Abstract
Objective
Valproic acid (VPA), as known as histone deacetylase inhibitor, has neuroprotective effects. This study investigated the histological changes and functional recovery from spinal cord injury (SCI) associated with VPA treatment in a rat model.
Methods
Locomotor function was assessed according to the Basso-Beattie-Bresnahan scale for 2 weeks in rats after receiving twice daily intraperitoneal injections of 200 mg/kg VPA or the equivalent volume of normal saline for 7 days following SCI. The injured spinal cord was then examined histologically, including quantification of cavitation.
Results
Basso-Beattie-Bresnahan scale scores in rats receiving VPA were significantly higher than in the saline group (p<0.05). The cavity volume in the VPA group was significantly reduced compared with the control (saline-injected) group (p<0.05). The level of histone acetylation recovered in the VPA group, while it was significantly decreased in the control rats (p<0.05). The macrophage level was significantly decreased in the VPA group (p<0.05).
Conclusion
VPA influences the restoration of hyperacetylation and reduction of the inflammatory reaction resulting from SCI, and is effective for histology and motor function recovery.
Keywords: Valproic acid, Spinal cord injury, Clip compression model, Acetylation, HDAC inhibitor
INTRODUCTION
Traumatic spinal cord injury (SCI) is a serious and complex medical condition that bestows significant and catastrophic dysfunction and disability35,40,41,51). Therapy has aimed at treatment and even recovery from SCI has been a research goal for a long time. Recent advancements in the knowledge of the pathophysiology, including the potential of stem cell therapy, prepare to take off for recovery from SCI.
SCI pathophysiology consists of two temporally-related events. Initial mechanical trauma results in the direct injury of the neural elements, the primary injury. Secondary injury is based largely on the primary event and a subsequent series of secondary degenerative processes that lead to apoptosis1,2,4,47). Although secondary injury should, in principle, be preventable, no innovative and effective treatment options exist41). To date, only steroids have been clinically approved for the drug-related treatment of SCI. However, at high-doses, steroids may produce complications which can be detriment to their long-term use8,9,18,23,39). Efforts to develop alternative treatments such as minocycline, erythropoietin, and statins, have focused on the reduction of secondary degeneration and recovery of neurological function. While these alternate compounds have been at least partially effective, questions remain concerning their benefits versus risk11,13,21,31,50,53).
Valproic acid (VPA; 2-propylpentanoic acid) is a well-established drug in the long-term treatment of epilepsy5,22,43). VPA can directly inhibit histone deacetylase (HDAC), which is crucial in histone acetylation regulation, chromatin remodeling, and gene expression22,31,43). VPA-mediated enhanced acetylation of histones H3 and H4 and altered gene transcription19) can alleviate neuron death induced by lipopolysaccharide, excitotoxicity, or aging12,27-29). In addition, VPA-mediated neuroprotection has been demonstrated in various neurodegenerative diseases such as amyotrophic lateral sclerosis, spinal muscular atrophy, middle cerebral artery occlusion, intracerebral hemorrhage, traumatic brain injury, and sciatic nerve axotomy10,14,15,17,24,28,41,45,49).
This amply-demonstrated neuroprotection has spurred interest in VPA as the basis of a novel therapy for neurodegenerative diseases, including SCI36). However, little is known regarding the therapeutic potential of VPA in SCI. The present study employed a rat model of SCI to investigate 1) how the treatment of VPA has effects on the various histological changes including cavitation volumes, histon acetylation, and inflammatory reaction, and 2) whether it also helps the functional recovery after SCI.
MATERIALS AND METHODS
Animal model and drug administration
All animal experiments were performed in accordance with the National Institute of Health guidelines on animal care, and were approved by the Institutional Animal Care Committee. All efforts were made to minimize the number of animals used and animal suffering. Adult male Sprague-Dawley rats weighing 290-310 grams (Samtako Bio, Osan, Korea) were randomly and blindly allocated into three groups (n=12 per group). In group 1 (sham), laminectomy was performed. In group 2 (SCI-VPA), the animals received single doses of VPA (Sigma-Aldrich, St. Louis, MO, USA). In group 3 (SCI-saline), animals received 1.0 mL of the saline vehicle solution. Initially, rats were anesthetized intraperitoneally with a mixture of xylazine (10 mg/kg) and ketamine (60 mg/kg). After laminectomy at T9, the extradural plane between the dura and adjacent vertebrae was carefully dissected. A modified aneurysm clip with a closing force of 30 grams (Aesculap, Tuttlingen, Germany) was held in an applicator in the open position. The clip was rapidly released from the applicator and applied vertically onto the exposed spinal cord for a 2-minute compression. For the sham controls the same surgical procedure was followed, but clip compression was not applied. After surgery, the muscle, fascia, and skin were sutured using a 4-0 silk suture. The rectal temperature was maintained at 37.0±0.5℃ by a thermostatically-regulated heating pad during surgery, and during recovery, animals were placed overnight in a temperature- and humidity-controlled chamber. To reduce post-surgery isolation-induced stress, rats were housed in pairs at an ambient temperature of 22-25℃ in an alternating 12-hour light/dark cycle. Bladders were manually emptied twice daily until spontaneous voiding occurred (usually within 7-10 days). A dose of 200 mg/kg of VPA or normal saline as a vehicle control was intraperitoneally injected twice daily at 12 hours intervals for 7 days. The total daily VPA dose of 400 mg/kg/day was similar to doses used in previous studies15,52). To evaluate histological changes, the animals were sacrificed and the spinal cords were collected 2 weeks after SCI.
Locomotor and behavioral analyses
The rats were tested for functional deficits each week for 2 weeks after the surgery using the open field locomotor rating scale developed by Basso, Beattie, and Bresnahan (the BBB score)3). Two evaluators who were unaware of the group allocations and previous functional scores observed each animal for 1 minute. Functional scores for each hind limb were recorded and averaged.
Histopathological examination
Six rats in three groups were deeply anesthetized by intraperitoneal injection of ketamine prior to decapitation 14 days after SCI. Following decapitation, a 1.5 cm segment of the spinal cord centered at the injury site was immediately harvested from the vertebral canal and postfixed in 10% formalin overnight. The portion of the spinal cord was divided into seven segments at 2-mm intervals from the lesion epicenter, and seven segments were embedded in paraffin. The segments were (6 mm, 4 mm, and 2 mm rostral to the lesion; lesion epicenter; and 2 mm, 4 mm, and 6 mm caudal to the lesion). Seven spinal cords from each of the two injury groups and the sham group were randomly selected. Representative sections were sliced into 5 µm-thick sections on the horizontal plane and stained with hematoxylin-eosin. For quantitative evaluation of spared tissue and cavity areas, 20 sequential slides of the serial sections were obtained from representative segments. The tissues were examined and photographed using a Zeiss Axioplan microscope (Carl Zeiss Meditec Incorporation, Jena, Germany) with high power differential interference contrast (DIC) optics. The images were viewed on a computer monitor using a Zeiss Plan-Apochromat 5x objective and a Zeiss AxioCam HRc digital camera (Carl Zeiss). The area of cavitation and total spared tissue area of each section were traced and measured using Axio vision 4 software (Carl Zeiss). Due to variable shrinkage of the lesion cavity, we chose to measure the area of remaining white and gray matter, in addition to measuring the area of the lesion itself. Any necrotic tissue within the cavities was counted as part of the lesion. The total cavity volume was calculated by a summation of the measured cavity area of each section multiplied by the intersection distance.
Immunohistochemistry (IHC) analysis
Six rats in three groups were deeply anesthetized by an intraperitoneal injection of ketamin and were perfused intracardially with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB, pH=7.4). The thoracic spinal cord was excised, postfixed for 24 hours, and maintained overnight in 30% sucrose in 0.1 M PB. Spinal cord tissues were sectioned at a thickness of 30 µm on a cryostat, and sections were floated on the surface of 0.1 M PB. To detect ED-1 (marker for activated macrophages) and histone acetylation, spinal cord sections were blocked with 4% normal serum in 0.5% Triton X-100 for 1 hour at room temperature and incubated overnight at 4℃ with a 1 : 500 dilution of mouse monoclonal anti-rat ED-1 (Serotec, Oxford, UK) and a 1 : 1000 dilution of polyclonal anti-rat acetyl-histone H3 (K9, Ac-H3/K9; Cell Signaling Technology, Danvers, MA, USA) and a 1 : 1000 dilution of polyclonal anti-rat acetyl-histone H3 (K18, Ac-H3/K18; Cell Signaling Technology), and rinsed for 3×10 min in 0.1 M PB. Sections were then incubated in 0.1 M PB containing 4% normal serum and 0.5% Triton X-100 for 2 hours at 25℃ on a shaker, and then in primary antiserum in 0.1 M PB containing 4% normal serum and 0.5% Triton X-100 for 12 hours at 25℃. After rinsing (3×10 min) in 0.1 M PB, sections were incubated in a 1 : 200 dilution of biotinylated anti-mouse IgG (Sigma-Aldrich) and a 1 : 200 dilution of anti-rabbit IgG (Vector Laboratory, Burlingame, CA, USA) in 0.1 M PB containing 4% normal serum and 0.5% Triton X-100 at 25℃ for 2 hours. The sections were then incubated in a 1 : 50 dilution of avidin-biotinylated horseradish peroxidase (Vector Laboratory) in 0.1 M PB for 2 hours and rinsed (3×10 min) in 0.25 M Tris. Finally, staining was visualized by reaction with 3, 3'-diaminobenzidine tetrahydrochloride (DAB) and hydrogen peroxide in 0.25 M Tris for 3-10 min using a DAB reagent set (Kirkegaard & Perry, Gaithersburg, MD, USA). All the sections were then rinsed in 0.1 M PB and mounted on Superfrost Plus slides (Fisher, Pittsburgh, PA, USA) and dried overnight at 37℃. The mounted sections were then dehydrated with alcohol, cleared with xylene, and coverslipped with Permount mounting medium (Fisher).
The labeled cells were identified and counted with separation of each antibody from two sites at four tissues in six different animals. The labeled tissues were photographed using a Zeiss Axiopan microscope with high power DIC optics (Carl Zeiss). The images were viewed on a computer monitor using a Zeiss Plan-Apochromat 40x objective (Carl Zeiss) and a Zeiss AxioCam HRc digital camera (Carl Zeiss). For comparison, labeled cells were respectively counted in 48 sampled areas in both the gray and white matter (each 250×250 µm field). Enumeration of immune-positive cells used a Labworks, version 4.5, computer-assisted image analyzer (UVP, Upland, CA, USA).
Statistical analysis
All statistical comparisons were computed using SPSS 17.0 (SPSS, Chicago, IL, USA). Data are expressed as mean±standard error of the mean (SEM). Repeated measure ANOVA was used to compare groups. Significance was accepted for p-values <0.05.
RESULTS
Locomotor and behavioral analysis
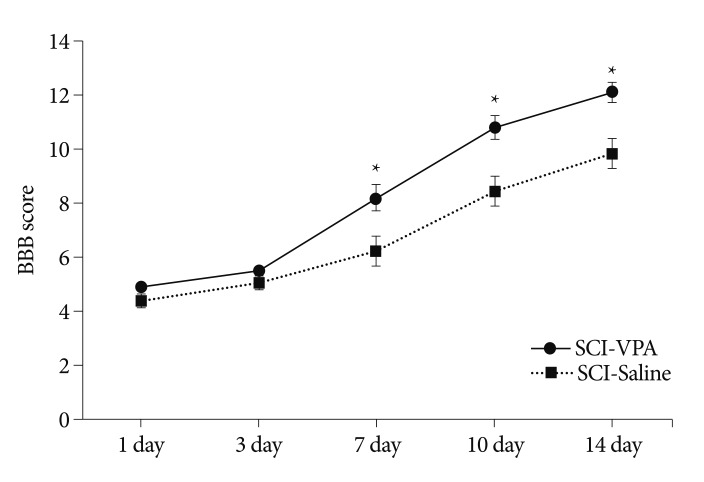
SCI in rats was followed by an injection regimen of VPA or saline (n=12/group). The injured rats were assessed for 2 weeks after surgery according to the open field motor testing using the BBB locomotor rating scale (Fig. 1). While all rats exhibited severe functional impairment the week following SCI, the motor function of the VPA-injected rats was markedly better than their saline-injected counterparts. The average BBB scores (mean±SEM) of the saline-injected rats were 4.33±0.27 on day 1, 5.06±0.21 on day 3, 6.22±0.54 on day 7, 8.44±0.53 on day 10, and 9.83±0.56 on day 4. The corresponding BBB scores of the VPA-injected rats were 4.9±0.19, 5.45±0.19, 8.20±0.47, 10.80±0.42, and 12.10±0.34. A difference in BBB scores at day 7 between the two groups was significant (p<0.05).
Fig. 1.
Neurological function of rats after SCI between VPA- and saline-injected groups, assessed by the BBB locomotor rating scale. VPA improved functional recovery after SCI. The error bars indicate the SEM. *p<0.05 on 7, 10, and 14 day (n=12/group). SCI : spinal cord injury, VPA : valproic acid, SEM : standard error of the mean, BBB : Basso, Beattie, and Bresnahan.
Lesion cavity
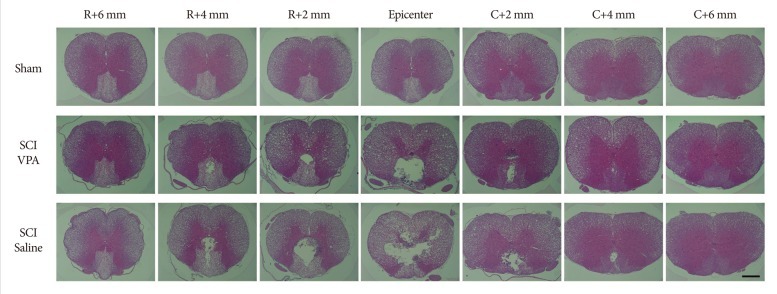
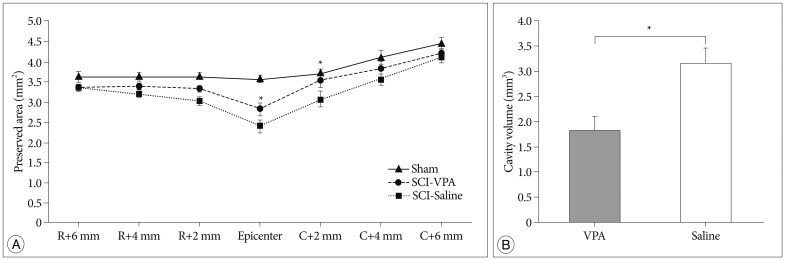
Two weeks following SCI, histological examination of the injured spinal cords revealed a central cavity with severe necrosis at the lesion epicenter. The lesions extended to over 4 mm rostrally and caudally, tapering gradually to cavities affecting the central and dorsal areas of the spinal cord gray and white matter (Fig. 2). In VPA-treated rats, the area of the preserved tissue was significantly increased compared to that of the rats that received saline (Fig. 3A). The spared area (µm2) of the spinal cord was 3.64±0.13 at 6 mm rostral section from epicenter, 3.65±0.10 at rostral 4 mm, 3.65±0.07 at rostral 2 mm, 3.57±0.08 at epicenter, 3.71±0.08 at caudal 2 mm, 4.09±0.19 at caudal 4 mm, and 4.43±0.15 at caudal 6 mm in the control rats. In VPA-treated rats, the corresponding values were 3.37±0.05, 3.40±0.70, 3.35±0.08, 2.84±0.16, 3.56±0.19, 3.81±0.11, and 4.19±0.11 µm2. In case of the saline-injected groups, the corresponding values were 3.37±0.09, 3.23±0.11, 3.05±0.11, 2.39±0.16, 3.08±0.21, 3.53±0.13, and 4.09±0.13 µm2. The cavitation volume was 3.17±0.28 µm3 and 1.83±0.27 µm3 in the saline- and VPA-treated group, respectively. The difference was significant (p<0.05) (Fig. 3B)
Fig. 2.
Representative spinal cord sections stained with hematoxylin and eosin showing the cavities at 2 weeks after T9 clip compression injury taken at the epicenter and at 2 mm increments rostral and caudal in a rat receiving saline vehicle and a rat treated five times with valproic acid (VPA). Larger cavitation is evident in saline-injected group than the VPA-injected group, a progressive diminishment with distance from the epicenter of the lesion. Scale bar=500 µm; 5× magnification. SCI : spinal cord injury.
Fig. 3.
VPA improves spinal cord tissue sparing after SCI. A : Measurements of the average area of preserved cord tissues at the injury epicenter and adjacent sections at an interval of 2 mm up to 6 mm rostrally and caudally. B : Histogram showing the cavitation volume of the spinal cord lesion in both groups. There was a considerable reduction of the cavity volumes in the VPA-treated group compared to the saline-injected group. The error bars indicate SEM. *p<0.05 for VPA-injected groups vs. saline-injected groups after SCI. R : rostral, C : caudal (n=6/group), SCI : spinal cord injury, VPA : valproic acid, SEM : standard error of the mean.
IHC analysis
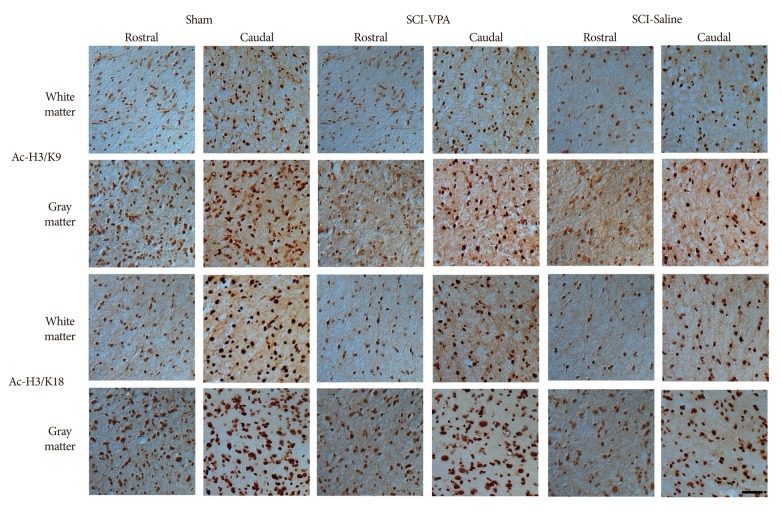
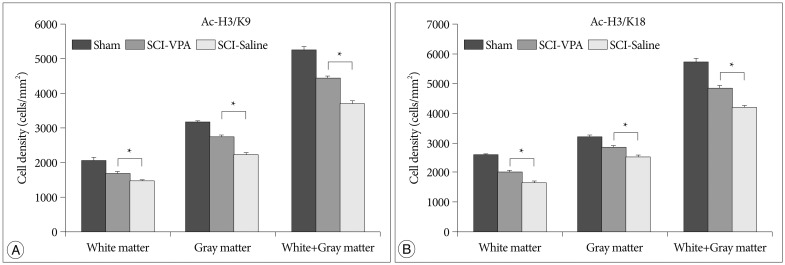
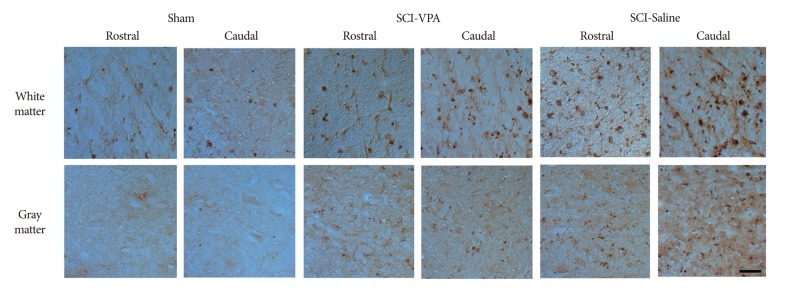
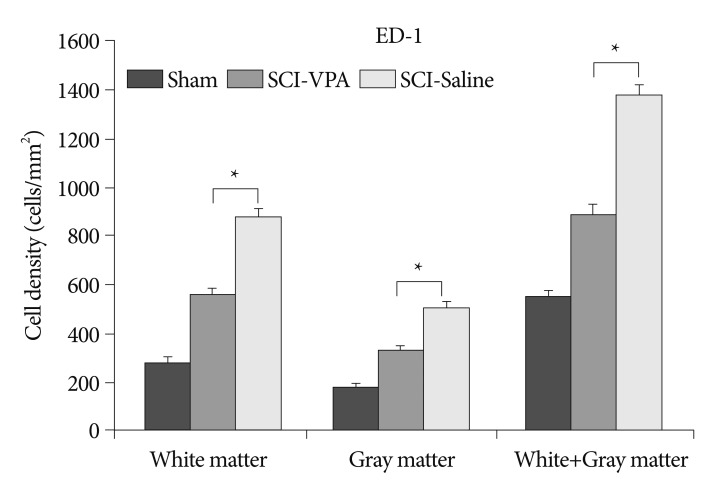
SCI causes significant hyperacetylation of histones. However, VPA injection significantly alleviated reduction of Ac-H3/K9, and Ac-H3/K18 at the 6 mm rostral and caudal segments from the lesion sites (Fig. 4, 5). Separate analysis of the gray and white matter revealed a quantitatively similar level of histone acetylation in the same group, but a significantly dissimilarity between the treated and control rats (p<0.05). In addition, decreased immunoreactivity of the ED-1 macrophage marker was evident in the VPA-injected group, while immunoreactivity was pronounced in the control group (Fig. 6, 7). Furthermore, the separate analyses of the gray and white matter revealed no disparity of immunoreactivity, although the number of ED-1 immunoreactive cells was considerably dissimilar in VPA-treated and control rats (p<0.05). Within 4 mm rostral and caudal from the injury site, expression of histone acetylation was too low and ED-1 expression was too great to allow comparison of the two groups. Besides, injured spinal cords had injury-induced cavities <4 mm from each epicenter. Thus, we compared IHC staining at distances further removed from the injury sites (6 mm).
Fig. 4.
Representative photographs of histone acetylation immunoreactive cells from SCI to sham animals at 6 mm both rostral and caudal to the lesion epicenter, 40×. There is a significantly restoration of the immunoreactivity of histone acetylation at both white and gray matter in VPA-injected group while in saline-injected groups, there are hyperacetylation at both white and gray matter. Scale bar=50 µm; 40× magnification. SCI : spinal cord injury, VPA : valproic acid.
Fig. 5.
Histogram of the quantification of the histone acetylation immunoreactive cells showing that VPA treatment increases the number of histone acetylation immunoreactive cells at 6 mm both rostral and caudal to the lesion epicenter. A : Acetyl-histon H3/K9. B : Acetyl-histon H3/K18. The error bars indicate SEM. *p<0.05 (n=6/group). SCI : spinal cord injury, VPA : valproic acid, SEM : standard error of the mean.
Fig. 6.
Representative photographs of ED-1 immunoreactive cells from SCI to sham animals at 6 mm both rostral and caudal to the lesion epicenter, 40×. Considerable decline of the immunoreactivity of ED-1 is evident in both white and gray matter in VPA-injected groups, while in saline-injected groups high immunoreactivity of ED-1 is evident. Scale bar=50 µm; 40× magnification. SCI : spinal cord injury, VPA : valproic acid.
Fig. 7.
Histogram of the quantification of the ED-1 immunoreactive cells showing that VPA treatment decreases the number of ED-1 immunoreactive cells at 6 mm both rostral and caudal to the lesion epicenter. The error bars indicate SEM. *p<0.05 (n=6/group). SCI : spinal cord injury, VPA : valproic acid, SEM : standard error of the mean.
DISCUSSION
Traumatic SCI results in durable or permanent neurological deficiencies in motor and sensory systems35,51). In addition, patients with traumatic SCI are at great risk of substantial morbidity and mortality. Alleviation of SCI has been one of the grand challenges in modern medical science. A variety of morphologic changes occurs after acute SCI, including petechial hemorrhage in the gray matter, small ruptures in the venules, increased size of the extracellular spaces in the gray and white matter, and an enlarged periaxonal space47). To overcome permanent damage after SCI, it is of paramount importance to cease the successive secondary injuries and restore the damaged spinal cord neural networks. Even stem cell therapy progresses from the theoretical to the possible, the restoration of an impaired neural network could remain technically elaborate and difficult. Thus, minimizing the secondary injury that drives rampant apoptosis is crucially important to overcome SCI. Drugs such as minocycline, erythropoietin, and statins are involved with neuroprotection in animal models of SCI11,21,34,50,53). The drug's effects relate mainly to apoptosis signaling, the core of the secondary injury after SCI. However, until recently, the capability of these drugs to diminish secondary injury has been unknown.
The present study, VPA considerably promoted functional recovery after SCI. Minutes to hours after SCI, the lesion is thought to spread centripetally, initially by the induction of necrotic cell death, with cavitation occurring. These events likely influence the serious dysfunction that results from SCI4,47). Also, it is reported that the amount of spared spinal cord tissue has been shown to be closely relevant to functional recovery after SCI6,55). Various trials have sought to diminish the cavitation caused by SCI. Erythropoietin, Nogo-66 receptor antagonist, and minocycline produce less scar tissue and tissue cavitation after SCI21,32,33,36,53). In present study, VPA also significantly diminished the cavitation volume resulting from SCI. Cavitation volume of VPA-injected groups was decreased approximate 42.27% compared to saline-injected groups. In particular, the cavitation volume was markedly decreased within both rostral and caudal 2 mm from the epicenter. We also confirmed that VPA considerably promotes functional recovery after SCI.
VPA is a well-established treatment for epilepsy and bipolar disorder43,46). Remodeling of chromatin is crucial in gene expression regulation, and results from the alteration of the acetylation and deacetylation of histone N-terminal tails, which interact between histones and DNA molecules in chromatin26,31). In general, hyperacetylation is intimately linked with repression. HDAC inhibitors such as VPA may modulate the expression of downstream target genes by regulating the activities of hyperacetylated transcription factors, which are non-histone substrates of HDAC7), providing the basis for VPA-mediated neuroprotection. In vitro, VPA can preserve rat cortical neurons form glutamate-induced excitotoxicity24) and hippocampal neurons from oxygen-glucose deprivation injury44). In addition, VPA has been linked with the prolonged life span of cultured cortical neurons27). Neuronal protection afforded by VPA treatment might be mediated through the extracellular signal-regulated kinase pathway and via the inhibition of proapoptotic molecules42). VPA involvement in neuroprotective genes such as Hsp70 and Bcl-2 has been described16,45,48,54). Since over-expression of the latter genes is associated with protection from cerebral ischemia20,30,37,56,57) and SCI49,57), the cellular neuroprotective mechanism of VPA is likely due to the upregulation of Hsp70 and Bcl-2 gene activity. Hsp70 over-expression ameliorates neurological deficits induced by transient focal ischemia45), which may be affiliated with the inhibition of the cytochrome c-dependent activation of caspase-338) and the attenuation of inflammation25).
The present results corroborate the findings of previous studies. The complex mechanisms related to VPA influence the reduction of secondary injury after SCI. Presently, VPA-treated rats displayed restored levels of histone acetylation compared to the control rats. In the case of Ac-H3/K9, this group showed a decline of only 15.4% from the sham group, while the saline-injected rats displayed a decline of 29.52% from the sham group. In the case of Ac-H3/K18, a decline of 15.72% was evident, while the saline-injected rats displayed a decline of 27.04%. VPA-treated rats also displayed decreased quantity of macrophages. This group showed an increase of 63.41% from the sham group, while rats treated only with saline displayed a far higher increase of 154.86%. The results indicate that VPA mitigates hyperacetylation and inflamatory reaction after SCI.
The use of various bioactive agents, neurotrophic factors, transplanted neuro-cellular, and other tissues for efficacy to limit the amount of secondary damage or promote healing and regeneration of the injured spinal cord have been studied. However, the complex mechanisms of healing and regeneration have proved very challenging to overcome. In the present study, VPA-treated rats displayed recovered cavitation volume and motor function. In addition, a high level of histone acetylation and decreased macrophage level was evident in VPA-treated rats, compared to saline-treated rats.
In conclusion, we investigated the effects of VPA, a HDAC inhibitor involved with secondary damage, to minimize secondary injury and promote the damaged motor functions after SCI. The results demonstrate the potential of VPA, which should spur further study.
CONCLUSION
VPA treatment enhances functional recovery after SCI by diminishing cavitation volume inflammatory reactions, and restoring the histon acetylations in injured spinal cords. VPA should be evaluated for use for clinical trials of SCI.
References
- 1.Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. [PubMed] [Google Scholar]
- 2.Balentine JD. Pathology of experimental spinal cord trauma. II. Ultrastructure of axons and myelin. Lab Invest. 1978;39:254–266. [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 4.Beattie MS. Inflammation and apoptosis : linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Blaheta RA, Nau H, Michaelis M, Cinatl J., Jr Valproate and valproate-analogues : potent tools to fight against cancer. Curr Med Chem. 2002;9:1417–1433. doi: 10.2174/0929867023369763. [DOI] [PubMed] [Google Scholar]
- 6.Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989;91:15–34. doi: 10.1016/0022-510x(89)90073-7. [DOI] [PubMed] [Google Scholar]
- 7.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 8.Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 9.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 10.Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006;59:970–975. doi: 10.1002/ana.20836. [DOI] [PubMed] [Google Scholar]
- 11.Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho DC, Cheong JH, Yang MS, Hwang SJ, Kim JM, Kim CH. The effect of minocycline on motor neuron recovery and neuropathic pain in a rat model of spinal cord injury. J Korean Neurosurg Soc. 2011;49:83–91. doi: 10.3340/jkns.2011.49.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui SS, Yang CP, Bowen RC, Bai O, Li XM, Jiang W, Zhang X. Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats. Brain Res. 2003;975:229–236. doi: 10.1016/s0006-8993(03)02699-4. [DOI] [PubMed] [Google Scholar]
- 15.Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE. 2010;5:e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- 17.Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155:567–572. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 19.Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I, et al. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res. 2009;65:35–43. doi: 10.1016/j.neures.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury : a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons : involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 25.Heneka MT, Gavrilyuk V, Landreth GE, O'Banion MK, Weinberg G, Feinstein DL. Noradrenergic depletion increases inflammatory responses in brain : effects on IkappaB and HSP70 expression. J Neurochem. 2003;85:387–398. doi: 10.1046/j.1471-4159.2003.01694.x. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Jeong MR, Hashimoto R, Senatorov VV, Fujimaki K, Ren M, Lee MS, et al. Valproic acid, a mood stabilizer and anticonvulsant, protects rat cerebral cortical neurons from spontaneous cell death : a role of histone deacetylase inhibition. FEBS Lett. 2003;542:74–78. doi: 10.1016/s0014-5793(03)00350-8. [DOI] [PubMed] [Google Scholar]
- 28.Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004;4:336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- 29.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 30.Khalatbary AR, Tiraihi T, Boroujeni MB, Ahmadvand H, Tavafi M, Tamjidipoor A. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010;1306:168–175. doi: 10.1016/j.brainres.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 31.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system : histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 32.Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. J Surg Res. 2011;166:280–289. doi: 10.1016/j.jss.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Nalivaeva NN, Belyaev ND, Turner AJ. Sodium valproate : an old drug with new roles. Trends Pharmacol Sci. 2009;30:509–514. doi: 10.1016/j.tips.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia : Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium. 2004;36:303–311. doi: 10.1016/j.ceca.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79:340–350. doi: 10.1002/jnr.20345. [DOI] [PubMed] [Google Scholar]
- 40.Penas C, Verdú E, Asensio-Pinilla E, Guzmán-Lenis MS, Herrando-Grabulosa M, Navarro X, et al. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. 2011;178:33–44. doi: 10.1016/j.neuroscience.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez M, Rojo AI, Wandosell F, Díaz-Nido J, Avila J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochem J. 2003;372:129–136. doi: 10.1042/BJ20021596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 44.Rekling JC. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci Lett. 2003;335:167–170. doi: 10.1016/s0304-3940(02)01193-x. [DOI] [PubMed] [Google Scholar]
- 45.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats : potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- 46.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 47.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies : promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 48.Shin YC, Choi KY, Kim WG. Cyclosporin A has a protective effect with induced upregulation of Hsp70 and nNOS on severe spinal cord ischemic injury in rabbits. J Invest Surg. 2007;20:113–120. doi: 10.1080/08941930701235833. [DOI] [PubMed] [Google Scholar]
- 49.Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;26:464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Stepień K, Tomaszewski M, Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005;57:561–569. [PubMed] [Google Scholar]
- 51.von Euler M, Seiger A, Sundström E. Clip compression injury in the spinal cord : a correlative study of neurological and morphological alterations. Exp Neurol. 1997;145:502–510. doi: 10.1006/exnr.1997.6481. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia : the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 54.Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008;210:531–542. doi: 10.1016/j.expneurol.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 55.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11(Suppl 1):13–22. [PubMed] [Google Scholar]
- 56.Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, et al. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- 57.Yune TY, Park HG, Lee JY, Oh TH. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]