Abstract
BACKGROUND
Green tea extract (GTE) has been shown to have antioxidative properties due to its high content of polyphenols and catechin gallates. Previous studies indicated that catechin gallates scavenge free radicals and attenuate the effects of reactive oxidative species (ROS). Cyclophosphamide (CP) produces ROS, which can have adverse effects on development, causing limb, digit, and cranial abnormalities. The current study was performed to determine if exposure to GTE can decrease teratogenic effects induced by CP in CD-1 mice.
METHODS
From gestation days (GD) 6–13, mated CD-1 mice were dosed with 400 or 800 mg/kg/d GTE; 100, 200, 400, or 800 mg/kg/d GTE + CP; CP alone, or the vehicle. GTE was given by gavage. CP (20 mg/kg) was given by intraperitoneal injection on GD 10. Dams were sacrificed on GD 17, and their litters were examined for adverse effects.
RESULTS
The highest GTE dose did not effectively attenuate, and in some cases exacerbated the negative effect of CP. GTE alone was also associated with an increased incidence of microblepharia. Conversely, moderate GTE doses (200 &/or 400 mg/kg/d) attenuated the effect of CP on fetal weight and (GTE 200 mg/kg/d) decreased the incidences of certain defects resulting from CP exposure.
CONCLUSIONS
Exposure of a developing mammal to moderate doses of GTE can modulate the effects of exposure to CP during development, possibly by affecting biotransformation, while a higher GTE dose tended to exacerbate the developmental toxicity of CP. GTE alone appeared to cause an adverse effect on eyelid development.
Keywords: green tea extract, cyclophosphamide, mice, development, teratogenicity, epigallocatechin-3-gallate, EGCG
INTRODUCTION
Exposure of the embryo or fetus to excessive amounts of oxidative stress, whether from exposure to drugs such as cyclophoshamide (CP) or from a maternal disease such as diabetes, is known to produce physiological abnormalities leading to in utero death or structural birth defects (Dicke, 1989; Wells et al., 2009). It is estimated that 10% of all birth defects are caused by prenatal exposure to a teratogen (Dicke, 1989). However, exposure to antioxidants, such as the catechins and polyphenols in green tea extract (GTE), may reduce the incidence and/or severity of oxidative stress.
CP is a drug commonly used to treat neoplastic disease and some autoimmune diseases. It is also a well-known and well-studied teratogen, causing a variety of birth defects in the fetuses of pregnant women treated with the drug (Latorre et al., 2007). Previous studies in rodents have shown that exposure to CP during organogenesis caused fetal resorption, limb, digit, head, and growth abnormalities, and skeletal malformations (Mirkes, 1985; Park et al., 2009).
In order to cause teratogenesis, CP must be bioactivated through a process involving oxidase enzymes that convert it into its active metabolites, phosphoramide mustard and acrolein (Kram et al., 1980; Mirkes, 1985). Phosphoramide mustard acts to inhibit DNA synthesis and causes cross-links in the existing DNA, resulting in cell death, and acrolein is thought to be responsible for some of the side effects of CP chemotherapy, such as cystitis. Although the mechanism of teratogenesis is still debated, it is believed that generation of reactive oxygen species (ROS) through these metabolites plays a role in CP-induced malformations (Mirkes, 1985; Hansen, 2006).
Free radicals or reactive oxidative species (ROS) are by-products of the breakdown of many drugs(Wells et al., 2009). For a thorough review of ROS generation, see Hansen (2006). An overabundance of ROS in the body causes a condition known as oxidative stress (Ornoy, 2007; Wells et al., 2009). The exposure of the embryo or fetus to ROS is normally carefully timed so that exposure occurs when antioxidant levels are also high, potentially decreasing the duration of the ROS signaland enabling the cell to repair damage to its DNA (Hansen, 2006; Dennery, 2007; Wells et al., 2009). However, exposure to excessive levels of ROS without sufficient antioxidant presence can cause brain and spinal cord defects, embryonic death, or skeletal malformations (Wells et al., 2009).
Oxidative stress can be prevented by antioxidants known to be effective in vitro for protection against conditions associated with oxidative damage through radical scavenging (Frei and Higdon, 2003; Navarova et al., 2005; Ornoy, 2007; Wells et al., 2009). Some antioxidants, such as glutathione and melatonin, are produced in the body; however, many, such as vitamins C and E, are obtained through dietary supplements or food (Chu et al., 2007; Fang et al., 2007).
In recent years, green tea extract (GTE) has gained mainstream attention for its anti-inflammatory, antibacterial, antioxidative, antiviral, and neuroprotective effects. The health-promoting effects of GTE have largely been attributed to its high concentration of polyphenols, particularly flavanols, which represent 30% of its dry leaf weight, and many of the benefits are attributed to the most abundant catechin, (−)-epigallocatechin-3-gallate (EGCG) (Picard, 1996; Chacko et al., 2010).
The chemical composition of green tea is complex. The major flavonoids of green tea are various catechins, which are found in greater amounts in green tea than in either black or oolong tea (Chacko et al., 2010). Green tea contains (+)-catechin (C), (−)-epicatechin (EC), (−)-gallocatechin (GC), (−)-epigallocatechin (EGC), (−)-catechin gallate (CG), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), and gallocatechin gallate (GCG) (Chu et al., 2006). The gallic acid ester EGCG is present in the highest concentration, comprising over 61% of the epicatechin derivatives by dry leaf weight. Caffeine makes up an additional 3%, along with trace amounts of theophylline, theobromine, and an amino acid unique to tea, theanine (Picard, 1996).
The antioxidant defense system includes enzymes such as SOD and catalase (Chacko et al., 2010). These agents are key elements in reducing molecular damage by ROS (Chacko et al., 2010). Intake of GTE increases the activity of SOD in serum, the expression of catalase in the aorta, and total plasma antioxidant activity in rats (Skrydlewska et al., 2002; Chu et al., 2006), and these enzymes are implicated in protection against ROS (Picard, 1996). Malondialdehyde, an oxidative stress marker, has also been observed to decrease in concentration following intake of GTE by rats (Yokozawa et al., 2002).
It is not unreasonable to expect that GTE could reduce the frequency and/or the severity of fetal abnormalities induced by a teratogen such as CP, as other studies have shown that CP-induced fetal malformations can be attenuated by antioxidants, including glutathione, cysteine, and indole-3-carbinol (Slott and Hales, 1987; Han et al., 1995; Winn and Wells, 1995; Bailey et al., 2005). Data regarding effects of GTE exposure prior to CP exposure during gestation have not been reported to date in mice. Thus the current study was conducted to determine if GTE would protect against the adverse effects of agents such as CP and if GTE might itself have any untoward effects during pregnancy.
MATERIALS AND METHODS
Animals and husbandry
Male and female CD-1 mice, obtained from Charles River Breeding Laboratories (Portage, MI), were housed in a USDA-approved animal facility in rooms maintained at 25 ± 2°C at 50% to 70% humidity with 12/12 hour light/dark cycles. Following a two-week acclimation period, animals were bred naturally, two females with one male. Observation of a copulatory plug designated gestation day (GD) 0. Mated females were individually housed in shoe-box type cages with recycled bedding and had ad libitum access to Harlan Teklad Rodent Diet (Harlan Teklad, Indianapolis, IN) and tap water. At least 20 mated females were assigned to each group, resulting in a minimum of 16 pregnancies (Table 1). Experiments were all approved by the Emporia State University Animal Care and Use Committee and not initiated until approval was granted (permit #ESU-ACUC-09-018).
Table 1.
Maternal and Litter Parameters of Mice Treated During Gestation With Cyclophosphamide (CP) With or Without Exposure to Green Tea Extract (GTE)
| Parameter
| ||||||
|---|---|---|---|---|---|---|
| Treatment and Dose (mg/kg/day) | Fetuses/litters examined* | Maternal weight gain (g ± SEM) | Fetalweight (g ± SEM) | Implantations (mean ± SEM) | Resorbed or dead fetuses (% ± SEM) | Litters with resorbed or dead fetuses [No.(%)] |
| Vehicle Control | 488/39 | 10.40 ± 0.47 | 1.01 ± 0.01 | 12.79 ± 0.39 | 1.37 ± 0.54 | 7 (18) |
| GTE 400 | 239/19 | 8.78 ± 0.64a | 1.07 ± 0.05 | 12.70± 0.39 | 1.71 ± 1.00 | 3 (16) |
| GTE 800 | 256/20 | 11.81 ± 0.80 | 1.05 ± 0.03 | 12.80 ± 0.79 | 0.55 ± 0.55 | 1 (5) |
| CP 20 | 430/40 | 10.75 ± 0.40 | 0.56 ± 0.02abd | 12.05 ± 0.41 | 14.37 ± 3.57ab | 23 (58) |
| GTE 100 + CP 20 | 236/20 | 10.86 ± 0.42 | 0.59 ± 0.04abd | 13.00 ± 0.63 | 8.35 ± 3.19 | 9 (45) |
| GTE 200 + CP 20 | 156/19 | 9.01 ± 0.80 | 0.73 ± 0.07abcefg | 11.25 ± 0.66 | 16.95 ± 7.39ab | 6 (32) |
| GTE 400 + CP 20 | 213/16 | 9.16 ± 0.49 | 0.59 ± 0.03abd | 12.26 ± 0.40 | 9.52 ± 3.91 | 7 (44) |
| GTE 800 + CP 20 | 195/20 | 10.44 ± 0.50 | 0.54 ± 0.04abd | 11.40 ± 0.78 | 16.30 ± 5.21ab | 11 (55) |
Live fetuses
Significantly different from vehicle control (P≤0.05).
Significantly different from GTE 400 and GTE 800 (P≤0.05).
Significantly different from GTE 100 + CP 20 (P≤0.05).
Significantly different from GTE 200 + CP 20 (P≤0.05).
Significantly different from GTE 400 + CP 20 (P≤0.05).
Significantly different from GTE 800 + CP 20 (P≤0.05).
Significantly different from CP 20 (P≤0.05).
Test Chemicals
Cyclophosphamide monohydrate (CP) was purchased from Sigma-Aldrich (St. Louis, MO). Green tea extract (GTE) was purchased from Health Genesis (Miami, FL). CP was dissolved in 0.9% saline to yield the appropriate concentrations for dosing. GTE was suspended in DI water to yield appropriate concentrations. All solutions were prepared daily at the time of dosing.
Treatments
The CP dose administered was based on the findings of a range-finding study (Lewis et al., 2000), in which a dose of 20 mg/kg was found to induce a wide range of malformations without causing embryo lethality or obvious maternal toxicity. Timing of CP administration was based on the results of that study and on the review by Mirkes (1985), which noted that CP administration in mice between GD 10 and 12 can cause dysmorphic effects.
Previously reported pharmacokinetic studies of catechins in rodents used widely differing dosages (between 50 and 1000 mg/kg of GTE) (Chen et al., 1997; Nakagawa and Miyazawa, 1997; Kim et al., 2000; Natsume et al., 2003; Chu et al., 2007), with EGCG showing the highest concentration of all the catechins in fetal tissues (Chu et al., 2007). A moderate dose of 400 mg/kg GTE was chosen as a starting dose and then was halved and doubled in order to evaluate a dose-dependency between CP teratogenicity and GTE supplementation. GTE powder was analyzed by HPLC at Emporia State University and was found to contain 0.1454 mg EGCG per mg. A 400 mg/kg/d GTE dose therefore provided 58.16 mg/kg/d EGCG.
Mated females were randomly assigned to one of eight treatment groups: 1) vehicle control (DI water), 2) 400 mg/kg/d GTE, 3) 800 mg/kg/d GTE, 4) 100 mg/kg/d GTE + 20 mg/kg CP, 5) 200 mg/kg/d GTE + 20 mg/kg CP, 6) 400 mg/kg/d GTE + 20 mg/kg CP, 7) 800 mg/kg/d GTE + 20 mg/kg CP, or 8) 20 mg/kg CP. GTE (suspended in DI water) and DI water were administered via gavage on GD 6–13. CP (dissolved in saline) was given by intraperitoneal (i.p.) injection on GD 10. This study was conducted in four sequential replicates.
Data Collection
On GD 17, pregnant females were euthanized by CO2 overdose (an AVMA-approved method). The uteri of pregnant dams were exposed, and the numbers of resorptions and dead or live fetuses were recorded. Live fetuses were removed from the uterus, weighed individually, and examined for gross malformations, such as cranial, limb, tail, or digit defects. Maternal body weight, minus the gravid uterine weight, was recorded. Litters were initially fixed in 70% ethanol and then cleared and stained by a double-staining technique described by Webb and Byrd (1994). Subsequently, fetuses were examined for skeletal malformations.
Data analysis
The litter was used as the experimental unit for statistical analysis. Malformations were grouped as follows, according to the affected anatomical area of the fetus: head (exencephaly or exencephalocele); tail (short or bent); talipes; limb (phocomelia or meromelia); digit (adactyly, brachydactyly, oligodactyly, oligosyndactyly, polydactyly, polysyndactyly, or syndactyly), macroglossia; eye (ablepharia or microblepharia); anasarca; or gastroschisis. The data from each study replicate were calculated independently, tested for homogeneity of variance by the Levene statistic, using SPSS (SPSS, Inc., Chicago, IL), and then pooled and analyzed to give the results reported. All tabular data are presented as the mean ± SEM. Data from each replicate were analyzed by one-way analysis of variance (ANOVA), followed by an LSD post-hoc test to determine specific differences (p ≤ 0.05).
RESULTS
Maternal Data
Maternal weight gain was significantly lowered in dams exposed to GTE 400 mg/k/d compared to control animals (p ≤ 0.05); however, this effect was not dosage-dependent, as dams in the GTE 800 mg/kg/d treatment group were unaffected (Table 1). No clinical signs of maternal toxicity were noted among any of the treatment groups.
Fetal Data
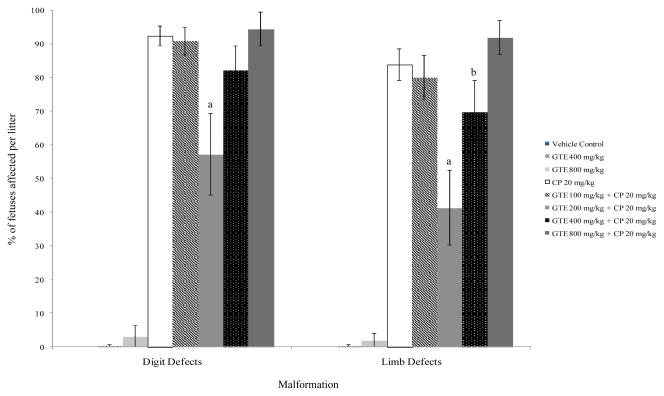
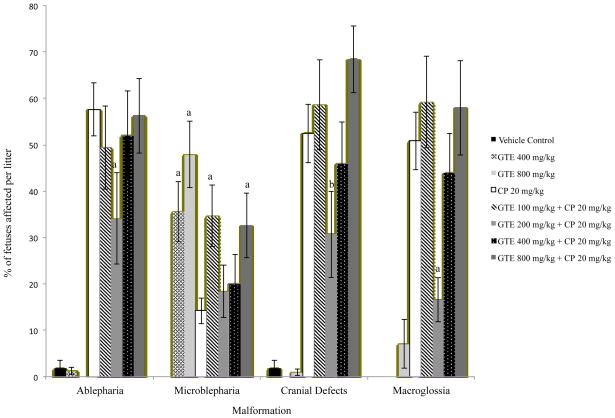
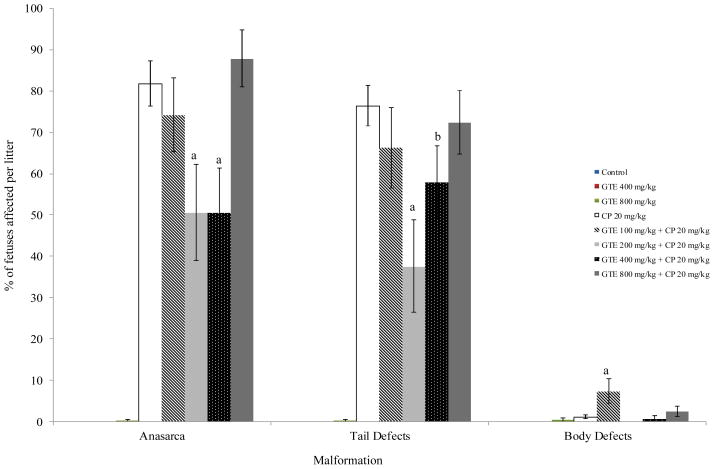
GTE alone did not have an effect on the incidence of prenatal demise, but treatment groups exposed to 20 mg/kg/d CP only, GTE 200 + CP, or GTE 800 + CP experienced a significant increased incidence in that parameter in comparison with the vehicle and GTE controls (p ≤ 0.05) (Table 1). GTE alone did not affect fetal weight as compared to the vehicle control. Fetuses in the GTE 200 mg/kg/d + CP group weighed significantly more than those from dams exposed to CP only. The litter mean percentages of fetuses displaying limb or tail defects in the GTE 200 mg/kg/d + CP and GTE 400 mg/kg/d + CP were significantly lower than those of fetuses in the CP only positive control group (p ≤ 0.05) (Figure 1). Treatment with 200 mg/kg/d GTE reduced the incidence of limb defects by over 50% (41% vs. 84% in the CP-only group). The incidence of digit and cephalic defects and macroglossia were also significantly lowered by exposure to GTE 200 mg/kg/d (p ≤ 0.05) (Figures 1 and 2). Exposure to either 200 or 400 mg/kg/d GTE prior to CP administration significantly lowered the incidence of anasarca compared to CP exposure only (p ≤ 0.01) (Figure 3). There was no significant reduction in the occurrence of talipes among any of the treatment groups (data not shown). A significantly higher percentage of fetuses in the GTE 100 mg/kg/d + CP group was affected by body wall defects (p ≤ 0.01) than was the case in all other treatment groups, but this effect did not appear to be dosage-dependent (Figure 3).
Fig. 1.
Incidence of digit and limb malformations in mouse fetuses following maternal exposure to cyclophosphamide (CP) during gestation, with or without exposure to green tea extract (GTE). aSignificantly different from CP 20 (P≤0.01); bSignificantly different from CP 20(P≤0.05).
Fig. 2.
Incidence of Cephalic Malformations in Mouse Fetuses Following Maternal Exposure to Cyclophosphamide (CP) During Gestation, With or Without Exposure to Green Tea Extract (GTE). aSignificantly different from CP 20 (P≤0.01); bSignificantly different from CP 20(P≤0.05).
Fig. 3.
Incidence of tail and body malformations in mouse fetuses following maternal exposure to cyclophosphamide (CP) during gestation, with or without exposure to green tea extract (GTE). aSignificantly different from CP 20 (P≤0.01); bSignificantly different from CP 20(P≤0.05).
The percentage of fetuses displaying ablepharia was significantly reduced by exposure to 200 mg/kg/d GTE compared to CP only (p ≤ 0.01). A significantly higher occurrence of microblepharia was observed in all GTE treatment groups compared to the vehicle control (p ≤ 0.01), and the GTE 400 mg/kg/d and GTE 800 mg/kg/d control groups reflected a possible dose-dependent effect. Only the GTE800 treatment among the GTE + CP combination groups increased the incidence of microblepharia compared to the CP only controls (Figure 2).
Reductions in skeletal malformations (fused or dumbbell-shaped vertebral centra) or variations (supernumerary or rudimentary ribs) were observed in fetuses exposed to GTE + CP compared to fetuses exposed to CP only, but the effects followed no apparent pattern and it is unclear whether the effect is truly biologically meaningful (data not shown).
DISCUSSION
GTE contains large amounts of polyphenols with antioxidant properties, including catechins. An ideal antioxidant should effectively inhibit free radical formation and scavenge free radicals and have a large volume of distribution and a long half-life (Chu et al., 2006). Catechins appear to possess these criteria due to their high reducing ability. They also protect neuronal cells by activation of protein kinase c and ERK1/2 to suppress pro-apoptotic gene expression. Additionally, they have good distribution to peripheral organs and potential for prolonged tissue retention (Chu et al., 2006).
It is not possible to identify which of the active compounds in GTE is responsible for the observed prophylactic effect; however, studies on pregnant rats have shown that EGCG is the most abundant catechin present in fetal tissue following oral administration of GTE, indicating its placental transfer rate is the highest among the catechins (Chu et al., 2006; Chu et al., 2007). Chu et al. (2006) indicated that catechins such as EGCG are eliminated through the bile and reabsorbed from the intestine through enterohepatic re-circulation, and the greater absorption of EGCG in the fetus indicates that it is selectively absorbed and retained. This identifies EGCG as a potential candidate for antioxidant supplementation to the fetus.
CP is bioactivated by P450 enzymes, specifically CYP3A (Klaassen, 2001). Green tea catechins have been found to inhibit CYP3A, with the most effective inhibitor being EGCG (Muto et al., 2001), providing a possible mechanism for influencing CP’s developmental toxicity. CP is detoxified by Phase II enzymes, which promote the excretion of potentially toxic or carcinogenic chemicals. Glutathione-S-transferases (GST) are detoxifying phase II enzymes that reduce the ability of ROS to react with nucleic acids and proteins. Previous rodent studies have indicated supplementation with green tea polyphenols, green tea leaves, and injections of EGCG intravenously increased GST activity in the liver and small intestines (Khan et al., 1992; Lin et al., 1998; Chou et al., 2000). Thus EGCG’s influence on Phase II biotransformation may provide an additional mechanism for modifying the effects of CP.
Along with the possible inhibition of P450 enzymes and the upregulation of GST, the catechins present in GTE have several structures necessary for radical scavenging activity. The structure of every catechin includes at least one ortho-dihydroxyl group in the B ring, which assists in electron delocalization and stability of the oxidizing radical. Gallocatechins and catechin gallates have additional hydroxyl groups that have been associated with increased antioxidant activity (Higdon and Frei, 2003). Other possible mechanisms for the antioxidant activity of flavonoids include chelation of metal ions, scavenging ROS via reactions with their hydroxyl groups, and /or increasing the activity of endogenous antioxidant enzymes (Picard, 1996). Additionally, Chu et al. (2006) reported that catechin gallates were the most readily absorbed of the catechins by the fetus but showed low maternal plasma levels (perhaps due to competition for absorption binding sites or carrier proteins or poorer solubility compared to other catechins). Chu et al’s. 2007 study showed that EGCG was always present in all fetal organs, indicating that its placental transfer rate is the greatest among the catechins. Therefore, it is possible that the major protective mechanism may not only be inhibition of CYP3A, but also significant ROS scavenging in the fetus itself.
GTE did not appear to cause embryotoxicity by itself, as reflected in the incidences of fetal mortality or effects on fetal weight. however, it also did not reduce the embryotoxicity of CP except for an apparent protective effect on fetal weight for the 200 mg/kg GTE dose. The results of this study indicate that moderate dosages of GTE did have protective effects against certain CP-induced malformations, however. For example, pre-treatment with 200 mg/kg/d GTE was effective in reducing the incidences of certain malformations induced by CP injection, including ablepharia and digit, limb, tail, and head malformations. GTE treatment itself, however does appear to have been associated with a specific eyelid defect, microblepharia. The results of this study suggest that there is an optimal dose (200 mg/kg/d) of GTE with regard to protective effects to the conceptus. All of the nuances and mechanisms of CP teratogenesis have not been fully elucidated; it is a complicated drug that manifests its effects on the fetuses in a myriad of ways (Ozolinš, 2010). Ours is the only study to date that has explored the influence of a range of dosages of GTE on CP teratogenesis, as the only other published report employed only a single dosage level (Park et al., 2009). According to the available literature, it appears that the catechins in GTE, primarily EGCG, affect many parts of the cyclophosphamide pathway, from bioactivation to detoxification, so that defining the exact mechanism or mechanisms of its actions is largely speculative.
Many of our findings are in apparent conflict with those of the recent rat study by Park et al. (2009). They found that prior GTE exposure to 100 mg/kg/d administered via gavage from GD 6–12 exacerbated the effects of 11 mg/kg CP administered i.p. on GD 12 on fetal weight, exencephaly, and micrognathia. As that study was performed in rats, it is possible that the difference in species could explain the difference in results from the findings of the current study. Additionally, GTE was administered for three days after CP exposure in our study, providing a longer period of bioavailability for the GTE and possibly allowing more radical scavenging to take place.
Interestingly, the occurrence of microblepharia was associated with both GTE treatment and CP exposure. Further study would be required to determine the mechanism by which eyelid development was delayed by GTE exposure.
The exposure levels of GTE in this study are considerably greater than those resulting from human ingestion of typical dietary supplements. A typical cup of brewed green tea has approximately 100 mg of catechins (Nakagawa et al., 1997). Humans consuming GTE in amounts equivalent to the dosages in the current study would need to consume 7,000 to 56,000 mg of GTE (1,017–4,071 mg of EGCG) daily for an average 70 kg adult. In certain populations where consumption of black or green tea is popular, as many as 10 cups of tea per day may be consumed, but this would still not provide the pharmacological levels of catechins used in this study (Nakagawa et al.,1997).
The exact mechanism(s) by which GTE influences the teratogenic effects of CP is not known at this time. This study demonstrates that exposure to GTE prior, during, and after the administration of a model teratogen could diminish the induction of certain malformations in fetuses. Such results in an animal suggest that pharmacological dosages of GTE might also have protective effects against teratogen-induced developmental defects in human fetuses. Of concern, however, is the occurrence of defects associated with GTE alone, albeit at relatively high exposures.
Acknowledgments
Grant Information: This project was made possible by NIH grant number P20 RR016475 from the INBRE Program of the National Center for Research Resources to Emporia State University. This project was also funded by an Emporia State University Faculty Research and Creativity Grant awarded to Melissa M. Bailey.
The authors would like to thank Erik Calderon, Jennifer Brady, Jill Hulse, Evan Janzen, and Kyle Wells for their invaluable assistance on this project. Many thanks also go to Dr. Malonne Davies in the Department of Physical Sciences at Emporia State University for conducting HPLC analysis on the green tea extract used in this study.
References
- Bailey MM, Sawyer RD, Behling JE, Boohaker JG, Hicks JG, O’Donnell MA, Stringer KR, Rasco JF, Hood RD. Prior exposure to indole-3-carbinol decreases the incidence of specific cyclophosphamide-induced developmental defects in mice. Birth Defects Res B. 2005;74:261–267. doi: 10.1002/bdrb.20046. [DOI] [PubMed] [Google Scholar]
- Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chinese Medicine. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution and elimination of tea polyphenols in rats. Drug Metabol Dispos. 1997;25:1045–1049. [PubMed] [Google Scholar]
- Chou FP, Chu YC, Hsu JD, Chiang HC, Wang CJ. Specific induction of glutathione-S-transferase GSTM2 subunit expression by epigallocatechin gallate in rat liver. Biochem Pharmacol. 2000;60:643–650. doi: 10.1016/s0006-2952(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Chu KO, Wang CC, Chu CY, Choy KW, Pang CP, Rogers MS. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Human Reprod. 2007;22:280–281. doi: 10.1093/humrep/del353. [DOI] [PubMed] [Google Scholar]
- Chu KO, Wang CC, Chu CY, Chan KP, Rogers MS, Choy KW, Pang CP. Pharmacokinetic studies of green tea catechins in maternal plasma and fetuses of rats. J Pharm Sci. 2006;95:1372–1381. doi: 10.1002/jps.20594. [DOI] [PubMed] [Google Scholar]
- Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C. 2007;81:155–162. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- Dicke JM. Teratology: principles and practice. Med Clin N Am. 1989;73:567–582. doi: 10.1016/s0025-7125(16)30658-7. [DOI] [PubMed] [Google Scholar]
- Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223–228. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Han SY, Shin JH, Kwon SC, Kang MO, Lee YM, Kim PG. Effects of enzyme inducers and glutathione on the embryotoxicity of cyclophosphamide in cultured rat embryos. Korean J Toxicol. 1995;11:31–36. [Google Scholar]
- Hansen JM. Oxidative stress as a mechanism of teratogenesis. Birth Defects Res C. 2006;78:293–307. doi: 10.1002/bdrc.20085. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992;52:4050–4052. [PubMed] [Google Scholar]
- Kim S, Lee MJ, Hong JG, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutri Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Casarett and Doull’s Toxicology. 6. New York: McGraw-Hill; 2001. [Google Scholar]
- Kram D, Bynum GD, Senula GC, Bickings CK, Schneider EL. In utero analysis of sister chromatid exchange: alterations in susceptibility to mutagenic damage as a function of fetal cell type and gestational age. PNAS. 1980;77:4784–4787. doi: 10.1073/pnas.77.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre AO, Hueza IM, Gorniak SL. Association of Ipomoea carnea and BCG reduces birth defects caused by cyclophosphamide in rats. Life Sci. 2007;80:430–435. doi: 10.1016/j.lfs.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Lewis EC, Unger AM, Hood RD. Comparison of fetal morphological exam and single cell gel electrophoresis as indicators of developmental toxicity in mouse embryos exposed in utero to cyclophosphamide. Teratology. 2000;61:487. (Abstr.) [Google Scholar]
- Lin YL, Cheng CY, Lin YP, Lau YW, Juan IM, Lin JK. Hypolipidemic effect of green tea leaves through induction of antioxidant and phase II enzymes including superoxide dismutase, catalase, and glutathione-S-transferase in rats. J Agric Food Chem. 1998;46:1893–1899. [Google Scholar]
- Mirkes PE. Cyclophosphamide teratogenesis: a review. Teratog Carcinog Mutagen. 1985;5:75–88. doi: 10.1002/tcm.1770050202. [DOI] [PubMed] [Google Scholar]
- Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Miyazawa T. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J Nutri Sci Vitaminol. 1997;43:679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Okuda S, Miyazawa T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci Biotechnol Biochem. 1997;61:1981–1985. doi: 10.1271/bbb.61.1981. [DOI] [PubMed] [Google Scholar]
- Natsume M, Osakabe N, Oyama M, Sasaki M, Baba S, Nakamura Y, Osawa T, Terao J. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: differences between human and rat. Radic Biol Medic. 2003;34:840–849. doi: 10.1016/s0891-5849(02)01434-x. [DOI] [PubMed] [Google Scholar]
- Navarova J, Ujhazy E, Dubovicky M, Mach M. Phenytoin induced oxidative stress in pre- and postnatal rat development: effect of Vitamin E on selective biochemical variables. Biomed Pap. 2005;149:325–328. doi: 10.5507/bp.2005.051. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ozolinš TRS. Cyclophosphamide and the Teratology Society: an awkward marriage. Birth Defects Res B. 2010;89:289–299. doi: 10.1002/bdrb.20255. [DOI] [PubMed] [Google Scholar]
- Park D, Jeon JH, Shin S, Joo SS, Kang DH, Moon SH, Jang MJ, Cho YM, Kim JW, Ji HJ, Ahn B, Oh KW, Kim YB. Green tea extract increases cyclophosphamide-induced teratogenesis by modulating the expression of cytochrome P-450 mRNA. Reprod Toxicol. 2009;27:79–84. doi: 10.1016/j.reprotox.2008.11.058. [DOI] [PubMed] [Google Scholar]
- Picard D. The biochemistry of green tea polyphenols and their potential application in human skin cancer. Altern Med Rev. 1996;1:31–42. [Google Scholar]
- Skrydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–238. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- Slott VL, Hales BF. Enhancement of embryotoxicity of acrolein, but not phosphoramide mustard, by glutathione depletion in rat embryos in vitro. Biochem Pharmacol. 1987;36:2019–2025. doi: 10.1016/0006-2952(87)90503-x. [DOI] [PubMed] [Google Scholar]
- Webb GN, Byrd RA. Simultaneous differential staining of cartilage and bone in rodent fetuses: an Alcian blue and alizarin red S procedure without glacial acetic acid. Biotech Histochem. 1994;69:181–185. doi: 10.3109/10520299409106284. [DOI] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJJ, Perstin J, Preston TJ, Wiley MJ, Wong AW. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- Winn LM, Wells PG. Free radical-mediated mechanisms of anticonvulsant teratogenicity. Eur J Neurol. 1995;2:5–29. [Google Scholar]
- Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002;50:3549–3552. doi: 10.1021/jf020029h. [DOI] [PubMed] [Google Scholar]