Abstract
Purpose
To develop an experimental approach for determining in vivo transverse relaxation rates (T2) of metabolites that are detected by spectral editing without using simulations, and to demonstrate this approach to measure the T2 of γ-aminobutyric acid (GABA).
Materials and Methods
The proposed method first determines the TE-dependence of the edited signals using measurements in a pure phantom solution (10 mM γ-aminobutyric acid; GABA); the phantom T2 is also determined. Once the editing echo time (TE) -modulation pattern is known, it can then be used to determine T2 in vivo. The method was applied to measure GABA T2 in the occipital lobe of five healthy adult subjects at 3T, using a J-difference editing method. Unwanted macromolecular contributions to the GABA signal were also measured.
Results
The in vivo T2 of edited GABA signal was 88 ± 12 ms; this preliminary result is somewhat shorter than other metabolite T2 values in the literature at this field strength.
Conclusion
Spectral editing methods are now widely used to detect low concentration metabolites, such as GABA, but to date no edited acquisition methods have been proposed for the measurement of transverse relaxation times (T2). The method described has been successfully applied to measuring the T2 of GABA.
Keywords: edited MR spectroscopy, transverse relaxation, T2, GABA, brain
Proton Magnetic Resonance spectroscopy (1H-MRS) is a useful technique to probe in vivo biochemical status noninvasively, which has been widely applied to study the brain in both clinical and neuroscience settings. If tissue water content is known, metabolite concentrations are often calculated from the relative intensities of metabolite and water signals (1), making appropriate corrections for longitudinal and transverse relaxation (characterized by the time constants T1 and T2, respectively). Thus, the measurement of metabolite relaxation parameters is an important prerequisite for absolute quantification of in vivo MR spectra.
In vivo transverse relaxation times (T2) are usually measured by making localized single-voxel measurements at a range of echo times (TE). For singlet resonances, such as N-acetyl aspartate (NAA), creatine (Cr) and choline (Cho), the TE-dependent decay in signal intensity can generally be modeled by a single exponential with time constant T2; metabolite T2s have been measured in this way at a range of field strengths (2–7). The TE-dependence of coupled spin systems is more complicated, because their signals are modulated by both T2 relaxation and scalar coupling evolution. The in vivo T2 of the coupled metabolite glutamate has been measured by modeling the TE dependence of the Glutamate signal (8). However, for many coupled metabolites (such as γ-aminobutyric acid [GABA] (9), glutathione (10), ascorbate (11), and NAAG (12)), low signal amplitude and signal overlap hamper their detection in vivo using conventional MRS methods, and it is usual to use spectral editing methods to detect them reliably. To the best of our knowledge, no method has been published to date that is able to measure the in vivo T2 of metabolites detected using spectral editing techniques.
There has been considerable recent interest in MRS measurements of the inhibitory neurotransmitter, GABA, investigating the role of GABA in disease, and the relationships between GABA and functional imaging (13–15) and behavior (16–19). However, although spectral editing methods have been extensively applied to investigate the individual and group differences in GABA levels, they have not been truly quantitative, as human in vivo relaxation parameters for GABA have not been previously measured. Relative measurements of this kind, often quantified in institutional units (i.u.), hamper inferences from separately published studies, both within and between centers. The most common method applied in these studies is J-difference editing (9), often implemented through the MEGA-PRESS technique (20), detecting GABA signals at an intermediate TE (~70 ms). Because this TE is of similar length to typical metabolite T2s (and therefore the anticipated T2 of GABA), measuring the in vivo T2 of GABA is an important step in making these measurements quantitative.
It is also known that the “GABA” signal detected at 3.0 ppm using standard J-difference techniques at 3 Tesla (T) contains an appreciable contribution from macromolecules (MM) which has a coupling partner at 1.7 ppm (20). The presence of this MM resonance needs to be accounted for, both for the determination of GABA T2 and for the estimation of “pure” GABA concentrations.
In this study, we present a framework for the determination of the in vivo T2 of edited metabolites, that is entirely experimental and does not rely upon simulation, and demonstrate the method to measure the in vivo T2 of GABA at 3T.
THEORY
The following discussion deals specifically with GABA measurements (for ease of description), but the methodological framework may be generalized to other metabolites detected by edited experiments. J-difference editing of GABA relies upon acquiring two sub-experiments: the ON experiment in which frequency selective editing pulses are applied to GABA spins at 1.9 ppm; and the OFF experiment in which they are either not applied, or are applied at another frequency that does not affect any of the GABA resonances. When applied, these editing pulses refocus the TE evolution of scalar couplings between the GABA spins at 1.9 ppm and those at 3 ppm. Thus at TE of around 70 ms, whereas the GABA pseudo-triplet at 3 ppm has the outer peaks inverted in the OFF experiment due to coupling evolution, the multiplet is in-phase in the ON experiment. The difference (DIFF) between these two experiments largely discards the center peak of the pseudo-triplet, along with all other signals that are not affected by the editing pulse. This subtraction results in an edited spectrum that separates the GABA signal of interest at 3ppm from the overlying creatine signal at the same chemical shift. The SUM spectrum contains similar information to an unedited PRESS spectrum (albeit with signals directly affected by the editing pulse (such as NAA) appearing at reduced intensity.
The TE dependence of the GABA DIFF signal SDIFF(TE) can be described as:
| [1] |
defining κ(TE), a function describing the intrinsic TE-dependence of the signal (due to coupling evolution), as separate from the exponential term representing transverse relaxation exp(−TE/T2). If the GABA signal at 3 ppm is approximated by a simple triplet with coupling constant J, then κ(TE) would be expected to be sin2(π J TE). This function oscillates from zero at TE = 0 (consistent with poor editing at short TE as there is no coupling evolution to refocus) to a maximum at TE = 1/2J (the usual editing time used for GABA) and back to zero at TE = 1/J.
The TE dependence of the GABA ON signal is much simpler:
| [2] |
There is no κ(TE) term because coupling evolution is refocused by the editing pulses at all TEs, and therefore only transverse relaxation modulates the signal.
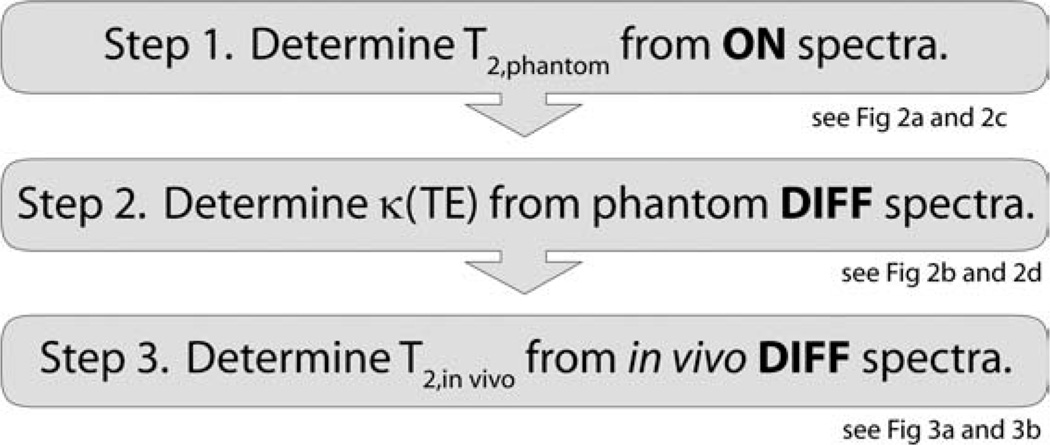
The proposed method for determining the T2 of GABA makes use of Eqs. [1] and [2] in a phantom containing a pure GABA solution. In this phantom the GABA signal can be observed directly without the need for spectral editing. From the ON spectra recorded as a function of TE, the phantom T2 can be determined from Eq. [2]. This can then be used to estimate κ(TE) using Eq. [1] from the DIFF spectra recorded as a function of TE. Once κ(TE) is known, Eq. [1] then can be applied to in vivo edited DIFF spectra to estimate GABA T2 in vivo. This process is depicted graphically in Figure 1.
Figure 1.
Workflow for determining of in vivo T2 of edited metabolites. Further details on individual components can be seen in Figures 2 and 3.
METHODS
All experiments were performed on a Philips Achieva 3T MRI scanner using the body coil for transmit and an eight-channel phased array head coil for receive.
Phantom Experimental
Measurements were made in a phantom containing 10 mM GABA. J-difference edited measurements of GABA were made at echo times (TE) of 70 to 280 ms in steps of 10 ms, and 315 ms and 350 ms using the MEGA-PRESS pulse sequence (20). The first slice-selective spin echo TE1 was fixed at 17 ms, and TE2 was changed to vary the total TE (TE1 + TE2). Other experimental parameters include: TR = 2 s; 64 transients of 2k datapoints acquired with a spectral width of 2 kHz; 15-ms editing pulse applied at 1.9 ppm (ON) and 7.46 ppm (OFF) in an interleaved manner; excited volume (3 cm)3; CHESS water suppression; B1,max 13.5 µT; slice selective refocusing bandwidth 1.3 kHz. Both ON and DIFF spectra were processed with 3 Hz exponential line broadening and zero-filling to 8k points. Signals were integrated from 2.7 ppm to 3.27 ppm after linear baseline correction. This full processing pipeline was automated in Matlab.
In Vivo Experimental
Five healthy volunteers were recruited to this study under local ethics committee approval (age, 39.9 ± 7.9 years; two male). In each subject, edited measurements were made at three TEs (70 ms, 100 ms, 180 ms), chosen as the minimum achievable value (70 ms), a second value relatively high on the first lobe of the κ-curve (see Fig. 2d), and a third value at high TE (180 ms) chosen to be slightly before the second local maximum of the κ-curve in anticipation of in vivo relaxation shifting that maximum to a lower TE (see Fig. 3b). Other experimental parameters matched phantom experiments with the exception of: 256 transients per measurement (8.5 min); excited volume (3 cm)3 in occipital lobe (as in Evans et al) (21); VAPOR water suppression (22). To separate GABA signal from co-edited macromolecular (MM) contributions to the edited signal (20), additional measurements were made at each TE, incorporating preinversion to null metabolite signals using a hyperbolic secant inversion pulse with 1.2 kHz bandwidth. The inversion time was set to 600 ms, based on an assumed value for the T1 of GABA of 1.1 s because no literature relaxation times exist for GABA at 3T. Making the further assumption that macromolecular signals are fully relaxed at this inversion time, one edited measurement (at each TE) contains signals from metabolites and macromolecules (met + MM) and the other only MM. The difference between these two scans is assumed to be GABA signal.
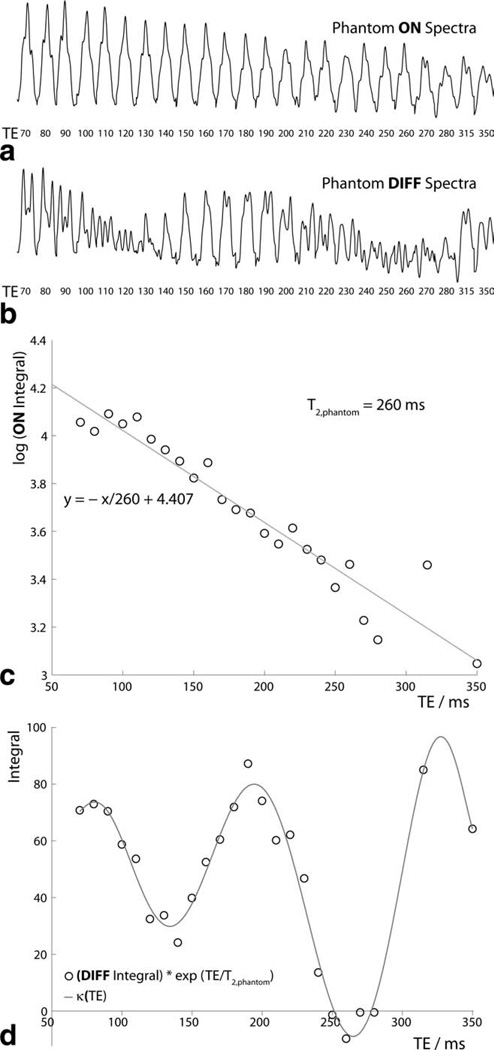
Figure 2.
Phantom experiments. a: ON spectra and (b) DIFF spectra are plotted from 2.89 ppm to 3.14 ppm for TEs from 70 ms to 350 ms. c: Plot of log of ON integrals against TE, allowing determination of T2,phantom. d: Plot of DIFF integrals (with T2-weighting removed) against TE, allowing determination of κ(TE).
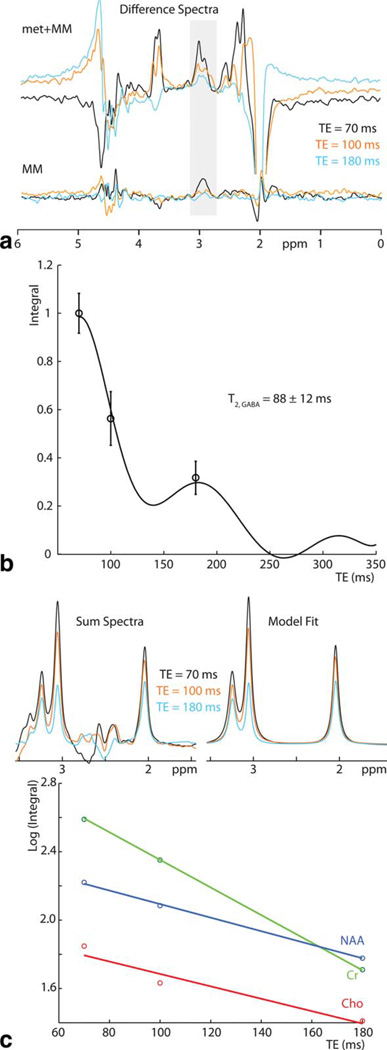
Figure 3.
In vivo experiments. a: In vivo edited spectra for one subject acquired at TEs of 70 ms, 100 ms, and 180 ms. Standard edited spectra (met+MM) are plotted above and metabolite-suppressed spectra are plotted below. b: Integrals of these data are modeled as κ(TE) exp(−TE/T2,in vivo); T2,in vivo is calculated as 137 ± 29 ms. Average integrals are plotted with error bars representing plus/minus the standard error on the mean. c: SUM spectra (after subtraction of metabolite-nulled spectra) are modeled by three Lorentzians, and a semi-log plot (below) plotted to determine T2 of NAA, Cr and Cho (being -1/slope).
DIFF and SUM spectra were processed with 5 Hz exponential line broadening. DIFF signals at 3 ppm were integrated after a baseline correction using a cubic spline. Numerical integration over a fixed frequency range (in csx3, local spectroscopy processing software available from http://godzilla.kennedykrieger.org/csx) was used for in vivo DIFF data, since it was found to give the most stable results across subjects. In vivo integral data for all subjects are fit by the model of Eq. [1], into which the phantom-measured κ(TE) is substituted to fit for T2,in vivo (the amplitude factor S0,DIFF is also included as a variable factor for fitting). A bootstrapping approach, implemented in Matlab (1000 samples), was used to estimate the uncertainty associated with the measurement. For each iteration, five values were selected from the five subjects’ data with replacement for each TE value. The same fitting analysis was run as for the original data, and the standard deviation of the 1000 iterations is reported as the error on the fitted T2 value.
The same process was applied to the raw MM measurements to estimate T2 of the macromolecular contribution—using the κ-function for GABA is not ideal, but appropriate for the MM signal which is known to have triplet character (23). After subtraction of metabolite-nulled SUM spectra, SUM spectra for each volunteer were modeled with three Lorentzians (fitting range 1.45 ppm to 3.54 ppm) to calculate transverse relaxation parameters for N-acetyl aspartate (NAA), creatine (Cr) and choline (Cho).
RESULTS
Phantom Experiments
Figure 2a,b shows the ON and DIFF phantom spectra at each echo time, plotted from 2.89 ppm to 3.14 ppm. The ON spectra demonstrate quite consistent multiplet form (the refocused pseudo-triplet), whereas the DIFF spectra show significant modulation as a function of TE, as expected. Figure 2c shows a plot of log of the ON integrals against TE, which is well fit by a linear model, suggesting that the integrals show monoexponential behavior as in Equation 2. The slope of this line of best fit is −1/T2,phantom; T2,phantom is thus measured as 260 ms. Figure 2d shows the plot of the DIFF integrals against TE. The function κ(TE) shown as a solid line is derived as an empirical fit to the integral data multiplied by exp(+TE/T2,phantom) to remove the effects of T2 relaxation. Fitting the κ(TE) curve to the phantom DIFF integrals for the TE values acquired in vivo (70 ms, 100 ms and 180 ms) resulted as a check on the process resulted in a phantom T2 value of 276 ms. The discrepancy (from the 260 ms value calculated from ON spectra) is caused by the small differences between the κ-model and the DIFF data values at those echo times that can be seen in Figure 2d.
In Vivo Experiments
Figure 3a shows typical in vivo spectra acquired for one volunteer, separating the standard edited spectra, marked met + MM, and the metabolite-suppressed MM scans. Two edited spectra (met + MM and MM) were acquired at all echo times in all subjects. Figure 3b shows mean data across all subjects overlaid with the model of best fit. For GABA, T2,in vivo is calculated as 88 ± 12 ms. The MM T2 is estimated to be 40 ± 7 ms.
Transverse relaxation parameters for NAA, Cr, and Cho from the SUM spectra are measured as: 198 ± 36 ms (NAA); 116 ± 9 ms (Cr); and 248 ± 60 ms (Cho), which are within error of gray matter values reported in the literature (7).
DISCUSSION
A framework has been proposed for the in vivo measurement of T2 for metabolites for which editing is required, and demonstrated for the inhibitory neurotransmitter GABA. The key step is the realization that the editing ON spectra can be described by simple exponential TE-dependence to measure T2,phantom and allow the function κ(TE) to be characterized (separate from T2 relaxation) from the phantom DIFF data. It is anticipated that this approach will be applicable to measure in vivo T2s for other metabolites for which edited detection is required. Although in principle κ(TE) can be simulated from the molecule’s chemical shifts and couplings (see below), the advantage of this experimental approach is that extremely accurate knowledge of the spin system in question is not required, and the effect of pulse sequence imperfections do not need to be modeled.
The measured in vivo GABA T2 of 88 ms is significantly shorter than other 3T in vivo metabolite T2s, which are typically measured as between 150 ms and 300 ms7. The 3T T2 of a similar coupled CH2 group in glutamate has been measured as 200 ms by Choi et al (8). This difference likely reflects the different cellular environments populated by GABA and glutamate, rather than a molecular difference. The transverse relaxation parameters calculated for NAA, Cr, and Cho are within error of those reported by Zaaraoui et al (7). This is important because the gradient timing of MEGA-PRESS (with gradient pairs separated to include editing pulses) could introduce some degree of diffusion weighting to the higher TE edited spectra; agreement with previously measured values signals that this is not a significant effect. Measurement of the NAA T2 from SUM spectra is not optimal, because the signal appears at half intensity (due to its suppression in the ON spectra), but this reduced signal does decay exponentially and can still be used for the purposes of this check.
The edited signal at 3.0 ppm contains significant contributions from so-called macromolecular species (which are coupled to spins at 1.7 ppm—within the range of the editing pulse) for this reason, a preinversion approach has been applied to subtract out MM contributions. This approach can only perfectly suppress signals that have a single T1-value and imperfect suppression of NAA signal can be seen in the MM-only spectrum of Figure 3a. However, despite the limitations of the method, it would not be possible to measure the T2 of GABA without accounting for the MM signal. Only small errors in NAA, Cr, and Cho T2 measurements arise from underlying MM signals as these signals are much larger relative to the MM baseline observed at typical minimum TEs. Measured T2 values for GABA would tend to be reduced by incomplete suppression of MM contamination, which might occur due to incomplete recovery of MM signal at the inversion time of 600 ms. T2 of GABA would be expected to depend upon the local environment of the GABA molecules, and one important unresolved question is the compartmentalization of the GABA detected by MRS, i.e., the weighting of intracellular, vesicular, synaptic extracellular, and extrasynaptic extracellular compartments. It should be noted that the T2 estimated for the MM signals (40 ms) is within error of the value measured by Behar et al at 2.1T (44 ± 4 ms (23)). Future studies may shed light on the different T1 and T2 pools, further separating macromolecular signal from GABA—by a combination of preinversion and symmetry-based editing suppression of MM signal at field strengths higher than 3T (24).
One limitation of this approach to measuring the T2 of edited GABA signal is the reliance on a small number and limited range of in vivo TEs. The range is restricted at the lower end by the minimum achievable TE for J-edited PRESS experiments, and at the upper end by T2 relaxation itself. For a total scanning session of approximately 1 hour, it was only possible to acquire six 8.5-minute measurements per session so that only three TEs were studied (each with and without metabolite suppression). In effect the necessary step of addressing MM contributions prevented a further three TEs from being sampled. However, it is relatively common in quantitative T2 imaging to use two-point measures of relaxation parameters, and three TE points is an acceptable compromise for this measurement. The high minimum TE (TEmin) is also not solely a restriction for these edited measurements—all in vivo metabolite T2 measurements are limited to some extent by a longer-than-preferable TEmin. PRESS-based measurements typically use a TEmin of ~30 ms, which while shorter than that used here, is still significantly longer than the ideal of TEmin = 0 ms. Therefore, it should be stressed that such measurements characterize a T2 value for those signals that are detected by localized measurements, and do not capture any very short-T2 pools that may exist. When time constraints limit the number of different TE values that can be acquired in vivo, the TE values should cover a range similar to the anticipate TE value, and also be chosen at TE values where κ(TE) is large (i.e., TE values where editing is efficient). The number of subjects in this study (five) is also relatively low for a study generating normative relaxation values, however the primary aim of this study is to demonstrate a new workflow for edited T2 measurements. It may also be useful to measure the T2 of GABA in white matter in addition to the gray matter-rich region of these studies, although given the reduced concentration of GABA in white matter, this would require longer experiments.
Another limitation of the current approach is the assumption that the ON spectra in the phantom exhibit no modulation due to scalar couplings. This will be the case in simple, weakly coupled spin systems (for instance of the type “AX”) where application of editing pulses selectively on “X” will fully remove any modulation of “A” in a spin-echo experiment. However, for more complex strongly coupled spin systems found in the brain (such as the aspartyl ABX spin system in NAA and N-acetyl aspartyl glutamate (12)), selective irradiation of one resonance may not remove all modulation from its coupling partners. In this case, the effect of these residual couplings may result in an underestimation of the true phantom T2, which in turn would propagate into an error in the in vivo T2.
Finally, as mentioned above, an alternative approach to the procedure described here would be to calculate κ(TE) using numerical simulations, e.g., Choi et al (8). This approach is expected to remove any uncertainties of the phantom T2 from the in vivo determination. Simulated LC Model basis sets have been used to model unedited spectra at 9.4T in rat brain (25), but this approach is not transferable to metabolites for which editing is necessary at 3T. Simulated approaches rely on the simulations accurately matching the experimental conditions, including any experimental effects on the modulation patterns due to RF pulse or gradient waveform imperfections, or chemical shift displacement effects (26), and on an accurate knowledge of the metabolite coupling values and chemical shifts. In this regard, it should be noted that two different sets of J-values of GABA have been published (27,28), and our own simulations (not presented here) based of either set of values results do not agree well with the experimentally determined κ(TE) curve. In contrast, the experimental approach proposed here uses the same conditions in the phantom and in vivo, so that the κ(TE) is expected to be very similar in both scans.
In conclusion, a practical method is described for measuring transverse relaxation times for coupled spin-systems detected in vivo by spectral editing. The method is demonstrated for GABA using the MEGA-PRESS editing experiment, but is expected to generalize for other molecules and other types of spectral- editing experiments. The current study is limited by a relatively small number of subjects, limited number of TE values used in vivo, and only one brain region covered. Future larger studies are required to definitively measure regional GABA T2 values in the brain at 3T. Knowledge of the GABA T2 is an important step in quantifying absolute GABA concentrations for in vivo spectral editing experiments.
ACKNOWLEDGMENTS
The authors thank Dr. C. John Evans for useful discussions.
REFERENCES
- 1.Soher BJ, Hurd RE, Sailasuta N, Barker PB. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magn Reson Med. 1996;36:335–339. doi: 10.1002/mrm.1910360302. [DOI] [PubMed] [Google Scholar]
- 2.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45:765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 3.Mlynarik V, Gruber S, Moser E. Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14:325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- 4.Posse S, Cuenod CA, Risinger R, Le Bihan D, Balaban RS. Anomalous transverse relaxation in 1H spectroscopy in human brain at 4 Tesla. Magn Reson Med. 1995;33:246–252. doi: 10.1002/mrm.1910330215. [DOI] [PubMed] [Google Scholar]
- 5.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 6.Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 7.Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human brain-structure resolved T(2) relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57:983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]
- 8.Choi C, Coupland NJ, Bhardwaj PP, et al. T2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56:971–977. doi: 10.1002/mrm.21055. [DOI] [PubMed] [Google Scholar]
- 9.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trabesinger AH, Weber OM, Duc CO, Boesiger P. Detection of glutathione in the human brain in vivo by means of double quantum coherence filtering. Magn Reson Med. 1999;42:283–289. doi: 10.1002/(sici)1522-2594(199908)42:2<283::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Terpstra M, Gruetter R. 1H NMR detection of vitamin C in human brain in vivo. Magn Reson Med. 2004;51:225–229. doi: 10.1002/mrm.10715. [DOI] [PubMed] [Google Scholar]
- 12.Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 15.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 20.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 22.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 24.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45:517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Xin L, Gambarota G, Mlynarik V, Gruetter R. Proton T2 relaxation time of J-coupled cerebral metabolites in rat brain at 9.4 T. NMR Biomed. 2008;21:396–401. doi: 10.1002/nbm.1205. [DOI] [PubMed] [Google Scholar]
- 26.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 27.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]