Abstract
Neuregulin 1 (NRG1) is a growth factor involved in neurodevelopment and plasticity. It is a schizophrenia candidate gene, and hippocampal expression of the NRG1 type I isoform is increased in the disorder. We have studied transgenic mice overexpressing NRG1 type I (NRG1tg-type I) and their wild-type littermates and measured hippocampal electrophysiological and behavioral phenotypes. Young NRG1tg-type I mice showed normal memory performance, but in older NRG1tg-type I mice, hippocampus-dependent spatial working memory was selectively impaired. Hippocampal slice preparations from NRG1tg-type I mice exhibited a reduced frequency of carbachol-induced gamma oscillations and an increased tendency to epileptiform activity. Long-term potentiation in NRG1tg-type I mice was normal. The results provide evidence that NRG1 type I impacts on hippocampal function and circuitry. The effects are likely mediated via inhibitory interneurons and may be relevant to the involvement of NRG1 in schizophrenia. However, the findings, in concert with those from other genetic and pharmacological manipulations of NRG1, emphasize the complex and pleiotropic nature of the gene, even with regard to a single isoform.
Keywords: gamma oscillation, hippocampus, neuregulin, schizophrenia, synaptic plasticity
Introduction
Neuregulin 1 (NRG1) is a member of the epidermal growth factor family. It signals through ErbB2–4 receptors and has diverse roles in development and plasticity (Buonanno and Fischbach 2001; Corfas et al. 2004; Rico and Marin 2011). Its pleiotropy reflects, in part, the generation of multiple isoforms, notably those resulting from alternative 5′ exons, called “types.” Classically, types I–III are recognized (Falls 2003), although types IV–VI have recently been identified (see Mei and Xiong 2008). Isoform-specific distributions, roles, and properties have been described, although they remain incompletely understood (Meyer et al. 1997; Flames et al. 2004; Michailov et al. 2004; Taveggia et al. 2005; Tan et al. 2007; Brinkmann et al. 2008; Chen et al. 2008).
Many studies of NRG1 have concentrated on early neurodevelopmental processes such as cell differentiation, synaptogenesis, and myelination, and studies initially focused on the peripheral rather than the central nervous system (CNS). However, NRG1 is also expressed throughout adulthood (Kerber et al. 2003; Law et al. 2004), and recent studies, discussed below, provide compelling evidence for its functionality in brain (for review, see Mei and Xiong 2008). A major psychiatric relevance of NRG1 emerges because its locus is implicated in neurodevelopmental disorders (Tabares-Seisdedos and Rubenstein 2009) and, in particular, from evidence that NRG1 is a schizophrenia susceptibility gene, in its own right (Stefansson et al. 2002; Li et al. 2006; Keri et al. 2009) and in epistasis with other genes in the NRG1 signaling pathway (Nicodemus et al. 2010). The expression and function of NRG1 is altered in schizophrenia and in people carrying NRG1 risk alleles (Hahn et al. 2006; Hall et al. 2006; Harrison and Law 2006; Chen et al. 2009; Nicodemus et al. 2009); notably, there is an isoform-specific increase in type I NRG1 messenger RNA in the disorder (Hashimoto et al. 2004; Law et al. 2006).
The genetic associations with schizophrenia have encouraged more studies of NRG1 in the adult CNS, including analysis of various heterozygous NRG1 knockout mice. The results show diverse functional effects, including “schizophrenia-like” behavioral and other phenotypes (Stefansson et al. 2002; Rimer et al. 2005; Karl et al. 2007; O’Tuathaigh et al. 2007; Chen et al. 2008; Ehrlichman et al. 2009; Duffy et al. 2010; Nason et al. 2011). However, very little is known about the consequences of genetically enhanced NRG1 signaling, nor the specific effects of the type I isoform, both of which might be of greater relevance to schizophrenia than are hypomorphs. Transgenic mice selectively overexpressing NRG1 type I (“NRG1tg-type I mice”) have been created primarily to study peripheral myelination (Michailov et al. 2004; Brinkmann et al. 2008). Analysis of their behavior is limited to an initial screen of ∼4-month-old mice, which revealed an increased startle response, impaired prepulse inhibition, a transient locomotor hypoactivity when first placed into activity cages, and no significant anxiety phenotype (Deakin et al. 2009). The mice also have a tremor, likely related to aberrant myelination (Michailov et al. 2004), which results in decreased performance on some motor tasks (Deakin et al. 2009).
Here, to help understand the consequences of overexpression of type I NRG1 for higher cognitive function, we report a detailed behavioral and electrophysiological characterization of NRG1tg-type I mice. We focused on the hippocampus, given its importance in cognition (Morris 2006) and schizophrenia (Harrison 2004), and since this is the region wherein type I NRG1 overexpression in the disorder was most clearly demonstrated (Law et al. 2006).
Materials and Methods
All experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986, and had local ethical approval.
Generation and Genotyping of NRG1tg-type I Mice
The generation and genotyping of NRG1tg-type I mice has been described (Michailov et al. 2004). The mice express NRG1 type I (β1a-isoform) under a Thy-1 promoter, with robust overexpression in multiple brain regions (Brinkmann et al. 2008), notably the pyramidal cell and granule cell layers of the hippocampus, deep layers of cerebral cortex, and brain stem nuclei, but not in cerebellum or striatum (Supplementary Fig. 1; see also Deakin et al. 2009). Expression of NRG1 types II and III isoforms is unaffected (see Supplementary Materials and Supplementary Table 1). The experiments reported here were performed in F6–F9 generations of backcross of heterozygous NRG1tg-type I males with wild-type (wt) C57BL/6J females, comparing NRG1tg-type I mice with their wt littermates.
Behavioral Phenotyping
Mice were group housed with same-sex littermates. A 12-h lighting scheme was maintained (lights on 7–19 h) and testing performed with mice from each genotype interleaved (9–17 h).
Locomotor activity was measured in transparent plastic cages (26 × 16 × 17 cm) containing a thin layer of bedding with 2 photocell beams crossing the bottom of the cage. The number of crossovers were recorded in a 2-h period (9–11 h). Locomotor activity was assessed in the same cohort of mice at 5, 7.5, 10, and 12.5 months.
For spatial memory tests, mice were maintained on a food-deprivation schedule of ≥85% free-feeding weight with ad libitum access to water. Mice were first habituated to drinking 50% condensed milk. Working memory was assessed using spatial nonmatching-to-place testing (rewarded alternation) in a T-maze (Deacon et al. 2002; Deacon and Rawlins 2006). Mice were run in batches of 5–6 with an intertrial interval of approximately 10 min and with 5 trials per day for 10 days. Short-term memory was also tested using a single-trial, spatial novelty preference (spontaneous alternation) task, performed as described (Sanderson et al. 2007). Spatial reference memory was assessed on an elevated Y-maze. The tests were performed in mice at 3–5 months and 10–11 months, as described in Results. For full details of behavioral tasks, see Supplementary Materials.
Synaptic Transmission and Long-term Potentiation in CA1
Recordings were performed in the stratum radiatum of CA1 on 400 μm sagittal hippocampal slices from mice aged 2.5–5 months and 10–13 months, as described (Romberg et al. 2009). For details, see Supplementary Materials. The magnitude of the field excitatory post-synaptic potential (fEPSP) response was measured by the slope of the central third of the rising phase. Stimulus response curves were investigated for stimulus strengths from 5 to 350 μA. Five repetitions of paired 50-μs stimuli, 50 ms apart, were presented for each of the stimulation strengths at 0.2 Hz. The mean fEPSP slope of the first stimulus in each pair was calculated. Paired pulse facilitation (PPF) was calculated by the ratio of the second to the first fEPSP slope of a stimuli pair at a stimulation strength in the exponential phase of the stimulus response curve.
Long-term potentiation (LTP) was induced by theta burst stimulation (TBS), which was 10 trains of 4 pulses at 100 Hz separated by 200 ms (Bjarnadottir et al. 2007). Stimuli were presented at 0.1 Hz at half the strength that induced the maximal fEPSP response (50% Vmax). If the baseline fEPSP slope was stable (<20% variation) for 15 min, TBS was presented. The magnitude of LTP was defined as the mean percent baseline fEPSP slope 46–50 min after TBS. Data from each mouse (the mean values from its slices) were analyzed with analysis of variance with between-subjects factors of genotype, age, and sex.
Electrophysiology: Oscillations and Cell Recordings
Carbachol-induced gamma oscillations (Fisahn et al. 1998) were studied using horizontal brain slices from mice aged 3–6 months (Oren et al. 2006). Rhythmic extracellular field activity was recorded in CA3b/c upon application of 5–20 μM carbachol (Sigma-Aldrich, UK) using an artificial cerebrospinal fluid (ACSF)-filled pipette (3–5 MΩ) placed at the stratum pyramidale–oriens border. Temperature in the submerged recording chamber was kept between 28 and 32 °C to reduce metabolic rate and enable sufficient oxygenation and to display pronounced and stable oscillatory cycles (Fisahn et al. 2009; Gulyas et al. 2010). Firing from individual neurons was detected using a second ACSF-filled pipette (4–8 MΩ) placed in the stratum pyramidale in close proximity to the field pipette. Currents underlying the generation of action potentials were recorded in voltage clamp mode for ∼30 to 60 s once seal resistance had reached >500 MΩ as loose cell patch. Recordings were digitally filtered using a bidirectional Igor Digital Signal Processing filter (Oren et al. 2006). Field recordings were low-pass filtered at 200 Hz, while cell-attached recordings were high-pass filtered at 1 Hz. The power of the field oscillations was estimated by means of power spectral density (PSD) analysis on ∼30- to 60-s-long epochs, taken at least 5 min following exposure to carbachol (see Supplementary Fig. 2). For this, a discrete fast Fourier transform was calculated on a sliding window of ∼1.5 s with consecutive sliding windows overlapping by 50%. The windows were multiplied by a Hanning window to minimize end effects. Oscillation frequencies were in the lower gamma frequency range (close to 25–30 Hz), typical of in vitro gamma models in submerged-style recording chambers (Mann et al. 2005; Oren et al. 2006; Fisahn et al. 2009; Hajos et al. 2009; Gulyas et al. 2010; Mann and Mody 2010). Hence, oscillatory power was sampled as the area under the PSD curve between 10 and 40 Hz. For analysis of the phase coupling of cell firing to the ongoing field potential oscillation, the field recording was transformed by Morlet wavelet and analyzed using circular statistics and Rayleigh probability. For full details of gamma oscillation detection and cellular recordings, see Supplementary Materials.
Results
NRG1tg-type I Mice Become Relatively Hyperactive and Develop a Spatial Working Memory Deficit with Age
Spontaneous locomotor activity monitoring showed an age-emergent hyperactivity in the NRG1tg-type I mice (age-by-genotype interaction, F3,108 = 3.68, P = 0.034; Fig. 1A and Supplementary Fig. 2), with a significant difference between genotypes emerging at 12 months of age, when NRG1tg-type I mice were relatively hyperactive (F1,36 = 9.09, P = 0.005), as a result of a decline in activity in the wt mice at this time point. At 5 months, NRG1tg-type I mice actually displayed a mild and transient locomotor hypoactivity when placed into the activity cages (see Supplementary Material and Supplementary Fig. 2), consistent with our previous observation in animals of similar age (Deakin et al. 2009). Perhaps related to the later hyperactivity, body mass showed an age-by-genotype interaction (F3,108 = 42.01, P < 0.001), with NRG1tg-type I mice being lighter at all time points beyond 5 months (all F1,36 > 28, P < 0.001; Supplementary Fig. 3).
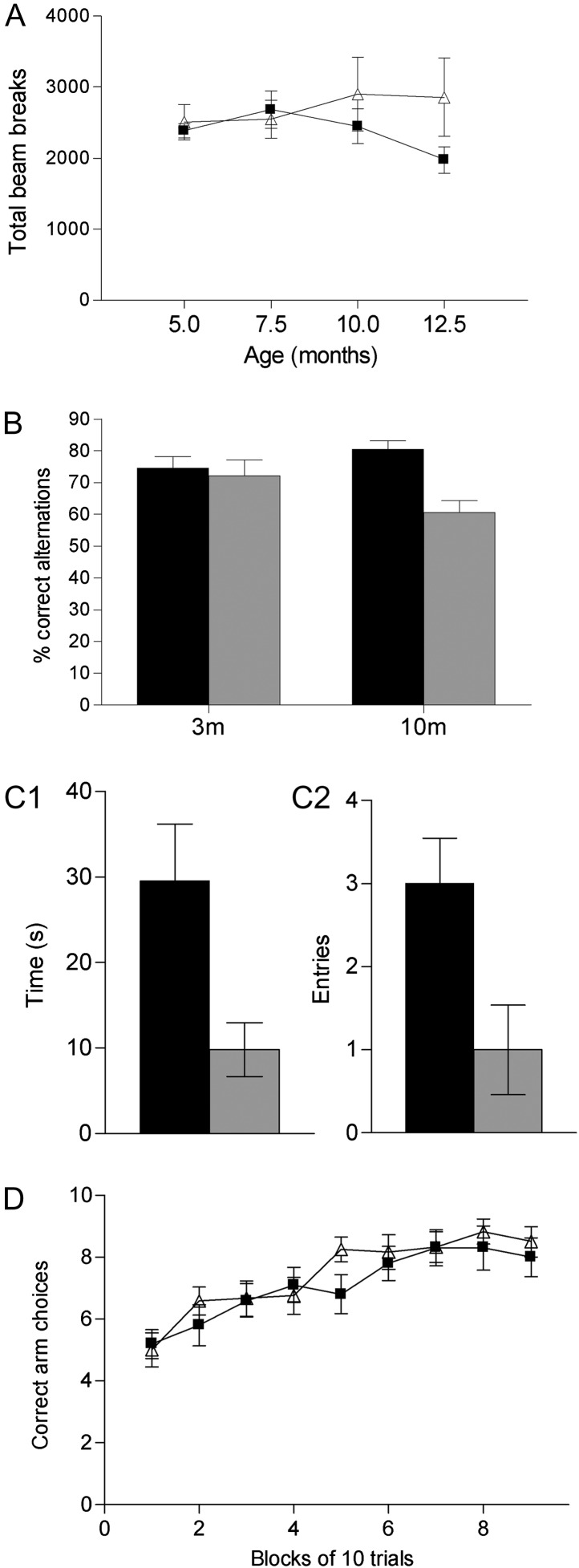
Figure 1.
Behavioral phenotypes in NRG1tg-type I mice: age-emergent locomotor hyperactivity and deficit in spatial short-term memory but not reference memory. (A) Spontaneous locomotor activity (total beam breaks in a 2-h session). NRG1tg-type I mice (triangles; male n = 9, female n = 8) were hyperactive at 12.5 months compared with wt mice (filled squares; male n = 10, female n = 13; P = 0.005). See also Supplementary Materials and Supplementary Figure 2. (B) Spatial working memory: rewarded alternation in the T-maze showing percent correct alternations from 50 trials. At 3–4 months (3 m), NRG1tg-type I mice (gray) did not differ from wt littermates (black), but at 10 months (10 m), NRG1tg-type I mice made fewer correct alternations than wt (P = 0.016). At both time points, wt male n = 10, female n = 14; NRG1tg-type I male n = 9, female n = 9. (C) Single-trial, spatial novelty preference Y-maze task in 10- to 11-month-old NRG1tg-type I (gray; n = 10) and wt (black; n = 16) mice. (C1) Time spent in novel arm minus time spent in the other arm. NRG1tg-type I mice spent relatively less time in the novel arm than wt (F1,22 = 4.21, P = 0.05). (C2) Number of entries into novel arm minus number of entries into other arms. NRG1tg-type I mice made relatively fewer entries into the novel arm than wt (F1,22 = 4.85, P < 0.05). For raw data, see Supplementary Table 2. (D) Spatial reference memory acquisition in an appetitive Y-maze task showing normal performance in 11-month-old NRG1tg-type I mice (triangles, n = 10) compared with wt littermates (filled squares, n = 12); main effect of genotype, F1,20 < 1; genotype by block, F7,140 < 1. Data shown as mean ± standard error of the mean.
Working memory was assessed with a discrete trial, spatial nonmatching-to-place (rewarded alternation) task on a T-maze (Fig. 1B). At 3–4 months, wt and NRG1tg-type I mice had similar rates of alternation (F1,38 = 0.36, P = 0.551), but at 10 months, NRG1tg-type I mice displayed a lower rate (F1,38 = 6.39, P = 0.016; age-by-genotype interaction F1,38 = 5.27, P = 0.027).
Having demonstrated this age-emergent impairment, 2 further tests were performed. First, spatial short-term memory in the older mice was assessed in a single-trial, spatial novelty preference task, which relies on the animal’s exploratory drive (Sanderson et al. 2007, 2008). Whereas wt mice spent more time in the novel arm of a Y-maze compared with recently visited, familiar arms and entered the novel arm more often, this novelty preference was reduced in NRG1tg-type I mice, confirming the presence of impaired spatial short-term memory (Fig. 1C and Supplementary Table 2). Second, spatial reference memory was tested in an appetitive Y-maze task and was unimpaired in NRG1tg-type I mice aged 11 months (Fig. 1D). Hence, the NRG1tg-type I mice have an age-emergent deficit, specifically in working/short-term memory, with intact associative reference memory. There were no sex-by-genotype interactions for any behavioral measure (not shown).
NRG1tg-type I Mice Have Normal Basal Synaptic Transmission and Hippocampal LTP
We next examined whether NRG1 type I overexpression affected hippocampal circuit functioning. We found no difference in basal synaptic transmission from CA3 to CA1 in young (Fig. 2A) or old (Fig. 2B) NRG1tg-type I mice. Increasing stimulation strength increased fEPSP slope response in CA1 (F10,300 = 181.84, P < 0.001), but there was no interaction with genotype, age, or sex (all F10,300 < 1.5, P > 0.2), nor main effects of genotype or age (all F1,30 < 1.1, P > 0.3). PPF was recorded as a measure of presynaptic plasticity and did not differ between genotypes (F1,32 = 0.27, P = 0.608), nor were there other main effects or interactions (F1,32 < 2.8, P > 0.1; young wt = 1.36 ± 0.08; young NRG1tg-type I = 1.43 ± 0.06; old wt = 1.18 ± 0.08; old NRG1tg-type I = 1.25 ± 0.11).
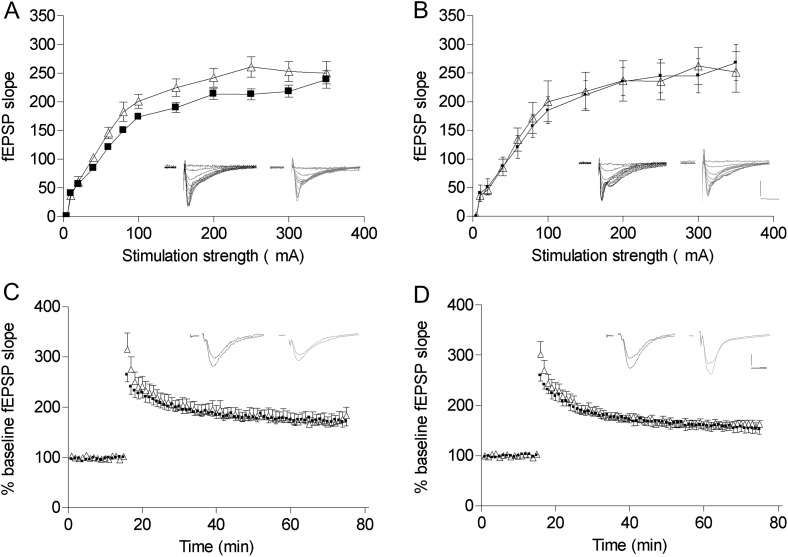
Figure 2.
Normal basal synaptic transmission and LTP in NRG1tg-type I mice. (A and C) Young mice (2.5–5 months old); (B and D) old mice (10–13 months old). Data are mean and standard error of the mean with example traces. Wt: black squares and black traces; NRG1tg-type I: open triangles and gray traces. (A and B) Stimulus response curves (A: wt, n = 8; NRG1tg-type I, n = 11; B: wt, n = 12, NRG1tg-type I, n = 11). Scale bars: 1 mV and 5 ms. (C and D) LTP (E: wt, n = 11; NRG1tg-type I, n = 12; F: wt, n = 12, NRG1tg-type I, n = 12). Scale bars: 5 mV and 5 ms. There are no differences between genotypes at either age.
LTP was induced with TBS, replicating the paradigm used in NRG1+/− mice (Bjarnadottir et al. 2007). LTP magnitude was not affected in NRG1tg-type I mice at either age, nor were there interactions between genotype, age, or sex (all F1,41 < 1.7, P > 0.2; Fig. 2C,D).
NRG1tg-type I Mice Show Altered Hippocampal Gamma Oscillatory Frequency and a Lower Threshold to Carbachol-Induced Epileptiform Activity
With LTP unaffected, we focused on oscillatory network activity in the gamma frequency range, which may be a correlate of memory encoding and retrieval (Engel et al. 2001; Montgomery and Buzsaki 2007; Herrmann et al. 2010; van Vugt et al. 2010). Robust gamma frequency oscillations were induced in CA3 by cholinergic activation (5–20 μM carbachol; Fig. 3A1). Oscillations in slices from wt mice (n = 19 slices from 9 mice) showed a mean frequency of 26.3 ± 1.1 Hz, in line with previous reports using these conditions (see Materials and Methods). Slices from NRG1tg-type I mice also presented stable oscillations (Fig. 3A2, Supplementary Fig. 4) but of lower peak frequency (22.0 ± 0.9 Hz; P = 0.005) (Fig. 3B1). Oscillatory power did not differ between genotypes (Fig. 3B2; P = 0.29).
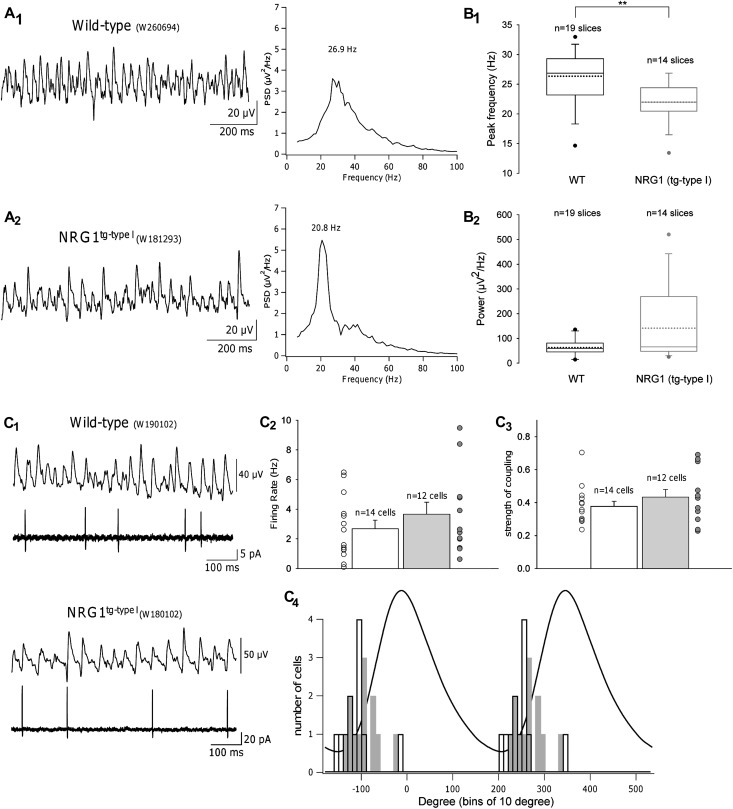
Figure 3.
Altered hippocampal gamma oscillations in NRG1tg-type I mice. (A). Cholinergic activation by carbachol (CCh) induces gamma frequency oscillations in hippocampal slices from wt and NRG1tg-type I mice. One-second-long sample traces (low-pass filtered at 200 Hz) of the recorded field potential oscillations in the hippocampal CA3 pyramidal cell layer and corresponding PSDs in a wt mouse (A1) and a NRG1tg-type I mouse (A2). PSDs, computed for the entire 60-s-long traces, confirm lower peak frequency in the NRG1tg-type I mice. (B) Summary box plots of all recordings demonstrate that peak frequencies (shown in B1) were consistently lower in NRG1tg-type I slices (mean peak frequency in wt: 26.2 ± 1.0 Hz [mean + standard error of the mean] vs. NRG1tg-type I: 22.0 ± 0.9 Hz; P = 0.006**). Whiskers indicate 90th and 10th percentiles of the data; outlying points are also plotted, dotted lines represent the mean. (B2) Mean oscillatory power did not differ between groups (wt: 107.0 ± 44.8 μV2/Hz vs. NRG1tg-type I: 141.4 ± 41.3 μV2/Hz; P = 0.44). (C) CA3 pyramidal cell firing during gamma oscillations. (C1) Representative extracellular field and corresponding cell-attached recordings from slices taken from wt (top) and NRG1tg-type I (bottom) mice. (C2) Histogram comparing the mean firing rate (Hertz) of individual pyramidal cells in wt (black) and NRG1tg-type I (gray) mice, with scatter plots corresponding to their firing rates. Statistical analysis revealed no difference between the groups (wt: 2.70 ± 0.56, NRG1tg-type I: 3.66 ± 0.81; P = 0.36). (C3) Histogram comparing the strength of pyramidal cell firing coupling to the field oscillation in wt (black) and NRG1tg-type I (gray) mice, plus scatter plots representing the coupling values of all individual cells recorded. The average vector length (see Supplementary Materials) did not differ between genotypes (wt: 0.38 ± 0.03; NRG1tg-type I mice: 0.43 ± 0.05; P = 0.38). Error bars in C2 and C3 are standard error of the mean. (C4) Distributions of the mean angles (degrees) at which cells fired relative to the field potential oscillation (wt: 12 cells from 5 mice; NRG1tg-type I: 12 cells from 7 mice). A representative cycle-averaged field oscillation is shown to illustrate phase definition. Firing of pyramidal cells is similarly phase locked to ascending phase of a field gamma cycle in both genotypes with no difference between groups (wt [black]: −111.4° ± 10.0°, NRG1tg-type I [gray]: −93.3° ± 9.2°; P = 0.106).
To probe action potential firing of individual pyramidal neurons during gamma oscillations, we performed loose cell-attached voltage clamp recordings from these cells (Fig. 3C1). Neither the firing rate (wt: 14 cells from 6 mice, NRG1 tg type I: 12 cells from 7 mice; P = 0.36; Fig. 3C2) nor the strength of coupling of the firing to the oscillation (P = 0.38; Fig. 3C3) differed between genotypes. In both groups, significantly coupled CA3 pyramidal cells (Rayleigh P < 0.05, wt: 12 cells from 5 mice, NRG1tg-type I: 12 cells from 7 mice) showed phase-coupling characteristic of gamma oscillations and fired preferentially at the start of the cycle, near the minimum of the field oscillation cycle (Fig. 3C4).
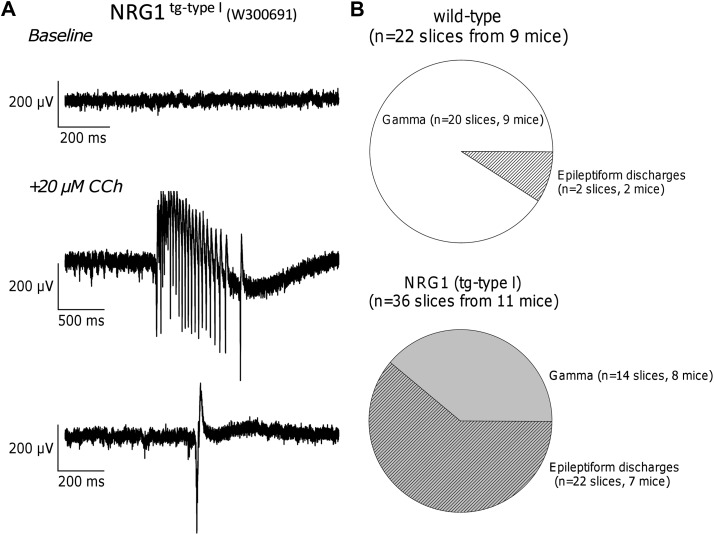
Figure 4.
Hippocampal slices from NRG1tg-type I mice are prone to carbachol-induced epileptiform activity. (A) Sample field potentials during baseline (top trace) and following application of 20 μM carbachol (CCh; middle and bottom traces) in slices from NRG1tg-type I mice. The lower 2 panels show examples of carbachol-evoked epileptiform field potential discharges resembling ictal and interictal-like bursts. (B) Pie charts illustrate the percentage of slices in which cholinergic activation (5–20 μM carbachol) results in robust gamma oscillations (empty segment) or immediate field epileptiform activity (filled segment). The proportion showing epileptiform activity is higher in slices from NRG1tg-type I mice than wt mice (Fisher’s exact test, P < 0.0001).
In addition to slower gamma oscillations, slices from NRG1tg-type I mice were more prone to develop epileptiform activity. Epileptiform discharges, detected by recordings in CA3, consisted of short interictal-like bursts and/or longer duration (>1 s) ictal-type episodes and occurred following wash-in of carbachol, but not at baseline (Fig. 4A). Over 60% of NRG1tg-type I slices (22 of 36 slices, from 7 of 11 mice) showed carbachol-induced epileptiform activity, compared with only 9% of wt slices (2 of 22 slices, from 2 of 9 mice) (Fisher’s exact test, P < 0.0001; Fig. 4B).
Discussion
NRG1 exists as multiple isoforms, which differ in their CNS expression and their signaling and processing characteristics (Falls 2003; Corfas et al. 2004; Mei and Xiong 2008). Understanding this complexity is important since the isoforms exhibit functional differences (Flames et al. 2004; Michailov et al. 2004; Taveggia et al. 2005; Brinkmann et al. 2008; Chen et al. 2008) and may contribute differentially to schizophrenia (Harrison and Law 2006; Chen et al. 2009), wherein type I NRG1 is increased (Hashimoto et al. 2004; Law et al. 2006). However, virtually nothing is known about this isoform in the brain. Here we studied NRG1 type I overexpressing mice and found a selective and age-emergent impairment of working memory, a reduced frequency of hippocampal gamma oscillations and increased epileptiform activity, but normal LTP. The results reveal roles of the type I isoform of NRG1 in hippocampal function and may have implications for the involvement of NRG1 type I in schizophrenia.
Age-Emergent Spatial Working Memory Deficits in NRG1tg-type I Mice
NRG1tg-type I mice were impaired on rewarded alternation, a spatial working memory task, at ∼10 months, a finding corroborated with a single-trial task and contrasting with their preserved spatial reference memory. The latter implies that nonspecific confounds (e.g., sensorimotor function or motivation) are unlikely to be responsible for the working/short-term memory deficit. These are to our knowledge the first data showing that NRG1 impacts upon hippocampus-dependent cognition, complementing the limited evidence for attentional or memory impairments in NRG1 hypomorphic mice (Chen et al. 2008; Duffy et al. 2010). Notably, young NRG1tg-type I mice performed entirely normally on the same spatial working memory task, indicating that the deficit emerges between 4 and 10 months of age, a time period later than that classically associated with NRG1 functions but consistent with the increasing focus upon roles of NRG1 in adulthood.
Although it is parsimonious to interpret the selective memory deficits as being directly related to the hippocampal overexpression of NRG1 type I, the mice also overexpress the gene in some other regions (Deakin et al. 2009; Supplementary Fig. 1). The behavioral phenotype clearly extends beyond the hippocampal domain, as evidenced by our earlier study (Deakin et al. 2009), and the hippocampus is not the only brain area that is important for performance on the spatial memory tasks used in the present paper. Nevertheless, the hippocampus is one brain area that is essential for spatial short-term memory (e.g., Deacon et al. 2002; Sanderson et al. 2007), and performance is likely to involve extended neural circuits, which include the hippocampus as one component.
Kato et al. (2010) recently described a separate line of NRG1tg-type I mice, in which the transgene was tagged to green fluorescent protein and driven by an elongation factor 1α promoter. They reported decreased social interactions and impaired contextual fear learning, as well as increased locomotor activity, and reductions in hippocampal dopaminergic markers. Extrapolation to the present findings is limited because Kato et al. (2010) did not use comparable cognitive tests and only examined young (8- to 12-week old) animals. Direct comparison of the lines would be of interest; some phenotypic differences might well be anticipated given the differing constructs and the magnitude and distribution of overexpression.
Electrophysiological Phenotype: Normal LTP, Altered Gamma Oscillations
Several studies have shown that hippocampal LTP is affected by genetic or pharmacological manipulation of NRG1. Acute administration of NRG1 peptide acutely suppresses (Huang et al. 2000; Pitcher et al. 2008; Chen et al. 2010) or reverses (Kwon et al. 2005, 2008) LTP, and LTP is decreased in NRG1 hypomorphs (Huang et al. 2000; Bjarnadottir et al. 2007). These results suggest an “inverted U” relationship between NRG1 signaling and LTP (Role and Talmage 2007) as well as between NRG1 “dose” and some other parameters (Li et al. 2007; Syed et al. 2010). Given these findings and the age-emergent working memory deficit, we anticipated an impairment of LTP in the old but perhaps not the young NRG1tg-type I mice, but in fact, LTP was normal at both ages. This may reflect the differing consequences of constitutive overexpression of NRG1 compared with the acute effects of exogenous NRG1. It also suggests that the type I isoform is not involved in NRG1 modulation of CA1 LTP and, by default, implicates type II or III NRG1, which are also expressed in the hippocampus (Law et al. 2006; Woo et al. 2007). Combining genetic and pharmacological manipulations of NRG1 will help clarify these issues.
Hippocampal gamma oscillations are generated by parvalbuminergic (PV+) interneurons (Mann et al. 2005; Bartos et al. 2007; Mann and Paulsen 2007; Gulyas et al. 2010). These are the primary ErbB4-expressing cell population (Krivosheya et al. 2008; Vullhorst et al. 2009; Fazzari et al. 2010), and NRG1–ErbB4 signaling regulates their synaptic development (Fazzari et al. 2010) and their release of γ-aminobutyric acid (GABA) (Woo et al. 2007; Wen et al. 2010). The frequency of hippocampal gamma oscillations is regulated by activation of interneuronal N-methyl-D-aspartate (NMDA) receptors (Mann and Mody 2010) and is influenced by genetic factors and correlated with GABAA receptor subunit expression (Heistek et al. 2010). Moreover, gamma oscillatory frequency in vivo is positively correlated with local GABA concentration, at least in human visual cortex (Muthukumaraswamy et al. 2009). These findings together strongly implicate PV+ interneurons, driven by enhanced or otherwise aberrant NRG1–ErbB4 signaling, as the basis for the alterations in gamma oscillatory frequency seen in the NRG1tg-type I mice. However, NRG1 acting via ErbB4 receptors stimulates interneuronal GABA release (Woo et al. 2007), and NRG1 overexpression might be expected to elevate this and so in turn be associated with an increased (not decreased) gamma frequency. It is also relevant that hippocampal gamma oscillations are altered by NRG1 peptides, but the effect is on power not frequency and occurs in response to kainate not carbachol (Fisahn et al. 2009). As with the LTP result, these findings together suggest a particular role of the type I isoform in the modulation of gamma oscillations; they also highlight that the duration and magnitude of NRG1 elevation are likely important determinants of its functional effects. As a first step toward characterization of PV+ cells in NRG1tg-type I mice, we have found no gross differences in hippocampal PV+ cell density in the mice (Supplementary Table 3 and Supplementary Fig. 5), but considerable further investigations are required to establish the functional status of these interneurons and to clarify the range of oscillatory responses to NRG1 isoforms in different circuits and experimental paradigms.
Whatever its cause, an altered frequency of gamma oscillations likely indicates a disturbed timing and suboptimal efficiency of hippocampal circuits and thereby an impairment of gamma-related hippocampal functions. It is of interest that a similar phenotype to that of NRG1tg-type I mice—that is, altered gamma oscillations, deficient spatial working memory, and intact reference memory—is seen in mice with conditional ablation in PV+ interneurons of GluA1 (Fuchs et al. 2007) or GluN1 (Korotkova et al. 2010) glutamate receptors. Impaired working memory is also seen in PV+ ErbB4 knockout mice (Wen et al. 2010). Notably, it has also recently been shown that functional removal of CA1 PV+ interneurons produces a selective impairment in spatial working memory but spared spatial reference memory (Murray et al. 2011), reminiscent of the pattern of effects observed here. These findings draw further attention to the hippocampal PV+ cell population as a contributor to the behavioral and electrophysiological phenotype of the NRG1tg-type I mice, albeit their working memory deficit did not arise until 10 months of age despite the oscillatory change already being present in younger mice. This temporal dissociation indicates either that the 2 aspects of phenotype are not in fact related or that the emergence of memory impairment in the NRG1tg-type I mice requires additional factors beyond the oscillopathy.
During hippocampal gamma oscillations, oxygen consumption is comparable to that of seizure-like events (Kann et al. 2011) and gamma activity is sensitive to hypoxia (Huchzermeyer et al. 2008). Fast-spiking interneurons are especially sensitive, with respiratory chain inhibition producing a markedly reduced frequency of inhibitory postsynaptic potentials, as well as decreased gamma power (Whittaker et al. 2011). The fact that hippocampal slices from NRG1tg-type I mice were predisposed to epileptiform activity, as well as exhibiting decreased gamma oscillatory frequency, may indicate that their mitochondrial function is impaired or that they have greater gamma-related metabolic demands than wt mice.
Implications for NRG1 in Schizophrenia
NRG1tg-type I mice are, to our knowledge, the first genetic mouse model of schizophrenia that also reproduces an isoform-selective change in expression of the gene that occurs in the disorder. It is thus of interest to consider the extent to which their phenotype recapitulates elements of schizophrenia.
Several features stand out in this regard. First, the focus upon PV+ interneurons is congruent with contemporary models, which envisage a critical role for these cells and their NMDA receptors in the pathogenesis of the disorder (e.g., Gonzalez-Burgos and Lewis 2008; Lisman et al. 2008; Lodge et al. 2009; Belforte et al. 2010; Woo et al. 2010). The present data suggest that NRG1 type I may contribute to this dysfunction (Banerjee et al. 2010; Buonanno 2010). At first sight, other findings in the NRG1tg-type I mice also appear broadly in keeping with schizophrenia, such as the spatial working memory impairment (Piskulic et al. 2007; Forbes et al. 2009) and altered gamma oscillatory frequency (Spencer et al. 2004). However, there is less congruence when these findings are considered more critically. Thus, in schizophrenia, memory impairment is present at, or before, the first episode (i.e., by early adulthood; Mesholam-Gately and Giuliano 2009), whereas it only emerged here in older mice. In any event, it is unclear whether spatial working memory as conceptualized and studied in humans is directly comparable to the term when applied to rodents (Sanderson and Bannerman 2011). And, apart from Spencer et al. (2004), most studies in schizophrenia have reported differences in gamma power not frequency, and all concern neocortical oscillations (Uhlhaas and Singer 2010). Moreover, preliminary data in NRG1tg-type I mice indicate hippocampal enlargement (I.H.D. and P.J.H., unpublished observations), whereas in schizophrenia, hippocampal volume is decreased (Harrison 2004). Thus, the results overall do not support the interpretation that NRG1tg-type I mice show a schizophrenia-like phenotype.
These considerations highlight the conceptual and interpretational limitations of genetic mouse models of psychiatric disorders (Desbonnet et al. 2009; Kellendonk et al. 2009; Arguello and Gogos 2010; Harrison et al. 2011) and show they apply even when, as here, the genetic manipulation reproduces an isoform-selective alteration in expression of a strong candidate gene and therefore has a relatively high construct validity (Nestler and Hyman 2010). Several factors, other than simply the complex genetic and environmental risk architecture of the disorder, may contribute to the “non–schizophrenia-like” aspects of the NRG1tg-type I phenotype. For example, the greater magnitude of overexpression compared with that observed in schizophrenia. And, since it is not known when the overexpression of type I NRG1 (or its pathogenic effects) begins in people who develop schizophrenia, we do not know whether the early onset and persistent overexpression in the mice is a good approximation to the disease state.
In summary, these data show that NRG1 type I impacts upon hippocampal function and circuitry. The findings also illustrate the manifold and partly isoform specific, contributions which NRG1 makes to the development and plasticity of the CNS, as well as potentially to its pathophysiology.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Wellcome Trust studentship to I.H.D.; Department of Pharmacology studentship to W.N.; UK Medical Research Council Career Development Fellowship to A.J.L.; Wellcome Trust Career Development Fellowship to K.P.L.; Wellcome Trust Senior Research Fellowship to D.M.B.; Stanley Medical Research Institute and Medical Research Council grants to P.J.H.
Supplementary Material
Acknowledgments
We thank A. Agrawal, I. Oren, A. Szabo, J. Seale-Finch, and J. Taylor for their contributions. Conflict of Interest : None declared.
References
- Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull. 2010;36:289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, MacDonald ML, Borgmann-Winter KE, Hahn C-G. Neuregulin 1-erb B4 pathway in schizophrenia: from genes to an interactome. Brain Res Bull. 2010;83:132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–U240. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1(+/−) knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Mueller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen Y-J, Zhang M, Yin D-M, Ting A, Wang P, Lu Y-S, Zhu X-H, Li S-J, Wu C-Y, Lai C, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-JJ, Johnson MA, Lieberman MD, Goodchild RE, Schoebel S, Lewandowski N, Rosoklija G, Liu R-C, Gingrich JA, Small S, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-JJ, Role LW, Talmage DA. Neuregulin 1 and schizophrenia. In: Javitt DC, Kantrowitz J, editors. Handbook of neurochemistry and molecular neurobiology: schizophrenia. 3rd ed. Vol. 27. New York: Springer; 2009. pp. 243–265. [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport. 2009;20:1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Waddington JL, O’Tuathaigh CM. Mice mutant for genes associated with schizophrenia: common phenotype or distinct endophenotypes? Behav Brain Res. 2009;204:258–273. doi: 10.1016/j.bbr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, Kreibich AS, Abel T, Brodkin ES, Hahn CG, et al. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins and the neuromuscular system: 10 years of answers and questions. J Neurocytol. 2003;32:619–647. doi: 10.1023/B:NEUR.0000020614.83883.be. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan LQ, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JLR, Marín O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, LeBeau FEN, Bannerman D, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Pritchett D, Stumpenhorst K, Betts JF, Nissen W, Schweimer J, Lane T, Burnet PWJ, Lamsa K, Sharp T, et al. Genetic mouse models relevant to schizophrenia: Taking stock and looking forward. Neuropharmacology. Forthcoming 2011 doi: 10.1016/j.neuropharm.2011.08.009. doi:10.1016/j.neuropharm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Heistek TS, Timmerman AJ, Spijker S, Brussard A, Mansvelder HD. GABAergic synapse properties may explain genetic variation in hippocampal network oscillations in mice. Front Cell Neurosci. 2010;4:18. doi: 10.3389/fncel.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel HJ, Otahl J, Taubenberger N, Heinemann U, Kovacs R, Kann O. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in the mitochondrial redox state. J Neurosci. 2008;28:1153–1162. doi: 10.1523/JNEUROSCI.4105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Huchzermeyer C, Kovacs R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–358. doi: 10.1093/brain/awq333. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–687. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS One. 2010;5(12):e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber G, Streif R, Schwaiger F, Kreutzberg G, Hager G. Neuregulin-1 isoforms are differentially expressed in the intact and regenerating adult rat nervous system. J Mol Neurosci. 2003;21:149–165. doi: 10.1385/JMN:21:2:149. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high risk of psychosis. Mol Psychiatry. 2009;14:118–122. doi: 10.1038/mp.2008.1. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Krivosheya D, Tapia L, Levinson JN, Huang K, Kang Y, Hines R, Ting AK, Craig AM, Mei L, Bamji SX, et al. ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J Biol Chem. 2008;283:32944–32956. doi: 10.1074/jbc.M800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens JÃ, Hernandez L, Vullhorst D, Buonanno A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Li B, Woo R-S, Mei L, Malinow R. The neuregulin-1 receptor ErbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–209. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hájos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately R, Giuliano AJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. Theories of hippocampal function. In: Andersen P, Morris R, Amaral A, Bliss T, O’Keefe J, editors. The hippocampus book. Oxford: Oxford University Press; 2006. pp. 581–714. [Google Scholar]
- Murray AJ, Sauer J-F, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacol. 2011;36:488–496. doi: 10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Luna A, Vakkalanka R, Straub RE, Kleinman JE, Weinberger DR. A 5′ promoter region SNP in NRG1 is associated with schizophrenia risk and type III isoform expression. Mol Psychiatry. 2009;14:741–743. doi: 10.1038/mp.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luma A, Kolachana B, Vakkalankka R, Rujescu D, Giegling I, Straub RE, McGee K, et al. NRG1, ERBB4 and AKT1 epistasis increases schizophrenia risk and is biologically validated via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67:991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hájos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2006;26:9923–9934. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CMP, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Harvey R, Waddington JL. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Res. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Marin O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21:262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Rimer M, Barrett DW, Maldonado MA, Vock V, Gonzalez-Lima F. Neuregulin-I immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- Role LW, Talmage DA. Neurobiology—new order for thought disorders. Nature. 2007;448:263–265. doi: 10.1038/448263a. [DOI] [PubMed] [Google Scholar]
- Romberg C, Raffel J, Martin L, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM, Paulsen O. Induction and expression of GluA1 (GluR-A)-independent LTP in the hippocampus. Eur J Neurosci. 2009;29:1141–1152. doi: 10.1111/j.1460-9568.2009.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Bannerman DM. The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus. 2011 doi: 10.1002/hipo.20896. doi: 10.1022/hipo.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res. 2008;169:159–178. doi: 10.1016/S0079-6123(07)00009-X. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RMJ, Seeburg PH, Sprengel R, Good MA, Rawlins JNP, Bannerman DM. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Rubenstein JLR. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14:563–589. doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- Tan W, Wang YH, Gold B, Chen JS, Dean M, Harrison PJ, et al. 2007. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J Biol Chem. 282:24343–24351. [DOI] [PubMed]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu XR, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Vazdarjanova A, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO. Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons. Brain. 2011 doi: 10.1093/brain/awr018. e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao YM, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Woo T-UW, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.