Abstract
Obesity is common in heart failure (HF) and associated with improved outcomes, often termed the “obesity paradox”. Although fat distribution varies by sex, the role of obesity in the outcomes of women compared to men with HF has not been well-studied. In a cohort of advanced systolic HF patients followed at a single university center, 2718 patients had body mass index (BMI) measured at baseline and 469 HF patients had waist circumference (WC) measured at baseline. Elevated BMI was defined as ≥25 kg/m2. High WC was defined as ≥88 cm in women and ≥102 cm in men. The primary outcome was death, urgent heart transplant, or ventricular assist device placement. Mean age was 53.0 ± 12.4, 25% of subjects were women, and LVEF was 22.9 ± 7.19. In men, 2-year event-free survival was better for high vs. normal BMI (63.2 vs. 53.5% p<0.001) and for high vs. normal WC (78.8% vs. 63.1%, p=0.01). In women, 2-year event-free survival was better in elevated vs. normal BMI groups (67.1% vs. 56.6%, p=0.01), but similar in WC groups. In multivariate analyses, normal BMI and normal WC were associated with higher risk of primary outcome in both men (BMI 1.34, WC 2.02) and women (BMI 1.38, WC 2.99). In advanced HF, high BMI and WC were associated with improved outcomes in both sexes. Further investigation of the interaction between body composition and sex in HF outcomes is warranted.
Keywords: Cardiomyopathy, mortality, adiposity, gender
Introduction
Heart failure (HF) affects 5.8 million individuals in the United States, including over 2.5 million women.1 Approximately one-half to two-thirds of HF patients are overweight or obese2. Obesity, quantified by anthropometric indices such body mass index (BMI) and waist circumference (WC), is associated with improved outcomes in HF, which has often been termed “the obesity paradox”.3-5 While fat distribution is known to vary by sex, the role of obesity in the outcomes of women compared to men with HF has not been well-studied.6 The primary aims of this study were to 1) assess the relationship between BMI and outcomes in men compared to women with HF and 2) assess the relationship between WC and outcomes in men and women with HF.
Methods
A total of 4089 patients were referred to a single university medical center for HF management and heart transplant evaluation between January 1983 and October 2011. All subjects were followed in a comprehensive HF management program as previously described.7 For the purposes of this study, patients were grouped into BMI and WC cohorts, which were not mutually exclusive. Since WC was only measured starting in May 2006, only 613 patients had WC measurements for inclusion in the WC group. Patients with left ventricular ejection fraction (LVEF) >40% (n=442) were excluded. Those without height or weight recorded (n=796) at time of initial evaluation were excluded from the BMI group. Furthermore, since underweight HF patients may have “cardiac cachexia,” known to be associated with worse prognosis, those classified as underweight (BMI <18.5 kg/m2, n=133) were excluded as well.8,9 Thus, the final study group consisted of 2718 BMI subjects (BMI cohort) and 469 WC subjects (WC cohort). Medical record review was approved by the Medical Institutional Review Board of the University of California—Los Angeles.
Height was recorded at the time of initial referral or at subsequent clinic visits. WC and body weight were measured at time of initial referral or within three months unless patients were deemed by physicians to be hypervolemic. In hypervolemic patients, WC and weight were recorded after hemodynamic optimization in order to remove the confounding effects of edema on these measurements. WC was measured at the midpoint between the lowest rib and the iliac crest; patients were classified as high or normal WC based on sex-specific thresholds for increased cardiometabolic risk (high WC in women ≥88 cm, men ≥102 cm).10 Patients were divided into BMI categories based on World Health Organization/National Institutes of Health guidelines: underweight (BMI <18.5 kg/m2 [excluded from study cohort]), healthy weight (BMI 18.5-24.9 kg/m2), overweight (BMI 25-29.9 kg/m2) and obese (BMI ≥30.0 kg/m2).9 Hemodynamic parameters and medical treatments were also recorded after invasive hemodynamic monitoring using Swan-Ganz catheterization, when necessary. Laboratory testing, echocardiography, and cardiopulmonary exercise tests all occurred within three months of the initial referral. Past medical history was extracted from medical record review.
Death, urgent status IA heart transplant (UT), or ventricular assist device (VAD) placement was the primary end point in this study. UT (status IA) and VAD placement were analyzed as HF death under the assumption that these patients would have died without an intervention.11,12 Death rather than UT or VAD implantation represented the majority of events (83%). Non-urgent transplants were censored and considered as nonfatal at the end of follow-up. All cause mortality was analyzed as a secondary endpoint.
For purposes of analysis, we stratified each cohort by sex and then considered two BMI groups: normal BMI (healthy weight; BMI 18.5-24.9 kg/m2) and high BMI (overweight and obese; BMI ≥25.0 kg/m2) and two WC groups: normal WC (WC in women <88 cm, men <102 cm) and high WC (WC in women ≥88 cm, men ≥102 cm). Actuarial survival curves for the male and female WC and BMI groups were calculated using the Kaplan-Meier estimate, and differences between curves were calculated using the log-rank statistic. Univariate survival analyses were performed with the likelihood ratio test, using the Cox model for baseline variables of WC and BMI. Multivariate analysis adjusting for known predictors in HF including sex, diabetes history, LVEF, HF etiology, and New York Heart Association (NYHA) class was performed by Cox proportional hazards regression analysis to estimate adjusted odds ratios and 95% confidence intervals for potential predictors of survival. The Cox model retained all independent variables with p<0.15. Statistics were calculated using the SPSS version 19.0 statistical package (IBM, Somers, NY).
Results
The BMI and WC cohorts shared similar baseline characteristics: both were approximately three-quarters male, and similar in terms of age, LVEF, WC, BMI, and prevalence of ischemic heart disease. Patients in the WC cohort were more likely to be managed with an aldosterone antagonist (60.0% versus 37.1%) and beta blocker (94.5% versus 57.3%), a difference attributable to changes in HF management practices between 1982 (when we began gathering BMI data) and 2006 (when we started to measure WC).
The baseline characteristics of the BMI and WC cohorts when stratified by sex are summarized in Table 1. Male sex was associated with higher WC, higher BMI, older age, ischemic etiology, and history of smoking in both BMI and WC cohorts. The baseline characteristics of the subject groups when stratified by both sex and high vs. normal BMI or WC are shown in Table 2. In both men and women, higher BMI was associated with younger age, increased peak oxygen consumption, increased prevalence of diabetes and smoking history, and decreased HDL.
Table 1. Baseline Characteristics by Sex in the BMI and WC Cohorts.
| Body Mass Index | Waist Circumference | |||||
|---|---|---|---|---|---|---|
| Men | Women | p Value* | Men | Women | p Value* | |
| n (%) | 2038 (75.0%) |
680 (25.0%) |
– | 344 (73.3%) |
125 (26.7%) |
– |
| Waist circumference (cm) |
104 ± 14.7 | 95.2 ± 17.9 |
<0.001 | 103 ± 14.6 | 94.8 ± 18.6 |
<0.001 |
| High waist circumference (≥88 cm in women, ≥102 cm in men) |
46.2% | 57.6% | 0.02 | 46.2% | 57.6% | 0.02 |
| Body mass index |
27.0 ± 5.2 | 27.5 ± 6.1 | 0.04 | 28.6 ± 5.7 | 27.6 ± 7.6 | 0.2 |
| High body mass index (≥25 kg/m2) |
60.2% | 58.7% | 0.3 | 69.5% | 53.5% | 0.004 |
| Age (yrs) | 53.7 ± 12.1 |
50.5 ± 12.9 |
<0.001 | 54.7 ± 12.7 |
51.8 ± 13.3 |
0.03 |
| Left ventricular ejection fraction (%) |
22.6 ± 7.1 | 23.9 ± 7.5 | <0.001 | 22.8 ± 7.7 | 24.5 ± 8.1 | 0.03 |
| Peak oxygen consumption (ml/min) |
1162 ± 456 |
879 ± 335 | <0.001 | 1232 ± 503 |
884 ± 384 | 0.2 |
| Peak oxygen consumption (ml/kg per min) |
13.9 ± 4.9 | 12.2 ± 4.3 | <0.001 | 14.0 ± 5.7 | 12.6 ± 5.5 | <0.001 |
| Coronary artery disease |
51.9% | 24.9% | <0.001 | 48.1% | 15.8% | <0.001 |
| Severe mitral regurgitation |
20.7% | 30.3% | 11.6% | 18.6% | 0.049 | |
| Severe tricuspid regurgitation |
11.6% | 15.8% | 0.007 | 5.4% | 14.4% | 0.004 |
| Left ventricular end-diastolic dimension (mm) |
70.2 ± 11.0 |
65.2 ± 11.0 |
<0.001 | 67.0 ± 11.7 |
59.1 ± 12.9 |
<0.001 |
| Left ventricular end-diastolic dimension index (mm/m2) |
35.3 ± 6.53 |
36.5 ± 7.07 |
<0.001 | 32.6 ± 6.42 |
33.0± 8.0 | 0.7 |
| New York Heart Association Class III or IV |
83.4% | 83.2% | 0.5 | 65.9% | 62.0% | 0.3 |
| Hypertension | 42.9% | 35.9% | 0.001 | 41.6% | 36.2% | 0.2 |
| Diabetes | 28.2% | 27.1% | 0.3 | 35.2% | 28.8% | 0.1 |
| Smokers (past and present) |
62.7% | 47.6% | <0.001 | 57.7% | 43.3% | 0.005 |
| Serum sodium (mmol/l) |
136.3 ± 4.6 |
136.5 ± 4.6 |
0.3 | 137.4 ± 3.94 |
136.5 ± 2.27 |
0.2 |
| Serum creatinine (mg/dl) |
1.55 ± 1.38 |
1.26 ± 0.88 |
<0.001 | 1.56 ± 1.90 |
1.30 ± 1.20 |
0.2 |
| Total cholesterol (mg/dl) |
166 ± 54.6 | 173 ± 59.3 | 0.02 | 149 ± 48.4 | 170 ± 48.6 | 0.001 |
| High density lipoprotein cholesterol (mg/dl) |
34.8 ± 13.3 |
41.4 ± 20.2 |
<0.001 | 36.5 ± 12.6 |
49.7 ± 29.9 |
<0.001 |
| Low density lipoprotein cholesterol (mg/dl) |
101.8 ± 41.7 |
102.0 ± 43.0 |
0.9 | 83.5 ± 35.0 |
96.9 ± 37.8 |
0.003 |
| Angiotensin- converting enzyme inhibitor or angiotensin IIreceptor blocker use |
85.2% | 86.2% | 0.3 | 85.4% | 86.4% | 0.5 |
| Aldosterone antagonist use |
36.0% | 40.5% | 0.03 | 60.1% | 60.0% | 0.5 |
| Beta blocker use |
58.0% | 55.1% | 0.1 | 95.3% | 92.0% | 0.1 |
Continuous variables are presented as the mean value ± SD, and categorical variables are presented as the percentage of patients.
Table 2. Baseline Characteristics by Sex Comparing Elevated to Normal Body Mass Index and Elevated to Normal Waist Circumference.
| Body Mass Index | Waist Circumference | |||||
|---|---|---|---|---|---|---|
| Elevated | Normal | p Value* | Elevated | Normal | p Value* | |
|
Men | ||||||
| n (%) | 1226 (60.2%) |
814 (39.8%) |
– | 159 (46.2%) |
185 (53.8%) |
– |
| Waist circumference (cm) |
110 ± 12.9 | 90.7 ± 8.37 |
<0.001 | 115 ± 11.5 | 92.7 ± 6.9 | <0.001 |
| High waist circumference (≥102 cm in men) |
65.9% | 10.3% | <0.001 | – | – | – |
| Body mass index |
30.1 ± 4.29 |
22.4 ± 1.70 |
<0.001 | 32.4 ± 5.18 |
25.0 ± 3.44 |
<0.001 |
| High body mass index (≥25 kg/m2) |
– | – | 93.8% | 46.3% | <0.001 | |
| Age (yrs) | 52.9 ± 11.4 |
55.0 ± 13.0 |
<0.001 | 53.1 ± 11.8 |
56.1 ± 13.3 |
0.03 |
| Left ventricular ejection fraction (%) |
22.9 ± 7.11 |
22.2 ± 6.95 |
0.04 | 23.8 ± 7.59 |
22.0 ± 7.79 |
0.03 |
| Peak oxygen consumption (ml/min) |
1264 ± 469 |
990 ± 376 | <0.001 | 1232 ± 503 |
884 ± 384 | 0.04 |
| Peak oxygen consumption (ml/kg per min) |
13.7 ± 4.6 | 14.2 ± 5.3 | 0.1 | 12.9 ± 4.4 | 15.0 ± 6.5 | 0.01 |
| Coronary artery disease |
51.0% | 53.3% | 0.2 | 42.3% | 53.1% | 0.3 |
| Severe mitral regurgitation |
17.0% | 26.0% | <0.001 | 10.9% | 12.3% | 0.4 |
| Severe tricuspid regurgitation |
9.0% | 15.2% | <0.001 | 2.2% | 8.3% | 0.2 |
| Left ventricular end-diastolic dimension (mm) |
70.6 ± 10.9 |
69.7 ± 11.2 |
0.1 | 67.4 ± 12.8 |
66.7 ± 10.8 |
0.6 |
| Left ventricular end-diastolic dimension index (mm/m2) |
33.4 ± 5.8 | 38.1 ± 6.6 | <0.001 | 29.9 ± 5.8 | 35.0 ± 6.0 | <0.001 |
| New York Heart Association Class III or IV |
80.3% | 88.1% | <0.001 | 69.2% | 63.0% | 0.2 |
| Hypertension | 47.6% | 35.7% | <0.001 | 50.0% | 34.6% | 0.003 |
| Diabetes | 32.4% | 21.5% | <0.001 | 39.6% | 31.4% | 0.07 |
| Smokers (past and present) |
63.8% | 61.2% | 0.1 | 58.6% | 56.5% | 0.4 |
| Serum sodium (mmol/l) |
136.7 ± 4.4 |
135.7 ± 4.9 |
<0.001 | 137.5 ± 3.8 |
137.2 ± 4.1 |
0.5 |
| Serum creatinine (mg/dl) |
1.54 ± 1.44 |
1.56 ± 1.29 |
0.7 | 1.62 ± 1.87 |
1.51 ± 1.93 |
0.6 |
| Total cholesterol (mg/dl) |
169 ± 55.5 | 162 ± 53.0 | 0.004 | 150 ± 45.5 | 148 ± 50.1 | 0.7 |
| High density lipoprotein cholesterol (mg/dl) |
34.1 ± 12.7 |
35.8 ± 14.2 |
0.01 | 35.4 ± 13.8 |
37.3 ± 11.4 |
0.2 |
| Low density lipoprotein cholesterol (mg/dl) |
102.6 ± 41.7 |
100.8 ± 41.8 |
0.4 | 84.1 ± 33.6 |
83.0 ± 36.1 |
0.8 |
| Angiotensin- converting enzyme inhibitor or angiotensin II- receptor blocker use |
86.2% | 83.5% | 0.7 | 88.6% | 82.7% | 0.08 |
| Aldosterone antagonist use |
39.4% | 30.6% | <0.001 | 62.9% | 57.6% | 0.2 |
| Beta blocker use |
62.9% | 50.3% | <0.001 | 97.5% | 93.5% | 0.07 |
|
Women | ||||||
| n (%) | 387 (58.7%) |
272 (41.2%) |
– | 53 (56.4%) | 41 (43.6%) | – |
| Waist circumference (cm) |
104 ± 17.3 | 82.7 ± 8.6 | <0.001 | 107 ± 14.8 | 78.3 ± 6.3 | <0.001 |
| High waist circumference (≥88 cm in women) |
86.8% | 22.0% | <0.001 | – | – | – |
| Body mass index |
31.3 ± 5.0 | 22.1 ± 1.9 | <0.001 | 32.2 ± 7.1 | 22 ± 2.8 | <0.001 |
| High body mass index (≥25 kg/m2) |
– | – | – | 83.6% | 15.9% | <0.001 |
| Age (yrs) | 49.3 ± 13.0 |
52.2 ± 12.6 |
0.005 | 50.2 ± 13.1 |
54 ± 13.4 | 0.1 |
| Left ventricular ejection fraction (%) |
24.0 ± 7.4 | 23.9 ± 7.8 | 0.9 | 24.7 ± 8.4 | 24.5 ± 7.8 | 0.9 |
| Peak oxygen consumption (ml/min) |
977 ± 321 | 748 ± 309 | <0.001 | 992 ± 254 | 774 ± 462 | 0.7 |
| Peak oxygen consumption (ml/kg per min) |
11.9 ± 3.7 | 12.5 ± 4.9 | 0.2 | 12 ± 2.8 | 13.2 ± 7.3 | 0.5 |
| Coronary artery disease |
24.6% | 25.4% | 0.4 | 16.2% | 15.4% | 0.6 |
| Severe mitral regurgitation |
31.0% | 29.2% | 0.4 | 21.9% | 14.3% | 0.2 |
| Severe tricuspid regurgitation |
15.5% | 16.1% | 0.5 | 9.7% | 20.4% | 0.1 |
| Left ventricular end-diastolic dimension (mm) |
66.0 ± 11.1 |
64.0 ± 10.7 |
0.04 | 59.1 ± 12.3 |
59.2 ± 13.7 |
0.9 |
| Left ventricular end-diastolic dimension index (mm/m2) |
34.5 ± 6.4 | 39.4 ± 6.9 | <0.001 | 29.9 ± 7 | 36.7 ± 7.7 | <0.001 |
| New York Heart Association Class III or IV |
84.9% | 80.8% | 0.1 | 67.2% | 55.3% | 0.1 |
| Hypertension | 41.0% | 28.7% | 0.001 | 38.5% | 33.3% | 0.4 |
| Diabetes | 32.3% | 19.8% | <0.001 | 41.7% | 11.3% | <0.001 |
| Smokers (past and present) |
48.3% | 46.5% | 0.4 | 48.5% | 36.5% | 0.1 |
| Serum sodium (mmol/l) |
136.4 ± 4.1 |
136.6 ± 5.1 |
0.5 | 137.6 ± 4.1 |
134.9 ± 18.4 |
0.3 |
| Serum creatinine (mg/dl) |
1.28 ± 0.82 |
1.22 ± 0.95 |
0.4 | 1.35 ± 1.21 |
1.23 ± 1.18 |
0.6 |
| Total cholesterol (mg/dl) |
178 ± 60.5 | 165 ± 56.9 | 0.1 | 174 ± 46.4 | 164.9 ± 51.6 |
0.4 |
| High density lipoprotein cholesterol (mg/dl) |
38.9 ± 15.9 |
44.68 ± 24.37 |
0.002 | 43.9 ± 16.8 |
57.1 ± 40 | 0.04 |
| Low density lipoprotein cholesterol (mg/dl) |
106.7 ± 43.5 |
95.9 ± 41.6 |
0.006 | 103.8 ± 38.3 |
88.4 ± 35.9 |
0.07 |
| Angiotensin- converting enzyme inhibitor or angiotensin II- receptor blocker use |
85.8% | 86.7% | 0.4 | 87.5% | 84.9% | 0.4 |
| Aldosterone antagonist use |
39.6% | 41.7% | 0.3 | 65.3% | 52.8% | 0.1 |
| Beta blocker use |
54.5% | 56.1% | 0.4 | 93.1% | 90.6% | 0.4 |
During two years of follow-up, 550 deaths, 379 UTs, and 59 VAD placements occurred in the BMI cohort, with 396 deaths, 322 UTs, and 50 VAD placements occurring in the first year. Of the total deaths, 144 were sudden, 187 were progressive HF deaths, 47 were multisystem organ failure, 9 were myocardial infarctions, and 163 were from unknown or other causes. In the WC cohort, 53 deaths, 35 UTs, and 4 VAD placements occurred, with 35 deaths, 29 UTs, and 2 VAD placements occurring in the first year. Of the total deaths, 8 were sudden, 21 were progressive HF deaths, 6 were multisystem organ failure, 1 was a myocardial infarction, and 17 were from unknown or other causes.
Outcomes were similar between men and women in both the BMI and WC cohorts. Overall survival free from death / UT / VAD for the BMI cohort at 2 years was 59.3% in men and 62.9% in women (p=0.2). Overall survival free from death / UT / VAD for the WC cohort at 2 years was 70.8% in men and 79.0% in women (p=0.08).
In men, both higher BMI and higher WC were associated with event-free survival (Table 3, Figure 1a and 1b). In women, higher BMI was associated with improved event-free survival (Table 3, Figure 1c). Women with high WC had a trend towards improved outcomes (Table 3, Figure 1d). There was not significant heterogeneity between high vs. normal WC outcomes comparing strata of men vs. women (p=0.06). For both men and women, higher BMI and WC were also associated with the secondary endpoint of improved survival free from all-cause mortality, although this finding was not significant for women with high WC (data not shown).
Table 3. WC, BMI and Survival at Two Years.
| Survivors | Univariate Relative Risk (95% Confidence Interval) |
p Value* | Multivariate Relative Risk (95% Confidence Interval)† |
p Value* | |
|---|---|---|---|---|---|
|
Men | |||||
| Waist circumference (high) |
78.8% | – | – | – | – |
| Waist circumference (normal) |
63.1% | 1.81 (1.12-2.92) | 0.01 | 2.02 (1.18-3.45) | 0.009 |
| Body mass index (high) |
63.2% | – | – | – | – |
| Body mass index (normal) |
53.5% | 1.36 (1.18-1.57) | <0.001 | 1.34 (1.13-1.58) | <0.001 |
|
Women | |||||
| Waist circumference (high) |
80.4% | – | – | – | – |
| Waist circumference (normal) |
76.9% | 1.26 (0.50-3.21) | 0.6 | 2.99 (0.90-4.8) | 0.4 |
| Body mass index (high) |
67.1% | – | – | – | – |
| Body mass index (normal) |
56.6% | 1.42 (1.09-1.85) | 0.01 | 1.38 (1.02-1.89) | 0.04 |
*p values reflect the likelihood ratio test by the Cox model. Variables are presented as the percentage of patients.
Multivariate analysis is adjusted for age, diabetes, left ventricular ejection fraction, New York Heart Association class, and heart failure etiology (ischemic vs. non-ischemic).
Figure 1.
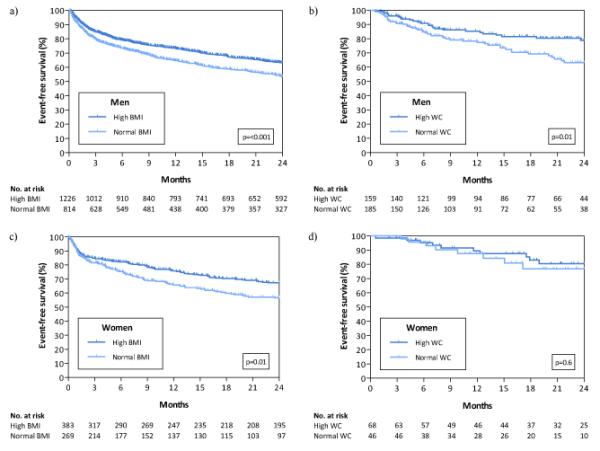
Survival at two years for the primary outcome of death/urgent heart transplant in men by BMI (a) and WC (b) and in women by BMI (c) and WC (d).
Multivariable analyses adjusting for LVEF, diabetes, NYHA, and HF etiology in cohorts of men and women were performed. On multivariable analysis, both men and women with normal WC and normal BMI were at substantially higher risk of adverse outcomes. Normal BMI compared to high BMI was associated with significantly worsened outcomes in both male and female cohorts, with 34% and 38% higher risk, respectively. Furthermore, multivariable analysis revealed normal WC compared to high WC also to be associated with higher risk of death / UT/ VAD in both sexes, with approximately doubling of risk in men and tripling of risk in women (Table 3).
Discussion
In our study of patients with advanced systolic HF, obesity as indexed by high BMI and high WC was associated with better outcomes in both men and women. Although in our female cohort the association between high WC and improved outcomes was not statistically significant, a trend towards improved outcomes was seen, without significant heterogeneity between the male and female cohorts. This finding was likely due to the smaller number of female subjects in this study; however, this is an area that deserves further investigation. To our knowledge, our study is the first to specifically demonstrate that the obesity paradox in terms of both BMI and WC is applicable to both men and women with HF.
Because systolic HF is more common in men, women make up only 28% of subjects in recent HF clinical trials.1,13,14 This selection bias extends into studies of the obesity paradox in HF, where women may be as little as 13 percent of a study population or the sex of participants may not even been reported.14,15
BMI is a surrogate measure of body fat and may also reflect lean body mass, while WC may most accurately measure visceral adiposity.10,16 The addition of WC to BMI thus predicts a greater variance in health risk than does BMI alone in general medical practice.17,18 BMI and WC have previously been identified as more strongly associated with cardiovascular morbidity and mortality in men than women – perhaps because women have less visceral fat overall.2,19,20 Metrics such as cardiopulmonary fitness have been suggested as better predictors than obesity of other types of CV risk in women21. Men generally store excessive fat in a visceral distribution, while women store fat in a peripheral subcutaneous distribution22. These differences have been shown to persist even after menopause, to a lesser degree.23,24 Visceral fat is associated with “adiposopathy”: existing fat cells hypertrophy and the local tissue environment may change, including increased circulating free fatty acids, tissue hypoxia, and inflammatory and immune response cascades. 25
Multiple competing and complimentary explanations have been proposed for the obesity paradox observed in HF populations. HF is a catabolic state and cachexia is associated with a poorer prognosis.8 Obesity may represent the other end of the same spectrum, where patients benefit from increased muscle mass and/or increased metabolic reserve in the form of fatty tissue26. The increased levels of serum lipoproteins associated with increased body fat may play an anti-inflammatory role, neutralizing circulating bacterial endotoxins or cytokines.27 Other studies have shown that low adiponectin levels and a decreased catecholamine response, both of which are seen in obese individuals, are linked to improved HF survival.28,29 Obese patients may also present at an early stage in their HF course due to increased symptoms and functional impairment caused by excess body weight.4,5 The present study suggests that these or other mechanisms are operative in both men and women with HF.
Our study has several strengths. The study involves a single center, allowing for accurate and thorough follow-up. We have eliminated the potential confounding variable of edematous weight gain by using WC and BMI measurements recorded after therapy aimed at hemodynamic optimization and euvolemia. Our research database includes numerous demographic, laboratory, echocardiographic, and hemodynamic variables, permitting detailed and adequately powered survival analysis and accounting for multiple potential confounders. However, our study also has limitations. Our cohort of women is smaller than our cohort of men, giving us relatively less power to detect differences in this group. Furthermore, the “n” of our WC cohort was significantly smaller than the BMI cohort. This was a selected group of patients referred for HF evaluation at a heart transplantation center and evaluated retrospectively. WC criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups30. There is also no consensus on an optimal site for measurement of WC. Furthermore, WC and BMI were assessed at referral, a single point in time (time of referral), while the extent of adiposity and/or loss of muscle mass may be an evolving process with progression of disease. We do not have direct adiposity quantification or distribution measures such as dual-energy x-ray absorptiometry (DEXA) or computed tomography. We also do not have data on cytokines, or adipokines, which would have been helpful in understanding the pathophysiology behind our observations. Residual measured or unmeasured confounding variables may influence some or all of the findings.
Acknowledgments
Funding Sources: Dr. Horwich was supported by NHLBI / NIH 1K23HL085097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow G, Norris C, McAlister F. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Horwich T, Fonarow G, Hamilton M, MacLellan W, Woo M, Tillisch J. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 5.Curtis J, Selter J, Wang Y, Rathore S, Jovin I, Jadbabaie F, Kosiborod M, Portnay E, Sokol S, Bader F, Krumholz H. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? Journal of the American College of Cardiology. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stevenson LW, Walden JA, Livingston NA, Steimle AE, Hamilton MA, Moriguchi J, Tillisch JH, Woo MA. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 8.Anker S, Ponikowski P, Varney S, Chua T, Clark A, Webb-Peploe K, Harrington D, Kox W, Poole-Wilson P, Coats A. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuczmarski R, Flegal K. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 10.Klein S, Allison D, Heymsfield S, Kelley D, Leibel R, Nonas C, Kahn R. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson K, Mancini D. Mortality remains high for outpatient transplant candidates with prolonged (>6 months) waiting list time. J Am Coll Cardiol. 1999;33:1189–1195. doi: 10.1016/s0735-1097(98)00697-4. [DOI] [PubMed] [Google Scholar]
- 12.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010;3:706–714. doi: 10.1161/CIRCHEARTFAILURE.110.944280. [DOI] [PubMed] [Google Scholar]
- 13.Hsich EM, Pina IL. Heart failure in women: a need for prospective data. Journal of the American College of Cardiology. 2009;54:491–498. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Howser R, Portner PM, Pierson RN., 3rd. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Hall JA, French TK, Rasmusson KD, Vesty JC, Roberts CA, Rimmasch HL, Kfoury AG, Renlund DG. The paradox of obesity in patients with heart failure. J Am Acad Nurse Pract. 2005;17:542–546. doi: 10.1111/j.1745-7599.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Corral A, Somers V, Sierra-Johnson J, Jensen M, Thomas R, Squires R, Allison T, Korinek J, Lopez-Jimenez F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28:2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 17.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze M, Overvad K, van der Schouw Y, Spencer E, Moons K, Tjønneland A, Halkjaer J, Jensen M, Stegger J, Clavel-Chapelon F, Boutron-Ruault M, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters P, May A, Bueno-de-Mesquita H, van Duijnhoven F, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós J, Key T, Bingham S, Khaw K, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 18.Bigaard J, Tjønneland A, Thomsen B, Overvad K, Heitmann B, Sørensen T. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 19.Levitan E, Yang A, Wolk A, Mittleman M. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2:202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy N, MacIntyre K, Stewart S, Hart C, Hole D, McMurray J. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 21.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. Jama. 2004;292:1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 23.Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, Allison DB, Heymsfield SB. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 25.Snijder M, van Dam R, Visser M, Seidell J. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 26.Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, Clark AL, Anker SD. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 27.Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, Coats AJ, Anker SD. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 29.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37:169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 30.Misra A, Wasir J, Vikram N. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition. 2005;21:969–976. doi: 10.1016/j.nut.2005.01.007. [DOI] [PubMed] [Google Scholar]


