Abstract
Learning-facilitated plasticity describes the ability of hippocampal synapses to respond with synaptic plasticity when weak afferent stimulation is coupled with a spatial learning event. Qualitative differences appear to influence whether long-term potentiation or long-term depression (LTD) are facilitated by spatial learning. At many hippocampal synapses, LTD is facilitated when rats actively explore a novel spatial context. We investigated whether learning-facilitated plasticity is expressed when an unconstrained but stationary rat observes a computer-generated spatial environment. Visual fields were separated. Novel object configurations were presented to one field; familiar constellations were presented to the other field. LTD was facilitated in the CA1 region of the hemisphere to which novel object constellations were presented. Familiar constellations had no effect. LTD facilitation was prevented by treatment with the protein translation inhibitor, anisomycin. LTD in the dentate gyrus was not facilitated by novel object constellations, suggesting that effects are not common to all hippocampal subfields. These data support a unique association of LTD in the CA1 region with learning about spatial context and indicate that rats can passively perceive space.
Keywords: CA1, in vivo, learning and memory, LTD, Schaffer collaterals, Stratum radiatum
Introduction
Although hippocampal synaptic plasticity is widely believed to comprise the cellular mechanism underlying many forms of memory, little evidence exists to date that incontrovertibly verifies synaptic plasticity as the means through which we learn. Idealized criteria such as experimental mimicry (Martin et al. 2003; Morris et al. 2003), whereby one shows that by causing synaptic plasticity in a population of synapses, a memory is created in the host individual that previously did not exist, are still far out of reach. Nonetheless, in recent years novel approaches have emerged whereby very tight correlations between specific forms of memory and specific forms of synaptic plasticity have been made. One example of this comprises the demonstration that fear memory activates the same cellular mechanisms as those induced by long-term potentiation (LTP), that these processes occlude one another, and that fear memory elicits small potentiations among certain synaptic populations in the CA1 region (Whitlock et al. 2003). Learning-facilitated plasticity is another approach that aims to identify associations between different forms of synaptic plasticity and different components of spatial memory (Kemp and Manahan-Vaughan 2007). Here, patterned afferent stimulation that is subthreshold for the induction of synaptic plasticity, when coupled with specific spatial learning tasks, leads to persistent synaptic plasticity that lasts for days or weeks in vivo. This facilitation of synaptic plasticity also occurs when spatial exploration is conducted in the absence of patterned afferent stimulation (test–pulse stimulation was given instead) (Manahan-Vaughan and Braunewell 1999). Facilitation of synaptic plasticity only occurs when the spatial experience is novel and when learning about the environment takes place (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2008; Lemon and Manahan-Vaughan 2006; Hagena and Manahan-Vaughan 2011). Two key questions emerge from these observations:
Is movement required for the facilitatory effect on synaptic plasticity to occur, that is, can this effect be seen when the animal is passive and arousal influences are minimized? In this case, the novelty of the spatial information becomes quite dominant in comparison to acquisition of information by active exploration. According to Lisman and Grace (2005), novelty should help drive the induction and persistence of synaptic plasticity.
Is the property of learning-facilitated plasticity confined to 3D space?
Elucidation of these points would not only help us understand to what extent rodents can passively acquire explicit memories but also provide insight into putative cellular mechanisms underlying the learning phenomenon. Clarification of the latter point could additionally comprise an important step in developing an experimental strategy to examine the consequences of virtual/digital media-derived sensory information (television, computer gaming) for information encoding in the “real” world. We unified these questions into a study that examined whether rats express learning-facilitated plasticity under passive circumstances where spatial information was provided by means of a computer screen.
Materials and Methods
All experiments were performed according to the guidelines of the German Animal Protection Law and were approved by the North Rhine Westphalia State Authority. Male Hooded Lister rats (Harlen Winkelmann, Germany, 7–8 weeks old) underwent implantation of hippocampal electrodes and a guide cannula under anesthesia, as described previously (Kemp and Manahan-Vaughan 2004).
Briefly, the animals were anaesthetized with sodium pentobarbitone (“Nembutal” [52 mg/kg body weight intraperitoneally]). The skull was uncovered, and holes for the electrodes and 2 anchor screws were drilled. The 2 anchor screws served as ground and reference electrode respectively. A monopolar recording electrode made of lacquer-coated stainless steel wire with an outer diameter of 0.1 mm was lowered into stratum radiatum in the right hemisphere at 2.8-mm posterior to bregma and 1.8-mm lateral to midline. A bipolar stimulating electrode was inserted into the ipsilateral schaffer collateral–commissural pathway (3.1-mm posterior to bregma and 3.1-mm lateral to midline). To obtain bilateral recordings, a second monopolar electrode was placed in stratum radiatum of the left hemisphere. Recordings from the dentate gyrus were obtained by placing a monopolar recording electrode in the granule cell layer (3.1-mm posterior to bregma and 1.9-mm lateral to the midline) and a bipolar electrode in the medial perforant path (6.9-mm posterior to bregma and 4.1-mm lateral to the midline for the stimulation electrode). To enable drug application, a guide cannula was inserted into the lateral cerebral ventricle. Throughout the implantation, evoked responses were monitored to ensure the correct position of the electrodes. The assembly was sealed and fixed with dental cement. The animals recovered for a period of 7–10 days before experiments commenced.
Measurement of Evoked Potentials
The field excitatory postsynaptic potential (fEPSP) was employed as a measure of excitatory synaptic transmission in the CA1 region. To obtain these measurements, an evoked response was generated in the stratum radiatum by stimulating at low frequency (0.025 Hz) with single biphasic square wave pulses of 0.2-ms duration per half wave, generated by a constant current isolation unit. For each time point measured during the experiments, 5 records of evoked responses were averaged. The first 6 time points, recorded at 5-min intervals, were used as baseline, and all points are shown in relation to the average of these 6 points. fEPSP changes were determined by means of assessing the maximal slope through the 5 steepest points obtained on the first negative deflection of the potential. By means of input/output curve determination (evaluation of 9 different stimulation intensities from 100 to 900 μA in 100-μA steps), the maximum fEPSP was found, and during experiments, all potentials employed as baseline criteria were evoked at a stimulus intensity that produced 40% of this maximum. To induce reliable short-term plasticity, 900 pulses at 1 Hz were applied (Manahan-Vaughan 2000). Before synaptic plasticity experiments were commenced, all animals were first assessed in a baseline experiment, where the stability of potentials evoked by test pulses (0.025 Hz) was assessed. Here, baseline stability was evaluated for a period of approximately 6 h followed by a further 1 h of measurements 24 h later. Only those animals that showed stable evoked responses (deviations of maximally ±10% from the mean baseline assessed during the first 30 min of the experiment) were included in subsequent experiments. Animals served as their own controls, that is, the responses of animals that were assessed for STD (using low-frequency stimulation [LFS] alone) were compared with the subsequent responses of the animals when, for example, LFS was given together with exposure to novel pictures.
To measure synaptic activity in the dentate gyrus, analysis of both the population spike (PS) amplitude and fEPSP slope was conducted. The maximal PS amplitude was determined by input–output curve analysis, as described above. PS was measured as the maximal slope through the 5 steepest points obtained on the first negative deflection of the PS. fEPSP was measured as the maximal slope through the 5 steepest points obtained on the first positive deflection of the potential.
The stimulus intensity used during experiments was set to evoke a potential that was 40% of the maximum obtained in the input–output analysis. For CA1 recordings, this was typically <100 μA, for the dentate gyrus recordings this was typically <250 μA. Cortical electroencephalography was continuously monitored throughout the experiments. Here, we could confirm that no disturbances were provoked by the experimental protocols.
Experimental Set-Up
To enable proper acclimatization, the animals were placed in the experiment room on the day before commencement of experimentation. The recording chamber measured 40 × 40 × 40 cm. It was constructed of gray Perspex except for a removable clear Perspex front wall. The box was open at the top. In the back wall, there was a 20-cm opening where a triangular annex was attached. The length was 22 cm. It ended in a 2-cm-wide opaque wall with a hole 6 cm above ground for a drinking bottle. The sides were partially covered with removable nonreflective glass panels. Parallel to each glass panel, a computer monitor was placed at a distance of 40 cm. Thus, when the animal was drinking the reward, one of its eyes faced one of the monitors (Fig. 1a), that is, the monitor was placed at an angle of approximately 180° respective to the eye. The implanted electrodes were connected via a flexible cable and swivel connector to the stimulation and recording equipment and thus the animal could move around freely in the recording chamber.
Figure 1.
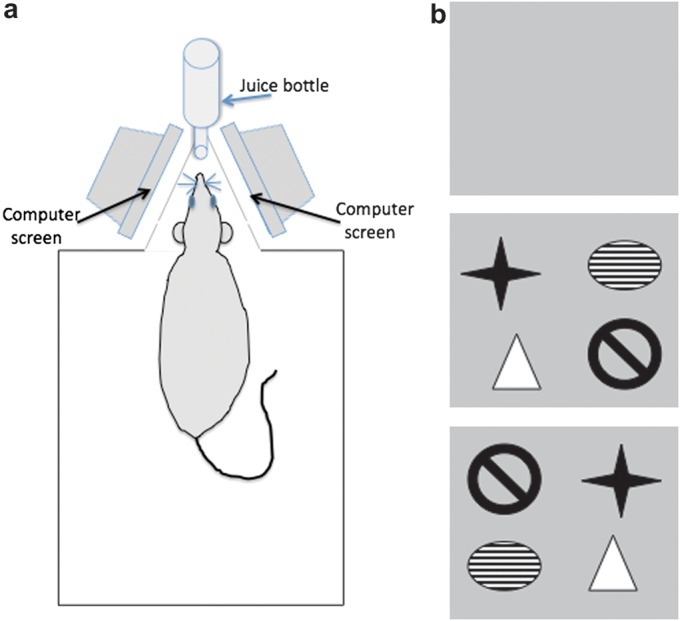
a) Schematic diagram of the recording chamber. The recording chamber measured 40 × 40 × 40 cm. In the back wall, a small triangular annex contained a hole in which the nozzle of the juice bottle could be inserted. The sides of the annex contained removable nonreflective glass panels through which 2 computer monitors could be seen. Thus, when the animal was drinking, each eye faced a separate monitor. When not drinking, the animal could move around freely in the recording chamber. (b) Examples of the visual imagery used. In control experiments, animals were exposed to a blank computer screen that consisted of a gray uniform color. Large, high contrast clip art was used for experiments that examined the effects of spatially arranged visual imagery on synaptic transmission and plasticity. Four images were shown on a gray background. The images were clearly distinct from one another. In the “reexposure” experiment, the same images in the same spatial locations were shown to the animal. In the “novel configuration” experiment, the same images were shown in new spatial locations.
After the initial 30 min of recording, a drinking bottle containing carrot juice was inserted into the hole in the back wall. When the rat started drinking, the LFS was commenced. During the 15 min of LFS, the animal had access to the juice.
The critical manipulations of the study comprised the following: In the first experiment, no pictures were shown on the monitors, which were however switched on. The animals simply viewed the gray background upon which images would later be placed. This served as a control experiment to evaluate if the reward itself influenced synaptic plasticity. In subsequent experiments, a configuration of 4 simple graphics (clipart from Corel Draw), on a gray background, was displayed on each monitor (Fig. 1b). One figure occupied each quadrant of the screen. The drawings were only displayed when the rat was sitting still and drinking, and during this time LFS was applied. To ensure an even illumination of the triangular annex, the gray background was displayed on the screens during the entire experiment.
In experiments where effects of the screen exposure were assessed during test–pulse stimulation only, screens were switched on after the initial 30 min of baseline recording. One set of animals was then exposed to the blank gray screen, and the other was exposed to the gray screen upon which a spatial arrangement of images was displayed for a 15-min period. Test–pulse stimulated controls were not exposed to the computer monitors.
Drugs
For injection, anisomycin (2.4 mg; Sigma-Aldrich) was first dissolved in 10 μl of 1-M HCl and then treated with 1-M NaOH to create a pH of 7.0. The solutions were subsequently made up to a 50-μl volume with 0.9% sodium chloride. Five microliters (240-μg anisomycin) was injected intracerebroventricularly (i.c.v) over a period of 5 min.
Data Analysis and Statistics
For each time point, an average was made of 5 consecutive evoked responses. Recordings were taken every 5 min until 30 min after LFS, and then subsequently at 15-min intervals until 4 h had elapsed. The following day, an additional 5 recordings were made. In the anisomycin experiment, recordings at 15-min intervals were commenced 15 min after injection.
The 30 min of recordings obtained prior to injection were averaged and served as baseline. All data were expressed as the mean percentage ± standard error of the mean of the baseline. Statistical evaluations were performed by analysis of variance (ANOVA) with repeated measures. ANOVA was conducted for the values obtained after the stimulation protocols. Student’s t-tests were used to assess individual time point differences. The level of significance was set at P < 0.05.
Results
Novel Visuospatial Information Is Correlated with Facilitation of LTD in Area CA1
To ensure that the animals were placed so that the pictures on the monitors were perceived in a monocular manner, juice was offered via a drinking tube inserted into a hole in the back wall of the chamber (Fig. 1a). This strategy was chosen as it circumvented having to deprive the rats of water: water deprivation is known to influence synaptic plasticity (Almaguer-Melian et al. 2003). When the animals drank the juice, they positioned their heads such that each eye looked directly through a glass panel at one computer screen.
In the first experiment, no pictures (“blank screen”) were shown on the screens during drinking. LFS that is known to elicit short-term depression (STD) (Manahan-Vaughan and Braunewell 1999) was applied during drinking (1 Hz, 600 pulses). LFS was commenced when the rats started drinking the juice for the first time. The juice was available throughout the duration of LFS (and was then removed). Application of LFS under these conditions elicited STD (Fig. 2). fEPSPs, recorded from the hippocampus ipsilateral to the stimulating electrode, expressed an initial depression of 72.5 ± 4.99% of basal fEPSPs (n = 12). Four hours later, the average value was 102.4 ± 5.16%. The fEPSPs obtained from the contralateral side also exhibited STD following LFS during juice drinking and the viewing of the blank screen. Five minutes after conclusion of LFS, fEPSP values on the contralateral side were 66.8 ± 4.98% of basal synaptic transmission (Fig. 2). Also in this case, values recovered after about 1 h to basal (control) levels.
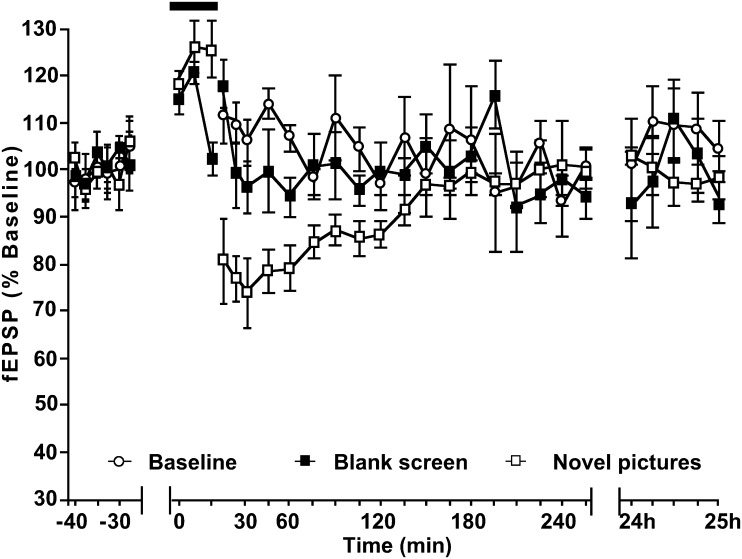
Figure 2.
Novel pictures displayed on a computer monitor facilitate LTD in the CA1 region. A stimulating electrode was placed in the Schaffer collaterals of the right hemisphere, and recording electrodes were placed in the Stratum radiatum of both hippocampi. Recordings were conducted from freely behaving rats, a minimum of 10 days after electrode implantation. LFS (1 Hz, 900 pulses) elicited STD in animals that viewed blank computer screens. When 4 novel pictures were displayed on the monitors, LFS resulted in LTD that lasted for over 24 h in both hemispheres. (a) Recordings from the hemisphere ipsilateral to the stimulation electrode. (b) Recordings from the contralateral hemisphere. Line breaks indicate change in time scale. Insets in (a): analog traces recorded before LFS (solid line), 5 min after LFS (dashed line) and 24 h after stimulation (dotted line). Analog traces on the left show evoked field potentials from the ipsilateral stratum radiatum in an experiment where LFS was applied alone, whereas analogs on the right represent an experiment where a configuration of 4 novel pictures were displayed during LFS. Insets in (b): signify field potentials evoked by the same stimulation as above but recorded in the contralateral hemisphere. Left: LFS only; right: LFS + 4 novel pictures. Horizontal scale bar: 2 mV, vertical scale bar: 5 ms.
In the subsequent experiment, 2 sets of 4 novel pictures (one set displayed to each eye), were shown to the animals during LFS. Exposure to the novel pictures facilitated STD into long-term depression (LTD) (>24 h) in both hemispheres (n = 12) (Fig. 2). Compared with the first experiment, fEPSPs recorded from the ipsilateral hemisphere were significantly reduced from 120 min after LFS onwards (ANOVA: F1,24 = 128.9, P < 0.0001, t = 120 min; t-test: P = 0.016). In the contralateral hemisphere, LTD was significant from t = 180 min (ANOVA: F1,24 = 47.85, P < 0.0001, t = 180 min; t-test: P = 0.016). The following day, the fEPSPs were 70.1 ± 6.11% and 84.3 ± 3.55% of average baseline values in the ipsilateral and contralateral hippocampi, respectively.
To examine whether exposure to the digital images has an independent effect on evoked potentials, we examined the effects of exposure to the blank screen on basal synaptic transmission. Here, stable responses were evoked by test–pulse stimulation (n = 6) that were consistent over the recording period (∼4 h) and did not differ from controls that received test–pulse stimulation without exposure to the monitors (n = 6) (ANOVA: F1,28 = 1.179, P = 0.249). We then examined the effect of exposure to a novel image constellation, 30 min after commencing recordings. The protocol was identical to that described above (for Fig. 2), except in this case only test–pulse stimulation (and no LFS) was given (Fig. 3). During exposure to the monitors, both animals that were exposed to the blank screen and animals exposed to the novel images showed a significant increase in the magnitudes of the fEPSPs (Fig. 3, P < 0.01) compared with preexposure baseline. At the end of this 15-min period, fEPSPs in the animals exposed to the blank screen were no longer significant from test-pulse–stimulated controls, whereas in the animals exposed to novel images, fEPSPs were significantly depressed. The depression was transient but significant (n = 6) (ANOVA: F1,22 = 13.99, P < 0.01). Evoked fEPSPs returned to basal levels by 105 min after commencement of exposure (Fig. 3).
Figure 3.
Exposure to a blank screen during test–pulse stimulation does not affect basal synaptic transmission, whereas exposure to novel image constellations elicits STD. Test–pulse stimulation of animals exposed to a blank (gray) computer screen has no lasting effect on basal synaptic transmission, compared with controls that received test–pulse stimulation in the absence of exposure to a monitor. In the presence of test–pulse stimulation, exposure to a novel set of spatially arranged visual imagery (black bar) elicits STD that persists for approximately 120 min. In both sets of animals exposed to either a blank or a image-containing monitor, an elevation of fEPSP magnitude was observed in the 15 min immediately after exposure was commenced. In animals exposed to a blank screen, values returned rapidly to control levels. In animals exposed to novel images, synaptic depression succeeded the initial potentiation. Line breaks indicate change in time scale.
Facilitation of LTD by Novel Visuospatial Information Is Localized to the Hemisphere That Receives the Novel Information
In a subset of animals, the experiment was repeated after LTD had declined, and fEPSP values were reached that were equivalent to those seen during basal synaptic transmission prior to LTD induction (>8 days). The same configuration of the now familiar pictures was displayed on both screens when the animals were drinking the reward. However, this time no facilitation of synaptic depression followed LFS (n = 5, Fig. 4). ANOVA of responses to familiar pictures was compared with blank screen: ANOVA (ipsilateral side): F1,24 = 2.015, P = 0.1566; ANOVA (contralateral side): F1,24 = 1.192, P = 0.2756. Subsequently, we presented novel information to the monocular visual field only. In rats, there is a near complete crossover at the optic chiasm. Thus, visual stimuli from the monocular visual field are predominantly projected to the contralateral hippocampus (i.e., contralateral to the stimulating electrode position). In the current experiment, an identical configuration of familiar pictures was displayed on the screen that was perceived by the eye that projects to the contralateral side (i.e., right eye, left hippocampus). On the computer screen that was perceived by the eye that projects to the hemisphere ipsilateral to the stimulating electrode, the familiar pictures were rearranged in a novel configuration (Fig. 4). On the contralateral side, which received the familiar configuration of pictures for the third time, no facilitation of LTD was elicited by LFS (ANOVA: F1,24 = 0.1562, P = 0.6931). On the ipsilateral side, the rearranged pictures elicited a facilitation of LTD following LFS (ANOVA: F1,24 = 156.5, P < 0.0001). Compared with the previous experiment where familiar pictures were used, significantly more synaptic depression was seen starting 90 min after LFS (t = 90 min, t-test: P = 0.0227).
Figure 4.
Familiar configurations of pictures displayed on a computer screen do not facilitate LTD. Animals were stationary and alert in the recording chamber. The left eye viewed a computer screen that could not be seen by the right eye, and vice versa. Reexposure to the same configuration of pictures (familiar pictures) did not facilitate LTD in CA1 in either the hippocampus ipsilateral to the stimulating electrode (a) or the contralateral hippocampus (b). In the next experiment, the same (familiar) pictures were displayed, in the same configuration as beforehand, to the eye that projects to the contralateral hemisphere (right eye, “3rd exposure”) whereas the eye that projects to the ipsilateral hemisphere (left eye, a) was shown a new configuration of the familiar images (“novel configuration”). This resulted in a facilitation of LTD in the ipsilateral hemisphere (a), whereas in the contralateral CA1 (b), no difference in the magnitude and duration of plasticity elicited was seen compared with STD elicited by LFS under control (blank screen) conditions.
Visuospatial Novelty Facilitated LTD Is Protein Synthesis Dependent
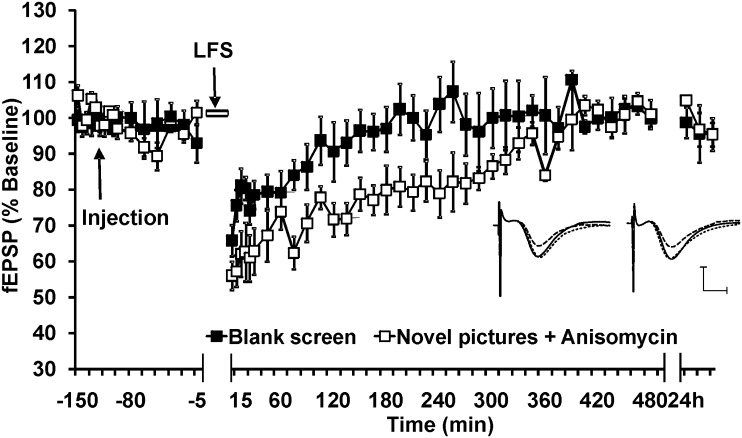
In the control experiment, vehicle was injected i.c.v. 2 h before LFS. During drinking, no pictures were shown on the screens (blank screens, Fig. 5, n = 8). Here, only STD occurred. In the next experiment, animals were treated 2 h before LFS with the protein translation inhibitor, anisomycin (240 μg, i.c.v). During LFS (in the presence of anisomycin), a configuration of 4 novel pictures was displayed. Recordings were made from both the ipsilateral and contralateral hemispheres. The protein translation inhibitor, anisomycin (240 μg, i.c.v) blocked the late but not the early phases of LTD (>4 h) in the hemisphere to which the novel images were displayed (ANOVA: F1,24 = 79.72, P < 0.0001). From 255 min after LFS, no significant difference was found between the LFS alone versus LFS + pictures groups (t-test: P = 0.0505). No significant effect of anisomycin on STD was seen in the hemisphere to which the “blank screen” was projected (not shown).
Figure 5.
Visuospatial novelty-facilitated LTD is protein synthesis dependent. (a) In the control experiment, no pictures were shown during LFS (blank screen). This induced a short-lasting synaptic depression (STD). Exposure to novel pictures facilitated LTD that lasted for over 24 h (see Fig. 2). When exposure to novel pictures during LFS occurred in the presence of the protein translation inhibitor, anisomycin (240 μg applied i.c.v), a significant inhibition occurred of the facilitation of LTD by novel pictures. Line breaks indicate change in time scale. Insets show original analog traces representing the field potentials evoked in the CA1 pre-LFS (solid line), 5 min (dotted line) and 24 h (dashed line) when the stimulation was blank screen (analogs on the left) and when novel pictures were shown to an animal treated with anisomycin (analogs on the right). Vertical scale bar corresponds to 2 mV, and horizontal scale bar corresponds to 5 ms.
LTD in the Dentate Gyrus Is Not Influenced by Perception of Novel Visuospatial Information
In an active spatial exploration test (Kemp and Manahan-Vaughan 2004, 2005, 2008; Hagena and Manahan-Vaughan 2011), LTD in the individual subregions of hippocampus was influenced by learning about different features of a novel spatial environment. Whereas LTD in the CA1 region is facilitated by discrete contextual features of a spatial environment, in the dentate gyrus, LTD is facilitated by highly visible landmark (or navigational) cues (Kemp and Manahan-Vaughan 2008). Given the potent effects of novel object constellations observed on CA1 LTD in the present study, we investigated whether this type of information also engages long-term plasticity in the dentate gyrus (Fig. 6).
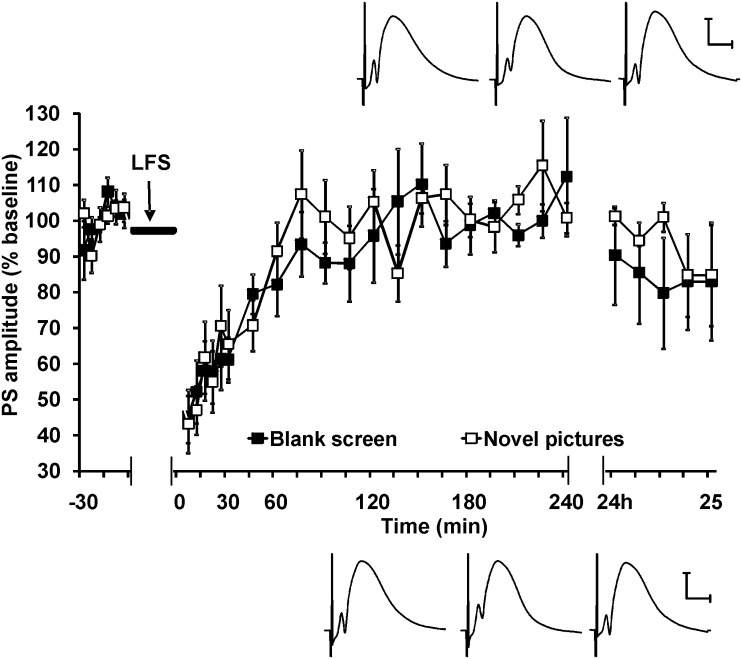
Figure 6.
Visuospatial novelty does not facilitate LTD in the dentate gyrus. In contrast to its effects in the CA1 region, viewing spatial configurations of novel pictures does not elicit a facilitation of LTD in the dentate gyrus. The figure shows recordings of the population spike amplitude. Equivalent effects occurred with regard to fEPSP (not shown). Line breaks indicate change in time scale. Insets show analog traces taken from a rat before LFS (left), 5 min after LFS (middle) and 24 h after LFS (right). The top row is from the control experiment (blank screen), and the bottom row is from the same rat exposed to novel pictures. Vertical scale bar corresponds to 2 mV, and horizontal scale bar corresponds to 5 ms.
In the control experiment, with free access to drinking during exposure to the blank computer screen, STD was induced in the dentate gyrus by LFS (n = 9) (ANOVA of effects on PS: F1,24 = 149.2, P < 0.0001; ANOVA of effects on fEPSP: F1,24 = 118.6, P < 0.0001). In contrast to the results from the CA1 region, no significant change in STD was induced when novel pictures were displayed during LFS. This suggests that the modulation of synaptic depression by passive spatial perception, at least under the conditions described here, is restricted to the CA1 region.
Discussion
In this study, we demonstrate for the first time that passive perception of spatial configurations of objects displayed on a computer screen facilitates LTD in adult rats. Strikingly, effects were restricted to the CA1 region: the dentate gyrus was not affected. LFS, given while the rats viewed a blank computer screen, resulted in STD in both hippocampal subregions. Test–pulse stimulation, given under the same conditions, had no effect on basal synaptic transmission. Exposure of the animals to spatially arranged visual imagery resulted in the facilitation of STD into LTD in the CA1 region but not dentate gyrus. Reexposure to the same configurations of the same imagery did not alter synaptic strength, whereas exposure to familiar images in new configurations facilitated CA1 LTD. Test–pulse stimulation given while the animals were exposed to spatially arranged visual imagery resulted in STD in the CA1 region. This demonstrates not only that passive learning influences synaptic plasticity but also that “2D” spatial imagery has a significant impact on synaptic plasticity. The data also support previous observations that LTD is modulated by spatial contextual learning. The finding that learning-facilitated LTD in the CA1 region is prevented by protein translation inhibition consolidates the link between this type of synaptic plasticity and learning.
In the present study, rats were not constrained, rather they learned that to access liquid, they needed to position themselves in a stationary manner in the recording chamber, such that each eye was exposed to information on 1 of 2 computer screens. This meant on the one hand that information perceived on one computer screen projected predominantly to the contralateral hemisphere and hippocampus, and on the other hand that we could implement an “in-animal” control whereby the effects of novel or familiar spatial information on hippocampal plasticity could be compared. In the first set of experiments, recordings from the CA1 region of both hippocampi were elicited by stimulation of the Schaffer collateral pathway of the right hippocampus. LFS at 1 Hz elicited STD that persisted for approximately 60 min. Coupling of this stimulation with a spatial object constellation, projected to both hemispheres simultaneously, resulted in LTD that persisted for over 24 h. Exposure of one eye to novel images and the other eye to the blank monitor resulted in LTD facilitation only in the hippocampus that received the novel image information. These data indicate that rats perceive 2D space and that this information influences synaptic plasticity processes in the hippocampus.
Test–pulse stimulation given during exposure to the test screen (screen on, gray color) had no significant effect on basal synaptic transmission, suggesting that changes in arousal derived from the new visual input and drinking activity have no lasting effects on basal synaptic transmission and do not elicit changes in synaptic strength. In contrast, test–pulse stimulation given during exposure to spatially arranged visual imagery resulted in STD. This finding is in line with reports that indicate that a certain level of afferent synaptic stimulation must coincide with the exposure to novel spatial constellations in order for persistent LTD to occur (Manahan-Vaughan and Braunewell 1999). Learning facilitation of LTD occurs when LFS as brief as 300 pulses is given (Popkirov and Manahan-Vaughan. 2011). These are useful observations, as they support that intensive afferent stimulation is not a prerequisite for the facilitation of LTD under circumstances where an animal can learn.
Test–pulse stimulation given in conjunction with image exposure does not provide adequate afferent stimulation to enable persistent LTD to be triggered. Here, only a transient synaptic depression occurs. This finding indicates that LTD elicited during image exposure is an active process, and it excludes that visual spatial perception merely elicits a persistent global depression of CA1 synapses. It was striking that during the initial 15-min exposure period, to either the blank or image-containing computer screen, excitability increased. This phenomenon has also been observed during exposure to spatial arrangements of physical objects during test pulses (Manahan-Vaughan and Braunewell 1999). The effect may relate to enhanced neuronal activity in the visual cortex during active visual processing (Tsanov and Manahan-Vaughan 2007a, 2007b) and an associated increase in hippocampal excitability (Tsanov and Manahan-Vaughan 2009). These excitability changes may reflect a lowering of synaptic plasticity thresholds in readiness for new synaptic information storage (Tsanov and Manahan-Vaughan 2008). In animals exposed to the blank screen, fEPSPs reverted rapidly to control levels, whereas the animals exposed to the spatially arranged visual imagery expressed significant synaptic depression that appeared immediately after the 15-min exposure period. It is tempting to speculate that the initial potentiation shares properties with LTP. In the physical environment, exposure to empty space facilitates LTP, whereas exposure to spatial content facilitates LTD (Kemp and Manahan-Vaughan 2007, 2008; Hagena and Manahan-Vaughan 2011). The fact that both experiences generally go hand in hand suggests that synaptic potentiation that occurs during a learning event may be pruned by subsequent LTD (triggered by salient spatial content) to enable an accurate and efficient spatial representation (Hagena and Manahan-Vaughan 2011). Our current finding that blank screen exposure does not cause lasting changes in test–pulse stimulated animals, whereas novel spatial imagery facilitates STD, suggests that it is not the exposure to visual information per se, but rather the saliency, presumably on a spatial level that leads to persistent changes in synaptic efficacy.
Hippocampal LTD is not facilitated by object novelty, rather it is the context of the object’s location in space that facilitates this form of plasticity (Kemp and Manahan-Vaughan 2004). Thus, the reexposure of an animal to a rearranged constellation of known objects is as effective as the first exposure to novel constellations of objects in facilitating LTD. However, no facilitation occurs if familiar objects are presented in familiar locations (Kemp and Manahan-Vaughan 2004). This property is shared by all synapses of the trisynaptic circuit (Manahan-Vaughan and Braunewell 1999; Lemon and Manahan-Vaughan 2006; Kemp and Manahan-Vaughan 2008; Hagena and Manahan-Vaughan 2011). Taken together, these findings comprise a very strong argument that it is the spatial component of the visual information that leads to facilitation of LTD. Furthermore, learning about the environment occurs in this paradigm: dipping and rearing behavior is significantly less frequent when the first exposure is compared with reexposure to familiar object configurations (Manahan-Vaughan and Braunewell 1999; Popkirov and Manahan-Vaughan 2011). We investigated if perception of virtual space shares this property. We observed that reexposure of the animals to the same spatial object configuration has no effect on LTD in either hemisphere. If however, we rearrange the objects on one screen, and on the other screen leave them in the original configuration, we now only see facilitation of LTD in the hippocampus to which the novel object constellation was projected. This suggests that rats can use 2D space for the creation of spatial representations and that this ability is strongly associated with the facilitation of hippocampal LTD. Furthermore, the finding that the facilitation of LTD was localized to the hemisphere that perceived the presentations of the rearranged objects suggests that LTD is confined to the synapses that participate in the encoding of the information.
These changes also occur on a molecular level: other studies have shown that perception of imagery on a computer screen alters immediate early gene expression in the hippocampus (Brown and Aggleton 2001; Aggleton et al. 2007). Here, spatially arranged images, but not single images caused an increase in the expression of c-fos in the hippocampus (Wan et al. 1999). It has also been shown that hippocampal lesions impair learning of image locations on a computer screen in rodents (Talpos et al. 2008). Immediate early genes trigger protein synthesis. As memory requires protein synthesis (Squire and Barondes 1970, 1972; Grecksh and Matthies 1980), it is believed that cellular mechanisms that underlie synaptic information storage and memory formation must share this property. This is because synaptic remodeling, synaptogenesis, the expression of additional or different neurotransmitter receptors, and even neurogenesis are believed to comprise elements of the acquisition, consolidation, and retention of new memories (Bruel-Jungerman et al. 2007). Hippocampal LTD, which is elicited solely by means of afferent stimulation, requires protein translation (Manahan-Vaughan et al. 2000). We therefore examined if learning-facilitated LTD, elicited by virtual spatial perception, requires protein synthesis. We found that treatment with anisomycin, a protein translation inhibitor, blocked the late but not the early phases of LTD (>4 h). This suggests that this phenomenon engages in synaptic processes that are common to both electrically induced synaptic plasticity and learning.
It has been reported that whereas the CA1 region responds with LTD when an animal explores small features of a spatial environment, LTD is facilitated in the dentate gyrus by large, presumably orientational, or navigational information (Kemp and Manahan-Vaughan 2008). The converse is not true: CA1 LTD is not facilitated by landmark information, and dentate gyrus LTD is not facilitated by small details of a spatial environment (Kemp and Manahan-Vaughan 2008). To establish the nature of the informational perception on the part of the rat, we examined whether LTD in the dentate gyrus is facilitated by presentation of the virtual object constellations. We observed no facilitation of LTD under these circumstances. This suggests that the facilitation of LTD in the CA1 region is similar to effects seen in a physical environment and that it relates to the processing of information about contextual features of an environment. It is interesting that the computer imagery is not processed as would be a cue card. Polarizing the environment with a novel cue card facilitates LTD in the dentate gyrus but not in CA1 (Kemp and Manahan-Vaughan 2008). It may be that LTD in the dentate gyrus is more tightly correlated to idiothetic cues than the CA1 region (Jacobs and Schenk 2003; Jacobs 2006). Metric information is primarily processed in the dentate gyrus whereas CA1 is involved in processing topological information (Goodrich-Hunsaker et al. 2008). Changing the “virtual” object constellation does not change the metric space but the topology is changed, which could explain why we observed changes in synaptic plasticity in the CA1 region only. Taken together, these data offer intriguing insights into information processing in the rat brain: they tell us that a rat is capable of perceiving a virtual environment generated by computer imagery and of building spatial associations between the imagery it perceives. Furthermore, they indicate that even in the absence of strong motion, arousal or motivational stimuli, LTD is facilitated by spatial information. This indicates that LTD may comprise a key mechanism for specific types of information processing in the hippocampus.
It is perhaps contraintuitive to expect that LTD contributes to learning processes, as the direct consequence of LTD is less synaptic transmission. On a generalized level, one might expect however that information from a neuronal network can be modified with greater precision if not all cells relay information by means of potentiated function. On a more specific hippocampal level, one could speculate that the Schaffer collateral input to CA1 via the trisynaptic network, and the direct entorhinal cortex (EC) input to CA1 (Remondes and Schuman 2002, 2003, 2004), may cooperate together in encoding spatial context. Whereas the Schaffer collateral input to CA1 conveys integrated information about space that has been modified as it progresses through the trisynaptic network, the EC input may convey an efference copy of the original information that was perceived by the cortex (Lisman and Otmakhova 2001; Vinogradova 2001; Rolls and Kesner 2006). Under conditions where spatial context is novel, the Schaffer collateral input to CA1 is depressed, and the EC input is active. Under conditions where spatial context is familiar, both inputs are active. By this means, the CA1 pyramidal cells receive a clear signal as to which spatial information is novel and which is not, which in turn enables accurate spatial representations to be created.
An unresolved and controversial issue, is the influence, particularly in children, of exposure to television and computer media on consolidation of recently learned semantic information (e.g., from the school environment). Whether 2D (or digital) spatial and sensory perception interferes with memory consolidation is an issue that it is difficult to resolve in psychological studies of humans. Our data indicate that rats engage in synaptic plasticity under conditions where spatial learning about digital information occurs. This finding will enable new experimental approaches that address possible interference of learning in the real world with learning in a virtual world.
Funding
German Research Foundation (Deutsche Forschungsgemeinschaft, SFB 874/TP B3) to D.M.-V.
Acknowledgments
We gratefully acknowledge the assistance of Jens Klausnitzer and Beate Krenzek. Conflict of Interest : None declared.
References
- Aggleton JP, Sanderson DJ, Pearce JM. Structural learning and the hippocampus. Hippocampus. 2007;17:723–734. doi: 10.1002/hipo.20323. [DOI] [PubMed] [Google Scholar]
- Almaguer-Melian W, Martínez-Martí L, Frey JU, Bergado JA. The amygdala is part of the behavioural reinforcement system modulating long-term potentiation in rat hippocampus. Neuroscience. 2003;119:319–322. doi: 10.1016/s0306-4522(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. Neuroscientist. 2007;13:492–505. doi: 10.1177/1073858407302725. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq271. PMID: 21493717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF. From movement to transitivity: the role of hippocampal parallel maps in configural learning. Rev Neurosci. 2006;17:99–109. doi: 10.1515/revneuro.2006.17.1-2.99. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Schenk F. Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychol Rev. 2003;110:285–315. doi: 10.1037/0033-295x.110.2.285. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex. 2005;15:1037–1043. doi: 10.1093/cercor/bhh204. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion of declarative memory processes. Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex. 2000;10:482–487. doi: 10.1093/cercor/10.5.482. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2003;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Molecular mechanisms contributing to long-lasting synaptic plasticity at the temporoammonic-CA1 synaps. Learn Mem. 2003;10:247–252. doi: 10.1101/lm.59103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Barondes SH. Actinomycin-D: effects on memory at different times after training. Nature. 1970;225:649–650. doi: 10.1038/225649a0. [DOI] [PubMed] [Google Scholar]
- Squire LR, Barondes SH. Variable decay of memory and its recovery in cycloheximide-treated mice. Proc Natl Acad Sci U S A. 1972;69:1416–1420. doi: 10.1073/pnas.69.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, Dias R, Bussey TJ, Saksida LM. Hippocampal lesions in rats impair learning and memory for locations on a touch-sensitive computer screen: the “ASAT” task. Behav Brain Res. 2008;192:216–225. doi: 10.1016/j.bbr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Intrinsic, light-independent and visual activity-dependent mechanisms cooperate in the shaping of the field response in rat visual cortex. J Neurosci. 2007a;27:8422–8429. doi: 10.1523/JNEUROSCI.1180-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. The adult visual cortex expresses dynamic synaptic plasticity that is driven by the light/dark cycle. J Neurosci. 2007b;27:8414–8421. doi: 10.1523/JNEUROSCI.1101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Synaptic plasticity from visual cortex to hippocampus: systems integration in spatial information processing. Neuroscientist. 2008;14:584–597. doi: 10.1177/1073858408315655. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Long-term plasticity is proportional to theta-activity. PLoS One. 2009;4:e5850. doi: 10.1371/journal.pone.0005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:5780598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2003;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]