Abstract
Obesity rates have increased dramatically among children in many parts of the world, especially in North America and several other English-speaking countries. The impact of obesity on pediatric health has become a major prevention initiative by the Obama administration and several public health organizations. Children with obesity are at increased risk for developing asthma, which is already one of the most common chronic diseases among children. The cause underlying obesity's impact on asthma risk is unknown. Commonly cited potential etiologies include airway smooth muscle dysfunction from thoracic restriction, obesity-related circulating inflammation priming the lung, and obesity-related comorbidities mediating asthma symptom development. Each of these theories does not fit precisely with all of the data that have accumulated over the last decade. In this review, I will explore other possible causes including: (1) dietary characteristics common in Westernized countries that might lead to both obesity and asthma; (2) reductions in physical activity; and (3) genetic alterations that increase the propensity to both obesity and asthma together. Next, I will review the current data on how obesity affects common characteristics of asthma such as airway inflammation, lung function, risk of exacerbation, atopy, and response to treatment. Obesity in children with asthma appears to be associated with greater airflow obstruction and a mildly diminished response to inhaled corticosteroids. Little objective evidence in children suggests that obesity significantly heightens the risk of exacerbation or worsens disease stability in children. Lastly, I will discuss the current literature that suggests that obese children with asthma generally should receive the same guidelines-based management as lean children. However, interventions that encourage daily physical activity, weight-loss, normalization of nutrient levels, and monitoring of common obesity-related sequelae should be considered by healthcare providers managing obese children with difficult-to-control asthma.
Introduction
Asthma is one of the most common significant chronic diseases affecting children. The number of people worldwide affected by asthma may be as high as 300 million.1 The highest prevalences have commonly been reported from traditionally Westernized, English-speaking countries. Asthma continues to pose an enormous burden on quality of life and healthcare costs. In the United States, asthma is a leading cause of hospitalization, emergency department visits, and missed school. The majority of pediatric asthma presents before age 5 and has strong familial aggregation. Despite the high degree of heritability, external factors such as air pollution, immune sensitization, nutrition, and obesity can affect disease risk.
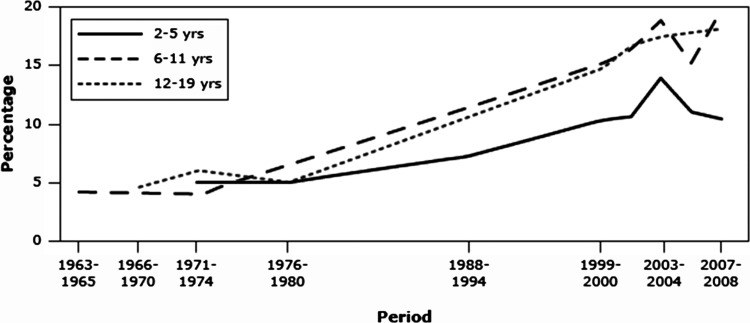
Obesity is now a serious public health problem and has been identified as an area of needed focus in order to improve the nation's health.2 Obesity is associated with reduced quality of life and excess risk for several chronic diseases. Obesity prevalence is highest among adults. However, the change in obesity prevalence in recent decades has been greatest among children (Fig. 1).3,4 It is also important to note that obesity in children is not a problem limited to North America. Worldwide there are now more than 40 million overweight or obese children below the age of 5.5,6 This fact makes the appreciation for the relationships between obesity, nutrition, and lung disease important for any clinician who cares for children in the fields of allergy, immunology, or pulmonology.
FIG. 1.
Prevalence of obesity during childhood by age group, United States, 1963–2008. Obesity is defined as body mass index ≥95th percentile for age and gender. Taken from Morbidity and Mortality Weekly Report (MMWR) January 21, 2011 Vol. 60, No. 2.
This article will discuss potential mechanisms that explain the evidence linking obesity with asthma risk in children. I will also discuss how obesity affects the characteristics (or endotype) of asthma in children. While covering the topics of asthma-risk and asthma-endotype, I will draw attention to how specific nutritional factors may contribute. Lastly, I will assess the latest data and discuss management considerations for obese children with difficult to control asthma.
If Obesity Leads to Asthma, What is the Mechanism?
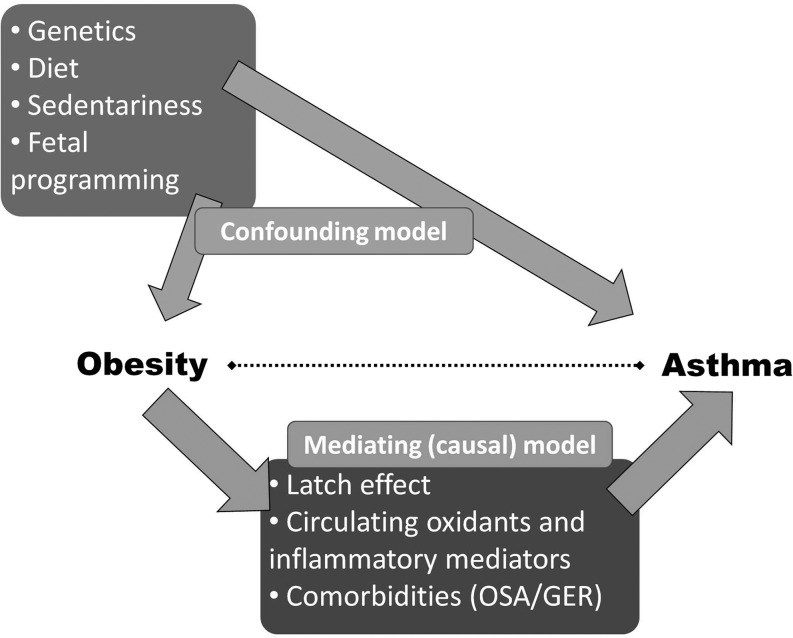
More than 15 longitudinal cohort studies have been conducted in adults7–14 and children.15–21 When lean and obese non-asthmatics are followed prospectively, almost universally obese participants are found to be at higher risk of developing true asthma, confirmed by specialist diagnosis22 and objective measures of disease.17 Several theories have been proposed that address the link between obesity and asthma risk.23–25 Theories that involve mediating factors stemming from the obese state include abnormal circulating inflammation and oxidant stress, chest restriction with airway narrowing, and obesity-related comorbidities (Fig. 2). Largely through the work of Shore et al., molecular and physiologic characteristics of the murine model of obese asthma are currently being elucidated.23,26–34 However, assessing lung inflammation and lung responses among obese children remains challenging and limited by current technologies. Studies in children involving bronchodilator responsiveness, bronchoconstricting agonists (e.g., methacholine challenge), and airway inflammatory markers have yet to show the same consistent pattern of hyperresponsiveness that have been demonstrated in mice. Therefore, discussions about the etiology of obesity-related asthma in children remain speculative.
FIG. 2.
Potential models of association between obesity and asthma. OSA: obstructive sleep apnea; GER: gastroesophageal reflux.
One hypothesis for the obesity–asthma link is that obesity-related systemic inflammation primes the lung for exaggerated responses to environmental triggers, leading to asthma-like symptoms. Adipose tissue releases pro-inflammatory ‘adipokines' (e.g., adiponectin, IL-6, TNFα, leptin) that influence multiple organ systems, including the lung's responses to external stimuli.29,33,35–37 Therefore, obesity-related inflammation may play a role in the development and severity of asthma.38 However, further work is needed in children.
Some data in adults suggest that obesity may lead to reduced lung volumes39,40 and greater airway responsiveness.41–43 These observations have led to the hypothesis that obesity-related chest wall restriction and the resultant breathing at low lung volume leads to airway obstruction, reactivity, and an asthma-like phenotype. These physiologic characteristics seen in some obese adults appear less commonly in children,44–49 perhaps due to a reduced duration of obesity in children relative to adults. Obesity's effects on airway mechanics and airway reactivity among asthmatic children require more investigation.
Lastly, obesity-related comorbidites such as gastroesophageal reflux and sleep apnea have yet to be conclusively linked to increased asthma risk in children.
A Case for Confounding?
The consistency of the obesity–asthma link suggests that this association is not spurious. However, two other explanations must be considered. These include reverse causality and the presence of a hidden third factor that is associated with obesity and that promotes asthma (e.g., a confounding variable). In the case of obesity and asthma, asthma could occur first and then lead to a higher risk for obesity due to reduced exercise or to frequent corticosteroid therapy. However, to date, there is not convincing evidence that this directionality (asthma causing obesity) is the best explanation, while there is ample evidence that obesity precedes and is a risk-factor for incident asthma.
Another consideration must be the possibility of an extrinsic factor that is closely related to obesity and constitutes the real driver of asthma risk. This factor would be said to be confounding the obesity–asthma relationship. Well-designed cohort studies attempt to anticipate potential confounding variables through statistical adjustment or using a stratified design. However, it can be hard to anticipate, measure, and adjust for all potential confounders. Several factors have been associated with obesity that could plausibly lead to asthma. These include dietary factors, sedentariness, fetal programming, and genetics.
Dietary factors
Diet constitutes an environmental factor that patients are exposed to literally every day, and by way of the maternal diet, it may be a major contributor to asthma development through the mechanism of fetal programming. There is evidence that a Western diet (high in calories, fats, and processed food, and relatively low in fresh fruits, vegetables, and fish) may contribute to both obesity and asthma. Obese children are more apt to have feeding disinhibition,50 greater fast-food and saturated fat consumption,51,52 and a diet with lower nutritional content.53 Reduced fruits, vegetables, and fish, and increased saturated fats, burgers, and fast food appear to increase the risk for asthma.54 Consistent with this, epidemiologic data suggest a relationship between low maternal vitamin E and vitamin D and infant asthma risk.55,56 It is biologically plausible for a high-fat, low-antioxidant diet to lead not only to obesity, but also to an increased risk for asthma. Biochemically, saturated fats may increase activation of the innate immune system and increase circulating mediators such as IL-6, TNFα, and leptin—factors shown to be important in inducing asthma. Within hours of eating a high fat load, NF-kB pathways are induced to create a pro-inflammatory state and excess oxidative stress. Toll-like receptors (TLR), which are known to reside on several types of airway inflammatory cells, serve to activate NF-kB, proinflammatory mediators, and the innate immune system. Importantly, TLR can also be activated by some dietary fatty acids. It should not be surprising that elevated dietary saturated fatty acids have been associated with asthma risk57,58 and asthma severity.59
Low antioxidant intake and serum levels have been associated with asthma risk and severity. Asthmatics have increased airway and circulating markers of oxidative stress.60 The antioxidant system, which is equipped to protect the lung from damaging oxidants, is impaired in asthma.61 Some antioxidants that neutralize reactive species can only be obtained in the diet (including carotenoids, vitamin E, and vitamin C). Children with low dietary intake of vitamins A, C, and E have significantly lower lung function,62,63 suggesting this may be a causative mechanism in obese asthma. These antioxidants are likely to work synergistically, suggesting that a diet consistently rich in fruits and vegetables may be most effective in maintaining lung health.
The effects of a diet high in fat may also depend on the relative composition of fatty acids. The typical Western high-fat diet contains up to 25 times more omega-6 (n-6) polyunsaturated fatty acids (PUFA) than n-3 PUFAs. n-6 PUFAs are found in eggs and most vegetable oils that are now commonly used in cooking, while n-3 PUFAs are contained in cold water fish (such as salmon, tuna, swordfish), mussels, and various seeds. The current 25:1 omega-6:omega-3 fatty acid ratio is much higher than the <2:1 ratio that was typically present during the majority of human evolution.64–66 The Western diet with abundant n-6 PUFA promotes a low leukocyte n-3:n-6 PUFA plasma membrane ratio and appears to increase cellular expression of 5-lipoxygenase pathway products (such as leukotrienes), TNFα, and other molecules important in asthma pathogenesis.67–70 Increases in dietary and inflammatory cell n-3:n-6 PUFA ratio have been associated with improvements in asthma outcomes.71–75 Interestingly, a Mediterranean diet higher in n-3 fatty acids and antioxidants appears to protect young children from recurrent wheezing.76
Obese children on average consume more n-6 dietary PUFAs, have a reduced n-3:n-6 ratio in their diet,77 and have reduced n-3:n-6 PUFA serum levels compared to similar leans.78 A high-fat meal can acutely induce inflammatory airway changes among healthy volunteers.79 Wood et al. recently showed that a high fat challenge can lead to excess inflammation in the airway and diminished lung function following bronchodilator administration.80 These observations suggest that a high fat diet consumed chronically may contribute to greater risk of both obesity and asthma.
A patient's vitamin D status also may confound the relationship between obesity status and asthma risk. Vitamin D sufficiency is associated with a healthy diet that is rich in fish and fortified whole grains, and is also associated with sun exposure during outdoor activity. We have found that hypovitaminosis D is highly prevalent in children with poorly controlled asthma, and that serum levels are significantly negatively correlated with BMI (Lang, unpublished). Low 25-hydroxy vitamin D (25(OH)D) levels might increase asthma risk through several biologically plausible mechanisms, including impaired clearance of respiratory pathogens and reduced maturation and functioning of suppressor T-cells and airway smooth muscle cells.81 Several birth cohorts evaluating 25(OH)D levels in the maternal diet or cord blood have suggested an increased risk in childhood asthma symptoms.82–84 It is possible that low 25(OH)D may be driving some of the asthma risk among obese children. However, further investigation is greatly needed.
Sedentariness
Sedentary behavior is a risk factor for obesity and is a plausible factor leading to asthma.85,86 Children who are overweight sustain less routine physical exertion than their lean counterparts.87 Repeated exercise promotes hyperventilation, cyclic airway smooth muscle stretch, and bronchodilation. Therefore, exercise and exhaustive play could protect against the development of asthma. Lucas et al. have raised the concern that past cohort studies have not adequately controlled for reduced physical activity and that reduced activity may be promoting asthma risk.88 Castro-Rodriguez has pointed out that precise measures of exercise and TV watching, plus other potential confounders (e.g. tobacco exposure and family history) may need to be measured more precisely to untangle the relationships among activity level, obesity, and asthma.89
Shared genetics
It would also be rational to hypothesize that both obesity and asthma stem from common genetic origins.25,90 Our current genetic understanding has stemmed from discovering associations between obesity and asthma phenotypes and candidate gene variants. For example, several promising genomic areas that contain genes connected with both obesity and asthma (5q23-32, 6p21-23, 11q13, and 12q13-24) have been identified.24,25,90–94 Only five genes have polymorphisms that have been associated with both obesity and asthma.95,96 These include β2-adrenergic receptor gene (ADRB2), the TNFα gene, the lymphotoxin-α (LTA) gene, vitamin D receptor (VDR) gene, and protein kinase C-α (PRKCA). Further interrogation of these and other genetic loci is needed among cohorts with and without obesity, and with and without asthma, in order to understand better the nature of the obesity–asthma link.
Obesity and Asthma Endotype in Children
Currently there is not a consensus among clinicians and researchers. does obesity affect asthma disease characteristics in children. I will explore current data on severity, atopy status, oxidation and inflammation in the airway, lung mechanics, and response to rescue and controller asthma therapy in obese and non-obese children.
Asthma severity
There is not currently a consensus about whether obese children with asthma experience greater disease severity. Severity describes the intrinsic intensity of the disease process and includes lifestyle impairment, risk of exacerbation, and level of therapy needed for symptom control.97 Several reports suggest that asthma is more severe among obese children,38,98–105 while others have found no real difference.106–111 Two large population-based studies have reported greater asthma severity among obese asthmatics based on either patient symptom reporting or physician reporting of diagnostic severity.38,103 Though these results come from excellent epidemiologic studies, they reflect subjective questionnaires or clinician diagnoses rather than objective measures of asthma and thus may be vulnerable to biases. Obese asthmatic children and adults do generally report reduced asthma-related quality of life compared to normal weight asthmatics.98,104,112,113 However, when well-phenotyped pediatric cohorts are examined for an effect of obesity on disease severity using objective measures, very little difference in asthma severity can be found.106,111,114 Recently, we described a cohort of school age children and adolescents with generally mild asthma.115 The obese adolescents had very similar symptom reporting compared to their non-obese counterparts, while the 6–11-year-old obese asthmatics had improved asthma symptoms compared to leans.
Asthma severity in the urgent care setting has been assessed among obese children in four recent reports.101,102,107,108 Obesity was associated with a significantly higher rate of hospitalization (34% vs. 25%).102 However, asthma severity using the Modified Pulmonary Index Score was the same in obese versus lean asthmatics. Ginde et al., using a similar index score, also saw no greater asthma severity among obese children in the setting of acute disease.107 Luder found that obese asthmatics were more likely to be on a greater number of asthma medications,105 while Vargas did not.100 We evaluated 10,291 lean and obese children diagnosed with asthma at four Nemours Children's Clinics pediatric asthma clinics over a 10-year period. We found a small but statistically significant increase in odds for severe asthma diagnosis and advanced treatment (EPR-3 treatment step 5 or higher) in obese versus lean children with asthma (Lang, unpublished). However, obese children with asthma seen in our clinic were not more likely to be experiencing an exacerbation. Overall, the data in children do not suggest that obesity leads to a higher risk for severe exacerbation.
The other major domain influencing asthma severity is the level of therapy required to control symptoms. Data suggest that obese asthmatics are at least as hard to control as their lean counterparts, and in some adult reports, are less responsive to conventional therapies.101,102,114,116–120 Evidence suggests that this reduced treatment efficacy may be rooted in true glucocorticosteroid resistance.116 Recently, Forno et al. showed that obese children may also be less responsive to inhaled corticosteroids.121 More investigation is needed to evaluate how obesity in children affects response to asthma medications.
Lung function
Obese adult asthmatics generally have similar lung function (or modest reductions in vital capacity) and bronchodilator responsiveness compared to lean asthmatics.114,122 Fewer studies have reported spirometric outcomes in lean and obese children with asthma. Unlike in adults, obesity does not appear to reduce vital capacity or total lung capacity substantially,98,104,106,109 and in some reports may be associated with greater volumes and capacity.123 However, obese children with asthma may have a mild obstructive impairment in airway flows,22,105,111,115,124 though this has not been a universal finding.98,106,109,125
Airways hyperresponsiveness (AHR) is excessive constriction triggered by innocuous stimuli, and is an important phenotypic component of true asthma. Both AHR and the response to bronchodilator correlate with airway inflammation126–128 and help assess asthma control and future exacerbation risk. Obese children with asthma appear to have similar or even reduced responsiveness to bronchoprovocation compared to lean asthmatic children.98 Additionally, obese children with asthma appear to have similar or even reduced response to bronchodilators104,111,125 compared to leans. These results provide some evidence that obesity-related risk for new asthma, and possible increases in asthma severity are not due to increases in traditional airway inflammation. Instead, it seems more likely that obesity-related symptoms in children are related to greater airflow obstruction.
Atopy
Though there are some reports of obesity being a risk factor for atopy among adolescent girls,129–131 obesity does not appear to consistently increase the risk for atopy. Both ISAAC and NHANES III data sets have shown no association between obesity and atopy.132,133
Airway inflammation
The measurement of airway inflammation in the obese asthmatic patient is an area in need of further study. Currently there is little evidence that obesity leads to greater allergic airway inflammation in children. Exhaled nitric oxide levels (a surrogate of eosinophilic inflammation) among obese asthmatic children may be the same or even reduced compared to similar leans.134–138 A few studies in adult obese asthmatics have shown reduced eosinophilic inflammation compared with similar leans.112,113 Obese asthmatic children did not have elevated airway LTB4.134 Recent adult studies suggest that obesity may enhance neutrophilic airway inflammation139 and systemic leukotriene production,140 though this has not been evaluated in children. Since inhaled steroids are generally ineffective against neutrophilic inflammation, the discovery of neutrophilic inflammation in obese asthmatics is consistent with findings of reduced corticosteroids efficacy in the obese.
Leukotriene-driven inflammation may play a more prominent role in the obese asthma phenotype. Leukotriene production may be upregulated in patients with obesity.140,141 There is also preliminary evidence that genes, more common among the obese, may affect the leukotriene pathway. We previously determined the allele frequencies of the addition/deletion promoter polymorphism in the ALOX5 gene among a population of lean and obese asthmatics.142,143 The relative risk of obesity in individuals carrying the variant allele was 2.04 compared to carriers of the wild type (p=0.0165; Lang, unpublished). It is rational to hypothesize that obese, non-asthmatic persons may be at enhanced risk for incident asthma due in part to greater upregulation of the leukotriene pathway.142,144 Lastly, data do suggest that relative response to montelukast (a leukotriene receptor antagonist) does increase with increasing BMI.145 Asthma and obesity are conditions involving excess oxidative stress. Airway 8-isoprostane, a marker of arachidonic acid oxidation, is elevated in obese adult asthmatics.146 Excess oxidative stress and injury is possibly a second mechanism making the obese–asthma phenotype less responsive to inhaled corticosteroid therapy.
Though the exact nature of the obese–asthma phenotype in children is far from clear, some patterns are emerging (Table 1). In addition, patient factors such as age, age of asthma onset, gender, race, and other factors may modify the effect of obesity on asthma phenotype.
Table 1.
Obese Asthma Endotype in Children
| Characteristic | Common allergic phenotype | Obese phenotype |
|---|---|---|
| Severity | Variable | Similar or increased symptom reporting; reduced quality of life |
| Onset | Early onset, usually before age 6 | Variable onset, can be throughout childhood |
| Atopy | Very common | less common |
| Airway inflammation | Eosinophilic, elevated FENO | More data needed; may be mixed with greater neutrophilic component/ reduced FENO |
| Lung function | Episodic airway obstruction; variable remodeling | Normal FVC, variable reduction in FEV1/FVC; remodeling unknown |
| Bronchodilator response | Very common | Common; slightly reduced compared to leans |
| Bronchoprovocation/AHR | Typically present | Often present, may be reduced compared to leans |
| Airflow perception | — | Greater in children;† reduced in adults† |
| Response to therapy | Steroid-resistance generally rare | Steroid-resistance may be more common; LTRA may be helpful |
| Common comorbidities | Allergic rhinitis, eczema, sinusitis, anxiety, GER | Hyperinsulinemia, elevated triglycerides, LDL; anxiety/depression, GER |
| Resolution | NAEPP/GINA guidelines | NAEPP/GINA guidelines, weight loss improves asthma control |
| Other considerations | Consider referral to registered dietician, evaluating Vitamin D intake and sufficiency; fat intake |
AHR: airway hyperresponsiveness; FENO: fractional exhalation nitric oxide; FVC: forced vital capacity; FEV: forced expiratory volume; LTRA: leukotriene receptor antagonist; GER: gastroesophageal reflux; LDL: low density lipoprotein; † compared to non-obese; NAEPP: National Asthma Education and Prevention Program; GINA: Global Initiative for Asthma.
Weight loss
Both adults and children who are overweight and have asthma are advised by current recommendations to lose weight. Data in adults are encouraging.147–151 However, no studies have been conducted in children determining the impact of weight loss on asthma. Of the adult studies, only a handful has focused on asthma as a primary outcome (Table 2). Most studies had methodological limitations, including small sample sizes and unclear diagnostic criteria for asthma cases. The most commonly noted improvements following weight loss were subjective reporting of asthma symptoms, rescue medication use, and quality of life.151 It may be important to note that surgical weight loss has yielded improvements in lung volumes and asthma symptoms, but has yet to show consistent improvement in asthma-specific outcomes such as airway reactivity or airflow obstruction. From the studies to date, it is difficult to decipher general improvements in cardiorespiratory health that typically follow weight loss from asthma-specific improvements. Dixon et al. recently reported on 23 adult asthmatics who underwent (primarily Roux-en-Y) weight loss surgery. Following surgery, patients on average had significant weight loss, improved asthma symptom and lung volumes, and reduced airway reactivity. Weight loss did not lead to significant changes in airflow obstruction measured by FEV1/FVC. Surgery-induced weight loss was also associated with increased bronchoalveolar lavage (BAL) lymphocytes and serum and BAL adiponectin. Interestingly, weight loss among asthma patients with elevated IgE did not improve airflow obstruction or airway reactivity, two central characteristics of asthma.
Table 2.
Key Weight Loss Studies in Patients with Asthma
| Subjects | Intervention | Weight loss | Asthma outcome | |
|---|---|---|---|---|
| Surgical interventions | ||||
| Macgregor (1993)174 | N=40, mostly females, aged 23–68 | Gastric bypass or gastroplasty | BMI mean went from 46 to 30 at 4 years | Patient reported improvements in symptom severity |
| Dixon (1999)175 | N=32, mostly females | Gastric banding | BMI mean went from 46 to 33 | Improved symptom scoring, need for asthma medications reduced |
| Eneli (2007)151 | Systematic review | 15 relevant studies involving bariatric surgery or calorie restriction | Significant | All 15 studies noted improvement in at least one outcome measure—mostly symptom reporting |
| Dixon (2011)176 | N=23, 21/23 females, | 20/23 Roux-en-Y | BMI decreased 27% (51 to 37) | Improved Lung volumes (FVC, FEV1), no change in airflow obstruction; improved symptoms; reduced airway reactivity in patients with normal IgE |
| Low energy diet interventions | ||||
| Hakala (2000)152 | N=14, mostly females | 8 weeks of <1800 kJ/day | BMI decreased 14%, 37 to 32 | Improved peak flows; Reduced PF variability and diurnal variation, raw and FEF/FVC. |
| Stenius-Aarniala (2000)149 | N=19, mostly females | 8 weeks of <1800 kJ/day | 14.5% weight reduction | Improved FEV1 and FVC, improved symptoms; reduced rescue medication and exacerbations |
| Aaron (2004)150 | N=24, mostly females | 6 months of 900 Kcal/day plus counseling | Mean weight loss of 20 kg at 6 months | Improved lung volumes (FVC, FEV1); no change in airflow obstruction or reactivity |
FEF, forced expiratory flow; PF, peak flow.
Fewer studies have been published assessing the effect of weight loss by calorie restriction on asthma outcomes. When obese asthma patients have lost weight with dietary restriction, studies have documented improved objective markers of airway stability and airflow obstruction,149,152 as well as asthma symptoms, rescue medication use, and risk for asthma exacerbation. These data are encouraging and suggest that either reduced calories or changes in particular components of the obese diet may improve asthma outcomes, separate from the beneficial effects of reduced weight. However, further work in this area, especially involving children and involving larger asthma cohorts with well-characterized disease, is greatly needed.
Though the impact of weight loss in obese children with asthma has not been adequately studied, weight loss is an Evidence B recommendation of the Expert Panel 3 Report (EPR-3).97
Management Considerations
Pharmacotherapeutics
Current research does not support major deviations from current GINA and NHLBI EPR-3 guidelines when considering the management of the obese asthmatic child. However, recent studies may provide important insights for the clinician caring for the obese asthmatic patient. Evidence suggests that obesity may blunt the response to inhaled corticosteroids116,118,145 and low-dose theophylline,114 and obese patients may respond more favorably (compared to the non-obese) to montelukast.145 Glucocorticoids inhibit inflammatory gene expression (in part) through activation of MAP-1 phosphatase, which negatively regulates mitogen-activated protein kinase signaling pathways. Therefore, MKP-1 is generally a reasonable marker of steroid signaling and responsiveness. Similar findings of poor steroid response were also seen among airway inflammatory cells. Little is known if the same mechanisms are present in children. A recent report suggests a possible genetic explanation for this pattern of reduced steroid response that may also be coupled with enhanced montelukast response.153 These findings in conjunction with evidence of superior adherence,154 suggest that montelukast may be particularly useful in the obese asthmatic population.
Comorbidities
Since the impairment domain of asthma assessment stems from subjective symptoms and quality of life, clinicians should attend to obesity-related sequelae that might interact with asthma symptoms and quality of life. Some obese patients with gastro-esophageal reflux and cough may improve with anti-reflux medications, though empiric treatment for “silent” gastro-esophageal reflux does not seem warranted.155 Clinicians should maintain a high-index of suspicion for sleep-disordered breathing and metabolic syndrome. Lastly, depression with anxiety is associated with both obesity and asthma, and is commonly overlooked.156 For these reasons, some children with obese asthma may need more intensive self-management education and help with coaching and adherence. Since mood can affect self-mastery, a home asthma management plan that jointly considers all of the patient's comorbidities may be most effective.
The most universally effective management plan for obese children with persistent asthma continues to involve weight loss, daily exercise, and repeated asthma education about an asthma control plan, inhaler technique, and trigger avoidance. Pharmacologic controller therapy with inhaled corticosteroids and leukotriene modifiers should be first-line therapies. Response variability will likely exist among obese asthmatics, as it does among lean asthmatics.157 Because of the flat dose–response curve and steroid resistance seen among obese asthmatics, initial step-up therapy for obese asthmatics with poor control should include ICS plus montelukast. However, regardless of the step-up therapy chosen, close patient follow-up is critically important to reassess and reiterate proper inhaler techniques, weight control, low-fat diet, daily exercise, and monitoring of asthma symptoms and medication side-effects.
Dietary interventions
The Centers for Disease Control and Prevention (CDC) and U.S. Department of Agriculture strongly recommend that children adhere to a balanced diet generally low in saturated fats and with half their meal plate consisting of fruits and vegetables.158 However, many children and families struggle to achieve these recommendations. In addition to supporting our primary care and public health colleagues and reminding asthma patients about these key health initiatives, we as asthma specialists should be aware of the impact diet and weight status may be playing in innate asthma severity and the response to therapy (Table 3).
Table 3.
Target Levels and Daily Dietary Intakes for Children and Adolescents
| Measures | School age children | Adolescents |
|---|---|---|
| Calories per day1 | 1600 | 2000 |
| BMI % | 15–85th percentile | 15–85th percentile |
| Daily activity2 | 60 minutes | 60 minutes |
| Saturated fats | 5–20% of daily energy intake | 5–20% of daily energy intake |
| High Omega-3 Fatty Acid containing fish3 | 8 ounces (2 servings) | 8 ounces (2 servings) |
| Omega-6 Fatty Acids | 10–12 g | 11–16 g |
| Vitamin D | 600 IU (15 mcg) | 600 IU (15 mcg) |
| Vitamin A | 1333 IU | 2000–3000 IU |
| Vitamin E | 7–10 IU | 11–15 IU |
| Vitamin C | 25 mcg | 45–75 mcg |
| Selenium | 30–40 mcg | 40–60 mcg |
Modified from the Food and Nutrition Board, The Institute of Medicine, National Academies of Sciences. 1: Daily caloric recommendations are based on age, sex, physical activity, body size, and other factors. The recommendation for the school age child is for an 8-year-old female exercising 30–60 minutes per day. The recommendation for the adolescent is for a 14-year-old female exercising 30–60 minutes per day. More specific recommendations can be accessed through http://www.choosemyplate.gov/. 2: Moderate-to-vigorous physical activity. For toddlers and small children to age the recommendation up to around 120 minutes per day. 3: Primarily salmon, tuna, trout, and swordfish.
There is evidence that suggests that maintaining key antioxidant at normal levels may be helpful in maintaining asthma control. Children with asthma and low antioxidant blood levels appear to have reduced symptom control. When intervention trials have been undertaken supplementing one vitamin alone, the results have been disappointing.159–161 However, intervention with a combination of antioxidants has been more successful.162,163 Similarly, vitamin A, C, and E supplementation together has been shown to ameliorate ozone-induced bronchoconstriction.164 It is too soon to recommend daily supplementation with antioxidant multivitamins for the treatment of asthma. However, encouraging patients with poor symptom control to commit to a healthier diet replete with the current recommendations for vitamins A, C, E, and selenium may be helpful for asthma control.
Vitamin D
Currently the data are encouraging for the potential future use of vitamin D supplementation as an adjuvant therapy for persistent asthma. Though more investigation is needed, basic research suggests that vitamin D may be important in viral defense,165,166 reduction of virally induced airway inflammation,167 and response to steroid therapy.168–170 Cross-sectional data in children suggest a significant negative association between 25(OH)D and asthma symptoms,171,172 and a significant positive association between 25(OH)D and both lung function and steroid response.169 One longitudinal study in children has shown an increased risk for asthma exacerbation among those with vitamin D insufficiency (25(OH)D ≤30 ng/ml). Researchers gave children with persistent asthma either inhaled steroid plus placebo or inhaled steroids plus 500 IU/day of vitamin D3 for 6 months. They found that the D3-treated patients had a significantly reduced rate of asthma exacerbation.173 However, at this time, the data are not yet strong enough to recommend supplementation beyond the standard daily allowance. However, many children with asthma have low vitamin D. For the many children with difficult-to-control persistent asthma, clinicians should consider hypovitaminosis D as a potential factor. It is for these difficult-to-control patients that adherence to the vitamin D Dietary Reference Intakes (600 IU/day for ages 1–18 years) and serum concentration of 25(OH)D >30 mg/ml (>50 nmol/L) may be most important (Table 4). The best dietary sources of vitamin D are fatty fish, fish liver oil, and fortified foods such as milk, orange juice, yogurt, and breakfast cereal.
Table 4.
Dietary Sources of Vitamin D
| Food | Amount | IU (% of RDA) |
|---|---|---|
| Tuna fish (canned in water) | 3 ounces | 154 (26) |
| Cooked salmon | 3 ounces | 447 (75) |
| Cooked mackerel | 3 ounces | 388 (65) |
| Breakfast cereal | 1 cup | 40 (7) |
| Eggs | 1 large with yolk | 41 (7) |
| Yoghurt | 6 ounces | 88 (15) |
| Milk (fortified) | 1 cup | 120 (20) |
| Cod liver oil | 1 tablespoon | 1,360 (226) |
Taken from the National Institutes of Health's Dietary Supplement Fact Sheet: Vitamin D, Office of Dietary Supplements (ODS), 2011. IU: International Units; RDA: Recommended Daily Allowance.
Conclusions
Obesity rates have increased dramatically among children in many parts of the world, especially in more Westernized regions. Children with obesity are at increased risk for developing asthma. The cause underlying obesity's impact on asthma risk is unknown and may involve one or more causes. It is likely that interactions between smooth muscle dysfunction and oxidative stress compounded by exercise and genetic and dietary factors are playing major roles. Obesity in children with asthma appears to be associated with greater airflow obstruction and a mildly diminished response to inhaled corticosteroids. Obesity does not appear to worsen risk of exacerbation or objective measures of asthma severity significantly. Obese children with asthma generally should receive the same guidelines-based management as lean children. However, interventions that encourage daily physical activity, weight loss, normalization of nutrient levels, and monitoring of common obesity-related sequelae should be considered by healthcare providers managing obese children with difficult-to-control asthma.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Masoli M. Fabian D. Holt S. Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (U.S.) Committee on Leading Health Indicators for Healthy People 2020. Leading health indicators for healthy people 2020: letter report. Washington, D.C.: National Academies Press; [PubMed] [Google Scholar]
- 3.Eaton DK. Kann L. Kinchen S, et al. Youth risk behavior surveillance—United States, 2007. MMWR Surveill Summ. 2008;57:1–131. [PubMed] [Google Scholar]
- 4.Ogden CL. Carroll MD. Curtin LR. McDowell MA. Tabak CJ. Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Selassie M. Sinha AC. The epidemiology and aetiology of obesity: a global challenge. Best Pract Res Clin Anaesthesiol. 25:1–9. doi: 10.1016/j.bpa.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.WHO. World Health Statistics 2011. Geneva, Switzerland: 2011. [Google Scholar]
- 7.Ford ES. Asthma, body mass index, and C-reactive protein among US adults. J Asthma. 2003;40:733–739. doi: 10.1081/jas-120023497. [DOI] [PubMed] [Google Scholar]
- 8.Nystad W. Meyer HE. Nafstad P. Tverdal A. Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol. 2004;160:969–976. doi: 10.1093/aje/kwh303. [DOI] [PubMed] [Google Scholar]
- 9.Camargo CA., Jr Weiss ST. Zhang S. Willett WC. Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 10.Beckett WS. Jacobs DR., Jr. Yu X. Iribarren C. Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y. Dales R. Tang M. Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 12.Stanley AH. Demissie K. Rhoads GG. Asthma development with obesity exposure: observations from the cohort of the National Health and Nutrition Evaluation Survey Epidemiologic Follow-up Study (NHEFS) J Asthma. 2005;42:97–99. doi: 10.1081/jas-51338. [DOI] [PubMed] [Google Scholar]
- 13.Huovinen E. Kaprio J. Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. 2003;97:273–280. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 14.Guerra S. Sherrill DL. Bobadilla A. Martinez FD. Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–1263. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 15.Guerra S. Wright AL. Morgan WJ. Sherrill DL. Holberg CJ. Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 16.Gilliland FD. Berhane K. Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158:406–415. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Rodriguez JA. Holberg CJ. Morgan WJ. Wright AL. Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 18.Gold DR. Damokosh AI. Dockery DW. Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36:514–521. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]
- 19.Mannino DM. Mott J. Ferdinands JM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY) Int J Obes (Lond) 2006;30:6–13. doi: 10.1038/sj.ijo.0803145. [DOI] [PubMed] [Google Scholar]
- 20.Taveras EM. Rifas-Shiman SL. Camargo CA, Jr, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J Allergy Clin Immunol. 2008;121:1161–1166. doi: 10.1016/j.jaci.2008.03.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinn S. Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax. 2001;56:845–850. doi: 10.1136/thorax.56.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang JE. Feng H. Lima JJ. Body mass index-percentile and diagnostic accuracy of childhood asthma. J Asthma. 2009;46:291–299. doi: 10.1080/02770900802712963. [DOI] [PubMed] [Google Scholar]
- 23.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. ; quiz 94–55. [DOI] [PubMed] [Google Scholar]
- 24.Weiss ST. Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169:963–968. doi: 10.1164/rccm.200303-403WS. [DOI] [PubMed] [Google Scholar]
- 25.Beuther DA. Weiss ST. Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston RA. Theman TA. Lu FL. Terry RD. Williams ES. Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 27.Johnston RA. Theman TA. Terry RD. Williams ES. Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol. 2007;102:149–156. doi: 10.1152/japplphysiol.00300.2006. [DOI] [PubMed] [Google Scholar]
- 28.Johnston RA. Zhu M. Rivera-Sanchez YM, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang JE. Williams ES. Mizgerd JP. Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1013–1020. doi: 10.1152/ajplung.00122.2007. [DOI] [PubMed] [Google Scholar]
- 30.Rivera-Sanchez YM. Johnston RA. Schwartzman IN, et al. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol. 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- 31.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shore SA. Rivera-Sanchez YM. Schwartzman IN. Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- 33.Shore SA. Terry RD. Flynt L. Xu A. Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Shore SA. Williams ES. Zhu M. No effect of metformin on the innate airway hyperresponsiveness and increased responses to ozone observed in obese mice. J Appl Physiol. 2008;105:1127–1133. doi: 10.1152/japplphysiol.00117.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry MA. Hargadon B. Shelley M, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 36.Shore SA. Schwartzman IN. Mellema MS. Flynt L. Imrich A. Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Lang JE. Williams E.S. Shore S.A. TNF-alpha contributes to innate airway hyperresponsiveness in murine obesity. J Appl Physiol. 2010;108:735–743. doi: 10.1152/japplphysiol.00749.2009. . PMID19875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelson PH. Williams LW. Benjamin DK. Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol. 2009;103:381–385. doi: 10.1016/S1081-1206(10)60356-0. [DOI] [PubMed] [Google Scholar]
- 39.Yap JC. Watson RA. Gilbey S. Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79:1199–1205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- 40.Sampson MG. Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- 41.Celedon JC. Palmer LJ. Litonjua AA, et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164:1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 42.Litonjua AA. Sparrow D. Celedon JC. DeMolles D. Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002;57:581–585. doi: 10.1136/thorax.57.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinn S. Jarvis D. Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcus CL. Curtis S. Koerner CB. Joffe A. Serwint JR. Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Bosisio E. Sergi M. di Natale B. Chiumello G. Ventilatory volumes, flow rates, transfer factor and its components (membrane component, capillary volume) in obese adults and children. Respiration. 1984;45:321–326. doi: 10.1159/000194638. [DOI] [PubMed] [Google Scholar]
- 46.Chaussain M. Gamain B. La Torre AM. Vaida P. de Lattre J. [Respiratory function at rest in obese children (author's transl)] Bull Eur Physiopathol Respir. 1977;13:599–609. [PubMed] [Google Scholar]
- 47.Boran P. Tokuc G. Pisgin B. Oktem S. Yegin Z. Bostan O. Impact of obesity on ventilatory function. J Pediatr (Rio J) 2007;83:171–176. doi: 10.2223/JPED.1609. [DOI] [PubMed] [Google Scholar]
- 48.Ho TF. Tay JS. Yip WC. Rajan U. Evaluation of lung function in Singapore obese children. J Singapore Paediatr Soc. 1989;31:46–52. [PubMed] [Google Scholar]
- 49.Inselma LS. Milanese A. Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol. 1993;16:130–137. doi: 10.1002/ppul.1950160209. [DOI] [PubMed] [Google Scholar]
- 50.Lawson OJ. Williamson DA. Champagne CM, et al. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes Res. 1995;3:153–161. doi: 10.1002/j.1550-8528.1995.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 51.Pereira MA. Kartashov AI. Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 52.Bray GA. Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 53.Puchau B. Zulet MA. de Echavarri AG. Hermsdorff HH. Martinez JA. Dietary total antioxidant capacity: a novel indicator of diet quality in healthy young adults. J Am Coll Nutr. 2009;28:648–656. doi: 10.1080/07315724.2009.10719797. [DOI] [PubMed] [Google Scholar]
- 54.Nagel G. Weinmayr G. Kleiner A. Garcia-Marcos L. Strachan DP. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65:516–522. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 55.Martindale S. McNeill G. Devereux G. Campbell D. Russell G. Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–128. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 56.Litonjua AA. Rifas-Shiman SL. Ly NP, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strom K. Janzon L. Mattisson I. Rosberg HE. Arborelius M. Asthma but not smoking-related airflow limitation is associated with a high fat diet in men: results from the population study “Men born in 1914”, Malmo, Sweden. Monaldi Arch Chest Dis. 1996;51:16–21. [PubMed] [Google Scholar]
- 58.Misso NL. Thompson PJ. Oxidative stress and antioxidant deficiencies in asthma: potential modification by diet. Redox Rep. 2005;10:247–255. doi: 10.1179/135100005X70233. [DOI] [PubMed] [Google Scholar]
- 59.Misso NL. Brooks-Wildhaber J. Ray S. Vally H. Thompson PJ. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J. 2005;26:257–264. doi: 10.1183/09031936.05.00006705. [DOI] [PubMed] [Google Scholar]
- 60.Denny SI. Thompson RL. Margetts BM. Dietary factors in the pathogenesis of asthma and chronic obstructive pulmonary disease. Curr Allergy Asthma Rep. 2003;3:130–136. doi: 10.1007/s11882-003-0025-6. [DOI] [PubMed] [Google Scholar]
- 61.Rahman I. Biswas SK. Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 62.Gilliland FD. Berhane KT. Li YF. Gauderman WJ. McConnell R. Peters J. Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol. 2003;158:576–584. doi: 10.1093/aje/kwg181. [DOI] [PubMed] [Google Scholar]
- 63.Harik-Khan RI. Muller DC. Wise RA. Serum vitamin levels and the risk of asthma in children. Am J Epidemiol. 2004;159:351–357. doi: 10.1093/aje/kwh053. [DOI] [PubMed] [Google Scholar]
- 64.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 65.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 66.Simopoulos AP. Overview of evolutionary aspects of omega 3 fatty acids in the diet. World Rev Nutr Diet. 1998;83:1–11. doi: 10.1159/000059674. [DOI] [PubMed] [Google Scholar]
- 67.Mantzioris E. Cleland LG. Gibson RA. Neumann MA. Demasi M. James MJ. Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr. 2000;72:42–48. doi: 10.1093/ajcn/72.1.42. [DOI] [PubMed] [Google Scholar]
- 68.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–358. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 69.James MJ. Gibson RA. Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 70.Caughey GE. Mantzioris E. Gibson RA. Cleland LG. James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 71.Emelyanov A. Fedoseev G. Krasnoschekova O. Abulimity A. Trendeleva T. Barnes PJ. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: a randomised clinical trial. Eur Respir J. 2002;20:596–600. doi: 10.1183/09031936.02.02632001. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto M. Mitsunobu F. Ashida K, et al. Effects of perilla seed oil supplementation on leukotriene generation by leucocytes in patients with asthma associated with lipometabolism. Int Arch Allergy Immunol. 2000;122:137–142. doi: 10.1159/000024369. [DOI] [PubMed] [Google Scholar]
- 73.Nagakura T. Matsuda S. Shichijyo K. Sugimoto H. Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000;16:861–865. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- 74.Villani F. Comazzi R. De Maria P. Galimberti M. Effect of dietary supplementation with polyunsaturated fatty acids on bronchial hyperreactivity in subjects with seasonal asthma. Respiration. 1998;65:265–269. doi: 10.1159/000029274. [DOI] [PubMed] [Google Scholar]
- 75.Dry J. Vincent D. Effect of a fish oil diet on asthma: results of a 1-year double-blind study. Int Arch Allergy Appl Immunol. 1991;95:156–157. doi: 10.1159/000235421. [DOI] [PubMed] [Google Scholar]
- 76.Castro-Rodriguez JA. Garcia-Marcos L. Alfonseda Rojas JD. Valverde-Molina J. Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152:823–828. doi: 10.1016/j.jpeds.2008.01.003. 8 e1–2. [DOI] [PubMed] [Google Scholar]
- 77.Daniel CR. McCullough ML. Patel RC, et al. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev. 2009;18:516–525. doi: 10.1158/1055-9965.EPI-08-0750. [DOI] [PubMed] [Google Scholar]
- 78.Karlsson M. Marild S. Brandberg J. Lonn L. Friberg P. Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity (Silver Spring) 2006;14:1931–1939. doi: 10.1038/oby.2006.225. [DOI] [PubMed] [Google Scholar]
- 79.Rosenkranz SK. Townsend DK. Steffens SE. Harms CA. Effects of a high-fat meal on pulmonary function in healthy subjects. Eur J Appl Physiol. 2010;109:499–506. doi: 10.1007/s00421-010-1390-1. [DOI] [PubMed] [Google Scholar]
- 80.Wood LG. Garg ML. Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 81.Paul G. Brehm J. Alcorn JF. Holguin F. Aujla S. Celedon JC. Vitamin D and Asthma. Am J Respir Crit Care Med. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Devereux G. Litonjua AA. Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 83.Miyake Y. Sasaki S. Tanaka K. Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35:1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 84.Camargo CA., Jr. Ingham T. Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 85.Rasmussen F. Lambrechtsen J. Siersted HC. Hansen HS. Hansen NC. Low physical fitness in childhood is associated with the development of asthma in young adulthood: the Odense schoolchild study. Eur Respir J. 2000;16:866–870. doi: 10.1183/09031936.00.16586600. [DOI] [PubMed] [Google Scholar]
- 86.Vlaski E. Stavric K. Seckova L. Kimovska M. Isjanovska R. Influence of physical activity and television-watching time on asthma and allergic rhinitis among young adolescents: preventive or aggravating? Allergol Immunopathol (Madr) 2008;36:247–253. doi: 10.1016/s0301-0546(08)75218-2. [DOI] [PubMed] [Google Scholar]
- 87.Belcher BR. Berrigan D. Dodd KW. Emken BA. Chou CP. Spruijt-Metz D. Physical activity in US youth: effect of race/ethnicity, age, gender, and weight status. Med Sci Sports Exerc. 2010;42:2211–2221. doi: 10.1249/MSS.0b013e3181e1fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lucas SR. Platts-Mills TA. Paediatric asthma and obesity. Paediatr Respir Rev. 2006;7:233–238. doi: 10.1016/j.prrv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Castro-Rodriguez JA. Asthma, obesity, sedentary lifestyle and physical activity: an important issue still unresolved? Allergol Immunopathol (Madr) 2008;36:245–256. doi: 10.1016/s0301-0546(08)75217-0. [DOI] [PubMed] [Google Scholar]
- 90.Weiss ST. Obesity: insight into the origins of asthma. Nat Immunol. 2005;6:537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- 91.Shore SA. Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Tantisira KG. Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001;56(Suppl 2):ii64–73. [PMC free article] [PubMed] [Google Scholar]
- 93.Howard TD. Meyers DA. Bleecker ER. Mapping susceptibility genes for asthma and allergy. J Allergy Clin Immunol. 2000;105:S477–481. doi: 10.1016/s0091-6749(00)90046-0. [DOI] [PubMed] [Google Scholar]
- 94.Yang W. Kelly T. He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 95.Litonjua AA. Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol. 2008;121:1075–1084. doi: 10.1016/j.jaci.2008.03.005. ; quiz 85–86. [DOI] [PubMed] [Google Scholar]
- 96.Murphy A. Tantisira KG. Soto-Quiros ME, et al. PRKCA: a positional candidate gene for body mass index and asthma. Am J Hum Genet. 2009;85:87–96. doi: 10.1016/j.ajhg.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—Summary report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 98.Pianosi PT. Davis HS. Determinants of physical fitness in children with asthma. Pediatrics. 2004;113:e225–229. doi: 10.1542/peds.113.3.e225. [DOI] [PubMed] [Google Scholar]
- 99.Tai A. Volkmer R. Burton A. Association between asthma symptoms and obesity in preschool (4–5-year-old) children. J Asthma. 2009;46:362–365. doi: 10.1080/02770900902759260. [DOI] [PubMed] [Google Scholar]
- 100.Vargas PA. Perry TT. Robles E, et al. Relationship of body mass index with asthma indicators in head start children. Ann Allergy Asthma Immunol. 2007;99:22–28. doi: 10.1016/S1081-1206(10)60616-3. [DOI] [PubMed] [Google Scholar]
- 101.Carroll CL. Bhandari A. Zucker AR. Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7:527–531. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 102.Carroll CL. Stoltz P. Raykov N. Smith SR. Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120:734–740. doi: 10.1542/peds.2007-0409. [DOI] [PubMed] [Google Scholar]
- 103.Cassol VE. Rizzato TM. Teche SP, et al. Obesity and its relationship with asthma prevalence and severity in adolescents from southern Brazil. J Asthma. 2006;43:57–60. doi: 10.1080/02770900500448597. [DOI] [PubMed] [Google Scholar]
- 104.van Gent R. van der Ent CK. Rovers MM. Kimpen JL. van Essen-Zandvliet LE. de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007;119:591–596. doi: 10.1016/j.jaci.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Luder E. Melnik TA. DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. J Pediatr. 1998;132:699–703. doi: 10.1016/s0022-3476(98)70363-4. [DOI] [PubMed] [Google Scholar]
- 106.Ross KR. Hart MA. Storfer-Isser A, et al. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatr Pulmonol. 2009;44:877–884. doi: 10.1002/ppul.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ginde AA. Santillan AA. Clark S. Camargo CA., Jr Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2009;21:480–488. doi: 10.1111/j.1399-3038.2009.00911.x. [DOI] [PubMed] [Google Scholar]
- 108.Hom J. Morley EJ. Sasso P. Sinert R. Body mass index and pediatric asthma outcomes. Pediatr Emerg Care. 2009;25:569–571. doi: 10.1097/PEC.0b013e3181b4f639. [DOI] [PubMed] [Google Scholar]
- 109.Kwong KY. Rhandhawa I. Saxena J. Morphew T. Jones CA. Ability to control persistent asthma in obese versus non-obese children enrolled in an asthma-specific disease management program (breathmobile) J Asthma. 2006;43:661–666. doi: 10.1080/02770900600925270. [DOI] [PubMed] [Google Scholar]
- 110.Mansell AL. Walders N. Wamboldt MZ, et al. Effect of body mass index on response to methacholine bronchial provocation in healthy and asthmatic adolescents. Pediatr Pulmonol. 2006;41:434–440. doi: 10.1002/ppul.20368. [DOI] [PubMed] [Google Scholar]
- 111.Tantisira KG. Litonjua AA. Weiss ST. Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Veen IH. Ten Brinke A. Sterk PJ. Rabe KF. Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 113.Lessard A. Turcotte H. Cormier Y. Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 114.Dixon AE. Shade DM. Cohen RI, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43:553–558. doi: 10.1080/02770900600859123. [DOI] [PubMed] [Google Scholar]
- 115.Lang JE. Hossain J. Dixon AE, et al. Does age impact the obese asthma phenotype?: Longitudinal asthma control, airway function and airflow perception among mild persistent asthmatics. Chest. 2011;140:1524–1533. doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- 116.Sutherland ER. Goleva E. Strand M. Beuther DA. Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boulet LP. Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 118.Camargo CA., Jr Boulet LP. Sutherland ER, et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;4 7:76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 119.Lavoie KL. Bacon SL. Labrecque M. Cartier A. Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med. 2006;100:648–657. doi: 10.1016/j.rmed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Saint-Pierre P. Bourdin A. Chanez P. Daures JP. Godard P. Are overweight asthmatics more difficult to control? Allergy. 2006;61:79–84. doi: 10.1111/j.1398-9995.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 121.Forno E. Lescher R. Strunk R. Weiss S. Fuhlbrigge A. Celedon JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boulet LP. Hamid Q. Bacon SL, et al. Symposium on obesity and asthma—November 2, 2006. Can Respir J. 2007;14:201–208. doi: 10.1155/2007/342618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lazarus R. Colditz G. Berkey CS. Speizer FE. Effects of body fat on ventilatory function in children and adolescents: cross-sectional findings from a random population sample of school children. Pediatr Pulmonol. 1997;24:187–194. doi: 10.1002/(sici)1099-0496(199709)24:3<187::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 124.Chu YT. Chen WY. Wang TN. Tseng HI. Wu JR. Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatr Pulmonol. 2009;44:472–479. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 125.Bibi H. Shoseyov D. Feigenbaum D, et al. The relationship between asthma and obesity in children: is it real or a case of over diagnosis? J Asthma. 2004;41:403–410. doi: 10.1081/jas-120026097. [DOI] [PubMed] [Google Scholar]
- 126.Laprise C. Laviolette M. Boutet M. Boulet LP. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J. 1999;14:63–73. doi: 10.1034/j.1399-3003.1999.14a12.x. [DOI] [PubMed] [Google Scholar]
- 127.Koskela HO. Hyvarinen L. Brannan JD. Chan HK. Anderson SD. Sensitivity and validity of three bronchial provocation tests to demonstrate the effect of inhaled corticosteroids in asthma. Chest. 2003;124:1341–1349. doi: 10.1378/chest.124.4.1341. [DOI] [PubMed] [Google Scholar]
- 128.Covar RA. Spahn JD. Martin RJ, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004;114:575–582. doi: 10.1016/j.jaci.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 129.Schachter LM. Peat JK. Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58:1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang SL. Shiao G. Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy. 1999;29:323–329. doi: 10.1046/j.1365-2222.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- 131.Hancox RJ. Milne BJ. Poulton R, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 132.von Mutius E. Schwartz J. Neas LM. Dockery D. Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.von Kries R. Hermann M. Grunert VP. von Mutius E. Is obesity a risk factor for childhood asthma? Allergy. 2001;56:318–322. doi: 10.1034/j.1398-9995.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 134.Leung TF. Li CY. Lam CW, et al. The relation between obesity and asthmatic airway inflammation. Pediatr Allergy Immunol. 2004;15:344–350. doi: 10.1111/j.1399-3038.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 135.Santamaria F. Montella S. De Stefano S, et al. Asthma, atopy, and airway inflammation in obese children. J Allergy Clin Immunol. 2007;120:965–967. doi: 10.1016/j.jaci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 136.Chow JS. Leung AS. Li WW. Tse TP. Sy HY. Leung TF. Airway inflammatory and spirometric measurements in obese children. Hong Kong Med J. 2009;15:346–52. [PubMed] [Google Scholar]
- 137.Wong GW. Liu EK. Leung TF, et al. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005;35:889–893. doi: 10.1111/j.1365-2222.2005.02263.x. [DOI] [PubMed] [Google Scholar]
- 138.Buchvald F. Baraldi E. Carraro S, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–1136. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 139.Scott HA. Gibson PG. Garg ML. Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38:594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 140.Giouleka P. Papatheodorou G. Lyberopoulos P, et al. Body mass index is associated with leukotriene inflammation in asthmatics. Eur J Clin Invest. 2011;4 1:30–38. doi: 10.1111/j.1365-2362.2010.02371.x. [DOI] [PubMed] [Google Scholar]
- 141.Kaaman M. Ryden M. Axelsson T, et al. ALOX5AP expression, but not gene haplotypes, is associated with obesity and insulin resistance. Int J Obes (Lond) 2006;30:447–452. doi: 10.1038/sj.ijo.0803147. [DOI] [PubMed] [Google Scholar]
- 142.Lima JJ. Zhang S. Grant A, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med. 2007;175:235, 242. doi: 10.1164/rccm.200603-416OC. [DOI] [PubMed] [Google Scholar]
- 144.Dwyer JH. Allayee H. Dwyer KM, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 145.Peters-Golden M. Swern A. Bird SS. Hustad CM. Grant E. Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 146.Komakula S. Khatri S. Mermis J, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simard B. Turcotte H. Marceau P, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 2004;14:1381–1388. doi: 10.1381/0960892042584021. [DOI] [PubMed] [Google Scholar]
- 148.Spivak H. Hewitt MF. Onn A. Half EE. Weight loss and improvement of obesity-related illness in 500 U.S. patients following laparoscopic adjustable gastric banding procedure. Am J Surg. 2005;189:27–32. doi: 10.1016/j.amjsurg.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 149.Stenius-Aarniala B. Poussa T. Kvarnstrom J. Gronlund EL. Ylikahri M. Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aaron SD. Fergusson D. Dent R. Chen Y. Vandemheen KL. Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 151.Eneli IU. Skybo T. Camargo CA., Jr. Weight loss and asthma: a systematic review. Thorax. 2008;63:671–676. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 152.Hakala K. Stenius-Aarniala B. Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 153.Mougey EB. Chen C. Tantisira K. Blake KV. Peters SP. Wise RA. Weiss ST. Lima JJ . Response to fluticasone and montelukast therapies are inversely associated with a common variant of the corticotropin releasing hormone receptor 1 (CRHR1) gene. Proc Am Thorac Soc—ATS 2011. 2011;183:A4080. [Google Scholar]
- 154.Price D. Musgrave SD. Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695–1707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 155.Mastronarde JG. Anthonisen NR. Castro M, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–1499. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bahreinian S. Ball GD. Colman I. Becker AB. Kozyrskyj AL. Depression is more common in girls with nonatopic asthma. Chest. 2011;40:1138–1145. doi: 10.1378/chest.11-0219. [DOI] [PubMed] [Google Scholar]
- 157.Lemanske RF., Jr. Mauger DT. Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.United States Dept of Health and Human Services, United States Dept of Agriculture, United States Dietary Guidelines Advisory Committee. Dietary guidelines for Americans. seventh. Washington, DC: GPO; 2010. [Google Scholar]
- 159.Pearson PJ. Lewis SA. Britton J. Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hasselmark L. Malmgren R. Zetterstrom O. Unge G. Selenium supplementation in intrinsic asthma. Allergy. 1993;48:30–36. [PubMed] [Google Scholar]
- 161.Kaur B. Rowe BH. Arnold E. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000993.pub3. CD000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Romieu I. Sienra-Monge JJ. Ramirez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 163.Trenga CA. Koenig JQ. Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001;56:242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 164.Romieu I. Meneses F. Ramirez M, et al. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med. 1998;158:226–232. doi: 10.1164/ajrccm.158.1.9712053. [DOI] [PubMed] [Google Scholar]
- 165.Griffin MD. Xing N. Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 166.Jartti T. Ruuskanen O. Mansbach JM. Vuorinen T. Camargo CA., Jr. Low serum 25-hydroxyvitamin D levels are associated with increased risk of viral coinfections in wheezing children. J Allergy Clin Immunol. 2010;126:1074–1076. doi: 10.1016/j.jaci.2010.09.004. , 6 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hansdottir S. Monick MM. Lovan N. Powers L. Gerke A. Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xystrakis E. Kusumakar S. Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Searing DA. Zhang Y. Murphy JR. Hauk PJ. Goleva E. Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sutherland ER. Goleva E. Jackson LP. Stevens AD. Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brehm JM. Celedon JC. Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chinellato I. Piazza M. Sandri M. Peroni D. Piacentini G. Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158:437–441. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 173.Majak P. Olszowiec-Chlebna M. Smejda K. Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294–1296. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 174.Macgregor AM. Rand CS. Gastric surgery in morbid obesity. Outcome in patients aged 55 years and older. Arch Surg. 1993;128:1153–1157. doi: 10.1001/archsurg.1993.01420220073010. [DOI] [PubMed] [Google Scholar]
- 175.Dixon JB. Chapman L. O'Brien P. Marked improvement in asthma after lap-band surgery for morbid obesity. Obes Surg. 1999;9:385–389. doi: 10.1381/096089299765552981. [DOI] [PubMed] [Google Scholar]
- 176.Dixon AE. Pratley RE. Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515. doi: 10.1016/j.jaci.2011.06.009. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]