Abstract
Background
The lung's ability to trap and clear foreign particles via the mucociliary elevator is an important mechanism for protecting the lung against respirable irritants and microorganisms. Although cigarette smoke (CS) exposure and particulate inhalation are known to alter mucociliary clearance, little is known about how CS and nanoparticles (NPs) modify cilia beating at the cytoskeletal infrastructure, or axonemal, level.
Methods
We used a cell-free model to introduce cigarette smoke extract (CSE) and NPs with variant size and surface chemistry to isolated axonemes and measured changes in ciliary motility. We hypothesized that CSE would alter cilia beating and that alterations in ciliary beat frequency (CBF) due to particulate matter would be size- and surface chemistry-dependent. Demembranated axonemes were isolated from ciliated bovine tracheas and exposed to adenosine triphosphate (ATP) to initiate motility. CBF was measured in response to 5% CSE, CSE filtrate, and carboxyl-modified (COOH), sulphate (SO4)-modified (sulfonated), or PEG-coated polystyrene (PS) latex NPs ranging in size from 40 nm to 500 nm.
Results
CSE concentrations as low as 5% resulted in rapid, significant stimulation of CBF (p<0.05 vs. baseline control). Filtering CSE through a 0.2-μm filter attenuated this effect. Introduction of sulphate-modified PS beads ∼300 nm in diameter resulted in a similar increase in CBF above baseline ATP levels. Uncharged, PEG-coated beads had no effect on CBF regardless of size. Similarly, COOH-coated particles less than 200 nm in diameter did not alter ciliary motility. However, COOH-coated PS particles larger than 300 nm increased CBF significantly and increased the number of motile points.
Conclusions
These data show that NPs, including those found in CSE, mechanically stimulate axonemes in a size- and surface chemistry-dependent manner. Alterations in ciliary motility due to physicochemical properties of NPs may be important for inhalational lung injury and efficient drug delivery of respirable particles.
Key words: cilia; axonemes, mucociliary clearance, cigarette smoke (CS), particulates, nanoparticles, pulmonary delivery
Introduction
Mucociliary clearance (MCC) is a crucial pulmonary defense mechanism that prevents entrance of foreign particles and microorganisms into the distal airways. Lining the conducting airways are cilia, the finger-like projections that beat in an orchestrated pattern to propel particulates from the bronchioles toward the gastrointestinal tract via the mucociliary elevator. The ciliary axoneme, a centriole-derived organelle composed of microtubule doublets in a 9+2 arrangement, is the structural component of cilia that causes it to bend via dynein and kinesin protein interactions.(1) Axonemes can be isolated from airway epithelium and are capable of beating ex situ when stimulated with adenosine triphosphate (ATP).(2)
The detrimental consequences of cigarette smoke (CS) exposure are well documented. CS damages airway epithelium and is the single most important exposure factor leading to chronic obstructive pulmonary disease (COPD).(3) Furthermore, the inflammatory changes resulting from exposure to CS are linked to lung cancer and atherosclerosis.(4) Additionally, CS is the most common cause of emphysema, leading to macrophage and neutrophil influx and obliteration of the alveolar wall.(5) Although millions of people are chronically exposed to CS and the resultant lung injury is clear, relatively little is known about how smoke exposure impacts motility at the axonemal level. The response to cigarette smoke on cilia depends on the system and the context. One of the important variables is the nature of the exposure with cigarette smoke extract (CSE) versus fresh whole inhaled cigarette smoke. Whether the CSE includes volatile, particulate, or soluble components must be taken into account. For example, acetaldehyde in fresh cigarette smoke has been shown to slow cilia. Another important factor is the chronicity of the smoke exposure. Because most noxious stimuli acutely cause increases in ciliary beat frequency (CBF) we expect that the acute context may be most relevant to axonemal effects.
Cilia beating and mucus clearance are key components of the innate host response to protect the lung from inhaled irritants and particles. CBF is a quantifiable measure of the speed of the bending cycle, which drives MCC. Several studies suggest that CS increases CBF. Zhou et al.(6) found that active smokers had an elevated CBF compared to nonsmokers. In vivo studies with ciliated epithelium similarly demonstrate a transient, but significant increase in baseline CBF in mice exposed to CS and cigarette smoke extract (CSE).(7,8) An initial and rapid increase in MCC was also observed in rats exposed to unfiltered CS.(9) Other studies, however, show significant decreases in CBF with exposure to CS. In vitro models of ciliated cells suggest that CSE slows ciliary beating,(10) thus predisposing the airways to infection and compromising lung sterility. Cohen et al.(11) found reductions in CBF and transepithelial chloride secretions with CS exposure. In addition to modifying CBF, CSE also disrupts ciliogenesis, leading to stunted cilia and a lower overall number of ciliated cells.(12) Thus, investigations on how CS exposure alters CBF have been both conflicting and variable, highlighting the complexity of the underlying axonemal physiology.
Alteration of MCC and CBF has been elicited from several biological and environmental compounds, including ethanol (EtOH),(13) sulfuric acid (H2SO4),(14,15) ozone,(16) and hog barn dust.(17) Short-term EtOH exposure specifically increases CBF in axonemes by releasing nitric oxide (NO),(18) an effect that is blunted by pretreatment with a nitric oxide synthase (NOS) inhibitor. Additionally, stimulation of axonemes above baseline CBF is regulated by both cAMP- and cGMP-dependent kinases (PKA and PKG).(19) Alternatively, protein kinase C (PKC) has been implicated in the slowing of CBF.(20) These biochemical pathways are important for understanding how axonemal CBF is regulated.
Because in vitro studies have been inconclusive on whether CSE exposure results in CBF stimulation or inhibition(7–9,11,21) we assayed CBF in a cell-free preparation of ciliary axonemes exposed to CSE. Given the high incidence of pulmonary disease in tobacco users, we hypothesized that CSE would alter CBF in a cell-free axoneme model.(2) To test this hypothesis, we exposed isolated axonemes to CSE filtered to remove large particulate matter and recorded CBF. We found that concentrations as low as 5% CSE caused CBF stimulation in axonemes, and this increase in motility occurred only when particulates with a diameter larger than 220 nm were present in the extract. To decipher whether particulate stimulation was due to a mechanically induced and/or physicochemical property of the particle, we tested both charged [sulfonated (SO4)- and COOH-modified] and near-neutral (PEGylated) polystyrene nanoparticles (NPs) over a size range of 40 to 500 nm. Introducing non-PEGylated NPs resulted in stimulation of CBF at the organelle level in both a size- and surface chemistry-dependent manner. These findings implicate cilia modulation as an important consideration in NP-based drug delivery to the lungs, and provide insight into the role particulates in cigarette smoke play and modifying ciliary function.
Materials and Methods
Materials
Research-grade cigarettes were purchased from the University of Kentucky College of Agriculture Reference Cigarette Program, Lexington, KY. Red fluorescent carboxyl-modified polystyrene nanoparticles (COOH-PS) were purchased from Molecular Probes (Invitrogen, Carlsbad, CA) in sizes ranging from 40 to 500 nm. Sulfonated polystyrene nanoparticles (SO4-modified particles; 300 nm) were purchased from Sigma (St. Louis, MO). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Nanoparticle conjugation and characterization
Nanoparticles were prepared as previously described.(22) Methoxy-PEG was conjugated to the polystyrene nanoparticles (PEG-PS) as previously described,(23) neutralizing the charge and increasing the diameter of the nanoparticle by approximately 15–20 nm. Nanoparticle size was quantified with dynamic light scattering as previously described.(24) Zetapotential, a measure of electrokinetic energy and surface charge, was determined by laser Doppler anemometry using a Zetasizer NanoZS (Malvern Instruments, Westborough, MA) (Table 1). The final concentration of COOH-modified particles was provided by Invitrogen and PEG-PS particle quantification was calculated using a standard curve. Sulfonated polystyrene latex nanoparticles (charged), PEGylated (neutral) nanoparticles, and COOH-modified (charged) nanoparticles were diluted with cilia resuspension buffer to a concentration of 1.828×1011 nanoparticles per well. To avoid aggregates, all nanoparticle suspensions were vortexed immediately prior to use. The nanoparticles were stored in the dark at 4°C and maintained on ice for the duration of the experiment.
Table 1.
Particle Characterization of Charged (COOH Modified; COOH) and Uncharged (PEGylated; PEG) Conjugated Polystyrene Beads
| Particle | Size (nm) | Zetapotential (mV) |
|---|---|---|
| 40 nm COOH | 49±4.2 | −47±0.7 |
| 100 nm COOH | 88±1.8 | −34±2.3 |
| 200 nm COOH | 250±5.4 | −32±1.0 |
| 500 nm COOH | 510±5.8 | −59±1.2 |
| 40 nm PEG | 67±1.3 | −11±0.4 |
| 100 nm PEG | 110±2.5 | −1.9±1.4 |
| 200 nm PEG | 290±9.0 | −2.8±0.3 |
| 500 nm PEG | 530±2.2 | −3.1±0.4 |
Ciliary axoneme extraction
Bovine ciliary axonemes were prepared using a previously described method.(19) Each axoneme experiment utilized a minimum of six different bovine tracheas (N=6). Fresh bovine tracheas were surgically removed and excess adipose and connective tissue excised. The tracheas were washed twice with 1×sterile filtered phosphate-buffered solution (PBS, pH 7.4). Following addition of 25 mL cilia extraction buffer (20 mM Tris-HCl, 50 mM NaCl, 10 mM CaCl2, 1 mM EDTA, 7 mM 2-mercaptoethanol, 100 mM Triton X-100, and 1 mM dithiothreitol), the proximal and distal ends were securely clamped with hemostats and the trachea agitated vigorously for 90 s. The extraction buffer containing the axoneme suspension was filtered through a 100-μm polypropylene mesh. The filtrate was centrifuged for 7 min at 17,250×g. The supernatant was discarded and the axoneme pellet brought to volume in cilia resuspension buffer [20 mM Tris-HCl, 50 mM KCl, 4 mM MgCl2, 0.5 mM dithiothreitol, 10 mM soybean trypsin inhibitor, and 25% sucrose (w/v)] to a final concentration of 1 mg/mL. Isolated axonemes were aliquoted and stored at −80°C for up to 6 months prior to experimental use.
Experimental preparation of axonemes
Isolated axoneme aliquots were thawed at 4°C and maintained on ice for a maximum of 4 h. The aliquots were diluted to 0.25 mg/mL with cilia resuspension buffer. Immediately prior to analysis, 10 μL of the axoneme sample was pipetted into a single well of a 48-well polystyrene tissue culture plate. Following addition of 10 μL of cilia resuspension buffer to the selected well, the plate was centrifuged in a Sorvall T6000D for 2 min at 400×g. After the plate was centrifuged, 10 μL of 2.5 mM ATP, 10 μL of cilia resuspension buffer, and 10 μL of the experimental condition agent were added to the well. The plate was then placed onto an Olympus IMT-2 inverted microscope and CBF directly measured at room temperature using the Sisson-Ammons Video Analysis system (SAVA; Ammons Engineering, Mt. Morris, MI).25 Volumes across experimental conditions were equilibrated with cilia resuspension buffer to standardize final concentrations. The cilia beat for at least 10 min before hydrolyzing most of the available ATP, providing a predictable and reproducible baseline (4.5 Hz) as well as a standard of evaluation for axoneme functionality.(19) Recordings were captured every minute for 10 min at 24°C and 85 frames per second. The total number of motile points were calculated per each field of view by Whole Field Analysis as described.(25) Data represent the average of a minimum of 10 separate whole fields per condition and each experiment was independently performed three times.
Preparation of volatile and filtered cigarette smoke extract
CSE was prepared fresh daily for experimental use. Two 3R4F reference cigarettes (filtered; University of Kentucky, Lexington, KY) or one 1R3F cigarette (unfiltered; University of Kentucky) were used to generate CSE for each experiment and produced similar results. The cigarettes were smoked using a peristaltic pump and bubbled into 25 mL of sterile-filtered 1×PBS (pH 7.4) for 6 min or to a butt length of 30 mm. The CSE was diluted prior to axoneme exposure using 25 μL CSE stock into 75 μL cilia resuspension buffer before the final experimental concentration of 5% CSE. In some cases, freshly prepared CSE was filtered through a sterile 0.22-μm hydrophilic polysulfone membrane before dilution in resuspension buffer to produce fresh, filtered 5% CSE. Cytotoxicity has been associated with cell exposure to a 15–20% dilution of this stock over time. A minimum-threshold dose that enlists a consistent response in our studies was with 5% cigarette smoke extract. Realistic exposure would thus be exposure to 5% of a single cigarette, with the understanding that cilia from a monolayer of airway epithelial cells in submerged culture are not protected by exhalation, cough, or mucus.
Mouse tracheal epithelial cell culture (MTEC) via air–liquid interface (ALI)
MTEC cultures were established using a modified method of Dossou et al.(26) Briefly, adult C57BL6 mice were euthanized with inhalation of isoflurane and pentobarbital overdose given i.p. (150 mg/kg; Sigma-Aldrich) as described.(2) Tracheas were dissected and immediately placed in chilled F-12 Nutrient Ham Mixture (Invitrogen Corp.). Tracheas were then placed in pronase (1.5 mg/mL; Roche Diagnostic, Mannheim, Germany) and F-12 Nutrient mixture solution and allowed to dissociate overnight at 4°C. The dissociated cells were harvested in 500 mL of MTEC basic media containing Dulbecco's Modified Minimal Essential Medium (DMEM; Invitrogen Corp.), F-12 Nutrient mixture, containing glutamine (200 mM/10 mL; Invitrogen Corp.), penicillin-streptomycin (10 units–μg/mL) and gentamycin (50 μg/mL; both purchased from Sigma-Aldrich), and amphotericin B (1.25 μg/mL; Pharmacia Corp, Kalamazoo, MI). Cells were allowed to incubate for 3.5 h at 37°C in air supplemented with 5% CO2 on 35×10 mm2 Falcon culture dishes (Fisher Scientific, Pittsburg, PA). Cells were then resuspended in 250 mL of MTEC supplemented media composed of MTEC basic medium, fetal bovine serum (Fisher Scientific), insulin (10 μg/mL), transferrin (5 μg/mL), recombinant human epidermal growth factor (5 mg/mL), bovine pituitary extract (each purchased from Lonza, Rockland, ME), cholera toxin (62 μg/mL) and retinoic acid (5% of volume; purchased from Sigma-Aldrich). Cells were seeded at a density of 106 cells/mm2 on 6.5 mm diameter Costar membrane inserts (Corning Life Sciences, Corning, NY), coated with rat tail collagen, type I (Becton Dickinson, Bedford, MA) and allowed to incubate at 37°C in air supplemented with 5% CO2. MTEC supplement media was replaced after 3 days of incubation. When the cultures were confluent (6 days in vitro), an ALI was established. For ALI, media was removed from the apical portion of the membrane resulting in the exposure of the cultures to air on the apical surface and media on the basal surface. The medium was replaced every 3–4 days. For experimental conditions, both PEGylated and COOH-modified particles were diluted in M199 media to a concentration of 1.828×1,011 nanoparticles per well and added (50 μL) to the apical surface of each ALI culture well. CBF was measured by SAVA.
Data analysis
Cilia samples were excluded from data analysis once the number of motile points in one 10-min recording dropped below 10% of the initial reading. Axonemes with a frequency of <2 Hz were not analyzed and considered nonmotile. For each experimental condition, a minimum of six separate fields were captured, analyzed, and expressed as a data point. One-way ANOVA with Tukey's post hoc testing was performed on each data point and considered significant with a p-value<0.05 using GraphPad Prism (San Diego, CA).
Results
CSE increases motility in isolated airway axonemes in a time-dependent manner
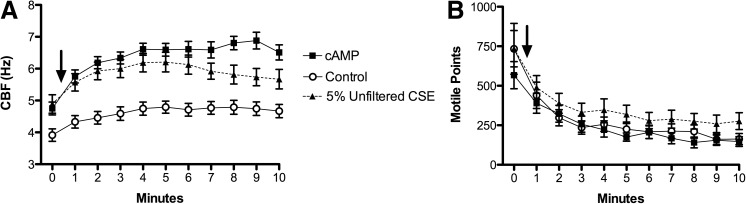
Using 10 μM cAMP in conjunction with 2.5 mM ATP as a positive control, the CBF increased to ∼6.5 Hz (Fig. 1A). At t=0, 5% CSE was introduced to the axonemes and CBF increased to ∼6 Hz, significantly above baseline CBF of ATP only (Fig. 1A). The stimulating effect of 5% CSE was both immediate and sustained throughout the 10-min time course (Fig. 1A). The number of motile points was similar across all conditions, indicating that 5% CSE was not injurious to the axonemal machinery and did not interfere with the typical consumption of ATP during this period of normal cilia beating (Fig. 1B). Thus, the CSE exposure levels did not induce toxicity, which would result in a severe reduction in the number of motile points.
FIG. 1.
CSE stimulates isolated axonemes in a time-dependent manner. (A) Time in minutes is represented on the x-axis, and ciliary beat frequency (CBF) measured in cycles/s (Hz) is represented on the y-axis. Axonemal beating was initiated with 2.5 mM adenosine-5′-triphosphate (ATP) and CBF recorded for 10 min (control). Five percent cigarette smoke extract (CSE) elevated CBF throughout the experimental time course. For a positive control, 10 μM 3′-5′ cyclic adenosine monophosphate (cAMP) was used to stimulate axoneme CBF. Baseline is time zero, and the addition of CSE or cAMP is indicated by an arrow. Each data point represents the mean±SEM of at least three experiments. p<0.05 for both cAMP and CSE compared to control from 1–10 min. (B) The y-axis represents total number of moving points within a whole field of analysis averaged over a minimum of 10 separate fields per treatment condition. The introduction of 5% CSE induced no significant changes to the total number of beating axonemes compared to control. Each data point represents the mean±SEM of at least three experiments.
Increased CBF in response to CSE is particle dependent
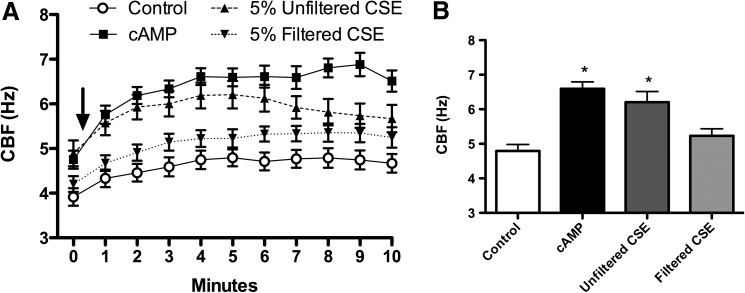
To deduce the source of CSE-generated stimulation of axonemes, we quantified the relative contributions of filterable particulates above and below a diameter of 220 nm. Studies of CS suggest that particles range from 90 to 270 nm, depending on whether the smoke is first-hand or passive.(27) Starting with this reference range, we filtered the particulate CSE through a sterile 0.22-μm hydrophilic polysulfone membrane, which resulted in a loss of stimulatory effect (Fig. 2A). This fresh-filtered CSE demonstrated the same baseline levels of axonemal CBF as control (2.5 mM ATP), significantly differing from the stimulated increases in CBF observed with nonfiltered CSE (Fig. 2B). Thus, filterable particles larger than 220 nm were responsible for the observed increase in axonemal CBF with exposure to CSE. These data suggest that the ciliostimulatory nature of CSE is dependent upon filterable particles larger than 220 nm.
FIG. 2.
CBF stimulation is dependent on filterable particulates in CSE. (A) Time in minutes is represented on the x-axis and CBF in Hz on the y-axis. ATP, at a concentration of 2.5 mM, was used as a baseline control as in Figure 1. CSE filtered through a 0.22-μm hydrophilic polysulfone membrane and then diluted 1:4 in resuspension buffer to create a final 5% fresh filtered solution (filtered CSE) did not cause a significant increase in CBF above control axonemes treated only with ATP. P<0.05 for both cAMP and unfiltered CSE compared to control or filtered CSE from 1–7 min. Baseline is time zero, and the addition of CSE or cAMP is indicated by arrow. (B) Experimental condition is on the x-axis and CBF (Hz) is on the y-axis. Axonemes were treated with 2.5 mM ATP (control), 10 μM cAMP, unfiltered 5% CSE or 0.22-μm filtered 5% CSE for 5 min and CBF determined. Each data point represents the mean±SEM of three experiments. *p<0.05 compared to filtered CSE.
Sulfonated polystyrene nanoparticles alone induce stimulation of CBF
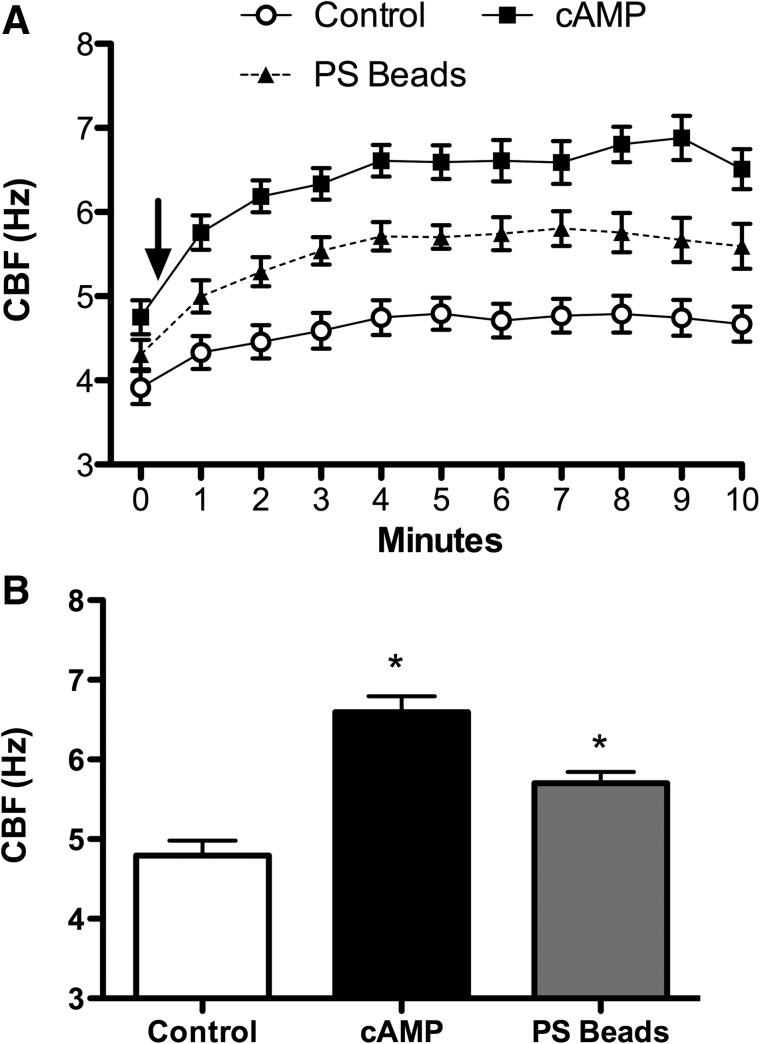
Given the rapid and sustained stimulation of cilia at the organelle level with unfiltered CSE that retained particles larger than 220 nm, we hypothesized that particles of this size, such as negatively charged 300 nm sulfonated polystyrene (PS) nanoparticles alone, would mechanically interact with the axonemes to increase beat frequency. Baseline ATP maintained a steady frequency of ∼4 Hz, whereas PS beads increased CBF significantly (Δ1.5 Hz, p<0.05) above control (Fig. 3A and B). As a control, the motion of the PS beads in the absence of axonemes was not detected by SAVA (data not shown). These data suggest that the sulfonated PS nanoparticle interacts with axonemal proteins to mechanically enhance cilia beating.
FIG. 3.
PS beads can induce increases in axoneme beat frequency mechanically. (A) Time in 1-min intervals is on the x-axis, while the y-axis represents CBF. Polystyrene (PS) beads significantly (p<0.05) stimulated increased CBF from 1–10 min when compared to 2.5 mM ATP only (control) as in Figure 1. This increase in CBF continued for the duration of the 10 min experiment. Although cAMP (10 μM) significantly (p<0.05) elevated CBF over baseline control at all time points, no significant difference existed between PS and cAMP treatment. Baseline is time zero, and the addition of beads or cAMP is indicated by arrow. (B) The x-axis is experimental condition while the y-axis is CBF in Hz. Axonemes were treated with 2.5 mM ATP (control), 10 μM cAMP, or 0.3-μm polystyrene beads (PS beads) for 5 min and CBF determined. Each data point is the mean±SEM of three experiments. *p<0.05 compared to control by one-way ANOVA.
PEG-coated (near neutrally charged) nanoparticles have no effect on axonemal beating
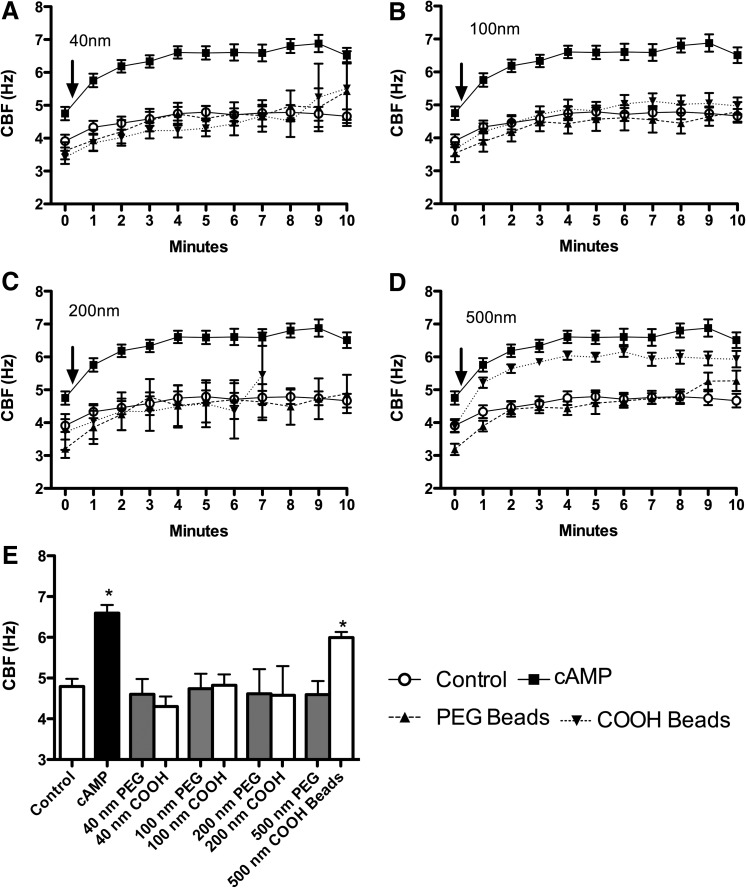
To determine if particle surface chemistry influences CBF, we introduced PEGylated nanoparticles ranging from 40 to 500 nm directly to the axonemes. PEGylated nanoparticles, regardless of diameter (40, 100, 200, or 500 nm), had no effect on CBF (Fig. 4A–D). Therefore, particles with a neutral surface chemistry did not stimulate axonemal beating regardless of particle size.
FIG. 4.
CBF stimulation of axonemes is both size and surface-coating dependent. (A–D) In all four panels, the x-axis is the time course (1-min increments) and the y-axis is CBF (Hz) following activation with 2.5 mM ATP (Control). cAMP (10 μM) is the positive control. Control and cAMP are the same data in each panel. Axonemes were exposed to neutral charge (PEG beads) or charged (COOH beads) NPs from 40–500 nm in diameter. At 40 nm (A), 100 nm (B), 200 nm (C), and 500 nm (D), uncharged particles had no effect on CBF. Smaller charged particles (40, 100, and 200 nm) similarly had no effect on CBF (A–C). However, 500 nm charged particles increased CBF significantly (p<0.05) from 1–10 min over control (D). Baseline is time zero, and the addition of beads or cAMP is indicated by arrow. (E) The y-axis is CBF and the x-axis is the indicated experimental condition. At 5 min, uncharged (PEGylated) particles had no effect on CBF regardless of size. Similarly, charged (COOH) particles up to 200 nm did not stimulate CBF compared to control axonemes (ATP only). Axonemes were treated with cAMP (10 μM) as a positive control. Each data point represents the mean±SEM of three experiments. *p<0.05 compared to control by one-way ANOVA.
The size of COOH-modified particles influences CBF in axonemes
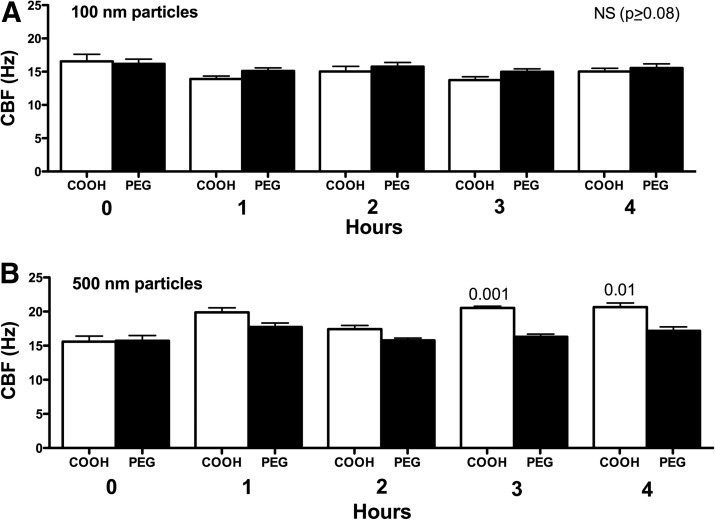
Physicochemical parameters of NPs, such as size and surface charge, have been suggested to influence both acute and chronic inflammatory responses in the lung.(28) We hypothesized that a size- and surface chemistry-dependent response could be observed in ciliary axoneme beating. Polystyrene nanoparticles conjugated with carboxy-modified surfaces (COOH), ranging from 40 to 500 nm (as above), were pipetted onto the axonemes. COOH-modified PS nanoparticles less than 200 nm resulted in no CBF changes above 2.5 mM ATP control (Fig. 4A–C). However, COOH-modified PS nanoparticles with a diameter of 500 nm, and consequently larger surface area, significantly increased CBF (Fig. 4D). The elevated CBF in response to 500 nm COOH-beads was ∼6 Hz, similar to the stimulation with 10 μM cAMP (Fig. 4D). As a control, the motion of the 500 nm COOH-beads in the absence of axonemes was not detected by SAVA (data not shown). Extending these findings to an intact cell model of cilia beat, MTEC were cultured under ALI conditions until motile cilia were expressed. Although intact cells demonstrated a higher baseline CBF than isolated axonemes, no change in CBF was observed when ciliated MTEC were treated with 100 nm particles (Fig. 5A). However, there was a significant (p<0.01) increase in CBF observed in response to 500 nm COOH-modified PS nanoparticles at 3- and 4-h exposure (Fig. 5B). Similar to axoneme particle exposure studies, 500 nm PEGylated nanoparticles failed to stimulate CBF at any time point measured.
FIG. 5.
Effect of particle surface coating and size on intact cell CBF stimulation. For panels A and B, the x-axis represents time in hr and the y-axis represents CBF in Hz. Intact mouse tracheal epithelial cells were grown to confluence and maintained in air–liquid interface culture until the majority of the cells expressed motile cilia. Cells were exposed to 100 nm (A) or 500 nm (B) PS particles with either a charge (COOH) or uncharged (PEG). Similar to isolated axoneme results, only 500 nm COOH-charged particles stimulated increases in CBF at 3 h (p<0.001 vs. 0 h) and 4 h (p<0.01 vs. 0 h).
In addition to CBF, charged nanoparticles (COOH- and SO4-modified) and near neutrally charged nanoparticles (possessing a dense PEG coating) physically interacted with the axonemes in drastically dissimilar manners. At t=0, both the PEGylated and COOH-modified nanoparticles flowed evenly over the beating axonemes. At t=1 min, the PEGylated beads remained suspended in buffer and moved in concert with the beating axonemes. However, by t=1 min, the 500-nm COOH-modified nanoparticles stuck to the axonemes, such that the nanoparticles did not beat in suspension but maintained physical attachment to the axoneme itself (see Video 1). This phenomenon occurred rapidly, as all non-PEGylated beads were fixed to axonemes by t=1 min and continued to be attached throughout the 10-min time course (data not shown).
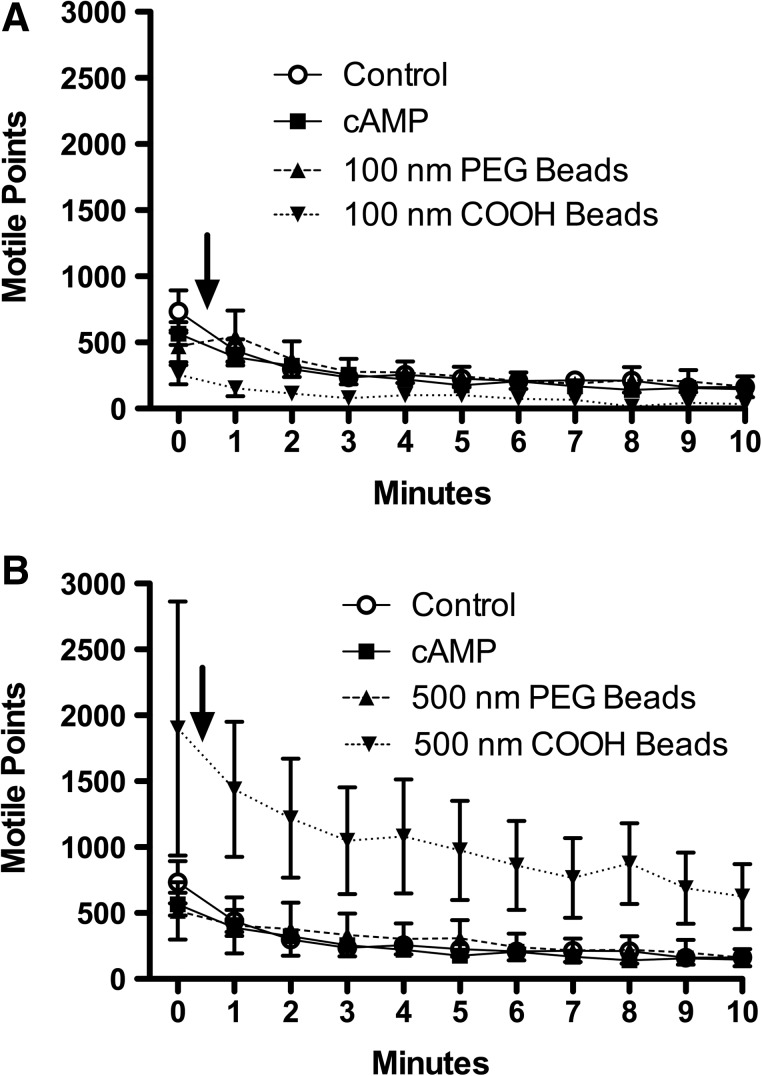
Large diameter carboxyl (COOH)-modified beads increase total motile points
The total number of motile points within the field of analysis was quantified to eliminate inconsistent axoneme sample size as an underlying variant of CBF changes that might elicit any correlating reductions or amplifications to CBF. Motile points are quantified on SAVA by measuring light and dark changes (motion) as the axonemes move through thousands of pixilated domains. With the addition of 100-nm COOH-modified beads, there was no significant change in the total number of motile points when compared to ATP only baseline control, 10 μM cAMP, or 100 nm PEGylated beads (Fig. 6A). When 500-nm COOH-modified beads were introduced to the axonemes, there was a significant increase in motile points (Fig. 6B). The COOH-modified 500 nm nanoparticles, which are visible on SAVA, can be observed attaching physically to the axoneme, resulting in multiple nanoparticles bending in concert with a single axoneme. The attachment of the 500-nm COOH-beads to the axonemes increases the number of pixels a single bending axoneme will move through, thus increasing the number of motile points within a single analysis field. Therefore, 500-nm COOH-modified beads impact the axoneme through two independent, but related mechanisms: by increasing CBF (Fig. 4D) and amplifying the motion of responsive motile cilia (Fig. 6B).
FIG. 6.
Charged beads larger than 500 nm increase total motile points. For Panels A and B, the x-axis represents time in min. The y-axis is the total number of moving points within a whole field of analysis averaged over a minimum of 10 separate fields per treatment condition. Pretreatment baseline is time zero, and the addition of beads or cAMP is indicated by arrow. (A) Exposing axonemes to non-PEGylated (COOH) particles in the 100 nm size range resulted in no significant differences in motile points compared to control (2.5 mM ATP), cAMP, or 200 nm neutral (PEGylated) beads. (B) Axonemes treated with 500 nm charged (COOH) particles generated a significant (p<0.01) increase in the total number of motile points across all time points, resulting in a nearly twofold increase versus all other treatment conditions. There was no significant difference in motile points between 2.5 mM ATP (control), cAMP, or 500 nm PEGylated beads. Each data point is the mean±SEM of three experiments.
Discussion
Axonemes provide the structural scaffold and dynein-driven bending by which cilia beat. Both 5% CSE and large-diameter COOH or SO4 surface modified-NPs rapidly stimulate CBF above baseline in axonemes. Although densely PEGylated particles under 0.22 μm in size had no effect on CBF, 500-nm COOH- or SO4-modified particles caused a significant increase in ciliary motility. These findings support the hypothesis that axonemes are mechanically sensitive to particulate matter in a surface chemistry- and size-dependent manner. The resultant changes in CBF due to particulate exposure have major implications for foreign particle deposition and clearance in the lung, leading to pulmonary dysfunction and disease. For example, CBF changes could lead to increased deposition of lung-colonizing microbes such as Streptococcus pneumoniae, especially in conjunction with ethanol consumption.(29)
The stimulation of axonemes by 5% CSE appears contrary to previously published in vitro and in vivo CBF studies of intact cilia.(30) However, CSE at the organelle level has not been examined to date. It is possible that CSE causes a delayed attenuation of CBF in axonemes; however, study limitations including ATP depletion and time restrictions on axoneme viability prevent data collection beyond the 10-min time course. An analysis of particle content in CS(27) demonstrates that primary smoke contains considerably larger particles than secondary smoke (a mean of 270 and 90 nm, respectively). The type of cigarette and presence of a filter were independent of particle size.(27) Our study, using a 220-nm filter, suggests that the size of particulates found in primary, or mainstream, CS is likely to alter axoneme responsiveness. Due to the limitation of our commercial filtration system, we were unable to test particle sizes smaller than 220 nm, that is, sizes more likely to be representative of sidestream, or secondhand, smoke. Presence of filter and brand of cigarette would be irrelevant variables in regard to these motility changes.(27)
The potential for nanomedicine to improve patient compliance and reduce side effects using respirable particles as drug carriers has made the lung an attractive target for both pulmonary and systemic pharmaceutical delivery.(31–33) The lung provides a relatively noninvasive route for inspirable drugs, among other advantages including a large surface area to deliver sustainable drug doses and achieve a relatively uniform drug distribution at the alveolar–capillary boundary.(32) For therapeutics to be distributed systemically by respirable particles, the intrinsic defense systems of the lung(34) must be eluded and the particle must escape mucociliary clearance. Effectively coating nanoparticles so as to minimize their interactions with cilia could potentially optimize particle delivery.
The size and charge of a particle can influence the efficacy of drug delivery. Although charge has been important in some in vitro studies, our results suggest that particle size and surface chemistry are key determinants of cilia–particle interaction. Particle diameter plays an influential role in optimizing a carrier that is capable of escaping host defenses, reducing inflammatory responses, and avoiding clearance by the mucociliary elevator. Many studies that investigate size alone do not consider the specific impact of surface chemistry on inspirability and mucociliary clearance. Dailey et al.,(35) for instance, demonstrates that larger PS particles caused greater infiltration of peripheral mononuclear cells, and Lauweryns et al.(36) reports that particles smaller than 260 nm are capable of evading uptake by macrophages. Meanwhile, Henning et al.(37,38) demonstrates that PS particles ranging from 50 to 6000 nm have no effect on clearance, that a physicochemical property other than particle size and total number of particles delivered affects MCC. Likewise, our study demonstrates that neutral, PEGylated particles do not impact CBF in axonemes regardless of size. Similarly, particles in the range of 40 to 200 nm do not elicit CBF changes in the organelle. However, COOH and SO4-coated particles larger than 500 nm significantly increase CBF. Thus, the ideal particle to evade MCC and successfully deliver drugs to targeted areas would be a neutral particle or small diameter particle, which might decrease immunological infiltrates in the lung, escape engulfment by alveolar macrophages, and evade entrapment in mucus and expulsion by beating cilia.
A barrier to drug uptake is the mucus blanket and periciliary layer that line the respiratory passages. Mucus is composed of mucins, which are heavily glycosylated, high molecular weight proteins with serine and threonine tandem repeats.(39,40) In order for NP carriers to transfer drugs of interest to airway cells, the particle must be able to penetrate the mucus layer. Effective transmission through the mucus barrier has been demonstrated for 200 and 500 nm polyethylene glycol-coated (PEGylated) NPs.(22,23,41) To further increase the attractiveness of neutral, small particles for drug transmission via the lung, PEGylated particles have been shown to increase time in the bloodstream and decrease hepatic toxicity when compared to non-PEGylated particles.(42) Our study shows that neutral particles do not increase CBF at the axoneme level; therefore, not only do PEGylated particles escape entrapment by mucins, but they also effect no changes in clearance by stimulating cilia beating. Conversely, large particles (500 nm COOH-modified) speed up CBF by direct mechanical interaction with the axoneme. These same particles would enhance motility should they come in contact with the beating cilia.
The signaling mechanism by which CBF is mechanically stimulated in intact cells and axonemes is an important focus for future research efforts. By investigating the biochemical pathways that stimulate or attenuate ciliary beating and axonemal bending, disease processes invoked by CS and particulates can be understood and treated. CS alone activates protein kinase C (PKC) in bronchial epithelial cells.(38) Ethanol, a biological agent shown to decrease beta agonist-stimulated CBF, is linked to cAMP-dependent protein kinase (PKA) desensitization in an in vivo model.(21) To maximally stimulate CBF in axonemes, both cAMP and cGMP are required to activate their respective protein kinase pathways (PKA and PKG).(19) Therefore, the NPs>200 nm and CS might initiate elevated CBF in the axoneme by activating PKA or PKG, even though signaling pathways in the intact cell may involve PKC-activated CBF decreases in response to CS. Importantly, the ability of particles to stimulate isolated demembranated axoneme implies a fundamental axoneme-associated beating regulation apparatus that does not depend on membrane receptors or intracellular signaling systems because neither is present on our system.
Another important moderator of CBF is epithelial-derived nitric oxide (NO).(13) We have previously demonstrated in these cell-free axoneme preparations that NO-regulable enzymes are colocalized in the basal body near the axoneme, suggesting an elaborate unit by which PKA and PKG activate CBF via NO release.(43) Protein analysis has confirmed that guanylate cyclase beta (GCβ), eNOS, PKA, and PKG are all located within the axonemal unit.(43) Such sequestering of signaling molecules and protein complexes near the axoneme suggests the intricacy by which targeted phosphorylation occurs in the intact cell to increase CBF. For initiation of ciliary beating, beta-I, beta-IV, and beta-V tubulin near the C terminus of the axoneme are necessary for ATP-activated bending.(44) The unexpectedly low number of motile points with 200 nm COOH beads is suggestive of a mechanical or charge disruption of normal protein interaction, such as the specific beta tubulins listed above. It is possible that the smaller size disrupts the beating apparatus and results in a cessation of axonemal bending in a time-dependent fashion. Alternatively, 500 nm beads, which induce a significantly higher CBF, may stimulate cyclic nucleotide-dependent processes in a more efficient and compelling manner, resulting in an enhanced signal and greater motility.
There is great appeal in identifying NP carriers that are successfully and dependably able to evade host defense mechanisms and deliver drug to target sites. Studies such as this identify the physicochemical parameters by which the surface of NPs can be modified to increase efficiency by escaping clearance via the mucociliary elevator. Our studies suggest that the use of nanoparticles that are well-coated with PEG should not cause changes in CBF and, therefore, should be considered lead candidates for inhalational drug delivery.
Supplementary Material
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Omaha VA Medical Center, Omaha, NE [Department of Veterans Affairs (VA Merit Review) to T.A.W.]. This work was supported by NIH-NIAAA (R37AA008769) to J.H.S., NIH-NIAAA (R01AA017993) to T.A.W., and NIH (R01EB003558 and P50ES015903) and Cystic Fibrosis Foundation (HANES07XX0) to J.H. The authors wish to acknowledge the expert editorial assistance of Ms. Lisa Chudomelka in the preparation of this manuscript.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- 1.Satir P. Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 2.Hastie AT. Dicker DT. Hingley ST. Kueppers F. Higgins ML. Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskel. 1986;6:25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- 3.Thorley AJ. Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:409–428. [PMC free article] [PubMed] [Google Scholar]
- 4.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 2008;59(Suppl 6):19–34. [PubMed] [Google Scholar]
- 5.Rennard SI. Togo S. Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc. 2006;3:703–708. doi: 10.1513/pats.200605-121SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H. Wang X. Brighton L. Hazucha M. Jaspers I. Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21:875–881. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simet SM. Sisson JH. Pavlik JA. Devasure JM. Boyer C. Liu X. Kawasaki S. Sharp JG. Rennard SI. Wyatt TA. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am J Respir Cell Mol Biol. 2010;43:635–640. doi: 10.1165/rcmb.2009-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott MK. Sisson JH. West WW. Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res. 2006;32:99–118. doi: 10.1080/01902140600710546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coote K. Nicholls A. Atherton HC. Sugar R. Danahay H. Mucociliary clearance is enhanced in rat models of cigarette smoke and lipopolysaccharide-induced lung disease. Exp Lung Res. 2004;30:59–71. doi: 10.1080/01902140490252885. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt TA. Schmidt SC. Rennard SI. Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med. 2000;225:91–97. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NA. Zhang S. Sharp DB. Tamashiro E. Chen B. Sorscher EJ. Woodworth BA. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 12.Tamashiro E. Xiong G. Anselmo-Lima WT. Kreindler JL. Palmer JN. Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23:117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 13.Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268:L596–L600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- 14.Lippmann M. Leikauf G. Spektor D. Schlesinger RB. Albert RE. The effects of irritant aerosols on mucus clearance from large and small conductive airways. Chest. 1981;80:873–877. doi: 10.1378/chest.80.6.873. [DOI] [PubMed] [Google Scholar]
- 15.Laube BL. Bowes SM., 3rd Links JM. Thomas KK. Frank R. Acute exposure to acid fog. effects on mucociliary clearance. Am Rev Respir Dis. 1993;147:1105–1111. doi: 10.1164/ajrccm/147.5.1105. [DOI] [PubMed] [Google Scholar]
- 16.Foster WM. Costa DL. Langenback EG. Ozone exposure alters tracheobronchial mucociliary function in humans. J Appl Physiol. 1987;63:996–1002. doi: 10.1152/jappl.1987.63.3.996. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt TA. Sisson JH. Von Essen SG. Poole JA. Romberger DJ. Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J. 2008;31:1249–1255. doi: 10.1183/09031936.00015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisson JH. Pavlik JA. Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res. 2009;33:610–616. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt TA. Forget MA. Adams JM. Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L546–L551. doi: 10.1152/ajplung.00107.2004. [DOI] [PubMed] [Google Scholar]
- 20.Slager RE. Allen-Gipson DS. Sammut A. Heires A. Devasure J. Von Essen SG. Romberger DJ. Wyatt TA. Hog barn dust slows airway epithelial cell migration in vitro through a PKC{alpha}-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1469–L1474. doi: 10.1152/ajplung.00274.2007. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt TA. Gentry-Nielsen MJ. Pavlik JA. Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang BC. Dawson M. Lai SK. Wang YY. Suk JS. Yang M. Zeitlin P. Boyle MP. Fu J. Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YY. Lai SK. Suk JS. Pace A. Cone R. Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytting E. Bur M. Cartier R. Bouyssou T. Wang X. Kruger M. Lehr CM. Kissel T. In vitro and in vivo performance of biocompatible negatively-charged salbutamol-loaded nanoparticles. J Control Release. 2010;141:101–107. doi: 10.1016/j.jconrel.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Sisson JH. Stoner JA. Ammons BA. Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 26.Dossou SJ. Bre MH. Hallworth R. Mammalian cilia function is independent of the polymeric state of tubulin glycylation. Cell Motil Cytoskeleton. 2007;64:847–855. doi: 10.1002/cm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becquemin MH. Bertholon JF. Attoui M. Roy F. Roy M. Dautzenberg B. Particle size in the smoke produced by six different types of cigarette] Rev Mal Respir. 2007;24:845–852. doi: 10.1016/s0761-8425(07)91386-8. [DOI] [PubMed] [Google Scholar]
- 28.Tetley TD. Health effects of nanomaterials. Biochem Soc Trans. 2007;35:527–531. doi: 10.1042/BST0350527. [DOI] [PubMed] [Google Scholar]
- 29.Vander Top EA. Wyatt TA. Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of streptococcus pneumoniae. Alcohol Clin Exp Res. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sisson JH. Tuma DJ. Vapor phase exposure to acetaldehyde generated from ethanol inhibits bovine bronchial epithelial cell ciliary motility. Alcoholism Clin Exp Res. 1994;18:1252–1255. doi: 10.1111/j.1530-0277.1994.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 31.Card JW. Zeldin DC. Bonner JC. Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansour HM. Rhee YS. Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai SK. Wang YY. Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azarmi S. Roa WH. Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 2008;60:863–875. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Dailey LA. Jekel N. Fink L. Gessler T. Schmehl T. Wittmar M. Kissel T. Seeger W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol. 2006;215:100–108. doi: 10.1016/j.taap.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Lauweryns JM. Baert JH. Alveolar clearance and the role of the pulmonary lymphatics. Am Rev Respir Dis. 1977;115:625–683. doi: 10.1164/arrd.1977.115.4.625. [DOI] [PubMed] [Google Scholar]
- 37.Henning A. Schneider M. Nafee N. Muijs L. Rytting E. Wang X. Kissel T. Grafahrend D. Klee D. Lehr CM. Influence of particle size and material properties on mucociliary clearance from the airways. J Aerosol Med Pulm Drug Deliv. 2010;23:233–241. doi: 10.1089/jamp.2009.0806. [DOI] [PubMed] [Google Scholar]
- 38.Elliott MK. Sisson JH. Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams OW. Sharafkhaneh A. Kim V. Dickey BF. Evans CM. Airway mucus: From production to secretion. Am J Respir Cell Mol Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voynow JA. Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 41.Lai SK. O'Hanlon DE. Harrold S. Man ST. Wang YY. Cone R. Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peracchia MT. Fattal E. Desmaele D. Besnard M. Noel JP. Gomis JM. Appel M. d'Angelo J. Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 43.Stout SL. Wyatt TA. Adams JJ. Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem. 2007;55:433–442. doi: 10.1369/jhc.6A7089.2007. [DOI] [PubMed] [Google Scholar]
- 44.Vent J. Wyatt TA. Smith DD. Banerjee A. Luduena RF. Sisson JH. Hallworth R. Direct involvement of the isotype-specific C-terminus of {beta} tubulin in ciliary beating. J Cell Sci. 2005;118:4333–4341. doi: 10.1242/jcs.02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.