Abstract
The present study examined infant negativity and maternal symptomatology by term status in a predominately low-income, rural sample of 132 infants (66 late-preterm) and their mothers. Late-preterm and term infants were group-matched by race, income, and maternal age. Maternal depression and anxiety symptoms were measured with the Brief Symptom Inventory 18 (BSI-18) when infants were 2 and 6 months of age. Also at 6 months, infant negativity was assessed by global observer ratings, maternal ratings, and microanalytic behavioral coding of fear and frustration. Results indicate that after controlling for infant age, late-preterm status predicted higher ratings of infant negativity by mothers, but not by global observers or microanalytic coding, despite a positive association in negativity across the three measures. Further, mothers of late-preterm infants reported more elevated and chronic co-morbid symptoms of depression and anxiety, which in turn, was related to concurrent maternal ratings of their infant’s negativity. Mothers response to late-preterm birth and partiality in the assessment of their infant’s temperament is discussed.
Keywords: Late-preterm birth, Temperament, Infant negativity, Maternal depression/anxiety
Preterm birth, defined as an infant born prior to 37 weeks gestation, is a prominent public health concern with more than 525,000 infants born preterm in the United States each year (NCHS, 2008). Infants born preterm present biological risk in the form of neurological immaturity, which may undermine the ability to appropriately organize and modulate physiological and behavioral responses to environmental stimuli (Als, Butler, Kosta, & McAnulty, 2005, IOM, 2003; DiPietro, Porges, & Uhly, 1992; Kopp, 1990). In the past two decades, there has been nearly a 30% increase in the incidence of prematurity, largely explained by a growing population of near-term or late-preterm infants born between the 34th and 37th week of gestation (Raju, Higgins, Stark, & Leveno, 2006). Late-preterm infants comprise 71% of preterm infants, yet the majority of the extant prematurity literature has focused on the mortality and morbidity of the highest risk births; “very preterm” infants born before the 32nd week of gestation (Petrini et al., 2008; Davidoff, et al. 2006). While decreasing gestational age typically corresponds to increasing morbidities, recent studies have shown that late-preterm birth status has an impact on physical health, such that relative to term infants, late-preterm infants have higher rates of respiratory distress, apnea, hypothermia, jaundice, and other neonatal morbidities (Engle et al., 2007; Raju et al., 2006; Wang, Dover, Fleming, & Catlin, 2004). However, the socio-emotional development of late-preterm infants and the effects of a late-preterm birth on parents emotional well-being have not been well-studied to date. The primary aim of the present study is to explore late-preterm infant temperament, specifically behavioral manifestations of infant negativity, and maternal symptoms of depression and anxiety.
Studies examining temperament among very preterm infants have generated considerable evidence to suggest that the nascent instability of autonomic, motor, and state systems places infants born before the 32nd week of gestation at a greater risk for behavioral disorganization manifested in a lower threshold for stimulation, and increased negativity, compared to term infants. Mothers have reported their very preterm infants to be more temperamentally difficult, describing their disposition as less rhythmic and adaptable and more negative in response to communicative bids (Spungen & Farran, 1986; Gennaro, Tulman, & Fawcett, 1990; Langkamp, Kim, & Pascoe, 1998; Hughes, et al., 2002). Further, behavioral coding by observers has shown very preterm infants to display more facial grimacing and negative arousal during interaction (Eckerman, Hsu, Molitor, Leung & Goldstein, 1999; Crnic, Ragozin, Greenberg, Robinson, & Basham, 1983), as well as, less soothability and organized behavioral states than term infants (Friedman, Jacobs, & Werthman, 1982; Field, 1977).
Other studies suggest that medical risk and age of assessment may account for preterm-term differences in negativity (Hughes et al., 2002; Bigsby et al., 1996). For example, it has been shown that very preterm infants who had longer stays in the neonatal intensive care unit (NICU) and more severe medical complications were rated by their mothers as less favorable in mood and less adaptable (Spungen & Farran, 1986). In addition, there has been some evidence to suggest that preterm-term differences in infant negativity dissipate over time, and in particular, after the preterm infant’s physiological stability has been met (Riese, 1988). In a longitudinal study of socioemotional functioning of preterm infants across the first 2 years, very preterm and term infants were rated similarly on emotional tone during a series of age appropriate play vignettes (Riese, 1988). No studies to date have examined the temperament of late preterm infants.
The term preterm parents is used to represent the idea that early birth is a risk factor for maternal psychological well-being and has been associated with difficult adaptation to parenthood (Bennett & Slade, 1991; Davis, Edwards, Mohay, & Wollin, 2003; Kersting et al., 2004; Teti, Hess, & O’Connell, 2005). Past research has shown that mothers of preterm infants are at an increased risk for depression and anxiety, which extends beyond discharge from the NICU (Whiffen, 1988; Miles, Holditch-Davis, Burchinal, & Nelson, 1999; Davis et al., 2003; Kersting et al., 2004; Teti, et al., 2005). For example, in comparison to mothers of term infants, a significantly greater proportion of very preterm mothers are likely to not only be depressed immediately post term (45% at hospital discharge), but to stay depressed (36% at 12 months) (Miles et al., 1999). Likewise, mothers of very preterm infants also report significantly higher levels of state anxiety postpartum (Zanardo, Freato, & Zacchello, 2003). This elevated risk for maternal symptomatology among very preterm mothers has been attributed to persistent concern regarding the infant’s health and well-being, and a view of the infant as highly vulnerable, even after medical issues have been resolved (Stern & Karraker, 1990). In addition, very preterm infants may be less responsive to communicative bids and exhibit arousal cues that are difficult to read, thus requiring fine-tuned responding on behalf of the parent, which may diminish parental self-efficacy and be emotionally taxing (Teti & Gelfand, 1991; Als et al., 2005; Schmucker et al., 2005). Despite the wealth of information on mothers of very preterm infants, no research has examined the emotional state of mothers of late-preterm infants.
The purpose of the present study was to: (1) determine if late-preterm and term infants differ in the temperamental dimension of infant negativity at 6 months of age, indexed by observer and maternal report in a group-matched sample by race, income, and maternal age; (2) address if late-preterm birth is associated with an increased risk for elevated symptoms of maternal depression and anxiety in the first 6 months; and (3) explore how maternal symptomatology affects mother’s perceptions of infant negativity. In comparison to very preterm infants, late-preterm infants, who are more physically mature at birth and generally present lower levels of medical risk, may exhibit increased physiological stability, and thus have improved state regulation. Therefore, it is plausible that late-preterm infants may not show increased behavioral displays of negativity relative to term infants. However, this may only be the case when infant negativity is assessed via observer ratings. Although the emotional effects of having a late-preterm infant on mothers are unclear, the preterm label may still generate concern, stress, and worry in preterm parents, and increase risk for symptoms of depression and anxiety, which in turn may increase their perception of their infant’s negativity. Presently, very little is known about the behavioral outcomes of late-preterm infants, thus no hypothesis was proposed regarding whether late-preterm and term infants differ with respect to infant negativity. However, we did expect that mothers of late-preterm infants would be at greater risk for experiencing elevated symptoms of depression and anxiety. Further, maternal emotional state may impact the perception of her late-preterm infant’s temperament, such that mothers who experience depressive or anxious symptoms are more likely, in their own negative emotional state, to perceive their infant’s behavior as more negative (McGrath, Records, & Rice, 2008). Thus, it was also hypothesized that maternal symptomatology would exacerbate perceptions of her infant’s negativity, particularly among late-preterm infants.
1. Method
1.1. Participants
This analysis of data is on a sub-sample of 132 infants (66 preterm) and their mothers drawn from the Family Life Project (N=1292), a longitudinal study examining how child characteristics, family life, and community context contribute to individual differences in the development of competence in young children living in two areas of concentrated rural child poverty in the United States: Appalachia and the Black South. Inclusion criteria restricted the sample to infants and his or her biological mother who participated in both the 2- and 6-month home visits.
In the present study, the criterion for preterm birth was infants born between 32 to 37 weeks gestation (mean gestation of preterms = 35.5 weeks)1. Since prematurity was not a principal interest in the larger study design of the Family Life Project, those preterm infants included in the current sample are categorized as late-preterm infants, who comparatively are of lower medical risk than very preterm infants born before 32 weeks completed gestation. For example, only 11 of the 66 preterm infants (17%) were placed in the neonatal intensive care unit (NICU) post birth. Further, the average hospital stay of preterms was 13 days (range=1 to 45 days).
Given that preterm birth disproportionately affects African-American, low-income, and teenage women (Kramer et al., 2001), we drew a group matched sample of term infants for comparison with preterm infants by income status, race, and maternal age (see Table 1). Of the total sample (N=132), 38% of the infants were African-American, 51% of the infants were female, and 68% were low-income. At the time of recruitment, mothers had a mean age of 26 years (range = 15 to 43 years), 84% had completed a high school degree or equivalent, and 41% were currently employed. Forty-eight percent of the mothers were married.
Table 1.
Infant and Family Demographic Characteristics of Late-Preterm and Full Term Infants
| Characteristics | Late-Preterm (n=66) |
Full Term (n=66) |
t/χ2 |
|---|---|---|---|
| Mean (SD) gestational age (weeks) | 35.58 (1.37) | 39.73 (1.40) | −19.88*** |
| Mean (SD) birth weight (m) | 2611.6 (505.14) | 3343.1 (505.33) | −8.32*** |
| % Male | 57.58 | 43.94 | 2.46 |
| % Infants African-Americana | 37.88 | 37.88 | 1.01 |
| Mean (SD) infant age at 6 month home visit | 7.65(1.13) | 7.06 (0.96) | 3.25** |
| Mean (SD) infant age at 6 month home visit, corrected for prematurity | 6.55 (1.02) | 7.06 (0.96) | −2.98** |
| Mean (SD) Maternal age | 26.63 (6.35) | 26.70 (6.70) | −0.07 |
| Maternal education | |||
| % high school degree or equivalent | 81.82 | 86.36 | 0.51 |
| % 4-year college degree | 18.18 | 13.64 | 0.51 |
| % Mothers Employed | 43.94 | 37.88 | 0.50 |
| % Married | 48.48 | 48.48 | 0.00 |
| % Mothers African-Americana | 39.39 | 33.33 | 1.64 |
| Mean (SD) Income-to-needs ratio | 1.80 (1.70) | 1.80 (1.57) | 0.01 |
| Mean (SD) Rurality index | 8.52 (0.79) | 8.28 (0.71) | 1.82 |
| % Pennsylvaniab | 45.45 | 51.52 | 0.48 |
p<.05,
p<.01,
p<.001
0=White, 1=African-American
0=North Carolina, 1=Pennsylvania
1.2. Procedure
The Family Life Project recruited families from three counties in the Appalachia region of Pennsylvania: Blair, Cambria, and Huntingdon, and three counties in the Black South region of North Carolina: Sampson, Wayne, and Wilson. English-speaking families, residing in one of the target counties, with no intent to relocate from the area in the next 3 years were recruited from 10 obstetric clinics. A cohort sampling method was used to over sample for low-income families and, specifically in the Black South, African-American families (with the expectation that families in Appalachia would be almost exclusively Caucasian). Of the 2,678 women who provided informed consent, a lottery system was employed to fill planned sampling cells (race, income, and site) and 1,553 families were formally invited to participate.
After hospital recruitment, infants and their families were visited in the home once when the infant was approximately 2 months of age, at which enrollment in the study was confirmed (N=1,292), and twice when their infant was approximately 6 months of age (age window 5.5–9 months). Each home visit lasted approximately 2–3 hours. During each visit, mothers completed questionnaires and interview assessments. In addition, at 6 months, infants participated in a series of fear and frustration inducing tasks designed to elicit emotion reactivity which were videotaped for later behavioral coding.
1.3. Measures
1.3.1. Demographic and socioeconomic status variables
Demographic data used in this study was collected via questionnaire at the 2 month home visit. All participants completed the K-fast literacy screener (Kaufman & Kaufman, 1994) and those who read at an eighth-grade reading level or beyond completed questionnaires independently (81% of total sample). For the remaining group that read below an eighth-grade reading level, all questionnaires were read to them (19% of total sample). Primary caregivers provided detailed information regarding infant sex, household composition, marital status, employment, and social address variables including race, education, and income. In addition, the 6 month demographic updates included a question on infant’s chronological age at the time of the visit. Site was dichotomized at the state level (PA or NC).
The Income-to-Needs Ratio was calculated by totaling all sources of reported household income at the 6 month home visit divided by the 2004 federal poverty threshold. The threshold is based on the number of people living in the household, further accounting for how many of those individuals are under the age of 18. For group matching of preterm and term infants, the household income-to-needs ratio was coded as a dichotomous low-income/non-low-income value. The low-income group was defined as a ratio value less than 2, representative of families living below 200% of the federal poverty line. Families with a ratio value equal or greater than 2 were defined as non low-income.
1.3.2. Infant birth status
The difference between infant birth date and due date, obtained during a recruitment screening questionnaire that biological mothers completed while in the hospital, was computed to represent gestational age. Preterm birth was defined as an infant born at less than 37 weeks gestation (N=80). Fourteen children born very preterm (<32 weeks) were excluded from the current preterm group as the extent of physical immaturity at birth and related medical risk is recognized to be qualitatively greater than that of preterm infants born after 32 weeks gestation (Nadeau, Tessier, Boivin, Lefebvre, & Robaey, 2003; Mouradian, Als, & Coster, 2000).
At the 2 month home visit, biological mothers completed the pregnancy and delivery module of the Missouri Assessment of Genetics Interview for Children (MAGIC; Todd, Joyner, Neuman, Heath, & Reich, 2003). Mothers reported their infant’s birth weight in pounds and ounces, which was converted to grams for data analysis. Although this was a retrospective report, past work shows that mothers recall of events during pregnancy are notably reliable and stable, especially within the first year post term, and particularly for those who experience delivery complications associated with low birth weight or prematurity (Sou, Chen, Hsieh, & Jeng, 2006; Reich, Todd, Joyner, Neuman, & Heath, 2003; Githens, Glass, Sloan, & Entman, 1993).
1.3.3. Maternal depression and anxiety symptoms
Brief symptom inventory 18 (BSI-18)
At the 2 and 6 month visits, mothers symptomatology was assessed using a composite of the depression and anxiety subscales of the Brief Symptom Inventory 18 (BSI-18) (Derogatis, 2000). The BSI-18 is a short, yet highly sensitive self-report screening index of psychological distress that has been widely used in the field (e.g. Aisenberg, 2001). The depression and anxiety subscales were each comprised of 6 items, scored using a 5-point Likert-type scale ranging from 0 (not at all) to 4 (extremely), targeting present feelings and symptoms. Raw item scores were summed to create depression and anxiety subscale total scores. The depression and anxiety subscales demonstrated good internal consistency in the current sample at 2 months (Dep. α= .80, Anx. α= .74) and 6 months (Dep. α= .80, Anx α= .81). In addition, depression and anxiety symptoms were significantly related in the current sample, r (132) = .65, p<.01. Standardized depression and anxiety t-scores were averaged at 2 and 6 months to derive a more general maternal symptomatology measure.
1.3.4. Infant negativity
Global observer report – Infant Behavior Record (IBR)
At both 6 month visits, two independent observers evaluated infant behavior across the duration of the visit and upon completion, rated infant temperament using an adapted version of the Infant Behavior Record (IBR) (Bayley, 1969; Stifter & Corey, 2001; Stifter, Willoughby, & Towe-Goodman, 2008). The adapted version used in the current study is comprised of 11 items including a 9-pt Likert-type subscale measuring irritability which was used in the present study to reflect global observer report of infant negativity. The internal consistency alpha for Irritability was .70, and home visitors showed moderate within-visit (r = .58–59) and cross-visit (r = .46) agreement. A mean negativity score across the two raters was calculated at each visit, standardized, and averaged across the two 6-month visits.
Maternal report – Infant Behavior Questionnaire (IBQ)
At the second 6 month home visit, a shortened 60 item version of the Infant Behavior Questionnaire (Rothbart, 1981) was administered to assess mothers report of their infant’s temperament. The distress to limitations and distress to novelty subscales, each 16 items rated on a 7-point Likert-type scale ranging from 1(never) to 7 (always), were averaged (r (132) = .44, p < .001) and used to index maternal ratings of infant negativity. Parents reported on specific infant behaviors during the previous week (e.g. When placed in an infant seat or car seat how often did the baby cry or get upset at first, then quiet down?). The IBQ has been widely validated, and the current sample generated comparable internal consistency alphas of .74 for distress to limitations and .87 for distress to novelty to previous studies (Rothbart, 1986).
Microanalytic behavioral coding
At the second 6 month home visit, infants were also videotaped during the administration of a set of developmentally appropriate emotional challenge tasks, including a presentation of 4 masks and a gentle arm restraint. Each of these tasks has been validated for use in the home environment (e.g. Kochanska, Coy, Tjbekes & Husarek, 1998). Prior to administration, mothers reported that the infant was fed and rested. Tasks were stopped early at the request of the mother or if the infant had been crying hard for a minimum of 20 consecutive seconds.
For the mask task (Lab-TAB, Goldsmith & Rothbart, 1996), infants were presented with four unusual masks in succession (a long-nosed woman, a Frankenstein, a goofy vampire, and a bald conehead). While wearing each mask for 10 seconds, the home visitor repeated the child’s name three times while moving from side to side and then leaning towards the child’s face. Mothers were seated next to the infant during this task. They were asked to maintain a neutral facial expression and to refrain from verbally interacting with their infant. Next, the mothers were asked to step out of the room during the gentle arm restraint of their infant (adapted from the Lab-TAB, Goldsmith & Rothbart, 1996). Infants were placed in a walker and the home visitors positioned themselves behind the walker and gently restrained the child’s arms for a period of up to 2 min or 20 s of hard crying. After releasing the infant’s arms, the home visitor and mother remained non-interactive for 1 min. Following this, the home visitor cued the mother to return and instructed her to soothe her infant as she normally would.
Infant emotional reactivity was coded second-by-second using Better Coding Approach software (Danville, Pennsylvania). Three levels of negative reactivity were coded: low, moderate, and high negative reactivity (Stifter & Braungart, 1995). For data reduction, a negativity reactivity intensity score was calculated using the respective proportion of time the infant spent at each level of negativity reactivity, quantified by a ratio of the total duration at a given level of reactivity relative to the task total duration, multiplied by the behavioral code. The mean negativity reactivity intensity score across the mask and gentle arm restraint tasks were used to index microanalytic behavioral coding of infant negativity. Interrater reliability was calculated on 15% of the sample, averaging 93% percent agreement and .89 by Cohen’s kappa.
2. Results
2.1. Preliminary analyses
Several variables, including maternal age, education, marital status, race, infant birth weight, infant sex, geographic site, rurality, and infant chronological age at time of 6 month visit, were determined to be possible covariates of the variables of interest in the current investigation. Differences between the late-preterm and term sample groups were examined via t-test, for continuous variables, and chi-square, for categorical variables. Due to group matching of term infants in sample selection, late-preterm and term infants were not expected to differ by maternal age, race, and poverty status. As presented in Table 1, across term status groups, there were no significant differences in maternal age, race, and income-to-needs ratio, as well as, maternal education, employment, marital status, rurality, site, and infant sex and race. As expected, significant group differences were found for infant gestational age and birth weight, and infant gestational age was highly related to infant birth weight r (132) = .74, p < .001. As such, infant birth weight was not included as a covariate in the primary analyses given the existing multicolinearity, which justifies infant gestational age as a proxy for birth weight in the current sample.
Since prematurity was not of principal interest in the larger study design, all infants were visited in the home for the 6 month visit during a chronological age window, between 5 to 9 months, that was not corrected for preterm status. To correct for prematurity in the current analyses the weeks/days born preterm was subtracted from the chronological age at the 6 month visit for preterm infants only. As seen in Table 1, late-preterm infants were significantly younger when corrected for gestational age at the 6 month visit in comparison to term infants. Moreover, infant age at the 6 month visit was related to the three measures of infant negativity (see Table 2). Therefore infant age at the 6 month home visit, corrected for preterm infants, was included as a covariate in the primary analyses.
Table 2.
Correlations Among Infant Gestational Age, Maternal Symptomatology, and 6 Month Infant Negativity Variables within the Total Sample
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Infant gestational age | -- | ||||||
| 2. Infant corrected age at 6 | 0.14 | -- | |||||
| months | |||||||
| 3. Maternal BSI at 2 months | −0.13 | −0.10 | -- | ||||
| 4. Maternal BSI at 6 months | −0.25** | −0.06 | 0.51*** | -- | |||
| 5. Global Observer Rating of Negativity | −0.10 | −0.29*** | 0.04 | 0.11 | -- | ||
| 6. Maternal Rating of Negativity | −0.26** | 0.27** | 0.16 | 0.24** | 0.20* | -- | |
| 7. Microanalytic Observer Coding of Negativity | 0.20* | 0.11 | −0.01 | 0.005 | 0.23* | 0.27* | -- |
p < .05.
p < .01.
p < .001
Intercorrelations among the main study variables were also calculated. As can be seen in Table 2, younger gestational age, indicating greater degree of prematurity was related to higher maternal rating of negativity. For all mothers in the sample, maternal symptoms of depression and anxiety were positively correlated across the 2 and 6 month measurements, r(132) = .51, p < .001. Further, there were positive interrelationships among the three measures of infant negativity. That is, infants who were observed to be more irritable and coded as more negative during the home visits were also rated by mothers as more negatively reactive. In addition, Table 3 lists mean values of study variables by term status.
Table 3.
Means, Standard Deviations, and Ranges for Maternal Symptomatology and Infant Negativity
| Full Term |
Preterm |
||
|---|---|---|---|
| M (SD) | M (SD) | Range | |
| Maternal Symptomatology | |||
| 2 Month BSI Depression/Anxiety | 44.64 (6.23) | 46.29 (8.06) | 39.00–68.50 |
| 6 Month BSI Depression/Anxiety | 44.88 (5.88) | 49.02 (8.62) | 39.00–70.50 |
| Infant Negativity | |||
| Global Observer Rating of Negativity | 2.97 (0.98) | 3.23 (1.14) | 1.25–6.50 |
| Maternal Rating of Negativity | 2.79 (0.62) | 3.18 (0.94) | 1.50–6.53 |
| Microanalytic Coding of Negativity | 0.43 (0.42) | 0.35 (0.36) | 0.00–1.68 |
2.2. Late-preterm status and infant negativity
To explore differences in infant negativity at the 6 month home visit, separate general linear models (Aiken & West, 1991) were conducted to test mean differences (see Table 4) in observer and maternal ratings of infant negativity by term status, controlling for corrected age at 6 months. The omnibus F-test for global observer ratings of infant negativity, F (2, 128) = 6.24, p < .01, was significant. However, individual parameter estimates indicate this overall effect is driven by the relation of 6 month infant age to global observer ratings of negativity. Controlling for corrected age at 6 months, there were no mean differences in global observer ratings of infant negativity for late-preterm and term infants. Likewise, controlling for corrected age at 6 months, the models for microanalytic coding of infant negativity, F (2, 109) = 0.95, p = .391, were non-significant. The model for maternal ratings of infant negativity, F (2, 122) = 12.88, p < .001 was significant. As expected, beyond the influence of corrected 6 month age mothers of late-preterm infants rated their infants significantly higher in negativity compared to mothers of term infants.
Table 4.
General Linear Model Analysis Predicting Infant Negativity at 6 Months
| β | t | p | ||
|---|---|---|---|---|
| Global Observer Rating of Infant Negativity | Step 1: Control variable entered Infant corrected age at 6 months | −.28 | −3.23 | .002** |
| Step 2: Test of main effects Preterm status | .09 | 0.52 | .607 | |
| Maternal Rating of Infant Negativity | Step 1: Control variable entered Infant corrected age at 6 months | .34 | 4.08 | <.001*** |
| Step 2: Test of main effects Preterm status | .66 | 3.90 | <.001*** | |
| Observer Microanalytic Behavior Coding of Infant Negativity | Step 1: Control variable entered Infant corrected age at 6 months | .09 | 0.89 | .378 |
| Step 2: Test of main effects Preterm status | −.15 | −0.78 | .439 |
p < .05,
p < .01,
p < .001
2.3. Late-preterm birth status and maternal symptomatology
To test whether mothers of late-preterm infants would have higher levels of symptomatology, separate linear models used to examine mean differences in the 2 and 6 month maternal symptoms composite of depression and anxiety symptomatology by term status, controlling for infant corrected age at 6 months. There was no difference in two month maternal symptomatology, t (131) = 1.07, p = .285, across mothers of term and late-preterm infants. However, there was a significant difference in 6 month maternal symptomatology, t (131) = 3.12, p < .01, such that mothers of late-preterm infants reported greater symptoms of depression and anxiety than mothers of term infants.
Mothers of late-preterms were expected to be more likely to experience elevated symptoms of depression and anxiety across the 2 and 6 month measurements. To examine this hypothesis, mothers with elevated symptoms in the top quartile at 2 (n = 23) or 6 months (n = 27) were identified. Further, 18 mothers reported chronic elevated symptoms in the top quartile at both 2 and 6 months. As can be seen in Table 5, at 2 months, there was no significant difference in the number of mothers with elevated symptomatology across late-preterm and term infants (p = .25). However, at 6 months the χ2 difference was significant, such that a greater number of mothers of late-preterms reported elevated symptomatology (p <.01). Moreover, a significant difference between the late-preterm and term groups was also found for chronicity, such that a greater number of mothers of late-preterm infants reported elevated symptomatology at 2 and 6 months (p < .05).
Table 5.
Chi-Square Test of Independence for Mothers’ of Late-Preterm and Full Term Infants Symptomatology
| Late-Preterm | Full Term | n total | χ2 | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Elevated 2M Symptoms | 14 | 21.0% | 9 | 14.0% | 23 | 1.32 |
| Elevated 6M Symptoms | 20 | 30.0% | 7 | 11.0% | 27 | 7.87** |
| Chronic Symptoms | 13 | 20.0% | 5 | 8.0% | 18 | 4.12* |
p < .05,
p < .01
2.4. Mediation of late-preterm status and maternal ratings of infant negativity by maternal symptoms
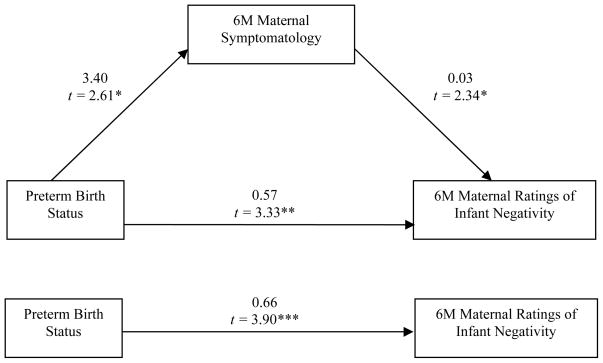
To test whether maternal symptomatology mediated the relationship between late-preterm status and maternal ratings of infant negativity, direct and indirect effects of this association were examined. Since neither the global observer ratings, nor the microanalytic coding, of infant negativity were predicted by late-preterm status, further analyses to test for the mediation of maternal depression and anxiety were not explored. However, late-preterm status, controlling for 6 month corrected age, was related to maternal ratings of infant negativity, t (123) = 3.90, p < .001, such that late-preterm infants were more likely to be rated as more negative by their mothers. Further, late-preterm status predicted higher maternal symptomatology at 6 months, t (123) = 2.60, p < .05, but not at 2 months. Therefore, the mediating effect of 6 month maternal symptomatology on the relationship between infant late-preterm status, controlling for infant age, and maternal ratings of infant negativity was tested. As seen in Figure 1, 6 month maternal symptomatology predicted higher concurrent ratings of infant negativity, controlling for infant age at the 6 month assessment and late-preterm status, t(122) = 2.34, p < .05. This indirect effect, .09, bias-corrected 95% C.I. = [.01, .27], was significant, as indicated by Preacher and Hayes (2008) non-parametric bootstrap method appropriate for smaller sample sizes with multivariate non-normality.
Figure 1.
Path diagram of the unstandardized beta weight estimates and t-values for the mediating effect and residual direct effect of preterm status on maternal ratings of infant negativity at 6 months, * p < .05, ** p < .01.
3. Discussion
The sum of past research on behavioral negativity of preterm infants suggests that preterm infants may be easily over-aroused and have difficulty with state transitioning, thus, they often display higher levels of negativity in infancy (Hughes et al., 2002; Bigsby et al., 1996). However, these studies have focused on high-risk births of very preterm infants born before 32 weeks completed gestation, who often present more medical complications and physiological immaturity. Comparatively much less is known about the socio-emotional development of late-preterm infants, who may exhibit less biological risk and instability at birth (Raju et al., 2006). The goal of the current study was to address this gap by examining the effect of late-preterm status on the infants negativity in the first six months of life and the mediating effect of maternal depression/anxiety on this relationship.
In the present study, the inclusion of multiple measures of infant negativity revealed that despite a positive association across measures, late-preterm-term differences in negativity were only found for maternal ratings. Mothers of infants born, on average, at 35 completed weeks gestation rated their infants as higher on negativity than mothers of term infants. This finding with a sample of late-preterm infants corroborates past research which suggests that mothers of very preterm infants find their infants to be more difficult, exhibiting more fussing and crying during social interaction (Spungen & Farran, 1986; Gennaro et al., 1990; Langkamp et al, 1998; Hughes et al., 2002). However, there was no relation between preterm status and global observer ratings or microanalytic coding of negativity. Thus, in the current sample of late-preterm infants, higher maternal ratings of preterms negativity may not be a reflection of observed infant affective behavior, per se, but more an indication of the degree of partiality in assessment (Stifter et al., 2008). In comparison to impartial observers, mothers may rate their late-preterm infants as more negative due to their perception of their infant’s vulnerability.
The findings of the current study also add to the literature concerning late-preterm mothers and the risk for persistent symptoms of depression and anxiety (Goldberg & DiVitto, 1983; Whiffen, 1988; Miles et al., 1999; Davis et al, 2003; Kersting et al., 2004; Schmucker et al., 2005). At 2 months postpartum, there were no group differences in maternal symptoms for mothers of preterm and term infants. Although this was contrary to expectations, at 2 months, postpartum feelings of depression and anxiety may be more common for all mothers in the current sample who are to varying degrees experiencing financial hardship, irrespective of prematurity (Rich-Edwards et al., 2006). Living in the context of rural poverty and bringing home a new infant may be stressful, as an additional member of the family may induce a greater degree of economic strain and challenges associated with employment and childcare provision (Evans & English, 2002). Thus, at 2 months, all mothers may be adjusting to the birth of a new baby, and adapting to make ends meet.
However, at 6 months, the combination of having a late-preterm infant reared in a low-income environment, referred to as double jeopardy, may begin to place an added strain on mothers (Aylward, 1992). As expected, differences emerged such that mothers of late-preterm infants reported greater symptoms of depression and anxiety. At this age, beyond postpartum depression, mothers of late-preterms may experience greater symptomatology related to ongoing concern about their infant’s fragility, growth, and achievement of age-appropriate developmental milestones (Miles et al., 1999; Teti et al., 2005). In addition, mothers may be feeling overwhelmed or down as they experience asynchronous interactions with their late-preterm infant, some of which may result in infant over-arousal and negativity (Bakeman & Brown, 1980; Crnic et al., 1983; Poehlman & Fiese, 2001; Muller-Nix et al., 2004). It is also noteworthy that in the current sample nearly three times as many mothers of a late-preterm infant experienced chronically elevated depression and anxiety symptoms across the first 6 months compared to mothers of a term infant.
Importantly, not only were mothers of late-preterm infants more likely to experience elevated and stable symptoms of depression and anxiety, but maternal emotional state had clear implications for concurrent maternal ratings of infant negativity (Leerkes & Crockenberg, 2003; Downey & Coyne, 1990; Whiffen, 1990). Findings from the present study suggest that mother’s concurrent emotional state may partly explain the relationship between infant preterm status and higher ratings of maternal infant negativity. Importantly, maternal depression and anxiety may exacerbate negative attributions about preterm infant behavior, even in late-preterm infants (Whiffen, 1990).
3.1. Limitations
Whereas the present study extends our understanding of the socio-emotional development of late-preterm infants, finding strength in the utilization of multiple measures to assess infant negativity in a diverse population of individuals living in rural poverty, there are several limitations to be noted. First, prematurity was not one of the central aims of the larger research study. Thus, it would be important in future work that correction for gestational age be applied prospectively when determining visit age windows. Late-preterm infants were younger than their term counterparts at the 6 month assessment, and as age is inextricably linked with behavioral manifestations of infant negativity, age proved to be a particularly influential predictor of the behavioral variance between late-preterm and term infants, perhaps masking potential group differences in observable behavior. In addition, the current study recruited mothers from hospitals after giving birth, thus there was no prenatal assessment of maternal depression or anxiety, which have been substantiated as important predictors of preterm birth (Wadhwa, Sandman, Porto, Dunkel-Schetter, & Garite, 1993). Prenatal maternal symptomatology may also predict postnatal symptoms, parenting behavior including sensitivity and ability to read infant cues and respond appropriately, and infant’s behavioral negativity (O’Connor, et al., 2002). Furthermore, due to group level matching of preterm and term infants, variables like race, marital status, and income were controlled for, and thus were not explored as potential contributors to overall cumulative risk. Given that the majority of the families were low-income, there may be an additional bias for depression and anxiety symptoms among mothers in this sample. Variations in income, for example, might elucidate important differences in maternal symptomatology and the effect of late-preterm birth. Finally, another direction for future research would be to include additional time points, both prior to and/or following 6 months of age, to assess infant behavior and maternal symptomatology. The longitudinal nature of these designs would be advantageous, as they would support questions of behavioral change in late-preterm infants, and further examination of stability or discontinuity in maternal perceptions of late-preterm infant negativity over time.
In summary, the findings of the present study suggest that in a late-preterm population of relatively low medical risk, observers do not note behavioral differences in infant negativity. However, this is not the case for mothers, since those that have a late-preterm infant indicate higher infant negativity than mothers with an infant born at term. This relationship is partly explained by the increased prevalence of maternal symptoms of depression and anxiety in mothers of late-preterm infants. These findings have implications for programs targeting late-preterm infants and their parents, highlighting social risk factors such as mothers adjustment to the birth of late-preterm infant, even if he or she is only a few weeks early, and experience with depression and anxiety symptomatology as key points for intervention.
Acknowledgments
We would like to thank the families who participated in this study. This research was supported by the National Institute of Child Health and Human Development Grant P01HD39667, with cofunding from the National Institute on Drug Abuse. The Family Life Project key investigators include Lynne Vernon-Feagans, Martha Cox, Clancy Blair, Peg Burchinal, Linda Burton, Keith Crnic, Ann Crouter, Patricia Garrett-Peters, Mark Greenberg, Stephanie Lanza, Roger Mills-Koonce, Debra Skinner, Cynthia Stifter, Emily Werner, and Michael Willoughby.
Footnotes
Eight moderately preterm infants, with a gestational age between 32 and 34 weeks gestation, are included in the sample of 66 late-preterm infants as there were no statistical differences in the study variables including length of hospital stay post birth, birth/medical complications, maternal symptoms, and infant negativity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristin M. Voegtline, Department of Human Development and Family Studies, Pennsylvania State University
Cynthia A. Stifter, Department of Human Development and Family Studies, Pennsylvania State University
Family Life Project Investigators, Pennsylvania State University and the University of North Carolina at Chapel Hill.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Als H, Butler S, Kosta S, McAnulty G. The assessment of preterm infants’ behavior (APIB): Furthering the understanding and measurement of neurodevelopmental competence in preterm and full-term infantsMental Retardation and Developmental Disabilities Research Reviews. Special Issue: NeuroDevelopmental Assessment of the Fetus and Young Infant. 2005;11:94–102. doi: 10.1002/mrdd.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R, Brown JV. Early interaction: Consequences for social and mental development at three years. Child Development. 1980;51:437–447. [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Mental Development. New York: Psychological Corporation; 1969. [Google Scholar]
- Bennett DE, Slade P. Infants born at risk: Consequences for maternal post-partum adjustment. British Journal of Medical Psychology. 1991;64:159–172. doi: 10.1111/j.2044-8341.1991.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Bigsby R, Coster W, Lester BM, Peucker MR. Motor behavioral cues of term and preterm infants at 3 months. Infant Behavior & Development. 1996;19:295–307. [Google Scholar]
- Crnic K, Ragozin AS, Greenberg MT, Robinson NM, Basham RB. Social interaction and developmental competence of preterm and full-term infants during the first year of life. Child Development. 1983;54:1199–1210. [PubMed] [Google Scholar]
- Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Seminars in Perinatology. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Davis L, Edwards H, Mohay H, Wollin J. The impact of very premature birth on the psychological health of mothers. Early Human Development. 2003;73:61–70. doi: 10.1016/s0378-3782(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Derogatis L. Brief Symptom Inventory. Vol. 18. Minneapolis, MN: NCS Pearson, Inc; 2000. [Google Scholar]
- DiPietro JA, Porges S, Uhly B. Reactivity and developmental competence in preterm and full-term infants. Developmental Psychology. 1992;28:831–841. [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: An integrative review. Psychological Bulletin. 1990;108:50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Eckerman CO, Hsu H, Molitor A, Leung EHL, Goldstein RF. Infant arousal in an en-face exchange with a new partner: Effects of prematurity and perinatal biological risk. Developmental Psychology. 1999;35:282–293. doi: 10.1037//0012-1649.35.1.282. [DOI] [PubMed] [Google Scholar]
- Engle WA, Tomashek KM, Wallman C the Committee on Fetus Newborn. “Late-preterm” infants: A population at-risk. Pediatrics. 2007;120:1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socio-emotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Field TM. Effects of early separation, interactive deficits, and experimental manipulations on infant-mother face-to-face interaction. Child Development. 1977;48:763–771. [Google Scholar]
- Friedman SL, Jacobs BS, Werthman MW. Preterms of low medical risk: Spontaneous behaviors and soothability at expected date of birth. Infant Behavior & Development. 1982;5:3–10. [Google Scholar]
- Gennaro S, Tulman L, Fawcett J. Temperament in preterm and full-term infants at three and six months of age. Merrill-Palmer Quarterly. 1990;36:201–215. [Google Scholar]
- Githens PB, Glass CA, Sloan FA, Entman SS. Maternal recall and medical records: An examination of events during pregnancy childbirth and early infancy. Birth. 1993;20:136–141. doi: 10.1111/j.1523-536x.1993.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Goldberg S, DiVitto BA. Born too soon: Preterm birth and early development. San Francisco: W.H. Freeman & Co; 1983. [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery, Locomotor version (Manual) 1996. [Google Scholar]
- Hughes MB, Shults J, McGrath J, Medoff-Cooper B. Temperament characteristics of premature infants in the first year of life. Journal of Developmental & Behavioral Pediatrics. 2002;23:430–435. doi: 10.1097/00004703-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Roundtable on Environmental Health Sciences, Research, and Medicine. Preterm birth and its consequences. In: Mattison DR, Wilson S, Coussens C, Gilbert D, editors. The Role of Environmental Hazards in Premature Birth. Washington, D.C.: The National Academies Press; 2003. pp. 13–27. [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Functional Academic Skills Test (K-FAST) Circle Pines, MN: American Guidance Service; 1994. [Google Scholar]
- Kersting A, Dorsch M, Wesselman U, et al. Maternal posttraumatic stress response after the birth of a very low-birth-weight infant. Journal of Psychosomatic Research. 2004;57:473–476. doi: 10.1016/j.jpsychores.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Tjebkes TL, Husarek SJ. Individual differences in emotionality in infancy. Child Development. 1998;64:375–390. [PubMed] [Google Scholar]
- Kopp CB. Risks in infancy: Appraising the research. Merrill-Palmer Quarterly. 1990;36:117–139. [Google Scholar]
- Kramer MS, Goulet L, Lydon J, Seguin L, McNamara H, Dassa C, et al. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatric and Perinatal Epidemiology. 2001;15:104–123. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Langkamp DL, Kim Y, Pascoe JM. Temperament of preterm infants at 4 months of age: Maternal ratings and perceptions. Journal of Developmental & Behavioral Pediatrics. 1998;19:391–396. doi: 10.1097/00004703-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Crockenberg SC. The impact of maternal characteristics and sensitivity on the concordance between maternal reports and laboratory observations of infant negative emotionality. Infancy. 2003;4:517–539. [Google Scholar]
- McGrath JM, Records K, Rice M. Maternal depression and infant temperament characteristics. Infant Behavior & Development. 2008;31:71–80. doi: 10.1016/j.infbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D, Burchinal P, Nelson D. Distress and growth outcomes in mothers of medically fragile infants. Nursing Research. 1999;48:129–140. doi: 10.1097/00006199-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Mouradian LE, Als H, Coster WJ. Neuro-behavioral functioning of healthy preterm infants of varying gestational ages. Journal of Developmental & Behavioral Pediatrics. 2000;21:408–416. doi: 10.1097/00004703-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother-child interactions. Early Human Development. 2004;79:145–158. doi: 10.1016/j.earlhumdev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Nadeau L, Tessier R, Boivin M, Lefebvre F, Robaey P. Extremely premature and very low birth weight infants: A double hazard population? Social Development. 2003;12:235–248. [Google Scholar]
- National Center for Health Statistics (NCHS) Final natality data. Retrieved September 19, 2008, from http://www.marchofdimes.com/peristats.
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. The British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raju TNK, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: A summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- Reich W, Todd R, Joyner CA, Neuman RJ, Heath AC. Reliability and stability of mothers reports about their pregnancies with twins. Twin Research. 2003;6:85–88. doi: 10.1375/136905203321536209. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. Journal of Epidemiology & Community Health. 2006;60:221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese ML. Temperament in full-term and preterm infants: Stability over ages 6 to 24 months. Journal of Developmental & Behavioral Pediatrics. 1988;9:6–11. [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- Rothbart MK. Longitudinal observation of infant temperament. Developmental Psychology. 1986;22:356–365. [Google Scholar]
- Schmücker G, Brisch K, Köhntop B, Betzler S, Österle M, Pohlandt F, et al. The influence of prematurity, maternal anxiety, and infants’ neurobiological risk on mother-infant interactions. Infant Mental Health Journal. 2005;26:423–441. doi: 10.1002/imhj.20066. [DOI] [PubMed] [Google Scholar]
- Sou S, Chen W, Hsieh W, Jeng S. Severe obstetric complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. Journal of Clinical Epidemiology. 1993;59:429–435. doi: 10.1016/j.jclinepi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Spungen LB, Farran AC. Effect of intensive care unit exposure on temperament in low birth weight infants. Journal of Developmental & Behavioral Pediatrics. 1986;7:288–292. doi: 10.1097/00004703-198610000-00002. [DOI] [PubMed] [Google Scholar]
- Stern M, Karraker KH. The prematurity stereotype: Empirical evidence and implications for practice. Infant Mental Health Journal. 1990;11:3–11. [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Stifter C, Corey J. Vagal regulation and observed social behavior in infancy. Social Development. 2001;10:189–201. [Google Scholar]
- Stifter CA, Willoughby M, Towe-Goodman N. Agree or agree to disagree: Assessing the convergence between parents and observers of infant temperament. Infant and Child Development. 2008;17:407–426. doi: 10.1002/icd.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti DM, Gelfand DM. Behavioral competence among mothers of infants in the first year: The mediational role of maternal self-efficacy. Child Development. 1991;62:918–929. doi: 10.1111/j.1467-8624.1991.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Teti DM, Hess CR, O’Connell M. Parental perceptions of infant vulnerability in a preterm sample: Prediction from maternal adaptation to parenthood during the neonatal period. Journal of Developmental & Behavioral Pediatrics. 2005;26:283–292. doi: 10.1097/00004703-200508000-00004. [DOI] [PubMed] [Google Scholar]
- Todd RD, Joyner CA, Heath AC, Neuman RJ, Reich W. Reliability and stability of a semi structured DSM-IV interview designed for family studies. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1460–1468. doi: 10.1097/00004583-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. American Journal of Obstetrics & Gynecology. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- Whiffen VE. Vulnerability to postpartum depression: A prospective multivariate study. Journal of Abnormal Psychology. 1988;97:467–474. doi: 10.1037//0021-843x.97.4.467. [DOI] [PubMed] [Google Scholar]
- Whiffen VE. Maternal depressed mood and perceptions of child temperament. Journal of Genetic Psychology. 1990;151:329–339. doi: 10.1080/00221325.1990.9914621. [DOI] [PubMed] [Google Scholar]
- Zanardo V, Freato F, Zacchello F. Maternal anxiety upon NICU discharge of high-risk infants. Journal of Reproductive and Infant Psychology. 2003;21:69–75. [Google Scholar]