Abstract
Background
Higher Lp-PLA2 activity is associated with increased risk of coronary heart disease (CHD), making Lp-PLA2 a potential therapeutic target. PLA2G7 variants associated with Lp-PLA2 activity could evaluate whether this relationship is causal.
Methods and Results
A meta-analysis including a total of 12 studies (5 prospective, 4 case-control, 1 case-only and 2 cross-sectional, n=26,118) was undertaken to examine the association of: (i) LpPLA2 activity vs. cardiovascular biomarkers and risk factors and CHD events (two prospective studies; n=4884); ii) PLA2G7 SNPs and Lp-PLA2 activity (3 prospective, 2 case-control, 2 cross-sectional studies; up to n=6094); and iii) PLA2G7 SNPs and angiographic coronary artery disease (2 case-control, 1 case-only study; n=4971 cases) and CHD events (5 prospective, 2 case-control studies; n=5523). Lp-PLA2 activity correlated with several CHD risk markers. Hazard ratio for CHD events top vs. bottom quartile of Lp-PLA2 activity was 1.61 (95%CI: 1.31, 1.99) and 1.17 (95%CI: 0.91, 1.51) after adjustment for baseline traits. Of seven SNPs, rs1051931 (A379V) showed the strongest association with Lp-PLA2 activity, VV subjects having 7.2% higher activity than AAs. Genotype was not associated with risk markers, angiographic coronary disease (OR 1.03 (95%CI 0.80, 1.32), or CHD events (OR 0.98 (95%CI 0.82, 1.17).
Conclusions
Unlike Lp-PLA2 activity, PLA2G7 variants associated with modest effects on Lp-PLA2 activity were not associated with cardiovascular risk markers, coronary atheroma or CHD. Larger association studies, identification of SNPs with larger effects, or randomised trials of specific Lp-PLA2 inhibitors are needed to confirm/refute a contributory role for Lp-PLA2 in CHD.
Keywords: genetics, epidemiology, risk factors, Mendelian randomization
Introduction
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is synthesised in macrophages and activated platelets, transported in the circulation on HDL and LDL cholesterol particles, and present in atheromatous plaques. Its effects could be proatherogenic, through the generation of lysophosphatidylcholine and oxidized fatty acids, or antiatherogenic through the hydrolysis of the pro-inflammatory mediator, platelet-activating factor and its analogues formed upon oxidation of LDL [reviewed in1].
To date, more than 25 prospective cohort studies (both of general populations or individuals with established coronary heart disease (CHD)) have reported a consistent association of circulating Lp-PLA2 mass or activity with an increased risk of CHD. A literature-based meta-analysis of 14 studies reported an odds ratio (OR) of 1.21 (95%CI: 1.11, 1.32) for a 1 standard deviation increase in the Lp-PLA2 mass and activity. 2 Lp-PLA2 is also associated with other cardiovascular risk factors. For example, individuals with higher levels of Lp-PLA2 tend to be older, to have higher levels of total-cholesterol, LDL cholesterol, triglycerides, apolipoprotein (apo) B, higher blood pressure and body mass index, and lower levels of HDL-cholestero.l3,4 However, it is uncertain whether one or more of these associated cardiovascular risk factors should be considered to be mediators or confounders of the Lp-PLA2-CHD association.
Darapladib, the first specific inhibitor of Lp-PLA2 activity, reduced the volume of the necrotic core of atheromatous plaque in a pig model 5 and in a randomised trial in humans where intravascular coronary ultrasound was used to image coronary atheroma. 6 This effect was achieved without an alteration in blood lipids. The effect of darapladib on cardiovascular events is currently being addressed in the ongoing STABILITY trial (NCT00799903), a phase-III outcome trial that will recruit up to 15,500 individuals.
In this study we investigated the relationship between common variants at PLA2G7 (encoding Lp-PLA2) with Lp-PLA2 activity, cardiovascular risk factors, angiographic CHD, as well as coronary events in a large collaborative analysis of European populations, exploiting the randomised allocation of alleles7 to better understand the nature of the association between Lp-PLA2 and CHD.
Methods
Study populations
Twelve studies were included in the analysis (full details from each study are provided in supplementary methods and supplementary Table 1). Ethical approval from relevant ethical committees was obtained for all studies.
Prospective studies
Four of the 5 prospective cohort studies used population-based sampling, the Second Northwick Park Heart Study (NPHS-II) 8, the European Prospective Investigation into Cancer and Nutrition study, Norfolk-UK component (EPIC-Norfolk)9, the Edinburgh Artery Study (EAS)10 and the Cyprus study,11 while the Whitehall-II Study (WH-II) was workplace based12. In EPIC-Norfolk, the analysis was based on a nested subset of CHD cases and controls matched for age, sex and study enrolment date.
Case-control studies
The Hypercoagulability and Impaired Fibrinolytic function MECHanisms predisposing to MI (HIFMECH) study is a European multicentre case-control study of MI. A total of 527 cases (non-fatal events) and 566 controls were available for genotyping.13 AtheroGene 3 had 1318 individuals with coronary artery disease (CAD) defined as a diameter stenosis ≥ 30% in at least one major coronary artery and a total of 485 healthy individuals. In the Ludwigshafen Risk and Cardiovascular Health Study (LURIC) a CAD case was defined as having ≥ 20% coronary stenosis in at least one of 15 coronary segments; a control was free of angiographic evidence of coronary stenosis (731 controls and 2581 cases).14 Information from the Wellcome Trust Case-Control study on CHD infarction (WTCCC-CHD) was requested for SNPs in strong linkage disequilibrium with the SNP rs1051931. This study contributed a total of 1988 CHD cases and 3004 controls.15
Case-only studies
For the Southampton Atherosclerosis Study (SAS) 1164 individuals with stenosis ≥ 50% in at least one major epicardial coronary artery were included for genotyping. For SAS, the NPHS-II prospective study was utilised as the control population for SNP-disease associations. Both studies are UK based from similar geographical areas of recruitment.16
Cross-sectional studies
The UCL DiAbetes and Cardiovascular Disease Study (UDACS) aimed to evaluate cardiovascular risk factors for CHD in subjects with type 2 diabetes T2D. Here only individuals of European descent (n=424) without CHD were included.17 For the THROMBOgenic Factors and Recurrent Coronary Events Study (THROMBO)18 of MI survivors, 529 individuals with DNA available were included in this analysis. Blood markers (including Lp-PLA2 activity) included in the THROMBO study and presented here were determined two months after the index MI. For the current analyses only the baseline information was utilised, since coronary events were recurrent instead of incident events.
Data collection
Following a pre-determined analysis plan, information was obtained on Lp-PLA2 activity, cardiovascular risk factors, PLA2G7 variants and cardiovascular events from 11 participating studies within the collaboration. Unified definitions of all relevant variables utilised in the analysis were used. For a sub-set of pre-defined variables (Lp-PLA2 activity, C-reactive protein, and triglycerides) natural-logarithmic transformations were used and these variables were analysed in the log-scale. Covariates included in the different multivariate models utilised to calculate risk of CHD were coded homogenously across all the studies. We used study specific definitions for cardiovascular events (See supplementary Table 1). Studies were then divided in two categories; those with CHD events as an outcome (EPIC-Norfolk, NPHS-II, WH-II, EAS, Cyprus, HIFMECH and WTCCC-CHD) and those with coronary artery disease defined by coronary angiography as an outcome (SAS, AtheroGene and LURIC). All the analyses were limited to individuals of identified European descent.
Laboratory analyses
Information on Lp-PLA2 activity was available from six of the studies. In order to reduce inter-study variability, plasma Lp-PLA2 activity in AtheroGene, EPIC-Norfolk, NPHS-II, UDACS and Cyprus, was determined in a single laboratory (Dr Ewa Ninio at INSERM UMRS937, Paris) using a radiometric assay as reported previously.3,19 For these studies a pool of plasma (always the same) was used to correct the data for inter-assay variability. For the THROMBO study, plasma Lp-PLA2 activity was determined by a commercial colorimetric assay (Cayman Chemical Co.) with 2-thio-PAF as substrate and according to the manufacturer’s directions18. For the LURIC study, Lp-PLA2 activity was measured by use of the Azwell Auto PAF-AH reagent set (Azwell). 20 The within-assay variability was <10%.
Genotyping of single nucleotide polymorphisms in the PLA2G7 gene
Within the NPHS-II study, 12 tagging SNPs (tSNPs) were identified in PLA2G7 based on a minor allele frequency (MAF) threshold >0.04 and r2 >80% (Supplementary Figure 1). In addition to rs1051931 (which encodes A379V) the following SNPs: rs974670, , rs1421378, rs1805017 (R92H) and rs9381475 were chosen for further investigation on the basis of initial association of Lp-PLA2 activity in NPHS-II (Supplementary Table 2) and the recent report by Sutton et al., which examined the association of PLA2G7 tSNPs and CHD risk.21 Genotype data on rs 1051931 was available for 11 studies. The SNPs rs974670, rs1421378, rs1805017 and rs9381475 were previously genotyped in EPIC-Norfolk, EAS, Cyprus, AtheroGene, UDACS and THROMBO. In addition, SNPs rs10948300 and rs2216465, were already genotyped in UDACS and EPIC-NORFOLK. For the WTCCC-CHD we utilize the SNP rs9472819 which is in complete LD (r2=1) with rs1051931. Details of the SNPs, genotyping methods, and MAFs are summarised in Table 1.
Table 1.
PLA2G7 SNPs included in the analyses.
| rs_number | NPHS-II | UDACS | EPIC-Norfolk | THROMBO | Cyprus | Atherogene | LURIC | WH-II | HIFMECH | EAS | SAS | WTCCC* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| † | n= 2725 | n= 424 | n= 4350 | n= 529 | n= 783 | n= 1776 | n= 3312 | n= 5592 | n= 1093 | n= 1098 | n= 1164 | n= 4992 |
| rs974670 | ||||||||||||
| HWE (P-value) | 0.55 | 0.12 | 0.51 | 0.13 | 0.17 | |||||||
| MAF | 0.40 | 0.39 | N/A | 0.36 | 0.26 | 0.38 | N/A | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs1805017 | ||||||||||||
| HWE (P-value) | 0.71 | 0.69 | 0.06 | 0.37 | 0.51 | 0.60 | 0.70 | |||||
| MAF | 0.27 | 0.24 | 0.25 | 0.29 | 0.31 | 0.22 | 0.26 ‡ | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs9381475 | ||||||||||||
| HWE (P-value) | 0.48 | 0.52 | 0.51 | 1.00 | 0.17 | 0.88 | ||||||
| MAF | 0.22 | 0.19 | 0.20 | 0.21 | 0.23 | 0.19 | N/A | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs10948300 | ||||||||||||
| HWE (P-value) | 0.80 | 1.00 | 0.59 | |||||||||
| MAF | 0.22 § | 0.17 | 0.20 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs2216465 | ||||||||||||
| HWE (P-value) | 0.69 | 0.28 | 0.03 | |||||||||
| MAF | 0.33 § | 0.28 | 0.32 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs1421378 | ||||||||||||
| HWE (P-value) | 1.00 | 0.68 | 0.04 | 0.10 | 0.20 | 0.92 | ||||||
| MAF | 0.4 § | 0.38 | 0.38 | 0.41 | 0.48 | 0.36 | N/A | N/A | N/A | N/A | N/A | N/A |
|
| ||||||||||||
| rs1051931 | ||||||||||||
| HWE (P-value) | 0.35 | 0.05 | 0.58 | 0.45 | 0.28 | 0.08 | 0.91 | 0.89 | 0.04 | 0.64 | 0.92 | 0.33 |
| MAF | 0.18 | 0.21 | 0.19 | 0.22 | 0.24 | 0.24 | 0.2 ‡ | 0.19 | 0.23 || | 0.19 | 0.19 # | 0.2 ** |
All genotyping was done using TaqMan assays unless otherwise stated.
WTCCC-CHD: the information correspond to the SNP rs9472819, which is in complete LD (r2=1) with rs1051931;
Number of DNA samples available, although genotype numbers might differ;
,RFLP/PCR;
Custom designed Illumina Bead Chip;
allele specific PCR ;
Affymetrix 500,
Statistical analyses
Association between Lp-PLA2 activity and CHD was assessed in the prospective NPHS-II and EPIC-Norfolk studies and pooled using random effects models. Within each study a hazard ratio (NPHS-II) and odds ratio (EPIC-Norfolk) and 95%CI for CHD by quartiles of Lp-PLA2 activity was obtained, using the bottom quartile as the reference group. Progressive adjustment for potential confounders was made in three separate models as follows: model 1 included age (continuous) and sex, plus enrolment date in EPIC-Norfolk and practice centre in NPHS-II. Model 2 included these variables together with BMI (continuous), smoking (current-ex vs. never), diabetes (yes vs. no), systolic blood pressure (continuous), fibrinogen (continuous), C-reactive protein (continuous log-transformed) and alcohol (continuous as units/week) and model 3 included all preceding covariates with the addition of total cholesterol (continuous), triglycerides (continuous log-transformed), Apo-B (continuous) and Apo-AI (continuous). Since complete genotype and phenotype data was not available for all 11 studies, the number included in the analysis of the relationship between PLA2G7 genotype to Lp-PLA2 activity, PLA2G7 genotype and other intermediate traits, and PLA2G7 genotype and CHD/angiographic CAD events differs. We detail for each analysis, the number of studies and individuals included. The mean difference in log-Lp-PLA2 activity for each PLA2G7 SNP was obtained from 7 studies (NPHS-II, EPIC-Norfolk, Cyprus, UDACS, and THROMBO, and for AtheroGene and LURIC only the control group was used in this analysis) including up to 6094 individuals. The effect on cardiovascular risk factors of each PLA2G7 SNP was evaluated in all studies except SAS and WTCCC-CHD (10 studies and 13,544 individuals).
The association between PLA2G7 SNPs and CHD events was assessed in 10 studies (NPHS-II, EPIC-Norfolk, WH-II, HIFMECH, Edinburgh Artery, Cyprus, LURIC, AtheroGene, SAS and WTCCC-CHD) a total of up to 10494 cases. The cross-sectional studies, UDACs and THROMBO, were not included in this analysis. Within each study a log-OR and standard error adjusted for age (continuous) and sex was obtained for each genotype comparison: subjects heterozygous vs. individuals homozygous for the common-allele (reference group), as well as those homozygous for rare-allele vs. those homozygous for the common-allele. For WTCCC-CHD study, genotype counts were utilised to calculate an unadjusted OR for the different genotype comparisons. For all previous analyses, results across studies were pooled using random effects models. No imputations for missing phenotype of genotype data was conducted. The outcome under evaluation (angiographic CAD vs. CHD events), and study design (prospective vs. case-control), were evaluated as potential sources of heterogeneity by meta-regression. With the combined dataset, comprising 4971 cases of angiographic CAD, 5523 CHD events and 15,624 controls we had 95% power at p=0.001 to detect a per allele odds ratio of 1.2 (http://pngu.mgh.harvard.edu/~purcell/gpc/). 22
Results
Lp-PLA2 activity, cardiovascular risk factors and risk of coronary heart disease
In two prospective studies (NPHS-II and EPIC-Norfolk) including 1030 cases and 3852 controls, BMI, blood pressure, total-cholesterol, LDL-cholesterol, Apo-B and triglycerides were all higher by quartiles of increasing Lp-PLA2 activity. By contrast, Lp-PLA2 activity was inversely correlated with HDL-cholesterol and Apo-AI. No clear associations were observed with the emerging cardiovascular risk factors fibrinogen and C-reactive protein (Figure 1).
Figure 1.
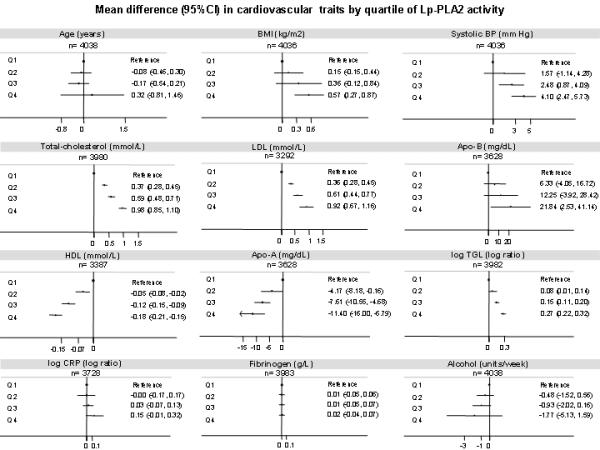
Mean differences in cardiovascular traits by quartiles of Lp-PLA2 activity in NPHS-II and EPIC-Norfolk. Subjects from the bottom quartile (Q1) were used as the reference group. Footnote: This analysis are based on data from the control group of the EPIC-Norfolk study (n = 1760) and the all participants from the prospective study NPHS-II (n= 2278) with the relevant information.
Higher Lp-PLA2 activity was associated with a graded increase in risk of CHD with no evidence of a threshold (Left panel, Figure 2). Individuals in the top quartile had a hazard ratio for CHD of 1.61 (95%CI: 1.31, 1.99) compared with individuals from the bottom quartile in a model adjusted for age, sex, and enrolment date (Model-1). The magnitude of the Lp-PLA2-CHD association slightly diminished with additional adjustment for BMI, smoking, diabetes, systolic blood pressure, C-reactive protein, fibrinogen and alcohol consumption (Model 2, HR of 1.56 (95%CI 1.24, 1.96) . When additional adjustment was made for total-cholesterol, Apo-B, Apo-AI and triglycerides, (Model 3) the hazard ratio for CHD fell further to 1.17 (95%CI: 0.91, 1.51) in individuals in the top quartile for Lp-PLA2 activity (Right panel, Figure 2).
Figure 2.
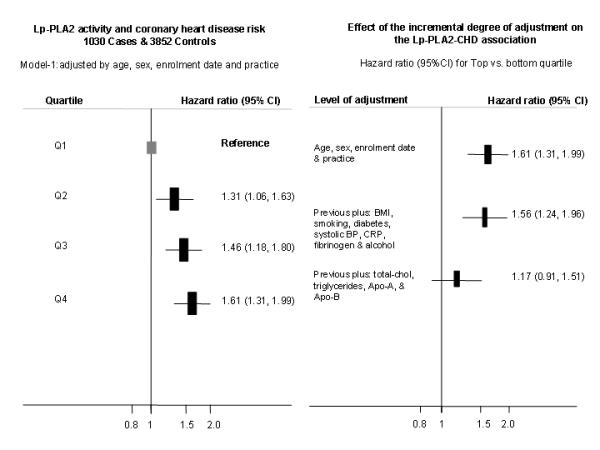
Hazard ratio (HR) of coronary heart disease risk by quartiles of Lp-PLA2 activity in NPHS-II and EPIC-Norfolk. Left panel describe the shape of the association using the minimally adjusted model (model-1). Right panel describe the effect on the HR (top vs. bottom quartile) of progressive levels of adjustment. Footnote: Hazard ratios for all the quartile with the different level of adjustment are reported in supplementary Table-4.
PLA2G7 variants
Of the 4 SNPs where genotype data was available from the 12 studies ( Table 1), only on four occasions was a borderline lack of Hardy Weinberg Equilibrium observed, with p=0.05 to 0.03. Since by chance alone we would expect 2 SNPs not to be in HWE, the borderline deviation from Hardy Weinberg proportions are unlikely to affect the pooled analysis.
PLA2G7 variants and Lp-PLA2 activity
The following studies, NPHS-II, EPIC-Norfolk, Cyprus, UDACS, AtheroGene, LURIC and THROMBO contributed information on one or more of seven PLA2G7 SNPs and Lp-PLA2 activity levels (expressed as a log-ratio) (Figure 3).
Figure 3.
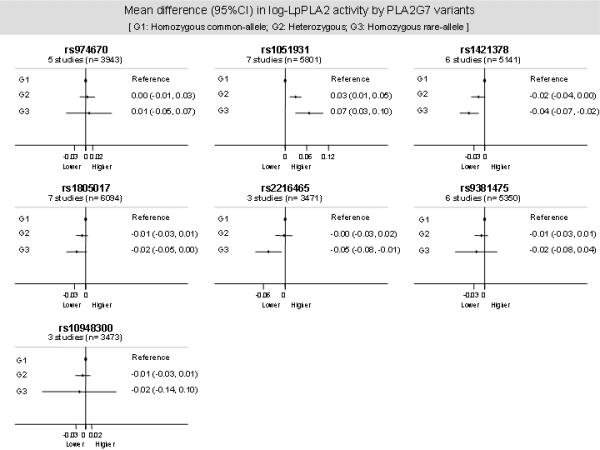
Mean differences by genotype in the Lp-PLA2 activity levels according the PLA2G7 variants evaluated. Data pooled from up to 7 studies (NPHS-II, EPIC-Norfolk, Cyprus, UDACS, AtheroGene, LURIC and THROMBO) including up to 6094 individuals.
Using individuals homozygous for the common allele in each case as the reference group (G1), we observed a statistically significant additive increase in Lp-PLA2 activity in individuals carrying rs1051931 (A379V, n=5801). Compared to those homozygous for the common allele (AA), individuals homozygous for the rare allele (VV) had a relative difference in Lp-PLA2 activity of 7.2%, and heterozygous subjects (AV) had a 3% relative difference. A trend was observed towards an inverse association with Lp-PLA2 activity for the following gene-variants: rs1421378 (n=5141), rs1805017 (R92H; n=6094) and rs2216465 (n=3471). None of these variants exhibited strong linkage disequilibrium (LD) (r2 <0.2) with the rs1051931. A null association with the levels of Lp-PLA2 activity was observed for the variants rs974670, rs9381475 and rs10948300 (Figure 3). For all previous analyses, the exclusion of the THROMBO study did not modify the results (data available on request).
PLA2G7 variants and cardiovascular risk factors
SNP rs1051931 with the largest effect on Lp-PLA2 activity was not associated with any of the cardiovascular risk factors correlated with Lp-PLA2 activity itself, despite a large dataset for BMI (n=4036), systolic blood pressure (n=4036), LDL-cholesterol (n=3292) and HDL-cholesterol (n=3387) (Figure 4). We observed no effect of the rs1051931 variant on additional cardiovascular risk factors such as diastolic blood pressure, glucose, and homocysteine (data available on request).
Figure 4.
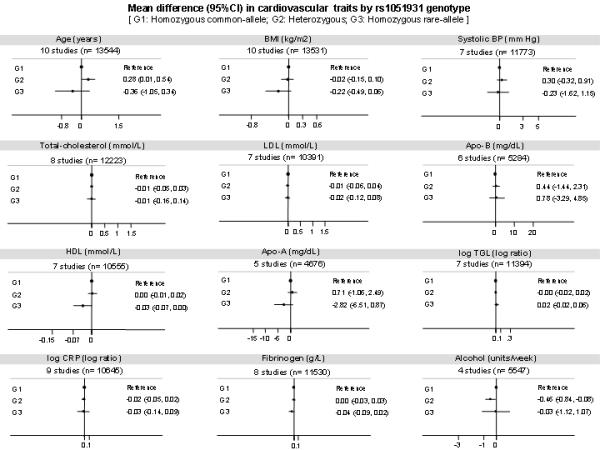
Mean differences in cardiovascular risk factors by rs1051931 genotype according to the PLA2G7 variants evaluated. Data pooled from up to 10 studies (all studies excluding SAS and WTCCC-CHD) and 13,544 individuals. The X-axes from the previous plots are the same as those in Figure 1, in order to increase visual comparability.
PLA2G7 gene variants and risk of coronary heart disease
In pooled analysis of data from 10 studies (NPHS-II, EPIC-Norfolk, Cyprus, AtheroGene, LURIC, THROMBO, WH-II, SAS, EAS and WTCCC-CHD) , with the total number of combined outcomes ranging from 1627 to 10,494, there was no clear association of any of the seven PLA2G7 variants with risk of CHD (Figure 5a), including rs1051931 (10,494 events) which was consistently associated with a modest difference in Lp-PLA2 activity (see Figure 3). The null association of the rs1051931 variant was preserved where CHD events and angiographically evaluated coronary artery disease were analysed separately. No significant heterogeneity was observed according to the outcome evaluated (CHD event vs. angiographic CAD) or according to study design (prospective vs. case-control). All P-values, derived from meta-regression, for the different genotype comparisons were > 0.1 (Figure 5b).
Figure 5a.

Relative odds of CHD associated with PLA2G7 variants. Data pooled from up to 10 studies (NPHS-II, EPIC-Norfolk, WH-II, HIFMECH, Edinburgh Artery, AtheroGene, LURIC, Cyprus, SAS and WTCCC-CHD) including up to 10,494 CHD events.
Figure 5b.
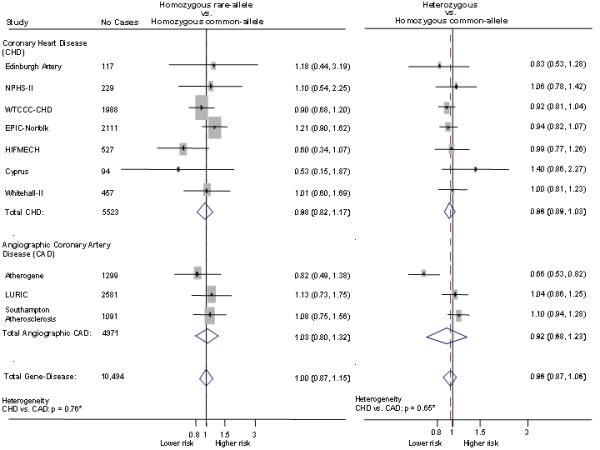
Effect of the rs1051931 variant on the risk of CHD stratified by outcome. CHD: Coronary heart disease includes fatal and non-fatal events. Specific definitions used within each studies are described in methods section. Angiographic-CAD: coronary artery disease defined by coronary angiography, specific study definitions reported in methods section. * P-values for heterogeneity between outcome (CHD vs. angiographic-CAD) were calculated by meta-regression.
Discussion
In this analysis of pooled data from 12 studies with 26,118 participants we identified a clear association of Lp-PLA2 activity with risk of CHD, but the effect size was sensitive to the degree of adjustment for co-variables including blood lipids, with which Lp-PLA2 activity is also associated. Several PLA2G7 variants that were associated with Lp-PLA2 activity, did not exhibit any association with a wide range of established and novel cardiovascular risk factors, suggesting that their association with CHD events or angiographic CHD should be less prone to confounding. Despite some of the variants showing association with Lp-PLA2 activity, none showed a clear association with angiographic coronary disease or CHD events. However, this null result needs to be interpreted in the light of the relatively weak influence of these SNPs on Lp-PLA2 activity, the available sample size and therefore the statistical power of the study.
The association of Lp-PLA2 activity with CHD risk was modified by the adjustment for other cardiovascular risk factors in a multivariate analysis with the attenuation in effect size being most marked when blood lipids were entered into the model. This involves a judgement on which variables should be considered as potential mediators of the association and which as confounders. Confounding factors should be balanced among groups of individuals categorised by genotype for common SNPs in PLA2G7 that affect Lp-PLA2 activity, because genotype is determined by randomised allocation at conception. We therefore identified variants in PLA2G7 associated with Lp-PLA2 activity and studied their effect on other cardiovascular risk factors and CHD events. A careful selection of the PLA2G7 SNPs was conducted to minimise the likelihood of a false-negative finding due to inadequate selection of genetic tools. This included the identification of common tag-SNPs for European-descent individuals which were initially evaluated in the NPHS-II study, and whose results were then complemented with published-evidence on the effects of PLA2G7 variants on Lp-PLA2 activity and disease risk21, enabling a final selection of the seven variants utilised in the genetic component of the analysis. Of the seven SNPs used, four (rs1051931, rs1421378, rs1805017 and rs2216465) exhibited association with Lp-PLA2 activity (Figure 3), although the magnitude of these associations was small to moderate. Indeed, rs1051931 which showed the strongest association, displayed only a relative difference on Lp-PLA2 activity of 7.2% and 3% for rare allele homozygotes and heterozygotes, respectively (see Figure 3); the other variants showed associations of similar or lower magnitude.
Lp-PLA2 activity itself was correlated with a range of blood lipids in the current analysis, and the findings are consistent with those of the Framingham Heart Study.23 However, we found that rs1051931, which displayed the largest effect on Lp-PLA2 activity, showed no association with blood lipids. This observation is consistent with data from the two short-term randomised trials in humans using the Lp-PLA2 inhibitor, darapladib.6,24 Even though Lp-PLA2 activity was reduced by about 60% (at a dose of 160 mg daily), darapladib did not modify the concentrations of the lipid particles (LDL-, HDL-cholesterol or triglycerides). These findings in combination suggest that blood lipids may be confounder rather than mediators for the association between Lp-PLA2 activity and CHD risk.
In the current study, variants that affect Lp-PLA2 activity were not associated with coronary atheroma detected by angiography. The absence of an effect on the degree of coronary stenosis could be consistent with the experimental studies in a pig model of diabetic hyperlipidaemia and a human trial of darapladib.5,6,24 In the pig study, 24 weeks treatment with darapladib led to only a modest reduction in coronary lesion size, but treated animals showed a significant alteration in their plaque composition with a smaller necrotic core populated by fewer inflammatory cells suggesting that darapladib treatment may reduce plaque composition and vulnerability, independent of blood lipid changes. Similarly, in the human studies of darapladib, there were detectable reductions in the area of necrotic core of the athermatous plaques in coronary arteries in patients receiving the drug.24 The hypothesis that this may translate to a reduction in plaque rupture and clinical events is now being tested in the STABILITY trial.
There was no clear signal for an effect of PLA2G7 variants on CHD, despite the inclusion of more than 10,494 cases (Figure 5a, b). However, given the small to modest effect of common alleles at the PLA2G7 locus on Lp-PLA2 activity, and considering the strength of the association of Lp-PLA2 activity and CHD risk (Figure 2), a per-allele OR as low as 1.05 (which we were insufficiently powered to detect) could still be compatible with a contributory role of Lp-PLA2 to CHD. Other genetic studies examining this question have yielded inconsistent findings.25,26 In a Taiwanese study of MIs before 45yrs, the rare allele of rs1051931 was associated with increased risk of an event (OR= 1.66 [95%CI: 1.14.1, 5.80]), despite being associated with lower Lp-PLA2 activity, while the V279F variant showed no association with enzyme activity or risk of MI.27 In a case-control study in Chinese subjects of the seven SNPs in PLA2G7 studied singly or as haplotypes, only the promoter SNP rs13210554 (not included in our analysis) was associated with increased CHD risk, while the V279F variant and rs1805018 (but not rs1805017 or rs1051931) showed association with lower Lp-PLA2 activity but were not associated with CHD risk.28 However, Sutton et al, reported a significant association of PLA2G7 variants, including rs1805017 and rs1051931 with CHD risk in two multi-ethnic studies.21
Lp-PLA2 activity levels are stable among healthy subjects or patients with stable coronary disease.29 It is important to consider whether other genetic variants might help delineate the mechanisms underlying the association between Lp-PLA2 activity and CHD. Genetic variants other than in PLA2G7 itself, in the genes MEF2A, CXCL12, VEGF, PTGIS and CD44 (presumably acting in trans) may influence Lp-PLA2 activity23. We also previously reported that variants in the APOE cluster explained ~4% of the variance in Lp-PLA2.30 However, variants in the vicinity of PLA2G7 itself (acting in cis) provide a much more specific genetic tool than variants located outside this gene which could influence other pathways. A number of approaches could be undertaken to overcome the problem that the PLA2G7 SNPs studied so far are weak genetic instruments, which compromises statistical power. These include expanding the dataset of European subjects included in a Mendelian randomisation analysis; resequencing of the PLA2G7 gene in individuals of European ancestry to identify less frequent variants with more extreme effects on Lp-PLA2 activity; and undertaking more studies in subjects of Asian ancestry among whom there is a common loss-of function variant (V279F) associated with a very substantial reduction in enzyme activity with a frequency of heterozygotes of 25% and homozygotes of 1-4%.31,32 The results from the association studies on this V279F variant have so far been inconclusive. Earlier hospital based studies have indicated that subjects carrying the mutant allele are at higher risk of arterial events (MI or stroke), as indicated in the study by Yamada et al. including up to 850 cases and 1684 controls33. However, more recently a Korean case-control study (532 cases: 670 controls) showed opposite results34 while the Chinese study of 827 cases and 947 controls did not find a significant association between the null variant and CHD.28
In summary, in a tagging SNP approach using common PLA2G7 variants in a large scale collaboration including approximately 26,000 European individuals, four SNPs were identified with small to moderate association with Lp-PLA2 activity, but with no effect on lipid variables or CHD risk. However, the modest effect of these variants on Lp-PLA2 activity means that their expected effect on CHD risk, were Lp-PLA2 to be contributory, would be predicted to be small, and even through the collaboration included over 10,400 cases, it may lack sufficient power to detect a genetic effect consistent with a causal role for Lp-PLA2 in CHD. The STABILITY trial should inform on the efficacy and safety of darapladib in particular and Lp-PLA2 inhibition in general for the prevention of CHD events in a number of ethnic groups.
Supplementary Material
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is transported in the circulation on HDL and LDL cholesterol particles, and present in atheromatous plaques where it may exert pro-atherogenic effects through generation of lysophosphatidylcholine and oxidized fatty acids. Higher Lp-PLA2 activity is associated with increased risk of coronary heart disease (CHD) in observational studies, making Lp-PLA2 a potential therapeutic target. However, higher Lp-PLA2 activity may simply mark alterations in blood lipids and other coronary heart disease (CHD) risk factors, reflect (rather than contribute to) plaque or even play an anti-atherogenic role through the hydrolysis of the pro-inflammatory mediator, platelet-activating factor and its analogues formed upon oxidation of LDL. We identified common variants of the PLA2G7 gene that encodes Lp-PLA2 associated with Lp-PLA2 activity and used these to clarify the nature of the association of Lp-PLA2 with other putative risk factors and CHD events using Mendelian randomisation. Genetic variants associated with modest effects on Lp-PLA2 activity were not associated with major alterations in cardiovascular risk factors, coronary atheroma or CHD events. Larger Mendelian randomisation analyses, perhaps using variants associated with larger effects on Lp-PLA2 activity and randomised trials of specific Lp-PLA2 inhibitors, will be needed to confirm/refute a contributory role for Lp-PLA2 in CHD.
Acknowledgements
We would like to thank Hervé Durand and Sofie Ashford for excellent technical assistance. This study makes use of data generated by the Wellcome Trust Case-Control Consortium 2. A full list of the investigators who contributed to the generation of the data is available from http://www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 085475.
Funding Sources In France, this work was supported by the Institut National de la Santé et de la Recherche Médicale, EN is Director of Research of Centre National de la Recherche Scientifique. This work was supported by an educational grant from GSK (PJT, MSS), and by the British Heart Foundation Programme rant RG05/014 and a senior fellowship to ADH, FS2005/125. SLR is funded by the British Heart Foundation. RS is a British Heart Foundation (Schillingford) Clinical Training Fellow (FS/07/011). The Cyprus study was supported by a joint Cyprus Research Promotion Foundation, Ministry of Health and Cyprus Heart Foundation grant (No 41/5PE). SY thanks support from the British Heart Foundation (PG98/183). The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Institute on Aging (AG13196), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health.
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karabina SA, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim Biophys Acta. 2006;1761:1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159–165. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 3.Blankenberg S, Stengel D, Rupprecht HJ, Bickel C, Meyer J, Cambien F, Tiret L, Ninio E. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res. 2003;44:1381–1386. doi: 10.1194/jlr.M300086-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulaiton. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 5.Wilensky RL, Shi Y, Mohler ER, III, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D’Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani A, Humphries S. Nature’s randomised trials. Lancet. 2005;366:1906–1908. doi: 10.1016/S0140-6736(05)67767-7. [DOI] [PubMed] [Google Scholar]
- 8.Miller GJ, Bauer KA, Barzegar S, Cooper JA, Rosenberg RD. Increased activation of the haemostatic system in men at high risk of fatal coronary heart disease. Thromb Haemost. 1996;75:767–771. [PubMed] [Google Scholar]
- 9.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 10.Wild SH, Byrne CD, Smith FB, Lee AJ, Fowkes FG. Low ankle-brachial pressure index predicts increased risk of cardiovascular disease independent of the metabolic syndrome and conventional cardiovascular risk factors in the Edinburgh Artery Study. Diabetes Care. 2006;29:637–642. doi: 10.2337/diacare.29.03.06.dc05-1637. [DOI] [PubMed] [Google Scholar]
- 11.Panayiotou A, Nicolaides A, Griffin M, Tyllis T, Georgiou N, Martin RM, Bond D, Tziakouri-Shiakalli C, Fessas C, Deltas C. Serum total homocysteine, folate, 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C-->T genotype and subclinical atherosclerosis. Expert Opin Ther Targets. 2009;13:1–11. doi: 10.1517/14728220802560281. [DOI] [PubMed] [Google Scholar]
- 12.Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 13.Juhan-Vague I, Morange PE, Frere C, Aillaud MF, Alessi MC, Hawe E, Boquist S, Tornvall P, Yudkin JS, Tremoli E, Margaglione M, Di Minno G, Hamsten A, Humphries SE. The plasminogen activator inhibitor-1 -675 4G/5G genotype influences the risk of myocardial infarction associated with elevated plasma proinsulin and insulin concentrations in men from Europe: the HIFMECH study. J Thromb Haemost. 2003;1:2322–2329. doi: 10.1046/j.1538-7836.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann MM, Winkler K, Renner W, Winkelmann BR, Seelhorst U, Wellnitz B, Boehm BO, Marz W. Genetic variants and haplotypes of lipoprotein associated phospholipase A2 and their influence on cardiovascular disease (The Ludwigshafen Risk and Cardiovascular Health Study) J Thromb Haemost. 2009;7:41–48. doi: 10.1111/j.1538-7836.2008.03216.x. [DOI] [PubMed] [Google Scholar]
- 15.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye S, Dunleavey L, Bannister W, Day LB, Tapper W, Collins AR, Day IN, Simpson I. Independent effects of the -219 G>T and epsilon 2/ epsilon 3/ epsilon 4 polymorphisms in the apolipoprotein E gene on coronary artery disease: the Southampton Atherosclerosis Study. Eur J Hum Genet. 2003;11:437–443. doi: 10.1038/sj.ejhg.5200983. [DOI] [PubMed] [Google Scholar]
- 17.Stephens JW, Hurel SJ, Acharya J, Humphries SE. An interaction between the interleukin-6 -174G>C gene variant and urinary protein excretion influences plasma oxidative stress in subjects with type 2 diabetes. Cardiovasc Diabetol. 2004;3:2. doi: 10.1186/1475-2840-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsetti JP, Rainwater DL, Moss AJ, Zareba W, Sparks CE. High lipoprotein-associated phospholipase A2 is a risk factor for recurrent coronary events in postinfarction patients. Clin Chem. 2006;52:1331–1338. doi: 10.1373/clinchem.2006.066845. [DOI] [PubMed] [Google Scholar]
- 19.Tselepis AD, Dentan C, Karabina SA, Chapman MJ, Ninio E. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler Thromb Vasc Biol. 1995;15:1764–1773. doi: 10.1161/01.atv.15.10.1764. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka T, Yamaguchi M, Soda Y, Kishimoto T, Tago A, Toyosato M, Mizuno K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin Chim Acta. 2000;296:151–161. doi: 10.1016/s0009-8981(00)00216-3. [DOI] [PubMed] [Google Scholar]
- 21.Sutton BS, Crosslin DR, Shah SH, Nelson SC, Bassil A, Hale AB, Haynes C, Goldschmidt-Clermont PJ, Vance JM, Seo D, Kraus WE, Gregory SG, Hauser ER. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Hum Mol Genet. 2008;17:1318–1328. doi: 10.1093/hmg/ddn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel R, Dupuis J, Larson MG, Lunetta KL, Robins SJ, Zhu Y, Rong J, Yin X, Stirnadel HA, Nelson JJ, Wilson PW, Keaney JF, Vasan RS, Benjamin EJ. Clinical and genetic factors associated with lipoprotein-associated phospholipase A2 in the Framingham Heart Study. Atherosclerosis. 2009;204:601–607. doi: 10.1016/j.atherosclerosis.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohler ER, III, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, Zalewski A. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 25.Abuzeid AM, Hawe E, Humphries SE, Talmud PJ. Association between the Ala379Val variant of the lipoprotein associated phospholipase A2 and risk of myocardial infarction in the north and south of Europe. Atherosclerosis. 2003;168:283–288. doi: 10.1016/s0021-9150(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 26.Ninio E, Tregouet D, Carrier JL, Stengel D, Bickel C, Perret C, Rupprecht HJ, Cambien F, Blankenberg S, Tiret L. Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Hum Mol Genet. 2004;13:1341–1351. doi: 10.1093/hmg/ddh145. [DOI] [PubMed] [Google Scholar]
- 27.Liu PY, Li YH, Wu HL, Chao TH, Tsai LM, Lin LJ, Shi GY, Chen JH. Platelet-activating factor-acetylhydrolase A379V (exon 11) gene polymorphism is an independent and functional risk factor for premature myocardial infarction. J Thromb Haemost. 2006;4:1023–1028. doi: 10.1111/j.1538-7836.2006.01895.x. [DOI] [PubMed] [Google Scholar]
- 28.Hou L, Chen S, Yu H, Lu X, Chen J, Wang L, Huang J, Fan Z, Gu D. Associations of PLA2G7 gene polymorphisms with plasma lipoprotein-associated phospholipase A2 activity and coronary heart disease in a Chinese Han population: the Beijing atherosclerosis study. Hum Genet. 2009;125:11–20. doi: 10.1007/s00439-008-0587-4. [DOI] [PubMed] [Google Scholar]
- 29.Wilensky RL, Macphee CH. Lipoprotein-associated phospholipase A(2) and atherosclerosis. Curr Opin Lipidol. 2009;20:415–420. doi: 10.1097/MOL.0b013e3283307c16. [DOI] [PubMed] [Google Scholar]
- 30.Drenos F, Talmud PJ, Casas JP, Smeeth L, Palmen J, Humphries SE, Hingorani AD. Integrated associations of genotypes with multiple blood biomarkers linked to coronary heart disease risk. Hum Mol Genet. 2009;18:2305–2316. doi: 10.1093/hmg/ddp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miwa M, Miyake T, Yamanaka T, Sugatani J, Suzuki Y, Sakata S, Araki Y, Matsumoto M. Characterization of serum platelet-activating factor (PAF) acetylhydrolase. Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest. 1988;82:1983–1991. doi: 10.1172/JCI113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafforini DM, Satoh K, Atkinson DL, Tjoelker LW, Eberhardt C, Yoshida H, Imaizumi T, Takamatsu S, Zimmerman GA, McIntyre TM, Gray PW, Prescott SM. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest. 1996;97:2784–2791. doi: 10.1172/JCI118733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada Y, Yoshida H, Ichihara S, Imaizumi T, Satoh K, Yokota M. Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Athero. 2000;150:209–216. doi: 10.1016/s0021-9150(99)00385-8. [DOI] [PubMed] [Google Scholar]
- 34.Jang Y, Kim OY, Koh SJ, Chae JS, Ko YG, Kim JY, Cho H, Jeong TS, Lee WS, Ordovas JM, Lee JH. The Val279Phe variant of the lipoprotein-associated phospholipase A2 gene is associated with catalytic activities and cardiovascular disease in Korean men. J Clin Endocrinol Metab. 2006;91:3521–3527. doi: 10.1210/jc.2006-0116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


