Abstract
Interest in pharmacological intervention to combat metabolic syndrome and its complications is increasing as the prevalence of obesity is reaching epidemic proportions. The potential efficacy of drugs is often tested in animal models; however, the method of drug delivery is frequently overlooked and may act as a confounder due to stress. We hypothesized that long-term orogastric gavage would negatively influence the development of hepatic steatosis and the metabolic syndrome in a murine model of diet-induced obesity. C57BL/6J male mice were fed a high fat diet and were gavaged with a vehicle once or twice daily for 9 weeks. A group without orogastric gavaging served as control. A similar experiment was performed using leptin deficient ob/ob mice that were fed a standard diet for 4 weeks. Food intake was monitored, insulin resistance determined, and steatosis was assessed by histology and quantified via magnetic resonance spectroscopy. After 9 weeks, control C57BL/6J mice exhibited significantly more weight gain, insulin resistance and hepatic steatosis, compared to mice that were gavaged daily, or twice daily. This effect was likely due to decreased food consumption associated with gavage-induced stress. In contrast, the phenotype of leptin deficient ob/ob mice was not affected by orogastric gavage. Therefore, we concluded that orogastric gavage may lead to increased stress, thereby affecting food intake and the development of diet-induced obesity in a murine model. The effects of what may seem to be trivial laboratory routines, such as orogastric gavage, should be taken into account when designing animal studies for drug development.
1. Introduction
Metabolic syndrome is a cluster of interrelated risk factors for diabetes and cardiovascular disease, including obesity, hyperglycemia, dyslipidemia and elevated blood pressure [1]. The prevalence of the metabolic syndrome is increasing worldwide, estimated to be 35–40% both in the US [1] and in the Middle East [2], depending on the criteria used. Risk factors for the metabolic syndrome are mainly related to a sedentary lifestyle and obesity. The prevalence of non-alcoholic fatty liver disease, which is considered to be the hepatic manifestation of the metabolic syndrome [3], is increasing as well, affecting up to 30% of individuals with the syndrome [4]. Non-alcoholic fatty liver disease is a spectrum of disease ranging from the simple build-up of fat in the liver (hepatic steatosis) to non-alcoholic steatohepatitis, cirrhosis, and ultimately liver failure [5]. The main goal of treating metabolic syndrome is to reduce obesity, especially abdominal girth, through a multimodality approach that includes diet, excise, and pharmacologic intervention [6]. Pharmacologic agents can be used to target both energy intake and energy expenditure, reduce food consumption, and to improve markers of insulin sensitivity, hypercholesterolemia and hepatic steatosis. Despite the availability of several classes of drugs such as antihyperglycemics, antihypertensives, and lipid lowering agents, further drug development is warranted to overcome current limitations, potentially leading to improved clinical management of the metabolic syndrome and its associated complications.
In order to test the efficacy of drugs on treating the metabolic syndrome, animal models are essential to investigate the relative influence of various treatments on energy intake, energy expenditure, and body weight. In animal models, drugs can be administered intravenously, intraperitoneally or via orogastric gavage, the last considered to be the least invasive route. However, even non-invasive laboratory routines such as personnel entering the animal housing room, cage maintenance, and body weight measurements may elicit an acute stress response [7].
More stressful procedures such as restraining the animal, may induce catabolism by activating the corticotrophin-releasing hormone system [8, 9]. Moreover, chronic activation of the stress response has been associated with altered energy homeostasis and reduction of body fat content [10]. Other reports, however, support a link between exposure to chronic stress and the development of obesity [11, 12]. To this day, it remains to be demonstrated whether an acute stressor such as repetitive orogastric gavage may by itself influence the metabolic phenotype of the model studied.
The aim of this study was to investigate the degree of stress induced by different frequencies of orogastric gavage on food intake, fat deposition, insulin resistance and hepatic steatosis in obesity-prone C57BL/6J and ob/ob mice. Using the classic model of diet-induced obesity as well as the transgenic model of leptin deficiency, we hypothesized that long-term, repetitive orogastric gavaging of mice would alter the development of the metabolic syndrome, and in particular, hepatic steatosis.
2. Material and Methods
2.1. Animals and general housing conditions
C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) fed a high fat diet ad libitum gradually develop visceral adiposity, hyperglycemia, insulin and leptin resistance, as well as hepatic steatosis, sharing many of the same obesity related phenotypes as humans [13]. B6.V-Lepob/J mice (Jackson Laboratories, Bar Harbor, ME), commonly referred to as ob/ob mice, are homozygous for the spontaneous mutation of the Lepob gene and exhibit obesity, hyperphagia, diabetes, and hepatic steatosis. They are both hypometabolic and hypothermic [14, 15]. Male 5-week-old mice were housed 4 (ob/ob) or 5 (C57BL/6J) animals per cage on paper chip bedding in a barrier room with regulated temperature (21°C ± 1 .6°C), humidity (45% ± 10%), and an alternating 12-hour light and dark cycle. All animals had free access to water and standard rodent chow pellets (Prolab Isopro, RMH 3000 #25; containing 14% fat, 26% protein, and 60% carbohydrate by calories; energy density 4.1 kcal/g; Prolabs Purina, Richmond, IN) for an acclimation period of 1 week prior to study initiation. During the study period, the animals were weighed twice a week and food intake was measured daily. Intake was measured per cage to avoid potential additional stress of individual housing. Animal protocols complied with the National Institutes of Health Animal Research Advisory Committee guidelines, and were approved by the Children's Hospital Boston Animal Care and Use Committee (protocol no. A06-08-065R).
2.2. Study diets
Study diets were stored at −80°C and provided fresh each day to avoid lipid peroxidation. In the high fat purified rodent diet (D12492; Research Diets, New Brunswick, NJ), the amount of calories derived from fat, protein, and carbohydrates were 60%, 20%, and 20%, respectively (energy density of 5.24 kcal/g). In the standard purified rodent diet (D12450B; Research Diets, New Brunswick, NJ), 10% of calories were derived from fat, 20% from protein, and 70% from carbohydrates (energy density of 3.85 kcal/g).
2.3. Intervention
Animals received 0.2 mL of a common vehicle, 0.45% (w/v) methylcellulose (Sigma-Aldrich, St. Louis, MO) in double distilled H2O, via orogastric gavage through a 30 mm 20 Gauge feeding needle (Fine Sciences Tools Inc., Foster City, CA). Mice were gavaged daily between 07:00 and 09:00 h, or if twice, again between 19:00 and 21:00 h. Control mice were disturbed and manually handled daily without gavaging. Orogastric gavage was routinely performed by one experienced investigator (VM) throughout the study, with 0% mortality.
2.3. Study design
After the one week acclimation period, fifteen C57BL/6J mice were placed on the high fat diet and randomized into three groups. In a separate study, twelve leptin deficient ob/ob mice were acclimatized for one week, were fed a standard purified rodent diet and randomized into three groups. In both experiments, animals in the control group (0x/day) were not gavaged and served as controls, whereas animals in the second (1x/day) and third group (2x/day) were gavaged daily, or twice daily, respectively, throughout the study period.
2.4. Specimen collection
At the end of the feeding experiments, mice were fasted for 6 hours. Glucose concentration was determined from tail vein blood using the OneTouch UltraSmart Blood Glucose Monitoring System (LifeScan, Milpitas, CA). Mice were then anesthetized with 2.5% Avertin (2,2,2-Tribromoethanol, Sigma-Aldrich, St. Louis, MO) by intraperitoneal injection. Blood was then collected via retro-orbital sinus puncture and centrifuged at 14000 rpm at 4°C for 10 min to obtain serum. Serum was delivered to the Clinical Laboratory at Children’s Hospital Boston for analysis of alanine aminotransferase (ALT), total cholesterol and triglyceride levels.
We then performed a midline laparotomy to observe, excise, and weigh the liver. The frontal lobe of the liver was fixed in 10% formalin at 4°C overn ight, washed with phosphate-buffered saline, and then embedded in paraffin. The left lateral lobe was excised and collected for magnetic resonance (MR) spectroscopy analysis. The remaining liver was immediately snap-frozen in liquid nitrogen and stored at −80°C.
White adipose tissue was dissected according to previously defined anatomic landmarks [16]. It was then weighed, snap-frozen in liquid nitrogen, and stored at −80°C. Inguinal (all subcutaneous fat between the lower part of the rib cage and mid thigh), mesenteric (all fat along the mesentery from the lesser curvature of the stomach to the sigmoid colon), retroperitoneal and epidydimal fat pads were weighed and expressed relative to eviscerated body weight. A white adipose tissue fat-index was calculated using the sum of the individual fat pads as a percentage of the eviscerated body weight [16].
2.5. Surrogate indexes of insulin sensitivity and resistance
Insulin levels were measured using a rat/mouse insulin ELISA kit (Linco Research, St. Charles, MO). Surrogate indexes for insulin sensitivity were calculated, including quantitative insulin-sensitivity check index (QUICKI), homeostasis model assessment (HOMA) and log(HOMA) [17]. The calculations were performed as follows: QUICKI=1/[log(I0)+log(G0)], where I0 is the fasting insulin (µU/mL) and G0 is the fasting glucose (mg/dL); HOMA=(G0xI0)/22.5 (with glucose expressed as mmol/L and insulin expressed as µU/mL); and log(HOMA).
2.6. Hepatic histology
Paraffin-embedded sections of the liver were stained with hematoxylin and eosin and periodic acid Schiff’s/diastase to examine cellular architecture, glycogen deposition and lipid accumulation. Frozen tissue sections embedded in tissue medium (Optimal Cutting Temperature OCT, Sakura Fenetek, Torrance, CA) were stained with Oil Red-O to detect fat droplets. A pathologist blinded to the treatment groups conducted a histological analysis of the liver sections.
2.7. Hepatic MR spectroscopy
MR spectroscopy was performed by the MR Laboratory at Beth Israel Deaconess Medical Center [18]. Samples were thawed at room temperature for 1 hour prior to analysis, blotted free of excess water and connective tissue, and placed in 5 mm diameter glass tubes for MR spectroscopy. An 8.5 T vertical bore magnet (DRX system, Bruker Instruments, Billerica, MA) was used for spectroscopic measurements of fat and water resonances. Specifically, a point resolved echo spectroscopic acquisition was applied to homogenous regions of liver, as identified from fast low angle shot images of the liver specimen. Voxel volumes interrogated spectroscopically with the point resolved echo spectroscopic sequence were 2 mm3. The repetition and echo times were 8 s and 12 ms, respectively, and 16 signal averages were acquired per spectrum. The water resonance and the methylene/methyl resonances were numerically integrated using the manufacturer supplied Paravision 4.0 software (Bruker Instruments, Billerica, MA). The methylene/methyl area was divided by the sum of the methylene/methyl area plus the water area to obtain the MR spectroscopy parameter representing hepatic fat fraction used for group comparisons.
2.8. Statistical analyses
Continuous data are expressed as means ± standard error of the mean (SEM). The Kolmogorov-Smirnov one sample test was used to check Gaussianity of the continuous data. Data sets involving more than two groups were assessed by analysis of variance (ANOVA), or if nonparametric, using the Kruskal-Wallis test. Repeated measures ANOVA was used to analyze body weight gain. If the ANOVA showed significant effects, group means were further compared using the unpaired two-tailed Student’s t test, or if nonparametric, by using the Mann-Whitney U test. P≤0.05 was considered statistically significant. All data were collected in a computerized Microsoft Excel database (Microsoft Inc., Redmond, WA). The analysis was performed with SPSS version 16.0 (SPSS Inc., Chicago, IL) statistical software, and figures were created using GraphPad Prism version 5.0 (GraphPad Software Inc., La Jolla, CA) software.
3. Results
3.1. C57BL/6J mice fed a 60% high fat diet for 9 weeks
3.1.1. Repetitive orogastric gavage of C57BL/6J mice on a high fat diet decreased body weight gain, energy intake, liver weights and adiposity, compared to non-gavaged animals
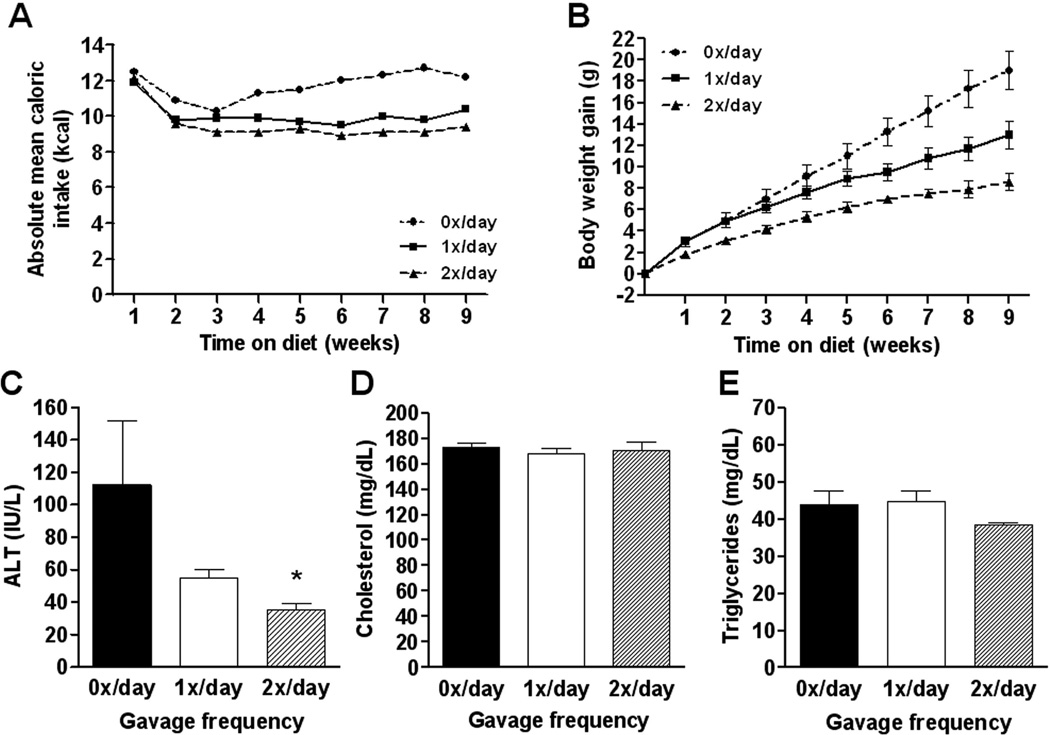
After 9 weeks of high fat feeding and a varying frequency of orogastric gavaging among groups, control (0x/day) animals gained significantly more weight compared to 1x/day and 2x/day animals (F(2,12)=17.87, P=0.0003) (Table 1; Figure 1). Compared to once (1x/day) or twice daily (2x/day) gavaged animals, the control group consumed more food throughout the study resulting in a higher caloric efficiency (Table 1; Figure 1). Liver weights, liver/body weight ratios and individual fat pad weights were all significantly higher in the non-gavaged, control animals (Table 1). After 9 weeks, control animals had a significantly higher white adipose tissue fat index compared to both 1x/day and 2x/day animals (Kruskal-Wallis statistic=10.50, P=0.0052) (Table 1). Repetitive orogastric gavage thus negatively affected the development of obesity in C57BL/6J mice fed a high fed diet, likely due to decreased energy consumption.
Table 1.
Body weights, tissue weights, caloric intake and surrogate markers for insulin resistance in C57BL/6J mice that were gavaged with different frequencies for 9 weeks.
| Gavage frequency |
||||||
|---|---|---|---|---|---|---|
| 0x/day | 1x/day | 2x/day | ||||
| Body weight gain | (g) | 19.0 ± 1.8 | 9.8 ± 1.3 | ** | 8.6 ± 0.8 | *** |
| Liver weight | (g) | 1.72 ± 017 | 1.25 ± 0.09 | * | 1.12 ± 0.04 | ** |
| Liver/body weight ratio | 0.045 ± 0.00 | 0.040 ± 0.00 | 0.040 ± 0.00 | |||
| Total caloric intake /mouse | (kcal) | 740 | 636 | 600 | ||
| Caloric efficiency | (g/kcal) | 0.0257 | 0.0154 | 0.0143 | ||
| Relative Inguinal fat mass | (%) | 6.92 ± 0.38 | 4.39 ± 0.70 | * | 2.91 ± 0.07 | *** |
| Mesenteric fat pad | (%) | 3.55 ± 0.14 | 2.39 ± 0.28 | ** | 1.89 ± 0.16 | *** |
| Retroperitoneal fat pad | (%) | 2.33 ± 0.16 | 1.61 ± 0.33 | 1.48 ± 0.04 | ** | |
| Epidydimal fat pad | (%) | 9.09 ± 0.38 | 7.20 ± 1.31 | 6.08 ± 0.14 | *** | |
| White adipose tissue fat index | 21.88 ± 0.80 | 15.58 ± 2.32 | * | 12.36 ± 0.22 | *** | |
| Fasting glucose | (mg/dL) | 406 ± 40 | 322 ± 18 | 351 ± 37 | ||
| Fasting insulin | (µU/mL) | 42.8 ± 3.2 | 28.0 ± 8.7 | 29.6 ± 3.2 | ||
| QUICKI | 0.24 ± 0.00 | 0.27 ± 0.01 | 0.26 ± 0.01 | |||
| HOMA | 43.0 ± 6.8 | 22.6 ± 7.4 | 28.0 ± 11.1 | |||
| Log(HOMA) | 1.62 ± 0.06 | 1.19 ± 0.23 | 1.29 ± 0.19 | |||
Values given are means ± SEM; 0x/day group was used as reference;
P<0.05;
P<0.01;
P<0.001. QUICKI indicates quantitative insulin-sensitivity check index; HOMA, homeostasis model assessment.
Figure 1.
Absolute mean caloric intake per C57BL/6J mouse per week (A) was calculated per group on a daily base. Body weight gain (B) was calculated relative to the weight of each individual animal before initiation of the experiment. Plasma alanine aminotransferase (ALT; C), total cholesterol (D) and triglyceride (E) levels in the different groups. Values represent the mean ± SEM. Statistical significance is calculated between the control animals (0x/day) and the difference between 1x/day animals and 2x/day animals (*, P<0.05).
3.1.2. Repetitive orogastric gavage of C57BL/6J mice on a high fat diet does not impair insulin sensitivity, compared to non-gavaged animals
In order to compare the effects of a varying frequency of orogastric gavage on insulin sensitivity we measured serum values for glucose and insulin and calculated surrogate markers (QUICKI, HOMA and log(HOMA)) for insulin sensitivity. Values of mice that were gavaged 1x/day and 2x/day were compared to those of control animals. Fasting blood glucose (F(2,12)=1.63, P=0.2365), insulin levels (F(2,12)=1.08, P=0.3692), and surrogate markers of insulin sensitivity (QUICKI: Kruskal-Wallis statistic=2.24, P=0.3263); HOMA: F(2,12)=1.50, P=0.2628; log(HOMA): F(2,12)=1.62, P=0.2384) were not significantly affected by repetitive orogastric gavage (Table 1).
3.1.3. Repetitive orogastric gavage of C57BL/6J mice on a high fat diet decreased ALT values, but did not affect triglyceride and cholesterol levels, compared to non-gavaged animals
ALT is used as a marker for evaluation of hepatic injury and is elevated in mice with hepatic steatosis. Liver enzymes were measured in all experimental groups (Figure 1). When compared to controls, 1x/day and 2x/day mice exhibited lower mean ALT values (Kruskal-Wallis statistic=7.75, P=0.0207), suggesting impaired progression of hepatic steatosis. Cholesterol (F(2,12)=0.22, P=0.8050) and triglyceride levels (Kruskal-Wallis statistic=1.83, P=0.3999) were not significantly affected by repetitive orogastric gavage (Figure 1). These data suggest that the liver injury associated with high fat feeding in the model of diet-induced obesity was significantly impaired by repetitive orogastric gavage.
3.1.4. Repetitive orogastric gavage of C57BL/6J mice on a high fat diet diminished hepatic steatosis as assessed by histology, compared to non-gavaged animals
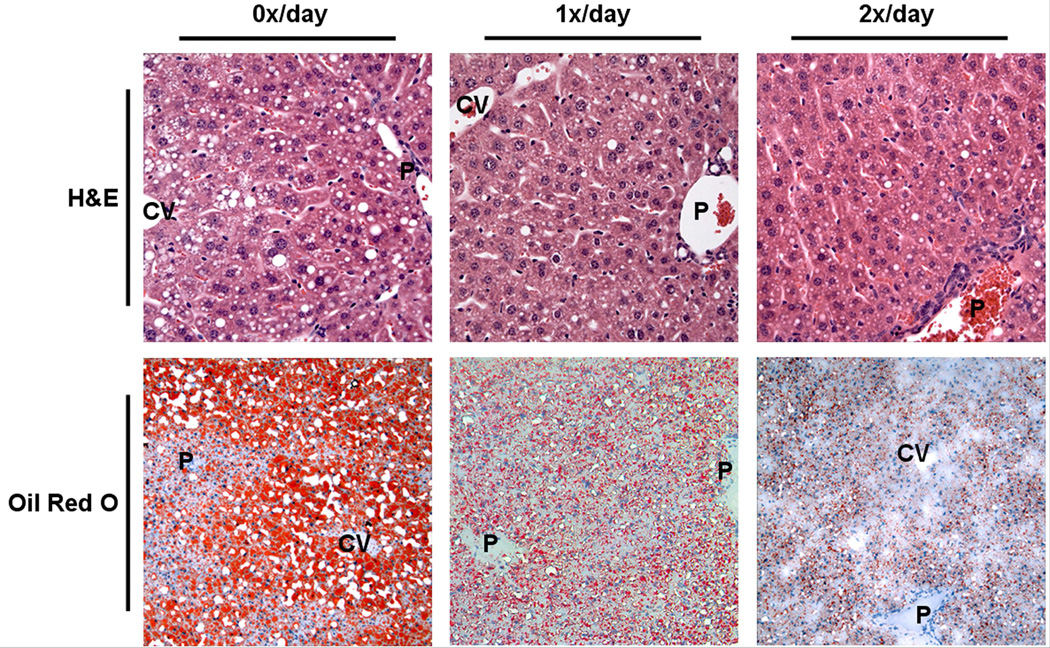
To further explore the potential effects of orogastric gavage on the development of hepatic steatosis in the diet-induced obesity model, we analyzed hematoxylin and eosin, and Oil Red-O stained liver sections (Figure 2). Liver sections from control mice showed fat throughout the liver parenchyma, including both macro- and microvesicular steatosis. Microvesicular and macrovesicular steatosis was present predominantly in the periportal and midzone areas, whereas occasional ballooned hepatocytes and macrovesicular steatosis were present in the central vein area. In contrast, liver sections from 1x/day and 2x/day animals showed moderate steatosis, predominantly microvesicular. Occasional macrovesicular hepatocytes were observed in the periportal zone. Analysis of periodic acid Schiff’s/diastase-stained liver sections excluded glycogen deposition as a cause of microvesicular changes in hepatocytes (data not shown). Steatohepatitis and acute inflammation were not observed in any of the experimental groups.
Figure 2.
Representative liver sections stained with hematoxylin and eosin (H&E; top panels; original magnification 400x), and Oil Red O (lower panels; original magnification 200x). Livers from C57BL/6J animals that had not been gavaged revealed extensive, microvesicular and macrovesicular steatosis after 9 weeks (left panels). Livers from animals that had been gavaged once (middle panels), or twice daily (right panels), exhibited moderate, predominantly microvesicular steatosis. P indicates portal tract; CV, central vein.
3.1.5. Repetitive orogastric gavage of C57BL/6J mice on a high fat diet diminished hepatic steatosis as assessed by MR spectroscopy, compared to non-gavaged animals
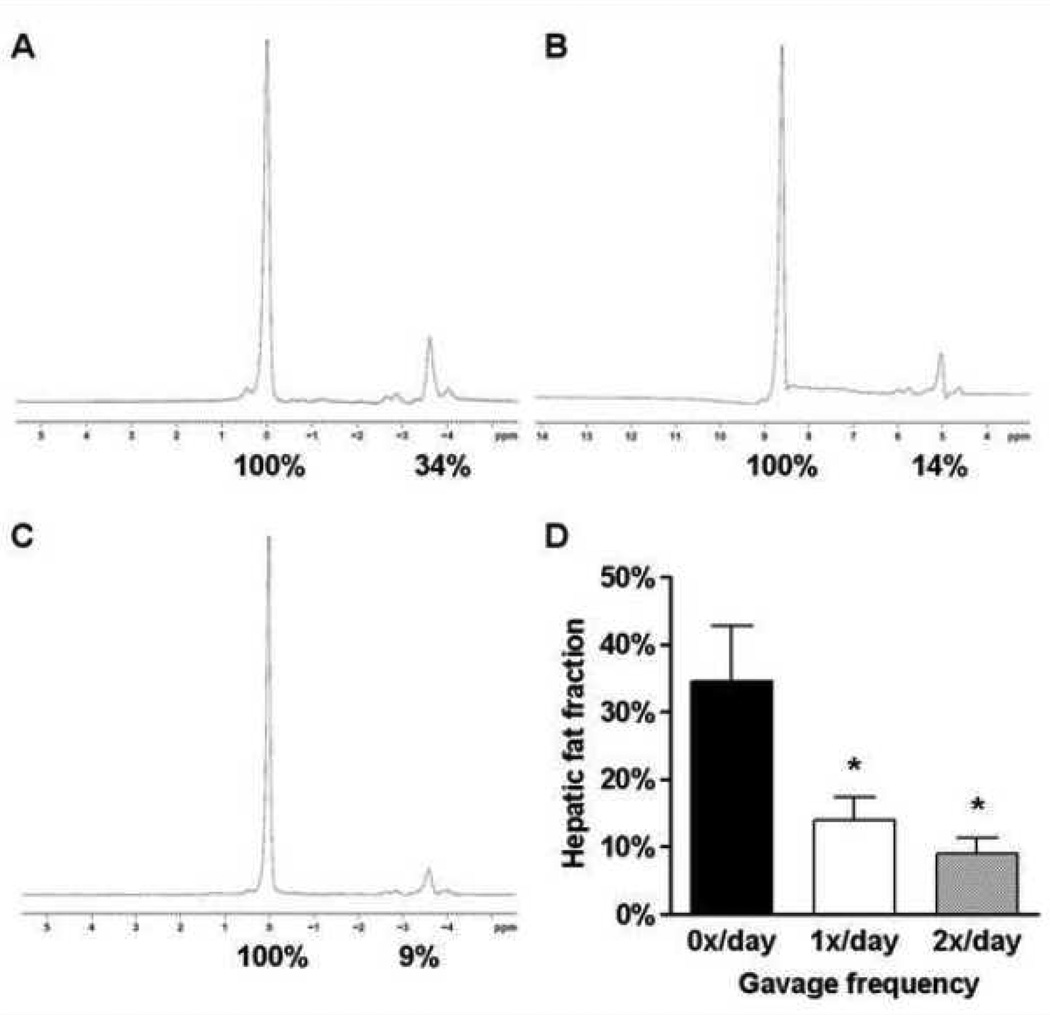
Hepatic fat content was quantified by using MR spectroscopy. Representative spectra of the different groups are shown (Figure 3). Mice that were gavaged once (1x/day) or twice daily (2x/day) exhibited a decreased liver fat content of 14.0±3.4% and 8.9±2.4%, respectively (F(2,12)=6.50, P=0.0122), when compared to non-gavaged mice (0x/day; 34.5±8.2%), demonstrating the effect of orogastric gavage on hepatic fat accumulation (Figure 3D). These data corroborate our histological findings that repetitive orogastric gavage of animals fed a high fat diet significantly affected the development of hepatic steatosis.
Figure 3.
Magnetic resonance spectra for 0x/day (A), 1x/day (B) and 2x/day (C) livers from C57BL/6J mice. Percent fat content was determined relative to water (100%) by numerical integration of the areas under the lipid and water peaks. Mean hepatic fat fraction as measured by magnetic resonance spectroscopy (D). Statistical significance is calculated between the 0x/day animals and the difference between 1x/day animals and 2x/day animals, respectively (*, P<0.05). Variance statistic is SEM.
3.2. Ob/ob mice fed a standard diet for 4 weeks
3.2.1. Repetitive orogastric gavage of ob/ob mice on a standard diet did not influence body weight gain, energy intake, liver and fat pad weights, and fasting glucose levels, compared to non-gavaged animals
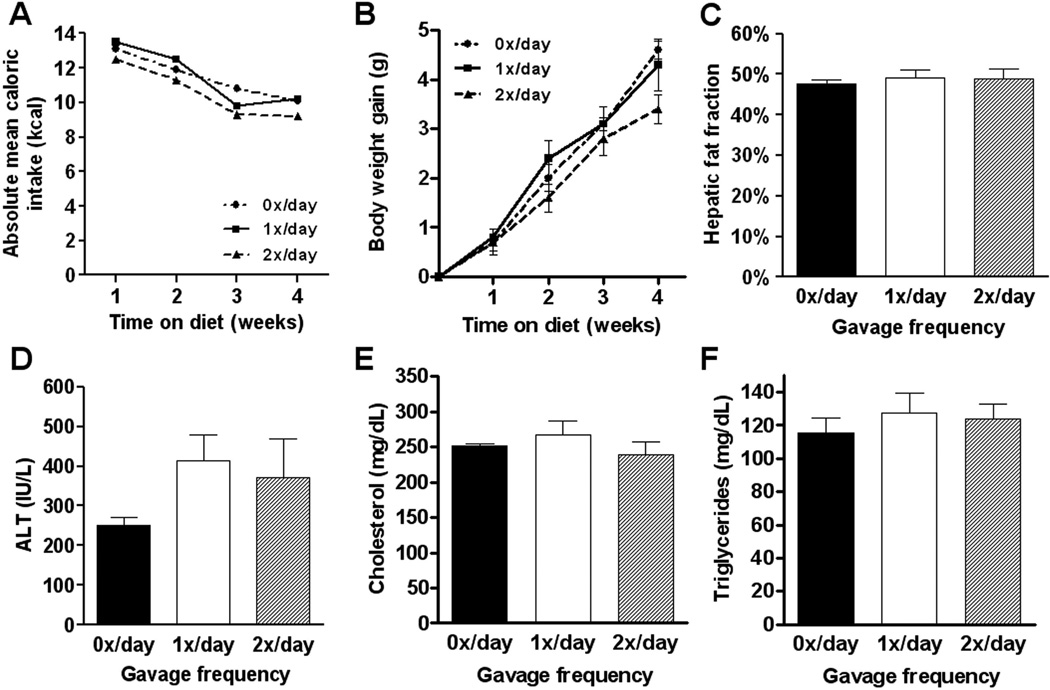
Since the confounding effects of orogastric gavage in the diet-induced obesity model appeared, at least in part, to be mediated by decreased food intake, we next investigated whether previously established obesity, insulin resistance and hepatic steatosis could be affected by orogastric gavage of leptin deficient ob/ob mice. Ob/ob mice exhibit profound hyperphagia, even on low fat diets, and may thus be less susceptible to gavage-induced stress. After 4 weeks, there were no significant differences in body weight gain (F(2,9)=0.38, P=0.6924), liver weights (F(2,9)=0.13, P=0.8769), adipose tissue weights (F(2,9)=0.18, P=0.8361) or fasting glucose levels (F(2,9)=1.15, P=0.3633) between control ob/ob mice and ob/ob mice that were gavaged once daily (1x/day) or twice daily (2x/day) (Table 2; Figure 4). More importantly, total caloric intake and caloric efficiency were similar among groups, indicating that repetitive orogastric gavage did not inhibit food intake in leptin deficient ob/ob mice (Table 2; Figure 4).
Table 2.
Body weights, tissue weights and caloric intake in ob/ob mice that were gavaged with different frequencies for 4 weeks.
| Gavage frequency |
||||
|---|---|---|---|---|
| 0x/day | 1x/day | 2x/day | ||
| Body weight gain | (g) | 4.7 ± 0.2 | 4.1 ± 0.3 | 4.3 ± 0.5 |
| Liver weight | (g) | 2.47 ± 0.12 | 2.41 ± 0.20 | 2.31 ± 0.26 |
| Liver/body weight ratio | 0.059 ± 0.00 | 0.056 ± 0.00 | 0.055 ± 0.00 | |
| Total caloric intake / mouse | (kcal) | 322 | 323 | 297 |
| Caloric efficiency | (g/kcal) | 0.0146 | 0.0127 | 0.0145 |
| Relative Inguinal fat mass | (%) | 16.32 ± 0.15 | 15.67 ± 0.53 | 16.31 ± 0.50 |
| Mesenteric fat pad | (%) | 5.07 ± 0.16 | 4.85 ± 0.10 | 4.78 ± 0.51 |
| Retroperitoneal fat pad | (%) | 9.60 ± 0.17 | 9.98 ± 0.24 | 9.10 ± 0.59 |
| Epidydimal fat pad | (%) | 12.96 ± 0.45 | 13.02 ± 0.33 | 12.76 ± 0.25 |
| White adipose tissue fat index | (%) | 43.96 ± 0.33 | 43.52 ± 0.56 | 42.95 ± 1.70 |
| Fasting glucose | (mg/dL) | 206 ± 10 | 200 ± 12 | 239 ± 29 |
Values given are means ± SEM; 0x/day group was used as reference.
Figure 4.
Absolute mean caloric intake per ob/ob mouse per week (A) was calculated per group on a daily base. Body weight gain (B) was calculated relative to the weight of each individual animal before initiation of the experiment, showing no difference. After 4 weeks, mean hepatic fat fraction as measured by magnetic resonance spectroscopy revealed no significant difference between groups (C). Plasma alanine aminotransferase (ALT; C), total cholesterol (D) and triglyceride (E) levels were not statistically different between groups after 4 weeks. Values represent the mean ± SEM. Statistical significance is calculated between the control animals (0x/day) and the difference between 1x/day animals and 2x/day animals (*, P<0.05).
3.2.2. Repetitive orogastric gavage of ob/ob mice on a standard diet did not affect the severity of hepatic steatosis, compared to non-gavaged animals
Liver enzymes were measured on all experimental groups to evaluate the effects of repetitive orogastric gavage on ob/ob mice (Figure 4). ALT (F(2,9)=1.12, P=0.3716), cholesterol (F(2,9)=0.77, P=0.4949) and triglyceride levels (F(2,12)=0.34, P=0.7231) were not significantly different among groups. Liver sections stained with hematoxylin and eosin and Oil Red-O were analyzed and demonstrated severe, predominantly macrovesicular steatosis throughout the parenchyma, with extensive hepatocyte ballooning but without inflammation, in all groups (data not shown). No differences were observed between control ob/ob mice and ob/ob mice that were gavaged once daily (1x/day) or twice daily (2x/day). MR spectroscopy was used to quantify the effect of orogastric gavage on hepatic fat accumulation (Figure 4). Again, no significant differences were seen among groups (F(2,9)=0.14, P=0.8689) thereby corroborating our histological findings that repetitive orogastric gavage of ob/ob mice did not affect the severity of hepatic steatosis.
4. Discussion
The prevalence of the metabolic syndrome and its associated complications, including hepatic steatosis, is reaching epidemic proportions. In addition to lifestyle modifications, pharmacologic interventions are being increasingly explored. Murine models of diet-induced obesity as well as transgenic models are widely used to investigate the potential efficacy of drugs on controlling the metabolic syndrome. However, methods of drug administration, even those that are perceived to be the least invasive, may induce stress. This can potentially introduce confounders that will affect outcomes. In this study we aimed to investigate the effect of various frequencies of orogastric gavaging on food intake, fat deposition, insulin resistance and hepatic steatosis in mice. Our results indicated that an acute stressor such as repetitive orogastric gavage influenced the development of the metabolic syndrome, in particular, hepatic steatosis, in diet-induced obese C57BL/6J mice, but not in transgenic ob/ob mice.
Orogastric gavage is a common laboratory procedure in toxicology, pharmacology and drug-development studies, used to deliver the experimental compounds. Gavaging involves the physical stress of handling and restraining the animal, followed by insertion of a rigid metal feeding needle from mouth to stomach. Orogastric gavage may lead to respiratory interference and stomach distension. Besides possible toxic effects of the agent studied, complications of orogastric gavage may include inadvertent tracheal cannulation/administration, reflux, aspiration pneumonia, esophageal perforation, hemothorax and death [7, 19]. Many of these complications are associated with restraint and incorrect placement of the feeding needle. In our study, however, orogastric gavage was routinely performed by one experienced investigator resulting in neither morbidity nor mortality.
In this study we demonstrated that orogastric gavage led to decreased food intake in the C57BL/6J mice, thereby altering the development of the metabolic syndrome. In particular, the severity of hepatic steatosis, which is increasingly recognized as the hepatic manifestation of the metabolic syndrome, was significantly reduced in gavaged C57BL/6J animals, as demonstrated by histology and confirmed by MR spectroscopy. In contrast, ob/ob mice which exhibit obesity, hyperphagia, diabetes and hepatic steatosis due to a spontaneous mutation in the Lepob gene, were not affected by daily or twice daily orogastric gavage. The difference might be due to their distorted leptin signal leading to hyperphagia, thereby maintaining their food intake level. We believe that this difference, combined with their hypometabolic and hypothermic physiology, may have counterbalanced the impact of orogastric gavage induced stress [14, 15].
Handling and restraining during repetitive orogastric gavage has been associated with an increased stress response, which may have led to the observed decreased food intake in the C57BL/6J mice [7]. In addition, although we did not study damage of the oropharynx upon necropsy, development of granulation tissue in the oropharynx following repeated gavage of rats has been reported [20]. This may indicate that repetitive orogastric gavage can lead to recurrent abrasion of the oropharynx, potentially leading to scarring and aversion to oral intake. However, the reported effects might have been strain-specific [21].
Although this study is the first of its kind to demonstrate the effects of orogastric gavage in mouse models of the metabolic syndrome, an important limitation warrants consideration. The acute stress response can be analyzed by measuring the plasma concentrations of corticosterone, the principal glucocorticoid produced by mice in the stressed state [22, 23]. Acute stress, such as restraining the animal, may also activate the corticotrophin-releasing hormone system, potentially inducing catabolism [8, 9]. In this study we did not directly measure plasma concentrations of stress related hormones. Although blood sampling to analyze hormone levels may be the most sensitive and accurate method of continual stress analysis, blood sampling is itself an invasive technique which has been shown to increase corticosterone levels [23]. An alternative method would have been the measurement of fecal corticosterone concentrations [24]; however, because the stressful event only lasted for a short time but was repeated over a longer period, the exact timing of sampling would have been difficult to assess.
In conclusion, this study demonstrates that long-term, repetitive orogastric gavage induced stress may affect food intake and, subsequently, the development of the metabolic syndrome and hepatic steatosis in diet-induced obese C57BL/6J mice, but not in leptin deficient ob/ob mice. The effects of laboratory routines such as orogastric gavage on the diet-induced obesity model should be taken into account when designing animal studies for drug development. Alternative, minimally invasive methods of drug administration, such as implantation of an mini-osmotic pump [25], may be more suitable for long-term drug administration in diet-induced obesity models by avoiding the recurrent gavage-related stress response and its associated complications.
Acknowledgements
The authors are grateful to Dr. Vânia Nosé (Brigham and Women Hospital, Boston, MA) for pathological evaluation and photography of the histology, and to M. Reza Akhavan-Sharif (Beth Israel Deaconess Medical Center, Boston, MA) for excellent technical assistance with the MR spectroscopy used in our study.
Dr. De Meijer was recipient of fellowships from the foundations Stichting Prof. Michaël-van Vloten Fonds (Venray, The Netherlands), VSBfonds (Utrecht, The Netherlands), Gerrit Jan Mulder Stichting (Rotterdam, The Netherlands), Prins Bernhard Cultuurfonds (Amsterdam, The Netherlands), and Dr. Saal van Zwanenberg Stichting (Oss, The Netherlands). Dr. Le was the recipient of the Joshua Ryan Rappaport Fellowship (Boston, MA). Dr. Puder was supported by the National Institutes of Health (grant DK069621-05) and the Children’s Hospital Surgical Foundation (Boston, MA). The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vincent E. de Meijer, Email: vincent.demeijer@childrens.harvard.edu.
Hau D. Le, Email: hau.le@childrens.harvard.edu.
Jonathan A. Meisel, Email: jmeisel@bidmc.harvard.edu.
Mark Puder, Email: mark.puder@childrens.harvard.edu.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Circulation. 2009 Oct 5; doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes care. 2009 Jun;32(6):1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001 Aug;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md. 2004 Dec;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. The New England journal of medicine. 2002 Apr 18;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2004 Nov;43(6):42–51. [PubMed] [Google Scholar]
- 8.Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biological psychiatry. 1990 May 15;27(10):1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 9.Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, et al. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proceedings of the National Academy of Sciences of the United States of America. 1999 Jul 6;96(14):8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel C, Duclos M, Cabanac M, Richard D. Chronic stress reduces body fat content in both obesity-prone and obesity-resistant strains of mice. Hormones and behavior. 2005 Aug;48(2):172–179. doi: 10.1016/j.yhbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, et al. Dynamic body weight and body composition changes in response to subordination stress. Physiology & behavior. 2007 Jul 24;91(4):440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PloS one. 2009;4(1):e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiology & behavior. 2004 Apr;81(2):243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science (New York, NY. 1995 Jul 28;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 15.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science (New York, NY. 1995 Jul 28;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 16.Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism: clinical and experimental. 1998 Sep;47(9):1089–1096. doi: 10.1016/s0026-0495(98)90283-9. [DOI] [PubMed] [Google Scholar]
- 17.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. American journal of physiology. 2008 Jan;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 18.Alwayn IP, Andersson C, Lee S, Arsenault DA, Bistrian BR, Gura KM, et al. Inhibition of matrix metalloproteinases increases PPAR-alpha and IL-6 and prevents dietary-induced hepatic steatosis and injury in a murine model. Am J Physiol Gastrointest Liver Physiol. 2006 Dec;291(6):G1011–G1019. doi: 10.1152/ajpgi.00047.2006. [DOI] [PubMed] [Google Scholar]
- 19.Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2000 Jan;39(1):17–21. [PubMed] [Google Scholar]
- 20.Germann PG, Ockert D. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a Fischer 344 rat carcinogenicity study. Laboratory animal science. 1994 Aug;44(4):338–343. [PubMed] [Google Scholar]
- 21.Germann PG, Ockert D, Heinrichs M. Pathology of the oropharyngeal cavity in six strains of rats: predisposition of Fischer 344 rats for inflammatory and degenerative changes. Toxicologic pathology. 1998 Mar-Apr;26(2):283–289. doi: 10.1177/019262339802600215. [DOI] [PubMed] [Google Scholar]
- 22.Hunt C, Hambly C. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiology & behavior. 2006 Mar 30;87(3):519–526. doi: 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Mostl E, Palme R. Hormones as indicators of stress. Domestic animal endocrinology. 2002 Jul;23(1–2):67–74. doi: 10.1016/s0739-7240(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 24.Good T, Khan MZ, Lynch JW. Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus) Physiology & behavior. 2003 Nov;80(2–3):405–411. doi: 10.1016/j.physbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Rowland RR, Reyes E, Chukwuocha R, Tokuda S. Corticosteroid and immune responses of mice following mini-osmotic pump implantation. Immunopharmacology. 1990 Nov-Dec;20(3):187–190. doi: 10.1016/0162-3109(90)90033-b. [DOI] [PubMed] [Google Scholar]