Abstract
We value skills we have learned intentionally, but equally important are skills acquired incidentally without ability to describe how or what is learned, referred to as implicit. Randomized practice schedules are superior to grouped schedules for long-term skill gained intentionally, but its relevance for implicit learning is not known. In a parallel design, we studied healthy subjects who learned a motor sequence implicitly under randomized or grouped practice schedule and obtained diffusion-weighted images to identify white matter microstructural correlates of long-term skill. Randomized practice led to superior long-term skill compared with grouped practice. Whole-brain analyses relating interindividual variability in fractional anisotropy (FA) to long-term skill demonstrated that 1) skill in randomized learners correlated with FA within the corticostriatal tract connecting left sensorimotor cortex to posterior putamen, while 2) skill in grouped learners correlated with FA within the right forceps minor connecting homologous regions of the prefrontal cortex (PFC) and the corticostriatal tract connecting lateral PFC to anterior putamen. These results demonstrate first that randomized practice schedules improve long-term implicit skill more than grouped practice schedules and, second, that the superior skill acquired through randomized practice can be related to white matter microstructure in the sensorimotor corticostriatal network.
Keywords: consolidation, contextual interference, diffusion tensor imaging, magnetic resonance imaging, motor learning, motor sequence, online learning
Introduction
In our daily lives, we spend a significant amount of time and energy learning new skills effortfully and intentionally. For such explicitly learned skills, a phenomenon termed the Contextual Interference (CI) effect has been identified in which random interleaving of different tasks rather than practicing them in grouped sets during training leads to superior long-term skill (Shea and Morgan 1979; Lee and Magill 1983; Shea and Zimny 1983; Lee et al. 1991, 1997; Porretta and O'Brien 1991; Hall et al. 1994; Immink and Wright 1998, 2001; Shea et al. 2001; Smith 2002; Brady 2004, 2008; Ste-Marie et al. 2004; Cross et al. 2007; Jones and French 2007; Zetou et al. 2007; Lin et al. 2008, 2009; Wymbs and Grafton 2009; Kantak et al. 2010; Tanaka et al. 2010). The CI effect is thought to have its basis in the greater effort spent for randomized practice schedules over grouped ones in constant reupdating within working memory of the parameters for the upcoming task (Lee and Magill 1983; Immink and Wright 1998; Cross et al. 2007). In line with this account, various brain regions involved in strategy and planning have been linked to the CI benefit of these explicit tasks (Kantak et al. 2010; Tanaka et al. 2010).
Unknown, however, is whether a similar benefit of randomized practice schedule would apply to a different class of learning that is effortless and incidental: implicit learning. Unlike explicit learning, persons do not set out to learn a new skill but rather acquire it incidentally simply by interacting with an environment (Reber 1993). Further, they show better performance for an implicitly learned skill without the ability to consciously recollect the learned material (Cleeremans and McClelland 1991). Research in amnesic and other neurologically impaired patient populations point to dissociations in neuroanatomical substrates for explicit and implicit learning and memory (Willingham 1997; Squire 2004). Therefore, it is possible that hypothesized benefits of randomized practice schedule on implicit learning may have different neuroanatomical correlates than those reported in working memory–dependent explicit learning.
Contrary to previous work that showed effects on explicit learning, here, we aimed to find out whether practice schedule could benefit long-term skill acquired implicitly and, if so, the neuroanatomical correlates of this effect. Specifically, we hypothesized that randomized practice would improve lasting skill acquired implicitly relative to grouped practice. To test this hypothesis, subjects were trained under either a randomized or a grouped practice schedule on an implicit motor sequencing task, in which a 12-unit pattern of key-press responses is learned in the absence of ability to consciously recollect the pattern (Nissen and Bullemer 1987), and then were retested after 1 week. We aimed to gain insight into the neuroanatomical basis of this benefit by regressing long-term skill at 1 week against indices of structural connectivity across the whole brain within each group of learners.
Specifically, we fitted a diffusion tensor model (DTI) to diffusion-weighted magnetic resonance imaging data collected prior to training and regressed 1 week skill against whole-brain measures of fractional anisotropy (FA). Water diffusion within voxels containing primarily white matter is relatively less restricted along the length of axon bundles (axial) compared with nonprimary directions (radial), and the degree of anisotropic water diffusion is quantified as FA. FA has been associated with white matter microstructure properties such as axonal fiber density, axonal diameter, and degree of myelination and hence represents a measure of structural connectivity strength (Johansen-Berg and Rushworth 2009). Previous work demonstrates that indices of structural connectivity within tracts connecting regions mediating task performance correlate strongly with behavioral measures (Boorman et al. 2007; Wahl et al. 2007; Della-Maggiore et al. 2009; Buch et al. 2010).
Materials and Methods
Participants
Fifty healthy volunteers participated in the study. All subjects gave their informed consent to the experimental procedure, which was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. All subjects were right-handed and naive to the task and had a normal neurological examination as assessed by a credentialed physician. Sleep quality was assessed with the Pittsburg Sleep Questionnaire Inventory (PSQI) (Buysse et al. 1989).
Experimental Design
Implicit Learning Task
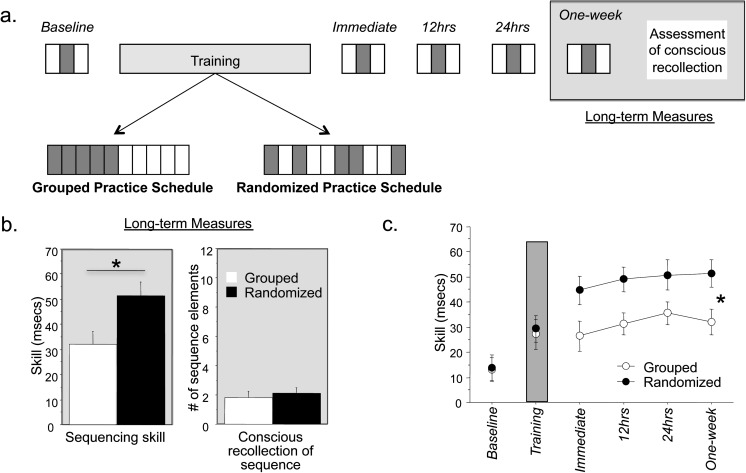
Subjects practiced the serial reaction time task (SRTT) with the right hand with either randomized (n = 21, 11 of them females, 27.5 ± 5.8 years old) or grouped (n = 29, 16 of them females; 28.4 ± 6.9 years old) practice schedules. In the SRTT, subjects made a key-press response to a target appearing in 1 of the 4 locations on the screen. Each target was a circle (1 cm diameter) spaced 3 cm apart (center to center) from the adjacent target, with the 4 targets occupying approximately 10° of the visual field (see Supplementary Fig. 1). Targets remained on-screen until the correct key-press response was made, which led to the appearance of the next target after 120 ms. Targets were organized in 12-unit sequences in pattern blocks and randomly in no-pattern blocks (Fig. 1a). At the end of each block, subjects were provided feedback of their reaction time (RT) and accuracy for 5 s followed by the next block. They were instructed to respond as quickly and accurately as possible but were told to focus more on accuracy if at any point during training accuracy dropped below 95% (Goedert and Willingham 2002).
Figure 1.
(a) Experimental design: Skill in pattern (gray) and no-pattern (white) blocks were tested at baseline, immediately after training, 12 h, 24 h, and 1 week after training in a grouped or randomized practice schedule. Long-term skill was assessed at 1 week after which conscious recollection of the sequence was assessed using the process dissociation procedure. For randomized practice, 5 pattern and 5 no-pattern blocks were randomly interleaved. For grouped practice, 5 pattern blocks were presented in a counterbalanced order with 5 no-pattern blocks. Subjects were instructed simply to respond as accurate and fast as possible and were not told of the existence of a repeating 12-unit pattern of key-presses. Thus, subjects did not know whether the practice schedule was randomized or grouped and further did not know the sequence they were learning. (b) Randomized practice schedules result in superior long-term skill as compared with grouped practice schedules: Long-term skill at 1 week was greater in randomized learners than grouped learners (left), although conscious recollection of the sequence was comparably minimal in both groups (right). On average, 2 of 12 sequence elements were identified in randomized and grouped learners. (c) Time course of skill gains: Subjects given randomized practice schedules (filled circles) also showed greater skill at earlier time points although skill was comparable at baseline and during training itself. *P < 0.05.
Each training sequence was randomly chosen for each subject from a corpus of 563 that met the following criteria: a key-press (or target) could not be repeated (e.g., 1332), each key-press appeared an equal number of times in the sequence, and all sequences excluded 4 sequential key-presses (e.g., 1234) or trills of 4 (e.g., 2424). In each pattern block, the training sequence was appended to itself 8 times (for a total of 96 trials). Four random key-press trials preceded each pattern block (Goedert and Willingham 2002). Test blocks were administered at baseline, immediately after training (immediate), and at different delays (12 h, 24 h, and 1 week; Fig. 1a).
Motor Sequencing Skill
Skill in test blocks was assessed as the difference between median RTs of pattern and flanking no-pattern blocks (Fig. 1a). Performance during training was assessed as the difference between median RTs of all pattern and all no-pattern blocks. Only correct trials were used for RT analysis.
Conscious Recollection of the Sequence
Conscious recollection of the sequence was determined only after the 1-week test blocks. At that time, subjects were informed of the existence of repeating patterns, and their recollection was tested with the process dissociation procedure (Destrebecqz et al. 2005). First, subjects were asked to reproduce on the keyboard the pattern they felt had repeated (inclusion block) and then they were asked to type randomly avoiding the pattern they felt had repeated (exclusion block). Comparative frequencies of generation of triplet chunks of the pattern between inclusion and exclusion blocks quantified conscious recollection according to previous work (Destrebecqz et al. 2005). Triplet chunks present in inclusion blocks ≥5% more often than in exclusion blocks were defined as consciously recollected sequence elements.
Statistical Analysis of Behavioral Data
For our primary hypothesis, 1-week measures of long-term skill and conscious recollection were compared between the 2 practice schedule groups using 2-sample unpaired t-tests. For all time points, the time course of skill changes was tested using a two-way Timebaseline, during training, immediate, 12 h, 24 h, 1 week × Schedulerandomized, grouped analysis of variancemixed design (ANOVAMD). If a main effect or interaction with Schedule was found, post hoc Tukey’s comparisons were used to compare skill between groups at each time point. Homogeneity of covariance was confirmed with Box’s M. Data are shown as group means ± standard error, and results were considered significant at P < 0.05.
DTI Processing
In a subset of subjects (n = 11 randomized, n = 14 grouped), prior to the training session, whole-brain, single-shot, echo-planar diffusion-weighted images (DWI) were acquired on a 3.0T GE Excite scanner using an 8-channel coil (GE Medical Systems, Milwaukee, WI) with the following parameters: time echo (TE)/time repetition (TR) = 76.4/18277.2 ms, 2.5 × 2.5 mm2 in-plane resolution, zero-filled to 1.875 × 1.875 mm2 resolution, with 60 slices at 2.5 mm thickness per volume and a total of 120 brain volumes (110 non-collinear directions; b = 100 [10 volumes], 300 [10], 500 [10], 800 [30] or 1100 [50] s/mm2) plus 10 volumes without diffusion weighting (b = 0 s/mm2). Array spatial sensitivity encoding technique acceleration factor = 2, no cardiac gating was performed. Structural T2-weighted (T2W) fast spin echo images were acquired for each subject with TE/TR = 122.304/8333.33 ms, field of view (FOV) = 240 mm2, acquisition matrix 512 × 512, 1.5 mm slice thickness. Also T1-weighted images were acquired for final stage registration into standard space (magnetization-prepared rapid gradient echo, TE/TR = 2.672/6.256 s, 0.9375 × 0.9375 mm2 in-plane resolution, with 198 slices at 1 mm thickness per volume, acquisition matrix = 256 × 256, FOV = 240 × 240 mm). Preprocessing of the DWIs was performed with algorithms included in the TORTOISE software package (www.tortoisedti.org). DWIs were corrected for motion and eddy current distortion (Rohde et al. 2004) and included proper reorientation of the b-matrix to account for the rotational component of the subject rigid body motion. In addition, B0 susceptibility–induced echo-planar imaging distortions were corrected for using an image registration based b-spline approach (Wu et al. 2008). Tensor model fitting and determination of FA values was performed using the DTIFIT software from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain’s (FMRIB’s) diffusion toolbox (FDT) from FMRIB’s Software Library (FSL; http://www.fmrib.oxac.uk/fsl/).
Tract-Based Spatial Statistics and Probabilistic Tractography
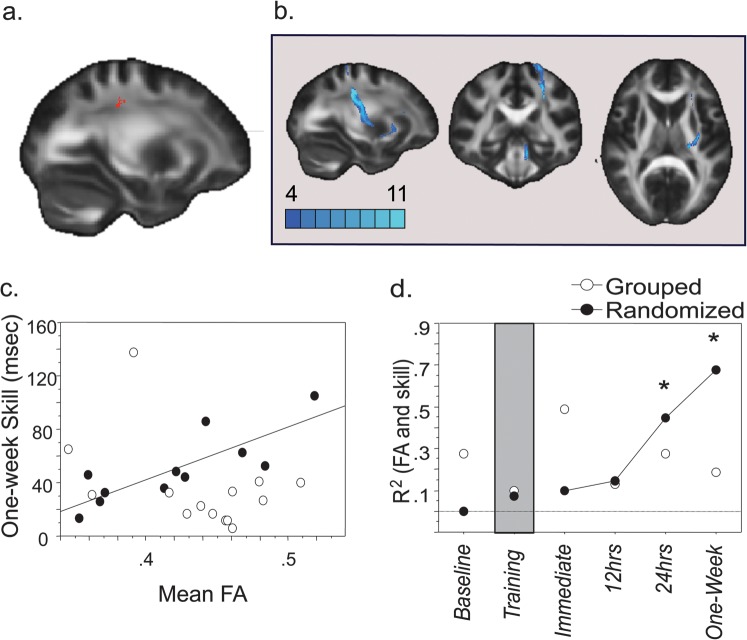
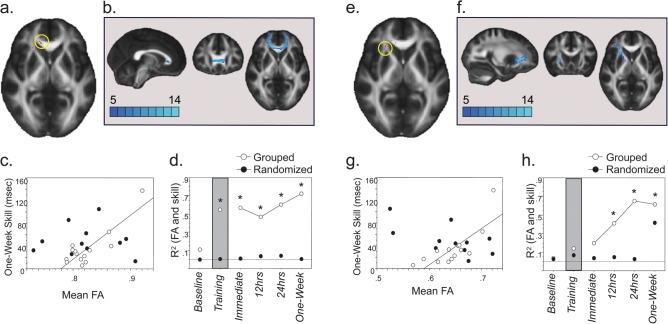
We regressed skill at 1 week (long-term, the primary focus of interest), against FA values using tract-based spatial statistics from FSL, which enables statistical comparison of FA values from homologous regions of the FA map across subjects, and used a statistical threshold of P < 0.005 uncorrected, followed by a cluster threshold of 25 voxels to identify correlated clusters (Boorman et al. 2007; Buch et al. 2010). Correlated clusters were used as seed masks for probabilistic diffusion tractography, using BEDPOSTx and PROBTRACKx software from the FDT. BEDPOSTx estimates a probability distribution function (PDF) on fiber direction at each voxel, and a 2-fiber model is fitted to the diffusion data at each voxel, allowing for the tracing of fibers through regions of fiber crossing or complexity (Behrens et al. 2003). This model is sufficient to resolve 2 crossing fibers with the data acquisition parameters used here. We drew 25 000 streamline samples from our seeded voxels through these PDFs to form an estimate of the probability distribution of connections from each seeded voxel to all other voxels in the brain. For the elimination of spurious connections, tractography in individual subjects was thresholded to include only voxels through which at least 1000 streamlines had passed (out of 25 000). These individual tracts were then binarized, transformed to standard space, and summed across subjects to produce group probability maps for each pathway. These group probabilistic maps were thresholded to display paths that were present in a minimum of one-third of subjects (color scale in Figs 2b and 3b,f indicate the number of subjects) (Boorman et al. 2007).
Figure 2.
White matter microstructure correlates for long-term skill in randomized learners: (a) Skill at 1 week in randomized learners (n = 11) correlated with FA in a cluster underlying the L SMC (cluster size = 27 voxels, t value and coordinates peak voxel = 3.83 and −28, −28, 38), part of the corticostriatal tract connecting L SMC to posterior putamen (shown in b). Group probabilistic map was thresholded to display paths present in at least one-third of the subjects (color scale indicates the number of subjects). (c) Scatterplot showing mean FA in the cluster identified in Figure 2a (L SMC) plotted against long-term skill at 1 week in randomized and grouped learners. Note a positive correlation between 1-week skill and mean FA in L SMC only in randomized learners (correlation line, r10 = 0.822, P < 0.002). (d) Time course of the positive correlation between mean FA in the cluster identified in Figure 2a (L SMC) and skill as a function of testing time in both groups. Note that mean FA in L SMC correlated positively with long-term skill (*P < 0.05) at 24 h and 1 week only in randomized learners (connected line).
Figure 3.
White matter microstructure correlates for long-term skill in grouped learners: Skill at 1 week in grouped learners (n = 14) correlated with FA in 2 clusters. (a) First, a cluster in the right forceps minor (cluster size = 34 voxels, t value and coordinates peak voxel = 3.59 and 13, 33, 1), part of a transcallosal tract connecting homologous regions of the lateral and medial PFC (shown in b). Group probabilistic maps were thresholded to display paths present in at least one-third of the subjects (color scale indicates the number of subjects). (c) Scatterplot showing mean FA in the cluster identified in Figure 3a (forceps minor) plotted against long-term skill at 1 week in both groups. Note a positive correlation between 1-week skill and mean FA in the right forceps minor only in grouped learners (correlation line, r13 = 0.851, P < 0.0001). (d) Time course of the positive correlation between mean FA in the cluster identified in Figure 3a (right forceps minor) and skill as a function of testing time in both groups. Note that mean FA in right forceps minor correlated positively with skill at all times during training and posttraining (*P < 0.05) only in grouped learners (connected line). (e) The second cluster was in the right anterior corona radiata (cluster size = 26 voxels, t value and coordinates peak voxel = 3.20 and 25, 25, 4), part of a corticostriatal tract that connects lateral PFC with anterior putamen (shown in f). (g) Scatterplot showing mean FA in the cluster identified in Figure 3e (right anterior corona radiata) plotted against long-term skill at 1 week in both groups. Note a positive correlation between 1-week skill and mean FA in the right anterior corona radiata in grouped (correlation line, r13 = 0.781, P < 0.001) but not in randomized learners (wherein the correlation was negative at 1 week). (h). Time course of the positive correlation between mean FA in the cluster identified in Figure 3e (right anterior corona radiata) and skill as a function of testing time in both groups. Note that mean FA in this location correlated positively with skill at all times after 12 h posttraining (*P < 0.05) only in grouped learners (connected line).
Results
Long-term Skill as a Function of Practice Schedule
Long-term skill assessed at 1 week was superior in randomized as compared with grouped learners (t48 = 2.54, P < 0.02). However, conscious recollection of the sequence assessed after the long-term skill test was minimal and comparable between the 2 groups (Fig. 1b). For skill at all time points, ANOVAMD showed a significant effect of Timebaseline, during training, immediate, 12 h, 24 h, 1 week (F5,240 = 16.1; Mean squared error (MSE) = 386.7, P < 0.0001), Schedulerandomized, grouped (F1,48 = 4.9; MSE = 2247.3, P < 0.03), and a Time × Schedule interaction (F5,240 = 2.2; MSE = 1871.6, P < 0.05) indicating a significant difference in the magnitude and time course of learning across practice schedule groups. Post hoc Tukey’s comparisons at other time points showed superior skill in randomized compared with grouped learners at immediate, 12, and 24 h posttraining in addition to at 1 week, with no differences at baseline or during training (Fig. 1c). Sleep quality as measured with the PSQI scale was comparable in randomized (4.5 ± 1.8) and grouped (5.3 ± 2.1) learners, as was accuracy during training (95.8 ± 1.8% and 96.4 ± 1.3%, respectively).
Relationship between Long-term Skill and Structural Connectivity as a Function of Practice Schedule
Long-term skill at 1 week in randomized learners correlated with FA in a cluster underlying the left sensorimotor cortex (L SMC) (Fig. 2a,c), localized with probabilistic tractography to the corticostriatal tract connecting L SMC to posterior putamen through the external capsule (Fig. 2b). The positive correlation between mean FA in L SMC seen at 1 week in randomized learners and skill became apparent from 24 h posttraining (P < 0.05; Fig. 2d).
Long-term skill at 1 week in grouped learners correlated with FA in 2 clusters. The first was the right forceps minor (Fig. 3a,c), which connects homologous regions of the lateral and medial prefrontal cortex (PFC; Fig. 3b). The positive correlation between mean FA in this cluster seen at 1 week in grouped learners started during training (P < 0.05; Fig. 3d). The second cluster, located in the right anterior corona radiata (Fig. 3e,g), was part of the corticostriatal tract connecting lateral PFC to anterior putamen through the internal capsule (Fig. 3f). The positive correlation between mean FA in this cluster seen at 1 week in grouped learners became apparent from 12 h posttraining (P < 0.05; Fig. 3h).
Discussion
Two main novel findings emerged from this study. First, for an implicitly learned motor sequence, a randomized practice schedule resulted in superior long-term (1 week) skill relative to a grouped schedule, as previously demonstrated for explicitly learned sequences. Second, long-term skill acquired through randomized practice was predicted by indices of structural connectivity in white matter tracts that link sensorimotor cortex (SMC) with posterior putamen.
Effects of Practice Schedule on Long-term Implicit Skill
We focused on long-term skill for 2 reasons: First, previous work showed that the CI benefit on explicit learning was maximal long after training ended (Lee and Magill 1983; Shea and Zimny 1983; Immink and Wright 1998; Cross et al. 2007; Wymbs and Grafton 2009; Kantak et al. 2010; Tanaka et al. 2010); and second, our interest is to understand the neural substrates of lasting implicit motor learning. Implicit learning is important in eliciting lasting skills in healthy humans (Rauch et al. 1995; Curran 1997; Shadmehr and Brashers-Krug 1997; Shadmehr et al. 1998; Willingham 1999; Poldrack et al. 2001; Shin and Ivry 2002; Schendan et al. 2003; Howard et al. 2004; Robertson et al. 2004; Fischer et al. 2006; Jimenez et al. 2006; Mazzoni and Krakauer 2006; Spencer et al. 2006) as well as in neurorehabilitation after stroke (Boyd and Winstein 2004, 2006; Vidoni and Boyd 2007; Dimyan and Cohen 2011).
Benefits of a randomized practice schedule reported here on implicit long-term skill were not apparent during training performance but appeared at posttraining retests, similar to reports of this effect on explicit learning (Shea and Morgan 1979). Of note, subjects in both practice groups showed minimal, and most importantly comparable, conscious recollection of only 2 of the 12 items in the sequence across groups (Fig. 1b). Thus, differences in long-term skill acquired through different practice schedules could not be explained by explicit contributions. While trials performed during retest may have reduced to some extent memory decay, this phenomenon could not explain differences in long-term skill identified across practice schedules. Additional analyses of sub-components contributing to sequencing skill will further enlighten the behavioral mechanisms of the benefits of practice schedule.
Relation between White Matter Microstructure and Long-term Skill Acquired through Different Practice Schedules
To address this issue, we related long-term skill to FA using DTI, a technique that provides information on white matter microstructural connectivity (Boorman et al. 2007; Wahl et al. 2007; Della-Maggiore et al. 2009; Buch et al. 2010). In randomized learners, we found that individuals with higher FA in white matter regions connecting the contralateral SMC and posterior putamen showed better long-term skill at 1 week (Fig. 2). This finding is consistent with previous reports on the role of the posterior putamen, part of the sensorimotor striatum (Nakahara et al. 2001), which is connected via corticostriatal loops to the SMC and the supplementary motor area (SMA) (Keele et al. 2003; Lehericy et al. 2004; Perez et al. 2007, 2008). These pathways are involved in long-term storage of skill (Miyachi et al. 1997; Nakahara et al. 2001; Keele et al. 2003). Interestingly, previous neuroimaging studies demonstrated a shift in blood oxygen level–dependent activity from the anterior to the posterior putamen with increased training (Lehericy et al. 2005). Consistently, lesions of the posterior putamen disrupt skill associated with overtrained tasks but not skill associated with newly trained motor behavior (Nakahara et al. 2001). In view of these results, it is possible that structural integrity of the sensorimotor corticostriatal loop between SMC and posterior putamen contributes to the superior long-term skill identified at 1 week posttraining with randomized practice.
This is in contrast to grouped learners, in whom skill at 1 week correlated with structural connectivity in different white matter regions: a portion of the corticostriatal tract that links the right lateral PFC to the anterior putamen and a transcallosal tract connecting PFC regions bilaterally (Fig. 3). Neuroimaging studies show anterior putamen involvement during early stages of motor training when skill is relatively poor (Lehericy et al. 2005), and lesions of the anterior striatum disrupt performance of newly encoded skill without affecting overtrained motor behavior (Miyachi et al. 1997). The anterior putamen, part of the associative striatum (Miyachi et al. 1997; Nakahara et al. 2001), is connected to the lateral PFC, the pre-SMA, and the frontal poles (Nakahara et al. 2001; Lehericy et al. 2004). Additionally, the lateral PFCs are anatomically connected through the forceps minor (Peltier et al. 2010). Further evidence for the functional role of connections in the forceps minor comes from a study that demonstrated a correlation between FA in this region and functional connectivity between lateral PFCs (Voineskos et al. 2010).
Within grouped learners, the correlation between skill and FA within the associative corticostriatal tract (lateral PFC–anterior putamen) (Fig. 3h) was significant only after 12 h, while within transcallosal prefrontal tracts it started during training (Fig. 3d). Previous work proposed the involvement of an associative prefrontal network in both implicit and explicit learning (Grafton et al. 1995; Pascual-Leone et al. 1996; Hazeltine et al. 1997; Robertson et al. 2001; Keele et al. 2003), which may reflect a shared resource for both learning types (Keele et al. 2003). These data further support the view that prefrontal regions linked by the forceps minor can be active during grouped training (Halsband and Lange 2006), while long-term skill in grouped learners can require engagement of prefrontal corticostriatal circuits (Doyon et al. 2003). Thus, one novel and intriguing finding in our study was that FA in different regions correlated with skill tested at different times depending on practice schedule. A possible explanation for these findings could be the existence of different neuroanatomical and physiological architectures supporting consolidation over time depending on practice schedule.
Thus, our results demonstrate that randomizing the order of practice rather than grouping it can substantially improve implicit learning and that interindividual differences in structural connectivity of white matter between SMC and posterior putamen may predict the magnitude of this superior long-term skill.
Supplementary Material
Supplementary Figures 1 and 2 can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health and utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health (http://biowulf.nih.gov).
Supplementary Material
Acknowledgments
Special thanks to Bina Bansinath, Steven Wise, Monica Perez, Mitsunari Abe, and Satoshi Tanaka for discussion and to Daniel Willingham for discussion and providing the corpus of sequences for the task. Conflict of Interest : None declared.
References
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J Neurol Phys Ther. 2006;30:46–57. doi: 10.1097/01.npt.0000282566.48050.9b. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem. 2004;11:388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady F. Contextual interference: a meta-analytic study. Percept Mot Skills. 2004;99:116–126. doi: 10.2466/pms.99.1.116-126. [DOI] [PubMed] [Google Scholar]
- Brady F. The contextual interference effect and sport skills. Percept Mot Skills. 2008;106:461–472. doi: 10.2466/pms.106.2.461-472. [DOI] [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cleeremans A, McClelland JL. Learning the structure of event sequences. J Exp Psychol Gen. 1991;120:235–253. doi: 10.1037//0096-3445.120.3.235. [DOI] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST. Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci. 2007;19:1854–1871. doi: 10.1162/jocn.2007.19.11.1854. [DOI] [PubMed] [Google Scholar]
- Curran T. Higher-order associative learning in amnesia: evidence from the serial reaction time task. J Cogn Neurosci. 1997;9:522–533. doi: 10.1162/jocn.1997.9.4.522. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Scholz J, Johansen-Berg H, Paus T. The rate of visuomotor adaptation correlates with cerebellar white-matter microstructure. Hum Brain Mapp. 2009;30:4048–4053. doi: 10.1002/hbm.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrebecqz A, Peigneux P, Laureys S, Degueldre C, Del Fiore G, Aerts J, Luxen A, Van Der Linden M, Cleeremans A, Maquet P. The neural correlates of implicit and explicit sequence learning: interacting networks revealed by the process dissociation procedure. Learn Mem. 2005;12:480–490. doi: 10.1101/lm.95605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Fischer S, Drosopoulos S, Tsen J, Born J. Implicit learning— explicit knowing: a role for sleep in memory system interaction. J Cogn Neurosci. 2006;18:311–319. [PubMed] [Google Scholar]
- Goedert KM, Willingham DB. Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn Mem. 2002;9:279–292. doi: 10.1101/lm.50102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Hall KG, Domingues DA, Cavazos R. Contextual interference effects with skilled baseball players. Percept Mot Skills. 1994;78:835–841. doi: 10.1177/003151259407800331. [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol Paris. 2006;99:414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120(Pt 1):123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging. 2004;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink M, Wright DL. Contextual interference: a response planning account. Q J Exp Psychol. 1998;51A:735–754. [Google Scholar]
- Immink MA, Wright DL. Motor programming during practice conditions high and low in contextual interference. J Exp Psychol Hum Percept Perform. 2001;27:423–437. doi: 10.1037//0096-1523.27.2.423. [DOI] [PubMed] [Google Scholar]
- Jimenez L, Vaquero JM, Lupianez J. Qualitative differences between implicit and explicit sequence learning. J Exp Psychol Learn Mem Cogn. 2006;32:475–490. doi: 10.1037/0278-7393.32.3.475. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Jones LL, French KE. Effects of contextual interference on acquisition and retention of three volleyball skills. Percept Mot Skills. 2007;105:883–890. doi: 10.2466/pms.105.3.883-890. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci. 2010;13:923–925. doi: 10.1038/nn.2596. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychol Rev. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Lee TD, Magill RA. The locus of contextual interference in motor-skill acquisition. J Exp Psychol Learn Mem Cogn. 1983;9:730–746. [Google Scholar]
- Lee TD, Swanson LR, Hall AL. What is repeated in a repetition? Effects of practice conditions on motor skill acquisition. Phys Ther. 1991;71:150–156. doi: 10.1093/ptj/71.2.150. [DOI] [PubMed] [Google Scholar]
- Lee TD, Wishart LR, Cunningham S, Carnahan H. Modeled timing information during random practice eliminates the contextual interference effect. Res Q Exerc Sport. 1997;68:100–105. doi: 10.1080/02701367.1997.10608871. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lin CH, Fisher BE, Winstein CJ, Wu AD, Gordon J. Contextual interference effect: elaborative processing or forgetting-reconstruction? A post hoc analysis of transcranial magnetic stimulation-induced effects on motor learning. J Mot Behav. 2008;40:578–586. doi: 10.3200/JMBR.40.6.578-586. [DOI] [PubMed] [Google Scholar]
- Lin CH, Fisher BE, Wu AD, Ko YA, Lee LY, Winstein CJ. Neural correlate of the contextual interference effect in motor learning: a kinematic analysis. J Mot Behav. 2009;41:232–242. doi: 10.3200/JMBR.41.3.232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Doya K, Hikosaka O. Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences—a computational approach. J Cogn Neurosci. 2001;13:626–647. doi: 10.1162/089892901750363208. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- Pascual-Leone A, Wassermann EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res. 1996;107:479–485. doi: 10.1007/BF00230427. [DOI] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, Deramond H, Pruvo JP, Le Gars D, Godefroy O. Microsurgical anatomy of the ventral callosal radiations: new destination, correlations with diffusion tensor imaging fiber-tracking, and clinical relevance. J Neurosurg. 2010;113:512–519. doi: 10.3171/2009.6.JNS081712. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci. 2008;28:9664–9669. doi: 10.1523/JNEUROSCI.3416-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Porretta DL, O'Brien K. The use of contextual interference trials by mildly mentally handicapped children. Res Q Exerc Sport. 1991;62:240–244. doi: 10.1080/02701367.1991.10608717. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Brown HD, Curran T, Alpert NM, Kendrick A, Fischman AJ, Kosslyn SM. A PET investigation of implicit and explicit sequence learning. Hum Brain Mapp. 1995;3:271–286. [Google Scholar]
- Reber AS. Implicit learning and tacit knowledge: an essay on the cognitive unconscious. New York: Oxford University Press; 1993. [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Tormos JM, Maeda F, Pascual-Leone A. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex. 2001;11:628–635. doi: 10.1093/cercor/11.7.628. [DOI] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time-dependent motor memory processes in amnesic subjects. J Neurophysiol. 1998;80:1590–1597. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea CH, Lai Q, Wright DL, Immink M, Black C. Consistent and variable practice conditions: effects on relative and absolute timing. J Mot Behav. 2001;33:139–152. doi: 10.1080/00222890109603146. [DOI] [PubMed] [Google Scholar]
- Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol Hum Learn. 1979;3:179–187. [Google Scholar]
- Shea JB, Zimny ST. Context effects in memory and learning movement information. In: Magill RA, editor. Memory and control of action. Amsterdam (The Netherlands): Elsevier; 1983. pp. 145–166. [Google Scholar]
- Shin JC, Ivry RB. Concurrent learning of temporal and spatial sequences. J Exp Psychol Learn Mem Cogn. 2002;28:445–457. doi: 10.1037//0278-7393.28.3.445. [DOI] [PubMed] [Google Scholar]
- Smith PJ. Applying contextual interference to snowboarding skills. Percept Mot Skills. 2002;95:999–1005. doi: 10.2466/pms.2002.95.3.999. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Curr Biol. 2006;16:1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Ste-Marie DM, Clark SE, Findlay LC, Latimer AE. High levels of contextual interference enhance handwriting skill acquisition. J Mot Behav. 2004;36:115–126. doi: 10.3200/JMBR.36.1.115-126. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Honda M, Hanakawa T, Cohen LG. Differential contribution of the supplementary motor area to stabilization of a procedural motor skill acquired through different practice schedules. Cereb Cortex. 2010;20:2114–2121. doi: 10.1093/cercor/bhp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA. Achieving enlightenment: what do we know about the implicit learning system and its interaction with explicit knowledge? J Neurol Phys Ther. 2007;31:145–154. doi: 10.1097/NPT.0b013e31814b148e. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Farzan F, Barr MS, Lobaugh NJ, Mulsant BH, Chen R, Fitzgerald PB, Daskalakis ZJ. The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol Psychiatry. 2010;68:825–831. doi: 10.1016/j.biopsych.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB. Systems of memory in the human brain. Neuron. 1997;18:5–8. doi: 10.1016/s0896-6273(01)80040-4. [DOI] [PubMed] [Google Scholar]
- Willingham DB. The neural basis of motor-skill learning. Curr Dir Psychol Sci. 1999;8:178–182. [Google Scholar]
- Wu M, Chang LC, Walker L, Lemaitre H, Barnett AS, Marenco S, Pierpaoli C. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. Med Image Comput Comput Assist Interv. 2008;11:321–329. doi: 10.1007/978-3-540-85990-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Grafton ST. Neural substrates of practice structure that support future off-line learning. J Neurophysiol. 2009;102:2462–2476. doi: 10.1152/jn.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetou E, Michalopoulou M, Giazitzi K, Kioumourtzoglou E. Contextual interference effects in learning volleyball skills. Percept Mot Skills. 2007;104:995–1004. doi: 10.2466/pms.104.3.995-1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.