | 0 | 0 | 0 |
| Subcutaneous tissue | 0 | 1 | 0 | 0 | 0 |
| Other | 5 | 10 | 1 | 0 | 0 |
| Worst Overall | 14 (20%) |
21 (30%) |
9 (13%) |
2 (3%) |
0 |
graded according to the Common Toxicity Criteria v 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf)
graded according to RTOG/EORTC criteria for late effects (http://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityS coringSchema.aspx); two patients had died before the late toxicity period began (i.e., within 90 days of treatment)
No grade 5 late toxicities were experienced.
Response Rates
Response rates at 2 months after treatment completion, scored according to RECIST, were as follows: 41% of patients (n=29) had CR and another 39% (n=28) had PR, for an overall response rate of 80%; another 10% of patients (n=7) had SD, 6% (n=4) had PD, and 4% (n=3) died before the 2-month post-treatment response could be evaluated.
Disease Failure Patterns
At the time of analysis, 8 patients (11%) were alive with no evidence of failure and 11 (15.5%) had died without evidence of failure. The site of first treatment failure was locoregional only in 14 patients (20%), distant only in 31 (44%), and both locoregional and distant in 5 (7%). Among the 36 patients who developed distant metastases, the most common sites were liver (27%) and bone (27%), followed by brain (24%), lung/pleura (14%), and adrenals (8%). Causes of death were disease-related in 46 patients (78%), protocol-related in 2 (4%), other in 7 (12%), and unknown in 4 (7%). Of the 43 patients who received PCI, 10 experienced brain metastases; this was no different than the proportion of patients with brain metastases who did not receive PCI 10 of 26).
Compliance with Radiotherapy and Chemotherapy Protocols
Fifty-eight patients (82%) were treated in compliance with the radiotherapy protocol. Nine patients (13%) had acceptable minor variations; only one patient had an unacceptable deviation. Three patients had incomplete radiotherapy: one patient died of sepsis and two others refused the twice-daily radiotherapy during the last 2 weeks of radiotherapy. Fifty-six patients (79%) received chemotherapy according to the protocol criteria. Of the 15 patients who did not, six had non-protocol dose modifications, seven discontinued chemotherapy (four patient refusals and three physician decisions), and two patients died while receiving treatment.
DISCUSSION
The primary purpose of this prospective phase II trial for limited SCLC was to improve OS without increasing acute severe esophagitis relative to the results of INT 0096.9 In that trial, twice-daily irradiation to 45 Gy produced a severe acute esophagitis rate of 27%, a 2-year OS rate of 47%, and a 5-year OS rate of 26%. In the current study, we found that delivery of 61.2 Gy in a twice-daily schedule produced severe acute esophagitis in 18% of patients and a 2-year OS rate of 36.6%. Hence the esophagitis rate was lower, but so was the 2-year survival rate.
The choice of dose fractionation for the current trial (RTOG 0239) was based on the results of RTOG 97-12,11 a prospective phase I trial of hyperfractionated, accelerated radiation therapy. Unfortunately the use of twice-daily radiation with concurrent chemotherapy is limited to local disease and carries the cost of significant toxicity.9 Because bulky hilar disease or mediastinal metastasis is present at presentation in about two-thirds of cases, the radiotherapy volume for SCLC necessarily includes much of the esophagus.15 However, the premise of the current trial was that the chemosensitivity of SCLC may allow us to reduce the amount of esophagus within the radiation fields during therapy, before the patients develop severe esophagitis, without compromising locoregional control.
The 2-year OS rate of 36.6% in the current study (RTOG 0239) was disappointing in that it did not meet the prespecified survival endpoint. However, as the INT 0096 results showed, the 2-year OS rate is not a good predictor of long-term survival. Although local control remains important, ultimately improving long-term survival will require more effective systemic therapy, because distant metastasis is still the dominant cause of failure (51% in this study compared with 27% local-regional recurrence).
The 18% severe acute esophagitis rate in this study was lower than the 27% experienced in the twice-daily arm of INT 0096 despite the higher radiation dose. The RTOG 0239 treatment-related death rate (2.8%, or 2/71) was similar to that of INT 0096 (2.7% [11/409], 6 in the twice-daily arm and 5 in the daily arm). The INT 0096 protocol called for starting thoracic radiation on day 1 of chemotherapy based on other studies showing that local control and survival were better when the radiation was started early relative to the chemotherapy.16,17
Other toxicities associated with the protocol tested here included the expected high rate of severe hematopoietic toxicity (90%; 15 grade 3 and 49 grade 4). Two patients experienced late grade 4 nonhematologic effects, one cardiac (myocardial infarction) and one pulmonary (reduction of diffusion capacity), and two patients died of treatment-related causes.
Conclusions
The principal finding from RTOG 0239, a 2-year OS rate of 36.6%, was no improvement over the results observed in INT 0096. However, the response rate (80%), the severe acute esophagitis rate (18%), and the treatment-related death rate (2.8%) compared favorably with INT 0096. The treatment strategy reported here is now one of three arms of a prospective randomized phase III trial of chemoradiation for limited SCLC (RTOG 0538/CALGB 30610).
ACKNOWLEDGMENTS
We gratefully acknowledge Christine Wogan, MS, ELS, of MD Anderson Cancer Center’s Division of Radiation Oncology, for her help in drafting and developing this manuscript.
Research Support: Supported in part by National Cancer Institute grants CA21661, CA32115, CA37422, and CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Aspects of this work have been presented at the 45th annual meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009; the 13th World Congress on Lung Cancer, International Association for the Study of Lung Cancer, San Francisco, CA, July 31-August 4, 2009; the 51st annual meeting of the American Society for Therapeutic Radiation Oncology, Chicago, IL, November 1-5, 2009; and the 14th World Conference on Lung Cancer, Amsterdam, The Netherlands, July 3-7, 2011.
Conflict of Interest Notification: The authors declare no conflicts of interest.
ClinicalTrials.gov identifier NCT00066222
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 2.Carney DN, Mitchell JB, Kinsella TJ. In vitro radiation and chemotherapy sensitivity of established cell lines of human small cell lung cancer and its large cell morphological variants. Cancer Res. 1983;43(6):2806–2811. [PubMed] [Google Scholar]
- 3.Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimens in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Arriagada R, Ihde D, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 5.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 992(10):890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 6.Pijls-Johannessma MC, De Ruysscher D, Lambin P, et al. Early vs late chest radiotherapy for limited stage small-cell lung cancer. Cochrane Database Syst Rev. 2005;(1):CD004700. doi: 10.1002/14651858.CD004700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–4845. doi: 10.1200/JCO.2004.01.178. [DOI] [PubMed] [Google Scholar]
- 8.De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomized controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. 2006;24:3823–3830. doi: 10.1093/annonc/mdj094. [DOI] [PubMed] [Google Scholar]
- 9.Turrisi AT, III, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 10.Choi NC, Herndon JE, 2nd, Rosenman J, et al. Phase I study to determine the maximum tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–3536. doi: 10.1200/JCO.1998.16.11.3528. [DOI] [PubMed] [Google Scholar]
- 11.Komaki R, Swann RS, Ettinger DS, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: RTOG protocol 97-12. Int J Radiat Oncol Biol Phys. 2005;62:342–350. doi: 10.1016/j.ijrobp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Fleming T. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1957;53:457–482. [Google Scholar]
- 15.Werner-Wasik M, Yorke E, Deasy J, et al. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S86–S93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray N, Coy P, Pater JL, et al. The National Cancer Institute of Canada Clinical Trials Group Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. J Clin Oncol. 1993;11(2):336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 17.Jeremic B, Shibamoto Y, Acimovic L. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol. 1997;15(3):893–900. doi: 10.1200/JCO.1997.15.3.893. at al. [DOI] [PubMed] [Google Scholar]