Abstract
NMDA and TRPV1 receptors that are expressed in sensory neurons have been independently demonstrated to play important roles in peripheral pain mechanisms. In the present study, we investigated whether the two receptor-channel systems form a functional complex that provides the basis for the development of mechanical hyperalgesia. In the masseter muscle, direct application of NMDA induced a time dependent increase in mechanical sensitivity, which was significantly blocked when the muscle was pretreated with a specific TRPV1 antagonist, AMG9810. The NR1 subunit of the NMDA receptor and TRPV1 were co-expressed in 32% of masseter afferents in trigeminal ganglia (TG). Furthermore, NR1 and NR2B formed protein-protein complexes with TRPV1 in TG as demonstrated by co-immunoprecipitation experiments. Calcium imaging analyses further corroborated that NMDA and TRPV1 receptors functionally interact. In TG culture, application of NMDA resulted in phosphorylation of serine, but not threonine or tyrosine, residues of TRPV1 in a time course similar to that of the development of NMDA-induced mechanical hyperalgesia. The NMDA-induced phosphorylation was significantly attenuated by CaMKII and PKC inhibitors, but not by a PKA inhibitor. Consistent with the biochemical data, the NMDA-induced mechanical hyperalgesia was also effectively blocked when the muscle was pretreated with a CaMKII or PKC inhibitor. Thus, NMDA receptors and TRPV1 functionally interact via CaMKII and PKC signaling cascades and contribute to mechanical hyperalgesia. These data offer novel mechanisms by which two ligand-gated channels in sensory neurons interact and reinforce the notion that TRPV1 functions as a “signal integrator” under pathological conditions.
1. Introduction
There is a large body of evidence demonstrating that endogenous release of glutamate in peripheral tissue following injury or inflammation can exacerbate pain conditions and modulate functional properties of nociceptors in skin, muscles, and joints [33,46,58]. A higher level of endogenous glutamate in the masseter muscle of temporomandibular disorder patients relative to healthy subjects further suggests the involvement of peripheral glutamate receptors (gluRs) in persistent muscle pain conditions [8]. Human and animal studies have consistently demonstrated the involvement of gluRs such as the NMDA receptor (NMDAR) and the metabotropic glutamate receptor 5 (mGluR5) in trigeminal sensory neurons in acute pain and mechanical hyperalgesia arising from orofacial muscle tissue [6,36,54,63]. However, the cellular mechanisms by which these gluRs lead to heightened mechanical sensitivity in deep craniofacial tissue are still unknown.
Mice lacking functional TRPV1 display impaired behavioral responses to noxious heat stimuli applied to cutaneous tissue while responses to noxious mechanical stimuli remain intact [9,10]. More recent studies, however, indicate that TRPV1 also contributes to the development of mechanical hyperalgesia. TRPV1 antagonists effectively reduce mechanical hyperalgesia in rats with spinal nerve ligation [13,14] as well as complete Freund’s adjuvant (CFA)-induced mechanical hyperalgesia [19,24,50]. Besides cutaneous tissue, TRPV1 has been suggested to play an important role in mechanical sensation in deep tissue such as the colon [29]. In muscle tissue, the direct injection of capsaicin significantly lowers mechanical thresholds in both humans and rats [1,56], and the blockade of TRPV1 attenuates mechanical hyperalgesia resulting from eccentric muscle contraction [17].
Activation of NMDARs results in an influx of Ca2+ and invokes Ca2+ calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), or protein kinase A (PKA) [18,22,41,68]. These intracellular kinases directly modulate TRPV1 function, and phosphorylation of TRPV1 by such intracellular kinases has been suggested as a major mechanism that accounts for sensitization of TRPV1 [44,45,57]. However, there is no data on whether NMDAR activation results in TRPV1 sensitization and phosphorylation in sensory neurons. These observations led us to hypothesize that the two important receptor/channel systems that have been independently implicated in muscle pain and hyperalgesia (i.e. NMDAR and TRPV1) functionally interact in nociceptors and these interactions constitute the peripheral mechanisms underlying the development of mechanical hyperalgesia. To test our hypothesis we specifically investigated whether (1) the activation of peripheral NMDARs leads to mechanical hyperalgesia via a TRPV1-dependent manner, (2) the activation of NMDARs enhances TRPV1 function in TG neurons, (3) NMDAR activation leads to TRPV1 phosphorylation in TG neurons, and (4) examined the intracellular mechanisms involved in NMDAR-TRPV1 interactions.
2. Materials and Methods
2.1 Animals
Adult male Sprague Dawley rats (150-350 g; Harlan, Indianapolis) were used. All animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Publication No. 80-23) and under a University of Maryland approved Institutional Animal Care and Use Committee protocol.
2.2 Drug Preparation
For behavioral experiments, NMDA, a specific agonist for the NMDAR, and AP5, a competitive NMDAR antagonist, were dissolved in phosphate buffered saline (PBS). AMG9810, a specific antagonist for TRPV1, was dissolved in dimethyl sulfoxide (DMSO) (100%). A PKC inhibitor, GF109203X, a specific CaMKII inhibitor, KN93, a PKA activator, forskolin, and a specific PKA inhibitor, KT5720, were dissolved in DMSO and diluted in PBS with final concentration of DMSO less than 1%. The concentration of mustard oil (MO) was 20%.
For Ca2+ imaging experiments, drugs were diluted to final concentrations in Ca2+ imaging buffer (CIB): NMDA (500 μM), glycine (10 μM), capsaicin (10 nM, 1 μM). Stock solutions were generated as follows: NMDA 100 mM, pH 7 in CIB, glycine 100 mM in H2O, and capsaicin 10 mM in ethanol. The final concentration of ethanol was less than 0.01%.
For biochemical experiments, KN92 and KN93 (10 μM), GF109203X (10 μM) and a PKA inhibitor, KT5720 (1 μM), were diluted to final concentrations in culture media from the stock solution dissolved in DMSO. The final concentration of DMSO was less than 0.1%.
NMDA, AP5, forskolin, MO and capsaicin were purchased from Sigma (St. Louis, MO), AMG9810, GF109203X, KN93 and KT5720 from Tocris Bioscience (Ellisvile, MO), and KN92 from Calbiochem (San Diego, CA).
2.3 Behavioral studies-Assessment of mechanical sensitivity in masseter muscle
Noxious chemical or mechanical stimulation of the masseter muscle evokes characteristic shaking of the ipsilateral hindpaw in lightly anesthetized rats. We have previously described the use of this behavior for testing mechanical sensitivity of the masseter muscle [55,56]. This lightly anesthetized rodent paradigm allows the delivery of a calibrated and reliable mechanical stimulus on the masseter muscle or temporomandibular joint before and after pharmacological manipulations, which is difficult in awake animals. Initially, rats (250-350 g) were anesthetized with an intraperitoneal injection of sodium pentobarbital (40 mg/kg). A level of ‘light’ anesthesia was determined by providing a noxious pinch to the tail or the hindpaw with a serrated forceps. Animals typically responded to the noxious pinch on the tail with an abdominal contraction and with a withdrawal reflex to the noxious pinch of a hindpaw within 30 min after the initial anesthesia. Once the animal reached this level a metal clip calibrated to produce 600 gm of force was applied 5 consecutive times to the tail, and experiments were continued only after the animals showed reliable reflex responses to every clip application. A tail vein was connected to an infusion pump (Harvard Apparatus, Pump11) for continuous infusion of pentobarbital. The rate of infusion was adjusted to maintain a relatively light level of anesthesia throughout the duration of the experiment (3–5 mg/h).
A baseline mechanical threshold for evoking the nocifensive responses was determined 15 min prior to drug injection using the electronic von Frey (VF) anesthesiometer (IITC Life Science, Inc, Woodland Hills, CA). A rigid tip (diameter = 2 mm) attached to the VF meter was applied to the masseter muscle until the animals responded with hindpaw shaking. The animal’s head was rested flat against the surface of the table when pressing the anesthesiometer on the masseter in order to provide stability. The threshold was defined as the lowest force needed to evoke a hindpaw response. Changes in masseter sensitivity were then assessed at 15, 30, 45, 60 and 90 min following drug treatments. In order to maintain the consistency of assessing behavioral responses all behavioral observations were made by one experimenter blinded to the experimental conditions.
To test whether the activation of NMDAR induces mechanical hypersensitivity, NMDA (10 mmol/40 μl) or the same volume of vehicle was administered into the masseter muscle. To determine that NMDA-induced behavioral responses are receptor mediated, the masseter muscle was pretreated with AP5 (1 μmol/20 μl) 5 min prior to the injections with NMDA in the same muscle. In order to investigate the involvement of TRPV1 in NMDA-induced mechanical hypersensitivity, AMG9810 (10, 100 nmol/10 μl), or the same volume of vehicle was administered in the masseter 5 min prior to the NMDA treatment. The high dose of AMG9810 (100 nmol) was administered in the muscle contralateral to the NMDA treatment to confirm that the antagonist’s effects are mediated by blocking local TRPV1 receptors. To rule out the possibility that the antagonist alone can produce analgesic responses, AMG9810 (100 nmol) was administered in the masseter muscle without NMDA. Separate groups of animals received intramuscular MO (20%, 20 μl) alone or in combination with AMG9810 (100 nmol) to test the specificity of AMG9810. For kinase experiments, GF109203X (100 nmol/20 μl), KN93 (0.1 and 1 mmol/20 μl), KT5720 (30 nmol/20 μl), or the same volume of vehicle was pre-administered in the masseter 5 min prior to NMDA injection in the same muscle. Finally, forskolin (50 nmol/20 μl) was administered with either KT5720 (30 nmol/20 μl) or vehicle. All injections were made directly in the mid-region of the masseter muscle with either a 25 μl or 50 μl Hamilton syringe over 5-10 s. Experimental and control groups were randomized and all groups consisted of 6-8 rats per group.
The percent changes in VF thresholds following drug treatment were calculated with respect to the baseline threshold and plotted against time. The time-dependent mean percent changes in mechanical thresholds normalized to the baseline threshold were analyzed with a two-way ANOVA with repeated measures. All multiple group comparisons were followed by a post hoc test (Bonferroni’s). The significance of all statistical analyses presented in this report was set to p < 0.05.
2.4 Immunohistochemistry for TRPV1 and NR1 in masseter afferents
Fast Blue (FB) (2%; 10 μl; Sigma) was injected in the masseter to retrogradely label TG muscle afferents in 3 rats. FB was injected into multiple sites in the masseter muscle using aseptic techniques. To avoid any leakage of the tracer the injection needle was left in place for 1-2 minutes before it was slowly retracted. The injection site was then covered with petroleum jelly. After allotting seven days for the FB to label the masseter afferents, the animals were transcardially perfused with cold phosphate buffer solution (PBS) followed by 4% paraformaldehyde in PBS (250 ml; pH 7.3-7.4; Sigma). The right TG from each rat was extracted and post-fixed for 90 min, placed in 30% sucrose solution at 4°C overnight and sectioned coronally at 12 μm. Every eighth section was collected and mounted on gelatin-coated slides for double-labeling immunohistochemistry. Following blocking, the sections were incubated overnight with primary antisera for the NR1 subunit of the NMDAR (1:100; goat polyclonal; Santa Cruz), and incubated with Daylight 594 donkey anti-goat antiserum for 60 min at 37°C for immunofluorescence. The NR1 antibody is directed against the human C-terminus of the NMDAζ1 receptor subtype. After staining for NR1, sections were incubated overnight with primary antisera for TRPV1 (1:1000; rabbit polyclonal; Neuromics), followed by 60 min with Daylight 488 goat anti-rabbit antiserum (1:250; Jackson Immunoresearch) for immunofluorescence. This antibody corresponds to amino acid residues 4-21 of TRPV1. The primary antibody for TRPV1 or NR1 was omitted from the processing of selected sections to control for non-specific background staining.
TRPV1, NR1 and FB positive cells were counted from 8 representative sections per ganglion from three TG. The somatotopic distribution of FB positive cells within the TG was assessed to ensure that the FB did not spread to other divisions of the TG. Trigeminal and facial motor nuclei were also evaluated as positive and negative controls for FB labeling, respectively. Only the labeled neurons that showed a clear nucleus were included in the counting. The percentages of masseter afferents labeled with TRPV1 and/or NR1 were calculated and presented as mean ± standard error of the mean (SE).
2.5 Calcium imaging
TG extracted from naïve male rats weighing between 150-200 g were minced in Dulbecco’s Modified Eagle Medium (DMEM)/F12 containing horse serum and penicillin/streptomycin/glutamine on ice, and were then incubated in media containing collagenase (type XI, Sigma) for 30 minutes at 37°C. Following mechanical dissociation, the cells were incubated for 2 minutes in trypsin (0.05%)/Ethylenediaminetetraacetic acid (EDTA) (0.1%) in PBS. The cells were then separated by Percoll and plated on poly-L-ornithine and laminin-coated glass coverslips. Two rats (4 TG) were used for one culture and cultures were used for experimentation 48 hr later.
Primary TG cultures were loaded with 20 μl of 1 mM Fura2-AM and 2 μl of 20% pluronic acid for 40 min at 37°C in a buffer containing NaCl 130, KCl 3, 0.5 MgCl2, CaCl2 0.9, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, sucrose 10, NaHCO3 1.2 (in mM, pH 7.45, 320 mOsm adjusted with mannitol). Following a 15 min wash period for de-esterification, dual images (510 nm emission) were collected every 2 s using NIS Elements (Nikon). The Fura response (F) was defined as the ratio of emissions measured during excitation at 340 and 380 nm. The CIB used during recording was as follows in mM: NaCl 130; KCl 3; CaCl2 2.5; HEPES 10; sucrose 10; NaHCO3 1.2; pH 7.45 and 320 mOsm. We omitted Mg2+ from the buffer to facilitate the response of NMDARs.
Recording protocol
An initial sub-maximal concentration of capsaicin (10 nM) was applied to assess the basal responses across individual cells and culture preparations. Next, vehicle (CIB) or 500 μM NMDA/10 μM glycine was applied for 3 min. One minute after the NMDAR agonist application, a second application of 10 nM capsaicin was made. A sub-maximal concentration of capsaicin was used to minimize the desensitizing effects of repeated capsaicin applications, and also to ensure that sensitization can be clearly observed while ceiling effects are avoided. In the control condition, after the second 10 nM capsaicin application NMDA/glycine was applied to identify NMDAR positive cells. At the end of the experiment, a supramaximal concentration of capsaicin (1 μM) was applied to confirm the identification of all TRPV1 neurons in both experimental and control conditions.
Data Analysis
We calculated the changes in Fura response (ΔF, F minus baseline) in every cell. Baseline was defined as the average of the five data points prior to a given stimulus application. A neuron was considered to be a responder if the Fura response to NMDA or any of the three capsaicin applications was above threshold.
Threshold was determined following a similar method as [15]. A histogram was generated from a population of neurons to a given stimulus protocol. The ΔF greater than two standard deviations from the peak ΔF of non-responding neurons was classified as responsive. The ΔF of the first and second capsaicin application were analyzed with two-way ANOVA with repeated measures, followed by Bonferroni’s test.
In order to confirm that the NMDA-induced Ca2+ responses are indeed mediated by functional NMDAR we treated cells with either AP5 (125 μM) or vehicle with NMDA (500 μM NMDA/10 μM glycine). The proportions of cells showing NMDA-induced responses were compared between AP5 and vehicle treated groups with the Chi square analysis.
We have also conducted additional calcium imaging experiments to test the specificity of AMG9810. In order to confirm that AMG9810 does not directly modulate NMDAR we treated the cells with AMG9810 (100 nmol) or vehicle with NMDA (500 μM NMDA/10 μM glycine). As a positive control we tested the same concentration of AMG9810 with capsaicin (100 nmol). The proportions of cells showing NMDA- or capsaicin-induced responses were compared between AMG9810 and vehicle targeted groups with the Chi square analysis.
2.6 Immunoprecipitation and co-immunoprecipitation
Dissociated TG cultures were lysed with RIPA lysis buffer (Cell signaling). The lysates were centrifuged at 12,000 rpm at 4°C for 20 min to remove cellular debris. The supernatant was incubated with anti-TRPV1 polyclonal antibody (Calbiochem) at 4°C overnight, and then with protein A/G-Sepharose beads (Santa Cruz) for 2 hours. LDS sample buffer including SDS was added to elute proteins from the protein A/G beads. Each sample was separated by 4-12% NuPAGE gel (Invitrogen) electrophoresis and subjected to immunoblotting. To determine the level of p-Ser, p-Thr or p-Tyr, the membranes were blocked and incubated with anti-phosphoserine (1:1000; Santa Cruz) or anti-phosphothreonine (1:1000; Calbiochem) or anti-phosphotyrosine (1:1000; Millipore), further washed and incubated with anti-mouse IgG HRP (1:5000), and then developed with the ECL detection kit (Amersham Bioscience). The membranes were stripped and re-probed with anti-TRPV1 (1:1000; Calbiochem) to determine the amount of immunoprecipitated proteins. After immunoblotting, the bands on the membrane were scanned and quantified with an image analyzer (Image J Software) and normalized to that of TRPV1, which served as the loading control. The data was subjected to either a one-way ANOVA or Kruskal-Walis analysis, depending on the outcome of the normality test. All multiple group comparisons were followed by a post-hoc test. Data are shown as mean ± SE obtained from 6-8 repeated experiments. For co-immunoprecipitation of NMDARs and TRPV1, intact TG were extracted and processed according to the protocol described above. For this experiment, the concentration of NP-40 (1%) in the lysis buffer was diluted to 0.5%, and the tissue incubation time was adjusted to 2 hrs for anti-NR1 and 4-6 hrs for anti-NR2B. The following antibodies were used: NR1 (1:500; Millipore) or NR2B (1:500; Millipore) and TRPV1 (1:1000; Calbiochem). The specificity of these antibodies was confirmed in previous studies [3, 47, 69]. Two TG were used for each co-IP experiment.
3. Results
3.1. NMDAR-mediated mechanical hyperalgesia involves TRPV1
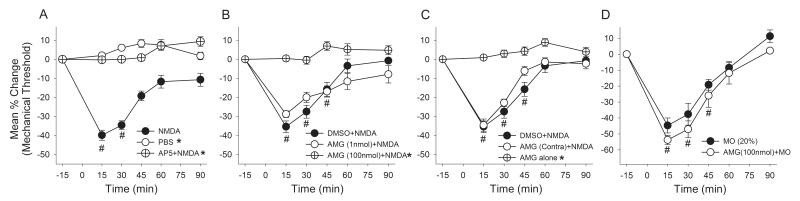
The baseline mechanical threshold that evoked the nocifensive response ranged from 501-582 g in lightly anesthetized rats, which was similar to those we previously reported [36,56]. There were no significant differences in the baseline mechanical thresholds between any of the groups we examined. We first confirmed that direct activation of NMDARs in the masseter muscle evokes a time dependent increase in mechanical sensitivity (Fig 1A). There was a significant group effect (F = 65.04, p < 0.001) and a significant time effect (F = 33.59, p < 0.001). Masseteric injection of NMDA (10 μmol/40 μl) significantly decreased the mechanical threshold reaching the peak effect at 15 min (−39% ± 2.6) and gradually returning to the baseline level within 2 h following the injection (Fig 1A). The vehicle injection in the same manner did not alter the mechanical sensitivity of the masseter muscle. A pretreatment of the muscle with a systemically low dose of AP5 (1 μmol) [54], a competitive NMDAR antagonist, completely prevented the development of NMDA-induced masseter hyperalgesia, indicating that the responses are produced specifically by the activation of local NMDARs.
Figure 1.
The effects of intramuscular injection of NMDA and AMG 9810 on masseter mechanical sensitivity. (A) Changes in mechanical sensitivity following NMDA, PBS or AP5 followed by NMDA administration in the masseter muscle. (B) NMDA-induced changes in mechanical sensitivity following pretreatment of the masseter with AMG 9810, a specific TRPV1 antagonist, or vehicle. (C) The effects on AMG9810 administered in the masseter contralateral to the NMDA treatment or AMG9810 alone on masseter mechanical sensitivity. (D) The effects of AMG9810 (100 nmol) on mustard oil-induced mechanical hyperalgesia. (*, p < 0.05 in group effects with respect to vehicle condition in two-way ANOVA; #, p < 0.05 to the baseline in post-hoc test; n = 6-8 for each group).
We then examined whether the NMDA-induced mechanical hyperalgesia was altered by pretreatment of the muscle with a specific TRPV1 antagonist, AMG9810. There was a significant group effect (F = 37.7, p < 0.001) and a significant time effect (F = 44.8, p < 0.001) (Fig 1B). The NMDA-induced mechanical hyperalgesia was significantly blocked when 100 nmol of AMG9810 was pre-administered in the masseter. A lower dose of AMG9810 (1 nmol) did not significantly block the NMDA-induced responses indicating a dose-dependent effect of the antagonist (Fig 1B). The vehicle did not did not alter the NMDA-induced mechanical hyperalgesia. The higher dose of AMG9810 (100 nmol) administered in the contralateral masseter muscle failed to attenuate the NMDA-induced masseter hypersensitivity, thus suggesting that the AMG9810 produced its effects via blocking local TRPV1 (Fig 1C). The same dose of AMG9810 injected alone did not alter masseter sensitivity. The pretreatment with all drugs was made 5-10 min prior to the NMDA injection.
Since it is possible that AMG9810 at 100 nmol can produce non-specific effects such as desensitization of nociceptive afferents we conducted additional experiments in which AMG9810 was used to block behavioral responses induced by MO (20%), a TRPA1 agonist. Figure 1D shows that there was only a significant time effect (F = 67.8, p < 0.001), without a significant group effect (F = 1.5, p > 0.05). The dose of AMG9810 that significantly attenuated the NMDA-induced responses failed to block the effects of MO suggesting that AMG9810 did not produce non-specific effects, but by specifically antagonizing TRPV1. Together these behavioral data showed that functional interactions between peripheral NMDARs and TRPV1 mediate the development of mechanical hyperalgesia.
3.2. NMDAR and TRPV1 are co-expressed in TG neurons and form protein-protein complexes
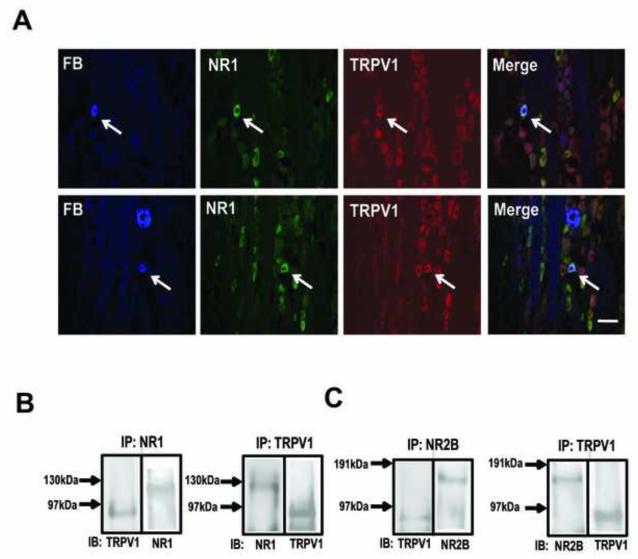
We have previously shown that NMDA receptor subunits NR1, NR2A, and NR2B are expressed in TG, and that TRPV1 is expressed in distinct populations of TG neurons that innervate masseter afferents [37,56]. Here, we sought to investigate the extent to which NMDARs and TRPV1 are co-expressed in masseter afferents. NR1 positive neurons were more abundant in TG as the NR1 was expressed in all size TG neurons compared to TRPV1 positive neurons that were predominantly expressed in small TG neurons. Of the 1827 TRPV1 positive TG neurons we examined, 96% co-expressed NR1. This data is consistent with the ubiquitous expression of NR1 in TG [26].
Of the 111 FB positive cells from 3 rats, 81.6 ± 4% were NR1 positive and 34 ± 2% TRPV1 positive. The percentage of FB positive masseter afferents that co-express NR1 and TRPV1 was 32 ± 3%. Figure 2A shows examples of masseter afferents that contain both NR1 and TRPV1. These data suggest that most masseter afferents that express TRPV1 also contain NR1, and that functional interactions between the two receptor systems can occur at the intracellular level.
Figure 2.
NR1 and TRPV1 expression and co-immunoprecipitation in TG. (A) immunohistochemical staining of TG sections showing FB-labeled muscle afferents, TRPV1- and NMDA-labeled neurons as indicated. The arrows indicate muscle afferents that co-express both NR1 and TRPV1. Scale bar: 50 μm. (B) (left) Immunoblot (IB) using anti-TRPV1 or anti-NR1 antibody following immunoprecipitation (IP) of TG extract with anti-NR1 antibody. (Right) Reverse IP with anti-TRPV1 antibody and IB with anti-NR1 or anti-TRPV1 antibody. (C) The same IB-IP protocols were used to show co-immunoprecipitation of TRPV1 and NR2B subunit.
We then utilized co-immunoprecipitation (Co-IP) techniques to demonstrate protein-protein interactions between NMDARs and TRPV1. Immunoprecipitation of TG extracts using a TRPV1 antibody resulted in a clear immunoblot stained with an NR1 antibody (Fig 2B). The membranes were then stripped and blotted with the TRPV1 antibody to confirm immunoprecipitation of TRPV1. The reverse immunoprecipitation in which TRPV1 was blotted following immunoprecipitation of TG extracts using the NR1 antibody confirmed the Co-IP of the two receptors. Since functional NMDARs require both NR1 and NR2 subunits we performed a similar experiment with a NR2B antibody. As with NR1, NR2B also co-immunoprecipitated with TRPV1 in TG extracts. These data provided evidence that NMDARs and TRPV1 are not only co-expressed in the same TG neurons but they also form protein-protein complexes.
3.3. NMDA potentiates capsaicin responses in a subset of trigeminal sensory neurons
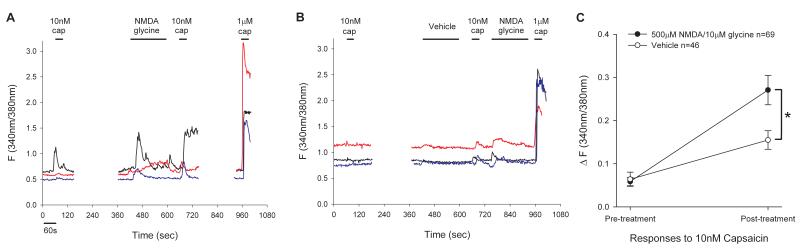
To further explore functional interactions between NMDARs and TRPV1 we examined whether NMDA can enhance capsaicin-induced Ca2+ responses in dissociated TG neurons. The application of a low concentration of capsaicin (10 nM) typically produced a small increase in Ca2+ responses in a subpopulation of neurons. To examine whether activation of NMDARs enhances capsaicin-evoked responses, we applied vehicle or NMDA with glycine prior to the second application of 10 nM of capsaicin and compared the relative amplitude of the responses following the first and second application of capsaicin. In the vehicle-treated group, we applied NMDA after the second application of capsaicin in order to identify neurons responding to both capsaicin and NMDA so that we could compare vehicle and NMDA-treated groups only in those subpopulations.
The proportions of neurons responding to NMDA only, capsaicin only, or both NMDA and capsaicin were similar across the two treatment groups: vehicle [2% (20), 88% (792) and 9% (84) of 896 cells], and NMDA [2% (18), 84% (821) and 14% (140) of 979 cells], respectively. Neurons responding to both capsaicin and NMDA showed mixed responses to the repeated application of capsaicin. The percentages of neurons showing increasing or decreasing responses were similar between vehicle, 55% (46/84) and 45% (38/84), respectively, and NMDA, 49% (69/140) and 51% (71/140), respectively, treated groups.
The cells with a low Ca2+ influx during the initial capsaicin treatment tended to show increasing responses to the second capsaicin treatment in both vehicle and NMDA-treated groups. Examples of cells showing this type of responses are shown in Fig 3A, B. Among the cells that showed increasing responses, responses to the initial application of capsaicin were similar between the vehicle (0.06 ± 0.02) and NMDA-treated groups (0.06 ± 0.01). However, the NMDA-treated group showed a significantly greater enhancement of the response following the second application of capsaicin compared to the vehicle treated group (Vehicle: 0.15 ± 0.02, NMDA: 0.27 ± 0.03, p < 0.001, Fig 3C).
Figure 3.
The effects of NMDA on capsaicin-induced responses in TG neurons. Representative traces show Fura ratio from TG neurons in NMDA (A) and vehicle (B) treated groups. (C) Averaged changes in Fura response by first and second application of 10 nM capsaicin in NMDA or vehicle-treated groups. *, p < 0.05 in two-way ANOVA.
The cells with higher initial Ca2+ responses tended to show decreasing responses in both vehicle and NMDA-treated groups. Among the cells that showed decreasing responses, responses to both the initial and second applications of capsaicin were not significantly different between the vehicle and NMDA-treated groups (Vehicle n = 38: cap1 0.27 ± 0.06, cap2 0.06 ± 0.02; NMDA n = 71: cap1 0.24 ± 0.04, cap2 0.09 ± 0.02, p = 0.993).
In order to confirm that NMDA-induced Ca2+ responses are receptor mediated we applied NMDA in the presence of a specific NMDAR antagonist, AP5 or its vehicle control. The percentage of cells responding to NMDA under vehicle and AP5 conditions were 20.78% of 255 cells, and 2.9% of 343 cells, respectively (χ2 = 39.4, p < 0.001), confirming that the NMDA-induced responses can be effectively blocked when NMDA receptors are pharmacologically inhibited. The AP5 application alone rarely produced responses.
We conducted additional experiments with NMDA application in the presence of a specific TRPV1 antagonist, AMG9810 (1 μmol) or its vehicle control to provide additional evidence for the specificity of AMG9810. The percentage of cells responding to NMDA under vehicle and AMG9810 conditions were 13.5% and 9.25% of 281 cells, respectively (χ2 = 2.0, p > 0.05). These data confirmed that AMG9810 does not directly modulate the NMDA-induced responses. The percentage of cells responding to capsaicin under vehicle and AMG9810 conditions were 65.5% of 183 cells and 36% of 259 cells, respectively (χ2 = 12.5, p < 0.001). Thus, the concentration of AMG9810 that failed to block NMDA responses effectively antagonized TRPV1 receptors. The AMG9810 application alone rarely produced responses.
These data reinforced the notion of functional interactions between NMDARs and TRPV1 in a subpopulation of TG neurons, and strengthened the observations made in behavioral experiments.
3.4. NMDA phosphorylates TRPV1 at specific residues in TG
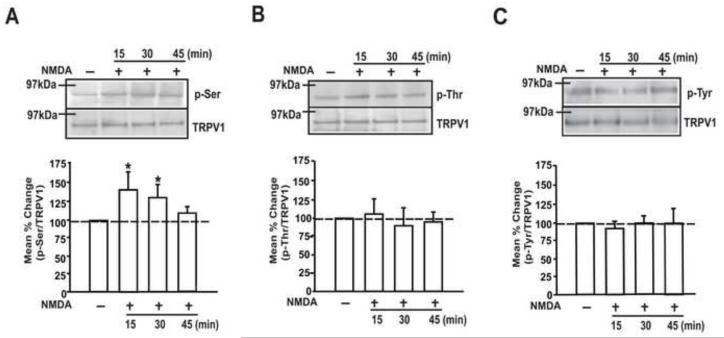
In order to examine whether the activation of NMDARs leads to the phosphorylation of TRPV1, we examined the changes in TRPV1 phosphorylation in sensory neurons following the application of NMDA. It is not feasible to perform such analysis at the primary afferent terminal level since the neural elements in the muscle tissue are too low to detect meaningful biochemical changes. Instead, we assessed the relative level of phosphorylated TRPV1 in cultured TG neurons 15, 30, and 45 min following the application of NMDA (200 μM), a time course comparable to our behavioral experiments.
The NMDA application caused a time-dependent elevation in serine phosphorylation (p-Ser) of TRPV1 (Fig 4A). A significant increase in p-Ser could be observed at 15 min and 30 min, the time points at which mechanical hyperalgesia was most prominent following NMDA injection in the in vivo condition. The total TRPV1 expression level did not change during this time course. The same concentration of NMDA did not produce significant alteration in phosphorylation of TRPV1 at either threonine or tyrosine residues (Fig 4B, C). These data provide support for NMDAR-TRPV1 interactions and that NMDAR activation leads to TRPV1 phosphorylation at specific sites.
Figure 4.
The effects of NMDA treatment on the phosphorylation of TRPV1 in cultured TG neurons. (A-C) Immunoblots using anti-p-Ser (A), p-Thr (B) or p-Tyr (C) antibody following IP using anti-TRPV1 antibody (upper). The samples were collected at the indicated time points following the treatment with vehicle or NMDA (200 μM). Immunoblots using anti-TRPV1 antibody in the same gel in the upper panel (lower). Averaged relative p-Ser/TRPV1, p-Thr/TRPV1 or p-Tyr/TRPV1 are also shown. (*, p < 0.05 in one-way ANOVA; n = 6-8 for each time point).
3.5. CaMKII and PKC mediate NMDAR-TRPV1 interactions in TG
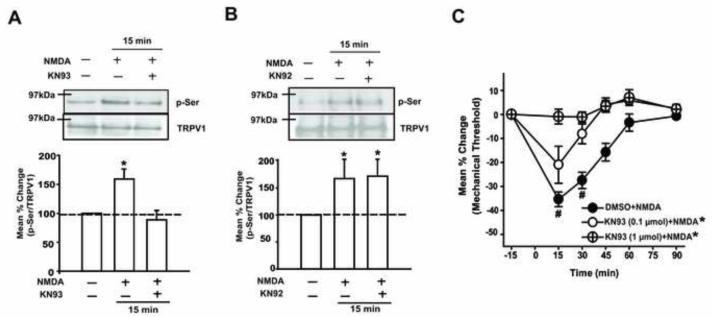
Serine phosphorylation of TRPV1 can be mediated by various protein kinases including CaMKII, PKC and PKA [5,16,30,51,53,65]. Since NMDAR activation has been shown to invoke a CaMKII mediated signaling pathway [18], we first examined whether the NMDA-mediated phosphorylation of serine residues in TRPV1 involves the CaMKII pathway. The significant increase in p-Ser of TRPV1 15 min following NMDA application was blocked when TG cultures were pretreated with a specific CaMKII inhibitor, KN93 (Fig 5A). The same concentration of KN92, an inactive analog of KN93, failed to block the NMDA-induced increase in p-Ser of TRPV1 (Fig 5B). Consistent with the biochemical data, the NMDA-induced mechanical hyperalgesia was dose-dependently blocked when KN93, but not vehicle, was pre-administered in the same muscle (Fig 5C). There was a significant group effect (F = 12.06, p < 0.01) and a significant time effect (F = 41.1, p < 0.001). The injection of KN93 alone did not alter the mechanical sensitivity in the muscle (data not shown). Collectively, these data provide evidence that activation of NMDARs invokes a CaMKII signaling cascade that results in serine phosphorylation of TRPV1 in TG.
Figure 5.
The involvement of CaMKII in NMDA-induced serine phosphorylation of TRPV1 and mechanical hyperalgesia. (A) Examples of immunoblots and averaged relative p-Ser/TRPV1 following the NMDA (200 μM) treatment with and without KN93 (10 μM). (B) Examples of immunoblots and averaged relative p-Ser/TRPV1 following the NMDA treatment with and without KN92 (10 μM), an inactive analog of KN93. The samples were collected at the 15 minute time point, during which the NMDA-induced serine phosphorylation of TRPV1 was most prominent (*, p < 0.05 in one-way ANOVA; n = 6-8 per group in A and B). (C) The effects of KN93 pretreatment on NMDA-induced mechanical hyperalgesia (*, p < 0.05 in group effects with respect to vehicle condition in two-way ANOVA; #, p < 0.05 to the baseline in post-hoc test; n = 6-8 per group).
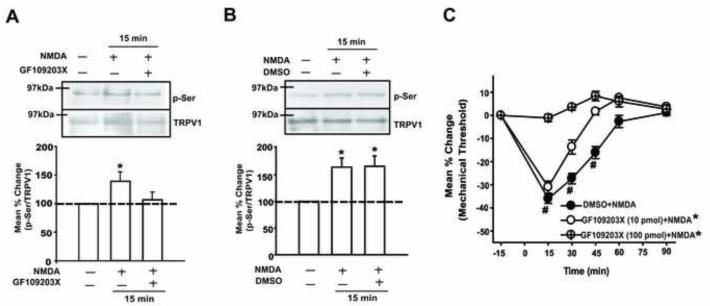
We performed similar experiments to examine whether PKC is also involved in NMDAR-TRPV1 interactions. The significant increase in p-Ser of TRPV1 15 min following NMDA application was blocked when TG cultures were pretreated with a PKC inhibitor, GF109203X (Fig 6A). The same concentration of vehicle failed to block the NMDA-induced increase in p-Ser of TRPV1 (Fig 6B). Again, consistent with the biochemical data, the NMDA-induced mechanical hyperalgesia was dose-dependently blocked when GF109203X, but not vehicle, was pre-administered in the same muscle (Fig 6C). There was a significant group effect (F = 23.02, p < 0.001) and a significant time effect (F = 41.39, p < 0.001). The injection of GF109203X compound alone did not alter the mechanical sensitivity (data not shown). These data provide evidence that, in addition to the CaMKII signaling pathway, activation of NMDARs also invokes a PKC signaling cascade that results in serine phosphorylation of TRPV1 in TG.
Figure 6.
The involvement of PKC in NMDA-induced serine phosphorylation of TRPV1 and mechanical hyperalgesia. (A) Examples of immunoblots and averaged relative p-Ser/TRPV1 following the NMDA (200 μM) treatment with and without GF109203X (10 μM). (B) Examples of immunoblots and averaged relative p-Ser/TRPV1 following the NMDA treatment with and without DMSO (0.1 %), the vehicle control for GF109203X. The samples were collected at the 15 minute time point (*, p < 0.05 in one-way ANOVA; n = 6-8 per group in A and B). (C) The effects of GF109203X pretreatment on NMDA induced mechanical hyperalgesia (*, p < 0.05 in group effects with respect to vehicle condition in two-way ANOVA; #, p < 0.05 to the baseline in post-hoc test; n = 6-8 per group).
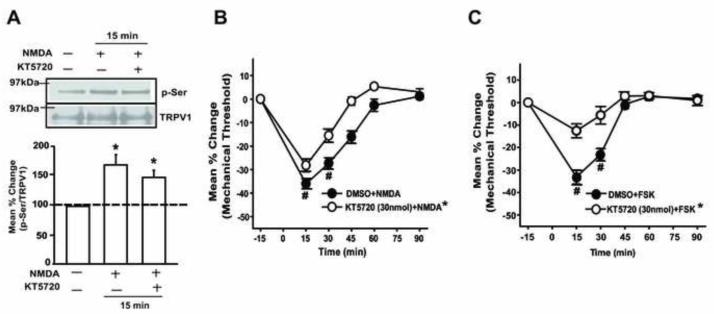
Finally, we examined whether NMDAR-TRPV1 interaction also involves a PKA signaling pathway. The NMDA-induced increase in p-Ser of TRPV1 was not significantly blocked when the TG was pretreated with a specific PKA inhibitor, KT5720 (Fig 7A). The dose of KT5720 was chosen based on the literature [59]. The NMDA-induced mechanical hyperalgesia was partially, but significantly reduced when the muscle was pretreated with KT5720 (Fig 7B). The same dose of KT5720 almost completely blocked the mechanical hyperalgesia induced by a direct injection of forskolin, a PKA activator (7C). These data suggest that NMDAR-induced mechanical hyperalgesia may involve PKA pathway, but the activation of that pathway does not result in TRPV1 phosphorylation in TG.
Figure 7.
PKA is not involved in NMDA-induced TRPV1 phosphorylation. (A) KT5720, a PKA inhibitor, or vehicle control failed to prevent the NMDA-induced elevation of p-Ser of TRPV1 (*, p < 0.05 in one-way ANOVA; n = 6-8 per group in A and B). (B, C) NMDA-induced mechanical hyperalgesia was only partially attenuated at the dose that completely blocked the forskolin induced mechanical hyperalgesia (*, p < 0.05 in group effects with respect to vehicle condition in two-way ANOVA; #, p < 0.05 to the baseline in post-hoc test; n = 6-8 per group).
4. Discussion
The current study demonstrated that the NMDAR and TRPV1 functionally interact in rat trigeminal sensory neurons and that such interactions are essential for the development of mechanical hyperalgesia in the masseter muscle. Several lines of evidence support this conclusion. First, the NR1 subunit of the NMDAR and TRPV1 are co-expressed in a subset of trigeminal afferents that innervate the masseter muscle, and form protein-protein complexes in TG neurons. Second, NMDAR activation enhances capsaicin-induced responses in a subset of TG neurons. Third, NMDAR activation leads to phosphorylation of specific residues in TRPV1 in TG via intracellular kinases that have been implicated in sensitization. Finally, NMDA-induced mechanical hyperalgesia in the masseter muscle requires TRPV1, and the inhibition of kinases that mediate TRPV1 phosphorylation in TG attenuates NMDA-induced mechanical hyperalgesia. Especially noteworthy is that the NMDA-induced phosphorylation of TRPV1, which we demonstrated in native sensory neurons, correlated with our behavioral responses, and that the pharmacological blockade of kinases produced corroborating biochemical and behavioral responses. These results strongly suggest that NMDARs and TRPV1 are functionally linked in peripheral terminals of nociceptors in the muscle tissue. These data offer novel mechanisms by which two distinct ligand-gated channels in nociceptors interact and form the underlying cellular basis for the development of mechanical hyperalgesia.
The significance of our data is that these two important ligand-gated ion channels, which have been independently implicated in muscle pain and hyperalgesia interact and may operate as “functional units”, which bears important scientific and clinical implications. Our data provide molecular mechanisms of possible interactions between glutamate receptors and capsaicin receptors that have been suggested in animal as well as human experimental muscle pain models. For example, injecting glutamate into craniofacial deep tissues significantly enhances capsaicin-induced nociceptor activity and lowers mechanical thresholds [32]. Similarly, capsaicin injections followed by glutamate in human tendon tissue significantly facilitate pain responses and decrease pressure pain thresholds [20].
An important implication of this data is that the NMDAR is activated upstream to TRPV1 activation under injury or inflammatory conditions. We conducted additional behavioral experiments to test the possibility that TRPV1 is activated upstream to NMDAR. The mechanical hyperalgesia induced by the direct injection of capsaicin in the masseter was significantly inhibited when the muscle was pre-treated with AP5 (data not shown), suggesting that the functional interactions are bi-directional. The directionality of interactions between TRPV1 and NMDARs could be governed by various factors such as the availability of endogenous ligands, the expression levels of TRPV1 and NMDARs, and the requisite intracellular machineries. It is likely that the intracellular mechanisms underlying the interactions in each direction are different from one another.
Therefore, while it is also plausible that the NMDAR functions as a down-stream target of TRPV1 activation, we felt the present data may more realistically reflect pathological conditions for the following reasons. First, it is well established that excess glutamate is released in peripheral tissue, including muscle tissue, upon injury or inflammation from numerous sources [33,34,42,46,48,49], which supports our data as physiologically tenable. Similar physiologically relevant endogenous agonists for TRPV1 in muscle tissue following injury may also be released, but they are yet to be fully characterized. Second, there is increasing evidence that TRPV1 functions as a downstream inflammatory signal integrator following the activation of G-protein coupled receptors (GPCR) [38]. Our data add to these observations in that signaling cascades invoked by activation of ligand-gated ion channels also converge onto TRPV1.
Functional interactions between glutamate receptors and TRPV1 have been suggested at the level of the spinal cord. Increased glutamate release due to enhanced pre-synaptic Ca2+ signaling following TRPV1 activation at the central terminals of nociceptors could result in prolonged activation of NMDARs in postsynaptic dorsal horn neurons [43,60]. The mechanisms described in the above studies, however, are intercellular rather than intracellular mechanisms. A recent study demonstrated that DAG produced upon mGluR5 activation directly activates TRPV1 in the same neuron in a membrane-delimited manner, a mechanism which has been proposed to contribute to the modulation of synaptic transmission in the substantia gelatinosa neurons of the spinal cord [31]. In this study, we demonstrated that NR1 subunits and TRPV1 co-express in a subset of trigeminal sensory neurons and that the two receptors form protein-protein complexes. At present, we do not know the precise nature of their association since co-IP data alone does not establish direct physical interactions. It is also not known whether the functional interactions between the two receptor systems require such physical interactions. Our data, however, provided novel information that NMDAR and TRPV1 reside in close proximity within the same cell, and suggest mechanisms relevant for functional interactions resulting from intracellular changes initiated by NMDAR in a micro domain.
Phosphorylation of TRPV1 has been considered as a major mechanism that accounts for TRPV1 sensitization and various second messenger pathways have been associated with TRPV1 phosphorylation [4,5,27,28,30,40,67]. It is well known that different kinases phosphorylate different residues of TRPV1. For example, PKC phosphorylates TRPV1 at Ser-502, Ser-800, and Thr-704 whereas PKA activation results in the phosphorylation of Ser-502, Ser-116, Thr-144 and Thr-370 residues [5,40,44,53]. CaMKII activation also leads to phosphorylation of TRPV1 at Ser-502, Thr-370 and Thr-704 [30]. It is also well known, that activation of GPCR, such as neurokinin, bradykinin, prostaglandin, and TrkA receptors sensitize TRPV1 via pathways involving PKC, PKA, and CaMKII [27,35,59,62,70]. However, it is unclear whether the activation of these receptors phosphorylates a specific residue in TRPV1 by invoking a specific kinase pathway. The present study revealed that activation of NMDARs results in phosphorylation of TRPV1 primarily at serine residues through the activation of PKC and CaMKII, but not PKA, pathways.
Activation of NMDARs increases the influx of Ca2+, which invokes multiple intracellular signaling cascades including activation of CaMKII [2,62,64]. The NMDAR-CaMKII cascade functionally coupled to acid sensing ion channels (ASICs) has been shown to contribute to acidotoxicity during ischemia [18]. Specifically, CaMKII-induced phosphorylation of a specific serine residue in ASICs plays an essential role in ischemia-induced cell death in the presence of excess glutamate, an example of CaMKII mediated mechanisms of “channel-channel” interactions. CaMKII is expressed in both peptidergic and non-peptidergic TRPV1 positive DRG neurons [7]. Blockade of CaMKII effectively reduces capsaicin-induced CGRP release in TG neurons [52]. Our behavioral data corroborated the biochemical data by demonstrating that NMDA-induced masseter hypersensitivity is attenuated by a CaMKII inhibitor. Together, these data allow us to postulate the NMDAR-CaMKII-TRPV1 cascade as an underlying factor for the development of masseter hypersensitivity.
Induction of the PKC signaling pathway following NMDAR activation has been widely demonstrated in the CNS [22,61,66]. In DRG neurons, activation of NMDARs enhances the activity of voltage dependent Ca2+ channels through PKC [11,39]. Our data provided additional evidence that NMDAR activation in TG sensory neurons invokes PKC, which targets TRPV1 at serine residues. We have previously shown that the activation of another glutamate receptor, mGluR5, in the masseter muscle results in mechanical hyperalgesia via PKC [36]. Thus, it seems that both the NMDAR and mGluR5 activated by excess glutamate released under pathological conditions can recruit PKC, which then modulates the activity of other pro-nociceptive molecules such as TRPV1.
Hu et al [25] showed mGluR5 activation in DRG neurons increases TRPV1 function in a PKA-, but not PKC-dependent manner. However, there are no data on whether the activation of NMDARs results in PKA activation in sensory neurons. The NMDA-induced mechanical hyperalgesia was partially attenuated by a PKA inhibitor at the dose that almost completely abolished forskolin-induced hyperalgesia. These data suggest that NMDAR activation may also recruit the PKA pathway. Although TRPV1 is a substrate for PKA, our biochemical data showed that NMDAR-mediated increase in TRPV1 phosphorylation does not involve PKA. Therefore, the NMDAR-PKA pathway may converge on other channels such as TRPA1 (unpublished observations). Collectively, our data suggest that the NMDAR engages TRPV1 in specific ways primarily by CaMKII and PKC, rather than PKA.
This work establishes that the two prominent channels in nociceptive circuitry cooperate as functional units. Our data also opens up the possibility that other non-specific cationic channels such as P2X and ASICs might also be communicating with TRPV1 via specific or common intracellular signaling pathways. Given that signals arising from GPCR converge onto TRPV1 and TRPA1 [38], our data further reinforce the notion that TRP channels are at the core of forming “functional units” in nociceptive signaling under pathological conditions. In a broader context, it would be interesting to explore whether similar interactions also take place in the CNS. Recently, TRPV1 is shown to be present in hippocampus, dentate gyrus and nucleus accumbens [12,21,23], brain areas enriched in glutamate receptors.
Acknowledgements
The authors thank Youping Zhang and Gregory Haynes for technical assistance. This study was supported by NIH Grant RO1 DE16062 (JYR). There is no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. Eur J Pain. 2008;12(5):661–70. doi: 10.1016/j.ejpain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aronowski J, Grotta JC, Waxham MN. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. J Neurochem. 1992;58(5):1743–53. doi: 10.1111/j.1471-4159.1992.tb10049.x. [DOI] [PubMed] [Google Scholar]
- [3].Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci. 2011;108(10):4206–11. doi: 10.1073/pnas.1101424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- [6].Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophys. 2003;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- [7].Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha-containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448(1):102–10. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- [8].Castrillon E, Ernberg M, Cairns B, Wang K, Sessle B, Arendt-Nielsen L, Svensson P. Interstitial glutamate concentration is elevated in the masseter muscle of myofascial temporomandibular patients. European Journal of Oral Sciences. 2010;24(4):350–360. [PubMed] [Google Scholar]
- [9].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [10].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [11].Chaban VV, Li J, Ennes HS, Nie J, Mayer EA, McRoberts JA. N-methyl-D-aspartate receptors enhance mechanical responses and voltage-dependent Ca2+ channels in rat dorsal root ganglia neurons through protein kinase C. Neuroscience. 2004;128(2):347–57. doi: 10.1016/j.neuroscience.2004.06.051. [DOI] [PubMed] [Google Scholar]
- [12].Chávez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nature Neuroscience. 2010;13:1511–8. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Christoph T, Gillen C, Mika J, Grünweller A, Schäfer MK, Schiene K, Frank R, Jostock R, Bahrenberg G, Weihe E, Erdmann VA, Kurreck J. Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int. 2007;50(1):281–90. doi: 10.1016/j.neuint.2006.08.017. [DOI] [PubMed] [Google Scholar]
- [14].Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350(1):238–43. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- [15].Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278(34):32037–46. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- [16].De Petrocellis L, Davis JB, Di Marzo V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506(3):253–6. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- [17].Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140(2):292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [18].Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [19].Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. JPET. 2005;313:474–84. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- [20].Gibson W, Arendt-Nielsen L, Sessle BJ, Graven-Nielsen T. Glutamate and capsaicin-induced pain, hyperalgesia and modulatory interactions in human tendon tissue. Exp Brain Res. 2009;194(2):173–82. doi: 10.1007/s00221-008-1683-3. [DOI] [PubMed] [Google Scholar]
- [21].Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–59. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Giordano G, Sanchez-Perez AM, Burgal M, Montoliu C, Costa LG, Felipo V. Chronic exposure to ammonia induces isoform-selective alterations in the intracellular distribution and NMDA receptor-mediated translocation of protein kinase C in cerebellar neurons in culture. J Neurochem. 2005;92:143–157. doi: 10.1111/j.1471-4159.2004.02852.x. [DOI] [PubMed] [Google Scholar]
- [23].Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neuroscience. 13:1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. JPET. 2005;314(1):410–21. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- [25].Hu HJ, Bhave G, Gereau RW., 4th Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22(17):7444–52. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ivanusic JJ, Beaini D, Hatch RJ, Staikopoulos V, Sessle BJ, Jennings EA. Peripheral N-methyl-d-aspartate receptors contribute to mechanical hypersensitivity in a rat model of inflammatory temporomandibular joint pain. Eur J Pain. 2011 Feb;15(2):179–85. doi: 10.1016/j.ejpain.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138(3):604–16. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phopshorylation and sensitization of TRPV1. Pain. 2009;146(3):301–7. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25(47):10981–9. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279(8):7048–54. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- [31].Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci. 2009;29:10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception I: activation and peripheral sensitization of deep craniofacial nociceptive afferents. Brain Res. 2009;1251:130–139. doi: 10.1016/j.brainres.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000;86:69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- [34].Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997;324:169–177. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- [35].Lee SY, Lee JH, Kang KK, Hwang SY, Choi KD, Oh U. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res. 2005;28(4):405–12. doi: 10.1007/BF02977669. [DOI] [PubMed] [Google Scholar]
- [36].Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience. 2007a;146:375–383. doi: 10.1016/j.neuroscience.2007.01.015. [DOI] [PubMed] [Google Scholar]
- [37].Lee JS, Ro JY. Differential regulation of excitatory amino acid receptor subunits following masseter inflammation in trigeminal ganglia. Neurosci Lett. 2007b;421:91–95. doi: 10.1016/j.neulet.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772(8):989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [39].Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- [40].Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- [41].Matsumura S, Kunori S, Mabuchi T, Katano T, Nakazawa T, Abe T, Watanabe M, Yamamoto T, Okuda-Ashitaka E, Ito S. Impairment of CaMKII activation and attenuation of neuropathic pain in mice lacking NR2B phosphorylated at Tyr147. European Journal of Neuroscience. 2010;32(5):798–810. doi: 10.1111/j.1460-9568.2010.07348.x. [DOI] [PubMed] [Google Scholar]
- [42].McAdoo DJ, Hughes MG, Xu GY, Robak G, de Castro R., Jr Microdialysis studies of the role of chemical agents in secondary damage upon spinal cord injury. J Neurotrauma. 1997;14(8):507–15. doi: 10.1089/neu.1997.14.507. [DOI] [PubMed] [Google Scholar]
- [43].Medvedeva YV, Kim MS, Usachev YM. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci. 2008;28(20):5295–311. doi: 10.1523/JNEUROSCI.4810-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- [45].Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proceedings of National Academy of Science. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- [47].Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;1:4–9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parpura V, Liu F, Jeftinija KV, Haydon PG, Jeftinija SD. Neuroligand-evoked calcium-dependent release of excitatory amino acids from Schwann cells. J Neurosci. 1995;15(8):5831–9. doi: 10.1523/JNEUROSCI.15-08-05831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Piani D, Frei K, Do KQ, Cuenod M, Fontana A. Murine brain macrophages induce NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- [50].Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. JPET. 2003;306(1):387–93. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- [51].Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408(6815):985–90. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- [52].Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22(11):4740–5. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ro JY, Nies M, Zhang Y. The role of peripheral N-methyl-D-aspartate receptors in muscle hyperalgesia. Neuroreport. 2005;16:485–489. doi: 10.1097/00001756-200504040-00013. [DOI] [PubMed] [Google Scholar]
- [55].Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res. 2007;1184:141–8. doi: 10.1016/j.brainres.2007.09.085. [DOI] [PubMed] [Google Scholar]
- [56].Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- [59].Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28(19):4904–17. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007;581:631–47. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994;63(4):1529–37. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- [63].Svensson P, Wang K, Arendt-Nielsen L, Cairns BE, Sessle BJ. Pain effects of glutamate injections into human jaw or neck muscles. J Orofac Pain. 2005;19:109–118. [PubMed] [Google Scholar]
- [64].Tang HB, Li YS, Miyano K, Nakata Y. Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons. Neuropharmacology. 2008;55(8):1405–11. doi: 10.1016/j.neuropharm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- [65].Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Varela JA, Hirsch SJ, Chapman D, Leverich LS, Greene RW. D1/D5 modulation of synaptic NMDA receptor currents. J Neurosci. 2009;29:3109–3119. doi: 10.1523/JNEUROSCI.4746-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, Lee CJ. Direct activation of transient receptor potential vanilloid 1 (TRPV1) by diacylglycerol (DAG) Mol Pain. 2008;4:42. doi: 10.1186/1744-8069-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xiaoping G, Xiaofang Z, Yaguo Z, Juan Z, Junhua W, Zhengliang M. Involvement of the spinal NMDA receptor/PKCγ signaling pathway in the development of bone cancer pain. Brain Res. 2010;1335:83–90. doi: 10.1016/j.brainres.2010.03.083. [DOI] [PubMed] [Google Scholar]
- [69].Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–72. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [70].Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34(4):689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]